1. Introduction
In the 1910s, the Swiss psychiatrist Eugen Bleuler introduced the term autism to refer to what he identified as one of the symptoms of schizophrenia (Bleuler 1911). A fundamental description of autism spectrum symptoms was later developed in the 1920s by Ssucharewa (Ssucharewa, 1926) followed by systematic case reports by Kanner (Kanner 1943) and Asperger (Asperger 1944) in the 1940s. Kanner and Asperger laid the foundation to make the distinction between autism and childhood schizophrenia (Vicedo 2024), later clarified in the 1980s with the 3rd edition of The Diagnostic and Statistical Manual of Mental Disorders (DSM-III) (American Psychiatric Association Staff and Spitzer 1980; Rosen et al. 2021). Over time, the apparent heterogeneous nature of autism in clinical presentation, etiology, underlying neurobiology, and degree of severity led to the adoption and common usage of the term autism spectrum disorder (ASD) (Masi et al. 2017). This conveys a shift from categorical concepts of autistic syndromes to a dimensional concept, within the larger framework of neurodevelopmental disorders (NDD). The current consensus, according to the National Institute of Mental Health (NIMH), defines ASD as “a neurological and developmental disorder that affects how people interact with others, communicate, learn, and behave” (NIMH 2024); while the current diagnostic criteria for ASD according to DSM-V (American Psychiatric Association and American Psychiatric Association 2013), states that a child must have persistent deficits in areas of social communication and interaction plus restricted repetitive behaviors and interests. Other disorders in addition to autism included within the umbrella of ASD consist of Asperger’s disorder, Rett’s disorder, Landau-Kleffner Syndrome, Fragile X syndrome, childhood disintegrative disorder (Heller's syndrome), and pervasive developmental disorder not otherwise specified (Irwin et al. 2011).
ASD is broadly characterized as a neuropsychiatric disorder, while its manifestation is that of a psychiatric syndrome (Salmond et al. 2003). Its most brutal manifestation would indicate, as Eugen Bleuler first identified, “a neonate’s tendency to turn away from reality and retire into a subjective world” (Bleuler 1951). Current real-world evidence documents that, in a significant number of cases, parents witness the occurrence of ASD behavioral phenotypes in normal babies who have reached all previous developmental milestones (Goldberg et al. 2003; Lord et al. 2004; Luyster et al. 2005; Werner and Dawson 2005; Barbaro and Dissanayake 2009). While former studies suggested that regressive forms of onset were not common in ASD, more recent investigations indicate that the rates are quite high and may be under-reported. For example, Ozonoff et al., (Ozonoff et al. 2010) report an 88% regressive ASD phenotype in a systematic investigation of infants with and without a family history of ASD. Changes in the postnatal or early developmental periods include dramatic abnormalities in social interaction and withdrawal (i.e., lack of pair-bonding, “avert eyes” (Davies et al. 1994), and lack of neonatal imitation (Rogers et al. 2005)), impairments in verbal and non-verbal communication, and a restricted repertoire of interests and activities, (Baron-Cohen 2004) as well as seeking and finding comfort in repetitive behaviors. Babies, adolescents, and adults with ASD are also reported to have difficulties in emotion processing, in particular, problems with recognizing and discriminating emotions in others (Carré et al. 2015).
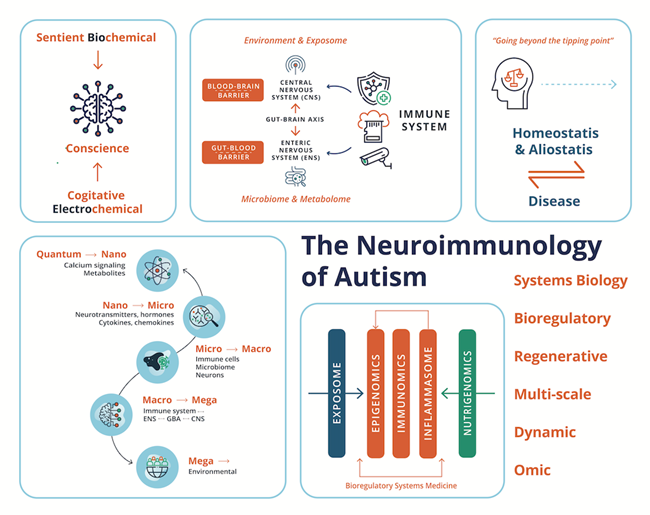
Growing evidence shows that much of what ASD involves is related not to fixed “autistic states of being” but rather to the consequences of an allostasis system malfunction. Principally mediated through environmental insult and deficiencies, leading to maladaptation and pathology of the immune system. Response to acute or chronic stress promotes allostasis or adaptation and promotes survival by protecting the body from damage and adaptive response for which it has immunologic memory (McEwen 1998a). However, when the allostatic systems are overworked beyond the “tipping point”, the capacity to respond acutely and appropriately is suppressed and, if the immunologic memory is for an inflammatory or autoimmune response within the nervous system, the shorter-term stress effect could exacerbate into a chronic disease process (McEwen 1998b), involving heterogeneous ASD behavioral phenotypes. This review aims to move beyond genetic determinism to epigenetic regulation and its modulation by acute and chronic environmental toxic exposure and insult to the immune system in individuals with ASD. Historically the documentation of psychological features largely preceded the documentation of physiological components of ASD. However, the pathological mechanisms we elaborate on, involve interacting sets of complex “whole-body” psychological and physiological processes along the immune-neuro-microbiota-axis, working through some individuals with ASD at different rates, with different equilibria or set points when compared to neurotypical people.
Real-world and clinical evidence of fever has been reported from the onset of ASD symptoms (Megremi 2013), which would indicate an inflammatory response in its etiology. Several immunological abnormalities have been detected both in the peripheral and central nervous system (CNS) for the pathogenesis of ASD (Gładysz et al. 2018). The role of toxic compounds and their accumulation in the CNS and their role in neurodegenerative and NDDs has been acknowledged for many decades (Kaur et al. 2021). However, ASD was not thought to be associated with any loss or maladaptation of the function of the immune system, as the CNS was understood to be shielded by barriers. Indeed, since the 1960´s the brain was almost axiomatically viewed as an immune-privileged site (Schwartz and Cahalon 2022). However, over the last decade, mounting evidence has uncovered the role of the immune system in CNS health and functioning and disease.
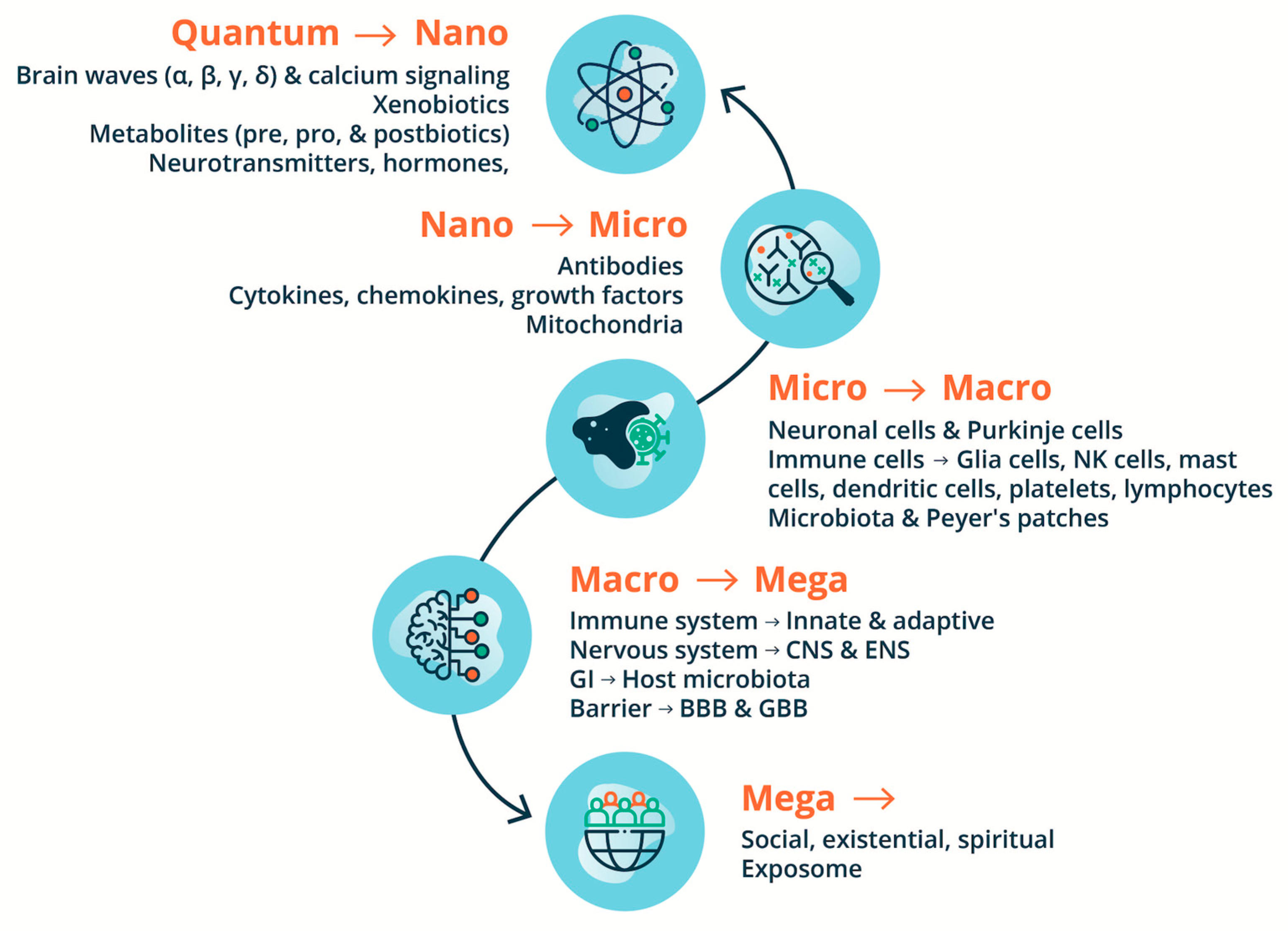
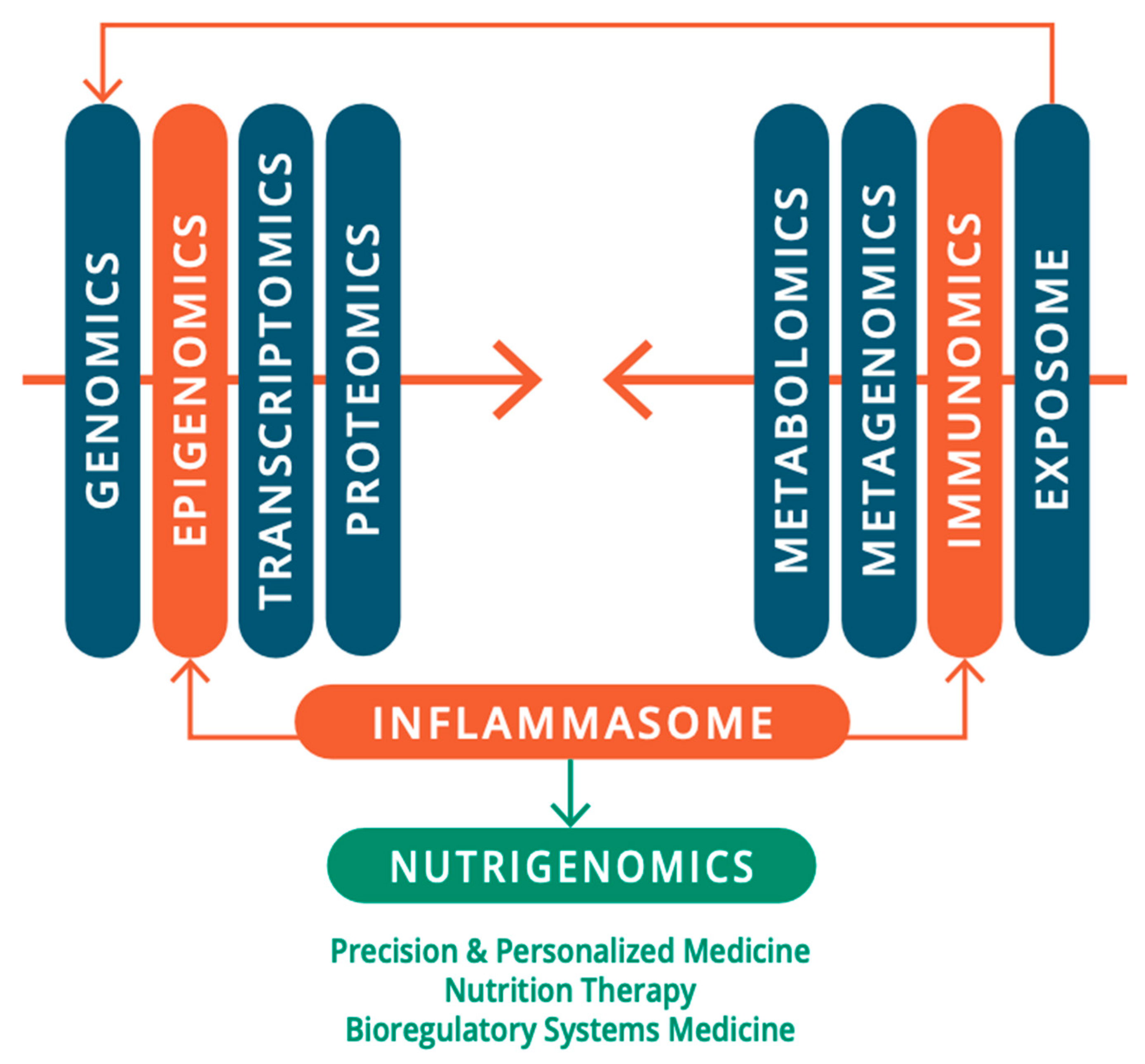
If the critical role of environmental insult in the pathogenesis of ASD is taken a priori, the role of the immune system becomes paramount in its pathology. As reviewed from the 2000s by Pardo and coworkers (Pardo et al. 2005; Pardo and Eberhart 2007), Anderson et al., (Anderson et al. 2008), and Ashwood and coworkers (Ashwood and Van de Water 2004; Onore et al. 2012). Indeed, alterations and maladaptation of the immune system including both adaptive and innate systems, remain some of the most controversial concepts in ASD. Building on this pioneering work, this review provides an updated and current commentary on the role of the immune system and of neuroimmunology within a broader environmental “exposome” in the pathology and pathophysiology of ASD. We highlight the current state-of-the-art detailing the interconnection, interdependence, and interference with or subjugation (as would be the case for autoinflammatory and autoimmune conditions) of the nervous system and host-microbiota by the immune system and its role in the pathogenesis of ASD. We begin with the epidemiological, genetic, and environmental underpinning of ASD, with attention to the current scientific discourse regarding the relationship of the immune system with the nervous system and host microbiota in homeostasis/allostasis, neurodevelopment, and psychological and physiological health and disease, before examining the role of the immune system in the etiology, pathogenesis, and pathophysiology of ASD. We also highlight the need for a multi-scale, holistic, and “middle-out” approach to understanding and developing future therapeutic modalities for ASD (Herbert 2014), accomplished through a multi-dimensional omics technology, and a bioregulatory systems (BrSYS) approach. Considering the heterogeneous nature of ASD and the vulnerable nature of individuals on the spectrum, we also endorse and highlight non-pharmacological, nutritional, and botanical therapeutic modalities, particularly considering the growing body of scientific literature documenting the impacts of these approaches on immune function. Optimally, multiple interventions with low-risk profiles would be synergistically woven into an individualized dynamic strategy individually tailored to the specific, usually multisystem, set of vulnerabilities of each person, so as to not only provide exceptional safety profiles but also go beyond a reductionist pharmacological medical paradigm, with the capacity to not only ameliorate the symptoms of ASD but also reverse its core phenotypes.
2. Epidemiological Preambles of ASD
ASD has complex heterogeneous clinical manifestations (Bauman 2010), has a strong bias toward males, and shares a broad crossover with numerous neuropsychiatric disorders. ASD phenotypes (social-communication impairments and restricted, repetitive patterns of behavior) are commonly associated with intellectual disabilities, anxiety, depression, epilepsy, attention deficit hyperactivity disorder (ADHD), sleep disorders, dyspraxia, and lack of verbal communication (Barlattani et al. 2023). The significant occurrence of physiological comorbidities in ASD is also highlighted (Khachadourian et al. 2023) with reports of increased rates of chronic physical health conditions across all organ systems in autistic children, adolescents, and adults (Ward et al. 2023). This can be broadly sub-grouped as related to metabolic disorders (Ghaziuddin and Al-Owain 2013); immune (allergies, infections, primary immunodeficiency, and autoimmune) disorders (Hughes et al. 2018); and motor disorders (Nordin et al. 2021; Gonzalez et al. 2024).
ASD is not strictly associated with mental retardation and some people on the ASD spectrum are exceedingly brilliant. Nonverbal people with ASD can also be articulate and expressive, through assistance and technologies that facilitate spelling and typing (Jaswal et al. 2024). A person unable to speak may be assumed to lack the capacity for symbolic thought that underlies language. However, verbal speech is only one means of expressive language, and literacy by non-verbal means has been extensively reported in nonspeaking people with ASD (Jaswal et al. 2024). For example, some have learned to communicate by pointing to alphabet letters. This method remains controversial because it requires the assistance of another person who could theoretically cue them to point to letters (Lilienfeld et al. 2014). In a recent study, Jaswal et al. (Jaswal et al. 2020) used head-mounted eye-tracking to investigate communicative agency in a sample of 9 nonspeaking autistic individuals. Researchers measured the speed and accuracy with which individuals looked at and pointed to letters as they responded to novel questions. Participants pointed to about one letter per second, rarely made spelling errors, and visually fixated on most letters for about half a second before pointing to them. Additionally, their response times reflected planning and production processes characteristic of fluent spelling in non-autistic typists. These findings by Jaswal et al. would render a cueing account of participants’ performance unlikely and a blanket dismissal of assisted autistic communication unwarranted.
While ASD can involve exquisite gifts and unusual qualities of perception and thought, the condition can also involve a great deal of suffering, for individuals on the spectrum as well as family and wider community. In a descriptive study, Weitlauf et al, (Weitlauf et al. 2014) report that up to 25% of 726 participants with ASD have severe disabilities and require substantial support and 24-hour-a-day care. Siegel et al., (Siegel et al. 2014) report that 11% of children with ASD are psychiatrically hospitalized in the USA before age twenty-one and Lui et al., report adolescents with ASD accessed emergency department services four times as often as adolescents without ASD (Liu et al. 2017). Depending on the degree of severity and intervention strategies accessed, some children with ASD may develop into independent and interdependent adults with full-time employment and self-sufficiency; however, this is seldom the case. Moreover, a clear need also becomes apparent, not only to address the core characteristics but also deeper existential suffering and loneliness for a growing and vulnerable adult population with ASD (Hickey et al. 2018). Such issues may include loss and change, freedom of choice versus loss of control, the dignity of the self, fundamental aloneness, altered quality of relationships, searching for meaning, mystery about what seems unknowable, and death anxiety (Kissane 2012; Cwik 2021).
ASD remained a relatively unknown disorder affecting less than 1 in 2,500 children up until the 1980s (C. Nevison et al. 2018). Current estimates by the CDC (Maenner 2021) and Dietz et al., (Dietz et al. 2020) indicate up to 1 in 36 children and 1 in 45 adults in the USA have ASD, respectively. This apparent dramatic increase in ASD prevalence over the past five decades has provoked a heated debate in the academic and medical community. While the broad clinical manifestations of ASD have remained largely unchanged since Kanner´s and Asperger´s first descriptions, speculations on the increasing prevalence of ASD have emerged with better and more expanded diagnosis in observational studies (Nevison and Blaxill 2017), as well as differing external methodological factors used in epidemiological research estimating the prevalence of ASD. A rising prevalence of ASD also coincides with an epidemic of chronic childhood diseases in the USA, each of which has its own set of diagnostic criteria. At present, the CDC reports up to 2 in 5 US students aged 6 to 17 have chronic health conditions such as asthma, diabetes, or epilepsy (CDC 2021). This again represents a dramatic increase from estimates of 18 in 1000 children reported to have conditions severe enough to interfere with usual daily activities in the 1960s (Perrin et al. 2014) and estimates of 1 in 100 to 1 in 25 children under 16 to have a severe chronic illness in the 1980s (Harkey 1983). In 2011 based on a 2007 National Survey of Children Health, Bethell et al. estimated that 54% of US children had at least 1 of 20 chronic health conditions or were at risk for developmental delays (Bethell et al. 2011). In 2021 Ullah and Kaelber (Ullah and Kaelber 2021) based on surveys conducted between 2016 – 2018, an estimated 40% of children and adolescents have at least one chronic disease including obesity, eczema, asthma, food allergies, ADHD, and hypertension. A rising, significant, and aging global population with ASD also has economic implications. For the individuals with ASD identified from 1990–2019, the lifetime social cost for the US was estimated to be more than $7 trillion in 2019 dollars (Cakir et al. 2020). As discussed by Cakir et al., even if one assumes that the rate of increase in the prevalence of ASD is static for the next decade (2020–2029), the projected cost estimate for ASD in the US will increase to $11.5 trillion in 2019 dollars. These numbers are not unique to the USA. Autism is often regarded internationally as the most expensive disability (Hurley-Hanson et al. 2020). In the United Kingdom, the total annual cost for children with ASD is $4.3 billion, while for adults it is reported to be $40.5 billion (Knapp et al. 2009); and the national cost of ASD in Australia is estimated to range from $4.5 to $7.2 billion (Horlin et al. 2014).
3. Underpinnings of ASD: From Genetic to Environmental Etiologies
The ongoing debate on the rise and prevalence of ASD over nearly half a century can be associated with the central scientific dogma that ASD is a highly heritable genetic disease of the brain. Indeed, the current professional standard of care emanates from the genetic narrative’s static encephalopathy-based model of autism. Yet although over $1 billion has been spent on genetic research in autism over the past 10 years by the NIH, Autism Speaks, and the Simons Foundation, unequivocal evidence that a genetic association is “hardwired” has not been established (Herbert 2012). Moreover, genetic investigations have failed to yield a single therapeutic to treat the core symptoms. Concordance (shared diagnosis) of 90% of monozygotic (identical twins) and 10% of dizygotic (fraternal twins), published in the 1970s, were initially used to justify an intensive focus on genetics to the exclusion of environmental influence (Folstein and Rutter 1977). These findings have yet to be corroborated., e.g., in 2011 Hallmayer et al. (Hallmayer et al. 2011) reported on the largest twin study to date and reported a lower monozygotic concordance and higher dizygotic concordance. Their results yielded a smaller gap between autism rates in identical as compared with fraternal twins, with 55% of the variance for strict autism and 58% for ASD explained by shared environmental factors, with moderate genetic heritability of 37-38% (Hallmayer et al. 2011). In 2014 using an epidemiological sample from Sweden, Gaugler et al., (Gaugler et al. 2014) concluded that autism's genetic architecture has a narrow-sense heritability of ≈52.4%, with most due to the common variation, and rare de novo mutations. A growing body of evidence implicates a strong interplay between environmental insults and epigenetics (Deth 2013) in the pathophysiology of ASD (Tordjman et al. 2014; Gropman and Sadle 2024), with chronic and acute multifactorial xenobiotic exposure occurring during peri- or postnatal or even preconception periods. Fuller et al. (Fuller et al. 2018) report up to nine million deaths per year (16% of all deaths worldwide) are attributed to air, water, and soil pollution alone. This is unsurprising when one considers that today’s typical contemporary diet and lifestyle are corrupted by ultra-processed and nutrient-poor food (Leo and Campos 2020; Miclotte and Van de Wiele 2020), and chemical exposure through cosmetics, home-care products, household construction materials, agrochemicals, and pharmaceuticals in the home, school, and workplace. Scientific literature spanning many decades implicates exposure to non-metabolic heavy metals such as mercury (Bernard et al. 2001; Geier et al. 2010; Kern et al. 2012) and aluminum (Exley 2013; C. A. Shaw et al. 2014; Exley and Clarkson 2020). Both have a long environmental legacy from mining in North America and have had widespread application as adjuvants in biologics recommended during pregnancy and to infants (Bernard et al. 2001; Blaxill et al. 2004; Kern et al. 2012; Hooker et al. 2014; Geier et al. 2015; Kern et al. 2017; Crépeaux et al. 2020). Other heavy metals implicated, again with no metabolic function in the human body, include cadmium, lead, and arsenic, all found in contaminated superfund sites. All these metals are highly neurotoxic and are known to cause neurodevelopmental deficits (Zota et al. 2016). Energy, porphyrin, and neurotransmitter homeostasis are the key metabolic pathways affected by heavy metal exposure. Other pharmaceuticals implicated in the etiology of ASD include antibiotics (Njotto et al. 2023), valproate (Zarate-Lopez et al. 2023), acetaminophen (Jones et al. 2024), antidepressants (Mezzacappa et al. 2017) and anticonvulsants and antiemetics taken by mothers during pregnancy (Chambers and Schaefer 2015). Fetal and neonatal exposure to toxins can also occur from industrial chemicals such as polyfluorinated substances, dioxins, polychlorinated biphenyls, alkylphenols, and plasticizers like phthalates and bisphenol A (Bjørklund et al. 2024). All these chemicals are known to be endocrine-disrupting chemicals and can disrupt normal immune function in the brain, leading to chronic or excessive neuroinflammation. Organophosphorus (OP) compounds are a class of acetylcholinesterase inhibitors used in agrochemicals (pesticides, insecticides, herbicides, and fungicides) and have a long history in chemical warfare as neurotoxins (Pu et al. 2020). Some chronic illnesses that manifest symptomatology to ASD such as Gulf War Illness have (at least in part) been attributed to OP exposure (Jaga and Dharmani 2007; Toomey et al. 2007; Zanchi et al. 2023). A proposed etiological role of glyphosate for ASD has recently been proposed by Seneff, Kyriakopoulos, and Nigh (Seneff et al. 2024). Glyphosate is a widely used active ingredient in agricultural herbicides, inhibiting the biosynthesis of aromatic amino acids in plants by targeting their shikimate pathway (Rueda-Ruzafa et al. 2019). The shikimate pathway is not present in mammals per se, however, the pathway is present in gut bacteria (Samsel and Seneff 2013). Walsh, Hill, and Ross (Walsh et al. 2023) further hypothesize and explore the potential of glyphosate to inhibit the growth or functionality of beneficial microbes in the gut. Moreover, glyphosate can substitute for glycine in protein synthesis, creating potential havoc. Studies spanning many decades have shown that exposure to low-level, low-frequency electromagnetic radiation (EMF) can break DNA chains, damage proteins, even increase the blood-brain barrier permeability, disturb sleep, and cause fatigue, memory, and concentration (ADHD) problems (Stein et al. 2015). Herbert and Sage (Herbert and Sage 2013a, 2013b) reviewed the pathophysiological damage to core cellular processes that are associated both with ASD and with the biological effects of EMF exposures that contribute to chronically disrupted homeostasis. Epidemiological studies have also shown a clear association between maternal immune activation (MIA) and schizophrenia or autism in the progeny (Estes and McAllister 2016). Emerging evidence suggests similar links for disorders like cerebral palsy and aging-associated neurodegenerative diseases, positioning MIA as a factor in the brain's responsiveness to cumulative lifetime exposure to environmental insults (Knuesel et al. 2014).
4. ASD from Biochemical & Psychiatric Standpoints to Correlates of Consciousness
From the biochemical standpoint, ASD lies on the other side of the “tipping point” and would represent a departure from brain homeostasis that appears to be generally permanent. From the perspective of ASD, the concept of “tipping point” refers to a critical threshold at which perturbations can shift a system into a new and often irreversible operation state (Simons et al. 2023). Homeostasis involves the maintenance and defense of vital physiological variables such as blood pressure and blood sugar. A first definition was provided by Walter Cannon in 1929, as the principle underlying physiological regulation (Fleming 1984). Later, in the late 1980´s Sterling and Eyer coined the term allostasis to reflect the process whereby adaptive organisms must be able to change the defended levels of one or more regulated parameters as needed to adjust to new or changing environments (McEwen 1998b). Allostasis refers to processes in which homeostasis leads to a new stable region of the system’s state space. Allostatic systems promote adaptation to stressful experiences and are generally most useful when rapidly mobilized and terminated. However, when they are prolonged without resolution, irreversible diseased states can be reached. Allostatic processes can undermine mental and physical health, primarily because of their effects on brain plasticity and immune and metabolic pathophysiology (Simons et al. 2023). The capacity for feeling can be perceived as closely related to the capacity to dynamically control the physiological processes of homeostasis and allostasis (Ramsay and Woods 2014). In 2002, Robertson elucidated an “astrocentric hypothesis” describing the essential role of astrocytes in consciousness and memory formation (Robertson 2002). Pereira further elaborates on the potential connection between the immune system and conscience using a neuro-astroglia interaction model of mental activity (Pereira and Furlan 2010). Pereira also speculates on transitioning from a neurocentric to an astrocentric perspective for cognitive and sensory processing (Pereira and Furlan 2009). Brain physiology and pharmacology research further indicate that wakefulness and sleep depend on astroglia calcium signaling (Bojarskaite et al. 2020; Pereira 2020).
From the psychiatric standpoint, the ASD syndrome can be integrally understood to involve fundamental perturbations of human consciousness. As the quintessential human attribute, consciousness has long fascinated philosophers, scientists, and psychiatrists (Lindhard 2019). The first perceptual awareness of the human organism is evident in the neural integration and prospective, primary sensorimotor intentions of the second-trimester fetus (Delafield-Butt and Ciaunica 2024). Developmental pediatrics identifies different degrees of consciousness that occur during human life, with self-consciousness emerging around the third year of life. Pereira distinguishes between sentience and cognitive consciousness, with a specific emphasis and application for the medical sciences (Pereira 2020). As part of this paradigm, sentience refers to feeling or sensation as distinguished from perception and thought. The physiological correlates of sentience are proposed to be the system underpinning the dynamic control of biochemical homeostasis and allostasis, emotions, and capacity to feel; while the correlates of cognitive consciousness are patterns of (bio)electrical activity in networks, cognition, and capacity to think. Based on the paradigm presented by Pereira, cognitive consciousness depends on sentience, but not vice versa. Pereira´s dynamic of sentience is adaptive, not restricted to the central nervous system (CNS) but composed of whole-body (Pereira and Wang 2016) interoceptive cycles that involve elaborate innervation of vital organs (e.g., the heart) (Azzalini et al. 2019) with their inputs from and outputs to the CNS and active striated motor with kinesthetic sensors. The distinction between the limbic system as an emotional center and the associative cortex prefrontal cortex, as the seat of reason and self-consciousness is common in neuropsychology. A paralimbic network is active, specific, and causal in self-awareness (Lou 2012). Its regions interact by gamma synchrony throughout infancy, childhood, and adolescence into adulthood and are regulated by dopamine and other neurotransmitters via γ-aminobutyric acid (GABA) interneurons. Khetrapal further explores the implication of the interaction of emotion with conscience, provides a framework for disturbed affective consciousness in ASD, and highlights the role of the amygdala (Khetrapal 2008). Baron-Cohen also proposes an “amygdala theory of autism” and presents plausible abnormalities of the amygdala as a cause of ASD based on a review of functional magnetic resonance imaging (fMRI) studies (Baron-Cohen et al. 2000).
Disorders of consciousness have been extensively researched in devastating neuropsychiatric disorders such as schizophrenia (Venkatasubramanian 2015). Like ASD, schizophrenia is a heterogeneous condition with no known or single specific etiology and is characterized by delusions, hallucinations, passivity phenomena, disordered thought processes, disorganized behavior, and progressive cognitive deficit. Tordjman et al. (Tordjman et al. 2019) discuss self-consciousness disorders in schizophrenia and ASD and how impairments of body-self can lead to impairments of self-other differentiation and its consequences on social communication, emotion, cognition, and empathy. Authors highlight how ASD provides a model for exploring the nature of self-consciousness, requiring the ability to reflect on mental states and the awareness of one’s own body and image as the recognition of oneself with awareness of one’s identity. Frith and Happé highlight experimental studies of normal and abnormal development, suggesting that the abilities to attribute mental states to self and others are closely related. (Frith and Happé 1999). Hill and Frith further outline the three main neurocognitive theories of ASD as; (a) theory of mind (ToM) deficit, (b) weak central coherence, and (c) weak executive dysfunction (Frith 1994). ToM refers to the ability to understand the mental states and behavior of oneself and others (Baron-Cohen 2000). Ploog discusses unresolved and critical issues surrounding ToM (Ploog 2023) and highlights the scientific “conundrum” of the “unobservable”, the logical fallacy of circular reasoning and reification, the lack of neuroscientific correlates of ToM, and the role of language as a prerequisite for ToM. Indeed, speech and language have historically been conflated and loss of speech in ASD doesn´t always mean lack of language. For example, in a retrospective study of 31 adolescents and adults with ASD, with limited or no speech, Jaswal et al. (Jaswal et al. 2024) objectively developed and assessed literacy with an iPad game. Researchers report that many autistic individuals who do not talk could learn how to spell or have already learned orthographic English conventions. Jaswal et al. further speculated that if they were given appropriate opportunities, individuals with ASD who lack verbal communication would be able to learn to communicate by typing.
A characteristic of normal information processing is the tendency to draw together diverse information to construct higher-level meaning in context, or “central coherence” (Happé 1994). Individuals with ASD have been reported not to show the normal bias towards processing certain types of information at a global level or, "the tendency to draw together diverse information to construct higher-level meaning in context" (Jarrold and Russell 1997). For example, “the gist of a story is easily recalled, while the actual surface form is quickly lost, and is effortful to retain” (Frith and Happé 1994). Frith and Happe suggest that weak central coherence in autism may be most appropriately viewed as a preference for a particular cognitive style, rather than a form of deficit or impairment. “Executive function” is an umbrella term, used to describe cognitive tasks such as planning, working memory, impulse control, inhibition, and shifting set, as well as for the initiation and monitoring of action (Hill 2004a; Rabinovici et al. 2015; Yngve and Lidström 2024). In an extensive review, Hill (Hill 2004b) acknowledges the complexity of investigating executive function in ASD. This would include the possible influence of IQ on executive performance in these groups, and the possibility of overlap between performance on tests of executive function in other NDDs that are likely to involve congenital deficits in the frontal lobes, such as ADHD and Tourette's syndrome. Additionally, there are considerably contradictory findings of people with ASD and diminished executive function by laboratory measures, operational tasks, and ratings-based measures, especially in preschoolers with ASD (Ko et al. 2024).
5. The Immune System: A System of Relations
In an abstract sense, the current dogma states that the immune system is tasked to monitor and interpret insults and potential threats (i.e., toxins, pathogens, and wounds) from the external world and mount appropriate defensive actions. This would include a sophisticated innate and adaptive defense system to counter environmental insults successfully. At the same time, it also monitors the states of self of internal organs–including the nervous system, facilitates resistance to stress and maintaining homeostasis, and has an essential role in allostasis and healing responses (Qi 2023). Of note, a general evolutionary trend indicates an inverse correlation between the ability to regenerate damaged/lost body parts and the development of an advanced immune system (Abnave and Ghigo 2019). The same abstraction given to the immune system also applies to the nervous system, particularly the CNS, which processes information from the external and internal worlds and commands reactions to external and internal stimuli to maintain homeostasis, allostasis, and survival. Homeostasis in the nervous tissue is controlled by glial cells (astrocytes, microglia) and mast cells; resident and invasive immune cells (e.g., T-cell and B-cell lymphocytes, neutrophils, dendritic cells, macrophages, mast cells); and immune signaling and inflammatory regulators (e.g., chemokines and cytokines). The immune system is essential for normal healthy functioning and the neuroinflammatory response (Abbaoui et al. 2023) and tumor immunosurveillance (Smyth et al. 2001). However, it can also act as a double-edged sword and exhibit complementary and inhibitory functions (Yong and Rivest 2009). For example, the chronic activation of the immune system has been highlighted to trigger self-reinforcing disease processes through failed shut-off of stress-responsive hormone systems (Epel et al. 1998).
The fundamental interplay between the nervous and the immune systems cannot be understated. Physically, every immune organ is innervated, and bi-directional communication between neural and immune system cells is established in numerous physiological systems (Maier and Watkins 1999). From a philosophical standpoint, the characteristics of self/oneself recognition, specificity, and memory are shared only by the immune and nervous systems (Ichim et al. 2007). Although the mechanisms by which peripheral immunity may influence neuronal function are largely unknown, recent findings suggest that the immune system influences behavior, such as spatial learning and memory (Ziv and Schwartz 2008). Filiano et al. (Filiano et al. 2016) implicate adaptive immune dysfunction in social dysfunction and suggest a co-evolutionary link between social behavior and an anti-pathogen immune response driven by interferon signaling. Immune dysfunction and neuroinflammatory components are also associated with several neurodegenerative diseases, including Alzheimer’s disease (Heppner et al. 2015; Lahiri et al. 2021). Psychiatric diseases associated with mitochondrial disorders include bipolar disorder, anxiety disorders, schizophrenia, and ASD (Manji et al. 2012). Hanaford and Johnson (Hanaford and Johnson 2022) review preclinical and clinical evidence for the immune system's role in the pathogenesis of mitochondrial dysfunction and disease.
As reviewed by Hiller-Sturmhöfel and Bartke (Hiller-Sturmhöfel and Bartke 1998) the CNS and the glandular endocrine system are intimately connected, forming the neuroendocrine system. Hormones can be produced by endocrine glands in the hypothalamus, pituitary gland, adrenal glands, and gonads (Kleine and Rossmanith 2016). In this system, the “neural part” can recognize its environment and can memorize it with influence on the endocrine system, acting as the “biochemical executor” (Csaba 2014). In tandem with, immune cells also synthesize, store, and secrete identical hormones to endocrine glands. These include the proopiomelanocortin hormones (e.g. endorphin), the thyroid system hormones, growth hormones, prolactin, melatonin, histamine, serotonin, and catecholamines (Csaba 2014). In the immune-endocrine axis, immune cells can recognize and store the information (memory) and execute the commands provoked by the recognition. Immune cells are also mobile, meaning they can appear in any place of the organism, under local factors that attract them, e.g. in the case of inflammation.
6. “The ASD Bone Is Connected to the Immune Bone, the Digestive Bone & the Brain Bone”
The role of microorganisms in disease, also known as “germ theory”, was established in the 19th century (Lederberg 2000) and the central role of microorganisms in childhood morbidity and mortality during the Industrial Revolution is well documented (Hill 1990). Infectious diseases in this period were principally mediated through poor sanitation, inadequate waste disposal systems and water supply, poverty, and deprivation (Konteh 2009). In juxtaposition, more recent discoveries over the last three decades have revealed that the human body harbors a diverse ecosystem of symbiotic microorganisms, including bacteria, viruses, and fungi, collectively known as the microbiota or microbiome, that are vital for normal health and well-being and prevention of disease (Dethlefsen et al. 2007). Complex, dynamic, and diversely composed microbiota reside in various niches of the human body which would include the oral cavity, nasal passages, lungs, skin, hair, bladder, and vagina (Costello et al. 2009). The ability of microorganisms to selectively colonize the human niche reflects their evolutionary adaptation and results in reciprocal interacting processes that form a single unified process (Baquero et al. 2021) and superorganism (or “holobiont”) (Margulis and Fester 1991; Stilling et al. 2014; Bordenstein and Theis 2015). The most studied microbiome of the human body resides in the gastrointestinal (GI) tract. GI mucosal surfaces are intimately associated with the most abundant and diverse microbial communities in the human body (Lynch and Pedersen 2016). The current consensus implicates four prominent bacteria phyla in the gut. I.e., Actinobacteria, Firmicutes, Proteobacteria, and Bacteroides (Costello et al. 2009). Stress can sway the balance of the microbiome away from homeostasis. Of note, while many triggers to disease have been identified, far less is known about the triggers of a natural return to homeostasis (Reid et al. 2011). The immune system learns to tolerate the dynamic evolution of commensal microbiota and respond appropriately to pathogens and antigens; in turn, the host microbiota is integral to educating the immune system to function correctly (Marques et al. 2016). From this viewpoint, the immune system represents the most conspicuous set of anti-exploitation adaptations involved in human–microbial symbiosis (Dethlefsen et al. 2007). Which mechanisms underlie the acquisition of microbial communities is still debated and controversial. It remains unclear whether pioneer colonization starts during fetal life (i.e., “in utero colonization” hypothesis) or whether it occurs during birth and the early postnatal period (i.e. “sterile womb paradigm”) (Perez-Muñoz et al. 2017; Stinson et al. 2017, 2019).
The gut-brain axis (GBA) (Mayer 2011; Cryan et al. 2019; Lai et al. 2022) provides an essential link to two important systems of the nervous system, namely the CNS and the enteric nervous system (ENS) and the gut microbiome and metabolome (Ramautar et al. 2013). The ENS represents an interdependent and extensive network of nerve cells within the gut. Its local complement component acts as a vigilant sentinel to a dynamic and evolving environment in the GI tract (Wu et al. 2024). Even though it is now considered a third branch of the autonomic nervous system (ANS), the ENS has been referred to as the “second brain” (Gershon 1999), based on its size, complexity, and similarity in neurotransmitters and signaling molecules with the brain (Mayer 2011). Since ENS neurons do not extend into the intestinal lumen, their ability to sense the microbiota is indirect, either by microbial molecules that have penetrated the epithelial barrier or by sensing through epithelial cells themselves (Macpherson et al. 2023). The ENS is viewed as a peripheral extension of the limbic system into the gut, where it is exposed closely to our complex internal environment, including powerful mechanical, (bio)chemical, and microbial influences (Wood et al. 1999). The GBA essentially represents the bidirectional communication between the CNS and the ENS, linking the emotional and cognitive centers of the brain with peripheral intestinal functions. The GBA has a critical role in the integration of external and internal changes in the environment and the maintenance of bodily homeostasis. Indeed, there is growing recognition that the GBA is a critical regulator of neurological functions and an intermediary to neurotoxicity by environmental stressors such as drugs, environmental contaminants, and dietary factors (Dempsey et al. 2019). Interactions represent a system with bidirectional communication channels and multiple feedback loops. GBA crosstalk occurs via multiple channels, with rapid neuronal signaling primarily by the vagus nerve, as well as the gut connectome (via the semi-autonomous ENS), and more delayed feedback being achieved through neuroendocrine and neuroimmune signals into the circulation (Mayer et al. 2022; Strandwitz 2018) in the form of gut-derived neurotransmitters (dopamine (DA), serotonin (5-HT), GABA and histamine), hormones and inflammatory mediators (i.e. cytokines). 90% of vagal fibers between the gut and brain are afferent, suggesting that the brain is more of a receiver than a transmitter concerning brain-gut communication (Berthoud and Neuhuber 2000).
Nonetheless, preclinical studies indicate the destabilization of microbial diversity and microbiome dysbiosis after acute brain trauma and chronic physiological stress (Karl et al. 2018; Kelly et al. 2021). The GBA is not only of importance for well-being but also a bridge to our understanding of homeostasis, adaptation, disease, and the body-mind connection (Strandwitz 2018). Mounting evidence implicates the GBA in the pathogenesis of multiple chronic diseases, including inflammatory bowel disease, coeliac disease, allergies, asthma, metabolic syndrome, cardiovascular disease, and obesity (Benakis et al. 2020), and pathology of numerous neurological, and psychiatric disorders. GI dysbiosis is also implicated in the etiology of ASD (Puricelli et al. 2022; Wakefield 2002), and children with ASD often experience chronic GI symptoms (e.g., diarrhea, constipation, bloating, and gastroesophageal reflux) (Wakefield et al. 2002). Moreover, a strong positive correlation has been observed between the severity of GI symptoms and the severity of ASD symptoms (Tomova et al. 2015). Indeed, in 2010, an expert panel of the American Academy of Pediatrics strongly recommended further investigation into the role of GI abnormalities in the pathophysiology of ASD (Buie et al. 2010).
Two important boundaries in the CNS and GBA are the blood-brain barrier (BBB) and the gut-blood barrier (GBB) (Macpherson et al. 2023). The integrity of both GBB and BBB has been reported to be impaired in ASD individuals (Theoharides and Doyle 2008). The BBB constitutes the largest exchange between the blood and the brain, separating the brain interstitial fluid from blood plasma (Abbott et al. 2010). In disease states, BBB breakdown and dysfunction lead to leakages of harmful blood components into the CNS, cellular infiltration, and aberrant transport and clearance of molecules. Like any other organ, the brain is vascularized from the surrounding vascular plexus during embryogenesis, and the BBB is tempered by the body’s immune system from embryonic development (Sweeney et al. 2019). Sweeney et al. (Sweeney et al. 2019) extensively review the involvement of BBB breakdown and dysfunction in neurological deficits and other pathologies for Alzheimer's disease, Parkinson’s disease, amyotrophic lateral sclerosis, multiple sclerosis, other neurodegenerative disorders; and acute CNS disorders such as stroke, traumatic brain injury, spinal cord injury, and epilepsy. The GBB, i.e., the intestinal epithelium, is a highly dynamic structure, is estimated to completely self-renew every 4–7 days, and plays an important role in homeostasis (Odenwald and Turner 2017). Weakened intestinal epithelial barrier function has recently been suggested as a possible source of chronic inflammation in schizophrenia (Odenwald and Turner 2017) and ASD (Heberling et al. 2013) and the development of autoimmune diseases (An et al. 2022). However, there are still major gaps in our understanding of how specific microbial compounds influence human conditions. The associations between circulating metabolite levels and brain function are difficult to interpret without knowledge of at least BBB permeability. In addition, many of the currently proposed mediators of microbiota-gut-brain signaling have no known receptor in the CNS.
7. The Pathophysiology of ASD
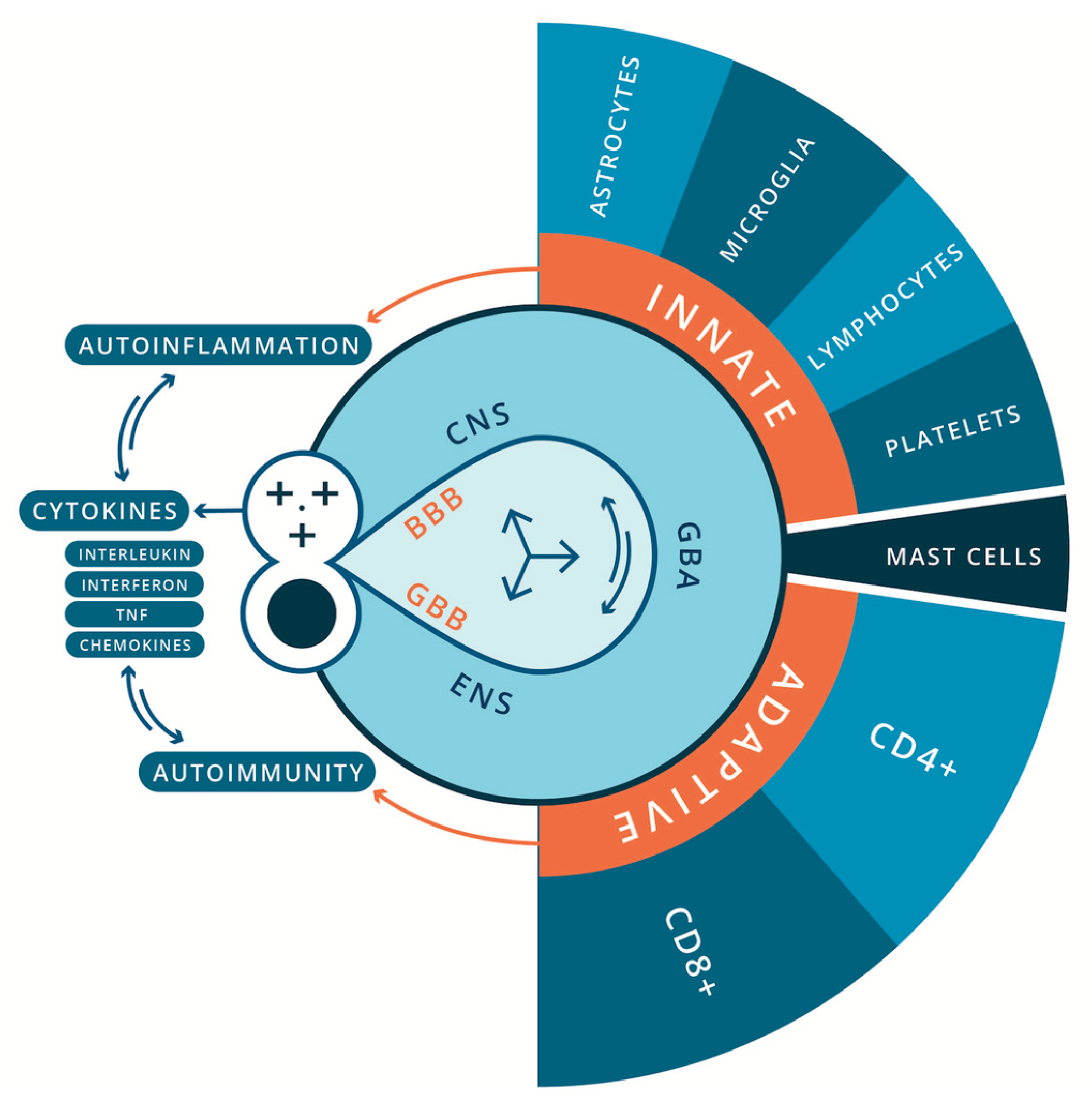
As discussed, recent progress in neuroimmunology has established that the nervous and the immune systems are two functionally related physiological systems with complementing cognitive and effector repertoires (Daëron 2022). From a pathophysiological perspective, maladaptation of inflammation processes is a common feature of many acute and chronic neurodegenerative (Amor et al. 2010; Heneka et al. 2015) and NDDs (Jiang et al. 2018). Immune abnormalities were first described in individuals with ASD in 1977 by Stubbs
et al. (Stubbs et al. 1977). Since then, a growing body of research spanning nearly half a century has implicated oxidative stress, mitochondrial dysfunction and inflammatory processes, and chronic and systemic immune dysregulation in the pathophysiology of ASD. This can be broadly characterized as neuroinflammation (encephalitis) and autoimmune encephalitis, involving the innate and adaptive immune systems respectively (Hamidreza and Morteza 2018; Mead and Ashwood 2015; Onore et al. 2012; Samsam et al. 2014) (
Figure 1). Alongside this, a growing body of evidence indicates the influence of the gut microbiota and microbial signaling molecules and metabolites (e.g., bile acids, short-chain fatty acids, and tryptophan metabolites) on neurodevelopment and behavior. Many studies have shown that early colonization, mode of delivery, and antibiotic usage significantly affect the development of gut microbiome and the onset of ASD (Taniya et al. 2022). Many authors speculate that gut-microbiota dysbiosis may be central to the etiology and pathological mechanisms of ASD (Buie et al. 2010; Strati et al. 2017; Ho and Ross 2017; Hughes et al. 2018; Pulikkan et al. 2019; Hazan et al. 2020; Taniya et al. 2022; Peralta-Marzal et al. 2024). However, as discussed by White (White 2003) and Beopoulos (Beopoulos et al. 2021), an alternative hypothesis also explored here is that it may be a secondary consequence of immune pathology in the GI tract and ENS (Brown and Mehl-Madrona 2011; Niesler et al. 2021; Obata and Pachnis 2016).
In the context of disorders of the CNS, immune cell abnormalities have been reported in brain resident innate and adaptive immune cells for children and adults diagnosed with ASD. This includes glial cells (i.e. microglia, astrocytes, and oligodendrocytes), monocytes, natural killer (NK) cells, thrombocytes, mast cells, and dendritic cells (Ashwood and Van de Water 2004; Pardo et al. 2005; Pardo and Eberhart 2007; Anderson et al. 2008); and T cells (e.g., CD4+ T-helper cells (Th) (major lineages including Th1, Th2, Th17, Treg) (Hirahara and Nakayama 2016) and CD8+ lymphocytes (Tc) (major lineages include Tc1, Tc2, Tc9, Tc17, Tc22) (Koh et al. 2023)) (Terrabuio et al. 2023), and regulatory B cell lymphocytes (Nadeem et al. 2022). Of note, immune memory was long thought to be restricted to the adaptive immune system; however, increasing evidence suggests that concepts of a trained immune system also apply to the innate immune system. Netea et al. (Netea et al. 2016) review the mounting evidence that cells of the innate immune system, which lack the antigen specificity, clonality, and longevity of T cell and B cells, do have some capacity to remember. This property would allow monocytes, macrophages, and NK cells to maintain homeostasis/allostasis by enhanced responsiveness when they reencounter xenobiotic insult. Many researchers have also investigated myriad chemical messengers and signaling cascades that regulate pro- and anti-inflammatory processes for their role in the etiology of ASD and as biomarkers of CNS pathology. They include, for example, cytokines such as interleukin (Il), interferon, tumor necrosis factor (TNF), and chemokines (Zhang and An 2007), neurotrophic factors (e.g., nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF)) (Liu et al. 2021) and insulin-like growth factor-1 (IGF-1) (Heemskerk et al. 1999)). In parallel, current research also highlights the role of neurotransmitters (e.g., serotonin, dopamine, norepinephrine, acetylcholine, oxytocin, endogenous opioid, cortisol, histamine, glutamate, and GABA) (Lam et al. 2006; Marotta et al. 2020) in the pathophysiology of ASD. All these molecules are not restricted to modulating only static neurons; they also modulate mobile immune cells. Their role from a neuroimmunological perspective would be important and evidence is provided for their involvement in the pathophysiology of ASD. That said, it is noteworthy that no universal or robust biological signature for ASD has been found. Finally, sex bias and the role of sex hormones in the pathogenesis of ASD (male bias) and autoimmune disorders (female bias) have been well-documented and are being further explored as a possible nexus between these disorders.
7.1. ASD, Inflammation & Oxidative Stress
Oxidative stress can be defined as an imbalance between pro-oxidants and antioxidants, resulting in a damaging action toward the cell caused by reactive oxygen species (ROS) and reactive free radicals (Singh et al. 2019). All these molecules are produced during the activity of peroxisomes, endoplasmic reticulum, proteasome, and mitochondria (Chirumbolo and Bjørklund 2017). Chirumbolo and Bjørklund (Chirumbolo and Bjørklund 2017) introduce the “PERM (proteasome, endoplasmic reticulum, and mitochondria) hypothesis”, to describe the complex dynamical system as a single master tuner of cellular decision-making. The authors highlighted that the ability of this system to adapt to stressors, insults, and stimuli may lie in its chaotic behavior, mainly formed by synchronized oscillatory mechanisms, involving calcium signing, ROS, and mitochondria polarization. Substantial percentages of ASD patients display peripheral markers of mitochondrial energy metabolism dysfunction, such as elevated lactate, pyruvate, and alanine levels in blood, urine, and cerebrospinal fluid (Frye et al. 2024); serum carnitine deficiency (Filipek et al. 2004; Frye et al. 2024); and enhanced oxidative stress (Palmieri and Persico 2010; Frye et al. 2024). Mitochondria can rapidly switch from primarily catabolic organelles generating ATP to anabolic organelles that generate both ATP and building blocks for macromolecule synthesis (Weinberg et al. 2015).
Recent evidence indicates that mitochondria lie at the heart of immunity and play a key role in innate and adaptive immune response (Weinberg et al. 2015; Mills et al. 2017; Angajala et al. 2018). I.e., mitochondrial signaling dictates macrophage polarization and function and is necessary for responses to activators of innate immune signaling. Mitochondrial ROS regulates Th (T helper) cell activation, differential metabolic pathways regulate CD4+ cell differentiation, and mitochondrial metabolism regulates CD8+ memory formation (Weinberg et al. 2015). Current knowledge highlights a disturbed mitochondrial function in immune cells for various immunological diseases (Faas and De Vos 2020). Several studies have suggested that redox imbalance and oxidative stress are integral parts of ASD pathophysiology (Rossignol and Frye 2012; Rossignol and Frye 2014; Anderson et al. 2008; Bjørklund et al. 2020; Usui et al. 2023) and inflammatory responses (Sies 2015). Recent evidence from post-mortem studies of autistic brains points toward abnormalities in mitochondrial function as possible downstream consequences of unreactive immunity and altered calcium signaling (Palmieri and Persico 2010). Children with ASD are considered more vulnerable to oxidative stress because of their imbalance in intracellular and extracellular glutathione levels and decreased glutathione reserve capacity (Bjørklund et al. 2020). Ghanizadeh et al., (Ghanizadeh et al. 2012) discuss the role of glutathione in the context of ASD, including its involvement in neuroprotection and against oxidative stress and neuroinflammation.
7.2. Innate Immune Deregulation, Encephalitis & ASD
Current research indicates that innate neuroimmune reactions play a major pathogenic role in an undefined proportion of autistic patients (Vargas et al. 2005). Glial cells (including astrocytes, microglia (Reemst et al. 2016), and oligodendrocytes (Shaw et al. 2021)), natural killer (NK) cells (Enstrom et al. 2009), mast cells (Chia et al. 2023), dendritic cells (Copf 2016) and platelets (Davenport and Sola-Visner 2022) play pivotal yet distinct roles at various developmental stages of the fetal and neonatal nervous system. Accordingly, the immune system would have explicit and important roles in the pathogenesis of any neurodevelopmental disease, especially those whose etiology is rooted in environmental xenobiotic insult. Moreover, in the developing brain, it is important to note that the energy requirement during the rapid postnatal growth period is due to a swift developmental progression, predominantly driven by the intricate maturation and refinement of existing neurons, as neurogenesis primarily occurs before birth. Cantando et al., (Cantando et al. 2024) elaborate on the interplay between astrocytes, microglia, and metabolic dysregulation in the context of ASDs, revealing a complex landscape for their potential contribution to the pathogenesis of these neurodevelopmental conditions.
7.2.1. The Pathophysiology of Glial Cells in ASD
Microglia are the major brain-resident macrophages that act as the first line of defense against injury and infection in the CNS (Matcovitch-Natan et al. 2016). In addition, microglia are reported to be the nervous system´s “electricians” (Graeber and Stre’rt 1990) and have an important function in forming a network of immune components within the CNS and ENS (Graeber and Streit 2010; Salter and Stevens 2017; Heiss and Olofsson 2019). New controversies have also emerged such as the question of whether microglia are active or reactive players in neurodegenerative disease conditions, or whether they may be “victims” themselves (Graeber and Streit 2010). The current consensus indicates that microglia play a prominent role in neurodevelopmental processes like synaptic pruning and neuronal network maturation (Mordelt and de Witte 2023) and are involved in neurodegenerative diseases including Parkinson's and Alzheimer’s disease (Graeber and Streit 2010). Microglia induce the formation of both inhibitory (I) and excitatory (E) synapses in the hippocampus as regulators of physiological homeostasis (Rosin and Kurrasch 2019). Moreover, using in-vivo imaging in rodent models, Haruwaka et al., (Haruwaka et al. 2019) demonstrate that microglia play a dual role in maintaining BBB integrity. Deng et al. (Deng et al. 2024) further elaborate on how gut metabolites can directly or indirectly regulate the functional states of microglia directly or via the GBA and their role in the pathogenesis of neurodegenerative diseases and NDDs. Microglial dysfunction in ASD is reported to be attenuated or excess synaptic pruning, impairment of the brain's excitation vs. inhibition (E vs. I or E/I) balance (Koyama and Ikegaya 2015; Petrelli et al. 2016; H. Kim et al. 2017; Xiong et al. 2023; Fan et al. 2023; Lebow et al. 2024; Luo and Wang 2024). Microglial abnormalities have been identified in ASD including in density, function, and morphology (Fan et al. 2023; Koyama and Ikegaya 2015), autophagy (Kim et al. 2017), and cascades of interaction with other brain resident glial cells (i.e., astrocytes and oligodendrocytes) (Petrelli et al. 2016; Xiong et al. 2023; Luo and Wang 2024) and associated with the phenotypic heterogeneity of ASD.
Astrocytes compose at least one-half of human brain tissue volume and up to a few decades ago were assumed to be primarily giving structural, metabolic, and functional support for neurons (Pereira and Furlan 2009). More recent discoveries implicate astrocytes to multiple functionalities beyond primary supportive roles, including homeostatic (molecular, cellular & network, systemic, organ, metabolic) and defensive processes of the CNS (Volterra and Meldolesi 2005; Verkhratsky and Nedergaard 2018). In the brain, astrocytes are involved in the control of synapse formation, neurogenesis, and brain vascular tone (Hussaini and Jang 2018). They may have not only harmful effects of aggravating neuro-inflammation and hindering synaptic sprouting or axon growth, but also beneficial effects of anti-inflammation, and neuroprotection (Dong and Benveniste 2001). Astrocytes are also deemed essential to the maturation, maintenance, and regulation of the BBB in the healthy brain (Dong and Benveniste 2001). However, as discussed by Pociūtė et al. (Pociūtė et al. 2024), little is known about the effects of astrocyte-secreted factors on the integrity of the BBB under physiological conditions. Alterations in the neuron–astrocyte partnership have begun to emerge and have been shown to underlie brain lesions in pathologies as varied as brain tumors, Alzheimer's disease, and amyotrophic lateral sclerosis (Pathak and Sriram 2023). Astrocytic excitation is chemically encoded and is revealed not through electrophysiology, as for neurons, but by assays of intracellular calcium concentration transients and oscillations (Sloan and Barres 2014). Astrocytes are seen as local communication elements within the CNS that can generate various signals, for example through the regulated release of ‘gliotransmitters’ including glutamate (Volterra and Meldolesi 2005). Alterations in astrocytic processing (neurogenesis, synaptogenesis, inflammation, myelination, glutamate) and number have been deemed a significant contribution to ASD pathophysiology (Gzielo and Nikiforuk 2021; Allen et al. 2022; Vakilzadeh and Martinez-Cerdeño 2023; Cantando et al. 2024).
Oligodendrocytes myelinate the brain and spinal cord to insulate axons electrically and provide neurons with trophic and metabolic factors (Baumann and Pham-Dinh 2001). Emerging evidence supports the notion that oligodendrogenesis and neural myelination may play a pivotal role in the pathophysiology of ASD and its clinical presentation (Galvez-Contreras et al. 2020). Steinman and Mankuta (Steinman and Mankuta 2013) provide evidence of the putative role of IGF-1 in the genesis of ASD. IGF-1 directly affects the rate at which oligodendrocytes promote myelination in the CNS (Steinman 2015). IGF-1 signaling pathways are crucial for adequate axonal myelination and oligodendrocyte differentiation, but, when disrupted, can lead to white matter alterations, learning challenges, ASD-like behaviors, and neurodevelopmental and neuropsychiatric disorders (Riikonen 2017; Réthelyi et al. 2023).
7.2.2. The Pathophysiology of NK Cells in ASD
NK cells were originally defined as effector lymphocytes of the innate immune system (Vivier et al. 2011) that control several types of tumors and microbial infections by limiting their spread and subsequent tissue damage (Herberman and Ortaldo 1981). Recent research highlights that NK cells are also regulatory cells engaged in reciprocal interactions with dendritic cells, macrophages, T cells, and endothelial cells (Vivier et al. 2008). In addition, there is increasing evidence that NK cells link innate and adaptive immunity and play an important role in the pathogenesis of autoimmune conditions (Gianchecchi et al. 2018; Liu et al. 2021). NK cells have been implicated in the pathology of neurological and behavioral disorders, including Tourette syndrome, schizophrenia, multiple sclerosis, neuromyelitis optica spectrum disorders, autoimmune encephalitis, Guillain-Barré Syndrome (GBS), chronic inflammatory demyelinating polyneuropathy, myasthenia gravis, and idiopathic inflammatory myopathy (Enstrom et al. 2009). NK cells are potent effectors of immune homeostasis, with receptors allowing them to sense the “non–self” or “missing-self” status of target cells. Over 30 years ago, Warren, Foster, and Margerten (Warren et al. 1987) reported altered NK activity in adults and children with ASD. Recently, Ebrahimi, Rostam-Abadia, and Rezaei (Ebrahimi et al. 2021) reviewed and highlighted a growing body of research detailing NK cell dysfunction in children with ASD as well as their parents. The authors discuss changes in the frequency, gene expressions, cytotoxicity features, and receptors of NK cells in children and adults with ASD. Highlighted studies include those of López-Cacho et al. (López-Cacho et al. 2016). Their investigation indicated an increase in the percentages of CD8+ Tc cells and B-cells, a decrease in NK cells, and a trend toward increased apoptosis in monocytes in patients with ASD. Vojdani et al., (Vojdani et al. 2008) further explored NK cell activity in 1027 blood samples from autistic children obtained from ten clinics in the USA and compared the results to 113 healthy controls. 45% of a subgroup of children with ASD suffered from low NK cell activity. The authors discuss the role of low intracellular levels of glutathione (Vojdani et al. 2008) as also underscored by Ghanizadeh et al., (Ghanizadeh et al. 2012).
7.2.3. The Pathophysiology of Mast Cells & Dendritic Cells in ASD
Mast cells are critical for allergic reactions but are also important in immune response and inflammation cascades in the nervous system (Kovacheva et al. 2024). Together with dendritic cells, they are the first line of defense in the immune system against invading pathogens (Nelissen et al. 2013). Mast cells are present in the brain and its meninges, including the area postrema, choroid plexus, and thalamic hypothalamic region (Hendriksen et al. 2017). Mast cell–neuron interactions can involve the ENS and regulation of the GBB permeability and GI pathophysiology (Theoharides and Doyle 2008). For example, mast cell activation by allergic, infectious, environmental, and stress-related triggers, especially perinatally, can release pro-inflammatory and neurotoxic molecules and disrupt the GBB and BBB, contributing to ASD pathogenesis and phenotype (Angelidou et al. 2011; Ribatti 2015). The role of mast cells in the pathophysiology of ASD has been extensively explored and discussed by Theoharides and coworkers (Theoharides et al. 2016; Theoharides et al. 2012, 2016, 2019). Researchers speculate that stress and environmental stimuli trigger cascades involving mast cells and microglia leading to abnormal synaptic pruning, and dysfunctional neuronal connectivity (Theoharides et al. 2016) leading to brain pathology. For example, processes at a cellular level in these systems could alter the “fear threshold” in the amygdala, and lead to an exaggerated “fight-or-flight” response.
As a further example, corticotropin-releasing factor is secreted from the hypothalamus under stress and, together with neurotensin, can stimulate brain mast cells to release inflammatory and neurotoxic mediators that disrupt BBB, stimulate microglia and cause focal inflammation (Theoharides et al. 2016, 2019). Also noteworthy, in allergy/immunology practice it is not unusual to find food allergies in an ASD child (Xu et al. 2018). Angelidou et al. (Angelidou et al. 2011) further speculate that subjects with hypersensitive mast cells may represent a unique subgroup of patients who are more likely to respond to environmental and stress triggers, leading to precipitating or worsening ASD. Jyonouchi highlights studies that indicate a high prevalence of non-IgE-mediated food allergies in young children with ASD and further speculates that food allergies may account for some but not all GI symptoms observed in children with ASD (Jyonouchi 2009). These findings also suggest that non-allergic autoimmune mechanisms involving mast cell activation, probably in response to environmental and stress triggers, could contribute to inflammation and pathogenesis of autism.
Alongside mast cells, dendritic cells have important functions in the modulation of the immune system and the phagocytosis of pathogens or debris, antigen presentation, activation of naïve T cells, induction of tolerance, and cytokine/chemokine production (D’Agostino et al. 2012). Dendritic integration plays a fundamental role in sensory processing, cognition, and conscious perception (Hameroff 2010) processes and is hypothesized to be impaired in individuals with ASD and NDDs. Impairments include problems with dendrite morphogenesis (Copf 2016), integration (Nelson and Bender 2021), and frequency (Martínez-Cerdeño 2017). Breece et al., (Breece et al. 2013) conducted a study of the frequencies of dendritic cells and their association with behavioral assessment and MRI measurements of amygdala volume. 57 patients with ASD were enrolled and compared to 29 typically developing controls. Researchers report a significant increase in the frequency of myeloid dendritic cells, which was also correlated with abnormal right and left amygdala enlargement, severity of GI symptoms, and increased repetitive behaviors. Alterations in dendritic cell frequency were corroborated in a study of 32 children with ASD vs. 30 healthy children by Saad et al., (Saad et al. 2017). Researchers enumerated data from flow cytometry of peripheral blood samples and indicated higher percentages of myeloid dendritic cells and plasmacytoid dendritic cells in the ASD group. Basheer et al. (Basheer et al. 2018) also reported elevated myeloid dendritic cells in a comprehensive evaluation of various peripheral immune cell subsets and associated serum cytokine levels in 30 children with ASD, compared to 30 typically developing children.
7.2.4. The Pathophysiology of Platelets in ASD
The classical role attributed to platelets is the maintenance of hemostasis; however, more recent evidence has highlighted a central role for platelets in the host inflammatory and immune responses (Jenne et al. 2013), by virtue of their high prevalence and ability to rapidly release a broad spectrum of immunomodulatory cytokines, chemokines, and other mediators, as circulating sentinels (Jenne et al. 2013). Moreover, platelets share common biological and molecular characteristics with neurons (calcium-dependent activation and secretion mechanism, cell surface receptor, and secretory vesicles with neurotransmitters such as serotonin, dopamine, glutamine, and GABA transporters) (Burnouf and Walker 2022). They also contribute to brain homeostasis (Leiter and Walker 2019), mediate protective neuroinflammation, and promote neuronal plasticity at the site of neuronal injury (Dukhinova et al. 2018). In general, dysfunction, abnormal activation, and morphological alteration of platelets have been implicated in many complex neurological disorders such as schizophrenia, migraine, Parkinson's disease, Alzheimer's disease, and ASD (Goubau et al. 2014). Clinical research by Farmer et al. (Farmer et al. 2021) suggests that elevation in BDNF may be partially explained by higher platelet counts in children with ASD. Although many studies reveal associations between platelet biomarkers and ASD, there is an important knowledge gap in linking these markers with ASD and explaining the altered platelet phenotypes detected in ASD patients (Padmakumar et al. 2019).
7.3. Adaptive Immune Dysfunction, Autoimmune Conditions, & the Pathophysiology of ASD
Alongside a decline in the incidence of most infectious diseases since the 1900s, there has been a steady rise in the incidence in the industrialized countries of chronic inflammatory disorders and autoimmune diseases (Bach 2018). Autoimmune diseases are caused by an inappropriate immune response against “self” antigens, resulting in local tissue-specific and systemic chronic cascades of inflammation and tissue damage. Autoimmune conditions are hypothesized to occur through exacerbation of the immunoregulatory deficiency. This deficiency is speculated to be due to the loss of microorganisms (dubbed the “hygiene hypothesis” or “Old Friends hypothesis”) from modern urban environments, with which humans have coevolved and tolerated and relied on for the development of immunoregulatory circuits (Rook 2012). Like other chronic diseases, autoimmune diseases exhibit shared alterations in the gut microbiota. Wang et al., (Wang et al. 2024) report microbial alterations among autoimmune diseases that were substantially more consistent compared with those of other diseases (cancer, metabolic disease, and neurological disease), with microbial signatures exhibiting notable discriminative power for disease prediction. Akdis (Akdis 2021) further discussed an epithelial (e.g., skin and gut) barrier hypothesis, which proposes that the increase in epithelial barrier-damaging agents linked to industrialization, urbanization, and modern life underlies the rise in allergic, autoimmune, and other chronic conditions, including ASD. One alternative hypothesis that ties infection with autoimmune disease is molecular mimicry (Albert and Inman 1999). Mechanisms of molecular mimicry essentially involve infectious agents containing an antigen cross-reactive to a host antigen in the body, a less specific activation of the innate immune response can also promote autoimmune disease (Albert and Inman 1999). No convincing or rigorous proof exists for the theory of molecular mimicry by pathogenic agents (Albert and Inman 2000; Albert and Inman 1999). However, as reviewed by Segal and Shoenfeld (Segal and Shoenfeld 2018), a growing body of research implicates mechanisms involving xenobiotics, including prophylactics in the development of autoimmune conditions. As for ASD, autoimmune diseases are heterogeneous in prevalence, manifestations, and pathogenesis. The CDC estimates as many as 50 million people in the USA have an autoimmune disease, making it the third most prevalent disease category, surpassed only by cancer and heart disease (NIEHS 2024). There are more than 80 autoimmune diseases including about 30 autoimmune disorders of the nervous system (Bhagavati 2021; Theofilopoulos et al. 2017).
Oxidative stress plays an important role in the pathogenesis of autoimmune diseases, and many environmental agents can participate in and amplify the cascade mechanisms involved (Khan and Wang 2018). Autoimmune diseases are typically defined by autoantibodies, produced by autoreactive B-cells and autoantigen-reactive Th cells against their host (Lleo et al. 2010). Natural autoantibodies provide immediate protection against infection and prevent inflammation by facilitating the clearance of oxidized lipids, oxidized proteins, and apoptotic cells (Lleo 2014). The role of autoantibodies in the development of autoimmunity is still unclear (Lleo et al. 2010) and the initial trigger for autoantibody production in patients with CNS autoimmunity is still widely unknown. However, autoantibody detection to neuronal or glial targets has resulted in a better understanding of CNS autoimmunity, and reclassification of some diseases previously thought to result from infectious, ‘idiopathic’ or psychogenic causes (Prüss 2021). Pathogens and commensals stimulate pattern recognition receptors, including toll-like receptors (TLRs), to protect against autoimmunity (Bach 2018). Pathogenic mechanisms of autoantibodies in autoimmune diseases include interaction with cell surface receptors, cell surface binding and lysis, immune complex-mediated damage, binding to extracellular molecules, and autoantibody transfer across the placenta (Lleo et al. 2010). One theory speculates that faults of the CD4+ T cell line (Zhu and Paul 2008) and reduced microbial stimulation of the TLRs in early life could lead to a weaker Th1 response and a stronger Th2 response to allergens. Th1 cells mainly develop following infections by intracellular bacteria and some viruses, whereas Th2 cells predominate in response to infestations by GI nematodes (Romagnani 1999). Th17 cells have also developed a reputation as a destructive element in several chronic diseases (Marwaha et al. 2012) and auto-inflammatory neurological disorders such as multiple sclerosis, Alzheimer's disease, Parkinson's disease, and schizophrenia (Tahmasebinia and Pourgholaminejad 2017). Regulatory T (Treg) cells are a population of T cells that can functionally suppress an immune response and are fundamental in maintaining T cell tolerance to self-antigens and immune homeostasis in healthy individuals (Keating et al. 2019). Mitochondrial-regulated Tregs are also hypothesized to be involved in the occurrence and progression of autoimmune diseases of the CNS (Han et al. 2023).
Antibodies against self-antigens are also found in cancer and during massive tissue damage (Bei et al. 2009). Cancer involves uncontrolled cell proliferation, whereas NDDs are connected to anomalies in the development of the nervous system. Wen and Herbert (Wen and Herbert 2017) discuss the overlap between ASD and cancer and speculate possible common mechanisms regarding signaling pathways related to metabolic alterations. Wen et al., (Wen et al. 2016) also conducted pathway network analyses for ASD, revealing multisystem involvement, major overlaps with other diseases, and convergence upon MAPK and calcium signaling pathways. In 2023 Yavyz et al., (Yavuz et al. 2023) published a large-scale study of de novo mutations in approximately 8000 samples with NDDs and approx. 10,000 tumor samples from The Cancer Genome Atlas. Mutations in NDDs tend to have a weaker functional impact and are more likely to influence differentiation compared to those in cancer. Although myriad publications implicate MIA in schizophrenia and ASD (Knuesel et al. 2014; Estes and McAllister 2016), an alternative and novel hypothesis for the etiology of ASD implicates autoimmune pathology involving a maladaptation of the immune system in the CNS (Edmiston et al. 2017). Money, Bobrow, and Clarke (Money et al. 1971) provide the first reports that autoimmunity of the CNS may be etiologically important in ASD. In a case report in 1971 authors describe a child with multiple diagnoses with a strong family history of autoimmune disorders. 35 years later, Edmiston, Ashwood, and Van de Water provided the first review of investigations, implicating autoimmunity and autoantibodies in individuals with ASD (Edmiston et al. 2017). Moreover, epidemiological studies document a significant association between ASD and autoimmune disorders such as celiac disease, type 1 diabetes, asthma, multiple sclerosis (Fasano 2012), epilepsy, and atopic disease (Donald and Finlay 2023; Theoharides et al. 2016).
In 2017, Ahmad et al., (Ahmad, Nadeem, et al. 2017) reported that children with ASD have imbalances between the anti- and pro-inflammatory milieu in blood leukocytes. Based on analysis of peripheral blood mononuclear cells of children comparing ASD with a typically developing control group, researchers reported increased pro-inflammatory cytokine production and decreased anti-inflammatory molecules. In a sequel paper, Ahmad et al. (Ahmad, Zoheir, et al. 2017) indicated dysregulation of Th1, Th2, Th17, and Treg cell-related transcription factor signaling in children with ASD. In the final study published in 2019, Ahmad et al., (Ahmad et al. 2019) used RT-PCR and western blotting to report elevated expression and significant mRNA and protein induction of IL-16 in children with ASD compared with typically developing controls. IL-16 is a chemoattractant for various CD4+ cell lines associated with proinflammatory processes and activation of glial cells. It is also reported to be closely involved in the pathology of multiple sclerosis and other inflammatory diseases in the CNS (Hridi et al. 2021). Nie et al. (Nie et al. 2023) also investigated CD4+ T cell subsets in 82 children with ASD and 50 healthy typically developing children from the Medical University of Yunnan Province. Overall, their data suggested an imbalance in inflammatory and regulatory immune responses in ASD. A higher effective T cell (Teff) to Treg ratio, was associated with more severe problematic behavioral symptoms. Researchers also report proinflammatory cytokine levels were higher in the plasma of children with ASD compared to typical controls. Molloy et al., (Molloy et al. 2006) report children with ASD had increased activation of both Th2 and Th1 arms of the adaptive immune response, with a Th2 predominance. Li et al. (Li et al. 2009) reported elevated immune responses in the brains of autistic patients with elevated proinflammatory cytokines. However, in disagreement with reports by Molloy et al., the Th1/Th2 ratio was significantly increased in ASD patients enrolled in this study. Basheer et al. (Basheer et al. 2018) report activated Th17 cells on analysis of blood serum from children with ASD. The role of Th17 cells in auto-inflammatory neurological disorders has been highlighted (Hridi et al. 2021). Moreover, Th17 cells increase the migration of other immune cells such as neutrophils into the inflamed CNS through the BBB and trigger inflammatory reactions that occasionally lead to irreversible neuronal damage (Pourgholaminejad and Tahmasebinia 2019). B cells play multiple roles, including the capacity to produce antibodies and cytokine milieu, and play fundamental roles in autoimmune diseases (Mauri and Bosma 2012). Nadeem et al., (Nadeem et al. 2022) report an imbalance in pro-inflammatory and anti-inflammatory cytokines in B cells of children with ASD. This study indicated that in ASD subjects, pro-inflammatory cytokines such as IL-6 and TNF-α were elevated in B cells while anti-inflammatory cytokine IL-10 was lowered. The elevation of TNF in individuals with ASD would indicate neuropathology involving neuronal signaling and homeostasis (Montgomery and Bowers 2012; Park and Bowers 2010). Cruz-Machado et al., (Cruz-Machado et al. 2021) report higher nocturnal saliva levels of TNF and IL-6 in 20 individuals with ASD compared to 20 normally developing individuals. Cruz-Machadet alo et al. speculate the involvement of immune-pineal axis activation, with elevated TNF but not IL-6 level, is associated with disrupted pineal melatonin release and sleep dysfunction in ASD. Further discussion of the brain-immune crosstalk in sleep is provided in a review by Marshall and Born (Marshall and Born 2002).
The limbic system, amygdala, and related structures have been extensively researched in the context of the pathophysiology of ASD (Sweeten et al. 2002). Limbic encephalitis is characterized by adaptive autoimmune inflammation of the limbic system. It has recently been identified as a major cause of temporal lobe epilepsy accompanied by progressive memory disturbance and emotional and behavioral changes (Bien and Elger 2007). This is noteworthy as it is now established and widely recognized that children with ASD are at high risk for developing epilepsy (Tuchman et al. 2009; Volkmar and Nelson 1990). Several researchers (Tuchman et al. 2009) provide further reflection on the autism-epilepsy phenotype and a growing body of evidence for shared neuronal networks that can account for both ASD and epilepsy. Epilepsy is a paroxysmal disorder characterized by abnormal electrical brain activity associated with a variety of behavioral manifestations. Autoimmune encephalitis and epilepsy have been linked to neural-specific autoantibodies targeting both intracellular and plasma membrane antigens (Toledano 2015). The autism-epilepsy phenotype has also recently been shown to be associated with macrocephaly, a pathologic condition due to accelerating brain growth in early development leading to ASD. Cerebellar alterations are often observed with epilepsy, including structural changes and modulation during seizures (Streng and Krook-Magnuson 2021). The role of cerebellar circuitry alterations in the pathology of sensorimotor and other ASD-related behaviors has been extensively highlighted (Becker and Stoodley 2013; D’Mello and Stoodley 2015; Mosconi et al. 2015; Su et al. 2021). Numerous studies have focused on cell pathology of the cerebella involving Purkinje cells for ASD (Fatemi et al. 2002). Purkinje neurons project into deep cerebellar nuclei and are the only output cells of the cerebellar cortex; and they play an important role in motor coordination, sensory processes, and cognition (D’Angelo 2018). They contain the inhibitory neurotransmitter GABA and the calcium-binding protein calbindin and their output from the cerebellar cortex is wholly inhibitory (Cellot and Cherubini 2014). Reports have indicated 35–95% fewer cerebellar Purkinje cells (Becker and Stoodley 2013) and a reduction of cell size in ASD brains compared to controls (Fatemi et al. 2002). The cerebellum can be a frequent target of autoimmune attacks. The underlying cause for this vulnerability is unclear. Hampe and Mitoma (Hampe and Mitoma 2022) speculate region-specific differences in BBB permeability, the high concentration of neurons in the cerebellum, and the presence of autoantigens on Purkinje cells. as potential explanations. Wills et al., examined plasma from children with ASD for antibodies directed against human cerebellar protein (Wills et al. 2009). Western blot analysis revealed that 13 of 63 subjects with ASD possessed autoantibodies that demonstrated specific reactivity to a cerebellar protein. Intense immunoreactivity to what was determined morphologically to be the Golgi cell of the cerebellum was noted for 7 of 34 subjects with ASD.
GBS is an autoimmune disease of the peripheral nervous system. Its annual incidence in children under 15 years old ranges from 0.34 to 1.34 per 100,000 (Willison et al. 2016). TLR and cytokines have been reported to have a critical role in the pathogenesis of the disease (Nyati and Prasad 2014). Despite having great clinical value, the interconnection between GBS and ASD has not been established and remains a mystery. Hasib et al., (Hasib et al. 2023) evaluated the differential expression pattern of genes from two RNA-seq data to discover potential common biomarkers for GBS and ASD. 17 common differentially expressed genes were identified for these two disorders. Common genes identified the protein-protein interaction (PPI) network and pathways associated with both disorders. PPI networks are distributed in complex diseases including cancer and autoimmune disorders (Safari-Alighiarloo et al. 2014).
Taken together, the findings of autoimmunity in families and the plethora of anti-brain antibodies reported for individuals with ASD suggest that in some patients, autoimmune encephalitis and autoantibodies that target the CNS and ENS may be a pathological or exacerbating factor in neuronal and ASD development.
7.4. Autoimmune Encephalitis, N-Methyl-D-Aspartate (NMDAR) Encephalitis & ASD
Autoimmune encephalitis comprises a group of CNS inflammatory disorders. The presentation of autoimmune encephalitis can be like encephalitis secondary to environmental insult. A study by the California Encephalitis Project, a center focused on the epidemiology and etiology of encephalitis, found that 63% of the patients remained without infectious etiology based on a battery of tests for 16 potential infectious agents. Of these patients, the most common etiology was immune-mediated (Gable et al. 2012). Encephalitis is indicated by acute or subacute symptoms of decreased or altered level of consciousness, lethargy, short-term memory loss, and personality changes such as apathy, irritability, agitation, and commonly, seizures (Serrano-Pozo 2018). In children, neurological manifestations such as seizure, movement disorders, and focal neurological deficits are more prominent at initial presentation than psychiatric or behavioral symptoms (Hardy 2022). When psychiatric symptoms do occur, they often manifest as temper tantrums, aggression, agitation, and rarely psychosis. Speech disorders are also a common complication of encephalitis in children (estimated 15-38% (Niyozovh et al. 2020)).
NMDAR encephalitis is the most common form of antibody-mediated limbic encephalitis in children and adults (Garg et al. 2020). NMDAR are voltage-dependent ionotropic glutamate receptors (Paoletti et al. 2013) and glutamate is a major excitatory neurotransmitter of the CNS. It is implicated in many basic neuronal functions and CNS processes including learning, memory, and synaptic plasticity (Niciu et al. 2012). The anti-NMDAR autoantibody is a typical synaptic protein that can bind to synaptic NMDA glutamate receptors, leading to dysfunctional glutamate neurotransmission in the brain that manifests as psychiatric symptoms (psychosis, hallucinations, and personality changes) (Kayser and Dalmau 2016). A growing body of case reports documents NMDAR antibodies in the serum and cerebrospinal fluid of children with developmental regression--particularly of social communication skills, mimicking an autistic regression (Hacohen et al. 2016; Khundakji et al. 2018). Kern et al., review the evidence for encephalitis and microglial and astrocytic activation, a unique and elevated proinflammatory profile of cytokines, and aberrant maladaptation of B cells (Kern et al. 2016). The authors speculate that at least 69% of individuals with an ASD diagnosis have encephalitis. Tzang et al., (Tzang et al. 2019) correlated ASD with dysfunctional autoimmunity and anti-NMDAR encephalitis. Authors also report autoantibodies, cytokines, decreased lymphocytes, serum immunoglobulin level imbalance, and T cell lineage to mediate immune responses as important biomarkers for autoimmune pathologies in ASD. Tarabeux et al., (Tarabeux et al. 2011) explored rare or de novo mutations in NDDs. Researchers sequenced the seven genes encoding for NMDARs in a large cohort of individuals affected with schizophrenia (n = 429) or ASD (n=428) vs. typical controls (n=568). Sequencing identified two de novo mutations in patients with sporadic schizophrenia and one de novo mutation in a patient with ASD. Data here further supports the hypothesis that rare de novo mutations could account for some cases of sporadic ASD. This study also further supports the importance of NMDARs in psychiatric disorders with neurodevelopmental origin. In NMDAR encephalitis unique EEG patterns of 20–30 Hz beta activity riding on rhythmic 1–3 Hz delta waves, called extreme delta brush, have been described (Bhagavati 2021). It remains to be seen if the relation between encephalitis and ASD is uni- or bidirectional–that is, whether children with ASD have an epigenetic diathesis to developing encephalitis (such as those mediated by the NMDAR), or conversely, if deranged or inflamed neuroreceptor processes are implicated in its development–or on occasion both.
7.5. GI Pathology, the Immune System & Pathophysiology of ASD
There is substantial evidence, which provides not only correlational but also a causal relationship between intestinal pathology, autoimmune (Forbes et al. 2016; Kharrazian et al. 2023), and neurodevelopmental diseases (Abo-Shaban et al. 2023). Notably, the largest component of the body’s immune system is gut-associated lymphoid tissue (GALT) comprising secretory lymphoid aggregates known as Peyer’s and caecal patches. GALT plays a central role in maintaining GI homeostasis including baseline levels of inflammation, prevention of microbial overgrowth, and neuroimmune interactions. Microfold (M) cells found in Peyer's patches actively transport luminal antigens to the underlying lymphoid follicles to initiate an immune response, leading to autoimmune diseases (Kobayashi et al. 2019) and plays a role at the interface between innate and adaptive immunity (Jung et al. 2010). M cells can engulf molecules in the intestinal mucosa and pass on information to the antigen-presenting cells such as macrophages and dendritic cells which further crosstalk with B cells for antibody production (Miller et al. 2007).
Because of their ability to transport luminal antigens and bacteria, Peyer's patches can be considered immune sensors in the intestine (Jung et al. 2010). The essential role of Peyer's patches includes their influence on gut microbiome composition, communication in the ENS, and influence of behavior as part of the GBA (Furness et al. 2014; Abo-Shaban et al. 2023). A high comorbidity of celiac disease, an immune-mediated, autoimmune reaction to gluten, and ASD has also been reported and extensively discussed in the context of ASD pathophysiology (Clappison et al. 2020; Di Liberto et al. 2020). Of note, the pathophysiology of celiac disease has been speculated to be neurological, involving loss of Purkinje cells and atrophy and gliosis of the cerebellum (Mulder and Mulder-Bos 2006). A commonly used restrictive dietary intervention for ASD is the gluten-free casein-free diet (GFCF) (Ciéslińska et al. 2017), which is based on the opioid excess theory (Tarnowska et al. 2023). Panksepp first proposed this theory to explain the pathogenesis of ASD and hypothesized that opioid peptides released from gluten and casein could pass through the mucosa and cross the blood-brain barrier, reaching the CNS and affecting brain function (Panksepp 1979).
With opioids, the neurotransmitter serotonin has also been extensively investigated for its role in the pathophysiology of ASD in the CNS (Kepser and Homberg 2015; Wegiel et al. 2024) and its ability to modulate neural plasticity and networks (Lesch and Waider 2012). Serotonin is detected in the first trimester and regulates neuronal growth, differentiation, migration, and survival (Daly et al. 2019). Serotonin also shares a strong relationship with the pathological cases of tumor, as well as pathogen infection, (Shajib et al. 2017) and is speculated to participate in the pathogenesis of autoimmune disease (Wan et al. 2020). In a systematic review and meta-analysis, Gabriele, Sacco, and Persico (Gabriele et al. 2014) report that approximately 30% of children with ASD show higher levels of serotonin in the blood in comparison to typically developing control groups. Serotonin signaling is also ubiquitous in the GI tract, as a neurotransmitter in the ENS, and is involved in epithelial proliferation and decreased injury from intestinal inflammation (Shah et al. 2021). Outside the CNS, serotonin is produced in the ENS and released predominantly by enterochromaffin cells (estimated to be up to 90% of the body's serotonin production (Geng et al. 2022)) of the gut mucosa and immune cells, with influence not only on the ENS but also on CNS via the GBA (D.-Y. Kim and Camilleri 2000). Not only does the gut secrete serotonin but it also expresses a kaleidoscopic abundance of serotonin receptors and serotonin transports (Gershon 2022). The impressive multi-functional nature of enteric serotonin has made the identification of physiological roles difficult and sometimes controversial. A novel hypothesis for documented GI pathology for ASD could be that immune-mediated insult damages the enteric innervation (enteric ganglionitis) with increased mucosal serotonin produced, predominantly by enterochromaffin cells of GI tract (De Giorgio et al. 2004).
7.6. Sex Hormones, the Pathogenesis of ASD & the Immune System
While sex hormones have long been recognized for their roles in reproductive functions, within the past two decades scientists have found that sex hormones are integral signaling modulators of the human immune system. Sex hormones are implicated in the immune response, with estrogens as enhancers at least of the humoral immunity and androgens and progesterone (and glucocorticoids) as natural immune suppressors (Cutolo et al. 2006). The sex hormonal status is also known to modulate the hypothalamic-pituitary-adrenal axis (HPA) with accumulating evidence indicating that estrogen has excitatory effects, while androgen has inhibitory effects on HPA function (McCormick et al. 1998). There is also compelling evidence to suggest that sex hormones are important in the maturation of the immune system during fetal life (McCruden and Stimson 1991). The inherent differences in sex-dependent neuroimmune development are predominantly orchestrated by a surge of gonadal hormones unique to males, specifically occurring within a critical perinatal window that significantly impacts brain development (Ardalan and Mallard 2024).
Fetal testosterone exposure is one of several hypotheses that attempt to explain the male preponderance of NDDs, especially in ASD (Ferri et al. 2018). Fetal testosterone is involved in many aspects of development and may interact with neurotransmitters, neuropeptides, or immune pathways to contribute to male vulnerability. Gonadal hormones may also impart male-biased behavioral vulnerabilities to immune activation via microglial mitochondrial function and directly influence neurons, as well as oligodendrocytes, endothelial cells, and astrocyte functioning. McCarthy and Wright review the role of microglia and astrocytes in directing brain masculinization. Authors further speculate that the natural process of brain masculinization puts males at risk of ASD by moving them closer to a vulnerability threshold, breached by refractory inflammation during critical periods of brain development (Werling 2016). Alternative hypotheses are provided by El-Ansary, Bhat, and Zayad (El-Ansary et al. 2020), who speculate that higher ASD phenotypes in males compared with females could be attributed to the protective effect of estrogen, the higher diversity and predominance of probiotics in females, and the lower liability of females to develop leaky gut, neuroinflammation, and excitotoxicity. Kushak and Winter (Kushak and Winter 2020) discuss the bidirectional relationship between sex hormones and intestinal microbiota and speculate sex bias in males may be associated with more significant changes in the intestinal microbiome than in affected girls. The sexually dimorphic prevalence of autoimmune disease for females (Denman 1991; Cutolo et al. 2006; Ackerman 2006; Zandman-Goddard et al. 2007; Knudsen 2009; Ngo et al. 2014; Moroni et al. 2012) and ASD for males (Werling 2016; McCarthy and Wright 2017; Ferri et al. 2018) remains one of the most intriguing clinical observations among both groups of these disorders. This raises the question of whether sex hormones could provide a bridge to understanding common etiologies of these conditions, in some neonates, occurring through differing gender-dependent mechanisms of pathogenesis.
8. Future Directions & Therapeutic Modalities for ASD
In the 1940s, Leo Kanner attributed the etiology of ASD to the emotional unavailability of an affected child’s mother (Harris 2018). While erroneous, this misconception originally and lastingly branded autism as a “brain-centered” psychiatric disorder, requiring psychiatric therapy and medications (Coury et al. 2012). In addition, there have been nearly eight decades of a common scientific consensus on a “static” genetic etiology. Only in the last decades have researchers taken “whole-body” pathologies for neonates and children diagnosed with ASD (M. Herbert and Weintraub 2013). Moreover, much like the issue of regressive autism, the idea that ASD for some is not a lifelong condition is a relatively recent addition to the knowledge of ASD. Isolated reports talking about children “growing out of autism” peppered the research literature in the 1980s and 1990s following some earlier initial interest in this topic (Whiteley et al. 2019).
A further limitation to our understanding of the ASD phenotype includes the reductionist paradigm, which views the human organism as a compilation of single targets for intervention and symptom alleviation without investigating its networks of interconnection. This paradigm has been central to modern healthcare models for over 150 years (Tillmann et al. 2015). Although it can offer a useful first step in understanding complex systems, the reductionist paradigm also tends to oversimplify non-communicable diseases and neglect the heterogeneity of their contributing factors. This makes it not only an ineffective tool for sufficiently understanding and treating the complex nature of heterogeneous disorders such as ASD, but an obstacle to the aspiration for the restoration of normal psychological and physiological well-being, which requires a non-linear dynamical systems approach. It is also clear that the non-linear, dynamic nature of the developing mind and the nervous and immune systems implies that potential deviations from typical development cannot be meaningfully captured with static, discrete, and linear parametric scales a priori imposed (Torres and Denisova 2016).
The debate continues as to the role of the immune system in ASD and whether the immune abnormality in ASD children is hyperactive or hypoactive (Brown and Mehl-Madrona 2011). In addition, the heterogeneous nature of ASD is reflected not only on a cellular level but also in whole brain autonomy (Pardo et al. 2005; Pretzsch et al. 2022) and disorders of the peripheral nervous systems (Brincker and Torres 2013). A need to empower and accelerate precision medicine (Hawgood et al. 2015) and personalized medicine approaches becomes apparent and essential for the efficacious treatment of ASD (Pretzsch et al. 2022). Moreover, modern therapeutic modalities usually take a reductionist approach and often use medication under the guise of a “magic bullet” that can eliminate a disease’s cause or symptom. This single-target perspective tends to neglect consideration of how these medications unintentionally impact the overall regulatory ability of the human organism from “whole-body” perspectives. Medications should be designed to mimic, modulate, or promote the body's natural resolution mechanisms instead of interfering with them. Given the current healthcare and medical challenges, it is evident that the single-molecule, single-target paradigm does not provide the specificity and sophistication that a multitargeting model has the potential to offer (Goldman et al. 2015).
8.1. ASD: Viewpoints from Environmental Exposomes, Omics Technologies & BrSYS Medicine
Critical windows of vulnerability include early pregnancy and childhood where the establishment of the immune system, host microbiome, nervous system, and endocrine system can proceed in a healthy or unhealthy direction depending on the exposome (Mallozzi et al. 2016). The window of vulnerability to fetal and neonatal neurodevelopment brings further specificity to the ASD phenotype. Recent research in various health disciplines demonstrates that deficiency and toxicity are common etiological determinants of ill health in the contemporary era (Genuis 2012). This totality of this exposure (and deficiency) can be described as the “human exposome” and refers to the variety of external and internal sources including chemical agents, biological agents, or radiation, from conception onward, over a complete lifetime (Vineis et al. 2020). The chemical exposome further captures the diversity and range of exposures to synthetic chemicals, dietary constituents, psychosocial stressors, physical factors, and their corresponding biological responses (Vermeulen et al. 2020). The exposome chemical space remains largely uncharted due to the sheer number of possible chemical structures, estimated at over 1060 unique forms (Samanipour et al. 2024). Individual responses to current exposures and disease susceptibility are influenced by multiple factors, including genetics, genomic variants (SNPs), epigenetics, physiology, and health status, which involve changes in biological pathways; these factors may devolve from present or past exposures (i.e., from a legacy of prior exposures or even ancestral exposures) (Karlsson 2023). Krausová et al. (Krausová et al. 2023) review the potential early-life impact of multi-factorial exposures that can influence the exposome during gestation or after birth and highlight the lack of large-scale studies covering a broad range of xenobiotics. Hertz-Picciotto et al., (Hertz-Picciotto et al. 2018) also report that exposure levels in pregnant women and infants are largely unknown for most contaminants, and that exposome-scale studies of large cohorts with a broader chemical space coverage are scarce. Recently, Tartaglione et al., (Tartaglione et al. 2024) conducted a systematic review of 24 case-control studies and indicated several pollutants within the first three years of life to be significantly associated with the risk of NDDs. However, the authors acknowledge that further studies are also needed to more accurately characterize the risk of NDDs by applying an “exposome approach”.
The exposome can be seen as a complement to the genome (Wild 2005) and encompasses all external and internal factors that influence functional proteins, metabolites, and gene expression (Balcells et al. 2024; Renz et al. 2017). It would be vital to consider individual exposomes as a basis for precision medicine (Karlsson 2023). Our understanding of the role of chemical exposomes in chronic non-communicable diseases has advanced considerably with high-throughput molecular-omics techniques (metagenomics, proteomics, metabolomics) (Higdon et al. 2015), which provide tools for a new emphasis on interdisciplinary research and personalized medicine for ASD. Maitre et al. (Maitre et al. 2022) have developed a blueprint of how multi-omics technologies can capture individual exposomes and establish signatures of disease. In a multi-center cohort of 1301 mother-child pairs, researchers associated individual exposomes of >100 chemical, outdoor, social, and lifestyle exposures during pregnancy and childhood. Of note, pregnancy exposures were predominantly associated with child DNA methylation changes. In contrast, childhood exposures are associated with features across all omics layers. A review by Higdon et al. (Higdon et al. 2015) elaborates on how distinct disease subtypes can be identified in ASD, through the integration and analysis of clinical and multi-omics data.
The science of omics technologies and the immune system can be further described as immunomics (Burns-Naas et al. 2006). Immunomics represents a bridge to elucidating the complex interactions between genes, environment, disease pathology, and the immune system. It also provides a new dimension to understanding immunopharmacology and immunotoxicology, with huge potential to impact disease etiology and pathogenesis and for the development of novel and personalized therapeutics. Inflammasomes are multimeric regulatory proteins that form in the cytosis, in response to danger signals that perturb cellular homeostasis and are involved in inflammatory responses, including the production of proinflammatory cytokines and programmed pyroptosis and autophagy (Schroder and Tschopp 2010). Inflammasome activity is closely associated with numerous human disorders, including autoinflammation, cardiovascular diseases, neurodegeneration, and cancer (Fu et al. 2024). Saresella et al. (Saresella et al. 2016) report that multiple inflammasome complexes are activated in ASD and suggest that therapeutic strategies targeting inflammasome activation could be useful in ASD. Considering the role of environmental insults in the etiology and pathogenesis of ASD, these early investigations provide a basis for a broad and multifactorial analysis of potential individual root causes of ASD, and strategies for precision and personalized therapy. Further direction to benchmark individual exposomes on a diagnosis of ASD in children would provide high-resolution inference and be essential to diminish any further escalation of disease states in the first instance.
Complex systems are now recognized to demonstrate “
emergent” behavior with functions arising from the totality of system interactions, which are often nonlinear and stochastic. With the application of omics technologies, a systems biology approach (Herbert 2014) further provides a “
middle-out”, dynamic, multi-scaled, and “
whole-body” approach to understanding ASD and the development of personalized therapeutics. This approach defines the human body as a complex biological network of interconnected components (molecules, cells, tissues, organs) (Randolph-Gips and Srinivasan 2012). This includes not only the immune system and the nervous system, but also the cardiovascular system, the digestive system, the endocrine system, the muscular system, the renal system, the reproductive system, the respiratory system, and the skeletal system. Goldman
et al. (Goldman et al. 2015) further extend the systems biology paradigm by merging it with regulatory and relevant empirical and treatment models which they designate as “
bioregulatory systems medicine”. BrSM is rooted in the idea that robust and effective solutions for complex diseases should optimize an individual's homeostatic and allostatic systems, and their interactions across all biological systems and emerges in part as a response to the limitations of the reductionist perspective model (Tillmann et al. 2015). A goal of BrSM would be to eventually develop biomarkers tailored to individual pathophysiological disease states, that predict the health-disease continuum and allow for therapeutic interventions to the specific stage of a patient's disease. A BrSM approach also incorporates a multi-scale approach and would also emphasize the simultaneous and complex dynamic interactions of activities across all biological scales, or different hierarchical levels of resolution
Figure 2 (Greenberg and Tobach 2014).
8.2. ASD Therapeutic Modalities: Standard of Care & State of the ART
The common phrase “children are not little adults” is even more true in drug development, with neonatal and children placed in the special category of a vulnerable population (Green et al. 2021). Clinical development of therapeutics for ASD poses challenges for the identification of suitable endpoints, lack of biomarkers for progression, and regulatory considerations (Ghosh et al. 2013; McCracken et al. 2021). Moreover, in nonverbal children or children with communication difficulties, side effects can be challenging to recognize, since children may exhibit different behaviors as side effects to the same therapeutic, (West, Brunssen, et al. 2009). Adverse events may also be camouflaged by comorbidities in individuals with ASD.
Doctors often use psychiatric drugs to address symptoms associated with ASDs. Risperidone and aripiprazole are both antipsychotics and are the only medications currently approved by the US FDA for the management of irritability in children with ASD (Choi et al. 2024). Other classes of pharmaceuticals commonly prescribed off-label include stimulants (e.g., amphetamines and methylphenidates), selective serotonin reuptake inhibitors (SSRIs), α2-adrenergic agonist inhibitors (e.g., clonidine and guanfacine), and selective norepinephrine reuptake inhibitors (Gupta and Gupta 2024; Lacivita et al. 2017; Politte et al. 2015). West et al., (West, Waldrop, et al. 2009) found that current pharmacological interventions used for ASD are only moderately useful in treating symptoms associated with ASD, such as attention, aggression, or repetitive movements, and they are only minimally effective in addressing the core deficits of ASD. All these classes of pharmaceuticals carry specific side effects and require professional long-term monitoring and management of adverse effects (Martin et al. 2004; Üçok and Gaebel 2008; West, et al. 2009; Alsayouf 2024). As an example, King et al. (King et al. 2009) conducted a randomized control trial of 149 individuals with ASD who received either citalopram (n = 76) or placebo (n = 76) over the 12-week therapeutic period. Clinical results indicated no significant difference in the rate of positive response. Moreover, citalopram use was significantly more likely to be associated with adverse events, particularly increased energy level, impulsiveness, decreased concentration, hyperactivity, stereotypy, diarrhea, insomnia, and dry skin or pruritus. Additionally, the vast majority of psychiatric drugs display a high potential for physiological dependence (Moncrieff 2006; Moncrieff et al. 2013) which would make testing and use in pediatric populations challenging and potentially risky, if not redundant.
Biologics and immunosuppressive drugs are the primary treatment tools for autoimmune disorders along with non-steroidal anti-inflammatory drugs and SSRIs (Gobin et al. 2014; Gianluca et al. 2017). Immunomodulating agents are designed to resemble antibodies, receptors, and other immunological factors. Emerging immunomodulating agents including biologics, many of which were developed only recently, may provide additional treatment options for ASD subjects. However, published data on clinical trials in ASD subjects are scant (Jyonouchi 2024). The use of anti-inflammatory drugs in ASD has been reviewed by Hafizi, Tabatabaei, and Lai (Hafizi et al. 2019). This included amantadine an antiviral and anti-Parkinson drug; celecoxib, a nonsteroidal anti-inflammatory drug; the corticosteroid, galantamine, an acetylcholinesterase inhibitor; intravenous immunoglobulin; lenalidomide, a derivative of thalidomide; memantine, an NMDA receptor blocker; minocycline, a tetracycline-class antibiotic with anti-inflammatory effect; the antioxidant N-acetylcysteine; pioglitazone, an antidiabetic medication; riluzole, an inhibitor of presynaptic release of glutamate; palmitoylethanolamide, a fatty acid amide with neuroprotective, anti-inflammatory, and anti-nociceptive properties; pentoxifylline, a xanthine derivative that has an inhibitory effect on inflammatory responses; spironolactone, an antagonist for aldosterone receptor; and topiramate, an anti-epilepsy medication. The authors concluded that for all pharmaceuticals reviewed, there was a lack of rigorous clinical trials and very few successful replications that did address the core characteristics or associated symptoms of ASD. Moreover, all therapeutic agents exhibited low safety profiles, including serious systemic side effects.
First-line treatments for autoimmune encephalitis include aggressive pharmaceuticals including intravenous steroids and immunoglobulin, plasma exchange, and immunoadsorption (Shin et al. 2018). Contradictory results in efficacy studies with these drugs, which can result in an immune-compromised state, putting the affected population at risk for serious infections or immune-related illnesses, malignancy, and cardiovascular conditions (Machado et al. 2023). Clinical investigations using stem cell products are currently being applied for a wide spectrum of conditions using a variety of stem cell types (Dimmeler et al. 2014). Siniscalco, Bradstreet, and Antonucci (Siniscalco et al. 2013) review the therapeutic potential of hematopoietic stem cells (HSC) in ASD-related inflammation. Researchers conclude that although HSCs are attractive candidates for potential restoration of ASD-related immune-mediated pathologies, concerns about several life-threatening risks were raised. These include abnormal trafficking of stem cells by the BBB into the CNS, resultant unintended consequences, the bidirectional nature of stem cells and potential contribution to the inflammatory state, and the risk of graft-versus-host disease (Leventhal et al. 2013).
Other novel interventions for ASD include microbial transplant therapy (MTT). Indeed, in 2019, the FDA recognized microbial transplant therapy with a “fast-track” designation for ASD treatment (Adams et al. 2019). MTT is a novel method to modify the microbial ecosystem in the GI tract of the hosts. MTT has indicated success in clinical trials (Kang et al. 2019) with significant improvements in GI symptoms, autism-related symptoms, and gut microbiota ecology. Kang et al. (Kang et al. 2019) report important changes in gut microbiota at the end of treatment remained at follow-up, including significant increases in bacterial diversity and relative abundances of Bifidobacteria and Prevotella. However, such interventions are intrusive, can have adverse effects, and come with several limitations regarding the source and uniformity of donor feces. In addition, adult fecal donors may correlate with the development of aging physiological function and unwanted immune reactions (Chen and Chiu 2022). Besides, there is a lack of clinical data in children regarding many facets of MTT, such as the uniformity of transplant protocols used, route of administration (oral, endoscopy, or enema), growth, and development after MTT.
8.3. ASD Therapeutics: Personalized & Precision Nutrition and Mind-Body Modalities
The maxims of ´let thy food be thy medicine´ accredited to Hippocrates (circa 400 BC) and ‘you are what you eat’ by Jean Anthelme Brillat-Savarin (in 1826) remain as pertinent today as they did then. In the contemporary era, research into these bioactive natural products originating from medicinal plants, fungi, and microorganisms is also based on ancient ethnopharmacological (including entheogenic) human practices (Modafferi et al. 2024; Yeung et al. 2020). Real-world evidence has provided ample evidence from parents of children with ASD and clinicians, of the utility of food and diet, for the treatment of symptoms and sporadic reversal of the core symptoms of ASD (Cekici and Sanlier 2019). These approaches principally involve an adjustment in dieting habits that begins with the removal of all ultra-processed foods and the encouragement of organic and whole-food diets. Indeed, the essential role of nutrition in optimizing, promoting, and recovering mental health is only now arising within medical practice and the scope of practice in psychiatry (Rucklidge et al. 2021). Peper and Shufor (Peper and Shufor 2024) coined the “grandmother therapy assessment”, in a recommendation that diet, and nutritional deficiency be the first line of assessment of ASD and ADHD. GI disorders in ASD and its treatment through nutritional therapy may also provide paths for modulating the expression of social and behavioral symptoms through the GBA. This includes not only the application of foods rich in probiotics (Shahbazi et al. 2021), but also prebiotics, as well as postbiotics (Zhang et al. 2024). The latter provides strategies to support natural gut healing and a shift in GI homeostasis from potentially pathogenic to beneficial and congenital microbial ecologies or, at least, to restore gut eubiosis. Indeed, GI microbiome restoration would be a prerequisite to the successful amendment of inflammation processes identified in any chronic disease (López-Varela et al. 2002; Luvián-Morales et al. 2022). van den Brink et al. (van den Brink et al. 2019) further elaborate on strategies to (re)gain inflammatory flexibility by regular mild metabolic triggers through perspectives of trained immunity, hormesis, and pro-resolution.
Modulating the neuro-immune-microbiome axis with “full-spectrum” functional foods and botanical plant-based medicines further provides a multitargeting paradigm and allows for a synergistic or “entourage effect”. The “entourage effect” essentially refers to the interaction of not only primary and secondary therapeutic agents (e.g., alkaloids, pre- and postbiotics, antioxidants, and microbial communities living in fermented foods) shown to have therapeutic potential. But in addition, there are myriad secondary metabolites in the “nutritional dark matter” in the “foodome” (Ribeiro 2018; Shahar et al. 2024; Yeung et al. 2020). Animals generally avoid these potentially toxic molecules, but our hominid ancestors elected to selectively consume foods rich in secondary metabolites (Lerer and Varia 2022) (i.e., polyphenols, flavonoids, carotenoids, phytosterols, polysaccharides, triterpenoids, tannins, saponins, and ash) as means of complementing their nutrition and which later formed the basis of natural and omnipresent archaic medicine practices and folklore. Wellness and therapeutic metabolites found in food and botanicals when taken in their natural matrix are generally safe even for chronic consumption with extremely low toxicity, present ease in uptake or breakdown by the body, have low retention, and present low potential for physiological dependency (Witkamp and van Norren 2018).
Despite considerable interest and real-world evidence for the utility of dietary interventions for ASD, no consensus exists regarding optimal nutritional therapy with much controversy regarding its effectiveness (Lange et al. 2015; Fraguas et al. 2019; Karhu et al. 2020; Monteiro et al. 2020; Keller et al. 2021; Doherty et al. 2024). As for pharmaceutical interventions, this is unsurprising, considering the heterogeneous nature of ASD. This further emphasizes that there is no “
one-size-fits-all” and that the need for a precision and personalized medicine paradigm that goes beyond the modern reductionist approach, through the application of multi-dimensional, multi-disciplinary nutritional therapy, is strong. One paradigm to address current pitfalls for successful nutritional intervention in ASD could be through the convergence of nutrition science with omics technology, or “
nutrigenomics” (van Ommen and Stierum 2002) (
Figure 3). The integration of nutritional systems with gene and protein expression profiles and metabolic fingerprints allows a more extensive analysis of the individual response to any nutritional intervention (Badimon et al. 2017).
“Nutrigenomics” provides a more global comprehensive understanding of how food components influence health status (Badimon et al. 2017) and new opportunities oriented toward personalized interventions (Badimon et al. 2017). A few studies of transcriptomic, proteomic, and metabolomic studies have demonstrated the ability of functional foods and their bioactive components to fight against oxidative damage (Moore and Weeks 2011) and to modulate the underlying dynamics of low-grade chronic, systemic inflammation, impaired metabolic flexibility, and dysregulation (Calçada et al. 2014).
Moving along from “body-mind” modalities, “mind-body” modalities have also been endorsed by health practitioners active in the field and there is modest clinical evidence supporting their efficacy for outcomes such as social communication and cognitive functions and anxiety (Akers et al. 2020; Huang et al. 2023). Non-pharmacological interventions that are standard of care include cognitive and behavioral therapies (CBT) to address issues of anxiety for children with ASD (Storch et al. 2013; J. J. Wood et al. 2020) and promote autonomy and emotional well-being (Misquita et al. 2024). CBT helps strengthen cognitive skills, such as mental flexibility and problem-solving, which are essential for an independent and fulfilling life (Misquita et al. 2024). Also of note, it has been speculated that approximately 90% of vagal fibers between the gut and brain are afferent, suggesting that the brain is more of a receiver than a transmitter concerning brain-gut communication (Berthoud and Neuhuber 2000). Nonetheless, this still establishes the role of non-pharmacological “mind-body” interventions for the restoration of normal neuro-immuno-microbiota functioning for individuals with ASD. Cutting-edge non-pharmacological technologies including breathwork training (Aideyan et al. 2020; Mitsea et al. 2022). The use of conscious breathing practices for physical, psychological, emotional, and spiritual healing has a long and varied history (Fincham et al. 2023). However, there has been little work to bring these practices into a coherent and unified form that contributes to the field of body psychotherapy (Victoria and Caldwell 2013). Overall, research findings indicate that breathwork may be efficacious for treating anxiety, depression, and PTSD (Aideyan et al. 2020). Physical benefits of breathwork can be summarized as improving immune function; regulating arousal; decreasing sinus problems; balancing hormones, enzymes, and neurotransmitters; stabilizing blood gasses; promoting digestion, circulation, and proper organ function; facilitating waste metabolism, aligning posture, decreasing muscle tension; and increasing motility, mobility and movement efficiency (Almahayni and Hammond 2024; Chavez and Zappaterra 2023; Victoria and Caldwell 2013). Despite preliminary evidence for breathwork's efficacy in treating common psychological distress, more research is needed to evaluate its utility for treating symptoms and core deficits of ASD.
9. Concluding Remarks
The explicit role of environmental (xenobiotic) insult in the etiology and pathogenesis of ASD has been speculated upon for over half a century and highlighted here. Chronic or acute xenobiotic activation of the immune system would trigger self-reinforcing disease processes through failed shut-off of stress-responsiveness. ASD therefore is an example of a situation that emerges as things “go beyond the tipping point” at various vulnerable states of neurological and immunological development. As highlighted by this review, the complex pathophysiology and heterogeneous ASD phenotype are hypothesized, for some individuals with ASD, to be related to deregulation of the immune system. This raises the question for some individuals diagnosed with ASD of whether the manifestation of immune cell pathology, and immune system dysregulation, are truly involved in the pathogenesis, or whether they are epiphenomena of the disorder; and whether their brain is truly and intrinsically electrochemically “defective” or instead biochemically “obstructed”? In support of the latter model recently D´Adamo et al., (D’Adamo et al. 2024) provided a case report of the reversal of autism symptoms in two dizygotic twins through a personalized lifestyle and environmental modification approach. These findings and other published cases of ASD reversal are encouraging. We would endorse more dialogue between parents, clinicians, and academics, with further documentation of regression and recovery. A crossover of ASD pathophysiology with autoinflammatory and autoimmune conditions further provides a novel hypothesis for understanding the pathogenesis of ASD. Moreover, omics and immunomics could provide game-changing platforms to elucidate the complex interactions between genes, environment, disease pathology, and the immune system leading to ASD phenotypes and the development of safe and effective personalized therapeutic solutions that address the core symptoms of ASD.
After reviewing the incredibly complex neuro-immuno-microbiota interactions implicated in clinical research, we believe that only once we understand that ASD is not genetically inevitable or a genetic tragedy but an environmental and physiological catastrophe, will we truly be able to grasp and address the root causes of the dramatic rise in its prevalence. Indeed, scientific evidence presented here would suggest that people with ASD are the “canaries in the coal mine” that is the most susceptible among us who are affected first by problems that might eventually reach us all. Moreover, it is quite plausible that this catastrophe goes beyond ASD. The rise of ASD may well to a significant extent evolve, or emerge, from overlapping vulnerability pathways, associated with systemic problems of other chronic diseases (Wen et al. 2016). The point henceforward becomes not just to support and seek full recovery for those diagnosed with ASD, but also how we as individuals, families, communities, and society in the contemporary era can most effectively protect future generations.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data Availability
No data was used for the research described in the article.
Acknowledgments
Maggie Humphries for graphical enhancement of figures.
Competing Interests
The author(s) declare none.
Credit Authorship Contribution Statement
Study’s conception and design by Hooker and Varia. The first draft of the manuscript by Varia, with further writing, review, and editing by Hooker, Herbert, and Varia. All authors have read and approved the final manuscript.
References
- Abbaoui, A., Fatoba, O., & Yamashita, T. (2023). Meningeal T cells function in the central nervous system homeostasis and neurodegenerative diseases. Frontiers in Cellular Neuroscience, 17. [CrossRef]
- Abbott, N. J., Patabendige, A. A. K., Dolman, D. E. M., Yusof, S. R., & Begley, D. J. (2010). Structure and function of the blood–brain barrier. Special Issue: Blood Brain Barrier, 37(1), 13–25. [CrossRef]
- Abnave, P., & Ghigo, E. (2019). Role of the immune system in regeneration and its dynamic interplay with adult stem cells. Planarian Regeneration, 87, 160–168. [CrossRef]
- Abo-Shaban, T., Sharna, S. S., Hosie, S., Lee, C. Y. Q., Balasuriya, G. K., & McKeown, S. J. (2023). Issues for patchy tissues: defining roles for gut-associated lymphoid tissue in neurodevelopment and disease. Journal of Neural Transmission, 130(3), 269–280. [CrossRef]
- Ackerman, L. S. (2006). Sex Hormones and the Genesis of Autoimmunity. Archives of Dermatology, 142(3), 371–376. [CrossRef]
- Adams, J. B., Borody, T. J., Kang, D.-W., Khoruts, A., Krajmalnik-Brown, R., & Sadowsky, M. J. (2019). Microbiota transplant therapy and autism: lessons for the clinic. Expert Review of Gastroenterology & Hepatology, 13(11), 1033–1037. [CrossRef]
- Ahmad, S. F., Ansari, M. A., Nadeem, A., Bakheet, S. A., AL-Ayadhi, L. Y., & Attia, S. M. (2019). Elevated IL-16 expression is associated with development of immune dysfunction in children with autism. Psychopharmacology, 236(2), 831–838. [CrossRef]
- Ahmad, S. F., Nadeem, A., Ansari, M. A., Bakheet, S. A., Attia, S. M., Zoheir, K. M. A., et al. (2017). Imbalance between the anti- and pro-inflammatory milieu in blood leukocytes of autistic children. Molecular Immunology, 82, 57–65. [CrossRef]
- Ahmad, S. F., Zoheir, K. M. A., Ansari, M. A., Nadeem, A., Bakheet, S. A., AL-Ayadhi, L. Y., et al. (2017). Dysregulation of Th1, Th2, Th17, and T regulatory cell-related transcription factor signaling in children with autism. Molecular Neurobiology, 54(6), 4390–4400. [CrossRef]
- Aideyan, B., Martin, G. C., & Beeson, E. T. (2020). A Practitioner’s Guide to Breathwork in Clinical Mental Health Counseling. Journal of Mental Health Counseling, 42(1), 78–94. [CrossRef]
- Akdis, C. A. (2021). Does the epithelial barrier hypothesis explain the increase in allergy, autoimmunity and other chronic conditions? Nature Reviews Immunology, 21(11), 739–751. [CrossRef]
- Akers, J. S., Davis, T. N., Gerow, S., & Avery, S. (2020). Decreasing motor stereotypy in individuals with autism spectrum disorder: A systematic review. Research in Autism Spectrum Disorders, 77, 101611. [CrossRef]
- Albert, L. J., & Inman, R. D. (2000). Gram-negative pathogens and molecular mimicry: is there a case for mistaken identity? Trends in Microbiology, 8(10), 444–445. [CrossRef]
- Albert L. J. & Inman R. D. (1999). Molecular Mimicry and Autoimmunity. New England Journal of Medicine, 341(27), 2068–2074. [CrossRef]
- Allen, M., Huang, B. S., Notaras, M. J., Lodhi, A., Barrio-Alonso, E., Lituma, P. J., et al. (2022). Astrocytes derived from ASD individuals alter behavior and destabilize neuronal activity through aberrant Ca2+ signaling. Molecular Psychiatry, 27(5), 2470–2484. [CrossRef]
- Almahayni, O., & Hammond, L. (2024). Does the Wim Hof Method have a beneficial impact on physiological and psychological outcomes in healthy and non-healthy participants? A systematic review. PLOS One, 19(3), e0286933. [CrossRef]
- Alsayouf, H. A. (2024). Growing evidence of pharmacotherapy effectiveness in managing attention-deficit/hyperactivity disorder in young children with or without autism spectrum disorder: a minireview. Frontiers in Psychiatry, 15. [CrossRef]
- American Psychiatric Association, D., & American Psychiatric Association, D. (2013). Diagnostic & statistical manual of mental disorders: DSM-5 (Vol. 5). American Psychiatric Association Washington, DC.
- American Psychiatric Association Staff, & Spitzer, R. L. (1980). Diagnostic & Statistical Manual of Mental Disorders (DSM-III). American Psychiatric Publishing.
- Amor, S., Puentes, F., Baker, D., & Van Der Valk, P. (2010). Inflammation in neurodegenerative diseases. Immunology, 129(2), 154–169. [CrossRef]
- An, J., Liu, Y., Wang, Y., Fan, R., Hu, X., Zhang, F., et al. (2022). The Role of Intestinal Mucosal Barrier in Autoimmune Disease: A Potential Target. Frontiers in Immunology, 13. [CrossRef]
- Anderson, M., Hooker, B. S., & Herbert, M. (2008). Bridging from cells to cognition in autism pathophysiology: biological pathways to defective brain function and plasticity. American Journal of Biochemistry & Biotechnology, 4 (2): 167-176, 4(PNNL-SA-54664). [CrossRef]
- Angajala, A., Lim, S., Phillips, J. B., Kim, J.-H., Yates, C., You, Z., & Tan, M. (2018). Diverse roles of mitochondria in immune responses: novel insights into immuno-metabolism. Frontiers in Immunology, 9, 1605. [CrossRef]
- Angelidou, A., Alysandratos, K.-D., Asadi, S., Zhang, B., Francis, K., Vasiadi, M., et al. (2011). Brief report:“allergic symptoms” in children with autism spectrum disorders. More than meets the eye? Journal of Autism & Developmental Disorders, 41, 1579–1585. [CrossRef]
- Ardalan, M., & Mallard, C. (2024). From hormones to behavior through microglial mitochondrial function. Brain, Behavior, & Immunity, 117, 471–472. [CrossRef]
- Ashwood, P., & Van de Water, J. (2004). A review of autism and the immune response. Clinical & Developmental Immunology, 11(2), 165.
- Asperger, H. (1944). Die „Autistischen psychopathen” im kindesalter. Archiv für Psychiatrie und Nervenkrankheiten, 117(1), 76–136. [CrossRef]
- Azzalini, D., Rebollo, I., & Tallon-Baudry, C. (2019). Visceral Signals Shape Brain Dynamics and Cognition. Trends in Cognitive Sciences, 23(6), 488–509. [CrossRef]
- Bach, J.-F. (2018). The hygiene hypothesis in autoimmunity: the role of pathogens and commensals. Nature Reviews Immunology, 18(2), 105–120. [CrossRef]
- Badimon, L., Vilahur, G., & Padro, T. (2017). Systems biology approaches to understand the effects of nutrition and promote health. British Journal of Clinical Pharmacology, 83(1), 38–45. [CrossRef]
- Balcells, C., Xu, Y., Gil-Solsona, R., Maitre, L., Gago-Ferrero, P., & Keun, H. C. (2024). Blurred lines: Crossing the boundaries between the chemical exposome and the metabolome. Current Opinion in Chemical Biology, 78, 102407. [CrossRef]
- Baquero, F., Coque, T. M., Galán, J. C., & Martinez, J. L. (2021). The Origin of Niches and Species in the Bacterial World. Frontiers in Microbiology, 12. [CrossRef]
- Barbaro, J., & Dissanayake, C. (2009). Autism spectrum disorders in infancy and toddlerhood: a review of the evidence on early signs, early identification tools, and early diagnosis. Journal of Developmental & Behavioral Pediatrics, 30(5), 447–459. [CrossRef]
- Barlattani, T., D’Amelio, C., Cavatassi, A., De Luca, D., Di Stefano, R., Di Berardo, A., et al. (2023). Autism spectrum disorders and psychiatric comorbidities: a narrative review. Journal of Psychopathology. [CrossRef]
- Baron-Cohen, S., Ring, H. A., Bullmore, E. T., Wheelwright, S., Ashwin, C., & Williams, S. C. R. (2000). The amygdala theory of autism. Neuroscience & Biobehavioral Reviews, 24(3), 355–364. [CrossRef]
- Baron-Cohen, S. (2000). Theory of mind and autism: A review. In International Review of Research in Mental Retardation (Vol. 23, pp. 169–184). Academic Press. [CrossRef]
- Baron-Cohen, S. (2004). The cognitive neuroscience of autism. Journal of Neurology, Neurosurgery & Psychiatry, 75(7), 945–948. [CrossRef]
- Basheer, S., Venkataswamy, M. M., Christopher, R., Van Amelsvoort, T., Srinath, S., Girimaji, S. C., & Ravi, V. (2018). Immune aberrations in children with Autism Spectrum Disorder: a case-control study from a tertiary care neuropsychiatric hospital in India. Psychoneuroendocrinology, 94, 162–167. [CrossRef]
- Baumann, N., & Pham-Dinh, D. (2001). Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiological Reviews, 81(2), 871–927. [CrossRef]
- Bauman, M. L. (2010). Medical comorbidities in autism: challenges to diagnosis and treatment. Neurotherapeutics, 7, 320–327. [CrossRef]
- Becker, E. B. E., & Stoodley, C. J. (2013). Chapter One - Autism Spectrum Disorder and the Cerebellum. In G. Konopka (Ed.), International Review of Neurobiology (Vol. 113, pp. 1–34). Academic Press. [CrossRef]
- Bei, R., Masuelli, L., Palumbo, C., Modesti, M., & Modesti, A. (2009). A common repertoire of autoantibodies is shared by cancer and autoimmune disease patients: Inflammation in their induction and impact on tumor growth. Cancer Letters, 281(1), 8–23. [CrossRef]
- Benakis, C., Martin-Gallausiaux, C., Trezzi, J.-P., Melton, P., Liesz, A., & Wilmes, P. (2020). The microbiome-gut-brain axis in acute and chronic brain diseases. Neurobiology of Disease, 61, 1–9. [CrossRef]
- Beopoulos, A., Gea, M., Fasano, A., & Iris, F. (2021). Autonomic nervous system neuroanatomical alterations could provoke and maintain gastrointestinal dysbiosis in autism spectrum disorder (ASD): a novel microbiome–host interaction mechanistic hypothesis. Nutrients, 14(1), 65. [CrossRef]
- Bernard, S., Enayati, A., Redwood, L., Roger, H., & Binstock, T. (2001). Autism: a novel form of mercury poisoning. Medical Hypotheses, 56(4), 462–471. [CrossRef]
- Berthoud, H.-R., & Neuhuber, W. L. (2000). Functional and chemical anatomy of the afferent vagal system. Autonomic Neuroscience: Basic & Clinical, 85(1), 1–17. [CrossRef]
- Bethell, C. D., Kogan, M. D., Strickland, B. B., Schor, E. L., Robertson, J., & Newacheck, P. W. (2011). A national and state profile of leading health problems and health care quality for US children: key insurance disparities and across-state variations. Academic Pediatrics, 11(3), S22–S33. [CrossRef]
- Bhagavati, S. (2021). Autoimmune disorders of the nervous system: pathophysiology, clinical features, and therapy. Frontiers in Neurology, 12, 664664. [CrossRef]
- Bien, C. G., & Elger, C. E. (2007). Limbic encephalitis: A cause of temporal lobe epilepsy with onset in adult life. Epilepsy & Behavior, 10(4), 529–538. [CrossRef]
- Bjørklund, G., Meguid, N. A., El-Bana, M. A., Tinkov, A. A., Saad, K., Dadar, M., et al. (2020). Oxidative Stress in Autism Spectrum Disorder. Molecular Neurobiology, 57(5), 2314–2332. [CrossRef]
- Bjørklund, G., Mkhitaryan, M., Sahakyan, E., Fereshetyan, K., Meguid, N. A., Hemimi, M., et al. (2024). Linking Environmental Chemicals to Neuroinflammation and Autism Spectrum Disorder: Mechanisms and Implications for Prevention. Molecular Neurobiology, 61(9), 6328–6340. [CrossRef]
- Blaxill, M. F., Redwood, L., & Bernard, S. (2004). Thimerosal and autism? A plausible hypothesis that should not be dismissed. Medical Hypotheses, 62(5), 788–794. [CrossRef]
- Bleuler, E. (1911). Dementia praecox: oder Gruppe der Schizophrenien. F. Deuticke.
- Bleuler, E. (1951). Autistic thinking. In Organization & pathology of thought: Selected sources (pp. 399–437). Columbia University Press.
- Bojarskaite, L., Bjørnstad, D. M., Pettersen, K. H., Cunen, C., Hermansen, G. H., & Åbjørsbråten, K. S. (2020). Astrocytic Ca2+ signaling is reduced during sleep and is involved in the regulation of slow wave sleep. Nature Communications, 11(1), 3240. [CrossRef]
- Bordenstein, S. R., & Theis, K. R. (2015). Host Biology in Light of the Microbiome: Ten Principles of Holobionts and Hologenomes. PLOS Biology, 13(8), e1002226. [CrossRef]
- Breece, E., Paciotti, B., Nordahl, C. W., Ozonoff, S., Van de Water, J. A., Rogers, S. J., et al. (2013). Myeloid dendritic cells frequencies are increased in children with autism spectrum disorder and associated with amygdala volume and repetitive behaviors. Inflammation & Mental Health, 31, 69–75. [CrossRef]
- Brincker, M., & Torres, E. B. (2013). Noise from the periphery in autism. Frontiers in Integrative Neuroscience, 7. [CrossRef]
- Brown, A. C., & Mehl-Madrona, L. (2011). Autoimmune and gastrointestinal dysfunctions: does a subset of children with autism reveal a broader connection? Expert Review of Gastroenterology & Hepatology, 5(4), 465–477. [CrossRef]
- Buie, T., Campbell, D. B., Fuchs, G. J., III, Furuta, G. T., Levy, J., VandeWater, J., et al. (2010). Evaluation, Diagnosis, and Treatment of Gastrointestinal Disorders in Individuals with ASDs: A Consensus Report. Pediatrics, 125(Supplement_1), S1–S18. [CrossRef]
- Burnouf, T., & Walker, T. L. (2022). The multifaceted role of platelets in mediating brain function. Blood, 140(8), 815–827. [CrossRef]
- Burns-Naas, L. A., Dearman, R. J., Germolec, D. R., Kaminski, N. E., Kimber, I., Ladics, G. S., et al. (2006). “Omics” Technologies and the Immune System. Toxicology Mechanisms & Methods, 16(2–3), 101–119. [CrossRef]
- Cakir, J., Frye, R. E., & Walker, S. J. (2020). The lifetime social cost of autism: 1990–2029. Research in Autism Spectrum Disorders, 72, 101502. [CrossRef]
- Calçada, D., Vianello, D., Giampieri, E., Sala, C., Castellani, G., de Graaf, A., et al. (2014). The role of low-grade inflammation and metabolic flexibility in aging and nutritional modulation thereof: a systems biology approach. Mechanisms of Ageing & Development, 136, 138–147. [CrossRef]
- Cantando, I., Centofanti, C., D’Alessandro, G., Limatola, C., & Bezzi, P. (2024). Metabolic dynamics in astrocytes and microglia during post-natal development and their implications for autism spectrum disorders. Frontiers in Cellular Neuroscience, 18, 1354259. [CrossRef]
- Carré, A., Chevallier, C., Robel, L., Barry, C., Maria, A.-S., Pouga, L., et al. (2015). Tracking social motivation systems deficits: the affective neuroscience view of autism. Journal of Autism & Developmental Disorders, 45, 3351–3363. [CrossRef]
- CDC. (2021). Managing Chronic Health Conditions. CDC Healthy Schools. https://www.cdc.gov/healthyschools/chronicconditions.htm. Accessed 1 June 2024.
- Cekici, H., & Sanlier, N. (2019). Current nutritional approaches in managing autism spectrum disorder: A review. Nutritional Neuroscience, 22(3), 145–155. [CrossRef]
- Cellot, G., & Cherubini, E. (2014). GABAergic Signaling as Therapeutic Target for Autism Spectrum Disorders. Frontiers in Pediatrics, 2. [CrossRef]
- Chambers, C., & Schaefer, C. (2015). 2.10 - Epilepsy and antiepileptic medications. In C. Schaefer, P. Peters, & R. K. Miller (Eds.), Drugs During Pregnancy and Lactation (Third Edition) (pp. 251–291). San Diego: Academic Press. [CrossRef]
- Chavez, J. A., & Zappaterra, M. (2023). Can Wim Hof Method breathing induce conscious metabolic waste clearance of the brain? Medical Hypotheses, 177, 111118. [CrossRef]
- Chen, C.-C., & Chiu, C.-H. (2022). Current and future applications of fecal microbiota transplantation for children. Biomedical Journal, 45(1), 11–18. [CrossRef]
- Chia, S. L., Kapoor, S., Carvalho, C., Bajénoff, M., & Gentek, R. (2023). Mast cell ontogeny: From fetal development to life-long health and disease. Immunological Reviews, 315(1), 31–53. [CrossRef]
- Chirumbolo, S., & Bjørklund, G. (2017). PERM hypothesis: the fundamental machinery able to elucidate the role of xenobiotics and hormesis in cell survival and homeostasis. International Journal of Molecular Sciences, 18(1), 165. [CrossRef]
- Choi, H., Kim, J. H., Yang, H. S., Kim, J. Y., Cortese, S., Smith, L., et al. (2024). Pharmacological and non-pharmacological interventions for irritability in autism spectrum disorder: a systematic review and meta-analysis with the GRADE assessment. Molecular Autism, 15(1), 7. [CrossRef]
- Ciéslińska, A., Kostyra, E., & Savelkoul, H. F. (2017). Treating autism spectrum disorder with gluten-free and casein-free diet: the underlying microbiota-gut-brain axis mechanisms. HSOA Journal of Clinical Immunology & Immunotherapy, 3.
- Clappison, E., Hadjivassiliou, M., & Zis, P. (2020). Psychiatric Manifestations of Coeliac Disease, a Systematic Review and Meta-Analysis. Nutrients, 12(1). [CrossRef]
- Copf, T. (2016). Impairments in dendrite morphogenesis as etiology for neurodevelopmental disorders and implications for therapeutic treatments. Neuroscience & Biobehavioral Reviews, 68, 946–978. [CrossRef]
- Costello, E. K., Lauber, C. L., Hamady, M., Fierer, N., Gordon, J. I., & Knight, R. (2009). Bacterial Community Variation in Human Body Habitats Across Space and Time. Science, 326(5960), 1694–1697. [CrossRef]
- Coury, D. L., Anagnostou, E., Manning-Courtney, P., Reynolds, A., Cole, L., McCoy, R., et al. (2012). Use of Psychotropic Medication in Children and Adolescents with Autism Spectrum Disorders. Pediatrics, 130(Supplement_2), S69–S76. [CrossRef]
- Crépeaux, G., Authier, F.-J., Exley, C., Luján, L., & Gherardi, R. K. (2020). The role of aluminum adjuvants in vaccines raises issues that deserve independent, rigorous and honest science. Journal of Trace Elements in Medicine & Biology, 62, 126632. [CrossRef]
- Cryan, J. F., O’Riordan, K. J., Cowan, C. S. M., Sandhu, K. V., Bastiaanssen, T. F. S., Boehme, M., et al. (2019). The Microbiota-Gut-Brain Axis. Physiological Reviews, 99(4), 1877–2013. [CrossRef]
- Csaba, G. (2014). Hormones in the immune system and their possible role. A critical review. Acta Microbiologica et Immunologica Hungarica, 61(3), 241–260. [CrossRef]
- Cutolo, M., Capellino, S., Sulli, A., Serioli, B., Secchi, M. E., Villaggio, B., & Straub, R. H. (2006). Estrogens and autoimmune diseases. Annals of the New York Academy of Sciences, 1089(1), 538–547. [CrossRef]
- Cwik, J. C. (2021). Spiritual needs of people with autism spectrum disorder. In Spiritual Needs in Research and Practice: The Spiritual Needs Questionnaire as a Global Resource for Health and Social Care (pp. 265–280). Springer.
- Cruz-Machado, S. S., Campos, L. M. G., Fadini, C. C., Anderson, G., Markus, R. P., & Pinato, L. (2021). Disrupted nocturnal melatonin in autism: Association with tumor necrosis factor and sleep disturbances. Journal of Pineal Research, 70(3), e12715. [CrossRef]
- Daëron, M. (2022). The immune system as a system of relations. Frontiers in Immunology, 13, 984678. [CrossRef]
- D’Agostino, P. M., Gottfried-Blackmore, A., Anandasabapathy, N., & Bulloch, K. (2012). Brain dendritic cells: biology and pathology. Acta Neuropathologica, 124(5), 599–614. [CrossRef]
- Daly, E., D. Tricklebank, M., & Wichers, R. (2019). Chapter Two - Neurodevelopmental roles and the serotonin hypothesis of autism spectrum disorder. In M. D. Tricklebank & E. Daly (Eds.), The Serotonin System (pp. 23–44). Academic Press. [CrossRef]
- Davenport, P., & Sola-Visner, M. (2022). Platelets in the neonate: Not just a small adult. Research & Practice in Thrombosis & Haemostasis, 6(3), e12719. [CrossRef]
- Davies, S., Bishop, D., Manstead, A. S., & Tantam, D. (1994). Face perception in children with autism and Asperger’s syndrome. Journal of Child Psychology & Psychiatry, 35(6), 1033–1057. [CrossRef]
- D’Adamo, C. R., Nelson, J. L., Miller, S. N., Rickert Hong, M., Lambert, E., & Tallman Ruhm, H. (2024). Reversal of Autism Symptoms among Dizygotic Twins through a Personalized Lifestyle and Environmental Modification Approach: A Case Report and Review of the Literature. Journal of Personalized Medicine, 14(6), 641. [CrossRef]
- D’Angelo, E. (2018). Physiology of the cerebellum. Handbook of Clinical Neurology, 154, 85–108. [CrossRef]
- De Giorgio, R., Guerrini, S., Barbara, G., Stanghellini, V., De Ponti, F., Corinaldesi, R., et al. (2004). Inflammatory neuropathies of the enteric nervous system. Gastroenterology, 126(7), 1872–1883. [CrossRef]
- Delafield-Butt, J., & Ciaunica, A. (2024). The sensorimotor foundations of bodily self-consciousness in utero. [CrossRef]
- Dempsey, J. L., Little, M., & Cui, J. Y. (2019). Gut microbiome: An intermediary to neurotoxicity. NeuroToxicology, 75, 41–69. [CrossRef]
- Deng, W., Yi, P., Xiong, Y., Ying, J., Lin, Y., Dong, Y., et al. (2024). Gut Metabolites Acting on the Gut-Brain Axis: Regulating the Functional State of Microglia. Aging & Disease, 15(2), 480.
- Denman, A. M. (1991). Sex hormones, autoimmune diseases, and immune responses. BMJ, 303(6793), 2–3. [CrossRef]
- Deth, R. C. (2013). Autism: A redox/methylation disorder. Global Advances in Health & Medicine, 2(6), 68–73. [CrossRef]
- Dethlefsen, L., McFall-Ngai, M., & Relman, D. A. (2007). An ecological and evolutionary perspective on human–microbe mutualism and disease. Nature, 449(7164), 811–818. [CrossRef]
- Di Liberto, D., D’Anneo, A., Carlisi, D., Emanuele, S., De Blasio, A., Calvaruso, G., et al. (2020). Brain Opioid Activity and Oxidative Injury: Different Molecular Scenarios Connecting Celiac Disease and Autistic Spectrum Disorder. Brain Sciences, 10(7). [CrossRef]
- Dietz, P. M., Rose, C. E., McArthur, D., & Maenner, M. (2020). National and state estimates of adults with autism spectrum disorder. Journal of Autism & Developmental Disorders, 50, 4258–4266. [CrossRef]
- Dimmeler, S., Ding, S., Rando, T. A., & Trounson, A. (2014). Translational strategies and challenges in regenerative medicine. Nature Medicine, 20(8), 814–821. [CrossRef]
- D’Mello, A. M., & Stoodley, C. J. (2015). Cerebro-cerebellar circuits in autism spectrum disorder. Frontiers in Neuroscience, 9. [CrossRef]
- Doherty, M., Foley, K.-R., & Schloss, J. (2024). Complementary and Alternative Medicine for Autism – A Systematic Review. Journal of Autism & Developmental Disorders. [CrossRef]
- Donald, K., & Finlay, B. B. (2023). Early-life interactions between the microbiota and immune system: impact on immune system development and atopic disease. Nature Reviews Immunology, 23(11), 735–748. [CrossRef]
- Dong, Y., & Benveniste, E. N. (2001). Immune function of astrocytes. Glia, 36(2), 180–190. [CrossRef]
- Dukhinova, M., Kuznetsova, I., Kopeikina, E., Veniaminova, E., Yung, A. W. Y., Veremeyko, T., et al. (2018). Platelets mediate protective neuroinflammation and promote neuronal plasticity at the site of neuronal injury. Brain, Behavior, and Immunity, 74, 7–27. [CrossRef]
- Ebrahimi M, S., Rostam-Abadi, Y., & Rezaei, N. (2021). Autism spectrum disorders and natural killer cells: a review on pathogenesis and treatment. Expert Review of Clinical Immunology, 17(1), 27–35. [CrossRef]
- Edmiston, E., Ashwood, P., & Van de Water, J. (2017). Autoimmunity, Autoantibodies, and Autism Spectrum Disorder. Biological Psychiatry, 81(5), 383–390. [CrossRef]
- El-Ansary, A., Bhat, R. S., & Zayed, N. (2020). Gut Microbiome and Sex Bias in Autism Spectrum Disorders. Current Behavioral Neuroscience Reports, 7(1), 22–31. [CrossRef]
- Enstrom, A. M., Lit, L., Onore, C. E., Gregg, J. P., Hansen, R. L., Pessah, I. N., et al. (2009). Altered gene expression and function of peripheral blood natural killer cells in children with autism. Brain, Behavior, & Immunity, 23(1), 124–133. [CrossRef]
- Epel, E. S., McEwen, B. S., & Ickovics, J. R. (1998). Embodying Psychological Thriving: Physical Thriving in Response to Stress. Journal of Social Issues, 54(2), 301–322. [CrossRef]
- Estes, M. L., & McAllister, A. K. (2016). Maternal immune activation: Implications for neuropsychiatric disorders. Science, 353(6301), 772–777. [CrossRef]
- Exley, C. (2013). Human exposure to aluminium. Environmental Science: Processes & Impacts, 15(10), 1807–1816. [CrossRef]
- Exley, C., & Clarkson, E. (2020). Aluminium in human brain tissue from donors without neurodegenerative disease: A comparison with Alzheimer’s disease, multiple sclerosis and autism. Scientific Reports, 10(1), 7770. [CrossRef]
- Faas, M., & De Vos, P. (2020). Mitochondrial function in immune cells in health and disease. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease, 1866(10), 165845. [CrossRef]
- Hamidreza, F., & Morteza, K. (2018). Overview of the Recent Advances in Pathophysiology and Treatment for Autism. (Formerly Current Drug Targets - CNS & Neurological Disorders), 17(8), 590–594. [CrossRef]
- Fan, G., Ma, J., Ma, R., Suo, M., Chen, Y., Zhang, S., et al. (2023). Microglia modulate neurodevelopment in autism spectrum disorder and schizophrenia. International Journal of Molecular Sciences, 24(24), 17297. [CrossRef]
- Farmer, C. A., Thurm, A. E., Honnekeri, B., Kim, P., Swedo, S. E., & Han, J. C. (2021). The contribution of platelets to peripheral BDNF elevation in children with autism spectrum disorder. Scientific Reports, 11(1), 18158. [CrossRef]
- Fasano, A. (2012). Leaky Gut and Autoimmune Diseases. Clinical Reviews in Allergy & Immunology, 42(1), 71–78. [CrossRef]
- Fatemi, S. H., Halt, A. R., Realmuto, G., Earle, J., Kist, D. A., Thuras, P., & Merz, A. (2002). Purkinje Cell Size Is Reduced in Cerebellum of Patients with Autism. Cellular & Molecular Neurobiology, 22(2), 171–175. [CrossRef]
- Ferri, S. L., Abel, T., & Brodkin, E. S. (2018). Sex Differences in Autism Spectrum Disorder: a Review. Current Psychiatry Reports, 20(2), 9. [CrossRef]
- Filiano, A. J., Xu, Y., Tustison, N. J., Marsh, R. L., Baker, W., Smirnov, I., et al. (2016). Unexpected role of interferon-γ in regulating neuronal connectivity and social behaviour. Nature, 535(7612), 425–429. [CrossRef]
- Filipek, P. A., Juranek, J., Nguyen, M. T., Cummings, C., & Gargus, J. J. (2004). Relative carnitine deficiency in autism. Journal of Autism and Developmental Disorders, 34, 615–623. [CrossRef]
- Fincham, G. W., Strauss, C., Montero-Marin, J., & Cavanagh, K. (2023). Effect of breathwork on stress and mental health: A meta-analysis of randomised-controlled trials. Scientific Reports, 13(1), 432. [CrossRef]
- Fleming, D. (1984). Walter B. Cannon and Homeostasis. Social Research, 51(3), 609–640. http://www.jstor.org/stable/40970955.
- Folstein, S., & Rutter, M. (1977). Genetic influences and infantile autism. Nature, 265(5596), 726–728. [CrossRef]
- Forbes, J. D., Van Domselaar, G., & Bernstein, C. N. (2016). The Gut Microbiota in Immune-Mediated Inflammatory Diseases. Frontiers in Microbiology, 7. [CrossRef]
- Fraguas, D., Díaz-Caneja, C., Pina-Camacho, L., Moreno, C., Durán Cutilla, M., Ayora, M., et al. (2019). Dietary Interventions for Autism Spectrum Disorder: A Meta-analysis. Pediatrics, 144, e20183218. [CrossRef]
- Frith, U. (1994). Autism and theory of mind in everyday life. Social Development, 3(2), 108–124. [CrossRef]
- Frith, U., & Happé, F. (1994). Autism: beyond “theory of mind.” Cognition, 50(1), 115–132. [CrossRef]
- Frith, U., & Happé, F. (1999). Theory of Mind and Self-Consciousness: What Is It Like to Be Autistic? Mind & Language, 14(1), 82–89. [CrossRef]
- Frye, R. E., Rincon, N., McCarty, P. J., Brister, D., Scheck, A. C., & Rossignol, D. A. (2024). Biomarkers of mitochondrial dysfunction in autism spectrum disorder: A systematic review and meta-analysis. Neurobiology of Disease, 106520. [CrossRef]
- Fu, J., Schroder, K., & Wu, H. (2024). Mechanistic insights from inflammasome structures. Nature Reviews Immunology, 24(7), 518–535. [CrossRef]
- Fuller, R., Rahona, E., Fisher, S., Caravanos, J., Webb, D., Kass, D., et al. (2018). Pollution and non-communicable disease: time to end the neglect. The Lancet Planetary Health, 2(3), e96–e98. [CrossRef]
- Furness, J. B., Callaghan, B. P., Rivera, L. R., & Cho, H.-J. (2014). The Enteric Nervous System and Gastrointestinal Innervation: Integrated Local and Central Control. In M. Lyte & J. F. Cryan (Eds.), Microbial Endocrinology: The Microbiota-Gut-Brain Axis in Health and Disease (pp. 39–71). New York, NY: Springer New York. [CrossRef]
- Gable, M. S., Sheriff, H., Dalmau, J., Tilley, D. H., & Glaser, C. A. (2012). The Frequency of Autoimmune N-Methyl-D-Aspartate Receptor Encephalitis Surpasses That of Individual Viral Etiologies in Young Individuals Enrolled in the California Encephalitis Project. Clinical Infectious Diseases, 54(7), 899–904. [CrossRef]
- Gabriele, S., Sacco, R., & Persico, A. M. (2014). Blood serotonin levels in autism spectrum disorder: A systematic review and meta-analysis. European Neuropsychopharmacology, 24(6), 919–929. [CrossRef]
- Galvez-Contreras, A. Y., Zarate-Lopez, D., Torres-Chavez, A. L., & Gonzalez-Perez, O. (2020). Role of oligodendrocytes and myelin in the pathophysiology of autism spectrum disorder. Brain Sciences, 10(12), 951. [CrossRef]
- Garg, D., Mohammad, S. S., & Sharma, S. (2020). Autoimmune Encephalitis in Children: An Update. Indian Pediatrics, 57(7), 662–670. [CrossRef]
- Gaugler, T., Klei, L., Sanders, S. J., Bodea, C. A., Goldberg, A. P., Lee, A. B., et al. (2014). Most genetic risk for autism resides with common variation. Nature Genetics, 46(8), 881–885. [CrossRef]
- Geier, D. A., Kern, J. K., & Geier, M. R. (2010). The biological basis of autism spectrum disorders: Understanding causation and treatment by clinical geneticists. Acta neurobiologiae experimentalis, 70(2), 209–226. [CrossRef]
- Geier, D. A., King, P. G., Hooker, B. S., Dórea, J. G., Kern, J. K., Sykes, L. K., & Geier, M. R. (2015). Thimerosal: clinical, epidemiologic and biochemical studies. Clinica Chimica Acta, 444, 212–220. [CrossRef]
- Geng, Z.-H., Zhu, Y., Li, Q.-L., Zhao, C., & Zhou, P.-H. (2022). Enteric Nervous System: The Bridge Between the Gut Microbiota and Neurological Disorders. Frontiers in Aging Neuroscience, 14. [CrossRef]
- Genuis, S. J. (2012). What′s Out There Making Us Sick? Journal of Environmental & Public Health, 2012(1), 605137. [CrossRef]
- Gershon, M. D. (1999). The Enteric Nervous System: A Second Brain. Hospital Practice, 34(7), 31–52. [CrossRef]
- Gershon, M. D. (2022). The Shaggy Dog Story of Enteric Signaling: Serotonin, a Molecular Megillah. In N. J. Spencer, M. Costa, & S. M. Brierley (Eds.), The Enteric Nervous System II (pp. 307–318). Cham: Springer International Publishing.
- Ghanizadeh, A., Akhondzadeh, S., Hormozi, M., Makarem, A., Abotorabi-Zarchi, M., & Firoozabadi, A. (2012). Glutathione-related factors and oxidative stress in autism, a review. Current Medicinal Chemistry, 19(23), 4000–4005. [CrossRef]
- Ghaziuddin, M., & Al-Owain, M. (2013). Autism spectrum disorders and inborn errors of metabolism: an update. Pediatric Neurology, 49(4), 232–236. [CrossRef]
- Ghosh, A., Michalon, A., Lindemann, L., Fontoura, P., & Santarelli, L. (2013). Drug discovery for autism spectrum disorder: challenges and opportunities. Nature Reviews Drug Discovery, 12(10), 777–790. [CrossRef]
- Gianchecchi, E., Delfino, D. V., & Fierabracci, A. (2018). NK cells in autoimmune diseases: Linking innate and adaptive immune responses. Autoimmunity Reviews, 17(2), 142–154. [CrossRef]
- Gładysz, D., Krzywdzińska, A., & Hozyasz, K. K. (2018). Immune abnormalities in autism spectrum disorder—could they hold promise for causative treatment? Molecular Neurobiology, 55, 6387–6435. [CrossRef]
- Gobin, V., Van Steendam, K., Denys, D., & Deforce, D. (2014). Selective serotonin reuptake inhibitors as a novel class of immunosuppressants. International Immunopharmacology, 20(1), 148–156. [CrossRef]
- Goldberg, W. A., Osann, K., Filipek, P. A., Laulhere, T., Jarvis, K., Modahl, C., et al. (2003). Language and other regression: assessment and timing. Journal of Autism & Developmental Disorders, 33, 607–616. [CrossRef]
- Goldman, A. W., Burmeister, Y., Cesnulevicius, K., Herbert, M., Kane, M., Lescheid, D., et al. (2015). Bioregulatory systems medicine: an innovative approach to integrating the science of molecular networks, inflammation, and systems biology with the patient’s autoregulatory capacity? Frontiers in Physiology, 6. [CrossRef]
- Goubau, C., Buyse, G. M., Van Geet, C., & Freson, K. (2014). The contribution of platelet studies to the understanding of disease mechanisms in complex and monogenetic neurological disorders. Developmental Medicine & Child Neurology, 56(8), 724–731. [CrossRef]
- Graeber, M. B., & Streit, W. J. (2010). Microglia: biology and pathology. Acta Neuropathologica, 119, 89–105. [CrossRef]
- Graeber, M. B., & Stre’rt, W. J. (1990). Microglia: immune network in the CNS. Brain Pathology, 1(1), 2–5. [CrossRef]
- Green, D. J., Park, K., Bhatt-Mehta, V., Snyder, D., & Burckart, G. J. (2021). Regulatory Considerations for the Mother, Fetus and Neonate in Fetal Pharmacology Modeling. Frontiers in Pediatrics, 9. [CrossRef]
- Greenberg, G., & Tobach, E. (2014). Behavioral Evolution & Integrative Levels: The T.C. Schneirla Conferences Series, Volume 1. Taylor & Francis.
- Gropman, A., & Sadle, C. J. (2024). Epigenetics of autism spectrum disorder. In Neuropsychiatric Disorders & Epigenetics (pp. 81–102). Elsevier. [CrossRef]
- Gupta, N., & Gupta, M. (2024). Off-label psychopharmacological interventions for autism spectrum disorders: strategic pathways for clinicians. CNS Spectrums, 29(1), 10–25. [CrossRef]
- Gzielo, K., & Nikiforuk, A. (2021). Astroglia in Autism Spectrum Disorder. International Journal of Molecular Sciences, 22(21). [CrossRef]
- Hacohen, Y., Wright, S., Gadian, J., Vincent, A., Lim, M., Wassmer, E., & Lin, J.-P. (2016). N-methyl-d-aspartate (NMDA) receptor antibodies encephalitis mimicking an autistic regression. Developmental Medicine & Child Neurology, 58(10), 1092–1094. [CrossRef]
- Hafizi, S., Tabatabaei, D., & Lai, M.-C. (2019). Review of Clinical Studies Targeting Inflammatory Pathways for Individuals With Autism. Frontiers in Psychiatry, 10. [CrossRef]
- Hallmayer, J., Cleveland, S., Torres, A., Phillips, J., Cohen, B., Torigoe, T., et al. (2011). Genetic heritability and shared environmental factors among twin pairs with autism. Archives of General Psychiatry, 68(11), 1095–1102. [CrossRef]
- Hameroff, S. (2010). The “conscious pilot”—dendritic synchrony moves through the brain to mediate consciousness. Journal of Biological Physics, 36(1), 71–93. [CrossRef]
- Hampe, C. S., & Mitoma, H. (2022). A Breakdown of Immune Tolerance in the Cerebellum. Brain Sciences, 12(3). [CrossRef]
- Han, A., Peng, T., Xie, Y., Zhang, W., Sun, W., Xie, Y., et al. (2023). Mitochondrial-regulated Tregs: potential therapeutic targets for autoimmune diseases of the central nervous system. Frontiers in Immunology, 14. [CrossRef]
- Hanaford, A., & Johnson, S. C. (2022). The immune system as a driver of mitochondrial disease pathogenesis: a review of evidence. Orphanet Journal of Rare Diseases, 17(1), 335. [CrossRef]
- Happé, F. G. E. (1994). Wechsler IQ Profile and Theory of Mind in Autism: A Research Note. Journal of Child Psychology & Psychiatry, 35(8), 1461–1471. [CrossRef]
- Hardy, D. (2022). Autoimmune Encephalitis in Children. Pediatric Neurology, 132, 56–66. [CrossRef]
- Harkey, J. (1983). The epidemiology of selected chronic childhood health conditions. Children’s Health Care, 12(2), 62. [CrossRef]
- Harris, J. (2018). Leo Kanner and autism: a 75-year perspective. International Review of Psychiatry, 30(1), 3–17. [CrossRef]
- Haruwaka, K., Ikegami, A., Tachibana, Y., Ohno, N., Konishi, H., Hashimoto, A., et al. (2019). Dual microglia effects on blood brain barrier permeability induced by systemic inflammation. Nature Communications, 10(1), 5816. [CrossRef]
- Hasib, R. A., Ali, Md. C., Rahman, M. H., Ahmed, S., Sultana, S., Summa, S. Z., et al. (2023). Integrated gene expression profiling and functional enrichment analyses to discover biomarkers and pathways associated with Guillain-Barré syndrome and autism spectrum disorder to identify new therapeutic targets. Journal of Biomolecular Structure and Dynamics, 1–23. [CrossRef]
- Hawgood, S., Hook-Barnard, I. G., O’Brien, T. C., & Yamamoto, K. R. (2015). Precision medicine: Beyond the inflection point. Science Translational Medicine, 7(300), 300ps17-300ps17. [CrossRef]
- Hazan, S., Spradling-Reeves, K. D., Papoutsis, A., & Walker, S. J. (2020). Shotgun metagenomic sequencing identifies dysbiosis in triplet sibling with gastrointestinal symptoms and ASD. Children, 7(12), 255. [CrossRef]
- Heberling, C. A., Dhurjati, P. S., & Sasser, M. (2013). Hypothesis for a systems connectivity model of autism spectrum disorder pathogenesis: Links to gut bacteria, oxidative stress, and intestinal permeability. Medical Hypotheses, 80(3), 264–270. [CrossRef]
- Heemskerk, V. H., Daemen, M. A., & Buurman, W. A. (1999). Insulin-like growth factor-1 (IGF-1) and growth hormone (GH) in immunity and inflammation. Cytokine & Growth Factor Reviews, 10(1), 5–14. [CrossRef]
- Heiss, C. N., & Olofsson, L. E. (2019). The role of the gut microbiota in development, function and disorders of the central nervous system and the enteric nervous system. Journal of Neuroendocrinology, 31(5), e12684. [CrossRef]
- Hendriksen, E., van Bergeijk, D., Oosting, R. S., & Redegeld, F. A. (2017). Mast cells in neuroinflammation and brain disorders. Neuroscience & Biobehavioral Reviews, 79, 119–133. [CrossRef]
- Heneka, M. T., Carson, M. J., El Khoury, J., Landreth, G. E., Brosseron, F., Feinstein, D. L., et al. (2015). Neuroinflammation in Alzheimer’s disease. The Lancet Neurology, 14(4), 388–405. [CrossRef]
- Heppner, F. L., Ransohoff, R. M., & Becher, B. (2015). Immune attack: the role of inflammation in Alzheimer disease. Nature Reviews Neuroscience, 16(6), 358–372. [CrossRef]
- Herberman, R. B., & Ortaldo, J. R. (1981). Natural killer cells: their roles in defenses against disease. Science, 214(4516), 24–30. [CrossRef]
- Herbert, M. R. (2012). 10 Autism: From Static Genetic Brain Defect to Dynamic Gene-Environment-Modulated Pathophysiology. In Genetic explanations: Sense & nonsense (pp. 122–146). Harvard University Press.
- Herbert, M. R. (2014). Translational implications of a whole-body approach to brain health in autism: how transduction between metabolism and electrophysiology points to mechanisms for neuroplasticity. In Frontiers in Autism Research: New Horizons for Diagnosis & Treatment, 515–556. [CrossRef]
- Herbert, M. R., & Sage, C. (2013a). Autism and EMF? Plausibility of a pathophysiological link – Part I. Pathophysiology, 20(3), 191–209. [CrossRef]
- Herbert, M. R., & Sage, C. (2013b). Autism and EMF? Plausibility of a pathophysiological link part II. Pathophysiology, 20(3), 211–234. [CrossRef]
- Herbert, M., & Weintraub, K. (2013). The Autism Revolution: Whole-Body Strategies for Making Life All It Can Be. Random House Publishing Group.
- Hertz-Picciotto, I., Schmidt, R. J., & Krakowiak, P. (2018). Understanding environmental contributions to autism: Causal concepts and the state of science. Autism Research, 11(4), 554–586. [CrossRef]
- Hickey, A., Crabtree, J., & Stott, J. (2018). ‘Suddenly the first fifty years of my life made sense’: Experiences of older people with autism. Autism, 22(3), 357–367. [CrossRef]
- Higdon, R., Earl, R. K., Stanberry, L., Hudac, C. M., Montague, E., Stewart, E., et al. (2015). The Promise of Multi-Omics and Clinical Data Integration to Identify and Target Personalized Healthcare Approaches in Autism Spectrum Disorders. OMICS: A Journal of Integrative Biology, 19(4), 197–208. [CrossRef]
- Hill, E. L. (2004a). Executive dysfunction in autism. Trends in Cognitive Sciences, 8(1), 26–32. [CrossRef]
- Hill, E. L. (2004b). Evaluating the theory of executive dysfunction in autism. Developmental Review, 24(2), 189–233. [CrossRef]
- Hill, K. (1990). The decline of childhood mortality.
- Hiller-Sturmhöfel, S., & Bartke, A. (1998). The endocrine system - An overview. Alcohol health & Research World, 22, 153–64.
- Hirahara, K., & Nakayama, T. (2016). CD4+ T-cell subsets in inflammatory diseases: beyond the T h 1/T h 2 paradigm. International Immunology, 28(4), 163–171. [CrossRef]
- Ho, P., & Ross, D. A. (2017). More than a gut feeling: the implications of the gut microbiota in psychiatry. Biological Psychiatry, 81(5), e35–e37. [CrossRef]
- Hooker, B., Kern, J., Geier, D., Haley, B., Sykes, L., King, P., & Geier, M. (2014). Methodological issues and evidence of malfeasance in research purporting to show Thimerosal in vaccines is safe. BioMed Research International, 2014(1), 247218. [CrossRef]
- Horlin, C., Falkmer, M., Parsons, R., Albrecht, M. A., & Falkmer, T. (2014). The cost of autism spectrum disorders. PLOS One, 9(9), e106552. [CrossRef]
- Hridi, S. U., Barbour, M., Wilson, C., Franssen, A. J., Harte, T., Bushell, T. J., & Jiang, H.-R. (2021). Increased Levels of IL-16 in the Central Nervous System during Neuroinflammation Are Associated with Infiltrating Immune Cells and Resident Glial Cells. Biology, 10(6). [CrossRef]
- Huang, J., Li, X., Chen, Z., Zou, L., Healy, S., Tse, C. Y. A., & Li, C. (2023). Effects of Mind-Body Exercises on Health-related Outcomes in Children and Adolescents with Autism Spectrum Disorder: A Systematic Review. Review Journal of Autism & Developmental Disorders. [CrossRef]
- Hughes, H. K., Mills Ko, E., Rose, D., & Ashwood, P. (2018). Immune dysfunction and autoimmunity as pathological mechanisms in autism spectrum disorders. Frontiers in Cellular Neuroscience, 12, 405. [CrossRef]
- Hughes, H. K., Rose, D., & Ashwood, P. (2018). The gut microbiota and dysbiosis in autism spectrum disorders. Current Neurology & Neuroscience Reports, 18, 1–15. [CrossRef]
- Hurley-Hanson, A. E., Giannantonio, C. M., Griffiths, A. J., Hurley-Hanson, A. E., Giannantonio, C. M., & Griffiths, A. J. (2020). The costs of autism. Springer. [CrossRef]
- Hussaini, S. M., & Jang, M. H. (2018). New roles for old glue: astrocyte function in synaptic plasticity and neurological disorders. International Neurourology Journal, 22(Suppl 3), S106.
- Ichim, T. E., Solano, F., Glenn, E., Morales, F., Smith, L., Zabrecky, G., & Riordan, N. H. (2007). Stem Cell Therapy for Autism. Journal of Translational Medicine, 5(1), 30. [CrossRef]
- Irwin, J. K., MacSween, J., & Kerns, K. A. (2011). History and evolution of the autism spectrum disorders. In International Handbook of Autism & Pervasive Developmental disorders (pp. 3–16). Springer.
- Jaga, K., & Dharmani, C. (2007). The interrelation between organophosphate toxicity and the epidemiology of depression and suicide. Reviews on Environmental Health, 22(1), 57–74. [CrossRef]
- Jarrold, C., & Russell, J. (1997). Counting Abilities in Autism: Possible Implications for Central Coherence Theory. Journal of Autism & Developmental Disorders, 27(1), 25–37. [CrossRef]
- Jaswal, V. K., Wayne, A., & Golino, H. (2020). Eye-tracking reveals agency in assisted autistic communication. Scientific reports, 10(1), 7882. [CrossRef]
- Jaswal, V. K., Lampi, A. J., & Stockwell, K. M. (2024). Literacy in nonspeaking autistic people. Autism, 13623613241230709. [CrossRef]
- Jenne, C. N., Urrutia, R., & Kubes, P. (2013). Platelets: bridging hemostasis, inflammation, and immunity. International Journal of Laboratory Hematology, 35(3), 254–261. [CrossRef]
- Jiang, N. M., Cowan, M., Moonah, S. N., & Petri, W. A. (2018). The impact of systemic inflammation on neurodevelopment. Trends in Molecular Medicine, 24(9), 794–804. [CrossRef]
- Jones, J. P., Williamson, L., Konsoula, Z., Anderson, R., Reissner, K. J., & Parker, W. (2024). Evaluating the Role of Susceptibility Inducing Cofactors and of Acetaminophen in the Etiology of Autism Spectrum Disorder. Life, 14(8). [CrossRef]
- Jung, C., Hugot, J.-P., & Barreau, F. (2010). Peyer′s Patches: The Immune Sensors of the Intestine. International Journal of Inflammation, 2010(1), 823710. [CrossRef]
- Jyonouchi, H. (2009). Food allergy and autism spectrum disorders: Is there a link? Current Allergy and Asthma Reports, 9(3), 194–201. [CrossRef]
- Jyonouchi, H. (2024). Autism spectrum disorder and a possible role of anti-inflammatory treatments: experience in the pediatric allergy/immunology clinic. Frontiers in Psychiatry, 15. [CrossRef]
- Kang, D.-W., Adams, J. B., Coleman, D. M., Pollard, E. L., Maldonado, J., McDonough-Means, S., et al. (2019). Long-term benefit of Microbiota Transfer Therapy on autism symptoms and gut microbiota. Scientific Reports, 9(1), 5821. [CrossRef]
- Kanner, L. (1943). Autistic disturbances of affective contact. Nervous Child, 2(3), 217–250.
- Karhu, E., Zukerman, R., Eshraghi, R. S., Mittal, J., Deth, R. C., Castejon, A. M., et al. (2020). Nutritional interventions for autism spectrum disorder. Nutrition Reviews, 78(7), 515–531. [CrossRef]
- Karl, J. P., Hatch, A. M., Arcidiacono, S. M., Pearce, S. C., Pantoja-Feliciano, I. G., Doherty, L. A., & Soares, J. W. (2018). Effects of Psychological, Environmental and Physical Stressors on the Gut Microbiota. Frontiers in Microbiology, 9. [CrossRef]
- Karlsson, O. (2023). Chemical safety and the exposome. Emerging Contaminants, 9(2), 100225. [CrossRef]
- Kaur, I., Behl, T., Aleya, L., Rahman, M. H., Kumar, A., Arora, S., & Akter, R. (2021). Role of metallic pollutants in neurodegeneration: effects of aluminum, lead, mercury, and arsenic in mediating brain impairment events and autism spectrum disorder. Environmental Science & Pollution Research, 28, 8989–9001. [CrossRef]
- Kayser, M. S., & Dalmau, J. (2016). Anti-NMDA receptor encephalitis, autoimmunity, and psychosis. Oxidative Stress & Inflammation in Schizophrenia, 176(1), 36–40. [CrossRef]
- Keating, B. A., Lees, J. G., & Moalem-Taylor, G. (2019). The Roles of Regulatory T Cells in Central Nervous System Autoimmunity. In H. Mitoma & M. Manto (Eds.), Neuroimmune Diseases: From Cells to the Living Brain (pp. 167–193). Cham: Springer International Publishing. [CrossRef]
- Keller, A., Rimestad, M. L., Friis Rohde, J., Holm Petersen, B., Bruun Korfitsen, C., Tarp, S., et al. (2021). The Effect of a Combined Gluten- and Casein-Free Diet on Children and Adolescents with Autism Spectrum Disorders: A Systematic Review and Meta-Analysis. Nutrients, 13(2). [CrossRef]
- Kelly, L. S., Apple, C. G., Gharaibeh, R., Pons, E. E., Thompson, C. W., Kannan, K. B., et al. (2021). Stress-related changes in the gut microbiome after trauma. Journal of Trauma & Acute Care Surgery, 91(1).
- Kepser, L.-J., & Homberg, J. R. (2015). The neurodevelopmental effects of serotonin: A behavioural perspective. Special Issue: Serotonin, 277, 3–13. [CrossRef]
- Kern, J., Geier, D., Audhya, T., King, P., Sykes, L., & Geier, M. (2012). Evidence of parallels between mercury intoxication and the brain pathology in autism. Acta Neurobiologiae Experimentalis, 72(2), 113–153. [CrossRef]
- Kern, J. K., Geier, D. A., Deth, R. C., Sykes, L. K., Hooker, B. S., Love, J. M., et al. (2017). Systematic assessment of research on autism spectrum disorder (ASD) and mercury reveals conflicts of interest and the need for transparency in autism research. Science & Engineering ethics, 23, 1691–1718. [CrossRef]
- Kern, J. K., Geier, D. A., Sykes, L. K., & Geier, M. R. (2016). Relevance of Neuroinflammation and Encephalitis in Autism. Frontiers in Cellular Neuroscience, 9. [CrossRef]
- Khachadourian, V., Mahjani, B., Sandin, S., Kolevzon, A., Buxbaum, J. D., Reichenberg, A., & Janecka, M. (2023). Comorbidities in autism spectrum disorder and their etiologies. Translational Psychiatry, 13(1), 71. [CrossRef]
- Khan, M. F., & Wang, G. (2018). Environmental agents, oxidative stress and autoimmunity. Oxidative Toxicology: Role of Reactive Oxygen Species (ROS) in Health & Disease: Mechanisms, Target Organ Toxicities, & Biomarkers, 7, 22–27. [CrossRef]
- Kharrazian, D., Herbert, M., & Lambert, J. (2023). The Relationships between Intestinal Permeability and Target Antibodies for a Spectrum of Autoimmune Diseases. International Journal of Molecular Sciences, 24(22). [CrossRef]
- Khetrapal, N. (2008). The framework for disturbed affective consciousness in autism. Neuropsychiatric Disease & Treatment, 4(3), 531–533.
- Khundakji, Y., Masri, A., & Khuri-Bulos, N. (2018). Anti-NMDA receptor encephalitis in a toddler: A diagnostic challenge. International Journal of Pediatrics & Adolescent Medicine, 5(2). [CrossRef]
- Kim, D.-Y., & Camilleri, M. (2000). Serotonin: A Mediator of The Brain–Gut Connection. Official journal of the American College of Gastroenterology ACG, 95(10). [CrossRef]
- Kim, H., Cho, M., Shim, W., Kim, J., Jeon, E., Kim, D., & Yoon, S. (2017). Deficient autophagy in microglia impairs synaptic pruning and causes social behavioral defects. Molecular psychiatry, 22(11), 1576–1584. [CrossRef]
- King, B. H., Hollander, E., Sikich, L., McCracken, J. T., Scahill, L., Bregman, J. D., et al. (2009). Lack of efficacy of citalopram in children with autism spectrum disorders and high levels of repetitive behavior: citalopram ineffective in children with autism. Archives of General Psychiatry, 66(6), 583–590. [CrossRef]
- Kissane, D. W. (2012). The relief of existential suffering. Archives of Internal Medicine, 172(19), 1501–1505. [CrossRef]
- Kleine, B., & Rossmanith, W. (2016). Hormones & the Endocrine System. [CrossRef]
- Knapp, M., Romeo, R., & Beecham, J. (2009). Economic cost of autism in the UK. Autism, 13(3), 317–336. [CrossRef]
- Knudsen, G. P. (2009). Gender bias in autoimmune diseases: X chromosome inactivation in women with multiple sclerosis. Journal of the Neurological Sciences, 286(1), 43–46. [CrossRef]
- Knuesel, I., Chicha, L., Britschgi, M., Schobel, S. A., Bodmer, M., Hellings, J. A., et al. (2014). Maternal immune activation & abnormal brain development across CNS disorders. Nature Reviews Neurology, 10(11), 643–660. [CrossRef]
- Ko, C.-L., Lin, C.-K., & Lin, C.-L. (2024). Relationship between executive function and autism symptoms in preschoolers with autism spectrum disorder. Research in Developmental Disabilities, 147, 104692. [CrossRef]
- Kobayashi, N., Takahashi, D., Takano, S., Kimura, S., & Hase, K. (2019). The Roles of Peyer’s Patches and Microfold Cells in the Gut Immune System: Relevance to Autoimmune Diseases. Frontiers in Immunology, 10. [CrossRef]
- Koh, C.-H., Lee, S., Kwak, M., Kim, B.-S., & Chung, Y. (2023). CD8 T-cell subsets: heterogeneity, functions, and therapeutic potential. Experimental & Molecular Medicine, 55(11), 2287–2299. [CrossRef]
- Konteh, F. H. (2009). Urban sanitation and health in the developing world: Reminiscing the nineteenth century industrial nations. Health & Place, 15(1), 69–78. [CrossRef]
- Kovacheva, E., Gevezova, M., Maes, M., & Sarafian, V. (2024). Mast Cells in Autism Spectrum Disorder—The Enigma to Be Solved? International Journal of Molecular Sciences, 25(5), 2651. [CrossRef]
- Koyama, R., & Ikegaya, Y. (2015). Microglia in the pathogenesis of autism spectrum disorders. Neuroscience research, 100, 1–5. [CrossRef]
- Krausová, M., Braun, D., Buerki-Thurnherr, T., Gundacker, C., Schernhammer, E., Wisgrill, L., & Warth, B. (2023). Understanding the Chemical Exposome During Fetal Development and Early Childhood: A Review. Annual Review of Pharmacology & Toxicology. Annual Reviews. [CrossRef]
- Kushak I. R., & Winter S. H. (2020). Gut Microbiota and Gender in Autism Spectrum Disorders. Current Pediatric Reviews, 16(4), 249–254. [CrossRef]
- Lacivita, E., Perrone, R., Margari, L., & Leopoldo, M. (2017). Targets for Drug Therapy for Autism Spectrum Disorder: Challenges and Future Directions. Journal of Medicinal Chemistry, 60(22), 9114–9141. [CrossRef]
- Lahiri, D. K., Maloney, B., Wang, R., Sokol, D. K., Rogers, J. T., & Westmark, C. J. (2021). How autism and Alzheimer’s disease are TrAPPed. Molecular Psychiatry, 26(1), 26–29. [CrossRef]
- Lai, Y., Dhingra, R., Zhang, Z., Ball, L. M., Zylka, M. J., & Lu, K. (2022). Toward Elucidating the Human Gut Microbiota–Brain Axis: Molecules, Biochemistry, and Implications for Health and Diseases. Biochemistry, 61(24), 2806–2821. [CrossRef]
- Lam, K. S., Aman, M. G., & Arnold, L. E. (2006). Neurochemical correlates of autistic disorder: a review of the literature. Research in Developmental Disabilities, 27(3), 254–289. [CrossRef]
- Lange, K. W., Hauser, J., & Reissmann, A. (2015). Gluten-free and casein-free diets in the therapy of autism. Current Opinion in Clinical Nutrition & Metabolic Care, 18(6). [CrossRef]
- Lebow, M., Kuperman, Y., & Chen, A. (2024). Prenatal-induced psychopathologies: All roads lead to microglia. In Stress: Immunology & Inflammation (pp. 199–214). Elsevier.
- Lederberg, J. (2000). Infectious History. Science, 288(5464), 287–293. [CrossRef]
- Leiter, O., & Walker, T. L. (2019). Platelets: The missing link between the blood and brain? Progress in Neurobiology, 183, 101695. [CrossRef]
- Leo, E. E. M., & Campos, M. R. S. (2020). Effect of ultra-processed diet on gut microbiota and thus its role in neurodegenerative diseases. Nutrition, 71, 110609. [CrossRef]
- Lerer, L., & Varia, J. (2022). A long trip into the universe: Psychedelics and space travel. Frontiers in Space Technologies, 3, 899159. [CrossRef]
- Lesch, K.-P., & Waider, J. (2012). Serotonin in the Modulation of Neural Plasticity and Networks: Implications for Neurodevelopmental Disorders. Neuron, 76(1), 175–191. [CrossRef]
- Leventhal, J., Miller, J., Abecassis, M., Tollerud, D. J., & Ildstad, S. T. (2013). Evolving Approaches of Hematopoietic Stem Cell–Based Therapies to Induce Tolerance to Organ Transplants: The Long Road to Tolerance. Clinical Pharmacology & Therapeutics, 93(1), 36–45. [CrossRef]
- Li, X., Chauhan, A., Sheikh, A. M., Patil, S., Chauhan, V., Li, X.-M., et al. (2009). Elevated immune response in the brain of autistic patients. Journal of Neuroimmunology, 207(1), 111–116. [CrossRef]
- Lilienfeld, S. O., Marshall, J., Todd, J. T., & Shane, H. C. (2014). The persistence of fad interventions in the face of negative scientific evidence: Facilitated communication for autism as a case example. Evidence-Based Communication Assessment and Intervention, 8(2), 62–101. [CrossRef]
- Lindhard, T. (2019). Consciousness from the Outside-In and Inside-Out Perspective. Journal of Consciousness Exploration & Research, 10, 135–150.
- Liu, G., Pearl, A. M., Kong, L., Leslie, D. L., & Murray, M. J. (2017). A Profile on Emergency Department Utilization in Adolescents and Young Adults with Autism Spectrum Disorders. Journal of Autism & Developmental Disorders, 47(2), 347–358. [CrossRef]
- Liu, M., Liang, S., & Zhang, C. (2021). NK cells in autoimmune diseases: protective or pathogenic? Frontiers in Immunology, 12, 624687. [CrossRef]
- Liu, S.-H., Shi, X.-J., Fan, F.-C., & Cheng, Y. (2021). Peripheral blood neurotrophic factor levels in children with autism spectrum disorder: a meta-analysis. Scientific Reports, 11(1), 15. [CrossRef]
- Lleo, A. (2014). Chapter 2 - What Is an Autoantibody? In Y. Shoenfeld, P. L. Meroni, & M. E. Gershwin (Eds.), Autoantibodies (Third Edition) (pp. 13–20). San Diego: Elsevier. [CrossRef]
- Lleo, A., Invernizzi, P., Gao, B., Podda, M., & Gershwin, M. E. (2010). Definition of human autoimmunity — autoantibodies versus autoimmune disease. Special Issue on The Environment Geoepidemiology & Autoimmune Diseases, 9(5), A259–A266. [CrossRef]
- López-Cacho, J. M., Gallardo, S., Posada, M., Aguerri, M., Calzada, D., Mayayo, T., et al. (2016). Characterization of immune cell phenotypes in adults with autism spectrum disorders. Journal of Investigative Medicine, 64(7), 1179–1185. [CrossRef]
- López-Varela, S., González-Gross, M., & Marcos, A. (2002). Functional foods and the immune system: a review. European Journal of Clinical Nutrition, 56(3), S29–S33. [CrossRef]
- Lord, C., Shulman, C., & DiLavore, P. (2004). Regression and word loss in autistic spectrum disorders. Journal of Child Psychology & Psychiatry, 45(5), 936–955. [CrossRef]
- Lou, H. C. (2012). Paradigm shift in consciousness research: the child′s self-awareness and abnormalities in autism, ADHD and schizophrenia. Acta Paediatrica, 101(2), 112–119. [CrossRef]
- Luo, Y., & Wang, Z. (2024). The impact of Microglia on Neurodevelopment and Brain function in Autism. Biomedicines, 12(1), 210. [CrossRef]
- Luvián-Morales, J., Varela-Castillo, F. O., Flores-Cisneros, L., Cetina-Pérez, L., & Castro-Eguiluz, D. (2022). Functional foods modulating inflammation and metabolism in chronic diseases: a systematic review. Critical Reviews in Food Science & Nutrition, 62(16), 4371–4392. [CrossRef]
- Luyster, R., Richler, J., Risi, S., Hsu, W.-L., Dawson, G., Bernier, R., et al. (2005). Early regression in social communication in autism spectrum disorders: A CPEA study. Developmental Neuropsychology, 27(3), 311–336. [CrossRef]
- Lynch S. V., & Pedersen O. (2016). The Human Intestinal Microbiome in Health and Disease. New England Journal of Medicine, 375(24), 2369–2379. [CrossRef]
- Machado, A. P., Ratliff, H., Abdelwahab, A., Vohra, M. H., Kuang, A., Shatila, M., et al. (2023). The Safety of Immunosuppressants Used in the Treatment of Immune-Related Adverse Events due to Immune Checkpoint Inhibitors: a Systematic Review. Journal of Cancer, 14(16), 2956–2963. [CrossRef]
- Macpherson, A. J., Pachnis, V., & Prinz, M. (2023). Boundaries and integration between microbiota, the nervous system, and immunity. Immunity, 56(8), 1712–1726. [CrossRef]
- Maenner, M. J. (2021). Prevalence and characteristics of autism spectrum disorder among children aged 8 years—autism and developmental disabilities monitoring network, 11 sites, United States, 2018. MMWR. Surveillance Summaries, 70. [CrossRef]
- Maier, S. F., & Watkins, L. R. (1999). Bidirectional communication between the brain and the immune system: implications for behaviour. Animal Behaviour, 57(4), 741–751. [CrossRef]
- Maitre, L., Bustamante, M., Hernández-Ferrer, C., Thiel, D., Lau, C.-H. E., Siskos, A. P., et al. (2022). Multi-omics signatures of the human early life exposome. Nature Communications, 13(1), 7024. [CrossRef]
- Mallozzi, M., Bordi, G., Garo, C., & Caserta, D. (2016). The effect of maternal exposure to endocrine disrupting chemicals on fetal and neonatal development: A review on the major concerns. Birth Defects Research Part C: Embryo Today: Reviews, 108(3), 224–242. [CrossRef]
- Manji, H., Kato, T., Di Prospero, N. A., Ness, S., Beal, M. F., Krams, M., & Chen, G. (2012). Impaired mitochondrial function in psychiatric disorders. Nature Reviews Neuroscience, 13(5), 293–307. [CrossRef]
- Margulis, L., & Fester, R. (1991). Symbiosis as a Source of Evolutionary Innovation: Speciation & Morphogenesis. MIT Press.
- Marotta, R., Risoleo, M. C., Messina, G., Parisi, L., Carotenuto, M., Vetri, L., & Roccella, M. (2020). The neurochemistry of autism. Brain Sciences, 10(3), 163. [CrossRef]
- Marques, R. E., Marques, P. E., Guabiraba, R., & Teixeira, M. M. (2016). Exploring the Homeostatic and Sensory Roles of the Immune System. Frontiers in Immunology, 7. [CrossRef]
- Marshall, L., & Born, J. (2002). Brain-Immune interactions in sleep. In International Review of Neurobiology (Vol. 52, pp. 93–131). Academic Press. [CrossRef]
- Martin, A., Scahill, L., Anderson, G. M., Aman, M., Arnold, L. E., McCracken, J., et al. (2004). Weight and Leptin Changes Among Risperidone-Treated Youths With Autism: 6-Month Prospective Data. American Journal of Psychiatry, 161(6), 1125–1127. [CrossRef]
- Martínez-Cerdeño, V. (2017). Dendrite and spine modifications in autism and related neurodevelopmental disorders in patients and animal models. Developmental Neurobiology, 77(4), 393–404. [CrossRef]
- Marwaha, A., Leung, N., McMurchy, A. N., & Levings, M. (2012). TH17 Cells in Autoimmunity and Immunodeficiency: Protective or Pathogenic? Frontiers in Immunology, 3. [CrossRef]
- Masi, A., DeMayo, M. M., Glozier, N., & Guastella, A. J. (2017). An overview of autism spectrum disorder, heterogeneity and treatment options. Neuroscience Bulletin, 33, 183–193. [CrossRef]
- Matcovitch-Natan, O., Winter, D. R., Giladi, A., Vargas Aguilar, S., Spinrad, A., Sarrazin, S., et al. (2016). Microglia development follows a stepwise program to regulate brain homeostasis. Science, 353(6301), aad8670. [CrossRef]
- Mauri, C., & Bosma, A. (2012). Immune Regulatory Function of B Cells. Annual Review of Immunology. Annual Reviews. [CrossRef]
- Mayer, E. A. (2011). Gut feelings: the emerging biology of gut–brain communication. Nature Reviews Neuroscience, 12(8), 453–466. [CrossRef]
- Mayer, E. A., Nance, K., & Chen, S. (2022). The Gut–Brain Axis. Annual Review of Medicine. Annual Reviews. [CrossRef]
- McCarthy, M. M., & Wright, C. L. (2017). Convergence of Sex Differences and the Neuroimmune System in Autism Spectrum Disorder. Biological Psychiatry, 81(5), 402–410. [CrossRef]
- McCormick, C. M., Furey, B. F., Child, M., Sawyer, M. J., & Donohue, S. M. (1998). Neonatal sex hormones have `organizational’ effects on the hypothalamic-pituitary-adrenal axis of male rats. Developmental Brain Research, 105(2), 295–307. [CrossRef]
- McCracken, J. T., Anagnostou, E., Arango, C., Dawson, G., Farchione, T., Mantua, V., et al. (2021). Drug development for Autism Spectrum Disorder (ASD): Progress, challenges, and future directions. Opportunities & Challenges in Drug Development for ASD: Product of ISCTM/ECNP Joint Working Group, 48, 3–31. [CrossRef]
- McCruden, A. B., & Stimson, W. H. (1991). Sex Hormones and Immune Function. In R. ADER, D. L. FELTEN, & N. COHEN (Eds.), Psychoneuroimmunology (pp. 475–493). Academic Press. [CrossRef]
- McEwen, B. S. (1998a). Protective and damaging effects of stress mediators. New England Journal of Medicine, 338(3), 171–179. [CrossRef]
- McEwen, B. S. (1998b). Stress, adaptation, and disease: Allostasis and allostatic load. Annals of the New York academy of sciences, 840(1), 33–44. [CrossRef]
- Mead, J., & Ashwood, P. (2015). Evidence supporting an altered immune response in ASD. Immunology Letters, 163(1), 49–55. [CrossRef]
- Megremi, A. S. (2013). Is fever a predictive factor in the autism spectrum disorders? Medical Hypotheses, 80(4), 391–398. [CrossRef]
- Mezzacappa, A., Lasica, P.-A., Gianfagna, F., Cazas, O., Hardy, P., Falissard, B., et al. (2017). Risk for Autism Spectrum Disorders According to Period of Prenatal Antidepressant Exposure: A Systematic Review and Meta-analysis. JAMA Pediatrics, 171(6), 555–563. [CrossRef]
- Miclotte, L., & Van de Wiele, T. (2020). Food processing, gut microbiota and the globesity problem. Critical Reviews in Food Science & Nutrition, 60(11), 1769–1782. [CrossRef]
- Miller, H., Zhang, J., Kuolee, R., Patel, G., & Chen, W. (2007). Intestinal M cells: The fallible sentinels? World Journal of Gastroenterology : WJG, 13, 1477–86.
- Mills, E. L., Kelly, B., & O’Neill, L. A. (2017). Mitochondria are the powerhouses of immunity. Nature Immunology, 18(5), 488–498. [CrossRef]
- Misquita, A. G., da Silva, C. N. F., Souza, D. R. O., dos Santos, D. N. B., Brito, I. de S. M., Ribeiro, J. de O. S., et al. (2024). Cognitive behavioral therapy: A valuable intervention in the autistic universe. International Seven Journal of Health Research, 3(2), 754–760. [CrossRef]
- Mitsea, E., Drigas, A., & Skianis, C. (2022). Cutting-Edge Technologies in Breathwork for Learning Disabilities in Special Education. Technium Social Sciences Journal, 34, 136–157. [CrossRef]
- Mitsea, E., Drigas, A., & Skianis, C. (2022). Cutting-Edge Technologies in Breathwork for Learning Disabilities in Special Education. Technium Social Sciences Journal, 34, 136–157. [CrossRef]
- Nordin, A. M., Ismail, J., & Nor, N. K. (2021). Motor development in children with autism spectrum disorder. Frontiers in Pediatrics, 9, 598276. [CrossRef]
- Molloy, C. A., Morrow, A. L., Meinzen-Derr, J., Schleifer, K., Dienger, K., Manning-Courtney, P., et al. (2006). Elevated cytokine levels in children with autism spectrum disorder. Journal of Neuroimmunology, 172(1), 198–205. [CrossRef]
- Moncrieff, J. (2006). Why is it so difficult to stop psychiatric drug treatment? It may be nothing to do with the original problem. Medical Hypotheses, 67(3), 517–523. [CrossRef]
- Moncrieff, J., Cohen, D., & Porter, S. (2013). The Psychoactive Effects of Psychiatric Medication: The Elephant in the Room. Journal of Psychoactive Drugs, 45(5), 409–415. [CrossRef]
- Money, J., Bobrow, N. A., & Clarke, F. C. (1971). Autism and autoimmune disease: A family study. Journal of Autism & Childhood Schizophrenia, 1(2), 146–160. [CrossRef]
- Monteiro, M., Santos, A., Gomes, L., & Rito, R. (2020). Autism Spectrum Disorder: A Systematic Review About Nutritional Interventions. Revista Paulista de Pediatria, 38. [CrossRef]
- Montgomery, S. L., & Bowers, W. J. (2012). Tumor Necrosis Factor-alpha and the Roles it Plays in Homeostatic and Degenerative Processes Within the Central Nervous System. Journal of Neuroimmune Pharmacology, 7(1), 42–59. [CrossRef]
- Moore, J. B., & Weeks, M. E. (2011). Proteomics and systems biology: current and future applications in the nutritional sciences. Advances in Nutrition, 2(4), 355–364. [CrossRef]
- Mordelt, A., & de Witte, L. D. (2023). Microglia-mediated synaptic pruning as a key deficit in neurodevelopmental disorders: Hype or hope? Current Opinion in Neurobiology, 79, 102674. [CrossRef]
- Gianluca, M., Giovanni, C., Devis, B., & Armando., G. (2017). Biologics in Inflammatory Immune-mediated Systemic Diseases. Current Pharmaceutical Biotechnology, 18(12), 1008–1016. [CrossRef]
- Moroni, L., Bianchi, I., & Lleo, A. (2012). Geoepidemiology, gender and autoimmune disease. Special Issue on Gender, Sex Hormones, Pregnancy & Autoimmunity, 11(6), A386–A392. [CrossRef]
- Mosconi, M. W., Wang, Z., Schmitt, L. M., Tsai, P., & Sweeney, J. A. (2015). The role of cerebellar circuitry alterations in the pathophysiology of autism spectrum disorders. Frontiers in Neuroscience, 9. [CrossRef]
- Mulder, S. J., & Mulder-Bos, G. C. (2006). Most probable origin of coeliac disease is low immune globulin A in the intestine caused by malfunction of Peyer’s patches. Medical Hypotheses, 66(4), 757–762. [CrossRef]
- Nadeem, A., Ahmad, S. F., Al-Harbi, N. O., Al-Ayadhi, L. Y., Sarawi, W., Attia, S. M., et al. (2022). Imbalance in pro-inflammatory and anti-inflammatory cytokines milieu in B cells of children with autism. Molecular Immunology, 141, 297–304. [CrossRef]
- Nelissen, S., Lemmens, E., Geurts, N., Kramer, P., Maurer, M., Hendriks, J., & Hendrix, S. (2013). The role of mast cells in neuroinflammation. Acta Neuropathologica, 125, 637–650. [CrossRef]
- Nelson, A. D., & Bender, K. J. (2021). Dendritic Integration Dysfunction in Neurodevelopmental Disorders. Developmental Neuroscience, 43(3–4), 201–221. [CrossRef]
- Netea, M. G., Joosten, L. A., Latz, E., Mills, K. H., Natoli, G., Stunnenberg, H. G., et al. (2016). Trained immunity: a program of innate immune memory in health and disease. Science, 352(6284), aaf1098. [CrossRef]
- evison, C., Blaxill, M., & Zahorodny, W. (2018). California autism prevalence trends from 1931 to 2014 and comparison to national ASD data from IDEA and ADDM. Journal of Autism & Developmental Disorders, 48, 4103–4117. [CrossRef]
- Nevison, C. D., & Blaxill, M. (2017). Diagnostic substitution for intellectual disability: A flawed explanation for the rise in autism. Journal of Autism & Developmental Disorders, 47, 2733–2742. [CrossRef]
- Ngo, S. T., Steyn, F. J., & McCombe, P. A. (2014). Gender differences in autoimmune disease. Sex Differences in Neurological & Psychiatric Disorders, 35(3), 347–369. [CrossRef]
- Niciu, M. J., Kelmendi, B., & Sanacora, G. (2012). Overview of glutamatergic neurotransmission in the nervous system. Glutamate Receptors, 100(4), 656–664. [CrossRef]
- Nie, Z.-Q., Han, D., Zhang, K., Li, M., Kwon, H.-K., Im, S.-H., et al. (2023). TH1/Treg ratio may be a marker of autism in children with immune dysfunction. Research in Autism Spectrum Disorders, 101, 102085. [CrossRef]
- NIEHS. (2024). Autoimmune Diseases. https://www.niehs.nih.gov/health/topics/conditions/autoimmune. Accessed 1 May 2024.
- Niesler, B., Kuerten, S., Demir, I. E., & Schäfer, K.-H. (2021). Disorders of the enteric nervous system—a holistic view. Nature reviews Gastroenterology & Hepatology, 18(6), 393–410. [CrossRef]
- NIMH. (2024). Autism Spectrum Disorder. https://www.nimh.nih.gov/health/topics/autism-spectrum-disorders-asd. Accessed 16 July 2024.
- Niyozovh, S., Djurabekova, A., & Igamova, S. (2020). Complication of encephalitis in children, an innovative approach for the treatment. International Journal of Pharmaceutical Research (09752366), 12(1). [CrossRef]
- Njotto, L. L., Simin, J., Fornes, R., Odsbu, I., Mussche, I., Callens, S., et al. (2023). Maternal and early-life exposure to antibiotics and the risk of autism and Attention-Deficit Hyperactivity Disorder in childhood: A Swedish population-based cohort study. Drug Safety, 46(5), 467–478. [CrossRef]
- Nyati, K. K., & Prasad, K. N. (2014). Role of Cytokines and Toll-Like Receptors in the Immunopathogenesis of Guillain-Barré Syndrome. Mediators of Inflammation, 2014(1), 758639. [CrossRef]
- Obata, Y., & Pachnis, V. (2016). The effect of microbiota and the immune system on the development and organization of the enteric nervous system. Gastroenterology, 151(5), 836–844. [CrossRef]
- Odenwald, M. A., & Turner, J. R. (2017). The intestinal epithelial barrier: a therapeutic target? Nature Reviews Gastroenterology & Hepatology, 14(1), 9–21. [CrossRef]
- Onore, C., Careaga, M., & Ashwood, P. (2012). The role of immune dysfunction in the pathophysiology of autism. Brain, Behavior, & Immunity, 26(3), 383–392. [CrossRef]
- Ozonoff, S., Iosif, A.-M., Baguio, F., Cook, I. C., Hill, M. M., Hutman, T., et al. (2010). A prospective study of the emergence of early behavioral signs of autism. Journal of the American Academy of Child & Adolescent Psychiatry, 49(3), 256–266. [CrossRef]
- Padmakumar, M., Van Raes, E., Van Geet, C., & Freson, K. (2019). Blood platelet research in autism spectrum disorders: In search of biomarkers. Research & Practice in Thrombosis & Haemostasis, 3(4), 566–577. [CrossRef]
- Palmieri, L., & Persico, A. M. (2010). Mitochondrial dysfunction in autism spectrum disorders: cause or effect? Biochimica et Biophysica Acta (BBA)-Bioenergetics, 1797(6–7), 1130–1137. [CrossRef]
- Panksepp, J. (1979). A neurochemical theory of autism. Trends in Neurosciences, 2, 174–177. [CrossRef]
- Paoletti, P., Bellone, C., & Zhou, Q. (2013). NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nature Reviews Neuroscience, 14(6), 383–400. [CrossRef]
- Pardo, C. A., & Eberhart, C. G. (2007). The neurobiology of autism. Brain Pathology, 17(4), 434–447. [CrossRef]
- Pardo, C. A., Vargas, D. L., & Zimmerman, A. W. (2005). Immunity, neuroglia and neuroinflammation in autism. International Review of Psychiatry, 17(6), 485–495. [CrossRef]
- Park, K. M., & Bowers, W. J. (2010). Tumor necrosis factor-alpha mediated signaling in neuronal homeostasis and dysfunction. Cellular Signalling, 22(7), 977–983. [CrossRef]
- Pathak, D., & Sriram, K. (2023). Neuron-astrocyte omnidirectional signaling in neurological health and disease. Frontiers in Molecular Neuroscience, 16, 1169320. [CrossRef]
- Peper, E., & Shufor, J. (2024). Reflections on the Increase in Autism, ADHD, Anxiety, and Depression: Part 2 – Exposure to Neurotoxins and Ultraprocessed Foods. NeuroRegulation, 11, 219–228. [CrossRef]
- Peralta-Marzal, L. N., Rojas-Velazquez, D., Rigters, D., Prince, N., Garssen, J., Kraneveld, A. D., et al. (2024). A robust microbiome signature for autism spectrum disorder across different studies using machine learning. Scientific Reports, 14(1), 814. [CrossRef]
- Pereira, A. (2020). Chapter Seven - Classical-quantum interfaces in living neural tissue supporting conscious functions. In R. R. Poznański & E. J. Brändas (Eds.), Advances in Quantum Chemistry (Vol. 82, pp. 213–252). Academic Press. [CrossRef]
- Pereira, A., & Furlan, F. A. (2009). On the role of synchrony for neuron–astrocyte interactions and perceptual conscious processing. Journal of Biological Physics, 35, 465–480. [CrossRef]
- Pereira, A., & Furlan, F. A. (2010). Astrocytes and human cognition: Modeling information integration and modulation of neuronal activity. Progress in Neurobiology, 92(3), 405–420. [CrossRef]
- Pereira, J. A. (2020). The Role of Sentience in the Theory of Consciousness and Medical Practice. [CrossRef]
- Pereira, J. A., & Wang, F. (2016). Neuromodulation, Emotional Feelings and Affective Disorders. Mens Sana Monographs, 0. [CrossRef]
- Perez-Muñoz, M. E., Arrieta, M.-C., Ramer-Tait, A. E., & Walter, J. (2017). A critical assessment of the “sterile womb” and “in utero colonization” hypotheses: implications for research on the pioneer infant microbiome. Microbiome, 5(1), 48. [CrossRef]
- Perrin, J. M., Anderson, L. E., & Van Cleave, J. (2014). The rise in chronic conditions among infants, children, and youth can be met with continued health system innovations. Health Affairs, 33(12), 2099–2105. [CrossRef]
- Petrelli, F., Pucci, L., & Bezzi, P. (2016). Astrocytes and microglia and their potential link with autism spectrum disorders. Frontiers in Cellular Neuroscience, 10, 21. [CrossRef]
- Ploog, B. O. (2023). Theory of Mind in Autism. In A. El Idrissi & D. McCloskey (Eds.), Neurobiology of Autism Spectrum Disorders (pp. 23–35). Cham: Springer International Publishing. [CrossRef]
- Pociūtė, A., Pivoriūnas, A., & Verkhratsky, A. (2024). Astrocytes dynamically regulate the blood-brain barrier in the healthy brain. Neural Regeneration Research, 19(4), 709–710.
- Politte, L. C., Howe, Y., Nowinski, L., Palumbo, M., & McDougle, C. J. (2015). Evidence-Based Treatments for Autism Spectrum Disorder. Current Treatment Options in Psychiatry, 2(1), 38–56. [CrossRef]
- Pourgholaminejad, A., & Tahmasebinia, F. (2019). The Role of Th17 Cells in Immunopathogenesis of Neuroinflammatory Disorders. In H. Mitoma & M. Manto (Eds.), Neuroimmune Diseases: From Cells to the Living Brain (pp. 83–107). Cham: Springer International Publishing. [CrossRef]
- Pretzsch, C. M., Schäfer, T., Lombardo, M. V., Warrier, V., Mann, C., Bletsch, A., et al. (2022). Neurobiological Correlates of Change in Adaptive Behavior in Autism. American Journal of Psychiatry, 179(5), 336–349. [CrossRef]
- Prüss, H. (2021). Autoantibodies in neurological disease. Nature Reviews Immunology, 21(12), 798–813. https://doi.org/10.1038/s41577-021-00543-w. [CrossRef]
- Pu, Y., Yang, J., Chang, L., Qu, Y., Wang, S., Zhang, K., et al. (2020). Maternal glyphosate exposure causes autism-like behaviors in offspring through increased expression of soluble epoxide hydrolase. Proceedings of the National Academy of Sciences, 117(21), 11753–11759. [CrossRef]
- Pulikkan, J., Mazumder, A., & Grace, T. (2019). Role of the gut microbiome in autism spectrum disorders. Reviews on Biomarker Studies in Psychiatric & Neurodegenerative disorders, 253–269. [CrossRef]
- Puricelli, C., Rolla, R., Gigliotti, L., Boggio, E., Beltrami, E., Dianzani, U., & Keller, R. (2022). The Gut-Brain-Immune Axis in Autism Spectrum Disorders: A State-of-Art Report. Frontiers in Psychiatry, 12. [CrossRef]
- Qi, H. (2023). Neuroimmunology: reviews and perspectives on recent advances. Cellular & Molecular Immunology, 20(11), 1257–1258. [CrossRef]
- Rabinovici, G. D., Stephens, M. L., & Possin, K. L. (2015). Executive Dysfunction. CONTINUUM: Lifelong Learning in Neurology, 21(3). [CrossRef]
- Ramautar, R., Berger, R., van der Greef, J., & Hankemeier, T. (2013). Human metabolomics: strategies to understand biology. Current Opinion in Chemical Biology, 17(5), 841–846. [CrossRef]
- Ramsay, D. S., & Woods, S. C. (2014). Clarifying the roles of homeostasis and allostasis in physiological regulation. Psychological Review, 121(2), 225–247. [CrossRef]
- Randolph-Gips, M., & Srinivasan, P. (2012). Modeling autism: a systems biology approach. Journal of Clinical Bioinformatics, 2(1), 17. [CrossRef]
- Reemst, K., Noctor, S. C., Lucassen, P. J., & Hol, E. M. (2016). The indispensable roles of microglia and astrocytes during brain development. Frontiers in Human Neuroscience, 10, 566. [CrossRef]
- Reid, G., Younes, J. A., Van der Mei, H. C., Gloor, G. B., Knight, R., & Busscher, H. J. (2011). Microbiota restoration: natural and supplemented recovery of human microbial communities. Nature Reviews Microbiology, 9(1), 27–38. [CrossRef]
- Renz, H., Holt, P. G., Inouye, M., Logan, A. C., Prescott, S. L., & Sly, P. D. (2017). An exposome perspective: Early-life events and immune development in a changing world. Journal of Allergy & Clinical Immunology, 140(1), 24–40. [CrossRef]
- Réthelyi, J. M., Vincze, K., Schall, D., Glennon, J., & Berkel, S. (2023). The role of insulin/IGF1 signalling in neurodevelopmental and neuropsychiatric disorders–evidence from human neuronal cell models. Neuroscience & Biobehavioral Reviews, 105330. [CrossRef]
- Ribatti, D. (2015). The crucial role of mast cells in blood–brain barrier alterations. Experimental Cell Research, 338(1), 119–125. [CrossRef]
- Ribeiro, S. (2018). Whole Organisms or Pure Compounds? Entourage Effect Versus Drug Specificity. In B. C. Labate & C. Cavnar (Eds.), Plant Medicines, Healing & Psychedelic Science: Cultural Perspectives (pp. 133–149). Cham: Springer International Publishing. [CrossRef]
- Riikonen, R. (2017). Insulin-like growth factors in the pathogenesis of neurological diseases in children. International Journal of Molecular Sciences, 18(10), 2056. [CrossRef]
- Robertson, J. M. (2002). The Astrocentric Hypothesis: proposed role of astrocytes in consciousness and memory formation. Journal of Physiology-Paris, 96(3), 251–255. [CrossRef]
- Rogers, S. J., Cook, I., & Meryl, A. (2005). Imitation and play in autism. In Handbook of Autism a& Pervasive Developmental Disorders (Vol. 1, pp. 382–405). Wiley Online Library.
- Romagnani, S. (1999). Th1/Th2 Cells. Inflammatory Bowel Diseases, 5(4), 285–294. [CrossRef]
- Rook, G. A. W. (2012). Hygiene Hypothesis and Autoimmune Diseases. Clinical Reviews in Allergy & Immunology, 42(1), 5–15. [CrossRef]
- Rosen, N. E., Lord, C., & Volkmar, F. R. (2021). The diagnosis of autism: From Kanner to DSM-III to DSM-5 and beyond. Journal of Autism & Developmental Disorders, 51, 4253–4270. [CrossRef]
- Rosin, J. M., & Kurrasch, D. M. (2019). Emerging roles for hypothalamic microglia as regulators of physiological homeostasis. Frontiers in Neuroendocrinology, 54, 100748. [CrossRef]
- Rossignol, D A., & Frye, R. E. (2012). A review of research trends in physiological abnormalities in autism spectrum disorders: immune dysregulation, inflammation, oxidative stress, mitochondrial dysfunction and environmental toxicant exposures. Molecular Psychiatry, 17(4), 389–401. [CrossRef]
- Rossignol, D. A., & Frye, R. E. (2014). Evidence linking oxidative stress, mitochondrial dysfunction, and inflammation in the brain of individuals with autism. Frontiers in Physiology, 5, 150. [CrossRef]
- Rucklidge, J. J., Johnstone, J. M., & Kaplan, B. J. (2021). Nutrition Provides the Essential Foundation for Optimizing Mental Health. Evidence-Based Practice in Child & Adolescent Mental Health, 6(1), 131–154. [CrossRef]
- Rueda-Ruzafa, L., Cruz, F., Roman, P., & Cardona, D. (2019). Gut microbiota and neurological effects of glyphosate. NeuroToxicology, 75, 1–8. [CrossRef]
- Saad, K., Zahran, A. M., Elsayh, K. I., Abdel-Rahman, A. A., Al-Atram, A. A., Hussein, A., & El-Gendy, Y. G. (2017). Frequency of Dendritic Cells and Their Expression of Costimulatory Molecules in Children with Autism Spectrum Disorders. Journal of Autism & Developmental Disorders, 47(9), 2671–2678. [CrossRef]
- Safari-Alighiarloo, N., Taghizadeh, M., Rezaei tavirani, M., Goliaei, B., & Peyvandi, A. (2014). Protein-Protein Interaction Networks (PPI) and complex diseases. Gastroenterology & Hepatology From Bed to Bench, 7, 17–31.
- Salmond, C., De Haan, M., Friston, K., Gadian, D., & Vargha-Khadem, F. (2003). Investigating individual differences in brain abnormalities in autism. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 358(1430), 405–413. [CrossRef]
- Salter, M. W., & Stevens, B. (2017). Microglia emerge as central players in brain disease. Nature Medicine, 23(9), 1018–1027. [CrossRef]
- Samanipour, S., Barron, L. P., van Herwerden, D., Praetorius, A., Thomas, K. V., & O’Brien, J. W. (2024). Exploring the Chemical Space of the Exposome: How Far Have We Gone? JACS Au, 4(7), 2412–2425. [CrossRef]
- Samsam, M., Ahangari, R., & Naser, S. A. (2014). Pathophysiology of autism spectrum disorders: revisiting gastrointestinal involvement and immune imbalance. World journal of gastroenterology: WJG, 20(29), 9942.
- Samsel, A., & Seneff, S. (2013). Glyphosate’s suppression of cytochrome P450 enzymes and amino acid biosynthesis by the gut microbiome: pathways to modern diseases. Entropy, 15(4), 1416–1463. [CrossRef]
- Saresella, M., Piancone, F., Marventano, I., Zoppis, M., Hernis, A., Zanette, M., et al. (2016). Multiple inflammasome complexes are activated in autistic spectrum disorders. Brain, Behavior, & Immunity, 57, 125–133. [CrossRef]
- Schroder, K., & Tschopp, J. (2010). The Inflammasomes. Cell, 140(6), 821–832. [CrossRef]
- Schwartz, M., & Cahalon, L. (2022). The vicious cycle governing the brain–immune system relationship in neurodegenerative diseases. Current Opinion in Immunology, 76, 102182. [CrossRef]
- Segal, Y., & Shoenfeld, Y. (2018). Vaccine-induced autoimmunity: the role of molecular mimicry and immune crossreaction. Cellular & Molecular Immunology, 15(6), 586–594. [CrossRef]
- Seneff, S., Kyriakopoulos, A. M., & Nigh, G. (2024). Is autism a PIN1 deficiency syndrome? A proposed etiological role for glyphosate. Journal of Neurochemistry, n/a(n/a). [CrossRef]
- Serrano-Pozo, A. (2018). Encephalopathy. In The Wiley Handbook on the Aging Mind and Brain (pp. 553–590). [CrossRef]
- Shah, P. A., Park, C. J., Shaughnessy, M. P., & Cowles, R. A. (2021). Serotonin as a Mitogen in the Gastrointestinal Tract: Revisiting a Familiar Molecule in a New Role. Cellular & Molecular Gastroenterology & Hepatology, 12(3), 1093–1104. [CrossRef]
- Shahar, O., Botvinnik, A., Shwartz, A., Lerer, E., Golding, P., Buko, A., et al. (2024). Effect of chemically synthesized psilocybin and psychedelic mushroom extract on molecular and metabolic profiles in mouse brain. Molecular Psychiatry. [CrossRef]
- Shahbazi, R., Sharifzad, F., Bagheri, R., Alsadi, N., Yasavoli-Sharahi, H., & Matar, C. (2021). Anti-Inflammatory and Immunomodulatory Properties of Fermented Plant Foods. Nutrients, 13(5). [CrossRef]
- Shajib, Md. S., Baranov, A., & Khan, W. I. (2017). Diverse Effects of Gut-Derived Serotonin in Intestinal Inflammation. ACS Chemical Neuroscience, 8(5), 920–931. [CrossRef]
- Shaw, C. A., Seneff, S., Kette, S. D., Tomljenovic, L., Oller Jr, J. W., & Davidson, R. M. (2014). Aluminum-Induced Entropy in Biological Systems: Implications for Neurological Disease. Journal of Toxicology, 2014(1), 491316. [CrossRef]
- Shaw, J. C., Crombie, G. K., Palliser, H. K., & Hirst, J. J. (2021). Impaired oligodendrocyte development following preterm birth: promoting GABAergic action to improve outcomes. Frontiers in Pediatrics, 9, 618052. [CrossRef]
- Shin, Y.-W., Lee, S.-T., Park, K.-I., Jung, K.-H., Jung, K.-Y., Lee, S. K., & Chu, K. (2018). Treatment strategies for autoimmune encephalitis. Therapeutic Advances in Neurological Disorders, 11, 1756285617722347. [CrossRef]
- Siegel, M., Milligan, B., Chemelski, B., Payne, D., Ellsworth, B., Harmon, J., et al. (2014). Specialized inpatient psychiatry for serious behavioral disturbance in autism and intellectual disability. Journal of Autism & Developmental Disorders, 44, 3026–3032. [CrossRef]
- Sies, H. (2015). Oxidative stress: a concept in redox biology and medicine. Redox Biology, 4, 180–183. [CrossRef]
- Gonzalez, S. M., Ni, G., Lam, V., & Demopoulos, C. (2024). Beyond words: an investigation of fine motor skills and the verbal communication spectrum in autism. Frontiers in Psychiatry, 15, 1379307. [CrossRef]
- Simons, M., Levin, J., & Dichgans, M. (2023). Tipping points in neurodegeneration. Neuron, 111. [CrossRef]
- Singh, A., Kukreti, R., Saso, L., & Kukreti, S. (2019). Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules, 24(8). [CrossRef]
- Siniscalco, D., Bradstreet, J. J., & Antonucci, N. (2013). Therapeutic Role of Hematopoietic Stem Cells in Autism Spectrum Disorder-Related Inflammation. Frontiers in Immunology, 4. [CrossRef]
- Sloan, S. A., & Barres, B. A. (2014). Mechanisms of astrocyte development and their contributions to neurodevelopmental disorders. Current Opinion in Neurobiology, 27, 75–81. [CrossRef]
- Smyth, M. J., Godfrey, D. I., & Trapani, J. A. (2001). A fresh look at tumor immunosurveillance and immunotherapy. Nature Immunology, 2(4), 293–299. [CrossRef]
- Ssucharewa, G. E. (1926). Die schizoiden Psychopathien im Kindesalter. (Part 1 of 2). European Neurology, 60(3–4), 235–247. [CrossRef]
- Stein, Y., Hänninen, O., Huttunen, P., Ahonen, M., & Ekman, R. (2015). Electromagnetic Radiation and Health: Human Indicators. In R. H. Armon & O. Hänninen (Eds.), Environmental Indicators (pp. 1025–1046). Dordrecht: Springer Netherlands. [CrossRef]
- Steinman, G. (2015). Plausible etiology of brain dysconnectivity in autism – Review and prospectus. Medical Hypotheses, 85(4), 405–407. [CrossRef]
- Steinman, G., & Mankuta, D. (2013). Insulin-like growth factor and the etiology of autism. Medical Hypotheses, 80(4), 475–480. [CrossRef]
- Stilling, R. M., Bordenstein, S. R., Dinan, T. G., & Cryan, J. F. (2014). Friends with social benefits: host-microbe interactions as a driver of brain evolution and development? Frontiers in Cellular & Infection Microbiology, 4. [CrossRef]
- Stinson, L. F., Boyce, M. C., Payne, M. S., & Keelan, J. A. (2019). The Not-so-Sterile Womb: Evidence That the Human Fetus Is Exposed to Bacteria Prior to Birth. Frontiers in Microbiology, 10. [CrossRef]
- Stinson, L. F., Payne, M. S., & Keelan, J. A. (2017). Planting the seed: Origins, composition, and postnatal health significance of the fetal gastrointestinal microbiota. Critical Reviews in Microbiology, 43(3), 352–369. [CrossRef]
- Storch, E. A., Arnold, E. B., Lewin, A. B., Nadeau, J. M., Jones, A. M., De Nadai, A. S., et al. (2013). The Effect of Cognitive-Behavioral Therapy Versus Treatment as Usual for Anxiety in Children With Autism Spectrum Disorders: A Randomized, Controlled Trial. Journal of the American Academy of Child & Adolescent Psychiatry, 52(2), 132-142.e2. [CrossRef]
- Strandwitz, P. (2018). Neurotransmitter modulation by the gut microbiota. Where the gut meets the brain, 1693, 128–133. [CrossRef]
- Strati, F., Cavalieri, D., Albanese, D., De Felice, C., Donati, C., Hayek, J., et al. (2017). New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome, 5, 1–11. [CrossRef]
- Streng, M. L., & Krook-Magnuson, E. (2021). The cerebellum and epilepsy. Epilepsy & Behavior, 121. [CrossRef]
- Stubbs, E. G., Crawford, M. L., Burger, D. R., & Vandenbark, A. A. (1977). Depressed lymphocyte responsiveness in autistic children. Journal of Autism & Childhood Schizophrenia, 7(1), 49–55. [CrossRef]
- Su, L.-D., Xu, F.-X., Wang, X.-T., Cai, X.-Y., & Shen, Y. (2021). Cerebellar Dysfunction, Cerebro-cerebellar Connectivity and Autism Spectrum Disorders. Neuroscience, 462, 320–327. [CrossRef]
- Sweeney, M. D., Zhao, Z., Montagne, A., Nelson, A. R., & Zlokovic, B. V. (2019). Blood-Brain Barrier: From Physiology to Disease and Back. Physiological Reviews, 99(1), 21–78. [CrossRef]
- Sweeten, T. L., Posey, D. J., Shekhar, A., & McDougle, C. J. (2002). The amygdala and related structures in the pathophysiology of autism. Functional Role of Specific Systems Within the Extended Amygdala & Hypothalamus, 71(3), 449–455. [CrossRef]
- Tahmasebinia, F., & Pourgholaminejad, A. (2017). The role of Th17 cells in auto-inflammatory neurological disorders. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 79, 408–416. [CrossRef]
- Taniya, M. A., Chung, H.-J., Al Mamun, A., Alam, S., Aziz, M. A., Emon, N. U., et al. (2022). Role of gut microbiome in autism spectrum disorder and its therapeutic regulation. Frontiers in Cellular & Infection Microbiology, 12, 915701. [CrossRef]
- Tarabeux, J., Kebir, O., Gauthier, J., Hamdan, F. F., Xiong, L., Piton, A., et al. (2011). Rare mutations in N-methyl-D-aspartate glutamate receptors in autism spectrum disorders and schizophrenia. Translational Psychiatry, 1(11), e55–e55. [CrossRef]
- Tartaglione, A. M., Camoni, L., Calamandrei, G., Chiarotti, F., & Venerosi, A. (2024). The contribution of environmental pollutants to the risk of autism and other neurodevelopmental disorders: A systematic review of case-control studies. Neuroscience & Biobehavioral Reviews, 164, 105815. [CrossRef]
- Tarnowska, K., Gruczyńska–Sękowska, E., Kowalska, D., Majewska, E., Kozłowska, M., & Winkler, R. (2023). The opioid excess theory in autism spectrum disorders - is it worth investigating further? Critical Reviews in Food Science & Nutrition, 63(19), 3980–3993. [CrossRef]
- Terrabuio, E., Zenaro, E., & Constantin, G. (2023). The role of the CD8+ T cell compartment in ageing and neurodegenerative disorders. Frontiers in Immunology, 14, 1233870. [CrossRef]
- Theofilopoulos, A. N., Kono, D. H., & Baccala, R. (2017). The multiple pathways to autoimmunity. Nature Immunology, 18(7), 716–724. [CrossRef]
- Theoharides, T. C., Angelidou, A., Alysandratos, K.-D., Zhang, B., Asadi, S., Francis, K., et al. (2012). Mast cell activation and autism. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease, 1822(1), 34–41. [CrossRef]
- Theoharides, T. C., & Doyle, R. (2008). Autism, gut-blood-brain barrier, and mast cells. Journal of Clinical Psychopharmacology, 28(5), 479–483. [CrossRef]
- Theoharides, T. C., Kavalioti, M., & Tsilioni, I. (2019). Mast cells, stress, fear and autism spectrum disorder. International Journal of Molecular Sciences, 20(15), 3611. [CrossRef]
- Theoharides, T. C., Stewart, J. M., Panagiotidou, S., & Melamed, I. (2016). Mast cells, brain inflammation and autism. European Journal of Pharmacology, 778, 96–102. [CrossRef]
- Theoharides, T., Tsilioni, I., Patel, A., & Doyle, R. (2016). Atopic diseases and inflammation of the brain in the pathogenesis of autism spectrum disorders. Translational Psychiatry, 6(6), e844–e844. [CrossRef]
- Tillmann, T., Gibson, A. R., Scott, G., Harrison, O., Dominiczak, A., & Hanlon, P. (2015). Systems Medicine 2.0: Potential Benefits of Combining Electronic Health Care Records with Systems Science Models. J Med Internet Res, 17(3), e64. [CrossRef]
- Toledano, M. P., Sean J. (2015). Autoimmune Epilepsy. Seminars in Neurology, 35(03), 245–258. [CrossRef]
- Tomova, A., Husarova, V., Lakatosova, S., Bakos, J., Vlkova, B., Babinska, K., & Ostatnikova, D. (2015). Gastrointestinal microbiota in children with autism in Slovakia. Physiology & Behavior, 138, 179–187. [CrossRef]
- Toomey, R., Kang, H. K., Karlinsky, J., Baker, D. G., Vasterling, J. J., Alpern, R., et al. (2007). Mental health of US Gulf War veterans 10 years after the war. British Journal of Psychiatry, 190(5), 385–393. [CrossRef]
- Tordjman, S., Celume, M. P., Denis, L., Motillon, T., & Keromnes, G. (2019). Reframing schizophrenia and autism as bodily self-consciousness disorders leading to a deficit of theory of mind and empathy with social communication impairments. Neuroscience & Biobehavioral Reviews, 103, 401–413. [CrossRef]
- Tordjman, S., Somogyi, E., Coulon, N., Kermarrec, S., Cohen, D., Bronsard, G., et al. (2014). Gene× Environment interactions in autism spectrum disorders: role of epigenetic mechanisms. Frontiers in Psychiatry, 5, 53. [CrossRef]
- Torres, E. B., & Denisova, K. (2016). Motor noise is rich signal in autism research and pharmacological treatments. Scientific Reports, 6(1), 37422. [CrossRef]
- Tuchman, R., Moshé, S. L., & Rapin, I. (2009). Convulsing toward the pathophysiology of autism. Brain & Development, 31(2), 95–103. [CrossRef]
- Tzang, R.-F., Chang, C.-H., Chang, Y.-C., & Lane, H.-Y. (2019). Autism Associated With Anti-NMDAR Encephalitis: Glutamate-Related Therapy. Frontiers in Psychiatry, 10. [CrossRef]
- Üçok, A., & Gaebel, W. (2008). Side effects of atypical antipsychotics: a brief overview. World Psychiatry, 7(1), 58–62. [CrossRef]
- Ullah, F., & Kaelber, D. C. (2021). Using large aggregated de-identified electronic health record data to determine the prevalence of common chronic diseases in pediatric patients who visited primary care clinics. Academic Pediatrics, 21(6), 1084–1093. [CrossRef]
- Usui, N., Kobayashi, H., & Shimada, S. (2023). Neuroinflammation and oxidative stress in the pathogenesis of autism spectrum disorder. International Journal of Molecular Sciences, 24(6), 5487. [CrossRef]
- Vakilzadeh, G., & Martinez-Cerdeño, V. (2023). Pathology and astrocytes in autism. Neuropsychiatric Disease & Treatment, 841–850. [CrossRef]
- van den Brink, W., van Bilsen, J., Salic, K., Hoevenaars, F. P., Verschuren, L., Kleemann, R., et al. (2019). Current and future nutritional strategies to modulate inflammatory dynamics in metabolic disorders. Frontiers in Nutrition, 6, 129. [CrossRef]
- van Ommen, B., & Stierum, R. (2002). Nutrigenomics: exploiting systems biology in the nutrition and health arena. Current Opinion in Biotechnology, 13(5), 517–521. [CrossRef]
- Vargas, D. L., Nascimbene, C., Krishnan, C., Zimmerman, A. W., & Pardo, C. A. (2005). Neuroglial activation and neuroinflammation in the brain of patients with autism. Annals of Neurology: Official Journal of the American Neurological Association & the Child Neurology Society, 57(1), 67–81. [CrossRef]
- Venkatasubramanian, G. (2015). Understanding Schizophrenia as a Disorder of Consciousness: Biological Correlates and Translational Implications from Quantum Theory Perspectives. Clinical Psychopharmacology & Neuroscience, 13(1), 36–47. [CrossRef]
- Verkhratsky, A., & Nedergaard, M. (2018). Physiology of astroglia. Physiological Reviews, 98(1), 239–389. [CrossRef]
- Vermeulen, R., Schymanski, E. L., Barabási, A.-L., & Miller, G. W. (2020). The exposome and health: Where chemistry meets biology. Science, 367(6476), 392–396. [CrossRef]
- Vicedo, M. (2024). Moving beyond the search for the first discoverer of autism. Frontiers in Psychiatry, 15, 1266486. [CrossRef]
- Victoria, H. K., & Caldwell, C. (2013). Breathwork in body psychotherapy: Clinical applications. Body, Movement & Dance in Psychotherapy, 8(4), 216–228. [CrossRef]
- Vineis, P., Robinson, O., Chadeau-Hyam, M., Dehghan, A., Mudway, I., & Dagnino, S. (2020). What is new in the exposome? Environment International, 143, 105887. [CrossRef]
- Vivier, E., Raulet, D. H., Moretta, A., Caligiuri, M. A., Zitvogel, L., Lanier, L. L., et al. (2011). Innate or adaptive immunity? The example of natural killer cells. Science, 331(6013), 44–49. [CrossRef]
- Vivier, E., Tomasello, E., Baratin, M., Walzer, T., & Ugolini, S. (2008). Functions of natural killer cells. Nature Immunology, 9(5), 503–510. [CrossRef]
- Vojdani, A., Mumper, E., Granpeesheh, D., Mielke, L., Traver, D., Bock, K., et al. (2008). Low natural killer cell cytotoxic activity in autism: the role of glutathione, IL-2 and IL-15. Journal of Neuroimmunology, 205(1–2), 148–154. [CrossRef]
- Volkmar, F. R., & Nelson, D. S. (1990). Seizure Disorders in Autism. Journal of the American Academy of Child & Adolescent Psychiatry, 29(1), 127–129. [CrossRef]
- Volterra, A., & Meldolesi, J. (2005). Astrocytes, from brain glue to communication elements: the revolution continues. Nature Reviews Neuroscience, 6(8), 626–640. [CrossRef]
- Wakefield, A. (2002). The Gut–Brain Axis in Childhood Developmental Disorders. Journal of Pediatric Gastroenterology & Nutrition, 34 Suppl 1, S14-7. [CrossRef]
- Wakefield, A. J., Puleston, J. M., Montgomery, S. M., Anthony, A., O’Leary, J. J., & Murch, S. H. (2002). The concept of entero-colonic encephalopathy, autism and opioid receptor ligands. Alimentary Pharmacology & Therapeutics, 16(4), 663–674. [CrossRef]
- Walsh, L., Hill, C., & Ross, R. P. (2023). Impact of glyphosate (RoundupTM) on the composition and functionality of the gut microbiome. Gut Microbes, 15(2), 2263935. [CrossRef]
- Wan, M., Ding, L., Wang, D., Han, J., & Gao, P. (2020). Serotonin: A Potent Immune Cell Modulator in Autoimmune Diseases. Frontiers in Immunology, 11. [CrossRef]
- Wang, T., Sternes, P. R., Guo, X.-K., Zhao, H., Xu, C., & Xu, H. (2024). Autoimmune diseases exhibit shared alterations in the gut microbiota. Rheumatology, 63(3), 856–865. [CrossRef]
- Ward, J. H., Weir, E., Allison, C., & Baron-Cohen, S. (2023). Increased rates of chronic physical health conditions across all organ systems in autistic adolescents and adults. Molecular Autism, 14(1), 35. [CrossRef]
- Warren, R. P., Foster, A., & Margaretten, N. C. (1987). Reduced natural killer cell activity in autism. Journal of the American Academy of Child & Adolescent Psychiatry, 26(3), 333–335. [CrossRef]
- Wegiel, J., Chadman, K., London, E., Wisniewski, T., & Wegiel, J. (2024). Contribution of the serotonergic system to developmental brain abnormalities in autism spectrum disorder. Autism Research, 17(7), 1300–1321. [CrossRef]
- Weinberg, S. E., Sena, L. A., & Chandel, N. S. (2015). Mitochondria in the regulation of innate and adaptive immunity. Immunity, 42(3), 406–417.
- Weitlauf, A. S., Gotham, K. O., Vehorn, A. C., & Warren, Z. E. (2014). Brief report: DSM-5 “levels of support:” A comment on discrepant conceptualizations of severity in ASD. Journal of Autism & Developmental Disorders, 44(2), 471–476. [CrossRef]
- Wen, Y., Alshikho, M. J., & Herbert, M. R. (2016). Pathway network analyses for autism reveal multisystem involvement, major overlaps with other diseases and convergence upon MAPK and calcium signaling. PLOS One, 11(4), e0153329. [CrossRef]
- Wen, Y., & Herbert, M. R. (2017). Connecting the dots: Overlaps between autism and cancer suggest possible common mechanisms regarding signaling pathways related to metabolic alterations. Medical Hypotheses, 103, 118–123. [CrossRef]
- Werling, D. M. (2016). The role of sex-differential biology in risk for autism spectrum disorder. Biology of Sex Differences, 7(1), 58. [CrossRef]
- Werner, E., & Dawson, G. (2005). Validation of the phenomenon of autistic regression using home videotapes. Archives of general psychiatry, 62(8), 889–895. [CrossRef]
- West, L., Brunssen, S. H., & Waldrop, J. (2009). Review of the Evidence for Treatment of Children with Autism with Selective Serotonin Reuptake Inhibitors. Journal for Specialists in Pediatric Nursing, 14(3), 183–191. [CrossRef]
- West, L., Waldrop, J., & Brunssen, S. (2009). Pharmacologic Treatment for the Core Deficits and Associated Symptoms of Autism in Children. Journal of Pediatric Health Care, 23(2), 75–89. [CrossRef]
- White, J. F. (2003). Intestinal pathophysiology in autism. Experimental Biology & Medicine, 228(6), 639–649. [CrossRef]
- Whiteley, P., Carr, K., & Shattock, P. (2019). Is autism inborn and lifelong for everyone? Neuropsychiatric disease & Treatment, 2885–2891. [CrossRef]
- Wild, C. P. (2005). Complementing the Genome with an “Exposome”: The Outstanding Challenge of Environmental Exposure Measurement in Molecular Epidemiology. Cancer Epidemiology, Biomarkers & Prevention, 14(8), 1847–1850. [CrossRef]
- Willison, H. J., Jacobs, B. C., & van Doorn, P. A. (2016). Guillain-Barré syndrome. The Lancet, 388(10045), 717–727. [CrossRef]
- Wills, S., Cabanlit, M., Bennett, J., Ashwood, P., Amaral, D. G., & Van de Water, J. (2009). Detection of autoantibodies to neural cells of the cerebellum in the plasma of subjects with autism spectrum disorders. Brain, Behavior, & Immunity, 23(1), 64–74. [CrossRef]
- Witkamp, R. F., & van Norren, K. (2018). Let thy food be thy medicine…. when possible. European Journal of Pharmacology, 836, 102–114. [CrossRef]
- Wood, J. D., Alpers, D. H., & Andrews, P. L. R. (1999). Fundamentals of neurogastroenterology. Gut, 45(suppl 2), II6. [CrossRef]
- Wood, J. J., Kendall, P. C., Wood, K. S., Kerns, C. M., Seltzer, M., Small, B. J., et al. (2020). Cognitive Behavioral Treatments for Anxiety in Children With Autism Spectrum Disorder: A Randomized Clinical Trial. JAMA Psychiatry, 77(5), 474–483. [CrossRef]
- Wu, M., Zheng, W., Song, X., Bao, B., Wang, Y., Ramanan, D., et al. (2024). Gut complement induced by the microbiota combats pathogens and spares commensals. Cell, 187. [CrossRef]
- Xiong, Y., Chen, J., & Li, Y. (2023). Microglia and astrocytes underlie neuroinflammation and synaptic susceptibility in autism spectrum disorder. Frontiers in Neuroscience, 17, 1125428. [CrossRef]
- Xu, G., Snetselaar, L. G., Jing, J., Liu, B., Strathearn, L., & Bao, W. (2018). Association of Food Allergy and Other Allergic Conditions with Autism Spectrum Disorder in Children. JAMA Network Open, 1(2), e180279–e180279. [CrossRef]
- Yavuz, B. R., Arici, M. K., Demirel, H. C., Tsai, C.-J., Jang, H., Nussinov, R., & Tuncbag, N. (2023). Neurodevelopmental disorders and cancer networks share pathways, but differ in mechanisms, signaling strength, and outcome. NPI Genomic Medicine, 8(1), 37. [CrossRef]
- Yeung, A. W. K., Heinrich, M., Kijjoa, A., Tzvetkov, N. T., & Atanasov, A. G. (2020). The ethnopharmacological literature: An analysis of the scientific landscape. Journal of Ethnopharmacology, 250, 112414. [CrossRef]
- Yngve, M., & Lidström, H. (2024). Implementation of information and communication technology to facilitate participation in high school occupations for students with neurodevelopmental disorders. Disability & Rehabilitation: Assistive Technology, 19(5), 2017–2025. [CrossRef]
- Yong, V. W., & Rivest, S. (2009). Taking Advantage of the Systemic Immune System to Cure Brain Diseases. Neuron, 64(1), 55–60. [CrossRef]
- Zanchi, M. M., Marins, K., & Zamoner, A. (2023). Could pesticide exposure be implicated in the high incidence rates of depression, anxiety and suicide in farmers? A systematic review. Environmental Pollution, 331, 121888. [CrossRef]
- Zandman-Goddard, G., Peeva, E., & Shoenfeld, Y. (2007). Gender and autoimmunity. Autoimmunity Reviews, 6(6), 366–372. [CrossRef]
- Zarate-Lopez, D., Torres-Chávez, A. L., Gálvez-Contreras, A. Y., & Gonzalez-Perez, O. (2023). Three decades of valproate: a current model for studying autism spectrum disorder. Current Neuropharmacology, 22(2), 260.
- Zhang, J.-M., & An, J. (2007). Cytokines, inflammation, and pain. International Anesthesiology Clinics, 45(2), 27–37.
- Zhang, S., Han, F., Wang, Q., & Fan, F. (2024). Probiotics and Prebiotics in the Treatment of Autism Spectrum Disorder: A Narrative Review. JIN, 23(1), 20-null. [CrossRef]
- Zhu, J., & Paul, W. E. (2008). CD4 T cells: fates, functions, and faults. Blood, 112(5), 1557–1569. [CrossRef]
- Ziv, Y., & Schwartz, M. (2008). Immune-based regulation of adult neurogenesis: Implications for learning and memory. Brain, Behavior, & Immunity, 22(2), 167–176. [CrossRef]
- Zota, A. R., Riederer, A. M., Ettinger, A. S., Schaider, L. A., Shine, J. P., Amarasiriwardena, C. J., et al. (2016). Associations between metals in residential environmental media and exposure biomarkers over time in infants living near a mining-impacted site. Journal of Exposure Science & Environmental Epidemiology, 26(5), 510–519. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).