1. Introduction
Targeted alpha therapy (TAT) has recently come to the forefront of clinical nuclear medicine as commercial sources of alpha-emitting nuclides such as
212Pb (t
1/2 = 10.6 h, Alpha particle energy E = 6.1 MeV (36%) and 8.8 MeV (64%)) and
225Ac (half-life t
1/2 = 9.9 d, E = 5.8 MeV, 6.3 MeV, 7.1 MeV and 8.4 MeV) are being established [
1,
2]. Chelators such as TCMC (1,4,7,10-tetrakis(carbamoylmethyl)-1,4,7,10-tetraazacyclododecane) [
3], lead-specific chelator (PSC) [
4], and macropa (MCP) [
5] have been developed which form highly stable complexes with the parent nuclides and, very importantly, with some of the daughter nuclides (
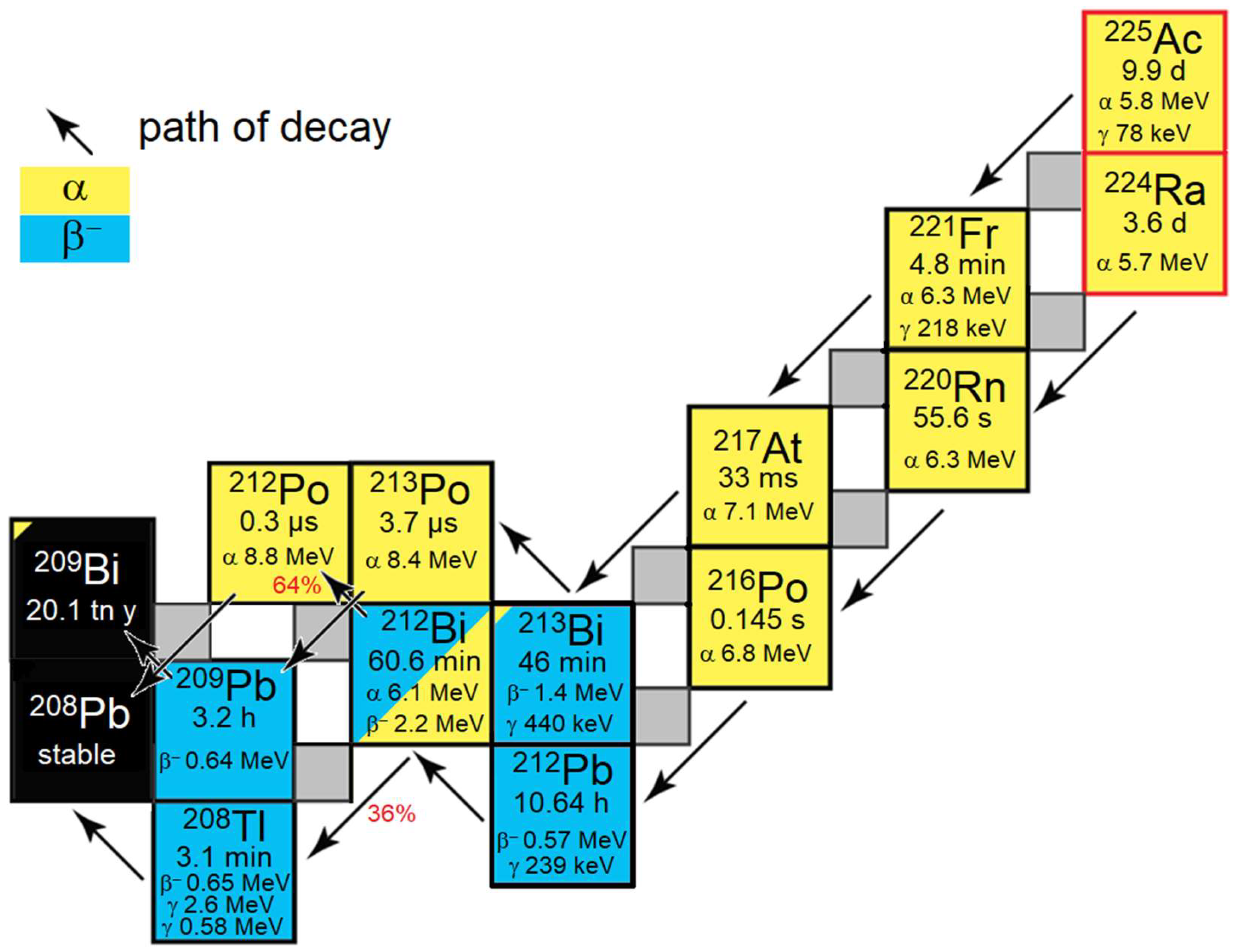
Figure 1). The newly developed chelators outperform the gold standard chelator DOTA (1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid) [
6] in terms of stability and labelling conditions. This has led to the clinical translation of preclinical results [
4,
7] and the introduction of new quality control standards [
8,
9].
The ejection of three alpha particles from
225Ac in relatively short time dramatically reduces the activity used for peptide receptor-targeted therapy by a factor of 1000 [
10] and the ejection of in sum one alpha particle from
212Pb reduces the activity by a factor of 20–30 [
11], while maintaining or even improving the therapeutic outcome. The cytotoxic nature of the alpha particles is mainly due to destruction of Golgi, endoplasmic reticulum, mitochondria or else structures within the cell plasma rather than direct DNA double strand breaks as often cited in the literature [
12], since the
212Pb- and
225Ac-labelled peptides certainly not reach the cell nuclei, but are able to pass the cell membrane at least to some extent. Commercially available DOTA-conjugated peptides used for radiotherapy can be commonly labelled with
177Lu or
90Y but practically also with
225Ac. The advantage is clear: lower activities for the patient are associated with lower doses for the personnel and less shielding material. However, the inadvertent use of alpha-emitting nuclides is linked with a significantly higher dose to personnel compared to beta-emitting nuclides. The only obstacle to widely clinical use of
225Ac is the lack of sources.
225Ac can be produced by cyclotrons using
226Ra targets and bombardment with protons [
1]. Once this defile is overcome, a wide field of
225Ac therapy is open.
A noteworthy characteristic of purified alpha-emitting nuclides mentioned in this work is, that their daughter nuclides are permanent formed during radiosynthesis and quality control and interfere with the determination of true values for the radiochemical purity (RCP) of radiopharmaceuticals measured by standard methods like thin-layer chromatography (TLC) and high-performance liquid chromatography (HPLC). While the RCP cannot be measured by HPLC because the free daughter nuclides always show signals at the beginning of a chromatogram [
7], TLC is very well suited because the paper sheets can be set aside after development to de-cay of the free daughter nuclides until the true RCP of the radiopharmaceuticals can be measured [
13]. A second characteristic is that the purification of these radiopharmaceuticals leads to the separation of non-chelated daughter nuclides from the product and therefore to a different measured activity on dose calibrators compared to the measured activity in equilibrium of parent and daughter nuclides. This affects the calculated radiochemical yield (RCY), which then appears to be lower than the true RCY. Here, certain time-dependent normalization factors for local dose calibrators are useful, but these can only be determined experimentally by comparison with dose measurements performed with a high-purity Germanium (HPGe) detector.
The MiniScanPRO+ is a scintillation counting system that mechanically moves a TLC strip under a collimated detector. During this process, the system records the count rate as a function of position along the strip. Different types of scintillation detectors can be used with the MiniScanPRO+ to make it possible to detect alpha- (ZnS(Ag)), beta- (plastic), and gamma (NaI(Tl)) radiation. The ionising radiation interacts with the scintillator, which produces light pulses that can be detected by a photomultiplier tube. In addition, the instrument can be equipped with a multi-channel analyser (MCA) and a corresponding spectroscopy quality NaI(Tl) detector for nuclide identification.
Compared to the MiniScanPRO+, the AR-2000 is a position sensitive gas filled proportional counter. During the measurement, a thin wire is continuously flushed with an ionisation gas while a high voltage is applied to the wire. Ionising radiation can interact with the gas molecules to form ion pairs. Free electrons from these ion pairs are accelerated towards the anode by the electric field created by the high voltage applied to the wire. As they move, more gas molecules are ionised, creating more ion pairs. This process generates a large number of ion pairs, resulting in a detectable current pulse, that can be correlated to the position of the event along the wire. The AR-2000 allows separate counting of alpha and beta emissions by adjusting the operating voltage. Due to the significantly higher ionisation produced by alpha particles (more than 10 times that of beta particles), alphas can be selectively counted at a reduced high voltage setting. By reducing the high voltage to 1000 V, the AR-2000 effectively excludes beta-emitting isotopes, allowing only alpha particles to be detected. At the standard high voltage of 1500 V, both alpha and beta emissions are measured. To isolate beta emissions, alpha particles can be easily blocked by using a simple shield, such as paper or a thin layer of plastic (e.g. parafilm). As the AR-2000 uses the imaging of an entire TLC lane compared to the scanning of the MiniScanPRO+, it is possible to visualise a single TLC strip in less than a minute with almost 100 times the sensitivity of the MiniScanPRO+.
In this work the results of two different TLC scanners (MiniScanPRO+ and AR-2000; Eckert&Ziegler), were compared on the suitability of RCP measurements of 212Pb- and 225Ac-labelled radiopharmaceuticals for clinical application.
2. Results
Radiochemistry
The radiolabelling of the different precursors was performed with >95% radiochemical yield (RCY) by using previously optimised reaction conditions. Considering this fact, the measurement of the radiochemical purity (RCP) by TLC should only show discrepancies due to different probe development times and measurement times. The following test parameters were used:
Amount of activity required on TLC (1–100 kBq)
Wait time for true RCP after TLC development immediately after synthesis
Wait time for true RCP after TLC development 24 h after synthesis
Wait time for true activity after synthesis and purification for calculation of true RCY
Acceptance criteria: Radiochemical purity >90% right after synthesis and TLC development (prospective), >97% 2–5 h after TLC development (retrospective)
True activity (in Becquerel) for 225Ac-labelled peptides was measured 2 h after the end of synthesis. 1 h after synthesis, the measured activity value was >95% of the true activity. True activity for 212Pb-labelled peptides was measured 4 h after synthesis. 2 h after synthesis the measured activity value was >85% of the true activity. The relatively short half-life of 212Pb leads to significantly lower activity at the equilibrium (e.g. 10 MBq at the end of synthesis are only 7.7 MBq at equilibrium 4 h later). Therefore, the true activity of the freshly purified product should always be calculated using a normalisation factor rather than waiting for equilibrium.
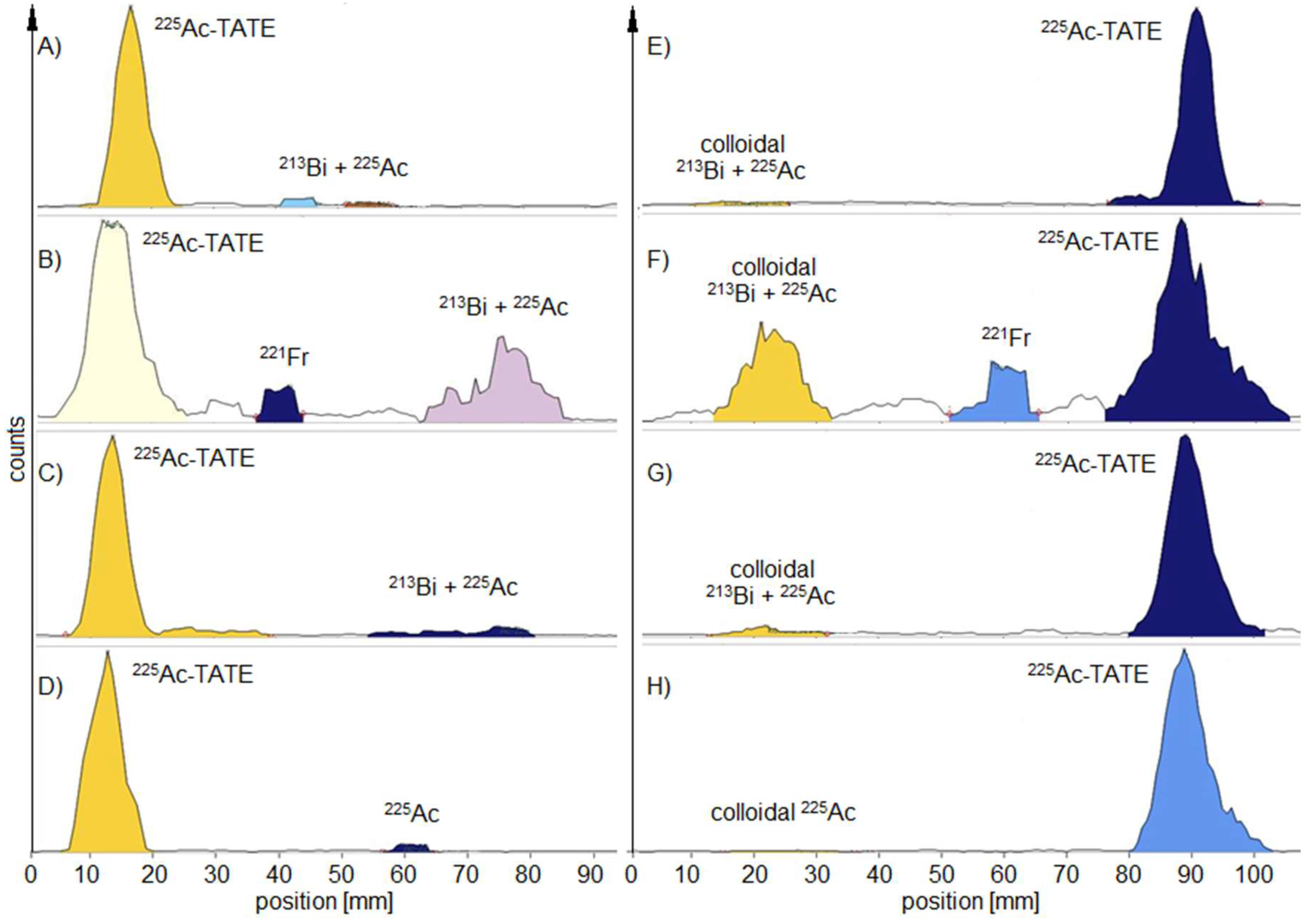
For
225Ac-labelled peptides there was found a rule for determining the true RCP using the MiniScanPRO+: 2 h after TLC development the values of RCP are within the acceptance criteria regardless of when the TLC probe was taken within the first 30 minutes after radiosynthesis (
Table 1). If the TLC probe was taken 24 h after radiosynthesis, the true value of RCP within the acceptance criteria was not measured until 5 h after TLC development (
Figure 2).
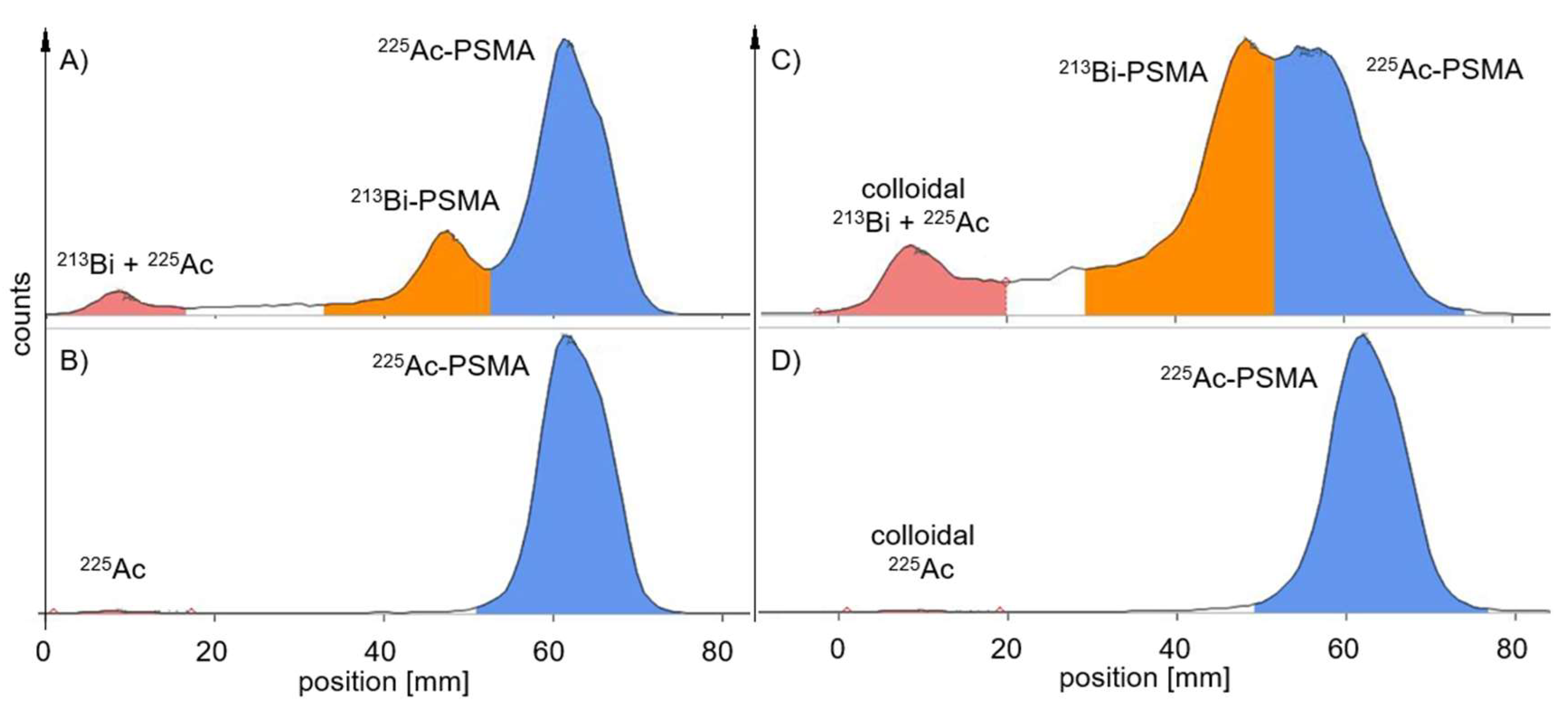
With the AR-2000, the waiting time for true RCP from a probe taken up to 24 h later was reduced to <2 h after development. Furthermore, the AR-2000 measured RCP values within the acceptance criteria for the first 2 hours of TLC probe sampling when applying alpha detection. In addition,
213Bi-labelled PSMA was also found on the TLC with a higher signal when using beta detection compared to alpha detection measurements (
Figure 3).
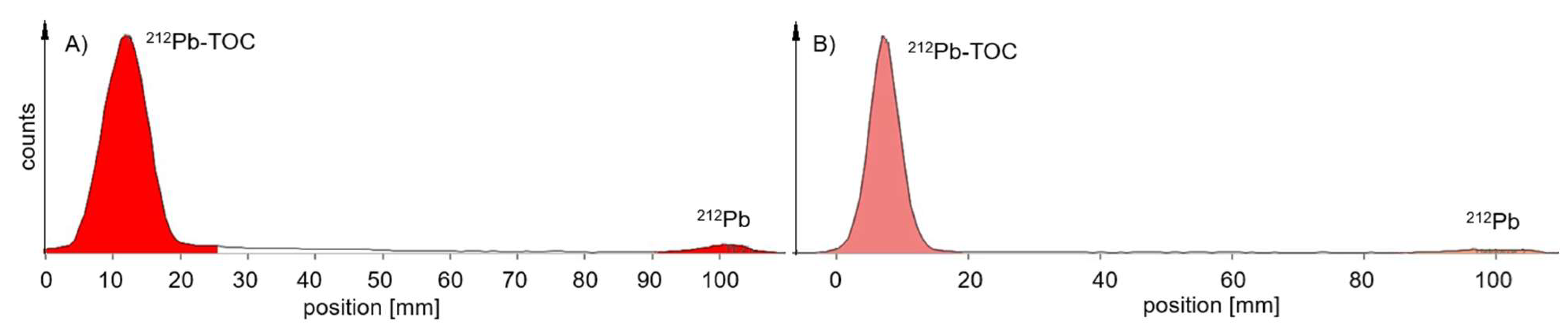
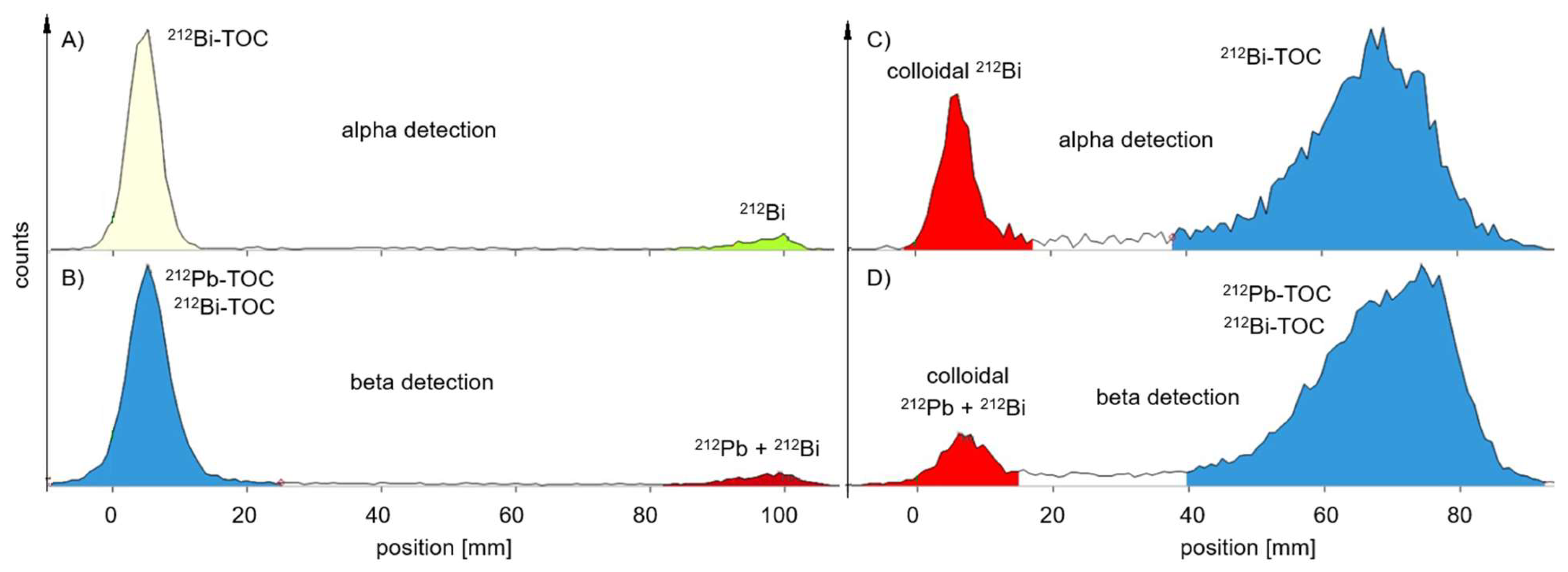
For
212Pb-labelled peptides, the probe sampling did not have such an effect on the values of RCP (
Table 2) for the MiniScanPRO+ and AR-2000 (
Figure 4). A difference was observed when measuring the TLC with the AR-2000 in different modes. In alpha detection, values of RCP were measured within the acceptance criteria on TLC with citrate, when the probe was taken immediately after synthesis up to 2 h after synthesis. In beta detection, values of RCP were measured within the acceptance criteria on TLC with ammonium acetate/methanol (NH
4Ac), when the probe was taken immediately after synthesis up to 2 h after synthesis (
Figure 5). Interestingly, on NH
4Ac sheets the daughter nuclide
212Bi showed a high signal out of the acceptance criteria in alpha detection, whereas in beta detection the sum of free
212Pb and free
212Bi was measured and the result was within the acceptance criteria. Therefore, it can be assumed, that the out of specification impurity (up to 25%) in alpha detection is mainly consisting of colloidal
212Bi, which was not efficiently chelated during the reaction (
Figure 5C). However, most of the
212Pb can be found in the product peak, when measuring the TLC in beta detection (
Figure 5D).
3. Discussion
The term “true radiochemical purity” is defined in this paper as the RCP of the product measured when all daughter nuclides are in equilibrium. Kelly et al. made the same definition for 26 h after TLC development [
13]. However, for
225Ac, mostly a waiting time of one to two half-lives of
213Bi (46–92 minutes) after TLC development is usually sufficient to find radiochemical purities >97%. Therefore, the synthesis protocol has to have two quality requirements for RCP for safety reasons. In routine synthesis, the first quality requirement is RCP >90% (assigned as prospective), because generally all products which reached this quality in the experiments, had RCPs >97% after 2 h waiting time and remeasurement of the TLC (
Table 1). The second quality requirement is for measured product RCPs <90% (assigned as retrospective), which needs to be >97% RCP after 2 h. This has the following consequence for routine quality control: if the measured RCP is >90% (prospective), a second measurement is unnecessary and the quality control can be finished. If the measured RCP is <90%, the quality control has to be elongated to >2 h and the TLC has to be remeasured (retrospective), after the daughter nuclides are decayed. In most of the experiments an RCP <90% was due to later probe sampling more than 5 minutes after the radiosyntheses were finished.
The AR-2000 had a better signal-to-noise ratio for every activity tested on the TLC. It was possible to determine the true radiochemical purity >97% immediately after synthesis without waiting time. On the MiniScanPRO+ it was possible to determine radiochemical purity >90% immediately after synthesis. A short waiting time of 20–40 minutes after TLC development was required to obtain radiochemical purities >95%. Longer waiting times >2 h resulted in the measurement of true radiochemical purities of >97%.
Measurement of each compound was possible with each dilution tested (10 kBq, 100 kBq) and system (MiniScanPRO+ and AR-2000). In addition, the AR-2000 produced readable spectra even at 1 kBq per TLC, whereas the MiniScanPRO+ had a disturbingly high background at this low activity level. The optimal MiniScanPRO+ measuring time was set to 5 mm/s. A slower measuring time might produce better spectra but would not reach the high sensitivity of the AR-2000.
The AR-2000 requires an ionization gas for measurement and lacks an MCA. One flask of this gas holds at least two years, if measurements are performed twice a week. Therefore, the operation of the AR-2000 with ionisation gas is very convenient.
Interesting is the fact, that
225Ac-labelled radiopharmaceuticals showed not such a high radiolysis in the final diluted and sterile solutions (
Table 1, 24 h values) as speculated by the literature [
14]. Of course, if on
225Ac nuclide decays, the ejected alpha particle or its repulsion could hit the rest of the molecule and destroys it by a certain percentage. Since the alpha particle can eject in every possible direction of a sphere, the possibility that it hits directly the molecule it is attached to or else molecules which are in the solution is very low. However, if the
225Ac nuclide decays, it is for sure away from the molecule. But the long half-life of
225Ac means, that on nuclide could stay intact for up to 9.9 days. Fact is, that the strong dilution (8–10 mL) of a very small number of radiolabelled molecules (e.g. 5 MBq
225Ac-PSMA-I&T consists of 50 µg or 33 nmol PSMA-I&T molecules) in the final solution leads to a very low radiolysis, which was observed experimentally in this work. Therefore, a centered production of
225Ac-radiophamarceuticals and distribution for over 24 h might be possible. With up to 5 h waiting time for determination of the true RCP by TLC after 24 h, the
225Ac-radiopharmaceuticals could also be tested for quality on arrival in the clinic in the early morning for application to patients during the daytime. Further, new chelators like PSC and MCP are known to bind the respective radionuclides even at room temperature. Therefore, a certain safety amount of unlabelled precursor within the final solution could lead to a rechelation of daughter nuclides like
212Bi (to PSC) or
213Bi (to MCP). Here, it would be interesting to know, if the strong dilution of the final solution interferes with the theoretical rechelating process. However, a lower dilution could lead to a higher radiolysis. The optimal volume for low radiolysis and high rechelating rate within the final solution will be examined experimentally in the near future.
For
225Ac-labelled radiopharmaceuticals, DTPA may be added to the final solution to prevent a high fraction of free daughter nuclides like
213Bi within the final solution, especially, when the quality control is elongated to >2 h by the above-mentioned procedure. The bounded
213Bi-DTPA might pass faster through the kidneys as compared to free
213Bi, which binds to the kidney physiologically [
15]. However, without DTPA in the final solution, most of the patients show good renal function up to the fourth cycle of
225Ac-therapy in our clinic. However, some patients show less renal function already before the fourth cycle of
225Ac-treatment and adding DTPA to the final solution might elongate the good renal function for those patients. In the literature, a DTPA content of 0.1% should be within the final solution [
10], which either can be added to the dilution agent during automated syntheses [
8] or directly into the final product vial, which might be the better choice. DTPA within the dilution solution might act as scavenger for free
225Ac and could chelate bounded free
225Ac from the purification cartridge and rinse it as
225Ac-DTPA into the product solution. Then, the quality control by TLC would show more
225Ac in the non-product fraction resulting in a wrong true RCP and a complicated situation.
The MiniScanPRO+ has the ability to measure a gamma spectrum with a spectroscopic grade NaI(Tl) detector (
Figure 6). This feature measurement is important, if radio nuclidic purity have to be measured for approval of the product. The sensitivity of the MCA is adequate to differentiate between important γ signals of
212Pb and
225Ac, but actually this test is unnecessary for quality control in most of the cases, since the radio nuclidic purity is also given by the certificate of quality and purity provided by the distributors of the radionuclides.
Theoretically, spectra of 225Ac-peptides that are measured at the start of TLC (e.g. high percentage 225Ac-TATE only when developed with citrate) and the front of TLC (e.g. low percentage free 225Ac and high percentage free 221Fr + 213Bi) might differ in the shape and intensity of the individual gamma signals at 78 keV (225Ac), 218 keV (221Fr) and 440 keV (213Bi). Experimentally, no difference in the peak height of the signals between start and front of the TLC were found, even with high activity in the range of ~37 MBq, as the difference between bound and free 225Ac must be highest. Therefore, with the MCA of the MiniScanPro+, it was not possible to skip the waiting time for determination of the true RCP by performing calculations on the percentage of the product peak by a certain nuclide quotient obtained from the gamma spectrum. Here, only a HPGe might have sufficient sensitivity to detect differences in the peak height of the γ signals between start and front fractions of the TLCs.
To date, the measurement of the radio nuclidic purity is only needed, if it is a requirement by the regulatory authorities for production of alpha-emitting radiopharmaceuticals. To date, there is no such monography for alpha-emitting radiopharmaceuticals, but this would change within the next years as the use of alpha-emitting radiopharmaceuticals in clinic is rising and companies already working on approvals for such alpha-emitting radiopharmaceuticals. An example for beta-emitting radionuclides is 177Lu, which needs the approval of >99.9% radio nuclidic purity, since 177mLu is an important impurity during production of 177Lu and should not exceed 0.1% (Ph. Eur. 11.0 07/2017:2798 corrected 9.3).
4. Materials and Methods
All reagents and solvents were purchased in highest purity from commercial suppliers and were used without further purification. PSC-PEG2-TOC (VMT-α-NET) and 224Ra/212Pb-generator (VMT-α-GEN) were obtained from Perspective Therapeutics Inc., Coralville, Iowa, USA. RCP was monitored by thin-layer chromatography (TLC) on iTLC-SG plates (Agilent, Santa Clara, California, USA). Measurement of the radio nuclidic purity (RNP) and evaluation of the radio-TLC was performed with a thin-layer scanner (MiniScanPRO+, Eckert&Ziegler Eurotope GmbH, Berlin, Germany) equipped with a Model 43-2 alpha detector ZnS(Ag) scintillator (Ludlum Measurements, Sweetwater, Texas, USA) and a build-in multi-channel analyser (MCA) for gamma spectroscopy or with a proportional gas-counter (AR-2000, Eckert&Ziegler Eurotope GmbH, Berlin, Germany) equipped with an ionisation gas (90% Ar / 10% CH4 or 90% Ar / 10% CO2). CORGON-10 was purchased from Linde Gas (Dresden, Germany). Radio-HPLC was performed on a Shimadzu HPLC system (Thermo Scientific, Dreieich, Germany), equipped with a reverse phase column (Analytical: Merck Chromolith RP-18e; 100 × 4.6 mm plus a guard column 5 × 4.6mm and a UV-diode array detector (220 nm). The solvent system used was a gradient of acetonitrile:water (containing 0.05% TFA) (0–13 min: 0–60% MeCN) at a flow rate of 1.6 mL/min unless otherwise stated. The pH was measured with a reflection photometer (QUANTOFIX Relax, Macherey-Nagel GmbH & Co. KG, Düren, Germany).
4.1. Radiochemistry
Automatic
225Ac-radiolabelling of DOTA-TATE or DOTAGA-PSMA-I&T was performed according to an established protocol [
8]. Solid 225Ac was dissolved in 100–500 µl of 0.04 M HClsuprapure. Briefly, 10–20 µg/MBq of precursor in H2Osuprapure was added to a 5 mL conical vial together with 2 mL 0.2 M NaAc/AcOH buffer (pH 5.7, 99,99% trace metal).
Automatic 212Pb-radiolabelling of PSC-PEG2-TOC was performed according to an established protocol (DOI : 10.1186/s41181-024-00305-8). In brief, 0.1 µg/MBq of precursor in H2Osuprapure was added to a 5 mL conical vial together with 100 µL EtOHabsolute, 290 µL 1 M NaAc/AcOH buffer (pH 4, 99,99 % trace metal) and 2 mg sodium ascorbate (Ph.Eur.) in 100 µl H2Osuprapur.
4.2. Thin-Layer Chromatography
RCP was determined by TLC either by cutting the developed TLC in two pieces and measuring their activity using a surface contamination monitor (CoMo-170, Nuvia Instruments, Dresden, Germany) or by TLC scanner.
For the detection of colloidal 225Ac hydroxide, TLC was performed in 1 M NH4Ac:MeOH 1:1 on silica gel-aluminum sheets for 225Ac-PSMA-I&T and on ITLC-SG for 225Ac-TATE.
For the detection of free 225Ac TLC was performed on silica gel-aluminum sheets in 0.1 M citrate buffer (pH 5.0)
For the detection of colloidal 212Pb hydroxide, TLC was performed in 1 M NH4Ac:MeOH 1:1 on ITLC-SG
For the detection of free 212Pb, TLC was performed on ITLC-SG in 0.1 M citrate buffer (pH 5.0)
RNP and exact volume activity were determined using an HPGe detector
10 µL of the product solution was diluted with 990 µL sterile water (1:100) and used for endotoxin level determination using EndoSafe PTS
The pH was determined using a Quantofix pH meter
5. Conclusions
Two TLC scanners were compared for their ability to measure the RCP of radiopharmaceuticals radiolabelled with 212Pb and 225Ac. Both scanners have their advantages and disadvantages. The MiniScanPRO+ is fast, does not require additional equipment and can also measure the gamma spectrum, which may be important for some radiopharmaceutical production sites and regulatory authorities. Unfortunately, measurements of the gamma spectrum revealed no difference in the peak area between high and low percentages of 225Ac in the individual spots at start and front of the TLC, presumably due to the low sensitivity of the MCA.
The AR-2000 requires ionization gas to operate. With independent alpha and beta detection, it was possible to produce clearer TLCs with a better signal-to-noise ratio than the MiniScanPRO+. This eliminated the need for additional waiting time after TLC development. Quality control may be faster than with the MiniScanPRO+ if the waiting time for probe sampling is longer than five minutes after radiosynthesis. However, the AR-2000 cannot measure a gamma spectrum, but as mentioned above, this additional measurement may not be required by regulatory authorities as the radionuclides themselves already have a certificate of quality and purity when they are delivered.
In our opinion, the best quality control setup would consist of both scanners as they support each other. Otherwise the decision depends on the local regulatory authorities and the head of quality control, who have to consider which scanner would better fit into a safe routine quality control of alpha radiopharmaceuticals.
Author Contributions
Conceptualization, M.P., J.W., and H.H.; methodology, M.P., H.H. and J.W.; software, J.W.; validation, M.P. and J.W.; formal analysis, M.P., and J.W.; investigation, M.P., J.W., and H.H.; resources, J.W., R.F. and J.K.; data curation, M.P., J.W.; writing—original draft preparation, M.P.; writing—review and editing, J.W., R.F., R.A.B, and E.M.; visualization, M.P.; supervision, M.P., R.F., and E.M.; project administration, R.A.B, J.K. and E.M; funding acquisition, M.P., R.A.B, J.K. and E.M.. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
All data can be referred to on request to the corresponding author.
Acknowledgments
The authors want to thank Petra Gehlmann and Kerstin Wetzig for their excellent technical assistance. Moreover, we thank Lisa Hübinger for the extensive proofreading of the manuscript. Further, the authors also acknowledge support of Perspective Therapeutics in form of providing the 212Pb-generator VMT-α-GEN and the VMT-α-NET precursor.
Conflicts of Interest
The MiniScanPRO+ and AR-2000 were provided by Eckert&Ziegler especially for evaluation of α-particles on TLC. J.W. is employed by Eckert&Ziegler, which has financial interest in distribution of TLC scanners. The authors declare no further conflict of interest.
References
- Apostolidis, C.; Molinet, R.; McGinley, J.; Abbas, K.; Mollenbeck, J.; Morgenstern, A. Cyclotron production of Ac-225 for targeted alpha therapy. Appl. Radiat. Isot. 2005, 62, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Pruszyński, M.; Walczak, R.; Rodak, M.; Bruchertseifer, F.; Morgenstern, A.; Bilewicz, A. Radiochemical separation of 224Ra from 232U and 228Th sources for 224Ra/212Pb/212Bi generator. Appl. Radiat. Isot. 2021, 172, 109655. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, J.C.; Schäfer, M.; Bauder-Wüst, U.; Lehnert, W.; Leotta, K.; Morgenstern, A.; Kopka, K.; Haberkorn, U.; Mier, W.; Kratochwil, C. Development and dosimetry of 203Pb/212Pb-labelled PSMA ligands: bringing "the lead" into PSMA-targeted alpha therapy? Eur. J. Nucl. Med. Mol. Imaging. 2019, 46, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Baumhover, N.J.; Liu, D.; Cagle, B.S.; Boschetti, F.; Paulin, G.; Lee, D.; Dai, Z.; Obot, E.R.; Marks, B.M. , et al. Preclinical evaluation of a lead specific chelator (PSC) conjugated to radiopeptides for 203Pb and 212Pb-Based theranostics. Pharmaceutics 2023, 15. [Google Scholar] [CrossRef]
- Thiele, N.A.; Brown, V.; Kelly, J.M.; Amor-Coarasa, A.; Jermilova, U.; MacMillan, S.N.; Nikolopoulou, A.; Ponnala, S.; Ramogida, C.F.; Robertson, A.K.H. , et al. An Eighteen-Membered Macrocyclic Ligand for Actinium-225 Targeted Alpha Therapy. Angew Chem Int Ed Engl 2017, 56, 14712–14717. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, X.; Quinn, T.P.; Lee, D.; Liu, D.; Kunkel, F.; Zimmerman, B.E.; McAlister, D.; Olewein, K.R.; Menda, Y. , et al. Automated cassette-based production of high specific activity [203/212Pb] peptide-based theranostic radiopharmaceuticals for image-guided radionuclide therapy for cancer. Appl. Radiat. Isot. 2017, 127, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Reissig, F.; Bauer, D.; Zarschler, K.; Novy, Z.; Bendova, K.; Ludik, M.C.; Kopka, K.; Pietzsch, H.J.; Petrik, M.; Mamat, C. Towards targeted alpha therapy with Actinium-225: Chelators for mild condition radiolabeling and targeting PSMA - A proof of concept study. Cancers 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Pretze, M.; Kunkel, F.; Runge, R.; Freudenberg, R.; Braune, A.; Hartmann, H.; Schwarz, U.; Brogsitter, C.; Kotzerke, J. Ac-EAZY! Towards GMP-compliant module syntheses of 225Ac-labeled peptides for clinical application. Pharmaceuticals 2021, 14. [Google Scholar] [CrossRef]
- Pretze, M.; Michler, E.; Runge, R.; Wetzig, K.; Tietze, K.; Brandt, F.; Schultz, M.K.; Kotzerke, J. Influence of the Molar Activity of 203/212Pb-PSC-PEG2-TOC on Somatostatin Receptor Type 2-Binding and Cell Uptake. Pharmaceuticals (Basel) 2023, 16, 1605. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Bruchertseifer, F.; Giesel, F.L.; Weis, M.; Verburg, F.A.; Mottaghy, F.; Kopka, K.; Apostolidis, C.; Haberkorn, U.; Morgenstern, A. 225Ac-PSMA-617 for PSMA-targeted α-radiation therapy of metastatic castration-resistant prostate cancer. J. Nucl. Med. 2016, 57, 1941–1944. [Google Scholar] [CrossRef] [PubMed]
- Delpassand, E.S.; Tworowska, I.; Esfandiari, R.; Torgue, J.; Hurt, J.; Shafie, A.; Núnez, R. Targeted α-emitter therapy with 212Pb-DOTAMTATE for the treatment of metastatic SSTR-expressing neuroendocrine tumors: first-in-humans dose-escalation clinical trial. J. Nucl. Med. 2022, 63, 1326–1333. [Google Scholar] [CrossRef] [PubMed]
- Graf, F.; Fahrer, J.; Maus, S.; Morgenstern, A.; Bruchertseifer, F.; Venkatachalam, S.; Fottner, C.; Weber, M.M.; Huelsenbeck, J.; Schreckenberger, M. , et al. DNA double strand breaks as predictor of efficacy of the alpha-particle emitter Ac-225 and the electron emitter Lu-177 for somatostatin receptor targeted radiotherapy. PLoS One 2014, 9, e88239. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.M.; Amor-Coarasa, A.; Sweeney, E.; Wilson, J.J.; Causey, P.W.; Babich, J.W. A suitable time point for quantifying the radiochemical purity of 225Ac-labeled radiopharmaceuticals. EJNMMI Radiopharm. Chem. 2021, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Hamisu, A.; Khiter, O.; Al-Zhrani, S.; Haridh, W.S.B.; Al-Hadeethi, Y.; Sayyed, M.I.; Tijani, S.A. The use of nanomaterial polymeric materials as ionizing radiation shields. Radiatiation Physics and Chemistry 2024, 216. [Google Scholar] [CrossRef]
- Essler, M.; Gartner, F.C.; Neff, F.; Blechert, B.; Senekowitsch-Schmidtke, R.; Bruchertseifer, F.; Morgenstern, A.; Seidl, C. Therapeutic efficacy and toxicity of 225Ac-labelled vs. 213Bi-labelled tumour-homing peptides in a preclinical mouse model of peritoneal carcinomatosis. Eur J Nucl Med Mol Imaging 2012, 39, 602–612. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).