Submitted:
06 November 2024
Posted:
07 November 2024
You are already at the latest version
Abstract
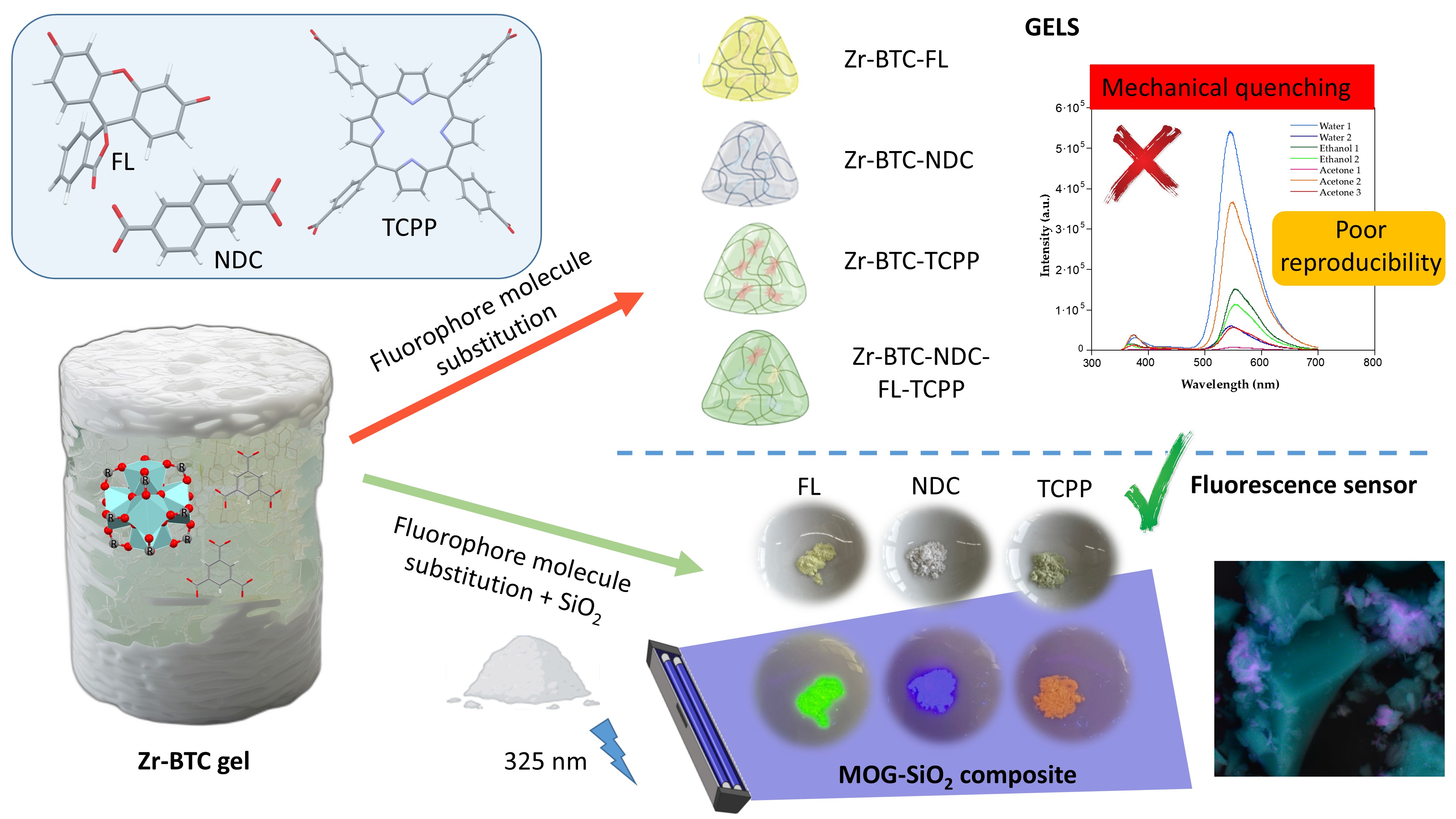
Keywords:
1. Introduction
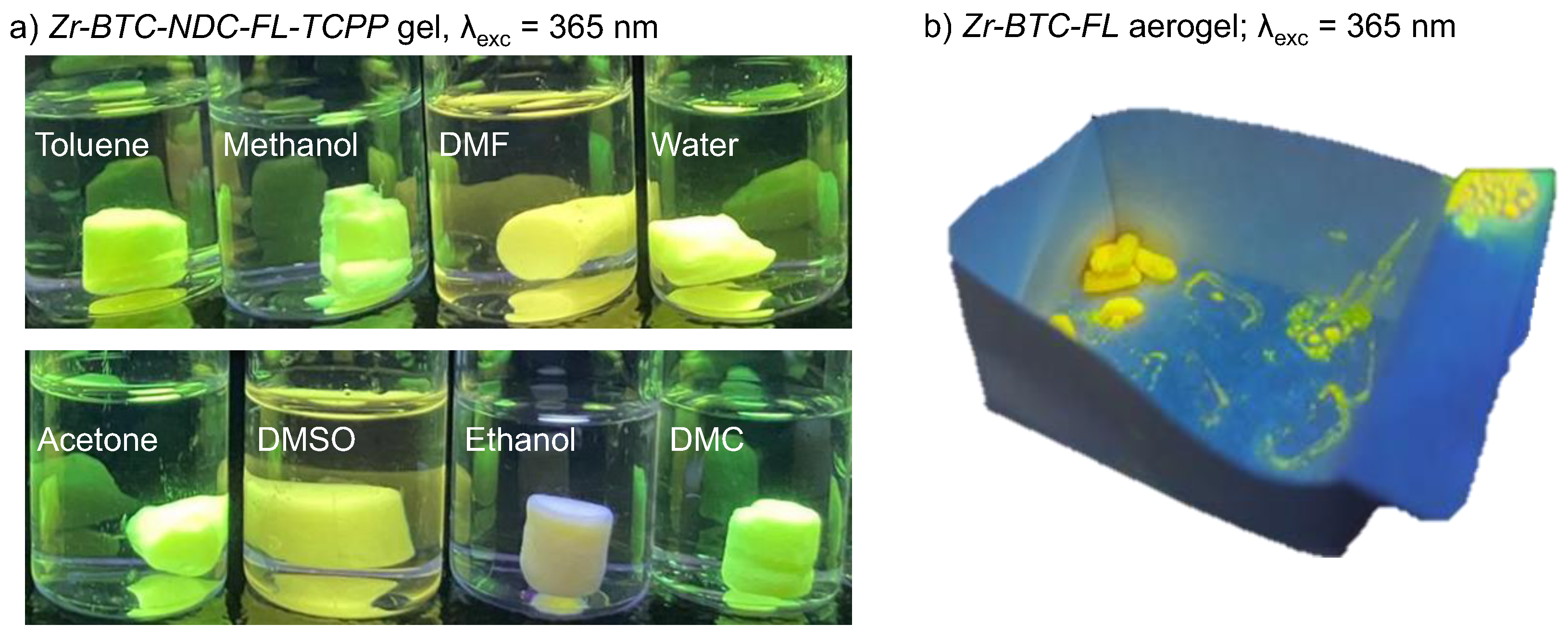
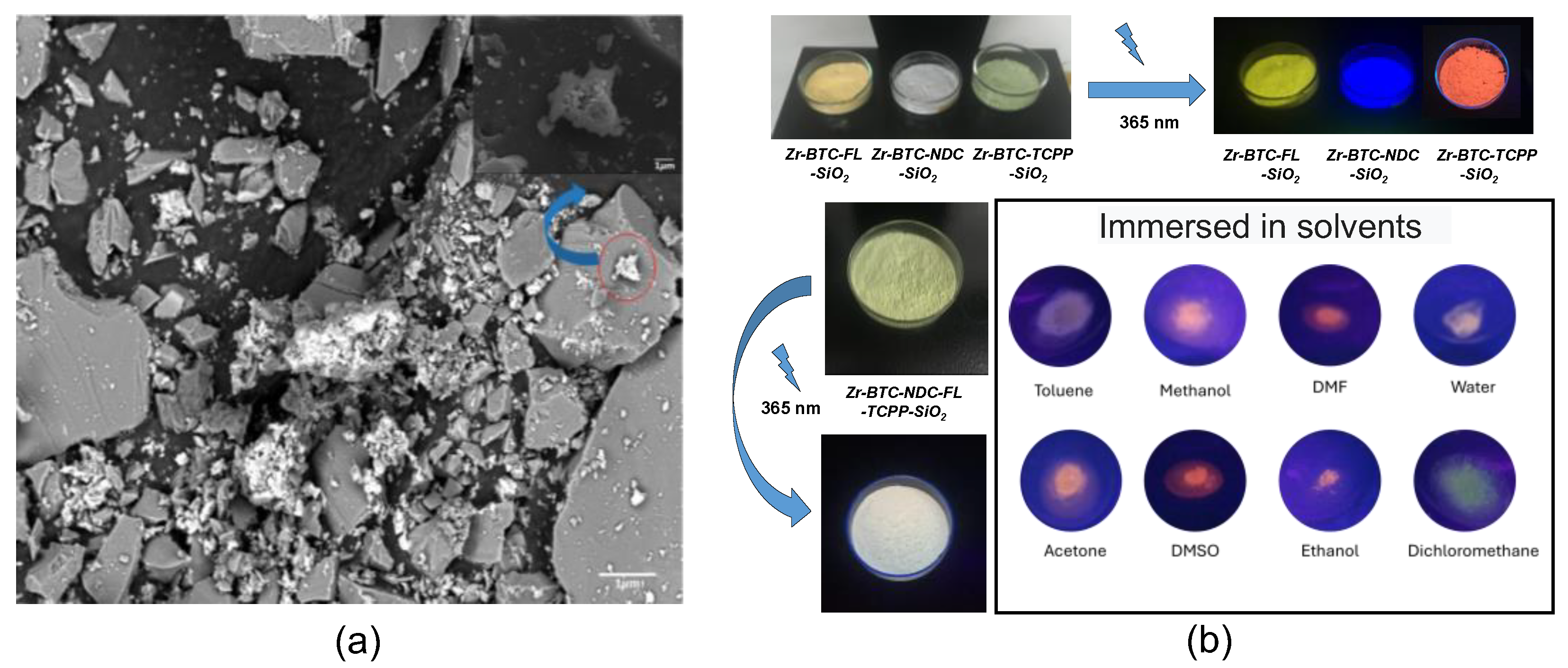
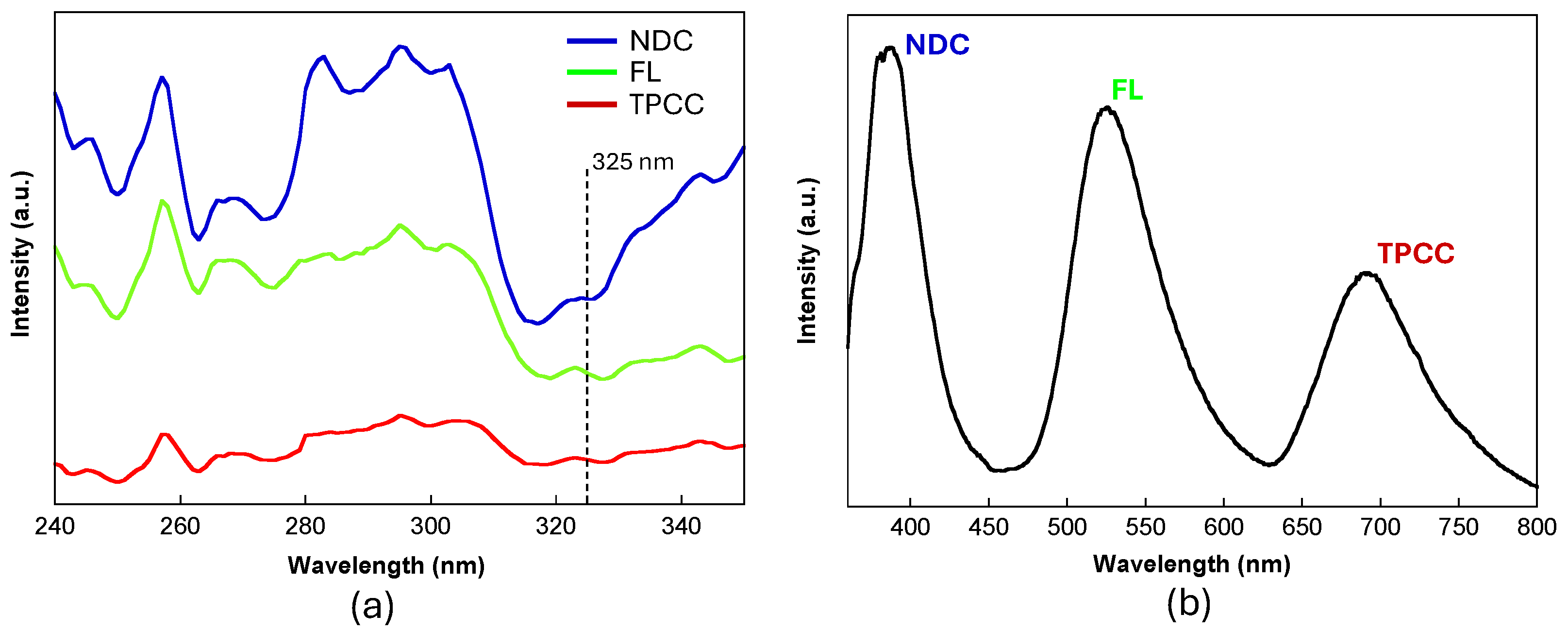
2. Results and Discussion
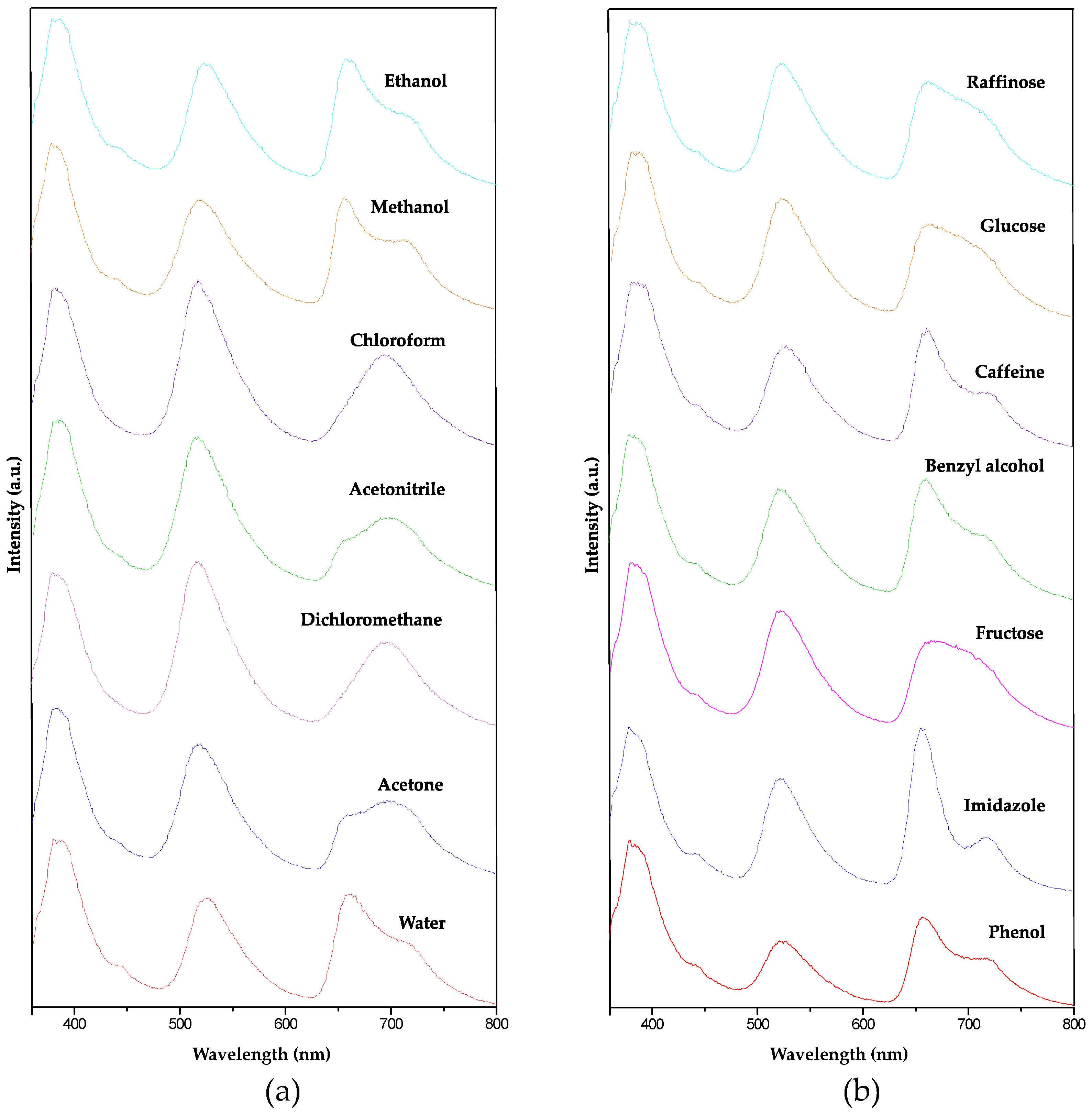
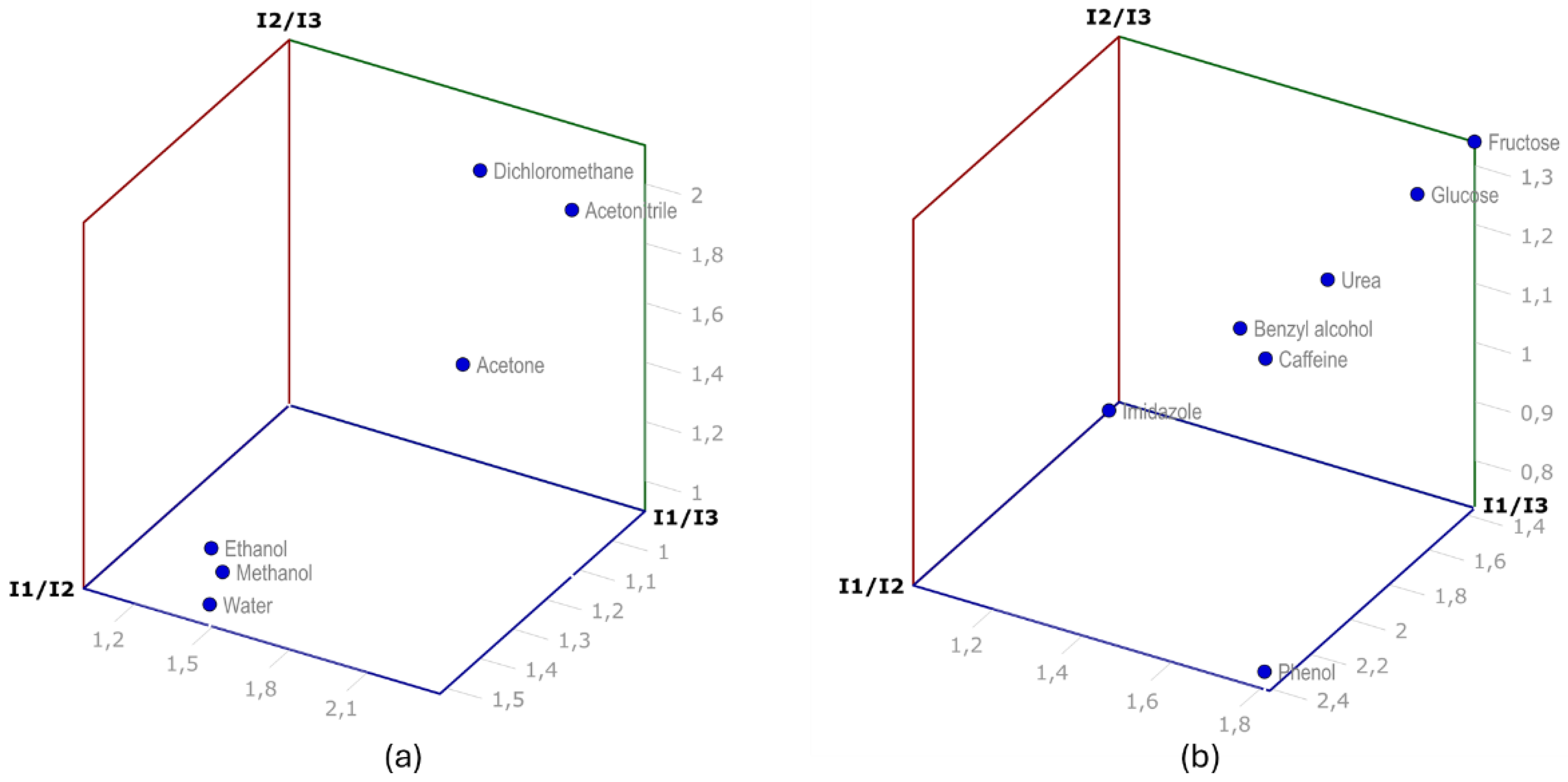
3. Conclusions
4. Materials and Methods
4.1. Synthesis of MOGs and MOG-SiO2 Composites Functionalized with Fluorophore Molecules
4.2. Fluorescence Measurements
4.3. Physical Measurements
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cao, W.; Lin, Z.; Zheng, D.; Zhang, J.; Heng, W.; Wei, Y.; Gao, Y.; Qian, S. Metal-Organic Gels: Recent Advances in Their Classification, Characterization, and Application in the Pharmaceutical Field. J. Mater. Chem. B 2023, 11, 10566–10594. [Google Scholar] [CrossRef] [PubMed]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A New Zirconium Inorganic Building Brick Forming Metal Organic Frameworks with Exceptional Stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, K.; Mandal, M.; Maiti, D.K. A Review on Zirconium-Based Metal-Organic Frameworks: Synthetic Approaches and Biomedical Applications. Mater. Adv. s 2023, 5, 51–67. [Google Scholar] [CrossRef]
- Angulo-Ibáñez, A.; Beobide, G.; Castillo, O.; Luque, A.; Pérez-Yáñez, S.; Vallejo-Sánchez, D. Aerogels of 1D Coordination Polymers: From a Non-Porous Metal-Organic Crystal Structure to a Highly Porous Material. Polymers 2016, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Yang, W.; Han, Y.; Luo, X.; Tang, W.; Yue, T.; Li, Z. A Straightforward Strategy to Synthesize Supramolecular Amorphous Zirconium Metal-Organic Gel for Efficient Pb(II) Removal. Chem. Eng. J. 2021, 407, 126744. [Google Scholar] [CrossRef]
- Shaiful Bahari, A.M.; Othman, S.Z.; Mohamad Fadli, M.F.; Zulkifli, M.Z.A.; Biyamin, S.A.; Islam, M.A.; Aspanut, Z.; Amin, N.; Misran, H. Facile Synthesis of Zr-Based Metal-Organic Gel (Zr-MOG) Using “Green” Sol-Gel Approach. Surf. Interfaces 2021, 27, 101469. [Google Scholar] [CrossRef]
- Liu, W.S.; Yang, Y.; Zhong, Q.K.; Xu, Z.P.; Zhang, J.Z.; Yao, B. Ben; Lian, X.; Niu, H.L. Zr4+-Based Metal Organic Gel as a Fluorescent “Turn on-off” Sensing Platform for the Selective Detection and Adsorption of CrO42-. Mater. Chem. Front. 2021, 5, 1932–1941. [Google Scholar] [CrossRef]
- Wang, J.; Xiao, X.; Lu, Y.; Wang, Y.; Chen, C.; Pang, H. Zirconium-Based Materials for Electrochemical Energy Storage. ChemElectroChem 2019, 6, 1949–1968. [Google Scholar] [CrossRef]
- Tan, C.; Xu, Z.; Zhang, L.; Lei, M.; Lei, J.; Duan, T.; Liu, W. Introducing Zirconium Organic Gels for Efficient Radioiodine Gas Removal. Inorg. Chem. 2022, 61, 4818–4824. [Google Scholar] [CrossRef]
- Santos-Lorenzo, J.; San José-Velado, R.; Albo, J.; Beobide, G.; Castaño, P.; Castillo, O.; Luque, A.; Pérez-Yáñez, S. A Straightforward Route to Obtain Zirconium Based Metal-Organic Gels. Micropor. Mesopor. Mat 2019, 284, 128–132. [Google Scholar] [CrossRef]
- Li, Y.; Young, D.J.; Loh, X.J. Fluorescent Gels: A Review of Synthesis, Properties, Applications and Challenges. Mater. Chem. Front. 2019, 3, 1489–1502. [Google Scholar] [CrossRef]
- Sun, S.; Wei, C.; Xiao, Y.; Li, G.; Zhang, J. Zirconium-Based Metal-Organic Framework Gels for Selective Luminescence Sensing. RSC Adv. 2020, 10, 44912–44919. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Xia, S.; Liu, Z.; Ma, T.; Liu, Z.; Li, Y.; Zou, D. Luminescent Porous Metal-Organic Gels for Efficient Adsorption and Sensitive Detection of Chlortetracycline Hydrochloride Assisted by Smartphones and a Test Paper-Based Analytical Device. Inorg. Chem. Front. 2022, 9, 1722–1734. [Google Scholar] [CrossRef]
- Aliev, S.B.; Gurskiy, S.I.; Zakharov, V.N.; Кustov, L. Synthesis of Novel Nanoporous Metal-Organic Gels with Tunable Porosity and Sensing of Aromatic Compounds. Micropor. and Mesopor. Mat. 2018, 264, 112–117. [Google Scholar] [CrossRef]
- Sun, S.; Wei, C.; Xiao, Y.; Li, G.; Zhang, J. Zirconium-Based Metal-Organic Framework Gels for Selective Luminescence Sensing. RSC Adv. 2020, 10, 44912–44919. [Google Scholar] [CrossRef]
- Zou, C.; Xie, M.H.; Kong, G.Q.; Wu, C. De Five Porphyrin-Core-Dependent Metal-Organic Frameworks and Framework-Dependent Fluorescent Properties. CrystEngComm 2012, 14, 4850–4856. [Google Scholar] [CrossRef]
- Li, Y.; Chai, B.L.; Xu, H.; Zheng, T.F.; Chen, J.L.; Liu, S.J.; Wen, H.R. Temperature- and Solvent-Induced Reversible Single-Crystal-to-Single-Crystal Transformations of TbIII-Based MOFs with Excellent Stabilities and Fluorescence Sensing Properties toward Drug Molecules. Inorg. Chem. Front. 2022, 9, 1504–1513. [Google Scholar] [CrossRef]
- Hou, J.; Sapnik, A.F.; Bennett, T.D. Metal-Organic Framework Gels and Monoliths. Chem. Sci. 2020, 11, 310–323. [Google Scholar] [CrossRef]
- Shaiful Bahari, A.M.; Othman, S.Z.; Mohamad Fadli, M.F.; Zulkifli, M.Z.A.; Biyamin, S.A.; Islam, M.A.; Aspanut, Z.; Amin, N.; Misran, H. Facile Synthesis of Zr-Based Metal-Organic Gel (Zr-MOG) Using “Green” Sol-Gel Approach. Surfaces and Interfaces 2021, 27, 101469. [Google Scholar] [CrossRef]
- Massimi, S.E.; Metzger, K.E.; McGuirk, C.M.; Trewyn, B.G. Best Practices in the Characterization of MOF@MSN Composites. Inorg. Chem. 2022, 61, 4219–4234. [Google Scholar] [CrossRef]
- Tella, A.C.; Owalude, S.O.; Omotoso, M.F.; Olatunji, S.J.; Ogunlaja, A.S.; Alimi, L.O.; Popoola, O.K.; Bourne, S.A. Synthesis, Crystal Structures and Luminescence Properties of New Multi-Component Co-Crystals of Isostructural Co(II) and Zn(II) Complexes. J. Mol. Struct. 2018, 1157, 450–456. [Google Scholar] [CrossRef]
- Gangu, K.K.; Dadhich, A.S.; Mukkamala, S.B. Synthesis, Crystal Structure and Fluorescent Properties of Two Metal-Organic Frameworks Constructed from Cd(II) and 2,6-Naphthalene Dicarboxylic Acid. Inorg. Nano-Met. Chem. 2017, 47, 313–319. [Google Scholar] [CrossRef]
- Ding, Y.; Pan, L.; Li, S.; He, H.; Zhang, D.; Hu, X.; Zhang, R.; Wang, Y.; Zhai, X.; Meng, Q. Fabrication and Investigation of 26NCA Films Exhibiting Tunable Blue Fluorescence Based on LVPVDM. J. Nanomater. 2018, 2018, 5426427. [Google Scholar] [CrossRef]
- Seybold, P.G.; Gouterman, M.; Callis, J. Calorimetric, Photometric and Lifetime Determinations of Fluorescence Yields of Fluorescein Dyes. Photochem. and Photobiol. 1969, 9, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Quiroz-Segoviano, R.I.Y.; Serratos, I.N.; Rojas-González, F.; Tello-Solís, S.R.; Sosa-Fonseca, R.; Medina-Juárez, O.; Menchaca-Campos, C.; García-Sánchez, M.A. On Tuning the Fluorescence Emission of Porphyrin Free Bases Bonded to the Pore Walls of Organo-Modified Silica. Molecules 2014, 19, 2261–2285. [Google Scholar] [CrossRef]
- Makarska-Bialokoz, M. Spectroscopic Study of Porphyrin-Caffeine Interactions. J. Fluoresc. 2012, 22, 1521–1530. [Google Scholar] [CrossRef]
- Rajasree, S.S.; Li, X.; Deria, P. Physical Properties of Porphyrin-Based Crystalline Metal‒organic Frameworks. Commun. Chem. 2021, 4, 1–14. [Google Scholar] [CrossRef]
- Thorseth, A. Photometry — The CIE System of Physical Photometry. ISO/CIE 2023, 23539.
- Qi, H.; Zhang, T.; Jing, C.; Zhang, Z.; Chen, Y.; Chen, Y.; Deng, Q.; Wang, S. Metal-Organic Gel as a Fluorescence Sensing Platform to Trace Copper(II). Anal. Methods 2022, 14. [Google Scholar] [CrossRef]
- Feng, X.; Zeng, L.; Zou, D.; Zhang, Z.; Zhong, G.; Peng, S.; Liu, L.; Chen, L.; Zhang, J. Trace-Doped Metal-Organic Gels with Remarkably Enhanced Luminescence. RSC Adv. 2017, 7, 37194–37199. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Yang, Y.S.; Wang, W.; Jiao, Q.C.; Zhu, H.L. Fluorescent Sensors for the Detection of Hydrazine in Environmental and Biological Systems: Recent Advances and Future Prospects. Coord. Chem. Rev. 2020, 417, 213367. [Google Scholar] [CrossRef]
- New, A.A.; Bowyer, E.J. Fluorescent Platforms for Environmental Sensing. In Fluorescent Chemosensors; James, L.W.A.C.S.X.-P.H.T.D., Ed.; Royal Society of Chemistry, 2023; pp. 378–405 ISBN 978-1-83916-386-9.
- Fakayode, S.O.; Lisse, C.; Medawala, W.; Brady, P.N.; Bwambok, D.K.; Anum, D.; Alonge, T.; Taylor, M.E.; Baker, G.A.; Mehari, T.F.; et al. Fluorescent Chemical Sensors: Applications in Analytical, Environmental, Forensic, Pharmaceutical, Biological, and Biomedical Sample Measurement, and Clinical Diagnosis. Appl. Spectrosc. Rev. 2024, 59, 1–89. [Google Scholar] [CrossRef]
- Nam, D.; Kim, J.; Choe, W. Evolution of Zr Nodes in Metal–Organic Frameworks. Trends Chem. 2023, 5, 339–352. [Google Scholar] [CrossRef]

| Solvents |
I1 (NDC) |
I2 (FL) |
I3 (TCPP) |
I1/I2 | I1/I3 | I2/I3 |
| Water | 513(2) | 337(4) | 344(10) | 1.52(2) | 1.49(4) | 0.97(3) |
| Acetone | 553(9) | 437(4) | 257(6) | 1.27(2) | 2.15(6) | 1.70(4) |
| Methanol | 624(4) | 431(12) | 431(21) | 1.45(4) | 1.45(7) | 1.00(6) |
| Ethanol | 511(4) | 377(10) | 396(14) | 1.36(4) | 1.29(5) | 0.95(4) |
| Acetonitrile | 583(13) | 521(2) | 245(6) | 1.12(3) | 2.38(8) | 2.13(5) |
| Dichloromethane | 405(5) | 437(12) | 228(2) | 0.93(3) | 1.78(1) | 1.92(3) |
| Aqueous solutions |
I1 (NDC) |
I2 (FL) |
I3 (TCPP) |
I1/I2 | I1/I3 | I2/I3 |
| Glucose | 514(8) | 372(13) | 302(13) | 1.38(4) | 1.70(8) | 1.23(6) |
| Urea | 520(2) | 364(7) | 343(22) | 1.43(3) | 1.52(10) | 1.06(7) |
| Fructose | 516(6) | 379(8) | 283(7) | 1.36(3) | 1.82(5) | 1.34(4) |
| Imidazole | 521(7) | 369(9) | 512(14) | 1.41(4) | 1.02(3) | 0.72(3) |
| Benzyl alcohol | 507(5) | 358(9) | 385(9) | 1.42(4) | 1.32(3) | 0.93(3) |
| Caffeine | 477(9) | 314(27) | 337(9) | 1.52(14) | 1.42(17 | 0.93(14) |
| Phenol | 434(6) | 180(2) | 239(2) | 2.41(4) | 1.81(3) | 0.75(1) |
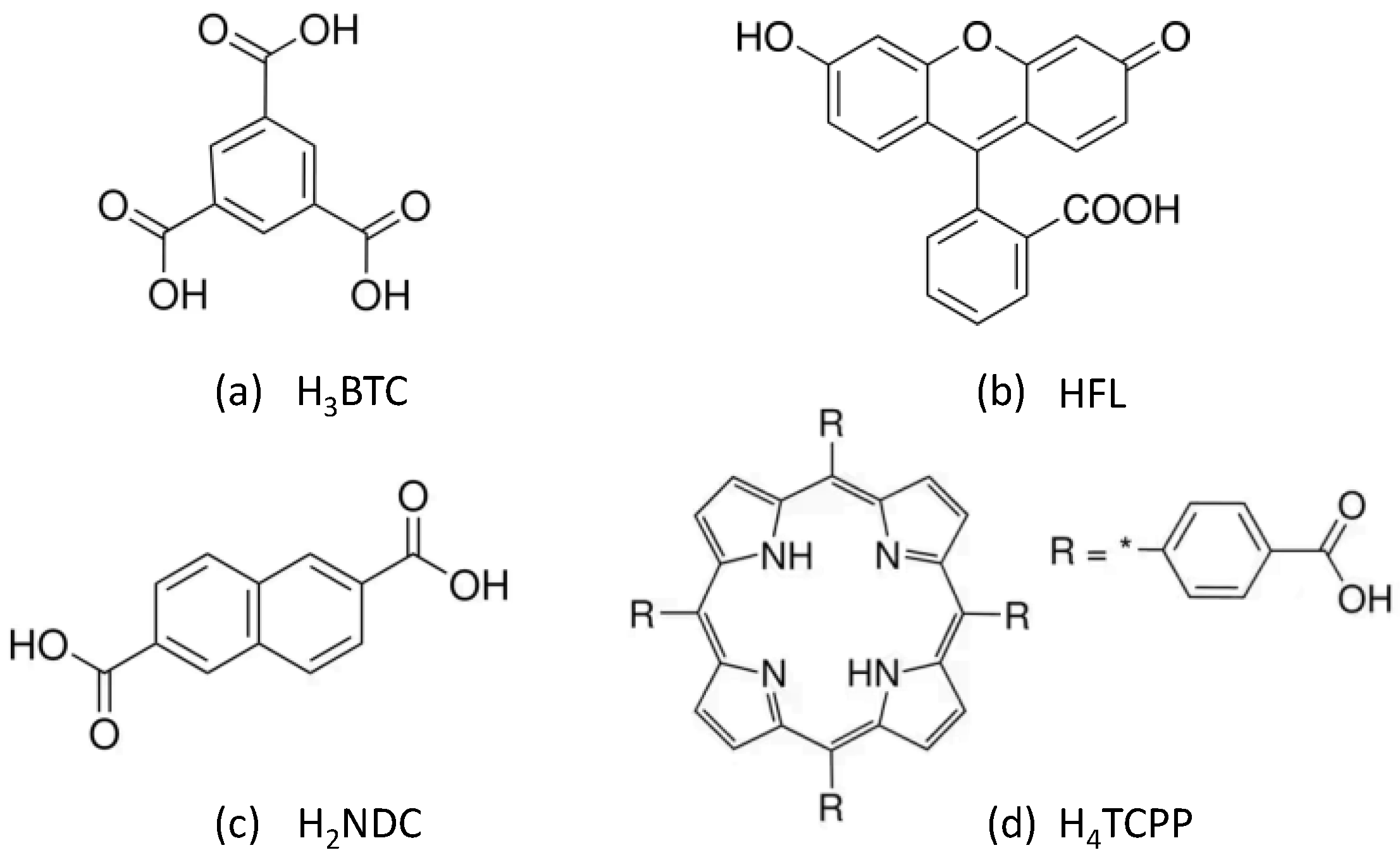
| Code | H3BTC (mmol) | HFL (mmol) | H2NDC (mmol) | H4TCPP (mmol) |
| Zr-BTC-FL | 1.090 | 0.050 | - | - |
| Zr-BTC-NDC | 0.560 | - | 0.820 | - |
| Zr-BTC-TCPP | 1.105 | - | - | 0.001 |
| Zr-BTC-NDC-FL-TCPP | 1.069 | 0.050 | 0.030 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





