1. Introduction
Cancer metastases remain to be a major cause of death in cancer patients and significant challenge to public health worldwide [
1,
2]. Metastasis is a multi-step process which includes local tumor cell invasion, entry into the vasculature followed by the exit of carcinoma cells from the circulation and colonization at the distal sites [
3]. Migration and invasion capacity of breast cancer cells has been linked to the reversible process of epithelial-to-mesenchymal transition (EMT), where the epithelial phenotype is lost and mesenchymal phenotype is gained, leading to metastasis [
4]. During EMT, epithelial cadherin (E-cadherin), which regulates cell adhesion is lost and mesenchymal cadherin (M-cadherin) is induced. Loss of E-cadherin has been associated with increased resistance to epidermal growth factor receptor (EGFR) inhibitors, resistance to radiotherapy and increased circulating tumour cells [
5,
6,
7,
8]. Class 1 histone deacetylase (HDACs) (HDAC 1,2,3 and 8) play an important role in regulating E-cadherin. Expression of E-cadherin has been reported to be suppressed by HDACs [
9,
10,
11]. Therefore, inhibition of HDACs by HDACi is, in theory, expected to reverse the repression of E-cadherin. But controversy remains around the mechanisms through which HDACi affect the EMT. Several studies have reported reversal of EMT while others have observed induction of EMT in different types of solid tumours including cholangiocarcinoma, prostate, lung, ductal pancreatic and breast, to name a few, using different classes of HDACi [
6,
12,
13,
14,
15,
16]. Similarly, in breast cancer, conflicting reports exist on the effect of the different HDACi, including SAHA, on migration and invasion [
17,
18,
19]. Taken together, it is tempting to conclude that the role of EMT is dependent on the type of cell and the class of HDACi used. Nevertheless, the role EMT in breast cancer metastasis has been brought under question and remains a matter of discussion [
20].
Another point of ongoing discussions is the fact that radiation promote metastasis. Ample evidence has supported that non-curative doses of photon radiation induce migration [
21,
22,
23,
24,
25,
26,
27,
28,
29,
30,
31]. However, more recent reports suggest that the clinical significance of this process is small when curative doses of radiation are given, but can be significant after non-curative doses of photon radiation and in irradiated normal tissues [
32]. The suggested mechanisms of radiation-induced migration include vascular damage, presence of hypoxia, epithelial-mesenchymal transition, cytokine expression and modulation of matrix-degrading enzymes [
22,
33,
34,
35,
36,
37]. Treatment with carbon ion beams was subsequently suggested as one of the strategies to counteract malignant cell migration and invasion after radiotherapy treatment [
22]. Both carbon ion and proton therapies have a superior dose distribution compared to X-rays that allows most of the dose to be delivered at the Bragg peak, but carbon ions have an exit dose due to nuclear fragmentation [
38]. Also, treatment centers that offer carbon ion are very limited compared to proton treatment centers. Existing reports on the effect of proton therapy on migration and invasion suggest that it is cell line dependent. For example, proton irradiation induced migration and invasion in human glioma cells, but inhibited migration and invasion in melanoma cells and HT1080 human fibrosarcoma cells [
39,
40,
41]. All considered, the study set out to determine the effectiveness of pan-HDAC inhibitor SAHA and multi-target inhibitor CUDC-101 in reducing migration and invasion of MCF-7, MDA-MB-231 and MCF-10A breast cell lines.
2. Materials and Methods
2.1. Cell Cultures
MCF-7 and MCF-10A (gifted by the Physiology Department, University of Pretoria) cells were cultured in Dulbecco’s Modified Eagle’s Medium F-12 (DMEM-F12; GibcoTM, Thermo Fisher Scientific, Sandton, South Africa) and Ham’s F-12 (GibcoTM, Thermo Fisher Scientific, Sandton, South Africa) supplemented with 10% foetal bovine serum (FBS) (GibcoTM, Thermo Fisher Scientific, Sandton, South Africa), 100 μg/mL penicillin (GibcoTM, Thermo Fisher Scientific, Sandton, South Africa), and 100 μg/mL streptomycin for bacterial contamination. MCF-10A medium was further supplemented with epidermal growth factor (EGF) (20 ng/mL final concentration) (GibcoTM, Thermo Fisher Scientific, Sandton, South Africa) and hydrocortisone (0.5 mg/mL final concentration) (Sigma-Aldrich, St. Louis, MO, USA).
MDA-MB-231 cells (gifted by the Department of Natural Sciences, University of Western Cape) were cultured in Roswell Park Memorial Institute (RPMI) 1640 (GibcoTM, Thermo Fisher Scientific, Sandton, South Africa) supplemented with 10% FBS, 100 μg/L penicillin and 100 μg/mL streptomycin (Sigma-Aldrich, St. Louis, MO, USA).
All cell lines were cultured in T275 or T75 cell culture flasks (Thermo Fisher Scientific, Sandton, South Africa) under standard conditions in a humidified incubator at 37 °C, 5% CO2 (Forma series 3 water jacketed incubator, Thermo Fisher Scientific, Waltham, MA, USA). Cell growth was assessed over 24 h intervals and sub-cultured once 80% confluence was reached.
2.2. Histone Deacetylase Inhibitors
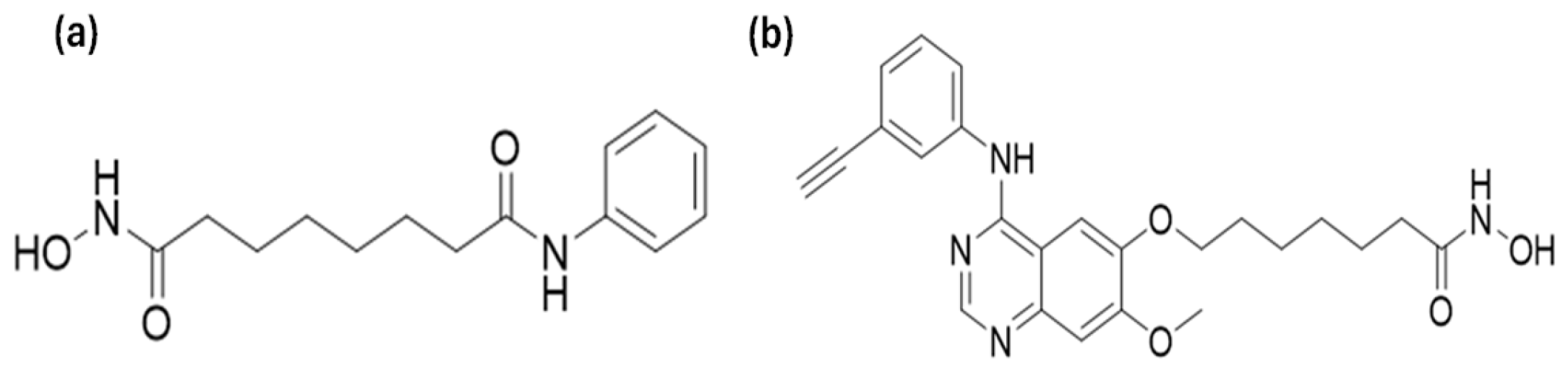
SAHA (molecular weight of 264.32) and CUDC-101 (molecular weight of 434.49)
Figure 1, were purchased from Sigma Aldrich (Sigma-Aldrich, Missouri, USA) and 1 mM stock solution was prepared according to the manufacturer’s instructions (5 mg of CUDC-101 was resolved in 11.5077 mL dimethylsulfide (DMSO) (Biotechnology Hub, Johannesburg, South Africa) and stored at-20° for short term storage and at -80° for long term storage.
2.3. Irradiations
Photon irradiations were performed using the 250 kVp X-Rad 320 unit (Precision X-ray, Madison, WI, USA) at a mean dose rate of 0.69 Gy/min at a Source Surface Distance (SSD) of 50 cm. Calibrations of the unit were performed according to the Technical Report Series-398 (TRS-398) protocol, with a Farmer 117 chamber for which a chamber calibration factor has been obtained from the National Metrology Institute of South Africa (NMISA).
Proton irradiations were performed at the Trento Institute for Fundamental Physics and Application (TIFPA). An SOBP beam of 2.5 cm has been produced, as detailed in Tommasino et al. through a 2D rang modulator applied to a beam with initial energy of 148 MeV/u and enlarged with a dual ring system to a lateral profile maintaining a 98% dose uniformity across a 6 cm diameter. The beam was calibrated with EBT gafchromic film and Markus chamber measurements. The cells were exposed after 11 cm of solid water slabs, corresponding to 11.45 cm of water [
42]. For both X-ray and proton irradiations, cells were irradiated in 5 mL media in T25 flasks.
2.4. Wound Healing Assays
Wound healing assays were performed using the 2 well cell culture-Insert (ibidi, Gräfelfing, Germany). Cells were harvested and seeded in each well of the inserts and allowed to attach and reach 80% confluency. Cells were treated with IC50 concentrations of the HDACi for 24 hours and irradiated with protons or X-rays. Immediately after irradiation, the inserts were removed and imaging using CytoSMARTTM 2 live imaging system (Whitehead scientific, Cape Town, South Africa) was done at 0hrs (immediately) and every 4 hours for 24 hours. Images were analysed using the ImageJ processing software (Version 1.54i, National Institutes of Health, Bethesda, MD, United States of America.
2.5. Trans-Well Invasion Assays
Cell invasion was assessed using 8µm pore trans-well inserts in 24 well plates (Greiner bio-one®, North Carolina, United States of America). Geltrex basement matrix (Gibco, Thermo Fisher Scientific, South Africa) was removed from freezer and thawed in ice in the fridge at 4-8°C. Geltrex (50µl) was carefully added to the upper chamber of the insert using pre-cooled pipette tips and the plates were incubated at 37°C for 30 minutes to allow the Geltrex to solidify. Cells were harvested using serum free media and 5000 cells were seeded in the upper chamber of the insert. Complete media (600µl) with 10% fetal bovine serum (FBS) were added at the bottom of the bottom wells of the 24 well plate and the trans-well insert was placed in the media containing plate. The plates were incubated for a further 24 hours at 37°C to allow cell invasion. After 24 hours, trans-well inserts were washed in PBS and cells were fixed with 4% paraformaldehyde (Merck Life Sciences, Johannesburg, South Africa) and stained using 2% crystal violet (Inqaba Biotechnical Industries, Pretoria, South Africa). Cells that did not invade were carefully removed from the upper chamber using a wet cotton swab and the inserts were allowed to dry for a minimum of 24 hours. Four quadrants of the inserts were imaged at 10X magnification using the CytoSMARTTM 2 live imaging system (Whitehead Scientific, Cape Town, South Africa).
2.6. Statistical Analysis
Statistical analysis was performed using Graphpad Prism version 10.2. All data was expressed as the mean the mean ± SD of three independent experiments (n=3). Statistical significance was determined using two-tailed Student’s t-test, and p < 0.05 was considered statistically significant.
3. Results
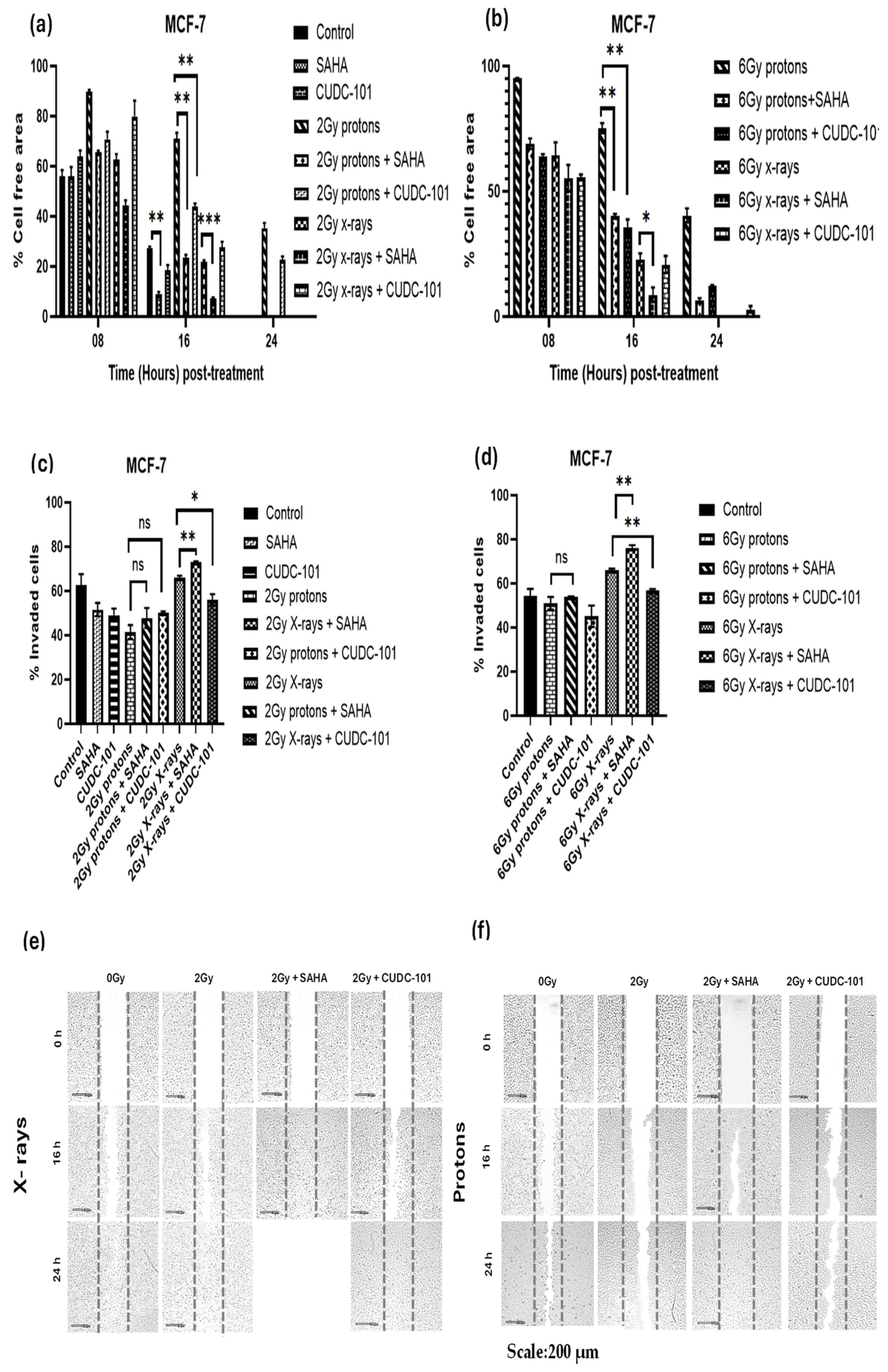
Wound healing (migration) and trans-well invasion assays were performed to assess the effect of HDACi SAHA and CUDC-101 on the migration and invasion capacity of MCF-7, MDA-MB-231 and spontaneously immortalised MCF-10A cell lines. Cells were treated with half maximal inhibitory concentration (IC50) concentrations of SAHA or CUDC-101 and 24 hours after drug treatment; cells were irradiated with either 2Gy or 6Gy X-rays or protons. In the MCF-7 cell line at 16 hours post treatment, significantly increased migration was seen after treatment with SAHA monotherapy compared to the untreated control (p = 0.0008). Significantly increased migration was also evident in SAHA pre-treated cells after 2Gy (p = 0.0016) and 6Gy (p = 0.0018) protons, as well as after 2Gy (p = 0.0005) and 6Gy (p = 0. 0341) X-rays (Figures 1 a and b). Similarly, from the results of the trans-well invasion assays, compared to X-ray alone, a significantly increased number of invaded cells were seen in SAHA pre-treated cells after 2Gy (p = 0.0091) and 6Gy (p = 0.0088) respectively. A non-significant difference in the number of invaded cells was observed in cells treated with SAHA and 2Gy proton (p = 0.24) or 6Gy protons (p = 0.2979) (Figures 1 c-d). Taken together, the results suggest that SAHA increases migration and invasion in the MCF-7 cell line. Treatment with protons alone statistically significantly reduced migration compared to combination treatment of protons and CUDC-101 with p = 0.0054 and p = 0.0018 after 2Gy and 6Gy protons respectively. Treatment with protons also statistically significantly reduced migration compared to X-rays with p = 0.0014 and p = 0. 0017 after 2Gy and 6Gy respectively. A similar pattern of results was seen after combination treatments where significant reduction in migration was seen in CUDC-101 pre-treated cells after treatment with 2Gy protons compared to 2Gy X-rays (p = 0.0115, as well as after 6Gy protons compared to 6Gy X-rays (p = 0.0454). Reduction in migration in the proton-irradiated cells was still evident at 24 hours post treatment (Figures 1a-b). The latter result suggests that proton irradiation alone reduces migration and invasion to a greater degree compared to the combination treatment of protons and CUDC-101 in the MCF-7 cell lines. Representative images are shown in Figures 1(e)-(f).
Figure 1.
Effect of SAHA and CUDC-101 on migration (a)-(b) and invasion (c)-(d) of MCF-7 cells. Representative images are showed in (e) and (f). Samples were treated with SAHA (1.2µM) and CUDC-101 (0.3µM) and irradiated with either protons or X-rays. Histograms show the mean ± SD of three independent experiments, (n=3). Comparisons were conducted using unpaired two-tailed Student t test, p < 0.05 was considered significant.
Figure 1.
Effect of SAHA and CUDC-101 on migration (a)-(b) and invasion (c)-(d) of MCF-7 cells. Representative images are showed in (e) and (f). Samples were treated with SAHA (1.2µM) and CUDC-101 (0.3µM) and irradiated with either protons or X-rays. Histograms show the mean ± SD of three independent experiments, (n=3). Comparisons were conducted using unpaired two-tailed Student t test, p < 0.05 was considered significant.
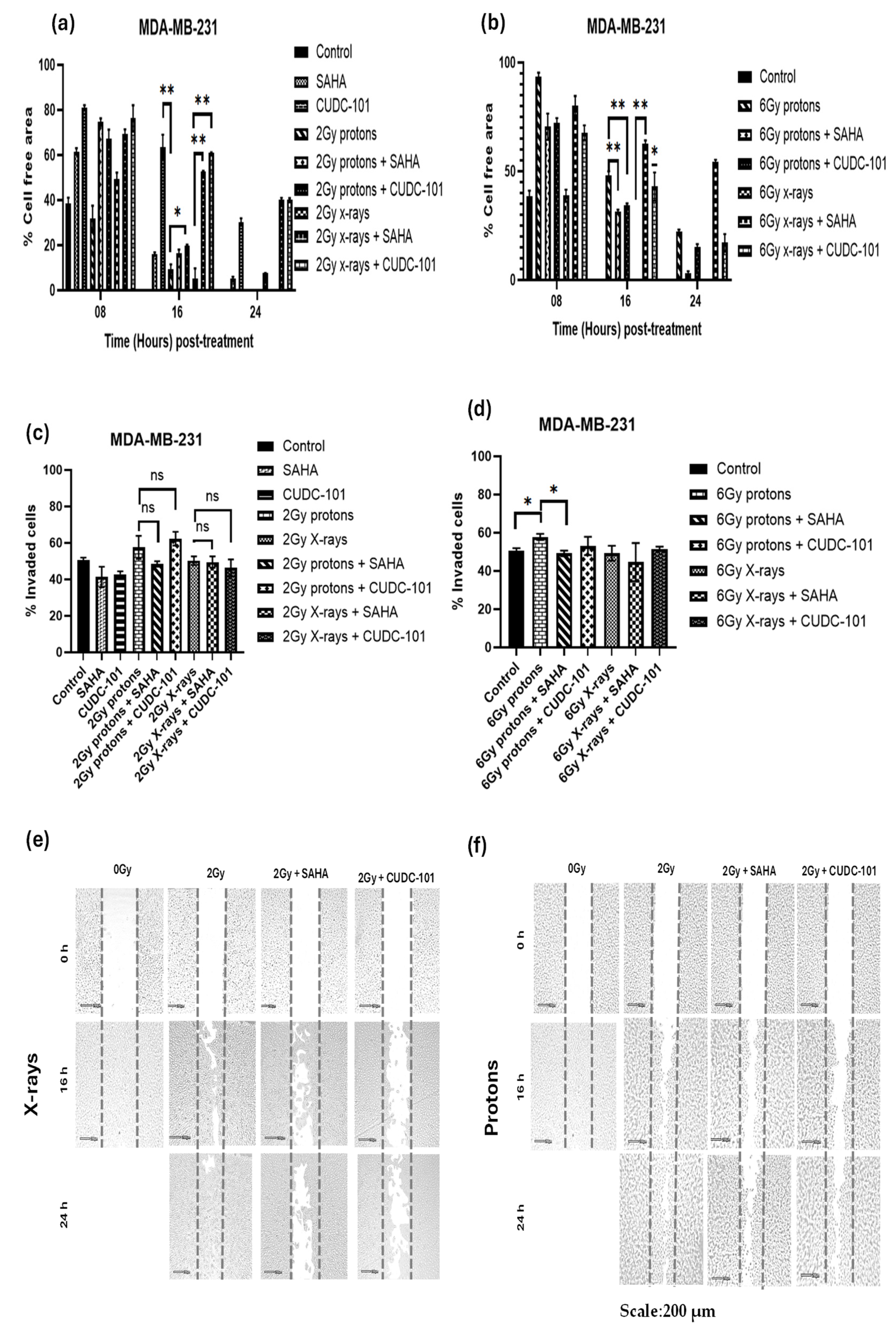
In the MDA-MB-231 cell line, significantly reduced migration was observed after treatment with CUDC-101 monotherapy compared to either proton irradiation (
p = 0.0056) or X-rays (
p = 0.0072). Compared to radiation alone, significantly reduced migration was seen after combination treatment of CUDC-101 and 2Gy protons (
p = 0.0183) or 6Gy protons (
p = 0.0095), as well as after 2Gy X-rays (
p = 0.0015) and 6Gy X-rays (
p = 0.0115 (Figures 2a-b). Contrary to the observations made in the MCF-7 cell line, proton irradiation (2Gy or 6Gy) alone did not reduce migration (
Figure 2 a-b). Comparison of migration after 2Gy protons and combination treatment of SAHA and 2Gy protons yielded a non-significant result (
p = 0.571). However, a significant reduction in migration was seen when SAHA was combined with 2Gy X-rays (
p = 0.0022), 6Gy X-rays (
p = 0.004) and 6Gy protons (p = 0.0066). Taken together, the results highlight the effect of CUDC-101 in reducing migration as monotherapy or in combination with X-rays. Also, the results suggest that SAHA augments the effect of X-rays in reducing migration but does not augment the effect of protons. Reduction in migration was evident at 24 hours after treatment with CUDC-101 alone, 2Gy X-rays and CUDC-101 as well as with 2Gy X-rays and SAHA (Figures 2 a-b). These observations were, however, not supported by the results of the trans-well invasion assay. Nevertheless, a significant difference in the number of invaded cells was seen after treatment with SAHA and 6Gy protons compared to protons alone (p=0.0294).
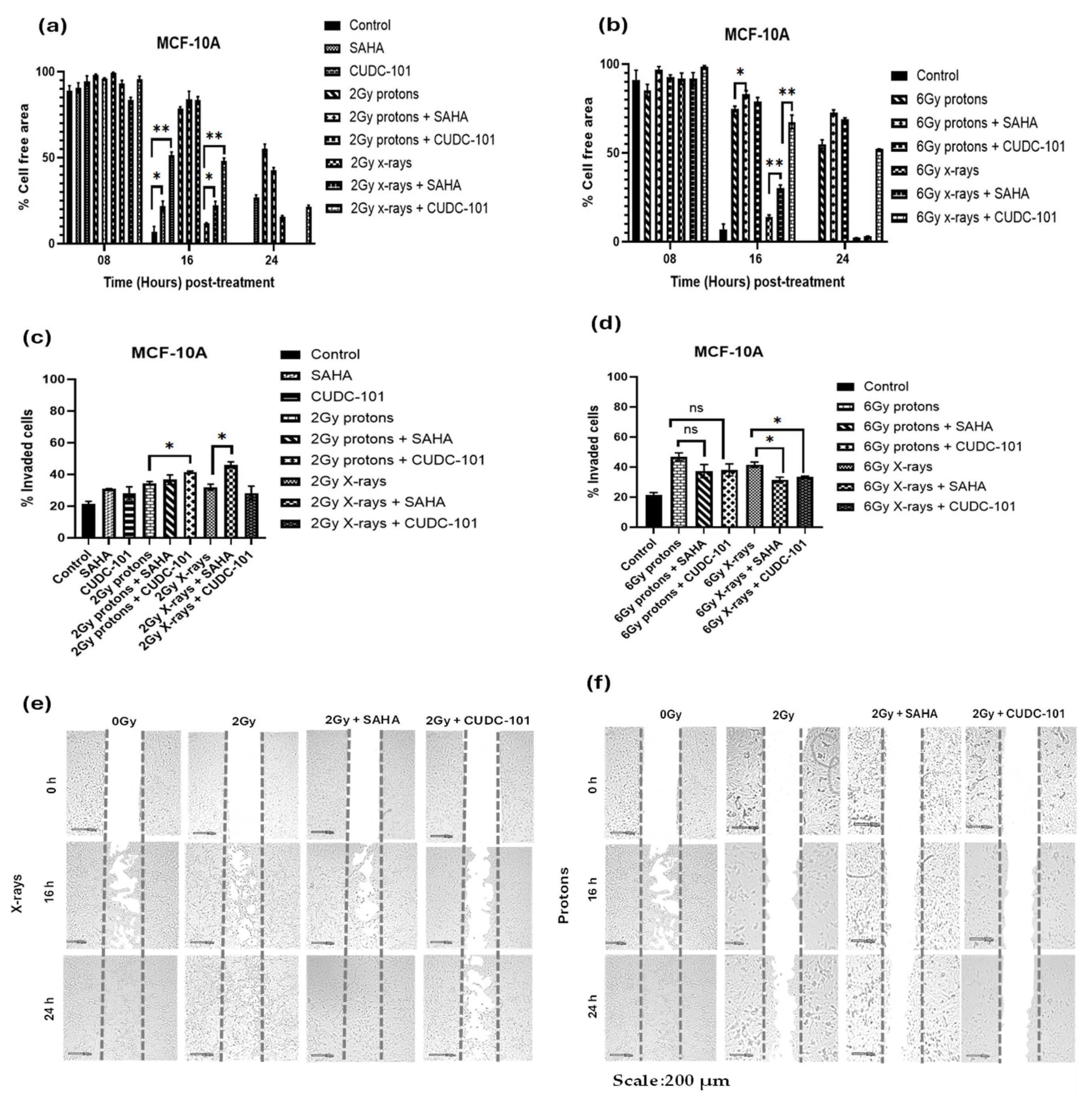
In the MCF-10A cell line, treatment with CUDC-101 alone significantly reduced migration compared to the control (p = 0.0032). This observation was supported by the results of the trans-well invasion assays which showed significantly reduced number of invaded cells after treatment with CUDC-101 compared with the control (p = 0.0373). Proton irradiation alone also significantly reduced migration compared to X-rays, p = 0.0002 and p = 0.0005 after 2Gy and 6Gy, respectively. However, the results of the trans-well invasion assay showed a non-significant difference between protons and X-rays, p = 0.3629 after 2Gy and p = 0.1400 after 6Gy protons. Compared to radiation alone, combination treatment of both HDACi and proton irradiation (2Gy or 6Gy) yielded a non-significant result, p = 0.2598 and p = 0.0.0712 in SAHA and CUDC-101 pre-treated cells, respectively, suggesting that addition of both HDACi did not enhance the effect of protons in reducing migration. A significant difference in the number of invaded cells was observed after combination treatment of both HDACi and X-irradiation, p = 0.0295 and p = 0.0012 in SAHA and CUDC-101 pre-treated cells respectively. Similarly, at higher doses (6Gy), pre-treatment with both HDACi significantly reduced migration when combined with X-rays, p = 0.0064 and p = 0.0027 in SAHA and CUDC-101 pre-treated cells, respectively.
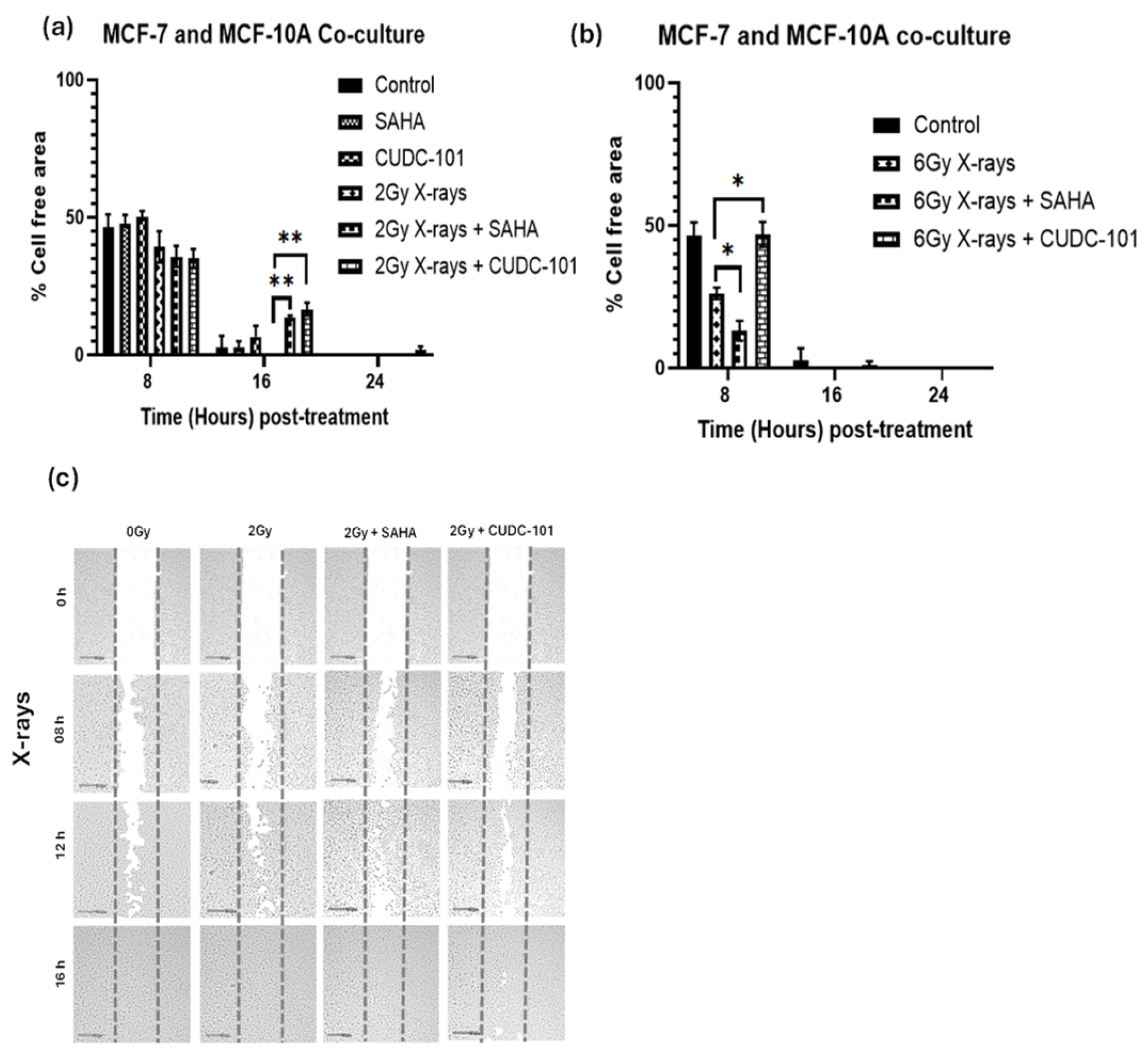
MCF-7 and MCF-10A were co-cultured, pre-treated with SAHA or CUDC-101 and irradiated with 2Gy or 6Gy X-rays. Wound healing assays were performed 24 hours after drug treatment. Comparison of the untreated controls at 16 hours showed increased migration (wound closure) when MCF-7 and MCF-10A cells were co-cultured compared to the untreated control of MCF-7 cells (
p = 0.0134). Comparison of the untreated co-culture control to the untreated control of the MCF-10A yielded a non-significant result (
p = 0.3858). The result implies that the increased migration observed with the co-seeding might be due to increased migration of the MCF-10A rather than the MCF-7 cells. Compared to 2Gy X-rays alone, decreased migration was seen in HDACi pre-treated cells with
p = 0. 0013 and
p = 0.0099 in SAHA and CUDC-101 respectively. Increased migration was seen after 6Gy doses compared to lower doses (2Gy); complete wound closure occurred before 16hrs (
Figure 4 a-b). At 8 hours post irradiation with 6Gy X-rays, significantly reduced migration was observed compared to combination treatment of 6Gy X-rays and SAHA (
p = 0.0439). However, treatment with 6Gy X-rays and CUDC-101 showed significant reduction in migration compared to 6Gy X-rays (
p = 0.0259). The former result suggests that increased migration might be from SAHA treated MCF-7 cells. The reduced migration seen after CUDC-101 might be due the effect of CUDC-101 on MCF-10A cells (
Figure 4a-b).
Figure 2.
Effect of SAHA and CUDC-101 on migration (a)-(b) and invasion potential (c)-(d) of MDA-MB-231 cells. Representative images are showed in (e) and (f). Samples were treated with SAHA (2µM) and CUDC-101 (0.6µM) and irradiated with either protons or X-rays. Histograms show the mean ± SD of three independent experiments (n=3). Comparisons were conducted using unpaired two-tailed Student t test where p < 0.05 was considered significant.
Figure 2.
Effect of SAHA and CUDC-101 on migration (a)-(b) and invasion potential (c)-(d) of MDA-MB-231 cells. Representative images are showed in (e) and (f). Samples were treated with SAHA (2µM) and CUDC-101 (0.6µM) and irradiated with either protons or X-rays. Histograms show the mean ± SD of three independent experiments (n=3). Comparisons were conducted using unpaired two-tailed Student t test where p < 0.05 was considered significant.
Figure 3.
Effect of SAHA and CUDC-101 on migration (a)-(b) and invasion potential (c)-(d) of MCF-10A cells. Representative images are showed in (e) and (f). Samples were treated with SAHA (6.3µM) and CUDC-101 (2.7µM) and irradiated with either protons or X-rays. Histograms show the mean ± SD of three independent experiments, (n=3). Comparisons were conducted using unpaired two-tailed Student t test, p < 0.05 was considered significant.
Figure 3.
Effect of SAHA and CUDC-101 on migration (a)-(b) and invasion potential (c)-(d) of MCF-10A cells. Representative images are showed in (e) and (f). Samples were treated with SAHA (6.3µM) and CUDC-101 (2.7µM) and irradiated with either protons or X-rays. Histograms show the mean ± SD of three independent experiments, (n=3). Comparisons were conducted using unpaired two-tailed Student t test, p < 0.05 was considered significant.
Figure 4.
Effect of SAHA and CUDC-101 on migration (a) and invasion potential (b) of co-cultured MCF7 and MCF-10A cells. Representative images are showed in (e) and (f). Samples were treated with of SAHA (2µM) and CUDC-101 (0.6µM) and irradiated with either protons or X-rays. Histograms show the mean ± SD of three independent experiments (n=3). Comparisons were conducted using unpaired two-tailed Student t test, p < 0.05 was considered significant.
Figure 4.
Effect of SAHA and CUDC-101 on migration (a) and invasion potential (b) of co-cultured MCF7 and MCF-10A cells. Representative images are showed in (e) and (f). Samples were treated with of SAHA (2µM) and CUDC-101 (0.6µM) and irradiated with either protons or X-rays. Histograms show the mean ± SD of three independent experiments (n=3). Comparisons were conducted using unpaired two-tailed Student t test, p < 0.05 was considered significant.
4. Discussion
Migration of tumour cells is a required for invasion and metastasis [
43]. Migration is facilitated by the dynamic process of epithelial mesenchymal transition (EMT). The effect of HDCAi on EMT in different solid tumours, remains a matter of discussion. Several reports supported the notion that HDACi inhibits migration and invasion in human breast cell lines including MCF-7, MDA-MB-231, BT549 and rat breast cell lines (MT-450 [
44,
45]. Chiu et al. also reported inhibition of migration in 4T1 breast cancer cells after treatment with 600nM SAHA [
46]. In another study, SAHA was observed to significantly inhibit leptin stimulated migration in MCF-7 and MDA-MB-231 cell lines [
47]. To the contrary, Wu et al. observed that SAHA induced EMT via HDAC8/FOXA1 and promoted migration in MDA-MB-231 and BT549 breast cells [
18]. In a recent study by Hu et al. treatment with 2.5 and 5 µM SAHA and LBH589 were reported to promote migration in BT549 and MDA-MB-231 breast cell lines by elevating the pro-metastasis gene NEDD9 [
19]. The authors concluded that monotherapy with pan-HDACi should be avoided in breast cancer [
19]. Further, in a phase II trial, SAHA monotherapy failed to show success in 14 patients with metastatic breast cancer [
48]. The results of this Phase II trial are congruent with the findings of the later studies. To that effect, in the current study, SAHA was combined with proton or X-radiation. Treatment with 1.2µM SAHA was observed to increase migration and invasion in the MCF-7 cell line when used as monotherapy but not in the MDA-MB-231 cell lines as reported by Hu et al. [
19]. The migration promoting effect of SAHA was also evident when SAHA was combined with X-rays but was repressed when SAHA was combined with protons (Figures 1a-d). Further, in the MCF-7 cell line, proton irradiation alone (2Gy and 6Gy) inhibited migration more effectively than combination therapy with either SAHA (p=0.0016) or CUDC-101 (p= 0.0053) after 2Gy and p= 0.0018 and p=0.0044 after 6Gy. Indeed, the migration inhibiting capacity of protons was previously reported in the MCF-7 and MDA-MB-231 cell lines [
40,
41,
49].
Contrary to the observations made in the MCF-7 cell line, at lower doses (2Gy), protons irradiation alone did not reduce migration in the MDA-MB-231 cell line,
Figure 2a. However, significant reduction was seen after 6Gy protons. This result agrees with previous studies that reported a dose-dependent inhibition of migration by protons in the MDA-MB-231 cell line [
49,
50]. In opposition to the observations of Wu et al. and Hu et al. that reported increased migration after SAHA treatment, in the MDA-MB-231 cell line, SAHA modestly inhibited migration when used as monotherapy and when combined with X-rays (Figures 2 a-d). This observation is in line with the observations from our previous paper where we reported increased γ-H2AX foci retention at 24 hours post irradiation after treatment with 2Gy X-rays and SAHA [
51]. The MDA-MB-231 cell line possess a mesenchymal phenotype and express low levels of E-cadherin[
5]. Consistent with this, Wang et al. reported a slight increase in E-cadherin that indicates reversal of EMT in SAHA-treated MDA-MB-231[
5]. Furthermore, Shah et al. also reported reversal of EMT through reversal of repression of E-cadherin in the MDA-MB-231 cell line [
17]. Taken together, the latter studies also support the modest inhibition in migration that was observed in the current study. Notwithstanding, in the triple negative cell line MDA-MB-468, although SAHA inhibited migration, mesenchymal marker N-cadherin was observed to be overexpressed [
6]. This brings into question whether EMT markers alone can provide a reliable measure of migration and invasion capacity [
52]. This could also explain the conflicting reports when SAHA is used in triple negative breast cell lines. In both the MCF-7 and MDA-MB-231 repression of migration by protons has been associated with reduction in cyclooxygenase-2 (COX-2) and matrix metalloproteinase-9 (MMP-9 [
49,
53]. Indeed, molecular profiling of MCF-7 cells after proton irradiation revealed increased sensitivity of this cell line to proton irradiation compared to MDA-MB-231 and MCF-10A cell lines [
54]. This is in line with the observed sensitivity to 2Gy protons as well as 6Gy in the current study,
Figure 1a. COX-2 has been found to be overexpressed in a number of tumour types including breast tumour [
49,
55,
56,
57]. In the MCF-10A cell line, significant reduction in migration was seen after proton irradiation alone (Figures 3 a-d). Although previous reports indicated that HDACi have little effect on normal cells due to active G2 checkpoint which is defective in malignant cells, modest reduction in migration was seen when SAHA was combined with X-rays in the current study (Figures 3 a-d) [
13,
58,
59,
60,
61].
CUDC-101 is a multi-target inhibitor of HDACs, EGFR and human epidermal growth factor receptor 2 (HER2). Data on CUDC-101 in breast cancer is very limited. Schlaff et al. reported inhibition of HER2, EGFR and HDAC in MDA-MB-231 cells after treatment with 0.5µM CUDC-101 [
62]. In the current study, reduction in migration and invasion were observed after 2Gy protons combined with CUDC-101 in the MCF-7 cell line. In the MDA-MB-231 cell line, significantly reduced migration was observed after monotherapy with CUDC-101 and 2Gy X-rays combined with CUDC-101 (
Figure 2 a-d). The reduction in migration after treatment with CUDC-101 in the MDA-MB-231 is thought to be due to inhibition of EGFR [
63,
64]. In support, Wang et al. also observed significant increase in E-cadherin after treatment with CUDC-101 compared to treatment with SAHA in MDA-MB-231 cell line [
5]. In the MCF-10A cell line, reduced migration was observed after CUDC-101 monotherapy, as well as after combination therapies in CUDC-101 pre-treated cells. (
Figure 3a, b). Previous studies had alluded that HDACi have little effect on healthy cells. Most of these studies were conducted with SAHA which is a pan-HDACi. The effect of CUDC-101 in the MCF-10A cell line could also be attributed to inhibition of EGFR, which is crucial for maintenance of MCF-10A cell line [
65]. The reduced migration in MCF-10A cell line implies increased side effects in clinical practice.
Previous reports have indicated that the migration capacity of malignant cells is influenced by the presence of normal cells [
66]. To that effect, MCF-7 and MCF-10A cells were co-cultured and treated with HDACi or X-rays as well as combination treatment of HDACi and X-rays. In general, increased migration was observed compared to when the MCF-7 or MCF-10A cells were cultured individually (Figures 4 a-b) The MCF-10A showed increased migration speed towards the MCF-7(
Figure 3 a-b). The observed differences in migration speed between the MCF-7 and MCF-10A cells could be explained by the facts that MCF-7 has lower metastatic potential, and low migration speed. Also, the differences in basal adenosine triphosphate (ATP) between these cell lines are different. Normal cells have higher levels of ATP , which is needed for migration, whereas in malignant cells, ATP production is reduced due to the Warburg effect [
66].
5. Conclusions
Metastasis is a complex multi-step process, and despite extensive research, efficacious therapies are yet to be found. The focus of most studies on HDACi has been placed on their ability to enhance radiation response, as well as in unraveling the mechanisms that underlie their radiation enhancing effect. The current study explored the systematic effects of HDACi when combined not only with X-rays but also with protons. The results highlighted that although pan-HDACi SAHA was previously proved to enhance radiation response, the observed in vitro migration promoting effects in the MCF-7 cell line, nullifies the previously reported benefits. The observed migration and invasion reducing capacity of proton irradiation alone in both malignant MCF-7 and MDA-MB-231 cell lines at 2Gy dose, which is commonly used in clinical, is encouraging in light of the increasing number of protons centers around the world. Also, the migration inhibiting effect protons higher doses (6Gy), bears relevance to the hypofractionation schedule, which is increasing in use in the treatment of breast cancers. Of note is also the significant reduction in migration seen in the normal MCF-10A, which infers slower wound healing in normal tissues. The results also highlighted the potential of CUDC-101 either as monotherapy or in combination with radiation, as therapeutic strategy in reducing metastasis in triple negative breast cancers. The results of this in vitro study needs to be validated in in vivo studies.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Figure S1: Representative images for apoptosis profiles; Figure S2: Representative images of the cell cycle profile after different treatment conditions.
Author Contributions
Conceptualization, E.N.S, S.N., C.V. and A.J.; methodology, E.N.S, S.N., C.V. and A.J.; formal analysis, E.N.S, S.N., C.V. and A.J.; investigation, E.N.,S.N., C.V.,A.B.,A.J.,S.; resources, X.X.; data curation, E.N.S.; writing—original draft preparation, E.N.S; writing—review and editing, E.N.S, S.N., C.V.,A.B. and A.J.; supervision, S.N., C.V. and A.J.; funding acquisition, E.N.S, S.N., C.V.,A.B.,A.J. All authors have read and agreed to the published version of the manuscript.”. Authorship must be limited to those who have contributed substantially to the work reported.
Funding
This research was funded by NRF iThemba Laboratories and Department of Higher Education and Training, South Africa. The APC was funded by the University of Pretoria, Cape Peninsula University of Technology, NRF iThemba Laboratories and GSI Helmholtzzentrum für Schwerionenforschung.
Conflicts of Interest
The authors Elsie Neo Seane, Charlot Vandevoorde, Alessandra Bisio and Anna Joubert declare no conflicts of interest. Author Shankari Nair is currently employed by the company Bayer AG. The authors confirm that no financial contribution was received from BAYER AG and the company was not involved in any way in the study. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Liu, Z.; Chen, J.; Ren, Y.; Liu, S.; Ba, Y.; Zuo, A.; Luo, P.; Cheng, Q.; Xu, H.; Han, X. Multi-stage mechanisms of tumor metastasis and therapeutic strategies. Signal Transduction and Targeted Therapy 2024, 9, 270. [Google Scholar] [CrossRef] [PubMed]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular principles of metastasis: a hallmark of cancer revisited. Signal Transduction and Targeted Therapy 2020, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- van Zijl, F.; Krupitza, G.; Mikulits, W. Initial steps of metastasis: cell invasion and endothelial transmigration. Mutat Res 2011, 728, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, Y.; Li, Y.; Shen, Y.; Dong, S.; Tan, J. A Novel Class I HDAC Inhibitor, AW01178, Inhibits Epithelial-Mesenchymal Transition and Metastasis of Breast Cancer. Int J Mol Sci 2024, 25. [Google Scholar] [CrossRef]
- Wang, J.; Pursell, N.W.; Samson, M.E.S.; Atoyan, R.; Ma, A.W.; Selmi, A.; Xu, W.; Cai, X.; Voi, M.; Savagner, P.; et al. Potential advantages of CUDC-101, a multitargeted HDAC, EGFR, and HER2 inhibitor, in treating drug resistance and preventing cancer cell migration and invasion. Molecular Cancer Therapeutics 2013, 12, 925–936. [Google Scholar] [CrossRef]
- Wawruszak, A.; Gumbarewicz, E.; Okon, E.; Jeleniewicz, W.; Czapinski, J.; Halasa, M.; Okla, K.; Smok-Kalwat, J.; Bocian, A.; Rivero-Muller, A.; et al. Histone deacetylase inhibitors reinforce the phenotypical markers of breast epithelial or mesenchymal cancer cells but inhibit their migratory properties. Cancer Manag Res 2019, 11, 8345–8358. [Google Scholar] [CrossRef] [PubMed]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol 2014, 15, 178–196. [Google Scholar] [CrossRef] [PubMed]
- Francart, M.E.; Lambert, J.; Vanwynsberghe, A.M.; Thompson, E.W.; Bourcy, M.; Polette, M.; Gilles, C. Epithelial-mesenchymal plasticity and circulating tumor cells: Travel companions to metastases. Dev Dyn 2018, 247, 432–450. [Google Scholar] [CrossRef]
- Aghdassi, A.; Sendler, M.; Guenther, A.; Mayerle, J.; Behn, C.O.; Heidecke, C.D.; Friess, H.; Büchler, M.; Evert, M.; Lerch, M.M.; et al. Recruitment of histone deacetylases HDAC1 and HDAC2 by the transcriptional repressor ZEB1 downregulates E-cadherin expression in pancreatic cancer. Gut 2012, 61, 439–448. [Google Scholar] [CrossRef]
- Serrano-Gomez, S.J.; Maziveyi, M.; Alahari, S.K. Regulation of epithelial-mesenchymal transition through epigenetic and post-translational modifications. Molecular Cancer 2016, 15, 18. [Google Scholar] [CrossRef]
- Choi, S.Y.; Kee, H.J.; Kurz, T.; Hansen, F.K.; Ryu, Y.; Kim, G.R.; Lin, M.Q.; Jin, L.; Piao, Z.H.; Jeong, M.H. Class I HDACs specifically regulate E-cadherin expression in human renal epithelial cells. J Cell Mol Med 2016, 20, 2289–2298. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Lee, E.J.; Ji, M.; Park, S.M. HDAC inhibitors, trichostatin A and valproic acid, increase E-cadherin and vimentin expression but inhibit migration and invasion of cholangiocarcinoma cells. Oncol Rep 2018, 40, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.H.; Kim, S.-N.; Kim, Y.K. Involvement of HDAC1 in E-cadherin expression in prostate cancer cells; its implication for cell motility and invasion. Biochemical and Biophysical Research Communications 2011, 404, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Kakihana, M.; Ohira, T.; Chan, D.; Webster, R.B.; Kato, H.; Drabkin, H.A.; Gemmill, R.M. Induction of E-Cadherin in Lung Cancer and Interaction with Growth Suppression by Histone Deacetylase Inhibition. Journal of Thoracic Oncology 2009, 4, 1455–1465. [Google Scholar] [CrossRef]
- Schiedlauske, K.; Deipenbrock, A.; Pflieger, M.; Hamacher, A.; Hänsel, J.; Kassack, M.U.; Kurz, T.; Teusch, N.E. Novel Histone Deacetylase (HDAC) Inhibitor Induces Apoptosis and Suppresses Invasion via E-Cadherin Upregulation in Pancreatic Ductal Adenocarcinoma (PDAC). Pharmaceuticals 2024, 17, 752. [Google Scholar] [CrossRef]
- Law, M.; Corsino, P.; Jahn, S.; Davis, B.; Chen, S.; Patel, B.; Pham, K.; Lu, J.; Sheppard, B.; Norgaard, P.; et al. Glucocorticoids and histone deacetylase inhibitors cooperate to block the invasiveness of basal-like breast cancer cells through novel mechanisms. Oncogene 2012, 32. [Google Scholar] [CrossRef]
- Shah, P.; Gau, Y.; Sabnis, G. Histone deacetylase inhibitor entinostat reverses epithelial to mesenchymal transition of breast cancer cells by reversing the repression of E-cadherin. Breast Cancer Res Treat 2014, 143, 99–111. [Google Scholar] [CrossRef]
- Wu, S.; Luo, Z.; Yu, P.J.; Xie, H.; He, Y.W. Suberoylanilide hydroxamic acid (SAHA) promotes the epithelial mesenchymal transition of triple negative breast cancer cells via HDAC8/FOXA1 signals. Biol Chem 2016, 397, 75–83. [Google Scholar] [CrossRef]
- Hu, Z.; Wei, F.; Su, Y.; Wang, Y.; Shen, Y.; Fang, Y.; Ding, J.; Chen, Y. Histone deacetylase inhibitors promote breast cancer metastasis by elevating NEDD9 expression. Signal Transduct Target Ther 2023, 8, 11. [Google Scholar] [CrossRef]
- Bill, R.; Christofori, G. The relevance of EMT in breast cancer metastasis: Correlation or causality? FEBS Letters 2015, 589, 1577–1587. [Google Scholar] [CrossRef]
- Vilalta, M.; Rafat, M.; Graves, E.E. Effects of radiation on metastasis and tumor cell migration. Cell Mol Life Sci 2016, 73, 2999–3007. [Google Scholar] [CrossRef] [PubMed]
- Moncharmont, C.; Levy, A.; Guy, J.B.; Falk, A.T.; Guilbert, M.; Trone, J.C.; Alphonse, G.; Gilormini, M.; Ardail, D.; Toillon, R.A.; et al. Radiation-enhanced cell migration/invasion process: a review. Crit Rev Oncol Hematol 2014, 92, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-C.; Liu, J.-Y.; Li, J.; Zhang, J.; Xu, Y.-Q.; Zhang, H.-W.; Qiu, L.-B.; Ding, G.-R.; Su, X.-M.; Mei, S.; et al. Ionizing Radiation Promotes Migration and Invasion of Cancer Cells Through Transforming Growth Factor-Beta–Mediated Epithelial–Mesenchymal Transition. International Journal of Radiation Oncology, Biology, Physics 2011, 81, 1530–1537. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, D.M.; Han, R.; Yu, Y.; Deng, S.H.; Liu, T.; Zhang, T.; Xu, Y. Low-Dose Radiation Promotes Invasion and Migration of A549 Cells by Activating the CXCL1/NF-κB Signaling Pathway. Onco Targets Ther 2020, 13, 3619–3629. [Google Scholar] [CrossRef]
- De Bacco, F.; Luraghi, P.; Medico, E.; Reato, G.; Girolami, F.; Perera, T.; Gabriele, P.; Comoglio, P.M.; Boccaccio, C. Induction of MET by ionizing radiation and its role in radioresistance and invasive growth of cancer. JNCI: Journal of the National Cancer Institute 2011, 103, 645–661. [Google Scholar] [CrossRef]
- Kaplan, H.S.; Murphy, E.D. The effect of local roentgen irradiation on the biological behavior of a transplantable mouse carcinoma; increased frequency of pulmonary metastasis. J Natl Cancer Inst 1949, 9, 407–413. [Google Scholar]
- Anderson, P.; Dische, S. Local tumor control and the subsequent incidence of distant metastatic disease. International Journal of Radiation Oncology*Biology*Physics 1981, 7, 1645–1648. [Google Scholar] [CrossRef]
- Fagundes, H.; Perez, C.A.; Grigsby, P.W.; Lockett, M.A. Distant metastases after irradiation alone in carcinoma of the uterine cervix. Int J Radiat Oncol Biol Phys 1992, 24, 197–204. [Google Scholar] [CrossRef]
- Wild-Bode, C.; Weller, M.; Rimner, A.; Dichgans, J.; Wick, W. Sublethal irradiation promotes migration and invasiveness of glioma cells: implications for radiotherapy of human glioblastoma. Cancer Res 2001, 61, 2744–2750. [Google Scholar]
- Sheldon, P.W.; Fowler, J.F. The effect of low-dose pre-operative X-irradiation of implanted mouse mammary carcinomas on local recurrence and metastasis. Br J Cancer 1976, 34, 401–407. [Google Scholar] [CrossRef]
- Strong, M.S.; Vaughan, C.W.; Kayne, H.L.; Aral, I.M.; Ucmakli, A.; Feldman, M.; Healy, G.B. A randomized trial of preoperative radiotherapy in cancer of the oropharynx and hypopharynx. Am J Surg 1978, 136, 494–500. [Google Scholar] [CrossRef] [PubMed]
- von Essen, C.F. Radiation enhancement of metastasis: a review. Clin Exp Metastasis 1991, 9, 77–104. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.W.; Hwang, S.Y.; Hwang, J.S.; Oh, E.S.; Park, S.; Han, I.O. Ionising radiation induces changes associated with epithelial-mesenchymal transdifferentiation and increased cell motility of A549 lung epithelial cells. Eur J Cancer 2007, 43, 1214–1224. [Google Scholar] [CrossRef] [PubMed]
- López-Novoa, J.M.; Nieto, M.A. Inflammation and EMT: an alliance towards organ fibrosis and cancer progression. EMBO Mol Med 2009, 1, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Moeller, B.J.; Cao, Y.; Li, C.Y.; Dewhirst, M.W. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: role of reoxygenation, free radicals, and stress granules. Cancer Cell 2004, 5, 429–441. [Google Scholar] [CrossRef]
- Moeller, B.J.; Dreher, M.R.; Rabbani, Z.N.; Schroeder, T.; Cao, Y.; Li, C.Y.; Dewhirst, M.W. Pleiotropic effects of HIF-1 blockade on tumor radiosensitivity. Cancer Cell 2005, 8, 99–110. [Google Scholar] [CrossRef]
- Derer, A.; Frey, B.; Fietkau, R.; Gaipl, U.S. Immune-modulating properties of ionizing radiation: rationale for the treatment of cancer by combination radiotherapy and immune checkpoint inhibitors. Cancer Immunol Immunother 2016, 65, 779–786. [Google Scholar] [CrossRef]
- Ioakeim-Ioannidou, M.; Rose, M.; Chen, Y.-L.; MacDonald, S.M. The Use of Proton and Carbon Ion Radiation Therapy for Sarcomas. Seminars in Radiation Oncology 2024, 34, 207–217. [Google Scholar] [CrossRef]
- Zaboronok, A.; Isobe, T.; Yamamoto, T.; Sato, E.; Takada, K.; Sakae, T.; Tsurushima, H.; Matsumura, A. Proton beam irradiation stimulates migration and invasion of human U87 malignant glioma cells. J Radiat Res 2014, 55, 283–287. [Google Scholar] [CrossRef]
- Jasińska-Konior, K.; Pochylczuk, K.; Czajka, E.; Michalik, M.; Romanowska-Dixon, B.; Swakoń, J.; Urbańska, K.; Elas, M. Proton beam irradiation inhibits the migration of melanoma cells. PLOS ONE 2017, 12, e0186002. [Google Scholar] [CrossRef]
- Ogata, T.; Teshima, T.; Kagawa, K.; Hishikawa, Y.; Takahashi, Y.; Kawaguchi, A.; Suzumoto, Y.; Nojima, K.; Furusawa, Y.; Matsuura, N. Particle irradiation suppresses metastatic potential of cancer cells. Cancer Res 2005, 65, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Tommasino, F.; Rovituso, M.; Bortoli, E.; La Tessa, C.; Petringa, G.; Lorentini, S.; Verroi, E.; Simeonov, Y.; Weber, U.; Cirrone, P.; et al. A new facility for proton radiobiology at the Trento proton therapy centre: Design and implementation. Phys Med 2019, 58, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Entschladen, F.; Drell, T.L.t.; Lang, K.; Joseph, J.; Zaenker, K.S. Tumour-cell migration, invasion, and metastasis: navigation by neurotransmitters. Lancet Oncol 2004, 5, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, L.V.; Tate, C.R.; Segar, H.C.; Burks, H.E.; Phamduy, T.B.; Hoang, V.; Elliott, S.; Gilliam, D.; Pounder, F.N.; Anbalagan, M.; et al. Suppression of triple-negative breast cancer metastasis by pan-DAC inhibitor panobinostat via inhibition of ZEB family of EMT master regulators. Breast Cancer Res Treat 2014, 145, 593–604. [Google Scholar] [CrossRef]
- Göttlicher, M.; Minucci, S.; Zhu, P.; Krämer, O.H.; Schimpf, A.; Giavara, S.; Sleeman, J.P.; Lo Coco, F.; Nervi, C.; Pelicci, P.G.; et al. Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. Embo j 2001, 20, 6969–6978. [Google Scholar] [CrossRef]
- Chiu, H.W.; Yeh, Y.L.; Wang, Y.C.; Huang, W.J.; Chen, Y.A.; Chiou, Y.S.; Ho, S.Y.; Lin, P.; Wang, Y.J. Suberoylanilide hydroxamic acid, an inhibitor of histone deacetylase, enhances radiosensitivity and suppresses lung metastasis in breast cancer in vitro and in vivo. PLoS One 2013, 8, e76340. [Google Scholar] [CrossRef]
- Feng, X.; Han, H.; Zou, D.; Zhou, J.; Zhou, W. Suberoylanilide hydroxamic acid-induced specific epigenetic regulation controls Leptin-induced proliferation of breast cancer cell lines. Oncotarget 2017, 8, 3364–3379. [Google Scholar] [CrossRef]
- Luu, T.H.; Morgan, R.J.; Leong, L.; Lim, D.; McNamara, M.; Portnow, J.; Frankel, P.; Smith, D.D.; Doroshow, J.H.; Wong, C.; et al. A phase II trial of vorinostat (suberoylanilide hydroxamic acid) in metastatic breast cancer: a California Cancer Consortium study. Clin Cancer Res 2008, 14, 7138–7142. [Google Scholar] [CrossRef]
- Kyu-Shik, L.; Jin-Young, M.; Kyung-Soo, N.; Yun-Hee, S. Inhibition of Metastatic Activities in Human Breast Cancer Cells Irradiated by a Proton Beam. Journal of The Korean Physical Society—J KOREAN PHYS SOC 2011, 59. [Google Scholar] [CrossRef]
- Kwon, Y.-S.; Lee, K.-S.; Chun, S.-Y.; Jang, T.J.; Nam, K.-S. Suppressive effects of a proton beam on tumor growth and lung metastasis through the inhibition of metastatic gene expression in 4T1 orthotopic breast cancer model. Int J Oncol 2016, 49, 336–342. [Google Scholar] [CrossRef]
- Seane, E.N.; Nair, S.; Vandevoorde, C.; Bisio, A.; Joubert, A. Multi-Target Inhibitor CUDC-101 Impairs DNA Damage Repair and Enhances Radiation Response in Triple-Negative Breast Cell Line. Pharmaceuticals 2024, 17, 1467. [Google Scholar] [CrossRef]
- Wawruszak, A.; Borkiewicz, L.; Okon, E.; Kukula-Koch, W.; Afshan, S.; Halasa, M. Vorinostat (SAHA) and Breast Cancer: An Overview. Cancers (Basel) 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Lee, D.H.; Chun, S.Y.; Nam, K.S. Metastatic potential in MDA-MB-231 human breast cancer cells is inhibited by proton beam irradiation via the Akt/nuclear factor-κB signaling pathway. Mol Med Rep 2014, 10, 1007–1012. [Google Scholar] [CrossRef] [PubMed]
- Bravatà, V.; Cammarata, F.P.; Minafra, L.; Pisciotta, P.; Scazzone, C.; Manti, L.; Savoca, G.; Petringa, G.; Cirrone, G.A.P.; Cuttone, G.; et al. Proton-irradiated breast cells: molecular points of view. J Radiat Res 2019, 60, 451–465. [Google Scholar] [CrossRef]
- Atula, T.; Hedström, J.; Ristimäki, A.; Finne, P.; Leivo, I.; Markkanen-Leppänen, M.; Haglund, C. Cyclooxygenase-2 expression in squamous cell carcinoma of the oral cavity and pharynx: association to p53 and clinical outcome. Oncol Rep 2006, 16, 485–490. [Google Scholar] [CrossRef]
- Tucker, O.N.; Dannenberg, A.J.; Yang, E.K.; Zhang, F.; Teng, L.; Daly, J.M.; Soslow, R.A.; Masferrer, J.L.; Woerner, B.M.; Koki, A.T.; et al. Cyclooxygenase-2 expression is up-regulated in human pancreatic cancer. Cancer Res 1999, 59, 987–990. [Google Scholar]
- Kang, J.H.; Song, K.H.; Jeong, K.C.; Kim, S.; Choi, C.; Lee, C.H.; Oh, S.H. Involvement of Cox-2 in the metastatic potential of chemotherapy-resistant breast cancer cells. BMC Cancer 2011, 11, 334. [Google Scholar] [CrossRef]
- Lee, J.H.; Choy, M.L.; Ngo, L.; Foster, S.S.; Marks, P.A. Histone deacetylase inhibitor induces DNA damage, which normal but not transformed cells can repair. Proc Natl Acad Sci U S A 2010, 107, 14639–14644. [Google Scholar] [CrossRef]
- Namdar, M.; Perez, G.; Ngo, L.; Marks, P.A. Selective inhibition of histone deacetylase 6 (HDAC6) induces DNA damage and sensitizes transformed cells to anticancer agents. Proceedings of the National Academy of Sciences 2010, 107, 20003–20008. [Google Scholar] [CrossRef]
- Qiu, L.; Burgess, A.; Fairlie, D.P.; Leonard, H.; Parsons, P.G.; Gabrielli, B.G. Histone deacetylase inhibitors trigger a G2 checkpoint in normal cells that is defective in tumor cells. Mol Biol Cell 2000, 11, 2069–2083. [Google Scholar] [CrossRef]
- Huang, L.; Pardee, A.B. Suberoylanilide Hydroxamic Acid as a Potential Therapeutic Agent for Human Breast Cancer Treatment. Molecular Medicine 2000, 6, 849–866. [Google Scholar] [CrossRef] [PubMed]
- Schlaff, C.; Arscott, W.; Gordon, I.; Camphausen, K.; Tandle, A. Human EGFR-2, EGFR and HDAC triple-inhibitor CUDC-101 enhances radiosensitivity of GBM cells. Biomedical Research Journal 2015, 2, 105. [Google Scholar] [CrossRef]
- Masuda, K.; Horinouchi, H.; Tanaka, M.; Higashiyama, R.; Shinno, Y.; Sato, J.; Matsumoto, Y.; Okuma, Y.; Yoshida, T.; Goto, Y.; et al. Efficacy of anti-PD-1 antibodies in NSCLC patients with an EGFR mutation and high PD-L1 expression. J Cancer Res Clin Oncol 2021, 147, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Hossein-Nejad-Ariani, H.; Althagafi, E.; Kaur, K. Small Peptide Ligands for Targeting EGFR in Triple Negative Breast Cancer Cells. Scientific Reports 2019, 9, 2723. [Google Scholar] [CrossRef]
- Tang, Wai Ying, Y.; Beckett, Alison J.; Prior, Ian A.; Coulson, Judy M.; Urbé, S.; Clague, Michael J. Plasticity of Mammary Cell Boundaries Governed by EGF and Actin Remodeling. Cell Reports 2014, 8, 1722–1730. [CrossRef]
- Kim, B.; Lopez, A.T.; Thevarajan, I.; Osuna, M.F.; Mallavarapu, M.; Gao, B.; Osborne, J.K. Unexpected Differences in the Speed of Non-Malignant versus Malignant Cell Migration Reveal Differential Basal Intracellular ATP Levels. Cancers (Basel) 2023, 15. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).