Introduction
The domestic dog (Canis lupus familiaris) is, like many mammalian species, a competent host for Mycobacterium (M.) bovis infection (Snider et al. 1971; Ellis et al. 2006; Broughan et al. 2013a). Although clinical cases of tuberculosis (TB) due to infection with M. bovis, or other members of the M. tuberculosis-complex (MTBC), are considered rare in the dog and generally only occur sporadically (Broughan et al. 2013b), we previously investigated a fulminant disease outbreak caused by M. bovis infection within a large group of kennel-housed working Foxhounds in the UK (O’Halloran et al. 2018). Investigation of this outbreak was impeded in its speed, and by the lack of available ante-mortem diagnostic tests for identifying M. bovis infections in domestic dogs (Snider et al. 1971; Snider et al. 1975; Broughan et al. 2013b). The tests employed therefore had to be adapted and interpreted in real time, based on extrapolation of performance data from non-canine species.
Interferon gamma (IFN-γ) release assay (IGRA) tests have been developed on the principle of quantitatively evaluating antigen-specific IFN-γ production by peripheral circulating effector memory T-cells following in vitro stimulation (Adams 2001; de la Rua-Domenach et al. 2006; Schiller et al. 2009). The first IGRA was designed to increase the sensitivity of M. bovis TB testing in cattle (Wood et al. 1990), and the resulting BOVIGAM® assay holds World Organisation for Animal Health (WOAH) validation, with a reported in-field sensitivity in GB of 90% (Schiller et al., 2009) and specificity of 96.5% (Vordermeier et al., 2006). IGRA tests have subsequently been adapted to identify active and latent TB in human patients with at least equivalent sensitivity and increased specificity when compared to the intradermal tuberculin skin test (TST) (Kim, 2011; Zhou, 2011; Eisenhut, 2014; Thillai, 2014). Similarly, IGRA test protocols have been developed for use in domestic cats with a high sensitivity and specificity for the detection of MTBC infections (Mitchell et al., 2021) and for the detection of M. tuberculosis infections in dogs - while the same study found the intradermal TST to be unreliable in the dog (Parsons et al. 2012).
The IGRA used in this study was specifically developed to test dogs for subclinical M. bovis infection, and was based on the protocol used for testing domestic cats (Mitchell et al 2021); the antigens used were purified protein derivative (PPD) from M. avium (PPDA), PPD from M. bovis (PPDB) and a cocktail of peptides derived from the immunodominant proteins 6kDa early secreted antigenic target (ESAT-6) and 10kDa culture filtrate protein (CFP-10). PPDA is frequently used with PPDB as a comparator in both IGRA and TST tests to assess and mitigate for exposure/sensitisation to, or infection with, environmental mycobacteria species. Infection with a MTBC mycobacteria is confirmed if the IFN-γ response of an animal is greater to PPDB than to PPDA (Wood, 2001; Vordermeier et al. 2007).
The proteins ESAT-6 and CFP-10 are both encoded on the RD-1 region of most MTBC, including M. bovis, and a small number of non-tuberculous mycobacteria (NTM) (Harboe et al. 1996; Brosch et al. 2002). They are secreted proteins that form a heterodimeric complex in a 1:1 ratio which has been consistently associated with phagocyte lysis and pathogen virulence (Gao et al. 2004; Guinn et al. 2004; Junqueira-Kipins et al. 2006). The presence of a concurrent response to the synthetic peptide combination of ESAT-6/CFP-10, as well as PPDB, indicates infection with an RD-1+ MTBC mycobacteria (i.e. it excludes infection with M. microti or previous vaccination with M. bovis-BCG) (Harboe et al. 1996; Buddle et al. 2001; Brosch et al. 2002).
A number of studies have tried to improve the sensitivity and/or specificity of IGRA tests. The overall performance of an IGRA, when used to test cattle for M. bovis or humans for M. tuberculosis infection, can be increased by the measurement of additional cytokines secreted by antigen specific cells within the blood (Jones; 2010; Elnagger et al. 2017). This includes tumour necrosis factor-alpha (TNF-α), which has a well described role in the mammalian host immune response to mycobacteria (Allie et al. 2013, Leem et al. 2018).
The bovine (BOVIGAM®) and human (QuantiFERON-TB® Gold) IGRA assays are both performed on whole blood without first needing to isolate peripheral blood mononuclear cells (PBMC), as is required for IGRA testing of cats, camelids and the human T-SPOT.TB® test (Hesketh, J. B. et al. 1994; Gutierrez, M. et al. 1998; Vordermeier et al. 2007; Rhodes et al. 2008a; Igari et al. 2018; Leem et al. 2018). The use of whole blood rather than PBMC makes the former assays quicker and cheaper to conduct and so would be an ideal development for canine TB testing.
IGRA and TST assays evaluate the cell mediated adaptive immune response (CMI) of the subject which dominates the reaction to intracellular pathogens, including mycobacteria (Allie et al. 2013). However, as above, skin test responses in dogs have been shown to be unreliable (Parsons et al. 2012). Diagnostic serological assays, while generally recognised as having lower sensitivity than CMI assays, have been used both experimentally and commercially for the diagnosis of MTBC infections in a number of species (Min et al. 2011; Rhodes et al., 2012 El-Seedy et al. 2013; Casal et al. 2014; Roos et al. 2016; Waters et al., 2017, Chambers et al., 2010, Barton et al., 2023). It is recognised that combining serological and CMI diagnostic tests may provide added value by increasing infection detection, e.g. Bezos et al. (2018) showed an increase in overall diagnostic sensitivity for both cattle and goats with MTBC infections.
M. bovis is an endemic pathogen of major significance to the UK farming industry as the causative agent of bovine tuberculosis (bTB) (Vordermeier et al. 2007). In England the counties with the highest prevalence of M. bovis, as defined by the incidence of bovine infections, comprise the high-risk area (HRA), and lower but still significant disease prevalence occurs within counties of the edge area (EA). Control measures implemented for cattle infections amount to an estimated cost of £100 million annually, and while the latest government statistics show a decrease in herd incidence across GB, the number of cattle slaughtered due to bovine TB remains high and eradication challenging (DEFRA, 2024).
Non-bovine incidents are increasingly being investigated for their potential role in the epidemiology of the disease; either as possible reservoirs of infection or as sentinel species, but species-specific diagnostic tests are often lacking which impedes such investigations (Buddle et al. 2000; Delahay et al. 2002) – in the case of canids this was highlighted by the fulminant outbreak of M. bovis infection in a pack of foxhounds (O’Halloran et al., 2018).
The aim of this study was to compare available diagnostic tests and assays to samples collected from individual dogs considered to be at high, low or no risk of M. bovis infection, to gauge their potential usefulness going forward.
2. Materials and Methods
2.1. Study Dog Samples
Diagnostic blood samples from animals considered to be at high risk of sub-clinical infection with
M. bovis (high risk group, HRG) were those taken from 164 kennel-housed foxhounds during an outbreak of TB due to
M. bovis which occurred between December 2016 and July 2017 (
Table 1) (O’Halloran
et al. 2018). Whole blood and PBMC stimulation assays were conducted at the time of sampling, whilst serological assays were performed subsequently on aliquots of serum separated and frozen at -80ºC within 24 hours of collection. We also tested a total of 119 opportunistically collected blood samples (
i.e. remnant blood from samples taken for a clinical reason unrelated to this project); 77 from presumed TB-free dogs (TB-free group) and 42 dogs with some degree of potential exposure to cats or dogs with a mycobacterial infection (low risk group, LRG).
The LRG comprised 45 serum samples and 74 PBMC samples (there were no LRG dogs where both serum and PBMC were available). Remnant samples were only included in this study if, prior to blood sample collection, the dog had not been treated with immunomodulatory medications e.g. non-steroidal anti-inflammatories (NSAIDs), chemotherapeutic agents or corticosteroids within 14 days prior to sample collection. Dogs were excluded if they were pre-treated with antibiotics with efficacy against mycobacteria, including fluoroquinolones, macrolides/azides or doxycycline within the same 14 day period. Dogs were not excluded if they had been treated with antimicrobial agents if these would be ineffective against mycobacteria, such as a penicillin or cephalosporin. Similarly, dogs were not excluded if they had been treated with non-immunomodulatory analgesic medications e.g. opioids.
This study was conducted following approval from the School of Veterinary Medicine Ethical Review Committee at the University of Edinburgh; all relevant guidelines and regulations were adhered to throughout.
2.2. IGRA Using Isolated PBMC
IGRA assays for the 164 dogs in the HRG were conducted as previously reported (O’Halloran
et al. 2018). The same assay was used to test PBMC isolates from 74 dogs in the LRG (PBMC samples,
Table 1). The basic assay protocol has been validated and previously published for use in domestic cats (Mitchell et al., 2021). A sample of up to 5ml heparinised whole blood was taken from each animal and transported to the laboratory at ambient temperature within 18 hours. Upon receipt, blood was diluted 1:1 with Hanks Balanced Salt Solution (HBSS, Gibco, UK) and layered over Histopaque 1077 (Sigma, UK) before centrifugation at 800 x
g for 40 minutes at room temperature. PBMC were removed from the interface, washed with HBSS and re-suspended in complete culture media (RPMI 1640 containing 100µg/ml L-glutamine, 10% foetal bovine serum, 100µg/ml penicillin, 100U/ml streptomycin, 5x10
-5M 2-mercaptoethanol and non-essential amino acids) to 2x10
6/ml. 100μl of PBMC suspension were stimulated in duplicate with PPDA or PPDB (Lelystad, Prionics, Netherlands); both at a final dilution of 1:100, as well as a peptide cocktail of ESAT-6/CFP-10 at a final concentration of 5ug/ml (Lionex, Germany), a mitogen positive control of phorbol myristate acetate plus calcium ionophore (PMA/Ca, Sigma, UK, 50ng/ml and 1µg/ml respectively), and finally a culture medium only, i.e. unstimulated negative control was included for each animal.
Cells were incubated for four days at 37oC/5% CO2, after which the supernatants were removed, and duplicates pooled, for quantification of IFN-γ by ELISA. Supernatants were either directly assayed or stored at -80oC until required. The IFN-γ ELISA was conducted using a commercially available canine specific ELISA kit (DY781B, R&D Systems, Europe Ltd., UK) according to the manufacturer’s instructions.
Supernatant from each cell culture condition was assayed in duplicate. Optical density (OD) values were measured at a wavelength of 450nm and 630nm; the replicate OD (450nm-630nm) values for each condition were averaged to give the final OD values and standard deviations were calculated. Where replicates differed by more than 30% from the mean, the test was considered invalid.
During the foxhound outbreak, IGRA test cut-offs had been set to identify positive dogs (O’Halloran et al. 2018). It was determined that a statistically significant response to any of the three test antigens (PPDB, PPDA or ESAT6/CFP10) above the negative condition response was indicative of a biologically significant T-cell response. The responsiveness threshold for each dog was defined as the mean OD value of the sample media negative control plus two standard deviations (2SD). The mean antigen-specific response minus 2SD must exceed this for a positive test (i.e. non-overlapping 2SD). Similarly a PPDB-biased response was one where the mean PPDB response minus 2SD was greater than the mean PPDA response plus 2SD. For the test to be considered valid, the sample positive control response must also be positive by the same criteria when compared to the negative sample control. As many of the PBMC positive control and antigen specific response values were above the linear range of the test kit standard curve (recombinant IFN-γ), the OD values, rather than IFN-γ concentration, were used for test result interpretation.
2.3. TNF-α Assay Using PBMC
TNF-α was measured in stimulated (with media-only, PPDA, PPDB or ESAT-6/CFP10 and PMA/CA as above) cell supernatants from a randomly selected subset of 45 dogs from the HRG by commercial ELISA (DY1507, R&D Systems, Europe Ltd., UK) according to the manufacturer’s instructions. Since PMA/Ca stimulation did not work well as a TNF-a positive control (i.e. did not induce TNF-α in any dog sample tested, even where antigen-specific TNF-α responses were observed), concanavalin A (ConA) was used as a positive control mitogen in one supporting experiment using 3 healthy dogs (from the blood donor subset,
Table 1). PBMC were isolated and incubated in the same conditions as described above but with the addition of ConA at a final concentration of 25µg/ml. After incubation for four days at 37ºC/5% CO
2, the culture supernatant was assayed for TNF-α by ELISA. Samples were interpreted as positive where the mean antigen-specific response minus 2SD was greater than the mean media sample negative control plus 2SD. Similarly a PPDB-biased response was one where the mean PPDB response minus 2SD was greater than the mean PPDA response plus 2SD.
2.4. IGRA Using Whole Blood
To compare the diagnostic usefulness of whole blood versus PBMC stimulation methods, a whole blood assay was conducted on the same subset of 45 dogs in the HRG tested in
Section 2.3. Whole blood stimulation was performed using heparinised whole blood. One millilitre of heparinised whole blood was added to each of five separate wells of a 12-well tissue culture plate (ThermoFisher Scientific, USA) to which the following had been added; (a) 25µL phosphate-buffered saline (PBS, pH 7.2) (b) 25µL PMA/Ca (Sigma, UK) to a final concentration of 50ng/ml and 1µg/ml respectively (c) PPDA or PPDB, to a final dilution of 1:100 (Lelystad, Prionics, Netherlands), (d) 25µL of purified ESAT-6 to a final concentration of 5µg/ml and (e) 25µL of combined ESAT-6/CFP-10, to a final concentration of 5µg/ml each. The plates were incubated overnight (approximately 16 hours) at 37ºC/5% CO
2. The following day, the plates were centrifuged at 800 x
g for 15 minutes and the plasma was removed from the cellular fraction before being stored frozen at -80ºC. The concentration of IFN-γ in the samples was subsequently determined by ELISA (DY781B, R&D Systems, Europe Ltd., UK). Plasma samples from each condition were assayed in duplicate. The mean replicate OD (450-630nm) values were used to determine the concentration of IFN-γ against a recombinant canine IFN-γ standard curve (R&D Systems) and values are reported in pg/ml.
2.5. Serum Antibody Testing
DPP VetTB lateral flow assay: Sera from each of the 164 HRG dogs and the 45 sera from TB-free dogs (
Table 1) were tested using the DPP VetTB lateral flow test (Chembio Diagnostic Systems, Inc., Medfords, New York, USA) as previously reported (O’Halloran
et al. 2018). Briefly 30μl of serum was applied to a single cassette and washed across two antigen lines (MPB83 and ESAT-6/CFP-10) with the kit buffer. After 5 minutes a further buffer application was applied to wash the colloidal gold detecting reagent across the antigen and test control lines. After 15 minutes each cassette is visually inspected for QC purposes (a visible positive control line) and the antigen-specific antibody binding quantified by inserting the cassette into an optical reader (Optricon DPP Reader, Chembio) which measures the reflectance of the response produced to both antigen lines individually as relative light units (RLU).
IDEXX M. bovis ELISA: Sera from the same animals (HRG and TB-free) were also tested using the cattle Idexx M. bovis antibody (Ab) ELISA kit (Idexx Laboratories, Inc., Westbrook, ME), performed with minor modifications to detect canine antibodies. The kit secondary anti-bovine detection antibody was retained for the kit-ELISA plate positive and negative bovine controls only, while antigen-bound canine antibodies were detected using Protein-G conjugated to horseradish peroxidase (HRP) (which binds the Fc region of antibodies of numerous species). Serum samples were diluted 1:50 and added to the ELISA plate in duplicate, together with plate positive and negative controls, and ELISA plates were incubated for one hour at room temperature. Plates were washed with wash buffer (supplied with the kit), and then incubated with the secondary detection reagents (Protein-G-HRP for canine samples and kit anti-bovine reagent for plate controls) for 30 minutes at room temperature and then washed again. Substrate (supplied with kit) was added to each well (100 µL/well), the plates were developed for 15 minutes at room temperature, and the reaction was stopped by adding 100 µL/well of stop buffer (supplied with kit). Absorbance values were read at 450nm as per the manufacturer’s instructions and the mean of each sample replicate calculated as a final OD value test result.
The HRG and TB-free group results were compared by Mann Whitney U test with a p-value of less than 0.05 considered to be significant.
Comparative PPD ELISA: A comparative antibody ELISA was conducted using a method similar to that used previously for cats (Mitchell et al, 2023) ELISA test plates (NUNC Maxisorp Immmunoplate F96, Scientific Laboratory Supplies Ltd., UK) were coated with 100µL of test antigen (PPDA or PPDB) diluted to 1:100 in carbonate-bicarbonate buffer (pH 9.6, Sigma Aldrich, UK). Plates were sealed and incubated at room temperature (RT) overnight. Plates were washed with PBS containing 0.05% Tween 20 and were blocked for one hour at RT with blocking buffer comprised of 4% bovine serum albumin (BSA; Sigma Aldrich, UK) in PBS. Serum from each dog (HRG, n=163 and LRG, n=45,
Table 1) was diluted 1:50 in 2% BSA in PBS. After the plated were washed 50µL of diluted serum was added to test wells in duplicate, blocking buffer was added to two wells on each plate (blank control). Plates were sealed and incubated at RT for two hours. Plates were washed again and 50µL of detection antibody (HRP conjugated goat-anti-canine IgG; Euroimmun, Lübeck, Germnany) was added to each well and incubated for 45 minutes at RT. Following incubation, plates were again washed and 50µL of 3,3’,5,5’-tetramethylbenzidine (TMB; Sigma Aldrich, UK) added. After a 15 minute incubation, 25µL of 2M H
2SO
4 stop solution was added.
Absorbance was read at 450nm and 630nm, test results for each dog were calculated as mean OD (450-630nm) of replicate wells with the mean OD (450-630nm) of blank control wells from that plate subtracted. For the comparative test, the final OD value for PPDA was subtracted from the value for PPDB, giving an overall ΔPPD OD value. Results were compared using Mann Whitney U test with a P value of less than 0.05 considered to be significant.
2.6. Serum IFN-γ and TNF-α ELISA
Neat serum from sampled dogs (HRG, n=163 and LRG, n=45) was assayed for IFN-γ and TNF-α using commercial ELISA kits as described above (
Section 2.2 and 2.3); 50µL of serum was assayed in duplicate for each dog. The calculated OD value (mean of the replicate OD (450-630nm) values) was used to determine the concentration of IFN-γ or TNF-α respectively, against the standard curves. Values were calculated in pg/ml; the results were statistically compared by Mann Whitney U test with a P value of less than 0.05 considered to be significant.
3. Results
3.1. IGRA and TNF-α PBMC Stimulation Assays
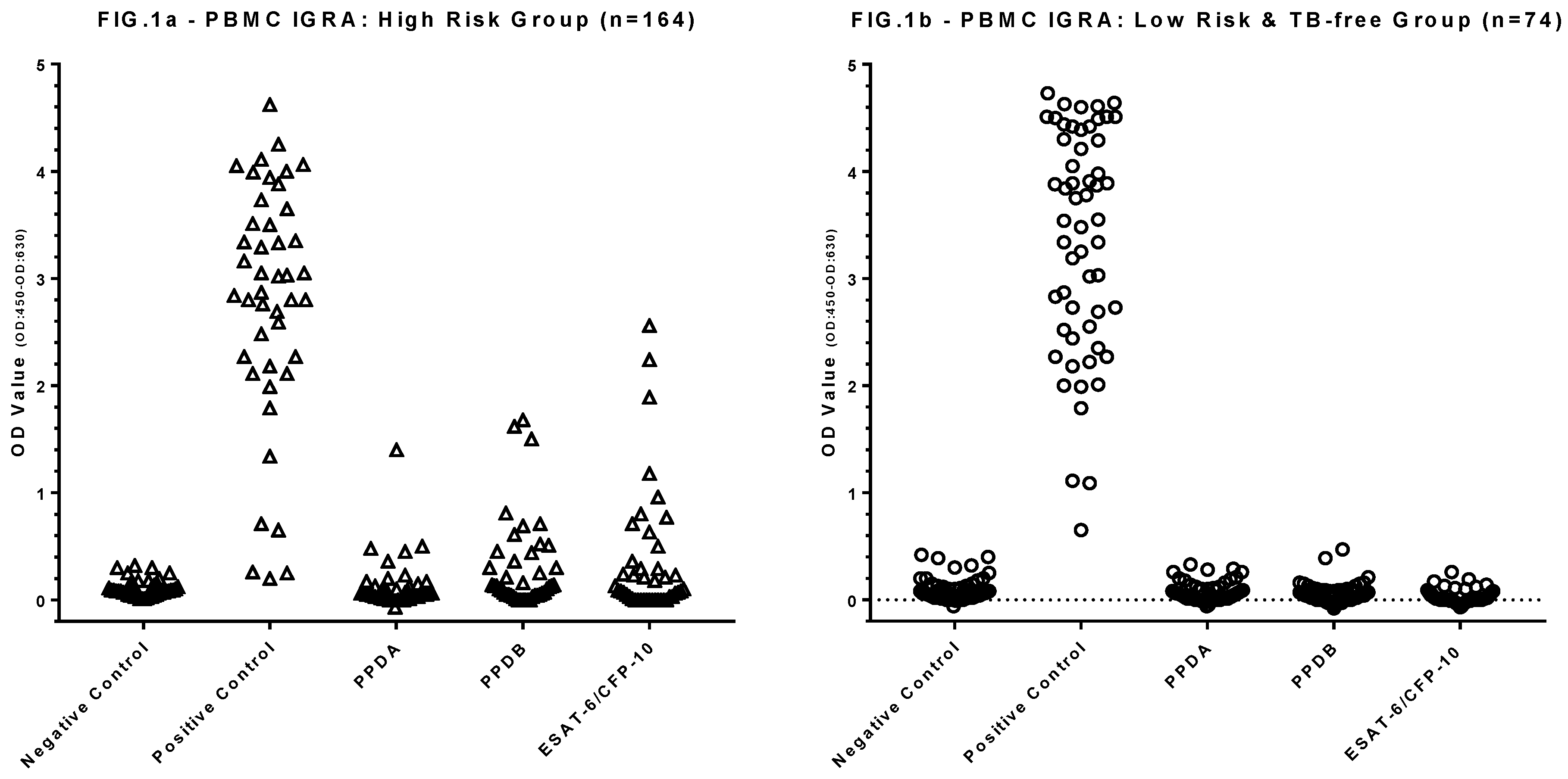
In total 164 dogs at high risk of subclinical
M. bovis infection were tested by PBMC IGRA, results are shown in
Figure 1a. Only 11 tests failed and required repetition to provide a viable result; the most frequent reason for this (n=6) was that there was a statistically insignificant response to the PMA/Ca positive control; in some cases there was clotting of the blood sample which precluded the isolation of PBMC (n=4), and one test required repetition due to poor replicate ELISA values.
Of the 164 high risk dogs, 85 (52.0%) showed positive responses to either of PPDA, PPDB or ESAT-6/CFP-10). Seventy-seven (90.1%) of these 85 dogs displayed a significant response bias to PPDB over the PPDA response, i.e. 47% of all HRG dogs tested displayed a response pattern indicative of MTBC infection. Within this group of 77 dogs, 48 (62.3%) also responded to the ESAT-6/CFP-10 peptide cocktail, i.e. a response pattern highly suggestive of M. bovis infection. In addition, five test positive individuals responded to ESAT-6/CFP-10 and no other test antigens whilst another three responded only to PPDA.
The IGRA results for the 74 LRG and TB-free dogs are shown in
Figure 1b. Among these dogs 14 (19.4%) showed positive responses to either of PPDA, PPDB or ESAT-6/CFP-10). However, in contrast to the HRG, 12 (85.7% of these 14) of these responses were biased to PPDA compared to PPDB, suggestive of exposure to/infection with environmental mycobacteria rather than MTBC species. Only two animals in this group showed a positive bias to PPDB above PPDA, indicative of MTBC infection, suggesting a test specificity of 97.3%. Neither of these dogs responded to the ESAT-6/CFP-10 peptides. Retrospective investigation identified that these two dogs had been previously cohabitant with the HRG and/or been raw fed. Were these dogs to be removed as dangerous contacts the test specificity would therefore be higher at ~100%.
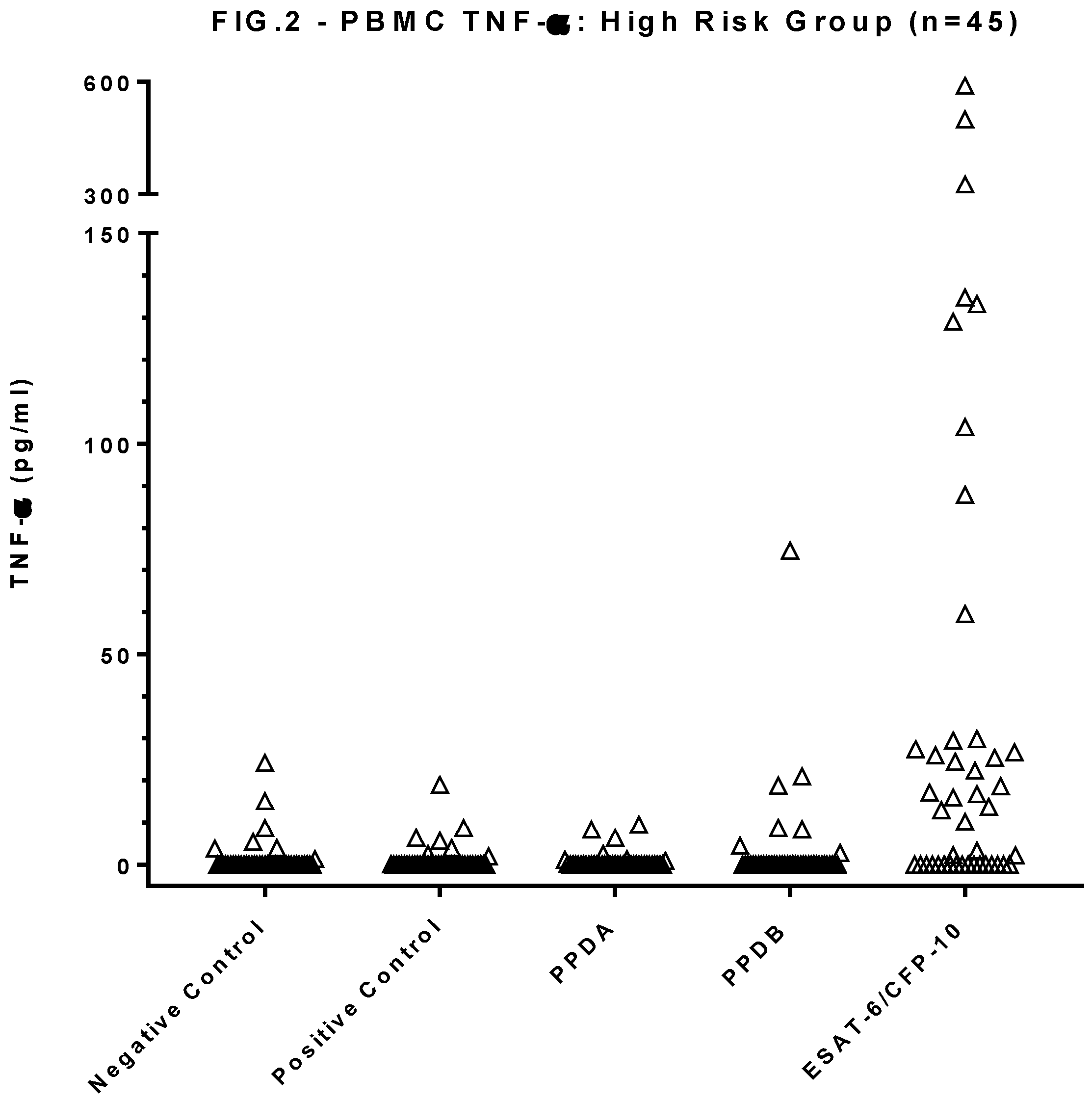
PBMC culture supernatants from 45 of the HRG dogs were assessed for antigen-specific TNF-α (
Figure 2). Three of the 45 dogs had statistically significant quantities of TNF-α in the supernatant of cells stimulated with PPDB compared to the negative control condition. The same three animals, along with an additional 25 individuals, were found to have significant levels of TNF-α in the supernatant of PBMC stimulated with the ESAT-6/CFP-10 peptide cocktail.
Comparing the concentration of ESAT6/CFP10-specific TNF-α production with the overall IGRA positivity (i.e. either a PPDB bias or positive ESAT-6/CFP-10 response), showed that these two tests, interpreted in parallel, identified 29 (64.4%) test positive and 16 test negative individuals. These tests showed very strong agreement (Cohen’s kappa coefficient, κ, =0.83).
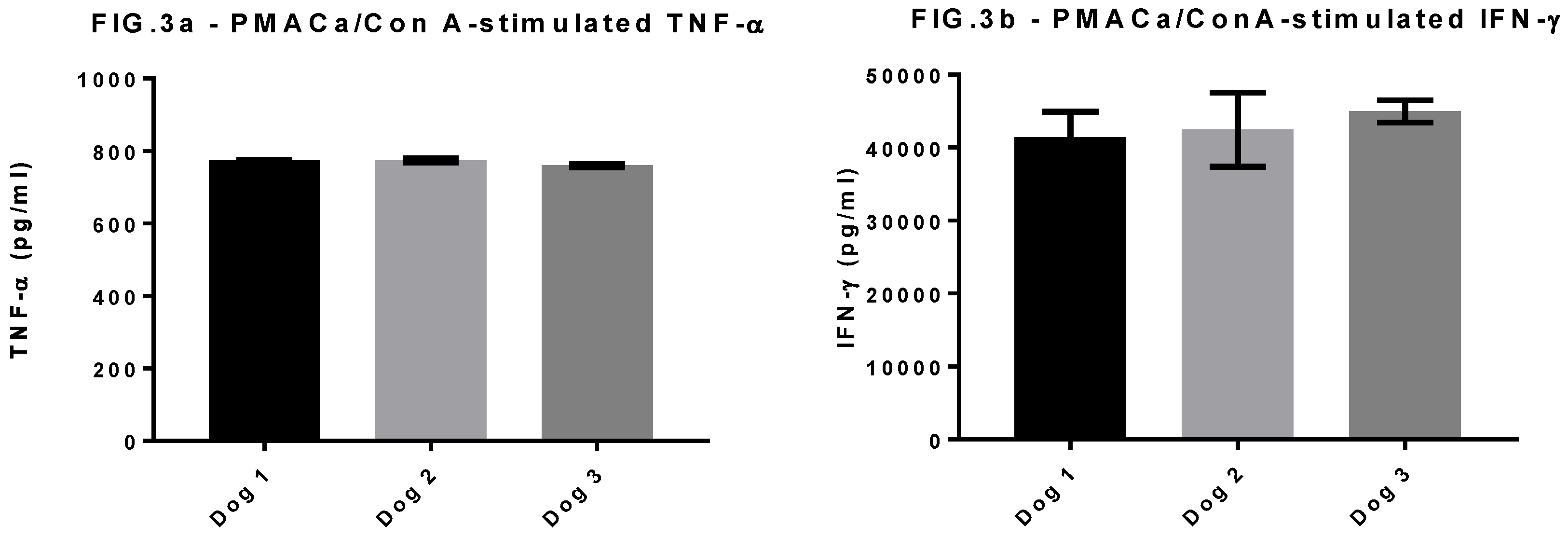
However, the mitogen PMA/Ca used as a sample positive control for the IGRA test did not stimulate canine TNF-α production. As we could not re-run the HRG dog stimulations to include an additional mitogen, we compared the IFN-γ and TNF-α production from 3 healthy dog PBMC samples stimulated with a cocktail of PMA/Ca and Concanavalin A (ConA). In this instance TNF-α and IFN-γ were both produced (
Figure 3). Therefore it was felt that the lack of TNF-α response to the PMA/Ca positive control in the HRG group did not invalidate the positive antigen-specific responses observed to PPDB and ESAT-6/CFP10, rather it indicates that the positive control was not optimal for canine samples and that future assays to measure this, and potentially other cytokines should consider various mitogen options for sample positive controls.
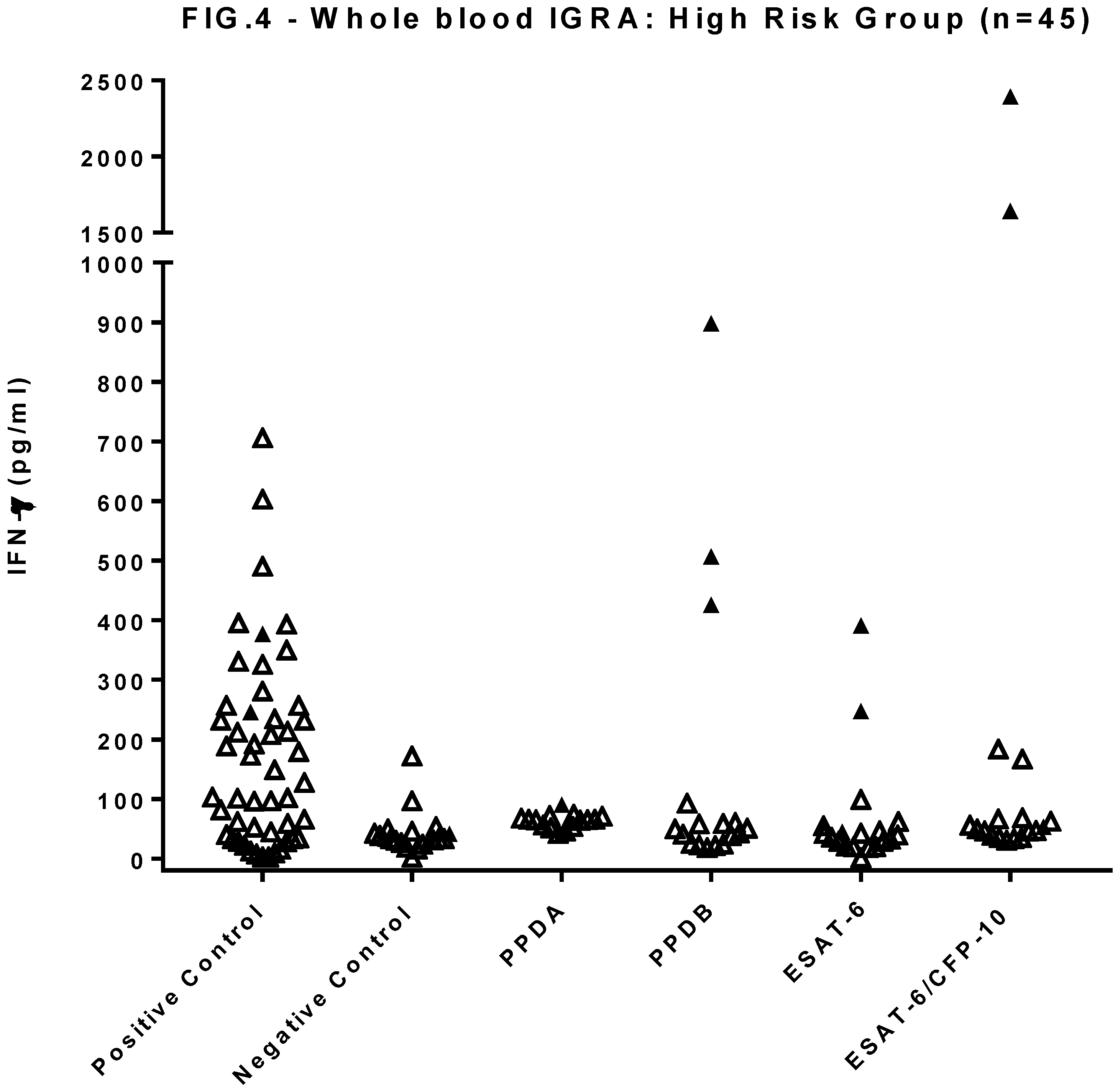
The same subset of 45 HRG dogs was tested using a whole blood IGRA (
Figure 4). Overall responses were low, with 3 individuals showing responses indicative of MTBC infection (PPDB>PPDA), two of which also showed responses to peptide cocktail and ESAT-6 protein. Comparing these results with those from the PBMC IGRA showed poor agreement between the two tests (κ= 0.2).
3.2. Serum Antibody Assays
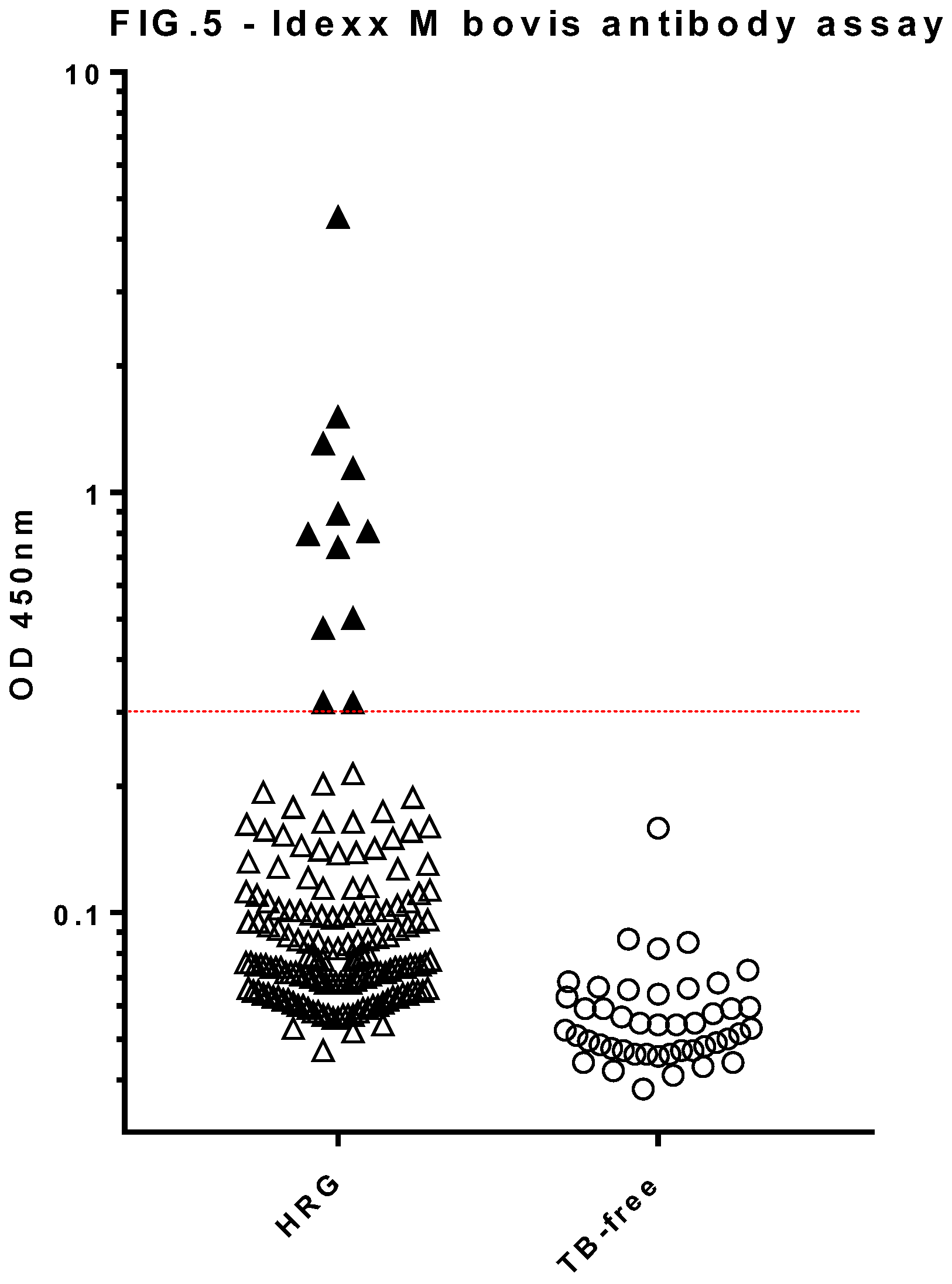
The
Idexx M. bovis Ab ELISA (
Figure 5) showed a statistically significant difference between the OD values for the HRG (n=163) and TB-free (n=45) dog groups (Mann Whitney U = 880.5, p<0.001). In deciding upon a preliminary test cut-off for the canine Idexx assay, we took into account both the cattle Idexx test protocol, and the modified Idexx test as applied to camelid TB testing at APHA, both of which approximate to an OD cut-off value of
~0.3 to provide tests of high specificity. Application of a cut off value of 0.3 to the canine Idexx test data resulted in 100% test specificity for this TB-free group, and identified 12 test-positive dogs within the HRG (7.4%).
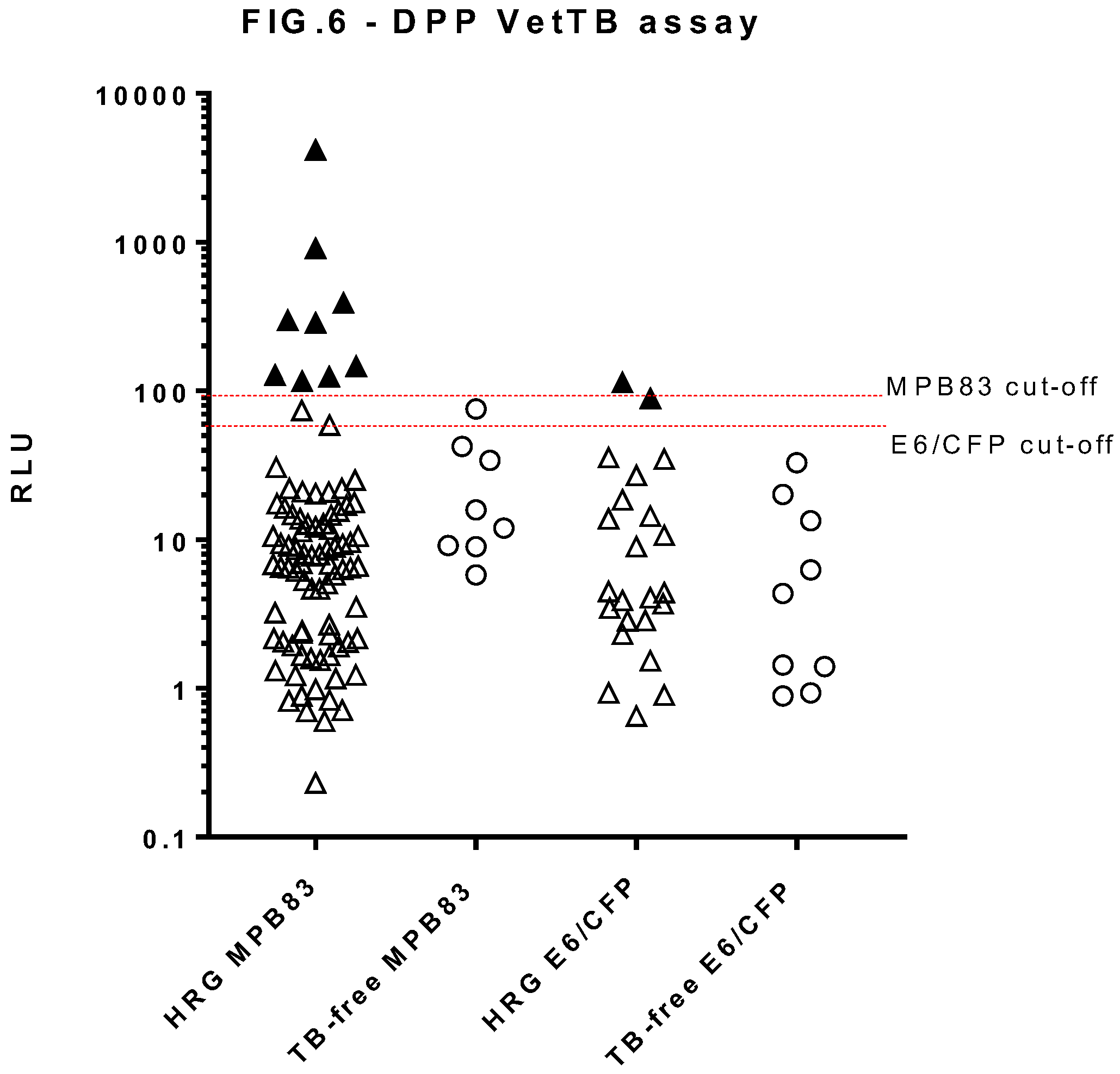
The
DPP VetTB assay (
Figure 6) showed significantly higher RLU values for the HRG compared to the TB-free dogs (Mann Whitney U = 2731, P=0.003). In deciding upon preliminary cut-offs for the MPB83 and ESAT6/CFP-10 antigens in this test, a preliminary ROC analysis for the MPB83 RLU values suggested a cut-off of >95 to provide a specificity of 100%. A similar analysis for ESAT-6/CFP-10 was not viable due to the very low number (n=2) of sero-positives; however, there was a clear divide between these two sero-positives and test-negatives, of between 40-80 RLU, therefore >60 RLU was chosen as the cut-off for the ESAT-/CFP-10 antigen line for the purpose of this study. Applying these cut-offs to the study cohorts provided a 100% specificity, and identified 11 HRG dogs (6.7%) as test-positive; 9 of these were positive to MPB83 only, and two were positive to ESAT-6/CFP-10 only.
The comparative PPD ELISA (
Figure 7) found generally higher responses to PPDA than to PPDB in the LRG population. The test cut-off was calculated as the mean OD [PPDB-PPDA] of the TB-free group plus 3 standard deviations, providing a cut-off value of 0.14. Using this threshold gave a 100% specificity and identified 27 test-positive HRG dogs (16.6%).
3.3. Serum IFN-γ ELISA & TNF-α ELISA Assays
No statistical difference was found between the quantities of either TNF-α or IFN-γ present in the peripheral circulation of the dogs comprising the HRG and LRG (data not shown).
3.4. Haematology and Serum Biochemistry
Standard haematological and serum biochemical analysis was conducted on samples collected from half (n=83) of the HRG dogs, selected at random. The only haematological abnormality identified frequently was an increase in the total leukocyte count (in 22 [26.5%] HRG dogs); in most cases this was due to the presence of a mature neutrophilia (in 18/22 of these HRG dogs) while band neutrophils were also present in a minority of cases (in 4/22 of these HRG dogs). One dog was found to have each of eosinophilia, monocytosis and reticulocytosis.
Only five dogs of the 83 HRG dogs tested (6.0%) were found to have all serum biochemical tests within the reference interval (RI). The most frequently detected abnormality was elevated serum urea which was above RI in 44/83 dogs (53.0%), three of which also had elevated bile acid concentrations. Total calcium was low in 18/83 HRG dogs (21.7%) whilst alkaline phosphatase (ALP) activity was reduced in 25/83 (30.1%) HRG dogs but increased in 6/83 HRG dogs. More than a quarter (22/83 of HRG dogs were found to have elevated globulin levels; 20 of them were elevated enough to increase the total protein concentration of the serum to greater than RI with normal albumin concentrations. No association was found between these changes and the results of the other tests.
4. Discussion
Almost every species of mammal, including the domestic dog, is susceptible to infection with MTBC organisms, including M. bovis (Brosch, 2002; Drewe, 2010; Miller, 2012; Parsons, 2013; Angkawanish, 2013). Canine disease due to M. bovis has been documented in a number of clinical case reports over many decades, with several reports demonstrating inter-species transmission, including to and from humans (Snider, 1971; Liu, 1980; Gay, 2000; Ayele, 2004; Ellis, 2006; Van Der Bürgt, 2009; Shrikrishna, 2009; Posthaus, 2011; Pesciaroli, 2014; Park, 2016, Szaluś-Jordanow, 2016;). Despite this, ante-mortem diagnostic testing for TB in the dog is limited to a single study describing the use of an IGRA for the detection of M. tuberculosis in a group of 40 dogs living in close contact with sputum-positive pulmonary TB owners (Parsons et al. 2012). To our knowledge, no other tests have been evaluated as effective for the diagnosis of canine MTBC infections; intradermal TST for both M. bovis and M. tuberculosis is unreliable, as are complement fixation and haemagglutination methods (Snider et al. 1971; Snider et al. 1975; Parsons et al. 2012 Broughan et al. 2013b).
In GB, M. bovis infection is endemic, with the highest prevalence in the counties comprising the High Risk Area of England and High TB Area of Wales, and lower but still significant prevalence within Intermediate or Edge Area counties. In these areas, species other than cattle may also be infected with M. bovis and it was from within the Edge Area that we previously reported an outbreak of M. bovis disease in a pack of kennelled foxhounds (Delahay et al. 2002, O’Halloran et al 2018). The lack of reliable diagnostic assays meant that the prevalence of subclinical M. bovis infection within the group of exposed animals was challenging to determine, and potentially led to the outbreak lasting longer, and costing more to bring under control, than if such a test or tests had been available.
To begin to address this shortcoming, we have in this study applied commercially available tests produced for other species (Idexx for cattle, DPP VetTB for cervids) with an in-house serology test, and cellular cytokine release assays (IFN-γ and TNF-α) using commercial reagents and kits, for their ability to detect subclinical infections in dogs from both high and low expected prevalence populations. All of the tests chosen evaluate the host immune response rather than relying on direct pathogen identification or isolation. Previous studies have demonstrated that the major immunological consequences of MTBC infections are broadly conserved across host species (Cousins and Florisson, 2005; Broughan, et al. 2013b). Once mycobacteria are phagocytosed, macrophages and dendritic cells present antigen from lysed bacilli to CD4+ and CD8+ αβ as well as γδ T-cell subsets in the context of cell surface MHC II molecules. The reciprocal activation between cells of these immunophenotypes, through the secretion of IFN-γ and TNF-α along with other cytokines, generates the classical cell mediated response required for the control of an intracellular pathogen.
The first diagnostic test we assessed was an adapted IGRA, identifying animals with IFN-γ producing antigen-specific T-cells. Similar to domestic cats, lion and New World Camelids, we found that it was necessary to isolate PBMC from heparinised blood samples prior to antigenic stimulation in order to reliably generate interpretable test data under these conditions (Hesketh et al. 1994; Gutierrez et al. 1998; Cousins and Florisson, 2005; Rhodes et al. 2008a; Leem et al. 2018; Rhodes et al., 2012). This was due to the failure of cells within the whole blood of these species to respond significantly to either positive control or antigen stimulation, unlike the whole blood responses observed in cattle, goats and humans (Vordermeier et al. 2006; Vordermeier et al. 2007; Bezos et al. 2018; Leem et al. 2018).
Examining the responses generated by the HRG dogs, we found that 77 (47.0%) showed a PPDB biased response indicating subclinical MTBC infection; compared to the LRG/TB-free groups where only two animals (2.7%) showed this response (interestingly, these were the two dogs most in-contact with the HRG hounds). These results suggest this test method and interpretation has a high specificity of 97.3-100%, which is within the range of 90-100% consistently reported for IGRA assays in cattle, cats and goats (Vordermeier et al. 2006; Rhodes et al. 2012; Bezos et al. 2018). Of the 77 HRG dogs showing a PPDB biased response, 62% simultaneously showed a significant response the ESAT-6/CFP-10, strongly suggestive of infection with an RD-1+ MTBC organism; this is slightly lower than the proportion reported for both cattle (~75% in the high specificity IGRA where both PPD and peptide must be positive, and 82% for peptide alone as a diagnostic, Vordermeier et al., 2016) and cats (80% but too few cats for significance, Rhodes et al., 2011). This difference may reflect a lesser immunological significance of these antigens in canine infections and/or may be an artefact of the unknown incubation time from infection to blood sampling in the HRG dogs; had infections been allowed to continue to incubate, the number of dogs responding to these peptides may have increased.
A further five dogs in the HRG only responded to the peptide cocktail ESAT-6/CFP-10. With a lack of an antigen-specific PPDB response, it is questionable whether these animals are truly infected with M. bovis or not, since some NTM (notably M. kansasii and M. leprae, among others), encode homologs of these proteins, and as the HRG in this study comprised working foxhounds there was significant potential for them to be exposed to/infected with confounding NTM. However, infected cattle are known to respond to ESAT6/CFP-10 in the absence of a positive PPD response, and in a setting of confirmed M. bovis infection such as the HRG dogs, a positive response to these peptides suggests an individual at high risk of being infected. Furthermore, no animals in the LRG/TB-free groups demonstrated ESAT-6/CFP-10 responses, despite many having similar opportunity for NTM sensitisation as the HRG. So, whilst the potential for confounding influence of NTM is important for interpreting individual animal responses, at a group level the data presented here would suggest that a response to ESAT-6/CFP-10, even alone, is sufficient to indicate high risk of M. bovis challenge.
In our previous assessment of test responses, individual dogs with a significant response to any test antigen were considered test-positive (O’Halloran et al. 2018); this included three dogs that responded to PPDA alone. Analysis of the LRG tests found that this response occurred in 12 of the 74 dogs (16.2%) suggesting that this should not be seen as indicative of being at risk of M. bovis infection. Therefore, the definition of IGRA-positive animals in the context of subclinical M. bovis infections, can be redefined to consider only individuals who show a statistically significant response above the media control to PPDB that is greater than the PPDA response, and/or those who respond significantly to the ESAT-6/CFP-10 peptide cocktail.
The diagnostic sensitivity of assessing antigen-specific TNF-α responses has only recently been tested clinically on relatively small populations of M. tuberculosis-infected people, with inconclusive results; however, it shows promise for discriminating between active and latent disease (Harari et al. 2011; Wang et al. 2013). The approach we used, combining two ELISA cytokine measurements (IFN-γ and TNF-α) on the same stimulated cell supernatants, identified additional test-positive individuals suggesting that a dual cytokine test approach may have a higher diagnostic sensitivity without the need to take any additional blood from the animals being tested. However going forward there would need to be a modification of the positive mitogen control to include ConA, since PMA/Ca alone did not stimulate canine TNF-α release.
The detection of specific antibody responses has been useful in the development of new diagnostic tests for mycobacterial infections, particularly for species where repeat capture of the same individual at a fixed time point is difficult, and therefore precludes the use of TST (Gao et al. 2007; Lesellier et al. 2008; Bezos et al. 2018) or where reagents for IFN-γ are not available, and/or an IFN-γ test might be viewed as disproportionate given the scale of problem. We therefore examined the antibody responses of dogs using a combination of serological tests already in use for other species.
As expected, the total number of test-positive animals identified by the serological assays was significantly lower than for the PBMC IGRA; 16.5%, 7.4% and 6.7% for PPD ELISA, Idexx ELISA and DPP VetTB tests respectively, compared to 52% IGRA-positive in the HRG dogs). The comparative PPD and Idexx M. bovis Ab ELISAs and the DPP VetTB assay all identified small numbers of animals not found to be positive by IGRA. Overall, there was generally weak agreement between the results of cellular and serological tests. However the simplicity of the antibody tests compared with the IGRA test does provide relative speed and a lower test cost compared to the IGRA, which could facilitate an initial blood test screening of large numbers of animals in confirmed breakdown situations, and/or provide a serological option where blood sampling and transport with PBMC remaining viable for an IGRA may not be possible.
Of the 3 antibody tests the comparative PPD ELISA found a much higher number of test-positive dogs, 27 compared to 12 (Idexx) and 11 (DPP VetTB). Whether this is due to PPD providing the largest epitope repertoire of all assays tested, or whether the test cut-off is not optimal remains to be explored. Many LRG/TB-free dogs had a PPDA-biased response resulting in a very low test-cut-off (0.14) compared to the Idexx ELISA (0.3).
Evaluating all of the test responses for each individual dog, it became clear that a small minority of individuals showed much higher responses to the tests than the rest of the test positive population on each assay. Further examination showed that this was consistently due to three individuals for the cell based and cytokine assays, and a single individual for the antibody based assays. The relevance of the magnitude of this test response is unclear. There is conflicting evidence in the scientific literature as to whether the concentration of cytokine in serum and/or produced in in vitro diagnostic assays is proportionate to the severity or progression of clinical disease in vivo (Hasan et al. 2009; Ruhwald et al. 2009; Jonnalagadda et al. 2013; Xu et al. 2018), whilst there is some evidence indicating that ESAT-6/CFP-10-specific IFN-γ responses correlate with disease severity in cattle (Vordermeier et al. 2002) and that the titre of detectable antibody correlates with the occurrence of a lesioned phenotype (Roos et al. 2016). This may be true for dogs; however, data in this study did not support this view, possibly because of to the subclinical nature of the HRG tested.
Haematological abnormalities were identified infrequently in the 83 dogs in the HRG that were tested. The high total leukocyte counts, mature and immature neutrophilia seen in a small number of dogs did not correlate with any of the mycobacteria-specific diagnostic tests; they probably reflect an unknown inflammatory stimulus in these dogs.
Serum biochemistry of the same dogs revealed multiple frequent abnormalities. The most frequent, elevated urea concentration, likely reflects the high protein diet of the working hounds as they were fed raw meat, rather than any underlying pathology. Half of the 22 dogs with hyperglobulinaemia were also found to be positive to at least one of the serology assays indicating that in some cases hyperglobulinaemia may have been related to the presence of antigen-specific antibodies. However, the overall specificity of hyperglobulinaemia with respect to the remainder of the tests was poor.
This study was unable to evaluate test sensitivity as too few confirmed infections were identified – in total seven confirmed clinical cases of M. bovis infection occurred during the course of the kennel outbreak. Due to the severity of the disease, these affected animals were euthanized on welfare grounds before diagnostic blood samples could be taken. The diagnosis of M. bovis infection in these animals was confirmed by mycobacterial culture of visibly lesioned tissues collected post-mortem in each of these cases. The rate of false negative test results (type II error) in this study is therefore also unknown.
5. Conclusion
In summary, this study demonstrates, for the first time, the utility of two cellular (IGRA and TNF-α) and three serological (DPP VetTB, Idexx and comparative PPD ELISA) assays for detecting sub-clinical M. bovis infections of dogs. Whilst the data suggest high test specificity for all assays evaluated, further work is needed to robustly validate the specificity of the tests, and investigate test sensitivity using sufficient numbers of dogs of confirmed infection status.
Acknowledgements
We wish to thank all of the staff and management team of the kennels affected by this outbreak for facilitating the investigation and giving permission for its findings to be published. Dr Shelley Rhodes was until recently a Senior Scientist at the Animal and Plant Health Agency, UK and provided technical advice throughout this study for which we are extremely grateful. One of the authors, Conor O’Halloran, was supported by a Biotechnology and Biological Sciences Research Council (BBSRC) studentship (BB/M014894/1). Jayne Hope is funded by BBSRC Institute Strategic Programme funding (BB/P013740/1 & BBS/E/D/20002174). The remaining authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
The authors declare no conflicts of interest.
References
- Adams L.G. (2001) In vivo and in vitro diagnosis of Mycobacterium bovis infection. Revue Scientifique et Technique de L’Office Internationale Des Epizooies 20, 304-324. [CrossRef]
- Ayele, W. Y., et al. (2004). Bovine tuberculosis: An old disease but a new threat to Africa. International Journal of Tuberculosis and Lung Disease 8(8): 924-937.
- Barton P. et al., (2023). Evaluation of Antibody Tests for Mycobacterium bovis Infection in Pigs and Deer. Veterinary Science 10. [CrossRef]
- Bezos J. et al. (2018) The use of serological tests in combination with the intradermal tuberculin test maximizes the detection of tuberculosis infected goats. Veterinary Immunology and Immunopathology 199:43-52. [CrossRef]
- Bezos, J., et al. (2014). Current ante-mortem techniques for diagnosis of bovine tuberculosis. Research in Veterinary Science 97(S): S44-S52. [CrossRef]
- Bossuyt, P.T. et al. (2015) STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ 351:h5527. [CrossRef]
- Brosch, R., et al. (2002). A new evolutionary scenario for the Mycobacterium tuberculosis complex. Proceedings of the National Academy of Sciences of the United States of America 99(6): 3684-3689. [CrossRef]
- Broughan, J. M., et al. (2013a). Mycobacterium bovis infections in domesticated non-bovine mammalian species. Part 1: Review of epidemiology and laboratory submissions in Great Britain 2004-2010. Veterinary Journal 198(2): 339-345. [CrossRef]
- Broughan, J. M., et al. (2013b). Mycobacterium bovis infections in domesticated non-bovine mammalian species. Part 2: A review of diagnostic methods. Veterinary Journal 198: 346-351. [CrossRef]
- Buddle B.M. et al. (2001) Use of ESAT-6 in the interferon-gamma test for diagnosis of bovine tuberculosis following skin testing. Veterinary Microbiology. 80: 37-46. [CrossRef]
- Buddle, B.M. et al (2000) Immunological approaches to the control of tuberculosis in wildlife reservoirs Veterinary Immunology and Immunopathology, 74 (1-2), pp. 1-16. [CrossRef]
- Casal, C.et al. (2014) Strategic use of serology for the diagnosis of bovine tuberculosis after intradermal skin testing. Veterinary Microbiology, 170 (3-4), pp. 342-351. [CrossRef]
- Cousins D.V., Florisson, N. (2005) A review of tests available for the use in the diagnosis of tuberculosis in non-bovine species. Revue Scientifique et Technique de L’Office Internationale Des Epizooies 24, 1039-1059.
- Department for the Environment, Farming and Rural Affairs [DEFRA] (2024) https://www.gov.uk/government/statistics/incidence-of-tuberculosis-tb-in-cattle-in-great-britain.
- De la Rua-Domenech, R., et al. (2006) Ante mortem diagnosis of tuberculosis in cattle: A review of the tuberculin tests, gamma-interferon assay and other ancillary diagnostic techniques. Research in Veterinary Science 81, 190-210. [CrossRef]
- Delahay, R.J. et al (2001) Wildlife disease reservoirs: The epidemiology of Mycobacterium bovis infection in the Eeuropean badger (Meles meles) and other British mammals. Tuberculosis, 81 (1-2), pp. 43-49. [CrossRef]
- Delahay, R.J. et al. (2003) Vaccinating badgers (Meles meles) against Mycobacterium bovis: The ecological considerations. Veterinary Journal, 166 (1), pp. 43-51. [CrossRef]
- Delahay, R.J.et al. (2002) The status of Mycobacterium bovis infection in UK wild mammals: A review. Veterinary Journal, 164 (2), pp. 90-105. [CrossRef]
- Eisenhut, M. (2014). The evidence for greater sensitivity of interferon-γ release assays compared to tuberculin skin test in diagnosing latent mycobacterium tuberculosis infection. Clinical Pediatrics 53(14): 1413. [CrossRef]
- Ellis, M. D., et al. (2006). Mycobacterium bovis infection in a dog. Veterinary Record 159(2): 46-48. [CrossRef]
- Elnaggar M.M. et al. (2017) Evaluation of antigen specific interleukin-1β as a biomarker to detect cattle infected with Mycobacterium bovis. Tuberculosis 105: 53-59. [CrossRef]
- El-Seedy, F.R. et al. (2013) The correlation between M. bovis isolation and ELISA using PPD-B and ESAT6-CFP10 mixture on the sera of tuberculin reactor cattle and buffaloes. 2013) Journal of Food, Agriculture and Environment, 11 (1), pp. 489-494. [CrossRef]
- Gao, L. Y., et al. (2004). A mycobacterial virulence gene cluster extending RD1 is required for cytolysis, bacterial spreading and ESAT-6 secretion. Molecular Microbiology 53(6): 1677-1693. [CrossRef]
- Gao, M.Q. et al. (2007) The clinical significance of serum tuberculosis specific antigen antibody in the diagnosis of tuberculosis. Chinese journal of tuberculosis and respiratory diseases, 30 (12), pp. 918-920.
- Gay, G., et al. (2000). Pulmonary mycobacterium bovis infection in a dog. New Zealand Veterinary Journal 48(3): 78-81. [CrossRef]
- Guinn, K. M., et al. (2004). Individual RD1 -region genes are required for export of ESAT-6/CFP-10 and for virulence of Mycobacterium tuberculosis. Molecular Microbiology 51(2): 359-370. [CrossRef]
- Gutierrez, M. et al. (1998) Evaluation of cellular and serological diagnostic tests for the detection of Mycobacterium bovis infected goats. Veterinary Microbiology 62: 281-290. [CrossRef]
- Harai, A. et al. (2010) Dominant TNF-alpha+ Mycobacterium tuberculosis-specific CD4+ T cell responses discriminate between latent infection and active disease. Nature Medicine 17: 372-376. [CrossRef]
- Hasan, Z. et al. (2009) Relationshp between circulating levels of IFN-γ, IL-10, CXCL9 and CCL2 in pulmonary and extrapulmonary tuberculosis is dependent on disease severity. Clinical Immunology, 69 pp. 259-267. [CrossRef]
- Hesketh, J. B. et al. (1994) Development of a diagnostic blood test for tuberculosis in alpacas (Lama pacos). New Zealand Veterinary Journal 42: 104-109. [CrossRef]
- Hope, J.C. and Villarreal-Ramos, B. (2008) Bovine TB and the development of new vaccines. Comparative Immunology, Microbiology and Infectious Diseases, 31 (2-3), pp. 77-100. [CrossRef]
- Houlihan, M. G. et al. (2008) Outbreak of bovine tuberculosis featuring anergy to the skin test, udder lesions and milkborne disease in young calves. Veterinary Record 163: 357-361. [CrossRef]
- Jonnalagadda, S.R. et al. (2013) Predictive value of interferon-gamma release assays for postpartum active tuberculosis in HIV-1-infected women. International Journal of Tuberculosis and Lung Disease, 17 (12), pp. 1552-1557+i-iv. [CrossRef]
- Junqueira-Kipnis, A. P., et al. (2006). Mycobacteria lacking the RD1 region do not induce necrosis in the lungs of mice lacking interferon-γ. Immunology 119(2): 224-231. [CrossRef]
- Kim, Y. Y., et al. (2011). Sensitivity of whole-blood interferon-gamma release assay according to the severity and the location of disease in patients with active tuberculosis. Tuberculosis and Respiratory Diseases 70(2): 125-131. [CrossRef]
- Leem, A.Y et al. (2018) Changes in cytokine responses to TB antigens ESAT-6, CFP-10 and TB 7.7 and inflammatory markers in peripheral blood during therapy(2018) Scientific Reports, 8 (1), art. no. 1159. [CrossRef]
- Leonard, D. (2017) Bovine TB controls in the UKVeterinary Record, 181 (9), p. 241. [CrossRef]
- Lesellier, S. et al. (2008) Antigen specific immunological responses of badgers (Meles meles) experimentally infected with Mycobacterium bovis. Veterinary Immunology and Immunopathology, 122 (1-2), pp. 35-45. [CrossRef]
- Liu, S., et al. (1980). Canine tuberculosis. Journal of the American Veterinary Medical Association 177(2): 164-167.
- Marassi, C.D. et al. (2014) Detection of specific antibodies in cows after injection of PPD. Pesquisa Veterinaria Brasileira, 34 (3), pp. 241-243. [CrossRef]
- Min, F. et al. (2011) Serum antibody responses to 10 Mycobacterium tuberculosis proteins, purified protein derivative, and old tuberculin in natural and experimental tuberculosis in rhesus monkeys. Clinical and Vaccine Immunology, 18 (12), pp. 2154-2160. [CrossRef]
- O’Halloran, C. et al. (2018) An outbreak of tuberculosis due to Mycobacterium bovis infection in a pack of English foxhounds (2016-2017). Transboundary and Emerging Diseases. [CrossRef]
- Office International des Epizooties [OIE] (2016) Manual of Diagnostic Tests for Aquatic Animals Chapter 1.1.2.
- Park, H. A., et al. (2016). Pulmonary Mycobacterium tuberculosis infection with giant tubercle formation in a dog: A case report. Veterinarni Medicina 61(2): 102-109. [CrossRef]
- Parsons, S. D. C., et al. (2012). Detection of Mycobacterium tuberculosis infection in dogs in a high-risk setting. Research in Veterinary Science 92(3): 414-419. [CrossRef]
- Parsons, S. D. C., et al. (2013). Novel cause of tuberculosis in meerkats, South Africa. Emerging Infectious Diseases 19(12): 2004-2007. [CrossRef]
- Pesciaroli, M., et al. (2014). Tuberculosis in domestic animal species. Research in Veterinary Science 97(S): S78-S85. [CrossRef]
- Pollock, J.M. and Neill, S.D (2002) Mycobacterium bovis infection and tuberculosis in cattle. Veterinary Journal 163: 115-127. [CrossRef]
- Posthaus, H., et al. (2011). Accidental infection of veterinary personnel with Mycobacterium tuberculosis at necropsy: A case study. Veterinary Microbiology 149(3-4): 374-380. [CrossRef]
- Rhodes, S. G., et al. (2008a). Adaptation of IFN-gamma ELISA and ELISPOT tests for feline tuberculosis. Veterinary Immunology and Immunopathology 124(3-4): 379-384. [CrossRef]
- Rhodes, S. G., et al. (2008b). Interferon-γ test for feline tuberculosis. Veterinary Record 162(14): 453-455. [CrossRef]
- Rhodes, S., Holder, T., Clifford, D., Dexter I., Brewer, J., 1, Smith, N., Waring, L., Crawshaw, T., Gillgan, S., Lyashchenko, K., Lawrence, J., Clarke, J., de la Rua-Domenech, and H.M. Vordermeier. 2012. Evaluation of IFNg and antibody TB tests in alpacas. Clin. Vaccine Immunol., 19(10): 1677-1683. [CrossRef]
- Roos, E.O. et al. (2016) Test performance of three serological assays for the detection of Mycobacterium bovis infection in common warthogs (Phacochoerus africanus. Veterinary Immunology and Immunopathology, 182, pp. 79-84. [CrossRef]
- Ruhwald, M. et al (2009)Biomarkers of latent TB infection. Expert Review of Respiratory Medicine, 3 (4), pp. 387-401. [CrossRef]
- Schiller, I., et al. (2009). Optimization of a whole-blood gamma interferon assay for detection of Mycobacterium bovis-infected cattle. Clinical and Vaccine Immunology 16(8): 1196-1202.
- Shrikrishna, D. et al. (2009). Human and canine pulmonary Mycobacterium bovis infection in the same household: re-emergence of an old zoonotic threat? Thorax 64, 89-91. [CrossRef]
- Snider, W. R. (1971). Tuberculosis in canine and feline populations. Review of the literature. American Review of Respiratory Disease 104(6): 877-887. [CrossRef]
- Szaluś-Jordanow, O., et al. (2016). Intracardiac tuberculomas caused by Mycobacterium tuberculosis in a dog. BMC Veterinary Research 12(1). [CrossRef]
- Thillai, M., et al. (2014). Interferon-gamma release assays for tuberculosis: Current and future applications. Expert Review of Respiratory Medicine 8(1): 67-78. [CrossRef]
- Trost, B. et al.(2016) Investigation of the cause of geographic disparities in IDEXX ELISA sensitivity in serum samples from Mycobacterium bovis-infected cattle. Scientific Reports, 6, art. no. 22763. [CrossRef]
- Vordermeier, M., Whelan, A., Ewer, K., Goodchild, T., Clifton-Hadley, R., Williams, J. and R.G. Hewinson (2006) The BOVIGAM assay as an ancillary test to the tuberculin skin test. Gov. Vet. J., vol. 16(1), 72-80.
- Vordermeier, M., Chambersw, M.A, Cockle, P.J., Whelan, A.O., Simmons, J. and Hewison, R.G. (2002) Correlation of ESAT-6-specific gamma interferon production with pathology in cattle following Mycobacterium bovis BCG vaccination against experimental bovine tuberculosis. Infect. Immunol. 70 (6) 3026-3032. [CrossRef]
- Vordermeier, H.M., Jones, G.J., Buddle, B.M. and R.G. Hewinson. 2016. Development of immunological reagents to diagnose bovine tuberculosis in cattle. Vet. Immunol. Immunopathol., 181:10-14. [CrossRef]
- Wang, G., et al (2013) Mycobacterium tuberculosis-specific TNF-α is a potential biomarker for the rapid diagnosis of active tuberculosis disease in Chinese population. PLOS One 8(11): e79431. [CrossRef]
- Wood P R, Corner L A and Plackett P. (1990) Development of a simple, rapid in vitro cellular assay for bovine tuberculosis based on the production of γ-interferon. Res Vet Sci 1990; 49: 46-49. [CrossRef]
- Wood, P. R. and S. L. Jones (2001). BOVIGAM™: An in vitro cellular diagnostic test for bovine tuberculosis. Tuberculosis 81(1-2): 147-155. [CrossRef]
- Xu, J.-C.et al. (2018) More significance of TB-IGRA except for the diagnose of tuberculosis. Journal of Clinical Laboratory Analysis, 32 (1), art. no. e22183. [CrossRef]
- Yearsley, D. et al. (1998) An evaluation of an anamnestic ELISA for the detection of tuberculous cattle. Irish Veterinary Journal, 51 (6), pp. 303-306.
- Zhou, Q., et al. (2011). Diagnostic accuracy of T-cell interferon-γ release assays in tuberculous pleurisy: A meta-analysis. Respirology 16(3): 473-480. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).