1. Introduction
In the United States and Europe, peanut allergy has become an escalating public health concern, with prevalence increasing 3.5-fold over the past two decades to affect approximately 1.4–2% of the population [
1]. Characterized by an abnormal IgE mediated immune response to peanut proteins, this allergy can lead to life-threatening anaphylaxis and has a significant impact on the quality of life for affected individuals and their families [
2]. IgE mediated peanut allergy is associated with a significantly higher number of deaths caused by anaphylaxis among patients treated in emergency departments compared to other IgE mediated food allergies [
2].
Current traditional treatment and management options for patients with IgE mediated peanut allergy include peanut avoidance, auto administrated epinephrine carriage, and prompt treatment of allergic symptoms [
3]. These practices however are limited by the rising cost and burden of self-carrying epinephrine and significant anxiety associated with food avoidance and surveillance. There is significant anxiety associated with peanut allergy and the social impact it has on patients’ lives [
4]. As a result, there has been an increasing focus on developing therapies that can change the course of the disease, provide long-term relief, and improve the quality of life by reducing the reliance on emergency medical treatments and alleviating food-related anxiety [
5].
Recent advancements in immunotherapy and novel therapeutic approaches offer new hope for individuals suffering from this potentially life-threatening condition [
6,
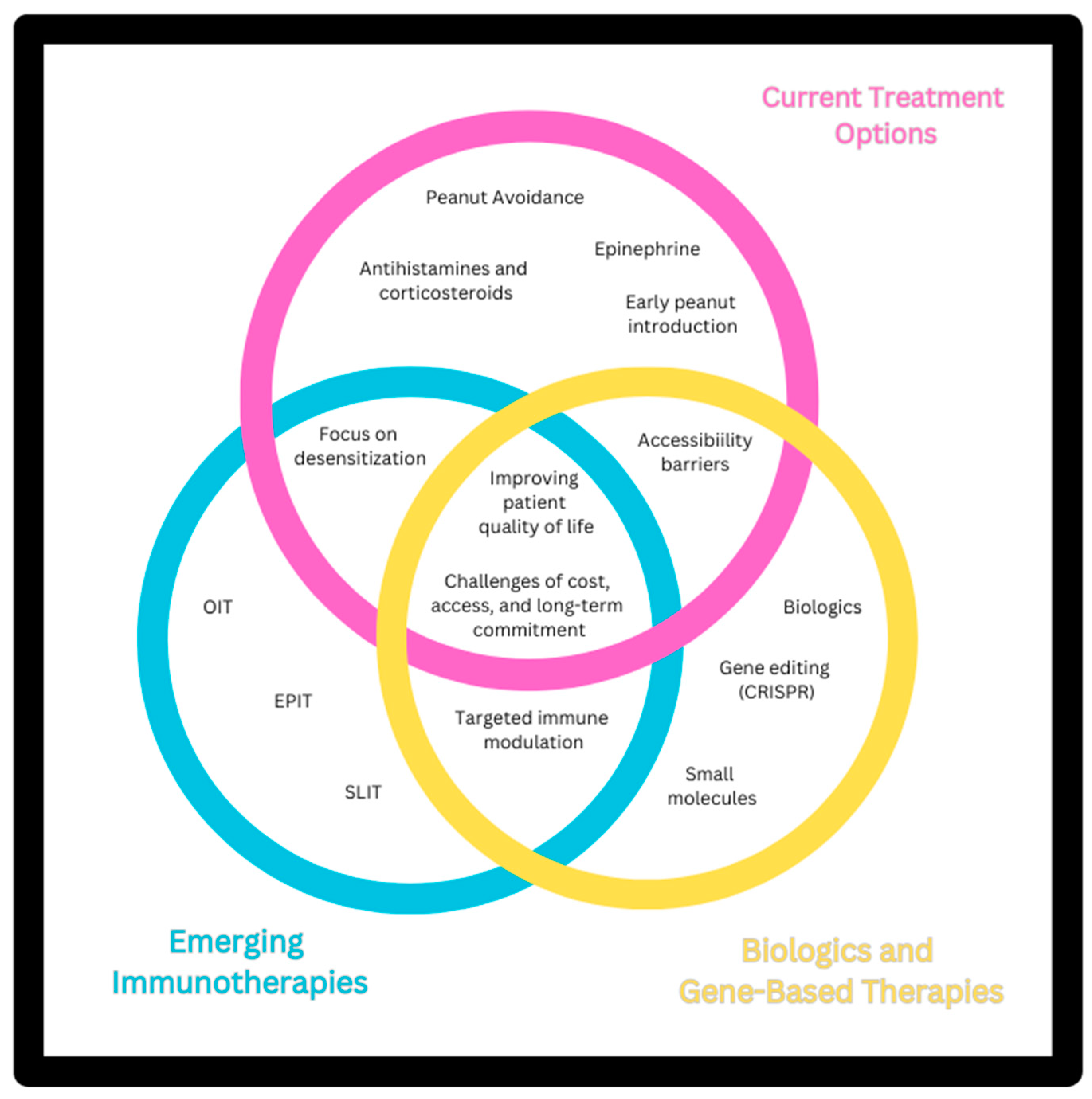
7]. This article explores the latest developments in emerging treatments for peanut allergy, including advances in immunotherapy, biologic agents, and novel vaccine strategies, as seen in
Figure 1. By examining recent clinical trials, efficacy data, and underlying mechanisms, we aim to provide a comprehensive overview of how these innovative therapies are reshaping the landscape of peanut allergy management and offer insights into their potential impact on patient outcomes and future research directions.
2. Current Treatment Options
Current standard of care in peanut allergy involves a multi-strategy approach with avoidance of the allergen as the mainstay of therapy. The goal of allergen avoidance is to reduce the risk of anaphylaxis in those with already diagnosed food allergies. It is important to note that current guidelines support early introduction of peanut products in infancy for all patients, particularly at 4-6 months of age, as this is associated with a lower risk of peanut allergy and should be encouraged to prevent allergy [
8,
9,
10,
11]. For those already with peanut allergy, the risk of life-threatening anaphylaxis has led to food labeling requirements and even peanut-free schools, though the data on the effectiveness of these measures is mixed. Allergen avoidance sounds simple but is challenging for several reasons and leads to decreased quality of life for those with peanut allergy. For example, in the United States, food labeling does not have standardized guidelines when an allergen is present in the manufacturing process or when cross contamination is possible. Only when an allergen is a direct ingredient is there standardized labeling. Often, foods contain vague unregulated non-standardized phrases such as, “may contain peanuts” without other explanation. This leads to inconsistent labeling and may lead patients to limit more than is truly necessary, or worse, could result in accidental ingestion [
12]. Many restaurants or manufacturers may be hesitant to completely reassure that the facility is peanut free even when it is. Food at restaurants may not be labeled properly or restaurants may not be able to ensure safety with cross-contamination or even understand that they need to take precautions to ensure no cross-contamination. Even when patients ask about allergens at restaurants, they can still end up accidentally ingesting their known allergens. Additionally, particularly for children, the risk of unintentional ingestion is a concern for both parents and children, leading to heightened anxiety and worse quality of life including restricted participation at social events [
13,
14]. Given the limitations of allergen avoidance, additional strategies have been devised to manage allergy symptoms and minimize allergic reactions, while research into new therapies continues.
When those with peanut allergy have an anaphylactic reaction, the recommended is removal of antigen and rapid administration of intramuscular epinephrine. However, even this standard treatment is changing since in August 2024 the United States Food and Drug Administration (FDA) approved the first intranasal version of epinephrine to treat anaphylaxis in patients over 30 kg, which has been shown to have equally effective systemic absorption in clinical trials [
15]. Only after administration of epinephrine should adjunctive therapies such as H1 or H2 antagonists or corticosteroids be considered, because initial use of these medications leads to suppression of allergic symptoms and delay of epinephrine treatment, which is associated with poor outcomes [
16]. Epinephrine is the only effective definitive treatment for anaphylaxis. After effective epinephrine treatment, antihistamines can be used for cutaneous symptom relief. Corticosteroids may have some benefit in decreasing hospital length of stay, but offer no prevention of biphasic reactions, do not prevent repeat ER visits and have limited benefit in acute anaphylaxis despite popular use [
17]. Additionally, antihistamines and corticosteroids should not be used to prevent anaphylaxis prior to a possible antigen exposure [
17].
To prevent risk of anaphylaxis, immunotherapies have been created to treat patients with peanut allergies by inducing desensitization, which can protect against severe reactions to accidental ingestions. In some patients, sustained remission may also be possible. The first peanut immunotherapy was delivered subcutaneously, first reported in 1992, but was discontinued due to a high rate of systemic reactions [
18,
19]. Similarly, a rectal vaccine for peanut allergy was trialed but halted due to an unsatisfactory rate of adverse systemic reactions and poor efficacy [
20].
Currently, trials of sublingual (SLIT), oral (OIT), and epicutaneous immunotherapy (EPIT) are promising with OIT being the only FDA approved therapy so far. SLIT has shown to be effective in desensitization and has a low rate of systemic reactions [
21,
22,
23], however, this modality requires smaller allergen doses for practical reasons and the desensitization outcomes may be outperformed by OIT [
24,
25]. Several studies have demonstrated the efficacy of peanut OIT in children and young adults, with best results in young children [
20,
26,
27,
28,
29]. For example, in the IMPACT trial, OIT in children less than 4 years old produced 71% desensitization and 21% achieved remission [
20]. Side effects of OIT are common across all trials. Most reactions produce mild to moderate side effects most commonly oropharyngeal or gastrointestinal symptoms, but severe reactions and systemic symptoms treated effectively with epinephrine occur in approximately 5-10% of study participants depending on the study.
EPIT via “peanut patch” placed on skin is currently being studied in clinical trials with most preliminary studies showing desensitization in pediatric patients [
30,
31]. The peanut patch is overall well-tolerated with lower rates of severe reactions than reported in OIT trials [
32].
Biologics have also been used in the treatment of peanut allergy. Biologics were first trialed in 2003 for peanut allergy and produced desensitization [
33]. Recently, Omalizumab monotherapy delivered subcutaneously has been shown to cause desensitization in those with food allergies including peanut allergy [
34]. There is also ongoing research into use of omalizumab in combination with immunotherapy [
34] with early trials showing rapid oral desensitization with combination therapy [
35]. Current treatment options for peanut allergy are compared in
Table 1.
Overall, we are witnessing an exciting period of growth and transformation in the approach to treating and preventing peanut allergies.
3. Emerging Treatment Outcomes
3.1. Immunotherapy Approaches
3.1.1. Oral Immuntherapy (OIT)
Allergen Immunotherapy modulates the immune response to increase the allergic reaction threshold by gradually exposing allergic individuals to increasing doses of an allergen. Among the different approaches, Oral Immunotherapy (OIT) is the most widely used type of immunotherapy for the treatment of IgE-mediated food allergies. During OIT, the allergen exposure reduces basophil sensitivity and decreases effector cell reactivity, resulting in desensitization [
20,
29,
36].
Treatment begins with a low dose of the allergen administered orally, followed by an updosing phase, until the maintenance dose is achieved, which is then continued for a variable duration. The aim of this treatment, as with all forms of food allergen immunotherapy, is to reduce the severity of allergic symptoms if an accidental exposure occurs [
20,
29].
OIT is the most studied type of immunotherapy for IgE-mediated peanut allergy. Multiple studies have been conducted, including numerous randomized trials. The trials conclude that this therapy is capable of achieving desensitization in most of the patients (60-80%) and increasing the tolerance threshold [
20,
24,
27,
28,
36,
37,
38]. Peanut is the only food for which an Oral Immunotherapy, Palforzia, has been approved by the Food and Drug Administration (FDA) for patients aged 4 to 17 years old [
27].
Adverse events associated with OIT include systemic allergic reactions, ranging from mild to severe anaphylaxis; skin-related side effects include pruritus, urticarial rash, and angioedema; and, gastrointestinal symptoms which are usually the most common, and include dysphagia, abdominal pain, gastritis, mouth pruritus, nausea, vomiting, and eosinophilic esophagitis. Side effects can result in discontinuation of OIT or be dose limiting. Gastrointestinal symptoms usually resolve within a few weeks following discontinuation [
20,
29,
39].
OIT treatment faces many challenges, including the adverse reactions discussed and long-term patient commitment. A subset of patients is unable to achieve sustained unresponsiveness, for unknown reasons, and there is a risk of regaining clinical reactivity to peanut once therapy is discontinued or even reduced. Patients may also present with psychological stress associated with potential reactions. Psychological stressors may pose a barrier to initiating OIT treatment. mise but involves high rates of adverse reactions, especially gastrointestinal. The Food and Drug Administration and European Medicines Agency have approved Palforzia® for patients aged 4-17, and studies are working to improve OIT tolerability and standardize protocols [
40].
3.1.2. Sublingual Immuntherapy (SLIT)
Sublingual Immunotherapy (SLIT) achieves desensitization by administering small amounts of glycerinated liquid allergen extract under the tongue. These antigens are captured by oral Langerhans cells, which initiate the immune response that promotes desensitization. The protocol is similar to OIT, requiring daily administration, starting with a low initial dose, followed by a dose escalation phase, and then a maintenance dose [
41]. In contrast to OIT, this immunotherapy is typically dosed in micrograms up to low milligrams [
21,
42,
43].
For OIT, initial escalation is usually done in a clinical setting due to higher doses and reaction risk, while maintenance often continues at home. SLIT uses much lower doses, allowing more frequent home dosing with minimal in-office monitoring [
41]. Anaphylaxis risk is higher with OIT, especially in early phases, though it decreases during maintenance. SLIT has a lower risk overall, with mainly mild throat symptoms and fewer severe reactions [
41]. Additionally, multiple studies have highlighted its favorable safety profile as another significant benefit. The most common side effect is oropharyngeal pruritus, which is generally mild and transient. Gastrointestinal and systemic symptoms are rare [
21,
23,
24,
42,
44].
Despite these advantages, SLIT faces several limitations. Similar to OIT, it requires long-term treatment commitment and treatment protocols are highly variable. SLIT has been found to be less effective compared to OIT, achieving lower levels of desensitization. In a retrospective comparison, participants undergoing OIT were three times more likely to pass a 12-month food challenge than those on SLIT, with OIT participants tolerating up to 5,000 mg of peanut protein versus 2,500 mg for SLIT participants [
45]. In a double-blind study with peanut allergic children, OIT participants showed a 141-fold increase in tolerance threshold, compared to only a 22-fold increase in the SLIT group [
24].
Long-term use of peanut SLIT has been shown to increase the tolerated dose of peanuts, with stronger tolerance also associated with higher treatment doses [
21,
23]. Despite developing tolerance, only a subset of patients will achieve sustained unresponsiveness. Currently, there is no data on how to predict which patients will achieve sustained unresponsiveness, similar to what is observed with OIT [
23,
44].
3.1.3. Epicutaneous Immuntherapy (EPIT)
Epicutaneous Immunotherapy (EPIT) induces desensitization by the application of allergens through the skin. The allergen is processed by dermal dendritic cells and Langerhans cells, which then migrate to the lymph nodes to initiate antigen-specific immune modulation. EPIT differs significantly from OIT and SLIT in its method and dosage. EPIT uses a Peanut patch that administers allergen microgram doses on intact skin, specifically designed to avoid systemic circulation and thereby minimize the risk of severe reactions associated with higher allergen exposure in OIT [
46]. By comparison, OIT delivers milligram doses of allergen orally, involving ingestion that leads to a broader, systemic immune response [
32]. SLIT uses milligram-level doses placed under the tongue, which leads to broader immune activation and a stronger desensitization effect but also carries a higher risk of mild adverse reactions [
47]. EPIT primarily aims to induce desensitization through cutaneous pathways, utilizing the immune properties of the skin to maintain localized activation of regulatory T cells, which is less likely to provoke anaphylaxis compared to the more invasive OIT and SLIT methods [
30].
There are multiple types of epicutaneous delivery systems. The most used and studied is the Viaskin (DBV Technologies, Montrouge, France) Peanut 250 μg (VP250) patch. Dry powdered peanut allergen is sprayed onto a transparent plastic membrane by electrostatic forces. In contact with the epidermis, Viaskin creates an enclosed chamber and increases skin permeability by utilizing transepidermal water losses. The amount of allergen in the patch remains constant throughout the treatment, while the daily application time of the patch gradually increases [
31,
32,
48,
49,
50,
51].
Other innovative types of EPIT delivery systems are being studied and they include the Ablative Fractional Laser (AFL) and the microneedle delivery system. The precise laser epidermal system (P.L.E.A.S.E.) is a type of AFL that enhances transcutaneous drug delivery, particularly for biomacromolecules, by creating microchannels in the dermal or epidermal layers. This is followed by the application of a patch coated with powdered allergen. The goal of this treatment is to enhance the amount of allergen delivered without systemic passage [
52,
53,
54].
The microneedle delivery system involves coated-allergen microneedles that penetrate the epidermis, creating micropores in the stratum corneum. These pores allow macromolecules to cross the epidermis, thereby enhancing drug penetration [
55,
56,
57].
Among the treatments discussed, only the EPIT Viaskin has published human studies for the treatment of food allergies [
30,
31,
32,
46,
48,
49]. The patch has been studied in children as young as one year old [
30]. The study supports that higher doses in epicutaneous immunotherapy (EPIT) lead to greater desensitization. Participants treated with 250 µg of Viaskin Peanut showed a higher success rate (48%) and a greater increase in peanut tolerance (130 mg) compared to those receiving 100 µg (46% success, 43 mg increase) and placebo (12% success, no increase). This demonstrates that increasing the dose enhances treatment efficacy [
49]. The most common adverse event is a local patch site reaction, including pruritus, edema, and redness [
30,
31,
32,
46,
48,
49]. Long-term studies have shown that daily treatment leads to sustained response in some patients [
58,
59]. Emerging immunotherapy approaches are compared in
Table 2.
3.2. Biologic Therapies
An important adjuvant in peanut oral immunotherapy for enhancing both efficacy and safety is the use of anti-IgE, which helps reduce reactions during oral desensitization. Omalizumab, an anti-IgE drug approved by the FDA in 2003, is administered subcutaneously and blocks the IgE receptor binding site, rapidly lowering the concentration of total free IgE for peanut and all other specific IgE [
60].
In 2024, a meta-analysis of 36 studies involving 1,000 subjects was conducted [
61]. The use of Omalizumab as a monotherapy or in combination with oral immunotherapy significantly increased the tolerated dose of foods, allowing the consumption of multiple foods at higher reactivity thresholds. Additionally, in patients with severe and persistent food allergies, Omalizumab aided in successful desensitization, though it did not significantly alter the cumulative tolerated dose.
In further analysis from the PRROTECT trial, IgE blockade by omalizumab allowed for a faster escalation of peanut doses during the build-up phase of peanut OIT, leading to fewer reported adverse reactions, fewer dose-related reactions requiring treatment, and fewer doses needed to reach maintenance. It also resulted in a more prolonged reduction in peanut-specific IgE levels [
62]. Additionally, there was a reported decrease in episodes of anaphylaxis in patients with recurrent and/or unprovoked anaphylactic reactions, offering additional benefits, particularly for patients at high risk of severe anaphylaxis related to food allergies [
61].
While omalizumab continues to be a significant option, next-generation anti-IgE antibodies and agents targeting alarmins are demonstrating distinct advantages. Dupilumab, which targets IL-4 and IL-13, also shows potential as dual therapy. The introduction of antialarmins expands the range of treatment options for food allergies [
63]. Next-generation antibodies such as ligelizumab and UB-221 exhibit increased potency and novel mechanisms, offering promise for food allergy treatment. Anti-IL-33 (etokimab) and anti-TSLP (tezepelumab), has yielded promising results, with etokimab showing early success in peanut allergy trials [
64]. However, additional studies are required to fully evaluate their efficacy and increased benefits over substantial risks.
3.3. Gene Editing and CRISPR Technology
New strategies have made gene editing easy and highly efficient in comparison to the well-known process of homologous recombination [
65]. The clustered regularly interspaced short palindromic repeats (CRISPR) gene editing technology has been utilized to identify genes that are considered the culprit of allergy disease, alter genes to manipulate triggering allergens to reduce allergenicity and disease and develop genes knockouts. This technology provided outstanding of benefits such as simple design, specificity, reproducibility, and cost efficiency [
66].
Through the use of inhibitors and gene silencing technology, scientists have identified CYP11A1 as a key regulator of IL-13 production and a crucial molecule in the development of peanut-induced intestinal anaphylaxis in mice [
67]. In a study involving activated peripheral blood CD4+ T cells from peanut-allergic children, CYP11A1 gene expression was found to be approximately 50 times higher, and the percentage of CD4+ CYP11A1+ T cells was 20 times greater compared to non-allergic controls [
68]. Targeting CYP11A1 has shown a reduction in its gene expression by more than 50%, along with a decrease in IL-13 production, suggesting that CYP11A1 may be a promising target for therapeutic intervention in children with peanut allergies [
67].
Given the ethical concerns surrounding gene editing in humans, another approach involves using gene editing to manipulate causative allergens directly. In one study, Dodo et al. developed allergen-reduced peanuts by using RNA interference to silence the Ara h 2 gene. This resulted in significantly reduced or even undetectable levels of Ara h 2 in several genetically modified peanut seeds, and a corresponding decrease in IgE binding from peanut-allergic patient serum compared to wild-type seeds [
69]. These innovative approaches offer a rapid and effective means to better understand gene functions and potentially reduce peanut allergy. However, it is important to know that these technologies lack human studies and are experimental, hence many years away from being implemented in general practice.
3.4. Novel Drug Therapies
3.4.1. Small Molecules
Recent advances in molecular immunology have revolutionized the management of atopic dermatitis (AD) and related conditions, with the development of Janus kinase (JAK) inhibitors and other pathway-specific therapies. These targeted treatments modulate the immune response by interfering with key signaling pathways, including JAK/STAT and spleen tyrosine kinase (SYK), central to the pathogenesis of inflammatory and allergic conditions.
The JAK/STAT pathway regulates the activity of multiple cytokines involved in AD and food allergies, such as IL-4, IL-5, and IL-13. By inhibiting this pathway, JAK inhibitors reduce inflammation and allergic responses. Abrocitinib, upadacitinib, and baricitinib are FDA-approved oral JAK inhibitors for moderate-to-severe AD, offering significant symptomatic relief and quality-of-life improvements [
70,
71]. Clinical trials and real-world studies demonstrate that abrocitinib and upadacitinib effectively reduce eczema severity, itch, and systemic inflammation, with manageable safety profiles [
72]. These inhibitors have also shown potential in improving allergen-specific basophil activation, providing a rationale for further research in food allergies. Meta-analyses indicate a manageable infection risk associated with JAK inhibitors in dermatological applications, emphasizing the importance of monitoring during treatment [
73].
SYK inhibitors block the IgE-mediated activation of mast cells, preventing the release of inflammatory mediators. Fostamatinib, approved for other immune-mediated conditions, has shown promise in preclinical studies for food allergy and allergic anaphylaxis [
74]. However, their progression to advanced clinical trials for atopic conditions is limited by toxicity and formulation challenges.
4. Challenges and Limitations in Emerging Treatments
Emerging treatments for peanut allergies face several challenges and limitations. One major issue is the risk of adverse reactions, including anaphylaxis, during immunotherapy, which can cause discomfort or potentially life-threatening responses for patients. Long-term commitment to these therapies is necessary, which can be challenging due to the psychological burden of anticipating reactions and the frequent need for medical supervision. Additionally, treatment standardization is lacking, as protocols vary between institutions, making outcomes and safety inconsistent. Cost and access also present significant barriers, as many therapies are expensive and not universally covered by insurance. Finally, while some patients achieve desensitization, sustained unresponsiveness remains elusive for a subset of individuals, complicating the long-term success of these therapies.
Author Contributions
T.S.: Conceptualization and overall coordination; A.X.A., M.W., K.B.C., D.C., P.T.: Drafting and revising the manuscript; G.K., M.G.: Supervision, critical review, and approval of the final manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study.
References
- L. Lange et al., “White paper on peanut allergy – part 1: Epidemiology, burden of disease, health economic aspects,” Allergo J. Int., vol. 30, no. 8, p. 261, Sep. 2021. [CrossRef]
- Z. Husain and R. A. Schwartz, “Peanut allergy: an increasingly common life-threatening disorder,” J. Am. Acad. Dermatol., vol. 66, no. 1, pp. 136–143, Jan. 2012. [CrossRef]
- E. M. Abrams, E. S. Chan, and S. Sicherer, “Peanut Allergy: New Advances and Ongoing Controversies,” Pediatrics, vol. 145, no. 5, p. e20192102, May 2020. [CrossRef]
- M. Tsoumani et al., “Allergy to Peanuts imPacting Emotions And Life (APPEAL): The impact of peanut allergy on children, teenagers, adults and caregivers in the UK and Ireland,” PLoS ONE, vol. 17, no. 2, p. e0262851, Feb. 2022. [CrossRef]
- M. Bublin and H. Breiteneder, “Developing Therapies for Peanut Allergy,” Int. Arch. Allergy Immunol., vol. 165, no. 3, p. 179, Dec. 2014. [CrossRef]
- Q. S. Cook and E. H. Kim, “Update on peanut allergy: Prevention and immunotherapy,” Allergy Asthma Proc., vol. 40, no. 1, pp. 14–20, Jan. 2019. [CrossRef]
- E. H. Kim, C. Patel, and A. W. Burks, “Immunotherapy approaches for peanut allergy,” Expert Rev. Clin. Immunol., vol. 16, no. 2, pp. 167–174, Feb. 2020. [CrossRef]
- G. Du Toit et al., “Randomized trial of peanut consumption in infants at risk for peanut allergy,” N. Engl. J. Med., vol. 372, no. 9, pp. 803–813, Feb. 2015. [CrossRef]
- G. Du Toit et al., “Follow-up to Adolescence after Early Peanut Introduction for Allergy Prevention,” NEJM Evid., vol. 3, no. 6, p. EVIDoa2300311, Jun. 2024. [CrossRef]
- K. Logan et al., “Early introduction of peanut reduces peanut allergy across risk groups in pooled and causal inference analyses,” Allergy, vol. 78, no. 5, pp. 1307–1318, May 2023. [CrossRef]
- A. Togias et al., “Addendum guidelines for the prevention of peanut allergy in the United States: Report of the National Institute of Allergy and Infectious Diseases–sponsored expert panel,” Ann. Allergy. Asthma. Immunol., vol. 118, no. 2, pp. 166-173.e7, Feb. 2017. [CrossRef]
- R. Gupta, M. Kanaley, O. Negris, A. Roach, and L. Bilaver, “Understanding Precautionary Allergen Labeling (PAL) Preferences Among Food Allergy Stakeholders,” J. Allergy Clin. Immunol. Pract., vol. 9, no. 1, pp. 254-264.e1, Jan. 2021. [CrossRef]
- R. M. King, R. C. Knibb, and J. O. Hourihane, “Impact of peanut allergy on quality of life, stress and anxiety in the family,” Allergy, vol. 64, no. 3, pp. 461–468, Mar. 2009. [CrossRef]
- K. M. Roy and M. C. Roberts, “Peanut allergy in children: relationships to health-related quality of life, anxiety, and parental stress,” Clin. Pediatr. (Phila.), vol. 50, no. 11, pp. 1045–1051, Nov. 2011. [CrossRef]
- D. A. Dworaczyk et al., “A 13.2 mg epinephrine intranasal spray demonstrates comparable pharmacokinetics, pharmacodynamics, and safety to a 0.3 mg epinephrine autoinjector,” J. Allergy Clin. Immunol. Glob., vol. 3, no. 2, p. 100200, May 2024. [CrossRef]
- A. Navalpakam, N. Thanaputkaiporn, and P. Poowuttikul, “Management of Anaphylaxis,” Immunol. Allergy Clin. North Am., vol. 42, no. 1, pp. 65–76, Feb. 2022. [CrossRef]
- M. S. Shaker et al., “Anaphylaxis-a 2020 practice parameter update, systematic review, and Grading of Recommendations, Assessment, Development and Evaluation (GRADE) analysis,” J. Allergy Clin. Immunol., vol. 145, no. 4, pp. 1082–1123, Apr. 2020. [CrossRef]
- J. J. Oppenheimer, H. S. Nelson, S. A. Bock, F. Christensen, and D. Y. Leung, “Treatment of peanut allergy with rush immunotherapy,” J. Allergy Clin. Immunol., vol. 90, no. 2, pp. 256–262, Aug. 1992. [CrossRef]
- H. S. Nelson, J. Lahr, R. Rule, A. Bock, and D. Leung, “Treatment of anaphylactic sensitivity to peanuts by immunotherapy with injections of aqueous peanut extract,” J. Allergy Clin. Immunol., vol. 99, no. 6 Pt 1, pp. 744–751, Jun. 1997. [CrossRef]
- S. M. Jones et al., “Efficacy and safety of oral immunotherapy in children aged 1-3 years with peanut allergy (the Immune Tolerance Network IMPACT trial): a randomised placebo-controlled study,” Lancet Lond. Engl., vol. 399, no. 10322, pp. 359–371, Jan. 2022. [CrossRef]
- E. H. Kim et al., “Open-label study of the efficacy, safety, and durability of peanut sublingual immunotherapy in peanut-allergic children,” J. Allergy Clin. Immunol., vol. 151, no. 6, pp. 1558-1565.e6, Jun. 2023. [CrossRef]
- E. H. Kim et al., “Desensitization and remission after peanut sublingual immunotherapy in 1- to 4-year-old peanut-allergic children: A randomized, placebo-controlled trial,” J. Allergy Clin. Immunol., vol. 153, no. 1, pp. 173-181.e10, Jan. 2024. [CrossRef]
- E. H. Kim et al., “Long-term sublingual immunotherapy for peanut allergy in children: Clinical and immunologic evidence of desensitization,” J. Allergy Clin. Immunol., vol. 144, no. 5, pp. 1320-1326.e1, Nov. 2019. [CrossRef]
- S. D. Narisety et al., “A randomized, double-blind, placebo-controlled pilot study of sublingual versus oral immunotherapy for the treatment of peanut allergy,” J. Allergy Clin. Immunol., vol. 135, no. 5, pp. 1275-1282.e1–6, May 2015. [CrossRef]
- C. A. Keet et al., “The safety and efficacy of sublingual and oral immunotherapy for milk allergy,” J. Allergy Clin. Immunol., vol. 129, no. 2, pp. 448–455, 455.e1–5, Feb. 2012. [CrossRef]
- L. Soller et al., “First Real-World Effectiveness Analysis of Preschool Peanut Oral Immunotherapy,” J. Allergy Clin. Immunol. Pract., vol. 9, no. 3, pp. 1349-1356.e1, Mar. 2021. [CrossRef]
- PALISADE Group of Clinical Investigators et al., “AR101 Oral Immunotherapy for Peanut Allergy,” N. Engl. J. Med., vol. 379, no. 21, pp. 1991–2001, Nov. 2018. [CrossRef]
- J. O’B Hourihane et al., “Efficacy and safety of oral immunotherapy with AR101 in European children with a peanut allergy (ARTEMIS): a multicentre, double-blind, randomised, placebo-controlled phase 3 trial,” Lancet Child Adolesc. Health, vol. 4, no. 10, pp. 728–739, Oct. 2020. [CrossRef]
- J. A. Bird et al., “Efficacy and Safety of AR101 in Oral Immunotherapy for Peanut Allergy: Results of ARC001, a Randomized, Double-Blind, Placebo-Controlled Phase 2 Clinical Trial,” J. Allergy Clin. Immunol. Pract., vol. 6, no. 2, pp. 476-485.e3, 2018. [CrossRef]
- M. Greenhawt et al., “Phase 3 Trial of Epicutaneous Immunotherapy in Toddlers with Peanut Allergy,” N. Engl. J. Med., vol. 388, no. 19, pp. 1755–1766, May 2023. [CrossRef]
- H. A. Sampson et al., “Effect of Varying Doses of Epicutaneous Immunotherapy vs Placebo on Reaction to Peanut Protein Exposure Among Patients With Peanut Sensitivity: A Randomized Clinical Trial,” JAMA, vol. 318, no. 18, pp. 1798–1809, Nov. 2017. [CrossRef]
- J. A. Pongracic et al., “Safety of Epicutaneous Immunotherapy in Peanut-Allergic Children: REALISE Randomized Clinical Trial Results,” J. Allergy Clin. Immunol. Pract., vol. 10, no. 7, pp. 1864-1873.e10, Jul. 2022. [CrossRef]
- J. Fowler and J. Lieberman, “Update on clinical research for food allergy treatment,” Front. Allergy, vol. 4, p. 1154541, Jul. 2023. [CrossRef]
- R. A. Wood et al., “Omalizumab for the Treatment of Multiple Food Allergies,” N. Engl. J. Med., vol. 390, no. 10, pp. 889–899, Mar. 2024. [CrossRef]
- A. J. MacGinnitie et al., “Omalizumab facilitates rapid oral desensitization for peanut allergy,” J. Allergy Clin. Immunol., vol. 139, no. 3, pp. 873-881.e8, Mar. 2017. [CrossRef]
- V. Bajzik et al., “Oral desensitization therapy for peanut allergy induces dynamic changes in peanut-specific immune responses,” Allergy, vol. 77, no. 8, pp. 2534–2548, Aug. 2022. [CrossRef]
- K. Anagnostou et al., “Assessing the efficacy of oral immunotherapy for the desensitisation of peanut allergy in children (STOP II): a phase 2 randomised controlled trial,” Lancet Lond. Engl., vol. 383, no. 9925, pp. 1297–1304, Apr. 2014. [CrossRef]
- T. Reier-Nilsen et al., “Feasibility of desensitizing children highly allergic to peanut by high-dose oral immunotherapy,” Allergy, vol. 74, no. 2, pp. 337–348, Feb. 2019. [CrossRef]
- Y. V. Virkud et al., “Novel baseline predictors of adverse events during oral immunotherapy in children with peanut allergy,” J. Allergy Clin. Immunol., vol. 139, no. 3, pp. 882-888.e5, Mar. 2017. [CrossRef]
- S. Foti Randazzese et al., “Current state and advances in desensitization for peanut allergy in pediatric age,” Pediatr. Allergy Immunol. Off. Publ. Eur. Soc. Pediatr. Allergy Immunol., vol. 35, no. 4, p. e14127, Apr. 2024. [CrossRef]
- W. Zhang, S. B. Sindher, V. Sampath, and K. Nadeau, “Comparison of sublingual immunotherapy and oral immunotherapy in peanut allergy,” Allergo J. Int., vol. 27, no. 6, p. 153, Jun. 2018. [CrossRef]
- E. H. Kim et al., “Sublingual immunotherapy for peanut allergy: clinical and immunologic evidence of desensitization,” J. Allergy Clin. Immunol., vol. 127, no. 3, pp. 640-646.e1, Mar. 2011. [CrossRef]
- D. M. Fleischer et al., “Sublingual immunotherapy for peanut allergy: a randomized, double-blind, placebo-controlled multicenter trial,” J. Allergy Clin. Immunol., vol. 131, no. 1, pp. 119-127.e1–7, Jan. 2013. [CrossRef]
- A. W. Burks et al., “Sublingual immunotherapy for peanut allergy: Long-term follow-up of a randomized multicenter trial,” J. Allergy Clin. Immunol., vol. 135, no. 5, pp. 1240-1248.e1–3, May 2015. [CrossRef]
- S. J. Chin et al., “Sublingual versus oral immunotherapy for peanut-allergic children: A retrospective comparison,” J. Allergy Clin. Immunol., vol. 132, no. 2, p. 476, Mar. 2013. [CrossRef]
- S. M. Jones et al., “Safety of epicutaneous immunotherapy for the treatment of peanut allergy: A phase 1 study using the Viaskin patch,” J. Allergy Clin. Immunol., vol. 137, no. 4, pp. 1258-1261.e10, Apr. 2016. [CrossRef]
- A. M. Scurlock et al., “Epicutaneous immunotherapy for treatment of peanut allergy: Follow-up from the Consortium for Food Allergy Research,” J. Allergy Clin. Immunol., vol. 147, no. 3, p. 992, Dec. 2020. [CrossRef]
- D. M. Fleischer et al., “Effect of Epicutaneous Immunotherapy vs Placebo on Reaction to Peanut Protein Ingestion Among Children With Peanut Allergy: The PEPITES Randomized Clinical Trial,” JAMA, vol. 321, no. 10, pp. 946–955, Mar. 2019. [CrossRef]
- S. M. Jones et al., “Epicutaneous immunotherapy for the treatment of peanut allergy in children and young adults,” J. Allergy Clin. Immunol., vol. 139, no. 4, pp. 1242-1252.e9, Apr. 2017. [CrossRef]
- L. Mondoulet et al., “Epicutaneous Immunotherapy Compared with Sublingual Immunotherapy in Mice Sensitized to Pollen (Phleum pratense),” ISRN Allergy, vol. 2012, p. 375735, 2012. [CrossRef]
- J. A. Bird, M. Sánchez-Borges, I. J. Ansotegui, M. Ebisawa, and J. A. Ortega Martell, “Skin as an immune organ and clinical applications of skin-based immunotherapy,” World Allergy Organ. J., vol. 11, no. 1, p. 38, 2018. [CrossRef]
- M. N. K. Kumar, C. Zhou, and M. X. Wu, “Laser-facilitated epicutaneous immunotherapy to IgE-mediated allergy,” J. Control. Release Off. J. Control. Release Soc., vol. 235, pp. 82–90, Aug. 2016. [CrossRef]
- X. Chen, D. Shah, G. Kositratna, D. Manstein, R. R. Anderson, and M. X. Wu, “Facilitation of transcutaneous drug delivery and vaccine immunization by a safe laser technology,” J. Control. Release Off. J. Control. Release Soc., vol. 159, no. 1, pp. 43–51, Apr. 2012. [CrossRef]
- C. H. Tripp et al., “Laser-assisted epicutaneous immunization to target human skin dendritic cells,” Exp. Dermatol., vol. 30, no. 9, pp. 1279–1289, Sep. 2021. [CrossRef]
- H. Amani, M.-A. Shahbazi, C. D’Amico, F. Fontana, S. Abbaszadeh, and H. A. Santos, “Microneedles for painless transdermal immunotherapeutic applications,” J. Control. Release Off. J. Control. Release Soc., vol. 330, pp. 185–217, Feb. 2021. [CrossRef]
- A. K. Shakya et al., “Microneedles coated with peanut allergen enable desensitization of peanut sensitized mice,” J. Control. Release Off. J. Control. Release Soc., vol. 314, pp. 38–47, Nov. 2019. [CrossRef]
- J. J. Landers et al., “Targeted allergen-specific immunotherapy within the skin improves allergen delivery to induce desensitization to peanut,” Immunotherapy, vol. 14, no. 7, pp. 539–552, May 2022. [CrossRef]
- T. F. Brown-Whitehorn et al., “Sustained unresponsiveness to peanut after long-term peanut epicutaneous immunotherapy,” J. Allergy Clin. Immunol. Pract., vol. 9, no. 1, pp. 524–526, Jan. 2021. [CrossRef]
- D. M. Fleischer et al., “Long-term, open-label extension study of the efficacy and safety of epicutaneous immunotherapy for peanut allergy in children: PEOPLE 3-year results,” J. Allergy Clin. Immunol., vol. 146, no. 4, pp. 863–874, Oct. 2020. [CrossRef]
- T. S. Hong, A. Hu, G. Fahim, and E. R. Hermes-DeSantis, “Emerging Therapies for Peanut Allergy,” J. Pharm. Pract., vol. 35, no. 2, pp. 289–297, Apr. 2022. [CrossRef]
- T. Zuberbier et al., “Omalizumab in IgE-Mediated Food Allergy: A Systematic Review and Meta-Analysis,” J. Allergy Clin. Immunol. Pract., vol. 11, no. 4, pp. 1134–1146, Apr. 2023. [CrossRef]
- A. J. Stranks et al., “IgE blockade during food allergen ingestion enhances the induction of inhibitory IgG antibodies,” Ann. Allergy Asthma Immunol. Off. Publ. Am. Coll. Allergy Asthma Immunol., vol. 122, no. 2, pp. 213–215, Feb. 2019. [CrossRef]
- S. B. Sindher, A. Fiocchi, T. Zuberbier, S. Arasi, R. A. Wood, and R. S. Chinthrajah, “The Role of Biologics in the Treatment of Food Allergy,” J. Allergy Clin. Immunol. Pract., vol. 12, no. 3, pp. 562–568, Mar. 2024. [CrossRef]
- J. P. Schuetz, B. Anderson, and S. B. Sindher, “New biologics for food allergy,” Curr. Opin. Allergy Clin. Immunol., vol. 24, no. 3, pp. 147–152, Jun. 2024. [CrossRef]
- Y. Wang et al., “Gene-edited pigs: a translational model for human food allergy against alpha-Gal and anaphylaxis,” Front. Immunol., vol. 15, p. 1358178, 2024. [CrossRef]
- Y. Xu and Z. Li, “CRISPR-Cas systems: Overview, innovations and applications in human disease research and gene therapy,” Comput. Struct. Biotechnol. J., vol. 18, pp. 2401–2415, 2020. [CrossRef]
- M. Wang, M. Schedel, and E. W. Gelfand, “Gene editing in allergic diseases: Identification of novel pathways and impact of deleting allergen genes,” J. Allergy Clin. Immunol., vol. 154, no. 1, pp. 51–58, Jul. 2024. [CrossRef]
- M. Wang et al., “Expression and activation of the steroidogenic enzyme CYP11A1 is associated with IL-13 production in T cells from peanut allergic children,” PloS One, vol. 15, no. 6, p. e0233563, 2020. [CrossRef]
- H. W. Dodo, K. N. Konan, F. C. Chen, M. Egnin, and O. M. Viquez, “Alleviating peanut allergy using genetic engineering: the silencing of the immunodominant allergen Ara h 2 leads to its significant reduction and a decrease in peanut allergenicity,” Plant Biotechnol. J., vol. 6, no. 2, pp. 135–145, Feb. 2008. [CrossRef]
- A. Wollenberg et al., “Longer-term safety and efficacy of baricitinib for atopic dermatitis in pediatric patients 2 to <18 years old: a randomized clinical trial of extended treatment to 3.6 years,” J. Dermatol. Treat., vol. 35, no. 1, p. 2411834, Dec. 2024. [CrossRef]
- J. C. Armario-Hita et al., “Treatment of atopic dermatitis with abrocitinib in real practice in Spain: efficacy and safety results from a 24-week multicenter study,” Int. J. Dermatol., vol. 63, no. 11, pp. e289–e295, 2024. [CrossRef]
- H. Zirpel, R. J. Ludwig, H. Olbrich, K. Kridin, S. Ständer, and D. Thaçi, “Comparison of safety profile in patients with atopic dermatitis treated with dupilumab or conventional systemic treatment: real world data from the US network,” J. Dermatol. Treat., vol. 35, no. 1, p. 2421429, Dec. 2024. [CrossRef]
- P. A. Ireland, M. Verheyden, N. Jansson, D. Sebaratnam, and J. Sullivan, “Infection risk with JAK inhibitors in dermatoses: a meta-analysis,” Int. J. Dermatol., Oct. 2024. [CrossRef]
- C. Perricone et al., “Sudden improvement of alopecia universalis and psoriatic arthritis while receiving upadacitinib: a case-based review,” Reumatismo, Oct. 2024. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).