1. Introduction
Nowadays, physical and chemical technologies are applied in the study, conservation and restoration of ancient artifacts. Restoration treatments were designed to slow down the aging effects of cultural artifacts, However, it is of paramount importance to acquire expertise in selecting and performing restoration treatments that will preserve the artifact’s unique characteristics. In this regard, the development of non-destructive techniques to assess an artifact’s condition before and after treatment is a crucial step to achieve this goal. Spectroscopic techniques, in particular, have played a pivotal role, particularly in the field of book heritage [
1,
2,
3,
4,
5,
6,
7,
8,
9].
The natural degradation process of paper over time results in both chemical and physical alterations. The primary constituent of paper, cellulose, undergoes two main degradation mechanisms through hydrolytic and oxidative reactions [
10]. Hydrolysis can occur in both acidic and basic environments causing depolymerization, whereas oxidation causes functional groups to bind to the cellulose, also disrupting its structure. The rate at which these processes occur is affect by several factors:
Intrinsic characteristics of the paper, including the raw materials used during production and manufacturing methods.
Nature of materials applied to the paper such as inks, pigments, binders, etc.
Possible presence of pathogens and air pollutants in the storage environment, as well as light exposure.
To preserve valuable artwork, it is essential to slow down and prevent degradation phenomena by implementing controlled restoration and protection techniques. Among the various paper restoration operations, cleaning plays a fundamental role. The aim of cleaning is to remove all cellulose degradation products as well as contaminant particles from the paper surface—in other words, materials that can alter the paper appearance and cause further aging.
In this context, cleaning treatments involving immersion in water are widely employed, as water effectively solubilizes most of the foreign particles on the surface, penetrating deeply in between cellulose fibers. Moreover, water promotes the formation of hydrogen bonds between cellulose fibrils, improving the bending resistance of paper sheets. However, washing treatments bring up some concerns: the uncontrolled water flow may come in contact with more degradable constituents or trigger unwanted processes, potentially causing irreversible alterations to the appearance of the artifact and the historical information it conveys.
Lately, materials of a certain texture such as hydrogels have been used to control volume and speed of water flow. Hydrogels are not reactive and retain large volumes of water and/or other solvents, releasing them on the paper surface upon contact. These solvents dissolve the target substances which are subsequently re-adsorbed by the hydrogels together with the solvents used.
The use of water alone in hydrogels has proven highly effective to clean water-soluble materials commonly found in book artifacts, such as polysaccharides (e.g., starch, gum arabic) and protein-based substances (e.g., gelatin, animal glues). For the removal of non-water-soluble substances, alternative solvents can be employed. Nevertheless, hydrogels make localized treatments on specific areas of paper possible, significantly reducing exposure of operators to potentially harmful vapors. The use of hydrogels as supporting materials is well-documented in the literature [
11,
12].
In this study, we employed a poly(acrylic acid) (PAAc) hydrogel, commercially available under the trade name Carbopol, loaded with TiO₂ nanoparticles for restoration purposes. Derived from polyacrylic acid, Carbopol can forms gels with good viscosity already at low concentrations (1–1.5% w/v) [
13,
14], which guarantees reduced adhesive capacity and, hence, easy removal. The incorporation of TiO₂ nanoparticles enhances the hydrogel's cleaning efficacy due to their photocatalytic properties. TiO₂ nanoparticles enable the inactivation of bacteria, fungi, and algae, as well as the removal of organic contaminants [
15,
16,
17,
18]. Moreover, TiO₂ is considered safe and harmless to humans.
The synthesis of TiO₂ thin coatings typically employs high temperatures or mechanical conditions incompatible with paper substrates [
19]. In a previous study, TiO₂-cellulose nanocomposite was deposited via spray techniques and was employed as paper consolidant to inhibit aging effects induced by UV light, air pollutants, and microbial activity [
20].
In this study, we incorporated laser-synthesized TiO₂ nanoparticles with high chemical purity into a PAAc matrix to create a composite hydrogel that could be applied on various paper samples [
13,
21] using a spatula. The photocatalytic process of TiO₂ was activated via UV irradiation at 385 nm.
Two experimental approaches were then followed. In the first, the CBP/TiO₂ hydrogel was used as a cleaning agent. When in contact with the porous surface of paper, the gel gradually releases water, facilitating cleaning. We optimized contact time to ensure removal of oxidation products and contaminants without compromising the structural integrity of the paper, considering restoration needs. We conducted experiments with varying contact times to determine the optimal cleaning conditions.
The effects of the cleaning procedures were analyzed by confocal Raman spectroscopy and confocal laser microscopy. In a previous study [
7], we identified the following paper aging markers based on Raman measurements:
Where I and A indicate intensity and area of peaks in the selected Raman shift range respectively.
These markers account separately for the hydrolysis and oxidation processes on cellulose, the main constituents of paper structure. Hydrolysis, which shortens cellulose chains, leads to a decrease of the
RH marker, proportional to the degree of polymerization, and of the crystallinity index
CI [
7]. Oxidation processes, which involve the addition of functional groups to the cellulose backbone, lead to an increase of the
OT marker, followed by an increase of the
OI marker, which is directly proportional to the area of carbonyl bands (A
1640-1850), representing the final oxidation stages of cellulose and, therefore, giving insight on the advancement state of the whole oxidation process.
Their variation in percentage was used to evaluate the cleaning procedure efficiency.
After removal of the gel, residues can be found on the treated surfaces. Their presence was assessed via confocal Raman spectroscopy by surface scanning and via Confocal Laser Scanning Microscopy (CLSM).
Comparisons between treatments involving the CBP/TiO₂ composite gel activated with UV irradiation and the unmodified CBP gel showed a significant reduction in oxidation markers, whereas polymerization degree and crystallinity markers remained unaffected, showing that paper integrity was preserved.
In a second experimental procedure, a thin layer of CBP/TiO₂ gel was applied to the paper without removal after UV activation. This approach aimed to evaluate its performance as a protective coating against light-induced aging.
Our measurements demonstrated that the CBP/TiO₂ coating effectively slowed down hydrolysis and prolonged the decrease of oxidation markers.
2. Results
2.1. Characterization of Cleaning Process with Raman Micro-Spectroscopy and Confocal Laser Microscopy
In our experiments, we analyzed different paper samples.
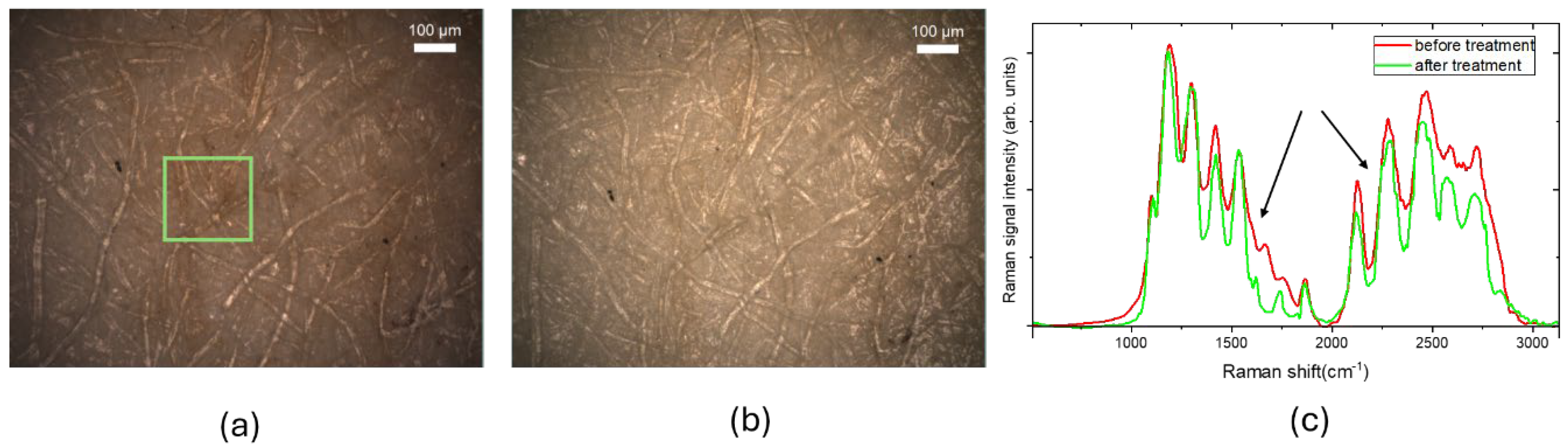
Figure 1 shows white light optical images of a 19th-century paper sample before (a) and after (b) the cleaning treatment. The green square marks the 200 µm × 200 µm area scanned via Raman spectral imaging mode.
Figure 1.c shows the typical Raman spectra detected in a point of the square.
The main peaks observed in the Raman spectra are to be attributed to vibrational modes of carbon-based species. These include C–C stretching and C–O–H and C–C–H vibrations in the 1000–1350 cm⁻¹ range, as well as lower intensity in-plane bending vibrations of H–C–H and O–C–H at 1425 cm⁻¹ [
7,
9,
22,
23]. Following the cleaning treatment, a decrease in intensity was observed for wavenumbers greater than 1500 cm⁻¹, due to formation of oxidized molecular groups (excluding CH-CH₂ stretching peaks at 2850–3000 cm⁻¹).
In particular, the bands at 1640 and 1740 cm⁻¹ are respectively ascribed to C=O stretching in carbonyl and carboxyl groups. The band at 1550 cm⁻¹ is ascribed to symmetric C=C stretching in C=C–O structures, while the peak at 1850 cm⁻¹ represents an overlap between C=O stretching and allene C=C=C stretching. Vibrations in the 2000–2100 cm⁻¹ region are attributed to C=C=O stretching of ketene groups. Finally, the peaks around 2550 cm⁻¹ are associated with overtones and combinations of carboxylic group frequencies.
The cleaning treatments were carried out by applying the hydrogel onto the paper samples, with contact times of up to 1 hour. Some additional tests were performed with a contact time of 2 hours, but the changes in the markers were not significantly different from those observed after 1 hour and transferring and excessive amount of water onto the paper surface was also a risk. During the cleaning treatment involving the CBP/TiO₂ nanoparticles, the gel was irradiated with UV light for the duration of the treatment.
For each spectrum in the Raman map, both before and after gel application, the values of the markers defined in (1) – (4) were calculated, obtaining a distribution of marker values for each map.
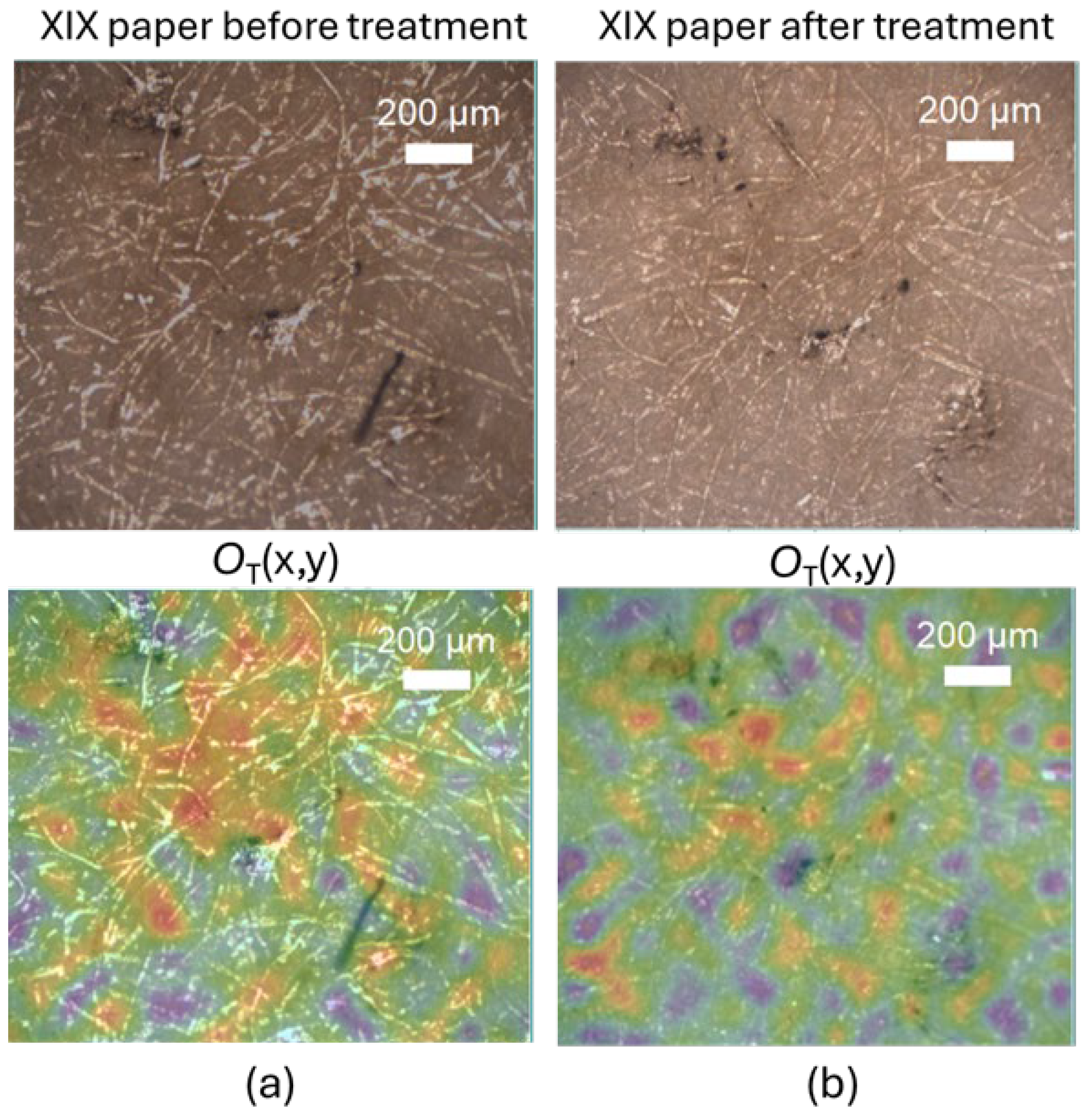
As an example,
Figure 2 shows the
OT marker maps, in false colors from lower values in green to higher values in yellow - red, co-localized with the optical image of the XIX century paper sample: a large dark stain is visible in
Figure 2.a, which becomes lighter after cleaning, as shown in
Figure 2.b. Contact time was 1 hour. By monitoring the peaks associated with the formation of oxidized molecular groups using the
OT marker, we can clarify the activity of CBP. Following the cleaning treatment, the yellow-red areas of the paper, corresponding to higher
OT values, decreased.
For each marker map, we calculated the average marker values before and after the CBP cleaning treatments, with and without the addition of TiO₂ nanoparticles.
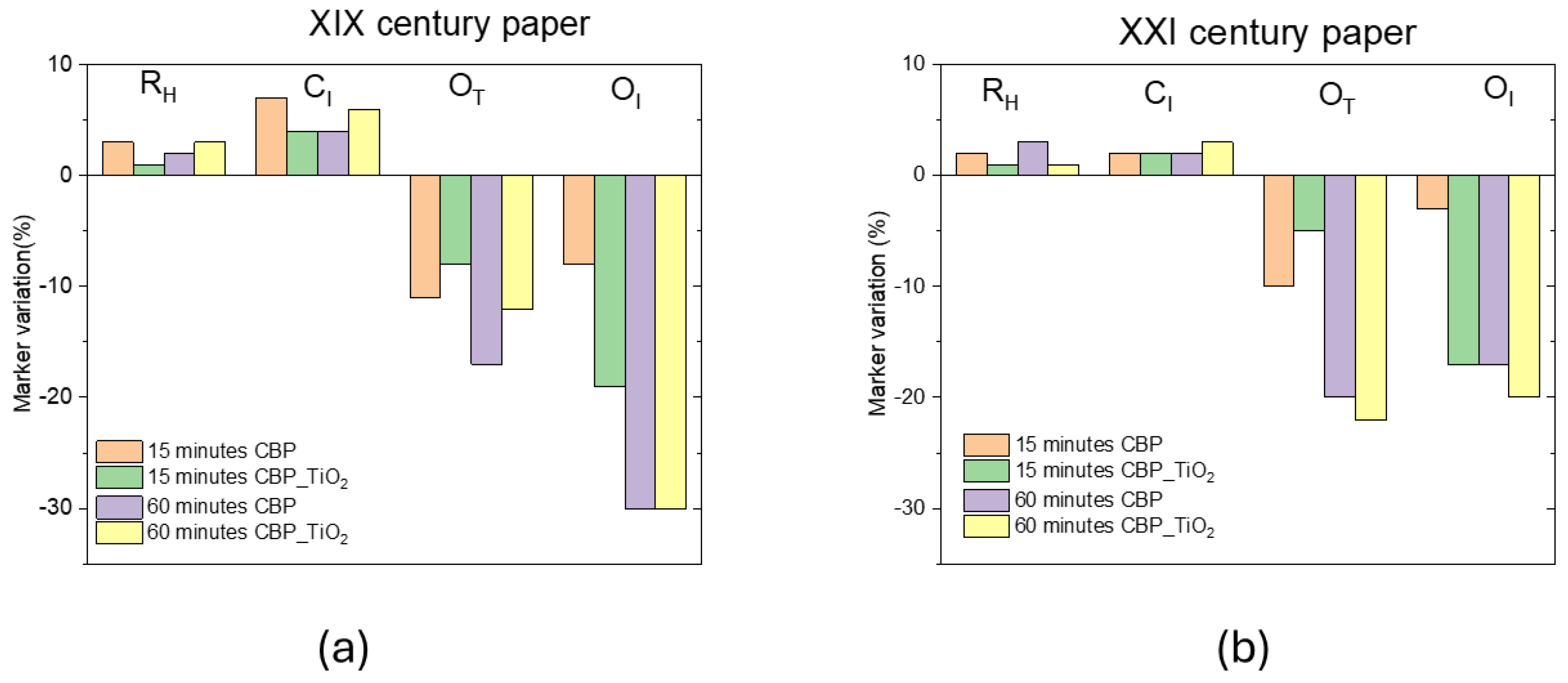
Figure 3 illustrates the observed changes in the marker values for the two paper samples used in our study: a XIX century paper sample as well as a 2021 laser printer paper which had been exposed to ambient laboratory light for three years. Although the most pronounced effects on marker values were observed in the XIX century paper, both treatments resulted in an increase in the
CI and
RH markers. This can be attributed to the formation of hydrogen bonds between cellulose fibers facilitated by the hydrogel’s water content. It was shown that after washing and drying processes the formation of additional hydrogen bonds can occur, enhancing some mechanical properties of the paper, such as bending resistance.
Oxidation markers decreased significantly (-20% OT and -30% OI after 1 h of treatment with CBP with and without TiO2 addition). The reduction in OI, which is proportional to the carbonyl content, suggests that oxidation products containing C=O groups were primarily removed. This is particularly noteworthy because such carbonyl-containing groups contribute to the formation of chromophore molecules responsible for the yellowish color typical of aged paper.
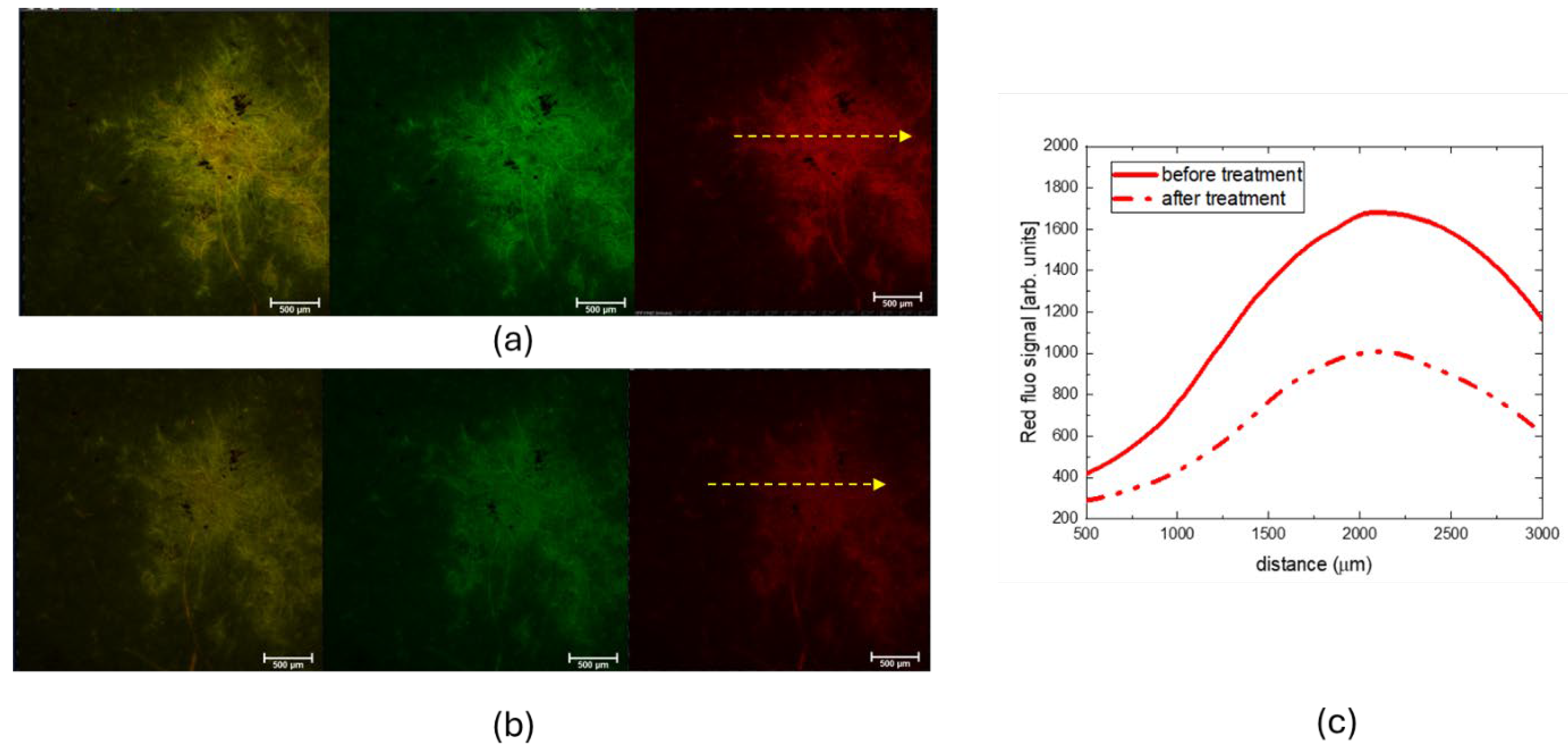
Figure 4 shows the CLSM images obtained in fluorescence mode, showing both red and green fluorescence channels, as well as their merge, from a selected region of the 19th-century paper sample before (a) and after (b) the CBP/TiO₂ cleaning treatment. The graph on the right displays the intensity profiles of the red fluorescence signal, measured along the yellow arrows.
It is known that one of the effects of cellulose aging is the increased luminescence intensity of paper, due to the formation of products like simple sugars, cellulose oligomers, and phenolic compounds originating from the degradation of cellulose, hemicellulose, and lignin [
24,
25]. Therefore, the observed decrease in fluorescence intensity, as evidenced by the CLSM measurements, confirms the cleaning efficiency of CBP/TiO₂ treatment.
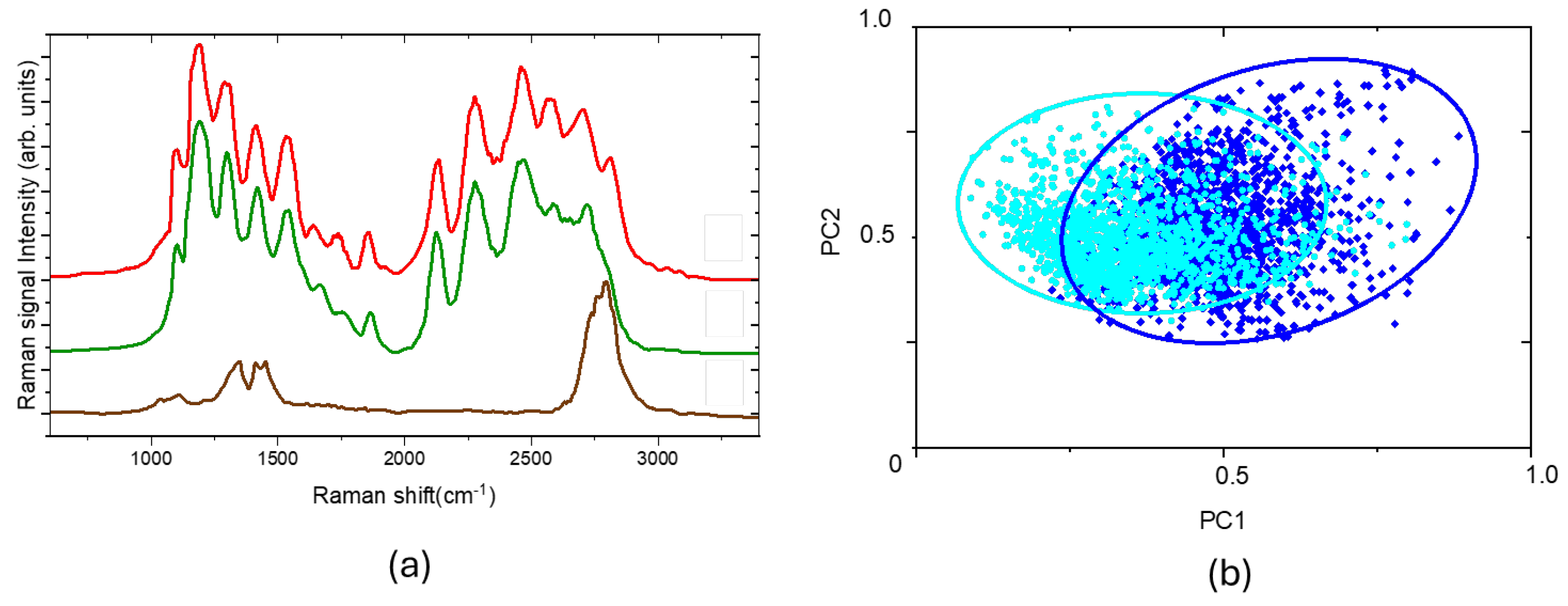
In all the tests conducted, we removed the gel residues with a spatula and assessed its efficacy by using the Classical Least Square (CLS) fitting. The CLS fitting allows the detected spectra to be represented as a linear combination of suitable reference Raman spectra (components). These spectra are used as components to attribute the coefficients (scores) that represent the contributions of each component in the linear combination.
In our case, the Raman spectrum (red curve) shown in
Figure 5.a, measured from the cleaned XIX century paper sample, can be seen as a linear combination of the CBP/TiO₂ gel (PC1 component, green curve) and the paper (PC2 component, brown curve) spectra.
The corresponding score plot was constructed by plotting the linear combination coefficients (scores) for each Raman spectrum of the acquired map. This score plot can be employed for the analysis of large datasets between two conditions, helping to quickly identify the most meaningful changes and providing a measure of statistical significance.
Figure 5.b shows the score plot of the maps acquired before (blue points) and after (cyan points) the gel removal with the spatula. The ellipse containing the scores shifts toward smaller values of the PC1 (gel) component, allowing us to monitor and evaluate the effectiveness of gel removal and determine whether it was sufficient or not.
Although the application of the CBP/TiO₂ hydrogel leads to a noticeable decoloration of the paper (see
Figure 1 and
Figure 2), there is no great improvement in the decrease of oxidation markers with the use of the composite hydrogel. However, the presence of TiO₂ nanoparticles is expected to provide a biocidal effect, and any residual gel left on the paper could prolong the protective activity against aging agents. In this regard, additional tests were performed by applying a thin layer of the CBP/TiO₂ gel onto the paper, aiming to evaluate its performance as protective layer, as discussed in the following paragraph.
2.2. Characterization of Protective Effect of CBP/TiO2 Coating
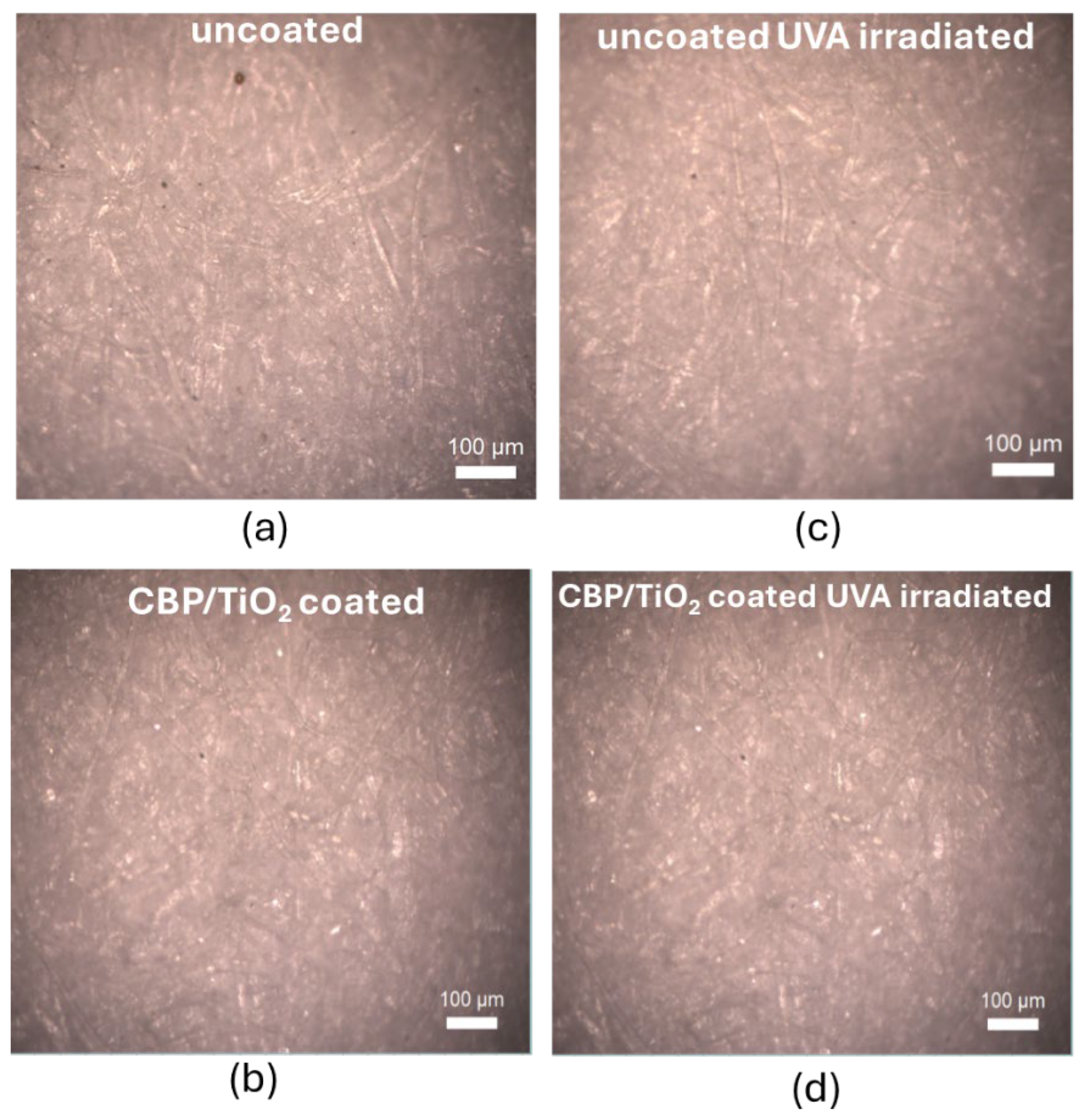
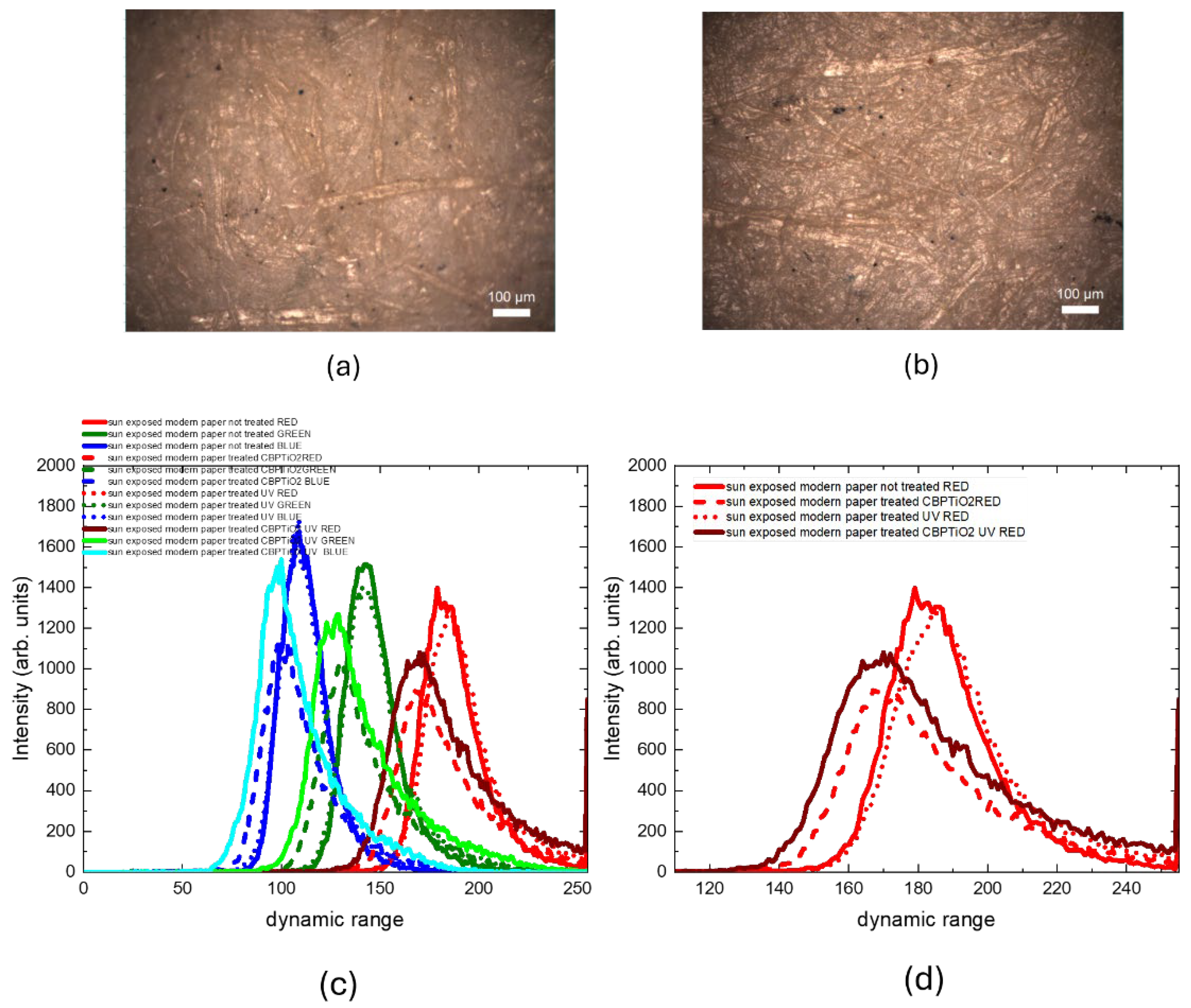
To evaluate the suitability of the CBP/TiO₂ coating as a protective layer for paper, we used two samples of modern (2024) laser printer paper. One sample was coated with a thin layer of CBP/TiO₂ gel applied with a spatula, while the other remained uncoated. Both samples were aged by UVA irradiation for 31 hours. The optical images, shown in
Figure 6, were subsequently analyzed using ImageJ software to evaluate RGB value distributions of the images.
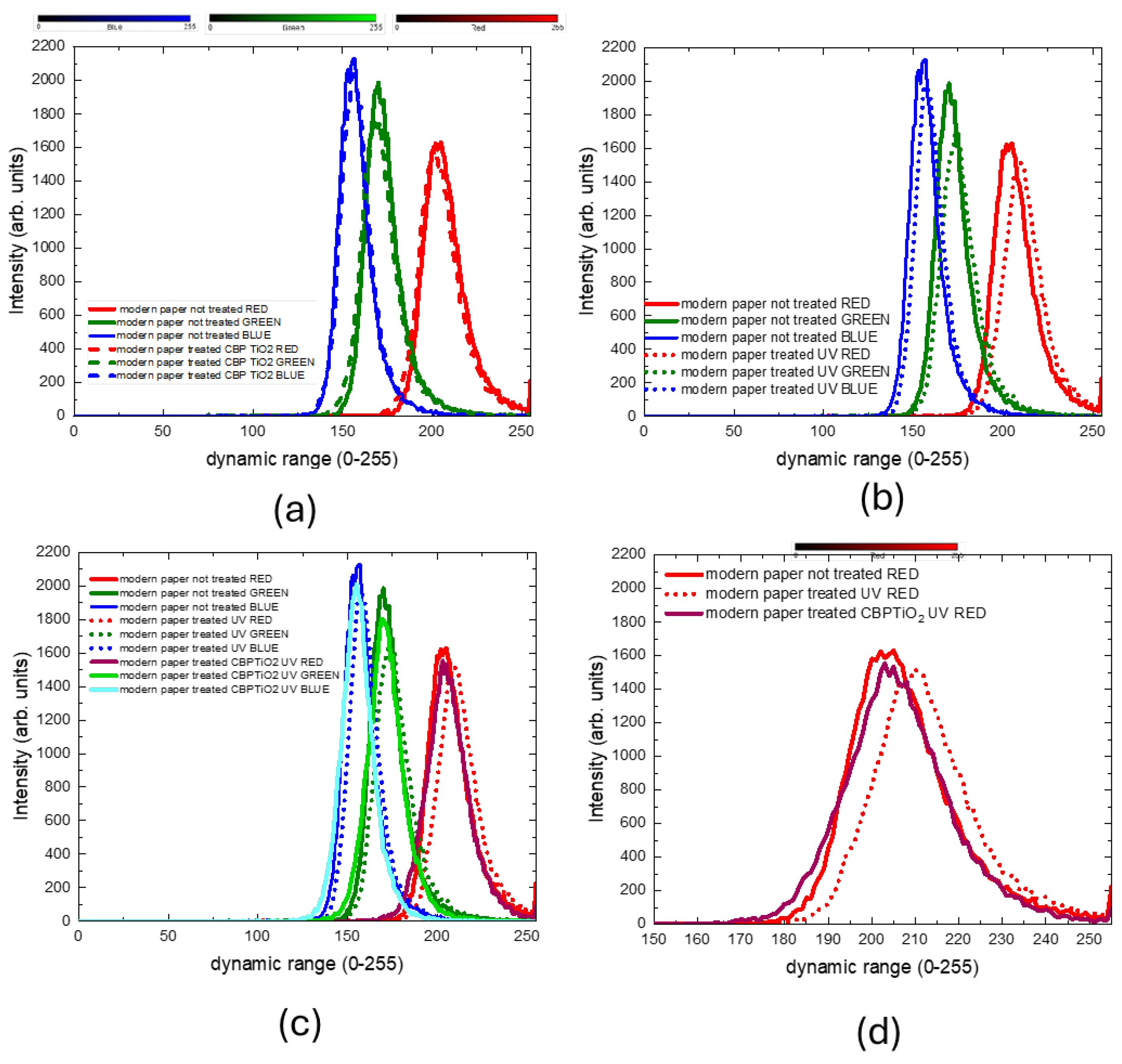
The RGB histogram of the coated and uncoated samples, shown in
Figure 7.a, indicates that the CBP/TiO₂ coating is a transparent layer that does not significantly alter the color of the paper.
As anticipated, prolonged exposure to UVA irradiation, see
Figure 7.b, leads to a color change in the uncoated paper, as demonstrated by a shift towards higher RGB values. In contrast, RGB values of the coated paper before and after UVA irradiation (see
Figure 7.c,d) remain similar, showing that the CBP/TiO₂ coating effectively preserves the paper from light-induced yellowing.
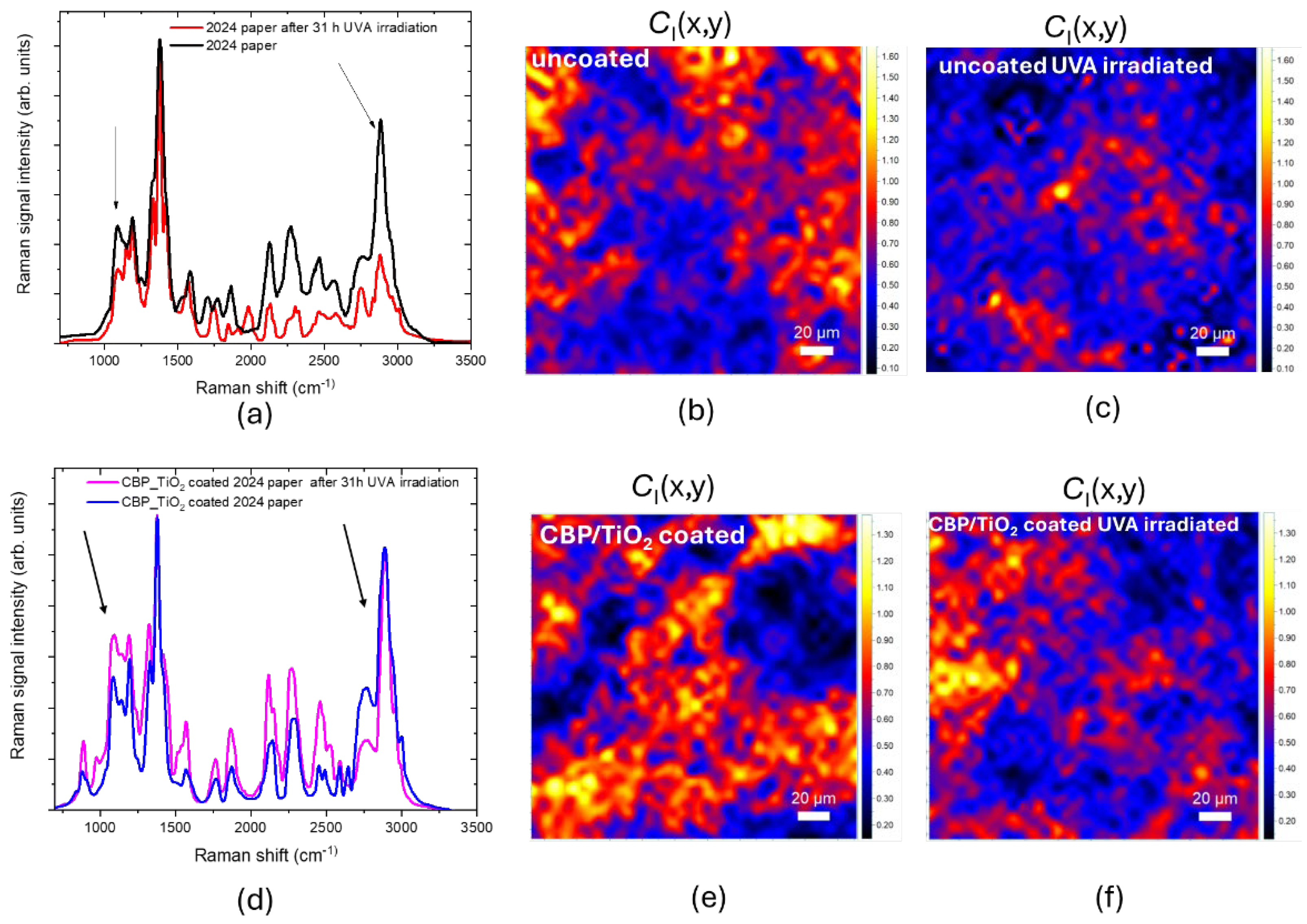
As shown in
Figure 8.a, the Raman spectra acquired from the 2024 paper before and after 31 hours of UVA irradiation exhibit a significant difference, particularly in the C–O–C and CH₂ peaks, which are notably reduced after UVA exposure. This finding suggests light-induced degradation of cellulose via hydrolysis. The shortening of the cellulose chains is accompanied by an increase in molecular disorder, as evidenced by the decrease in the C
I marker, shown in
Figure 8.b.
Differently, for the 2024 coated paper, the decrease in the C–O–C and CH₂ peaks is less pronounced. (
Figure 8.d–f)
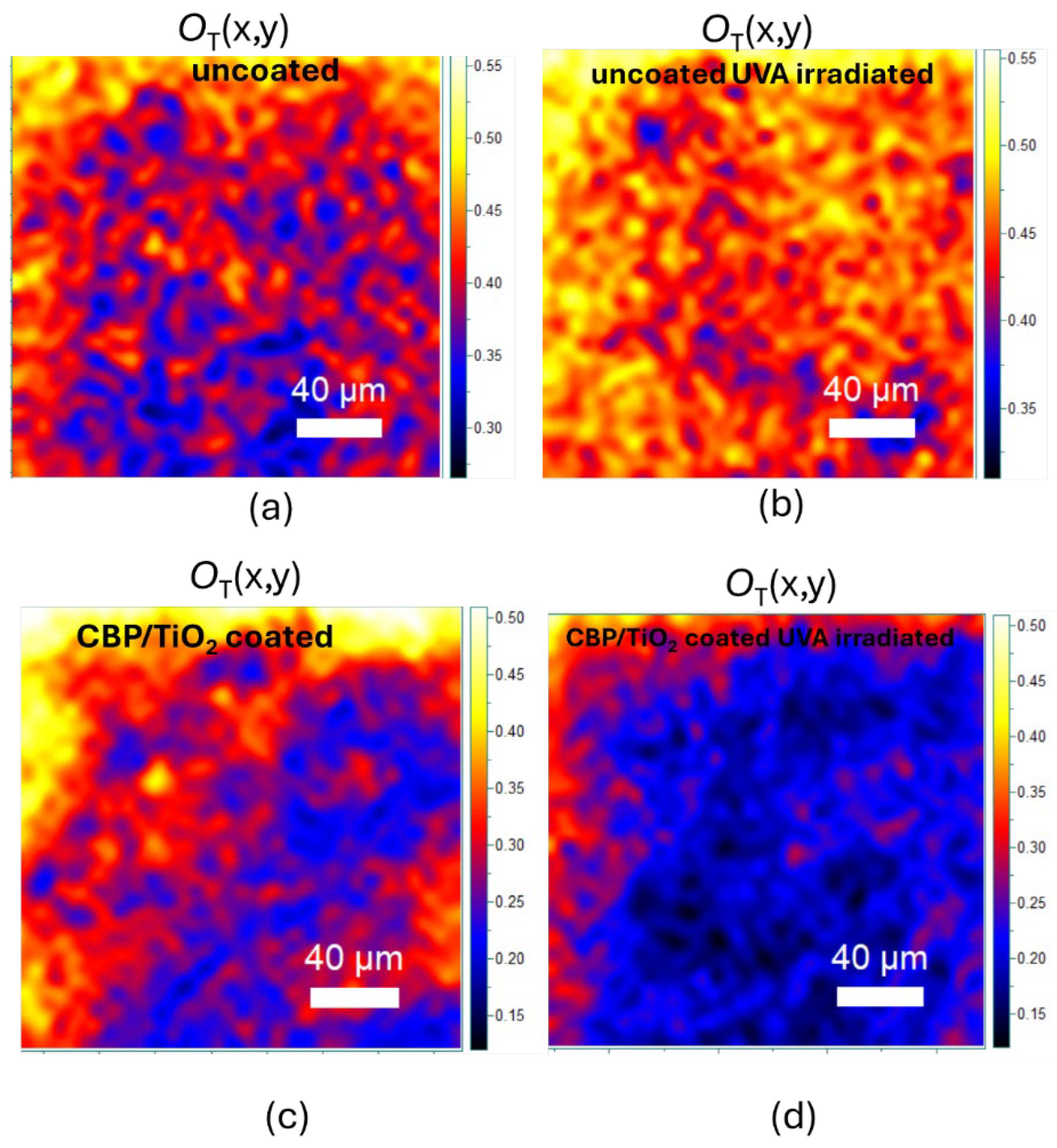
The protective effect of the coating against cellulose oxidation was also assessed. As shown in
Figure 9.a,b, after UVA exposure, the O
T marker increases, due to the incorporation of oxidized functional groups into the cellulose backbone. As for the coated paper, the O
T marker decreases (
Figure 9.c,d), suggesting that the UVA irradiation may prolong the cleaning action of the gel.
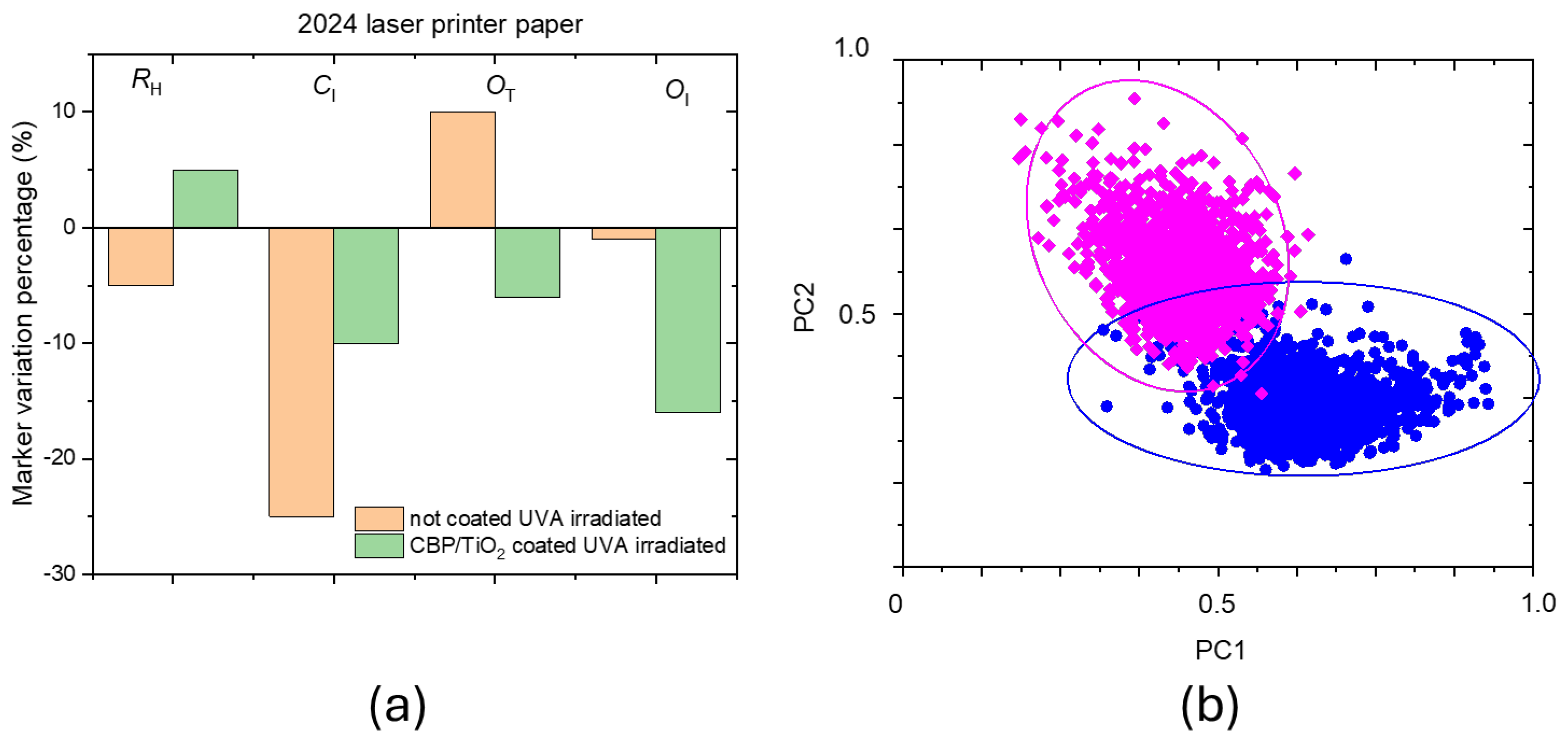
The marker variation before and after 31 hours of UVA irradiation is shown in percentage in
Figure 10 for both uncoated and coated 2024 paper samples. The CBP/TiO₂ coating not only inhibits the oxidation and hydrolysis of cellulose but also inhibits the decrease of C
I.
Figure 10b illustrates the score plots for coated samples before (blue dots) and after (fuchsia dots) UVA irradiation. The observed reduction in gel scores may be attributed to the gel depletion on the paper surface caused by UVA exposure. However, as observed in previous experiments [
13], an alternative hypothesis to consider is the penetration of the gel layer beneath the outer fiber of the sample surface
The same procedure was performed on the 2021 paper that had undergone the natural aging processes in the laboratory environment (see
Figure 11 a―b). In this case, as shown in
Figure 11 c―d, the clearing effect of CBP/TiO
2 was preserved when exposed to UV radiation.
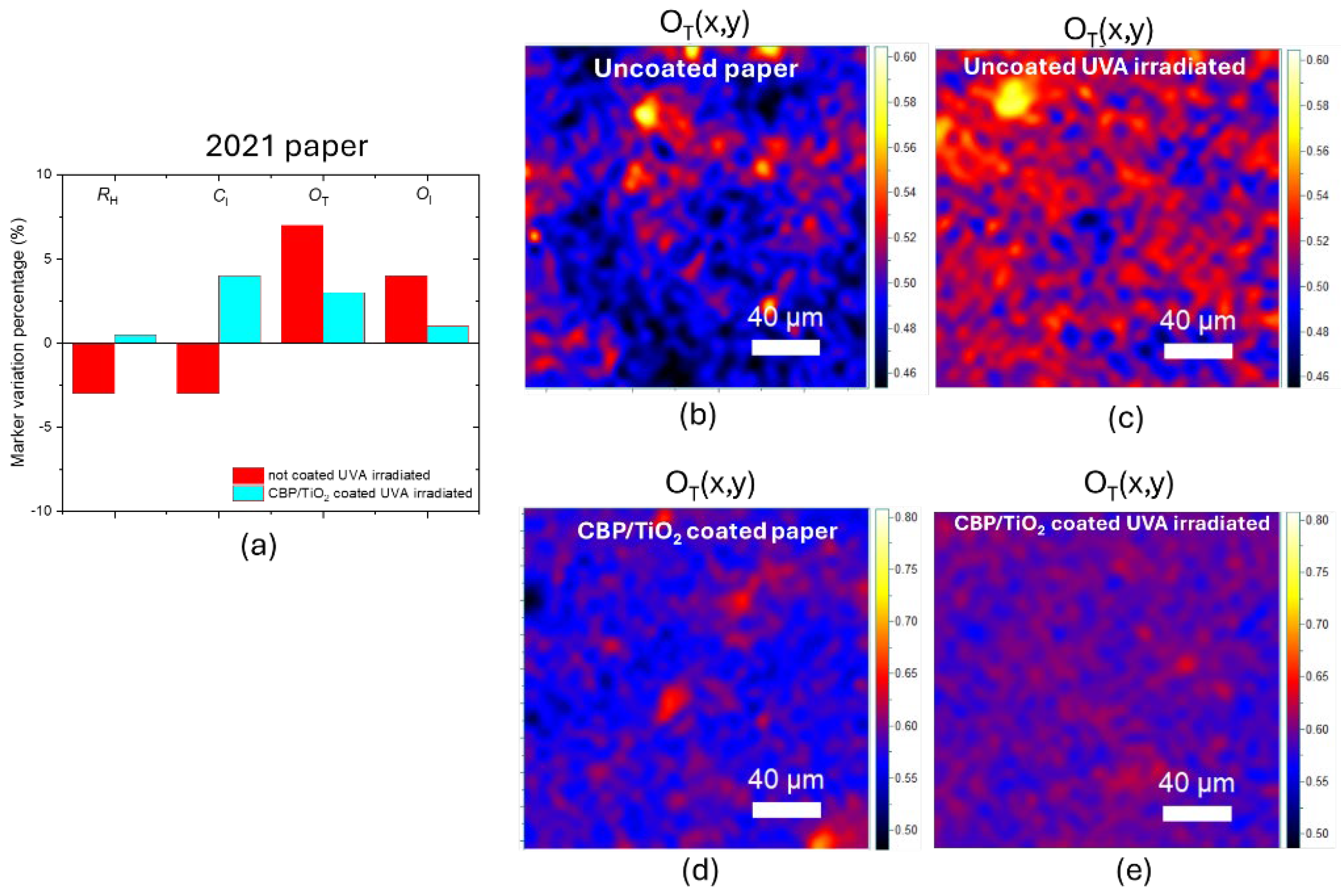
The marker variation shown in
Figure 12a highlights minimal degradation for samples coated with the CBP/TiO₂ composite. Specifically, the CBP/TiO₂ coating induces only a 3% increase in the O
T marker following UVA irradiation. In comparison, the uncoated 2021 paper exhibits a 7% increase in the O
T marker, which is slightly lower than the value observed for modern paper (
Figure 12a―b). The difference can be explained by the fact that the 2021 paper sample had already been exposed to ambient light for three years, in which the oxidation process progressed rapidly during its initial stage and later slowed down, as it is typical.
To further assess the protective properties of the composite gel, an additional 2021 paper sample was prepared and coated with a thicker layer of CBP/TiO₂.
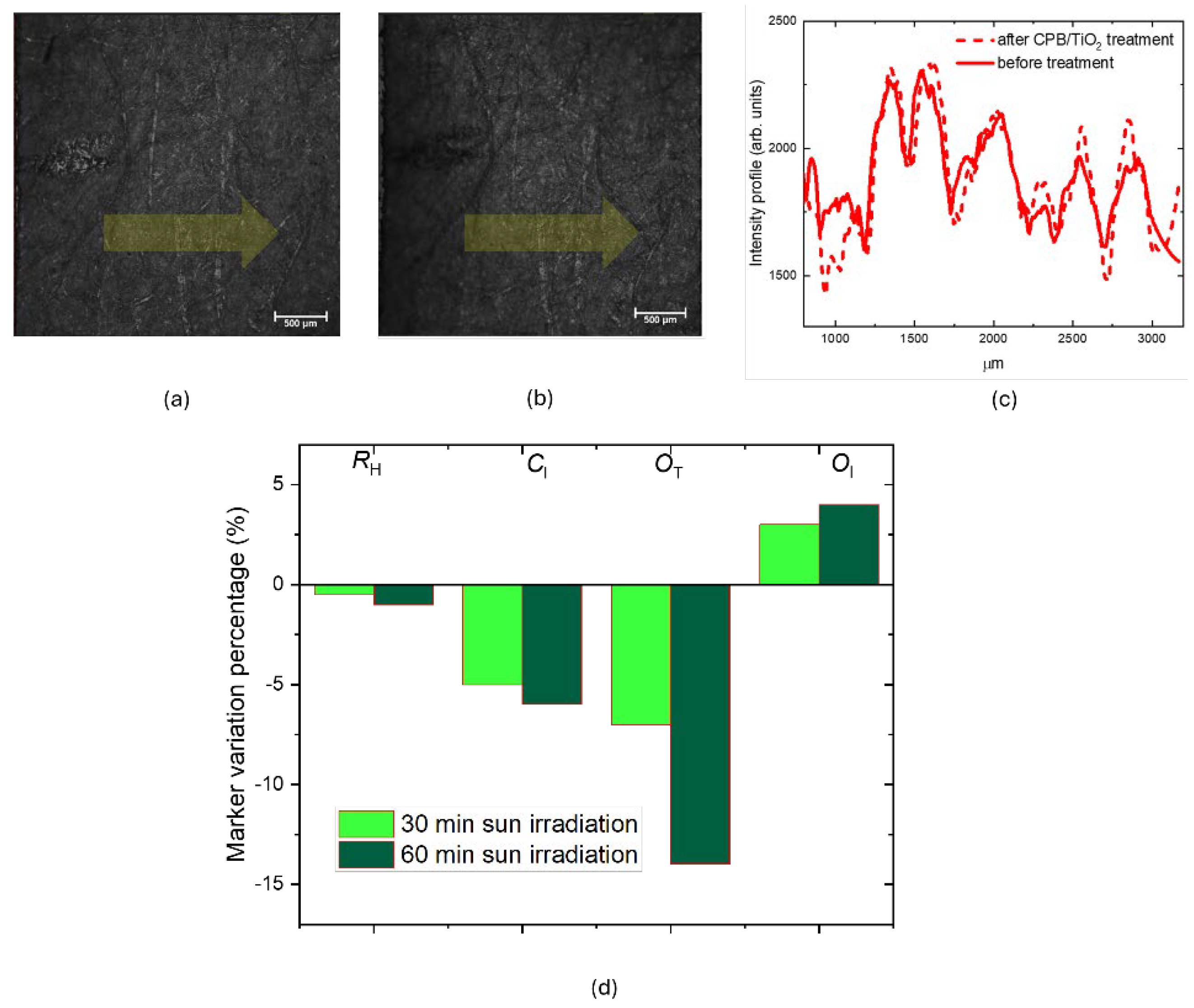
Figure 13a shows CLSM images, in reflection mode, comparing coated (a) and uncoated (b) paper samples under identical acquisition conditions. A short pencil line, visible on the left side of the images, was used as spatial reference to facilitate microscope observation of the same area before and after treatment.
The slightly increased thickness of the coating layer does not alter the optical image resolution of the paper, as confirmed by the intensity profiles measured along the yellow arrow shown in
Figure 13c. The samples were exposed to outdoor sunlight (22 W/m
2, May 16th, 2022) for 30 minutes and 1 hour . A slight but progressive decrease in the R
H index was observed, due to hydrolysis processes. The O
T marker showed a progressive decrease with increased irradiation time. On the other hand, the O
I marker exhibited an increase, suggesting that although the photoactivated TiO₂ decomposes organic oxidizing acids, the oxidation process continues, forming additional C=O bonds [
7].
3. Discussion
The aim of the present study was to evaluate the effectiveness of cleaning treatments using a CBP/TiO₂ composite gel activated by UV light, through a Raman spectroscopy-based diagnostic protocol. Compared to the application of CBP alone, the addition of TiO₂ slightly improves the removal of oxidizing functional groups, while also ensuring biocidal activity. Moreover, by using CLS fitting of Raman spectra, we were able to monitor the effectiveness of gel manual removal.
Existing literature suggests the potential of titanium dioxide nanoparticles for protecting artworks from light-induced degradation. To assess the ability of the CBP/TiO₂ coating to shield paper surfaces from light aging, we exposed various paper samples, coated and uncoated, to UVA radiation.
Our measurements show that, in the coated samples, hydrolysis is slowed down, and oxidizing agents are progressively removed through a self-cleaning process, even when exposed to outdoor sunlight.
Additionally, the presence of the coating layer does not alter the original color of the paper and helps prevent light-induced yellowing.
Figure 13.
CLSM images obtained in reflection mode of the 2021 paper. (a) uncoated; (b) coated; (c) the intensity profiles measured along the yellow arrow; (d) Marker variation percentage for 30 and 60 minutes of irradiation. .
Figure 13.
CLSM images obtained in reflection mode of the 2021 paper. (a) uncoated; (b) coated; (c) the intensity profiles measured along the yellow arrow; (d) Marker variation percentage for 30 and 60 minutes of irradiation. .
4. Materials and Methods
4.1. Nano-Composite Hydrogel Preparation
Carbopol® Ultrez 10 (CAS No. 9003-01-4) from Lubrizol (Wickliffe, OH, USA) has a high level of purity. The following specifications were reported by the manufacturer: loss on drying ≤ 2%, heavy metals ≤ 10 ppm, and residual solvents ≤ 0.45%.
A 3.33 wt% solution of Carbopol® in water was used for the hydrogel matrix.
Since Carbopol® reaches maximum viscosity at pH 7, a 20 wt% NaOH solution was employed as a neutralizing agent [
26,
27,
28]. To achieve a homogeneous texture, TiO₂ nanoparticles were incorporated into the mixture, followed by sonication to ensure uniform dispersion. The resulting gel proves to be suitable for easy application on substrates. Furthermore, it holds long-term stability and retains its properties even at elevated temperatures, provided it is stored in a sealed container shielded from light.
The primary preparation conditions for the composite hydrogel are summarized in
Table 1 and the resulting hydrogel structure is illustrated in
Figure 14.
Titania nanoparticles employed were synthesized via CO₂ laser-induced pyrolysis reactions in a titanium(IV) isopropoxide aerosol, following a previously established protocol [M1_20]. This method ensures high chemical purity of the nanoparticles, as the reactor walls remain cold and non-reactive throughout the process. An estimation carried out throught BET measurements shows a mean powder diameter of 20 nm.
When irradiated with photons of energy greater than its band gap, TiO₂ undergoes excitation and generates free electron-hole pairs:
These electron-hole pairs persist briefly before recombination, leaving the molecule in an excited state long enough to allow them to interact with chemical species adsorbed on the NP surface. Organic contaminants are therefore removed by redox reactions [De Filpo et al., 2015].
4.1. Paper Samples
Paper samples were cut from 2024 and 2021 laser printing paper. The 2021 sample was exposed for three years to ambient light. Additionally, we examined non printed areas of an ancient book (Brehm, Vita degli Animali, vol. 6, Torino 1896). The book was positioned on the sample holder.
4.1. Gel Application Procedure
The samples were divided into two subsamples and treated with CBP and CB10_TiO2 composite gel. The gels were applied as a homogeneous layer using a spatula. In the case of the CB10_TiO2 composite gel, the sample was then placed under a UV light source (LED lamp) to activate the TiO2 nanoparticles. After treatment, the gel was easily removed with the use of a spatula. Contact times for both cleaning procedures were set at 15 minutes and 1 hour.
4.2. UV Source
The photocatalytic process of titania nanoparticles was activated with a high power UV LED source emitting a peak wavelenght at 385 nm. The LED was used by mounting a collimation optics obtaining a closely collimated beam with a power of 120 mW on a beam area of 1450 mm2. This UV LED was used also for light ageing process.
4.3. Raman Confocal Spectrometer
Raman spectra were acquired using a confocal micro-Raman spectrometer (Horiba XploRA Plus) with a 532 nm laser wavelength. The Raman signals were collected through a microscope equipped with 5x, 10x, 50x, and 100x objectives. Laser power was adjusted using neutral density filters. After preliminary studies, optimal conditions for both laser power and acquisition times were selected to achieve good signal-to-noise ratio while ensuring safe operating conditions for the samples. Raman spectra were recorded point-by-point across a selected area, following a predefined grid defining a 2D map and defining a 2D spectra array.
The fluorescence background was automatically subtracted from each Raman spectrum, for which the software also calculated the intensity, peak area, and peak width, which were used as contrast parameters. In the case of partially overlapping bands, the software performed spectral deconvolution, obtaining the spectrum as the best fit of a superposition of Gaussian curves. For each defined contrast parameter, a 2D Raman map was generated and associated with the optical image collected.
4.4. Confocal Laser Scanning Optical Microscope
Optical images were acquired using a confocal laser scanning microscope (CLSM) Nikon 80i-C1, operating in both fluorescence and reflection modes [
29]. In fluorescence mode, the samples were illuminated with a continuous 445-nm laser (nominal output power of 1.5 mW), and the spectrally integrated PL signal was detected by a system of two photomultiplier tubes, which separately and independently acquired signals in two distinct visible spectral ranges (red and green), selected using an optical filtering system: a long-pass filter at 560 nm for the red signal and a filter transmitting from 500 nm to 530 nm for the green signal. In reflection mode, the samples were illuminated using a 532-nm laser (nominal output power of 3 mW), and the reflected signal was detected by a PMT.
5. Conclusions
Ancient books, artworks, and important documents preserved in archives are subject to chemical and biological deterioration processes, which can lead to irreversible damage.
Several hydrogel cleaning treatments are being studied to slow down these processes without altering the properties of the paper. In this study we investigated the use of CBP/TiO2 as protective agent for paper artworks, employing a non-invasive diagnostic protocol based on Raman spectroscopy. Prolonged exposure to UVA radiation was used to simulate ageing and/or damaging effects.
It has been observed that papers coated with the composite gel layer exhibit better color stability, compared to uncoated papers, when exposed to UVA radiation; a lower content of oxidizing agents content was observed as well as inhibition of hydrolysis process.
Author Contributions
Conceptualization, S.B. and F.B.; methodology, S.B. and F.B.; validation, S.B., F.B.; data curation, S.B., F.B., J.R., M.G.S.; writing—original draft preparation, S.B.; writing—review and editing, S.B., F.B., J.R. All authors have read and agreed to the published version of the manuscript.”
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable
Data Availability Statement
All data are available from the corresponding authors upon reasonable request.
Acknowledgments
S.B and F.B thank the IAEA Coordinated Research Project (CRP) F22082 “Development and Implementation of Cultural Heritage Preservation using Ionizing Radiation Technology.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Manso, M.; Carvalho, M.L. Application of spectroscopic techniques for the study of paper documents: a survey. Spectrochim. Acta Part B At. Spectrosc. 2009, 64, 482–490. [Google Scholar] [CrossRef]
- Bitossi, G.; Giorgi, R.; Mauro, M.; Salvadori, B. Spectroscopic techniques on cultural heritage conservation: a survey. Appl. Spectrosc. Rev. 2005, 40, 187–228. [Google Scholar] [CrossRef]
- Chiriu, D.; Ricci, P.C.; Cappellini, G.; Carbonaro, C.M. ; Ancient and modern paper: study on ageing and degradation process by means of portable NIR μ-Raman spectroscopy. Microchemical Journal 2018, 138, 26–34. [Google Scholar] [CrossRef]
- Librando, V.; Minniti, Z.; Lorusso, S. Ancient and modern paper characterization by FTIR and Micro-Raman Spectroscopy. Conservation Science in Cultural Heritage 2011, 11, 249–268. [Google Scholar]
- Balakhnina, I.A.; Brandt, N.N.; Chikishev, A.Y.; Rebrikova, N.L. Raman microspectroscopy of old paper samples with foxing. Appl. Spec. 2018, 68, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Lojewska, J.; Miskowiec, P.; Lojesewski, T.; Proniewicz, L.M. Cellulose oxidative and hydrolytic degradation: in situ FTIR approach. Polym. Degrad. Stabil. 2005, 88, 512–520. [Google Scholar] [CrossRef]
- Botti, S.; Bonfigli, F.; Nigro, V.; Rufoloni, A.; Vannozzi, A. Evaluating the Conservation State of Naturally Aged Paper with Raman and Luminescence Spectral Mapping: Toward a Non-Destructive Diagnostic Protocol. Molecules 2022, 27, 1712. [Google Scholar] [CrossRef] [PubMed]
- Refugio Martínez, J.; Nieto-Villena, A.; De la Cruz-Mendoza, J.A.; Ortega-Zarzosa, G.; Lobo Guerrero, A. Monitoring the natural aging degradation of paper by fluorescence. J. Cult. Herit. 2017, 26, 22–27. [Google Scholar] [CrossRef]
- Chiriu, D.; Ricci, P.C.; Cappellini, G.; Salis, M.; Loddo, G.; Carbonaro, C.M. Ageing of ancient paper: A kinetic model of cellulose degradation from Raman spectra. J. Raman Spectrosc. 2018, 49, 1802–1811. [Google Scholar] [CrossRef]
- Teygeler, R. Preserving paper: recent advances. In Managing preservation for libraries and archives. Current Practice and future developments, ed. J. Feather, Aldershot: Ashgate, 2004.
- Mazzuca, C.; Micheli, L.; Carbone, M.; Basoli, F.; Cervelli, E.; Iannuccelli, S.; Sotgiu, S.; Palleschi, A. Gellan hydrogel as a powerful tool in paper cleaning process: a detailed Study. Journal of Colloid and Interface Science 2014, 416, 205–211. [Google Scholar] [CrossRef]
- De Filipo, G.; Palermo A., M; Tolmino, R.; Formoso, P.; Nicoletta F., P. Gellan gum hybrid hydrogels for the cleaning of paper artworks contaminated with Aspergillus versicolor. Cellulose 2016, 23, 3265–3279. [Google Scholar] [CrossRef]
- Botti, S.; Bonfigli, F.; D’Amato, R.; Rodesi, J.; Santonicola, M.G. Colorimetric Sensors Based on Poly(acrylic Acid)/TiO2 Nanocomposite Hydrogels for Monitoring UV Radiation Exposure. Gels 2023, 9, 797-1–797-12. [Google Scholar] [CrossRef]
- Talarico, F.; Caldi, C.; Valanzuela, M.; Zaccheo, C.; Zampa, A.; Nugari, M. P. Applicazione dei gel come supportanti nel restauro”, Bollettino ICR, n. 3, 2001, 101-118.
- De Filpo, G.; Palermo, A.M.; Tolmino, R.; Formoso, P.; Nicoletta, F.P. Gellan gum hybrid hydrogels for the cleaning of paper artworks contaminated with Aspergillus versicolor. Cellulose 2016, 23, 3265–3279. [Google Scholar] [CrossRef]
- De Filpo, G.; Mormile, S.; Nicoletta, F.P.; Chidichimo, G. Fast, self-supplied, all-solid photoelectrochromic film. J. Power Sources 2010, 195, 4365–4369. [Google Scholar] [CrossRef]
- De Filpo, G.; Palermo, A.M.; Rachiele, F. , Nicoletta, F.P. Preventing fungal growth in wood by titanium dioxide nanoparticles. Int Biodeterior Biodegrad. 2013, 85, 217–222. [Google Scholar] [CrossRef]
- De Filpo, G.; Palermo, A.M.; Munno, R.; Molinaro, L.; Formoso, P.; Nicoletta, F.P. Gellan gum/titanium dioxide nanoparticle hybrid hydrogels for the cleaning and disinfection of parchment. Int Biodeterior Biodegrad. 2015, 103, 51–58. [Google Scholar] [CrossRef]
- J.T. Rantala, J.T. A.H.O. Kärkkäinen, A.H.O. Optical properties of spin-on deposited low temperature titanium oxide thin films, Optic Express 2003, 11, 1406–1410.
- Afsharpour, M.; Rad, F.T.; Malekian, H. New cellulosic titanium dioxide nanocomposite as a protective coating for preserving paper-art-works. J Cult Herit. 2011, 12, 380–383. [Google Scholar] [CrossRef]
- D’Amato, R.; Falconieri, M.; Gagliardi, S.; Popovici, E.; Serra, E.; Terranova, G.; Borsella, E. Synthesis of ceramic nanoparticles by laser pyrolysis: from research to applications. Journal of Analytical and Applied Pyrolysis 2013, 104, 461–469. [Google Scholar] [CrossRef]
- Wiley, J.H.; Atalla, R.H. Band assignments in the Raman spectra of celluloses. Carbohydrate Research 1987, 160, 113–129. [Google Scholar] [CrossRef]
- Botti, S.; Di Lazzaro, P.; Flora, F.; Mezi, L.; Murra, D. Raman spectral mapping reveal molecular changes in cellulose aging induced by ultraviolet and extreme ultraviolet radiation. Cellulose 2024, 31, 749–758. [Google Scholar] [CrossRef]
- Kacík, F.; Kacíková, D.; Jablonsky, M.; Katuscak, S. Cellulose degradation in newsprint paper ageing. Polym. Deg. Stab. 2009, 94, 1509–1514. [Google Scholar] [CrossRef]
- Malesi, J.; Kolar, J.; Stirlic, M.; Kocar, D.; Fromageot, D.; Lemaire, J. Photoinduced degradation of cellulose. Polym. Deg. Stab. 2005, 89, 64–69. [Google Scholar] [CrossRef]
- Lubrizol. Optimizing Performance of Carbopol®ETD 2020 and Ultrez 10 Polymers with Partial Neutralization of Polymer Dispersions. Technical Data Sheet TDS-243, Ed. Available online: https://www.lubrizol.com/-/media/Lubrizol/Health/TDS/TDS243. OptimizingPerformanceCarbopolETD2020Ultrez10PartialNeutralizationPolymerDispersions.pdf (accessed on 15 October 2007).
- Lubrizol. Viscosity of Carbopol®Polymers in Aqueous Systems. Technical Data Sheet TDS-730, Ed. Available online: https://www.lubrizol.com//media/Lubrizol/Health/TDS/TDS730_Viscosity_Carbopol_in_Aqueous-Systems.pdf (accessed on 13 August 2010).
- [Gutowski, I.A.; Lee, D.; de Bruyn, J.R.; Frisken, B.J. Scaling and mesostructure of Carbopol dispersions. Rheol. Acta 2012, 51, 441–450. [Google Scholar] [CrossRef]
- Diaspro, A. Confocal and Two-Photon Microscopy. Foundations, Applications and Advances; Diaspro, A., Ed.; Wiley-Liss: New York, NY, USA, 2002.
Figure 1.
Optical images in bright field of XIX paper. The green rectangle indicates the area of 200µm x 200µm scanned with a step size of 5 µm in Raman spectral imaging mode using green excitation (λ = 532 nm) and 10X objective (a) before the cleaning treatment; (b) after the cleaning treatment; (c) examples Raman spectra acquired before and after the cleaning treatment. .
Figure 1.
Optical images in bright field of XIX paper. The green rectangle indicates the area of 200µm x 200µm scanned with a step size of 5 µm in Raman spectral imaging mode using green excitation (λ = 532 nm) and 10X objective (a) before the cleaning treatment; (b) after the cleaning treatment; (c) examples Raman spectra acquired before and after the cleaning treatment. .
Figure 2.
Optical images of XIX paper (upper panels) and OT marker value colocalized maps. (a) Before and (b) after 1 h CBP cleaning treatment.
Figure 2.
Optical images of XIX paper (upper panels) and OT marker value colocalized maps. (a) Before and (b) after 1 h CBP cleaning treatment.
Figure 3.
(a) Ageing marker variation in percentage for the XIX century paper (b) the same for the XXI century paper. This laser printer 2021 paper was exposed to ambient light for three years.
Figure 3.
(a) Ageing marker variation in percentage for the XIX century paper (b) the same for the XXI century paper. This laser printer 2021 paper was exposed to ambient light for three years.
Figure 4.
CLSM images (objective 4x) of a selected spot on Brehm paper. (a) Before cleaning treatment; (b) After cleaning treatment with CBP/TiO2. The figures show red and the green fluorescence channels and their overlay; (c) Graph of the red fluorescence signal intensity profiles detected along the yellow ar-rows of the spot before and after CBP/TiO2 treatment.
Figure 4.
CLSM images (objective 4x) of a selected spot on Brehm paper. (a) Before cleaning treatment; (b) After cleaning treatment with CBP/TiO2. The figures show red and the green fluorescence channels and their overlay; (c) Graph of the red fluorescence signal intensity profiles detected along the yellow ar-rows of the spot before and after CBP/TiO2 treatment.
Figure 5.
(a) Raman spectra of cleaned XIX century paper sample (red curve), XIX century paper sample before gel application (green curve), CBP/TiO2 (brown curve); (b) score plot related to map of cleaned paper before (blue dots) and after gel removal (cyan dots).
Figure 5.
(a) Raman spectra of cleaned XIX century paper sample (red curve), XIX century paper sample before gel application (green curve), CBP/TiO2 (brown curve); (b) score plot related to map of cleaned paper before (blue dots) and after gel removal (cyan dots).
Figure 6.
Optical images of 2024 modern paper: (a) uncoated and non-irradiated; (b) coated with a thin layer of CBP/TiO2; (c) uncoated and UVA irradiated; (d) coated with a thin layer of CBP/TiO2 and UVA irradiated.
Figure 6.
Optical images of 2024 modern paper: (a) uncoated and non-irradiated; (b) coated with a thin layer of CBP/TiO2; (c) uncoated and UVA irradiated; (d) coated with a thin layer of CBP/TiO2 and UVA irradiated.
Figure 7.
RGB value distributions of 2024 modern paper comparing uncoated and non-irradiated paper samples with CBP/TiO2 coated and UVA irradiated ones.
Figure 7.
RGB value distributions of 2024 modern paper comparing uncoated and non-irradiated paper samples with CBP/TiO2 coated and UVA irradiated ones.
Figure 8.
(a) Raman spectra acquired from 2024 paper before and after 31h of UVA irradiation. (b), (c) CI marker value maps of uncoated 2024 paper non-irradiated and UVA irradiated, respectively. (d) Raman spectra acquired from 2024 paper CBP/TiO2 coated before and after 31h of UVA irradiation. (e), (f) CI marker value maps of CBP/TiO2 coated 2024 paper, non-irradiated and UVA irradiated, respectively.
Figure 8.
(a) Raman spectra acquired from 2024 paper before and after 31h of UVA irradiation. (b), (c) CI marker value maps of uncoated 2024 paper non-irradiated and UVA irradiated, respectively. (d) Raman spectra acquired from 2024 paper CBP/TiO2 coated before and after 31h of UVA irradiation. (e), (f) CI marker value maps of CBP/TiO2 coated 2024 paper, non-irradiated and UVA irradiated, respectively.
Figure 9.
(a), (b) OT marker value maps of uncoated 2024 paper before and after 31h of UVA irradiation, respectively. (c), (d) OT marker value maps of CBP/TiO2 coated 2024 paper before and after 31h of UVA irradiation, respectively.
Figure 9.
(a), (b) OT marker value maps of uncoated 2024 paper before and after 31h of UVA irradiation, respectively. (c), (d) OT marker value maps of CBP/TiO2 coated 2024 paper before and after 31h of UVA irradiation, respectively.
Figure 10.
a) Marker variation in percentage after 31 h of UVA irradiation for the uncoated and coated 2024 paper. (b) Score plots of coated paper before (blue points) and after irradiation (fuchsia points). The PC1 component is the CBP/TiO2 gel Raman spectrum, while PC2 component is the paper one.
Figure 10.
a) Marker variation in percentage after 31 h of UVA irradiation for the uncoated and coated 2024 paper. (b) Score plots of coated paper before (blue points) and after irradiation (fuchsia points). The PC1 component is the CBP/TiO2 gel Raman spectrum, while PC2 component is the paper one.
Figure 11.
Optical images of 2021 paper naturally aged in laboratory. (a) uncoated; (b) coated with a thin layer of CBP/TiO2.
Figure 11.
Optical images of 2021 paper naturally aged in laboratory. (a) uncoated; (b) coated with a thin layer of CBP/TiO2.
Figure 12.
(a) Marker variation in percentage after 31 h of UVA irradiation for the uncoated and coated 2021 paper exposed to ambient light of laboratory for three years. OT marker value maps for (b) uncoated paper, (c) UVA irradiated uncoated paper, (d) coated paper, (e) UVA irradiated coated paper.
Figure 12.
(a) Marker variation in percentage after 31 h of UVA irradiation for the uncoated and coated 2021 paper exposed to ambient light of laboratory for three years. OT marker value maps for (b) uncoated paper, (c) UVA irradiated uncoated paper, (d) coated paper, (e) UVA irradiated coated paper.
Figure 14.
Photo of hydrogel. On the left CBP gel (1-2-3) on the right CBP/TiO2 gel.
Figure 14.
Photo of hydrogel. On the left CBP gel (1-2-3) on the right CBP/TiO2 gel.
Table 1.
Materials for hydrogel preparation.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).