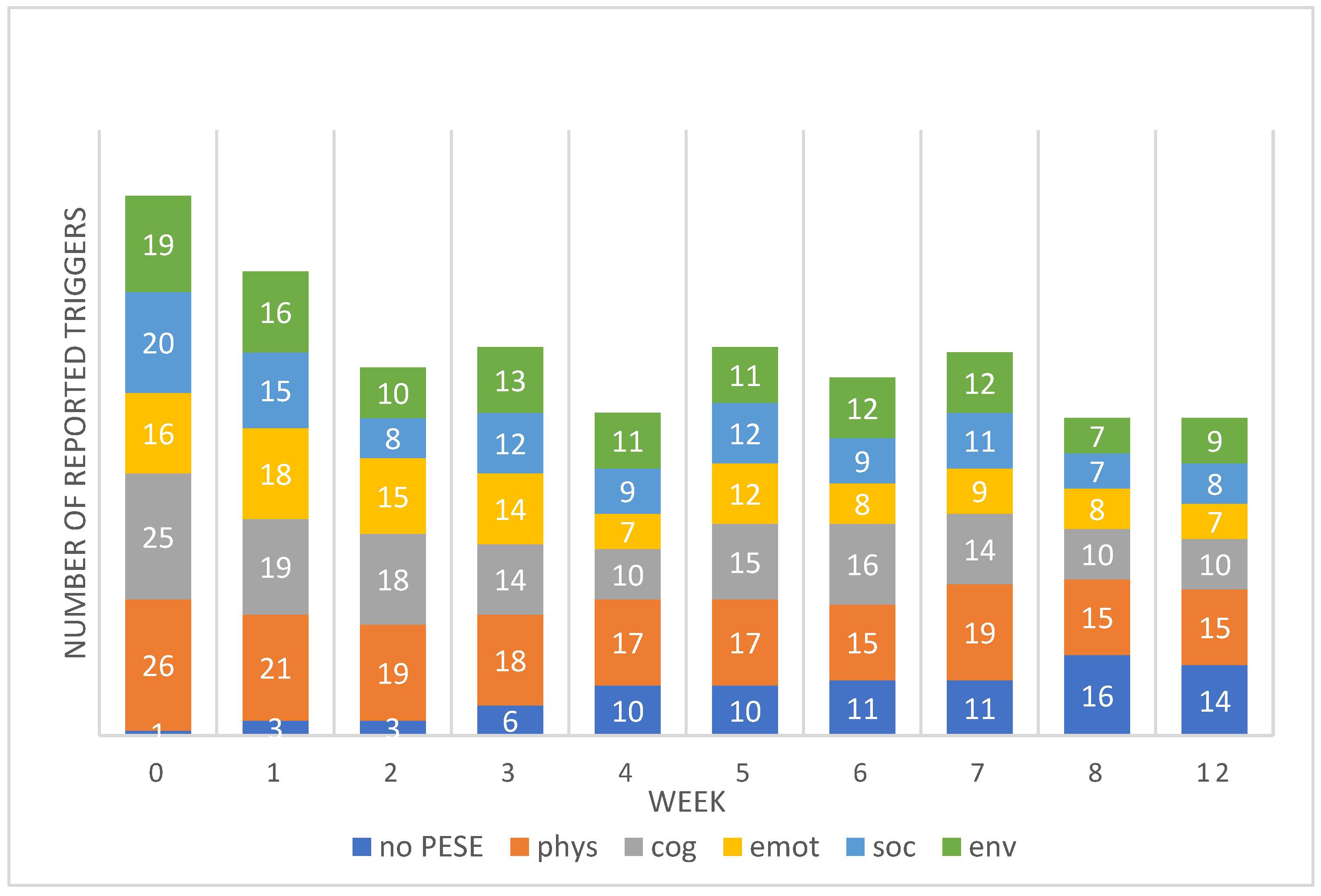1. Introduction
It is now over four years since COVID-19 became a global pandemic and we saw the first signs of Post Covid Syndrome (PCS), or long COVID (LC). Defined as signs and symptoms that develop during or after an infection consistent with COVID-19, which continue for more than 12 weeks and are not explained by an alternative diagnosis, [
1] LC continues to affect millions of people worldwide. Office for National Statistics (ONS) data from March 2023 estimated that 1.9 million people in the UK had self reported LC, with those in the 35-69 age group reporting symptoms most frequently [
2] A further ONS study from the winter of 2023-24 reported the figure for England and Scotland as 2 million, or 3.3% of the population. Of those people, 71% reported having had symptoms for at least one year, 51% at least two years, and 31% at least three years. 74.7% of respondents reported an adverse impact on day to day activities and 19.2% stated their day to day activities were ‘limited a lot’ [
3].
Of the many symptoms reported, Post Exertional Symptom Exacerbation (PESE), or Post Exertional Malaise (PEM) has emerged as one of the most common and debilitating, with 86% of respondents to a TUC survey in 2022 reporting it as a feature of their LC [
4]. PESE is also a defining feature of Myalgic Encephalomyelitis / Chronic Fatigue Syndrome (ME/CFS) therefore much of what we know comes from the literature associated with this condition. PESE is characterised by worsening fatigue, pain, cognitive function and other symptoms in response to any form of exertion/activity [
5,
6,
7]. Symptoms can start soon after activity or have delayed onset of up to 72 hours, lasting for days, weeks or longer [
8,
9,
10,
11]. Symptom severity is disproportionate to perceived level of exertion, with both the severity and behaviour of symptoms being variable between patients and indeed within the same patient [
12]. This makes PESE difficult to predict and manage, creating challenges for patients and clinicians alike, given the already fluctuating nature of LC symptoms. For some, fear of an exacerbation becomes so great they avoid activity wherever possible, risking isolation, deconditioning and declining overall health. Due to the numbers affected by LC and the prevalence of PESE within the condition, there is a growing disability burden amongst patients and a potentially devastating effect on their roles at home, work and in communities. The associated impact on healthcare, economies and society is of great concern due to the vast number of working age people affected by LC and the numbers continuing to report lack of full recovery, to the point that in some it has become a long term condition [
13,
14,
15,
16,
17].
There is currently no medical/pharmacological treatment for PESE, therefore effective symptom management strategies are needed [
9,
11,
18]. Strategies for PESE management generally involve pacing, which should not be confused with traditional graded exercise as this can be detrimental to those experiencing PESE [
19]. Effective pacing encourages patients to be as active as possible within the limits of their symptoms, also known as their energy envelope [
20,
21]. It aims to enable manageable and consistent levels of activity rather than ‘boom-bust’ patterns, whereby patients push themselves to continue despite fatigue and other symptoms, only to trigger debilitating ‘crashes’ [
5,
18,
22,
23]. Currently, there is lack of consensus on how to pace effectively therefore more work is required [
24]. A scoping review of pacing in 2023 found a lack of studies, especially in the years before the pandemic, and that the quality of research was generally low to moderate. Many studies gathered patient opinion regarding pacing, rather than actually implementing it, and use of patient reported outcome measures (PROMS) was low. Effective pacing methods involved activity planning, consistency, energy management techniques and avoiding activity progression, but the authors concluded that the low quality and quantity of research indicated that further work is urgently needed [
5].
The World Health Organisation Borg CR-10 (WHO Borg CR-10) pacing algorithm has five phases of activity to monitor and adjust exertion levels. Users are encouraged to use the algorithm as a reference tool, matching phase of activity to current functional ability, only progressing the the next phase if they achieve a PESE-free period, and reverting to an easier phase during times of symptom exacerbation [
25]. We, in our previous study, tested a 6-week structured WHO Borg CR-10 pacing protocol, the results of which showed a significant reduction in PESE episodes and improved quality of life in a cohort of LC patients [
25]. However, clinicians in the service had observed that patients often struggled with the notion of rest. Many were not prioritising it and were unsuccessfully using sleep as a strategy for PESE. The pacing protocol therefore needed inclusion of guidance on active rest, and to be tested over a slightly longer period than our previous study (i.e., 8 weeks). We wanted to know whether using the WHO Borg CR-10 algorithm to pace effectively helped in the management of PESE, enabling patients to cope with greater activity, and whether this in turn, improved their LC symptoms.
2. Materials and Methods
Patients over the age of 18 years were recruited from the LC community rehabilitation service and were invited to take part in the service evaluation if PESE was a significant symptom. They were given written information and completed a consent form and baseline EQ-5D-5L and C19YRSm questionnaires, both of which are outcome measures used regularly in the service. Those unable to commit to 8 weekly phone calls plus a follow-up call 4 weeks later, and those without sufficient capacity to undertake the study were excluded.
2.1. Pacing Programme
Patients received information and guidance on the WHO Borg CR-10 pacing protocol (Supplemental Material 1) [
25]. This encompasses five incremental phases of activity, alongside a Borg CR-10 Rating of Perceived Exertion (RPE) of 0-10, with 0 being complete rest and 10 being maximal perceived exertion, providing patients with a simple subjective assessment of effort level during an activity. Examples of activities and effort levels were provided to participants.
The pacing protocol helped patients gauge effort levels during activity and assess which phase of activity felt appropriate in the context of symptoms. Patients also identified and introduced an active rest activity, incorporating this into their daily routine (Supplemental material 1). The term active rest describes activities aimed at stimulating a parasympathetic response, such as resonant breathing exercises or meditation techniques. To help the adoption of active rest, patients were asked to (if feasible) remain in activity phase two for the first week of the study, meaning their Borg score stayed at no more than 3 out of 10. This, it was hoped, would help patients adopt the notion of restorative, rather than passive, rest.
Patients monitored PESE symptoms in response to use of the protocol, either progressing, regressing, or remaining at the same phase of activity each week. This was intended to promote autonomy and assist in building confidence to adjust activity levels when necessary, following the principle of remaining as active as possible within the limits of symptoms.
2.2. Outcome Measures
Patients completed the Leeds PESE Questionnaire (LPQ), a 4 question Likert scale, C19YRSm and EQ-5D-5L outcome measures at the start of the programme (baseline). They then engaged with 8 weekly phone calls with a clinician, which included completing the LPQ and Likert scale and discussing any significant issues or events. The C19YRSm and EQ-5D-5L were completed again at week 8, and patients self-managed independently for 4 weeks before a follow-up call at week 12 to complete the LPQ, Likert scale, C19YRSm and EQ-5D-5L for the final time. Patients were also asked to complete a short qualitative questionnaire to record their experience of the study and the effect of the programme on PESE and LC symptoms.
2.2.1. EQ-5D-5L
The EuroQol EQ-5D-5L is a health-related quality of life measure with five domains: Mobility, Usual Activities, Selfcare, Pain / Discomfort, and Anxiety / Depression. Each item has five response categories ranging from 1 (no problems) to 5 (severe problems). Responses to each item are collated into a profile score which is converted into a health utility or index score using a country-specific algorithm (tariff or value set). The utility score reflects societal preference for health state and is measured on a metric from 0 (dead) to 1 (perfect health). The EQ-5D-5L scores are mapped onto the EQ-5D-3L (an alternative version of the instrument with 3 response categories advocated by the National Institute for Health and Care Excellence, NICE) using a standard mapping crosswalk algorithm to derive UK utility values [
26].
2.2.2. C19-YRSm
The COVID-19 Yorkshire Rehabilitation Scale (C19-YRS) was literature’s first condition specific PROM developed to measure the symptoms, functioning and disability associated with COVID-19. C19-YRSm is a modified version of the original C19-YRS with 17 items and four sub-scales. Each item has a 4-point response category: 0, no problem to 3, severe problem [
27]. The subscales (range) are: Symptom Severity (0-30), Functional Disability (0-15), Other Symptoms (0-25), and Overall Health (0-10). The evaluation of psychometric properties of C19-YRSm revealed it is a valid, reliable and responsive measure [
28]. The Minimal Clinical Important Difference (MCID) has been estimated to be 4 points for the Symptom Severity subscale and 4 points for the Functional Disability subscales.
2.2.3. PESE Characteristics
The standard Leeds PESE Questionnaire (LPQ) recorded the number and nature of PESE episodes over the past 7 days. The Likert scale comprised 3 questions adopted from the Multidimensional Assessment of Interoceptive Awareness (MAIA) V2 2018 [
29] and a 4th relating to confidence in completing diaphragmatic breathing technique. This was included as it related to established practice informed by literature on resonant breathing [
30] and by the HEARTLOC study [
31]. The LPQ and Likert scale can be found in Supplemental Materials 2.
2.3. Statistical Analysis
Patient age, EQ-5D-5L, and C19-YRSm measures were presented as mean (SD), duration of LC was presented as median (IQR), and categorical characteristics as the number of participants in each category (%). Participant characteristics at baseline (week 0) are presented descriptively by intervention group, for comparison.
Mixed-effects linear regression was used to compare outcomes at baseline (week 0) with the end of intervention (week 8) and end of follow-up (week 12) for EQ-5D-5L utility scores and visual analogue scales, C19YRSm symptom severity scores, functional disability scores, and overall health scores, and C19YRSm PESE scores, adjusting for age and gender.
Week-on-week change in weekly process measures (measured weekly during the intervention weeks 0 to 8 and at final follow-up week 12) were modelled, adjusting for age and gender, and taking account of the serial time measures within each patient. Mixed-effects Poisson regression was used to model the number of PESE episodes per week, and the number of symptoms per week, with random slopes over time (weeks 0 to 12), with estimates presented as percentage change in incidence per week. Mixed-effects linear regression was used to model symptom severity, and duration of episodes, activity phase, and active resting score over time, with estimates presented as absolute change in outcome per week.
3. Results
A total of 47 patients were invited to take part in the programme. Three were no longer eligible to participate because their symptoms had improved sufficiently prior to the study, seven could not be contacted or did not return consent, and six were unable to complete because of acute illness. This left a total of 31 patients who received the pacing programme.
Demographic and clinical characteristics at baseline week 0 are shown in
Table 1. The mean (SD) age of participants was 47 (11) years, with more females (65%) than males. Nearly half (45%) of participants were not in full-time paid employment. On clinical measures, the median (IQR) duration of LC symptoms was >2 years (29 months).
3.1. Changes in EQ-5D-5L
On completion of the intervention (week 8) there was no evidence of improvement in EQ-5D-5L utility (change = .00, 95% CI -.04 to .05, p=0.9) or VAS scores (4, -1 to 9, p=0.1) (8 points, 95% CI 4 to 11) after adjusting for age and gender. At the end of follow-up (week 12) there was no evidence of improvement in EQ-5D-5L utility (.04, -.01 to .09, p=0.1) but there was evidence of improvement in the EQ-5D-5L visual analogue scale (8 points, 4 to 11, p<0.001) (
Table 2).
3.2. Changes in C19YRSm
There was evidence of improvement in C19YRSm functional disability (-.9, -1.7 to -.2, p=0.01) and C19YRSm PESE subscore (-.5, -.7 to -.2, p<0.001) on completion of the intervention at 8 weeks, and improvement in all C19YRS measures at 12 weeks follow-up (
Table 2)
3.3. Changes in PESE Characteristics
Weekly changes in process measures over time within the intervention group are shown in Supplemental Material 3 for weeks 0 to 8 of the intervention, then week 12 after the intervention had completed. There was evidence of improvement across process measures during the intervention (
Table 3) with the number of PESE episodes decreasing gradually each week (15% fewer each week, 95% CI: 11% to 20%, p<0.001), episodes of shorter duration, with fewer symptoms, and of milder severity each week (
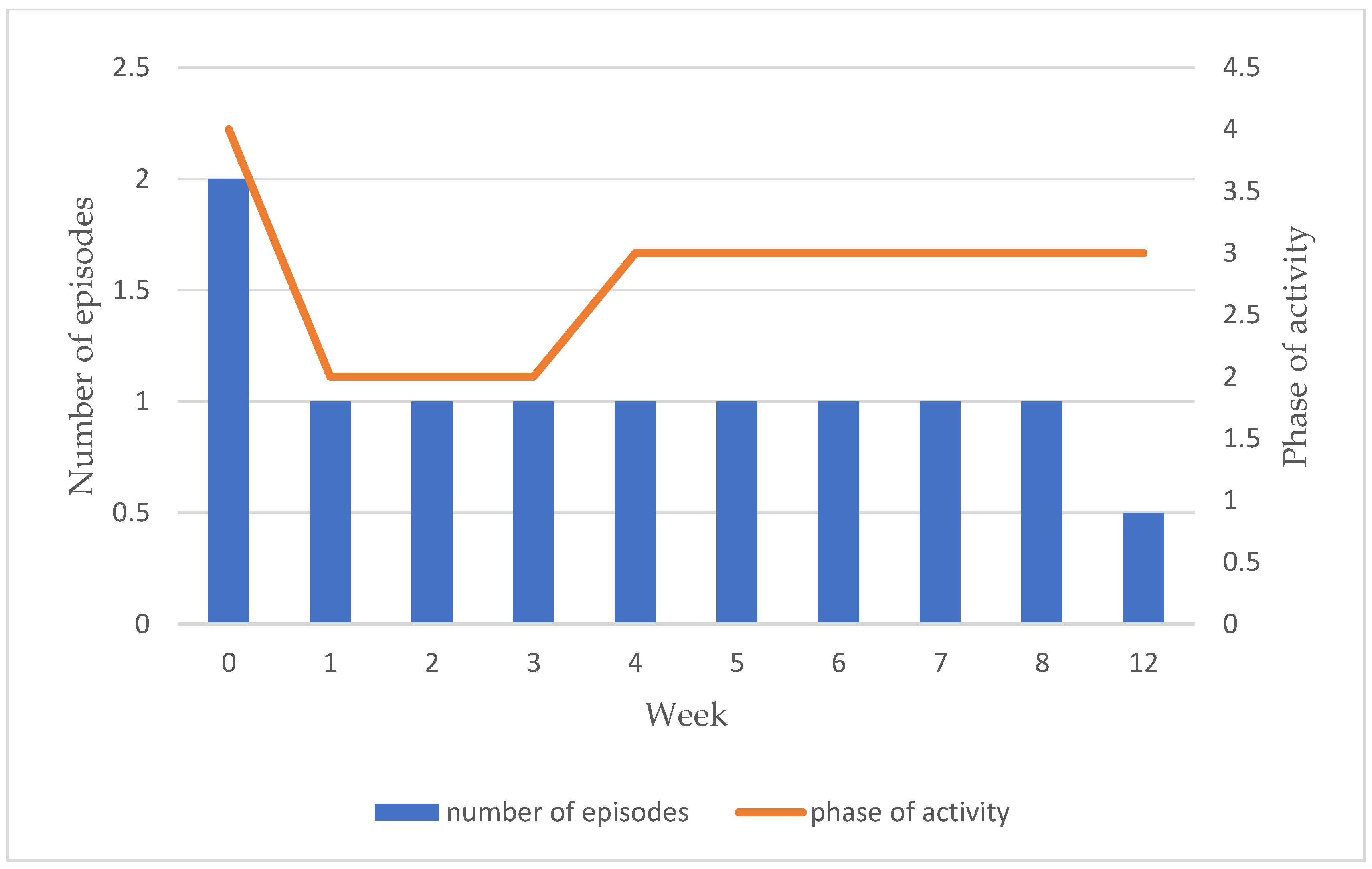
Table 3). There was no evidence of overall change in Borg activity phase, reflecting the initial reduction in activity during pacing, before gradual increases over the remaining 8 weeks until activity returned close to its previous levels, whilst maintaining decreased numbers of PESE episodes, and symptoms, reduced symptom severity, and shorter duration of episodes. Active resting score also improved over time within the pacing intervention group.
Total numbers of reported triggers of PESE reduced by 54% over the weeks with a total of 107 reported triggers at week 0 (baseline), 47 at week 8 and 49 at week 12. The number of patients reporting no PESE each week (and therefore no triggers), rose from 1 (3%) at week 0 (baseline) to 16 at week 8 (51%) and 14 (45%) at week 12. A full breakdown of reported triggers per week is given in
Figure 1.
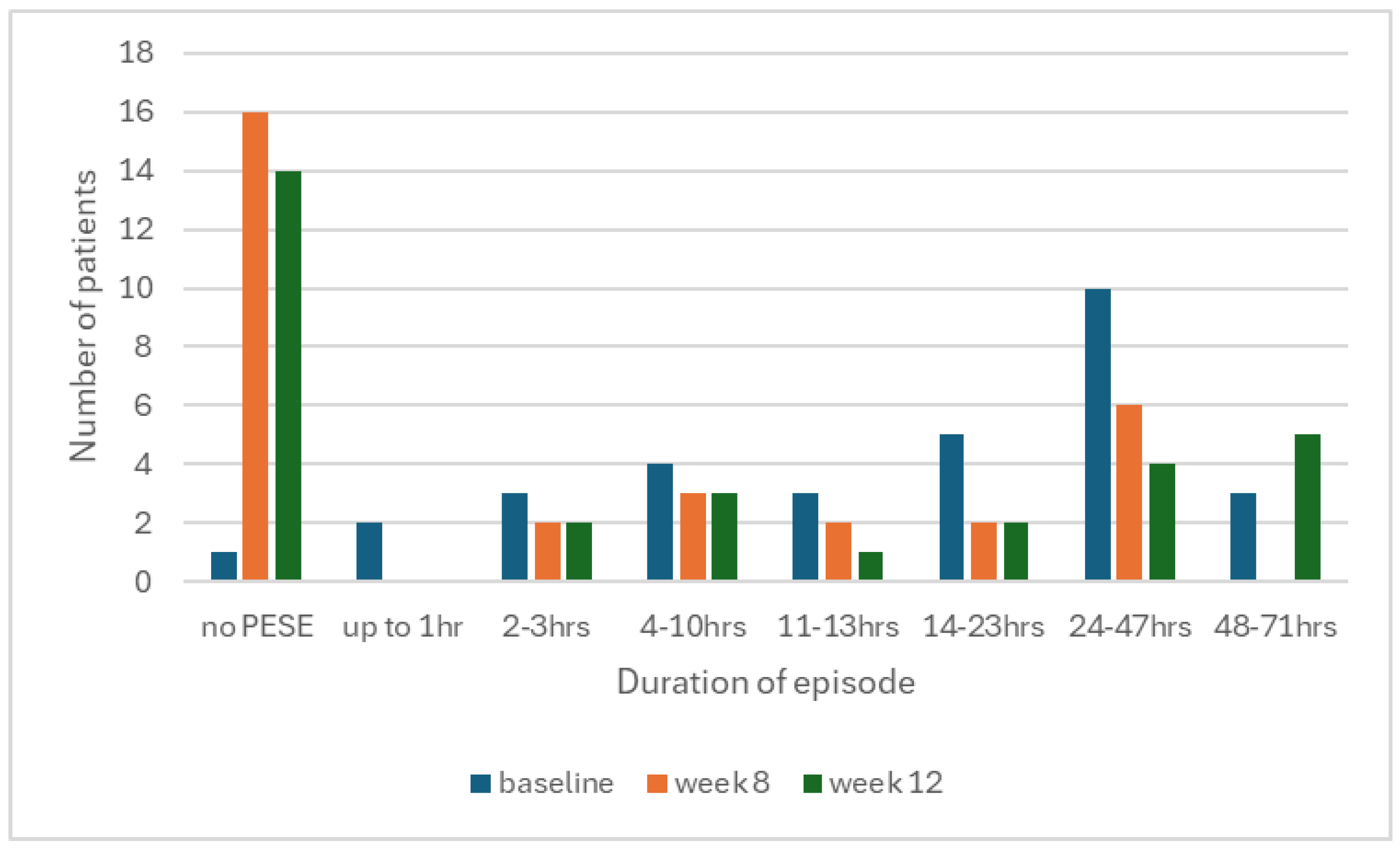
When PESE did occur, patients reported slightly fewer symptoms with a median of 3 at baseline to 2 at week 8, and reduced severity, with a median of 2.5 out of 3 at baseline to 1 out of 3 at week 8. We also observed less episodes of longer duration by week 8, resulting in a statistically significant improvement overall (
Figure 2,
Table 3).
Overall, no significant increase in activity phase was seen from baseline to week 12. However, patients intentionally reduced their phase of activity between baseline and week 1 whilst they began the process of adopting the programme protocol, before gradually re-building them as they felt able. Activity levels steadily returned to near previous levels over the course of the programme, whilst decreased numbers of PESE episodes and symptoms, reduced symptom severity and shorter duration of episodes were maintained (
Figure 3).
Active resting score (Likert) also showed statistically significant change over time, increasing from a median of 23 at baseline to 30 at weeks 8 (0.7, 0.4, 0.9, p<0.001) and 12 (0.5, 0.3, 0.6, p<0.001). This shows self reported improvements in both interoceptive awareness and ability to carry out diaphragmatic breathing. The data is enhanced by some of the qualitative data collected at the end of the study, (
Table 4) though it should be noted it was beyond the scope of this study to perform full qualitative analysis.
4. Discussion
Our service evaluation demonstrates that use of the WHO Borg CR10 pacing protocol over 8 weeks is associated with decreased PESE episodes, a reduction in the number of longer episodes, fewer symptoms, and milder symptom severity. This was reflected in statistically significant improvements in C19YRSm scores and EQ-5D-5L VAS, but the improvement in EQ-5D-5L utility score was not statistically significant.
The lack of improvement in EQ-5D-5L utility score over the intervention period is in contrast to our earlier work [
25], which reported statistically significant improvement across all domains after 6 weeks of using the WHO Borg CR-10 pacing protocol. This may be related to participants with shorter duration of symptoms (17 months) in our earlier study, compared to substantially longer duration of LC symptoms in the current study (29 months). We know that longer duration of symptoms is associated with poorer prognosis in ME/CFS [
32], and that lack of full recovery is being seen in LC when studied over time [
13,
14,
16,
17]. However, we still observed improvements in some measures which is encouraging to see, despite the sample being an uncontrolled cohort. Also, it seemed in this study that change continued over the follow-up period and by week 12 there was a greater change in scores. The EQ-5D-5L VAS, C19YRSm symptom severity, functional disability and overall health scores, were not statistically significant at baseline to week 8, but were so at week 12. This suggests a slower pace of change with increased duration of symptoms, which has implications both for further research and clinical practice.
The data regarding triggers for PESE may offer additional insight into the pace of change in EQ-5D-5L and C19YRSm scores. We asked patients to consider social and environmental triggers as well as physical, cognitive and emotional causes, thereby encompassing most aspects of daily life. Many identified several triggers within their episodes, perhaps recognising overlaps and patterns not previously perceived despite how long they had been experiencing symptoms. We wonder whether the impact of PESE as a stubborn and complex symptom, present for over two years, was overwhelming, and that this provoked a negative response initially. This would align with previous work showing increased symptom burden and psychological distress in ME/CFS patients who experience PESE [
33,
34].
We believe there is an implication for practice when comparing the differences in EQ-5D-5L and C19YRSm scores in our study to those of our previous work [
25]. Specifically, the WHO Borg CR10 pacing protocol may be most effective when implemented as early as possible following onset of symptoms, meaning early referral is key. For those with prolonged symptom duration, we need to recognise they may now have transitioned to a long term condition and allow for slower, more gradual change. We would argue pacing is no less important for this group as it remains a cornerstone of fatigue and PESE management, but we should not expect to see rapid changes. Instead our focus should be on individualised self management support for patients, using the protocol to help maximise activity levels (within the context of symptoms), minimise avoidance and fear, and promote quality of life.
Interestingly, median C19YRSm PESE scores were statistically significant by week 8 of the intervention, in contrast to Symptom Severity score which was significant only at 12. This sits alongside statistically significant changes in PESE characteristics throughout the programme, namely improvements in number of episodes, number of symptoms, severity of symptoms and duration of episodes. Improvement in Likert scores was also statistically significant by the end of week 8, showing patients became more interoceptively aware and more confident to practise resonant breathing. We feel it is possible that improvements in interoceptive awareness and time spent on active rest may have helped address the boom-bust cycle when combined with commitment to manageable effort levels via use of the Borg scale and activity phases. There are two aspects to this. Firstly, emphasising the importance of balancing activity with quality rest, alongside weekly telephone calls, may have led to greater consistency of activity. In a previous study, activity consistency was associated with lower depression, lower avoidance and increased function in a group of chronic pain patients [
35]. We feel a similar effect may have been experienced in our group, whereby planning time for active rest and consicously allowing oneself to remain at a manageable effort level resulted in an overall sense of coping more effectively with PESE. In this way we would hope that use of the programme over time could lead the way to increased and/or more meaningful activity, rather than focussing on symptom reduction. Secondly, the rest aspect of pacing has previously been described as: sleep, relaxation, inactivity, active restoration and self regulation [
36]. However, there is often little explanation beyond that, and in the context of PESE, fatigue and brain fog – all associated LC symptoms – it is possible that some patients do not have the energy to really think about, or engage with, what meaningful rest might mean to them. Our focused work on active rest activities and how to incorporate these into daily routines was generally well received and the associated changes in Likert scores are encouraging. Overall, we feel that promoting active rest added to the efficacy of the intervention and that further investigation of this concept is warranted. This could be especially poignant in the context of vocational rehabilitation given the numbers of people whose ability to work has been affected by LC [
4], but we feel there is scope for transfer to any activity as part of an individualised multidisciplinary rehabilitation programme.
There are several limitations to our service evaluation, including small sample size and lack of diversity in our patient group. We were unable to include a comparable control group, which would have added to the quality of our data greatly. Clinicians were not blinded in any way and we were very conscious of opportunities for bias. That said, we feel there are lessons to learn from our results, not least that taking the time to rest well and maintain manageable activity levels can lead to a reduction in the frequency, impact and duration of PESE. The intervention was generally easy to implement in terms of clinical time and space, and did not require expensive resources. Many patients reported preferring frequent short phone calls to lengthy clinic appointments as it was less burdensome on energy levels and meant that issues could be addressed quickly and easily. For us as a clinical rehabilitation service, these are important observations and we aim to continue developing pacing within our practice.
5. Conclusion
PESE has become a hallmark of LC and for many, their symptoms are becoming synonymous with ME/CFS. Effective pacing remains one of the only recommended management strategies for PESE, and although simple in principle, it can be difficult to master. We have made minor refinements to the pacing programme by including active rest and extending our period of observation, with some degree of success. We feel the non-pharmacological nature of pacing and its potential be used alongside technology mean it is worth pursuing as an area of research for both LC and ME/CFS, but we need to be mindful that changes to overall reported health outcomes may take time and be small, and that only by measuring change over a prolonged period of time will we have any indication of impact. For that reason, we would support further and larger studies with robust design (clinical trials). From a clinical perspective, NHS resources to support long term conditions are stretched, and people are struggling to maintain their day to day activities, especially work. A pacing intervention which can be delivered virtually, in shorter appointments, but which results in real life tangible changes in quality of life, provides a valuable and cost effective intervention that is easily deployable throughout the LC community, and therefore we advocate its use.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Supplementary materials 1: WHO Borg 10-CR pacing protocol, Supplementary materials 2: Leeds PESE questionnaire and 4-item Likert scale for active resting, Supplementary materials 3: Intervention process measures across time points.
Author Contributions
B.G., J.S., S.W., R.B., R.T. and M.S. contributed towards conceptualisation, methodology, investigation, writing, review and editing. M.S. provided supervision. B.G., J.S. and S.W. collected and formulated the data. B.G. undertook data curation and analysis; D.C.G provided statistical analysis and guidance. B.G. prepared the original draft. All authors have read and agreed to the published version of the manuscript.
Funding
This study received no external funding. Co-authors B.G.; J.S., S.W., R.B. and R.T were supported by internal LCH RCF (Research Capability Funding).
Institutional Review Board Statement
This was a service evaluation study that collected. Data from patients receiving care in the service. We completed the NHS online HRA toolkit with study information, and the decision was that this was not research and only local trust approvals were needed. The service evaluation was approved by the participating NHS trust (Leeds Community Healthcare NHS Trust) as service evaluation as per standard requirements.
Informed Consent Statement
Informed consent in writing was obtained from all participants in the service evaluation.
Data Availability Statement
Anonymised data can be obtained by contacting the corresponding author.
Acknowledgments
The authors would like to thank clinicians and patients in the service for their support and time in relation to this service evaluation. Also to the research team at LCH for providing support and encouragement to carry out research activity.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- COVID-19 rapid guideline: managing the long-term effects of COVID-19 – NICE Guideline. Updated January 2024. Available online: https://www.nice.org.uk/guidance/ng188 (accessed on 20 March 2024).
- Prevalence of Ongoing Symptoms Following Coronavirus (COVID-19) Infection in the UK – Office for National Statistics. March 2023. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/prevalenceofongoingsymptomsfollowingcoronaviruscovid19infectionintheuk/30march2023 (accessed on 20 March 2024).
- Self-reported coronavirus (COVID-19) infections and associated symptoms; England and Scotland: November 2023 to March 2024 – Office for National Statistics. Available online: Self-reported coronavirus (COVID-19) infections and associated symptoms, England and Scotland - Office for National Statistics. (accessed on day month year).
- TUC Joint Report by TUC and Long COVID Support, March 2023. Available online: https://www.tuc.org.uk/research-analysis/reports/workers-experience-long-covid (accessed on 20 March 2024).
- Sanal-Hayes, N.E.M.; Mclaughlin M.; Hayes, L.D.; Mair, J.L.; Ormerod, J.; Carless, D.; Hilliard, N.; Meach, R.; Ingram, J.; Sculthorpe, N.F. A scoping review of ‘Pacing’ for management of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): lessons learned for the long COVID pandemic,” Journal of Translational Medicine 2023, 21, 720. [CrossRef]
- Weigel, B.; Eaton-Fitch, N.; Thapaliya, K.; Marshall-Gradisnik, S. Illness presentation and quality of life in myalgic encephalomyelitis/chronic fatigue syndrome and post COVID-19 condition: a pilot Australian cross-sectional study. Quality of Life Research 2024, 33, 2489–2507. [Google Scholar] [CrossRef] [PubMed]
- Stussman, B.; Williams, A.; Snow, J.; Gavin, A.; Scott, R.; Nath, A.; Wallitt, B. Characterization of Post-exertional Malaise in Patients with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Frontiers in Neurology 2020, 11, 1025. [Google Scholar] [CrossRef]
- Clinical management of COVID-19: living guideline. World Health Organization (WHO). August 2023. Available online: Guideline Clinical management of COVID-19: living guideline. (accessed on 21 March 2024).
- Myalgic Encephalomyelitis / Chronic Fatigue Syndrome – Strategies to Prevent Worsening of Symptoms. May 2024. Available online: Strategies to Prevent Worsening of Symptoms | ME/CFS | CDC. (accessed on 17 September 2024).
- Wormgoor, M.E.A.; Rodenburg, S.C. Focus on post-exertional malaise when approaching ME/CFS in specialist healthcare improves satisfaction and reduces deterioration. Frontiers in Neurology 2023, 14, 1247698. [Google Scholar] [CrossRef] [PubMed]
- Vernon, S.D.; Hartle, M.; Sullivan, K.; Bell, J.; Abbaszadeh, S.; Unutmaz, D.; and Bateman, L. Post-exertional malaise among people with long COVID compared t0 myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) Work. 2023, 74, 1179–1186. [CrossRef]
- Ghali, A.; Richa, P.; Lacout, C.; Gury, A.; Beucher, A.; Homedan, C.; Lavigne, C.; Urbanski, G. Epidemiological and clinical factors associated with post-exertional malaise severity in patients with myalgic encephalomyelitis/ chronic fatigue syndrome. Journal of Translational Medicine 2020, 18, 246. [Google Scholar] [CrossRef] [PubMed]
- Hurt, R.T.; Yadav, S.; Schroeder, D.R.; Croghan, I.T.; Mueller, M.R.; Grach, S.L.; Aakre, C.A.; Gilman, E.A.; Stephenson, C.R.; Overgaard, J.; Collins, N.M.; Lawson, D.K.; Thompson, A.M.; Natividad, L.T.; Elfadil, O.M.; Ganesh, R. Longitudinal Progression of Patients with Long COVID Treated in a Post-COVID Clinic: A Cross-Sectional Survey. Journal of Primary Care & Community Health 2024, 15, 1–11. [Google Scholar] [CrossRef]
- Hastie, C.E.; Lowe, D.J.; McAuley, A.; Andrew J. Winter, A.J.; Mills, N.L.; Black, C.; Scott, J.T.; O’Donnell, C.A.; Blane, D.N.; Browne, S.; Ibbotson, T.R.; Pell, J.P. Outcomes among confirmed cases and a matched comparison group in the Long-COVID in Scotland study. Nature Communications 2022,13, 5663. [CrossRef]
- Demko, Z.O.; Yu, T.; Mullapudi, S.K.; Varela Heslin, M.G.; Dorsey, C.A.; Payton, C.B.; Blair, P.W.; Mehta, S.H.; Thomas, D.L.; Manabe, Y.C.; et al. Two-Year Longitudinal Study Reveals That Long COVID Symptoms Peak and Quality of Life Nadirs at 6–12 Months Postinfection. Open Forum Infect. Dis. 2024, 11, ofae027. [Google Scholar] [CrossRef] [PubMed]
- Sivan, M.; Greenwood, D.; Smith, A.; Rocha Lawrence, R.; Osborne, T.; Goodwin, M. A National Evaluation of Outcomes in Long COVID Services Using Digital PROM Data from the ELAROS Platform. LOCO-MOTION and ELAROS, Published by NHS England, 2023. Available online: https://locomotion.leeds.ac.uk/wpcontent/uploads/sites/74/2023/10/National-Evaluationof-LC-Service-Outcomes-using-ELAROS-Data-09-10-23.pdf (accessed on 3 October 2024).
- Bodey, R.; Grimaldi, J.; Tait, H.; Godfrey, B.; Witton, S.; Shardha, J.; Tarrant, R,; Sivan, M. How Long Is Long COVID? Evaluation of Long-Term Health Status in Individuals Discharged from a Specialist Community Long COVID Service. Journal of Clinical Medicine 2024, 13, 5187. [Google Scholar] [CrossRef]
- Ghali, A.; Lacombe, V.; Ravaiau, C.; Delattre, E.; Ghali, M.; Urbanski, G.; Lavigne, C. The relevance of pacing strategies in managing symptoms of post-COVID-19 syndrome. Journal of Translational Medicine. 2023, 21, 375. [Google Scholar] [CrossRef] [PubMed]
- Myalgic encephalomyelitis (or encephalopathy)/chronic fatigue syndrome: diagnosis and management - NICE guideline. October 2021. Available online: www.nice.org.uk/guidance/ng206 (accessed on 16 July 2024).
- Kos, D,; van Eupen, I. ; Meirte, J.; Van Cauwenbergh, D.; Moorkens, G.; Meeus, M.; Nijs, J. Activity Pacing Self-Management in Chronic Fatigue Syndrome: A Randomized Controlled Trial. The American Journal of Occupational Therapy 2015, 69, 5. [Google Scholar]
- Jason, L.A.; Brown, M.; Brown, A.; Evans, M.; Flores, S.; Grant-Holler, E.; Sunnquist, M. Energy Conservation/Envelope Theory Interventions to Help Patients with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Fatigue 2013, 14, 27–42. [Google Scholar] [CrossRef]
- Antcliff, D.; Keenan, A.; Keeley, P.; Woby, S.; McGowan, L. Testing a newly developed activity pacing framework for chronic pain/fatigue: a feasibility study. BMJ Open 2021, 11, e045398. [Google Scholar] [CrossRef] [PubMed]
- Pacing for people with, M.E.; A detailed guide to managing energy, rest and activity for adults with mild/moderate M.E. Action for M.E 2022. Available online: https://www.actionforme.org.uk/uploads/pdfs/Pacing-for-people-with-ME-2022.pdf (accessed on 17 September 2024).
- Hunt, J.; Blease, C.; Geraghty, K.J. Long Covid at the crossroads: Comparisons and lessons from the treatment of patients with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Journal of Health Psychology 2022, 27, 3106–3120. [Google Scholar] [CrossRef]
- Parker, M.; Brady Sawant, H.; Flannery, T.; Tarrant, R.; Shardha, J.; Bannister, R.; Ross, D.; Halpin, S.; Greenwood, D.C.; Sivan, M. Effect of using a structured pacing protocol on post-exertional symptom exacerbation and health status in a longitudinal cohort with the post-COVID-19 syndrome. Journal of Medical Virology, 2837. [Google Scholar] [CrossRef]
- Van Hout, B.; Janssen, M.F.; Feng, Y.-S.; Kohlmann, T.; Busschbach, J.; Golicki, D.; Lloyd, A.; Scalone, L.; Kind, P.; Pikard, S. Interim Scoring for the EQ-5D-5L: Mapping the EQ-5D-5L to EQ-5D-3L Value Sets. Value Health 2012, 15, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Sivan, M.; Preston, N.; Parkin, A.; Makower, S.; Gee, J.; Ross, D.; Tarrant, R.; Davison, J.; Halpin, S.; O’Connor, R.; Horton, M. The modified COVID-19 Yorkshire Rehabilitation Scale (C19-YRSm) patient-reported outcome measure for Long COVID or Post-COVID-19 syndrome. J. Med. Virol. 2022, 94, 4253–4269. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Greenwood, D.; Horton, M.; Osborne, T.; Goodwin, M.; Lawrence, R.R.; Winch, D.; Williams, P.; Milne, R.; Sivan, M. Psychometric analysis of the modified COVID-19 Yorkshire Rehabilitation Scale (C19-YRSm) in a prospective multicentre study. BMJ Open Respir. Res. 2024, 11, e002271. [Google Scholar] [CrossRef]
- Mehling, W.E.; Acree, M.; Stewart, A.; Silas, J.; Jones, A. The Multidimensional Assessment of Interoceptive Awareness, Version 2 (MAIA-2). PLoS ONE 2018, 13, e0208034. [Google Scholar] [CrossRef] [PubMed]
- Polizzi, J.; Tosto-Mancuso, J.; Tabacof, L. ’ Wood, J.; Putrino, D. Resonant breathing improves self-reported symptoms and wellbeing in people with Long COVID. Frontiers in Rehabilitation Science 2024, 5, 1411344. [Google Scholar] [CrossRef]
- Corrado, J.; Iftekhar, N.; Halpin, S.; Li, M.; Tarrant, R.; Grimaldi, J.; Simms, A.; O’Connor, R.J.; Casson, A.; Sivan, M. HEART Rate Variability Biofeedback for LOng COVID Dysautonomia (HEARTLOC): Results of a Feasibility Study. Advances in Rehabilitation Science and Practice 2024, 13, 1–8. [Google Scholar] [CrossRef]
- Ghali, A.; Lacout, C.; Fortrat, J.; Depres, K.; Ghali, M.; Lavigne, C. Factors Influencing the Prognosis of Patients with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Diagnostics 2022, 12, 2540. [Google Scholar] [CrossRef] [PubMed]
- König, R.S.; Paris, D.H.; Sollberger, M.; Tschopp, R. Identifying the mental health burden in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) patients in Switzerland: A pilot study. Heliyon 2024, 10, e27031. [Google Scholar] [CrossRef]
- May, M.; Milrad, S.F.; Perdomo, D.M.; Czaja, S.J. ; Fletcher, M,A.; Jutagir, D.R.; Hall, D.L.; Klima, N.; Antoni, M.H. Post-Exertional Malaise is Associated with Greater Symptom Burden and Psychological Distress in Patients Diagnosed with Chronic Fatigue Syndrome. J Psychosom Res. 1098. [Google Scholar] [CrossRef]
- Antcliff, D,; Campbell, M.; Woby, S.; Keeley, P. Activity Pacing is Associated With Better and Worse Symptoms for Patients With Long-term Conditions. Clin J Pain 2017, 33, 205–214. [CrossRef]
- Barakou, I.; Hackett, K.L.; Finch, T.; Hettinga, F.J. Self-regulation of effort for a better health-related quality of life: a multidimensional activity pacing model for chronic pain and fatigue management. Annals of Medicine 2023, 2, 2270688. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).