Submitted:
28 November 2024
Posted:
29 November 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
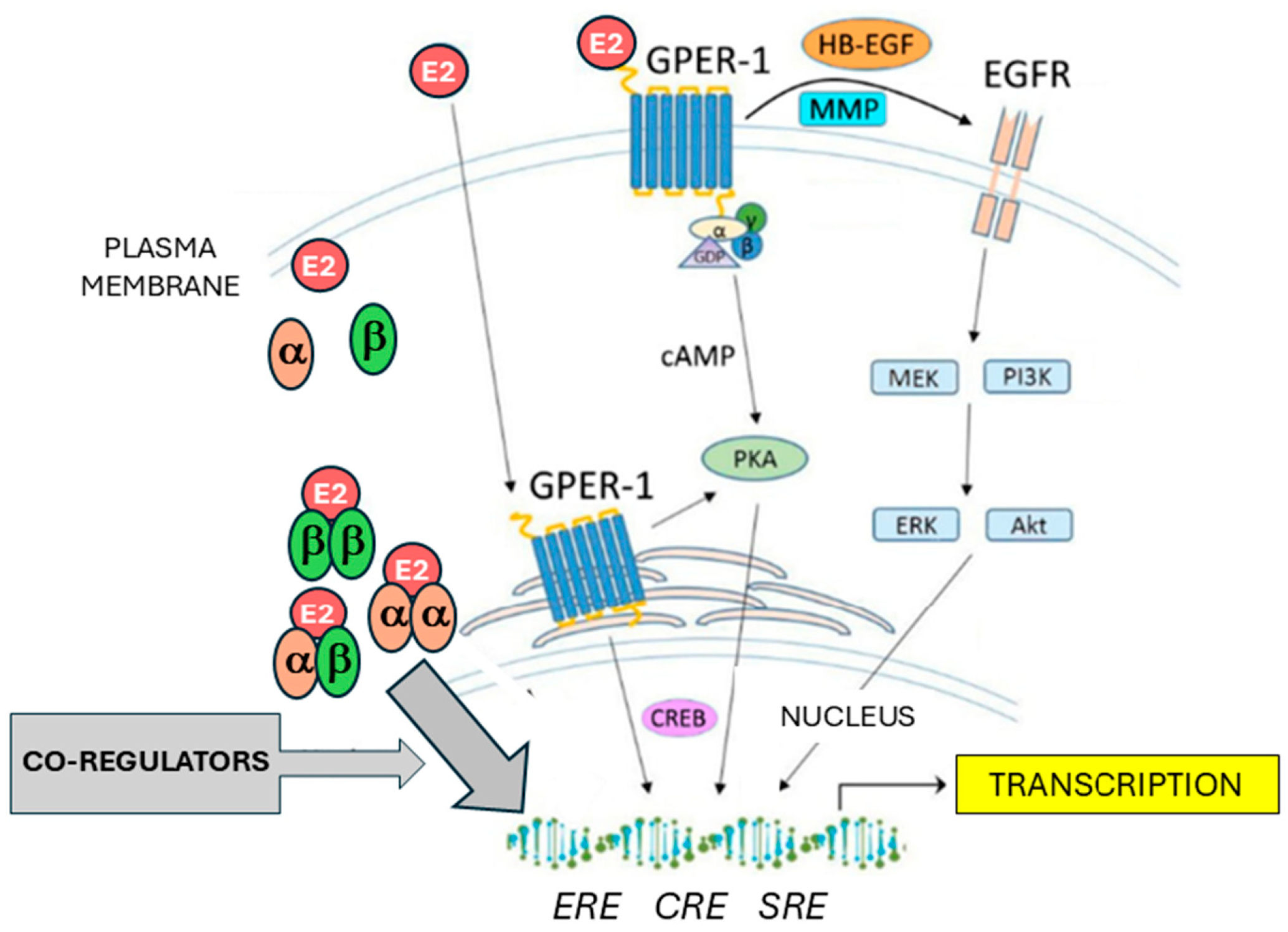
2. Estrogen Signaling
2.1. Role of Estrogen Signaling in Ovarian Cancer
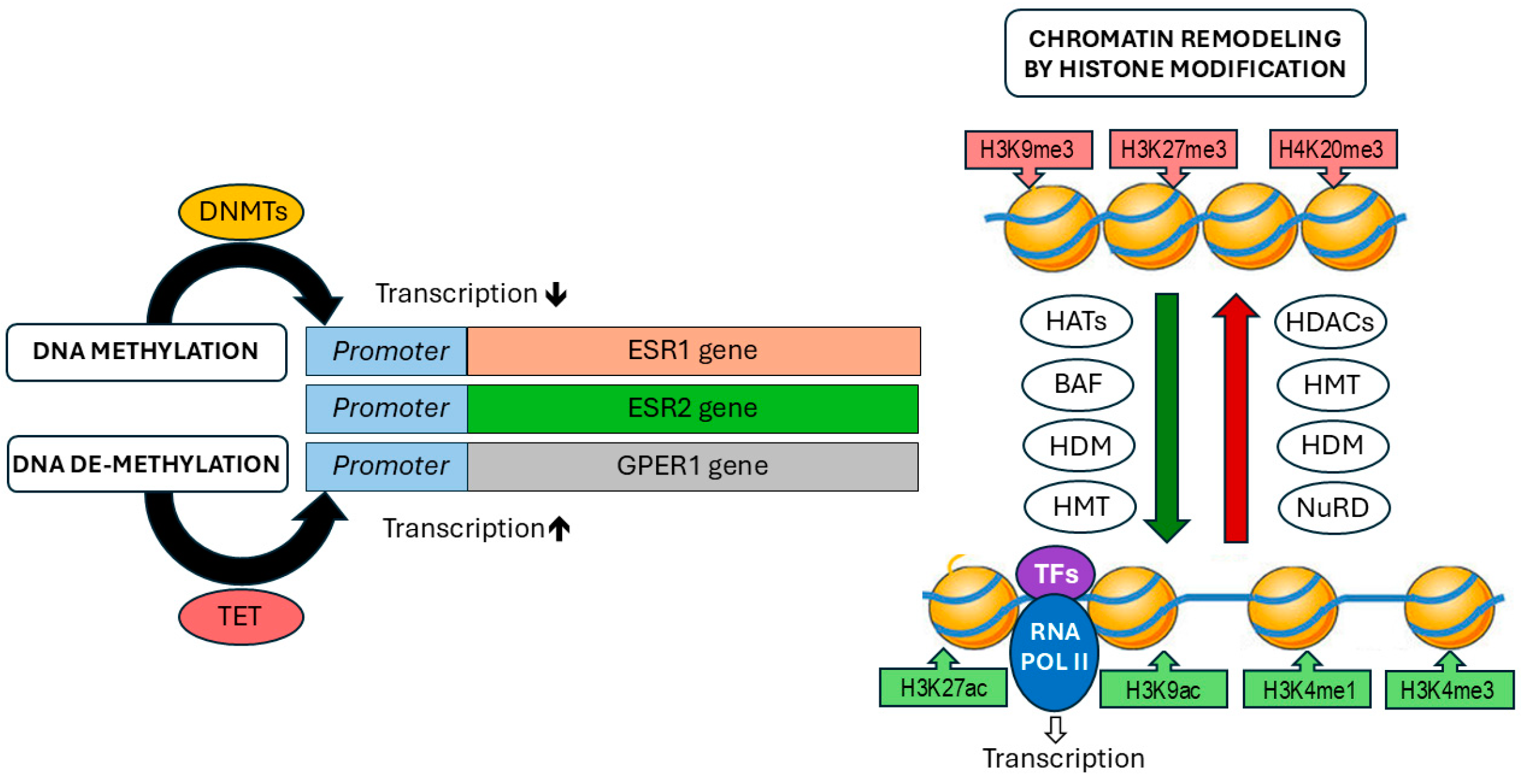
3. Epigenetic Mechanisms
3.1. Epigenetic Mechanisms Involved in Estrogen Signaling in Ovarian Cancer
3.1.1. Epigenetic Regulation of ERα Expression and Activity in Ovarian Cancer
3.1.2. Epigenetic Regulation of ERβ Expression and Transcriptional Activity in Ovarian Cancer
3.1.3. Epigenetic Regulation of GPER1 Expression and Activity in Ovarian Cancer
3.1.4. Epigenetic Regulation of Estrogen Receptor Target Genes in Ovarian Cancer
| Epigenetic mechanism | Gene | References |
|---|---|---|
| Promoter methylation |
ESR1 ESR2 GPER1 CDH1 CCND1 PTEN |
[86,87,88,89] [99,100] [59] [116,117] [114] [118] |
| Histone modification |
ESR1 ESR2 GPER1 TFF1 FOXA1 GREB1 CCND1 BCL2 |
[89], [91,92] [98,99] [109,112] [90] [90,121] [90] [113] [119] |
4. Drugs with Epigenetic Functions: Are they Promising Tools for OC Therapy?
Author Contributions
Funding
Conflicts of Interest
References
- Cabasag, C.J.; Fagan, P.J.; Ferlay, J.; Vignat, J.; Laversanne, M.; Liu, L.; van der Aa, M.A.; Bray, F.; Soerjomataram, I. OC today and tomorrow: A global assessment by world region and Human Development Index using GLOBOCAN 2020. Int J Cancer 2022. [Google Scholar] [CrossRef]
- Munoz-Galvan, S.; Carnero, A. Leveraging Genomics, Transcriptomics, and Epigenomics to Understand the Biology and Chemoresistance of Ovarian Cancer. Cancers (Basel) 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Kurman, R.J.; Shih Ie, M. The Dualistic Model of Ovarian Carcinogenesis: Revisited, Revised, and Expanded. Am J Pathol 2016, 186, 733–747. [Google Scholar] [CrossRef] [PubMed]
- Rojas, V.; Hirshfield, K.M.; Ganesan, S.; Rodriguez-Rodriguez, L. Molecular Characterization of Epithelial Ovarian Cancer: Implications for Diagnosis and Treatment. Int J Mol Sci 2016, 17. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, I.; Matsuura, T. Screening and Prevention for High-Grade Serous Carcinoma of the Ovary Based on Carcinogenesis-Fallopian Tube- and Ovarian-Derived Tumors and Incessant Retrograde Bleeding. Diagnostics (Basel) 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Liberto, J.M.; Chen, S.Y.; Shih, I.M.; Wang, T.H.; Wang, T.L.; Pisanic, T.R., 2nd. Current and Emerging Methods for OC Screening and Diagnostics: A Comprehensive Review. Cancers (Basel) 2022, 14. [Google Scholar]
- Wang, Y.; Huang, Z.; Li, B.; Liu, L.; Huang, C. The Emerging Roles and Therapeutic Implications of Epigenetic Modifications in Ovarian Cancer. Front Endocrinol (Lausanne) 2022, 13, 863541. [Google Scholar] [CrossRef]
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. OC statistics, 2018. CA Cancer J Clin 2018, 68, 284–296. [Google Scholar] [CrossRef]
- Pignata, S.; Cannella, L.; Leopardo, D.; Pisano, C.; Bruni, G.S.; Facchini, G. Chemotherapy in epithelial ovarian cancer. Cancer Lett 2011, 303, 73–83. [Google Scholar] [CrossRef]
- Williams, C.; Simera, I.; Bryant, A. Tamoxifen for relapse of ovarian cancer. Cochrane Database Syst Rev 2010, 2010, CD001034. [Google Scholar] [PubMed]
- Borella, F.; Fucina, S.; Mangherini, L.; Cosma, S.; Carosso, A.R.; Cusato, J.; Cassoni, P.; Bertero, L.; Katsaros, D.; Benedetto, C. Hormone Receptors and Epithelial Ovarian Cancer: Recent Advances in Biology and Treatment Options. Biomedicines 2023, 11. [Google Scholar] [CrossRef] [PubMed]
- Heublein, S.; Page, S.; Mayr, D.; Schmoeckel, E.; Trillsch, F.; Marme, F.; Mahner, S.; Jeschke, U.; Vattai, A. Potential Interplay of the Gatipotuzumab Epitope TA-MUC1 and Estrogen Receptors in Ovarian Cancer. Int J Mol Sci 2019, 20. [Google Scholar] [CrossRef]
- Singh, N.; Jayraj, A.S.; Sarkar, A.; Mohan, T.; Shukla, A.; Ghatage, P. Pharmacotherapeutic treatment options for recurrent epithelial ovarian cancer. Expert Opin Pharmacother 2022. [Google Scholar] [CrossRef]
- Gu, S.; Lheureux, S.; Sayad, A.; Cybulska, P.; Hogen, L.; Vyarvelska, I.; Tu, D.; Parulekar, W.R.; Nankivell, M.; Kehoe, S.; Chi, D.S.; Levine, D.A.; Bernardini, M.Q.; Rosen, B.; Oza, A.; Brown, M.; Neel, B.G. Computational modeling of OC dynamics suggests optimal strategies for therapy and screening. Proc Natl Acad Sci U S A 2021, 118. [Google Scholar]
- Hira, M.T.; Razzaque, M.A.; Angione, C.; Scrivens, J.; Sawan, S.; Sarker, M. Integrated multi-omics analysis of OC using variational autoencoders. Sci Rep 2021, 11, 6265. [Google Scholar] [CrossRef]
- Zeller, C.; Dai, W.; Steele, N.L.; Siddiq, A.; Walley, A.J.; Wilhelm-Benartzi, C.S.; Rizzo, S.; van der Zee, A.; Plumb, J.A.; Brown, R. Candidate DNA methylation drivers of acquired cisplatin resistance in OC identified by methylome and expression profiling. Oncogene 2012, 31, 4567–4576. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Li, B.; Ou-Yang, L. Role of estrogen receptors in health and disease. Front Endocrinol (Lausanne) 2022, 13, 839005. [Google Scholar] [CrossRef]
- Russell, J.K.; Jones, C.K.; Newhouse, P.A. The Role of Estrogen in Brain and Cognitive Aging. Neurotherapeutics 2019, 16, 649–665. [Google Scholar] [CrossRef]
- Fuentes, N.; Silveyra, P. Estrogen receptor signaling mechanisms. Adv Protein Chem Struct Biol 2019, 116, 135–170. [Google Scholar]
- Klinge, C.M. Estrogen receptor interaction with estrogen response elements. Nucleic Acids Res 2001, 29, 2905–2919. [Google Scholar] [CrossRef]
- Feng, Q.; O'Malley, B.W. Nuclear receptor modulation--role of coregulators in selective estrogen receptor modulator (SERM) actions. Steroids 2014, 90, 39–43. [Google Scholar] [CrossRef]
- Onate, S.A.; Tsai, S.Y.; Tsai, M.J.; O'Malley, B.W. Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science 1995, 270, 1354–1357. [Google Scholar] [CrossRef]
- Johnson, A.B.; O'Malley, B.W. Steroid receptor coactivators 1, 2, and 3: critical regulators of nuclear receptor activity and steroid receptor modulator (SRM)-based cancer therapy. Mol Cell Endocrinol 2012, 348, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Ma, H.; Hong, H.; Koh, S.S.; Huang, S.M.; Schurter, B.T.; Aswad, D.W.; Stallcup, M.R. Regulation of transcription by a protein methyltransferase. Science 1999, 284, 2174–2177. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Lin, R.J.; Schiltz, R.L.; Chakravarti, D.; Nash, A.; Nagy, L.; Privalsky, M.L.; Nakatani, Y.; Evans, R.M. Nuclear receptor coactivator ACTR is a novel histone acetyltransferase and forms a multimeric activation complex with P/CAF and CBP/p300. Cell 1997, 90, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.D.; Evans, R.M. A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature 1995, 377, 454–457. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Silva, C.D.; Villegas-Pineda, J.C.; Pereira-Suarez, A.L. Expression and Role of the G Protein-Coupled Estrogen Receptor (GPR30/GPER) in the Development and Immune Response in Female Reproductive Cancers. Front Endocrinol (Lausanne) 2020, 11, 544. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.L.; Huang, Z.Y.; Yu, K.; Li, J.; Fu, X.W.; Deng, S.L. Estrogen Biosynthesis and Signal Transduction in Ovarian Disease. Front Endocrinol (Lausanne) 2022, 13, 827032. [Google Scholar] [CrossRef] [PubMed]
- Longuespee, R.; Boyon, C.; Desmons, A.; Vinatier, D.; Leblanc, E.; Farre, I.; Wisztorski, M.; Ly, K.; D'Anjou, F.; Day, R.; Fournier, I.; Salzet, M. OC molecular pathology. Cancer Metastasis Rev 2012, 31, 713–732. [Google Scholar] [CrossRef]
- Koziel, M.J.; Piastowska-Ciesielska, A.W. Estrogens, Estrogen Receptors and Tumor Microenvironment in Ovarian Cancer. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef]
- Pujol, P.; Rey, J.M.; Nirde, P.; Roger, P.; Gastaldi, M.; Laffargue, F.; Rochefort, H.; Maudelonde, T. Differential expression of estrogen receptor-alpha and -beta messenger RNAs as a potential marker of ovarian carcinogenesis. Cancer Res 1998, 58, 5367–5373. [Google Scholar]
- Li, A.J.; Baldwin, R.L.; Karlan, B.Y. Estrogen and progesterone receptor subtype expression in normal and malignant ovarian epithelial cell cultures. Am J Obstet Gynecol 2003, 189, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, P.R.; Cajander, S.; Backstrom, T.; Gustafsson, J.A.; Makela, S.; Olofsson, J.I. Estrogen and progesterone receptors in ovarian epithelial tumors. Mol Cell Endocrinol 2004, 221, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Halon, A.; Nowak-Markwitz, E.; Maciejczyk, A.; Pudelko, M.; Gansukh, T.; Gyorffy, B.; Donizy, P.; Murawa, D.; Matkowski, R.; Spaczynski, M.; Lage, H.; Surowiak, P. Loss of estrogen receptor beta expression correlates with shorter overall survival and lack of clinical response to chemotherapy in OC patients. Anticancer Res 2011, 31, 711–718. [Google Scholar] [PubMed]
- Sieh, W.; Kobel, M.; Longacre, T.A.; Bowtell, D.D.; deFazio, A.; Goodman, M.T.; Hogdall, E.; Deen, S.; Wentzensen, N.; Moysich, K.B.; Brenton, J.D.; Clarke, B.A.; Menon, U.; Gilks, C.B.; Kim, A.; Madore, J.; Fereday, S.; George, J.; Galletta, L.; Lurie, G.; Wilkens, L.R.; Carney, M.E.; Thompson, P.J.; Matsuno, R.K.; Kjaer, S.K.; Jensen, A.; Hogdall, C.; Kalli, K.R.; Fridley, B.L.; Keeney, G.L.; Vierkant, R.A.; Cunningham, J.M.; Brinton, L.A.; Yang, H.P.; Sherman, M.E.; Garcia-Closas, M.; Lissowska, J.; Odunsi, K.; Morrison, C.; Lele, S.; Bshara, W.; Sucheston, L.; Jimenez-Linan, M.; Driver, K.; Alsop, J.; Mack, M.; McGuire, V.; Rothstein, J.H.; Rosen, B.P.; Bernardini, M.Q.; Mackay, H.; Oza, A.; Wozniak, E.L.; Benjamin, E.; Gentry-Maharaj, A.; Gayther, S.A.; Tinker, A.V.; Prentice, L.M.; Chow, C.; Anglesio, M.S.; Johnatty, S.E.; Chenevix-Trench, G.; Whittemore, A.S.; Pharoah, P.D.; Goode, E.L.; Huntsman, D.G.; Ramus, S.J. Hormone-receptor expression and OC survival: an Ovarian Tumor Tissue Analysis consortium study. Lancet Oncol 2013, 14, 853–862. [Google Scholar] [CrossRef]
- Bogush, T.A.; Basharina, A.A.; Bogush, E.A.; Ryabinina, O.M.; Tjulandina, A.S.; Tjulandin, S.A. Estrogen Receptors alpha and beta in Ovarian Cancer: Expression Level and Prognosis. Dokl Biochem Biophys 2018, 482, 249–251. [Google Scholar] [CrossRef]
- Fixemer, T.; Remberger, K.; Bonkhoff, H. Differential expression of the estrogen receptor beta (ERbeta) in human prostate tissue, premalignant changes, and in primary, metastatic, and recurrent prostatic adenocarcinoma. Prostate 2003, 54, 79–87. [Google Scholar] [CrossRef]
- Foley, E.F.; Jazaeri, A.A.; Shupnik, M.A.; Jazaeri, O.; Rice, L.W. Selective loss of estrogen receptor beta in malignant human colon. Cancer Res 2000, 60, 245–248. [Google Scholar] [PubMed]
- Park, B.W.; Kim, K.S.; Heo, M.K.; Ko, S.S.; Hong, S.W.; Yang, W.I.; Kim, J.H.; Kim, G.E.; Lee, K.S. Expression of estrogen receptor-beta in normal mammary and tumor tissues: is it protective in breast carcinogenesis? Breast Cancer Res Treat 2003, 80, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Bossard, C.; Busson, M.; Vindrieux, D.; Gaudin, F.; Machelon, V.; Brigitte, M.; Jacquard, C.; Pillon, A.; Balaguer, P.; Balabanian, K.; Lazennec, G. Potential role of estrogen receptor beta as a tumor suppressor of epithelial ovarian cancer. PLoS One 2012, 7, e44787. [Google Scholar] [CrossRef]
- O'Donnell, A.J.; Macleod, K.G.; Burns, D.J.; Smyth, J.F.; Langdon, S.P. Estrogen receptor-alpha mediates gene expression changes and growth response in OC cells exposed to estrogen. Endocr Relat Cancer 2005, 12, 851–866. [Google Scholar] [CrossRef]
- Treeck, O.; Pfeiler, G.; Mitter, D.; Lattrich, C.; Piendl, G.; Ortmann, O. Estrogen receptor beta1 exerts antitumoral effects on SK-OV-3 OC cells. J Endocrinol 2007, 193, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Schuler-Toprak, S.; Moehle, C.; Skrzypczak, M.; Ortmann, O.; Treeck, O. Effect of estrogen receptor beta agonists on proliferation and gene expression of OC cells. BMC Cancer 2017, 17, 319. [Google Scholar] [CrossRef]
- Bardin, A.; Hoffmann, P.; Boulle, N.; Katsaros, D.; Vignon, F.; Pujol, P.; Lazennec, G. Involvement of estrogen receptor beta in ovarian carcinogenesis. Cancer Res 2004, 64, 5861–5869. [Google Scholar] [CrossRef]
- Lazennec, G. Estrogen receptor beta, a possible tumor suppressor involved in ovarian carcinogenesis. Cancer Lett 2006, 231, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Langdon, S.P.; Hirst, G.L.; Miller, E.P.; Hawkins, R.A.; Tesdale, A.L.; Smyth, J.F.; Miller, W.R. The regulation of growth and protein expression by estrogen in vitro: a study of 8 human ovarian carcinoma cell lines. J Steroid Biochem Mol Biol 1994, 50, 131–135. [Google Scholar] [CrossRef]
- Langdon, S.P.; Crew, A.J.; Ritchie, A.A.; Muir, M.; Wakeling, A.; Smyth, J.F.; Miller, W.R. Growth inhibition of oestrogen receptor-positive human ovarian carcinoma by anti-oestrogens in vitro and in a xenograft model. Eur J Cancer 1994, 30A, 682–686. [Google Scholar] [CrossRef]
- Fujiwara, S.; Terai, Y.; Kawaguchi, H.; Takai, M.; Yoo, S.; Tanaka, Y.; Tanaka, T.; Tsunetoh, S.; Sasaki, H.; Kanemura, M.; Tanabe, A.; Yamashita, Y.; Ohmichi, M. GPR30 regulates the EGFR-Akt cascade and predicts lower survival in patients with ovarian cancer. J Ovarian Res 2012, 5, 35. [Google Scholar] [CrossRef]
- Smith, H.O.; Arias-Pulido, H.; Kuo, D.Y.; Howard, T.; Qualls, C.R.; Lee, S.J.; Verschraegen, C.F.; Hathaway, H.J.; Joste, N.E.; Prossnitz, E.R. GPR30 predicts poor survival for ovarian cancer. Gynecol Oncol 2009, 114, 465–471. [Google Scholar] [CrossRef]
- Ignatov, T.; Modl, S.; Thulig, M.; Weissenborn, C.; Treeck, O.; Ortmann, O.; Zenclussen, A.; Costa, S.D.; Kalinski, T.; Ignatov, A. GPER-1 acts as a tumor suppressor in ovarian cancer. J Ovarian Res 2013, 6, 51. [Google Scholar] [CrossRef] [PubMed]
- Schuler-Toprak, S.; Skrzypczak, M.; Ignatov, T.; Ignatov, A.; Ortmann, O.; Treeck, O. G protein-coupled estrogen receptor 1 (GPER-1) and agonist G-1 inhibit growth of OC cells by activation of anti-tumoral transcriptome responses: impact of GPER-1 mRNA on survival. J Cancer Res Clin Oncol 2020, 146, 3175–3188. [Google Scholar] [CrossRef] [PubMed]
- Fraungruber, P.; Kaltofen, T.; Heublein, S.; Kuhn, C.; Mayr, D.; Burges, A.; Mahner, S.; Rathert, P.; Jeschke, U.; Trillsch, F. G Protein-Coupled Estrogen Receptor Correlates With Dkk2 Expression and Has Prognostic Impact in OC Patients. Front Endocrinol (Lausanne) 2021, 12, 564002. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Chen, Z.; Wang, Y.; Zhao, H.; Du, Y. The Role of Cancer-Associated Fibroblasts in Ovarian Cancer. Cancers (Basel) 2022, 14. [Google Scholar] [CrossRef]
- Yousefzadeh, Y.; Hallaj, S.; Baghi Moornani, M.; Asghary, A.; Azizi, G.; Hojjat-Farsangi, M.; Ghalamfarsa, G.; Jadidi-Niaragh, F. Tumor associated macrophages in the molecular pathogenesis of ovarian cancer. Int Immunopharmacol 2020, 84, 106471. [Google Scholar] [CrossRef] [PubMed]
- Somasundaram, A.; Rothenberger, N.J.; Stabile, L.P. The Impact of Estrogen in the Tumor Microenvironment. Adv Exp Med Biol 2020, 1277, 33–52. [Google Scholar]
- Esteller, M. Epigenetics in cancer. N Engl J Med 2008, 358, 1148–1159. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Peng, M.; Tan, S.; Oyang, L.; Lin, J.; Xia, L.; Wang, J.; Wu, N.; Jiang, X.; Peng, Q.; Zhou, Y.; Liao, Q. The roles and molecular mechanisms of non-coding RNA in cancer metabolic reprogramming. Cancer Cell Int 2024, 24, 37. [Google Scholar] [CrossRef] [PubMed]
- Good, D.J. Non-Coding RNAs in Human Health and Diseases. Genes (Basel) 2023, 14. [Google Scholar] [CrossRef]
- Esteller, M. Cancer epigenomics: DNA methylomes and histone-modification maps. Nat Rev Genet 2007, 8, 286–298. [Google Scholar] [CrossRef]
- Rossello-Tortella, M.; Bueno-Costa, A.; Martinez-Verbo, L.; Villanueva, L.; Esteller, M. DNA methylation-associated dysregulation of transfer RNA expression in human cancer. Mol Cancer 2022, 21, 48. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Yang, Y.; Li, L.; Tang, J.; Zhang, R. DNA methylation profiles in cancer: functions, therapy, and beyond. Cancer Biol Med 2023, 21, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Tajbakhsh, J.; Mortazavi, F.; Gupta, N.K. DNA methylation topology differentiates between normal and malignant in cell models, resected human tissues, and exfoliated sputum cells of lung epithelium. Front Oncol 2022, 12, 991120. [Google Scholar] [CrossRef] [PubMed]
- Davalos, V.; Esteller, M. Cancer epigenetics in clinical practice. CA Cancer J Clin 2022. [Google Scholar] [CrossRef] [PubMed]
- Mattei, A.L.; Bailly, N.; Meissner, A. DNA methylation: a historical perspective. Trends Genet 2022, 38, 676–707. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Z.; Ding, Y.; Wang, L.; Wang, S.; Wang, H.; Qin, Y. DNA Methylation: From Cancer Biology to Clinical Perspectives. Front Biosci (Landmark Ed) 2022, 27, 326. [Google Scholar] [CrossRef]
- Zhao, S.; Allis, C.D.; Wang, G.G. The language of chromatin modification in human cancers. Nat Rev Cancer 2021, 21, 413–430. [Google Scholar] [CrossRef] [PubMed]
- Kouzarides, T. Chromatin modifications and their function. Cell 2007, 128, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Chi, P.; Allis, C.D.; Wang, G.G. Covalent histone modifications--miswritten, misinterpreted and mis-erased in human cancers. Nat Rev Cancer 2010, 10, 457–469. [Google Scholar] [CrossRef]
- Zhuang, J.; Huo, Q.; Yang, F.; Xie, N. Perspectives on the Role of Histone Modification in Breast Cancer Progression and the Advanced Technological Tools to Study Epigenetic Determinants of Metastasis. Front Genet 2020, 11, 603552. [Google Scholar] [CrossRef] [PubMed]
- Geng, R.; Chen, T.; Zhong, Z.; Ni, S.; Bai, J.; Liu, J. The m6A-Related Long Noncoding RNA Signature Predicts Prognosis and Indicates Tumor Immune Infiltration in Ovarian Cancer. Cancers (Basel) 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Weng, H.; Chen, J. m(6)A Modification in Coding and Non-coding RNAs: Roles and Therapeutic Implications in Cancer. Cancer Cell 2020, 37, 270–288. [Google Scholar] [CrossRef]
- Shulman, Z.; Stern-Ginossar, N. The RNA modification N(6)-methyladenosine as a novel regulator of the immune system. Nat Immunol 2020, 21, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Frye, M.; Harada, B.T.; Behm, M.; He, C. RNA modifications modulate gene expression during development. Science 2018, 361, 1346–1349. [Google Scholar] [CrossRef]
- Gu, J.; Bi, F. Significance of N6-Methyladenosine RNA Methylation Regulators in Immune Infiltrates of Ovarian Cancer. Front Genet 2021, 12, 671179. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Meng, D.; Wan, M.; Xu, N.; Xu, Y.; Yuan, K.; Liu, P.; Fang, H.; Hu, H.; Lan, S. m6A-Related lncRNAs Predict Overall Survival of Patients and Regulate the Tumor Immune Microenvironment in Osteosarcoma. Comput Intell Neurosci 2022, 2022, 9315283. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Feng, J.; Wu, J.; Zhong, W.; Zouxu, X.; Huang, W.; Huang, X.; Yi, J.; Wang, X. Prognostic value of comprehensive typing based on m6A and gene cluster in TNBC. J Cancer Res Clin Oncol 2022. [Google Scholar] [CrossRef]
- Alam, S.; Giri, P.K. Emerging role of m6A modification in ovarian cancer: progression, drug resistance, and therapeutic prospects. Front Oncol 2024, 14, 1366223. [Google Scholar] [CrossRef] [PubMed]
- Mann, M.; Cortez, V.; Vadlamudi, R.K. Epigenetics of estrogen receptor signaling: role in hormonal cancer progression and therapy. Cancers (Basel) 2011, 3, 1691–1707. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Shang, Y. Estrogen and cancer. Annu Rev Physiol 2013, 75, 225–240. [Google Scholar] [CrossRef]
- Carroll, J.S.; Meyer, C.A.; Song, J.; Li, W.; Geistlinger, T.R.; Eeckhoute, J.; Brodsky, A.S.; Keeton, E.K.; Fertuck, K.C.; Hall, G.F.; Wang, Q.; Bekiranov, S.; Sementchenko, V.; Fox, E.A.; Silver, P.A.; Gingeras, T.R.; Liu, X.S.; Brown, M. Genome-wide analysis of estrogen receptor binding sites. Nat Genet 2006, 38, 1289–1297. [Google Scholar] [CrossRef]
- Li, W.; Hu, Y.; Oh, S.; Ma, Q.; Merkurjev, D.; Song, X.; Zhou, X.; Liu, Z.; Tanasa, B.; He, X.; Chen, A.Y.; Ohgi, K.; Zhang, J.; Liu, W.; Rosenfeld, M.G. Condensin I and II Complexes License Full Estrogen Receptor alpha-Dependent Enhancer Activation. Mol Cell 2015, 59, 188–202. [Google Scholar] [CrossRef]
- Kirn, V.; Strake, L.; Thangarajah, F.; Richters, L.; Eischeid, H.; Koitzsch, U.; Odenthal, M.; Fries, J. ESR1-promoter-methylation status in primary breast cancer and its corresponding metastases. Clin Exp Metastasis 2018, 35, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Quintas-Granados, L.I.; Cortes, H.; Carmen, M.G.; Leyva-Gomez, G.; Bustamante-Montes, L.P.; Rodriguez-Morales, M.; Villegas-Vazquez, E.Y.; Lopez-Reyes, I.; Alcaraz-Estrada, S.L.; Sandoval-Basilio, J.; Soto-Reyes, E.; Sharifi-Rad, J.; Figueroa-Gonzalez, G.; Reyes-Hernandez, O.D. The high methylation level of a novel 151-bp CpG island in the ESR1 gene promoter is associated with a poor breast cancer prognosis. Cancer Cell Int 2021, 21, 649. [Google Scholar] [CrossRef] [PubMed]
- Giacinti, L.; Claudio, P.P.; Lopez, M.; Giordano, A. Epigenetic information and estrogen receptor alpha expression in breast cancer. Oncologist 2006, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bos, M.K.; Deger, T.; Sleijfer, S.; Martens, J.W.M.; Wilting, S.M. ESR1 Methylation Measured in Cell-Free DNA to Evaluate Endocrine Resistance in Metastatic Breast Cancer Patients. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef]
- Imura, M.; Yamashita, S.; Cai, L.Y.; Furuta, J.; Wakabayashi, M.; Yasugi, T.; Ushijima, T. Methylation and expression analysis of 15 genes and three normally-methylated genes in 13 OC cell lines. Cancer Lett 2006, 241, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Gong, G.; Lin, T.; Yuan, Y. Integrated analysis of gene expression and DNA methylation profiles in ovarian cancer. J Ovarian Res 2020, 13, 30. [Google Scholar] [CrossRef] [PubMed]
- Kirn, V.; Shi, R.; Heublein, S.; Knabl, J.; Guenthner-Biller, M.; Andergassen, U.; Fridrich, C.; Malter, W.; Harder, J.; Friese, K.; Mayr, D.; Jeschke, U. Estrogen receptor promoter methylation predicts survival in low-grade ovarian carcinoma patients. J Cancer Res Clin Oncol 2014, 140, 1681–1687. [Google Scholar] [CrossRef] [PubMed]
- Salvati, A.; Gigantino, V.; Nassa, G.; Giurato, G.; Alexandrova, E.; Rizzo, F.; Tarallo, R.; Weisz, A. The Histone Methyltransferase DOT1L Is a Functional Component of Estrogen Receptor Alpha Signaling in OC Cells. Cancers (Basel) 2019, 11. [Google Scholar]
- Liu, Z.; Merkurjev, D.; Yang, F.; Li, W.; Oh, S.; Friedman, M.J.; Song, X.; Zhang, F.; Ma, Q.; Ohgi, K.A.; Krones, A.; Rosenfeld, M.G. Enhancer activation requires trans-recruitment of a mega transcription factor complex. Cell 2014, 159, 358–373. [Google Scholar] [CrossRef]
- Yang, F.; Ma, Q.; Liu, Z.; Li, W.; Tan, Y.; Jin, C.; Ma, W.; Hu, Y.; Shen, J.; Ohgi, K.A.; Telese, F.; Liu, W.; Rosenfeld, M.G. Glucocorticoid Receptor:MegaTrans Switching Mediates the Repression of an ERalpha-Regulated Transcriptional Program. Mol Cell 2017, 66, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jaiswal, S.K.; Kaur, R.; Alsaadi, D.; Liang, X.; Drews, F.; DeLoia, J.A.; Krivak, T.; Petrykowska, H.M.; Gotea, V.; Welch, L.; Elnitski, L. Differential gene expression identifies a transcriptional regulatory network involving ER-alpha and PITX1 in invasive epithelial ovarian cancer. BMC Cancer 2021, 21, 768. [Google Scholar] [CrossRef]
- Schuler-Toprak, S.; Skrzypczak, M.; Grundker, C.; Ortmann, O.; Treeck, O. Role of Estrogen Receptor beta, G-Protein Coupled Estrogen Receptor and Estrogen-Related Receptors in Endometrial and Ovarian Cancer. Cancers (Basel) 2023, 15. [Google Scholar]
- Rody, A.; Holtrich, U.; Solbach, C.; Kourtis, K.; von Minckwitz, G.; Engels, K.; Kissler, S.; Gatje, R.; Karn, T.; Kaufmann, M. Methylation of estrogen receptor beta promoter correlates with loss of ER-beta expression in mammary carcinoma and is an early indication marker in premalignant lesions. Endocr Relat Cancer 2005, 12, 903–916. [Google Scholar] [CrossRef] [PubMed]
- Manente, A.G.; Pinton, G.; Zonca, S.; Tavian, D.; Habib, T.; Jithesh, P.V.; Fennell, D.; Nilsson, S.; Moro, L. KDM6B histone demethylase is an epigenetic regulator of estrogen receptor beta expression in human pleural mesothelioma. Epigenomics 2016, 8, 1227–1238. [Google Scholar] [CrossRef]
- Salahuddin, A.; Ghanem, H.; Omran, G.A.; Helmy, M.W. Epigenetic restoration and activation of ERbeta: an inspiring approach for treatment of triple-negative breast cancer. Med Oncol 2022, 39, 150. [Google Scholar] [CrossRef] [PubMed]
- Walton, T.J.; Li, G.; Seth, R.; McArdle, S.E.; Bishop, M.C.; Rees, R.C. DNA demethylation and histone deacetylation inhibition co-operate to re-express estrogen receptor beta and induce apoptosis in prostate cancer cell-lines. Prostate 2008, 68, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Venkata, P.P.; Jayamohan, S.; He, Y.; Alejo, S.; Johnson, J.D.; Palacios, B.E.; Pratap, U.P.; Chen, Y.; Liu, Z.; Zou, Y.; Lai, Z.; Suzuki, T.; Viswanadhapalli, S.; Weintraub, S.T.; Palakurthi, S.; Valente, P.T.; Tekmal, R.R.; Kost, E.R.; Vadlamudi, R.K.; Sareddy, G.R. Pharmacological inhibition of KDM1A/LSD1 enhances estrogen receptor beta-mediated tumor suppression in ovarian cancer. Cancer Lett 2023, 575, 216383. [Google Scholar] [CrossRef]
- Yap, O.W.; Bhat, G.; Liu, L.; Tollefsbol, T.O. Epigenetic modifications of the Estrogen receptor beta gene in epithelial OC cells. Anticancer Res 2009, 29, 139–144. [Google Scholar] [PubMed]
- Suzuki, F.; Akahira, J.; Miura, I.; Suzuki, T.; Ito, K.; Hayashi, S.; Sasano, H.; Yaegashi, N. Loss of estrogen receptor beta isoform expression and its correlation with aberrant DNA methylation of the 5'-untranslated region in human epithelial ovarian carcinoma. Cancer Sci 2008, 99, 2365–2372. [Google Scholar] [CrossRef] [PubMed]
- Dalal, H.; Dahlgren, M.; Gladchuk, S.; Brueffer, C.; Gruvberger-Saal, S.K.; Saal, L.H. Clinical associations of ESR2 (estrogen receptor beta) expression across thousands of primary breast tumors. Sci Rep 2022, 12, 4696. [Google Scholar] [CrossRef] [PubMed]
- Hartman, J.; Strom, A.; Gustafsson, J.A. Current concepts and significance of estrogen receptor beta in prostate cancer. Steroids 2012, 77, 1262–1266. [Google Scholar] [CrossRef]
- Heublein, S.; Lenhard, M.; Vrekoussis, T.; Schoepfer, J.; Kuhn, C.; Friese, K.; Makrigiannakis, A.; Mayr, D.; Jeschke, U. The G-protein-coupled estrogen receptor (GPER) is expressed in normal human ovaries and is upregulated in ovarian endometriosis and pelvic inflammatory disease involving the ovary. Reprod Sci 2012, 19, 1197–1204. [Google Scholar] [CrossRef]
- Heublein, S.; Mayr, D.; Friese, K.; Jarrin-Franco, M.C.; Lenhard, M.; Mayerhofer, A.; Jeschke, U. The G-protein-coupled estrogen receptor (GPER/GPR30) in ovarian granulosa cell tumors. Int J Mol Sci 2014, 15, 15161–15172. [Google Scholar] [CrossRef]
- Tirado-Garibay, A.C.; Falcon-Ruiz, E.A.; Ochoa-Zarzosa, A.; Lopez-Meza, J.E. GPER: An Estrogen Receptor Key in Metastasis and Tumoral Microenvironments. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Rong, J.; Xie, X.; Niu, Y.; Su, Z. Correlation between the RNA Expression and the DNA Methylation of Estrogen Receptor Genes in Normal and Malignant Human Tissues. Curr Issues Mol Biol 2024, 46, 3610–3625. [Google Scholar] [CrossRef]
- Manjegowda, M.C.; Gupta, P.S.; Limaye, A.M. Hyper-methylation of the upstream CpG island shore is a likely mechanism of GPER1 silencing in breast cancer cells. Gene 2017, 614, 65–73. [Google Scholar] [CrossRef]
- Weissenborn, C.; Ignatov, T.; Nass, N.; Kalinski, T.; Dan Costa, S.; Zenclussen, A.C.; Ignatov, A. GPER Promoter Methylation Controls GPER Expression in Breast Cancer Patients. Cancer Invest 2017, 35, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, G.W.; Kwon, S.H.; Lee, J.S. Broad domains of histone H3 lysine 4 trimethylation in transcriptional regulation and disease. FEBS J 2020, 287, 2891–2902. [Google Scholar] [CrossRef]
- Dawson, M.A.; Kouzarides, T. Cancer epigenetics: from mechanism to therapy. Cell 2012, 150, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Chen, Z.; Wu, D.; Zhang, L.; Lin, X.; Su, J.; Rodriguez, B.; Xi, Y.; Xia, Z.; Chen, X.; Shi, X.; Wang, Q.; Li, W. Broad H3K4me3 is associated with increased transcription elongation and enhancer activity at tumor-suppressor genes. Nat Genet 2015, 47, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Han, N.; Heublein, S.; Jeschke, U.; Kuhn, C.; Hester, A.; Czogalla, B.; Mahner, S.; Rottmann, M.; Mayr, D.; Schmoeckel, E.; Trillsch, F. The G-Protein-Coupled Estrogen Receptor (GPER) Regulates Trimethylation of Histone H3 at Lysine 4 and Represses Migration and Proliferation of OC Cells In Vitro. Cells 2021, 10. [Google Scholar] [CrossRef]
- Schneider, L.; Herkt, S.; Wang, L.; Feld, C.; Wesely, J.; Kuvardina, O.N.; Meyer, A.; Oellerich, T.; Haupl, B.; Seifried, E.; Bonig, H.; Lausen, J. PRMT6 activates cyclin D1 expression in conjunction with the transcription factor LEF1. Oncogenesis 2021, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Ivan, C.; Hu, W.; Bottsford-Miller, J.; Zand, B.; Dalton, H.J.; Liu, T.; Huang, J.; Nick, A.M.; Lopez-Berestein, G.; Coleman, R.L.; Baggerly, K.A.; Sood, A.K. Epigenetic analysis of the Notch superfamily in high-grade serous ovarian cancer. Gynecol Oncol 2013, 128, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Cheung, L.W.; Wong, A.S.; Leung, P.C. Estrogen regulates Snail and Slug in the down-regulation of E-cadherin and induces metastatic potential of OC cells through estrogen receptor alpha. Mol Endocrinol 2008, 22, 2085–2098. [Google Scholar] [CrossRef] [PubMed]
- Moselhy, S.S.; Kumosani, T.A.; Kamal, I.H.; Jalal, J.A.; Jabaar, H.S.; Dalol, A. Hypermethylation of P15, P16, and E-cadherin genes in ovarian cancer. Toxicol Ind Health 2015, 31, 924–930. [Google Scholar] [CrossRef] [PubMed]
- Sawada, K.; Mitra, A.K.; Radjabi, A.R.; Bhaskar, V.; Kistner, E.O.; Tretiakova, M.; Jagadeeswaran, S.; Montag, A.; Becker, A.; Kenny, H.A.; Peter, M.E.; Ramakrishnan, V.; Yamada, S.D.; Lengyel, E. Loss of E-cadherin promotes OC metastasis via alpha 5-integrin, which is a therapeutic target. Cancer Res 2008, 68, 2329–2339. [Google Scholar] [CrossRef]
- Ho, C.M.; Lin, M.C.; Huang, S.H.; Huang, C.J.; Lai, H.C.; Chien, T.Y.; Chang, S.F. PTEN promoter methylation and LOH of 10q22-23 locus in PTEN expression of ovarian clear cell adenocarcinomas. Gynecol Oncol 2009, 112, 307–313. [Google Scholar] [CrossRef]
- Zhao, R.; Han, C.; Eisenhauer, E.; Kroger, J.; Zhao, W.; Yu, J.; Selvendiran, K.; Liu, X.; Wani, A.A.; Wang, Q.E. DNA damage-binding complex recruits HDAC1 to repress Bcl-2 transcription in human OC cells. Mol Cancer Res 2014, 12, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Xiu, Y.L.; Chen, X.; Sun, K.X.; Chen, S.; Wu, D.D.; Liu, B.L.; Zhao, Y. The transcription factor FOXA1 induces epithelial OC tumorigenesis and progression. Tumour Biol 2017, 39, 1010428317706210. [Google Scholar]
- Lou, T.; Liu, C.; Qu, H.; Zhang, Z.; Wang, S.; Zhuang, H. FOXA1 can be modulated by HDAC3 in the progression of epithelial ovarian carcinoma. J Transl Med 2022, 20, 19. [Google Scholar] [CrossRef] [PubMed]
- Matthews, B.G.; Bowden, N.A.; Wong-Brown, M.W. Epigenetic Mechanisms and Therapeutic Targets in Chemoresistant High-Grade Serous Ovarian Cancer. Cancers (Basel) 2021, 13. [Google Scholar] [CrossRef]
- Wilson, A.J.; Lalani, A.S.; Wass, E.; Saskowski, J.; Khabele, D. Romidepsin (FK228) combined with cisplatin stimulates DNA damage-induced cell death in ovarian cancer. Gynecol Oncol 2012, 127, 579–586. [Google Scholar] [CrossRef]
- Helland, O.; Popa, M.; Bischof, K.; Gjertsen, B.T.; McCormack, E.; Bjorge, L. The HDACi Panobinostat Shows Growth Inhibition Both In Vitro and in a Bioluminescent Orthotopic Surgical Xenograft Model of Ovarian Cancer. PLoS One 2016, 11, e0158208. [Google Scholar] [CrossRef] [PubMed]
- Garrett, L.A.; Growdon, W.B.; Rueda, B.R.; Foster, R. Influence of a novel histone deacetylase inhibitor panobinostat (LBH589) on the growth of ovarian cancer. J Ovarian Res 2016, 9, 58. [Google Scholar] [CrossRef]
- Ma, Y.Y.; Lin, H.; Moh, J.S.; Chen, K.D.; Wang, I.W.; Ou, Y.C.; You, Y.S.; Lung, C.C. Low-dose LBH589 increases the sensitivity of cisplatin to cisplatin-resistant OC cells. Taiwan J Obstet Gynecol 2011, 50, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.J.; Gupta, V.G.; Liu, Q.; Yull, F.; Crispens, M.A.; Khabele, D. Panobinostat enhances olaparib efficacy by modifying expression of homologous recombination repair and immune transcripts in ovarian cancer. Neoplasia 2022, 24, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Ovejero-Sanchez, M.; Gonzalez-Sarmiento, R.; Herrero, A.B. Synergistic effect of Chloroquine and Panobinostat in OC through induction of DNA damage and inhibition of DNA repair. Neoplasia 2021, 23, 515–528. [Google Scholar] [CrossRef]
- Rodrigues Moita, A.J.; Bandolik, J.J.; Hansen, F.K.; Kurz, T.; Hamacher, A.; Kassack, M.U. Priming with HDAC Inhibitors Sensitizes OC Cells to Treatment with Cisplatin and HSP90 Inhibitors. Int J Mol Sci 2020, 21. [Google Scholar]
- Booth, L.; Roberts, J.L.; Rais, R.; Poklepovic, A.; Dent, P. Valproate augments Niraparib killing of tumor cells. Cancer Biol Ther 2018, 19, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Joshi, J.; Yeh, I.J.; Doughman, Y.; Blankenberg, D.; Wald, D.; Montano, M.M. Re-Expression of ERalpha and AR in Receptor Negative Endocrine Cancers via GSK3 Inhibition. Front Oncol 2022, 12, 824594. [Google Scholar] [CrossRef] [PubMed]
- Dizon, D.S.; Blessing, J.A.; Penson, R.T.; Drake, R.D.; Walker, J.L.; Johnston, C.M.; Disilvestro, P.A.; Fader, A.N. A phase II evaluation of belinostat and carboplatin in the treatment of recurrent or persistent platinum-resistant ovarian, fallopian tube, or primary peritoneal carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol 2012, 125, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Meteran, H.; Knudsen, A.O.; Jorgensen, T.L.; Nielsen, D.; Herrstedt, J. Carboplatin plus Paclitaxel in Combination with the Histone Deacetylate Inhibitor, Vorinostat, in Patients with Recurrent Platinum-Sensitive Ovarian Cancer. J Clin Med 2024, 13. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





