1. Introduction
Vitamin D has a regulatory effect on the immune system [
1,
2], including innate and adaptive immunity in COVID-19 [
3,
4]. Low vitamin D levels are associated with the morbidity and severity of COVID-19 [
5,
6]; however, few studies have examined the association between blood vitamin D levels and COVID-19 vaccine immunogenicity. Saroha et al. (2024) analyzed the mRNA dynamics of peripheral blood mononuclear cells (PBMCs) and suggested that vitamin D supplementation improves the efficacy of the viral vector vaccine ChAdOx1 nCoV-19 by enhancing T-cell memory responses [
7].
In contrast, Lavell et al. reported no association between baseline precursor vitamin D concentrations and humoral or cellular immune responses to mRNA vaccines [
8].
The precursor vitamin D, 25-hydroxyvitamin D, reflects the total amount of vitamin D [
9], and its blood concentration shows high variability, most likely due to the amount of ultraviolet radiation exposure; the precursor vitamin D levels in summer are more than twice those in winter [
10]. This high variability suggests the limitations of studies focusing on precursor vitamin D as the concentrations of precursor and active forms of vitamin D do not correlate [
2]. The active form, 1, 25-(OH)
2 vitamin D, is produced via hydroxylation of 25(OH) vitamin D by 1α-hydroxylase (CYP27B1) in the kidneys [
9], and parathyroid hormone keeps its blood levels constant at a range of 120–140 pmol/L (50–58 pg/mL) [
10,
11]. Active vitamin D binds to vitamin D receptors expressed in immune cells, migrates into the nucleus, and binds directly to genomic DNA, thereby regulating the expression of genes involved in the immune system [
12], including monocytes [
13], macrophages [
14], dendritic cells [
15], and T and B lymphocytes [
16,
17].
Studies on the relationship between active vitamin D and COVID-19 immunity are lacking. To address this gap, we examined the association between baseline blood active vitamin D levels and the humoral and cellular immunogenicity of COVID-19 mRNA vaccines in the general Japanese population.
2. Materials and Methods
This study was approved by the Ethics Committee for Clinical Research at the School of Medicine, Saga University, Saga, Japan (No. R2-44 and R3-9). All the participants provided written informed consent before undergoing any study procedure.
2.1. Study Design
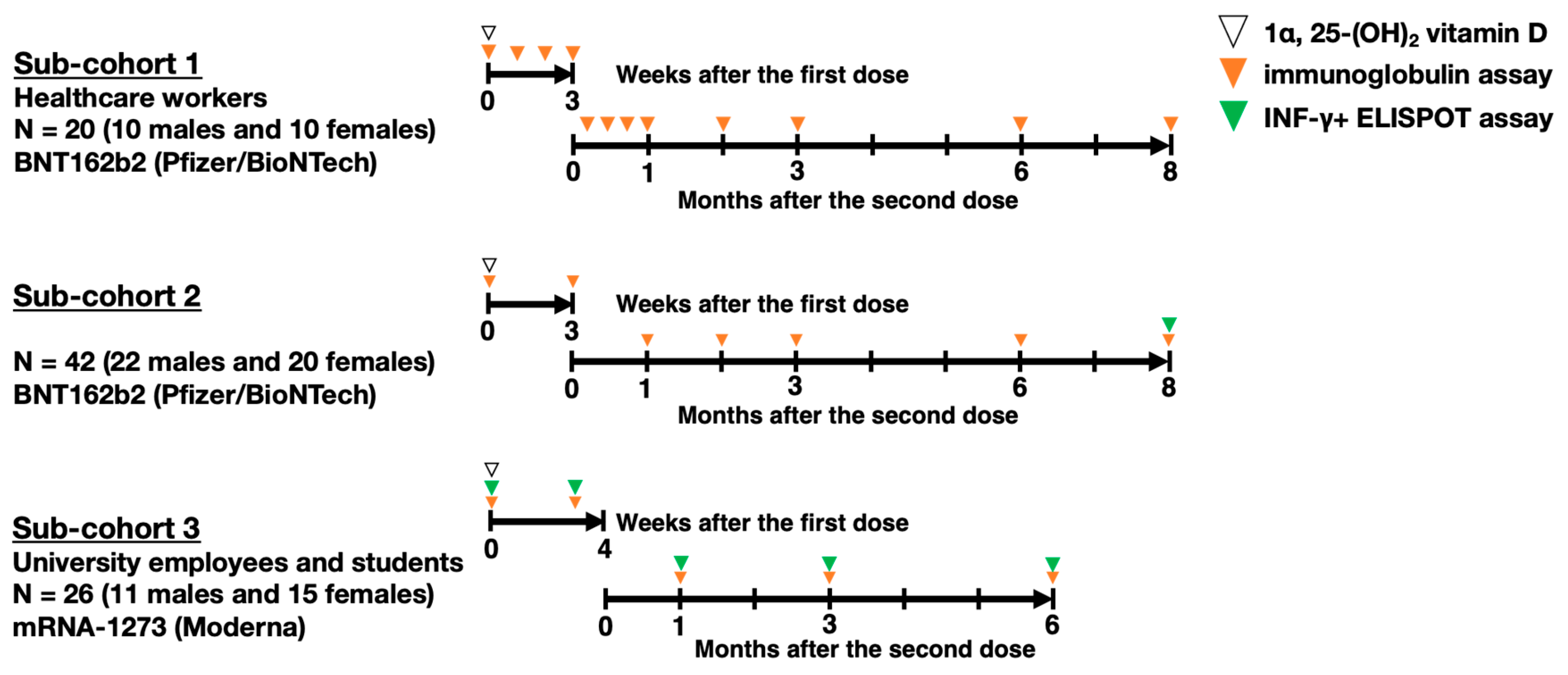
The study group included 88 participants (43 males and 45 females) from hospitals and a university in Saga Prefecture who voluntarily received two doses of a COVID-19 mRNA vaccine. As shown in
Figure 1, 62 participants in sub-cohorts 1 and 2 (20 healthcare workers: 10 males, 10 females; and 42 students: 22 males, 20 females) received two doses of BNT162b2 (Pfizer Inc., New York, NY, USA/BioNTech SE, Mainz, Germany) (30 µg). Sub-cohort 3, comprising 26 university employees and students (11 males, 15 females), received two doses of mRNA-1273 (Moderna Inc., Cambridge, MA, USA/Takeda Pharmaceutical Co., Ltd., Tokyo, Japan) (100 µg). The first dose was administered in April and May 2021 for sub-cohorts 1 and 2, and in August for sub-cohort 3, with the second dose given 21 days after the first for BNT162b2 and 28 days after for mRNA-1273. None of the participants had a history of COVID-19 at baseline or were pregnant. Data were excluded if SARS-CoV-2 infection was suspected during follow-up based on specific antibody and lymphocyte levels.
Blood samples were collected from the participants at multiple time points to monitor their immune responses to the COVID-19 mRNA vaccine. Initial samples were collected prior to the administration of the first vaccine dose to establish baseline measurements. Subsequent samples were collected, as shown in
Figure 1, briefly, at weekly intervals for 3 weeks following the first dose in sub-cohort 1 or only 3 weeks later in sub-cohorts 2 and 3. After the second dose, blood samples were collected at weeks 1, 2, and 3 only in sub-cohort 1 and at 1 3-month intervals up to 8 months following the second dose in all sub-cohorts.
2.2. Serological Tests
Serum was extracted on the drawing day and stored at −80 ℃ until analysis. The active form of vitamin D, i.e., 1α, 25-(OH)
2 vitamin D, was quantified in a radioimmunoassay. A high-sensitivity chemiluminescent enzyme immunoassay platform (Sysmex Co., Kobe, Japan) was used to measure anti-SARS-CoV-2 antibodies, the S1 subunit of the anti-spike protein (S1 subunit) IgG, anti-S1 IgM, and anti-nucleocapsid protein (N) IgG to monitor SARS-CoV-2 viral infection [
18]. The units for anti-S1 IgG, anti-IgM, and anti-N IgG were binding antibody units per milliliter (BAU/mL), Sysmex units per mL (SU/mL), and SU/mL, respectively. The BAU was calibrated using the WHO International Standards.
2.3. Enzyme-Linked Immunospot Assay
The numbers of antigen-specific INF-γ-releasing lymphocytes were determined as previously described [
19]. Briefly, deep-frozen peripheral blood mononuclear cells donated from 26 participants in sub-cohort 3 were subjected to an Enzyme-Linked ImmunoSpot (ELISPOT) assay, followed by 19 additional ELISPOT assays, depending on the availability of research funding, for a subset of participants in sub-cohort 2, 8 months following the second dose. A human IFN-γ ELISPOT kit (Mabtech AB, Nacka Strand, Sweden) was used to evaluate cellular immunity against SARS-CoV-2 using lyophilized 315-peptide mixtures of SARS-CoV-2 spike glycoprotein, PepMixTM SARS-CoV-2 (JPT Peptide Technologies, Berlin, Germany), the original strain (PM-WCPV-S-2) as antigens. The peptide mixture was dissolved in DMSO at a concentration of 0.5 ug/peptide; DMSO was used as a negative control, while an NF-kB activator, phorbol myristate acetate, and ionomycin were positive controls. The assay was performed according to the manufacturer's instructions using a spot counter, ImmunoSpot S6 VERSA (Cellular Technology Limited, Shaker Heights, OH, USA).
2.4. Self-Administered Questionnaire
A self-administered questionnaire was used, as previously described [
20]. Briefly, the participants were asked about cigarette smoking and habitual drinking. Alcohol consumption was calculated based on the amount consumed over the previous six months, adjusted for a body weight of 60 kg, and categorized into four groups: less than 1 g/day, 1 g/day or more, less than 20 g/day, and 20 g/day or more. Exercise habits were assessed by asking, “Do you usually exercise?” with response options of no habit, less than 1 day/week, 1 to 3 days/week, and 3 or more days/week. Perceived stress was evaluated using the question “Do you feel psychological stress?” on a 5-point scale: no (0), mostly no (1), unsure (2), quite often (3), and yes (4). The presence of steroid use, allergic diseases, and dyslipidemia over the past 3 years.
2.5. Genotyping of rs671
The East Asian-specific variant, a single nucleic polymorphism of
Aldehyde dehydrogenase 2 (
ALDH2) rs671, was identified as previously described [
20]. In brief, DNA was extracted from blood clots as follows: Approximately 0.1 mL of blood clots were incubated overnight at 56 °C in 0.4 mL of proteinase K solution (1–10 U/mL proteinase K in 0.01 M Tris-HCl, pH 8, with 0.01 M EDTA and 0.5% sodium dodecyl sulfate). Subsequently, TE-saturated phenol (0.5 mL) was added. After vigorous mixing for 20 s, the samples were placed on ice for 10 min, followed by centrifugation at 16,000×
g for 5 min at room temperature (20–25 °C). The aqueous layer was separated, mixed with 0.5 mL of 95–100% ethanol, and incubated at room temperature for 10 min. Following centrifugation at 12,000 rpm for 10 min, the DNA precipitate was collected and the supernatant was discarded. The DNA pellets were washed with 0.25 mL of 70% ethanol, dried, and dissolved in 20–200 µL of DNase-free water. The DNA samples were genotyped using the TaqMan® SNP genotyping assay system according to the manufacturer’s instructions (Thermo Fisher Scientific, Waltham, MA, USA).
2.6. Statistical Analyses
Statistical analyses were performed using SAS9.4 TS Level 1M5 for Windows (SAS Institute, Cary, NC, USA). Statistical significance was set at
p < 0.05. To approximate the normal distribution, IgG, IgM, and INF-γ, primary outcomes, were log-transformed. 1α, 25-(OH)
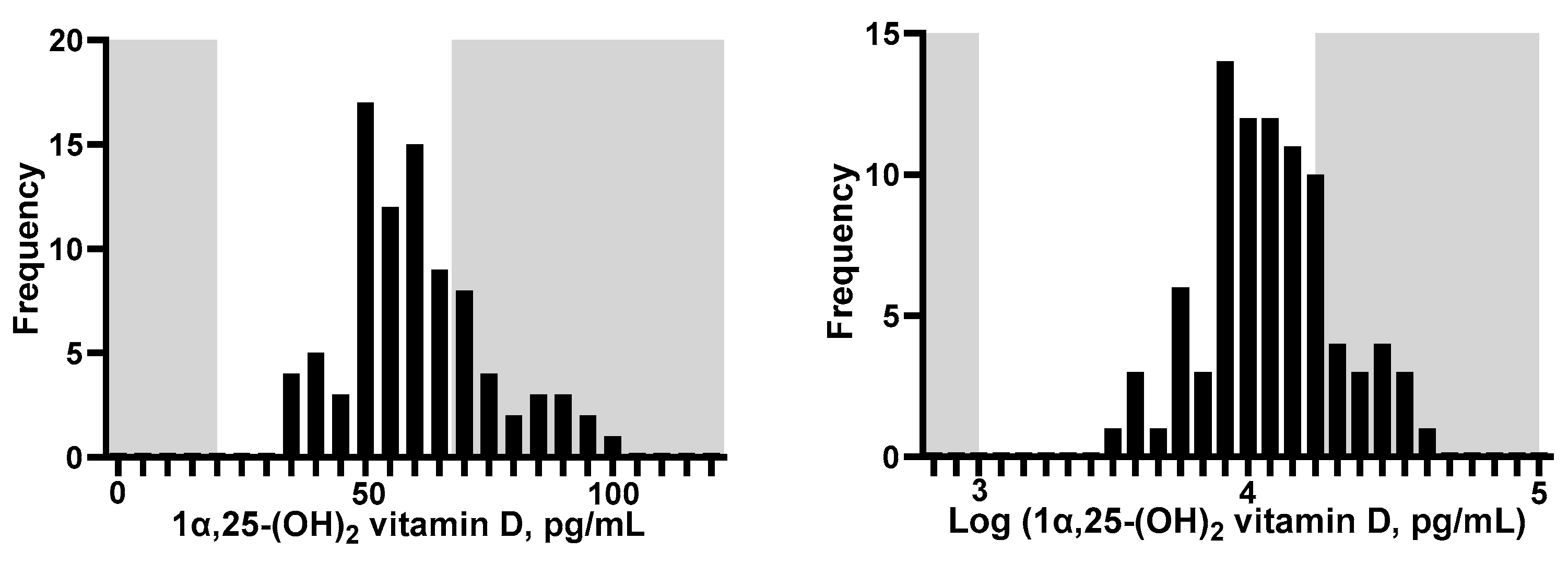
2 vitamin D level, the primary explanatory variable, was also log-transformed (
Figure 2).
2.6.1. Covariates
The following covariates suspected to be associated with immune response or vitamin D level were included in the statistical models: sex and age [
21], rs671 (
ALDH2 gene variant) [
19], height, smoking status [
22,
23], alcohol intake [
24,
25], exercise habits [
26,
27], perceived stress [
28], and medical history including allergic disease [
29] and steroid use [
30,
31].
2.6.2. Mixed Model
Under the assumption of a linear regression between baseline blood levels of 1α, 25-(OH)₂ vitamin D and immune response to the COVID-19 mRNA vaccine, associations were estimated using mixed models that accounted for repeated measures, subpopulation as a random effect, and fixed effects of relevant covariates (proc mixed).
To further validate this association and explore non-linear relationships between vitamin D levels and immune responses, least squares geometric means and standard errors were calculated for three vitamin D quantiles (Q1: ≤52.4, Q2: 53.7–64, and Q3: ≥64.6). Mixed models for estimation included interactive terms between weeks post-vaccination (categorical) and categorical vitamin D levels.
3. Results
3.1. Baseline Characters of the Total Participants
Histograms of 1α, 25-(OH)
2 vitamin D concentration are shown in
Figure 2. The vitamin D ranged from 33.7 and 99.8 pg/mL. None of the participants showed levels that fell below the general reference range for the Japanese population (20–60 pg/mL) [
32], and 60.2% showed levels above the highest value within the reference range.
Table 1 lists the baseline characteristics by tertile of 1α, 25-(OH)
2 vitamin D. Blood vitamin D levels were not associated with any of the variables. Four participants reported current smoking habits, and none of them had altered their smoking habits in the past year. Two participants had current steroid use, and the other participants had not reported steroid use in the previous 3 years: one participant reported current dyslipidemia and had the second-lowest vitamin D level of 37.3 pg/mL, while the other had no history of dyslipidemia in the past 3 years.
3.2. Anti-S1 IgG Production
In the BNT162b2 group, anti-S1 IgG antibody titers peaked 2 weeks after the second dose (median of each tertile ranged from 1,118–2,383 BAU/mL,
Table S1). In the mRNA-1273 group, the highest titers were in the first blood collection after the second dose (2680–3209 BAU/mL;
Table S1). No consistent relationship was found between vitamin D and anti-S1 IgG levels in the comparison of medians.
Table 2 lists the results of the multivariate-adjusted regression model, including all observations in a single mixed model. When the model was adjusted for time course and vaccine type, a negative trend was observed for vitamin D and anti-S1 IgG levels (AIC = 1302,
p = 0.15). With the addition of biological characteristics (sex, age, rs671, an East Asian-specific immune-related variant, and height), the fit improved (AIC = 1292), and a marginal and stronger negative association was estimated (
p = 0.04). This trend was further strengthened in model 3, which included lifestyle and medical history (AIC = 1276,
p = 0.0001). As shown in
Table S2, this negative association was more robustly estimated in the sensitivity analyses that excluded one participant with dyslipidemia (
p = 0.012, <0.001, and < 0.001 for models 1, 2, and 3, respectively).
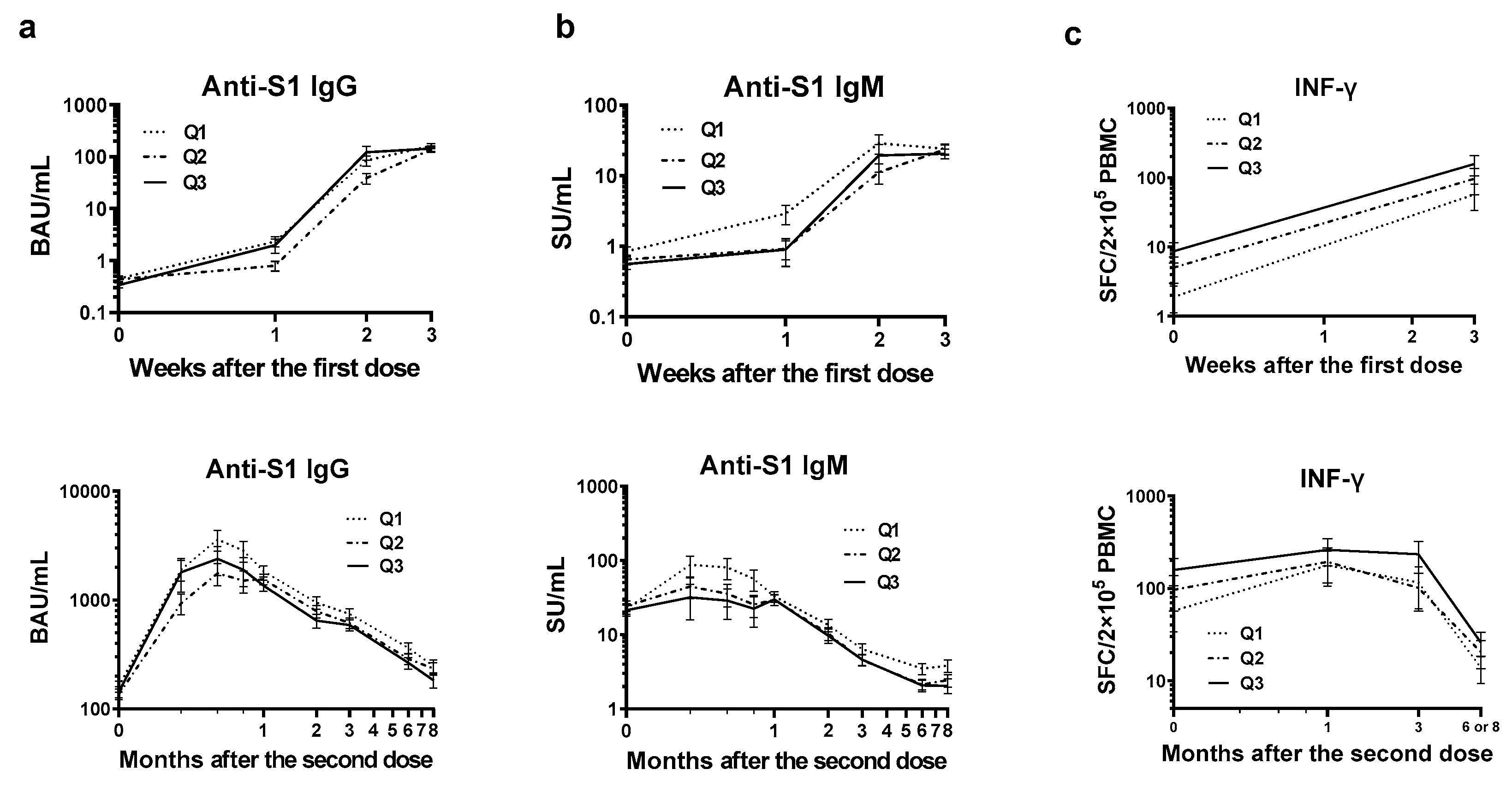
Next, to depict the non-linear relationship between vitamin D and anti-S1 IgG levels, we estimated the adjusted means of IgG values for the three quantile groups of vitamin D. As shown in
Figure 3a, the highest IgG levels were consistently observed in the Q1 group (the lowest vitamin D level) following the second dose. The orders of the Q2 and Q3 groups were inconsistent.
3.3. Anti-S1 IgM Production
Anti-S1 IgM peak was observed 1 week after the second dose in the BNT162b2 group (median for each of the three quantiles ranged from to 22–63 SU/mL), 1 week earlier than IgG, although it was not observed in the mRNA-1273 group due to lack of frequent sampling. No consistent relationship was found between the quantile order of vitamin D and the median IgM titers (
Table S3).
As shown in
Table 3, model 1 estimated a trend toward a negative correlation between IgM and vitamin D levels (
p = 0.196, AIC = 1753). The addition of biological characteristics gave a stronger negative estimate with a lower
p-value (0.037) with a better model fit (AIC = 1749, model 2), and the further inclusion of lifestyle and medical history resulted in the best-fitting model (AIC = 1724), estimating the strongest association with the lowest
p-value (< 0.001) among these models. As listed in
Table S4, sensitivity analyses that excluded one participant with dyslipidemia estimated robust negative correlations with low
p-values (0.004, < 0.001, and < 0.001 for models 1, 2, and 3, respectively).
The non-linear vitamin D—IgM relationship is depicted in
Figure 3b by the quantiles of vitamin D. The highest IgM levels were consistently observed in the Q1 group (lowest vitamin D levels) following the second dose.
3.4. SARS-CoV-2 Spike Protein-Specific INF-γ Producing Cell Counts
Repeated observation of ELISPOT counts of SARS-CoV-2 spike protein-specific INF-γ-producing cells was analyzed for 26 participants, with 122 observations in sub-cohort 3 and 19 participants with 19 observations in sub-cohort 2. The highest median values in the Q1-3 groups were observed for Q1 and Q3 (
Table S5).
As listed in
Table 4, model 1 showed no effect on baseline vitamin D levels (
p = 0.48, AIC = 420). Although the model fit worsened in model 2 (AIC = 427), including biological characteristics revealed a positive effect of vitamin D (
p = 0.036). Model 3 showed a stronger positive effect of vitamin D with a lower probability of ɑ error (
p = 0.003, AIC = 426).
The non-linear relationship between vitamin D and ELISPOT is illustrated in
Figure 3c by estimating the corresponding adjusted means for each time course by the three quantile groups. The highest values were consistently estimated for Q3, which had the highest vitamin D.
4. Discussion
To the best of our knowledge, this study is the first to report the effects of baseline blood levels of active vitamin D, 1α, 25-(OH)2 vitamin D, on humoral and cellular immune reaction to COVID-19 mRNA vaccination. The participants showed high vitamin D levels, from normal to above the reference range, within which vitamin D levels were negatively associated with humoral immunity and positively with cellular immunity.
Active vitamin D is a ligand of a nuclear receptor found in immune cells, such as dendritic cells, macrophages, and T and B cells [
33]. After transmigration into the nucleus, activated vitamin D receptors bind to vitamin D response sequences in the genomic DNA and regulate the expression of various target genes, including cell cycle regulators [
24,
34]. Active vitamin D suppresses the proliferation of B cells and the production of IgG and IgM [
35,
36], consistent with the findings in this study. Since active vitamin D increases the expression of p27 in B lymphocytes and reduces the mRNA levels of cell division growth factors, such as cyclin-dependent kinases 4 and 6 and cyclin D [
37], high levels of blood active vitamin D may result in the inhibition of B cell proliferation, potentially suppressing antibody production, as observed in this study.
Active vitamin D suppresses Th1-mediated inflammatory responses [
35,
38] and Th1 are the primary cells to produce INF-γ in response to antigen exposure [
39]. However, we found consistently higher counts of INF-γ-producing cells in the Q3 group, which had the highest levels of active vitamin D. One hypothesis to bridge this gap may be the recent finding regarding memory T cells in human intervention studies; Saroha et al. supplemented the intervention group with precursor vitamin D [
7], confirmed an increase in active 1α, 25-(OH)
2 vitamin D concentration in supplemented group, and found upregulations in the expression of memory T cell-related genes through comprehensive analysis of PBMC mRNA. The hypothesis that vitamin D favors the preservation of memory T cells but not the proliferation of effector T cells may clarify why we did not observe a notable vaccine-induced increase in the INF-producing cell count linked to vitamin D in our study. We observed slightly higher cell counts, which were consistently estimated in Q3 from the baseline.
Lavell et al. reported no association between baseline precursor vitamin D and SARS-CoV-2 Spike protein-specific INF-γ release [
8,
37]. These results do not contradict our findings, because the concentrations of vitamin D in the precursor and active forms are not proportional [
40]. In addition, since the ELISPOT assay used in our study is an advanced technology developed to increase sensitivity [
41,
42], the ELISA used in their study might not have been sensitive enough to detect the effects.
One of the greatest challenges of COVID-19 vaccines is breakthrough infections caused by immune escape due to mutations in the receptor-binding domain (RBD) of the spike protein, which reduces the affinity between the antibody and the RBD [
43]. In contrast, cell-mediated immunity is less susceptible to mutations in viral strains as T-cell receptors recognize the epitopes of polyclonal spike proteins and RBDs [
44]. Maintaining a high concentration of active vitamin D in the blood and a strong cellular immune system may contribute to the long-term reduction of severe COVID-19.
This study has some limitations. First, the small number of participants limited statistical power. Although we adopted a mixed model accounting for repeated measurements and sub-cohorts to minimize the problem, there may be associations that could not be detected. In particular, the evaluation of cellular immunity was largely limited by research funding (141 observations from 45 participants). Second, the lowest active vitamin D level (33.7 pg/mL) in the current cohort was above the lowest reference range (20 pg/mL). Active vitamin D levels below 20 pg/mL have been shown to increase the incidence of severe illness and mortality [45-48]. We could not verify the effects on the immunogenicity of the COVID-19 vaccine. Third, the extent to which the immunogenicity effects observed in this study influenced actual COVID-19 infections. To further elucidate the role of vitamin D in enhancing vaccine-induced immunity, a longitudinal cohort study with a larger number of participants will be required.
5. Conclusions
The active form of vitamin D, 1α, 25-(OH)₂ vitamin D, may suppress increased antibody titers while promoting cellular immune responses following COVID-19 mRNA vaccination. Maintaining blood levels of active vitamin D above the reference range could reduce the risk of breakthrough infections and severe disease by enhancing cellular immunity.
Supplementary Materials
Table S1. Anti-SARS-CoV-2 S1 IgG levels (BAU/mL), Table S2. Sensitivity analysis: fixed effects of baseline characteristics on log-transformed anti-S1 IgG, BAU/mL, Table S3. Anti-SARS-CoV-2 S1 IgM levels (SU/mL), Table S4. Sensitivity analysis: fixed effects of baseline characteristics on log-transformed anti-S1 IgM, SU/mL, Table S5.IFN-γ ELISPOT count (SFC/2.0 x10⁵ PBMC).
Author Contributions
Conceptualization, M.H., Y.H., and A.M.; Methodology, M.H. and A.M.; Formal Analysis, T.H., G.Y., M.T. and A.M.; Investigation, G.Y., M.T., T.F., C.I., T.S., M.A.K., M.H. and A.M.; Resources, M.H., T.T., and A.M.; Data Curation, M.H. and A.M.; Writing—Original Draft Preparation, T.H., G.Y., M.T., T.T., and A.M.; Writing—Review and Editing, All authors; Visualization, T.H., G.Y. and A.M.; Supervision, M.H. and A.M.; Project Administration, M.H.; Funding Acquisition, Y.H. This research was part of a dissertation submitted by the first author (T.H.) for partial fulfilment of a Ph.D. degree. All authors provided consent. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by a research grant for Research on Emerging and Re-emerging Infectious Diseases, Health and Labor Science Research Grants from the Ministry of Health, Labor and Welfare, Japan (R2-SHINKOGYOSEI-SHITEI-003 and 20HA2001), and the JSPS KAKENHI Grant (No. P21K19652) of the Japanese Ministry of Education, Culture, Sports, Science and Technology. The funding organizations did not influence the study design, data collection, analysis, interpretation, or writing of the manuscript.
Institutional Review Board Statement
This study was approved by the Research Review Committee and Human Genome Ethics Review Committee of the Faculty of Medicine, Saga University, Japan (approval numbers: R2-24, R2-44, R3-4, R3-9, and R3-39), and conducted in accordance with the Declaration of Helsinki.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets generated and/or analyzed in the current study are not publicly available to protect the privacy of the participants; however, they are available from the corresponding author upon reasonable request.
Acknowledgments
We thank Mikako Horita, Miyuki Fuchigami, Kazuhiro Kawamoto, and M. Said Ashenagar for their administrative and technical support. We also extend our thanks to the students, healthcare workers, and employees of Saga University for their participation in the present study and for donating the blood samples.
Conflicts of Interest
Yoshio Hirota was employed by SOUSEIKAI Medical Group (Medical Co., LTA). The authors declare that the research was conducted in the absence of commercial or financial relationships that could be construed as potential conflicts of interest.
References
- Lemire, J. M., D. C. Archer, L. Beck and H. L. Spiegelberg. "Immunosuppressive actions of 1,25-dihydroxyvitamin d3: Preferential inhibition of th1 functions." J Nutr 125 (1995): 1704s-08s. [CrossRef]
- Jeffery, L. E., F. Burke, M. Mura, Y. Zheng, O. S. Qureshi, M. Hewison, L. S. Walker, D. A. Lammas, K. Raza and D. M. Sansom. "1,25-dihydroxyvitamin d3 and il-2 combine to inhibit t cell production of inflammatory cytokines and promote development of regulatory t cells expressing ctla-4 and foxp3." J Immunol 183 (2009): 5458-67. [CrossRef]
- Cesur, F., Z. Atasever and Y. Özoran. "Impact of vitamin d3 supplementation on covid-19 vaccine response and immunoglobulin g antibodies in deficient women: A randomized controlled trial." Vaccine 41 (2023): 2860-67. [CrossRef]
- Bychinin, M. V., T. V. Klypa, I. A. Mandel, G. M. Yusubalieva, V. P. Baklaushev, N. A. Kolyshkina and A. V. Troitsky. "Effect of vitamin d3 supplementation on cellular immunity and inflammatory markers in covid-19 patients admitted to the icu." Sci Rep 12 (2022): 18604. [CrossRef]
- Balla, M., G. P. Merugu, V. M. Konala, V. Sangani, H. Kondakindi, M. Pokal, V. Gayam, S. Adapa, S. Naramala and S. V. Malayala. "Back to basics: Review on vitamin d and respiratory viral infections including covid-19." J Community Hosp Intern Med Perspect 10 (2020): 529-36. [CrossRef]
- Grant, W. B., H. Lahore, S. L. McDonnell, C. A. Baggerly, C. B. French, J. L. Aliano and H. P. Bhattoa. "Evidence that vitamin d supplementation could reduce risk of influenza and covid-19 infections and deaths." Nutrients 12 (2020). [CrossRef]
- Saroha, H. S., S. Bhat, L. Das, P. Dutta, M. F. Holick, N. Sachdeva and R. K. Marwaha. "Calcifediol boosts efficacy of chadox1 ncov-19 vaccine by upregulating genes promoting memory t cell responses." NPJ Vaccines 9 (2024): 114. [CrossRef]
- Lavell, A. H. A., A. E. Schramade, J. J. Sikkens, K. van der Straten, K. A. van Dort, M. A. Slim, B. Appelman, L. A. van Vught, A. P. J. Vlaar, N. A. Kootstra, et al. "25-hydroxyvitamin d concentrations do not affect the humoral or cellular immune response following sars-cov-2 mrna vaccinations." Vaccine 42 (2024): 1478-86. [CrossRef]
- Zhu, J. G., J. T. Ochalek, M. Kaufmann, G. Jones and H. F. Deluca. "Cyp2r1 is a major, but not exclusive, contributor to 25-hydroxyvitamin d production in vivo." Proc Natl Acad Sci U S A 110 (2013): 15650-5. [CrossRef]
- Zhang, Y., F. Fang, J. Tang, L. Jia, Y. Feng, P. Xu and A. Faramand. "Association between vitamin d supplementation and mortality: Systematic review and meta-analysis." Bmj 366 (2019): l4673. [CrossRef]
- Zelini, P., P. d'Angelo, E. Cereda, C. Klersy, P. Sabrina, R. Albertini, G. Grugnetti, A. M. Grugnetti, C. Marena, S. Cutti, et al. "Association between vitamin d serum levels and immune response to the bnt162b2 vaccine for sars-cov-2." Biomedicines 10 (2022). [CrossRef]
- MacDonald, P. N., T. A. Baudino, H. Tokumaru, D. R. Dowd and C. Zhang. "Vitamin d receptor and nuclear receptor coactivators: Crucial interactions in vitamin d-mediated transcription." Steroids 66 (2001): 171-6. [CrossRef]
- Matias, M. L., M. Romao-Veiga, V. R. Ribeiro, P. R. Nunes, V. J. Gomes, A. C. Devides, V. T. Borges, G. G. Romagnoli, J. C. Peracoli and M. T. Peracoli. "Progesterone and vitamin d downregulate the activation of the nlrp1/nlrp3 inflammasomes and tlr4-myd88-nf-κb pathway in monocytes from pregnant women with preeclampsia." J Reprod Immunol 144 (2021): 103286. [CrossRef]
- Fernandez, G. J., J. M. Ramírez-Mejía, J. A. Castillo and S. Urcuqui-Inchima. "Vitamin d modulates expression of antimicrobial peptides and proinflammatory cytokines to restrict zika virus infection in macrophages." Int Immunopharmacol 119 (2023): 110232. [CrossRef]
- Saul, L., I. Mair, A. Ivens, P. Brown, K. Samuel, J. D. M. Campbell, D. Y. Soong, N. Kamenjarin and R. J. Mellanby. "1,25-dihydroxyvitamin d(3) restrains cd4(+) t cell priming ability of cd11c(+) dendritic cells by upregulating expression of cd31." Front Immunol 10 (2019): 600. [CrossRef]
- Behm, C., A. Blufstein, J. Gahn, B. Kubin, A. Moritz, X. Rausch-Fan and O. Andrukhov. "Pleiotropic effects of vitamin d(3) on cd4(+) t lymphocytes mediated by human periodontal ligament cells and inflammatory environment." J Clin Periodontol 47 (2020): 689-701. [CrossRef]
- Mariz, H. A., E. I. Sato, P. R. G. Cardoso, R. Gonçalves, A. Duarte, M. J. B. de Melo Rego, I. da Rocha Pitta and M. G. da Rocha Pitta. "Vitamin d presented in vitro immunomodulatory property on t lymphocyte-related cytokines in systemic lupus erythematosus." Inflammation 46 (2023): 730-38. [CrossRef]
- Ashenagar, M. S., A. Matsumoto, H. Sakai, M. Tokiya, M. Hara and Y. Hirota. "Comparison of cleia and elisa for sars-cov-2 virus antibodies after first and second dose vaccinations with the bnt162b2 mrna vaccine." Vaccines (Basel) 10 (2022). [CrossRef]
- Bogahawaththa, S., M. Hara, T. Furukawa, C. Iwasaka, T. Sawada, G. Yamada, M. Tokiya, K. Kitagawa, Y. Miyake, M. A. Kido, et al. "Asian flush gene variant enhances cellular immunogenicity of covid-19 vaccine: Prospective observation in the japanese general population." Vaccines (Basel) 12 (2024). [CrossRef]
- Matsumoto, A., M. Hara, M. S. Ashenagar, M. Tokiya, T. Sawada, C. Iwasaka, T. Furukawa, K. Kitagawa, Y. Miyake and Y. Hirota. "Variant allele of aldh2, rs671, associates with attenuated post-vaccination response in anti-sars-cov-2 spike protein igg: A prospective study in the japanese general population." Vaccines (Basel) 10 (2022). [CrossRef]
- Notarte, K. I., J. A. Catahay, J. V. Velasco, A. Pastrana, A. T. Ver, F. C. Pangilinan, P. J. Peligro, M. Casimiro, J. J. Guerrero, M. M. L. Gellaco, et al. "Impact of covid-19 vaccination on the risk of developing long-covid and on existing long-covid symptoms: A systematic review." EClinicalMedicine 53 (2022): 101624. [CrossRef]
- Mori, Y., M. Tanaka, H. Kozai, K. Hotta, Y. Aoyama, Y. Shigeno, M. Aoike, H. Kawamura, M. Tsurudome and M. Ito. "Antibody response of smokers to the covid-19 vaccination: Evaluation based on cigarette dependence." Drug Discov Ther 16 (2022): 78-84. [CrossRef]
- Bozinovski, S., H. J. Seow, S. P. Chan, D. Anthony, J. McQualter, M. Hansen, B. J. Jenkins, G. P. Anderson and R. Vlahos. "Innate cellular sources of interleukin-17a regulate macrophage accumulation in cigarette- smoke-induced lung inflammation in mice." Clin Sci (Lond) 129 (2015): 785-96. [CrossRef]
- Gonzalez-Quintela, A., R. Alende, F. Gude, J. Campos, J. Rey, L. M. Meijide, C. Fernandez-Merino and C. Vidal. "Serum levels of immunoglobulins (igg, iga, igm) in a general adult population and their relationship with alcohol consumption, smoking and common metabolic abnormalities." Clin Exp Immunol 151 (2008): 42-50. [CrossRef]
- Frank, K., S. Abeynaike, R. Nikzad, R. R. Patel, A. J. Roberts, M. Roberto and S. Paust. "Alcohol dependence promotes systemic ifn-γ and il-17 responses in mice." PLoS One 15 (2020): e0239246. [CrossRef]
- Silva, B. R., F. R. Monteiro, K. Cezário, J. B. D. Amaral, V. Paixão, E. B. Almeida, C. Santos, G. R. Amirato, D. B. L. Oliveira, E. L. Durigon, et al. "Older adults who maintained a regular physical exercise routine before the pandemic show better immune response to vaccination for covid-19." Int J Environ Res Public Health 20 (2023). [CrossRef]
- Zamani, A., I. Salehi and M. Alahgholi-Hajibehzad. "Moderate exercise enhances the production of interferon-γ and interleukin-12 in peripheral blood mononuclear cells." Immune Netw 17 (2017): 186-91. [CrossRef]
- Segerstrom, S. C. and G. E. Miller. "Psychological stress and the human immune system: A meta-analytic study of 30 years of inquiry." Psychol Bull 130 (2004): 601-30. [CrossRef]
- Shamji, M. H., R. Valenta, T. Jardetzky, V. Verhasselt, S. R. Durham, P. A. Würtzen and R. J. J. van Neerven. "The role of allergen-specific ige, igg and iga in allergic disease." Allergy 76 (2021): 3627-41. [CrossRef]
- Settipane, G. A., R. K. Pudupakkam and J. H. McGowan. "Corticosteroid effect on immunoglobulins." J Allergy Clin Immunol 62 (1978): 162-6. [CrossRef]
- John, M., S. J. Hirst, P. J. Jose, A. Robichaud, N. Berkman, C. Witt, C. H. Twort, P. J. Barnes and K. F. Chung. "Human airway smooth muscle cells express and release rantes in response to t helper 1 cytokines: Regulation by t helper 2 cytokines and corticosteroids." J Immunol 158 (1997): 1841-7.
- Akiruno. "1,25-(oh)2 vitamin d." 2024. https://test-directory-en.srl.info/akiruno_en/test/detail/024790200. Accessed 31-10 2024.
- Carlberg, C. "Molecular endocrinology of vitamin d on the epigenome level." Mol Cell Endocrinol 453 (2017): 14-21. [CrossRef]
- Christakos, S., P. Dhawan, A. Verstuyf, L. Verlinden and G. Carmeliet. "Vitamin d: Metabolism, molecular mechanism of action, and pleiotropic effects." Physiol Rev 96 (2016): 365-408. [CrossRef]
- Zhang, Z., F. Chen, J. Li, F. Luo, T. Hou, J. Xu and D. Sun. "1,25(oh)(2)d(3) suppresses proinflammatory responses by inhibiting th1 cell differentiation and cytokine production through the jak/stat pathway." Am J Transl Res 10 (2018): 2737-46.
- Baeke, F., T. Takiishi, H. Korf, C. Gysemans and C. Mathieu. "Vitamin d: Modulator of the immune system." Curr Opin Pharmacol 10 (2010): 482-96. [CrossRef]
- Chen, S., G. P. Sims, X. X. Chen, Y. Y. Gu, S. Chen and P. E. Lipsky. "Modulatory effects of 1,25-dihydroxyvitamin d3 on human b cell differentiation." J Immunol 179 (2007): 1634-47. [CrossRef]
- Boonstra, A., F. J. Barrat, C. Crain, V. L. Heath, H. F. Savelkoul and A. O'Garra. "1alpha,25-dihydroxyvitamin d3 has a direct effect on naive cd4(+) t cells to enhance the development of th2 cells." J Immunol 167 (2001): 4974-80. [CrossRef]
- Swanson, M. A., W. T. Lee and V. M. Sanders. "Ifn-gamma production by th1 cells generated from naive cd4+ t cells exposed to norepinephrine." J Immunol 166 (2001): 232-40. [CrossRef]
- Tang, J. C. Y., S. Jackson, N. P. Walsh, J. Greeves and W. D. Fraser. "The dynamic relationships between the active and catabolic vitamin d metabolites, their ratios, and associations with pth." Sci Rep 9 (2019): 6974. [CrossRef]
- Czerkinsky, C., G. Andersson, H. P. Ekre, L. A. Nilsson, L. Klareskog and O. Ouchterlony. "Reverse elispot assay for clonal analysis of cytokine production. I. Enumeration of gamma-interferon-secreting cells." J Immunol Methods 110 (1988): 29-36. [CrossRef]
- Ewen, C. and M. E. Baca-Estrada. "Evaluation of interleukin-4 concentration by elisa is influenced by the consumption of il-4 by cultured cells." J Interferon Cytokine Res 21 (2001): 39-43. [CrossRef]
- Cao, Y., J. Wang, F. Jian, T. Xiao, W. Song, A. Yisimayi, W. Huang, Q. Li, P. Wang, R. An, et al. "Omicron escapes the majority of existing sars-cov-2 neutralizing antibodies." Nature 602 (2022): 657-63. [CrossRef]
- Edelman, A., E. R. Boniface, V. Male, S. T. Cameron, E. Benhar, L. Han, K. A. Matteson, A. Van Lamsweerde, J. T. Pearson and B. G. Darney. "Association between menstrual cycle length and covid-19 vaccination: Global, retrospective cohort study of prospectively collected data." BMJ Med 1 (2022). [CrossRef]
- Szarpak, L., Z. Rafique, A. Gasecka, F. Chirico, W. Gawel, J. Hernik, H. Kaminska, K. J. Filipiak, M. J. Jaguszewski and L. Szarpak. "A systematic review and meta-analysis of effect of vitamin d levels on the incidence of covid-19." Cardiol J 28 (2021): 647-54. [CrossRef]
- Ghasemian, R., A. Shamshirian, K. Heydari, M. Malekan, R. Alizadeh-Navaei, M. A. Ebrahimzadeh, M. Ebrahimi Warkiani, H. Jafarpour, S. Razavi Bazaz, A. Rezaei Shahmirzadi, et al. "The role of vitamin d in the age of covid-19: A systematic review and meta-analysis." Int J Clin Pract 75 (2021): e14675. [CrossRef]
- Chiu, S. K., K. W. Tsai, C. C. Wu, C. M. Zheng, C. H. Yang, W. C. Hu, Y. C. Hou, K. C. Lu and Y. C. Chao. "Putative role of vitamin d for covid-19 vaccination." Int J Mol Sci 22 (2021). [CrossRef]
- Teshome, A., A. Adane, B. Girma and Z. A. Mekonnen. "The impact of vitamin d level on covid-19 infection: Systematic review and meta-analysis." Front Public Health 9 (2021): 624559. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).