1. Introduction
This review aims to address a critical issue in the diagnosis of vestibular disorders by comprehensively examining the role of imaging modalities, such as computed tomography (CT) and magnetic resonance imaging (MRI), in various vestibular pathologies. By clarifying the utility and limitations of these techniques, this article seeks to improve diagnostic accuracy, optimize patient care, and inform clinical decision-making. While these symptoms often originate from the vestibular system, accurate diagnosis can be challenging for healthcare practitioners due to the complex interplay of clinical presentation, diagnostic tests, and imaging findings. Despite the increasing availability of advanced imaging technologies, there remains a knowledge gap regarding their appropriate use in evaluating vestibular disorders.
Dizziness and vertigo are common complaints encountered by primary care physicians, neurologists, and ENT specialists. It seems that vertigo and dizziness affect 15% to over 20 % yearly in adult large population-based studies [
1]. The two symptoms have a prevalence of 20 to 56% in general population [
2]. The prevalence of dizziness, vertigo and balance disorders increases with age [
3]. These symptoms originate usually from lesions within the vestibular system[
4] or pathological changes in the central nervous system [
5]. Diagnosing the exact site and nature of these lesions involves a thorough history, an attentive clinical examination, functional vestibular testing and different imaging modalities [
6].
The initial and most important step in diagnosing vertigo and dizziness is a comprehensive anamnesis. The history allows experienced physicians to correctly diagnose two-thirds of cases based solely on the information about the symptoms, the characteristics, and the evolution of the disease [
7]
,[
8,
9]. A physical examination can identify the affected side by assessing the alteration in the function of the vestibulo-ocular and the vestibulo-spinal reflexes[
10,
11,
12]. Abnormalities in the vestibulo-ocular reflex often manifest as nystagmus. Nystagmus is an involuntary, rhythmic movement of the eyes that can be congenitally or acquired by lesions in the vestibular organs or the central nervous system [
13]. A vestibular nystagmus has two phases: a rapid phase that characterizes the sense of the nystagmus and a slow phase toward the side with the vestibular deficit. Nystagmus in vestibular disorders can be spontaneous or triggered by positional changes. In order to assess the patient with vestibular disorders caloric, or rotational stimuli may be applied to elicit a vestibular nystagmus [
14]
Laboratory examinations, the third diagnostic step, include functional testing using electro- or video-oculography with positional, caloric, and rotational assessments[
15]. Electro or video-oculography is widely used to measure eye movement in clinical environment providing information about nystagmus in different conditions. The vestibular test battery often comprises the video-head impulse test (vHIT), vestibular evoked myogenic potentials (VEMP), and posturography [
16]. The vHIT is used to quantitatively assess the vestibulo-ocular reflex by measuring the speed of the eye movements during head movements. VEMP is performed stimulating one ear with repetitive pulse or click sounds while collecting EMG responses over selected muscles. VEMP is useful in detecting superior canal dehiscence, but it can provide information for the holistic examination of the vestibular function. Posturography is a method of examining the patient’s posture while standing for differentiating the contribution of vestibular, visual, and proprioceptive inputs to maintain equilibrium.
Radiology is seldom mentioned in the literature as a diagnostic modality for the evaluation of vestibular disorders. Although imaging is not explicitly emphasized in guidelines for diagnosing vestibular disorders, many physicians recommend MRI or CT scans during the initial consultation. However, it is estimated that 50% of imaging tests in general medicine are unnecessary [
17]. Specifically, it seems that only 5-10% of MRI scans in patients with vestibular disorders reveal findings directly related to the pathological process [
18].
2. Materials and Methods
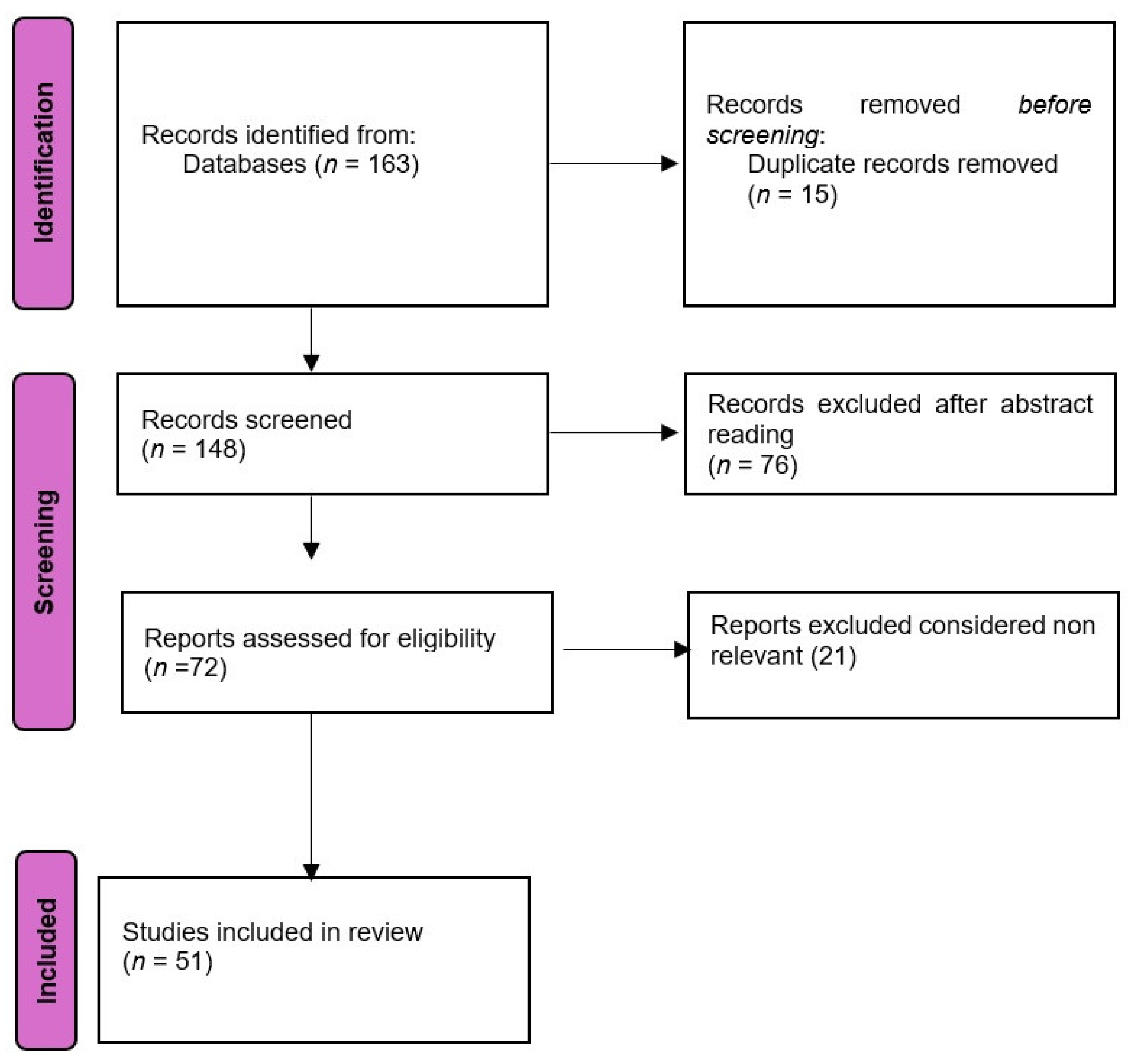
We performed a search in Pubmed database and Cochrane Library using the Boolean terms (((vestibular disorders) AND (MRiI) AND (CT) AND (diagnosis) which yielded 163 titles (153 in Pubmed + 4 in Cochrane library).We searched titles from 1986 until 2024. The inclusion criteria were narrative and systematic reviews, clinical trials, meta-analyses, clinical case studies, and randomized control studies. Exclusion criteria were letters to editorials of magazines, abstracts to conferences, and unpublished materials. The flow chart of the search is presented in
Figure 1. The search results were evaluated by two groups of independent reviewers in order to evaluate the quality and relevance of each title. Any disagreements were resolved by a third reviewer was consulted in order to limit possible bias.
3. Imaging Modalities in Vestibular Disorder Diagnosis
There are imaging tools available for evaluating patients with vestibular disorders. To accurately diagnose vestibular disorders, a detailed visualization of the inner ear, the trajectory of the vestibulo-cochlear nerves, and central nervous system pathways can be useful [
19]. Taking into account that most of the vestibular disorders originate from the peripheral vestibular system, a detailed image of the vestibular end organs is required. The size of the inner ear and of the vestibular structures involved in maintaining balance necessitates an imaging modality able to visualize small-scale details. The imaging modalities usually used in the assessment of vestibular disorders are computed tomography and magnetic resonance imaging. In present, none of these methods provide enough information about minute details of the anatomy of the vestibular end organs in order to assess their modifications in different vestibular disorders because of the fact that the resolution is insufficient. Physicians usually refer patients for imagistic evaluation to diagnose a possible central vestibular disorder. A progressive hearing loss associated with vestibular symptoms is a good indication for a radiologic evaluation because it can indicate an acoustic neuroma. A clinician should consider imagistic exploration whenever the patient has neurologic symptoms, cerebrovascular disease risk factors, or unilateral progressive hearing loss[
20].
Computed Tomography (CT): CT uses X-rays and a computer to create detailed images of tissues of varying densities. It is a very good method used to characterize the bony anatomy of the labyrinth. It is used to view erosions, fractures, displacement of prostheses. While iodine contrast agents can enhance resolution, CT is generally insufficient for detecting inner ear abnormalities associated with vestibular disorders and its contribution to the clinical diagnosis of vertigo is limited. CT is often used in the emergency department to visualize hemorrhage or infraction in the brain structures. However, it is considered that CT and CT angiograms have low diagnostic yield in the diagnosis of vertigo [
21]. New data signalize the possibility of identifying the side of the vestibular deficit by using the head CT evaluating a sign called “Vestibular eye sign”- VES defined as an eye deviation that correlates with the slow phase of the nystagmus [
22].
Magnetic Resonance Imaging (MRI): MRI employs strong magnetic fields and radiofrequency waves to generate images based on tissue signal intensities. It does not use the damaging X-ray radiation [
23]. MRI is significantly better than CT in evaluating soft tissue modifications [
24]. The MRI is required to image the structures of the inner ear with the perilymphatic and the endolymphatic spaces and the vestibular nerves. Although MRI with contrast agents like gadolinium and the special sequences provide better visualization of the inner ear and vestibular nerve, it often lacks sufficient detail for comprehensive assessment. MRI is the imaging of choice for brain lesions and that is why it is better for the differential diagnosis between the peripheral and central vestibular disorders. It is the main method of investigation used to characterize stroke and space occupying lesions [
25]. DWI is used for the assessment of routine ischemia, while gadolinium is used for characterizing inflammatory and tumoral lesions. Nonetheless, both MRI and CT are instrumental in distinguishing peripheral from central vestibular disorders [
18].
3.1. Specific Vestibular Disorders and Imaging Utility
3.1.1. Vestibular Neuritis
Is a peripheral vestibular disorder considered to be caused by an inflammation of the vestibular portion of the eighth nerve presenting with vertigo, nausea and gait imbalance. It is also known as vestibular neuronitis. The Barany Society suggests renaming it “acute unilateral vestibulopathy” [
26] This peripheral vestibular disorder involves damage to the vestibular nerve fibers, possibly due to a viral infection, although a vascular etiology cannot be totally rejected. Nowadays, the viral theory is accepted and a reactivation of the HSV-1 latent in the vestibular ganglia is incriminated [
27]. The annual incidence is reported as 3,5-15,5 for 100000 persons [
28]. It manifests as an acute vestibular syndrome due to sudden loss of the vestibular function without auditive symptoms. Symptoms include acute vertigo, imbalance, nausea, and vomiting, typically resolving slowly with central compensation. Symptoms are accentuated, not triggered by head movement. Usually, it lasts for several days, but it can last weeks to months until the complete resolution of symptoms. At the clinical exam, patients exhibit horizontal or horizontal-rotatory nystagmus towards the unaffected ear and deviation of the body towards the diseased side. The nystagmus is always unidirectional with the slow phase toward the diseased ear and follows the law of Alexander (it is more intense when the patient is looking to the healthy side). The Head impulse test (HIT) is positive on the lesioned side. Video HIT, VNG with calorics and VEMP tests show deficits on the affected side and are capable of detecting which division of the vestibular nerve is involved. Auditory function is usually preserved. Imaging has limited utility in diagnosing vestibular neuritis, as MRI and CT generally lack the resolution to detect such fine details. The diagnosis of vestibular neuritis is based on anamnesis and physical examination. MRI with contrast can sometimes show enhanced signals in the vestibular nerve[
29] [
30]. If the symptoms do not show signs of improvement in 48 hours and there are vascular risk factors, an imagistic evaluation is needed. In this case MRI is superior to CT in ruling out a stroke [
31].
3.1.2. Meniere’s Disease
It is also known as idiopathic endolymphatic hydrops because the presumed pathophysiologic mechanism is the increment of the pressure in the endolymphatic system. Although the disease was described by Dr Prosper Meniere in 1861 [
32], controversies regarding the pathophysiology still exist and the disease has unclear pathophysiology [
33]. Meniere’s disease is idiopathic by definition, but Meniere syndrome occurs secondary to various conditions that interfere with the production or resorption of endolymph such as autoimmune disfunction, endocrine abnormalities, electrolyte imbalance, medication. Meniere’s diseases is characterized by spontaneous vertigo attacks (lasting 20 minutes to 24 hours) accompanied by nausea, vomiting, fluctuating hearing loss, tinnitus and a sensation of ear pressure. It affects 20 to 200 per 100000 persons per year, most frequently between 40-60 years of age [
34]. The prevalence of the diseases varies widely from 15 per 100000 in US to 157 per 10000 in UK. There are different guidelines for the diagnosis of the disease issued by different medical societies. The American Society of Otolaryngology and Head and Neck Surgery bases the diagnosis of Meniere solely on clinical symptoms [
35]. According to the guideline published in 2020, Meniere is a clinical condition defined by spontaneous attacks of vertigo (lasting from 20 minutes to 12 hours), documented sensorineural hearing loss (before, during, or after the attack), and other fluctuating aural symptoms such as tinnitus or ear fulness. The physical examination findings are not remarkable or specific to the disease because the status may vary depending on the phase. Audiometric testing may show low to mid frequencies hearing loss[
36]. Different electrophysiological tests have been used as diagnostic modalities for the disease but none of them reached international consensus. The electrophysiologic tests or other functional tests that have been proposed as a diagnostic procedure are: ECoG, VNG, VEMP, or otoacoustic emissions, but none of them is pathognomonic [
37]. Electrocochleography measures the ratio between the summation potential (possibly produced by the displacement of the basilar membrane) and the nerve action potential, hydrops being suggested by an elevation of this ratio bigger than 35%. Videonystagmography records and measures the nystagmus, but the direction may vary during or after an attack and it is not a reliable indicator of the diseased side. In general, the nystagmus has a fast phase away from the affected ear because Meniere’s diminishes the reactions of the diseased ear, however an irritative phase may appear during the attack.
The guideline of the AAO-HNS states in the guideline that physicians may offer as an option magnetic resonance imaging (MRI) of the internal auditory canal (IAC) and posterior fossa in patients with possible Meniere’s disease and audiometrically verified asymmetric hearing loss [
35]. Imaging, particularly high-resolution CT and MRI, has a controversial utility [
38]. CT may show some modifications compatible with Meniere’s disease. A narrowed vestibular aqueduct is encountered more frequently in affected ears [
39,
40]. Non-contrast enhanced MRI can show in advanced cases elongation of the saccule, diminished fluid in the cochlear aqueduct, or invisibility of the endolymphatic sac and duct [
41,
42]. One of the most important steps in the imaging diagnosis of endolymphatic hydrops has been the MRI with gadolinium contrast. Initially, it was administered intratympanically with delayed acquisition. Later it was reported the fact that endolymphatic hydrops can be visualised also after intravenous administration of contrast [
43]. The gadolinium based contrast agent accumulates in the perilymphatic space, allowing the visualization of the less permeable endolymphatic space. In 3D FLAIR MRI his space appears as signal voids surrounding hyperintense perilymph, so it highlights changes in the inner ear consistent with endolymphatic hydrops. These changes can be enlargement of the cochlear endolymphatic space, an increment of the vestibular endolymphatic space occupying over one-third of the vestibular space (50% should be significant), and saccule larger than utricle [
44,
45]. In
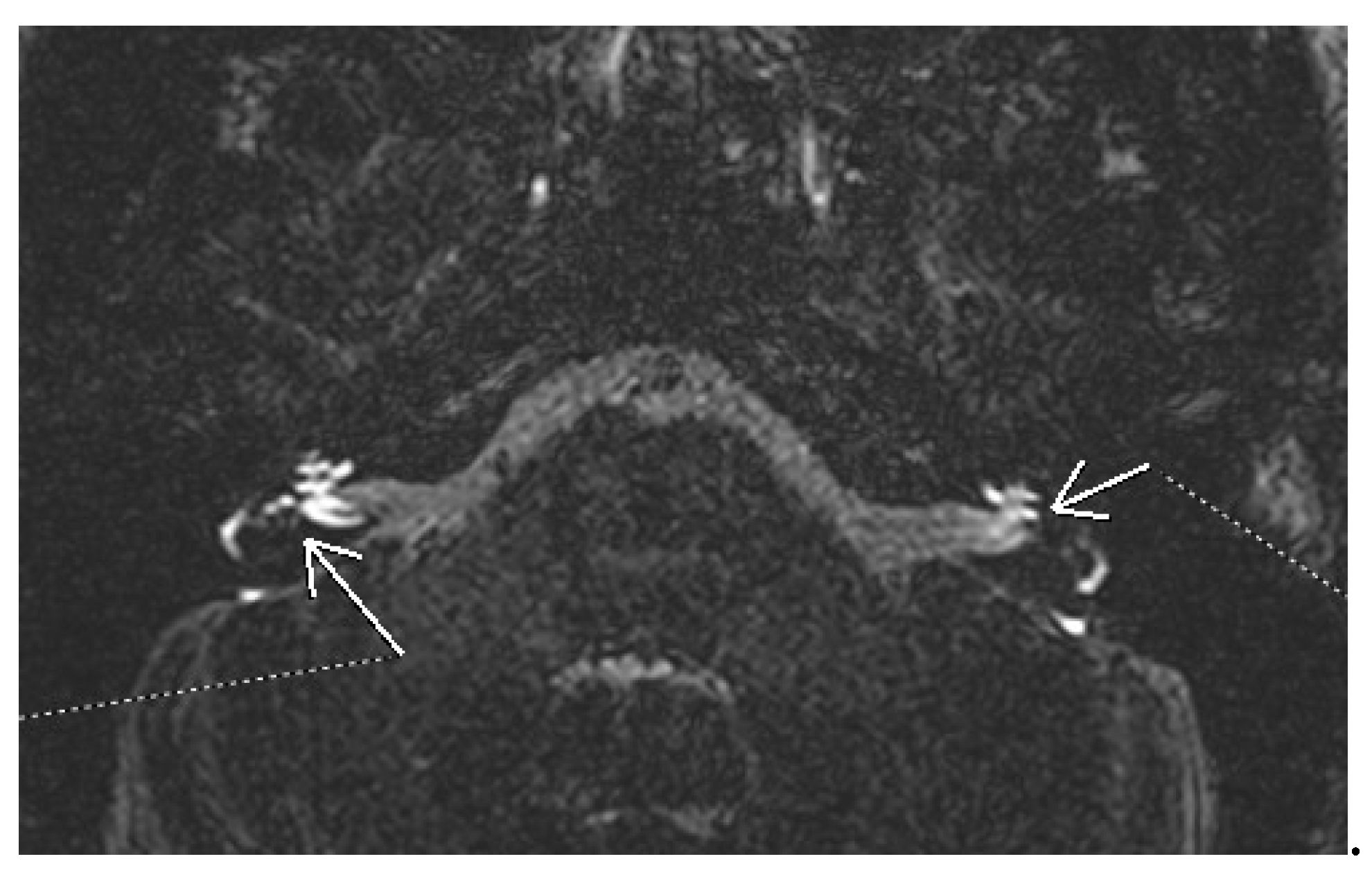
Figure 1 there is the image of bilateral hydrops seen on a 3D FLAIR sequence evaluated 4 hours after iv administration of gadolinium. Different qualitative and semiquantitative criteria for describing the endolymphatic hydrops have been proposed over the years. The most well-known is the Nakashima criteria. It defines vestibular hydrops as an endolymph/peryplimph ratio of >33%. For the cochlear hydrops, any visual hydrops of the cochlear duct is considered hydropic [
46]. Later Barath modified this classification into a 3-point scale categorizing the cochlear and vestibular hydrops into none, grade 1, and grade 2 [
47]. Except for hydrops, there are other MRI features compatible with Meniere’s. The non-visualization of saccule has been described in some patients. The proposed explanation is either the collapse or the fistulation noticed in some histopathologic studies [
48]. Recently a “round window sign” has been described as a hyperintense signal in the region of the round window on delayed 3D FLAIR. This has been hypothesized as a sign of perilymphatic fistula [
49]. Further research is needed to establish the role of MRI in the diagnosis of Meniere disease, to improve and validate the techniques in acquisition and interpretation.
Figure 1.
3D FLAIR MRI of bilateral cochlear and vestibular hydrops.
Figure 1.
3D FLAIR MRI of bilateral cochlear and vestibular hydrops.
3.1.3. Benign Paroxysmal Positional Vertigo (BPPV)
BPPV is the most common peripheral vestibular disorder accounting for more than half of the cases [
50]. Although it can occur at any age, the peak incidence of BPPV is between 50-70 years [
51]. It is considered that a minimum of 20% of the patients presenting with vertigo have BPPV. The pathophysiology of the disease consists in dislodged otoliths from the utricular membrane entering the semicircular canals. The otoliths modify the rheology of the endolymph in the canal during head movements generating vertigo. The diagnosis of BPPV is based on the history and the physical examination. The disease is characterized by short, recurrent, paroxysmal episodes of vertigo triggered by movements of the head. The gold standard for the diagnosis is the positivity of the Dix-Hallpike test for the posterior canal BPPV or the supine-roll test for the horizontal canal BPPV [
52]. Laboratory tests are not indicated for the diagnosis of BPPV. The otoliths, ranging from 1 to 20 μm in size, are too small to be detected by CT, which has a spatial resolution limit of 125 μm [
53]. The American Academy of Otolaryngology advises against radiographic imaging for BPPV diagnosis unless atypical symptoms are present [
54]. Imaging tests are ordered only to rule out other vestibular disorders.
3.1.4. Acoustic Neuroma
This benign tumor develops from the Schwann cell sheath of the eighth nerve in the internal auditory canal invading the cerebellopontine angle in its growth. The WHO classifies schwannomas as grade I benign tumors. It seems that most of the time it develops from the inferior division of the vestibular component of the nerve. Usually, it is a unilateral sporadic tumor. Bilateral vestibular schwannomas are suggestive of type 2 neurofibromatosis, a genetic autosomal dominant disorder [
55]. The NF2 gene on the 22 chromosome is responsible for the appearance of the tumors in sporadic or syndromic schwannoma development. From a histopathologic point of view, the schwannomas are classified into classic schwannomas (with 2 distinct regions Antoni A and B), cellular schwannomas (relatively uncommon), plexiform schwannomas (associated with schwanomatosis, NF2 and other syndromes), and melanotic schwannomas (potentially malignant neoplasm). The incidence of vestibular schwannomas is 1,2 cases per 100000 persons/year [
56]. It accounts for 6-8% of intracranial tumors and 80% of cerebello-pontine angle tumors. Schwannomas may exist for years without any sign because it has a slow growth. The tumors often cause progressive hearing loss and nonpulsatile tinnitus. The vestibular symptoms are less pronounced due to gradual tumor growth and central compensation [
57]. Neurologic symptoms may appear when the tumor is large and invades the cerebello pontine angle and presses on the CNS structures. Facial nerve palsy is rare as a first presentation sign. It is a benign tumor, but it can be dangerous on account of the extension and the compression at the level of the adjacent structures[
58] [
59]. Assessing the location, size, and growth rate is mandatory for identifying and establishing the treatment approach. Most of the tumors have an intra-canalicular component enlarging the porus acusticus (“trumpeted internal acoustic canal”), extracanalicular extension may result in an “ice cream cone “aspect; a minority of tumors are only extracanalicular. The Koos grading system is used in order to classify the size of the tumor with impact on the hearing preservation rate after surgery [
60]. This is the reason why imaging is of paramount importance in the diagnosis of the neuroma. It can differentiate acoustic neuromas from other cerebellopontine tumors such as meningiomas, metastases, cholesteatomas, vascular lesions, epidermoid cysts, exophytic gliomas [
61]. Vestibular schwannomas are well-circumscribed, encapsulated tumors, separated from the nerve fibers. Small tumors tend to be solid, while large ones may have cystic degeneration or hemorrhage. Typically, there are no calcifications.
For the radiologic evaluation of vestibular schwannomas MRI and CT scans are considered. MR is the preferred technique use to characterize, therapeutic planning and post therapeutic evaluation for acoustic neuromas. CT is used whenever MRI is contraindicated for different reasons. CT may reveal internal acoustic canal enlargement or bone erosion, with contrast enhancing the lesion. In some cases, enhancement can be underwhelming especially in large, cystic tumors [
62]. The lesion is hard to see especially on account of the adjacent petrous bone artifacts. MRI, particularly with contrast, is the “gold standard” [
63], showing the tumor [
62]as hypointense or isointense on T1 and hyperintense on T2 images, with intense contrast enhancement [
64]. In
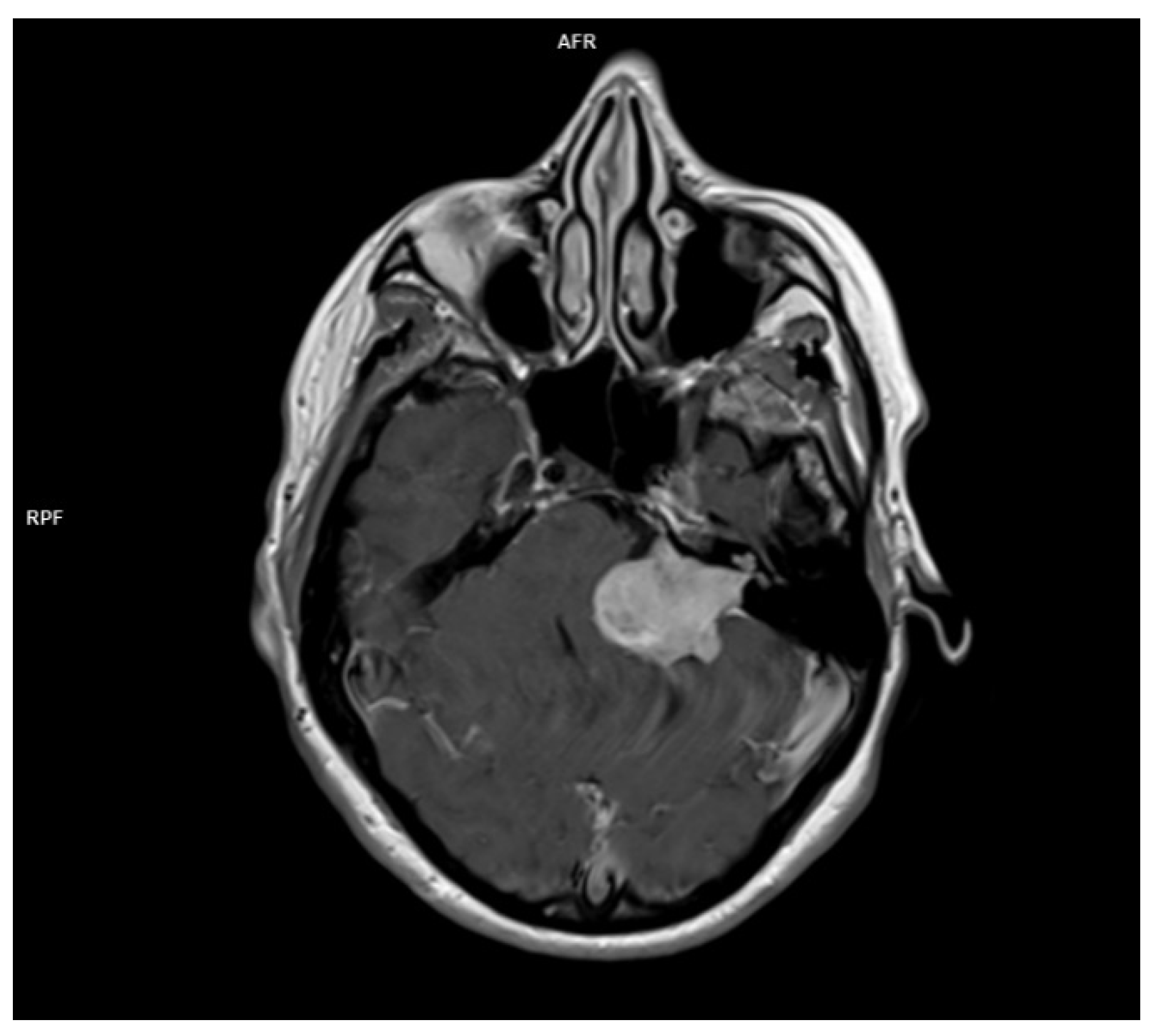
Figure 2. there is the image of a huge vestibular schwannoma seen on a T1 sequence with contrast invading the cerebello-pontine angle and compressing the CNS adjacent structures. A target sign (a T2 hyperintensity circumscribing a central hypointensity) is a specific sign for a peripheral nerve sheath tumor, but it is not specific for a schwannoma.
Figure 2.
MRI T1 with contrast of a left acoustic neuroma occupying the internal acoustic canal, the pontocerebellar angle compressing the CNS adjacent structures.
Figure 2.
MRI T1 with contrast of a left acoustic neuroma occupying the internal acoustic canal, the pontocerebellar angle compressing the CNS adjacent structures.
In the last period, due to the accessibility of the MRI, a large number of schwannomas are discovered. Many of these tumors do not necessitate surgery immediately or other treatment modalities and a “wait and scan” attitude is adopted. MRI is indeed instrumental in assessing tumor characteristics, including size, location, and growth rate. The decision to proceed with surgery is a complex one, influenced by various factors such as tumor growth rate, patient symptoms, and overall health. Regular MRI follow-up is crucial to monitor tumor progression and determine the optimal timing for surgical intervention. MRI is essential in deciding the moment of the surgery and in monitoring patients postoperatively, especially in patients with cochlear implantation following the removal of the tumor [
65].
Another issue to discuss is the fact that one of the initial presentations of acoustic neuroma can be sudden sensory neural hearing loss (SSNHL)[
66] or an acute vertigo mimicking a vestibular neuritis [
67]. These atypical presentations underscore the importance of considering vestibular schwannoma in the differential diagnosis and prompt initiation of imaging studies, such as MRI, to rule out this potentially life-changing condition.
3.1.5. Superior Canal Dehiscence
This rare disorder in which a bone defect creates a third window in the labyrinth [
68]. The disease is characterized by vertigo triggered by loud sounds or pressure, hearing loss, autophonia, and tinnitus [
69]. The vestibular symptoms induced by loud sounds are called the Tullio phenomenon because he was the first to describe it [
70]. The vestibular symptoms induced by the increment of the pressure in the external auditory canal are called Hennebert sign [
71]. Minor found a series of patients with both Tullio and Hennebert signs and related the findings with an anatomic defect of the superior canal bone [
72]. There is no single gold standard for the diagnosis of superior canal dehiscence, it is diagnosed through anamnesis, audiograms, VEMP (vestibular evoked myogenic potentials), and imaging [
73],[
74]. The typical audiogram in superior canal dehiscence is a combination of increased air conduction thresholds with lowered bone conduction thresholds at low frequencies [
75]. VEMP are myogenic potentials collected either on the sternocleidomastoidian muscle or the inferior oblique muscle as a response to the stimulation of the inner ear with loud sounds or galvanic stimulation, or a mechanical head tap. Although VEMP evaluates the integrity of the entire reflex pathway it is usually considered a test for the otolithic organs (saccule and utricle). cVEMP evaluates the saccule and the integrity of its connection through the inferior vestibular nerve. oVEMP measures the utricular and the superior nerve function. Consecutively to the apparition of a third window in the labyrinth, VEMP amplitudes increase, and thresholds decrease in case of superior canal dehiscence [
76].
Imaging studies are critical for the diagnosis of superior canal dehiscence syndrome. High-resolution CT scans with thin slices can detect bony defects in the superior semicircular canal at the level of arcuate eminence. If the slices are obtained at 0,5 mm instead of 1 mm the accuracy is increased from 50% to 90%[
68]. Although MRI is not traditionally used for the detection of superior canal dehiscence, recent research suggests its superior sensitivity and specificity, making it a valuable “rule-out” test. In a study published in 2013 MRI had a sensitivity of 96,5%, a positive predicting value of 61,1%, and a negative predictive value of 100% [
77].
In
Table 1 we present an overview of common vestibular disorders, highlighting key symptoms, diagnostic methods, and the role of imaging in their diagnosis. This table provides a concise summary of the information discussed.
3.1.6. Differential Diagnosis with Central Vertigo
Vertigo is a common symptom in posterior circulation stroke. Although usually, in a stroke of posterior circulation vertigo is associated with other neurologic signs or symptoms, it is possible that a small infarct is manifested only as vertigo [
78]. Acute stroke presenting only as vertigo can be easily mistaken as peripheral vestibulopathy. Roughly 25% of patients with acute vestibular syndrome have a potentially life-threatening disease and stroke is encountered in 4-15 % of cases [
79]. Differential diagnosis between central and peripheral vertigo is critical in the case of acute vestibular syndrome. It is based on clinical history, targeted physical examination and radiological evaluation.
The physical examination of the patient with acute vestibular syndrome is based on a battery of tests aimed to rapidly orientate the diagnosis in the emergency room. The HINTS battery of tests is used to assess the patient with acute vestibular syndrome in bedside examination. It is composed of three different tests: HIT (head impulse test), N (nystagmus), and S (test of skew). HIT assesses how the eyes move in response to rapid movement of the head; in case of a positive test, it is not possible to keep the eyes on the target and a corrective saccade is noticed. The direction of the nystagmus can change in relation to the position of the eyes in case of central pathology. The misalignment of the eyes called skew deviation is typical to central vestibular disorders [
80]. The combination of the three signs positive HIT, direction-changing nystagmus, and presence of a skew deviation is indicative of a central vestibular disorder. CT is widely used in the emergency room, for the evaluation of a stroke patient CT because it is available, fast, non-sensitive to movement. CT can be helpful in ruling-out cerebellar hemorrhage in patients with acute vestibular syndrome but hemorrhage is a rare cause of isolated vertigo and dizziness [
81]. A posterior fossa ischemic stroke, which is more susceptible to manifest only as vertigo, is difficult to evaluate with CT and might be missed. MRI has a higher soft tissue contrast and is superior to CT in acute and hyperacute phases. A negative MRI-DWI is useful in ruling out posterior fossa stroke earlier than CT and other MR sequences, although false-negative findings are still possible [
82]. There are studies showing that 50% of the MRI neuroimaging obtained in emergency situation is false negative [
79]. MRI-DWI misses 15-20% of posterior fossa infarction in the first 24 hours. HINTS test is considered more sensitive than MRI in diagnosing stroke in patients presenting with acute vertigo [
83,
84] [
85].
The present study has limitations mainly on account of the fact it is not a systematic review or a meta-analysis. The heterogeneity of the vestibular disorders in the review did not allow a detailed analysis of each topic. Some of the articles included in the study were not available in full text-open access.
4.1. Conclusions
Dizziness and vertigo are symptoms often encountered in clinical settings, with their incidence rising with age. These symptoms originated in either peripheral or central vestibular dysfunction. The diagnosis of these disorders necessitates a complex diagnostic approach using patient history, clinical examination, vestibular function tests, and imaging modalities.
While a detailed anamnesis remains the basis for diagnosing dizziness and vertigo, functional vestibular testing like video-oculography, VEMP, and computer posturography bring additional information for the evaluation of vestibular function. Imaging, particularly MRI, plays an important role in differentiating between peripheral and central causes of vestibular disorders, but exhibits a limited ability to detect fine vestibular anomalies.
Specific disorders such as vestibular neuritis, Meniere’s disease, BPPV, and acoustic neuromas each have distinct diagnostic profiles. For example, vestibular neuritis is primarily diagnosed through clinical evaluation and functional tests, with imaging reserved for ruling out central causes. Meniere’s disease, though primarily a clinical diagnosis, benefits from advanced MRI techniques to visualize endolymphatic hydrops. BPPV diagnosis relies heavily on clinical maneuvers like the Dix-Hallpike test, with imaging used to exclude other pathologies. Acoustic neuromas, conversely, require imaging for accurate localization and characterization, critical for guiding management.
Emerging imaging techniques, such as high-resolution CT for detecting superior canal dehiscence and advanced MRI sequences for visualizing endolymphatic hydrops, are increasing our ability to understand the pathophysiology and diagnose vestibular disorders. But these modalities complement rather than replace traditional diagnostic approaches.
Given the limitations of nowadays imagistic modalities a future development could be the use of artificial intelligence which is a promising emerging direction [
86]. Artificial intelligence can enhance the possibility of detecting subtle abnormalities of the inner ear or brain structures.
In summary, diagnosing dizziness and vertigo is a complex process that requires a special approach, combining clinical examination with advanced investigation modalities. Continued imaging and functional testing advancements will ensure accurate and earlier detection of vestibular disorders, thus improving patient outcomes. Future research should concentrate on developing these diagnostic tools and exploring new modalities to better diagnose and treat vestibular conditions.
Authors contributions
conceptualization, GCM, AAMM, CPT; methodology AM, OM: writing-original draft preparation GCM, AAMM, OM, CPT, MP; Writing-review of editing GCM, CPT. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed consent statement
Not applicable.
Acknowledgments
Publication of this article was supported by the ‘Carol Davila’ University of Medicine and Pharmacy (Bucharest, Romania), through the institutional program ‘Publish not Perish’.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Neuhauser H K, “Handbook of clinical neurology.”.
- R. Teggi et al., “Prevalenza dei sintomi vertigine e instabilità in un campione di 2672 soggetti e correlazione con il sintomo cefalea,” Acta Otorhinolaryngologica Italica, vol. 36, no. 3, pp. 215–219, May 2016. [CrossRef]
- H. W. Lin and N. Bhattacharyya, “Balance disorders in the elderly: Epidemiology and functional impact,” Laryngoscope, vol. 122, no. 8, pp. 1858–1861, Aug. 2012. [CrossRef]
- M. Strupp and T. Brandt, “Peripheral vestibular disorders,” Curr Opin Neurol, vol. 26, no. 1, pp. 81–89, Feb. 2013. [CrossRef]
- T. Brandt and M. Dieterich, “The dizzy patient: don’t forget disorders of the central vestibular system,” Nat Rev Neurol, vol. 13, no. 6, pp. 352–362, Jun. 2017. [CrossRef]
- V. Renga, “Clinical Evaluation of Patients with Vestibular Dysfunction,” Neurol Res Int, vol. 2019, pp. 1–8, Feb. 2019. [CrossRef]
- M. Strupp and V. Arbusow, “Acute vestibulopathy,” Curr Opin Neurol, vol. 14, no. 1, pp. 11–20, Feb. 2001. [CrossRef]
- J.-Y. Choi and J.-S. Kim, “Nystagmus and central vestibular disorders,” Curr Opin Neurol, vol. 30, no. 1, pp. 98–106, Feb. 2017. [CrossRef]
- B. M. Seemungal, “Neuro-otological emergencies,” Curr Opin Neurol, vol. 20, no. 1, pp. 32–39, Feb. 2007. [CrossRef]
- A. G. Feldman and L. Zhang, “Eye and head movements and vestibulo-ocular reflex in the context of indirect, referent control of motor actions,” J Neurophysiol, vol. 124, no. 1, pp. 115–133, Jul. 2020. [CrossRef]
- M. Bronstein, M. Patel, and Q. Arshad, “A brief review of the clinical anatomy of the vestibular-ocular connections—how much do we know?,” Eye, vol. 29, no. 2, pp. 163–170, Feb. 2015. [CrossRef]
- J. A. Edlow and D. Newman-Toker, “Using the Physical Examination to Diagnose Patients with Acute Dizziness and Vertigo,” J Emerg Med, vol. 50, no. 4, pp. 617–628, Apr. 2016. [CrossRef]
- A. Serra, “Diagnostic value of nystagmus: spontaneous and induced ocular oscillations,” J Neurol Neurosurg Psychiatry, vol. 73, no. 6, pp. 615–618, Dec. 2002. [CrossRef]
- S. D. Z. Eggers et al., “Classification of vestibular signs and examination techniques: Nystagmus and nystagmus-like movements,” Journal of Vestibular Research, vol. 29, no. 2–3, pp. 57–87, Jul. 2019. [CrossRef]
- M. M. Ganança, H. H. Caovilla, and F. F. Ganança, “Eletronistagmografia versus videonistagmografia,” Braz J Otorhinolaryngol, vol. 76, no. 3, pp. 399–403, Jun. 2010. [CrossRef]
- S. A. Zuniga and M. E. Adams, “Efficient Use of Vestibular Testing,” Otolaryngol Clin North Am, vol. 54, no. 5, pp. 875–891, Oct. 2021. [CrossRef]
- V. M. Rao and D. C. Levin, “The Overuse of Diagnostic Imaging and the Choosing Wisely Initiative,” Ann Intern Med, vol. 157, no. 8, p. 574, Oct. 2012. [CrossRef]
- S. E. J. Connor and N. Sriskandan, “Imaging of dizziness,” Clin Radiol, vol. 69, no. 2, pp. 111–122, Feb. 2014. [CrossRef]
- Bakous and Douglas, Vertigo and Disequilibrium: A Practical Guide to Diagnosis and Management, Second edition. 2017.
- D. Patkar, G. Yevankar, and R. Parikh, “Radiology in Vertigo and Dizziness,” An International Journal of Otorhinolaryngology Clinics, vol. 4, no. 2, pp. 86–92, Aug. 2012. [CrossRef]
- A. Guarnizo, K. Farah, D. A. Lelli, D. Tse, and N. Zakhari, “Limited usefulness of routine head and neck CT angiogram in the imaging assessment of dizziness in the emergency department,” Neuroradiol J, vol. 34, no. 4, pp. 335–340, Aug. 2021. [CrossRef]
- R. Farhat et al., “The ‘Vestibular Eye Sign’—‘VES’: a new radiological sign of vestibular neuronitis can help to determine the affected vestibule and support the diagnosis,” J Neurol, vol. 270, no. 9, pp. 4360–4367, Sep. 2023. [CrossRef]
- H.-M. Shi, H.-C. Sun, and F.-H. Ju, “Recommendations for reducing exposure to medical X-ray irradiation (Review),” Medicine International, vol. 2, no. 4, p. 22, Jul. 2022. [CrossRef]
- A. E. CHANG et al., “Magnetic Resonance Imaging Versus Computed Tomography in the Evaluation of Soft Tissue Tumors of the Extremities,” Ann Surg, vol. 205, no. 4, pp. 340–348, Apr. 1987. [CrossRef]
- S. Vadera and D. Smith, “MRI brain (summary),” in Radiopaedia.org, Radiopaedia.org, 2015. [CrossRef]
- M. Strupp et al., “Acute unilateral vestibulopathy/vestibular neuritis: Diagnostic criteria,” Journal of Vestibular Research, vol. 32, no. 5, pp. 389–406, Oct. 2022. [CrossRef]
- S. Himmelein et al., “Differential Involvement during Latent Herpes Simplex Virus 1 Infection of the Superior and Inferior Divisions of the Vestibular Ganglia: Implications for Vestibular Neuritis,” J Virol, vol. 91, no. 14, Jul. 2017. [CrossRef]
- Adamec, M. Krbot Skorić, J. Handžić, and M. Habek, “Incidence, seasonality and comorbidity in vestibular neuritis,” Neurological Sciences, vol. 36, no. 1, pp. 91–95, Jan. 2015. [CrossRef]
- M. Eliezer et al., “Detection of intralabyrinthine abnormalities using post-contrast delayed 3D-FLAIR MRI sequences in patients with acute vestibular syndrome,” Eur Radiol, vol. 29, no. 6, pp. 2760–2769, Jun. 2019. [CrossRef]
- H. Byun, J. H. Chung, S. H. Lee, C. W. Park, D. W. Park, and T. Y. Kim, “Clinical value of 4-hour delayed gadolinium-Enhanced 3D FLAIR MR Images in Acute Vestibular Neuritis,” Laryngoscope, vol. 128, no. 8, pp. 1946–1951, Aug. 2018. [CrossRef]
- B. Navi et al., “Rate and Predictors of Serious Neurologic Causes of Dizziness in the Emergency Department,” Mayo Clin Proc, vol. 87, no. 11, pp. 1080–1088, Nov. 2012. [CrossRef]
- R. W. Baloh, “Prosper Ménière and His Disease,” Arch Neurol, vol. 58, no. 7, p. 1151, Jul. 2001. [CrossRef]
- H. Thai-Van, M.-J. Bounaix, and B. Fraysse, “Meni??re???s Disease,” Drugs, vol. 61, no. 8, pp. 1089–1102, 2001. [CrossRef]
- Y. Watanabe, K. Mizukoshi, H. Shojaku, I. Watanabe, M. Hinoki, and M. Kitahara, “Epidemiological and Clinical Characteristics of Meniere’s Disease in Japan,” Acta Otolaryngol, vol. 115, no. sup519, pp. 206–210, Jan. 1995. [CrossRef]
- G. J. Basura et al., “Clinical Practice Guideline: Ménière’s Disease,” Otolaryngology–Head and Neck Surgery, vol. 162, no. S2, Apr. 2020. [CrossRef]
- K. Sharma, “Audiological Assessment in Meniere’s Disease,” in Up to Date on Meniere’s Disease, InTech, 2017. [CrossRef]
- A. Ciorba, P. H. Skarżyński, V. Corazzi, C. Bianchini, C. Aimoni, and S. Hatzopoulos, “Assessment Tools for Use in Patients with Ménière Disease: An Update,” Medical Science Monitor, vol. 23, pp. 6144–6149, Dec. 2017. [CrossRef]
- Kay-Rivest, D. R. Friedmann, and J. T. Roland, “Imaging for Menière Disease,” American Journal of Neuroradiology, vol. 41, no. 11, pp. 1964–1965, Nov. 2020. [CrossRef]
- H. Yamane et al., “Practical 3DCT imaging of the vestibular aqueduct for Meniere’s disease,” Acta Otolaryngol, vol. 135, no. 8, pp. 799–806, Aug. 2015. [CrossRef]
- T. Miyashita, Y. Toyama, R. Inamoto, and N. Mori, “Evaluation of the vestibular aqueduct in Ménière’s disease using multiplanar reconstruction images of CT,” Auris Nasus Larynx, vol. 39, no. 6, pp. 567–571, Dec. 2012. [CrossRef]
- A. Venkatasamy et al., “Imaging of the saccule for the diagnosis of endolymphatic hydrops in Meniere disease, using a three-dimensional T2-weighted steady state free precession sequence: accurate, fast, and without contrast material intravenous injection,” Eur Radiol Exp, vol. 1, no. 1, p. 14, Dec. 2017. [CrossRef]
- J.-H. Park, A. Shen, S. Keil, N. Kraemer, and M. Westhofen, “Radiological findings of the cochlear aqueduct in patients with Meniere’s disease using high-resolution CT and high-resolution MRI,” European Archives of Oto-Rhino-Laryngology, vol. 271, no. 12, pp. 3325–3331, Dec. 2014. [CrossRef]
- T. Nakashima et al., “Endolymphatic hydrops revealed by intravenous gadolinium injection in patients with Meniere’s disease,” Acta Otolaryngol, pp. 1–6, 2009. [CrossRef]
- A. Bernaerts, “MRI in Menière’s Disease,” J Belg Soc Radiol, vol. 102, no. S1, Nov. 2018. [CrossRef]
- G. Conte et al., “MR imaging of endolymphatic hydrops in Ménière’s disease: not all that glitters is gold,” Acta Otorhinolaryngologica Italica, vol. 38, no. 4, pp. 369–376, Aug. 2018. [CrossRef]
- T. Nakashima et al., “Grading of endolymphatic hydrops using magnetic resonance imaging,” Acta Otolaryngol, vol. 129, no. sup560, pp. 5–8, Jan. 2009. [CrossRef]
- Baráth, B. Schuknecht, A. M. Naldi, T. Schrepfer, C. J. Bockisch, and S. C. A. Hegemann, “Detection and Grading of Endolymphatic Hydrops in Menière Disease Using MR Imaging,” American Journal of Neuroradiology, vol. 35, no. 7, pp. 1387–1392, Jul. 2014. [CrossRef]
- Eliezer et al., “Clinical and radiological characteristics of patients with collapse or fistula of the saccule as evaluated by inner ear MRI,” Acta Otolaryngol, vol. 140, no. 4, pp. 262–269, Apr. 2020. [CrossRef]
- Dubrulle, V. Chaton, M. Risoud, H. Farah, Q. Charley, and C. Vincent, “The round window sign: a sensitive sign to detect perilymphatic fistulae on delayed postcontrast 3D-FLAIR sequence,” Eur Radiol, vol. 30, no. 11, pp. 6303–6310, Nov. 2020. [CrossRef]
- D. A. FROEHLING, M. D. SILVERSTEIN, D. N. MOHR, C. W. BEATTY, K. P. OFFORD, and D. J. BALLARD, “Benign Positional Vertigo: Incidence and Prognosis in a Population-Based Study in Olmsted County, Minnesota,” Mayo Clin Proc, vol. 66, no. 6, pp. 596–601, Jun. 1991. [CrossRef]
- P. Hilton and D. K. Pinder, “The Epley (canalith repositioning) manoeuvre for benign paroxysmal positional vertigo,” Cochrane Database of Systematic Reviews, Dec. 2014. [CrossRef]
- R. B. Halker, D. M. Barrs, K. E. Wellik, D. M. Wingerchuk, and B. M. Demaerschalk, “Establishing a Diagnosis of Benign Paroxysmal Positional Vertigo Through the Dix-Hallpike and Side-Lying Maneuvers,” Neurologist, vol. 14, no. 3, pp. 201–204, May 2008. [CrossRef]
- D. Bell, “Benign paroxysmal positional vertigo,” in Radiopaedia.org, Radiopaedia.org, 2017. [CrossRef]
- Bhattacharyya et al., “Clinical Practice Guideline: Benign Paroxysmal Positional Vertigo (Update),” Otolaryngology–Head and Neck Surgery, vol. 156, no. S3, Mar. 2017. [CrossRef]
- L. Kluwe, “Molecular study of frequency of mosaicism in neurofibromatosis 2 patients with bilateral vestibular schwannomas,” J Med Genet, vol. 40, no. 2, pp. 109–114, Feb. 2003. [CrossRef]
- R. Babu, R. Sharma, J. H. Bagley, J. Hatef, A. H. Friedman, and C. Adamson, “Vestibular schwannomas in the modern era: epidemiology, treatment trends, and disparities in management,” J Neurosurg, vol. 119, no. 1, pp. 121–130, Jul. 2013. [CrossRef]
- C. Matthies and M. Samii, “Management of 1000 Vestibular Schwannomas (Acoustic Neuromas): Clinical Presentation,” Neurosurgery, vol. 40, no. 1, pp. 1–10, Jan. 1997. [CrossRef]
- X. HUANG et al., “Clinical features of intracranial vestibular schwannomas,” Oncol Lett, vol. 5, no. 1, pp. 57–62, Jan. 2013. [CrossRef]
- A. Venkatasamy, C. Nicolas-Ong, H. Vuong, A. Charpiot, and F. Veillon, “Extension patterns of vestibular schwannomas towards the middle ear: three new cases and review of the literature,” European Archives of Oto-Rhino-Laryngology, vol. 276, no. 4, pp. 969–976, Apr. 2019. [CrossRef]
- W. T. Koos, J. D. Day, C. Matula, and D. I. Levy, “Neurotopographic considerations in the microsurgical treatment of small acoustic neurinomas,” J Neurosurg, vol. 88, no. 3, pp. 506–512, Mar. 1998. [CrossRef]
- L. R. Gentry, C. G. Jacoby, P. A. Turski, L. W. Houston, C. M. Strother, and J. F. Sackett, “Cerebellopontine angle-petromastoid mass lesions: comparative study of diagnosis with MR imaging and CT.,” Radiology, vol. 162, no. 2, pp. 513–520, Feb. 1987. [CrossRef]
- Deng and F. Gaillard, “Vestibular schwannoma,” in Radiopaedia.org, Radiopaedia.org, 2008. [CrossRef]
- E. Hofmann and L. Choné, “Neuroradiologische Bildgebung des Akustikusneurinoms (Vestibularisschwannoms),” HNO, vol. 59, no. 1, pp. 9–15, Jan. 2011. [CrossRef]
- T. H. Mulkens et al., “Acoustic schwannoma: MR findings in 84 tumors.,” American Journal of Roentgenology, vol. 160, no. 2, pp. 395–398, Feb. 1993. [CrossRef]
- V. Dallari et al., “Cochlear Implantation Following Transcanal Infrapromontorial Approach for Vestibular Schwannoma: A Case Series,” Audiol Res, vol. 13, no. 1, pp. 1–11, Dec. 2022. [CrossRef]
- M. Song et al., “Sudden sensorineural hearing loss as the initial symptom in patients with acoustic neuroma,” Front Neurol, vol. 13, Aug. 2022. [CrossRef]
- J. Y. Park and C.-H. Kim, “Vestibular Schwannoma Presenting as Acute Vertigo Mimicking Vestibular Neuritis,” Case Rep Neurol, vol. 14, no. 3, pp. 464–468, Nov. 2022. [CrossRef]
- C. J. Belden, N. Weg, L. B. Minor, and S. J. Zinreich, “CT Evaluation of Bone Dehiscence of the Superior Semicircular Canal as a Cause of Sound- and/or Pressure-induced Vertigo,” Radiology, vol. 226, no. 2, pp. 337–343, Feb. 2003. [CrossRef]
- M. Palma Diaz, J. Cisneros Lesser, and A. Vega Alarcón, “Superior Semicircular Canal Dehiscence Syndrome – Diagnosis and Surgical Management,” Int Arch Otorhinolaryngol, vol. 21, no. 02, pp. 195–198, Apr. 2017. [CrossRef]
- J. Addams-Williams, K. Wu, and J. Ray, “The experiments behind the Tullio phenomenon,” J Laryngol Otol, vol. 128, no. 3, pp. 223–227, Mar. 2014. [CrossRef]
- A. G. Shuman, S. S. Rizvi, C. W. Pirouet, and K. D. Heidenreich, “Hennebert’s sign in superior semicircular canal dehiscence syndrome: A Video Case Report,” Laryngoscope, vol. 122, no. 2, pp. 412–414, Feb. 2012. [CrossRef]
- Minor L B, “Superior canal dehiscence syndrome,” Am J Otol . vol. 1, no. 21, pp. 9–19, 2000.
- W. W. Chien, J. P. Carey, and L. B. Minor, “Canal dehiscence,” Curr Opin Neurol, vol. 24, no. 1, pp. 25–31, Feb. 2011. [CrossRef]
- B. K. Ward et al., “Superior semicircular canal dehiscence syndrome: Diagnostic criteria consensus document of the committee for the classification of vestibular disorders of the Bárány Society,” Journal of Vestibular Research, vol. 31, no. 3, pp. 131–141, May 2021. [CrossRef]
- Y. S. Cheng, S. Raufer, X. Guan, C. F. Halpin, D. J. Lee, and H. H. Nakajima, “Superior Canal Dehiscence Similarly Affects Cochlear Pressures in Temporal Bones and Audiograms in Patients,” Ear Hear, vol. 41, no. 4, pp. 804–810, Jul. 2020. [CrossRef]
- K. S. Noij and S. D. Rauch, “Vestibular Evoked Myogenic Potential (VEMP) Testing for Diagnosis of Superior Semicircular Canal Dehiscence,” Front Neurol, vol. 11, Jul. 2020. [CrossRef]
- Browaeys, T. L. Larson, M. L. Wong, and U. Patel, “Can MRI Replace CT in Evaluating Semicircular Canal Dehiscence?,” American Journal of Neuroradiology, vol. 34, no. 7, pp. 1421–1427, Jul. 2013. [CrossRef]
- J. S. Kim and H. Lee, “Posterior Circulation Stroke and Vestibular Syndromes,” in Oxford Textbook of Vertigo and Imbalance, Oxford University Press, 2013, pp. 251–266. [CrossRef]
- A. Zwergal and M. Dieterich, “Vertigo and dizziness in the emergency room,” Curr Opin Neurol, vol. 33, no. 1, pp. 117–125, Feb. 2020. [CrossRef]
- M. Gottlieb, G. D. Peksa, and J. N. Carlson, “Head impulse, nystagmus, and test of skew examination for diagnosing central causes of acute vestibular syndrome,” Cochrane Database of Systematic Reviews, vol. 2023, no. 11, Nov. 2023. [CrossRef]
- S. Datar and A. A. Rabinstein, “Cerebellar Hemorrhage,” Neurol Clin, vol. 32, no. 4, pp. 993–1007, Nov. 2014. [CrossRef]
- T. Bulut, A. Yildirim, B. Ekmekci, N. Eskut, and H. P. Gunbey, “False-Negative Diffusion-Weighted Imaging in Acute Stroke and its Frequency in Anterior and Posterior Circulation Ischemia,” J Comput Assist Tomogr, vol. 38, no. 5, pp. 627–633, 2014. [CrossRef]
- D. Sankalia, S. Kothari, and D. Phalgune, “Diagnosing Stroke in Acute Vertigo: Sensitivity and Specificity of HINTS Battery in Indian Population,” Neurol India, vol. 69, no. 1, p. 97, 2021. [CrossRef]
- T. Qiu, X. Dai, X. Xu, G. Zhang, L. Huang, and Q. Gong, “A prospective study on the application of HINTS in distinguishing the localization of acute vestibular syndrome,” BMC Neurol, vol. 22, no. 1, p. 378, Oct. 2022. [CrossRef]
- C. L. Warner, L. Bunn, N. Koohi, G. Schmidtmann, J. Freeman, and D. Kaski, “Clinician’s perspectives in using head impulse-nystagmus-test of skew (HINTS) for acute vestibular syndrome: UK experience,” Stroke Vasc Neurol, vol. 7, no. 2, pp. 172–175, Apr. 2022. [CrossRef]
- I.-A. Taciuc et al., “Applications and challenges of neural networks in otolaryngology (Review),” Biomed Rep, vol. 20, no. 6, p. 92, Apr. 2024. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).