Submitted:
28 November 2024
Posted:
29 November 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. GC-MS Chromatographic Analysis
2.2. Antibacterial Activity
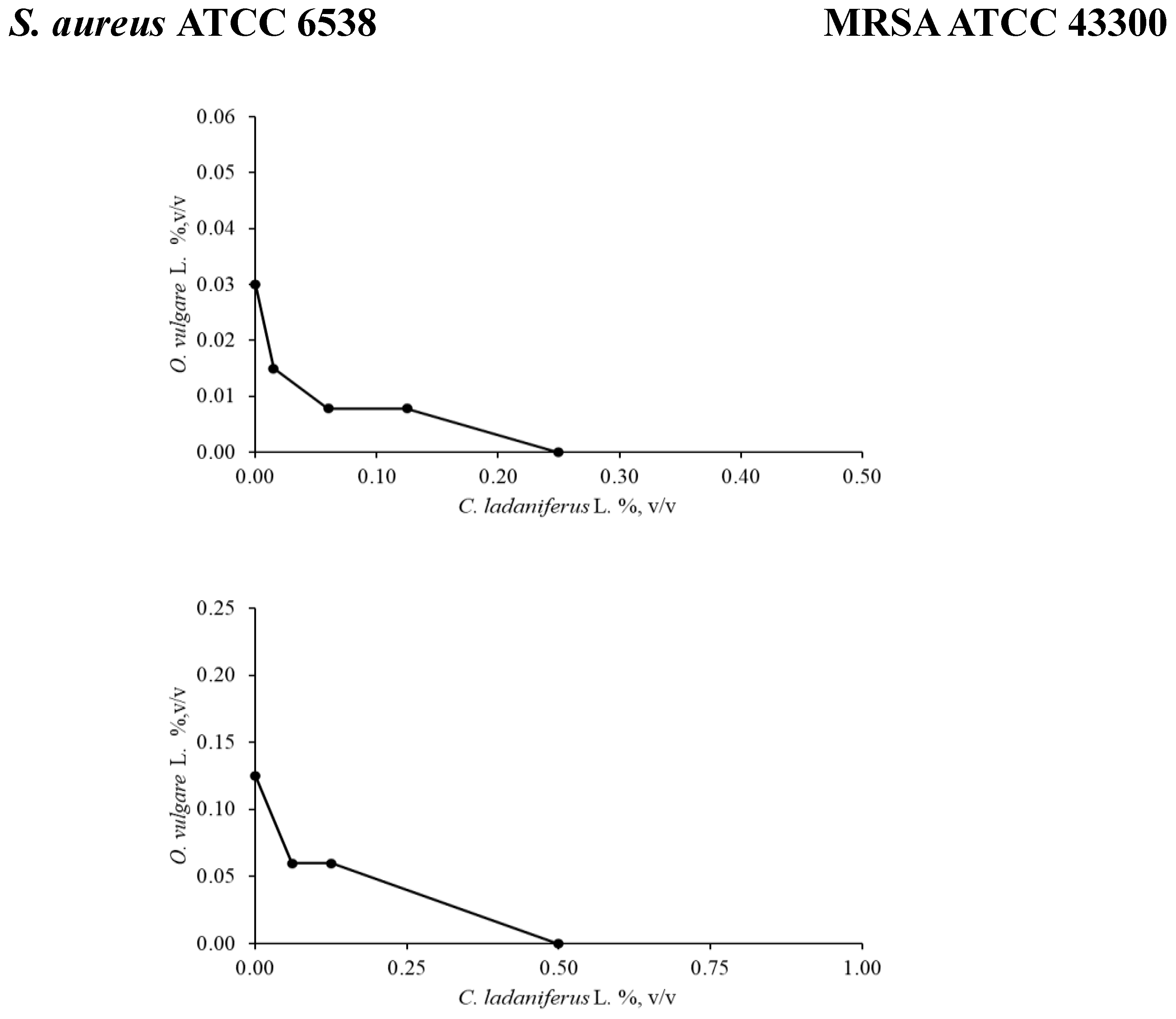
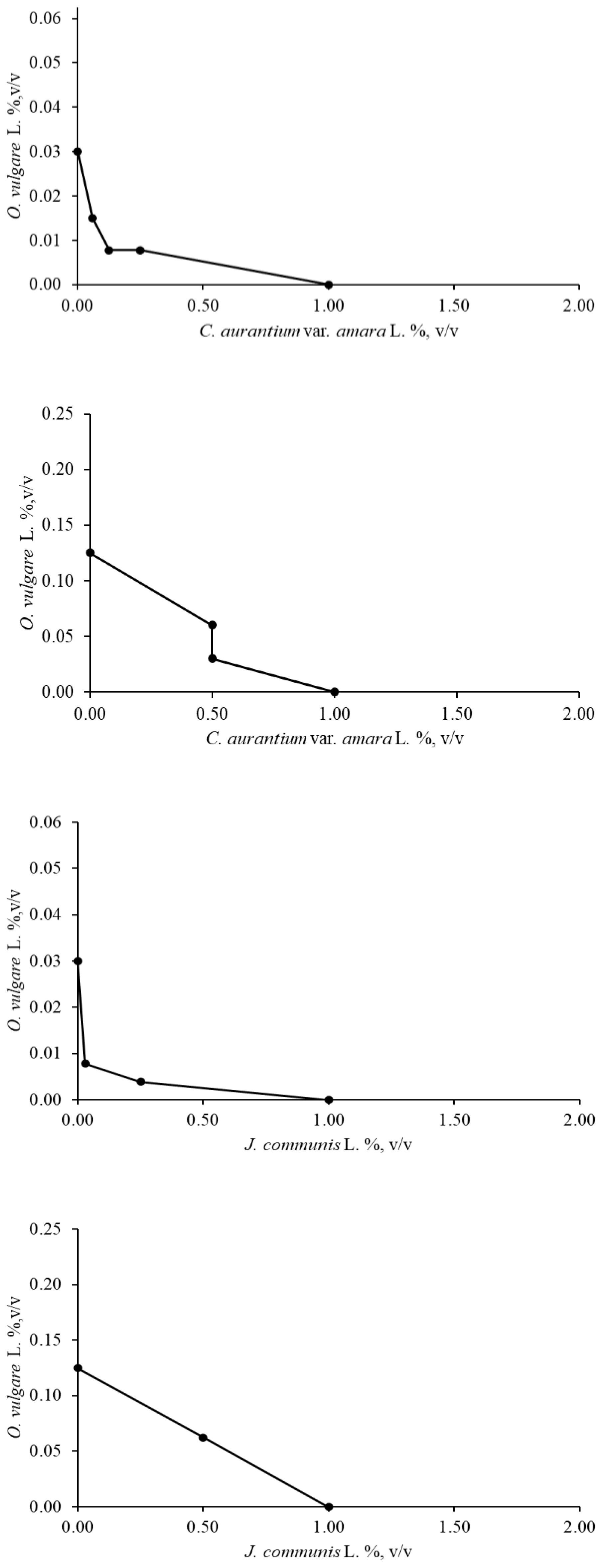
2.2.1. Checkerboard Assay
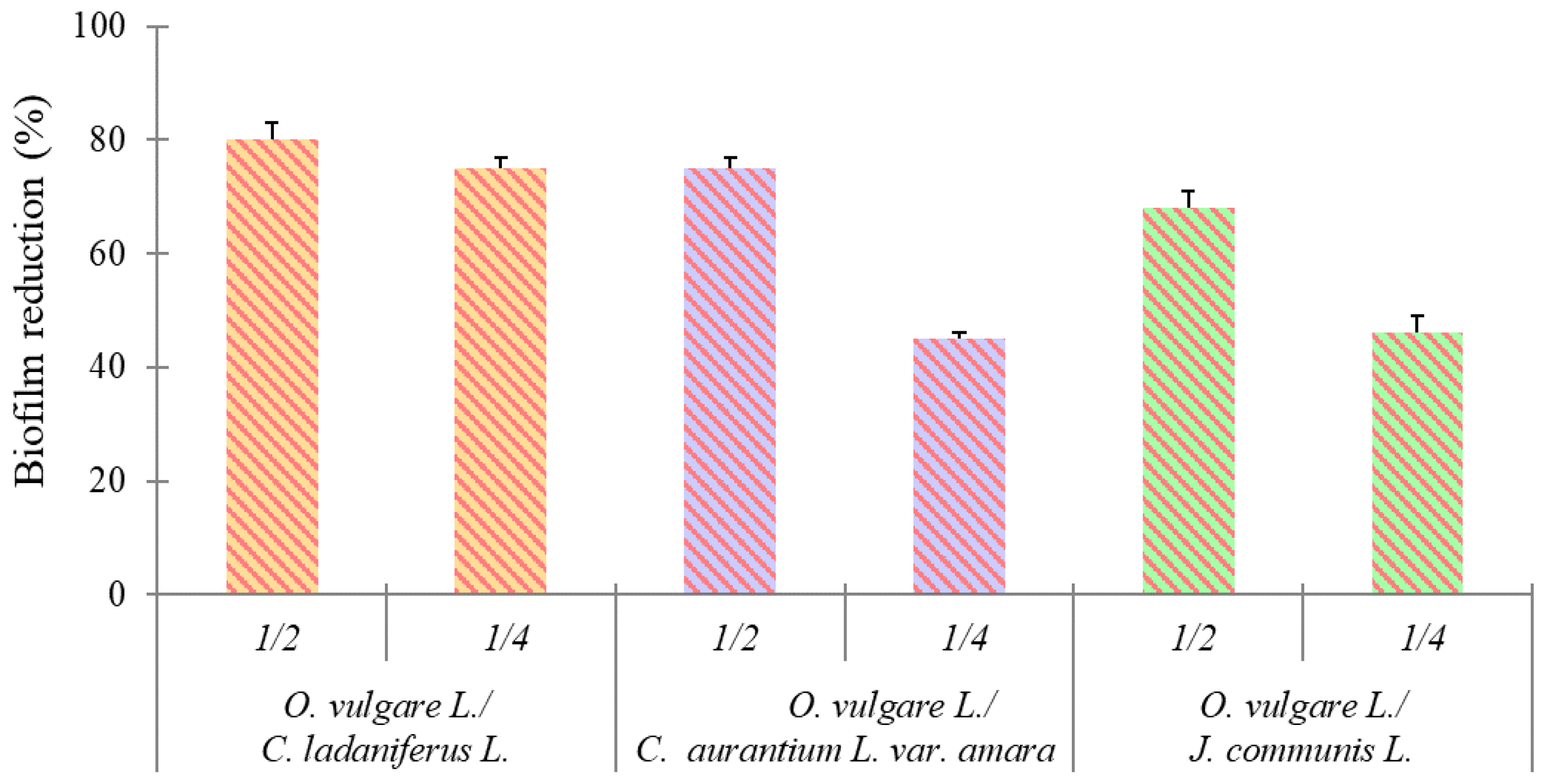
2.2.2. Effect on Biofilm Formation
2.3. Antioxidant Activity
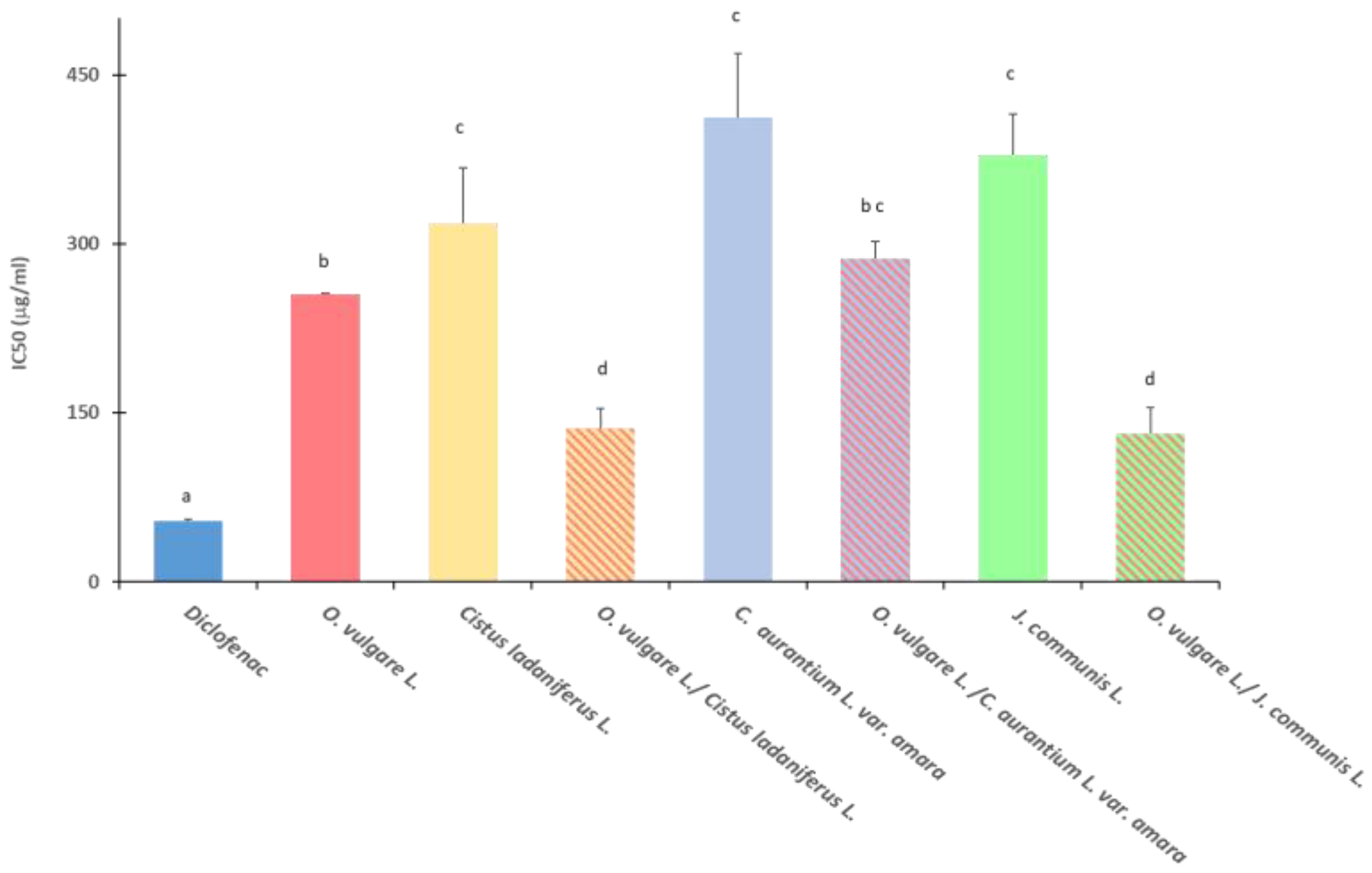
2.4. Anti-Inflammatory Activity
2.5. Antioxidant and Anti-Inflammatory Activities of EO Combinations
3. Discussion
4. Materials and Methods
4.1. Essential Oils Sampling
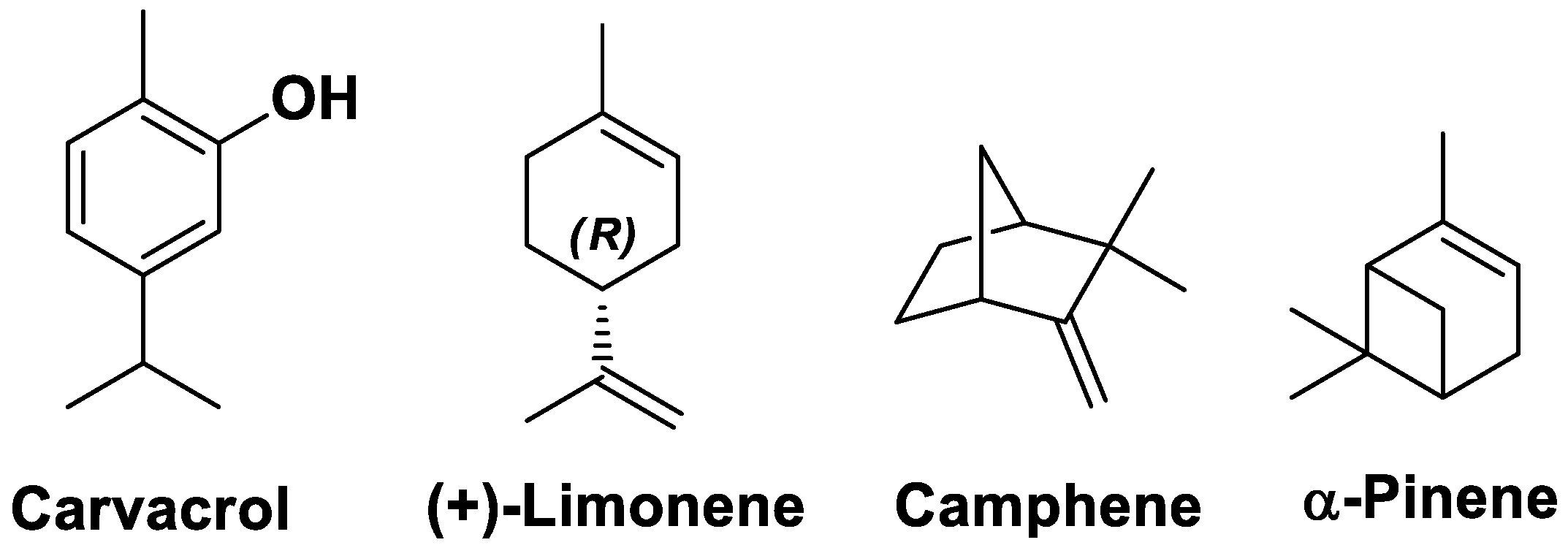
4.2. GC-MS Chromatoghrphic Analysis
4.3. Antibacterial Activity
4.3.1. Bacterial Strains and Culture Conditions
4.3.2. MIC and MBC Determination
4.3.3. Checkerboard Assay
4.3.4. Effect on Biofilm Formation
4.4. Antioxidant Activity
4.4.1. 2,2-. diphenyl-1-picrylhydrazyl (DPPH) Test
4.4.2. 2,2’-. azinobis-(3-ethyl-benzothiazolin-6-sulfonic acid (ABTS) Assay
4.4.3. Ferric Reducing/Antioxidant Power (FRAP) Assay
4.5. Anti-Inflammatory Activity
4.6. Antioxidant and Anti-Inflammatory Activities of EOs Combinations
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Velazquez-Meza, M.E.; Galarde-López, M.; Carrillo-Quiróz, B.; Alpuche-Aranda, C.M. Antimicrobial Resistance: One Health Approach. Vet. World 2022, 743–749. [Google Scholar] [CrossRef]
- Jin, M.; Osman, M.; Green, B.A.; Yang, Y.; Ahuja, A.; Lu, Z.; Cazer, C.L. Evidence for the Transmission of Antimicrobial Resistant Bacteria between Humans and Companion Animals: A Scoping Review. One Health 2023, 17, 100593. [Google Scholar] [CrossRef]
- Palma, E.; Tilocca, B.; Roncada, P. Antimicrobial Resistance in Veterinary Medicine: An Overview. Int. J. Mol. Sci. 2020, 21, 1914. [Google Scholar] [CrossRef]
- Jugreet, B.S.; Suroowan, S.; Rengasamy, R.R.K.; Mahomoodally, M.F. Chemistry, Bioactivities, Mode of Action and Industrial Applications of Essential Oils. Trends Food Sci. Technol. 2020, 101, 89–105. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, Y.; Chi, P.; Liu, H.; Jing, Z.; Cao, H.; Du, Y.; Zhao, Y.; Qin, X.; Zhang, W.; et al. Essential Oils: Chemical Constituents, Potential Neuropharmacological Effects and Aromatherapy - A Review. Pharmacol. Res. - Mod. Chin. Med. 2023, 6, 100210. [Google Scholar] [CrossRef]
- Zuzarte, M.; Salgueiro, L. Essential Oils Chemistry. In Bioactive Essential Oils and Cancer; De Sousa, D.P., Ed.; Springer International Publishing: Cham, 2015; pp. 19–61. ISBN 978-3-319-19143-0. [Google Scholar]
- Mandal, U.; Panda, M.; Mahalik, G. Traditional Uses of Essential Oils in Aromatherapy; 2021; ISBN 978-81-940943-7-1.
- Manniche, L. Sacred Luxuries: Fragrance, Aromatherapy and Cosmetics in Ancient Egypt; Opus: London, 1999; ISBN 978-0-9535546-0-7. [Google Scholar]
- De Sousa, D.P.; Damasceno, R.O.S.; Amorati, R.; Elshabrawy, H.A.; De Castro, R.D.; Bezerra, D.P.; Nunes, V.R.V.; Gomes, R.C.; Lima, T.C. Essential Oils: Chemistry and Pharmacological Activities. Biomolecules 2023, 13, 1144. [Google Scholar] [CrossRef] [PubMed]
- Raina, R.; Verma, P.K.; Peshin, R.; Kour, H. Potential of Juniperus Communis L as a Nutraceutical in Human and Veterinary Medicine. Heliyon 2019, 5, e02376. [Google Scholar] [CrossRef] [PubMed]
- Benali, T.; Bouyahya, A.; Habbadi, K.; Zengin, G.; Khabbach, A.; Achbani, E.H.; Hammani, K. Chemical Composition and Antibacterial Activity of the Essential Oil and Extracts of Cistus Ladaniferus Subsp. Ladanifer and Mentha Suaveolens against Phytopathogenic Bacteria and Their Ecofriendly Management of Phytopathogenic Bacteria. Biocatal. Agric. Biotechnol. 2020, 28, 101696. [Google Scholar] [CrossRef]
- Dosoky, N.S.; Setzer, W.N. Biological Activities and Safety of Citrus Spp. Essential Oils. Int. J. Mol. Sci. 2018, 19, 1966. [Google Scholar] [CrossRef]
- Leyva-López, N.; Gutiérrez-Grijalva, E.; Vazquez-Olivo, G.; Heredia, J. Essential Oils of Oregano: Biological Activity beyond Their Antimicrobial Properties. Molecules 2017, 22, 989. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.-Y.; Jiang, J.-G.; Zhu, W.; Ou-Yang, Q. Anti-Inflammatory Effect of Essential Oil from Citrus Aurantium L. Var. Amara Engl. J. Agric. Food Chem. 2017, 65, 8586–8594. [Google Scholar] [CrossRef]
- Rivera, D.; Verde, A.; Fajardo, J.; Obón, C.; Consuegra, V.; García-Botía, J.; Ríos, S.; Alcaraz, F.; Valdés, A.; Moral, A.D.; et al. Ethnopharmacology in the Upper Guadiana River Area (Castile-La Mancha, Spain). J. Ethnopharmacol. 2019, 241, 111968. [Google Scholar] [CrossRef] [PubMed]
- Bouabidi, M.; Salamone, F.L.; Gadhi, C.; Bouamama, H.; Speciale, A.; Ginestra, G.; Pulvirenti, L.; Siracusa, L.; Nostro, A.; Cristani, M. Efficacy of Two Moroccan Cistus Species Extracts against Acne Vulgaris: Phytochemical Profile, Antioxidant, Anti-Inflammatory and Antimicrobial Activities. Molecules 2023, 28, 2797. [Google Scholar] [CrossRef]
- Zalegh, I.; Akssira, M.; Bourhia, M.; Mellouki, F.; Rhallabi, N.; Salamatullah, A.M.; Alkaltham, M.S.; Khalil Alyahya, H.; Mhand, R.A. A Review on Cistus Sp.: Phytochemical and Antimicrobial Activities. Plants 2021, 10, 1214. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.; Tab, N.; Karim, A.; Mekhfi, H.; Bnouham, M.; Ziyyat, A.; Melhaoui, A.; Legssyer, A. Relaxant Effect of Aqueous Extract of Cistus Ladaniferus on Rodent Intestinal Contractions. Fitoterapia 2006, 77, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Belmokhtar, M.; Bouanani, N.E.; Ziyyat, A.; Mekhfi, H.; Bnouham, M.; Aziz, M.; Matéo, P.; Fischmeister, R.; Legssyer, A. Antihypertensive and Endothelium-Dependent Vasodilator Effects of Aqueous Extract of Cistus Ladaniferus. Biochem. Biophys. Res. Commun. 2009, 389, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Guinoiseau, E.; Luciani, A.; Serra, D.D.R.; Quilichini, Y.; Berti, L.; Lorenzi, V. Primary Mode of Action of <I>Cistus Ladaniferus</I> L. Essential Oil Active Fractions on <I>Staphylococcus Aureus</I> Strain. Adv. Microbiol. 2015, 05, 881–890. [Google Scholar] [CrossRef]
- De Moraes Pultrini, A.; Almeida Galindo, L.; Costa, M. Effects of the Essential Oil from Citrus Aurantium L. in Experimental Anxiety Models in Mice. Life Sci. 2006, 78, 1720–1725. [Google Scholar] [CrossRef]
- Azanchi, T.; Shafaroodi, H.; Asgarpanah, J. Anticonvulsant Activity of Citrus Aurantium Blossom Essential Oil (Neroli): Involvment of the GABAergic System. Nat. Prod. Commun. 2014, 9, 1615–1618. [Google Scholar]
- Gumral, N.; Kumbul, D.D.; Aylak, F.; Saygin, M.; Savik, E. Juniperus Communis Linn Oil Decreases Oxidative Stress and Increases Antioxidant Enzymes in the Heart of Rats Administered a Diet Rich in Cholesterol. Toxicol. Ind. Health 2015, 31, 85–91. [Google Scholar] [CrossRef]
- Akdogan, M.; Koyu, A.; Çiriş, M.; Yildiz, K. Anti-Hypercholesterolemic Activity of Juniperus Communis Lynn Oil in Rats: A Biochemical and Histopathological Investigation. 2012, 23, 321–328.
- Lombrea, A.; Antal, D.; Ardelean, F.; Avram, S.; Pavel, I.Z.; Vlaia, L.; Mut, A.-M.; Diaconeasa, Z.; Dehelean, C.A.; Soica, C.; et al. A Recent Insight Regarding the Phytochemistry and Bioactivity of Origanum Vulgare L. Essential Oil. Int. J. Mol. Sci. 2020, 21, 9653. [Google Scholar] [CrossRef] [PubMed]
- Kovačević, Z.; Kladar, N.; Čabarkapa, I.; Radinović, M.; Maletić, M.; Erdeljan, M.; Božin, B. New Perspective of Origanum Vulgare L. and Satureja Montana L. Essential Oils as Bovine Mastitis Treatment Alternatives. Antibiotics 2021, 10, 1460. [Google Scholar] [CrossRef] [PubMed]
- Fratini, F.; Giusti, M.; Mancini, S.; Pisseri, F.; Najar, B.; Pistelli, L. Evaluation of the in Vitro Antibacterial Activity of Some Essential Oils and Their Blends against Staphylococcus Spp. Isolated from Episodes of Sheep Mastitis. Rendiconti Lincei Sci. Fis. E Nat. 2021, 32, 407–416. [Google Scholar] [CrossRef]
- Naccari, V.; Orlandella, B.M.; Naccari, C. Effectiveness of Thymus Vulgaris Essential Oil on Ovine Mammary Pustular Dermatitis. Atti Della Accad. Peloritana Dei Pericolanti - Cl. Sci. Medico-Biol. 2019, 107, 1–8. [Google Scholar] [CrossRef]
- Vaillancourt, K.; LeBel, G.; Yi, L.; Grenier, D. In Vitro Antibacterial Activity of Plant Essential Oils against Staphylococcus Hyicus and Staphylococcus Aureus, the Causative Agents of Exudative Epidermitis in Pigs. Arch. Microbiol. 2018, 200, 1001–1007. [Google Scholar] [CrossRef]
- Amat, S.; Magossi, G.; Rakibuzzaman, A.; Holman, D.B.; Schmidt, K.N.; Kosel, L.; Ramamoorthy, S. Screening and Selection of Essential Oils for an Intranasal Spray against Bovine Respiratory Pathogens Based on Antimicrobial, Antiviral, Immunomodulatory, and Antibiofilm Activities. Front. Vet. Sci. 2024, 11, 1360398. [Google Scholar] [CrossRef] [PubMed]
- Braga Paiano, R.; Bonilla, J.; Moro De Sousa, R.L.; Micke Moreno, A.; Sampaio Baruselli, P. Chemical Composition and Antibacterial Activity of Essential Oils against Pathogens Often Related to Cattle Endometritis. J. Infect. Dev. Ctries. 2020, 14, 177–183. [Google Scholar] [CrossRef]
- Naccari, C.; Cicero, N.; Orlandella, B.M.; Naccari, V.; Palma, E. Antimicrobial Activity of Essential Oils ( Citrus Bergamia Risso & Poiteau, Melaleuca Alternifolia and Chenopodium Botrys) on Pathogen Strains Isolated in Milk Samples from Mastitic Sheep. Nat. Prod. Res. 2024, 1–7. [Google Scholar] [CrossRef]
- Diniz, A.F.; Santos, B.; Nóbrega, L.M.M.O.; Santos, V.R.L.; Mariz, W.S.; Cruz, P.S.C.; Nóbrega, R.O.; Silva, R.L.; Paula, A.F.R.; Santos, J.R.D.A.; et al. Antibacterial Activity of Thymus Vulgaris (Thyme) Essential Oil against Strains of Pseudomonas Aeruginosa, Klebsiella Pneumoniae and Staphylococcus Saprophyticus Isolated from Meat Product. Braz. J. Biol. 2023, 83, e275306. [Google Scholar] [CrossRef]
- Ebani, V.V.; Mancianti, F. Use of Essential Oils in Veterinary Medicine to Combat Bacterial and Fungal Infections. Vet. Sci. 2020, 7, 193. [Google Scholar] [CrossRef]
- Vavala, E.; Passariello, C.; Pepi, F.; Colone, M.; Garzoli, S.; Ragno, R.; Pirolli, A.; Stringaro, A.; Angiolella, L. Antibacterial Activity of Essential Oils Mixture against PSA. Nat. Prod. Res. 2016, 30, 412–418. [Google Scholar] [CrossRef]
- Iseppi, R.; Condò, C.; Messi, P. Synergistic Inhibition of Methicillin-Resistant Staphylococcus Aureus (MRSA) by Melaleuca Alternifolia Chell (Tea Tree) and Eucalyptus Globulus Labill. Essential Oils in Association with Oxacillin. Antibiotics 2023, 12, 846. [Google Scholar] [CrossRef]
- Sateriale, D.; Forgione, G.; De Cristofaro, G.A.; Facchiano, S.; Boscaino, F.; Pagliuca, C.; Colicchio, R.; Salvatore, P.; Paolucci, M.; Pagliarulo, C. Towards Green Strategies of Food Security: Antibacterial Synergy of Essential Oils from Thymus Vulgaris and Syzygium Aromaticum to Inhibit Escherichia Coli and Staphylococcus Aureus Pathogenic Food Isolates. Microorganisms 2022, 10, 2446. [Google Scholar] [CrossRef] [PubMed]
- Acharya, V.V.; Chaudhuri, P. Modalities of Protein Denaturation and Nature of Denaturants. Int. J. Pharm. Sci. Rev. Res. 2021, 69. [Google Scholar] [CrossRef]
- Silvestrini, B.; Silvestrini, M. Medical Implications of the Relationships among Protein Denaturation, Necrosis and Inflammation: An Intriguing Story. In Tendons - Trauma, Inflammation, Degeneration, and Treatment; Rosenberg, N., Ed.; IntechOpen, 2023 ISBN 978-1-83768-185-3.
- Agarwal, P.; Sebghatollahi, Z.; Kamal, M.; Dhyani, A.; Shrivastava, A.; Singh, K.K.; Sinha, M.; Mahato, N.; Mishra, A.K.; Baek, K.-H. Citrus Essential Oils in Aromatherapy: Therapeutic Effects and Mechanisms. Antioxidants 2022, 11, 2374. [Google Scholar] [CrossRef]
- De Carvalho, F.O.; Silva, É.R.; Gomes, I.A.; Santana, H.S.R.; Do Nascimento Santos, D.; De Oliveira Souza, G.P.; De Jesus Silva, D.; Monteiro, J.C.M.; De Albuquerque Júnior, R.L.C.; De Souza Araújo, A.A.; et al. Anti-inflammatory and Antioxidant Activity of Carvacrol in the Respiratory System: A Systematic Review and Meta-analysis. Phytother. Res. 2020, 34, 2214–2229. [Google Scholar] [CrossRef]
- Salehi, B.; Upadhyay, S.; Erdogan Orhan, I.; Kumar Jugran, A.; L.D. Jayaweera, S.; A. Dias, D.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic Potential of α- and β-Pinene: A Miracle Gift of Nature. Biomolecules 2019, 9, 738. [CrossRef]
- Chen, X.; Ding, Y.; Guan, H.; Zhou, C.; He, X.; Shao, Y.; Wang, Y.; Wang, N.; Lv, G.; Chen, S.-H. The Pharmacological Effects and Potential Applications of Limonene From Citrus Plants: A Review. Nat. Prod. Commun. 2024, 19. [Google Scholar] [CrossRef]
- Nostro, A.; Blanco, A.R.; Cannatelli, M.A.; Enea, V.; Flamini, G.; Morelli, I.; Sudano Roccaro, A.; Alonzo, V. Susceptibility of Methicillin-Resistant Staphylococci to Oregano Essential Oil, Carvacrol and Thymol. FEMS Microbiol. Lett. 2004, 230, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Sela, F.; Karapandzova, M.; Stefkov, G.; Cvetkovikj, I.; Trajkovska-Dokik, E.; Kaftandzieva, A.; Kulevanova, S. Chemical Composition and Antimicrobial Activity of Leaves Essential Oil of Juniperus Communis (Cupressaceae) Grown in Republic of Macedonia. Maced. Pharm. Bull. 2013, 59, 23–32. [Google Scholar] [CrossRef]
- Ellouze, I.; Abderrabba, M.; Sabaou, N.; Mathieu, F.; Lebrihi, A.; Bouajila, J. Season’s Variation Impact on Citrus Aurantium Leaves Essential Oil: Chemical Composition and Biological Activities. J. Food Sci. 2012, 77. [Google Scholar] [CrossRef] [PubMed]
- Suntar, I.; Khan, H.; Patel, S.; Celano, R.; Rastrelli, L. An Overview on Citrus Aurantium L.: Its Functions as Food Ingredient and Therapeutic Agent. Oxid. Med. Cell. Longev. 2018, 2018, 7864269. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef] [PubMed]
- Rúa, J.; Del Valle, P.; De Arriaga, D.; Fernández-Álvarez, L.; García-Armesto, M.R. Combination of Carvacrol and Thymol: Antimicrobial Activity Against Staphylococcus Aureus and Antioxidant Activity. Foodborne Pathog. Dis. 2019, 16, 622–629. [Google Scholar] [CrossRef]
- Silva, F.V.; Guimarães, A.G.; Silva, E.R.S.; Sousa-Neto, B.P.; Machado, F.D.F.; Quintans-Júnior, L.J.; Arcanjo, D.D.R.; Oliveira, F.A.; Oliveira, R.C.M. Anti-Inflammatory and Anti-Ulcer Activities of Carvacrol, a Monoterpene Present in the Essential Oil of Oregano. J. Med. Food 2012, 15, 984–991. [Google Scholar] [CrossRef]
- Landa, P.; Kokoska, L.; Pribylova, M.; Vanek, T.; Marsik, P. In Vitro Anti-Inflammatory Activity of Carvacrol: Inhibitory Effect on COX-2 Catalyzed Prostaglandin E2 Biosynthesisb. Arch. Pharm. Res. 2009, 32, 75–78. [Google Scholar] [CrossRef]
- Caelli, M.; Porteous, J.; Carson, C.F.; Heller, R.; Riley, T.V. Tea Tree Oil as an Alternative Topical Decolonization Agent for Methicillin-Resistant Staphylococcus Aureus. J. Hosp. Infect. 2000, 46, 236–237. [Google Scholar] [CrossRef] [PubMed]
- Edwards-Jones, V.; Buck, R.; Shawcross, S.G.; Dawson, M.M.; Dunn, K. The Effect of Essential Oils on Methicillin-Resistant Staphylococcus Aureus Using a Dressing Model. Burns 2004, 30, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Tang, X.; Dong, W.; Sun, N.; Yuan, W. A Review of Biofilm Formation of Staphylococcus Aureus and Its Regulation Mechanism. Antibiotics 2022, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Baj, T.; Kowalska, G.; Kowalski, R.; Szymańska, J.; Kai, G.; Coutinho, H.D.M.; Sieniawska, E. Synergistic Antioxidant Activity of Four—Component Mixture of Essential Oils: Basil, Cedarwood, Citronella and Thyme for the Use as Medicinal and Food Ingredient. Antioxidants 2023, 12, 577. [Google Scholar] [CrossRef]
- Mapeka, T.M.; Sandasi, M.; Viljoen, A.M.; Van Vuuren, S.F. Optimization of Antioxidant Synergy in a Polyherbal Combination by Experimental Design. Molecules 2022, 27, 4196. [Google Scholar] [CrossRef] [PubMed]
- Crespo, Y.A.; Bravo Sánchez, L.R.; Quintana, Y.G.; Cabrera, A.S.T.; Bermúdez Del Sol, A.; Mayancha, D.M.G. Evaluation of the Synergistic Effects of Antioxidant Activity on Mixtures of the Essential Oil from Apium Graveolens L., Thymus Vulgaris L. and Coriandrum Sativum L. Using Simplex-Lattice Design. Heliyon 2019, 5, e01942. [Google Scholar] [CrossRef] [PubMed]
- Benyoucef, F.; Dib, M.E.A.; Arrar, Z.; Costa, J.; Muselli, A. Synergistic Antioxidant Activity and Chemical Composition of Essential Oils From Thymus Fontanesii, Artemisia Herba-Alba and Rosmarinus Officinalis. J. Appl. Biotechnol. Rep. 2018, 5, 151–156. [Google Scholar] [CrossRef]
- Sharma, K.; Guleria, S.; Razdan, V.K.; Babu, V. Synergistic Antioxidant and Antimicrobial Activities of Essential Oils of Some Selected Medicinal Plants in Combination and with Synthetic Compounds. Ind. Crops Prod. 2020, 154, 112569. [Google Scholar] [CrossRef]
- Padilla-Camberos, E.; Sanchez-Hernandez, I.M.; Torres-Gonzalez, O.R.; Gallegos-Ortiz, M.D.R.; Méndez-Mona, A.L.; Baez-Moratilla, P.; Flores-Fernandez, J.M. Natural Essential Oil Mix of Sweet Orange Peel, Cumin, and Allspice Elicits Anti-Inflammatory Activity and Pharmacological Safety Similar to Non-Steroidal Anti-Inflammatory Drugs. Saudi J. Biol. Sci. 2022, 29, 3830–3837. [Google Scholar] [CrossRef]
- Djouahri, A.; Saka, B.; Boudarene, L.; Benseradj, F.; Aberrane, S.; Aitmoussa, S.; Chelghoum, C.; Lamari, L.; Sabaou, N.; Baaliouamer, A. In Vitro Synergistic/Antagonistic Antibacterial and Anti-Inflammatory Effect of Various Extracts/Essential Oil from Cones of Tetraclinis Articulata (Vahl) Masters with Antibiotic and Anti-Inflammatory Agents. Ind. Crops Prod. 2014, 56, 60–66. [Google Scholar] [CrossRef]
- Arooj, B.; Asghar, S.; Saleem, M.; Khalid, S.H.; Asif, M.; Chohan, T.; Khan, I.U.; Zubair, H.M.; Yaseen, H.S. Anti-Inflammatory Mechanisms of Eucalyptol Rich Eucalyptus Globulus Essential Oil Alone and in Combination with Flurbiprofen. Inflammopharmacology 2023, 31, 1849–1862. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Virgous, C.; Si, H. Synergistic Anti-Inflammatory Effects and Mechanisms of Combined Phytochemicals. J. Nutr. Biochem. 2019, 69, 19–30. [Google Scholar] [CrossRef]
- Basavegowda, N.; Baek, K.-H. Synergistic Antioxidant and Antibacterial Advantages of Essential Oils for Food Packaging Applications. Biomolecules 2021, 11, 1267. [Google Scholar] [CrossRef] [PubMed]
- Bassolé, I.H.N.; Juliani, H.R. Essential Oils in Combination and Their Antimicrobial Properties. Molecules 2012, 17, 3989–4006. [Google Scholar] [CrossRef] [PubMed]
- Lambert, R.J.W.; Skandamis, P.N.; Coote, P.J.; Nychas, G.-J.E. A Study of the Minimum Inhibitory Concentration and Mode of Action of Oregano Essential Oil, Thymol and Carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar] [CrossRef]
- De Azeredo, G.A.; Stamford, T.L.M.; Nunes, P.C.; Gomes Neto, N.J.; De Oliveira, M.E.G.; De Souza, E.L. Combined Application of Essential Oils from Origanum Vulgare L. and Rosmarinus Officinalis L. to Inhibit Bacteria and Autochthonous Microflora Associated with Minimally Processed Vegetables. Food Res. Int. 2011, 44, 1541–1548. [Google Scholar] [CrossRef]
- Nostro, A.; Papalia, T. Antimicrobial Activity of Carvacrol: Current Progress and Future Prospectives. Recent Patents Anti-Infect. Drug Disc. 2012, 7, 28–35. [Google Scholar] [CrossRef]
- Leite-Sampaio, N.F.; Gondim, C.N.F.L.; Martins, R.A.A.; Siyadatpanah, A.; Norouzi, R.; Kim, B.; Sobral-Souza, C.E.; Gondim, G.E.C.; Ribeiro-Filho, J.; Coutinho, H.D.M. Potentiation of the Activity of Antibiotics against ATCC and MDR Bacterial Strains with (+)-α-Pinene and (-)-Borneol. BioMed Res. Int. 2022, 2022, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Jeyakumar, E.; Lawrence, R. Journey of Limonene as an Antimicrobial Agent. J. Pure Appl. Microbiol. 2021, 15, 1094–1110. [Google Scholar] [CrossRef]
- Hachlafi, N.E.; Aanniz, T.; Menyiy, N.E.; Baaboua, A.E.; Omari, N.E.; Balahbib, A.; Shariati, M.A.; Zengin, G.; Fikri-Benbrahim, K.; Bouyahya, A. In Vitro and in Vivo Biological Investigations of Camphene and Its Mechanism Insights: A Review. Food Rev. Int. 2023, 39, 1799–1826. [Google Scholar] [CrossRef]
- De Freitas, B.C.; Queiroz, P.A.; Baldin, V.P.; Do Amaral, P.H.; Rodrigues, L.L.; Vandresen, F.; R Caleffi-Ferracioli, K.; De L Scodro, R.B.; Cardoso, R.F.; Siqueira, V.L. (-)-Camphene-Based Derivatives as Potential Antibacterial Agents against Staphylococcus Aureus and Enterococcus Spp. Future Microbiol. 2020, 15, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- Ameryckx, A.; Thabault, L.; Pochet, L.; Leimanis, S.; Poupaert, J.H.; Wouters, J.; Joris, B.; Van Bambeke, F.; Frédérick, R. 1-(2-Hydroxybenzoyl)-Thiosemicarbazides Are Promising Antimicrobial Agents Targeting d-Alanine-d-Alanine Ligase in Bacterio. Eur. J. Med. Chem. 2018, 159, 324–338. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Kuang, W.; Jin, L.; Wang, R.; Niu, L.; Xie, C.; Ding, J.; Liao, Y.; Wang, L.; Wan, H.; et al. Carvacrol Protects Mice against LPS-Induced Sepsis and Attenuates Inflammatory Response in Macrophages by Modulating the ERK1/2 Pathway. Sci. Rep. 2023, 13, 12809. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.D.S.; Quintans-Júnior, L.J.; De Santana, W.A.; Martins Kaneto, C.; Pereira Soares, M.B.; Villarreal, C.F. Anti-Inflammatory Effects of Carvacrol: Evidence for a Key Role of Interleukin-10. Eur. J. Pharmacol. 2013, 699, 112–117. [Google Scholar] [CrossRef]
- Bakhtazad, S.; Ghotbeddin, Z.; Tabandeh, M.R.; Rahimi, K. Alpha-Pinene Ameliorate Behavioral Deficit Induced by Early Postnatal Hypoxia in the Rat: Study the Inflammatory Mechanism. Sci. Rep. 2024, 14, 6416. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-S.; Lee, H.-J.; Jeon, Y.-D.; Han, Y.-H.; Kee, J.-Y.; Kim, H.-J.; Shin, H.-J.; Kang, J.; Lee, B.S.; Kim, S.-H.; et al. Alpha-Pinene Exhibits Anti-Inflammatory Activity Through the Suppression of MAPKs and the NF-κB Pathway in Mouse Peritoneal Macrophages. Am. J. Chin. Med. 2015, 43, 731–742. [Google Scholar] [CrossRef]
- Bach, H.; Bach, H. Antimicrobial and Anti-Inflammatory Activities of Commercial Aromatizing Fragrances. Future Sci. OA 2021, 7, FSO704. [Google Scholar] [CrossRef]
- Kummer, R.; Fachini-Queiroz, F.C.; Estevão-Silva, C.F.; Grespan, R.; Silva, E.L.; Bersani-Amado, C.A.; Cuman, R.K.N. Evaluation of Anti-Inflammatory Activity of Citrus Latifolia Tanaka Essential Oil and Limonene in Experimental Mouse Models. Evid. Based Complement. Alternat. Med. 2013, 2013, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Elbouzidi, A.; Taibi, M.; Laaraj, S.; Loukili, E.H.; Haddou, M.; El Hachlafi, N.; Naceiri Mrabti, H.; Baraich, A.; Bellaouchi, R.; Asehraou, A.; et al. Chemical Profiling of Volatile Compounds of the Essential Oil of Grey-Leaved Rockrose (Cistus Albidus L.) and Its Antioxidant, Anti-Inflammatory, Antibacterial, Antifungal, and Anticancer Activity in Vitro and in Silico. Front. Chem. 2024, 12, 1334028. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.; De Fina, M.R.; Valentino, M.R.; Crupi, M.L.; Mondello, L. APPLICATION OF A NEW GC-MS LIBRARY, DESIGNED WITH A RETENTION INDEX FILTER TOOL, TO THE ANALYSIS OF THE ESSENTIAL OIL OF CISTUS LADANIFER. Acta Hortic. 2009, 271–276. [Google Scholar] [CrossRef]
- Weinstein, M.P.; Patel, J.B. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically: M07-A11; Documents / Clinical and Laboratory Standards Institute; 11. edition.; Committee for Clinical Laboratory Standards: Wayne, PA, 2018; ISBN 978-1-56238-836-2. [Google Scholar]
- Marini, E.; Di Giulio, M.; Magi, G.; Di Lodovico, S.; Cimarelli, M.E.; Brenciani, A.; Nostro, A.; Cellini, L.; Facinelli, B. Curcumin, an Antibiotic Resistance Breaker against a Multiresistant Clinical Isolate of Mycobacterium Abscessus. Phytother. Res. 2018, 32, 488–495. [Google Scholar] [CrossRef] [PubMed]
- EUCAST Terminology Relating to Methods for the Determination of Susceptibility of Bacteria to Antimicrobial Agents. Clin. Microbiol. Infect. 2000, 6, 503–508. [CrossRef] [PubMed]
- Mulyaningsih, S.; Sporer, F.; Zimmermann, S.; Reichling, J.; Wink, M. Synergistic Properties of the Terpenoids Aromadendrene and 1,8-Cineole from the Essential Oil of Eucalyptus Globulus against Antibiotic-Susceptible and Antibiotic-Resistant Pathogens. Phytomedicine 2010, 17, 1061–1066. [Google Scholar] [CrossRef]
- Nostro, A.; Roccaro, A.S.; Bisignano, G.; Marino, A.; Cannatelli, M.A.; Pizzimenti, F.C.; Cioni, P.L.; Procopio, F.; Blanco, A.R. Effects of Oregano, Carvacrol and Thymol on Staphylococcus Aureus and Staphylococcus Epidermidis Biofilms. J. Med. Microbiol. 2007, 56, 519–523. [Google Scholar] [CrossRef]
- Dehimi, K.; Speciale, A.; Saija, A.; Dahamna, S.; Raciti, R.; Cimino, F.; Cristani, M. Antioxidant and Anti-Inflammatory Properties of Algerian Thymelaea Microphylla Coss. and Dur. Extracts. Pharmacogn. Mag. 2016, 12, 203. [Google Scholar] [CrossRef] [PubMed]
- Chelly, S.; Chelly, M.; Occhiuto, C.; Cimino, F.; Cristani, M.; Saija, A.; Molonia, M.S.; Ruberto, G.; D’Angelo, V.; Germanò, M.P.; et al. Evaluation of Antioxidant, Anti-Inflammatory and Antityrosinase Potential of Extracts from Different Aerial Parts of Rhanterium Suaveolens from Tunisia. Chem. Biodivers. 2021, 18, e2100316. [Google Scholar] [CrossRef] [PubMed]
- Chelly, M.; Chelly, S.; Occhiuto, C.; Cimino, F.; Cristani, M.; Saija, A.; Muscarà, C.; Ruberto, G.; Speciale, A.; Bouaziz-Ketata, H.; et al. Comparison of Phytochemical Profile and Bioproperties of Methanolic Extracts from Different Parts of Tunisian Rumex Roseus. Chem. Biodivers. 2021, 18, e2100185. [Google Scholar] [CrossRef] [PubMed]
- Belkhodja; Meddah; Sidelarbi; Bouhadi; Medjadel; Brakna IN VITRO AND IN VIVO ANTI-INFLAMMATORY POTENTIAL OF EUCALYPTUS GLOBULUS ESSENTIAL OIL. 2022. [CrossRef]
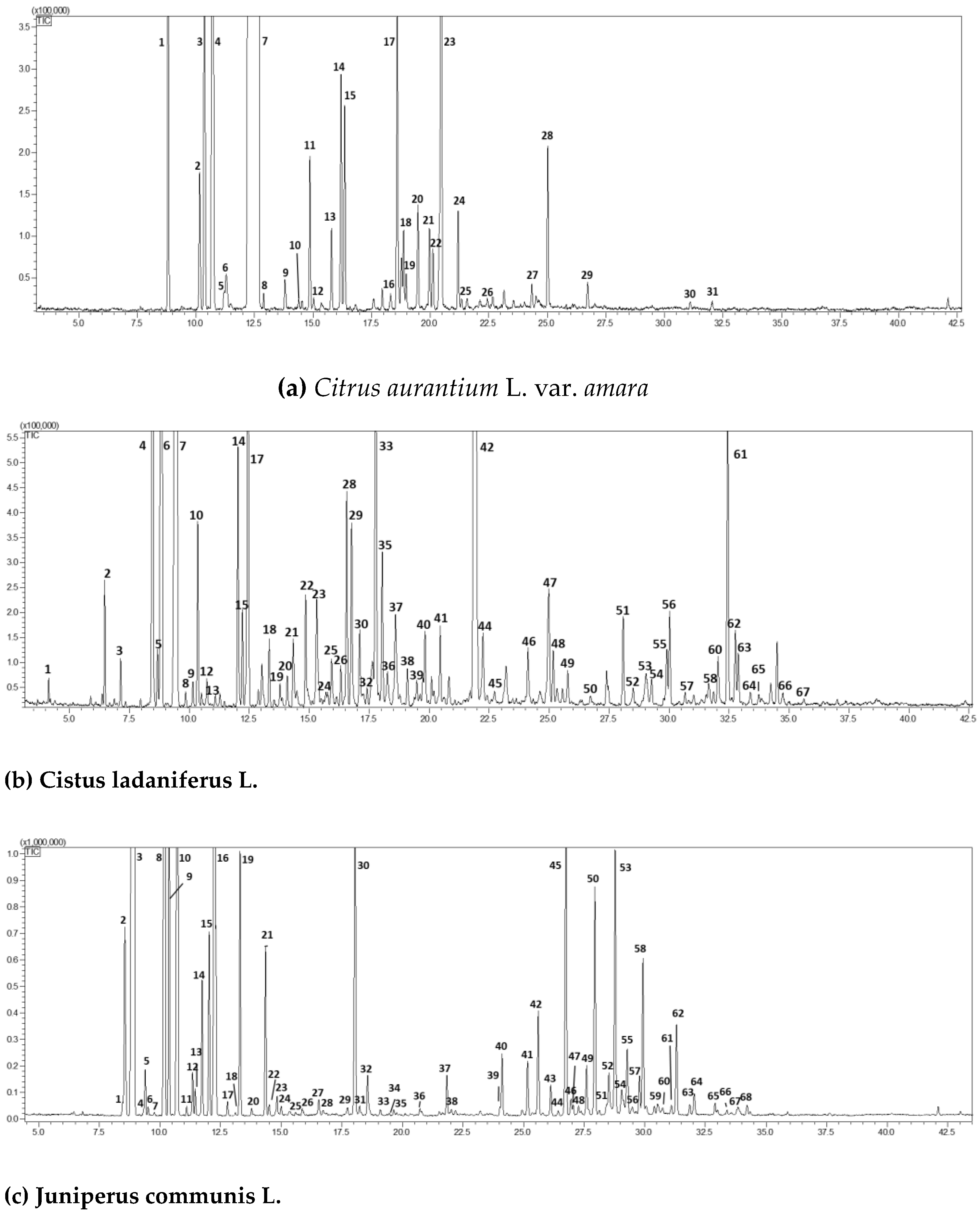
| (a) Citrus aurantium L. var. amara. | ||||||
| Peak | Compound | RI exp | RI pub | Area (%)Mean (n = 3) | std.dev | |
| 1 | a-pinene | 930 | 932 | 0.88 | 0.03 | |
| 2 | sabinene | 971 | 972 | 0.26 | 0.02 | |
| 3 | b-pinene | 973 | 974 | 1.12 | 0.13 | |
| 4 | myrcene | 990 | 991 | 3.45 | 0.11 | |
| 5 | octanal | 995 | 998 | 0.02 | 0.01 | |
| 6 | p-mentha-1(7),8-diene | 1001 | 1003 | 0.10 | 0.02 | |
| 7 | limonene | 1027 | 1030 | 87.87 | 0.55 | |
| 8 | (E)-b-ocimene | 1042 | 1044 | 0.03 | 0.01 | |
| 9 | octanol | 1060 | 1063 | 0.06 | 0.01 | |
| 10 | terpinolene | 1084 | 1086 | 0.03 | 0.01 | |
| 11 | linalool | 1095 | 1095 | 0.30 | 0.03 | |
| 12 | nonanal | 1098 | 1100 | 0.03 | 0.01 | |
| 13 | trans-p-2,8-menthadien-1-ol | 1120 | 1122 | 0.14 | 0.02 | |
| 14 | cis-limonene oxide | 1149 | 1152 | 0.45 | 0.02 | |
| 15 | trans-limonene oxide | 1158 | 1160 | 0.34 | 0.03 | |
| 16 | trans-isocarveol | 1187 | 1189 | 0.04 | 0.01 | |
| 17 | α-terpineol | 1192 | 1195 | 0.59 | 0.03 | |
| 18 | decanal | 1198 | 1201 | 0.12 | 0.02 | |
| 19 | octyl acetate | 1207 | 1211 | 0.06 | 0.01 | |
| 20 | trans-carveol | 1213 | 1215 | 0.20 | 0.03 | |
| 21 | cis-carveol | 1224 | 1226 | 0.16 | 0.01 | |
| 22 | neral | 1233 | 1235 | 0.11 | 0.01 | |
| 23 | linalyl acetate | 1250 | 1254 | 1.84 | 0.11 | |
| 24 | geranial | 1261 | 1264 | 0.18 | 0.02 | |
| 25 | perillaldehyde | 1275 | 1278 | 0.02 | 0.00 | |
| 26 | perilla alcohol | 1295 | 1299 | 0.03 | 0.00 | |
| 27 | neryl acetate | 1356 | 1359 | 0.06 | 0.01 | |
| 28 | geranyl acetate | 1378 | 1379 | 0.27 | 0.03 | |
| 29 | (E)-caryophyllene | 1416 | 1417 | 0.06 | 0.01 | |
| 30 | (E)-nerolidol | 1561 | 1561 | 0.02 | 0.00 | |
| 31 | caryophyllene oxide | 1580 | 1582 | 0.02 | 0.01 | |
| 98.84 | 0.58 | |||||
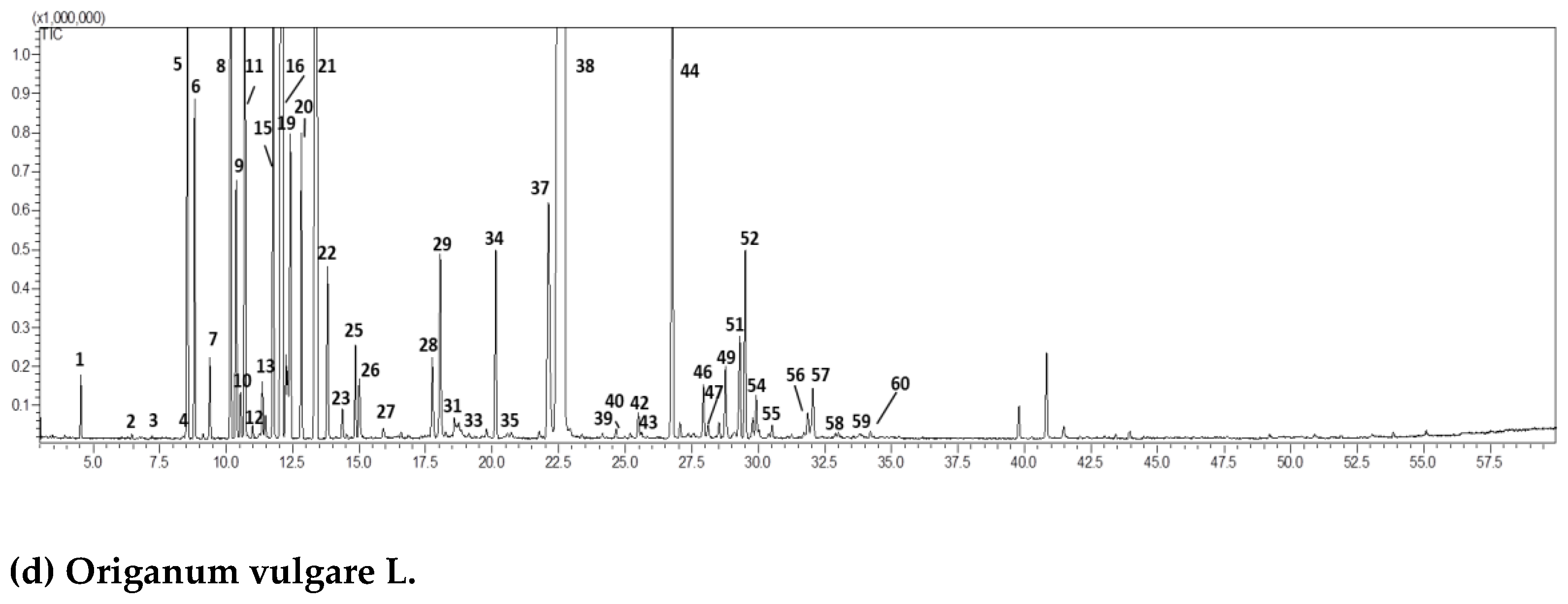
| (b) Cistus ladaniferus L. | ||||||
| Peak | Compound | RI exp | RI pub | Area (%)Mean (n = 3) | std.dev | |
| 1 | 1,2,3-trimethylcyclopentene | 828 | 822 | 0.05 | 0.00 | |
| 2 | 1,2,4,4-tetramethylcyclopentene | 882 | 895 | 0.32 | 0.02 | |
| 3 | 2-methyl-1-propenylcyclopentane | 905 | 915 | 0.13 | 0.01 | |
| 4 | tricyclene | 920 | 921 | 5.08 | 0.13 | |
| 5 | 1,3-dimethylcyclohexanol | 926 | 934 | 0.17 | 0.00 | |
| 6 | α-pinene | 932 | 932 | 13.68 | 0.31 | |
| 7 | camphene | 947 | 946 | 37.04 | 0.15 | |
| 8 | 2-methyl-1-hepten-6-one | 947 | 958 | 0.05 | 0.01 | |
| 9 | sabinene | 970 | 972 | 0.08 | 0.01 | |
| 10 | β-pinene | 975 | 974 | 0.52 | 0.03 | |
| 11 | 6-methyl-5-hepten-2-one | 989 | 986 | 0.05 | 0.01 | |
| 12 | trans-dehydroxylinalool oxide | 992 | 991 | 0.12 | 0.02 | |
| 13 | cis-dehydroxylinalool oxide | 1008 | 1006 | 0.06 | 0.00 | |
| 14 | p-cymene | 1023 | 1025 | 0.72 | 0.05 | |
| 15 | limonene | 1029 | 1030 | 0.30 | 0.02 | |
| 16 | 1,8-cineole | 1031 | 1031 | 0.04 | 0.00 | |
| 17 | 2,2,6-trimethylcyclohexanone | 1036 | 1035 | 3.95 | 0.10 | |
| 18 | seudenone | 1057 | 1055 | 0.18 | 0.04 | |
| 19 | cis-linalool oxide | 1072 | 1069 | 0.08 | 0.01 | |
| 20 | 2-methylcyclopentanone | 1078 | 1075 | 0.12 | 0.01 | |
| 21 | camphenilone | 1082 | 1078 | 0.29 | 0.03 | |
| 22 | linalool | 1098 | 1095 | 0.31 | 0.05 | |
| 23 | 3,4-dimethylcyclohexanol | 1108 | 1105 | 0.51 | 0.03 | |
| 24 | exo-fenchol | 1121 | 1118 | 0.05 | 0.00 | |
| 25 | α-campholenal | 1129 | 1126 | 0.23 | 0.02 | |
| 26 | 3-nonen-2-one | 1142 | 1137 | 0.15 | 0.01 | |
| 27 | nopinone | 1145 | 1139 | 0.04 | 0.01 | |
| 28 | trans-pinocarveol | 1147 | 1141 | 0.77 | 0.08 | |
| 29 | camphor | 1149 | 1141 | 0.73 | 0.03 | |
| 30 | camphene hydrate | 1155 | 1156 | 0.31 | 0.04 | |
| 31 | trans-pinocamphone | 1158 | 1158 | 0.05 | 0.00 | |
| 32 | isoborneol | 1166 | 1165 | 0.08 | 0.01 | |
| 33 | borneol | 1170 | 1173 | 1.65 | 0.16 | |
| 34 | cis-pinocamphone | 1178 | 1176 | 0.04 | 0.01 | |
| 35 | terpinen-4-ol | 1182 | 1180 | 0.60 | 0.08 | |
| 36 | p-cymen-8-ol | 1193 | 1189 | 0.18 | 0.03 | |
| 37 | α-terpineol + myrtenal | 1197 | 1195 | 0.58 | 0.09 | |
| 38 | verbenone | 1213 | 1208 | 0.15 | 0.01 | |
| 39 | trans-carveol | 1228 | 1223 | 0.09 | 0.01 | |
| 40 | isobornyl formate | 1235 | 1235 | 0.30 | 0.03 | |
| 41 | linalyl acetate | 1256 | 1254 | 0.25 | 0.03 | |
| 42 | bornyl acetate | 1290 | 1287 | 21.93 | 0.15 | |
| 43 | isobornyl acetate | 1293 | 1287 | 0.09 | 0.01 | |
| 44 | trans-pinocarvyl acetate | 1299 | 1296 | 0.28 | 0.02 | |
| 45 | cis-pinocarvyl acetate | 1308 | 1311 | 0.06 | 0.01 | |
| 46 | α-cubebene | 1345 | 1349 | 0.19 | 0.05 | |
| 47 | cyclosativene | 1365 | 1367 | 0.57 | 0.04 | |
| 48 | α-copaene | 1374 | 1374 | 0.17 | 0.03 | |
| 49 | sativene | 1386 | 1390 | 0.14 | 0.01 | |
| 50 | (E)-caryophyllene | 1415 | 1417 | 0.03 | 0.00 | |
| 51 | alloaromadendrene | 1462 | 1458 | 0.27 | 0.05 | |
| 52 | γ-muurolene | 1477 | 1478 | 0.08 | 0.01 | |
| 53 | viridiflorene | 1493 | 1496 | 0.22 | 0.01 | |
| 54 | α-muurolene | 1502 | 1500 | 0.14 | 0.02 | |
| 55 | δ-cadinene | 1516 | 1518 | 0.25 | 0.02 | |
| 56 | trans-calamenene | 1522 | 1521 | 0.35 | 0.03 | |
| 57 | α-calacorene | 1542 | 1544 | 0.06 | 0.01 | |
| 58 | palustrol | 1569 | 1567 | 0.14 | 0.02 | |
| 59 | spathulenol | 1572 | 1577 | 0.05 | 0.02 | |
| 60 | caryophyllene oxide | 1581 | 1582 | 0.20 | 0.02 | |
| 61 | viridiflorol | 1589 | 1592 | 1.03 | 0.06 | |
| 62 | ledol | 1596 | 1602 | 0.26 | 0.03 | |
| 63 | copaborneol | 1615 | 1613 | 0.27 | 0.04 | |
| 64 | 1-epicubenol | 1625 | 1627 | 0.06 | 0.00 | |
| 65 | α-cadinol | 1638 | 1641 | 0.05 | 0.01 | |
| 66 | cadalene | 1672 | 1675 | 0.05 | 0.01 | |
| 67 | 10-nor-Calamenen-10-one | 1699 | 1702 | 0.03 | 0.01 | |
| TOTAL | 97.08 | 0.48 | ||||
| (c) Juniperus communis L. | ||||||
| Peak | Compound | RI exp | RI pub | Area (%)Mean (n = 3) | std.dev | |
| 1 | tricyclene | 920 | 921 | 0.07 | 0.01 | |
| 2 | α-thujene | 923 | 924 | 1.35 | 0.08 | |
| 3 | α-pinene | 932 | 932 | 42.01 | 0.12 | |
| 4 | α-fenchene | 945 | 948 | 0.02 | 0.00 | |
| 5 | camphene | 947 | 946 | 0.26 | 0.03 | |
| 6 | thuja-2,4(10)-diene | 955 | 953 | 0.04 | 0.01 | |
| 7 | verbenene | 960 | 961 | 0.02 | 0.01 | |
| 8 | sabinene | 970 | 972 | 11.66 | 0.12 | |
| 9 | β-pinene | 973 | 974 | 2.38 | 0.27 | |
| 10 | myrcene | 990 | 991 | 10.72 | 0.23 | |
| 11 | δ–2-carene | 998 | 1000 | 0.05 | 0.01 | |
| 12 | α–phellandrene | 1001 | 1002 | 0.26 | 0.02 | |
| 13 | δ–3-carene | 1005 | 1008 | 0.17 | 0.02 | |
| 14 | α–terpinene | 1015 | 1018 | 0.89 | 0.08 | |
| 15 | p-cymene | 1023 | 1025 | 1.04 | 0.11 | |
| 16 | limonene | 1029 | 1030 | 6.36 | 0.17 | |
| 17 | (E)-β-ocimene | 1043 | 1044 | 0.10 | 0.02 | |
| 18 | pentyl isobutyrate | 1048 | 1049 | 0.02 | 0.01 | |
| 19 | γ-terpinene | 1054 | 1054 | 1.65 | 0.12 | |
| 20 | cis-sabinene hydrate | 1065 | 1069 | 0.04 | 0.01 | |
| 21 | terpinolene | 1083 | 1086 | 1.16 | 0.08 | |
| 22 | p-cymenene | 1087 | 1089 | 0.09 | 0.01 | |
| 23 | linalool | 1090 | 1095 | 0.17 | 0.01 | |
| 24 | isopentyl isovalerate | 1101 | 1102 | 0.09 | 0.01 | |
| 25 | β-thujone | 1117 | 1118 | 0.02 | 0.00 | |
| 26 | cis-p-menth-2-en-1-ol | 1120 | 1124 | 0.07 | 0.01 | |
| 27 | trans-pinocarveol | 1139 | 1141 | 0.16 | 0.02 | |
| 28 | trans-verbenol | 1144 | 1145 | 0.06 | 0.01 | |
| 29 | isoborneol | 1163 | 1165 | 0.08 | 0.01 | |
| 30 | terpinen-4-ol | 1171 | 1174 | 2.97 | 0.14 | |
| 31 | p-cymen-8-ol | 1178 | 1179 | 0.11 | 0.01 | |
| 32 | α-terpineol | 1183 | 1186 | 0.37 | 0.03 | |
| 33 | verbenone | 1201 | 1204 | 0.07 | 0.01 | |
| 34 | trans-carveol | 1214 | 1215 | 0.03 | 0.01 | |
| 35 | citronellol | 1220 | 1223 | 0.02 | 0.01 | |
| 36 | methyl citronellate | 1255 | 1257 | 0.07 | 0.01 | |
| 37 | isobornyl acetate | 1279 | 1283 | 0.33 | 0.02 | |
| 38 | 2-undecanone | 1291 | 1293 | 0.05 | 0.01 | |
| 39 | α-terpinyl acetate | 1344 | 1346 | 0.05 | 0.01 | |
| 40 | α-cubebene | 1342 | 1345 | 0.47 | 0.04 | |
| 41 | α-copaene | 1372 | 1374 | 0.38 | 0.02 | |
| 42 | β-elemene | 1388 | 1389 | 0.78 | 0.05 | |
| 43 | sibirene | 1398 | 1400 | 0.24 | 0.01 | |
| 44 | longifolene | 1406 | 1407 | 0.04 | 0.01 | |
| 45 | (E)-caryophyllene | 1415 | 1417 | 1.89 | 0.13 | |
| 46 | γ-elemene | 1430 | 1432 | 0.14 | 0.01 | |
| 47 | β-copaene | 1428 | 1430 | 0.08 | 0.01 | |
| 48 | cis-thujopsene | 1432 | 1433 | 0.08 | 0.01 | |
| 49 | (E)-β-farnesene | 1452 | 1452 | 0.38 | 0.03 | |
| 50 | γ-humulene | 1454 | 1454 | 1.79 | 0.09 | |
| 51 | trans-cadina-1(6),4-diene | 1472 | 1475 | 0.10 | 0.02 | |
| 52 | γ-muurolene | 1478 | 1478 | 0.44 | 0.04 | |
| 53 | germacrene D | 1481 | 1480 | 2.04 | 0.10 | |
| 54 | valencene | 1493 | 1492 | 0.23 | 0.03 | |
| 55 | bicyclogermacrene | 1501 | 1500 | 0.64 | 0.06 | |
| 56 | β-bisabolene | 1507 | 1505 | 0.09 | 0.01 | |
| 57 | γ-cadinene | 1515 | 1512 | 0.34 | 0.02 | |
| 58 | δ-cadinene | 1520 | 1518 | 1.20 | 0.12 | |
| 59 | selina-4(15),7(11)-diene | 1542 | 1540 | 0.11 | 0.02 | |
| 60 | selina-3,7(11)-diene | 1546 | 1545 | 0.10 | 0.01 | |
| 61 | (E)-nerolidol | 1563 | 1561 | 0.09 | 0.01 | |
| 62 | germacrene B | 1565 | 1559 | 0.79 | 0.06 | |
| 63 | spathulenol | 1580 | 1577 | 0.09 | 0.01 | |
| 64 | caryophyllene oxide | 1583 | 1582 | 0.20 | 0.03 | |
| 65 | humulene epoxide II | 1610 | 1608 | 0.10 | 0.01 | |
| 66 | 1-epicubenol | 1627 | 1627 | 0.04 | 0.01 | |
| 67 | τ-muurolol | 1641 | 1640 | 0.11 | 0.02 | |
| 68 | α-cadinol | 1652 | 1652 | 0.12 | 0.03 | |
| TOTAL | 98.22 | 1.80 | ||||
| (d) Origanum vulgare L. | ||||||
| Peak | Compound | RI exp | RI pub | Area (%)Mean (n = 3) | std.dev | |
| 1 | methyl 2-methylbutyrate | 768 | 769 | 0.10 | 0.02 | |
| 2 | (3Z)-hexenol | 850 | 853 | 0.01 | 0.01 | |
| 3 | 3-heptanone | 886 | 885 | 0.01 | 0.01 | |
| 4 | tricyclene | 920 | 921 | 0.01 | 0.01 | |
| 5 | α-thujene | 923 | 924 | 1.81 | 0.02 | |
| 6 | α-pinene | 932 | 932 | 0.74 | 0.07 | |
| 7 | camphene | 947 | 946 | 0.18 | 0.01 | |
| 8 | sabinene | 970 | 972 | 1.43 | 0.12 | |
| 9 | 1-octen-3-ol | 980 | 978 | 0.59 | 0.03 | |
| 10 | 3-octanone | 986 | 986 | 0.11 | 0.02 | |
| 11 | myrcene | 990 | 991 | 1.97 | 0.08 | |
| 12 | 3-octanol | 997 | 999 | 0.02 | 0.01 | |
| 13 | α–phellandrene | 1001 | 1002 | 0.16 | 0.02 | |
| 14 | δ–3-carene | 1005 | 1008 | 0.05 | 0.00 | |
| 15 | α–terpinene | 1015 | 1018 | 1.23 | 0.12 | |
| 16 | p-cymene | 1023 | 1025 | 11.06 | 0.34 | |
| 17 | limonene | 1029 | 1030 | 0.21 | 0.04 | |
| 18 | β-phellandrene | 1031 | 1031 | 0.16 | 0.03 | |
| 19 | (Z)-β-ocimene | 1033 | 1032 | 0.87 | 0.11 | |
| 20 | (E)-β-ocimene | 1043 | 1044 | 0.68 | 0.04 | |
| 21 | γ-terpinene | 1054 | 1054 | 13.71 | 0.24 | |
| 22 | cis-sabinene hydrate | 1065 | 1069 | 0.46 | 0.05 | |
| 23 | terpinolene | 1083 | 1086 | 0.08 | 0.01 | |
| 24 | p-cymenene | 1087 | 1089 | 0.02 | 0.00 | |
| 25 | linalool | 1090 | 1095 | 0.26 | 0.03 | |
| 26 | trans-sabinene hydrate | 1097 | 1098 | 0.18 | 0.02 | |
| 27 | cis-p-menth-2-en-1-ol | 1115 | 1118 | 0.02 | 0.00 | |
| 28 | borneol | 1170 | 1173 | 0.28 | 0.01 | |
| 29 | terpinen-4-ol | 1171 | 1174 | 0.57 | 0.07 | |
| 30 | p-cymen-8-ol | 1178 | 1179 | 0.01 | 0.00 | |
| 31 | α-terpineol | 1183 | 1186 | 0.18 | 0.01 | |
| 32 | (Z)-dihydrocarvone | 1210 | 1207 | 0.03 | 0.01 | |
| 33 | (E)-dihydrocarvone | 1217 | 1215 | 0.02 | 0.00 | |
| 34 | carvacryl methyl ether | 1243 | 1239 | 0.52 | 0.07 | |
| 35 | pulegone | 1245 | 1241 | 0.02 | 0.01 | |
| 36 | carvone | 1250 | 1246 | 0.03 | 0.01 | |
| 37 | thymol | 1290 | 1289 | 1.13 | 0.14 | |
| 38 | carvacrol | 1315 | 1317 | 56.43 | 0.57 | |
| 39 | α-cubebene | 1342 | 1345 | 0.02 | 0.01 | |
| 40 | carvacrol acetate | 1369 | 1370 | 0.03 | 0.01 | |
| 41 | α-copaene | 1372 | 1374 | 0.03 | 0.01 | |
| 42 | β-bourbonene | 1384 | 1382 | 0.08 | 0.01 | |
| 43 | β-elemene | 1388 | 1389 | 0.03 | 0.01 | |
| 44 | (E)-caryophyllene | 1415 | 1417 | 1.65 | 0.29 | |
| 45 | β-copaene | 1428 | 1430 | 0.05 | 0.01 | |
| 46 | α-humulene | 1454 | 1454 | 0.17 | 0.02 | |
| 47 | ε-muurolene | 1455 | 1453 | 0.05 | 0.01 | |
| 48 | γ-muurolene | 1481 | 1478 | 0.05 | 0.01 | |
| 49 | germacrene D | 1481 | 1480 | 0.28 | 0.04 | |
| 50 | γ-amorphene | 1496 | 1495 | 0.04 | 0.01 | |
| 51 | (E,E)-α-farnesene | 1505 | 1505 | 0.39 | 0.04 | |
| 52 | β-bisabolene | 1507 | 1505 | 0.60 | 0.04 | |
| 53 | γ-cadinene | 1515 | 1512 | 0.07 | 0.01 | |
| 54 | δ-cadinene | 1520 | 1518 | 0.17 | 0.03 | |
| 55 | (E)-α-bisabolene | 1542 | 1540 | 0.04 | 0.01 | |
| 56 | spathulenol | 1580 | 1577 | 0.12 | 0.04 | |
| 57 | caryophyllene oxide | 1583 | 1582 | 0.20 | 0.03 | |
| 58 | humulene epoxide II | 1610 | 1608 | 0.01 | 0.01 | |
| 59 | τ-muurolol | 1641 | 1640 | 0.02 | 0.01 | |
| 60 | α-cadinol | 1652 | 1652 | 0.03 | 0.01 | |
| TOTAL | 99.34 | 0.30 | ||||
| Strains |
Cistus Ladaniferus L. |
Citrus auratium L. var. amara |
Juniperus communis L. |
Origanum vulgare L. |
||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| (%, v/v) | ||||||||
| S. aureus ATCC 6538 | 0.25 | 0.5 | 1 | >1 | 1 | >1 | 0.0312 | 0.0625 |
| S. aureus (MRSA) ATCC 43300 | 0.5 | 1 | 1 | >1 | 1 | >1 | 0.125 | 0.25 |
| E. coli ATCC 10536 | 0.5 | 1 | 1 | >1 | 1 | >1 | 0.0625 | 0.125 |
| Strains | Checkerboard | Best combinationa | ||
|---|---|---|---|---|
| FIC | FICI | Effect | ||
|
S. aureus ATCC 6538 |
O. vulgare L./C. ladaniferus L. | 0.250/0.250 | 0.5 | Synergy |
| O. vulgare L./C. aurantium L.var. amara | 0.250/0.125 | 0.375 | Synergy | |
| O. vulgare L./J. Communis L. | 0.250/0.062 | 0.312 | Synergy | |
|
S. aureus (MRSA) ATCC 43300 |
O. vulgare L./C. ladaniferus L. | 0.5/0.125 | 0.625 | Additive |
| O. vulgare L./C. aurantium L. var. amara | 0.250/0.5 | 0.75 | Additive | |
| O. vulgare L./J. Communis L. | 0.5/0.5 | 1 | Additive | |
|
E. coli ATCC 10536 |
O. vulgare L./C. ladaniferus L. | 1/0.5 | 1.5 | Indifference |
| O. vulgare L./C. aurantium L.var. amara | 1/0.5 | 1.5 | Indifference | |
| O. vulgare L./J. Communis L. | 1/0.25 | 1.25 | Indifference | |
| Species | EC50 (mg/ml) | ||
|---|---|---|---|
| DPPH | ABTS | FRAP | |
| C. ladaniferus L. | a 804.4 ± 49.4 | a 647.1 ± 85.7 | a 954.1 ± 50.9 |
| C. aurantiumL. var. amara | b 924.2 ± 25.6 | b 1077.3 ± 65.3 | b 1062.0 ± 53.9 |
| J. communis L. | c 720.5 ± 89.7 | c 786.1 ± 18.7 | c728.8 ± 50.9 |
| O. vulgare L. | d188.5 ± 37.8 | d407.0 ± 72.4 | d556.1 ± 63.6 |
| Trolox | 149.6 ± 35.7 | 61.3 ± 8.5 | |
| FeSO4 | 66.73 ± 9.9 | ||
| Combinations | EC50 (μg/ml) | |||
|---|---|---|---|---|
| DPPH | ABTS | FRAP | ||
| O. vulgareL./C. ladaniferusL. | a 144.3 ± 33.2 | a b 291.0 ± 68.6 | a 338.8 ± 27.4 | |
| O. vulgareL./C. aurantium L. var. amara | b 275.3 ± 24.9 | b366.2 ± 50.5 | b 421.1 ± 31.3 | |
| O. vulgareL./J. communis L. | c 207.7 ± 21.6 | a 265.3 ± 30.8 | a 298.6 ± 21.7 | |
| Combination | DPPH | ABTS | FRAP | |||
|---|---|---|---|---|---|---|
| ΣFIC | Effect | ΣFIC | Effect | ΣFIC | Effect | |
| O. vulgareL./C. ladaniferusL. | 0.945 | Additive | 1.165 | Indifference | 0.964 | Additive |
| O. vulgareL./C. aurantium L. var. amara | 1.758 | Indifference | 1.240 | Indifference | 1.154 | Indifference |
| O. vulgareL./J. communis L. | 1.390 | Indifference | 0.989 | Additive | 0.947 | Additive |
| Combination | BSA | |
|---|---|---|
| ΣFIC | Interaction | |
| O. vulgare L./C. ladaniferusL. | 0.964 | Additive |
| O. vulgare L./C. aurantium L. var. amara | 1.774 | Indifference |
| O. vulgare L./J. communis L. | 0.862 | Additive |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





