Submitted:
05 November 2024
Posted:
06 November 2024
You are already at the latest version
Abstract
Background: An accurate diagnosis of thyroid nodules is crucial for avoiding unnecessary surgical procedures and making timely treatment possible. The objective of the present study was to evaluate the diagnostic accuracy of FNAB (fine-needle aspiration biopsy) using histopathological findings as the reference standards. Patients with the diagnostic categories (DCs) III, IV, V were subjected to a special analysis. In addition, the authors assessed whether other factors, i.e. age, gender, BMI (body mass index), obesity and histopathologically confirmed lymphocytic thyroiditis had an impact on the occurrence of malignant tumors. Methods: A retrospective analysis was conducted on 535 patients who underwent thyroid surgery between October 2022 and September 2023. To assess the FNAB reliability, the result obtained using the Bethesda classification was compared with the histopathological result. Results: The ROM (risk of malignancy) values for DCs I-VI were 38.1%, 15.6%, 29.8%, 18.6%, 91.0%, 93.2%, respectively. DC V (OR 62.34, p< 0.0001) and age≤50 (OR=2.31, p<0.006) had a statistically significant effect on the risk of thyroid cancer. DCs III and IV were not statistically significantly associated with the risk of malignancy (OR=1.68,p=0.16; OR=1.51,p=0.3,respectively). There were no statistically significant differences in sex, BMI and obesity between the patients with benign and malignant lesions. Conclusion: DC V is associated with a high likelihood of malignancy, especially in patients under 50 years of age, and therefore surgery is indicated in this category of subjects. In the DCs III and IV categories, the risk of malignancy is lower and conservative management with active clinical and ultrasound surveillance can be considered. In patients < 50 years of age, with the Bethesda categories III and IV, surgical treatment should be considered.
Keywords:
1. Introduction
2. Materials and Methods
2.1. Patients and Methods
2.2. Diagnosis and Evaluation
2.3. Statistical Analysis
3. Results
3.1. Results Obtained from the Entire Study Group of Patients (n=521)
3.2. Results of Patients in DCs III, IV and V According to Bethesda (n=221)
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Maso, L.D.; Vaccarella, S. Global trends in thyroid cancer incidence and the impact of overdiagnosis. Lancet Diabetes Endo 2020, 8, 468–70. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.M.; Chang, K.P.; Pitman, M.; Faquin, W.C.; Randolph, G.W. Thyroid fine-needle aspiration biopsy: variability in reporting. Thyroid. 2009, 19, 717–23. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Gupta, S.; Gupta, V.B. Correlation of fine needle aspiration cytology with histopathology in the diagnosis of solitary thyroid nodule. J Thyroid Res. 2010, 2010, 379051. [Google Scholar] [CrossRef]
- Russ, G.; Bonnema, S.J.; Erdogan, M.F.; Durante, C.; Ngu, R.; Leenhardt, L. European Thyroid Association Guidelines for Ultrasound Malignancy Risk Stratification of Thyroid Nodules in Adults: The EU-TIRADS. Eur Thyroid J. 2017, 6, 225–237. [Google Scholar] [CrossRef]
- Cibas, E.S.; Ali, S.Z. The 2017 Bethesda System for Reporting Thyroid Cytopathology. J Am Soc Cytopathol. 2017, 6, 217–222. [Google Scholar] [CrossRef]
- Pusztaszeri, M.; Rossi, E.D.; Auger, M.; Baloch, Z.; Bishop, J.; Bongiovanni, M.; Chandra, A.; Cochand-Priollet, B.; Fadda, G.; Hirokawa, M.; Hong, S.; Kakudo, K.; Krane, J.F.; Nayar, R.; Parangi, S.; Schmitt, F.; Faquin, W.C. The Bethesda System for Reporting Thyroid Cytopathology: Proposed Modifications and Updates for the Second Edition from an International Panel. Acta Cytol. 2016, 60, 399–405. [Google Scholar] [CrossRef]
- Baloch ZW, Cooper DS, Gharib H, Alexander EK (2018) Overview of diagnostic terminology and reporting. In: Syed Z,Ali ESC (eds) The Bethesda system for reporting cytopathology: definitions, criteria and explanatory notes. Springer, Cham, pp 1–6.
- Krajewska, J.; Chmielik, E.; Dedecjus, M.; Jarząb, B.; Hubalewska-Dydejczyk, A.; Karbownik-Lewińska, M.; Kos-Kudła, B.; Lewiński, A.; Ruchała, M. Diagnosis and treatment of thyroid cancer in adult patients - Recommendations of Polish Scientific Societies and the National Oncological Strategy. Update of the 2022. Endokrynol Pol. 2022, 73, 799–802. [Google Scholar] [CrossRef]
- Kobaly, K.; Kim, C.S.; Mandel, S.J. Contemporary Management of Thyroid Nodules. Annu Rev Med. 2022, 73, 517–528. [Google Scholar] [CrossRef]
- EUROCRINE: https://eurocrine.eu; last accessed at April 28, 2024.
- Jung, C.K.; Bychkov, A.; Kakudo, K. Update from the 2022 World Health Organization Classification of Thyroid Tumors: A Standardized Diagnostic Approach. Endocrinol Metab (Seoul). 2022, 37, 703–718. [Google Scholar] [CrossRef]
- R Core Team (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/.
- Osseis, M.; Jammal, G.; Kazan, D.; Noun, R. Comparison between Fine Needle Aspiration Cytology with Histopathology in the Diagnosis of Thyroid Nodules. J Pers Med. 2023, 13, 1197. [Google Scholar] [CrossRef] [PubMed]
- Sherman, S.I. Thyroid carcinoma. Lancet. 2003, 361, 501–11. [Google Scholar] [CrossRef] [PubMed]
- McCoy KL, Jabbour N, Ogilvie JB, Ohori NP, Carty SE, Yim JH, et al. The incidence of cancer and rate off alse-negative cytology in thyroid nodules greater than or equal to 4 cm in size. Surgery 2007, 142, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed]
- Hegedüs, L. The thyroid nodule. New Engl J Med 2004, 351, 1764–71. [Google Scholar] [CrossRef]
- Rai, K.; Park, J.; Gokhale, S.; Irshaidat, F.; Singh, G. Diagnostic Accuracy of the Bethesda System for Reporting Thyroid Cytopathology (TBSRTC): An Institution Experience. Int J Endocrinol. 2023, 2023, 9615294. [Google Scholar] [CrossRef]
- Garg, S.; Desai, N.J.; Mehta, D.; Vaishnav, M. To Establish Bethesda System for Diagnosis of Thyroid Nodules on the Basis of Fnac with Histopathological Correlation. J Clin Diagn Res. 2015, 9, EC17–EC21. [Google Scholar] [CrossRef]
- Nandedkar, S.S.; Dixit, M.; Malukani, K.; Varma, A.V.; Gambhir, S. Evaluation of Thyroid Lesions by Fine-needle Aspiration Cytology According to Bethesda System and its Histopathological Correlation. Int J Appl Basic Med Res. 2018, 8, 76–82. [Google Scholar] [CrossRef]
- Patel, K.A.; Anandani, G.; Sharma, B.S.; Parmar, R.A. Study of Fine Needle Aspiration Cytology (FNAC) of Thyroid Gland According to the Bethesda System. Cureus. 2023, 15, e37371. [Google Scholar] [CrossRef]
- Mondal, S.K.; Sinha, S.; Basak, B.; Roy, D.N.; Sinha, S.K. The Bethesda system for reporting thyroid fine needle aspirates: A cytologic study with histologic follow-up. J Cytol. 2013, 30, 94–9. [Google Scholar] [CrossRef]
- Inabnet WB 3rd, Palazzo F, Sosa JA, Kriger J, Aspinall S, Barczynski M, Doherty G, Iacobone M, Nordenstrom E, Scott-Coombes D, Wallin G, Williams L, Bray R, Bergenfelz A. Correlating the Bethesda System for Reporting Thyroid Cytopathology with Histology and Extent of Surgery: A Review of 21,746 Patients from Four Endocrine Surgery Registries Across Two Continents. World J Surg. 2020, 44, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Anand, B.; Ramdas, A.; Ambroise, M.M.; Kumar, N.P. The Bethesda System for Reporting Thyroid Cytopathology: A Cytohistological Study. J Thyroid Res. 2020, 2020, 8095378. [Google Scholar] [CrossRef] [PubMed]
- Zarif, H.A.; Ghandurah, S.E.; Al-Garni, M.A.; Binmahfooz, S.K.; Alsaywid, B.S.; Satti, M.B. Thyroid Nodules Cytopathology Applying the Bethesda System with Histopathological Correlation. Saudi J Med Med Sci. 2018, 6, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Alshathry, A.H.; Almeshari, N.Z.; Alarifi, A.S.; Aleidy, A.M.; Aldhahri, S. The Prevalence of Thyroid Papillary Microcarcinoma in Patients With Benign Thyroid Fine Needle Aspiration. Cureus. 2020, 12, e11820. [Google Scholar] [CrossRef]
- Gan TRX, Nga ME, Lum JHY, Wong WM, Tan WB, Parameswaran R, et al. Thyroid cytology—nuclear versus architectural atypia within the “Atypia of undetermined significance/follicular lesion of undetermined significance” Bethesda category have significantly different rates of Malignancy. Cancer Cytopathology 2017, 125, 245–56. [Google Scholar] [CrossRef]
- Mosca, L.; Silva, L.F.F.D.; Carneiro, P.C.; Chacon, D.A.; Araujo-Neto, V.J.F.; Araujo-Filho, V.J.F.; Cernea, C.R. Malignancy rates for Bethesda III subcategories in thyroid fine needle aspiration biopsy (FNAB). Clinics (Sao Paulo). 2018, 73, e370. [Google Scholar] [CrossRef]
- Kim, S.J.; Roh, J.; Baek, J.H.; Hong, S.J.; Shong, Y.K.; Kim, W.B.; Song, D.E. Risk of malignancy according to sub-classification of the atypia of undetermined significance or follicular lesion of undetermined significance (AUS/FLUS) category in the Bethesda system for reporting thyroid cytopathology. Cytopathology. 2017, 28, 65–73. [Google Scholar] [CrossRef]
- Erdogan-Durmus, S.; Balta, H.; Demirtas, R.; Kurt, A. Malignancy Rates of Atypia of Undetermined Significance/Follicular Lesion of Undetermined Significance (AUS/FLUS) Cases: A Tertiary Center Study. Acta Endocrinol (Buchar). 2021, 17, 77–82. [Google Scholar] [CrossRef]
- Yang, W.; Fananapazir, G.; LaRoy, J.; Wilson, M.; Campbell, M.J. Can the American Thyroid Association, K-Tirads, and Acr-Tirads Ultrasound Classification Systems Be Used to Predict Malignancy in Bethesda Category IV Nodules? Endocr Pract. 2020, 26, 945–952. [Google Scholar] [CrossRef]
- Marina, M.; Zatelli, M.C.; Goldoni, M.; Del Rio, P.; Corcione, L.; Martorana, D.; Percesepe, A.; Bonatti, F.; Mozzoni, P.; Crociara, A.; Ceresini, G. Combination of ultrasound and molecular testing in malignancy risk estimate of Bethesda category IV thyroid nodules: results from a single-institution prospective study. J Endocrinol Invest. 2021, 44, 2635–2643. [Google Scholar] [CrossRef]
- Ha, S.M.; Baek, J.H.; Na, D.G.; Jung, C.K.; Suh, C.H.; Shong, Y.K.; Sung, T.Y.; Song, D.E.; Lee, J.H. Assessing the diagnostic performance of thyroid biopsy with recommendations for appropriate interpretation. Ultrasonography. 2021, 40, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Velsen, E. , Peeters R., Stegenga M, van Kemenade F., van Ginhoven T, Verburg F., Visser E, The influence of age on disease outcome in 2015 ATA high-risk differentiated thyroid cancer patients. European Journal of Endocrinology 2021, 185, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Raparia, K.; Min, S.K.; Mody, D.R.; Anton, R.; Amrikachi, M. Clinical outcomes for “suspicious” category in thyroid fine-needle aspiration biopsy: patient's sex and nodule size are possible predictors of Malignancy. Arch Pathol Lab Med 2009, 133, 787–90. [Google Scholar] [CrossRef]
- Konturek, A.; Barczyński, M.; Wierzchowski, W.; Stopa, M.; Nowak, W. Coexistence of papillary thyroid cancer with Hashimoto thyroiditis. Langenbecks Arch Surg. 2013, 398, 389–94. [Google Scholar] [CrossRef] [PubMed]
- Zosin, I.; Balaş, M. Clinical, ultrasonographical and histopathological aspects in Hashimoto's thyroiditis associated with malignant and benign thyroid nodules. Endokrynol Pol. 2013, 64, 255–62. [Google Scholar] [CrossRef] [PubMed]
| Total (N=521) |
benign (N=324) |
cancer (N=197) |
p-value | |
|---|---|---|---|---|
| Sex | 0.94 | |||
| Female | 425 (81.6%) | 264 (81.5%) | 161 (81.7%) | |
| Male | 96 (18.4%) | 60 (18.5%) | 36 (18.3%) | |
| BMI | 0.52 | |||
| Mean (SD) | 27.3 (4.87) | 27.4 (4.76) | 27.1 (5.08) | |
| Median [Q1-Q3] | 26.6 [23.5-30.5] | 26.7 [23.8-30.5] | 26.4 [23.2-30.8] | |
| Missing | 98 (18.8%) | 57 (17.6%) | 41 (20.8%) | |
| Obesity (BMI ≥ 30) | 0.76 | |||
| no | 302 (58.0%) | 192 (59.3%) | 110 (55.8%) | |
| yes | 121 (23.2%) | 75 (23.1%) | 46 (23.4%) | |
| Missing | 98 (18.8%) | 57 (17.6%) | 41 (20.8%) | |
| Age | < 0.0001 | |||
| Mean (SD) | 52.3 (15.2) | 55.3 (14.7) | 47.4 (14.9) | |
| Median [Q1-Q3] | 53.0 [40.0-65.0] | 57.0 [43.8-67.0] | 45.0 [36.0-59.0] | |
| Cytology | < 0.0001 | |||
| I | 21 (4.0%) | 13 (4.0%) | 8 (4.1%) | |
| II | 180 (34.5%) | 152 (46.9%) | 28 (14.2%) | |
| III | 84 (16.1%) | 59 (18.2%) | 25 (12.7%) | |
| IV | 70 (13.4%) | 57 (17.6%) | 13 (6.6%) | |
| V | 67 (12.9%) | 6 (1.9%) | 61 (31.0%) | |
| VI | 59 (11.3%) | 4 (1.2%) | 55 (27.9%) | |
| Not performed | 40 (7.7%) | 33 (10.2%) | 7 (3.6%) | |
| Lymphocytic thyroiditis | < 0.0001 | |||
| no | 442 (84.8%) | 292 (90.1%) | 150 (76.1%) | |
| yes | 79 (15.2%) | 32 (9.9%) | 47 (23.9%) | |
| Malignancy | ||||
| PTC | 172 (33.0%) | 172 (87.3%) | ||
| FTC | 16 (3.1%) | 16 (8.1%) | ||
| MTC | 5 (1.0%) | 5 (2.5%) | ||
| ATC | 1 (0.2%) | 1 (0.5%) | ||
| OTC | 2 (0.4%) | 2 (1.0%) | ||
| Lymphoma | 1 (0.2%) | 1 (0.5%) | ||
| Thyroid operation | ||||
| Total thyroidectomy | 480 (92.1%) | 295 (91%) | 185 (94%) | |
| Unilateral lobectomy | 30 (5.7%) | 22 (6.8%) | 8 (4.1%) | |
| Other operation on thyroid gland | 11 (2.1%) | 7 (2.2%) | 4 (2.0%) | |
| Lymph nodes | ||||
| Bilateral central lymph node dissection | 191 (36.7%) | 89 (27.5%) | 102 (51.8%) | |
| Central lymph node dissection AND one-sided lat. Lymph node dissection | 18 (3.5%) | 2 (0.6%) | 16 (8.1%) | |
| Unilateral central lymph node dissection | 80 (15.4%) | 40 (12.3%) | 40 (20.3%) | |
| One-sided lateral lymph node dissection | 7 (1.3%) | 2 (0.6%) | 5 (2.5%) | |
| Bilateral lateral lymph node dissection | 2 (0.4%) | 0 (0%) | 2 (1%) | |
| None | 223 (42.8%) | 191 (59.0%) | 32 (16.2%) |
| Cytology | Total, n (%) | Histopathology | |||
|---|---|---|---|---|---|
| Benign, n (%) | Cancer, n (%) | ||||
| I | 21 (4.0%) | FA | 2 (15.4%) | FTC | 2 (25%) |
| GD | 1 (7.7%) | PTC | 6 (75%) | ||
| NG | 9 (69.2%) | ||||
| Other diagnosis | 1 (7.7%) | ||||
| II | 180 (34.5%) | FA | 15 (9.9%) | FTC | 4 (14.3%) |
| FT-UMP | 1 (0.7%) | MTC | 3 (10.7%) | ||
| GD | 1 (0.7%) | PTC | 21 (75%) | ||
| OA | 1 (0.7%) | ||||
| LT | 1 (0.7%) | ||||
| NG | 130 (85.5%) | ||||
| NIFTP | 2 (1.3%) | ||||
| III | 84 (16.1%) | FA | 14 (23.7%) | FTC | 7 (28.0%) |
| FT-UMP | 2 (3.4%) | OTC | 1 (4.0%) | ||
| OA | 1 (1.7%) | PTC | 17 (68.0%) | ||
| LT | 3 (5.1%) | ||||
| NG | 35 (59.3%) | ||||
| NIFTP | 2 (3.4%) | ||||
| WDT-UMP | 2 (3.4%) | ||||
| IV | 70 (13.4%) | FA | 15 (26.3%) | OTC | 1 (7.7%) |
| FT-UMP | 5 (8.8%) | PTC | 12 (92.3%) | ||
| OA | 13 (22.8%) | ||||
| LT | 2 (3.5%) | ||||
| NG | 18 (31.6%) | ||||
| NIFTP | 2 (3.5%) | ||||
| WDT-UMP | 2 (3.5%) | ||||
| V | 67 (12.9%) | FA | 1 (16.7%) | ATC | 1 (1.6%) |
| NG | 3 (50.0%) | FTC | 2 (3.3%) | ||
| NIFTP | 1 (16.7%) | PTC | 58 (95.1%) | ||
| Other diagnosis | 1 (16.7%) | ||||
| VI | 59 (11.3%) | NG | 2 (50.0%) | FTC | 1 (1.8%) |
| Other diagnosis | 2 (50.0%) | Lymphoma | 1 (1.8%) | ||
| MTC | 2 (3.6%) | ||||
| PTC | 51 (92.7%) | ||||
|
Not performed |
40 (7.7%) | FA | 4 (12.1%) | PTC | 7 (100%) |
| GD | 3 (9.1%) | ||||
| OA | 2 (6.1%) | ||||
| LT | 1 (3.0%) | ||||
| NG | 19 (57.6%) | ||||
| NIFTP | 3 (9.1%) | ||||
| Cytology | No. of malignant cases (all) | ROM (%) |
|---|---|---|
| I | 8 (21) | 38.1 |
| II | 28 (180) | 15.6 |
| III | 25 (84) | 29.8 |
| IV | 13 (70) | 18.6 |
| V | 61 (67) | 91 |
| VI | 55 (59) | 93.2 |
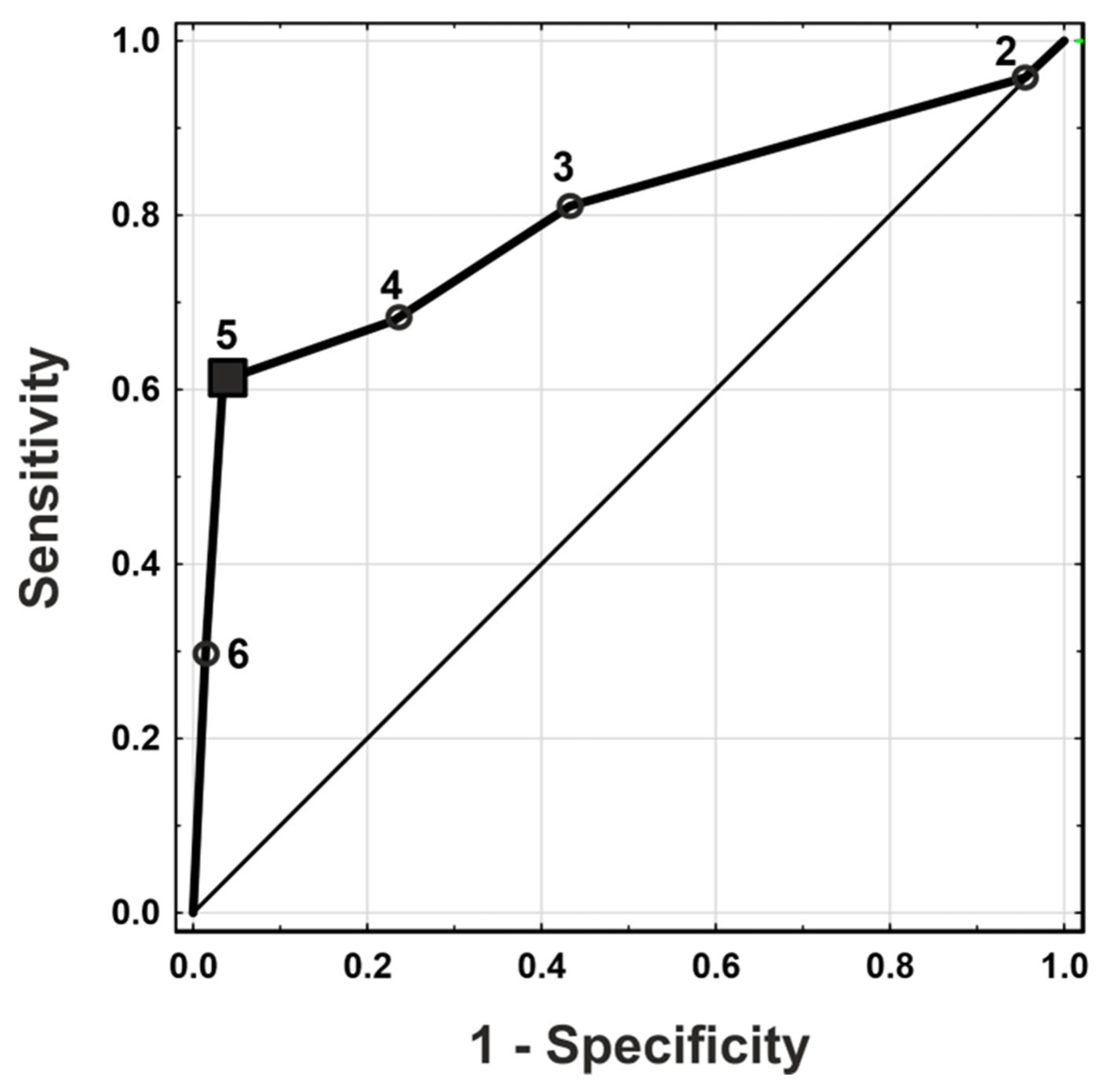
| Criterion | TP | FP | FN | TN | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|---|---|---|
| 6 | 55 | 4 | 135 | 287 | 0.29 (0.23, 0.36) | 0.99 (0.97, 1.00) | 0.93 (0.84, 0.98) | 0.68 (0.63, 0.72) |
| 5 | 116 | 10 | 74 | 281 | 0.61 (0.54, 0.68) | 0.97 (0.94, 0.98) | 0.92 (0.86, 0.96) | 0.79 (0.75, 0.83) |
| 4 | 129 | 67 | 61 | 224 | 0.68 (0.61, 0.74) | 0.77 (0.72, 0.82) | 0.66 (0.59, 0.72) | 0.79 (0.73, 0.83) |
| 3 | 154 | 126 | 36 | 165 | 0.81 (0.75, 0.86) | 0.57 (0.51, 0.62) | 0.55 (0.49, 0.61) | 0.82 (0.76, 0.87) |
| 2 | 182 | 278 | 8 | 13 | 0.96 (0.92, 0.98) | 0.04 (0.02, 0.08) | 0.40 (0.35, 0.44) | 0.62 (0.38, 0.82) |
| Total (N=401) |
II (N=180) |
III (N=84) |
IV (N=70) |
V (N=67) |
p-value | |
|---|---|---|---|---|---|---|
| Sex | 0.39 | |||||
| Female | 326 (81.3%) | 140 (77.8%) | 69 (82.1%) | 60 (85.7%) | 57 (85.1%) | |
| Male | 75 (18.7%) | 40 (22.2%) | 15 (17.9%) | 10 (14.3%) | 10 (14.9%) | |
| Age | 0.00001 | |||||
| Mean (SD) | 53.6 (14.7) | 55.2 (14.4) | 54.6 (12.0) | 55.8 (16.3) | 45.8 (14.3) | |
| Median [Q1-Q3] | 54.0 [42.0-66.0] | 56.0 [44.0-67.0] | 57.0 [44.0-65.3] | 58.0 [42.0-68.0] | 42.0 [35.5-56.0] | |
| Histological main diagnosis | < 0.0001 | |||||
| Benign | 274 (68.3%) | 152 (84.4%) | 59 (70.2%) | 57 (81.4%) | 6 (9.0%) | |
| Cancer | 127 (31.7%) | 28 (15.6%) | 25 (29.8%) | 13 (18.6%) | 61 (91.0%) | |
| Lymphocytic thyroiditis | < 0.0001 | |||||
| no | 340 (84.8%) | 163 (90.6%) | 74 (88.1%) | 62 (88.6%) | 41 (61.2%) | |
| yes | 61 (15.2%) | 17 (9.4%) | 10 (11.9%) | 8 (11.4%) | 26 (38.8%) |
| Bethesda categories | Malignancy | |
|---|---|---|
| n(%)/ n(%) | p value | |
| III vs II | 25 (29.8%)/ 28 (15.6%) | 0.019 |
| III vs IV | 25 (29.8%)/ 13 (18.6%) | 0.161 |
| III vs V | 25 (29.8%)/ 61 (91.0%) | <0.0001 |
| II vs IV | 28 (15.6%)/ 13 (18.6%) | 0.572 |
| II vs V | 28 (15.6%)/ 61 (91.0%) | <0.0001 |
| IV vs V | 13 (18.6%)/ 61 (91.0%) | <0.0001 |
| Bethesda categories | Lymphocytic thyroiditis | |
|---|---|---|
| n(% )/ n(%) | p value | |
| III vs II | 10 (11.9%)/ 17 (9.4%) | 0.98 |
| III vs IV | 10 (11.9%)/ 8 (11.4%) | 0.99 |
| III vs V | 10 (11.9%)/ 26 (38.8%) | 0.0008 |
| II vs IV | 17 (9.4%)/ 8 (11.4%) | 0.98 |
| II vs V | 17 (9.4%)/ 26 (38.8%) | < 0.0001 |
| IV vs V | 8 (11.4%)/ 26 (38.8%) | 0.0009 |
| Variable | OR per | Univariate | Multivariate* | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |||
| Sex | Female/male | 0.87 (0.51-1.51) | 0.63 | 1.02 (0.47-2.18) | 0.96 | |
| Lymphocytic thyroiditis | yes/no | 2.85 (1.63-4.96) | 0.0002 | 1.31 (0.55-3.11) | 0.54 | |
| Bethesda III | yes/no | 0.89 (0.53-1.51) | 0.67 | 1.68 (0.81-3.47) | 0.16 | |
| Bethesda IV | yes/no | 0.43 (0.23-0.83) | 0.011 | 1.51 (0.69-3.32) | 0.30 | |
| Bethesda V | Bethesda V | yes/no | 41.28 (17.11-99.61) | < 0.0001 | 62.34 (20.16-192.8) | < 0.0001 |
| Bethesda V | ||||||
| Age<=50 | yes/no | 2.67 (1.74-4.12) | < 0.0001 | 2.31 (1.27-4.19) | 0.006 | |
| Obesity (BMI>=30) | yes/no | 0.90 (0.53-1.51) | 0.68 | 1.2 (0.62-2.31) | 0.59 | |
| Bethesda category |
TBSRTC [6] first edition |
TBSRTC [8] second edition |
ROM of our study n=521 |
ROM Inabnet et al. [24] n= 1,746 |
ROM Anand et al. [25] n=646 |
ROM Zarif at al. [26] n=373 |
|---|---|---|---|---|---|---|
| I – nondiagnostic II – benign III – AUS/FLUS IV - FN/SFN V – SFM VI -malignant |
1-4% 0-3% 5-15% 15-30% 60-75% 97-99% |
5-10% 0-3% 10-30% 25-40% 50-75% 97-99% |
38.8% 15.6% 29.8% 18.6% 91.0% 93.2% |
19.2% 12.7% 31.9% 31.4% 77.8% 96.0% |
8.5% 66.7% 63.6% 100% 100% |
34.6% 15.6% 50% 52% 95.7% 100% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





