Submitted:
06 November 2024
Posted:
06 November 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Patient and Methods
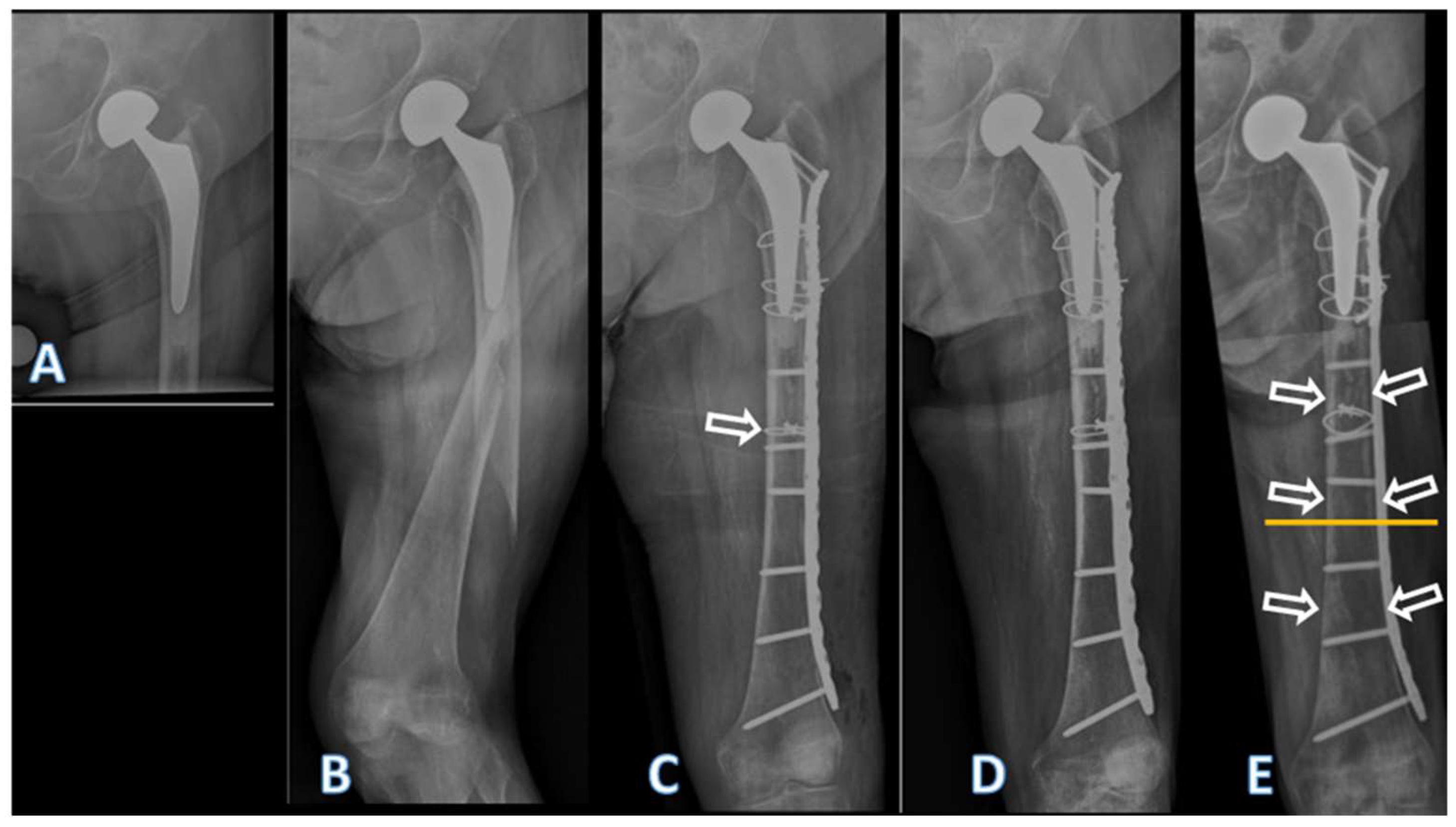
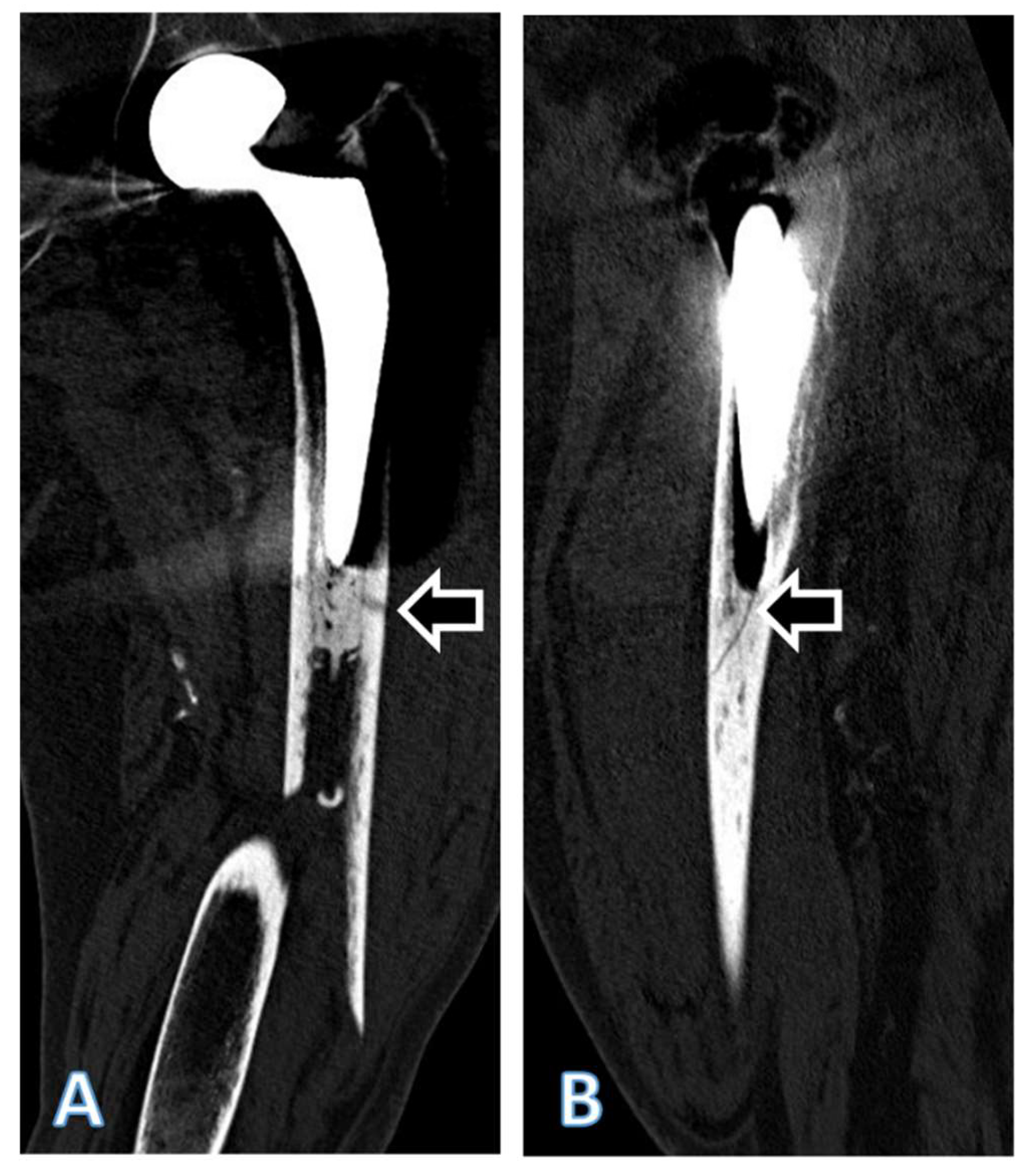
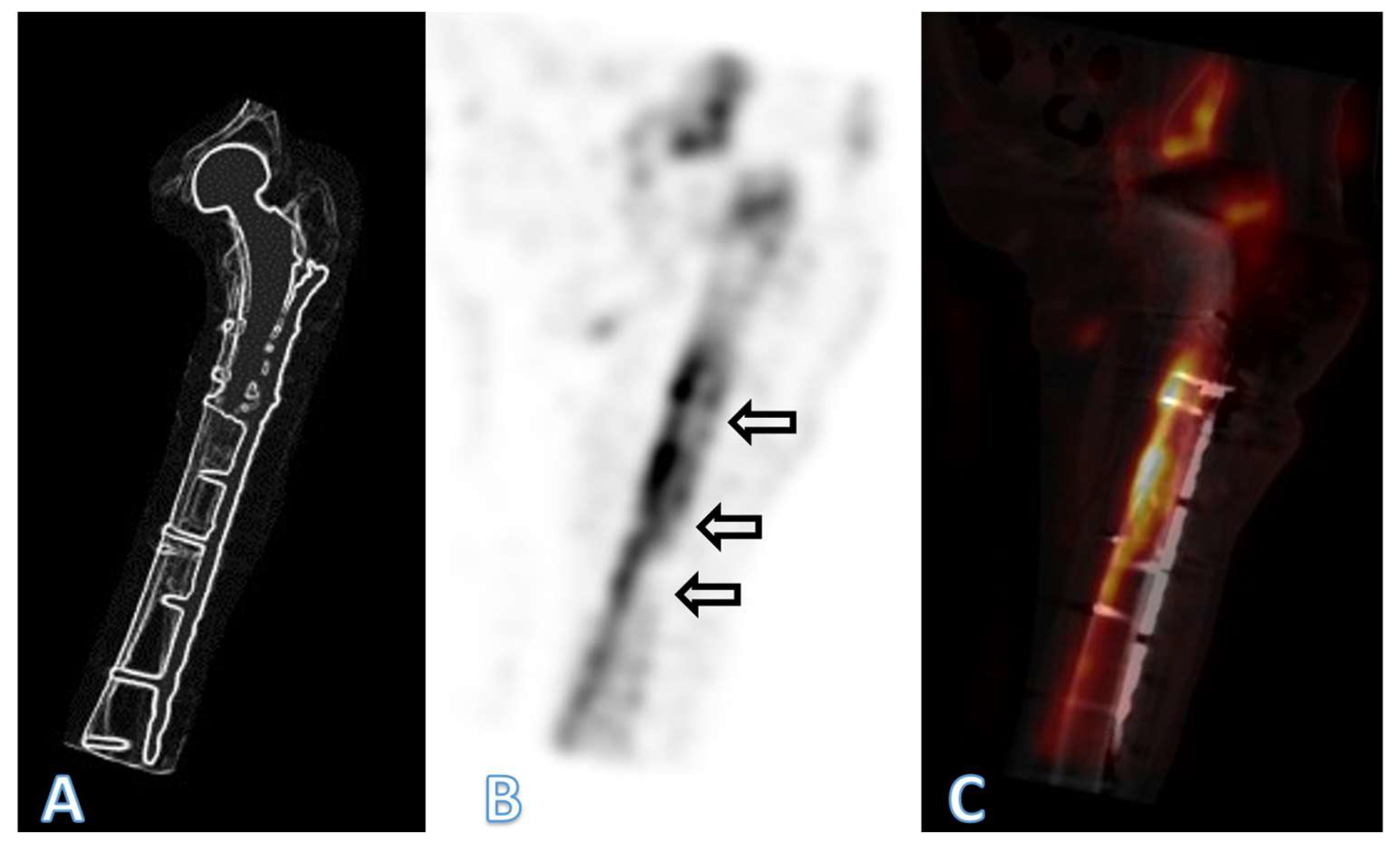
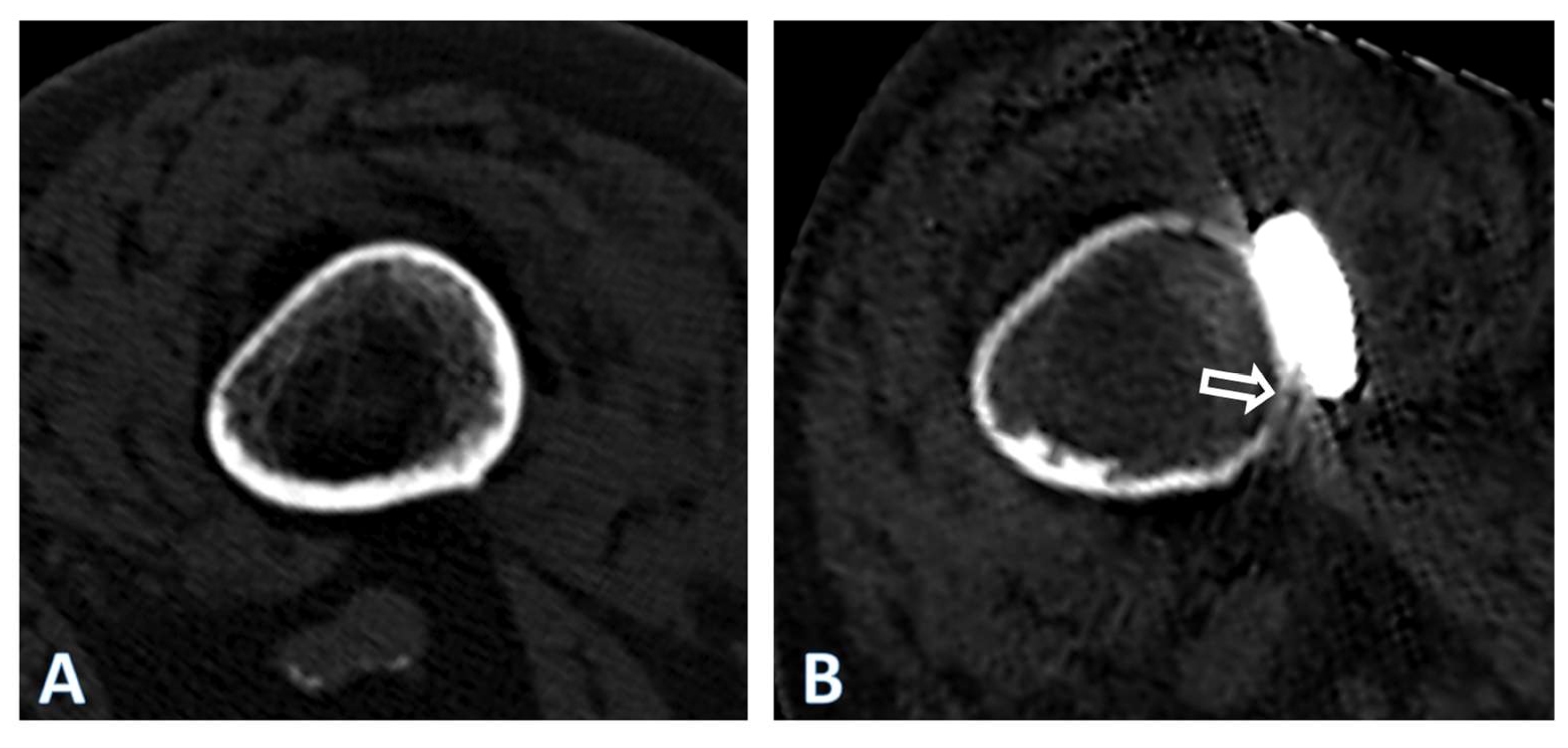
3. Results
4. Discussion
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdel, M.; Watts, C.; Houdek, M.; Lewallen, D.; Berry, D. Epidemiology of periprosthetic fracture of the femur in 32 644 primary total hip arthroplasties: a 40-year experience. The bone & joint journal 2016, 98, 461-467.
- Stoffel, K.; Horn, T.; Zagra, L.; Mueller, M.; Perka, C.; Eckardt, H. Periprosthetic fractures of the proximal femur: beyond the Vancouver classification. EFORT open reviews 2020, 5, 449-456.
- SIRIS. Swiss National Hip&Knee Joint Registry Annual Report 2023; 2023.
- Moloney, G.B.; Westrick, E.R.; Siska, P.A.; Tarkin, I.S. Treatment of periprosthetic femur fractures around a well-fixed hip arthroplasty implant: span the whole bone. Archives of orthopaedic and trauma surgery 2014, 134, 9-14.
- Xue, H.; Tu, Y.; Cai, M.; Yang, A. Locking compression plate and cerclage band for type B1 periprosthetic femoral fractures: preliminary results at average 30-month follow-up. The Journal of Arthroplasty 2011, 26, 467-471. e461.
- Buttaro, M.; Farfalli, G.; Núñez, M.P.; Comba, F.; Piccaluga, F. Locking compression plate fixation of Vancouver type-B1 periprosthetic femoral fractures. JBJS 2007, 89, 1964-1969.
- Ehlinger, M.; Adam, P.; Moser, T.; Delpin, D.; Bonnomet, F. Type C periprosthetic fractures treated with locking plate fixation with a mean follow up of 2.5 years. Orthopaedics & Traumatology: Surgery & Research 2010, 96, 44-48.
- Froberg, L.; Troelsen, A.; Brix, M. Periprosthetic Vancouver type B1 and C fractures treated by locking-plate osteosynthesis: fracture union and reoperations in 60 consecutive fractures. Acta orthopaedica 2012, 83, 648-652.
- González-Martín, D.; Pais-Brito, J.L.; González-Casamayor, S.; Guerra-Ferraz, A.; Ojeda-Jiménez, J.; Herrera-Pérez, M. Treatment algorithm in Vancouver B2 periprosthetic hip fractures: osteosynthesis vs revision arthroplasty. EFORT Open Reviews 2022, 7, 533.
- Graham, S.M.; Moazen, M.; Leonidou, A.; Tsiridis, E. Locking plate fixation for Vancouver B1 periprosthetic femoral fractures: a critical analysis of 135 cases. Journal of Orthopaedic Science 2013, 18, 426-436.
- Perren, S.M. Evolution of the internal fixation of long bone fractures: the scientific basis of biological internal fixation: choosing a new balance between stability and biology. The Journal of bone and joint surgery. British volume 2002, 84, 1093-1110.
- Jacobs, R.; Rahn, B.; Perren, S. Effects of plates on cortical bone perfusion. The Journal of trauma 1981, 21, 91-95.
- Field, J.R.; Hearn, T.C.; Caldwell, C.B. Bone plate fixation: an evaluation of interface contact area and force of the dynamic compression plate (DCP) and the limited contact-dynamic compression plate (LC-DCP) applied to cadaveric bone. Journal of orthopaedic trauma 1997, 11, 368-373.
- Gautier, E.; Rahn, B.; Perren, S. Vascular remodelling. Injury 1995, 26, B11-B19.
- Perren, S.M.; Cordey, J.; Rahn, B.A.; Gautier, E.; Schneider, E. Early temporary porosis of bone induced by internal fixation implants. A reaction to necrosis, not to stress protection? Clin Orthop Relat Res 1988, 139-151.
- Bottlang, M.; Doornink, J.; Lujan, T.J.; Fitzpatrick, D.C.; Marsh, J.L.; Augat, P.; von Rechenberg, B.; Lesser, M.; Madey, S.M. Effects of construct stiffness on healing of fractures stabilized with locking plates. JBJS 2010, 92, 12-22.
- Duncan, C.; Haddad, F. The Unified Classification System (UCS): improving our understanding of periprosthetic fractures. The bone & joint journal 2014, 96, 713-716.
- ASTM. ASTM Volume 13.01: Medical And Surgical Materials And Devices (I): E667–F2477 2023.
- Gonzalez-Martin, D.; Hernández-Castillejo, L.E.; Herrera-Pérez, M.; Pais-Brito, J.L.; Gonzalez-Casamayor, S.; Garrido-Miguel, M. Osteosynthesis versus revision arthroplasty in Vancouver B2 periprosthetic hip fractures: a systematic review and meta-analysis. European Journal of Trauma and Emergency Surgery 2022, 1-20.
- Patsiogiannis, N.; Kanakaris, N.K.; Giannoudis, P.V. Periprosthetic hip fractures: an update into their management and clinical outcomes. EFORT Open Reviews 2021, 6, 75.
- Mondanelli, N.; Troiano, E.; Facchini, A.; Ghezzi, R.; Di Meglio, M.; Nuvoli, N.; Peri, G.; Aiuto, P.; Colasanti, G.B.; Giannotti, S. Treatment algorithm of periprosthetic femoral fracturens. Geriatric Orthopaedic Surgery & Rehabilitation 2022, 13, 21514593221097608.
- Niikura, T.; Lee, S.Y.; Sakai, Y.; Nishida, K.; Kuroda, R.; Kurosaka, M. Treatment results of a periprosthetic femoral fracture case series: treatment method for Vancouver type b2 fractures can be customized. Clinics in Orthopedic Surgery 2014, 6, 138-145.
- Spina, M.; Scalvi, A. Vancouver B2 periprosthetic femoral fractures: a comparative study of stem revision versus internal fixation with plate. European Journal of Orthopaedic Surgery & Traumatology 2018, 28, 1133-1142.
- Stoffel, K.; Blauth, M.; Joeris, A.; Blumenthal, A.; Rometsch, E. Fracture fixation versus revision arthroplasty in Vancouver type B2 and B3 periprosthetic femoral fractures: a systematic review. Archives of Orthopaedic and Trauma Surgery 2020, 140, 1381-1394.
- Verdonschot, N. Philosophies of stem designs in cemented total hip replacement. Orthopedics 2005, 28, S833-S840.
- Scheerlinck, T.; Casteleyn, P.-P. The design features of cemented femoral hip implants. The Journal of Bone and Joint Surgery. British volume 2006, 88, 1409-1418.
- Beel, W.; Klaeser, B.; Kalberer, F.; Meier, C.; Wahl, P. The Effect of a Distal Centralizer on Cemented Femoral Stems in Arthroplasty Shown on Radiographs and SPECT/CT: A Case Report. JBJS Case Connector 2021, 11, e20.
- Rhinelander, F.W. The normal microcirculation of diaphyseal cortex and its response to fracture. JBJS 1968, 50, 784-800.
- Gautier, E.; Perren, S. Limited Contact Dynamic Compression Plate (LC-DCP)--biomechanical research as basis to new plate design. Der Orthopade 1992, 21, 11-23.
- Gautier, E.; Sommer, C. Guidelines for the clinical application of the LCP. Injury 2003, 34, 63-76.
- Bottlang, M.; Doornink, J.; Byrd, G.D.; Fitzpatrick, D.C.; Madey, S.M. A nonlocking end screw can decrease fracture risk caused by locked plating in the osteoporotic diaphysis. JBJS 2009, 91, 620-627.
- Perren, S.; Mane, K.; Pohler, O.; Predieri, M.; Steinemann, S.; Gautier, E. The limited contact dynamic compression plate (LC-DCP). Archives of orthopaedic and trauma surgery 1990, 109, 304-310.
- Gautier, E.; Perren, S.; Cordey, J. Strain distribution in plated and unplated sheep tibia an in vivo experiment. Injury 2000, 31, 37-93.
- Gautier, E.; Perren, S.; Cordey, J. Effect of plate position relative to bending direction on the rigidity of a plate osteosynthesis. A theoretical analysis. Injury 2000, 31, 14-92.
- Disegi, J. Implant Materials. Titanium - 6% Aluminum - 7% Niobium. 2008, Second Edition.
- Zimmer. Periprosthetic Bone Plate. 7 juni 2010 2011.
- Niinomi, M. Mechanical properties of biomedical titanium alloys. Materials Science and Engineering: A 1998, 243, 231-236.
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants–a review. Progress in materials science 2009, 54, 397-425.
- Dickenson, R.; Hutton, W. The mechanical properties of bone in osteoporosis. The Journal of Bone & Joint Surgery British Volume 1981, 63, 233-238.
- Rodriguez, E.K.; Zurakowski, D.; Herder, L.; Hall, A.; Walley, K.C.; Weaver, M.J.; Appleton, P.T.; Vrahas, M. Mechanical construct characteristics predisposing to non-union after locked lateral plating of distal femur fractures. Journal of Orthopaedic Trauma 2016, 30, 403-408.
- Moreta, J.; Aguirre, U.; de Ugarte, O.S.; Jáuregui, I.; Martínez-De Los Mozos, J.L. Functional and radiological outcome of periprosthetic femoral fractures after hip arthroplasty. Injury 2015, 46, 292-298.
- Eickhoff, A.M.; Cintean, R.; Fiedler, C.; Gebhard, F.; Schütze, K.; Richter, P.H. Analysis of partial weight bearing after surgical treatment in patients with injuries of the lower extremity. Archives of orthopaedic and trauma surgery 2022, 142, 77-81.
- Vasarhelyi, A.; Baumert, T.; Fritsch, C.; Hopfenmüller, W.; Gradl, G.; Mittlmeier, T. Partial weight bearing after surgery for fractures of the lower extremity–is it achievable? Gait & posture 2006, 23, 99-105.
- Bentler, S.E.; Liu, L.; Obrizan, M.; Cook, E.A.; Wright, K.B.; Geweke, J.F.; Chrischilles, E.A.; Pavlik, C.E.; Wallace, R.B.; Ohsfeldt, R.L. The aftermath of hip fracture: discharge placement, functional status change, and mortality. American journal of epidemiology 2009, 170, 1290-1299.
- Mobily, P.R.; Kelley, L.S. Iatrogenesis in the elderly: factors of immobility. 1991, 17, 5-9.
- Osnes, E.; Lofthus, C.; Meyer, H.; Falch, J.; Nordsletten, L.; Cappelen, I.; Kristiansen, I.S. Consequences of hip fracture on activities of daily life and residential needs. Osteoporosis international 2004, 15, 567-574.
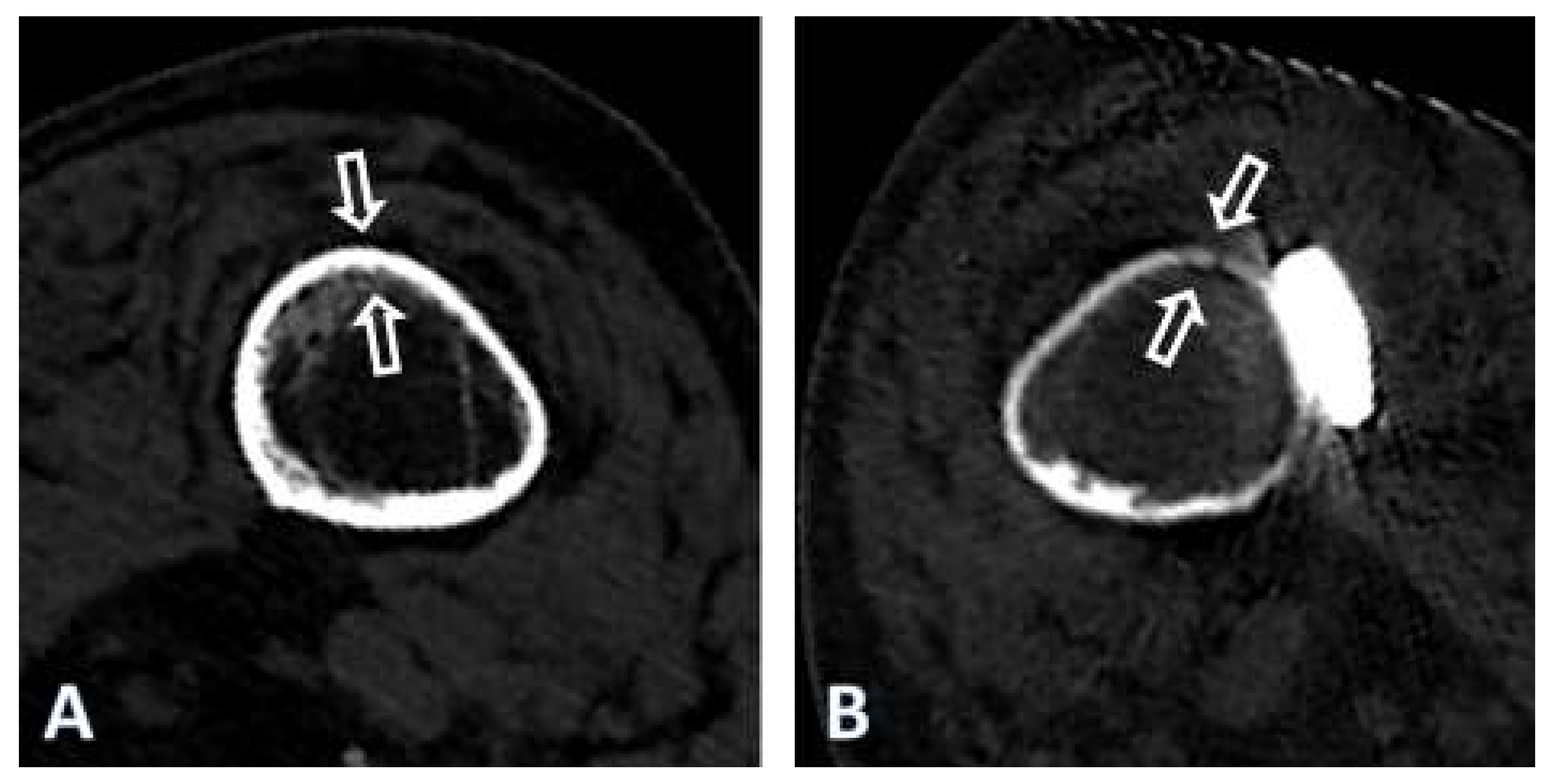
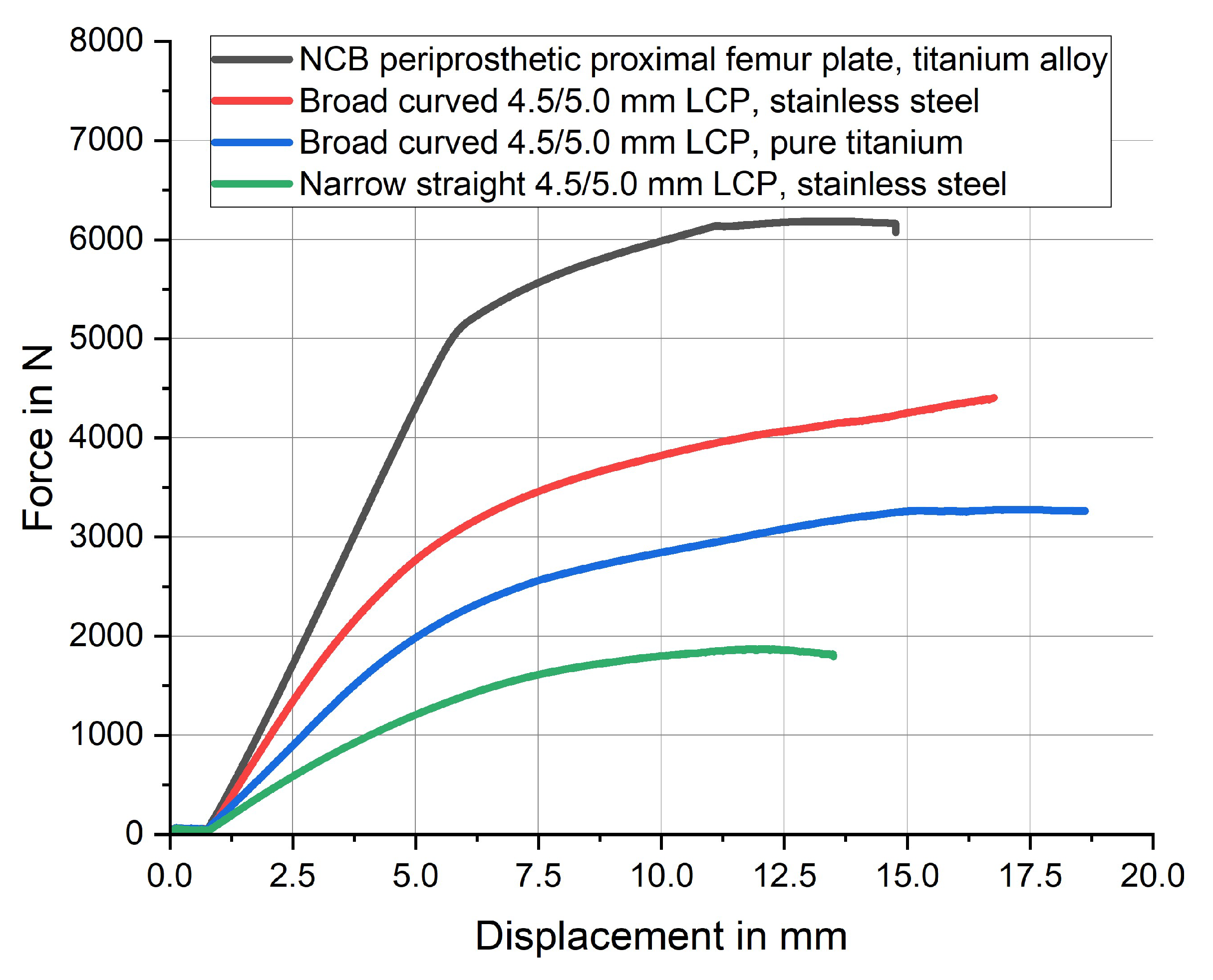
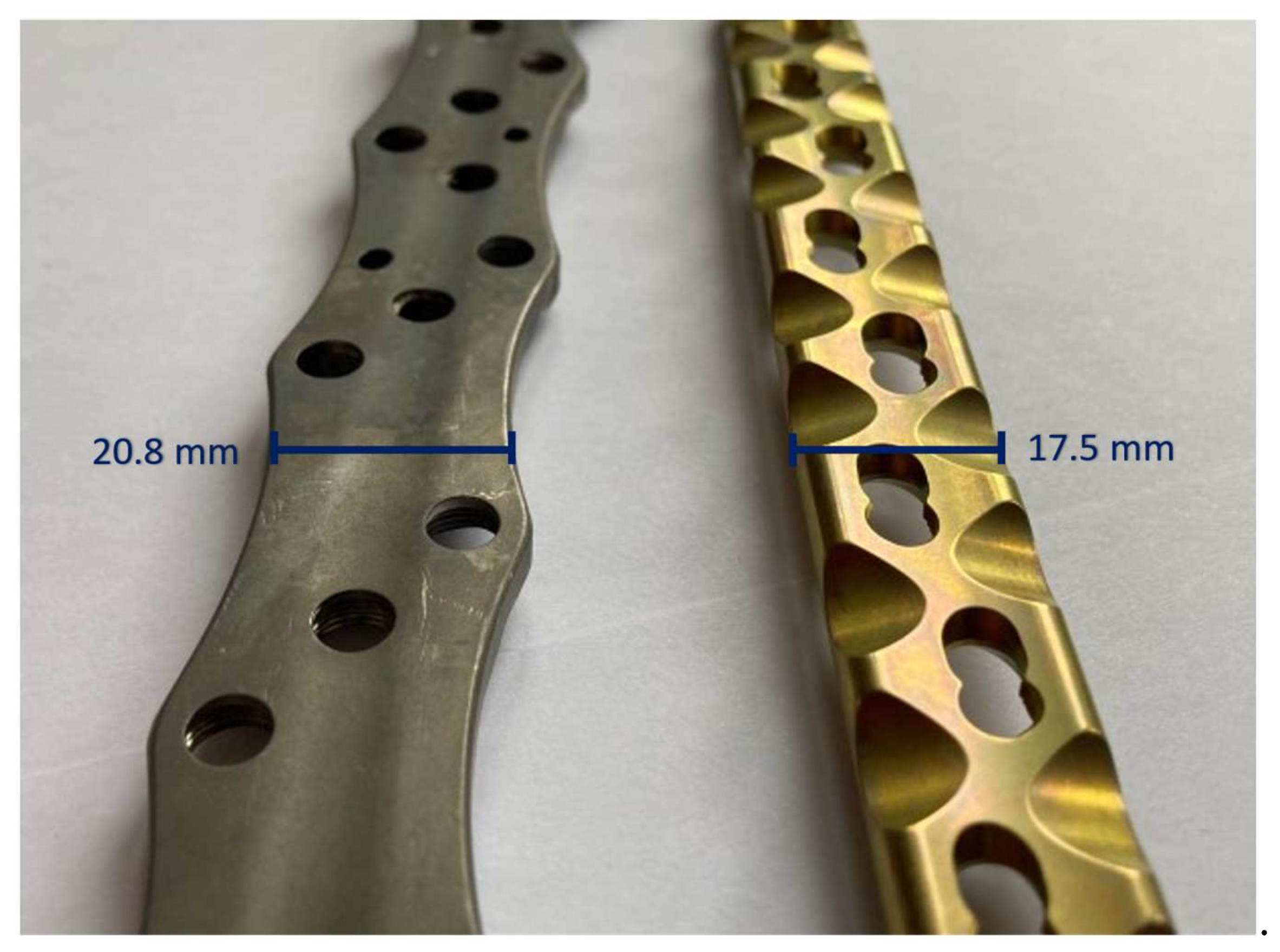
| Plate type | Material | Bending stiffness | Fmax |
|---|---|---|---|
| NCB periprosthetic proximal femur plate | titanium-aluminum-vanadium alloy | 903.1 [N/mm2] | 6185 [N] |
| 4.5/5.0 mm broad curved LCP | stainless steel | 786.7 [N/mm2] | 4407 [N] |
| 4.5/5.0 mm broad curved LCP | pure titanium | 481.5 [N/mm2] | 3279 [N] |
| 4.5/5.0 mm narrow straight LCP | stainless steel | 327.8 [N/mm2] | 1871 [N] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





