Submitted:
02 December 2024
Posted:
05 December 2024
You are already at the latest version
Abstract
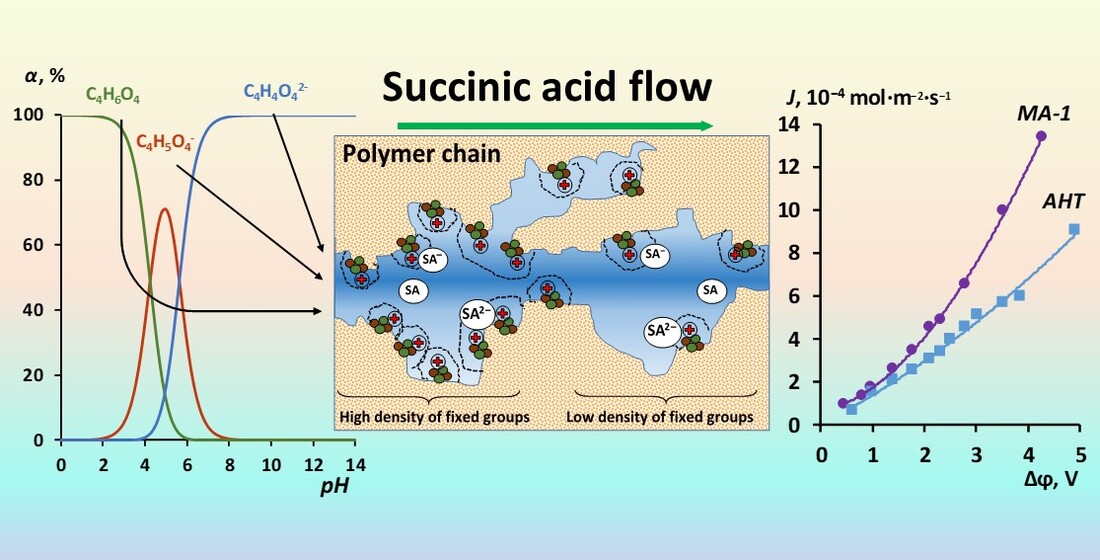
Keywords:
1. Introduction
2. Materials and Methods
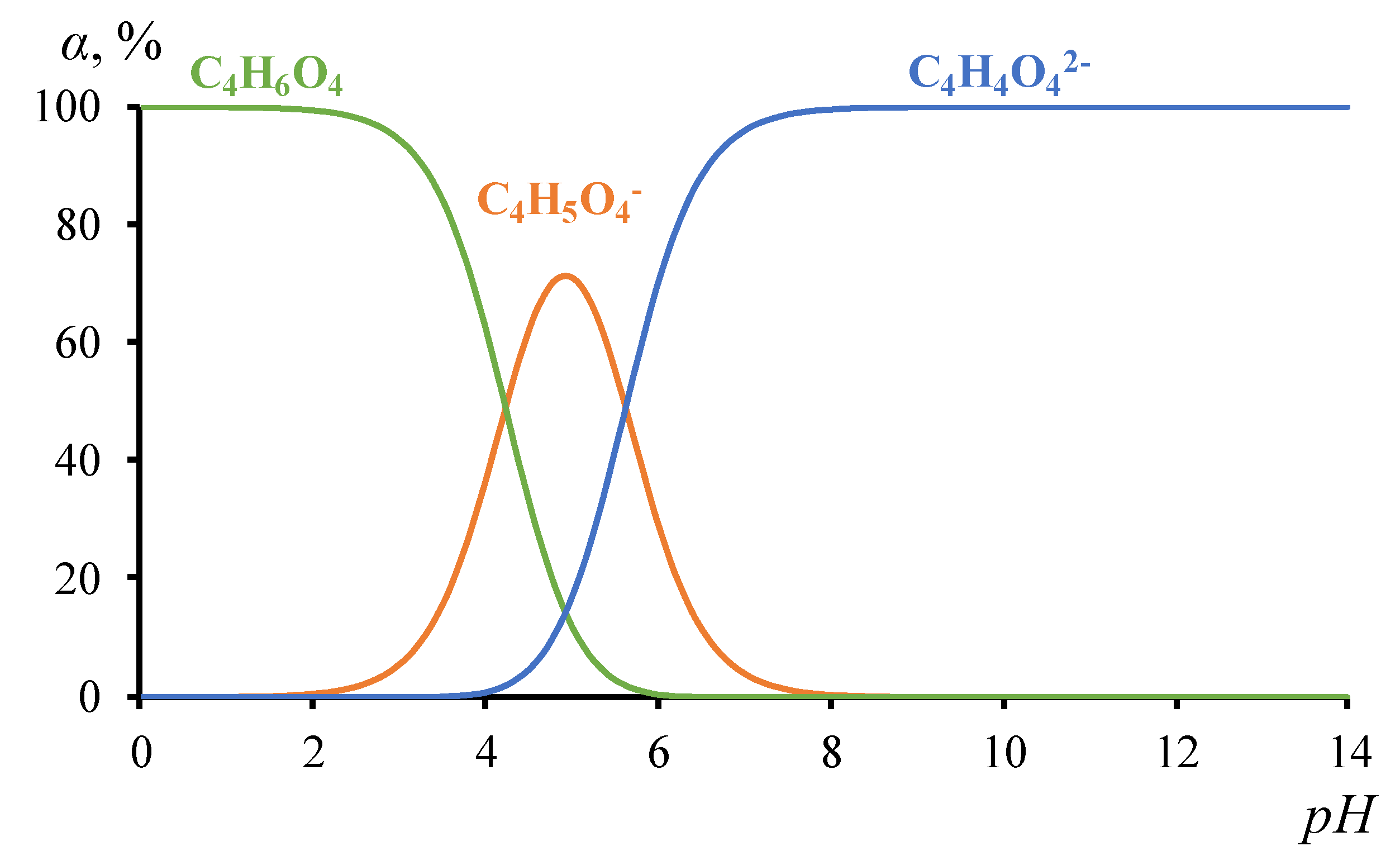
Objects of the Study


2.4. Investigation of the physico-mechanical and transport-structural characteristics of anion exchange membranes
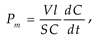
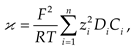
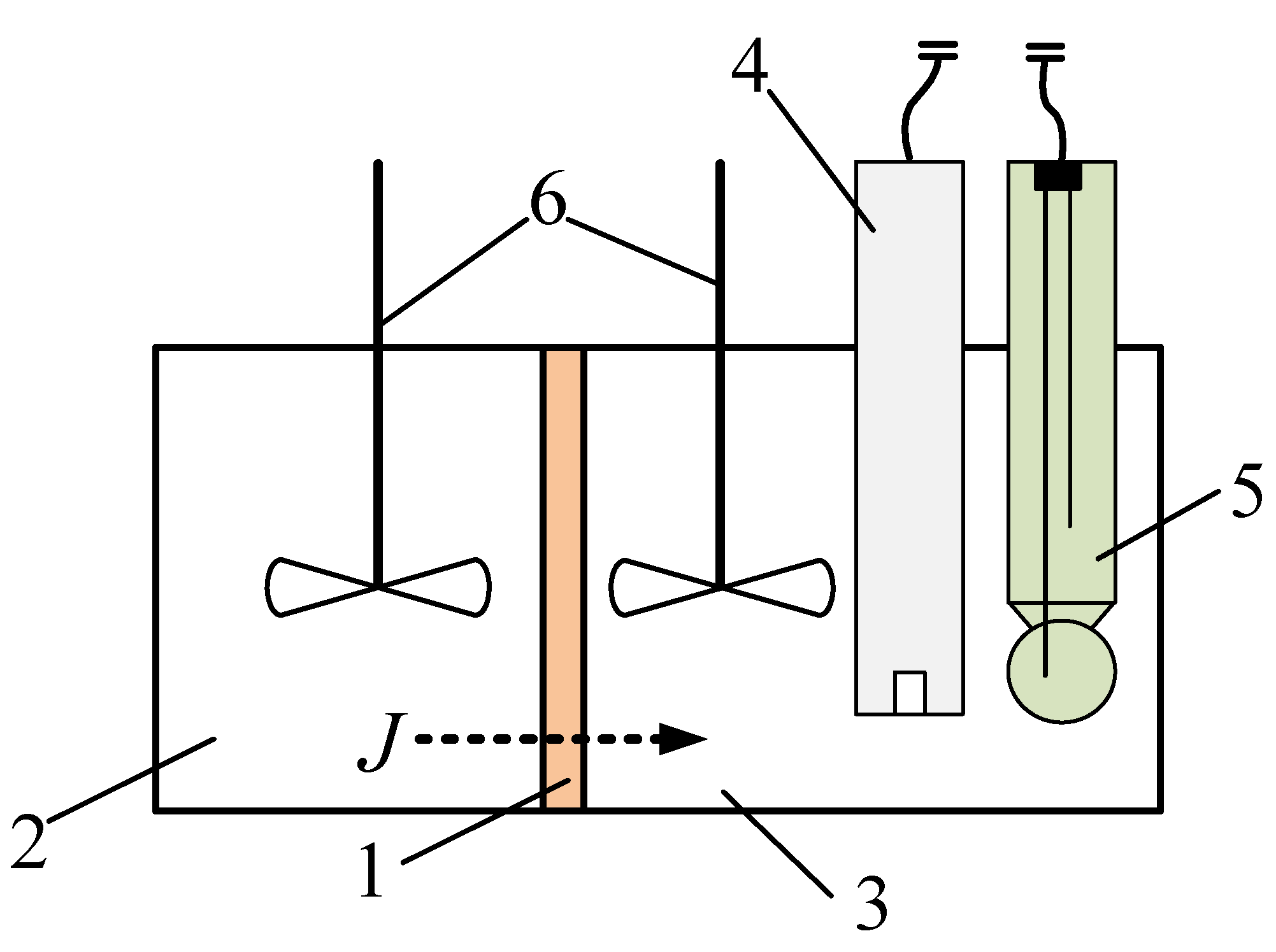
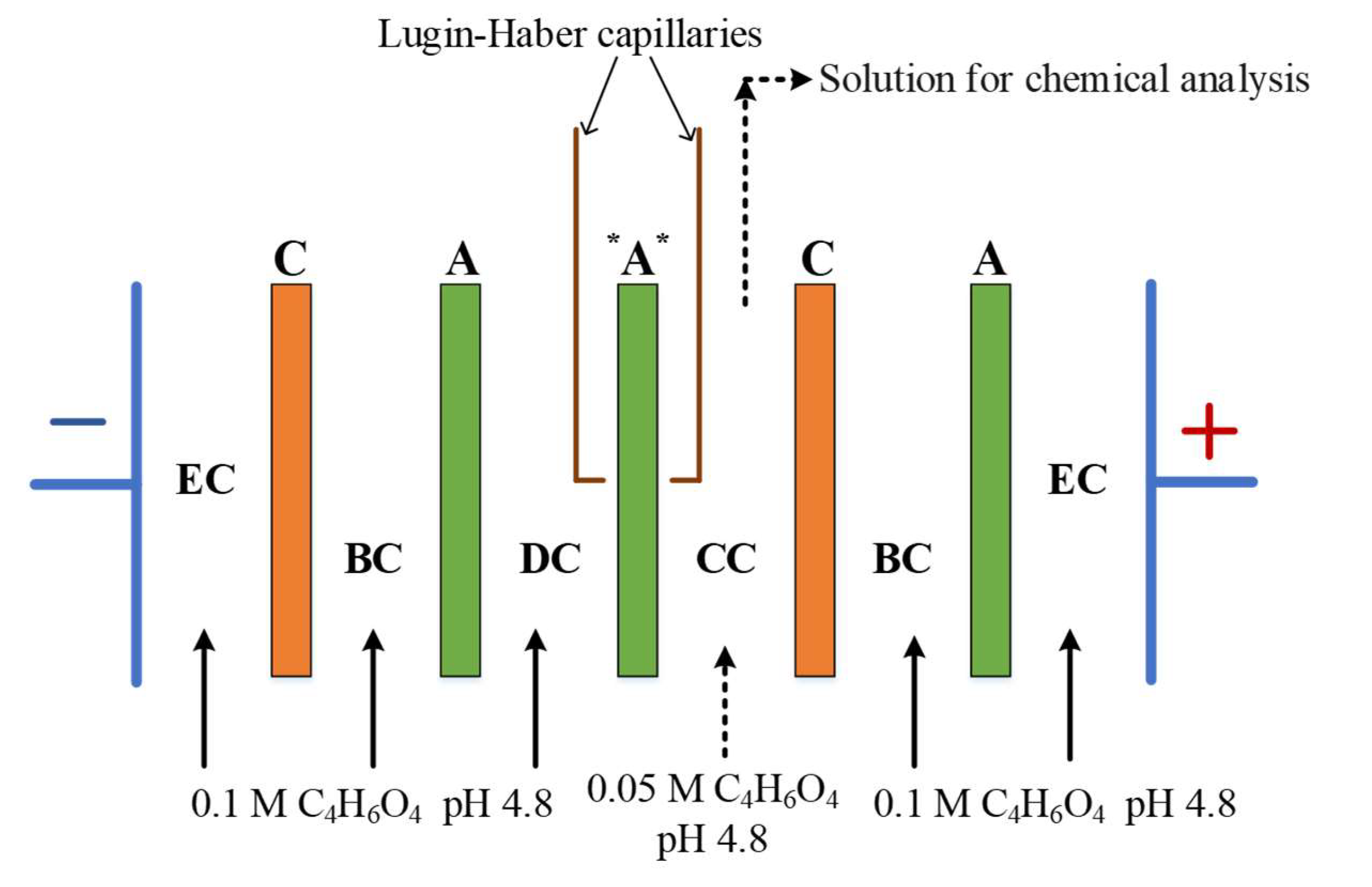
2.5. Investigation of the volt-ampere characteristics of anion exchange membranes and electromass transfer in solutions of sodium succinate
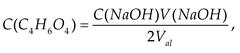
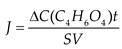
3. Results and discussion
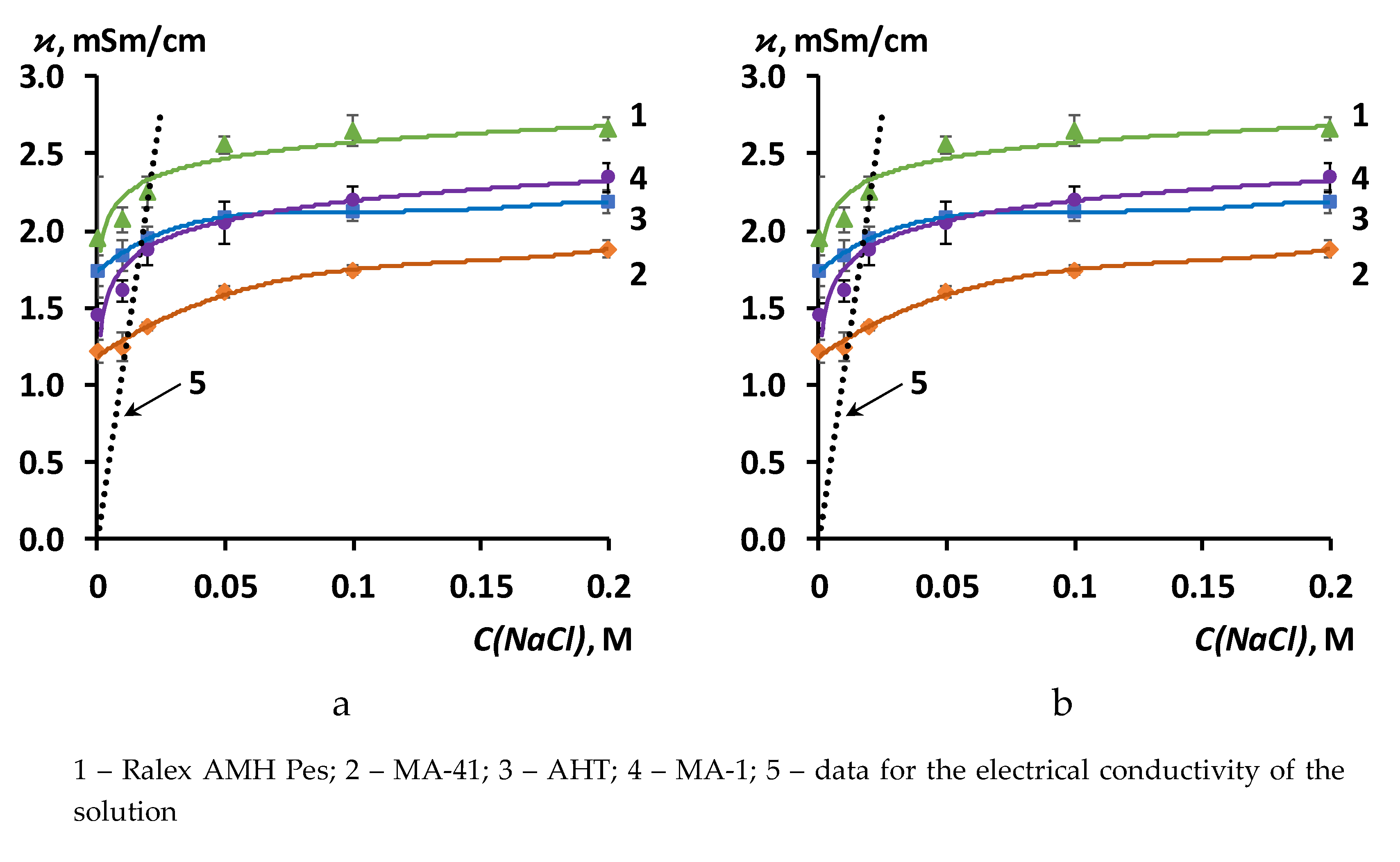
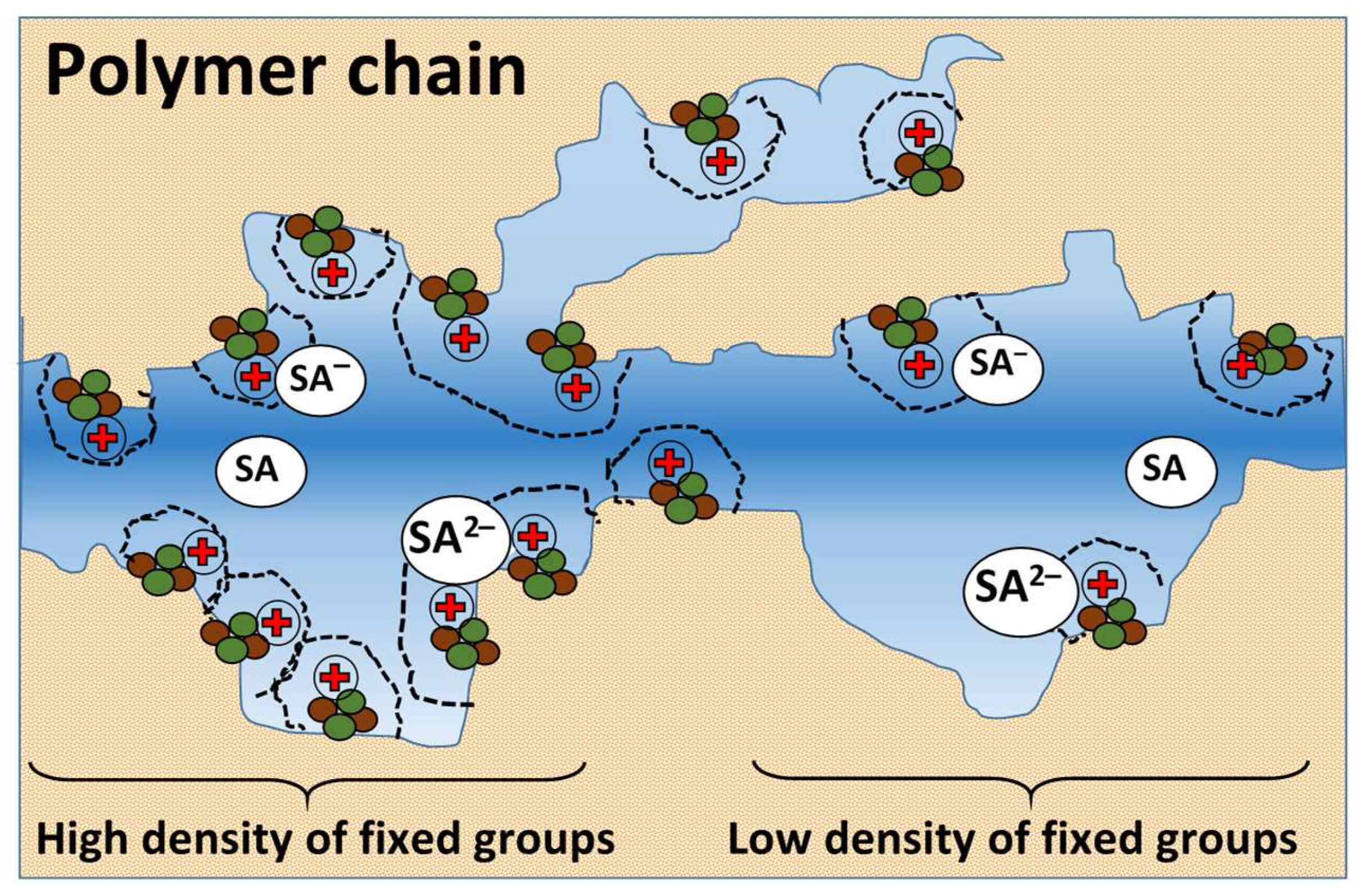
3.1. The results of the study of the physico-mechanical and transport-structural characteristics of anion exchange membranes
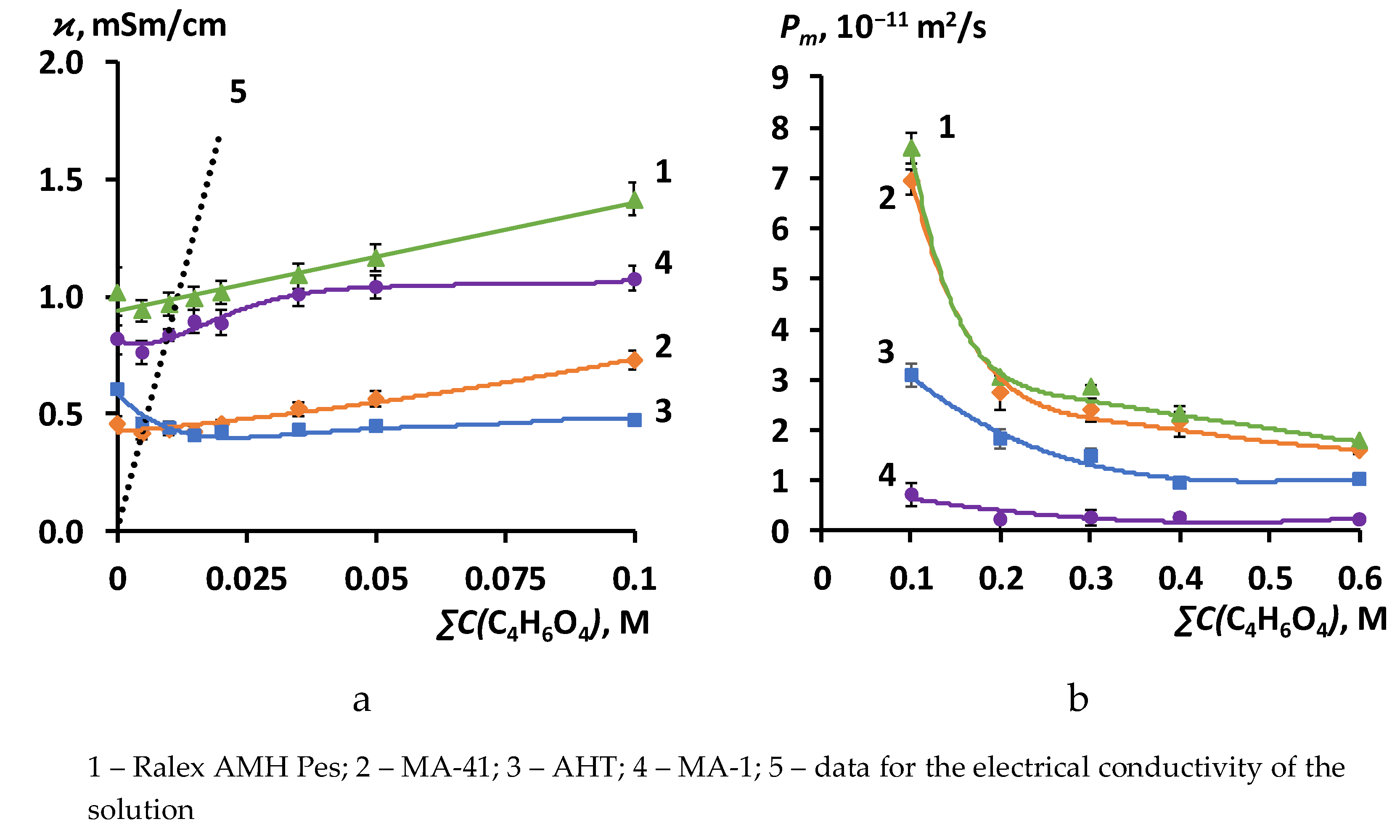
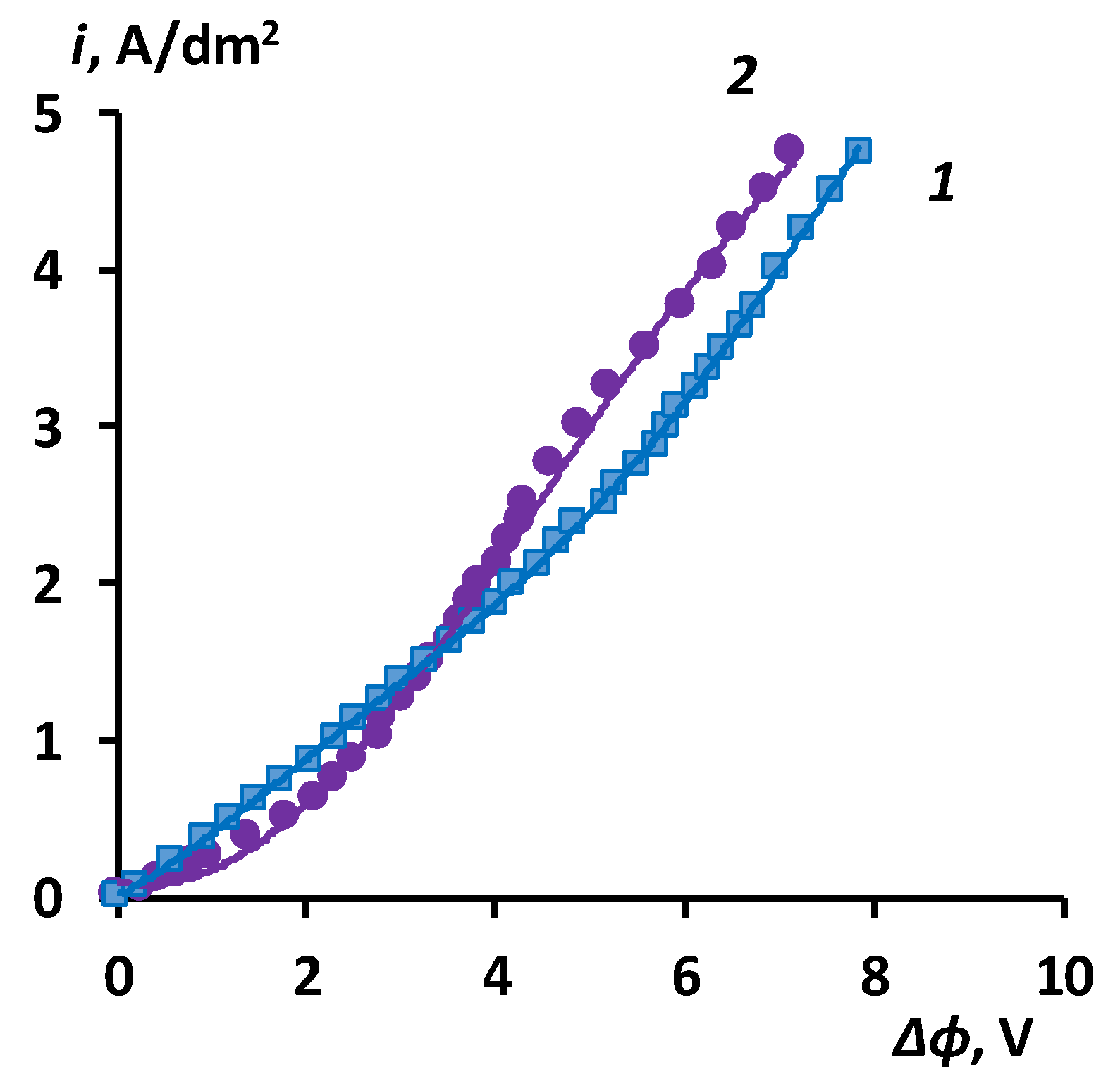
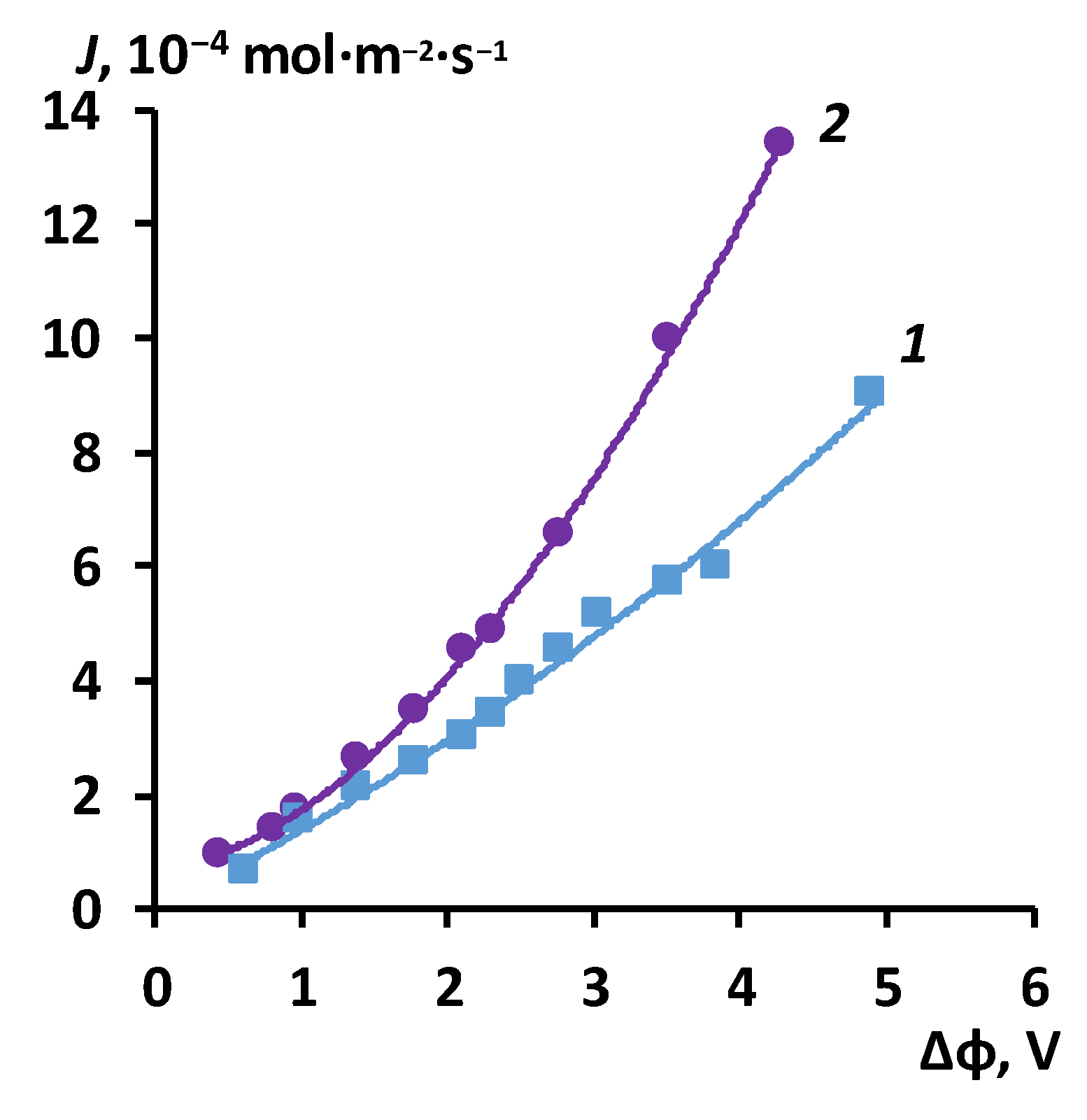
3.2. The results of the study of the volt-ampere characteristics of anion exchange membranes and electromass transfer in solutions of sodium succinate
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nghiem Nhuan, P. , Kleff Susanne, Schwegmann Stefan Succinic Acid: Technology Development and Commercialization. Fermentation 2017, 3. [Google Scholar] [CrossRef]
- Saxena, R.K. , Saran S., Isar J., K.R. 27 - Production and Applications of Succinic Acid. In Current Developments in Biotechnology and Bioengineering; 2017; pp. 601–630.
- Smirnov, A.V. , Nesterova, O.B., Golubev, R.V. Succinic acid and its application in medicine. Part I. Succinic acid: Metabolite and regulator of metabolism of the human body. Nephrol. (In Russ.) 2014, 18, 33–41. [Google Scholar]
- Smirnov, A.V. , Nesterova, O.B., Golubev, R.V. Succinic acid and its application in medicine. Part II. Application of succinic acid in medicine. Nephrol. (In Russ.) 2014, 18, 12–24. [Google Scholar]
- Kirk- Othme Encyclopedia of chemical technology. 5th ed.; Wiley Blac.; New york, 1991;
- Itziar, A. Escanciano и др. Modeling the Succinic Acid Bioprocess: A Review. Fermentation 2022, 8. [Google Scholar] [CrossRef]
- McKinlay J., B. , Vieille C., Z.J.G. Prospects for a bio-based succinate industry. Appl. Microbiol. Biotechnol. 2007, 76, 727–740. [Google Scholar] [CrossRef]
- Cao, Y. , Zhang R., Sun C., Cheng T., Liu Y., X.M. Fermentative Succinate Production: An Emerging Technology to Replace the Traditional Petrochemical Processes. Biomed. Res. Ind. 2013. [Google Scholar] [CrossRef]
- Jiang, M.; Ma, J.; Wu, M.; Liu, R.; Liang, L.; Xin, F.; Zhang, W.; Jia, H.; Dong, W. Progress of succinic acid production from renewable resources: Metabolic and fermentative strategies. Bioresour. Technol. 2017, 245, 1710–1717. [Google Scholar] [CrossRef]
- Kurzrock, T.; Weuster-Botz, D. Recovery of succinic acid from fermentation broth. Biotechnol. Lett. 2010, 32, 331–339. [Google Scholar] [CrossRef]
- Kumar, R.; Basak, B.; Jeon, B.-H. Sustainable production and purification of succinic acid: A review of membrane-integrated green approach. J. Clean. Prod. 2020, 277, 123954. [Google Scholar] [CrossRef]
- Lin, S.K.C.; Du, C.; Blaga, A.C.; Camarut, M.; Webb, C.; Stevens, C. V.; Soetaert, W. Novel resin-based vacuum distillation-crystallisation method for recovery of succinic acid crystals from fermentation broths. Green Chem. 2010, 12, 666. [Google Scholar] [CrossRef]
- Alexandri, M.; Vlysidis, A.; Papapostolou, H.; Tverezovskaya, O.; Tverezovskiy, V.; Kookos, I.K.; Koutinas, A. Downstream separation and purification of succinic acid from fermentation broths using spent sulphite liquor as feedstock. Sep. Purif. Technol. 2019, 209, 666–675. [Google Scholar] [CrossRef]
- Fu, L.; Gao, X.; Yang, Y.; Aiyong, F.; Hao, H.; Gao, C. Preparation of succinic acid using bipolar membrane electrodialysis. Sep. Purif. Technol. 2014, 127, 212–218. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, S.; Zhang, X.; Zheng, Y.; Xiu, Z. Ionic liquid-based sugaring-out and salting-out extraction of succinic acid. Sep. Purif. Technol. 2018, 204, 133–140. [Google Scholar] [CrossRef]
- Szczygiełda, M.; Antczak, J.; Prochaska, K. Separation and concentration of succinic acid from post-fermentation broth by bipolar membrane electrodialysis (EDBM). Sep. Purif. Technol. 2017, 181, 53–59. [Google Scholar] [CrossRef]
- Lightfoot, E.N.; Friedman, I.J. Ion Exchange Membrane Purification of Organic Electrolytes. Ind. Eng. Chem. 1954, 46, 1579–1583. [Google Scholar] [CrossRef]
- Vásquez-Garzón, M.L.; Bonotto, G.; Marder, L.; Zoppas Ferreira, J.; Bernardes, A.M. Transport properties of tartrate ions through an anion-exchange membrane. Desalination 2010, 263, 118–121. [Google Scholar] [CrossRef]
- Yazicigil, Z.; Oztekin, Y. Boron removal by electrodialysis with anion-exchange membranes. Desalination 2006, 190, 71–78. [Google Scholar] [CrossRef]
- Vasil’eva, V.I. , Shaposhnik, V.A., Zemlyanukhina, I.A., Grigorchuk, O.V. Facilitated diffusion of amino acids in ion-exchange membranes. Russ. J. Phys. Chem. A. 2003, 77, 1017–1019. [Google Scholar]
- Melnikova, E.D. , Tsygurina, K.A., Pismenskaya, N.D., Nikonenko, V.V. Influence of protonation–deprotonation reactions on the diffusion of ammonium chloride through anion-exchange membrane. Membr. Membr. Technol. 2021, 11, 324–333. [Google Scholar] [CrossRef]
- Pismenskaya, N.; Nikonenko, V.; Auclair, B.; Pourcelly, G. Transport of weak-electrolyte anions through anion exchange membranes. J. Memb. Sci. 2001, 189, 129–140. [Google Scholar] [CrossRef]
- Belashova, E.D.; Pismenskaya, N.D.; Nikonenko, V.V.; Sistat, P.; Pourcelly, G. Current-voltage characteristic of anion-exchange membrane in monosodium phosphate solution. Modelling and experiment. J. Memb. Sci. 2017, 542, 177–185. [Google Scholar] [CrossRef]
- Melnikova, E.D.; Pismenskaya, N.D.; Bazinet, L.; Mikhaylin, S.; Nikonenko, V. V. Effect of ampholyte nature on current-voltage characteristic of anion-exchange membrane. Electrochim. Acta 2018, 285, 185–191. [Google Scholar] [CrossRef]
- Rybalkina, O.; Tsygurina, K.; Melnikova, E.; Mareev, S.; Moroz, I.; Nikonenko, V.; Pismenskaya, N. Partial fluxes of phosphoric acid anions through anion-exchange membranes in the course of NaH2PO4 solution electrodialysis. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef] [PubMed]
- Gally, C. , García-Gabaldón, M, Ortega, E, Bernardes, A.M. Chronopotentiometric study of the transport of phosphoric acid anions through an anion-exchange membrane under different pH values. Sep. Purif. Technol. 2020, 238. [Google Scholar] [CrossRef]
- Chandra, A.; E, B.; Chattopadhyay, S. A critical analysis on ion transport of organic acid mixture through an anion-exchange membrane during electrodialysis. Chem. Eng. Res. Des. 2022, 178, 13–24. [Google Scholar] [CrossRef]
- Martí-Calatayud, M.C.; Evdochenko, E.; Bär, J.; García-Gabaldón, M.; Wessling, M.; Pérez-Herranz, V. Tracking homogeneous reactions during electrodialysis of organic acids via EIS. J. Memb. Sci. 2020, 595. [Google Scholar] [CrossRef]
- Eliseeva, T. V.; Shaposhnik, V.A. Short communications. Russ. J. Electrochem. 2000, 36, 902–905. [Google Scholar] [CrossRef]
- Eliseeva, T. V; Krisilova, E. V; Vasilevsky, V.P.; Novitsky, E.G. Electrodialysis of solutions of tartaric acid and its salts. Pet. Chem. 2013, 52, 609–613. [Google Scholar] [CrossRef]
- Shaposhnik, V.A.; Eliseeva, T. V. Barrier effect during the electrodialysis of ampholytes. J. Memb. Sci. 1999, 161, 223–228. [Google Scholar] [CrossRef]
- Kozaderova, O.A.; Niftaliev, S.I.; Kim, K.B. Ionic Transport in Electrodialysis of Ammonium Nitrate. Russ. J. Electrochem. 2018, 54, 363–367. [Google Scholar] [CrossRef]
- Zabolotskiy, V.I.; Bespalov, A. V.; Bondarev, D.A.; Gornyaeva, Y.A.; Strelkov, V.D. Perspective modifiers for anionexchange membranes based on polymers with quaternary nitrogen atoms, which included in five- and six-membered heterocyclic rings. Polythematic Online Sci. J. Kuban State Agrar. Univ. 2016. [Google Scholar] [CrossRef]
- Apelblat, A. Dissociation Constants and Limiting Conductances of Organic Acids in Water. J. Mol. Liq. 2002, 95, 99–145. [Google Scholar] [CrossRef]
- Berezina, N.P.; Kononenko, N.A.; Dyomina, O.A.; Gnusin, N.P. Characterization of ion-exchange membrane materials: Properties vs structure. Adv. Colloid Interface Sci. 2008, 139, 3–28. [Google Scholar] [CrossRef] [PubMed]
- Shutkina, E.A.; Nevakshenova, E.E.; Pismenskaya, N.D.; Mareev, S.A.; Nikonenko, V.V. Diffusion permeability of the anion-exchange membranes in sodium dihydrogen phosphate solution. Kondens. sredy i mezhfaznyye granitsy (In Rus) 2015, 17, 566–578. [Google Scholar]
- Belova, E.I.; Lopatkova, G.Y.; Pismenskaya, N.D.; Nikonenko, V.V.; Larchet, C.; Pourcelly, G. Effect of anion-exchange membrane surface properties on mechanisms of overlimiting mass transfer. J. Phys. Chem. B 2006, 110, 13458–13469. [Google Scholar] [CrossRef]
- David Harvey Modern Analytical Chemistry; The McGraw.; 2000; ISBN 0-07-237547-7.
- Demina, O.A.; Falina, I. V.; Kononenko, N.A. Model description of conductivity of ion–exchange membranes in a wide range of concentrations of electrolyte solution. Russ. J. Electrochem. 2015, 51, 561–565. [Google Scholar] [CrossRef]
- Belashova, E.D. , Minakova E.A., Kharchenko O.A., Pismenskaya N.D. Influence of structural changes on current-voltage characteristics of the anion exchange membrane after their long contact with an ampholyte solution // Sorbtsionnye i khromatograficheskie p.
- Melnikov, S.; Kolot, D.; Nosova, E.; Zabolotskiy, V. Peculiarities of transport-structural parameters of ion-exchange membranes in solutions containing anions of carboxylic acids. J. Memb. Sci. 2018, 557, 1–12. [Google Scholar] [CrossRef]
- Pismenskaya, N.; Laktionov, E.; Nikonenko, V.; El Attar, A.; Auclair, B.; Pourcelly, G. Dependence of composition of anion-exchange membranes and their electrical conductivity on concentration of sodium salts of carbonic and phosphoric acids. J. Memb. Sci. 2001, 181, 185–197. [Google Scholar] [CrossRef]
- Sarapulova, V.; Nevakshenova, E.; Pismenskaya, N.; Dammak, L.; Nikonenko, V. Unusual concentration dependence of ion-exchange membrane conductivity in ampholyte-containing solutions: Effect of ampholyte nature. J. Memb. Sci. 2015, 479, 28–38. [Google Scholar] [CrossRef]
- Franck-Lacaze, L. , Sistat Ph., H.P.J. Determination of the pKa of poly (4-vinylpyridine)-based weak anion exchange membranes for the investigation of the side proton leakage. J. Memb. Sci. 2009, 326, 650–658. [Google Scholar] [CrossRef]
- Kozaderova, O. , Kozaderov O. , N.S. Electromass Transfer in the System “Cation Exchange Membrane—Ammonium Nitrate Solution.” Membranes (Basel). 2022, 12. [Google Scholar] [CrossRef]
- Pismenskaya, N.; Sarapulova, V.; Klevtsova, A.; Mikhaylin, S.; Bazinet, L. Adsorption of anthocyanins by cation and anion exchange resins with aromatic and aliphatic polymer matrices. Int. J. Mol. Sci. 2020, 21. [Google Scholar] [CrossRef] [PubMed]
- Bald, A.; Kinart, Z. Volumetric Properties of Some Aliphatic Mono- and Dicarboxylic Acids in Water at 298.15 K. J. Solution Chem. 2011, 40, 1–16. [Google Scholar] [CrossRef]
- Vasil’eva, V.I.; Vorob’eva, E.A. Dynamics of the separation of amino acid and mineral salt in the stationary dialysis of solutions with an MK-40 profiled sulfo group cation exchange membrane. Russ. J. Phys. Chem. A 2012, 86, 1726–1731. [Google Scholar] [CrossRef]
- Nikonenko, V.V.; Pismenskaya, N.D.; Belova, E.I.; Sistat, P.; Huguet, P.; Pourcelly, G.; Larchet, C. Intensive current transfer in membrane systems: Modelling, mechanisms and application in electrodialysis. Adv. Colloid Interface Sci. 2010, 160, 101–23. [Google Scholar] [CrossRef]
- Pismenskaya, N.D.; Rybalkina, O.A.; Kozmai, A.E.; Tsygurina, K.A.; Melnikova, E.D.; Nikonenko, V. V. Generation of H+ and OH− ions in anion-exchange membrane/ampholyte-containing solution systems: A study using electrochemical impedance spectroscopy. J. Memb. Sci. 2020, 601, 117920. [Google Scholar] [CrossRef]
- Bondarev, D.; Melnikov, S.; Zabolotskiy, V. New homogeneous and bilayer anion-exchange membranes based on N,N-diallyl-N,N-dimethylammonium chloride and ethyl methacrylate copolymer. J. Memb. Sci. 2023, 675, 121510. [Google Scholar] [CrossRef]
- Bondarev, D.A.; Samoilenko, A.A.; Mel’nikov, S.S. Comparison of Homogeneous Anion-Exchange Membrane Based on Copolymer of N,N-Diallyl-N,N-dimethylammonium Chloride and Commercial Anion-Exchange Membranes in Electrodialysis Processing of Dilute Sodium Chloride Solutions. Membr. Membr. Technol. 2024, 6, 171–180. [Google Scholar] [CrossRef]
| Membrane | Ralex AMH Pes | MA-41 | AHT | MA-1 | ||
| Exchange capacity, mmol/g(wet) | 1.05±0.06 | 0.74±0.04 | 0.49±0.05 | 0.34±0.01 | ||
| Density, g/cm3 | 1.15±0.02 | 1.14±0.02 | 1.03±0.02 | 1.16±0.02 | ||
| Moisture capacity, % | NaCl | 62.6±0.80 | 72.3±0.60 | 80.5±0.30 | 53.1±0.90 | |
| С4Н6О4 | 40.0±0.90 | 32.9±0.20 | 22.8±0.30 | 44.3±0.20 | ||
| Swelling (change in linear dimensions), % | NaCl | thickness | 23.3±0.10 | 10.91±0.09 | 11.42±0.10 | 14.3±0.10 |
| length | 3.24±0.08 | 5.7±0.10 | 4.05±0.04 | 12.4±0.10 | ||
| С4Н6О4 | thickness | 21.9±0.10 | 9.9±0.10 | 8.1±0.10 | 5.8±0.10 | |
| length | 2.08±0.04 | 3.15±0.06 | 1.9±0.10 | 6.7±0.10 | ||
| Membrane | ϰ iso, mSm/cm | f2 | α | G, 10– 15 m5∙mol –1∙s –1 |
| Ralex AMH Pes | 2.20 | 0.12 | 0.39 | 7.86 |
| MA-41 | 1.30 | 0.15 | 0.28 | 1.37 |
| AHT | 1.90 | 0.06 | 0.38 | 4.55 |
| MA-1 | 1.70 | 0.12 | 0.51 | 6.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





