Submitted:
20 November 2024
Posted:
21 November 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. PFAS as Emerging Obesogens
2.1. Integrative Approaches to Understanding PFAS Toxicity and Obesogenicity
2.2. PFAS Associated Maternal and Childhood Obesity
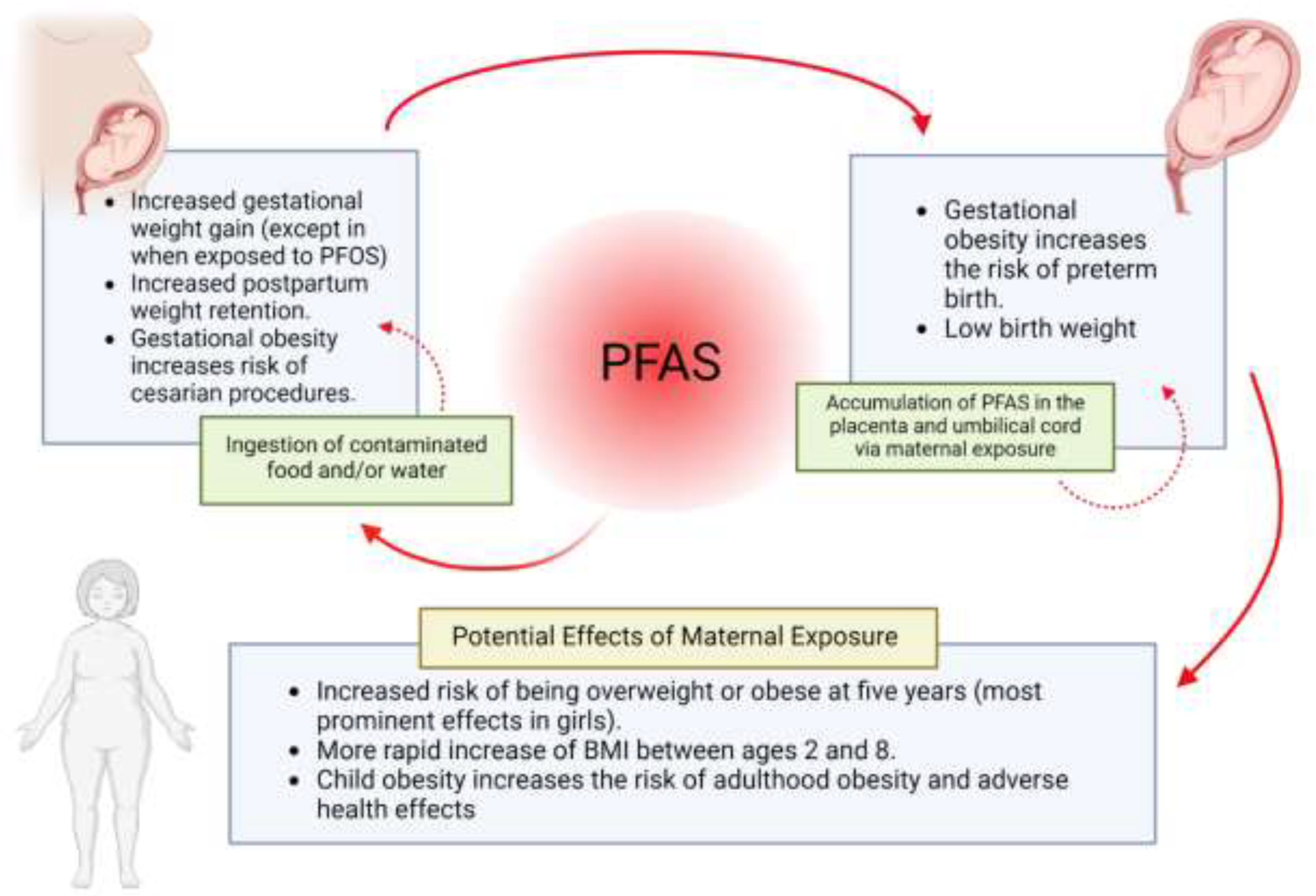
2.3. Maternal PFAS Exposure and Offspring Outcomes
2.4. In Vitro and In Vivo Insights: PFAS’s Role in Adipogenesis
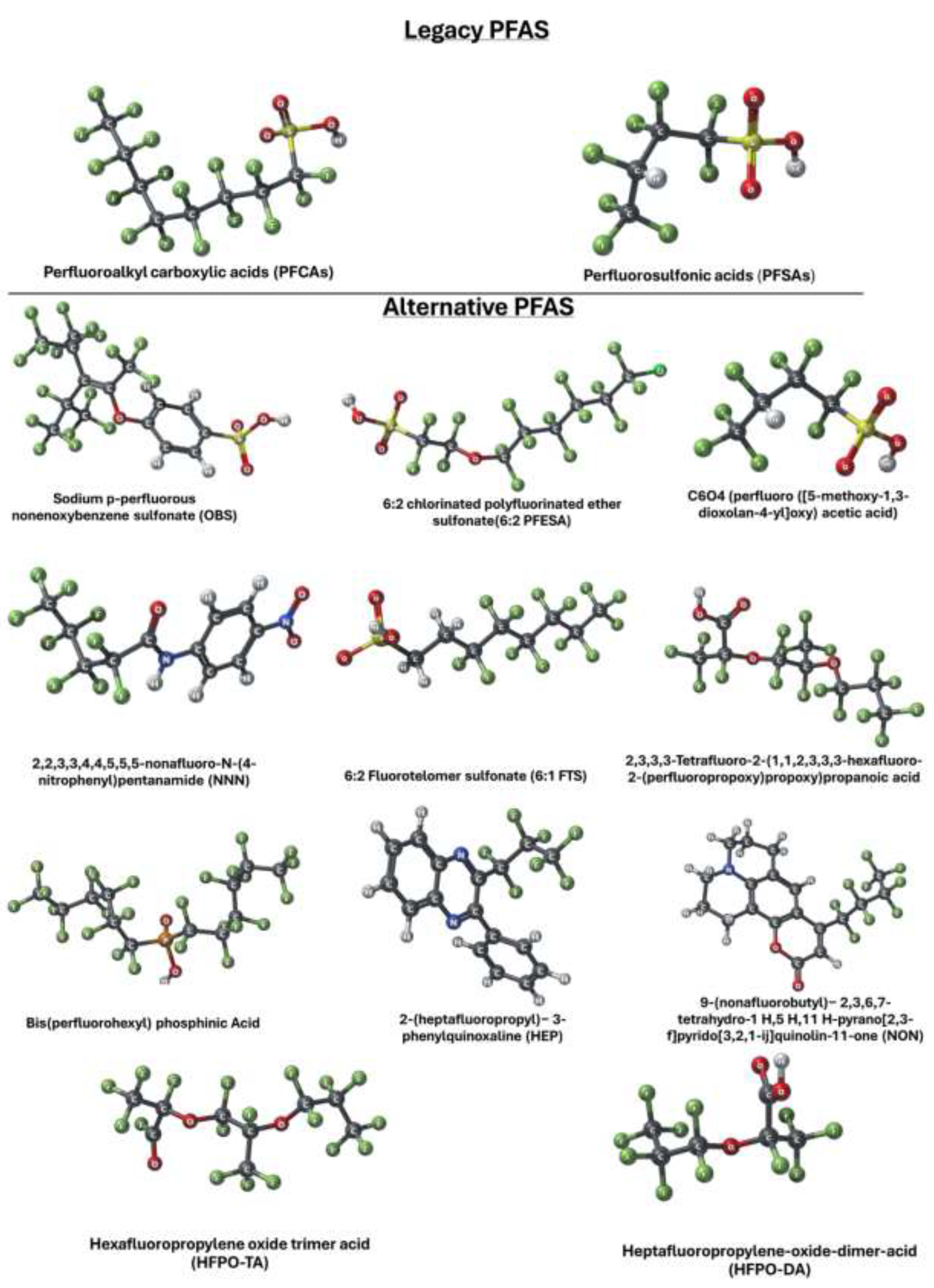
| Title | Focus | Findings | Methodology | Organism | Experimental techniques | Reference |
|---|---|---|---|---|---|---|
| In vitro Studies | ||||||
| In vitro activity of a panel of per- and polyfluoroalkyl substances (PFAS), fatty acids, and pharmaceuticals in peroxisome proliferator-activated receptor (PPAR) alpha, PPAR gamma, and estrogen receptor assays. | HFPO-DA and HFPO-DA-AS were the most potent of all PFAS in rat and human PPAR- assays. |
- Many PFAS compounds activate both PPARα and PPARγ receptors in human and rat assays, with HFPO-DA, HFPO-DA-AS, and NBP2 being the most potent. - A few PFAS compounds (PFHxS, 8:2 FTOH, 6:2 FTOH) also exhibited agonism of the human estrogen receptor. - The activation of PPARα and PPARγ receptors by PFAS may be a molecular initiating event contributing to their in vivo effects. |
- In vitro assays with human or rat PPARα or PPARγ ligand binding domains - Evaluation of 16 PFAS, 3 endogenous fatty acids, and 3 pharmaceuticals - Testing for human estrogen receptor (hER) transcriptional activation - Evaluation of receptor activation and relative potencies using EC20, pmaxtop, and AUC |
humans, rats |
- In vitro assays with human or rat PPARα ligand binding domains - In vitro assays with human or rat PPARγ ligand binding domains - Human estrogen receptor (hER) transcriptional activation assays - Evaluation of receptor activation and relative potencies using EC20, pmaxtop, and AUC |
[37] |
| Characterization of Per- and Polyfluorinated Alkyl Substances Present in Commercial Anti-fog Products and Their In vitro Adipogenic Activity | Characterization of PFAS in anti-fog products and their adipogenic activity | PFAS compounds, including FTOHs and FTEOs, were found in anti-fog products. Significant cytotoxicity and adipogenic activity were observed in murine 3T3-L1 cells. FTEOs were identified as a major contributor to adipogenic activity. | GC–HRMS, LC–MS/MS, HPLC–HRMS, in vitro adipogenesis assay | Murine 3T3-L1 preadipocytes | Gas chromatography, liquid chromatography, high-performance liquid chromatography, cell viability and proliferation assays, fluorescence microscopy | [38] |
| PFAS Environmental Pollution and Antioxidant Responses: An Overview of the Impact on Human Field | The cellular antioxidant defense system is activated by PFAS. |
- PFAS are a group of over 4,600 man-made chemicals that are toxic to both animals and humans, with PFOA and PFOS being the most widespread organic pollutants. - PFAS exposure is associated with oxidative stress, which can lead to various adverse health effects in humans such as diabetes, cardiovascular disease, and cancer. - PFAS are endocrine disruptors that can compromise many physiological processes and alter the redox environment. |
The methodology involves summarizing available data from epidemiological studies, in vitro and in vivo research on PFAS exposure and its effects on oxidative stress and human health. It includes results from biomonitoring studies and clinical examinations of occupationally exposed workers. | humans |
- Measurement of reactive oxygen species (ROS) formation - Lipid peroxidation assays - Enzymatic assays for superoxide dismutase (SOD) - Enzymatic assays for catalase (CAT) - Enzymatic assays for glutathione peroxidase (GPx) - Enzymatic assays for glutathione reductase (GR) - Measurement of glutathione (GSH) levels - Quantitative Real-Time PCR (qRT-PCR) for gene expression analysis |
[39] |
| Thyroid Disrupting Effects of Old and New Generation PFAS | Per- and polyfluoroalkyl substances are persistent pollutants accumulating in waters and soil and recoverable in foods due to their release by food packaging. |
- PFAS, including long-chain, short-chain, and newly emerging compounds, can have detrimental effects on thyroid function based on in vitro and animal studies. - Epidemiological studies have shown associations between PFAS exposure and changes in thyroid function parameters in exposed workers, the general population, and pregnant women/infants, though the results are not entirely consistent. - Further research is needed to fully understand the clinical relevance of PFAS-induced thyroid disruption, especially regarding potential impacts on fetal and child development. |
- Review of recent data on old and new generation PFAS effects on thyroid homeostasis. - Collection of information from in vitro studies, animal models, and in vivo data on exposed workers, general population, and pregnant women. - Laboratory studies on thyroid cell cultures to assess thyroid-disrupting effects. - Review of clinical studies on the relationship between PFAS exposure and thyroid dysfunction, especially during pregnancy. - Use of tables to summarize types of PFAS compounds and recent data from maternal cohorts. |
humans, rats, mice, zebrafish, Xenopus laevis, cats, Atlantic walruses |
- Thyroid cell cultures - Luciferase reporter assay - Iodide accumulation assays - cAMP production assays - Oral administration of PFAS - Assaying circulating maternal thyroid hormones - Blood transcriptomic analysis - Transmission electron microscopy |
[40] |
| Prenatal and childhood exposure to per-/polyfluoroalkyl substances (PFASs) and its associations with childhood overweight and/or obesity: a systematic review with meta-analyses | Positive associations were evidenced between prenatal PFNA and BMI in children who were 3 or less years. |
- Positive associations were found between prenatal exposure to PFNA and childhood BMI/waist circumference, and between prenatal PFOA exposure and BMI in children over 3 years old. - Negative associations were found between prenatal PFOS exposure and BMI in children 3 years or younger, between prenatal PFHxS exposure and risk of overweight, and between childhood exposure to PFOA, PFOS, and PFNA and BMI, especially PFOS in boys. |
- Conducted a systematic review with meta-analysis. - Searched PubMed and Embase using specific text strings. - Included biomonitoring studies in pregnant women or children up to 18 years assessing BMI, WC, or fat mass. - Conducted meta-analysis when at least three studies reported estimates of associations. - Developed a method to convert different effect estimates for comparability. - Stratified meta-analyses by sex and age. - Performed sensitivity analyses. - Retrieved 826 articles initially, included 49 in the review, and 30 in the meta-analyses. |
humans |
- Search on bibliographic databases (PubMed and Embase) - Biomonitoring studies - Meta-analysis - Stratification by sex and age - Sensitivity analyses |
[41] |
| Perfluoroalkyl Substances (PFAS) and Their Effects on the Placenta, Pregnancy, and Child Development: a Potential Mechanistic Role for Placental Peroxisome Proliferator–Activated Receptors (PPARs) | Perfluoroalkyl substances are associated with increased incidence of gestational diabetes, childhood obesity, preeclampsia, and fetal growth restriction. |
- PFAS exposure is associated with negative health outcomes during pregnancy, birth, and child development, including gestational diabetes, childhood obesity, preeclampsia, and fetal growth restriction. - The mechanisms involve PFAS interaction with PPARs, which regulate lipid metabolism and placental functions important for healthy pregnancies and child development. - PFAS interfere with trophoblast lipid homeostasis, inflammation, and invasion, which could be mediated by PFAS-PPAR interactions and other biological mechanisms. |
- Review of existing studies - Includes human population-based associations - Includes in vitro-based experimental data |
humans |
A review of In vitro-based experimental data and - Human population-based association studies - Molecular biology techniques (e.g., studying interactions with PPARs, lipid homeostasis, inflammation, and invasion) |
[42] |
| Evaluation of Per- and Polyfluoroalkyl Substances (PFAS) in vitro toxicity testing for developmental neurotoxicity | Evaluates the developmental neurotoxicity (DNT) of 160 PFAS using in vitro high-throughput screening assays. | 42 out of 160 PFAS decreased measures of neural network connectivity and neurite length. PFAS with longer perfluorinated carbon chains (≥8) and higher carbon:fluorine ratios were more likely to be bioactive. |
DNT new approach methods (NAMs) battery including microelectrode array neuronal network formation assay (NFA) and high-content imaging (HCI) assays to evaluate proliferation, apoptosis, and neurite outgrowth. Chemical concentration-response data analyzed using the ToxCast Pipeline (tcpl). |
In vitro (rat cortical cells, human neural progenitor cells, human glutamatergic enriched neurons) |
Microelectrode array (MEA) network formation assay (NFA) High-content imaging (HCI) assays Statistical and bioinformatics analysis using R and ToxCast Pipeline |
[43] |
| In vitro screening of per- and polyfluorinated substances (PFAS) for interference with seven thyroid hormone system targets across nine assays | Screening for interference of PFAS with thyroid hormone system targets | Evaluated activity of 136 PFAS at seven key molecular initiating events (MIE) using nine in vitro assays. Identified 85 PFAS with sufficient activity to produce an EC50 in at least one assay. Several PFAS had strong potency towards transthyretin binding. | Nine in vitro assays: enzyme inhibition assays (hDIO1, hDIO2, hDIO3, xDIO3, hIYD, xIYD), fluorescence-based assays (hTPO, hTTR, hTBG). | Human, Xenopus | Colorimetric endpoint using Sandell-Kolthoff reaction, fluorescence-based assays | [44] |
| PFAS and Potential Adverse Effects on Bone and Adipose Tissue Through Interactions With PPARγ | Investigating the effects of PFAS on bone and adipose tissue through interactions with PPARγ | PFAS exposure may lead to several adverse outcomes including altered cell differentiation, bone development issues, increased adipogenesis, metabolic disorders, and bone weakness. PFAS can trigger multiple molecular initiating events through interactions with nuclear receptors like PPARγ. | Literature review, evaluation of epidemiological and toxicological studies on PFAS, PPARγ interaction mechanisms | Human, Mouse, Rat |
Review of existing in vitro and in vivo studies. In vitro studies assessing PPARγ activation, bone development anomalies, and adipogenesis, using human mesenchymal stem cells and animal models. Mechanistic exploration of PPARγ's role in MSC differentiation to adipocytes versus osteoblasts. |
[45] |
| In vivo Studies | ||||||
| Per- and polyfluoroalkyl substance mixtures and gestational weight gain among mothers in the Health Outcomes and Measures of the Environment study | Investigating the influence of PFAS mixtures on gestational weight gain (GWG) among mothers | Each doubling in serum concentrations of PFOA, PFOS, and PFNA was associated with a small increase in GWG. The association of PFNA with GWG was stronger among women with BMI≥25 kg/m2. There was little association between PFAS and GWG z-scores. | Mass spectrometry, multivariable linear regression, weighted quantile sum regression, restricted cubic splines | Human (pregnant women) | Serum PFAS quantification using mass spectrometry, data analysis using multivariable linear regression, and weighted quantile sum regression | [46] |
| Environmental toxicants and placental function | The impact of environmental toxicants on placental function and fetal development | Environmental toxicants, such as toxic trace elements, PFAS, and environmental phenols, can cross the placenta and impact fetal development through endocrine disruption, oxidative stress, and epigenetic changes. These toxicants may lead to adverse outcomes such as preterm birth, low birth weight, and pregnancy loss. | Literature review, meta-analysis, systematic review | Human (pregnant women and fetuses) | Biomonitoring, epidemiological studies, gene expression analysis, epigenetic analysis | [47] |
| Umbilical cord serum concentrations of perfluorooctane sulfonate, perfluorooctanoic acid, and the body mass index changes from birth to 5 1/2 years of age | Investigating the impact of prenatal exposure to PFAS on the BMI trajectory of children from birth to 5 1/2 years | Prenatal exposure to PFOS and PFOA was associated with lower BMI SDS during infancy but an increase in BMI SDS in later childhood, particularly among girls. | Growth curve modeling, high-performance liquid chromatography (HPLC), tandem mass spectrometry (MS/MS) | Human (children from the Hamamatsu Birth Cohort) | BMI measurements, log10-transformed PFAS concentrations, statistical analysis using STATA and Mplus | [33] |
| Maternal serum levels of perfluoroalkyl substances and organochlorines and indices of fetal growth: a Scandinavian study | The associations between prenatal exposure to endocrine disruptive chemicals (EDCs) and fetal growth | Prenatal exposure to PFOA, PCB 153, and HCB was associated with higher odds for SGA birth among Swedish women, with stronger associations in male offspring. No significant associations were found in the Norwegian cohort. | Case-cohort study, linear and logistic regression with 95% confidence intervals (CIs) | Human (mother-child pairs) | Measurement of PFASs and OCs in maternal serum, statistical analysis using linear and logistic regression | [33] |
| Pregnancy Per- and Polyfluoroalkyl Substance Concentrations and Postpartum Health in Project Viva: A Prospective Cohort | Associations between PFAS plasma concentrations during pregnancy and postpartum anthropometry, blood pressure, and blood biomarkers | Pregnancy concentrations of certain PFAS were associated with greater adiposity, higher systolic blood pressure, and adverse changes in blood biomarkers at 3 years postpartum. | Prospective cohort study, multivariable regression analysis | Human (pregnant women and postpartum women) | Plasma PFAS quantification using online solid-phase extraction HPLC-MS/MS, measurement of anthropometric data, blood pressure, and blood biomarkers | [21] |
| Exposure to Per- and Polyfluoroalkyl Substances and Adiposity at Age 12 Years: Evaluating Periods of Susceptibility | Assessing the associations of repeated pre- and postnatal serum PFAS concentrations with adolescent adiposity and risk of overweight/obesity | Serum PFOA and PFHxS concentrations during pregnancy were associated with modest increases in central adiposity and risk of overweight/obesity, with no consistent pattern for postnatal concentrations. | Longitudinal cohort study, multiple informant models, generalized estimating equations | Human (mother-offspring pairs) | Serum PFAS quantification using online solid-phase extraction HPLC-MS/MS, anthropometry, dual-energy X-ray absorptiometry | [18] |
| Early-life exposure to perfluoroalkyl substances in relation to serum adipokines in a longitudinal birth cohort | Assessing the relationship between early-life PFAS exposure and serum adipokine concentrations in children | Significant associations between PFAS exposure at 18 months and 5 and 9 years with changes in leptin, leptin receptor, and resistin levels at age 9. No significant association for PFAS exposure at birth. | Longitudinal cohort study, multivariable linear regression models, Bayesian kernel machine regression (BKMR) | Human (mother-child pairs) | Serum PFAS quantification using online solid-phase extraction HPLC-MS/MS, serum adipokine measurements using ELISA kits | [48] |
| Prenatal exposure to perfluoroalkyl substances modulates neonatal serum phospholipids, increasing risk of type 1 diabetes | The study examines the impact of prenatal exposure to perfluoroalkyl substances (PFAS) on neonatal serum phospholipids and the subsequent risk of developing type 1 diabetes (T1D). |
- High PFAS exposure during pregnancy is associated with decreased cord serum phospholipids. PFAS exposure correlates with progression to T1D-associated islet autoantibodies in offspring. Similar lipid profile changes were observed in both human and non-obese diabetic (NOD) mice models. |
- PFAS levels and metabolomic profiles were determined from pregnant mothers and newborn infants' cord serum. A combination of cohort studies (EDIA and DIABIMMUNE) and mouse models were used to validate findings. Techniques included ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) for PFAS analysis, and lipidomic and bile acid profiling. |
Human (mother-infant cohorts) and Non-obese diabetic (NOD) mice |
- Ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) Lipidomics and bile acid profiling Clustering and correlation analysis using R statistical programming |
[49] |
| Exposure to Perfluoroalkyl Substances and Glucose Homeostasis in Youth | Examines the associations between exposure to per- and polyfluoroalkyl substances (PFAS) and glucose metabolism in overweight/obese youth. |
- High PFHxS levels in females associated with dysregulated glucose metabolism beginning in late puberty. PFHxS exposure associated with 25-mg/dL higher 60-min glucose and 25% lower b-cell function postpuberty in females. No consistent associations observed in males or with other PFAS. |
- Longitudinal cohort study with annual visits. OGTT performed to estimate glucose metabolism and b-cell function. PFAS measured using liquid chromatography-high-resolution mass spectrometry (LC-HRMS). |
Overweight/obese adolescents and young adults |
- Oral Glucose Tolerance Test (OGTT) Liquid chromatography-high-resolution mass spectrometry (LC-HRMS) Linear mixed effects models and linear regression models Sensitivity analysis |
[50] |
| A Review of the Pathways of Human Exposure to Poly- and Perfluoroalkyl Substances (PFASs) and Present Understanding of Health Effects | Serum concentrations of legacy PFASs in humans are declining globally. |
- More than 4,000 PFAS chemicals have been manufactured, with hundreds detected in the environment. - Serum levels of legacy PFAS are declining globally, but exposures to newer PFAS compounds are not well characterized. - Significant associations have been found between PFAS exposure and adverse immune outcomes in children, as well as dyslipidemia. - Evidence for cancer and neurodevelopmental impacts is limited, but preliminary evidence suggests significant health effects from emerging PFAS chemicals. |
The study is a review of existing research on sources, trends, and health effects of PFAS exposure, including epidemiologic evidence from multiple studies. | humans | [51] | |
| Invited Perspective: PFAS and the Childhood Obesity Phenotype—Challenges and Opportunities | Per- and polyfluoroalkyl substances are a group of manmade chemicals. |
- Higher prenatal exposure to PFAS was associated with a slightly higher risk of overweight or obesity in children aged 2-5 years. - The association was not sex-specific, meaning it was similar in boys and girls. - The prevalence of overweight/obesity in the study population was around 20%, which is worryingly high and consistent with previous estimates in US and European children. |
- Data source: Environmental influences on Child Health Outcomes (ECHO) consortium - Exposure assessment: Maternal serum or plasma concentrations of PFAS - Study design: Examination of associations between prenatal PFAS exposure and childhood obesity - Data pooling: Eight prospective cohorts from various U.S. locations - Outcome measurement: Body mass index (BMI) defined as ≥85th percentile for age and sex - Specific chemicals assessed: Seven long-chain PFAS, including PFOS and PFOA |
humans |
- Maternal serum or plasma concentrations to assess prenatal exposure to PFAS - Body Mass Index (BMI) to define overweight/obesity |
[52] |
| Per- and Polyfluoroalkyl Substance Toxicity and Human Health Review: Current State of Knowledge and Strategies for Informing Future Research | The absence of toxicity data for PFAS is a concern. |
- Epidemiological studies have found associations between PFAS exposure and various health effects, including immune, thyroid, liver, metabolic, reproductive, and developmental issues, as well as cancer. - These findings are supported by concordant data from experimental animal studies. - More advanced approaches are needed to accelerate the development of toxicity information for the many PFAS lacking data. - An appropriate degree of precaution may be needed to protect human health given the known health effects of some PFAS. |
- Review of existing literature on toxicological effects of PFAS - Assessment of epidemiological studies revealing associations between PFAS exposure and health effects - Concordance with experimental animal data - Proposal of contemporary and high-throughput approaches (read-across, molecular dynamics, protein modeling) to accelerate toxicity information development |
humans, various animals |
- Epidemiological studies - Experimental animal studies - Read-across - Molecular dynamics - Protein modeling |
[53] [17] |
| PFAS exposure and overweight/obesity among children in a nationally representative sample. | Perfluoroalkyl substances are associated with intermediate cardiovascular disease outcomes among children. |
- There is an association between higher levels of PFOA exposure and increased risk of overweight/obesity in children. - Higher quartiles of PFOA exposure were associated with higher odds ratios for overweight/obese BMI z-score. |
- Aim: Explore the relationship between PFASs and overweight/obesity and abdominal obesity among children. - Sample: 2473 US children aged 12-18 years from NHANES 1999-2012. - Measures: PFOA and PFOS levels, BMI, and waist circumference. - Definitions: Overweight/obesity (BMI z-score ≥ 85th percentile), abdominal obesity (waist circumference ≥90th percentile). - Analysis: Dose-response relationships and multivariable adjustments to determine associations. |
humans |
- Statistical analysis of associations - Anthropometric measurements (BMI, waist circumference) - Use of standardized growth charts or reference data - Multivariable adjustment techniques - Data from the National Health and Nutrition Examination Survey (NHANES) |
[54] |
| Exposure to perfluoroalkyl and polyfluoroalkyl substances and pediatric obesity: a systematic review and meta-analysis | Prenatal exposures to four different types of PFAS were not statistically associated with changes in body mass index or waist circumference. |
- There was no evidence of a positive association between prenatal PFAS exposure and pediatric obesity. - Postnatal exposure to certain PFAS chemicals was inversely associated with changes in BMI in children. - The findings should be interpreted cautiously due to the small number of studies. |
- Systematic review to synthesize literature and explore heterogeneity - Searched six databases for relevant studies - Included studies with individual-level PFAS and anthropometric data from children up to 12 years old - Excluded studies evaluating obesity measures at birth - Full-text review and quality assessment using OHAT criteria - Created forest plots to summarize measures of association and assess heterogeneity - Used funnel plots to assess small-study effects - Identified 24 studies, 19 with cohort design, and included 13 in the meta-analysis |
humans |
- Systematic review - Database search - Full-text review - Quality assessment using OHAT criteria - Forest plots - Funnel plots - Trim and Fill method |
[55] |
| Assessing the human health risks of perfluorooctane sulfonate by in vivo and in vitro studies. | Exposure to PFOS has caused hepatotoxicity, neurotoxicity, reproductive toxicity, immunotoxicity, thyroid disruption, cardiovascular toxicity, pulmonary toxicity, and renal toxicity in laboratory animals and many in vitro human systems. |
- Exposure to PFOS has been shown to cause a variety of toxic effects in laboratory animals and human cell systems, including hepatotoxicity, neurotoxicity, reproductive toxicity, immunotoxicity, thyroid disruption, cardiovascular toxicity, pulmonary toxicity, and renal toxicity. - These findings, along with related epidemiological studies, confirm the human health risks of PFOS, especially from exposure through food and drinking water. - The main mechanisms of PFOS toxicity that have been widely studied are oxidative stress and disruption of physiological processes due to the similarity of PFOS to fatty acids. |
- Systematic review of in vivo and in vitro studies from 2008 to 2018 - Analysis of epidemiological studies |
laboratory animals, human cell systems |
- In vivo studies - In vitro studies |
[56] |
| Prenatal Exposure to Perfluorooctanoate and Risk of Overweight at 20 Years of Age: A Prospective Cohort Study | Low-dose developmental exposure to PFOA was positively associated with anthropometry at 20 years in female offspring. |
- In utero exposure to PFOA was positively associated with overweight and high waist circumference in female offspring at 20 years of age. - Maternal PFOA concentrations were positively associated with biomarkers of adiposity (insulin, leptin, leptin-adiponectin ratio) in female offspring. - The findings support the hypothesis that early-life exposure to endocrine disruptors, even at low concentrations, may contribute to the obesity epidemic. |
- Prospective cohort study with 665 pregnant women recruited in 1988-1989. - PFOA measured in maternal serum at gestational week 30. - Offspring follow-up at 20 years for BMI, waist circumference, and adiposity biomarkers. - Data collection included interviews, blood samples, and health records. - Follow-up involved web-based questionnaires and clinical exams. - Statistical analyses: linear regression for continuous outcomes, log-Poisson regression for dichotomous outcomes. - Adjustments for maternal age, education, smoking status, pre-pregnancy BMI, parity, infant birth weight, and offspring age. - Log-transformation of adiposity biomarkers due to skewed distributions. |
humans |
- Measurement of PFOA in serum samples - Recording of BMI and waist circumference - Collection and processing of blood samples (separation into serum, plasma, erythrocytes; freezing) - Time-resolved immunofluorometric assay for adiponectin and leptin - Commercial Insulin ELISA kit for plasma insulin - Linear regression for continuous outcomes - Log-Poisson regression for dichotomous outcomes - Division of maternal PFOA concentrations into quartiles for trend analysis |
[57] |
| Prenatal Perfluoroalkyl Substance Exposure and Child Adiposity at 8 Years of Age: The HOME Study | Prenatal perfluoroalkyl substance exposure and adiposity in children born to women who lived downstream from a fluoropolymer manufacturing plant. | humans | [58] | |||
| Perfluoroalkyl and Polyfluoroalkyl Substances and Body Size and Composition Trajectories in Midlife Women: The Study of Women’s Health Across the Nation 1999–2018 | Certain PFAS were positively associated with greater body size and body fat. |
- Higher concentrations of certain PFAS (PFOS, linear PFOA, EtFOSAA, MeFOSAA, PFHxS) were associated with greater body size and body fat at baseline and faster increases in body size and body fat over time in midlife women. - No significant associations were found between PFNA and body size or composition. |
- Examined associations of serum PFAS concentrations with body size and composition trajectories. - Included 1,381 midlife women with 15,000 repeated measures. - Follow-up period averaged 14.9 years (range: 0-18.6 years). - Body size and composition assessed using objective measurements and dual-energy X-ray absorptiometry. - Near-annual visits for assessments. - Used linear mixed models with piecewise linear splines to model non-linear trajectories. - Multivariable adjustments made for potential confounders. |
humans |
- Measurement of serum PFAS concentrations - Objective measurement of weight - Objective measurement of waist circumference (WC) - Dual-energy X-ray absorptiometry (DXA) for body composition - Linear mixed models with piecewise linear splines for data analysis |
[59] |
| Perfluoroalkyl and polyfluoroalkyl substances and human fetal growth: A systematic review | Higher PFOS and PFOA concentrations were associated with decreased average birth weight in most studies. |
- Higher PFOS and PFOA concentrations were associated with decreased average birth weight in most studies, but only some results were statistically significant. - The impact on public health is unclear, but the global exposure to PFASs warrants further investigation. |
- Systematic literature searches in MEDLINE and EMBASE - Inclusion of original studies on pregnant women with measurements of PFOA or PFOS in maternal blood or umbilical cord - Investigation of citations and references from included articles to find more relevant studies - Extraction of study characteristics and results into structured tables - Assessment of completeness of reporting, risk of bias, and confounding |
humans |
- Systematic literature searches in MEDLINE and EMBASE - Measurement of PFOA or PFOS in maternal blood or umbilical cord - Investigation of citations and references from included articles - Extraction of study characteristics and results to structured tables - Assessment of completeness of reporting, risk of bias, and confounding |
[59,60] |
| The Role of Persistent Organic Pollutants in Obesity: A Review of Laboratory and Epidemiological Studies | Persistent organic pollutants are potential obesogens that may affect adipose tissue development and functioning, thus promoting obesity. |
- Laboratory data demonstrate that POPs can contribute to obesity through mechanisms like dysregulation of adipogenesis regulators, affinity for nuclear receptors, epigenetic effects, and proinflammatory activity. - In vivo studies show the impact of POPs on adipogenesis is affected by factors like sex, age, and exposure duration. - Epidemiological data show a significant association between POP exposure and obesity, as well as obesity-related metabolic disturbances, though more research is needed. |
- Review of existing laboratory data - Review of in vivo studies - Review of epidemiological data - Discussion of mechanisms linking POPs to adipose tissue dysfunction and obesity |
humans |
- In vitro assays for dysregulation of adipogenesis regulators (PPARγ and C/EBPα) - Receptor binding assays - Epigenetic profiling techniques - Inflammation assays - In vivo studies in living organisms - Epidemiological studies |
[61] |
| Association of Perfluoroalkyl and Polyfluoroalkyl Substances With Adiposity | A higher plasma PFAS concentration was associated with increases in weight and hip girth over time. |
- Higher plasma PFAS concentrations were associated with increases in weight and hip girth over time, but this association was attenuated in the group that received a lifestyle intervention of diet and exercise. - The authors suggest that a lifestyle intervention of diet and exercise can mitigate the obesogenic effects of environmental chemicals like PFASs. |
- Prospective cohort study with 957 participants from the Diabetes Prevention Program (DPP) and its follow-up study (DPPOS). - Participants randomized into pharmacologic intervention (metformin), placebo, or lifestyle intervention groups. - Lifestyle intervention included training in diet, physical activity, and behavior modification. - Plasma concentrations of six PFASs measured at baseline and two years after randomization. - Weight, waist circumference, and hip girth measured at baseline and scheduled visits. - Blood samples analyzed using high-performance liquid chromatography-isotope dilution-tandem mass spectrometry. - Statistical analyses included adjusted linear regression models for cross-sectional associations and longitudinal mixed-effects regression models for prospective associations. |
humans |
- Online solid-phase extraction-high-performance liquid chromatography-isotope dilution-tandem mass spectrometry - Calibrated balance scale for weight measurement - Tape measure for waist circumference and hip girth - Lange skinfold calipers for skinfold thickness - Computed tomography for visceral and subcutaneous fat - Adjusted linear regression models - Longitudinal mixed-effects regression models |
[62] |
| Early life exposure to per- and polyfluoroalkyl substances (PFAS) and latent health outcomes: A review including the placenta as a target tissue and possible driver of peri- and postnatal effects. | Exposures to some PFAS in utero are associated with adverse outcomes for both mother and offspring. |
- PFAS exposure is associated with adverse health outcomes, including reduced kidney function, metabolic syndrome, thyroid disruption, and adverse pregnancy outcomes. - Exposure to PFAS during pregnancy is linked to hypertensive disorders of pregnancy (HDP), preeclampsia, and low birth weight in offspring. - The placenta is an understudied target of PFAS exposure, and placental dysfunction may contribute to the relationship between PFAS exposure and increased risk of chronic diseases in adulthood. |
- Review of existing literature - Synthesis of evidence on PFAS effects on thyroid function, kidney disease, and metabolic syndrome - Emphasis on the placenta as a target tissue and programming agent of adult disease |
humans | a review | [63] |
| Early-life perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS) exposure cause obesity by disrupting fatty acids metabolism and enhancing triglyceride synthesis in Caenorhabditis elegans. | Low concentrations of PFOA and PFOS induced obesity in Caenorhabditis elegans. |
- Low concentrations of PFOA and PFOS (0.1 and 1 μM) induced obesity in C. elegans, which was not due to increased feeding rate. - PFOA and PFOS exposure altered the fatty acid composition, decreasing saturated fatty acids and increasing polyunsaturated fatty acids. - Genes related to fatty acid desaturation (mdt-15, nhr-49, fat-6) and fatty acid/triglyceride synthesis (fasn-1, dgat-2) were associated with the increased body fat, triglycerides, and lipid droplet content in C. elegans exposed to PFOA and PFOS. |
- Used Caenorhabditis elegans as an in vivo model. - Investigated lipid accumulation, feeding behaviors, fatty acids composition, and genetic regulation. - Exposed C. elegans to low concentrations of PFOA and PFOS (0.1 and 1 μM). - Conducted mutant assay and mRNA levels analysis to study genetic regulation. |
nematode (Caenorhabditis elegans) |
- Use of Caenorhabditis elegans as an in vivo model - Chemical exposure experiments with PFOA and PFOS - Analysis of fatty acid composition - Mutant assays - Gene expression analysis (e.g., quantitative PCR) |
[64] |
| Prenatal Exposure to Perfluoroalkyl Substances and Adiposity in Early and Mid-Childhood | Prenatal exposure to perfluoroalkyl substances was associated with small increases in adiposity measurements in mid-childhood. |
- Prenatal exposure to perfluoroalkyl substances (PFASs) was associated with small increases in adiposity measurements in mid-childhood, but only among girls. - No associations were found between prenatal PFAS exposure and early-childhood adiposity measures, or for boys. |
- Measured plasma PFAS concentrations in 1,645 pregnant women at median 9.6 weeks gestation. - Assessed overall and central adiposity in children at median ages 3.2 years (early childhood) and 7.7 years (mid-childhood) using anthropometric and DXA measurements. - Fitted multivariable linear regression models to estimate exposure-outcome associations and evaluated effect modification by child sex. |
humans |
- Plasma analysis for PFAS concentrations - Anthropometric measurements - Dual X-ray absorptiometry (DXA) - Multivariable linear regression models |
[65] |
| Phenotypic dichotomy following developmental exposure to perfluorooctanoic acid (PFOA) in female CD-1 mice: Low doses induce elevated serum leptin and insulin, and overweight in mid-life | Low-dose effects of PFOA on body weight gain, as well as leptin and insulin concentrations in mid-life are important to explore. |
- Low doses of PFOA (0.01-0.3 mg/kg) during development increased body weight, serum insulin, and serum leptin in mid-life in female CD-1 mice. - The effects of in utero PFOA exposure on body weight were no longer detected at 18 months of age. - High doses of PFOA decreased white adipose tissue and spleen weights, but increased brown adipose tissue weight, in both intact and ovariectomized mice. |
- Study subjects: CD-1 mice - Exposure scenarios: (1) in utero exposure, (2) in utero exposure followed by ovariectomy (ovx), (3) adult exposure - Exposure duration: 17 days during pregnancy or as young adults - PFOA doses: 0, 0.01, 0.1, 0.3, 1, 3, or 5mg PFOA/kg BW - Measurements: body weight (postnatal day 1, weaning, mid-life, late life), serum insulin and leptin levels, weights of white adipose tissue, spleen, brown adipose tissue, and liver |
mice |
- Exposure to various doses of PFOA - Measurement of body weight at specific time points (postnatal day 1, weaning, mid-life) - Measurement of serum insulin and leptin levels - Ovariectomy (ovx) - Measurement of white adipose tissue weight - Measurement of spleen weight - Measurement of brown adipose tissue weight - Measurement of liver weight |
[66] |
| Diet as an Exposure Source and Mediator of Per- and Polyfluoroalkyl Substance (PFAS) Toxicity | Western diets enriched in high fat and high cholesterol containing foods may be an important human exposure route of PFAS. |
- PFAS exposure is associated with a range of health effects in both animals and humans, including hyperlipidemia and fatty liver disease. - There are inconsistencies between animal and human studies on the effects of PFAS on lipid metabolism and cardiometabolic profiles. - More research is needed using human-relevant animal models and on the toxicity of emerging PFAS, as well as the dietary modulation of PFAS toxicity. |
The methodology involves reviewing existing literature to outline dietary exposure sources of PFAS, describe associated metabolic health effects, and examine studies on dietary interactions with PFAS exposure. The review includes data from epidemiological studies, animal studies, and regulatory agencies. | mice, rats, monkeys |
- Oral gavage - Dietary exposure - Serum concentration measurement - Plasma lipid analysis - Hepatic histology - Gene expression analysis - Use of genetically engineered animal models |
[8] |
| Effect of Per and Poly-Fluoroalkyl Substances on Pregnancy and Child Development. | PFAS exposure occurs through the Peroxisome Proliferator-Activated Receptor, leading to increased fat deposition and profound health effects in child growth and development. |
- PFAS exposure during pregnancy disrupts placental health and breastfeeding, leading to impaired child growth and development. - PFAS exposure increases adipocyte number, alters lipid metabolism, and leads to increased adiposity and weight gain through activation of PPAR-γ and ER-α. - PFAS concentrations are positively correlated in maternal serum. |
- Detailed literature survey using online databases (Science Direct, Google Scholar, Scopus, Cochrane, PubMed) - Focus on effects of PFAS on maternal and child health, particularly neurological complications - Neurotoxicity testing using SH-SY5Y human-derived cell line (in vitro model) - In vivo studies in mice and human cell lines to investigate PPAR-γ and ER-α activation - Analysis of PFAS concentrations in maternal sera using liquid chromatography/quadrupole mass spectrometry |
humans, mice |
- SH-SY5Y human-derived cell line (in vitro model) - In vivo studies in mice - Human cell lines - Liquid chromatography/quadrupole mass spectrometry |
[67] |
| Halogenated bisphenol-A analogs act as obesogens in zebrafish larvae (Danio rerio). | Halogenated bisphenol-A analogs induced lipid accumulation in zebrafish larvae. |
- Halogenated BPA analogs like TBBPA and TCBPA are rapidly absorbed and metabolized by zebrafish, primarily through sulfation. - TBBPA and TCBPA act as agonists for both human and zebrafish PPAR-gamma, a key regulator of adipogenesis. - Exposure to TBBPA, TCBPA, and TBT during early zebrafish development leads to increased body mass index (BMI) in juvenile zebrafish at 1 month of age. |
- Zebrafish larvae were used as an in vivo model. - Embryonic exposure to TBBPA and TCBPA was analyzed for lipid accumulation using Oil Red-O staining. - Activation of human and zebrafish PPARγ was assessed in zebrafish and reporter cell lines. - Metabolic fate of TBBPA and TCBPA was analyzed using high-performance liquid chromatography (HPLC). - Zebrafish larvae were housed under controlled conditions and exposed to chemicals dissolved in DMSO. - GFP expression was quantified in transgenic zebrafish embryos to assess PPARγ activation. - Larvae were fed an egg yolk diet and treated with chemicals daily until 11 days post-fertilization (dpf). - Lipid accumulation was assessed by Oil Red-O staining, and larvae were imaged using microscopy. - Weight and length of juvenile zebrafish were recorded at 30 days post-fertilization (dpf) to calculate BMI. |
zebrafish (Danio rerio) |
- Oil Red-O staining - High-performance liquid chromatography (HPLC) - Use of transgenic zebrafish (Tg(hPPARγ-eGFP)) - Reporter cell lines stably transfected with PPARγ-LBD - Luminescence measurement using a plate reader - GFP quantification using a plate reader - 3D microscopy live imaging using Nikon AZ100M microscope - Solid-phase extraction (SPE) - Washing and staining of fixed larvae with Oil Red-O solution - Calculation of BMI as weight/(length)² |
[68] |
| Per- and polyfluoroalkyl substances and obesity, type 2 diabetes and non-alcoholic fatty liver disease: a review of epidemiologic findings | Causal links between per- and polyfluoroalkyl substances and obesity, diabetes, and non-alcoholic fatty liver disease/non-alcoholic steatohepatitis require further large-scale prospective cohort studies combined with mechanistic laboratory studies to better assess these associations. |
- There is a growing body of literature linking per- and polyfluoroalkyl substances (PFAS) exposure to obesity, type 2 diabetes, and non-alcoholic fatty liver disease/non-alcoholic steatohepatitis. - Approximately two-thirds of studies found positive associations between PFAS exposure and the prevalence of obesity and/or type 2 diabetes. - More research is needed to establish causal links between PFAS and these health outcomes. |
- Review of existing literature - Search of PubMed for human studies on obesity, diabetes, and non-alcoholic fatty liver disease/non-alcoholic steatohepatitis - Summary of historical use, chemistry, routes of exposure, and epidemiologic evidence |
humans | [69] | |
| A Review of Human Exposure to Microplastics and Insights Into Microplastics as Obesogens | Microplastic exposure in laboratory animals is linked to various forms of inflammation, immunological response, endocrine disruption, alteration of lipid and energy metabolism, and other disorders. |
- Microplastics are ubiquitous in the environment and human food chain, leading to widespread human exposure. - The increase in global obesity over the past 5 decades coincides with the rise in plastics production and use. - The authors hypothesize that exposure to microplastics and plastic additives (obesogens) may be contributing to the global obesity pandemic. |
- Compilation of data from various studies on MP concentrations in air, dust, drinking water, food, and beverages. - Use of spectroscopy-based methods (FTIR, Raman, X-ray photoelectron spectroscopy, energy dispersive x-ray spectroscopy, scanning electron microscopy) for identification and quantification. - Biomonitoring studies to provide direct evidence of MP exposure in humans. - Analysis of human and pet animal stool specimens for MP content. - Measurement of MP concentrations in human tissues such as lungs and placenta. |
humans, dogs, cats |
- FTIR (Fourier-transform infrared spectroscopy) - Raman spectroscopy - X-ray photoelectron spectroscopy - Energy dispersive X-ray spectroscopy - Scanning electron microscopy - Biomonitoring studies (analysis of human tissues and stool) |
[70] |
| Perfluoroalkyl substances and changes in body weight and resting metabolic rate in response to weight-loss diets: A prospective study | Higher baseline plasma perfluoroalkyl substance concentrations were associated with a greater weight regain, especially in women. |
- Higher baseline plasma PFAS concentrations were significantly associated with greater weight regain, especially in women. - Higher baseline plasma PFAS concentrations, particularly PFOS and PFNA, were significantly associated with a greater decline in resting metabolic rate during weight loss and a smaller increase in resting metabolic rate during weight regain. |
- Prospective analysis within the POUNDS Lost randomized clinical trial. - Participants: 621 overweight and obese individuals aged 30-70 years. - Intervention: Four energy-reduced diets designed to induce weight loss. - Measurements: Baseline plasma concentrations of major PFASs; body weight at baseline, 6, 12, 18, and 24 months; RMR and other metabolic parameters at baseline, 6 months, and 24 months. - Statistical analysis: Linear regression to examine associations between baseline PFAS levels and changes in body weight and RMR. |
humans |
- Body weight measurement - Resting metabolic rate (RMR) assessment using Deltatrac II Metabolic Monitor - Dual energy X-ray absorptiometry (DXA) for body fat mass and lean mass - Computed tomography (CT) scanner for visceral and subcutaneous abdominal fat - Online solid phase extraction and liquid chromatography coupled to a triple quadropole mass spectrometer for PFAS concentrations - Synchron CX7 and CX5 systems for glucose, insulin, cholesterol, and HbA1c - Ultrasensitive immunoassay for plasma leptin and soluble leptin receptor - Competitive electrochemiluminescence immunoassay for thyroid hormones - Direct hybridization using Illumina HumanHT-12 v3 Expression BeadChip for gene expression - Baecke physical activity questionnaire for physical activity assessment |
[71] |
| Perfluorooctanesulfonic acid (PFOS) and perfluorohexanesulfonic acid (PFHxS) alter the blood lipidome and the hepatic proteome in a murine model of diet-induced obesity. | Perfluorooctanesulfonic acid and perfluorohexanesulfonic acid increase the risk of metabolic and inflammatory disease induced by diet. |
- PFOS and PFHxS increased the expression of genes involved in lipid metabolism and oxidative stress in the liver of mice fed a high-fat, high-carbohydrate diet. - PFOS and PFHxS altered the blood lipidome, changing the levels of various lipid species, including phosphatidylcholines, phosphatidylethanolamines, plasmogens, sphingomyelins, and triglycerides. - PFOS and PFHxS led to an increase in oxidized lipid species in the blood lipidome of mice fed a high-fat, high-carbohydrate diet. |
- Male C57BL/6J mice were used. - Mice were fed either a low-fat diet or a high fat high carbohydrate (HFHC) diet. - PFOS or PFHxS were included in the feed at 0.0003% w/w for 29 weeks. - Lipidomic, proteomic, and gene expression profiles were determined. - Effects on lipid metabolism and oxidative stress were measured in the liver and blood. |
mice |
- Lipidomic profiling - Proteomic profiling - Gene expression profiling |
[72] |
| Associations of Prenatal Per- and Polyfluoroalkyl Substance (PFAS) Exposures with Offspring Adiposity and Body Composition at 16–20 Years of Age: Project Viva | Higher prenatal PFAS concentrations were associated with higher obesity risk in late adolescence. |
- Higher prenatal PFAS exposures, particularly PFOS, PFOA, and PFNA, were associated with increased risk of obesity in late adolescence. - There was an interaction between PFOA and PFOS, where the positive association between PFOS and obesity was stronger when PFOA levels were lower. - The PFAS mixture as a whole was associated with increased obesity risk and higher BMI. - Children with higher prenatal PFOS, EtFOSAA, and MeFOSAA had higher rates of BMI increase starting from 9-11 years of age. |
- Studied 545 mother–child pairs from Project Viva cohort. - Measured six PFAS in maternal early pregnancy plasma samples. - Assessed anthropometric measures and body composition in late adolescence. - Used bioelectrical impedance analysis and dual-energy X-ray absorptiometry for body composition. - Analyzed associations with obesity/adiposity using multivariable Poisson and linear regression models. - Evaluated PFAS mixture effects using Bayesian kernel machine regression and quantile g-computation. - Assessed BMI trajectories using fractional-polynomial models. |
humans |
- Measurement of PFAS in maternal plasma samples - Bioelectrical impedance analysis - Dual-energy X-ray absorptiometry - Multivariable Poisson regression models - Linear regression models - Bayesian kernel machine regression (BKMR) - Quantile g-computation - Fractional-polynomial models |
[73] |
| Exposure to Perfluoroalkyl Chemicals and Cardiovascular Disease: Experimental and Epidemiological Evidence | Legacy and new PFAS can be incorporated in platelet cell membranes giving a solid rationale to the observed increase risk of cardiovascular events in the populations exposed to PFAS by directly promoting thrombus formation. |
- Exposure to PFAS may increase the risk of cardiovascular disease through worsening of cardiovascular risk factors and a direct prothrombotic effect on platelets. - Mechanistic studies suggest PFAS can accumulate in platelet membranes and alter their function, leading to increased platelet activation and thrombus formation. - These platelet-mediated effects may help explain the observed increase in cardiovascular events in PFAS-exposed populations. |
- Review of epidemiological studies on PFAS exposure and cardiovascular disease. - Selection criteria for studies: sample size, study design (longitudinal preferred), intensity of exposure. - Analysis of mechanistic studies on PFAS incorporation in platelet membranes and thrombus formation. - Summarized data in tables on clinical, epidemiological, and experimental studies. |
humans |
- Liquid chromatography/mass-mass spectrometry (LC-MS/MS) - Thrombin receptor activator peptide 6 (TRAP-6) stimulation - Microfluidic biochip pre-coated with collagen - Measurement of large microvesicles expressing C41 and binding annexin V - Bilayer fluidity-sensitive probe Merocyanin 540 - Platelet aggregation under flow conditions with/without acetylsalicylic acid |
[74] |
| Per/poly fluoroalkyl substances induce lipid accumulation via the serotonergic signaling pathway in | Perfluorononanoic acid, perfluorooctanesulfonamide, and perfluorooctane sulfonate promote fat accumulation in Caenorhabditis elegans. |
- Exposure to PFNA, PFOSA, and PFOS significantly increased lipid accumulation in C. elegans, with PFNA showing the highest level of lipid accumulation. - PFNA, PFOSA, and PFOS downregulated the expression of genes involved in serotonin production and beta-oxidation, and upregulated the expression of a gene involved in triacylglycerol synthesis. - The study demonstrates that PFNA, PFOSA, and PFOS promote fat accumulation through the serotonin-involved pathway and lipogenesis, leading to an obesogenic effect. |
- Model organism: Caenorhabditis elegans - Exposure concentration: 1 µM PFNA, PFOSA, and PFOS - Lipid accumulation measurement: bodipy 493/503 and nile red staining methods - Food intake measurement: pharyngeal pumping rate - Gene expression evaluation: tph-1, mod-1, nhr-76, atgl-1, and dgat-2 |
Caenorhabditis elegans (C. elegans) |
- Use of Caenorhabditis elegans as a model organism - Bodipy 493/503 staining - Nile red staining - Measurement of pharyngeal pumping rate - Gene expression analysis |
[75] |
| Do perfluoroalkyl substances aggravate the occurrence of obesity-associated glucolipid metabolic disease? | Perfluoroalkyl substances are aggravating the occurrence of obesity-associated glucolipid metabolic disease. |
- Both obesity and PFASs exposure can independently cause disruptions in glucose and lipid metabolism. - Obesity is a crucial factor that increases the incidence of GLMD induced by PFASs. - PFASs are exacerbating the development of obesity-associated GLMD, such as diabetes, cardiovascular disease, and liver disease. |
- Summarized epidemiological studies on PFASs and obesity-related GLMD - Reviewed relevant experimental evidence - Proposed three research programs to explore the synergistic mechanism of PFASs and obesity - Recommended three suggestions to mitigate the harm of PFASs pollutants to humans |
humans |
- Epidemiological surveys Experimental studies on animal models Statistical analysis of literature data |
[76] |
| Reduced Birth Weight and Exposure to Per- and Polyfluoroalkyl Substances: A Review of Possible Underlying Mechanisms Using the AOP-HelpFinder | Prenatal exposure to per- and polyfluorinated substances may impair fetal growth. |
- PFAS are associated with oxidative stress, which triggers increased PPARγ expression and activation of growth signaling pathways, leading to hyperdifferentiation of pre-adipocytes and reduced adipose tissue weight, which may reduce birth weight. - PFAS may also impair fetal growth through endocrine effects, including estrogenic effects and thyroid-damaging effects that are associated with decreased body and organ weight in animal studies. |
- Used the Adverse Outcome Pathway (AOP)-helpFinder tool to search PubMed - Focused on studies examining PFAS exposure in relation to birth weight, oxidative stress, hormones/hormone receptors, or growth signaling pathways - Initial search yielded 1880 articles - Screened down to 106 experimental studies after abstract screening |
animals |
- In vivo animal studies - In vitro studies - Measurement of reactive-oxygen species (ROS) generation - Measurement of peroxisome proliferator-activated receptor (PPAR)γ expression - Assays for hormone levels - Gene expression analysis related to thyroid function |
[77] |
| Association between gestational PFAS exposure and Children's adiposity in a diverse population. | Perfluoroundecanoic acid was associated with their children having higher waist circumference z-score. |
- There were more non-Hispanic Black and Hispanic children with overweight/obesity compared to non-Hispanic white and Asian/Pacific Islander children. - Among women without obesity, higher levels of perfluoroundecanoic acid (PFUnDA) were associated with their children having higher waist circumference, fat mass, and body fat percentage. - The associations between PFAS and children's adiposity varied significantly by maternal race-ethnicity, although the direction of the associations was inconsistent. - Among children of women with obesity, higher levels of PFOS, perfluorononanoic acid, and perfluorodecanoic acid were associated with less adiposity. |
- Estimated associations between gestational PFAS concentrations and childhood adiposity. - Measured six PFAS in first trimester blood plasma using ultra-high-performance liquid chromatography with tandem mass spectrometry. - Sample: non-smoking women with low-risk singleton pregnancies (n = 803). - Adiposity measures in children aged 4-8 years: BMI, waist circumference, fat mass, fat-free mass, % body fat. - Adjusted for confounders. |
humans |
- Ultra-high-performance liquid chromatography with tandem mass spectrometry - Body mass index (BMI) - Waist circumference (WC) - Fat mass - Fat-free mass - % body fat |
[5,78] |
| Exposure to Polyfluoroalkyl Chemicals and Cholesterol, Body Weight, and Insulin Resistance in the General U.S. Population | Polyfluoroalkyl chemicals are used commonly in commercial applications and are detected in humans and the environment worldwide. |
- Serum concentrations of PFOS, PFOA, and PFNA were positively associated with total cholesterol and non-HDL cholesterol levels in the general U.S. population. - Serum concentrations of PFHxS were negatively associated with total cholesterol and non-HDL cholesterol levels, in contrast to the other PFCs studied. - The association between PFNA and cholesterol levels was the strongest and most consistent, despite lower serum concentrations of PFNA compared to PFOS and PFOA. |
- Data source: 2003–2004 NHANES - Participants: 12–80 years old - Sampling design: Complex multistage probability sampling - Measurements: Blood and urine samples at a mobile examination center - PFC measurement: Automated solid-phase extraction coupled to isotope dilution/high-performance liquid chromatography/tandem mass spectrometry - Analysis: Linear regression controlling for covariates - Outcomes: Cholesterol, body size, insulin resistance - Exposure modeling: Quartiles of PFC concentration - Statistical software: SAS version 9.1 Proc SURVEYREG - Adjustments: Relevant covariates instead of NHANES sampling weights |
humans |
- Linear regression - Automated solid-phase extraction coupled to isotope dilution/high-performance liquid chromatography/tandem mass spectrometry - Enzymatic measurement of total cholesterol (TC) and high-density lipoprotein (HDL) - Calculation of non-HDL cholesterol - Estimation of low-density lipoprotein (LDL) using the Friedewald formula - Homeostatic model assessment (HOMA) method - Enzymatic measurement of plasma insulin and glucose - SAS version 9.1 Proc SURVEYREG procedure for statistical analysis - Identification and exclusion of influential points and outliers using studentized residuals, predicted values, and scatter plots |
[79] |
| Perfluorooctane sulfonate (PFOS), perfluorooctanoic acid (PFOA) and their salts Scientific Opinion of the Panel on Contaminants in the Food chain. | Perfluorochemicals in residents of the United States in 2001 through 2002. | mice, rats, cynomolgus monkeys |
- High-Performance Liquid Chromatography (HPLC) Electrospray Mass Spectrometry - Liquid Chromatography coupled to High-Resolution Mass Spectrometry |
[80] | ||
| PFAS health effects database: Protocol for a systematic evidence map. | Regulators, scientists, and citizens need to stay informed on the growing health and toxicology literature related to PFAS. |
- The goal of this study is to identify and organize the available literature on the health and toxicological effects of 29 PFAS of emerging concern. - The study will search the PubMed database for primary research studies investigating the link between PFAS and health effects, toxicology, or biological mechanisms. - The extracted and coded information from the included studies will be visualized in a publicly available, interactive database, and the results will be published in a narrative summary. |
- Search PubMed for health or toxicological studies on 29 PFAS of emerging concern. - Include studies with primary research linking PFAS to health, toxicological, or biological endpoints. - Title and abstract screening by a single reviewer for inclusion; two independent reviewers for exclusion. - No study quality assessment. - Extract and code study characteristics, checked by a second reviewer. - Visualize data in a publicly available, interactive database on Tableau Public. - Publish results in a narrative summary. |
humans |
- Literature search in PubMed - Title and abstract screening - Full text review - Data extraction and coding - Data visualization using Tableau Public |
[81] |
| Prenatal Per- and Polyfluoroalkyl Substance (PFAS) Exposures, Individually and as a Mixture, Are Associated With Obesity Risk at 16-20 Years in the Project Viva Prospective Cohort: Implications for PFAS as Hazardous Substances for Developmental Health | Prenatal PFAS exposures may have long-lasting, intergenerational obesogenic effects. |
- Prenatal exposure to higher levels of PFOS and PFNA was associated with a greater risk of obesity in adolescence. - There was an interaction between PFOS and PFOA, where the positive association between PFOS and obesity was stronger when PFOA levels were lower, and PFOA had a negative association with obesity when PFOS levels were higher. - Exposure to a mixture of higher concentrations of PFAS was associated with a greater risk of obesity in a dose-dependent manner. |
- Prospective pre-birth cohort study (Project Viva) - Measured PFAS in maternal plasma samples collected in the first trimester - Measured child BMI at mid-adolescent visit (median: 17.4 years) - Defined obesity as BMI ≥ 95th percentile for age and sex based on CDC Growth Charts - Used Poisson regression with robust variance estimates for individual PFAS associations - Used Bayesian kernel machine regression (BKMR) for PFAS mixtures associations - Adjusted for maternal age, education, pre-pregnancy BMI, race/ethnicity, parity, and smoking status during pregnancy |
humans |
- Measurement of PFAS in maternal plasma samples - Measurement of child BMI - Poisson regression with robust variance estimates - Bayesian kernel machine regression (BKMR) |
[82] |
| Effects of triphenyl phosphate exposure during fetal development on obesity and metabolic dysfunctions in adult mice: Impaired lipid metabolism and intestinal dysbiosis. | Fetal exposure to triphenyl phosphate can promote the development of obesity and metabolic dysfunctions in adult mice. |
- Fetal exposure to triphenyl phosphate (TPHP) led to increased obesity, metabolic dysfunction, and altered lipid metabolism and gut microbiome in adult mice. - TPHP exposure during fetal development promoted the development of obesity and related metabolic disorders in adult mice. - Fetal TPHP exposure modulated gut microbiome composition and host-gut co-metabolism, which may contribute to the observed metabolic dysfunctions. |
- Exposure to TPHP during fetal development and lactation at three doses (10, 100, and 1000 μg/kg BW) - Evaluation in adult male mice fed a low-fat diet (LFD) or high-fat diet (HFD) - Examination of body weight, liver weight, histopathology, blood biochemistry, gene expression, and gut microbiota compositions and metabolic functions - Gas chromatography-mass spectrometry (GC-MS) for fatty acid composition analysis - 16S rRNA gene sequencing and 1H NMR based fecal metabolomics for gut microbiome composition and host-gut co-metabolism |
mice |
- Body weight measurement - Liver weight measurement - Histopathology - Blood biochemistry assays - Gene expression analysis - Gut microbiota analysis - Gas chromatography-mass spectrometry (GC-MS) - 16S rRNA gene sequencing - 1H NMR based fecal metabolomics |
[83] |
| Health-related toxicity of emerging per- and polyfluoroalkyl substances: Comparison to legacy PFOS and PFOA. | Evidence derived from both animal models and humans suggested PFAS may exert harmful impacts on both animals and humans. |
- Exposure to PFAS has been associated with a wide range of adverse health impacts, including effects on fertility, metabolism, endocrine function, lipid metabolism, hepatic and renal function, immune function, cardiovascular health, bone health, neurological function, and cancer risk. - However, the cause-and-effect relationships for many of these outcomes have not been clearly elucidated, and there are still limitations in our understanding of PFAS precursor kinetics, toxicity mechanisms, and the long-term effects of chronic PFAS exposure in humans. - Further investigation of the long-term-exposed population is required to better evaluate the biological toxicity of chronic PFAS exposure. |
- Critical review of recent research on PFAS exposure - Compilation and analysis of findings from multiple recent studies - Comparison of evidence from animal models and human studies - Evaluation of cause-and-effect relationships - Identification of gaps in current knowledge and need for further investigation |
humans, animals | [84] | |
| Verification of In Vivo Estrogenic Activity for Four Per- and Polyfluoroalkyl Substances (PFAS) Identified as Estrogen Receptor Agonists via New Approach Methodologies. | Exposure to FC8-diol, FC10-diol, and HFPO-DA caused concentration-dependent increases in the expression of transcript coding for vitellogenin and estrogen receptor alpha in fish exposed in vivo. |
- Exposure to FC8-diol, FC10-diol, and FC8-DOD caused concentration-dependent increases in the expression of vitellogenin and estrogen receptor alpha, and reduced expression of insulin-like growth factor and apolipoprotein eb, indicating estrogenic activity in vivo. - The rank order of estrogenic potency in vivo matched the previous in vitro screening results, after accounting for differences in bioconcentration. - These findings provide a screening-level benchmark for estimating the potential estrogenic hazards of PFAS and a basis for identifying structurally similar PFAS that may also have estrogenic activity. |
- Tiered testing strategy with high-throughput in vitro screening as the initial tier. - Evaluation of in vitro screening effectiveness by exposing fathead minnows to five PFAS for 96 hours. - Measurement of transcript expression for vitellogenin, estrogen receptor alpha, insulin-like growth factor, and apolipoprotein eb. - Comparison of in vivo results with in vitro findings to validate the screening method and establish potency rank order. |
Fathead minnows (Pimephales promelas) |
- Exposure of fathead minnows to PFAS - Measurement of transcript expression (vitellogenin, estrogen receptor alpha, insulin-like growth factor, apolipoprotein eb) - Bioconcentration analysis |
[85] |
| Early-Life Exposure to Perfluoroalkyl Substances and Childhood Metabolic Function | Children with higher PFAS concentrations had lower insulin resistance in mid-childhood. |
- Early-life exposure to PFASs was not associated with adverse metabolic effects in mid-childhood. - In fact, children with higher PFAS concentrations had lower insulin resistance. |
- Studied 665 mother-child pairs from Project Viva cohort (1999-2002) - Quantified PFAS concentrations in maternal plasma at first prenatal visit (median 9.6 weeks gestation) and in child plasma at mid-childhood (median 7.7 years) - Assessed leptin, adiponectin, and HOMA-IR in mid-childhood - Used covariate-adjusted linear regression models and stratified analyses by child sex |
humans |
- Quantification of PFAS concentrations in plasma - Biochemical assays for leptin, adiponectin, and HOMA-IR - Covariate-adjusted linear regression models - Stratified analyses by child sex |
[48] |
| Perfluoroalkyl and Polyfluoroalkyl Substances and Body Size and Composition Trajectories in Midlife Women: The Study of Women’s Health Across the Nation 1999–2018 | Certain PFAS were positively associated with large body size and body fat. |
- Certain PFAS (PFOS, linear PFOA, EtFOSAA, MeFOSAA, PFHxS) were positively associated with larger body size and higher body fat at baseline and over time in midlife women. - Women with the highest PFAS levels had significantly higher weight, waist circumference, fat mass, and proportion of fat compared to those with the lowest levels. - Higher PFAS levels were also associated with faster annual increases in weight, waist circumference, and fat mass over the 14.9 year follow-up period. |
- Examined associations of serum PFAS concentrations with body size and composition trajectories. - Included 1,381 midlife women with 15,000 repeated measures. - Follow-up period averaged 14.9 years. - Body size and composition assessed using objective measurements and dual-energy X-ray absorptiometry. - Near-annual visits for assessments. - Used linear mixed models with piecewise linear splines to model non-linear trajectories. - Multivariable adjustments made for confounders. |
humans |
- Measurement of serum PFAS concentrations - Objective measurement of weight - Objective measurement of waist circumference (WC) - Dual-energy X-ray absorptiometry (DXA) for body composition - Linear mixed models with piecewise linear splines for data analysis |
[59] |
| Perfluoroalkyl and polyfluoroalkyl substances (PFAS) and their effects on the ovary | PFAS exposures target the ovary and represent major risks for women's health. |
- PFAS are present in follicular fluid and can pass through the blood-follicle barrier. - Epidemiological studies have found associations between higher PFAS exposure and disruptions in ovarian function, such as later menarche, irregular menstrual cycles, earlier menopause, and reduced sex hormone levels. - Experimental studies have confirmed adverse effects of PFAS on ovarian folliculogenesis and steroidogenesis, potentially through various mechanisms. |
- The study is a review of human population and toxicological studies. - A comprehensive review was performed by searching PubMed. - Extensive search terms were used, including both general and specific keywords related to PFAS and ovarian function. |
humans |
- Activation of peroxisome proliferator-activated receptors - Disruption of gap junction intercellular communication - Induction of thyroid hormone deficiency - Antagonism of ovarian enzyme activities - Inhibition of kisspeptin signalling |
[87] |
| Exposure to perfluoroalkyl substances (PFAS) and liver injury: a systematic review and meta-analysis | Perfluoroalkyl substances are synthetic chemicals widely used in industry and consumer products that persist in the environment and bioaccumulate in food webs and human tissues. |
- There is consistent evidence from human and animal studies that exposure to certain PFAS (PFOA, PFOS, PFNA) is associated with liver injury, as indicated by increased levels of liver enzymes and liver steatosis. - PFOA exposure was specifically associated with increased levels of the liver enzymes AST and GGT in humans. - PFAS-exposed rodents showed increased ALT levels, liver steatosis, and liver weight compared to non-exposed rodents. |
- Systematic review of literature on PFAS exposure and liver injury. - Searched PubMed and Embase through January 27, 2021, using relevant keywords. - Data synthesis focused on two primary outcomes: serum alanine aminotransferase (ALT) and steatosis. - Included other measures of liver injury as secondary outcomes. - Synthesized evidence from at least three observational studies per PFAS using a weighted z-score approach for human studies. - Summarized direction and significance of exposure effects on hepatic enzyme abundance and activity for animal studies. |
humans, rodents |
- Literature search in PubMed and Embase - Measurement of serum alanine aminotransferase (ALT) - Measurement of steatosis - Weighted z-score approach for synthesizing observational study data - Synthesis of data on hepatic enzyme abundance and activity in animal studies |
[88] |
| Per- and Polyfluoroalkyl Substance Exposure, Gestational Weight Gain, and Postpartum Weight Changes in Project Viva | Investigates the association between PFAS exposure during pregnancy and subsequent gestational weight gain and postpartum weight changes. |
- Doubling of EtFOSAA associated with 0.37 kg more weight gain during pregnancy. Doubling of PFOA associated with 0.55 kg more weight retention at 1-year postpartum and 0.91 kg more weight gain at 3 years postpartum. Higher PFOS associated with more weight gain at 3 years postpartum. Stronger postpartum weight change associations in women with higher pre-pregnancy BMI. |
- Longitudinal cohort study with follow-ups at 1 and 3 years postpartum. PFAS levels measured in plasma using high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS). Analysis included multivariable linear regression and Bayesian Kernel Machine Regression (BKMR) for mixture analysis. |
Human (pregnant women and postpartum mothers) |
- High-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) Multivariable linear regression Bayesian Kernel Machine Regression (BKMR) |
[89] |
2.5. Epidemiological Evidence linking PFAS exposure to obesity and metabolic dysfunction
3. Toxicokinetic of PFAS in the Human Body
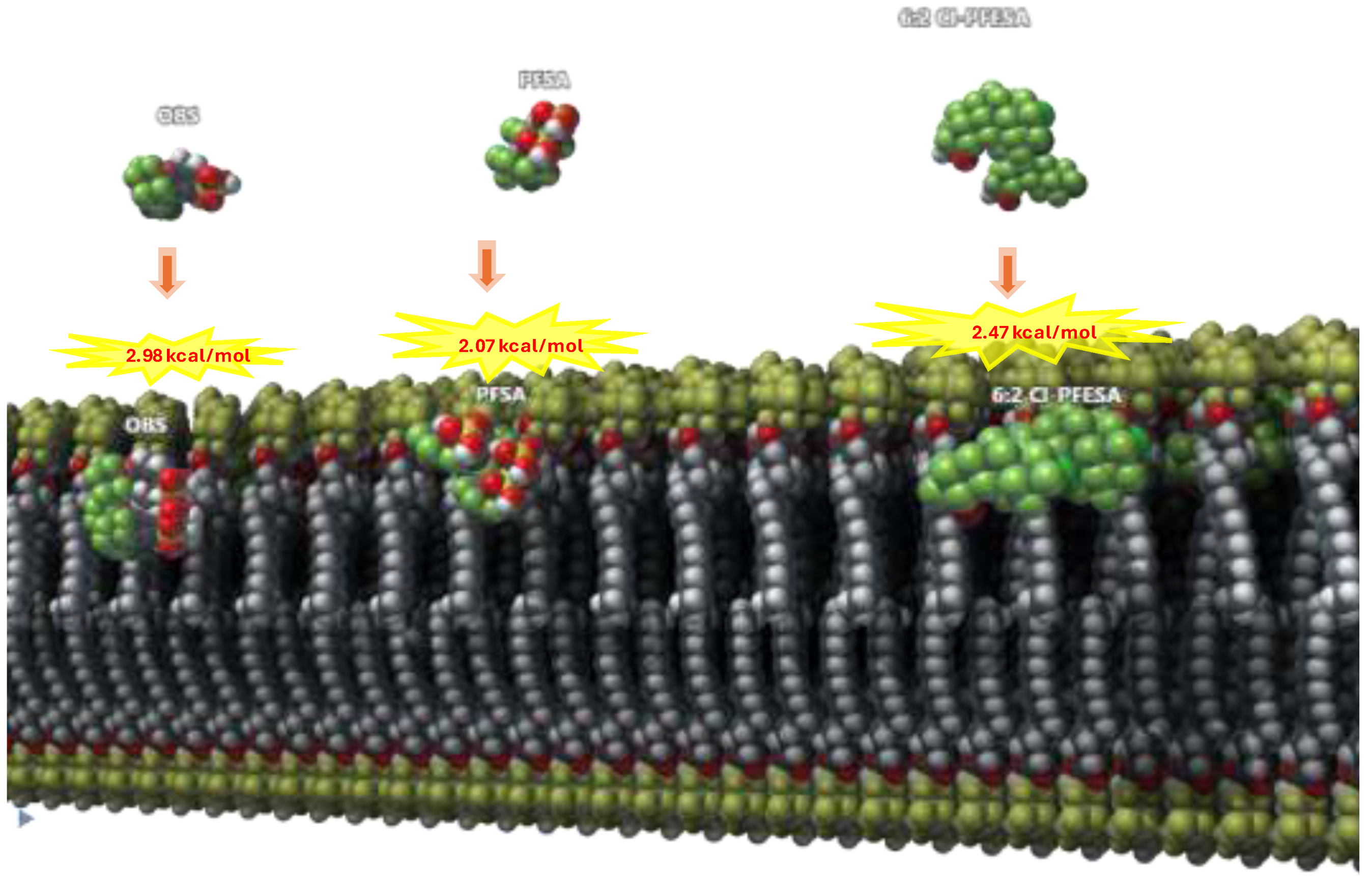
4. Regulatory Challenges and Risk Assessment of PFAS
5. Strategies and Challenges in PFAS Remediation and Detoxification
6. Insights from In Silico Studies of PFAS
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Silva, A.O.; Armitage, J.M.; Bruton, T.A.; Dassuncao, C.; Heiger-Bernays, W.; Hu, X.C.; Karrman, A.; Kelly, B.; Ng, C.; Robuck, A.; et al. PFAS Exposure Pathways for Humans and Wildlife: A Synthesis of Current Knowledge and Key Gaps in Understanding. Environ Toxicol Chem 2021, 40, 631-657. [CrossRef]
- Buck, R.C.; Franklin, J.; Berger, U.; Conder, J.M.; Cousins, I.T.; de Voogt, P.; Jensen, A.A.; Kannan, K.; Mabury, S.A.; van Leeuwen, S.P. Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr Environ Assess Manag 2011, 7, 513-541. [CrossRef]
- Jha, G.; Kankarla, V.; McLennon, E.; Pal, S.; Sihi, D.; Dari, B.; Diaz, D.; Nocco, M. Per- and Polyfluoroalkyl Substances (PFAS) in Integrated Crop-Livestock Systems: Environmental Exposure and Human Health Risks. Int J Environ Res Public Health 2021, 18. [CrossRef]
- Kowalska, D.; Sosnowska, A.; Bulawska, N.; Stepnik, M.; Besselink, H.; Behnisch, P.; Puzyn, T. How the Structure of Per- and Polyfluoroalkyl Substances (PFAS) Influences Their Binding Potency to the Peroxisome Proliferator-Activated and Thyroid Hormone Receptors-An In Silico Screening Study. Molecules 2023, 28. [CrossRef]
- Bloom, M.S.; Commodore, S.; Ferguson, P.L.; Neelon, B.; Pearce, J.L.; Baumer, A.; Newman, R.B.; Grobman, W.; Tita, A.; Roberts, J.; et al. Association between gestational PFAS exposure and Children's adiposity in a diverse population. Environmental Research 2022, 203. [CrossRef]
- Dickman, R.A.; Aga, D.S. A review of recent studies on toxicity, sequestration, and degradation of per- and polyfluoroalkyl substances (PFAS). Journal of Hazardous Materials 2022, 436, 129120-129120. [CrossRef]
- East, A.; Dawson, D.E.; Brady, S.; Vallero, D.A.; Tornero-Velez, R. A Scoping Assessment of Implemented Toxicokinetic Models of Per- and Polyfluoro-Alkyl Substances, with a Focus on One-Compartment Models. Toxics 2023, 11. [CrossRef]
- Roth, K.; Imran, Z.; Liu, W.; Petriello, M.C. Diet as an Exposure Source and Mediator of Per- and Polyfluoroalkyl Substance (PFAS) Toxicity. Frontiers in Toxicology 2020, 2. [CrossRef]
- Zhao, L.; Teng, M.; Zhao, X.; Li, Y.; Sun, J.; Zhao, W.; Ruan, Y.; Leung, K.M.Y.; Wu, F. Insight into the binding model of per- and polyfluoroalkyl substances to proteins and membranes. Environment International 2023, 175, 107951-107951. [CrossRef]
- Stahl, T.; Mattern, D.; Brunn, H. Toxicology of perfluorinated compounds. Environmental Sciences Europe 2011, 23, 1-52. [CrossRef]
- Khazaee, M.; Christie, E.; Cheng, W.; Michalsen, M.; Field, J.; Ng, C. Perfluoroalkyl Acid Binding with Peroxisome Proliferator-Activated Receptors α, γ, and δ, and Fatty Acid Binding Proteins by Equilibrium Dialysis with a Comparison of Methods. Toxics 2021, 9, 1-16. [CrossRef]
- Ul Hasan, A.; Rahman, A.; Kobori, H. Interactions between Host PPARs and Gut Microbiota in Health and Disease. International Journal of Molecular Sciences 2019, 20. [CrossRef]
- Lai, K.P.; Ng, A.H.M.; Wan, H.T.; Wong, A.Y.M.; Leung, C.C.T.; Li, R.; Wong, C.K.C. Dietary exposure to the environmental chemical, PFOS on the diversity of gut microbiota, associated with the development of metabolic syndrome. Frontiers in Microbiology 2018, 9. [CrossRef]
- Sanyaolu, A.; Okorie, C.; Qi, X.; Locke, J.; Rehman, S. Childhood and Adolescent Obesity in the United States: A Public Health Concern. Global Pediatric Health 2019, 6. [CrossRef]
- Pampel, F.C.; Krueger, P.M.; Denney, J.T. Socioeconomic disparities in health behaviors. Annual Review of Sociology 2010, 36, 349-370. [CrossRef]
- Cawley, J.; Biener, A.; Meyerhoefer, C.; Ding, Y.; Zvenyach, T.; Smolarz, G.; Ramasamy, A. Direct medical costs of obesity in the United States and the most populous states; 2021.
- Fenton, S.E.; Ducatman, A.; Boobis, A.; DeWitt, J.C.; Lau, C.; Ng, C.; Smith, J.S.; Roberts, S.M. Per- and Polyfluoroalkyl Substance Toxicity and Human Health Review: Current State of Knowledge and Strategies for Informing Future Research. Environmental Toxicology and Chemistry 2021, 40, 606-630. [CrossRef]
- Liu, Y.; Li, N.; Papandonatos, G.D.; Calafat, A.M.; Eaton, C.B.; Kelsey, K.T.; Chen, A.; Lanphear, B.P.; Cecil, K.M.; Kalkwarf, H.J.; et al. Exposure to Per- And Polyfluoroalkyl Substances and Adiposity at Age 12 Years: Evaluating Periods of Susceptibility. Environmental Science and Technology 2020, 54, 16039-16049. [CrossRef]
- Blake, B.E.; Fenton, S.E. Early life exposure to per- and polyfluoroalkyl substances (PFAS) and latent health outcomes: A review including the placenta as a target tissue and possible driver of peri- and postnatal effects. Toxicology 2020, 443, 152565-152565. [CrossRef]
- Friedman, C.; Dabelea, D.; Keil, A.P.; Adgate, J.L.; Glueck, D.H.; Calafat, A.M.; Starling, A.P. Maternal serum per- and polyfluoroalkyl substances during pregnancy and breastfeeding duration. Environmental Epidemiology 2023, 7, E260-E260. [CrossRef]
- Mitro, S.D.; Sagiv, S.K.; Fleisch, A.F.; Jaacks, L.M.; Williams, P.L.; Rifas-Shiman, S.L.; Calafat, A.M.; Hivert, M.F.; Oken, E.; James-Todd, T.M. Pregnancy per- And polyfluoroalkyl substance concentrations and postpartum health in project viva: a prospective cohort. Journal of Clinical Endocrinology and Metabolism 2020, 105, E3415-E3426. [CrossRef]
- Padula, A.M.; Ning, X.; Bakre, S.; Barrett, E.S.; Bastain, T.; Bennett, D.H.; Bloom, M.S.; Breton, C.V.; Dunlop, A.L.; Eick, S.M.; et al. Birth Outcomes in Relation to Prenatal Exposure to Per-and Polyfluoroalkyl Substances and Stress in the Environmental Influences on Child Health Outcomes (ECHO) Program. Environmental Health Perspectives 2023, 131. [CrossRef]
- Daraki, V.; Georgiou, V.; Papavasiliou, S.; Chalkiadaki, G.; Karahaliou, M.; Koinaki, S.; Sarri, K.; Vassilaki, M.; Kogevinas, M.; Chatzi, L. Metabolic Profile in Early Pregnancy Is Associated with Offspring Adiposity at 4 Years of Age: The Rhea Pregnancy Cohort Crete, Greece. PLoS ONE 2015, 10. [CrossRef]
- Dias, M.D.S.; Matijasevich, A.; Barros, A.J.D.; Menezes, A.M.B.; Schneider, B.C.; Hartwig, F.P.; Barros, F.C.; Wehrmeister, F.C.; Gonçalves, H.; Santos, I.S.; et al. Influence of maternal pre-pregnancy nutritional status on offspring anthropometric measurements and body composition in three Brazilian Birth Cohorts. Public Health Nutrition 2021, 24, 882-882. [CrossRef]
- Kato, R.; Kubota, M.; Yasui, Y.; Hayashi, Y.; Higashiyama, Y.; Nagai, A. Retrospective tracking of young obese children back to birth in Japan: special attention to the relationship with parental obesity. Asia Pacific journal of clinical nutrition 2014, 23, 641-650. [CrossRef]
- Do “Forever Chemicals” Have “Forever Impacts”? Study suggests link between higher prenatal PFAS exposures and offspring obesity risk in adolescence | Department of Population Medicine.
- Leddy, M.A.; Power, M.L.; Schulkin, J. The Impact of Maternal Obesity on Maternal and Fetal Health; 2008; pp. 170-178.
- Birru, R.L.; Liang, H.W.; Farooq, F.; Bedi, M.; Feghali, M.; Haggerty, C.L.; Mendez, D.D.; Catov, J.M.; Ng, C.A.; Adibi, J.J. A pathway level analysis of PFAS exposure and risk of gestational diabetes mellitus. Environmental Health: A Global Access Science Source 2021, 20. [CrossRef]
- Guo, J.; Wu, J.; He, Q.; Zhang, M.; Li, H.; Liu, Y. The Potential Role of PPARs in the Fetal Origins of Adult Disease. Cells 2022, 11. [CrossRef]
- Lauritzen, H.B.; Larose, T.L.; Øien, T.; Sandanger, T.M.; Odland, J.O.; Van De Bor, M.; Jacobsen, G.W. Prenatal exposure to persistent organic pollutants and child overweight/obesity at 5-year follow-up: A prospective cohort study. Environmental Health: A Global Access Science Source 2018, 17. [CrossRef]
- Mitro, S.D.; Sagiv, S.K.; Fleisch, A.F.; Jaacks, L.M.; Williams, P.L.; Rifas-Shiman, S.L.; Calafat, A.M.; Hivert, M.F.; Oken, E.; James-Todd, T.M. Pregnancy Per- and Polyfluoroalkyl Substance Concentrations and Postpartum Health in Project Viva: A Prospective Cohort. J Clin Endocrinol Metab 2020, 105, e3415-3426. [CrossRef]
- Romano, M.E.; Gallagher, L.G.; Eliot, M.N.; Calafat, A.M.; Chen, A.; Yolton, K.; Lanphear, B.; Braun, J.M. Per- and polyfluoroalkyl substance mixtures and gestational weight gain among mothers in the Health Outcomes and Measures of the Environment study. Int J Hyg Environ Health 2021, 231, 113660. [CrossRef]
- Horikoshi, T.; Nishimura, T.; Nomura, Y.; Iwabuchi, T.; Itoh, H.; Takizawa, T.; Tsuchiya, K.J. Umbilical cord serum concentrations of perfluorooctane sulfonate, perfluorooctanoic acid, and the body mass index changes from birth to 5 1/2 years of age. Scientific Reports 2021, 11. [CrossRef]
- Wang, S.; Lin, Y.; Gao, L.; Yang, Z.; Lin, J.; Ren, S.; Li, F.; Chen, J.; Wang, Z.; Dong, Z.; et al. PPAR-γ integrates obesity and adipocyte clock through epigenetic regulation of Bmal1. Theranostics 2022, 12, 1589-1606. [CrossRef]
- Rosenmai, A.K.; Taxvig, C.; Svingen, T.; Trier, X.; van Vugt-Lussenburg, B.M.; Pedersen, M.; Lesne, L.; Jegou, B.; Vinggaard, A.M. Fluorinated alkyl substances and technical mixtures used in food paper-packaging exhibit endocrine-related activity in vitro. Andrology 2016, 4, 662-672. [CrossRef]
- Bodin, J.; Groeng, E.C.; Andreassen, M.; Dirven, H.; Nygaard, U.C. Exposure to perfluoroundecanoic acid (PFUnDA) accelerates insulitis development in a mouse model of type 1 diabetes. Toxicology Reports 2016, 3, 664-672. [CrossRef]
- Evans, N.; Conley, J.M.; Cardon, M.; Hartig, P.; Medlock-Kakaley, E.; Gray, L.E., Jr. In vitro activity of a panel of per- and polyfluoroalkyl substances (PFAS), fatty acids, and pharmaceuticals in peroxisome proliferator-activated receptor (PPAR) alpha, PPAR gamma, and estrogen receptor assays. Toxicol Appl Pharmacol 2022, 449, 116136. [CrossRef]
- Herkert, N.J.; Kassotis, C.D.; Zhang, S.; Han, Y.; Pulikkal, V.F.; Sun, M.; Ferguson, P.L.; Stapleton, H.M. Characterization of Per- and Polyfluorinated Alkyl Substances Present in Commercial Anti-fog Products and Their In Vitro Adipogenic Activity. Environmental Science and Technology 2022, 56, 1162-1173. [CrossRef]
- Bonato, M.; Corrà, F.; Bellio, M.; Guidolin, L.; Tallandini, L.; Irato, P.; Santovito, G. PFAS Environmental Pollution and Antioxidant Responses: An Overview of the Impact on Human Field. Int J Environ Res Public Health 2020, 17. [CrossRef]
- Coperchini, F.; Croce, L.; Ricci, G.; Magri, F.; Rotondi, M.; Imbriani, M.; Chiovato, L. Thyroid Disrupting Effects of Old and New Generation PFAS. Frontiers in Endocrinology 2021, 11, 612320-612320. [CrossRef]
- Frigerio, G.; Ferrari, C.M.; Fustinoni, S. Prenatal and childhood exposure to per-/polyfluoroalkyl substances (PFASs) and its associations with childhood overweight and/or obesity: a systematic review with meta-analyses. Environmental Health: A Global Access Science Source 2023, 22, 1-42. [CrossRef]
- Szilagyi, J.T.; Avula, V.; Fry, R.C. Perfluoroalkyl Substances (PFAS) and Their Effects on the Placenta, Pregnancy, and Child Development: a Potential Mechanistic Role for Placental Peroxisome Proliferator–Activated Receptors (PPARs). Current Environmental Health Reports 2020, 7, 222-230. [CrossRef]
- Carstens, K.E.; Freudenrich, T.; Wallace, K.; Choo, S.; Carpenter, A.; Smeltz, M.; Clifton, M.S.; Henderson, W.M.; Richard, A.M.; Patlewicz, G.; et al. Evaluation of Per- and Polyfluoroalkyl Substances (PFAS) In Vitro Toxicity Testing for Developmental Neurotoxicity. Chem Res Toxicol 2023, 36, 402-419. [CrossRef]
- Degitz, S.J.; Olker, J.H.; Denny, J.S.; Degoey, P.P.; Hartig, P.C.; Cardon, M.C.; Eytcheson, S.A.; Haselman, J.T.; Mayasich, S.A.; Hornung, M.W. In vitro screening of per- and polyfluorinated substances (PFAS) for interference with seven thyroid hormone system targets across nine assays. Toxicol In Vitro 2024, 95, 105762. [CrossRef]
- Kirk, A.B.; Michelsen-Correa, S.; Rosen, C.; Martin, C.F.; Blumberg, B. PFAS and Potential Adverse Effects on Bone and Adipose Tissue Through Interactions With PPARγ. Endocrinology 2021, 162. [CrossRef]
- Romano, M.E.; Gallagher, L.G.; Eliot, M.N.; Calafat, A.M.; Chen, A.; Yolton, K.; Lanphear, B.; Braun, J.M. Per- and polyfluoroalkyl substance mixtures and gestational weight gain among mothers in the Health Outcomes and Measures of the Environment study. International Journal of Hygiene and Environmental Health 2021, 231. [CrossRef]
- Bloom, M.S.; Varde, M.; Newman, R.B. Environmental toxicants and placental function. Best Practice and Research: Clinical Obstetrics and Gynaecology 2022, 85, 105-120. [CrossRef]
- Shih, Y.H.; Blomberg, A.J.; Jørgensen, L.H.; Weihe, P.; Grandjean, P. Early-life exposure to perfluoroalkyl substances in relation to serum adipokines in a longitudinal birth cohort. Environmental Research 2022, 204. [CrossRef]
- McGlinchey, A.; Sinioja, T.; Lamichhane, S.; Sen, P.; Bodin, J.; Siljander, H.; Dickens, A.M.; Geng, D.; Carlsson, C.; Duberg, D.; et al. Prenatal exposure to perfluoroalkyl substances modulates neonatal serum phospholipids, increasing risk of type 1 diabetes. Environment International 2020, 143. [CrossRef]
- Goodrich, J.A.; Alderete, T.L.; Baumert, B.O.; Berhane, K.; Chen, Z.; Gilliland, F.D.; Goran, M.I.; Hu, X.; Jones, D.P.; Margetaki, K.; et al. Exposure to perfluoroalkyl substances and glucose homeostasis in youth. Environmental Health Perspectives 2021, 129. [CrossRef]
- Sunderland, E.M.; Hu, X.C.; Dassuncao, C.; Tokranov, A.K.; Wagner, C.C.; Allen, J.G. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J Expo Sci Environ Epidemiol 2019, 29, 131-147. [CrossRef]
- Stratakis, N.; Vrijheid, M. Invited Perspective: PFAS and the Childhood Obesity Phenotype-Challenges and Opportunities. Environ Health Perspect 2023, 131, 61301. [CrossRef]
- Fenton, S.E.; Ducatman, A.; Boobis, A.; DeWitt, J.C.; Lau, C.; Ng, C.; Smith, J.S.; Roberts, S.M. Per- and Polyfluoroalkyl Substance Toxicity and Human Health Review: Current State of Knowledge and Strategies for Informing Future Research. Environ Toxicol Chem 2021, 40, 606-630. [CrossRef]
- Geiger, S.D.; Yao, P.; Vaughn, M.G.; Qian, Z. PFAS exposure and overweight/obesity among children in a nationally representative sample. Chemosphere 2021, 268, 128852. [CrossRef]
- Frangione, B.; Birk, S.; Benzouak, T.; Rodriguez-Villamizar, L.A.; Karim, F.; Dugandzic, R.; Villeneuve, P.J. Exposure to perfluoroalkyl and polyfluoroalkyl substances and pediatric obesity: a systematic review and meta-analysis. International Journal of Obesity 2023 48:2 2023, 48, 131-146. [CrossRef]
- Zeng, Z.; Song, B.; Xiao, R.; Zeng, G.; Gong, J.; Chen, M.; Xu, P.; Zhang, P.; Shen, M.; Yi, H. Assessing the human health risks of perfluorooctane sulfonate by in vivo and in vitro studies. Environ Int 2019, 126, 598-610. [CrossRef]
- Halldorsson, T.I.; Rytter, D.; Haug, L.S.; Bech, B.H.; Danielsen, I.; Becher, G.; Henriksen, T.B.; Olsen, S.F. Prenatal exposure to perfluorooctanoate and risk of overweight at 20 years of age: a prospective cohort study. Environmental health perspectives 2012, 120, 668-673. [CrossRef]
- Braun, J.M.; Chen, A.; Romano, M.E.; Calafat, A.M.; Webster, G.M.; Yolton, K.; Lanphear, B.P. Prenatal perfluoroalkyl substance exposure and child adiposity at 8 years of age: The HOME study. Obesity (Silver Spring) 2016, 24, 231-237. [CrossRef]
- Ding, N.; Karvonen-Gutierrez, C.A.; Herman, W.H.; Calafat, A.M.; Mukherjee, B.; Park, S.K. Perfluoroalkyl and polyfluoroalkyl substances and body size and composition trajectories in midlife women: the study of women's health across the nation 1999-2018. Int J Obes (Lond) 2021, 45, 1937-1948. [CrossRef]
- Bach, C.C.; Bech, B.H.; Brix, N.; Nohr, E.A.; Bonde, J.P.; Henriksen, T.B. Perfluoroalkyl and polyfluoroalkyl substances and human fetal growth: a systematic review. Crit Rev Toxicol 2015, 45, 53-67. [CrossRef]
- Aaseth, J.; Javorac, D.; Djordjevic, A.B.; Bulat, Z.; Skalny, A.V.; Zaitseva, I.P.; Aschner, M.; Tinkov, A.A. The Role of Persistent Organic Pollutants in Obesity: A Review of Laboratory and Epidemiological Studies. Toxics 2022, 10. [CrossRef]
- Cardenas, A.; Hauser, R.; Gold, D.R.; Kleinman, K.P.; Hivert, M.F.; Fleisch, A.F.; Lin, P.I.D.; Calafat, A.M.; Webster, T.F.; Horton, E.S.; et al. Association of Perfluoroalkyl and Polyfluoroalkyl Substances With Adiposity. JAMA Network Open 2018, 1. [CrossRef]
- Blake, B.E.; Fenton, S.E. Early life exposure to per- and polyfluoroalkyl substances (PFAS) and latent health outcomes: A review including the placenta as a target tissue and possible driver of peri- and postnatal effects. Toxicology 2020, 443, 152565. [CrossRef]
- Lin, T.A.; Huang, C.W.; Wei, C.C. Early-life perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS) exposure cause obesity by disrupting fatty acids metabolism and enhancing triglyceride synthesis in Caenorhabditis elegans. Aquat Toxicol 2022, 251, 106274. [CrossRef]
- Mora, A.M.; Oken, E.; Rifas-Shiman, S.L.; Webster, T.F.; Gillman, M.W.; Calafat, A.M.; Ye, X.; Sagiv, S.K. Prenatal Exposure to Perfluoroalkyl Substances and Adiposity in Early and Mid-Childhood. Environ Health Perspect 2017, 125, 467-473. [CrossRef]
- Hines, E.P.; White, S.S.; Stanko, J.P.; Gibbs-Flournoy, E.A.; Lau, C.; Fenton, S.E. Phenotypic dichotomy following developmental exposure to perfluorooctanoic acid (PFOA) in female CD-1 mice: Low doses induce elevated serum leptin and insulin, and overweight in mid-life. Mol Cell Endocrinol 2009, 304, 97-105. [CrossRef]
- Kilari, T.; Singh, S.A.; Singh, A.; Begum, R.; Venkkatesh, P.; Vellapandian, C. Effect of Per and Poly-Fluoroalkyl Substances on Pregnancy and Child Development. Curr Pediatr Rev 2024. [CrossRef]
- Riu, A.; McCollum, C.W.; Pinto, C.L.; Grimaldi, M.; Hillenweck, A.; Perdu, E.; Zalko, D.; Bernard, L.; Laudet, V.; Balaguer, P.; et al. Halogenated bisphenol-A analogs act as obesogens in zebrafish larvae (Danio rerio). Toxicol Sci 2014, 139, 48-58. [CrossRef]
- Qi, W.; Clark, J.M.; Timme-Laragy, A.R.; Park, Y. Per- and Polyfluoroalkyl Substances and Obesity, Type 2 Diabetes and Non-alcoholic Fatty Liver Disease: A Review of Epidemiologic Findings. Toxicol Environ Chem 2020, 102, 1-36. [CrossRef]
- Kannan, K.; Vimalkumar, K. A Review of Human Exposure to Microplastics and Insights Into Microplastics as Obesogens. Front Endocrinol (Lausanne) 2021, 12, 724989. [CrossRef]
- Liu, G.; Dhana, K.; Furtado, J.D.; Rood, J.; Zong, G.; Liang, L.; Qi, L.; Bray, G.A.; DeJonge, L.; Coull, B.; et al. Perfluoroalkyl substances and changes in body weight and resting metabolic rate in response to weight-loss diets: A prospective study. PLoS medicine 2018, 15. [CrossRef]
- Pfohl, M.; Ingram, L.; Marques, E.; Auclair, A.; Barlock, B.; Jamwal, R.; Anderson, D.; Cummings, B.S.; Slitt, A.L. Perfluorooctanesulfonic Acid and Perfluorohexanesulfonic Acid Alter the Blood Lipidome and the Hepatic Proteome in a Murine Model of Diet-Induced Obesity. Toxicol Sci 2020, 178, 311-324. [CrossRef]
- Zhang, M.; Rifas-Shiman, S.L.; Aris, I.M.; Fleisch, A.F.; Lin, P.D.; Nichols, A.R.; Oken, E.; Hivert, M.F. Associations of Prenatal Per- and Polyfluoroalkyl Substance (PFAS) Exposures with Offspring Adiposity and Body Composition at 16-20 Years of Age: Project Viva. Environ Health Perspect 2023, 131, 127002. [CrossRef]
- Meneguzzi, A.; Fava, C.; Castelli, M.; Minuz, P. Exposure to Perfluoroalkyl Chemicals and Cardiovascular Disease: Experimental and Epidemiological Evidence. Frontiers in Endocrinology 2021, 12, 706352-706352. [CrossRef]
- Jiang, J.Y.; Wei, C.C. Per/poly fluoroalkyl substances induce lipid accumulation via the serotonergic signaling pathway in {Caenorhabditis elegans}. ISEE Conference Abstracts 2023, 2023.
- Liu, H.; Hu, W.; Li, X.; Hu, F.; Xi, Y.; Su, Z.; Huang, Y.; Liu, B.; Zhang, C. Do perfluoroalkyl substances aggravate the occurrence of obesity-associated glucolipid metabolic disease? Environ Res 2021, 202, 111724. [CrossRef]
- Gundacker, C.; Audouze, K.; Widhalm, R.; Granitzer, S.; Forsthuber, M.; Jornod, F.; Wielsøe, M.; Long, M.; Halldórsson, T.I.; Uhl, M.; et al. Reduced Birth Weight and Exposure to Per- and Polyfluoroalkyl Substances: A Review of Possible Underlying Mechanisms Using the AOP-HelpFinder. Toxics 2022, 10. [CrossRef]
- Bloom, M.S.; Commodore, S.; Ferguson, P.L.; Neelon, B.; Pearce, J.L.; Baumer, A.; Newman, R.B.; Grobman, W.; Tita, A.; Roberts, J.; et al. Association between gestational PFAS exposure and Children's adiposity in a diverse population. Environ Res 2022, 203, 111820. [CrossRef]
- Nelson, J.W.; Hatch, E.E.; Webster, T.F. Exposure to polyfluoroalkyl chemicals and cholesterol, body weight, and insulin resistance in the general U.S. population. Environ Health Perspect 2010, 118, 197-202. [CrossRef]
- Perfluorooctane sulfonate (PFOS), perfluorooctanoic acid (PFOA) and their salts Scientific Opinion of the Panel on Contaminants in the Food chain. Efsa j 2008, 6, 653. [CrossRef]
- Pelch, K.E.; Reade, A.; Wolffe, T.A.M.; Kwiatkowski, C.F. PFAS health effects database: Protocol for a systematic evidence map. Environ Int 2019, 130, 104851. [CrossRef]
- Zhang, M.; Rifas-Shiman, S.L.; Aris, I.; Fleisch, A.; Oken, E.; Hivert, M.-F. Abstract 64: Prenatal Per- and Polyfluoroalkyl Substance (PFAS) Exposures, Individually and as a Mixture, Are Associated With Obesity Risk at 16-20 Years in the Project Viva Prospective Cohort: Implications for PFAS as Hazardous Substances for Developmental Health. Circulation 2023, 147, A64-A64.
- Wang, D.; Yan, S.; Yan, J.; Teng, M.; Meng, Z.; Li, R.; Zhou, Z.; Zhu, W. Effects of triphenyl phosphate exposure during fetal development on obesity and metabolic dysfunctions in adult mice: Impaired lipid metabolism and intestinal dysbiosis. Environ Pollut 2019, 246, 630-638. [CrossRef]
- Jane, L.E.L.; Yamada, M.; Ford, J.; Owens, G.; Prow, T.; Juhasz, A. Health-related toxicity of emerging per- and polyfluoroalkyl substances: Comparison to legacy PFOS and PFOA. Environ Res 2022, 212, 113431. [CrossRef]
- Villeneuve, D.L.; Blackwell, B.R.; Cavallin, J.E.; Collins, J.; Hoang, J.X.; Hofer, R.N.; Houck, K.A.; Jensen, K.M.; Kahl, M.D.; Kutsi, R.N.; et al. Verification of In Vivo Estrogenic Activity for Four Per- and Polyfluoroalkyl Substances (PFAS) Identified as Estrogen Receptor Agonists via New Approach Methodologies. Environ Sci Technol 2023, 57, 3794-3803. [CrossRef]
- Ding, N.; Gutierrez, C.A.K.; Herman, W.H.; Calafat, A.M.; Mukherjee, B.; Park, S.K. Perfluoroalkyl and Polyfluoroalkyl Substances and Body Size and Composition Trajectories: the Study of Women’s Health Across the Nation 1999-2018. ISEE Conference Abstracts 2021, 2021.
- Ding, N.; Harlow, S.D.; Randolph, J.F., Jr.; Loch-Caruso, R.; Park, S.K. Perfluoroalkyl and polyfluoroalkyl substances (PFAS) and their effects on the ovary. Hum Reprod Update 2020, 26, 724-752. [CrossRef]
- Costello, E.; Rock, S.; Stratakis, N.; Eckel, S.; Walker, D.I.; Valvi, D.; Cserbik, D.; Jenkins, T.; Xanthakos, S.A.; Kohli, R.; et al. Exposure to perfluoroalkyl substances (PFAS) and liver injury: a systematic review and meta-analysis. ISEE Conference Abstracts 2021, 2021.
- Mitro, S.D.; Sagiv, S.K.; Rifas-Shiman, S.L.; Calafat, A.M.; Fleisch, A.F.; Jaacks, L.M.; Williams, P.L.; Oken, E.; James-Todd, T.M. Per- and Polyfluoroalkyl Substance Exposure, Gestational Weight Gain, and Postpartum Weight Changes in Project Viva. Obesity 2020, 28, 1984-1992. [CrossRef]
- Canova, C.; Barbieri, G.; Jeddi, M.Z.; Gion, M.; Fabricio, A.; Daprà, F.; Russo, F.; Fletcher, T.; Pitter, G. Associations between perfluoroalkyl substances and lipid profile in a highly exposed young adult population in the Veneto Region. Environment international 2020, 145. [CrossRef]
- Li, Y.; Barregard, L.; Xu, Y.; Scott, K.; Pineda, D.; Lindh, C.H.; Jakobsson, K.; Fletcher, T. Associations between perfluoroalkyl substances and serum lipids in a Swedish adult population with contaminated drinking water. Environmental Health: A Global Access Science Source 2020, 19, 1-11. [CrossRef]
- Lin, P.I.D.; Cardenas, A.; Hauser, R.; Gold, D.R.; Kleinman, K.P.; Hivert, M.F.; Fleisch, A.F.; Calafat, A.M.; Webster, T.F.; Horton, E.S.; et al. Per- and polyfluoroalkyl substances and blood lipid levels in pre-diabetic adults-longitudinal analysis of the diabetes prevention program outcomes study. Environment international 2019, 129, 343-353. [CrossRef]
- Kahn, L.G.; Philippat, C.; Nakayama, S.F.; Slama, R.; Trasande, L. Endocrine-disrupting chemicals: implications for human health. The lancet. Diabetes & endocrinology 2020, 8, 703-703. [CrossRef]
- He, X.; Liu, Y.; Xu, B.; Gu, L.; Tang, W. PFOA is associated with diabetes and metabolic alteration in US men: National Health and Nutrition Examination Survey 2003-2012. The Science of the total environment 2018, 625, 566-574. [CrossRef]
- Liu, H.S.; Wen, L.L.; Chu, P.L.; Lin, C.Y. Association among total serum isomers of perfluorinated chemicals, glucose homeostasis, lipid profiles, serum protein and metabolic syndrome in adults: NHANES, 2013-2014. Environmental pollution (Barking, Essex : 1987) 2018, 232, 73-79. [CrossRef]
- Per- and Polyfluoroalkyl Substances (PFAS).
- Lind, P.M.; Lind, L. Are Persistent Organic Pollutants Linked to Lipid Abnormalities, Atherosclerosis and Cardiovascular Disease? A Review. Journal of lipid and atherosclerosis 2020, 9, 334-348. [CrossRef]
- De Toni, L.; Radu, C.M.; Sabovic, I.; Di Nisio, A.; Dall’acqua, S.; Guidolin, D.; Spampinato, S.; Campello, E.; Simioni, P.; Foresta, C. Increased Cardiovascular Risk Associated with Chemical Sensitivity to Perfluoro–Octanoic Acid: Role of Impaired Platelet Aggregation. International Journal of Molecular Sciences 2020, Vol. 21, Page 399 2020, 21, 399-399. [CrossRef]
- Minuz, P.; De Toni, L.; Dall'Acqua, S.; Di Nisio, A.; Sabovic, I.; Castelli, M.; Meneguzzi, A.; Foresta, C. Interference of C6O4 on platelet aggregation pathways: Cues on the new-generation of perfluoro-alkyl substance. Environment international 2021, 154. [CrossRef]
- Li, L.; Shi, X.; Guo, X.; Li, H.; Xu, C. Ionic protein-lipid interaction at the plasma membrane: What can the charge do? Trends in Biochemical Sciences 2014, 39, 130-140. [CrossRef]
- Averina, M.; Brox, J.; Huber, S.; Furberg, A.S. Exposure to perfluoroalkyl substances (PFAS) and dyslipidemia, hypertension and obesity in adolescents. The Fit Futures study. Environmental Research 2021, 195. [CrossRef]
- Margolis, R.; Sant, K.E. Associations between exposures to perfluoroalkyl substances and diabetes, hyperglycemia, or insulin resistance: a scoping review. Journal of Xenobiotics 2021, 11, 115-129. [CrossRef]
- Tumova, J.; Andel, M.; Trnka, J. Excess of Free Fatty Acids as a Cause of Metabolic Dysfunction in Skeletal Muscle Obesity and circulating free fatty acids. Physiol. Res 2016, 65, 193-207. [CrossRef]
- White, S.S.; Fenton, S.E.; Hines, E.P. Endocrine disrupting properties of perfluorooctanoic acid. The Journal of steroid biochemistry and molecular biology 2011, 127, 16-26. [CrossRef]
- Barry, V.; Darrow, L.A.; Klein, M.; Winquist, A.; Steenland, K. Early life perfluorooctanoic acid (PFOA) exposure and overweight and obesity risk in adulthood in a community with elevated exposure. Environmental Research 2014, 132, 62-69. [CrossRef]
- Jin, R.; McConnell, R.; Catherine, C.; Xu, S.; Walker, D.I.; Stratakis, N.; Jones, D.P.; Miller, G.W.; Peng, C.; Conti, D.V.; et al. Perfluoroalkyl substances and severity of nonalcoholic fatty liver in Children: An untargeted metabolomics approach. Environ Int 2020, 134, 105220. [CrossRef]
- Braun, J.M.; Kalloo, G.; Chen, A.; Dietrich, K.N.; Liddy-Hicks, S.; Morgan, S.; Xu, Y.; Yolton, K.; Lanphear, B.P. Cohort Profile: The Health Outcomes and Measures of the Environment (HOME) study. International Journal of Epidemiology 2017, 46, 24-24. [CrossRef]
- Pérez, F.; Nadal, M.; Navarro-Ortega, A.; Fàbrega, F.; Domingo, J.L.; Barceló, D.; Farré, M. Accumulation of perfluoroalkyl substances in human tissues. Environment international 2013, 59, 354-362. [CrossRef]
- Kudo, N.; Kawashima, Y. Toxicity and toxicokinetics of perfluorooctanoic acid in humans and animals. The Journal of toxicological sciences 2003, 28, 49-57. [CrossRef]
- Cheng, W.; Doering, J.A.; LaLone, C.; Ng, C. Integrative computational approaches to inform relative bioaccumulation potential of per- and polyfluoroalkyl substances (PFAS) across species. Toxicological sciences : an official journal of the Society of Toxicology 2021, 180, 212-212. [CrossRef]
- Han, X.; Snow, T.A.; Kemper, R.A.; Jepson, G.W. Binding of perfluorooctanoic acid to rat and human plasma proteins. Chemical research in toxicology 2003, 16, 775-781. [CrossRef]
- Sheng, N.; Cui, R.; Wang, J.; Guo, Y.; Wang, J.; Dai, J. Cytotoxicity of novel fluorinated alternatives to long-chain perfluoroalkyl substances to human liver cell line and their binding capacity to human liver fatty acid binding protein. Archives of toxicology 2018, 92, 359-369. [CrossRef]
- Woodcroft, M.W.; Ellis, D.A.; Rafferty, S.P.; Burns, D.C.; March, R.E.; Stock, N.L.; Trumpour, K.S.; Yee, J.; Munrok, K. Experimental characterization of the mechanism of perfluorocarboxylic acids' liver protein bioaccumulation: the key role of the neutral species. Environmental toxicology and chemistry 2010, 29, 1669-1677. [CrossRef]
- Ducatman, A.; Luster, M.; Fletcher, T. Perfluoroalkyl substance excretion: Effects of organic anion-inhibiting and resin-binding drugs in a community setting. Environmental Toxicology and Pharmacology 2021, 85, 103650-103650. [CrossRef]
- Understanding PFAS Exposure and Your Body | Per- and Polyfluoroalkyl Substances (PFAS) and Your Health | ATSDR.
- Andersen, M.E.; Butenhoff, J.L.; Chang, S.C.; Farrar, D.G.; Kennedy, G.L.; Lau, C.; Olsen, G.W.; Seed, J.; Wallace, K.B. Perfluoroalkyl acids and related chemistries–toxicokinetics and modes of action. Toxicological sciences : an official journal of the Society of Toxicology 2008, 102, 3-14. [CrossRef]
- Solan, M.E.; Lavado, R. The use of in vitro methods in assessing human health risks associated with short-chain perfluoroalkyl and polyfluoroalkyl substances (PFAS). Journal of applied toxicology : JAT 2022, 42, 1298-1309. [CrossRef]
- Khazaee, M. Investigating the impacts of per-and polyfluoroalkyl substances (PFAS) on biological systems by complementary in vivo, in vitro, and in silico approaches. 2022.
- Ng, C.A.; Hungerbühler, K. Bioconcentration of perfluorinated alkyl acids: How important is specific binding? Environmental Science and Technology 2013, 47, 7214-7223. [CrossRef]
- Zhao, W.; Zitzow, J.D.; Weaver, Y.; Ehresman, D.J.; Chang, S.C.; Butenhoff, J.L.; Hagenbuch, B. Organic Anion Transporting Polypeptides Contribute to the Disposition of Perfluoroalkyl Acids in Humans and Rats. Toxicological sciences : an official journal of the Society of Toxicology 2017, 156, 84-95. [CrossRef]
- Jiao, X.; Shi, Q.; Gan, J. Uptake, accumulation and metabolism of PFASs in plants and health perspectives: A critical review. Critical Reviews in Environmental Science and Technology 2021, 51, 2745-2776. [CrossRef]
- Lv, G.; Sun, X. The molecular-level understanding of the uptake of PFOS and its alternatives (6:2 Cl-PFESA and OBS) into phospholipid bilayers. Journal of Hazardous Materials 2021, 417, 125991-125991. [CrossRef]
- Yuan, S.; Zhang, H.; Yuan, S. Theoretical insights into the uptake of sulfonamides onto phospholipid bilayers: Mechanisms, interaction and toxicity evaluation. Journal of Hazardous Materials 2022, 435, 129033-129033. [CrossRef]
- Willemsen, J.A.R.; Bourg, I.C. Molecular dynamics simulation of the adsorption of per- and polyfluoroalkyl substances (PFASs) on smectite clay. Journal of Colloid and Interface Science 2021, 585, 337-346. [CrossRef]
- Brennan, N.M.; Evans, A.T.; Fritz, M.K.; Peak, S.A.; von Holst, H.E. Trends in the Regulation of Per- and Polyfluoroalkyl Substances (PFAS): A Scoping Review. Int J Environ Res Public Health 2021, 18. [CrossRef]
- Lindstrom, A.B.; Strynar, M.J.; Libelo, E.L. Polyfluorinated compounds: past, present, and future. Environmental science & technology 2011, 45, 7954-7961. [CrossRef]
- Tachachartvanich, P.; Singam, E.R.A.; Durkin, K.A.; Furlow, J.D.; Smith, M.T.; La Merrill, M.A. In Vitro characterization of the endocrine disrupting effects of per- and poly-fluoroalkyl substances (PFASs) on the human androgen receptor. Journal of Hazardous Materials 2022, 429. [CrossRef]
- Abunada, Z.; Alazaiza, M.Y.D.; Bashir, M.J.K. An Overview of Per- and Polyfluoroalkyl Substances (PFAS) in the Environment: Source, Fate, Risk and Regulations. Water 2020, Vol. 12, Page 3590 2020, 12, 3590-3590. [CrossRef]
- Langenbach, B.; Wilson, M. Per- and Polyfluoroalkyl Substances (PFAS): Significance and Considerations within the Regulatory Framework of the USA. International Journal of Environmental Research and Public Health 2021, 18, 11142-11142. [CrossRef]
- All news - ECHA.
- Questions and Answers: Drinking Water Health Advisories for PFOA, PFOS, GenX Chemicals and PFBS | US EPA.
- List of Key New Pollutants for Control (2022 Edition) (Draft for Comment) Annex 2.
- Guidelines for Canadian Drinking Water Quality: Guideline Technical Document – Perfluorooctane Sulfonate (PFOS) - Canada.ca.
- Ahrens, L. Polyfluoroalkyl compounds in the aquatic environment: A review of their occurrence and fate. Journal of Environmental Monitoring 2011, 13, 20-31. [CrossRef]
- PFAS Information for Clinicians - 2024 | ATSDR.
- Per- and Polyfluoroalkyl Substances (PFAS) | State Legislation and Federal Action.
- Wee, S.Y.; Aris, A.Z. Revisiting the “forever chemicals”, PFOA and PFOS exposure in drinking water. npj Clean Water 2023, 6. [CrossRef]
- EPA proposes limits for ‘forever chemicals’ in drinking water.
- Persistent Chemicals: Technologies for PFAS Assessment, Detection, and Treatment | U.S. GAO.
- Per- and Polyfluoroalkyl Substances (PFAS) | FDA.
- Krafft, M.P.; Riess, J.G. Per- and polyfluorinated substances (PFASs): Environmental challenges. Current Opinion in Colloid & Interface Science 2015, 20, 192-212. [CrossRef]
- Land, M.; De Wit, C.A.; Bignert, A.; Cousins, I.T.; Herzke, D.; Johansson, J.H.; Martin, J.W. What is the effect of phasing out long-chain per- and polyfluoroalkyl substances on the concentrations of perfluoroalkyl acids and their precursors in the environment? A systematic review. Environmental Evidence 2018, 7, 1-32. [CrossRef]
- Production and emissions - OECD Portal on Per and Poly Fluorinated Chemicals.
- Our Current Understanding of the Human Health and Environmental Risks of PFAS | US EPA.
- Guelfo, J.L.; Marlow, T.; Klein, D.M.; Savitz, D.A.; Frickel, S.; Crimi, M.; Suuberg, E.M. Evaluation and Management Strategies for Per- and Polyfluoroalkyl Substances (PFASs) in Drinking Water Aquifers: Perspectives from Impacted U.S. Northeast Communities. Environmental health perspectives 2018, 126. [CrossRef]
- Bălan, S.A.; Mathrani, V.C.; Guo, D.F.; Algazi, A.M. Regulating PFAS as a Chemical Class under the California Safer Consumer Products Program. Environmental health perspectives 2021, 129, 1-9. [CrossRef]
- Hoover, G.; Kar, S.; Guffey, S.; Leszczynski, J.; Sepúlveda, M.S. In vitro and in silico modeling of perfluoroalkyl substances mixture toxicity in an amphibian fibroblast cell line. Chemosphere 2019, 233, 25-33. [CrossRef]
- Marciesky, M.; Aga, D.S.; Bradley, I.M.; Aich, N.; Ng, C. Mechanisms and Opportunities for Rational In Silico Design of Enzymes to Degrade Per- and Polyfluoroalkyl Substances (PFAS). Journal of Chemical Information and Modeling 2023, 63, 7299-7299. [CrossRef]
- Leonello, D.; Fendrich, M.A.; Parrino, F.; Patel, N.; Orlandi, M.; Miotello, A. Light-Induced Advanced Oxidation Processes as PFAS Remediation Methods: A Review. Applied Sciences 2021, Vol. 11, Page 8458 2021, 11, 8458-8458. [CrossRef]
- Panieri, E.; Baralic, K.; Djukic-Cosic, D.; Djordjevic, A.B.; Saso, L. PFAS Molecules: A Major Concern for the Human Health and the Environment. Toxics 2022, 10. [CrossRef]
- Ji, B.; Kang, P.; Wei, T.; Zhao, Y. Challenges of aqueous per- and polyfluoroalkyl substances (PFASs) and their foreseeable removal strategies. Chemosphere 2020, 250. [CrossRef]
- Genuis, S.J.; Birkholz, D.; Ralitsch, M.; Thibault, N. Human detoxification of perfluorinated compounds. Public health 2010, 124, 367-375. [CrossRef]
- Shi, Y.; Vestergren, R.; Xu, L.; Zhou, Z.; Li, C.; Liang, Y.; Cai, Y. Human Exposure and Elimination Kinetics of Chlorinated Polyfluoroalkyl Ether Sulfonic Acids (Cl-PFESAs). Environmental science & technology 2016, 50, 2396-2404. [CrossRef]
- National Academies of Sciences, E.; Medicine. Guidance on PFAS Exposure, Testing, and Clinical Follow-Up. Guidance on PFAS Exposure, Testing, and Clinical Follow-Up 2022. [CrossRef]
- Ross, I.; McDonough, J.; Miles, J.; Storch, P.; Kochunarayanan, P.T.; Kalve, E.; Hurst, J.; Dasgupta, S.S.; Burdick, J. A review of emerging technologies for remediation of PFASs. Remediation Journal 2018, 28, 101-126. [CrossRef]
- Sharma, N.; Kumar, V.; Sugumar, V.; Umesh, M.; Sondhi, S.; Chakraborty, P.; Kaur, K.; Thomas, J.; Kamaraj, C.; Maitra, S.S. A comprehensive review on the need for integrated strategies and process modifications for per- and polyfluoroalkyl substances (PFAS) removal: Current insights and future prospects. Case Studies in Chemical and Environmental Engineering 2024, 9. [CrossRef]
- Shia, Y.; Mua, H.; Youa, J.; Hana, C.; Chenga, H.; Wanga, J.; Hua, H.; Rena, H. Confined water encapsulated activated carbon for capturing short-chain perfluoroalkyl and polyfluoroalkyl substances from drinking water. Proceedings of the National Academy of Sciences of the United States of America 2023, 120. [CrossRef]
- Ahmed, M.B.; Alam, M.M.; Zhou, J.L.; Xu, B.; Johir, M.A.H.; Karmakar, A.K.; Rahman, M.S.; Hossen, J.; Hasan, A.T.M.K.; Moni, M.A. Advanced treatment technologies efficacies and mechanism of per- and poly-fluoroalkyl substances removal from water. Process Safety and Environmental Protection 2020, 136, 1-14. [CrossRef]
- Lutze, H.V.; Brekenfeld, J.; Naumov, S.; von Sonntag, C.; Schmidt, T.C. Degradation of perfluorinated compounds by sulfate radicals – New mechanistic aspects and economical considerations. Water Research 2018, 129, 509-519. [CrossRef]
- Chen, A.; Jandarov, R.; Zhou, L.; Calafat, A.M.; Zhang, G.; Urbina, E.M.; Sarac, J.; Augustin, D.H.; Caric, T.; Bockor, L.; et al. Association of perfluoroalkyl substances exposure with cardiometabolic traits in an island population of the eastern Adriatic coast of Croatia. Science of the Total Environment 2019, 683, 29-36. [CrossRef]
- Chen, X.; Yuan, T.; Yang, X.; Ding, S.; Ma, M. Insights into Photo/Electrocatalysts for the Degradation of Per- and Polyfluoroalkyl Substances (PFAS) by Advanced Oxidation Processes. Catalysts 2023, Vol. 13, Page 1308 2023, 13, 1308-1308. [CrossRef]
- Lewis, A.J.; Joyce, T.; Hadaya, M.; Ebrahimi, F.; Dragiev, I.; Giardetti, N.; Yang, J.; Fridman, G.; Rabinovich, A.; Fridman, A.A.; et al. Rapid degradation of PFAS in aqueous solutions by reverse vortex flow gliding arc plasma. Environmental Science: Water Research & Technology 2020, 6, 1044-1057. [CrossRef]
- Liu, J.Q.; Kurihara, T.; Ichiyama, S.; Miyagi, M.; Tsunasawa, S.; Kawasaki, H.; Soda, K.; Esaki, N. Reaction mechanism of fluoroacetate dehalogenase from Moraxella sp. B. The Journal of biological chemistry 1998, 273, 30897-30902. [CrossRef]
- Kowalska, D.; Sosnowska, A.; Bulawska, N.; Stępnik, M.; Besselink, H.; Behnisch, P.; Puzyn, T. How the Structure of Per- and Polyfluoroalkyl Substances (PFAS) Influences Their Binding Potency to the Peroxisome Proliferator-Activated and Thyroid Hormone Receptors—An In Silico Screening Study. Molecules 2023, 28. [CrossRef]
- Cheng, W.; Doering, J.A.; LaLone, C.; Ng, C.A. Estimating the Bioaccumulation Potential of Per- and Polyfluoroalkyl Substances (PFAS) across Species by Integrative in Silico Approaches. 2020. [CrossRef]
- Rogers, R.D.; Reh, C.M.; Breysse, P. Advancing per- and polyfluoroalkyl substances (PFAS) research: an overview of ATSDR and NCEH activities and recommendations. Journal of Exposure Science & Environmental Epidemiology 2021 31:6 2021, 31, 961-971. [CrossRef]
- Dharpure, R.; Pramanik, S.; Pradhan, A. In silico analysis decodes transthyretin (TTR) binding and thyroid disrupting effects of per- and polyfluoroalkyl substances (PFAS). Archives of Toxicology 2023, 97, 755-768. [CrossRef]
- Li, W.; Hu, Y.; Bischel, H.N. In-Vitro and In-Silico Assessment of Per- and Polyfluoroalkyl Substances (PFAS) in Aqueous Film-Forming Foam (AFFF) Binding to Human Serum Albumin. Toxics 2021, Vol. 9, Page 63 2021, 9, 63-63. [CrossRef]
- Al-Karmalawy, A.A.; Dahab, M.A.; Metwaly, A.M.; Elhady, S.S.; Elkaeed, E.B.; Eissa, I.H.; Darwish, K.M. Molecular Docking and Dynamics Simulation Revealed the Potential Inhibitory Activity of ACEIs Against SARS-CoV-2 Targeting the hACE2 Receptor. Frontiers in Chemistry 2021, 9, 661230-661230. [CrossRef]
- Priya Doss, C.G.; Chakraborty, C.; Chen, L.; Zhu, H. Integrating in silico prediction methods, molecular docking, and molecular dynamics simulation to predict the impact of ALK missense mutations in structural perspective. BioMed Research International 2014, 2014. [CrossRef]
- Zhang, J.; Zhang, M.; Tao, H.; Qi, G.; Guo, W.; Ge, H.; Shi, J. A QSAR–ICE–SSD Model Prediction of the PNECs for Per- and Polyfluoroalkyl Substances and Their Ecological Risks in an Area of Electroplating Factories. Molecules 2021, 26. [CrossRef]
- Li, C.H.; Ren, X.M.; Cao, L.Y.; Qin, W.P.; Guo, L.H. Investigation of binding and activity of perfluoroalkyl substances to the human peroxisome proliferator-activated receptor β/δ. Environmental Science: Processes & Impacts 2019, 21, 1908-1914. [CrossRef]
- Filipe, H.A.L.; Loura, L.M.S. Molecular Dynamics Simulations: Advances and Applications. Molecules 2022, Vol. 27, Page 2105 2022, 27, 2105-2105. [CrossRef]
- Mirzadeh, A.; Kobakhidze, G.; Vuillemot, R.; Jonic, S.; Rouiller, I. In silico prediction, characterization, docking studies and molecular dynamics simulation of human p97 in complex with p37 cofactor. BMC Molecular and Cell Biology 2022, 23, 1-12. [CrossRef]
- Dawson, D.; Lau, C.; Pradeep, P.; Judson, R.; Tornero-Velez, R.; Wambaugh, J. A quantitative structure-activity relationship (QSAR) model to estimate half-lives of perfluoro-alkyl substances (PFAS) in multiple species. 2021. [CrossRef]
- Sosnowska, A.; Bulawska, N.; Kowalska, D.; Puzyn, T. Towards higher scientific validity and regulatory acceptance of predictive models for PFAS. Green Chemistry 2023, 25, 1261-1275. [CrossRef]
- Cousins, I.T.; Dewitt, J.C.; Glüge, J.; Goldenman, G.; Herzke, D.; Lohmann, R.; Miller, M.; Ng, C.A.; Scheringer, M.; Vierke, L.; et al. Strategies for grouping per- and polyfluoroalkyl substances (PFAS) to protect human and environmental health. Environmental Science: Processes & Impacts 2020, 22, 1444-1460. [CrossRef]
- Approaches to Non-targeted Analyses of Per- and Polyfluoroalkyl Substances (PFAS) in Environmental Samples | Waters.
- Torralba-Sanchez, T.L.; Dmitrenko, O.; Toro, D.M.D.; Tratnyek, P.G. In Silico Prediction of Fate and Risk-Determining Properties of Per-and Polyfluoroalkyl Substances (PFAS). 2022.
- Rowan-Carroll, A.; Reardon, A.; Leingartner, K.; Gagné, R.; Williams, A.; Meier, M.J.; Kuo, B.; Bourdon-Lacombe, J.; Moffat, I.; Carrier, R.; et al. High-Throughput Transcriptomic Analysis of Human Primary Hepatocyte Spheroids Exposed to Per- and Polyfluoroalkyl Substances as a Platform for Relative Potency Characterization. Toxicological Sciences 2021, 181, 199-214. [CrossRef]
- Pouwer, M.G.; Pieterman, E.J.; Chang, S.C.; Olsen, G.W.; Caspers, M.P.M.; Verschuren, L.; Jukema, J.W.; Princen, H.M.G. Dose Effects of Ammonium Perfluorooctanoate on Lipoprotein Metabolism in APOE*3-Leiden.CETP Mice. Toxicological Sciences 2019, 168, 519-534. [CrossRef]
- Beggs, K.M.; McGreal, S.R.; McCarthy, A.; Gunewardena, S.; Lampe, J.N.; Lau, C.; Apte, U. The Role of Hepatocyte Nuclear Factor 4-Alpha in Perfluorooctanoic Acid- and Perfluorooctanesulfonic Acid-Induced Hepatocellular Dysfunction. Toxicology and applied pharmacology 2016, 304, 18-18. [CrossRef]
- Robarts, D.R.; Dai, J.; Lau, C.; Apte, U.; Corton, J.C. Hepatic Transcriptome Comparative In Silico Analysis Reveals Similar Pathways and Targets Altered by Legacy and Alternative Per- and Polyfluoroalkyl Substances in Mice. Toxics 2023, 11, 963-963. [CrossRef]
- Addicks, G.C.; Rowan-Carroll, A.; Reardon, A.J.F.; Leingartner, K.; Williams, A.; Meier, M.J.; Moffat, I.; Carrier, R.; Lorusso, L.; Wetmore, B.A.; et al. Per- and polyfluoroalkyl substances (PFAS) in mixtures show additive effects on transcriptomic points of departure in human liver spheroids. Toxicological Sciences 2023, 194, 38-52. [CrossRef]
- Rericha, Y.; Mary, L.S.; Truong, L.; McClure, R.S.; Martin, J.K.; Leonard, S.; Thunga, P.; Simonich, M.T.; Waters, K.M.; Field, J.A.; et al. Distinct transcriptomic responses to structurally diverse per-and polyfluoroalkyl substances (PFAS) precede developmental toxicity in zebrafish. Frontiers in Toxicology 6, 1425537-1425537. [CrossRef]
- Beccacece, L.; Costa, F.; Pascali, J.P.; Giorgi, F.M. Cross-Species Transcriptomics Analysis Highlights Conserved Molecular Responses to Per- and Polyfluoroalkyl Substances. Toxics 2023, 11, 567-567. [CrossRef]
- Rudzanová, B.; Thon, V.; Vespalcová, H.; Martyniuk, C.J.; Piler, P.; Zvonař, M.; Klánová, J.; Bláha, L.; Adamovsky, O. Altered Transcriptome Response in PBMCs of Czech Adults Linked to Multiple PFAS Exposure: B Cell Development as a Target of PFAS Immunotoxicity. Environmental Science and Technology 2024, 58, 90-98. [CrossRef]
- Feinstein, J.; ganesh, s.; Picel, K.; Peters, B.; Vazquez-Mayagoitia, A.; Ramanathan, A.; MacDonell, M.; Foster, I.; Yan, E. Uncertainty-Informed Deep Transfer Learning of PFAS Toxicity. 2021. [CrossRef]
- Lai, T.T.; Kuntz, D.; Wilson, A.K. Molecular Screening and Toxicity Estimation of 260,000 Perfluoroalkyl and Polyfluoroalkyl Substances (PFASs) through Machine Learning. Journal of chemical information and modeling 2022, 62, 4569-4578. [CrossRef]
- Dawson, D.E.; Lau, C.; Pradeep, P.; Sayre, R.R.; Judson, R.S.; Tornero-Velez, R.; Wambaugh, J.F. A Machine Learning Model to Estimate Toxicokinetic Half-Lives of Per- and Polyfluoro-Alkyl Substances (PFAS) in Multiple Species. Toxics 2023, 11. [CrossRef]
- Azhagiya Singam, E.R.; Tachachartvanich, P.; Fourches, D.; Soshilov, A.; Hsieh, J.C.Y.; La Merrill, M.A.; Smith, M.T.; Durkin, K.A. Structure-based virtual screening of perfluoroalkyl and polyfluoroalkyl substances (PFASs) as endocrine disruptors of androgen receptor activity using molecular docking and machine learning. Environmental Research 2020, 190, 109920-109920. [CrossRef]
- Pappalardo, F.; Russo, G.; Corsini, E.; Paini, A.; Worth, A. Translatability and transferability of in silico models: Context of use switching to predict the effects of environmental chemicals on the immune system. Computational and Structural Biotechnology Journal 2022, 20, 1764-1764. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





