Submitted:
20 November 2024
Posted:
22 November 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
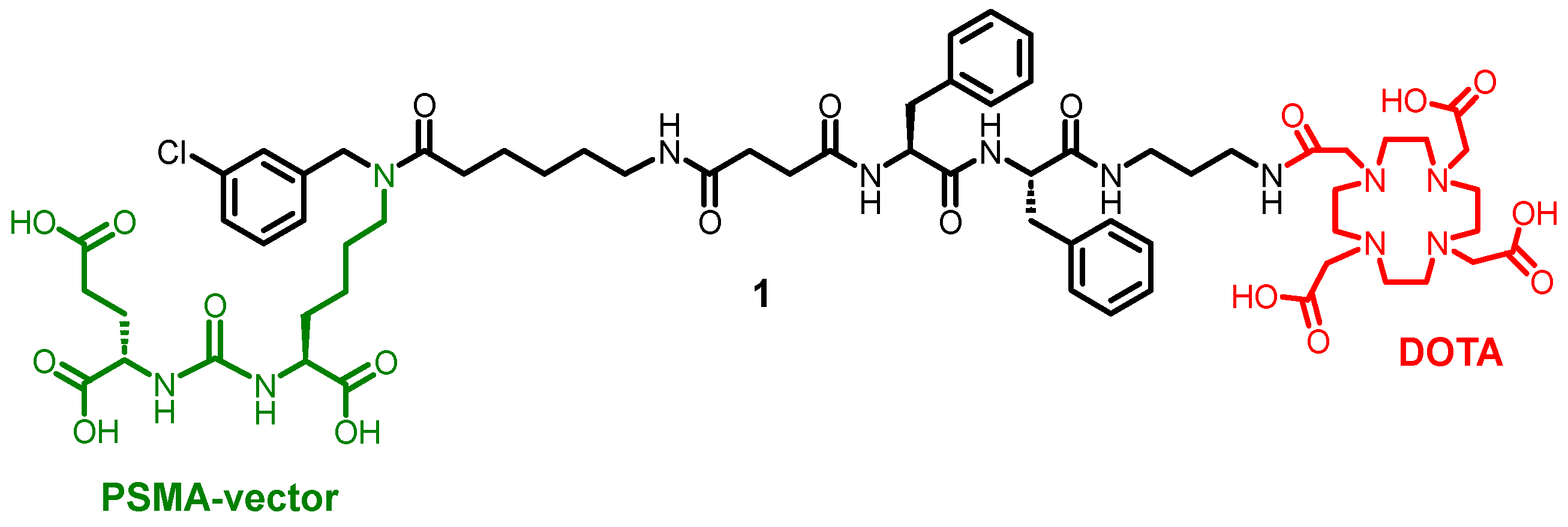
Labeling conditions
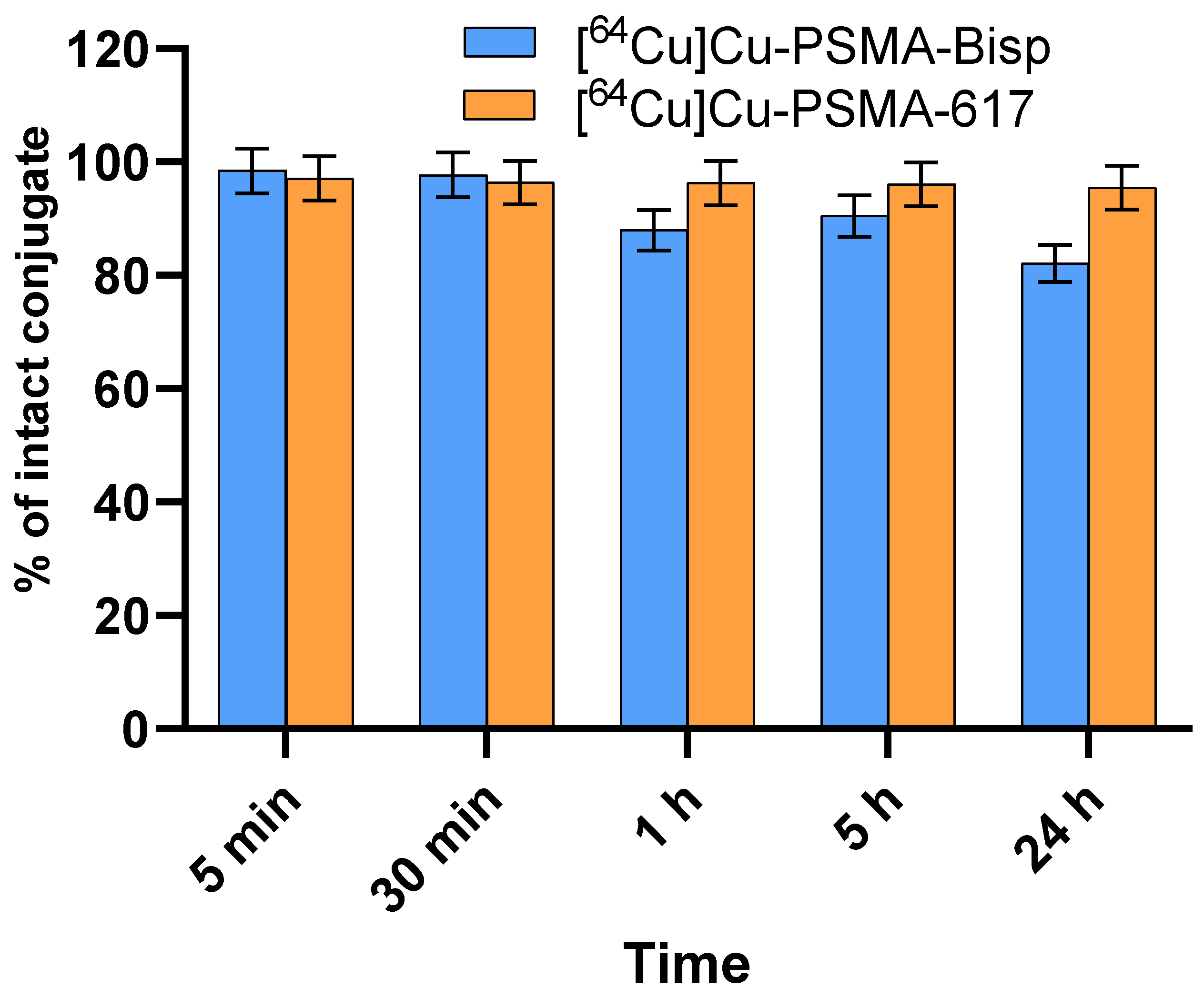
Stability
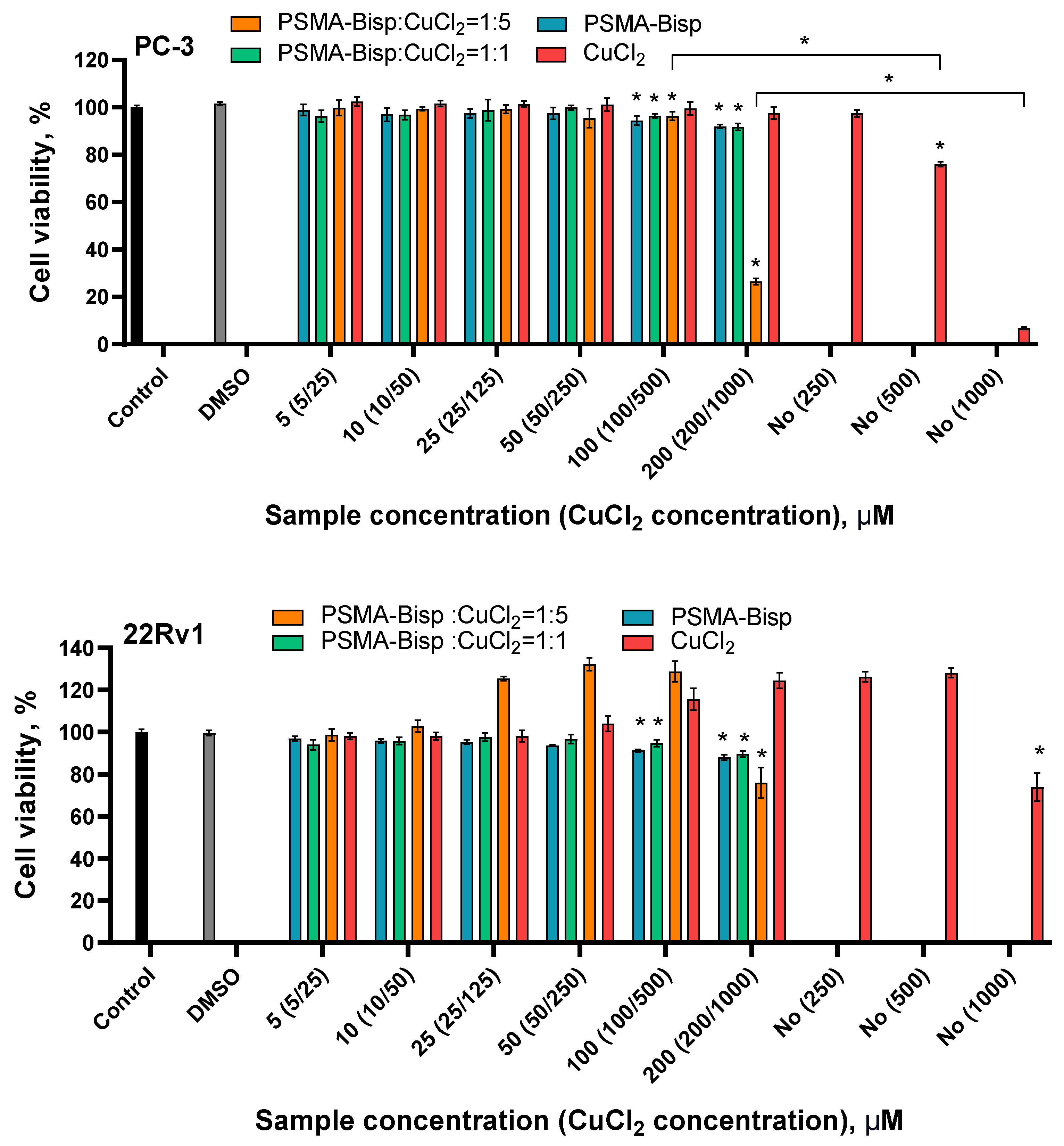
ROS level and cytotoxicity evaluation
3. Materials and Methods
3.1. General
3.2. Synthesis
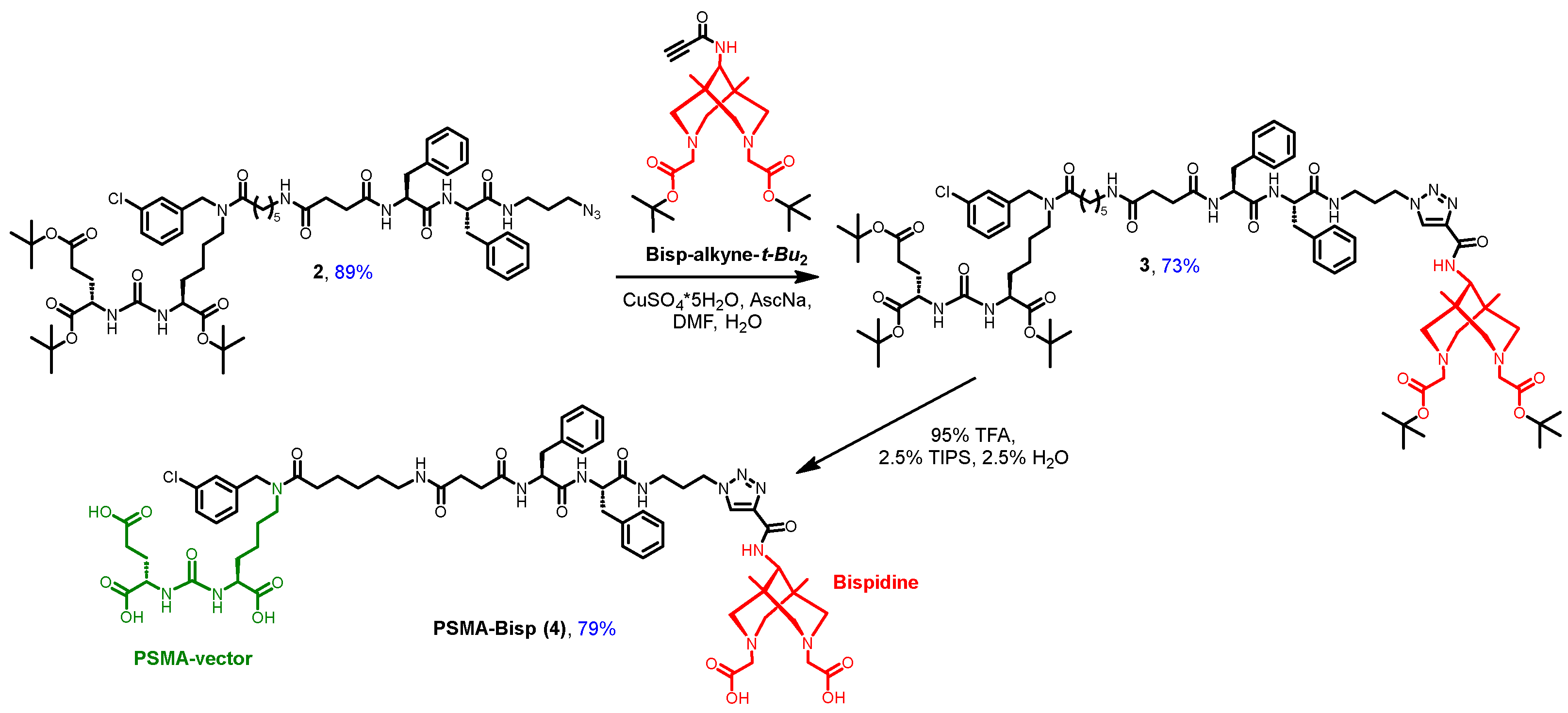
- Synthesis of compound 3.
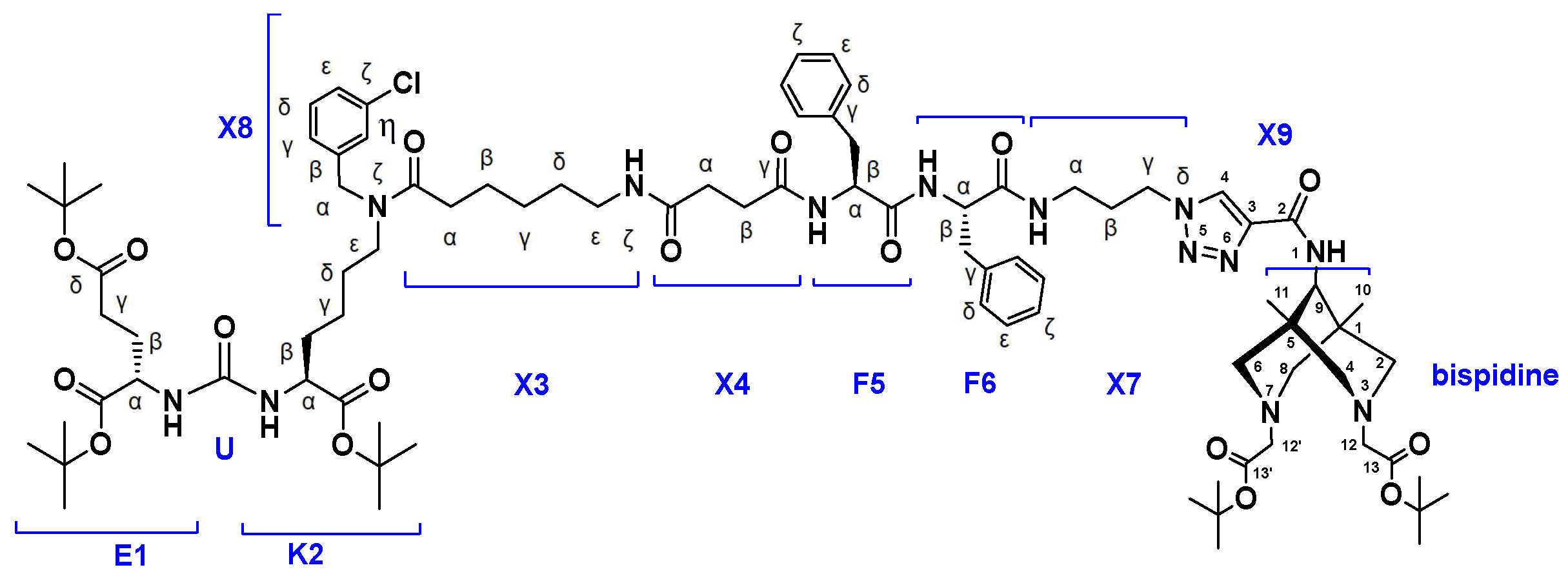
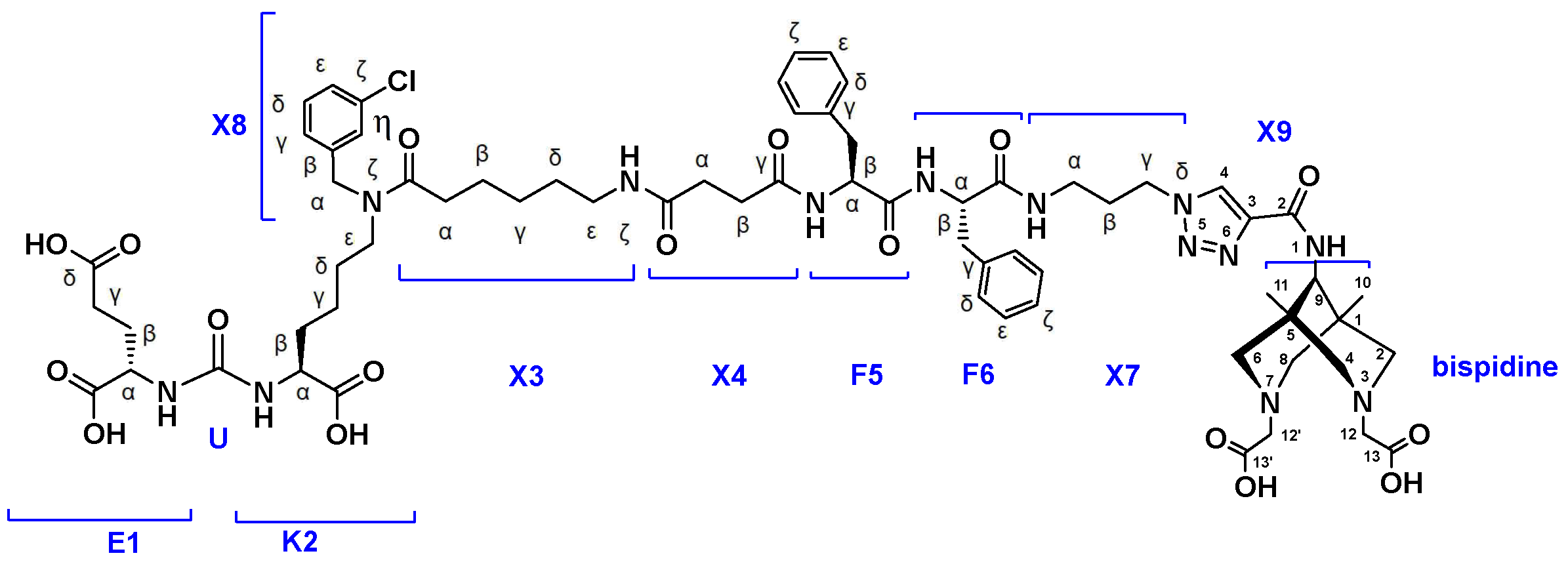
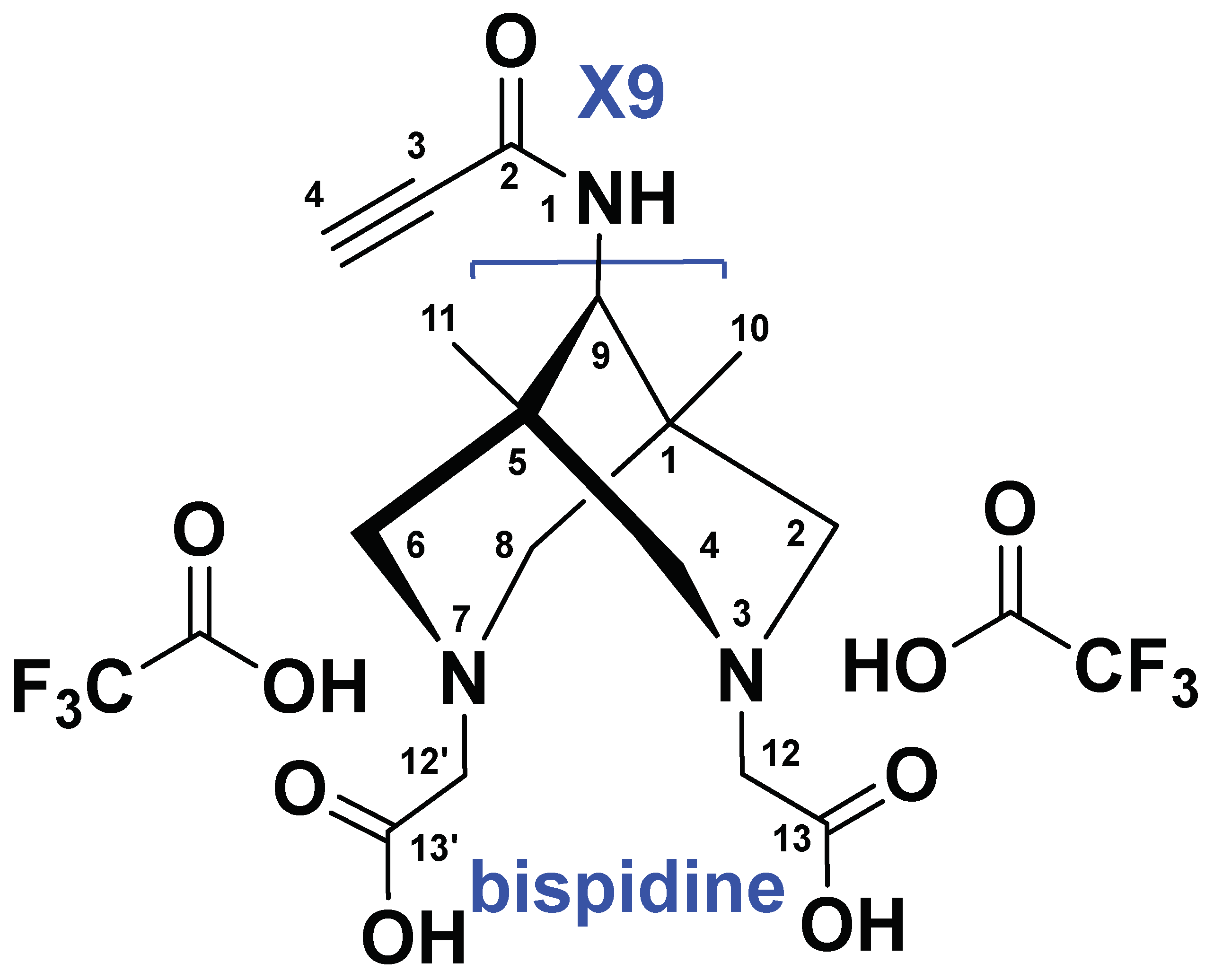
- Synthesis of compound 4 (PSMA-Bisp).
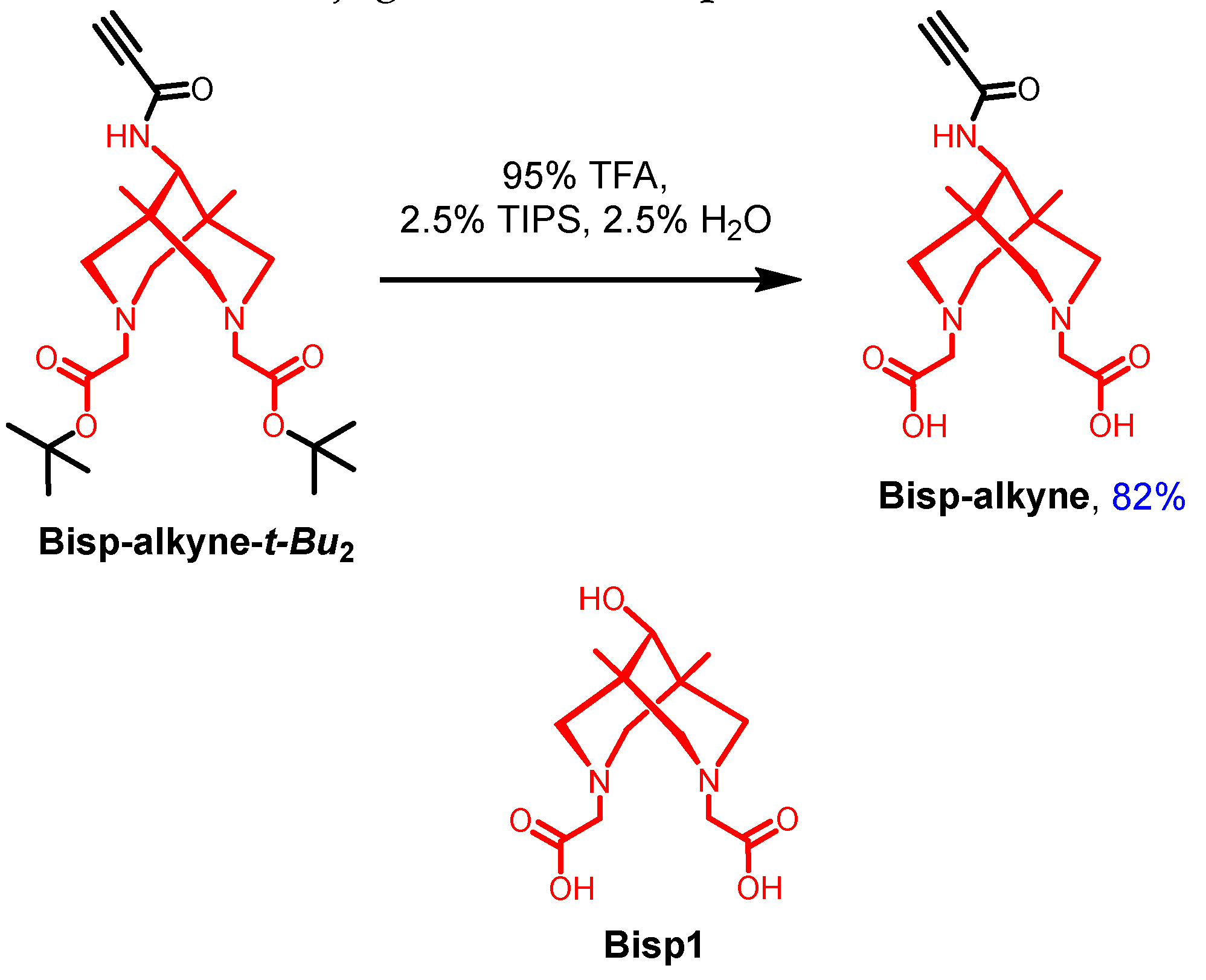
- Synthesis of compound Bisp-alkyne.
3.3. Isolation of 64Cu
3.4. Measurement of radioactivity
3.5. Labeling experiments
3.6. Thin-layer chromatography
3.7. In vitro evaluation
3.8. Cell Lines
3.9. Single cell ROS and Cu2+ Measurement by Using nanoelectrodes
3.10. Cytotoxicity
4. Conclusions
Author Contributions.
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Petrov, S.A.; Zyk, N.Y.; Machulkin, A.E.; Beloglazkina, E.K.; Majouga, A.G. PSMA-Targeted Low-Molecular Double Conjugates for Diagnostics and Therapy. Eur J Med Chem 2021, 225, 113752. [Google Scholar] [CrossRef]
- Sharifi, M.; Yousefnia, H.; Zolghadri, S.; Bahrami-Samani, A.; Naderi, M.; Jalilian, A.R.; Geramifar, P.; Beiki, D. Preparation and Biodistribution Assessment of 68Ga-DKFZ-PSMA-617 for PET Prostate Cancer Imaging. Nuclear Science and Techniques 2016, 27. [Google Scholar] [CrossRef]
- Giesel, F.L.; Cardinale, J.; Schäfer, M.; Neels, O.; Benešová, M.; Mier, W.; Haberkorn, U.; Kopka, K.; Kratochwil, C. 18F-Labelled PSMA-1007 Shows Similarity in Structure, Biodistribution and Tumour Uptake to the Theragnostic Compound PSMA-617. Eur J Nucl Med Mol Imaging 2016, 43, 1929–1930. [Google Scholar] [CrossRef]
- Afshar-Oromieh, A.; Hetzheim, H.; Kratochwil, C.; Benesova, M.; Eder, M.; Neels, O.C.; Eisenhut, M.; Kübler, W.; Holland-Letz, T.; Giesel, F.L.; et al. The Theranostic PSMA Ligand PSMA-617 in the Diagnosis of Prostate Cancer by PET/CT: Biodistribution in Humans, Radiation Dosimetry, and First Evaluation of Tumor Lesions. Journal of Nuclear Medicine 2015, 56, 1697–1705. [Google Scholar] [CrossRef]
- Hasnowo, L.A.; Larkina, M.S.; Plotnikov, E.; Bodenko, V.; Yuldasheva, F.; Stasyuk, E.; Petrov, S.A.; Zyk, N.Y.; Machulkin, A.E.; Vorozhtsov, N.I.; et al. Synthesis, 123I-Radiolabeling Optimization, and Initial Preclinical Evaluation of Novel Urea-Based PSMA Inhibitors with a Tributylstannyl Prosthetic Group in Their Structures. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Krasnovskaya, O.O.; Abramchuck, D.; Erofeev, A.; Gorelkin, P.; Kuznetsov, A.; Shemukhin, A.; Beloglazkina, E.K. Recent Advances in 64Cu/67Cu-Based Radiopharmaceuticals. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef]
- Hussain, M.; Qaim, S.M.; Spahn, I.; Aslam, M.N.; Neumaier, B. Copper Radionuclides for Theranostic Applications: Towards Standardisation of Their Nuclear Data. A Mini-Review. Front Chem 2023, 11. [Google Scholar] [CrossRef]
- Kubeil, M.; Neuber, C.; Starke, M.; Arndt, C.; Rodrigues Loureiro, L.; Hoffmann, L.; Feldmann, A.; Bachmann, M.; Pietzsch, J.; Comba, P.; et al. 64Cu Tumor Labeling with Hexadentate Picolinic Acid-Based Bispidine Immunoconjugates. Chemistry – A European Journal 2024, 30, e202400366. [Google Scholar] [CrossRef] [PubMed]
- Kopp, I.; Cieslik, P.; Anger, K.; Josephy, T.; Neupert, L.; Velmurugan, G.; Gast, M.; Wadepohl, H.; Brühlmann, S.A.; Walther, M.; et al. Bispidine Chelators for Radiopharmaceutical Applications with Lanthanide, Actinide, and Main Group Metal Ions. Inorg Chem 2023, 62, 20754–20768. [Google Scholar] [CrossRef] [PubMed]
- Kovács, A. Metal-Ligand Bonding in Bispidine Chelate Complexes for Radiopharmaceutical Applications. Struct Chem 2023, 34, 5–15. [Google Scholar] [CrossRef]
- Cieslik, P.; Kubeil, M.; Zarschler, K.; Ullrich, M.; Brandt, F.; Anger, K.; Wadepohl, H.; Kopka, K.; Bachmann, M.; Pietzsch, J.; et al. Toward Personalized Medicine: One Chelator for Imaging and Therapy with Lutetium-177 and Actinium-225. J Am Chem Soc 2022, 144, 21555–21567. [Google Scholar] [CrossRef] [PubMed]
- Comba, P.; Starke, M.; Wadepohl, H. Optimization of Hexadentate Bispidine Ligands as Chelators for 64CuII PET Imaging. Chempluschem 2018, 83, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Medved’ko, A. V; Egorova, B. V; Komarova, A.A.; Rakhimov, R.D.; Krut’ko, D.P.; Kalmykov, S.N.; Vatsadze, S.Z. Copper–Bispidine Complexes: Synthesis and Complex Stability Study. ACS Omega 2016, 1, 854–867. [Google Scholar] [CrossRef]
- Roux, A.; Gillet, R.; Huclier-Markai, S.; Ehret-Sabatier, L.; Charbonnière, L.J.; Nonat, A.M. Bifunctional Bispidine Derivatives for Copper-64 Labelling and Positron Emission Tomography. Org. Biomol. Chem. 2017, 15, 1475–1483. [Google Scholar] [CrossRef]
- Voráčová, I.; Vaněk, J.; Pasulka, J.; Střelcová, Z.; Lubal, P.; Hermann, P. Dissociation Kinetics Study of Copper(II) Complexes of DO3A, DOTA and Its Monosubstituted Derivatives. Polyhedron 2013, 61, 99–104. [Google Scholar] [CrossRef]
- Joyner, J.C.; Cowan, J.A. Target-Directed Catalytic Metallodrugs. Brazilian Journal of Medical and Biological Research 2013, 46, 465–485. [Google Scholar] [CrossRef]
- Lilly Thankamony, A.S.; Wittmann, J.J.; Kaushik, M.; Corzilius, B. Dynamic Nuclear Polarization for Sensitivity Enhancement in Modern Solid-State NMR. Prog Nucl Magn Reson Spectrosc 2017, 102–103, 120–195. [Google Scholar] [CrossRef]
- Rinne, S.S.; Leitao, C.D.; Mitran, B.; Bass, T.Z.; Andersson, K.G.; Tolmachev, V.; Ståhl, S.; Löfblom, J.; Orlova, A. Optimization of HER3 Expression Imaging Using Affibody Molecules: Influence of Chelator for Labeling with Indium-111. Sci Rep 2019, 9, 655. [Google Scholar] [CrossRef]
- Rinne, S.S.; Dahlsson Leitao, C.; Gentry, J.; Mitran, B.; Abouzayed, A.; Tolmachev, V.; Ståhl, S.; Löfblom, J.; Orlova, A. Increase in Negative Charge of 68Ga/Chelator Complex Reduces Unspecific Hepatic Uptake but Does Not Improve Imaging Properties of HER3-Targeting Affibody Molecules. Sci Rep 2019, 9, 17710. [Google Scholar] [CrossRef]
- Machulkin, A.E.; Petrov, S.A.; Bodenko, V.; Larkina, M.S.; Plotnikov, E.; Yuldasheva, F.; Tretyakova, M.; Bezverkhniaia, E.; Zyk, N.Yu.; Stasyuk, E.; et al. Synthesis and Preclinical Evaluation of Urea-Based Prostate-Specific Membrane Antigen-Targeted Conjugates Labeled with 177Lu. ACS Pharmacol Transl Sci 2024, 7, 1457–1473. [Google Scholar] [CrossRef] [PubMed]
- Machulkin, A.E.; Shafikov, R.R.; Uspenskaya, A.A.; Petrov, S.A.; Ber, A.P.; Skvortsov, D.A.; Nimenko, E.A.; Zyk, N.U.; Smirnova, G.B.; Pokrovsky, V.S.; et al. Synthesis and Biological Evaluation of PSMA Ligands with Aromatic Residues and Fluorescent Conjugates Based on Them. J Med Chem 2021, 64, 4532–4552. [Google Scholar] [CrossRef] [PubMed]
- Kalinin, M.A.; Medved’ko, A. V; Minyaev, M.E.; Vatsadze, S.Z. Synthesis of N,N′-Unsymmetrical 9-Amino-5,7-Dimethyl-Bispidines. J Org Chem 2023, 88, 7272–7280. [Google Scholar] [CrossRef] [PubMed]
- Mozhaitsev, E.S.; Ponomarev, K.Y.; Patrusheva, O.S.; Medvedko, A. V; Dalinger, A.I.; Rogachev, A.D.; Komarova, N.I.; Korchagina, D. V; Suslov, E. V; Volcho, K.P.; et al. Conjugates of Bispidine and Monoterpenoids as Ligands of Metal Complex Catalysts for the Henry Reaction. Russian Journal of Organic Chemistry 2020, 56, 1969–1981. [Google Scholar] [CrossRef]
- Cui, C.; Hanyu, M.; Hatori, A.; Zhang, Y.; Xie, L.; Ohya, T.; Fukada, M.; Suzuki, H.; Nagatsu, K.; Jiang, C.; et al. Synthesis and Evaluation of [ 64 Cu]PSMA-617 Targeted for Prostate-Specific Membrane Antigen in Prostate Cancer; 2017; Vol. 7.
- Vaneev, A.N.; Gorelkin, P. V.; Garanina, A.S.; Lopatukhina, H. V.; Vodopyanov, S.S.; Alova, A. V.; Ryabaya, O.O.; Akasov, R.A.; Zhang, Y.; Novak, P.; et al. In Vitro and In Vivo Electrochemical Measurement of Reactive Oxygen Species After Treatment with Anticancer Drugs. Anal Chem 2020, 92, 8010–8014. [Google Scholar] [CrossRef]
- Timoshenko, R. V.; Gorelkin, P. V.; Vaneev, A.N.; Krasnovskaya, O.O.; Akasov, R.A.; Garanina, A.S.; Khochenkov, D.A.; Iakimova, T.M.; Klyachko, N.L.; Abakumova, T.O.; et al. Electrochemical Nanopipette Sensor for In Vitro/In Vivo Detection of Cu 2+ Ions. Anal Chem 2024, 96, 127–136. [Google Scholar] [CrossRef]
- Tietze L.F.; Eicher T.; Diederichsen U.; Speicher A.; Schützenmeister N. Reactions and Syntheses: In the Organic Chemistry Laboratory; Wiley-VCH, 2015; ISBN 978-3-527-33814-6.
- Zyk, N.Y.; Ber, A.P.; Nimenko, E.A.; Shafikov, R.R.; Evteev, S.A.; Petrov, S.A.; Uspenskaya, A.A.; Dashkova, N.S.; Ivanenkov, Y.A.; Skvortsov, D.A.; et al. Synthesis and Initial in Vitro Evaluation of PSMA-Targeting Ligands with a Modified Aromatic Moiety at the Lysine ε-Nitrogen Atom. Bioorg Med Chem Lett 2022, 71, 128840. [Google Scholar] [CrossRef]
- Machulkin, A.; Shafikov, R.; Uspenskaya, A.; Petrov, S.; Ber, A.; Skvortsov, D.; Nimenko, E.; Zyk, N.; Smirnova, G.; Pokrovsky, V.; et al. Synthesis and Biological Evaluation of PSMA Ligands with Aromatic Residues and Fluorescent Conjugates Based on Them. J Med Chem 64, 4532–4552. [CrossRef]
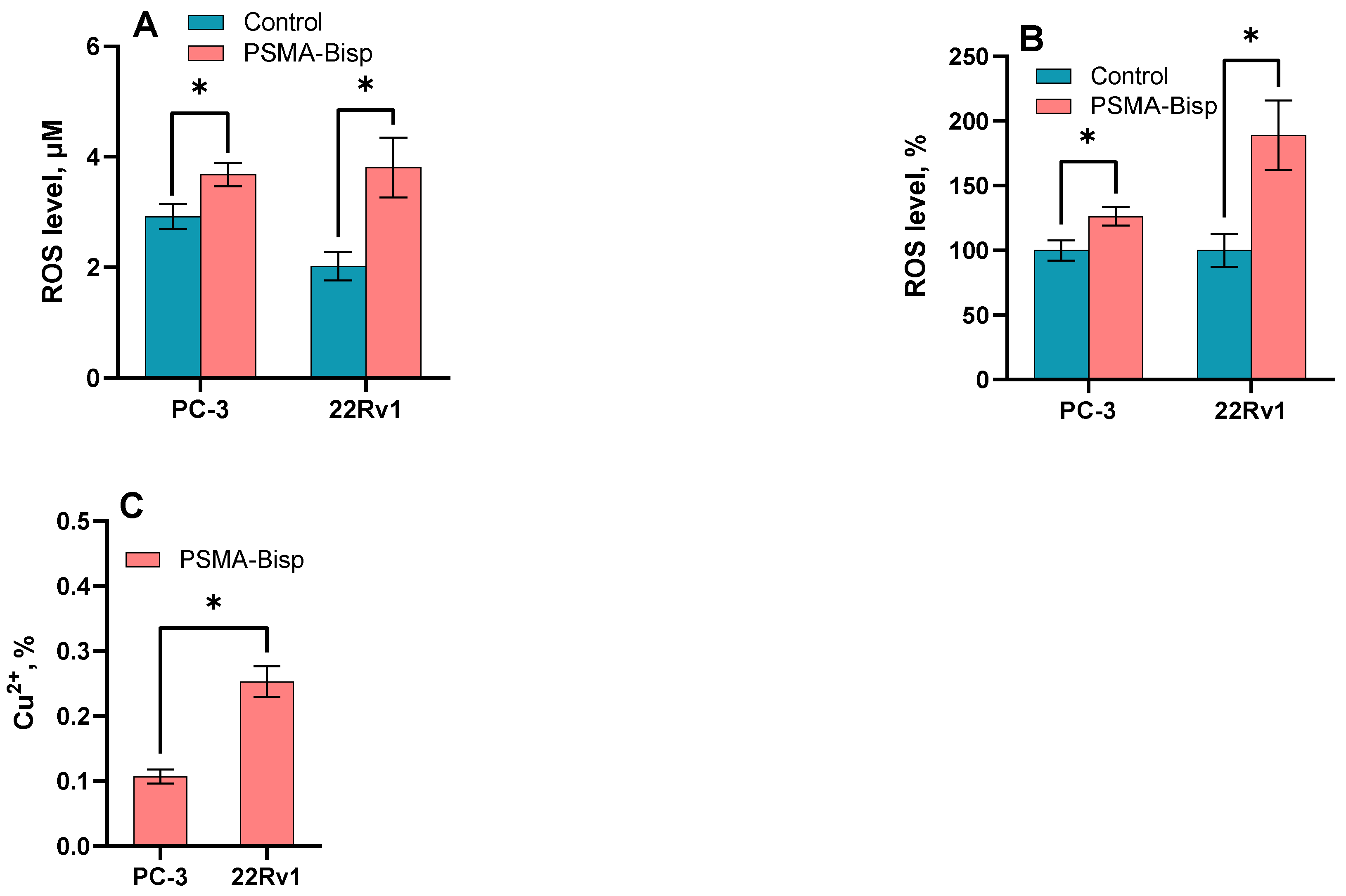
| c(L), µM | 10 | 20 | 50 | 100 | 200 | 500 | 1000 |
|---|---|---|---|---|---|---|---|
| [64Cu]Cu-Bisp1, % | - | 19 | 38 | 76 | 96 | 97 | 98 |
| [64Cu]Cu-Bisp-alkyne, % | 17 | 31 | 71 | 100 | 100 | 100 | - |
| [64Cu]Cu-PSMA-Bisp, % | - | - | 23 | 42 | - | 99 | 99 |
| % [64Cu]Cu-PSMA-Bisp | Mg2+ 10 mM |
Ca2+ 50 mM |
Zn2+ 1 mM |
Cu2+ 1 mM |
Fe3+ 1mM |
| γ-spectrometry | 98 ± 1 | 98 ± 1 | 93 ± 1 | 100 ± 1 | 100 ± 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





