1. Nrf2-Keap1 Signalling Pathway
The nuclear factor erythroid 2-related factor 2 (Nrf2) is a transcription factor encoded by the
NFE2L2 gene, known to be a master regulator of multiple cytoprotective pathways by inducing the expression of a variety of genes, including antioxidant, detoxifying and metabolic enzymes [
1].
Nrf2 levels are tightly regulated, and under normal physiological conditions, Nrf2 is maintained at low levels in the cytoplasm through its interaction with Kelch-like ECH-associated protein 1 (Keap1) (
Figure 1). Keap1 is a cysteine-rich protein that acts as a substrate adaptor for the Cullin (Cul3)-dependent E3 ubiquitin ligase complex, binding and targeting Nrf2 for ubiquitination and subsequent proteasomal degradation [
2]. This constitutive degradation pathway ensures tight regulation of Nrf2 activity, preventing excessive activation in the absence of stress stimuli. Keap1 acts as a crucial sensor for oxidative stress, and upon exposure to oxidative signals or electrophiles, the oxidation of Keap1’s highly reactive cysteine residues leads to conformational changes in the protein that disrupt its interaction with Nrf2, allowing Nrf2 to escape from Keap1-mediated degradation, accumulate and translocate to the nucleus [
3]. Nrf2 stabilisation can be also achieved by other mechanisms that facilitate Keap1 degradation or impair the Nrf2-Keap1 binding, such as the interaction of the autophagy-related protein p62 with the Nrf2-binding domain of Keap1 [
4]. Additionally, other ubiquitin-ligase systems have also been described to control Nrf2 degradation independently of Keap1, like the β-TrCP/Cul 1 system (
Figure 1). The glycogen synthase kinase-3 beta (GSK3-β) acts as a modulator of this route by phosphorylating Nrf2 and targeting it for degradation [
5]. Since GSK3-β activity, and therefore Nrf2 degradation, is regulated by survival pathways such as PI3K/Akt or Wnt, the interplay between Nrf2 and GSK3-β represents a key regulatory axis in receptor-mediated signal transduction, influencing cell survival under conditions of oxidative damage and increased metabolic demands.
Either way, once in the nucleus, Nrf2 heterodimerizes with small Maf proteins and binds to antioxidant response elements (ARE) located in the promoter regions of its target genes (
Figure 1). This binding triggers the transcriptional activation of a diverse array of genes encoding antioxidant enzymes (e.g., superoxide dismutase, catalase, glutathione peroxidase), phase II detoxification enzymes (e.g., NAD(P)H oxidoreductase 1 (NQO1), glutathione S-transferases, heme-oxygenase I (HO-1)), and other cytoprotective proteins [
6,
7].
1.1. Pharmacological Activation of Nrf2-Keap1 Signalling Pathway
Given its protective role, Nrf2 activation is an attractive therapeutic strategy for diseases characterised by mitochondrial dysfunction and oxidative stress [
8,
9]. Various pharmacological agents can induce Nrf2, as reviewed in [
10]. Most of these activators are electrophilic compounds that covalently modify Keap1's cysteine residues, causing structural changes that prevent Nrf2 degradation. Examples include sulforaphane, TBE-31, dimethyl fumarate (DMF), omaveloxolone, and several natural compounds. Efforts are underway to develop non-electrophilic compounds that specifically inhibit the Nrf2-Keap1 interaction without covalent modification [
10]. Some Nrf2 activators are in different stages of clinical development, and a few are already approved as disease-modifying treatments for various conditions [
10]. Notably, fumaric acid ester derivatives, such as DMF are approved for treating relapsing-remitting multiple sclerosis with long-term safety and efficacy [
11,
12], and omaveloxolone has been recently authorised in USA and Europe for the treatment of Friedreich’s ataxia [
13]. The fact that both are neurological disorders, highlights the key cytoprotective role of Nrf2 in the brain, both in physiology, and also as a pharmaceutical target.
2. Nrf2, the Cytoprotective Defence in the Brain
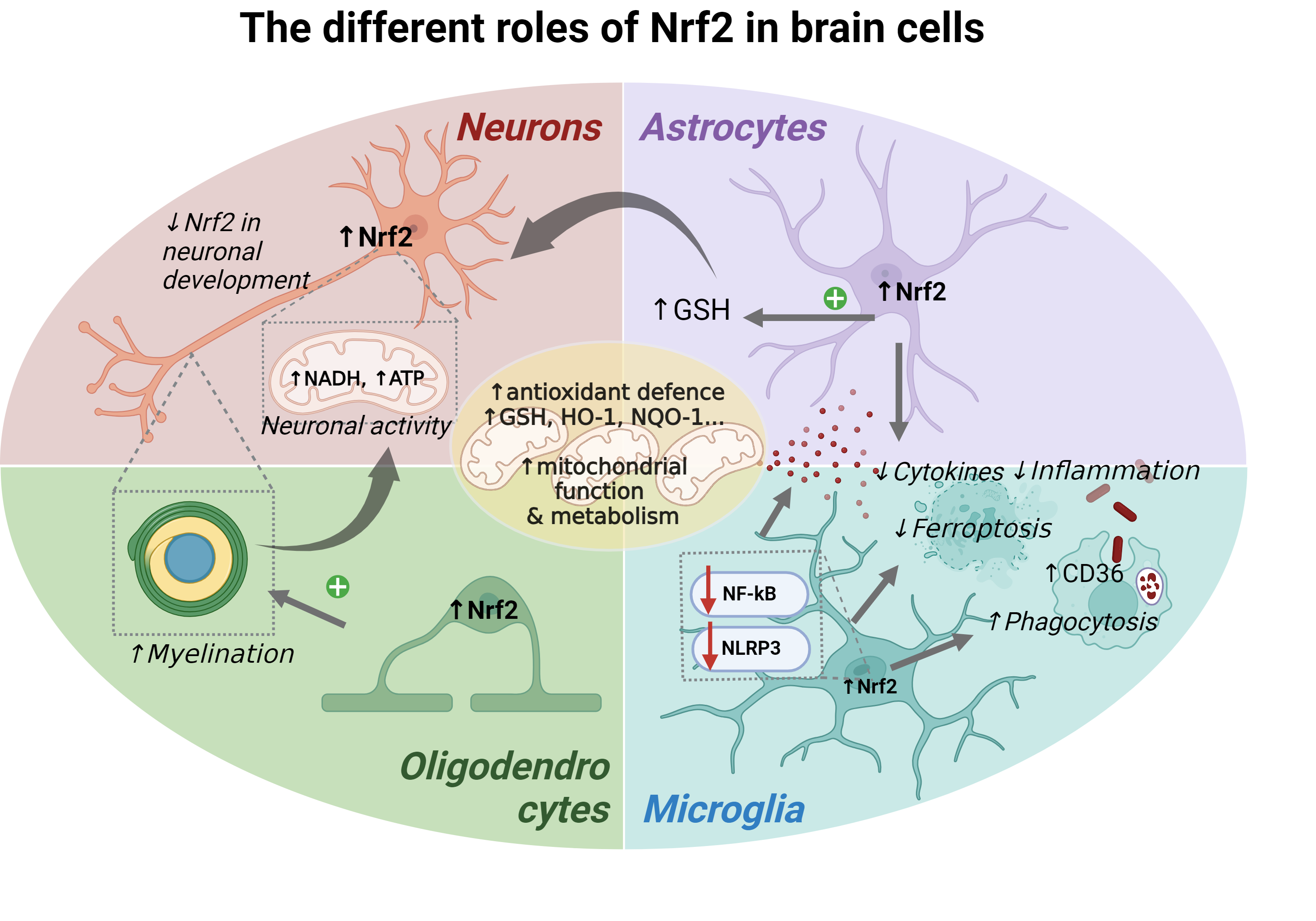
The brain is an exceptionally energy-demanding organ due to its unique functions, such as sustaining neuronal activity and synaptic transmission. These energy requirements make the brain a highly active metabolic organ which represents only about 2% of body weight but consumes up to 20 % of the total oxygen in the body, as it relies mostly on glucose oxidation for ATP production. As a consequence, a high amount of reactive oxygen species (ROS) are produced as a byproduct of mitochondrial respiration, which need to be neutralised to avoid oxidative damage (
Figure 2). This is essential, since the brain is particularly rich in polyunsaturated lipid membranes, prone to oxidation; and free iron, which acts as a catalyst in the formation of ROS, initiating a cycle of oxidative damage and metabolic failure finally leading to neuronal death [
14] (
Figure 2).
In this context, endogenous Nrf2 plays a pivotal role as one of the key cytoprotective mechanisms in the brain, acting as a master regulator of brain energy metabolism and defence against oxidative stress and inflammation, among others (
Figure 2). Nrf2 is differentially expressed in different brain regions and cell types within the brain, being more abundant in glial cells (including oligodendrocytes, astrocytes and microglia) rather than in neurons [
15] (
https://www.proteinatlas.org/ENSG00000116044-NFE2L2/single+cell+type). In the following sections, we will explore the current knowledge on the several aspects of Nrf2 function across the different brain cell types within the central nervous system (CNS), its impact on brain health and its potential implication in neurodegenerative diseases.
2.1. Nrf2 in Neurons
Neurons are highly specialised cells responsible for electro-chemical signalling transmission and are considered to consume about 80% of the brain’s energy. These exceptionally high energy requirements are largely due to the activity of Na
+/K
+ ATPase pumps, which are essential for restoring the ion gradients after excitatory synaptic signalling and action potentials propagation [
16]. To meet these needs, neurons rely heavily on oxidative metabolism, making mitochondria a key component of their energy production. This intense metabolic activity also results in a higher generation of ROS due to the leakage of electrons out of the mitochondrial electron transport chain in an oxygen-rich environment (
Figure 2). When not properly neutralised, ROS accumulation might lead to oxidative stress to which neurons are particularly vulnerable for different reasons, such as the high susceptibility of neuronal lipid membranes to oxidation [
14]. Therefore, the balance between large energy production and the management of oxidative stress is critical for maintaining neuronal health and preventing neurodegenerative damage.
2.1.1. Nrf2, Neuronal ROS and Neuronal Development
In this scenario, it appears paradoxical that neurons have a relatively weak antioxidant defence compared to other cell types in the brain. Isolated neurons are known to exhibit low levels of catalase and glutathione, two key endogenous antioxidants, both regulated by Nrf2, which also shows minimal basal activity in these cells [
17]. In fact, although Nrf2 is considered ubiquitous, results from multiple single cell RNA-seq and single nucleus RNA-seq datasheets consistently show that the
NFE2L2 gene is repressed in most of the mature neurons in the CNS [
18]. This downregulation appears to occur at the epigenetic level, where the hypo-acetylation of the histones associated with
NFE2L2 promoter prevents gene transcription by limiting chromatin accessibility [
18,
19]. Not only Nrf2 mRNA is downregulated in mature neurons, but also Nrf2 protein, which appears to be more unstable when compared to other brain cells, partially due to a parallel increase in Keap1 and Cul1 expression [
20]. In agreement, the mRNA and protein levels of some of Nrf2 target genes such as NQO1 or HO-1 among others are also lower in neurons than in other cell types, suggesting an overall weaker Nrf2 system in this cell type [
18,
20].
NFE2L2 repression occurs during the development and differentiation of neuronal precursors, when Nrf2 mRNA levels decline and Keap1 increase, shutting down the activity of this transcription factor during development [
18]. In contrast, Nrf2 is highly expressed in embryonic stem cells and plays a key role in their self-renewal capacity, proliferation, and differentiation [
21]. Likewise, neuronal stem cells and neuronal progenitor cells also show elevated Nrf2 mRNA levels [
19]. Indeed, Nrf2 plays an important role in their function both during the early stages of development, and in the regulation of the activity of the neuronal stem cells in the adult brain [
22]. These cells are responsible for the generation of new neurons and astrocytes during adult neurogenesis in specific brain niches such as the subventricular zone. It is known that their activity and neurogenic potential declines with age, and this occurs in correlation with an age-dependent decline in Nrf2 activity [
23]. Indeed, modulation of Nrf2 appears to restore their neurogenic and proliferative potential [
24]. However, even when Nrf2 activity is crucial for the function of precursor cells, its inactivation becomes critical for proper neuronal maturation as differentiation progresses, since redox signalling is essential for neuronal development [
19]. Although a disbalance in their levels is strongly associated with the onset of many diseases, ROS also play an important role in physiology and cell signalling. Among the different processes they regulate, an optimal level of brain ROS is necessary for the proper maturation of neurons [
25]. Among others, redox signalling regulates different pathways essential for development, such as Wnt, which can be potentiated by ROS [
26]. Nrf2, through its potent antioxidant capacity, prevents redox-dependent Wnt activation, suggesting that its shut off during development is essential to allow for a boost of Wnt signalling in this period [
19]. Indeed, forcing Nrf2 expression during early differentiation impairs neuronal maturation, including neurite outgrowth and synaptogenesis [
19]. It is still not clear why Nrf2 levels remain low in mature neurons, despite their higher antioxidant demands, but it is known that ROS signalling controls many aspects of neuronal function [
27,
28], so it could be possible that a basal chronic activation of Nrf2 in neurons could interfere with these processes. However, epigenetic de-repression of neuronal Nrf2 in mature neurons appears to enhance its cytoprotective effects under damaging circumstances [
29].
2.1.2. Nrf2, Neuronal ROS and Neurodegenerative Disorders
It has also been argued that Nrf2 reduced expression in neurons in physiological conditions could be due to an insufficient rate of ROS to trigger Nrf2 activation. Nevertheless, neuronal expression of Nrf2 also appears to be low in some neurodegenerative conditions associated with increased oxidative stress such as Alzheimer's disease (AD) [
30] or multiple sclerosis [
31], according to single nucleus RNA sequencing profiles. Conversely, studies at the subcellular level in human brain tissues from patients with various neurodegenerative conditions reveal distinct patterns depending on the disease. In AD, Nrf2 predominantly exhibits a cytoplasmic localization in hippocampal neurons affected by the pathology [
32], whereas in Parkinson's disease (PD), neurons in the substantia nigra show a pronounced nuclear localization of Nrf2, likely indicating a compensatory response to elevated oxidative stress [
32,
33]. This has also been found in
in vitro models of other neurodegenerative conditions. For example, induced neurons derived from amyotrophic lateral sclerosis (ALS) patients with the C9orf72 mutation showed higher Nrf2 nuclear location and activity, indicating an activation of the Nrf2 pathway under oxidative stress conditions [
34]. This partial activation of Nrf2 appeared insufficient to mitigate the elevated levels of ROS present in these cells. However, pharmacological activators of Nrf2, such as DMF, were able to significantly reduce oxidative stress [
34]. This finding suggests that, although endogenous Nrf2 activation was insufficient to protect neurons, external activation through compounds like DMF effectively enhanced the antioxidant response, offering improved defence against oxidative damage. This neuroprotective effect has been further demonstrated in several animal models of different disorders, either by genetic or pharmacological activation of the Nrf2 pathway, for detailed reviews [
7,
35].
2.1.3. Nrf2 and Neuronal Mitochondria
As previously mentioned, the processes involved in synaptic transmission significantly account for the majority of ATP consumed by neurons. Mitochondria are the primary source of neuronal ATP and play a critical role in maintaining synaptic homeostasis, by acting as localised calcium buffers during pre- and post-synaptic events, which involve large influxes of cytosolic calcium [
36]. Mitochondrial calcium homeostasis is closely linked to energy production; increased calcium uptake boosts ATP synthesis, helping neurons adapt to their energy needs. Additionally, calcium modulates the movement and positioning of mitochondria within neurons, directing them to areas with higher energy demands such as the axon terminals. Therefore, proper mitochondrial function is crucial for optimal neuron activity, and it is well established that Nrf2 regulates various aspects of mitochondrial activity, both in normal physiology and as a potential therapeutic target in pathological conditions [
37].
Although low compared to other cell types, basal neuronal Nrf2 expression has been described to play an important role in the neuronal mitochondrial function, as described in
in vitro and animal models of genetical Nrf2 deficiency. Isolated primary neurons of Nrf2 knockout mice present increased oxidative stress and mitochondrial bioenergetics dysfunction, including impaired respiration and a less efficient activity of the Krebs cycle, leading to reduced levels of mitochondrial NADH and ATP, compared to wild-type neurons [
38,
39]. This is accompanied by defects in neuronal dendritic arborization and synaptic gene expression and is associated with cognitive deficits in the Nrf2-deficient aged mice [
39,
40]. In contrast, in isolated neurons of Keap1 knock-down mice, genetic activation of Nrf2 enhances respiration efficiency by increasing the availability of substrates for the electron transport chain (ETC), leading to higher ATP production in the mitochondria compared with wild-type neurons [
38] (
Figure 2). Indeed, primary cultures from this mice model show that genetic Nrf2 activation enhances glucose uptake into neurons, which is preferentially metabolised in the glycolytic/oxidative phosphorylation pathway to produce energy [
41].
Beyond its physiological function, the stimulation of neuronal glucose metabolism by Nrf2 underlies some of the neuroprotective effects that its pharmacological activation has shown in different pathological contexts
. Neurons are very vulnerable to mitochondrial dysfunction and energy deprivation, which are a common hallmark of different neurodegenerative disorders. Among them, PD is characterised by an impairment of mitochondrial respiration due to insufficient substrate availability for the ETC, especially at complex I. This leads to reduced ATP production, which is particularly detrimental to the dopaminergic neurons in the substantia nigra, a region severely impacted by the disease. These mitochondrial effects, which resemble those observed in Nrf2 deficiency, can be reverted by the pharmacological activation of Nrf2, which has been shown to enhance mitochondrial substrates provision for the ETC [
42]. Activation of Nrf2 with sulforaphane or omaveloxolone (RTA-408), was shown to reverse bioenergetic deficits in cultured neurons within a PINK1-deficient PD model and prevent neuronal death, confirming the role of Nrf2 activation in supporting mitochondrial bioenergetics in neurons [
43]. The mitochondrial and bioenergetic effects of omaveloxolone have also been observed in Friedreich's ataxia, a hereditary, slowly progressive ataxia characterised at the molecular level by impaired mitochondrial function due to reduced substrate availability for ETC Complex I [
44]. Pharmacological activation of Nrf2 with omaveloxolone increased the availability of NADH for mitochondrial complex I in cerebellar granular neurons of mouse models of the disease and restored the mitochondrial respiration, preventing neuronal death [
44]. The neuroprotective effects of omaveloxolone were also demonstrated in both
in vivo and
in vitro models, preventing the onset of seizures [
45]. Prolonged seizure-like activity induces synchronised calcium oscillations, which lead to mitochondrial depolarization, depletion of ATP and GSH and neuronal damage. Administration of omaveloxolone after the onset of status epilepticus increased the reduced form of glutathione, GSH (γ-L-glutamyl-L-cysteinylglycine) and ATP levels, preventing associated neuronal damage and the spontaneous seizures that typically develop in the weeks following status epilepticus [
45]. Crucially, Nrf2 activation provided neuroprotection by enhancing mitochondrial bioenergetic function and supplying neuronal ATP, helping to meet the increased energy demands caused by high-frequency calcium oscillations during seizures. Overall, these findings suggest that modulating mitochondrial bioenergetics through Nrf2 activation is a promising therapeutic strategy for neurodegenerative diseases, given the significant energy demands of neurons and their dependence on mitochondria [
9].
In addition to improving neuronal mitochondrial function and preventing oxidative stress, numerous
in vivo studies have highlighted various aspects of the neuroprotective effects of the pharmacological activation of Nrf2 across different neuropathological conditions [
46,
47]. However, it is also important to consider the role of non-neuronal cells in this process. Indeed, it has been argued that the Nrf2-mediated neuronal protection can be attributed to astrocytic Nrf2 activation [
48], which appears to play a key role in the process as will be discussed in the next section.
2.2. Nrf2 in Astrocytes
Astrocytes, along with neurons, originate from neuroepithelial radial glia, through a process governed by different signals that determine the neural or glial fate of their progenitors. They are the most abundant glial cells in the human adult brain and play a crucial role in maintaining the CNS homeostasis [
49]. Among other functions, such as regulating neurotransmitter recycling or synapse maturation, they are known to play a role in providing support for neuronal functions, including energy homeostasis and antioxidant defence. In addition, in response to brain injury, astrocytes undergo molecular and functional modifications which can have both beneficial and harmful effects on neuronal function and survival [
49].
In contrast to neurons, astrocytes are known to produce elevated levels of antioxidants, with a major role for the glutathione, thioredoxin and catalase systems [
50] (
Figure 2). Likewise, Nrf2 pathway appears to be highly active in astrocytes, both at the mRNA and protein levels and by the abundance of some of its target genes [
20]. In this case, astrocytic Nrf2 activity is maintained throughout the maturation process, suggesting that the molecular mechanisms implicated in their differentiation are different to those of neurons, and probably less dependent on ROS signalling [
17].
Neuronal and astrocytic metabolic profiles also differ significantly as astrocytes are highly glycolytic and rely less on oxidative phosphorylation. Moreover, astrocytes are thought to supply neurons with lactate as an energy substrate via the astrocyte-neuron lactate shuttle [
51]. Similarly to neurons, Nrf2 activation increases glucose uptake in astrocytes and plays a role in modulating its metabolism, as shown in primary cultures from the Keap1 knock-down mice model [
41]. Once internalised, glucose is metabolised by two main metabolic pathways: either glycolysis, oriented to energy production, or the pentose phosphate pathway, which among others generates NADPH, a cofactor needed by many antioxidant enzymes. When glucose availability is limited, Nrf2 has been shown to prioritise the metabolism of glucose for energy production in astrocytes, with a smaller contribution to support redox reactions [
41]. This highlights the importance of the role of astrocytic Nrf2 in supporting brain energy metabolism, both in physiology and under conditions of energy deprivation.
2.2.1. Nrf2 and Neuronal-Astrocytic Crosstalk
Neuronal-astrocytic communication is key to couple neuronal needs and astrocytic support, and Nrf2 has been described to mediate some of the signalling cascades that allow this crosstalk. Among them, the molecular events occurring during synaptic transmission are inevitably linked to an increase in neuronal ROS, and coupling of neuronal activity with Nrf2 activation in astrocytes supports neurons with an adequate antioxidant defence [
52]. During neurotransmission, glutamate released has been shown to act not only in the post-synaptic neurons but also to stimulate metabotropic glutamatergic receptors in astrocytes [
53]. Activation of astrocytic glutamatergic receptors leads to the translocation of Nrf2 to the nucleus [
20,
53] and allows the transcription of genes involved in glutathione metabolism in astrocytes [
54]. These GSH precursors can be then released by astrocytes and used by the neighbouring neurons to synthetize GSH, thus protecting them from oxidative stress [
55] and coupling neuronal activity to astrocytic-induced neuroprotection [
53]. Nevertheless, neuronal activity has also been linked to the activation of some neuroprotective Nrf2-target genes independently of astrocytes and the Nrf2 pathway, which suggests the cooperation of different pathways in the process [
56,
57].
Adaptive astrocytic neuroprotective responses have also been shown to be triggered by other stimuli. For example, Bell et al showed that exposure of co-cultures of neurons and astrocytes to mild levels of oxidative stress (sub-toxic hydrogen peroxide levels) led to a neuroprotective response that depended on the activation of Nrf2 pathway in astrocytes [
58]. However, other studies have shown that endogenous astrocytic hydrogen peroxide does not activate the Nrf2 pathway, yet it can confer neuroprotection through Nrf2-independent mechanisms [
59]. Mitochondrial dysfunction has also been found to trigger Nrf2 activation in astrocytes [
60], while astrocytic-specific Nrf2 overexpression is able to protect neurons against mitochondrial-induced neurotoxicity in an
in-vivo model of mitochondrial complex II inhibition [
60].
Indeed, experiments in
in-vivo models of different neurodegenerative conditions such as PD [
48,
61], ALS [
62], spinal cord injury [
63] or vascular cognitive impairment [
64] support the role of astrocytic Nrf2 in neuroprotection. These studies show how selective overexpression of Nrf2 in astrocytes under a glial fibrillary acidic protein (GFAP) promoter protected dopaminergic neurons, motor-neurons, spinal cord and white matter respectively from degeneration [
48,
61,
62,
63,
64].
In addition to endogenous Nrf2, experiments in cellular cultures have shown that Nrf2 inducers preferentially target astrocytes [
65]. Therefore, when considering the neuroprotective role of Nrf2 pharmacological activation in the brain, it is important to pay attention to the specific role of astrocytes in the different neurodegenerative disorders, for a detailed review [
66].
2.2.2. Nrf2 and Astrocytic Function: Its Role in Inflammation
Some of the molecular pathways involved in the physiological and therapeutic astrocytic-mediated neuroprotection have to do with the role of astrocytes as mediators of the brain´s inflammatory response, with Nrf2 playing a key role in modulating this process [
67,
68]. Upon activation, Nrf2 reduces the expression of pro-inflammatory cytokines, thus shifting astrocytes toward an anti-inflammatory phenotype. This action limits excessive inflammation, which could otherwise lead to astrocytic dysfunction and contribute to pathological conditions such as neuroinflammation, which is a hallmark of various neurodegenerative diseases. Indeed, it is known that specific subpopulations of reactive astrocytes induce neurotoxicity in different neurological disorders [
69], and single cell RNAseq-based studies identified a reduced Nrf2 activity within those astrocytic populations as the underlying cause of the neuroinflammation observed in a multiple sclerosis model [
70]. Therefore, astrocytic Nrf2 is a critical component in protecting against neurodegenerative disorders, where chronic neuroinflammation plays a central role in disease progression [
71].
Nevertheless, the modulation of the inflammatory response in the CNS is not exclusive to astrocytes but is also a key function of other glial cells such as the microglia, and the interplay between them. The molecular pathways linked to this aspect of Nrf2 function will be described in detail in the following sections.
2.3. Nrf2 in Microglia
Microglia, the brain's resident immune cells, play crucial roles in both health and disease. They originate from yolk sac progenitors, migrate to the developing brain, and become isolated by the blood-brain barrier (BBB). Microglia have a complex “sensome” composed by surface receptors that allow them to detect and react to a variety of situations [
72]. In the attempt to react and respond to physiological and pathological contexts microglia acquire and coexist in various dynamic states named as phenotypes [
73,
74]. As resident efficient macrophages of the brain, microglia undergo crucial functions including, although not limited to, neurogenesis, synaptic remodelling, release of soluble factors, tissue repair or phagocytosis [
74]. Microglia display a higher expression of Nrf2 compared to neurons, as they show higher ARE activity and more Nrf2 transcripts [
75].
The role of Nrf2 modulating the innate immune system was first addressed using models of acute inflammation. One of the first pieces of evidence arises with a model of experimental sepsis where the absence of Nrf2 is associated with a more severe damage, higher lethality and increased proinflammatory markers [
76]. Using the lipopolysaccharide (LPS) model of acute inflammation, several authors have also shown how the absence of Nrf2 derives in higher inflammatory response (both
in vitro and
in vivo) shown by an increased cytokine release and changes in F4/80 immunostaining and morphology within the brain, pointing to a response mediated by microglia [
77]. Moreover, in the MPTP model of PD, the absence of Nrf2 leads to a higher cytokine release and a reduction in the expression of anti-inflammatory markers. This situation was associated with a higher neuronal vulnerability in basal ganglia [
78]. Similarly, in a model of tauopathy the microgliosis elicited by mutant tau can be prevented pharmacologically by Nrf2 inducers such as DMF [
79], whereas the effect is lost in Nrf2-/- animals. Several mechanisms reviewed below contribute to the effect of Nrf2 in controlling inflammation.
2.3.1. Nrf2 Inhibits NFkB Signalling Pathway
The molecular mechanism underlying this anti-inflammatory effect implies different players, including the transcription factor nuclear factor κB (NFκB) multicomplex (
Figure 2). NFκB is a pivotal mediator in the innate and adaptive immune response, orchestrating the expression of a number of pro-inflammatory cytokines and chemokines. Classical pro-inflammatory stimuli such as LPS, TNFα or IL-1β activate the canonical pathway. This pathway leads to the activation of the IKK complex, which promotes the phosphorylation of IκBα and degradation via the proteasome pathway. This allows the release of the RelA/p50 dimer, which translocates to the nucleus and participates in the expression of pro-inflammatory genes, including cytokines, chemokines and adhesion molecules, among others (
Figure 2). In the non-canonical pathway, the NIK (NFκB inducible kinase) phosphorylates the IκKα complex leading to subsequent release of p52/RelB complex. The effect of Nrf2 on NFκB signalling pathway was established by the observation that Nrf2 deficient animals showed increased NFκB activation (as well as downstream mediators such as cytokines and adhesion molecules) in a model of traumatic brain injury [
80]. Indeed, Nrf2 deficiency promotes IκBα phosphorylation and degradation, thus permitting a higher expression of NFκB and therefore promoting inflammation [
81]. Keap1 has been shown to be implicated in this process, as it is responsible also for the ubiquitination and subsequent degradation of IKKβ. Therefore, in the absence of Keap1 a stabilisation of IKKβ occurs which also leads to maintained inflammation [
82]. It should be noted that this regulation is not unidirectional. Besides, NFκB can also inhibit or prevent Nrf2 induction by several mechanisms including the competition with the coactivator CBP or recruiting HDAC3 which promotes histone hypoacetylation and impedes Nrf2 signalling [
83]
. Indeed, a number of anti-inflammatory agents can activate Nrf2 by inhibiting NFkB pathway [
84]
. Besides its action on NFκB, studies using chromatin immunoprecipitation (ChIP)-seq have revealed that Nrf2 binds near genes induced during inflammation (IL-6, IL-1β) and prevents their expression [
85].
2.3.2. Nrf2 Prevents the NLRP3 Inflammasome Formation
Another mechanism by which Nrf2 controls the inflammatory response is by interfering in the inflammasome formation. Inflammasomes serve as sensors of inflammatory signals, such as infectious agents or cell damage and in response to these signals they trigger an innate immune response [
86]. One of such inflammasomes is NOD-like receptor protein 3 (NLRP3) inflammasome, a multiprotein cytoplasmic complex which is crucial in the regulation of the inflammatory response [
87]
. Furthermore, inflammasome formation can induce cell death through pyroptosis, which also contributes to the propagation of inflammation and damage. Three components form this multicomplex structure: the NLRP3 protein which acts as sensor, an adaptor protein named ASC and an effector pro-caspase-1 protein [
88] (
Figure 2). Its activation requires 2 subsequent steps, first a priming step responsible for the transcription of NLRP3, pro-IL-1β and pro-IL-18 which is mediated by the translocation of NFκB to the nucleus. Subsequently, the activation step in which occurs the assembly of the inflammasome complex that finally activates caspase-1. This leads to the maturation and secretion of pro-inflammatory cytokines, including IL-1β and IL-18. Although classical triggers of NLRP3 formation are danger and pathogen molecular patterns, intracellular ROS can also induce its activation. As both, NLRP3 and Nrf2 can respond to oxidative stress, the accumulation of ROS acts as the link in the interplay between both signalling pathways. Under a situation of oxidative stress, the thioredoxin-interacting protein binds to NLRP3 and triggers its assembly [
89]. Another protein implicated in the inflammasome activation under oxidative conditions seems to be the NADPH oxidase 4 (NOX4), which is an important source of ROS catalysing the production of superoxide anion and hydrogen peroxide (
Figure 2). The blockade of this enzyme has been demonstrated to prevent NLRP3 formation in oxidative conditions, as NOX4-deficient animals and glial cells fail to produce IL-1β or IL-18 in different models associated to inflammatory and oxidative damage [
90]. Thus, both NLRP3 and Nrf2 get activated under the accumulation of oxidative stress and Nrf2 would elicit its antioxidant effect counteracting NLRP3 formation. Indeed, it has been widely reported how Nrf2 inducers can block NLRP3 assembly in microglia, including sulforaphane [
91], DMF [
92] or melatonin [
93,
94] among others. In the same line but with the opposite approach, Nrf2 deficient macrophages have been shown to upregulate NLRP3 and the subsequent production of cleaved caspase-1 [
95]. The inhibitory role of Nrf2 on NLRP3 inflammasome formation seems to happen in the priming step and can also derive from the role that Nrf2 has blocking NFκB signalling as well as its antioxidant capacity. As reviewed in a previous section, Nrf2 has a role inhibiting NFκB signalling, consequently blocking the expression of genes needed for NLRP3 formation. Nrf2 target genes such as HO-1 and NQO-1 have been demonstrated to be implicated in this process. For example, NQO1 overexpression in macrophages through activation of Nrf2 derives in a reduced expression of NLRP3 and caspase-1, and consequently, reduced release of IL-1β [
95].
Although all previous research points to an inhibitory role of Nrf2 on the NLRP3 formation, other authors have also shown that Nrf2 is needed for an appropriate activation. In an
in vivo model of atherosclerosis, the activation of Nrf2 by cholesterol crystals, leads to NLRP3 formation, whereas the ablation of Nrf2 in this model would be preventing the inflammatory damage [
96]. In the same line, it has been reported how Nrf2-deficient macrophages show a defect in the maturation and secretion of IL-1β and caspase-1 [
97]
, pointing to Nrf2 as a positive regulator in the formation of this complex.
2.3.3. Nrf2 Regulates Phagocytosis
Besides the role of Nrf2 in the control of inflammation, this transcription factor is also crucial for another function associated with phagocytes, which is the clearance of apoptotic cells, mediated by a process named as efferocytosis. The role of Nrf2 controlling this process has been shown in a myriad of contexts, both in the CNS and the periphery.
Experiments performed in the context of multiple sclerosis, where the uptake of myelin debris is fundamental, led to the observation that Nrf2 was also implicated in the process of phagocytosis. Using peripheral macrophages and primary microglia, Grajchen and collaborators showed that Nrf2 is necessary for the uptake of myelin debris [
98]
. In the cited paper, they show that Nrf2 controls the expression of the scavenger receptor CD36. In the absence of Nrf2, there is a deficit in the expression of this receptor which affects phagocytosis, specifically the subprocess of uptake of debris. Moreover, Nrf2 deficient microglia also showed impaired phagocytosis in basal conditions or after stimulation with alpha-synuclein as a model of PD [
99] and tauopathy [
100]. The molecular mechanism behind this dysfunction may imply mediators such as Axl or Mer which showed a decreased expression in Nrf2 deficient microglia. In this same line, the use of Nrf2 inducers such as sulforaphane increases CD36 expression which promotes phagocytosis of red blood cells by brain microglia in a model of intracerebral haemorrhage [
101].
Nevertheless, efferocytosis is a complex mechanism that not only implies the uptake, but also the digestion and elimination of the cargo [
102] and the involvement of Nrf2 at these levels still needs to be elucidated.
2.3.4. Nrf2 as Regulator of Immunometabolism in Microglia
As reviewed before, microglia counteract a myriad of functions, such as phagocytosis, proliferation, or cytokine release, which require continuous metabolic adjustments to support their activities. Whereas homeostatic microglia rely on oxidative phosphorylation for ATP production, they undergo metabolic reprogramming during inflammatory conditions. This shift favours anaerobic pathways, which, although less efficient, provide a more rapid energy supply to meet the high demands of microglial activation and produce intermediates necessary for cytokine production [
103]. A key player in all this immunometabolism regulation is itaconate, a mitochondrial metabolite generated by microglia and macrophages in response to inflammatory stimuli to control damage [
104]. The production of itaconate is catalysed by immune-responsive gene 1 (IRG1) in the mitochondrial matrix [
105]. Pro-inflammatory stimuli such as LPS or interferons activates the expression of IRG1 and itaconate which inhibits succinate dehydrogenase (or complex II) limiting ROS production and promoting anti-inflammatory phenotype in myeloid cells [
104]. Interestingly, Nrf2 also plays a role in this process, as itaconate has been shown to react with cysteine residues of Keap1, activating Nrf2 and promoting the expression of downstream genes [
106]. Thus, IRG1 and itaconate have an anti-inflammatory and protective role in microglia, which is lost in the absence of Nrf2 [
107].
Besides its role regulating metabolic reprogramming, Nrf2 is essential for maintaining mitochondrial functioning. Danger associated molecular patterns produce a microglial response that originates inflammation and ROS production. As phagocytic cells, microglia express high levels of NADPH oxidases which produce superoxide radicals. Thus, microglial ROS are primarily produced by these enzymes and are important for shaping microglial phenotypes and host defence. Different cell surface receptors in the microglia such as toll-like receptors (TLRs), complement receptor 3 (CR3), purinergic receptors or CD36 lead to the NOX-mediated oxidant production [
108]. Among NADPH oxidases, NOX2 seems to be highly expressed in microglia, localised in the plasma membrane, mostly associated with lipid rafts [
108], followed by NOX4. The production of ROS by NOX is necessary for microglial functions such as phagocytosis or inflammation, and they act as secondary messengers that may propagate inflammatory states and damage associated phenotypes [
109]. Nevertheless, it is important that antioxidant pathways such as Nrf2 modulate this oxidative environment to prevent deleterious propagation (
Figure 2). Thus, Nrf2 is essential to counteract oxidative damage, as reviewed in previous sections, regulating mitochondrial antioxidant defence in microglia to prevent ROS accumulation, and enhancing NADPH supply as reviewed elsewhere [
110].
2.3.5. Nrf2 Prevents Microglia from Ferroptosis
Ferroptosis is a mechanism of programmed cell death associated with a large accumulation of iron and lipid peroxidation firstly described by Dixon and collaborators [
111]. Microglia adjust iron transport and metabolism based on inflammatory signals, accumulating iron during inflammation by sequestering intracellular iron released from heme catabolism and extracellular iron taken up through transporters like divalent metal transporter (DMT1) [
112]. In this context, there is an increase in oxidative stress and lipid peroxidation which ultimately leads to this mechanism of programmed cell death. Ferroptosis process in microglia contributes to the release of pro-inflammatory signals and affects neighbouring cells, thus, the inhibition of ferroptosis in microglia may serve as target for neuroprotection. In this context, many downstream genes of Nrf2 are directly linked to the regulation of ferroptosis including GPX4 (GSH peroxidase 4) or SLC7A11 (12-channel transmembrane protein transporter vector family 7 member 11) [
113], this former promoting the generation of GSH. Moreover, as Nrf2 promotes an antioxidant environment, it reduces the sensitivity to ferroptosis. This mechanism has been shown in microglial cells, where the activation of Nrf2 promotes ferroptosis resistance attenuating the inflammatory response [
114].
Nevertheless, the downstream effector of Nrf2, HO-1, may play a deleterious role in the context of ferroptosis. Pro-inflammatory stimuli such as LPS, as well as ageing or pathology increases the expression of HO-1, which contributes to iron overload and ferroptosis, as it will be described in the following section. Indeed, it has been shown how the ablation/inhibition of HO-1 in microglia as well as the use of iron chelators can be protective in models associated with neuroinflammation [
115,
116].
2.3.6. Role of Microglia Heme-Oxygenase I in the Function of Nrf2
In the control of microglial functions elicited by Nrf2, it is noteworthy the role of HO-1 [
117]. HO-1 is an inducible 32 kDa protein that catalyses the degradation of heme group, releasing free iron (Fe[
2]
+), carbon monoxide (CO) and biliverdin (BV) (rapidly converted to bilirubin). Among the transcriptional network regulated by Nrf2, the expression of the
Hmox1 gene is under Nrf2 regulation, among other factors. HO-1 is ubiquitously expressed and localises within the cells especially in the endoplasmic reticulum and the cytosol [
118] although it can be also associated to other intracellular membranes, including in plasma caveoles and mitochondria [
119]. HO-1 has canonical effects (directly derived from the byproducts generated by its enzymatic activity) and non-canonical effects which are not correlated with its enzymatic activity. In both cases, HO-1 has been shown to counteract inflammation. Whereas heme group has pro-inflammatory functions, the byproducts of its degradation via HO-1 (BV, CO and Fe
2+) have shown direct anti-inflammatory effects [
120,
121]. Indeed, free Fe
2+ and bilirubin (which derive from BV) inhibit NFκB signalling and the production of pro-inflammatory cytokines. The non-canonical effects include protein-protein interaction (as HO-1 can directly interact with HO-2), subcellular localization (i.e.: translocation to the nucleus and other organelles) where its enzymatic activity is affected [
122].
Multiple evidence point to HO-1 as a key factor on the beneficial roles of Nrf2 in microglia. Indeed, the blockade of this enzyme prevents from the anti-inflammatory and antioxidant effects elicited by Nrf2 inductors either in microglial or organotypic cultures [
123,
124] and its absence prevents from mitochondrial biogenesis in glial cells [
125]. These beneficial effects have clinical consequences, as the induction of microglia HO-1 has been reported to protect in a model of brain haemorrhage [
125], whereas the protection exhibited by Nrf2 induction in a photothrombotic model of stroke is lost in animals lacking HO-1 [
126]. Nevertheless, an overactivation of this enzyme can also derive in deleterious effects, mainly due to Fe2+ overload, as described in the previous section [
115]. Indeed, in ageing and AD samples (both human and mice) there is an increased expression of HO-1 predominantly in microglia [
127], which could be a compensatory mechanism although it can also trigger harmful effects. Among them, it has been described how long-term overactivation of HO-1 can induce tau aggregation [
128,
129], increase microgliosis [
130], and derive in cognitive decline in models of tauopathy and AD [
131]. These beneficial and deleterious effects derived from HO-1 have been reviewed elsewhere [
132]. Altogether, these results highlight the importance of a tight regulation of Nrf2 and its downstream targets, as a sustained overexpression can lead to deleterious effects.
2.4. Nrf2 in Oligodendrocytes
Finally, although far less explored, Nrf2 also regulates the functionality of oligodendrocytes, which are defined as the myelin-producing cells of the CNS. They are crucial for myelination and myelin regeneration in the CNS and essential for reducing energy consumption and enhancing neuronal signal transmission. Oligodendrocytes also support neurons metabolically by transporting necessary metabolites and play a role in modulating neuroinflammation through interactions with microglia. They are characterised by high metabolic rates, and consume large amounts of oxygen and ATP, which ultimately leads to the production of ROS [
133]. Moreover, in the myelination process many of the enzymes are iron-dependent, thus having large stores of iron, which can ultimately produce lipid peroxidation [
133] (
Figure 2). Taken all together, oligodendrocytes are especially vulnerable to metabolic energy supply and oxidative stress, highlighting the potential importance of the Nrf2 system in this specific cell type. Indeed, among the different brain cell types they express the higher levels of Nrf2 according to transcriptomic analyses [
15], Human Cell Atlas). Here we will describe the current knowledge of Nrf2 in oligodendrocyte functionality.
2.4.1. Metabolic Support and Antioxidant Defence of Nrf2 in Oligodendrocytes
As stated before, oligodendrocytes are metabolically active cells which makes them especially vulnerable to oxidative damage. In this context, it has been shown in an oligodendroglial cell line subjected to sodium azide as mitochondrial inhibitor, how Nrf2 activation through the knock-down of Keap1, partly prevented from mitochondrial depolarization and increased metabolic activity. Opposite results were obtained when Nrf2 was knocked-down [
134]. In the same line, the use of pharmacological activators of Nrf2 in an oligodendroglial cell line prevented from cell death elicited by hydrogen peroxide, inducing the synthesis of GSH and counteracting oxidative stress [
135]. The oxidative damage associated with mitochondrial blockade in oligodendrocytes is connected with the expression of endoplasmic reticulum stress response, which has been demonstrated to be also mediated by Nrf2 [
136]. Not only does Nrf2 reduce the oxidative damage through the activation of antioxidant defences, but also promotes mitochondrial biogenesis through activation of PGC-1α [
137] permitting a higher metabolic rate which is essential for oligodendrocyte differentiation.
2.4.2. Nrf2 Participates in Myelination
Some studies have addressed the potential role of Nrf2 in the demyelination / remyelination process, mostly in the context of multiple sclerosis. Most of the studies presented in this section rely on the cuprizone model, which produces a transitory de-myelinization associated with oligodendrocyte loss. Using this model, it has been shown that the absence of Nrf2 produces an increased vulnerability [
138]. In the same line, different Nrf2 inducers have shown protection against demyelinating insults [
139,
140]. Nevertheless, the mechanism involved is still elusive and might not be related (at least exclusively) to the role of Nrf2 in oligodendrocytes but other cell types such as microglia and astrocytes. In this line, it has been recently shown that during remyelination astrocytes downregulate Nrf2 pathway, turning their metabolism towards cholesterol biosynthesis [
141], which they export to oligodendrocytes to regulate their survival and remyelination. Indeed, these authors use a model that constitutively overexpresses Nrf2 in astrocytes (GFAP-Nrf2) and they show an impairment in remyelination [
141]. On the contrary, opposite results have been obtained in other studies where the activation of Nrf2 in the astrocytes using GFAP-specific Keap1 deletion animals, showed lower oligodendrocyte loss and demyelination [
142]. These different approaches highlight the importance of further exploring this pathway and demonstrate the importance of a tight control on Nfr2 activity to maintain brain homeostasis and prevent damage.
Myelin is composed mainly by lipids, thus lipid homeostasis is key in the biology of oligodendrocytes. In this context, metabolomic studies showed that treatment of oligodendrocytes with DMF produced a reduction in most of the lipids (such as phosphatidylcholine, sphingomyelin, or free fatty acids) at the time-points that it exerted antioxidant and neuroprotective effects [
143]. Authors speculate that the reduction of lipid synthesis could be an initial response where all antioxidant enzymes need to be synthesised, whereas chronic treatment would swift towards metabolic reprogramming implying higher lipid synthesis for myelination.
Besides the molecular pathway, the fact that DMF is the first approved Nrf2-inducer in clinic and is used for the treatment of multiple sclerosis, demonstrates the importance of this factor for promoting myelination.
3. Conclusions and Future Perspectives
The transcription factor Nrf2 plays a pivotal role in maintaining brain health by regulating mitochondrial function, antioxidant defence, inflammation, and myelination, among other functions. Its activation across different brain cell types—neurons, astrocytes, microglia, and oligodendrocytes—demonstrates a complex, yet interdependent, role in cellular homeostasis and neuroprotection. Neurons, which are the most vulnerable cells to energy deprivation and oxidative stress, surprisingly present the lowest Nrf2 levels within the different cellular subtypes. However, even despite its low levels, Nrf2 still plays a vital role in supporting the mitochondrial function, mitigating oxidative stress, and preserving energy production in mature neurons. In astrocytes, it enhances antioxidant defence mechanisms, such as glutathione synthesis, which protects not only the astrocytes themselves but also adjacent neurons. Both astrocytes and microglia rely on Nrf2 for balancing their pro-inflammatory and anti-inflammatory responses, preventing chronic neuroinflammation while promoting tissue repair. Oligodendrocytes also benefit from Nrf2 activation, where it plays a role in the differentiation process and myelination, contributing to efficient neuronal signal transmission.
Despite these findings, there remain significant gaps in understanding the cell type-specific regulation of Nrf2 and its therapeutic potential in neurodegenerative diseases, such as Alzheimer’s and Parkinson’s disease, as well as in demyelinating disorders like multiple sclerosis. Future research should focus on elucidating the distinct Nrf2-dependent pathways within each cell type under both physiological and pathological conditions. Furthermore, the development of targeted Nrf2 modulators could offer new therapeutic strategies tailored to specific cell types or disease states. However, more detailed studies are required to unravel its multifaceted roles in different brain cell types and its broader impact on neurodegenerative and demyelinating diseases.
Author Contributions
EN and NE contributed equally to the work, wrote, and edited the manuscript and designed the figures.
Acknowledgments
This article is based upon work from COST Action CA20121 - Bench to bedside transition for pharmacological regulation of NRF2 in noncommunicable diseases (BenBedPhar) benbedphar.org, supported by COST (European Cooperation in Science and Technology). www.cost.eu. The work was supported by grants from the Spanish Ministry of Science, Innovation and Universities to EN (PID2022-139936OA-I00), and NE (PID2022-137011OA-I00/ AEI/10.13039/501100011033/ FEDER, EU) and a Ramon y Cajal Fellowship to NE (RYC2021-034267-I) funded by the Spanish Ministry of Science and Innovation MCIN/AEI/10.13039/501100011033 and the European Union “NextGenerationEU”/PRTR.
Conflicts of Interest
The authors declare no conflicts of interest.
Figure Legends:Graphical abstract - The different roles of Nrf2 in brain cells. The transcription factor Nrf2 coordinates a myriad of cytoprotective pathways across the different cell types within the brain. The illustration summarises the common and specific roles of Nrf2 in neurons, astrocytes, microglia and oligodendrocytes, as well as the crosstalk and interaction among them. Created in Biorender.
Abbreviations
AD - Alzheimer's disease, ALS - amyotrophic lateral sclerosis, ARE - antioxidant response elements, BBB - blood-brain barrier, BV – biliverdin, CNS - central nervous system, CO - carbon monoxide, CR3 - complement receptor 3, Cul – cullin, Cys – cysteine, DMT1 - divalent metal transporter, DMF - dimethyl fumarate, ETC - electron transport chain, Fe2+ - free iron, GFAP - glial fibrillary acidic protein, GSH - reduced glutathione (γ-L-glutamyl-L-cysteinylglycine), GPX4 - GSH peroxidase 4, GSK3-β - glycogen synthase kinase-3 beta, HO-1 - heme-oxygenase 1, IRG1 - immune-responsive gene 1, Keap1 - Kelch-like ECH-associated protein 1, LPS – lipopolysaccharide, NFkB - nuclear factor κB, NIK - NFkB inducible kinase, NLRP3 - NOD-like receptor protein 3, NOX - NADPH oxidase, NQO1 - NAD(P)H oxidoreductase 1, Nrf2 - nuclear factor erythroid 2-related factor 2, PD - Parkinson's disease, ROS - reactive oxygen species, SLC7A11 - 12-channel transmembrane protein transporter vector family 7 member 11, TLRs - toll-like receptors .
References
- Hayes:, J.D.; Dinkova-Kostova, A.T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem Sci 2014, 39, 199–218. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Wakabayashi, N.; Katoh, Y.; Ishii, T.; Igarashi, K.; Engel, J.D.; Yamamoto, M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev 1999, 13, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Muramatsu, A.; Saito, R.; Iso, T.; Shibata, T.; Kuwata, K.; Kawaguchi, S.I.; Iwawaki, T.; Adachi, S.; Suda, H.; et al. Molecular Mechanism of Cellular Oxidative Stress Sensing by Keap1. Cell Rep 2019, 28, 746–758.e744. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, M.; Kurokawa, H.; Waguri, S.; Taguchi, K.; Kobayashi, A.; Ichimura, Y.; Sou, Y.S.; Ueno, I.; Sakamoto, A.; Tong, K.I.; et al. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat Cell Biol 2010, 12, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Salazar, M.; Rojo, A.I.; Velasco, D.; de Sagarra, R.M.; Cuadrado, A. Glycogen synthase kinase-3beta inhibits the xenobiotic and antioxidant cell response by direct phosphorylation and nuclear exclusion of the transcription factor Nrf2. J Biol Chem 2006, 281, 14841–14851. [Google Scholar] [CrossRef]
- Ngo, V.; Duennwald, M.L. Nrf2 and Oxidative Stress: A General Overview of Mechanisms and Implications in Human Disease. Antioxidants (Basel) 2022, 11. [Google Scholar] [CrossRef]
- Heurtaux, T.; Bouvier, D.S.; Benani, A.; Helgueta Romero, S.; Frauenknecht, K.B.M.; Mittelbronn, M.; Sinkkonen, L. Normal and Pathological NRF2 Signalling in the Central Nervous System. Antioxidants (Basel) 2022, 11. [Google Scholar] [CrossRef]
- Cuadrado, A.; Rojo, A.I.; Wells, G.; Hayes, J.D.; Cousin, S.P.; Rumsey, W.L.; Attucks, O.C.; Franklin, S.; Levonen, A.L.; Kensler, T.W.; et al. Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat Rev Drug Discov 2019, 18, 295–317. [Google Scholar] [CrossRef]
- Esteras, N.; Dinkova-Kostova, A.T.; Abramov, A.Y. Nrf2 activation in the treatment of neurodegenerative diseases: a focus on its role in mitochondrial bioenergetics and function. Biol Chem 2016, 397, 383–400. [Google Scholar] [CrossRef]
- Robledinos-Antón, N.; Fernández-Ginés, R.; Manda, G.; Cuadrado, A. Activators and Inhibitors of NRF2: A Review of Their Potential for Clinical Development. Oxid Med Cell Longev 2019, 2019, 9372182. [Google Scholar] [CrossRef]
- Carlström, K.E.; Ewing, E.; Granqvist, M.; Gyllenberg, A.; Aeinehband, S.; Enoksson, S.L.; Checa, A.; Badam, T.V.S.; Huang, J.; Gomez-Cabrero, D.; et al. Therapeutic efficacy of dimethyl fumarate in relapsing-remitting multiple sclerosis associates with ROS pathway in monocytes. Nat Commun 2019, 10, 3081. [Google Scholar] [CrossRef] [PubMed]
- Gold, R.; Arnold, D.L.; Bar-Or, A.; Fox, R.J.; Kappos, L.; Mokliatchouk, O.; Jiang, X.; Lyons, J.; Kapadia, S.; Miller, C. Long-term safety and efficacy of dimethyl fumarate for up to 13 years in patients with relapsing-remitting multiple sclerosis: Final ENDORSE study results. Mult Scler 2022, 28, 801–816. [Google Scholar] [CrossRef]
- Boesch, S.; Indelicato, E. Approval of omaveloxolone for Friedreich ataxia. Nat Rev Neurol 2024, 20, 313–314. [Google Scholar] [CrossRef] [PubMed]
- Angelova, P.R.; Esteras, N.; Abramov, A.Y. Mitochondria and lipid peroxidation in the mechanism of neurodegeneration: Finding ways for prevention. Med Res Rev 2021, 41, 770–784. [Google Scholar] [CrossRef] [PubMed]
- Moubarak, M.M.; Pagano Zottola, A.C.; Larrieu, C.M.; Cuvellier, S.; Daubon, T.; Martin, O.C.B. Exploring the multifaceted role of NRF2 in brain physiology and cancer: A comprehensive review. Neurooncol Adv 2024, 6, vdad160. [Google Scholar] [CrossRef]
- Padamsey, Z.; Rochefort, N.L. Paying the brain's energy bill. Curr Opin Neurobiol 2023, 78, 102668. [Google Scholar] [CrossRef]
- Baxter, P.S.; Hardingham, G.E. Adaptive regulation of the brain's antioxidant defences by neurons and astrocytes. Free Radic Biol Med 2016, 100, 147–152. [Google Scholar] [CrossRef]
- Levings, D.C.; Pathak, S.S.; Yang, Y.M.; Slattery, M. Limited expression of Nrf2 in neurons across the central nervous system. Redox Biol 2023, 65, 102830. [Google Scholar] [CrossRef]
- Bell, K.F.; Al-Mubarak, B.; Martel, M.A.; McKay, S.; Wheelan, N.; Hasel, P.; Márkus, N.M.; Baxter, P.; Deighton, R.F.; Serio, A.; et al. Neuronal development is promoted by weakened intrinsic antioxidant defences due to epigenetic repression of Nrf2. Nat Commun 2015, 6, 7066. [Google Scholar] [CrossRef]
- Jimenez-Blasco, D.; Santofimia-Castaño, P.; Gonzalez, A.; Almeida, A.; Bolaños, J.P. Astrocyte NMDA receptors' activity sustains neuronal survival through a Cdk5-Nrf2 pathway. Cell Death Differ 2015, 22, 1877–1889. [Google Scholar] [CrossRef]
- Dai, X.; Yan, X.; Wintergerst, K.A.; Cai, L.; Keller, B.B.; Tan, Y. Nrf2: Redox and Metabolic Regulator of Stem Cell State and Function. Trends Mol Med 2020, 26, 185–200. [Google Scholar] [CrossRef] [PubMed]
- Robledinos-Antón, N.; Rojo, A.I.; Ferreiro, E.; Núñez, Á.; Krause, K.H.; Jaquet, V.; Cuadrado, A. Transcription factor NRF2 controls the fate of neural stem cells in the subgranular zone of the hippocampus. Redox Biol 2017, 13, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Corenblum, M.J.; Ray, S.; Remley, Q.W.; Long, M.; Harder, B.; Zhang, D.D.; Barnes, C.A.; Madhavan, L. Reduced Nrf2 expression mediates the decline in neural stem cell function during a critical middle-age period. Aging Cell 2016, 15, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Anandhan, A.; Kirwan, K.R.; Corenblum, M.J.; Madhavan, L. Enhanced NRF2 expression mitigates the decline in neural stem cell function during aging. Aging Cell 2021, 20, e13385. [Google Scholar] [CrossRef]
- Wilson, C.; Muñoz-Palma, E.; González-Billault, C. From birth to death: A role for reactive oxygen species in neuronal development. Semin Cell Dev Biol 2018, 80, 43–49. [Google Scholar] [CrossRef]
- Funato, Y.; Michiue, T.; Asashima, M.; Miki, H. The thioredoxin-related redox-regulating protein nucleoredoxin inhibits Wnt-beta-catenin signalling through dishevelled. Nat Cell Biol 2006, 8, 501–508. [Google Scholar] [CrossRef]
- Beckhauser, T.F.; Francis-Oliveira, J.; De Pasquale, R. Reactive Oxygen Species: Physiological and Physiopathological Effects on Synaptic Plasticity. J Exp Neurosci 2016, 10 (Suppl 1), 23–48. [Google Scholar] [CrossRef]
- Esteras, N.; Kopach, O.; Maiolino, M.; Lariccia, V.; Amoroso, S.; Qamar, S.; Wray, S.; Rusakov, D.A.; Jaganjac, M.; Abramov, A.Y. Mitochondrial ROS control neuronal excitability and cell fate in frontotemporal dementia. Alzheimers Dement 2022, 18, 318–338. [Google Scholar] [CrossRef]
- Baxter, P.S.; Márkus, N.M.; Dando, O.; He, X.; Al-Mubarak, B.R.; Qiu, J.; Hardingham, G.E. Targeted de-repression of neuronal Nrf2 inhibits α-synuclein accumulation. Cell Death Dis 2021, 12, 218. [Google Scholar] [CrossRef]
- Leng, K.; Li, E.; Eser, R.; Piergies, A.; Sit, R.; Tan, M.; Neff, N.; Li, S.H.; Rodriguez, R.D.; Suemoto, C.K.; et al. Molecular characterization of selectively vulnerable neurons in Alzheimer's disease. Nat Neurosci 2021, 24, 276–287. [Google Scholar] [CrossRef]
- Schirmer, L.; Velmeshev, D.; Holmqvist, S.; Kaufmann, M.; Werneburg, S.; Jung, D.; Vistnes, S.; Stockley, J.H.; Young, A.; Steindel, M.; et al. Neuronal vulnerability and multilineage diversity in multiple sclerosis. Nature 2019, 573, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, C.P.; Glass, C.A.; Montgomery, M.B.; Lindl, K.A.; Ritson, G.P.; Chia, L.A.; Hamilton, R.L.; Chu, C.T.; Jordan-Sciutto, K.L. Expression of Nrf2 in neurodegenerative diseases. J Neuropathol Exp Neurol 2007, 66, 75–85. [Google Scholar] [CrossRef]
- Delaidelli, A.; Richner, M.; Jiang, L.; van der Laan, A.; Bergholdt Jul Christiansen, I.; Ferreira, N.; Nyengaard, J.R.; Vægter, C.B.; Jensen, P.H.; Mackenzie, I.R.; et al. α-Synuclein pathology in Parkinson disease activates homeostatic NRF2 anti-oxidant response. Acta Neuropathol Commun 2021, 9, 105. [Google Scholar] [CrossRef]
- Au, W.H.; Miller-Fleming, L.; Sanchez-Martinez, A.; Lee, J.A.; Twyning, M.J.; Prag, H.A.; Raik, L.; Allen, S.P.; Shaw, P.J.; Ferraiuolo, L.; et al. Activation of the Keap1/Nrf2 pathway suppresses mitochondrial dysfunction, oxidative stress, and motor phenotypes in C9orf72 ALS/FTD models. Life Sci Alliance 2024, 7. [Google Scholar] [CrossRef] [PubMed]
- Brandes, M.S.; Gray, N.E. NRF2 as a Therapeutic Target in Neurodegenerative Diseases. ASN Neuro 2020, 12, 1759091419899782. [Google Scholar] [CrossRef] [PubMed]
- Esteras, N.; Abramov, A.Y. Mitochondrial Calcium Deregulation in the Mechanism of Beta-Amyloid and Tau Pathology. Cells 2020, 9. [Google Scholar] [CrossRef]
- Esteras, N.; Abramov, A.Y. Nrf2 as a regulator of mitochondrial function: Energy metabolism and beyond. Free Radic Biol Med 2022, 189, 136–153. [Google Scholar] [CrossRef]
- Holmström, K.M.; Baird, L.; Zhang, Y.; Hargreaves, I.; Chalasani, A.; Land, J.M.; Stanyer, L.; Yamamoto, M.; Dinkova-Kostova, A.T.; Abramov, A.Y. Nrf2 impacts cellular bioenergetics by controlling substrate availability for mitochondrial respiration. Biol Open 2013, 2, 761–770. [Google Scholar] [CrossRef]
- Zweig, J.A.; Caruso, M.; Brandes, M.S.; Gray, N.E. Loss of NRF2 leads to impaired mitochondrial function, decreased synaptic density and exacerbated age-related cognitive deficits. Exp Gerontol 2020, 131, 110767. [Google Scholar] [CrossRef]
- Zweig, J.A.; Brandes, M.S.; Brumbach, B.H.; Caruso, M.; Wright, K.M.; Quinn, J.F.; Soumyanath, A.; Gray, N.E. Loss of NRF2 accelerates cognitive decline, exacerbates mitochondrial dysfunction, and is required for the cognitive enhancing effects of Centella asiatica during aging. Neurobiol Aging 2021, 100, 48–58. [Google Scholar] [CrossRef]
- Esteras, N.; Blacker, T.S.; Zherebtsov, E.A.; Stelmashuk, O.A.; Zhang, Y.; Wigley, W.C.; Duchen, M.R.; Dinkova-Kostova, A.T.; Abramov, A.Y. Nrf2 regulates glucose uptake and metabolism in neurons and astrocytes. Redox Biol 2023, 62, 102672. [Google Scholar] [CrossRef] [PubMed]
- Bartolome, F.; Esteras, N.; Martin-Requero, A.; Boutoleau-Bretonniere, C.; Vercelletto, M.; Gabelle, A.; Le Ber, I.; Honda, T.; Dinkova-Kostova, A.T.; Hardy, J.; et al. Pathogenic p62/SQSTM1 mutations impair energy metabolism through limitation of mitochondrial substrates. Sci Rep 2017, 7, 1666. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Baird, L.; Holmström, K.M.; Meyer, C.J.; Abramov, A.Y. The spatiotemporal regulation of the Keap1-Nrf2 pathway and its importance in cellular bioenergetics. Biochem Soc Trans 2015, 43, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Abeti, R.; Baccaro, A.; Esteras, N.; Giunti, P. Novel Nrf2-Inducer Prevents Mitochondrial Defects and Oxidative Stress in Friedreich's Ataxia Models. Front Cell Neurosci 2018, 12, 188. [Google Scholar] [CrossRef]
- Shekh-Ahmad, T.; Eckel, R.; Dayalan Naidu, S.; Higgins, M.; Yamamoto, M.; Dinkova-Kostova, A.T.; Kovac, S.; Abramov, A.Y.; Walker, M.C. KEAP1 inhibition is neuroprotective and suppresses the development of epilepsy. Brain 2018, 141, 1390–1403. [Google Scholar] [CrossRef] [PubMed]
- Silva-Llanes, I.; Martín-Baquero, R.; Berrojo-Armisen, A.; Rodríguez-Cueto, C.; Fernández-Ruiz, J.; De Lago, E.; Lastres-Becker, I. Beneficial Effect of Dimethyl Fumarate Drug Repositioning in a Mouse Model of TDP-43-Dependent Frontotemporal Dementia. Antioxidants (Basel) 2024, 13. [Google Scholar] [CrossRef]
- Uruno, A.; Yamamoto, M. The KEAP1-NRF2 System and Neurodegenerative Diseases. Antioxid Redox Signal 2023, 38, (13–15). [Google Scholar] [CrossRef]
- Chen, P.C.; Vargas, M.R.; Pani, A.K.; Smeyne, R.J.; Johnson, D.A.; Kan, Y.W.; Johnson, J.A. Nrf2-mediated neuroprotection in the MPTP mouse model of Parkinson's disease: Critical role for the astrocyte. Proc Natl Acad Sci U S A 2009, 106, 2933–2938. [Google Scholar] [CrossRef]
- Rodríguez-Carreiro, S.; Nogales, M.; Jiménez-Galán, D.; Carmona-Lorenzo, S.; Caro-Martín, A.; Navarro, E.; Esteras, N. Role of non-neuronal cells in neurorepair: a focus on proteinopathy and neurodegeneration. Regen Med Rep. 2024, 1, 31–51. [Google Scholar] [CrossRef]
- Kim, Y.; Park, J.; Choi, Y.K. The Role of Astrocytes in the Central Nervous System Focused on BK Channel and Heme Oxygenase Metabolites: A Review. Antioxidants (Basel) 2019, 8. [Google Scholar] [CrossRef]
- Bonvento, G.; Bolaños, J.P. Astrocyte-neuron metabolic cooperation shapes brain activity. Cell Metab 2021, 33, 1546–1564. [Google Scholar] [CrossRef] [PubMed]
- Bolaños, J.P. Bioenergetics and redox adaptations of astrocytes to neuronal activity. J Neurochem 2016, 2 (Suppl Suppl 2), 115–125. [Google Scholar] [CrossRef] [PubMed]
- Habas, A.; Hahn, J.; Wang, X.; Margeta, M. Neuronal activity regulates astrocytic Nrf2 signaling. Proc Natl Acad Sci U S A 2013, 110, 18291–18296. [Google Scholar] [CrossRef] [PubMed]
- McGann, J.C.; Mandel, G. Neuronal activity induces glutathione metabolism gene expression in astrocytes. Glia 2018, 66, 2024–2039. [Google Scholar] [CrossRef] [PubMed]
- Shih, A.Y.; Johnson, D.A.; Wong, G.; Kraft, A.D.; Jiang, L.; Erb, H.; Johnson, J.A.; Murphy, T.H. Coordinate regulation of glutathione biosynthesis and release by Nrf2-expressing glia potently protects neurons from oxidative stress. J Neurosci 2003, 23, 3394–3406. [Google Scholar] [CrossRef] [PubMed]
- Deighton, R.F.; Márkus, N.M.; Al-Mubarak, B.; Bell, K.F.; Papadia, S.; Meakin, P.J.; Chowdhry, S.; Hayes, J.D.; Hardingham, G.E. Nrf2 target genes can be controlled by neuronal activity in the absence of Nrf2 and astrocytes. Proc Natl Acad Sci U S A 2014, 111, E1818–1820. [Google Scholar] [CrossRef] [PubMed]
- Habas, A.; Hahn, J.; Wang, X.; Margeta, M. Reply to Deighton et al.: Neuronal activity regulates distinct antioxidant pathways in neurons and astrocytes. Proc Natl Acad Sci U S A 2014, 111, E1821–1822. [Google Scholar] [CrossRef]
- Bell, K.F.; Al-Mubarak, B.; Fowler, J.H.; Baxter, P.S.; Gupta, K.; Tsujita, T.; Chowdhry, S.; Patani, R.; Chandran, S.; Horsburgh, K.; et al. Mild oxidative stress activates Nrf2 in astrocytes, which contributes to neuroprotective ischemic preconditioning. Proc Natl Acad Sci U S A 2011, 108, E1–2. [Google Scholar] [CrossRef]
- Haskew-Layton, R.E.; Payappilly, J.B.; Smirnova, N.A.; Ma, T.C.; Chan, K.K.; Murphy, T.H.; Guo, H.; Langley, B.; Sultana, R.; Butterfield, D.A.; et al. Controlled enzymatic production of astrocytic hydrogen peroxide protects neurons from oxidative stress via an Nrf2-independent pathway. Proc Natl Acad Sci U S A 2010, 107, 17385–17390. [Google Scholar] [CrossRef]
- Calkins, M.J.; Vargas, M.R.; Johnson, D.A.; Johnson, J.A. Astrocyte-specific overexpression of Nrf2 protects striatal neurons from mitochondrial complex II inhibition. Toxicol Sci 2010, 115, 557–568. [Google Scholar] [CrossRef]
- Gan, L.; Vargas, M.R.; Johnson, D.A.; Johnson, J.A. Astrocyte-specific overexpression of Nrf2 delays motor pathology and synuclein aggregation throughout the CNS in the alpha-synuclein mutant (A53T) mouse model. J Neurosci 2012, 32, 17775–17787. [Google Scholar] [CrossRef] [PubMed]
- Vargas, M.R.; Johnson, D.A.; Sirkis, D.W.; Messing, A.; Johnson, J.A. Nrf2 activation in astrocytes protects against neurodegeneration in mouse models of familial amyotrophic lateral sclerosis. J Neurosci 2008, 28, 13574–13581. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Gasterich, N.; Clarner, T.; Voelz, C.; Behrens, V.; Beyer, C.; Fragoulis, A.; Zendedel, A. Astrocytic Nrf2 expression protects spinal cord from oxidative stress following spinal cord injury in a male mouse model. J Neuroinflammation 2022, 19, 134. [Google Scholar] [CrossRef] [PubMed]
- Sigfridsson, E.; Marangoni, M.; Johnson, J.A.; Hardingham, G.E.; Fowler, J.H.; Horsburgh, K. Astrocyte-specific overexpression of Nrf2 protects against optic tract damage and behavioural alterations in a mouse model of cerebral hypoperfusion. Sci Rep 2018, 8, 12552. [Google Scholar] [CrossRef] [PubMed]
- Kraft, A.D.; Johnson, D.A.; Johnson, J.A. Nuclear factor E2-related factor 2-dependent antioxidant response element activation by tert-butylhydroquinone and sulforaphane occurring preferentially in astrocytes conditions neurons against oxidative insult. J Neurosci 2004, 24, 1101–1112. [Google Scholar] [CrossRef]
- Liddell, J.R. Are Astrocytes the Predominant Cell Type for Activation of Nrf2 in Aging and Neurodegeneration? Antioxidants (Basel) 2017, 6. [Google Scholar] [CrossRef]
- Linker, R.A.; Lee, D.H.; Ryan, S.; van Dam, A.M.; Conrad, R.; Bista, P.; Zeng, W.; Hronowsky, X.; Buko, A.; Chollate, S.; et al. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain 2011, 134 Pt 3, 678–692. [Google Scholar] [CrossRef]
- Nakano-Kobayashi, A.; Canela, A.; Yoshihara, T.; Hagiwara, M. Astrocyte-targeting therapy rescues cognitive impairment caused by neuroinflammation via the Nrf2 pathway. Proc Natl Acad Sci U S A 2023, 120, e2303809120. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- Wheeler, M.A.; Clark, I.C.; Tjon, E.C.; Li, Z.; Zandee, S.E.J.; Couturier, C.P.; Watson, B.R.; Scalisi, G.; Alkwai, S.; Rothhammer, V.; et al. MAFG-driven astrocytes promote CNS inflammation. Nature 2020, 578, 593–599. [Google Scholar] [CrossRef]
- Bhushan, B.; Singh, N.K. Role of Astrogliosis in the Pathogenesis of Parkinson's Disease: Insights into Astrocytic Nrf2 Pathway as a Potential Therapeutic Target. CNS Neurol Disord Drug Targets 2024, 23, 1015–1029. [Google Scholar] [CrossRef] [PubMed]
- Hickman, S.E.; Kingery, N.D.; Ohsumi, T.K.; Borowsky, M.L.; Wang, L.C.; Means, T.K.; El Khoury, J. The microglial sensome revealed by direct RNA sequencing. Nat Neurosci 2013, 16, 1896–1905. [Google Scholar] [CrossRef] [PubMed]
- Keren-Shaul, H.; Spinrad, A.; Weiner, A.; Matcovitch-Natan, O.; Dvir-Szternfeld, R.; Ulland, T.K.; David, E.; Baruch, K.; Lara-Astaiso, D.; Toth, B.; et al. A Unique Microglia Type Associated with Restricting Development of Alzheimer's Disease. Cell 2017, 169, 1276–1290.e1217. [Google Scholar] [CrossRef] [PubMed]
- Paolicelli, R.C.; Sierra, A.; Stevens, B.; Tremblay, M.E.; Aguzzi, A.; Ajami, B.; Amit, I.; Audinat, E.; Bechmann, I.; Bennett, M.; et al. Microglia states and nomenclature: A field at its crossroads. Neuron 2022, 110, 3458–3483. [Google Scholar] [CrossRef]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int J Mol Sci 2020, 21. [Google Scholar] [CrossRef]
- Thimmulappa, R.K.; Lee, H.; Rangasamy, T.; Reddy, S.P.; Yamamoto, M.; Kensler, T.W.; Biswal, S. Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J Clin Invest 2006, 116, 984–995. [Google Scholar] [CrossRef]
- Innamorato, N.G.; Rojo, A.I.; García-Yagüe, A.J.; Yamamoto, M.; de Ceballos, M.L.; Cuadrado, A. The transcription factor Nrf2 is a therapeutic target against brain inflammation. J Immunol 2008, 181, 680–689. [Google Scholar] [CrossRef]
- Rojo, A.I.; Innamorato, N.G.; Martín-Moreno, A.M.; De Ceballos, M.L.; Yamamoto, M.; Cuadrado, A. Nrf2 regulates microglial dynamics and neuroinflammation in experimental Parkinson's disease. Glia 2010, 58, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado, A.; Kügler, S.; Lastres-Becker, I. Pharmacological targeting of GSK-3 and NRF2 provides neuroprotection in a preclinical model of tauopathy. Redox Biol 2018, 14, 522–534. [Google Scholar] [CrossRef]
- Jin, W.; Wang, H.; Yan, W.; Xu, L.; Wang, X.; Zhao, X.; Yang, X.; Chen, G.; Ji, Y. Disruption of Nrf2 enhances upregulation of nuclear factor-kappaB activity, proinflammatory cytokines, and intercellular adhesion molecule-1 in the brain after traumatic brain injury. Mediators Inflamm 2008, 2008, 725174. [Google Scholar] [CrossRef]
- Cuadrado, A.; Martín-Moldes, Z.; Ye, J.; Lastres-Becker, I. Transcription factors NRF2 and NF-κB are coordinated effectors of the Rho family, GTP-binding protein RAC1 during inflammation. J Biol Chem 2014, 289, 15244–15258. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.F.; Kuo, H.P.; Liu, M.; Chou, C.K.; Xia, W.; Du, Y.; Shen, J.; Chen, C.T.; Huo, L.; Hsu, M.C.; et al. KEAP1 E3 ligase-mediated downregulation of NF-kappaB signaling by targeting IKKbeta. Mol Cell 2009, 36, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.H.; Qu, J.; Shen, X. NF-kappaB/p65 antagonizes Nrf2-ARE pathway by depriving CBP from Nrf2 and facilitating recruitment of HDAC3 to MafK. Biochim Biophys Acta 2008, 1783, 713–727. [Google Scholar] [CrossRef]
- Gao, W.; Guo, L.; Yang, Y.; Wang, Y.; Xia, S.; Gong, H.; Zhang, B.K.; Yan, M. Dissecting the Crosstalk Between Nrf2 and NF-κB Response Pathways in Drug-Induced Toxicity. Front Cell Dev Biol 2021, 9, 809952. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, E.H.; Suzuki, T.; Funayama, R.; Nagashima, T.; Hayashi, M.; Sekine, H.; Tanaka, N.; Moriguchi, T.; Motohashi, H.; Nakayama, K.; et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat Commun 2016, 7, 11624. [Google Scholar] [CrossRef]
- Yao, J.; Sterling, K.; Wang, Z.; Zhang, Y.; Song, W. The role of inflammasomes in human diseases and their potential as therapeutic targets. Signal Transduct Target Ther 2024, 9, 10. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, S.; Xiao, Y.; Zhang, W.; Wu, S.; Qin, T.; Yue, Y.; Qian, W.; Li, L. NLRP3 Inflammasome and Inflammatory Diseases. Oxid Med Cell Longev 2020, 2020, 4063562. [Google Scholar] [CrossRef]
- Tastan, B.; Arioz, B.I.; Genc, S. Targeting NLRP3 Inflammasome With Nrf2 Inducers in Central Nervous System Disorders. Front Immunol 2022, 13, 865772. [Google Scholar] [CrossRef]
- Zhou, R.; Tardivel, A.; Thorens, B.; Choi, I.; Tschopp, J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol 2010, 11, 136–140. [Google Scholar] [CrossRef]
- Palomino-Antolín, A.; Decouty-Pérez, C.; Farré-Alins, V.; Narros-Fernández, P.; Lopez-Rodriguez, A.B.; Álvarez-Rubal, M.; Valencia, I.; López-Muñoz, F.; Ramos, E.; Cuadrado, A.; et al. Redox Regulation of Microglial Inflammatory Response: Fine Control of NLRP3 Inflammasome through Nrf2 and NOX4. Antioxidants (Basel) 2023, 12. [Google Scholar] [CrossRef]
- Tufekci, K.U.; Ercan, I.; Isci, K.B.; Olcum, M.; Tastan, B.; Gonul, C.P.; Genc, K.; Genc, S. Sulforaphane inhibits NLRP3 inflammasome activation in microglia through Nrf2-mediated miRNA alteration. Immunol Lett 2021, 233, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Tastan, B.; Arioz, B.I.; Tufekci, K.U.; Tarakcioglu, E.; Gonul, C.P.; Genc, K.; Genc, S. Dimethyl Fumarate Alleviates NLRP3 Inflammasome Activation in Microglia and Sickness Behavior in LPS-Challenged Mice. Front Immunol 2021, 12, 737065. [Google Scholar] [CrossRef] [PubMed]
- Arioz, B.I.; Tastan, B.; Tarakcioglu, E.; Tufekci, K.U.; Olcum, M.; Ersoy, N.; Bagriyanik, A.; Genc, K.; Genc, S. Melatonin Attenuates LPS-Induced Acute Depressive-Like Behaviors and Microglial NLRP3 Inflammasome Activation Through the SIRT1/Nrf2 Pathway. Front Immunol 2019, 10, 1511. [Google Scholar] [CrossRef]
- Gao, Z.; Luo, K.; Hu, Y.; Niu, Y.; Zhu, X.; Li, S.; Zhang, H. Melatonin alleviates chronic stress-induced hippocampal microglia pyroptosis and subsequent depression-like behaviors by inhibiting Cathepsin B/NLRP3 signaling pathway in rats. Transl Psychiatry 2024, 14, 166. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, X.; Ding, Y.; Zhou, W.; Tao, L.; Lu, P.; Wang, Y.; Hu, R. Nuclear Factor E2-Related Factor-2 Negatively Regulates NLRP3 Inflammasome Activity by Inhibiting Reactive Oxygen Species-Induced NLRP3 Priming. Antioxid Redox Signal 2017, 26, 28–43. [Google Scholar] [CrossRef] [PubMed]
- Freigang, S.; Ampenberger, F.; Spohn, G.; Heer, S.; Shamshiev, A.T.; Kisielow, J.; Hersberger, M.; Yamamoto, M.; Bachmann, M.F.; Kopf, M. Nrf2 is essential for cholesterol crystal-induced inflammasome activation and exacerbation of atherosclerosis. Eur J Immunol 2011, 41, 2040–2051. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Gillette, D.D.; Li, X.; Zhang, Z.; Wen, H. Nuclear factor E2-related factor-2 (Nrf2) is required for NLRP3 and AIM2 inflammasome activation. J Biol Chem 2014, 289, 17020–17029. [Google Scholar] [CrossRef]
- Grajchen, E.; Wouters, E.; van de Haterd, B.; Haidar, M.; Hardonnière, K.; Dierckx, T.; Van Broeckhoven, J.; Erens, C.; Hendrix, S.; Kerdine-Römer, S.; et al. CD36-mediated uptake of myelin debris by macrophages and microglia reduces neuroinflammation. J Neuroinflammation 2020, 17, 224. [Google Scholar] [CrossRef]
- Lastres-Becker, I.; Ulusoy, A.; Innamorato, N.G.; Sahin, G.; Rábano, A.; Kirik, D.; Cuadrado, A. α-Synuclein expression and Nrf2 deficiency cooperate to aggravate protein aggregation, neuronal death and inflammation in early-stage Parkinson's disease. Hum Mol Genet 2012, 21, 3173–3192. [Google Scholar] [CrossRef]
- Castro-Sánchez, S.; García-Yagüe Á, J.; Kügler, S.; Lastres-Becker, I. CX3CR1-deficient microglia shows impaired signalling of the transcription factor NRF2: Implications in tauopathies. Redox Biol 2019, 22, 101118. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, G.; Ting, S.M.; Song, S.; Zhang, J.; Edwards, N.J.; Aronowski, J. Cleaning up after ICH: the role of Nrf2 in modulating microglia function and hematoma clearance. J Neurochem 2015, 133, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Romero-Molina, C.; Garretti, F.; Andrews, S.J.; Marcora, E.; Goate, A.M. Microglial efferocytosis: Diving into the Alzheimer's disease gene pool. Neuron 2022, 110, 3513–3533. [Google Scholar] [CrossRef] [PubMed]
- Bernier, L.P.; York, E.M.; MacVicar, B.A. Immunometabolism in the Brain: How Metabolism Shapes Microglial Function. Trends Neurosci 2020, 43, 854–869. [Google Scholar] [CrossRef]
- Lampropoulou, V.; Sergushichev, A.; Bambouskova, M.; Nair, S.; Vincent, E.E.; Loginicheva, E.; Cervantes-Barragan, L.; Ma, X.; Huang, S.C.; Griss, T.; et al. Itaconate Links Inhibition of Succinate Dehydrogenase with Macrophage Metabolic Remodeling and Regulation of Inflammation. Cell Metab 2016, 24, 158–166. [Google Scholar] [CrossRef]
- Xia, N.; Madore, V.; Albalakhi, A.; Lin, S.; Stimpson, T.; Xu, Y.; Schwarzschild, M.A.; Bakshi, R. Microglia-dependent neuroprotective effects of 4-octyl itaconate against rotenone-and MPP+-induced neurotoxicity in Parkinson's disease. Sci Rep 2023, 13, 15539. [Google Scholar] [CrossRef]
- Mills, E.L.; Ryan, D.G.; Prag, H.A.; Dikovskaya, D.; Menon, D.; Zaslona, Z.; Jedrychowski, M.P.; Costa, A.S.H.; Higgins, M.; Hams, E.; et al. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature 2018, 556, 113–117. [Google Scholar] [CrossRef]
- Ni, L.; Xiao, J.; Zhang, D.; Shao, Z.; Huang, C.; Wang, S.; Wu, Y.; Tian, N.; Sun, L.; Wu, A.; et al. Immune-responsive gene 1/itaconate activates nuclear factor erythroid 2-related factor 2 in microglia to protect against spinal cord injury in mice. Cell Death Dis 2022, 13, 140. [Google Scholar] [CrossRef]
- Haslund-Vinding, J.; McBean, G.; Jaquet, V.; Vilhardt, F. NADPH oxidases in oxidant production by microglia: activating receptors, pharmacology and association with disease. Br J Pharmacol 2017, 174, 1733–1749. [Google Scholar] [CrossRef] [PubMed]
- Simpson, D.S.A.; Oliver, P.L. ROS Generation in Microglia: Understanding Oxidative Stress and Inflammation in Neurodegenerative Disease. Antioxidants (Basel) 2020, 9. [Google Scholar] [CrossRef]
- Rojo, A.I.; McBean, G.; Cindric, M.; Egea, J.; López, M.G.; Rada, P.; Zarkovic, N.; Cuadrado, A. Redox control of microglial function: molecular mechanisms and functional significance. Antioxid Redox Signal 2014, 21, 1766–1801. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, R.C.; Sosa, J.C.; Gardeck, A.M.; Baez, A.S.; Lee, C.H.; Wessling-Resnick, M. Inflammation-induced iron transport and metabolism by brain microglia. J Biol Chem 2018, 293, 7853–7863. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Long, D. Nrf2 and Ferroptosis: A New Research Direction for Neurodegenerative Diseases. Front Neurosci 2020, 14, 267. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, Z.; Zhou, X.; Zhao, Z.; Zhao, R.; Xu, X.; Kong, X.; Ren, J.; Yao, X.; Wen, Q.; et al. Microglia and macrophage exhibit attenuated inflammatory response and ferroptosis resistance after RSL3 stimulation via increasing Nrf2 expression. J Neuroinflammation 2021, 18, 249. [Google Scholar] [CrossRef]
- Fernández-Mendívil, C.; Luengo, E.; Trigo-Alonso, P.; García-Magro, N.; Negredo, P.; López, M.G. Protective role of microglial HO-1 blockade in aging: Implication of iron metabolism. Redox Biol 2021, 38, 101789. [Google Scholar] [CrossRef]
- Xiang, Y.; Song, X.; Long, D. Ferroptosis regulation through Nrf2 and implications for neurodegenerative diseases. Arch Toxicol 2024, 98, 579–615. [Google Scholar] [CrossRef]
- Cuadrado, A.; Rojo, A.I. Heme oxygenase-1 as a therapeutic target in neurodegenerative diseases and brain infections. Curr Pharm Des 2008, 14, 429–442. [Google Scholar] [CrossRef]
- Wilks, A. Heme oxygenase: evolution, structure, and mechanism. Antioxid Redox Signal 2002, 4, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Dunn, L.L.; Midwinter, R.G.; Ni, J.; Hamid, H.A.; Parish, C.R.; Stocker, R. New insights into intracellular locations and functions of heme oxygenase-1. Antioxid Redox Signal 2014, 20, 1723–1742. [Google Scholar] [CrossRef]
- Canesin, G.; Hejazi, S.M.; Swanson, K.D.; Wegiel, B. Heme-Derived Metabolic Signals Dictate Immune Responses. Front Immunol 2020, 11, 66. [Google Scholar] [CrossRef]
- Ryter, S.W.; Choi, A.M. Targeting heme oxygenase-1 and carbon monoxide for therapeutic modulation of inflammation. Transl Res 2016, 167, 7–34. [Google Scholar] [CrossRef]
- Wu, Y.H.; Hsieh, H.L. Roles of Heme Oxygenase-1 in Neuroinflammation and Brain Disorders. Antioxidants (Basel) 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Navarro, E.; Buendia, I.; Parada, E.; León, R.; Jansen-Duerr, P.; Pircher, H.; Egea, J.; Lopez, M.G. Alpha7 nicotinic receptor activation protects against oxidative stress via heme-oxygenase I induction. Biochem Pharmacol 2015, 97, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Parada, E.; Buendia, I.; Navarro, E.; Avendaño, C.; Egea, J.; López, M.G. Microglial HO-1 induction by curcumin provides antioxidant, antineuroinflammatory, and glioprotective effects. Mol Nutr Food Res 2015, 59, 1690–1700. [Google Scholar] [CrossRef]
- Navarro, E.; Gonzalez-Lafuente, L.; Pérez-Liébana, I.; Buendia, I.; López-Bernardo, E.; Sánchez-Ramos, C.; Prieto, I.; Cuadrado, A.; Satrustegui, J.; Cadenas, S.; et al. Heme-Oxygenase I and PCG-1α Regulate Mitochondrial Biogenesis via Microglial Activation of Alpha7 Nicotinic Acetylcholine Receptors Using PNU282987. Antioxid Redox Signal 2017, 27, 93–105. [Google Scholar] [CrossRef]
- Parada, E.; Egea, J.; Buendia, I.; Negredo, P.; Cunha, A.C.; Cardoso, S.; Soares, M.P.; López, M.G. The microglial α7-acetylcholine nicotinic receptor is a key element in promoting neuroprotection by inducing heme oxygenase-1 via nuclear factor erythroid-2-related factor 2. Antioxid Redox Signal 2013, 19, 1135–1148. [Google Scholar] [CrossRef]
- Fernández-Mendívil, C.; Arreola, M.A.; Hohsfield, L.A.; Green, K.N.; Lopez, M.G. Aging and Progression of Beta-Amyloid Pathology in Alzheimer's Disease Correlates with Microglial Heme-Oxygenase-1 Overexpression. Antioxidants (Basel) 2020, 9. [Google Scholar] [CrossRef]
- Hui, Y.; Wang, D.; Li, W.; Zhang, L.; Jin, J.; Ma, N.; Zhou, L.; Nakajima, O.; Zhao, W.; Gao, X. Long-term overexpression of heme oxygenase 1 promotes tau aggregation in mouse brain by inducing tau phosphorylation. J Alzheimers Dis 2011, 26, 299–313. [Google Scholar] [CrossRef]
- Si, Z.; Wang, X.; Zhang, Z.; Wang, J.; Li, J.; Li, J.; Li, L.; Li, Y.; Peng, Y.; Sun, C.; et al. Heme Oxygenase 1 Induces Tau Oligomer Formation and Synapse Aberrations in Hippocampal Neurons. J Alzheimers Dis 2018, 65, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Albarral, J.A.; Salobrar-García, E.; Matamoros, J.A.; Fernández-Mendívil, C.; Del Sastre, E.; Chen, L.; de Hoz, R.; López-Cuenca, I.; Sánchez-Puebla, L.; Ramírez, J.M.; et al. Microglial Hemoxygenase-1 Deletion Reduces Inflammation in the Retina of Old Mice with Tauopathy. Antioxidants (Basel) 2022, 11. [Google Scholar] [CrossRef]
- Wang, D.; Hui, Y.; Peng, Y.; Tang, L.; Jin, J.; He, R.; Li, Y.; Zhang, S.; Li, L.; Zhou, Y.; et al. Overexpression of heme oxygenase 1 causes cognitive decline and affects pathways for tauopathy in mice. J Alzheimers Dis 2015, 43, 519–534. [Google Scholar] [CrossRef] [PubMed]
- Si, Z.; Wang, X. The Neuroprotective and Neurodegeneration Effects of Heme Oxygenase-1 in Alzheimer's Disease. J Alzheimers Dis 2020, 78, 1259–1272. [Google Scholar] [CrossRef] [PubMed]
- Bradl, M.; Lassmann, H. Oligodendrocytes: biology and pathology. Acta Neuropathol 2010, 119, 37–53. [Google Scholar] [CrossRef]
- Liessem-Schmitz, A.; Teske, N.; Scheld, M.; Nyamoya, S.; Zendedel, A.; Beyer, C.; Clarner, T.; Fragoulis, A. Nrf2 Signaling in Sodium Azide-Treated Oligodendrocytes Restores Mitochondrial Functions. J Mol Neurosci 2018, 66, 229–237. [Google Scholar] [CrossRef]
- Lim, J.L.; van der Pol, S.M.; Baron, W.; McCord, J.M.; de Vries, H.E.; van Horssen, J. Protandim Protects Oligodendrocytes against an Oxidative Insult. Antioxidants (Basel) 2016, 5. [Google Scholar] [CrossRef]
- Teske, N.; Liessem, A.; Fischbach, F.; Clarner, T.; Beyer, C.; Wruck, C.; Fragoulis, A.; Tauber, S.C.; Victor, M.; Kipp, M. Chemical hypoxia-induced integrated stress response activation in oligodendrocytes is mediated by the transcription factor nuclear factor (erythroid-derived 2)-like 2 (NRF2). J Neurochem 2018, 144, 285–301. [Google Scholar] [CrossRef]
- De Nuccio, C.; Bernardo, A.; Troiano, C.; Brignone, M.S.; Falchi, M.; Greco, A.; Rosini, M.; Basagni, F.; Lanni, C.; Serafini, M.M.; et al. NRF2 and PPAR-γ Pathways in Oligodendrocyte Progenitors: Focus on ROS Protection, Mitochondrial Biogenesis and Promotion of Cell Differentiation. Int J Mol Sci 2020, 21. [Google Scholar] [CrossRef]
- Nellessen, A.; Nyamoya, S.; Zendedel, A.; Slowik, A.; Wruck, C.; Beyer, C.; Fragoulis, A.; Clarner, T. Nrf2 deficiency increases oligodendrocyte loss, demyelination, neuroinflammation and axonal damage in an MS animal model. Metab Brain Dis 2020, 35, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Abd El Aziz, A.E.; Sayed, R.H.; Sallam, N.A.; El Sayed, N.S. Neuroprotective Effects of Telmisartan and Nifedipine Against Cuprizone-Induced Demyelination and Behavioral Dysfunction in Mice: Roles of NF-κB and Nrf2. Inflammation 2021, 44, 1629–1642. [Google Scholar] [CrossRef]
- Khaledi, E.; Noori, T.; Mohammadi-Farani, A.; Sureda, A.; Dehpour, A.R.; Yousefi-Manesh, H.; Sobarzo-Sanchez, E.; Shirooie, S. Trifluoperazine reduces cuprizone-induced demyelination via targeting Nrf2 and IKB in mice. Eur J Pharmacol 2021, 909, 174432. [Google Scholar] [CrossRef]
- Molina-Gonzalez, I.; Holloway, R.K.; Jiwaji, Z.; Dando, O.; Kent, S.A.; Emelianova, K.; Lloyd, A.F.; Forbes, L.H.; Mahmood, A.; Skripuletz, T.; et al. Astrocyte-oligodendrocyte interaction regulates central nervous system regeneration. Nat Commun 2023, 14, 3372. [Google Scholar] [CrossRef] [PubMed]
- Draheim, T.; Liessem, A.; Scheld, M.; Wilms, F.; Weißflog, M.; Denecke, B.; Kensler, T.W.; Zendedel, A.; Beyer, C.; Kipp, M.; et al. Activation of the astrocytic Nrf2/ARE system ameliorates the formation of demyelinating lesions in a multiple sclerosis animal model. Glia 2016, 64, 2219–2230. [Google Scholar] [CrossRef]
- Huang, H.; Taraboletti, A.; Shriver, L.P. Dimethyl fumarate modulates antioxidant and lipid metabolism in oligodendrocytes. Redox Biol 2015, 5, 169–175. [Google Scholar] [CrossRef]
Figure 1.
Molecular Regulation of Nrf2. The transcription factor Nrf2 is a master regulator of multiple cytoprotective pathways by inducing the expression of antioxidant, detoxification and metabolic enzymes. Under basal conditions, Nrf2 is targeted for continuous proteasomal degradation by different ubiquitin-ligase systems. The cullin 3 (Cul3) RING-box 1 (RBX1) ligase complex mediated degradation employs the Kelch-like ECH-associated protein 1, Keap1, as a substrate adaptor. Keap1 is a cysteine-rich adaptor protein that binds Nrf2, facilitating its ubiquitination and targeting it for degradation under basal conditions. Keap1 acts as an oxidative stress sensor, and in the presence of oxidative signals or electrophiles, Keap1 highly reactive Cys residues become oxidised, leading to a conformational change that prevents Nrf2 binding, allowing its stabilisation and translocation to the nucleus. Nrf2 stabilisation can also be achieved by several molecules, such as p62 or pharmacological inhibitors, which prevent Nrf2 binding to Keap1. The β-TrCP/Cul 1 ubiquitin ligase complex mediates the degradation of Nrf2 in a mechanism involving the glycogen synthase kinase 3 (GSK3β). In basal conditions, GSK3β-mediated phosphorylation of Nrf2 targets the protein for degradation through this system. When GSK3β is inhibited, such as after its phosphorylation by the PI3K/Akt survival pathway, Nrf2 degradation doesn´t occur, allowing its stabilization and translocation to the nucleus. By either way, once in the nucleus, Nrf2 binds to the antioxidant response elements (ARE) present in the promoter of its target genes, inducing their transcriptional activation. Created in Biorender.
Figure 1.
Molecular Regulation of Nrf2. The transcription factor Nrf2 is a master regulator of multiple cytoprotective pathways by inducing the expression of antioxidant, detoxification and metabolic enzymes. Under basal conditions, Nrf2 is targeted for continuous proteasomal degradation by different ubiquitin-ligase systems. The cullin 3 (Cul3) RING-box 1 (RBX1) ligase complex mediated degradation employs the Kelch-like ECH-associated protein 1, Keap1, as a substrate adaptor. Keap1 is a cysteine-rich adaptor protein that binds Nrf2, facilitating its ubiquitination and targeting it for degradation under basal conditions. Keap1 acts as an oxidative stress sensor, and in the presence of oxidative signals or electrophiles, Keap1 highly reactive Cys residues become oxidised, leading to a conformational change that prevents Nrf2 binding, allowing its stabilisation and translocation to the nucleus. Nrf2 stabilisation can also be achieved by several molecules, such as p62 or pharmacological inhibitors, which prevent Nrf2 binding to Keap1. The β-TrCP/Cul 1 ubiquitin ligase complex mediates the degradation of Nrf2 in a mechanism involving the glycogen synthase kinase 3 (GSK3β). In basal conditions, GSK3β-mediated phosphorylation of Nrf2 targets the protein for degradation through this system. When GSK3β is inhibited, such as after its phosphorylation by the PI3K/Akt survival pathway, Nrf2 degradation doesn´t occur, allowing its stabilization and translocation to the nucleus. By either way, once in the nucleus, Nrf2 binds to the antioxidant response elements (ARE) present in the promoter of its target genes, inducing their transcriptional activation. Created in Biorender.

Figure 2.
Main cytoprotective pathways of Nrf2 in the brain. Nrf2 orchestrates several essential cellular functions in the brain, including mitochondrial function and metabolism, antioxidant defence, and the modulation of the inflammatory response. Left panel: The brain primarily relies on glucose as an energy source. Glucose is taken up by cells via glucose transporters and metabolized through glycolysis, generating pyruvate, ATP, and NADH. Alternatively, glucose can be processed via the pentose phosphate pathway, which is critical to produce the cofactor NADPH. Pyruvate can either be converted into lactate during anaerobic respiration or enter the mitochondria to fuel the Krebs cycle. The Krebs cycle, a series of enzymatic reactions, produces NADH and FADH2, which donate electrons to the mitochondrial electron transport chain for ATP generation through oxidative phosphorylation. Nrf2 modulates glucose metabolism by increasing glucose uptake, enhancing the activity of enzymes involved in its metabolism, and improving the efficiency of the Krebs cycle, thereby increasing the availability of the substrates NADH and FADH2 for mitochondrial respiration and subsequently ATP production. Middle panel: Enzymatic sources, such as xanthine oxidase and NADPH oxidases, along with non-enzymatic sources like electron leakage from the mitochondria, trigger the production of reactive oxygen species (ROS). This occurs primarily through the conversion of O₂ into the superoxide anion (O₂•⁻), which is rapidly converted into hydrogen peroxide (H₂O₂), either spontaneously or by the action of superoxide dismutase (SOD). In the presence of iron, H₂O₂ can generate the hydroxyl radical (•OH) through the Fenton reaction. The hydroxyl radical (•OH) is extremely reactive and can initiate lipid peroxidation in cellular membranes, ultimately triggering ferroptosis. To limit the damage caused by ROS, H₂O₂ is detoxified into water (H₂O) by various antioxidant pathways, such as catalase and glutathione peroxidases (GPx), which catalyze the reduction of H₂O₂ (and other peroxides) using glutathione (GSH) as a co-substrate. GSH is synthesized in the cytosol from its constituent amino acids by the sequential actions of glutamate-cysteine ligase (GCL) and glutathione synthetase (GS). Another crucial player in antioxidant defense is the thioredoxin (Trx) system, which reduces disulfide bonds in oxidized proteins (Prot-SS). Cellular redox reactions are ultimately driven by NADPH, which is used to reduce oxidized thioredoxin (Trx-SS) and oxidized glutathione (GSSG) through thioredoxin reductase (TrxR) and glutathione reductase (GSR), respectively, regenerating reduced thioredoxin (Trx-SH) and GSH. Among other mechanisms, Nrf2 modulates the cellular antioxidant defense by upregulating the expression of key enzymes involved in the synthesis and regeneration of GSH (GCL, GS, GR) and the thioredoxin (TrxR) systems, while also enhancing the production of NADPH through the activation of the pentose phosphate pathway. Right panel: Various pro-inflammatory signals, including LPS, cytokines, damage-associated patterns, and reactive oxygen species (ROS), can activate specific receptors on microglial cells, thereby initiating the NF-kB and inflammasome pathways. Within the NF-kB pathway, IKK (in the canonical pathway) or NIK (in the non-canonical pathway) phosphorylate IKBα, leading to its degradation by the proteasome. This degradation results in the release of RelA/p50 or RelB/p52, which then translocate to the nucleus to activate the transcription of inflammatory mediators such as cytokines, chemokines, and adhesion molecules. Moreover, it also permits the expression of NLRP3 and pro-IL-1β, coordinating priming step in the NLRP3 (NOD-like receptor protein 3) inflammasome pathway. Afterwards, in the activation step the NLRP3 protein, the adaptor protein ASC, and pro-caspase-1 assemble the inflammasome complex and activates caspase-1, IL-1β and IL-18. The crosstalk between Nrf2 and NF-κB pathways underscores the intricate balance between antioxidant defenses and inflammatory responses in maintaining brain homeostasis. Created in Biorender.
Figure 2.
Main cytoprotective pathways of Nrf2 in the brain. Nrf2 orchestrates several essential cellular functions in the brain, including mitochondrial function and metabolism, antioxidant defence, and the modulation of the inflammatory response. Left panel: The brain primarily relies on glucose as an energy source. Glucose is taken up by cells via glucose transporters and metabolized through glycolysis, generating pyruvate, ATP, and NADH. Alternatively, glucose can be processed via the pentose phosphate pathway, which is critical to produce the cofactor NADPH. Pyruvate can either be converted into lactate during anaerobic respiration or enter the mitochondria to fuel the Krebs cycle. The Krebs cycle, a series of enzymatic reactions, produces NADH and FADH2, which donate electrons to the mitochondrial electron transport chain for ATP generation through oxidative phosphorylation. Nrf2 modulates glucose metabolism by increasing glucose uptake, enhancing the activity of enzymes involved in its metabolism, and improving the efficiency of the Krebs cycle, thereby increasing the availability of the substrates NADH and FADH2 for mitochondrial respiration and subsequently ATP production. Middle panel: Enzymatic sources, such as xanthine oxidase and NADPH oxidases, along with non-enzymatic sources like electron leakage from the mitochondria, trigger the production of reactive oxygen species (ROS). This occurs primarily through the conversion of O₂ into the superoxide anion (O₂•⁻), which is rapidly converted into hydrogen peroxide (H₂O₂), either spontaneously or by the action of superoxide dismutase (SOD). In the presence of iron, H₂O₂ can generate the hydroxyl radical (•OH) through the Fenton reaction. The hydroxyl radical (•OH) is extremely reactive and can initiate lipid peroxidation in cellular membranes, ultimately triggering ferroptosis. To limit the damage caused by ROS, H₂O₂ is detoxified into water (H₂O) by various antioxidant pathways, such as catalase and glutathione peroxidases (GPx), which catalyze the reduction of H₂O₂ (and other peroxides) using glutathione (GSH) as a co-substrate. GSH is synthesized in the cytosol from its constituent amino acids by the sequential actions of glutamate-cysteine ligase (GCL) and glutathione synthetase (GS). Another crucial player in antioxidant defense is the thioredoxin (Trx) system, which reduces disulfide bonds in oxidized proteins (Prot-SS). Cellular redox reactions are ultimately driven by NADPH, which is used to reduce oxidized thioredoxin (Trx-SS) and oxidized glutathione (GSSG) through thioredoxin reductase (TrxR) and glutathione reductase (GSR), respectively, regenerating reduced thioredoxin (Trx-SH) and GSH. Among other mechanisms, Nrf2 modulates the cellular antioxidant defense by upregulating the expression of key enzymes involved in the synthesis and regeneration of GSH (GCL, GS, GR) and the thioredoxin (TrxR) systems, while also enhancing the production of NADPH through the activation of the pentose phosphate pathway. Right panel: Various pro-inflammatory signals, including LPS, cytokines, damage-associated patterns, and reactive oxygen species (ROS), can activate specific receptors on microglial cells, thereby initiating the NF-kB and inflammasome pathways. Within the NF-kB pathway, IKK (in the canonical pathway) or NIK (in the non-canonical pathway) phosphorylate IKBα, leading to its degradation by the proteasome. This degradation results in the release of RelA/p50 or RelB/p52, which then translocate to the nucleus to activate the transcription of inflammatory mediators such as cytokines, chemokines, and adhesion molecules. Moreover, it also permits the expression of NLRP3 and pro-IL-1β, coordinating priming step in the NLRP3 (NOD-like receptor protein 3) inflammasome pathway. Afterwards, in the activation step the NLRP3 protein, the adaptor protein ASC, and pro-caspase-1 assemble the inflammasome complex and activates caspase-1, IL-1β and IL-18. The crosstalk between Nrf2 and NF-κB pathways underscores the intricate balance between antioxidant defenses and inflammatory responses in maintaining brain homeostasis. Created in Biorender.

|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).