1. Introduction
Wheat is one of the most important food crops in the world. Many studies have shown that wheat root-zone biocommunities are closely related to wheat growth and health [
1]. Soil microorganisms and nematodes are important components of soil biocommunities and play a vital role in ecosystem functions [
2]. Long-term application of inorganic fertilizers affects soil bacterial and fungal communities and inhibit the biomass accumulation of nematodes (especially fungivorous nematodes). It has been found that fertilization managements could greatly affect the soil microbial biomass and community structure [
3]. For example, the combined application of chemical and organic fertilizers increased soil nutrients and bacterial diversity compared with the application of chemical fertilizer [
4,
5]. Long-term application of organic fertilizers could maintain the bacterial diversity in low-productivity soils, which further increases the availability of nutrients in the soil. Soil quality is significantly affected by nitrogen application. Studies have shown that excessive nitrogen fertilization could acidify the soil, interfere with soil enzyme activity and nutrient cycling, and indirectly affect soil microbial communities [
6]. Besides, soil acidification may further leads to the reduction in the abundance and diversity of soil microorganisms [
7]. However, long-term nitrogen fertilization could significantly increase soil microbial biomass and promote the growth of soil fungi compared with the unfertilized control group [
8]. Therefore, it is of great significance to study the effects of chemical nitrogen use efficiency on wheat growth and soil biocommunity.
Due to inappropriate fertilization, especially excessive nitrogen application, the fertilizer use rate is very low, causing serious damage to the ecosystem. Therefore, to meet the needs of crop growth and increase crop yield, it is necessary to optimize nitrogen fertilization [
9]. However, due to the low thermal stability and high solubility [
10], nitrogen fertilizers are rapidly hydrolyzed after direct application to the soil, which affects the soil nitrogen availability in the later growth stage of crops. Polymers have been used to regulate soil moisture in recent years. Polymers can improve the soil moisture status to affect the transport of solutes in the soil, and can also adsorb nutrients in the soil and fertilizers. The combined application of polymers and nitrogen fertilizer can also increase the availability of nitrogen [
11]. At present, fertilization through drip irrigation systems is very popular in arid areas such as Xinjiang, China. However, Most polymers are solid and difficult to apply through drip irrigation systems. This leads to a difficulty for polymers to fully contact with fertilizers, affecting its application performance [
12]. Fortunately, a liquid modified polymer with water-retaining and slow-release functions was successfully developed (PPM, patent number CN105801297, composed of polyacrylamide, polyvinyl alcohol, and anganese sulfate-synthetic polymer compound).
Polymer application can promote the formation of large soil aggregates, increase soil water content, pH, EC, bulk density, porosity, and microbial community diversity, and inhibit nutrient loss compared with the control [
13,
14]. Soil microbial diversity is associated with soil quality and greatly impacts crop productivity [
15]. Therefore, polymers have a good function of improving the soil microenvironment. Many studies have shown that long-term excessive nitrogen application can stimulate many metabolic processes, especially carbohydrate and amino acid metabolism [
16]. Nitrogen fertilization in a certain amount can increase the abundance of genes related to carbohydrate metabolism compared with the control, but excessive nitrogen fertilization can increase the abundances of nitrate reductase activity genes in microbial communities [
17].
In summary, previous studies have explored the effects of nitrogen fertilizer use efficiency on crop growth and soil microorganisms, as well as the slow-release effect of polymers on soil nutrients. However, there is currently no in-depth analysis of the effects of polymers, especially PPM (a new water-soluble polymer), on crop nitrogen utilization and soil biocommunity. Therefore, in this study, the new polymer PPM was combined with different amounts of nitrogen fertilizer. The specific objectives were to clarify the effects of the combined application of PPM and nitrogen fertilizer on (1) soil microbial and nematode community diversity and abundance, (2) soil physicochemical properties, (3) soil enzyme activities, and (4) soil carbon and nitrogen metabolism characteristics in wheat fields in arid regions. This study will provide technical reference for nitrogen fertilizer reduction and nitrogen use efficiency enhancement in drip-irrigated wheat fields in arid regions.
2. Materials and Methods
2.1. Experimental Site
The experiment was conducted from March to July in 2021 in Experimental Farm Company No.2 of Shihezi University, Xinjiang, China (44.33°E, 86.00°N). The annual sunshine hours were 2,721-2,818 h, the average annual temperature was 2-15
oC, the average annual rainfall was 180-220 mm, and the average annual evaporation was 1000-1500 mm. The soil texture was loam. The physicochemical properties of the soil in the experimental area are shown in
Table 1.
2.2. Experimental Design
There were three treatments in the experiment, and a randomized complete block design was employed (
Table 2). Each treatment had three replicates/plots. The area of each plot was 10 m
2 (2 m × 5 m), and the plot spacing was 0.5 m. P
2O
5 of 120 kg/hm
2 and K
2O of 90 kg/hm
2 were applied in all treatments before sowing. The drip irrigation system was used, and the dripper spacing was 30 cm. One drip tape supplied water for four rows. Spring wheat (cultivar Xinchun 38) seeds were sown on March 28, 2023. Polymer (PPM) and different amounts of nitrogen fertilizer were dissolved in the fertilizer tank (
Table 2), and applied to the soil after wheat seedling emergence. The total irrigation amount in the whole growth period of wheat was 4500 m
3/hm
2. Other agricultural managements were consistent with those in local fields.
2.3. Sampling
Soil samples (0-20 cm soil layer) were collected during the flowering stage of wheat. Three soil samples were collected from each plot using a soil drill. After mixing the three soil samples, air-drying, and sieving (2 mm), the soil sample of each plot was divided into two parts. One part were stored at 4 oC for the determination of soil physicochemical properties and enzyme activity. Specifically, some of the soil samples were stored in aluminum boxes for soil bulk density (BD) analysis, while the remaining sol samples were stored in plastic containers, filtered through an 8 mm sieve, and air-dried for soil aggregate analysis. The other part was stored at -80 oC for analysis of soil microbiomes and metabolome.
2.4. Measurements
2.4.1. High-Throughput Sequencing
Genomic DNA was extracted from soil samples by a standard kit, and the DNA integrity and purity were determined using a 1% agarose gel. The concentration and purity of the DNA were then determined using Qubit (Thermo Fisher Scientific, USA) and Nanodrop (Thermo Fisher Scientific, Weihao, USA) instruments. Sequencing libraries were generated using the NEBNext Ultra DNA Library Prepkit for Illumina (New England BioLabs, MA, USA). Finally, the library was generated on the Illumina NovaSeq PE250 sequencing platform by Shanghai Paiseno Biotechnology Co., Ltd., and the required sequences were obtained. The bacterial, fungal, and nematode data were obained after amplification with specific primers.
2.4.2. Metabolome Measurement
Soil samples (200 mg) were vortexed and shaken for 30 s in a 2 mL EP tube with 0.6 mL of 2-chlorophenylalanine (4 ppm)-methanol (-20 oC) solution. Subsequently, 100 mg of glass beads were added, placed in the CBGT-48 high-throughput tissue grinder, and ground at 60 Hz for 90 s. Samples were sonicated for 30 min at room temperature and placed on ice for 30 min. The samples were centrifuged at 12,000 rpm at 4 oC for 10 min, and 300 uL of supernatant was collected and passed through a 0.22 μm filter. The filtrate was placed in a bottle. A 20 uL solution was taken from each sample and used to correct for deviation in the analysis results of the mixed samples, and the remaining samples were tested by liquid chromatography-tandem mass spectrometry (LC-MS). The liquid chromatograph Thermo Ultimate 3000 was used for liquid chromatography, and the Mass spectrometer Thermo Q Exactive HF-X was used for mass spectrometry.
2.4.3. Measurement of Soil Enzyme Activity
Soil catalase activity was determined by volumetric method [
18]. Soil urease activity was determined by indigophenol blue colorimetric method [
19]. Soil protease activity was determined by ninhydrin colorimetric assay [
20]. Soil nitrate reductase activity was determined by phenol disulfonic acid-colorimetry [
21]. Soil nitrite reductase activity was determined by α-namine-colorimetriy [
22]. Soil hydroxylamine reductase activity was determined by the ferric ammonium sulfate-phenthroline colorimetry [
23].
2.4.4. Measurement of Soil Microbial Biomass Carbon and Microbial Biomass Nitrogen
Soil microbial biomass carbon (MBC) and microbial biomass nitrogen (MBN) were measured by chloroform fumigation-extraction method [
24].
2.4.5. Measurement of Soil Aggregates
After the soil samples were brought back to the laboratory, small stones and animal and plant residues were removed from the samples, and the samples were divided into small blocks of different sizes along the natural profile. The soil blocks were placed in a ventilated place for air drying. Then, 150 g of air-dried soil samples were weighed and placed on sieves of 5.0, 2.0, 1.0, 0.5, 0.25, and 0.053 mm. After that, the samples were oscillated on an oscillator at 180 r/min for 5-10 min to obtain soil aggregates of different particle sizes. This step was repeated five times.
The content of soil aggregates was calculated by following equation:
Where SAC represents the mass percentage of aggregates at each particle size, MA represents the mass of aggregates of that particle size, and TA represents the total mass of aggregates.
The stability of soil aggregates was characterized by Mean Weight Diameter (MWD, mm) and Geometry Mean Diameter (GMD, mm) [
25]:
Where R0.25 is the content of aggregates with a diameter greater than 0.25 mm,
is the average diameter (mm) of soil aggregates within a certain particle size range, and
is the proportion of the mass of soil aggregates of size n.
2.5. Data Analysis
The Duncan’s test (p < 0.05, SPSS 22.0) was used to analyze the variance of the obtained data, and the data were plotted using Origin 2021 software. LDA (Linear Discriminant Analysis) > 2.4 was used to analyze the bacterial effect size (LefSe). Correlation analysis was conducted with the "psych" package in R software, and the results with a Pearson correlation coefficient greater than 0.9 were retained. The Gephi 0.9.2 (
https://gephi.org/) platform was used to plot the networks.
3. Results
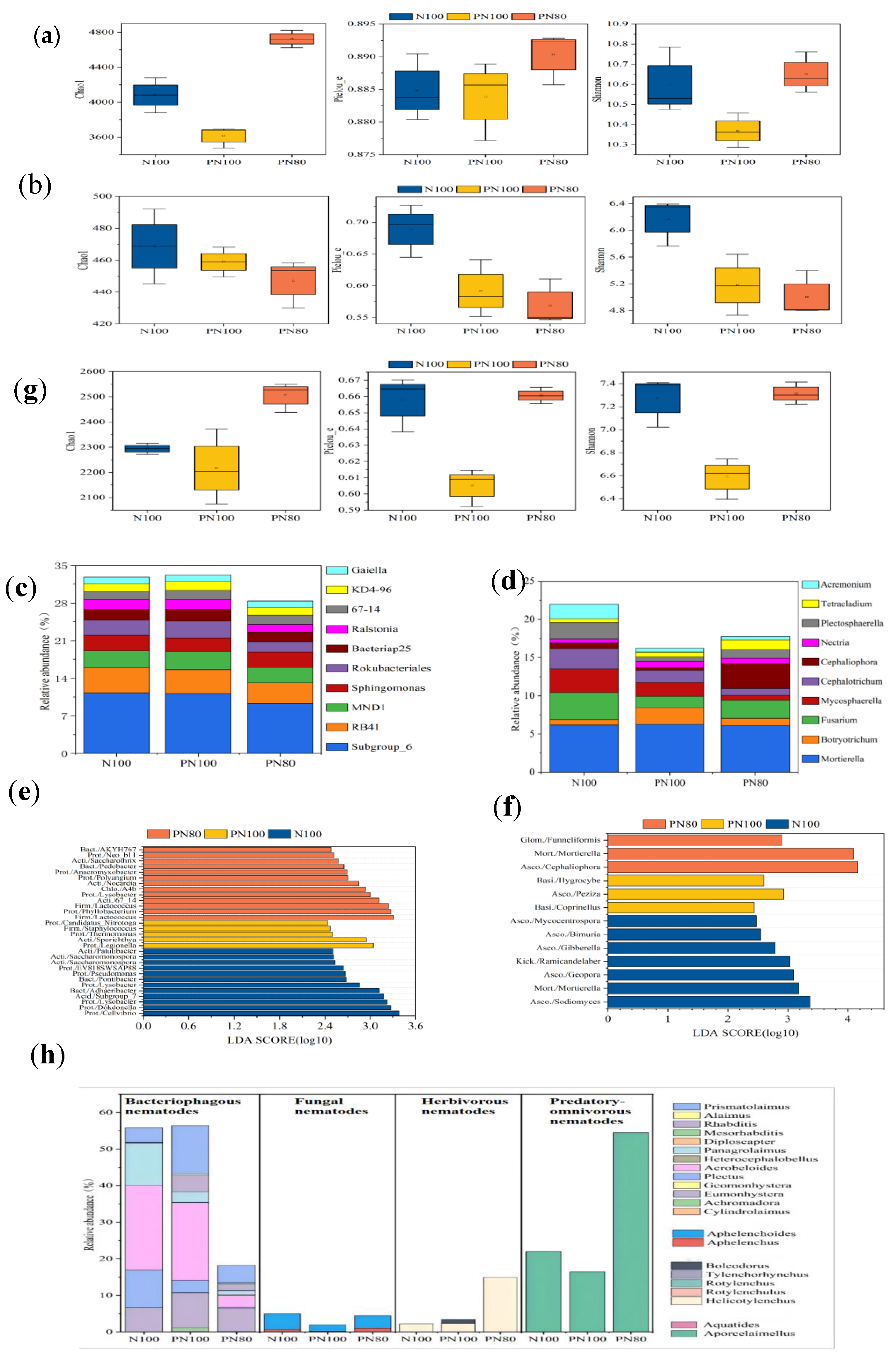
3.1. Effects of Combined Application of PPM and Nitrogen Fertilizer on the α-Diversity Indices of Soil Biocommunity
The combined application of PPM and nitrogen fertilizer had effects on the Chao1 and Shannon indices of soil microbial and nematode communities (
p < 0.05) (
Figure 1a). The Chao1 index of soil bacterial and nematode communities of the PN80 treatment increased by 30.63% and 10.68%, respectively compared with that of the N100 treatment (
p < 0.05) (
Figure 1a,g). However, there was no difference in the Chao1 index of soil fungal community between the treatments (
p > 0.05) (
Figure 1b). The Shannon index of soil bacterial and nematode communities of the PN80 treatment increased by 2.72% and 3.17%, respectively (
p < 0.05) compared with that of the PN100 treatment (
Figure 1a,g). The Pielo e and Shannon index of the PN100 treatment reduced by 21.14% and 23.34%, respectively (
p < 0.05), and those of the PN80 treatment reduced by 17.43% and 18.92%, respectively (
p < 0.05), compared with those of the N100 treatment (
Figure 1b).
3.2. Effects of Combined Application of PPM and Nitrogen Fertilizer on the Soil Biocommunity Structure and Abundance
The dominant soil bacterial genera of the three treatments were Subgroup_6, RB41 (Acidobacteria), MND1, Sphingomonas, Bacteriap25, Ralstonia (Proteobacteria), Rokubacteriales (Rokubacteria), KD4-96 (Chloroflexi), 67-14, and Gaiella (Actinobacteria) (
Figure 1c,d). The relative abundances of soil bacteria Subgroup_6, Rokubacteria (Rokubacteria), and Ralstonia (Proteobacteria) of the PN100 treatment reduced by 17.93%, 32.09%, and 18.79%, respectively (p < 0.05), but the relative abundance of KD4-96 (Chloroflexi) of the PN80 treatment increased by 19.70% (p < 0.05), compared with those of the N100 treatment.
The dominant soil fungal genera of the three treatments were Mortierella (Mortierellomycota), Botryotrichum, Fusarium, Mycosphaerella, Cephalotrichum, Cephaliophora, Nectria, Plectosphaerella, Tetracladium (Ascomycota), and Acremonium (
Figure 1d). The relative abundance of Fusarium of the PN100 and PN80 treatments reduced by 57.96% and 33.43%, respectively (p < 0.05) compared with that of the N100 treatment. Similarly, the relative abundances of Cephalotrichum, Plectosphaerella, and Acremonium of the PN100 treatment reduced by 39.21%, 74.32%, and 70.70%, respectively (p < 0.05), and those of the PN80 treatment reduced by 66.29%, 47.55%, and 77.83%, respectively (p < 0.05), compared with those of the N100 treatment. In addition, the relative abundances of Botryotrichum and Necria of the PN100 treatment increased by 207.47% and 50.12%, respectively (p < 0.05) compared with those of the N100 treatment. The relative abundance of Mycosphaerella of the PN80 treatment reduced by 79.22% (p < 0.05), and that of Cephaliophora increased by 365.76% (p < 0.05), compared with those of the N100 treatment.
A total of 22 nematode genera were identified in the soils of the three treatments, including 13 bacteriophagous nematodes, 2 fungivorous nematodes, 5 herbivorous nematodes, and 2 predatory/omnivorous nematodes. The relative abundances of the soil fungivorous nematode Aphelenchoides and the predatory/omnivorous nematode Aporcelaimellus of the PN100 treatment reduced by 153.6% and 34.1%, respectively (p < 0.05), and the relative abundance of the soil fungivorous nematode Prismatolamius increased by 229.5% (p < 0.05), compared with those of the N100 treatment. Surprisingly, the relative abundances of the herbivorous nematode Helicotylenchus and the predatory/omnivorous nematode Aporcelaimellus in the soil of the PN80 treatment increased by 577.3% and 147.05%, respectively (p < 0.05), compared with those of the N100 treatment.
3.3. Analysis of Differential Abundance Characteristics of Soil Bacterial and Fungal Communities
LEfSe analysis found that the number of soil bacteria and fungi of the PN100 and PN80 treatments increased compared with that of the N100 treatment (
p < 0.05). The bacterial genera with a larger LDA value of the N100 treatment were
Cellvibrio, Dokdonella, Lysobacter, Pseudomonas, EV818SWSAP88 (Proteobacteria), Adhaeribacter, Pontibacter (Bacteroidetes), Saccharomonospora, Patulibacter (Actinobacteria), and
Subgroup_7 (Acidobacteria). The bacterial genera with a larger LDA value of the PN100 treatment were
Legionella, Thermomonas, Candidatus_Nitrotoga (Proteobacteria), Staphylococcus (Firmicutes), and
Sporichthya (Actinobacteria). The bacterial genera with a larger LDA value of the PN80 treatment were
Phyllobacterium, Lysobacter, Polyangium, Anaeromyxobacter, Neo_b11 (Proteobacteria), Lactococcus (Firmicutes), A4b (Chloroflexi), Pedobacter, AKYH767 (Bacteroidetes), 67_14, Nocardia, and
Saccharothrix (Actinobacteria). In addition, the fungal genera with a larger LDA value of the N100 treatment were
Sodiomyces, Geopora, Gibberella, Bimuria, Mycocentrospora (Ascomycota), Coprinellus (Basidiomycota), Ramicandelaber (Kickxellomycota), and
Mortierella (Mortierellomycota). The fungal genera with a larger LDA value of the PN100 treatment were
Peziza (Ascomycota) and
Hygrocybe (Basidiomycota). The fungal genera with a larger LDA value of the PN80 treatment were
Cephaliophora (Ascomycota), Funneliformis (Glomeromycota), and
Mortierella (Mortierellomycota). Therefore, the soil microbial community structures of the PN100 and PN80 treatments were generally similar, but there were some differences in relative abundance (
Figure 1f).
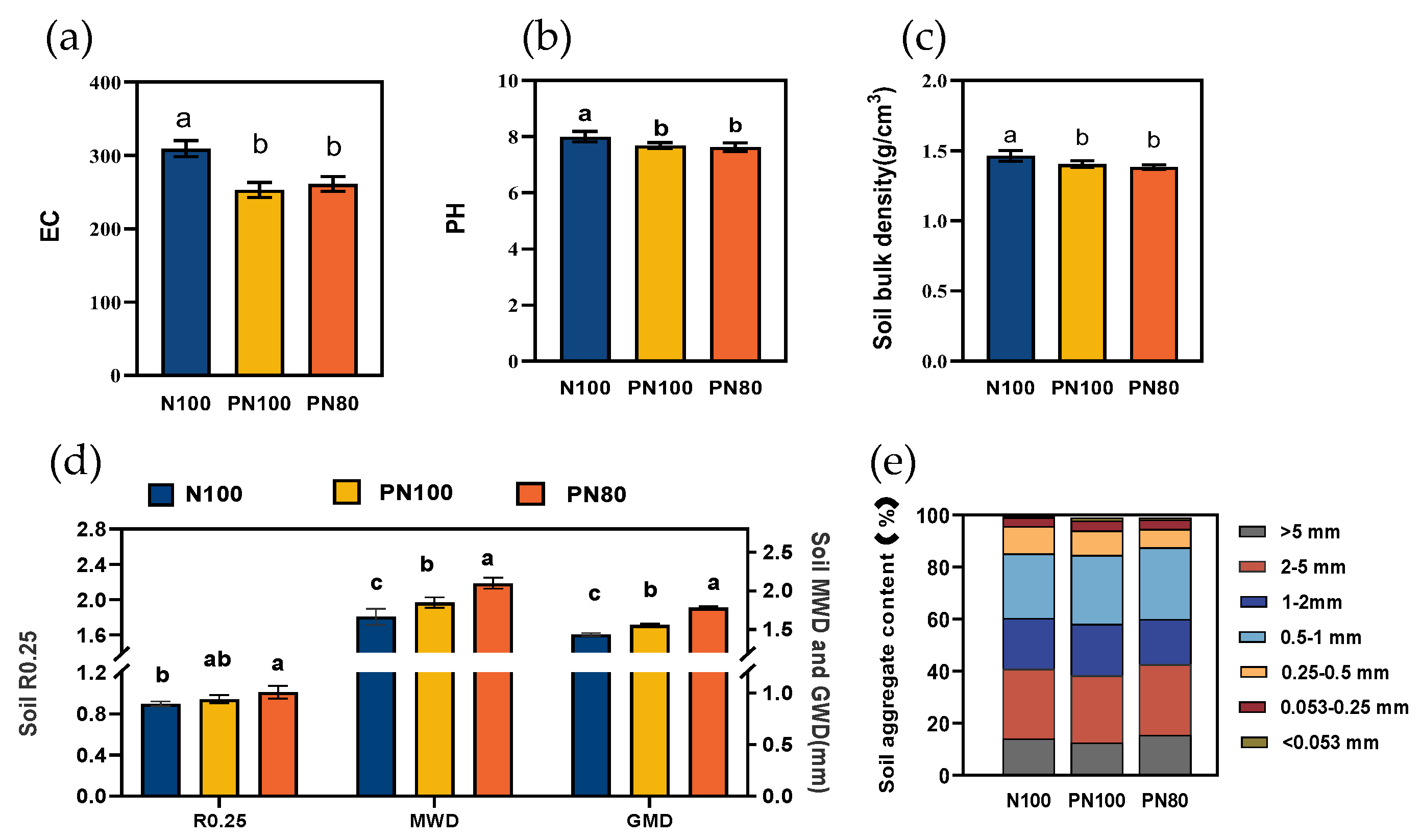
3.4. Effects of Combined Application of Polymer and Nitrogen Fertilizer on Soil Physicochemical Properties and Enzyme Activities
The combined application of PPM and nitrogen fertilizer significantly changed the particle size distribution of soil aggregates (
Figure 2e). The content of soil aggregates with a particle size of < 0.053 mm, 0.053-0.25 mm, and 0.5-1.0 mm of the PN80 and PN100 treatments increased by 35.9%-76.5%, 12.2%-14.0%, and 6.9%-11.4%, respectively (
p < 0.05) compared with those of the N100 treatment. In addition, the content of soil aggregates with a particle size of > 0.25 mm (R0.25), geometric mean diameter (GMD), and mean weight diameter (MWD) of the PN80 treatment increased by 5.1%, 6.7%, and 8.9%, respectively, and those of the PN100 treatment increased by 7.1%, 11.4%, and 11.1%, respectively, compared with those of the N100 treatment. However, the soil pH, EC, and bulk density of the PN80 and PN100 treatments showed opposite trends compared with those of the N100 treatment (
Figure 2).
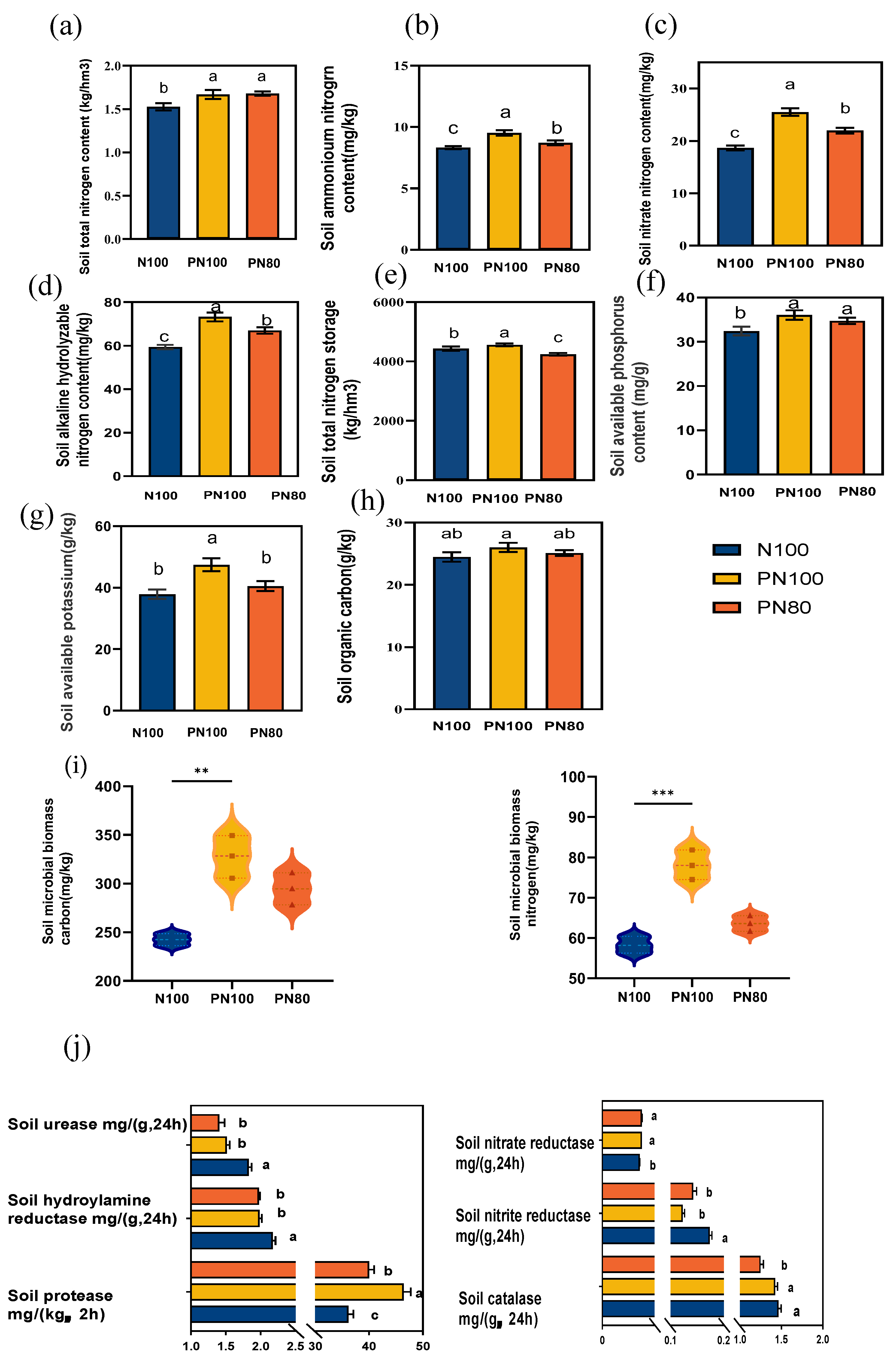
The soil TN, ammonium nitrogen, nitrate nitrogen, ANS, and AK contents of the PN100 treatment increased by 10.3%, 14.5%, 36.74%, 23.3%, and 21.7%, respectively (
p < 0.05), and those of the PN80 treatment increased by 9.0%, 4.7%, 17.8%, 12.8%, and 8.7%, respectively (
p < 0.05), compared with those of the N100 treatment. Besides, the SOC, MBC, and MBN contents of the PN80 and PN100 treatments increased compared with those of the N100 treatment, and the SOC, MBC, and MBN contents of the PN100 treatment increased by 6.3%, 35.2%, and 34.1%, respectively (
p < 0.05) (
Figure 3a–i).
The combined application of polymer and nitrogen fertilizer impacted the activities of soil enzymes. The nitrite reductase activity of the PN80 and PN100 treatments increased by 5.42% and 5.21%, respectively (
p < 0.05), and the soil protease activity increased by 29.31% and 11.53%, respectively (
p < 0.05), compared with those of the N100 treatment. On the contrary, the activities of soil urease, hydroxylamine reductase, nitrate reductase and catalase of the PN80 and PN100 treatments reduced (
p < 0.05) compared with those of the N100 treatment, and the soil catalase activity of the PN80 treatment decreased by 15.07% (
p < 0.05) (
Figure 3j).
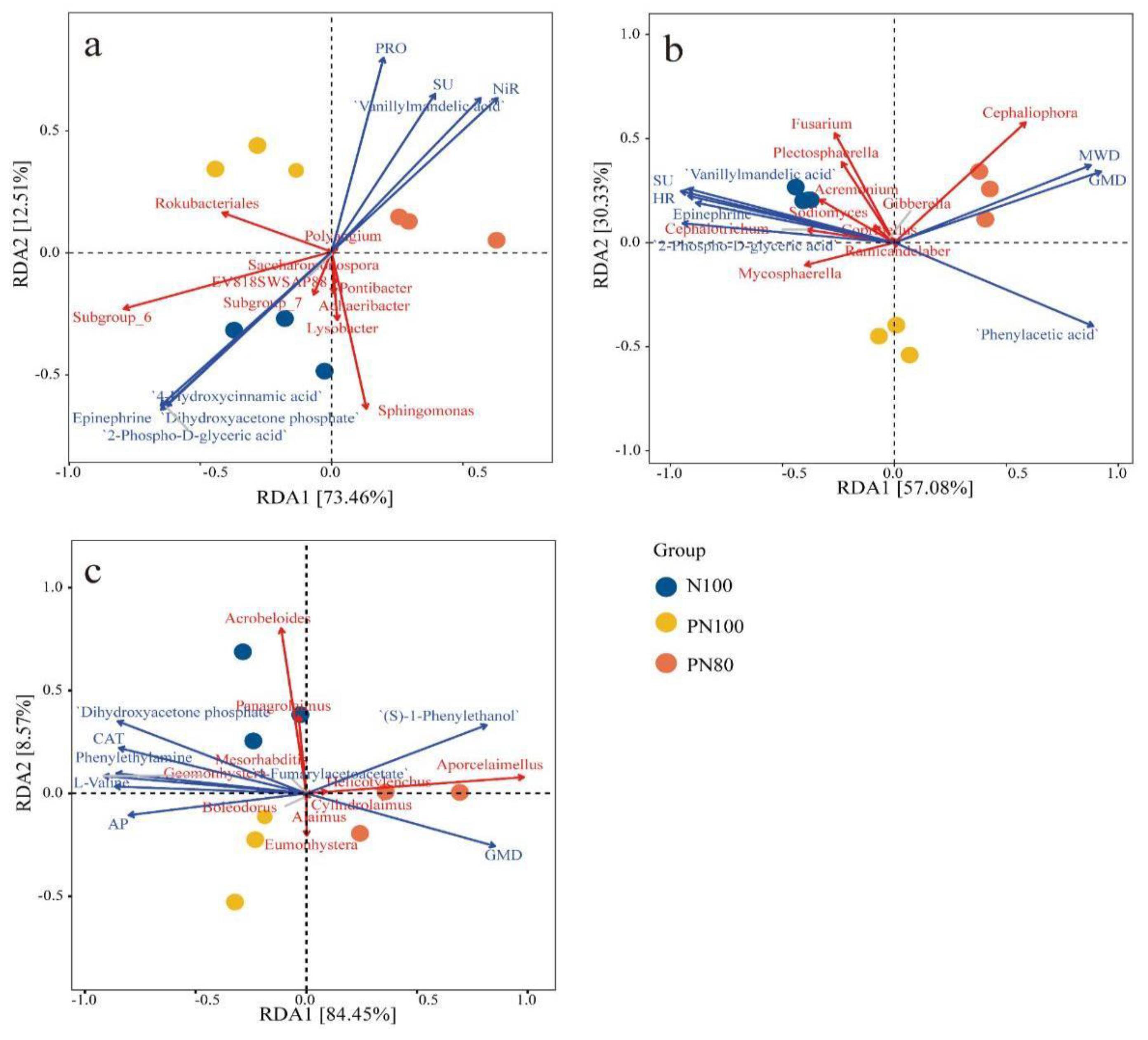
3.5. RDA of Soil Biocommunity and Soil Physicochemical Properties
The RDA1 and RDA2 axes explained 73.46% and 12.5% of the total variation in the soil bacterial communities in different treatments, respectively (
Figure 4a). The RDA1 axis separated N100 and PN100 treatments from PN80 treatment, while the RDA2 axis clearly separated N100 treatment from polymer treatments (PN100, PN80). Meanwhile, PN80 treatment had a significantly positive correlation with the activities of soil protease, urease, and nitrite reductase activities. The PN100 treatment mainly had a significant impact on
Rokubacteriales.
The RDA1 and RDA2 axes explained 57.08% and 30.33% of the total variation in the soil fungal communities in different treatments, respectively. The RDA1 axis separated N100 treatment from PN80 treatment, while the RDA2 axis separated PN100 treatment from N100 and PN80 treatments. Meanwhile, PN80 treatment mainly had a significant impact on MWD, GMD, and
Cephaliophora (p < 0.05) (
Figure 4b).
The RDA1 and RDA2 axes explained 84.45% and 8.57% of the total variation in the soil nematode communities in different treatments, respectively. The RDA1 axis separated N100 and PN100 treatments from PN80 treatment, and the RDA2 axis clearly separated N100 treatment from polymer treatments (PN100, PN80). The nematode community of the PN80 treatment was mainly affected by the predatory/omnivorous nematode
Aporcelaimellus and the metabolite (S)-1-Phenylthanol. The nematode community of the PN100 treatment was mainly affected by the
bacteriophagous nematode
Eumonhystera and the available phosphorus content in the soil (
Figure 4c).
3.6. Soil Metabolome Characteristics and Pathway Analysis
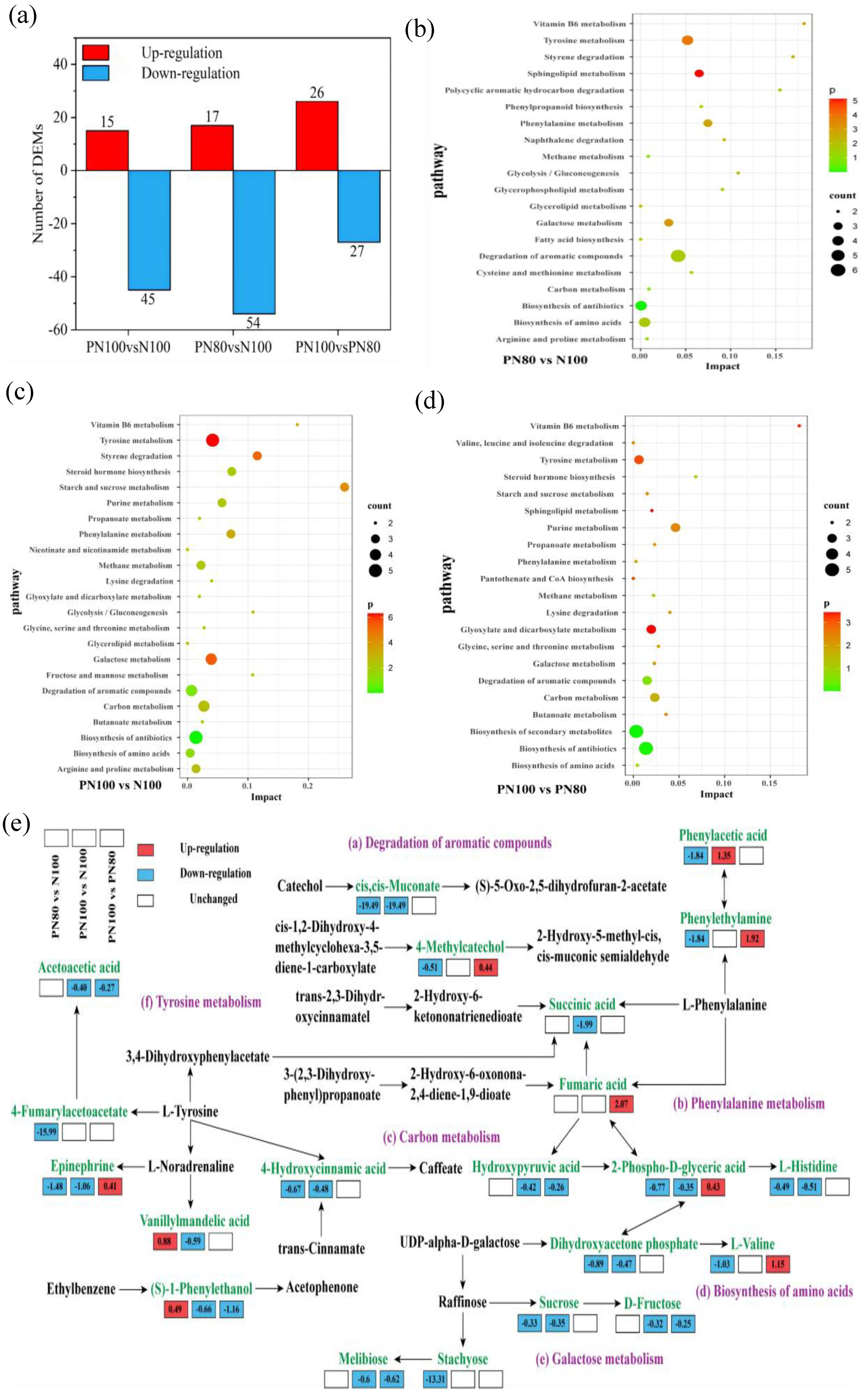
There were 60 DAMs in PN100 vs. N100, among which 15 metabolites were up-regulated and 45 metabolites were down-regulated. There were 71 DAMs in PN80 vs. N100, among which 17 metabolites were up-regulated and 54 metabolites were down-regulated (
Figure 5a). There were 53 DAMs in PN80 vs. PN100, among which 26 metabolites were up-regulated and 27 metabolites were down-regulated.
KEGG enrichment analysis (
Figure 5b–d) showed that the DAMs for PN80 vs. N100 were significantly enriched in the Degradation of aromatic compounds, Tyrosine metabolism, Biosynthesis of amino acids, and Biosynthesis of antibiotics pathways (count > 3). The DAMs for PN100 vs. N100 were significantly enriched in the Tyrosine metabolism, Biosynthesis of antibiotics, Galactose metabolism, Carbon metabolism, and Degradation of aromatic compounds pathways (count > 3). The DAMs for PN100 vs. PN80 were significantly enriched in the Biosynthesis of Biosynthesis of antibiotics and Biosynthesis of secondary metabolites pathways (count > 3).
In the Degradation of aromatic compounds pathway (
Figure 5e), the cis,cis-Muconate and 4-hydroxycinnamic acid metabolic pathways of the PN100 and PN80 treatments were significantly down-regulated compared with those of the N100 treatment, which slowed down the catabolism of aromatic compounds and led to the accumulation of intermediates. The 4-methylcatechol metabolic pathway of the PN80 treatment was significantly down-regulated and the (S)-1-phenylethanol metabolic pathway was significantly up-regulated, compared with those of the N100 treatment. The succinic acid and (S)-1-phenylethanol metabolic pathways of the PN100 treatment were significantly down-regulated compared with those of the N100 treatment. In the Phenylalanine metabolism pathway, the phenylethylamine metabolic pathway of the PN80 treatment was significantly down-regulated, and the phenylacetic acid metabolic pathway of the PN100 and PN80 treatments was significantly up-regulated, compared with those of the N100 treatment. In the carbon metabolism pathway, the hydroxypyruvic acid metabolic pathway of the PN100 treatment was significantly down-regulated, and the 2-phospho-D-glyceric acid and dihydroxyacetone phosphate metabolic pathways of the PN100 and PN80 treatments were also significantly down-regulated, compared with those of the N100 treatment. In the Biosynthesis of amino acids pathway, the L-Histidine and L-valine metabolic pathways of the PN100 and PN80 treatments were significantly down-regulated, compared with those of the N100 treatment. In the Tyrosine metabolism pathway, the epinephrine and vanillylmandelic acid metabolic pathways of the PN100 and PN80 treatments were significantly down-regulated, and the 4-fumarylacetoacetate metabolic pathway of the PN80 treatment and the Acetoacetic acid metabolic pathway of the PN100 treatment were also significantly down-regulated, compared with those of the N100 treatment.
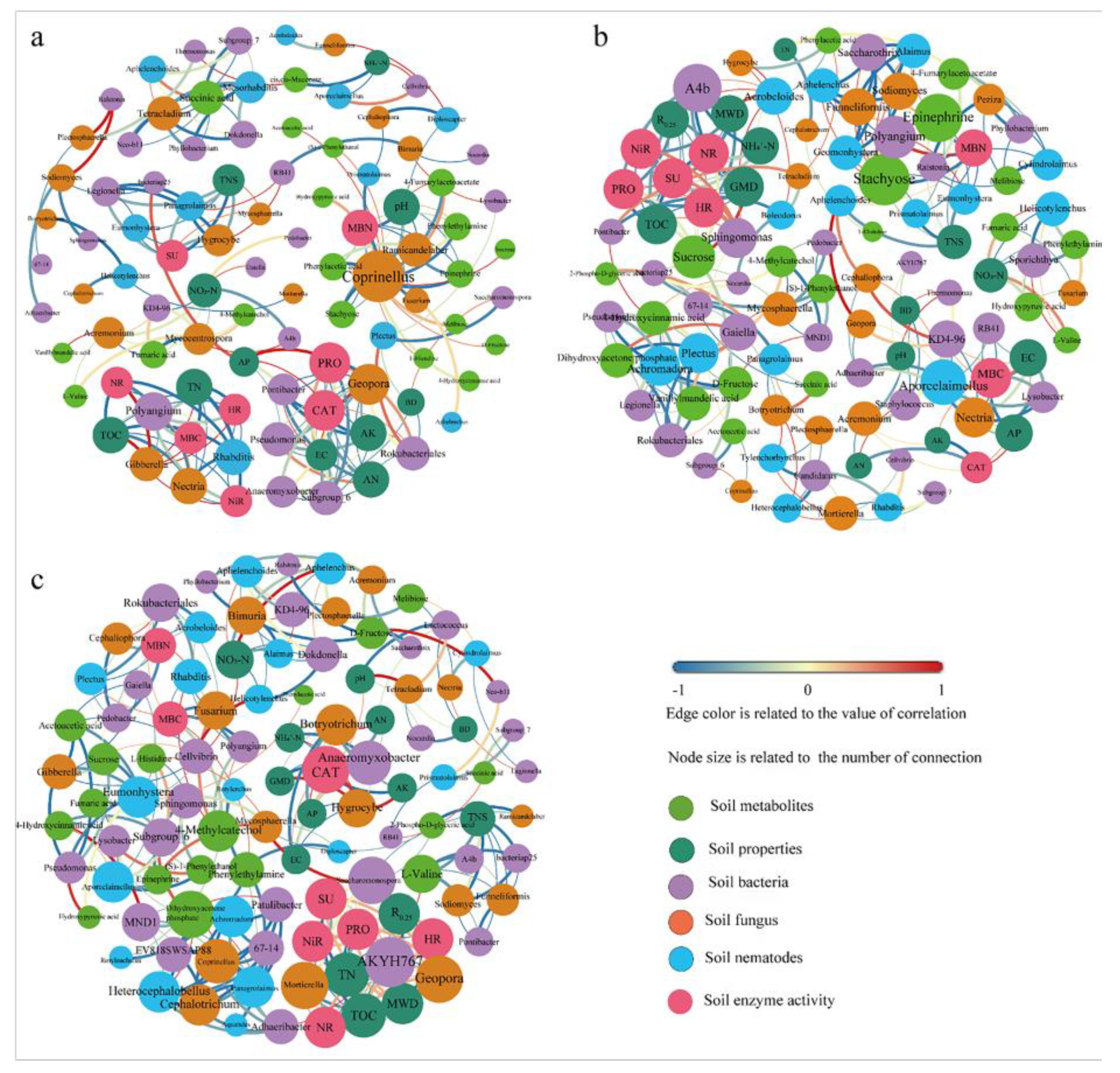
3.7. Correlation Network Analysis
The number of nodes and edges of the PN100 and PN80 treatments significantly increased compared with those of the N100 treatment (
Figure 6). In the N100 treatment (
Figure 6a), soil protease activity was the lowest, and soil catalase activity was the highest. This was due to the strong correlation with soil physicochemical properties (AN, AP, AK, EC) and the interactions with soil bacteria (
Pontibacter, Pseudomonas, Anaeromyxobacter, Subgroup_6, Rokubacteriales) and fungi
(Geopora). The soil TN, SOC, MBC, and MBN contents of the PN100 treatment increased and the activities of soil urease, hydroxylamine reductase, and nitrate reductase decreased, compared with those of the N100 treatment. This was mainly related to the metabolite Sucrose and the soil bacteria A4b and
Sphingomonas (
Figure 6b). The variations in the contents of TN and SOC of the PN80 treatment compared with the N100 treatment were not only related to soil bacteria (
AKYH767, Saccharomonospora) and fungi (
Mortierella, Geopora), but also to the activities of soil protease, urease, nitrate reductase, and hydroxylamine reductase (
Figure 6c).
4. Discussion
4.1. Effects of Combined Application of Polymer and Nitrogen Fertilizer on Soil Biocommunity Diversity and Structure
The combined application of PPM and nitrogen fertilizer (PN100 and PN80 treatments) significantly increased the diversity and species abundance of soil bacterial and omnivorous nematode communities compared with the N100 treatment, but had no significant effect on the structure and composition of soil fungal communities. The bacterial Chao1 and Shannon indices and the fungal and nematode Pielo-e and Shannon indices of the PN100 treatment significantly decreased compared with those of the N100 treatment. However, the bacterial Chao1 and Shannon indices of the PN80 treatment significantly increased, and the fungal Pielo-e and Shannon indices decreased. Meanwhile, studies have found that polymers do not have adverse effects on soil microbial environment [
26]. Therefore, the main reason for the decrease in soil bacterial species abundance (represented by Chao1 index) and diversity (represented by Shannon index) after the combined application of polymer and nitrogen fertilizer is the nitrogen addition. These findings are consistent with those of Sojka et al. [
27]. The relative abundance of soil bacteriophagous nematode
Prismatoliaimus of the PN100 treatment significantly increased compared with that of the N100 treatment (
Figure 1g). Therefore,
Prismatoliaimus, a bacteriophagous nematode that contributes greatly to soil nitrogen transformation, organic matter decomposition, and turnover [
28], actively responds to changes in soil environment. Besides, bacteriophagous nematodes (
Alamius, Geomonastera, Achromadora) and herbivorous nematodes (
Boleodorus) were only detected in the polymer treatments. Therefore, the addition of the polymer PPM could increase the abundance of soil omnivorous nematodes and enrich the food web of soil nematode communities. In addition, the combination of PPM and nitrogen reduction (PN80) can maintain a high bacterial and nematode community diversity in the soil.
4.2. Effects of Combined Application of Polymer and Nitrogen Fertilizer on Soil Biocommunity Diversity and Structure
The combined application of PPM and nitrogen fertilizer increased the content of soil nutrients (TN, ANS, nitrate nitrogen, ammonium nitrogen, SOC, MBC, and MBN) and protease and nitrite reductase activities by regulating the relative abundance of soil organisms, thus promoting the nitrification of soil nitrogen. The increases in soil MWD, GMD, and available phosphorus content were affected by soil protease, nitrite reductase,
Cephaliophora, and
Eumonhystera. Soil microorganisms play a crucial role in the soil carbon [
29] and nitrogen [
30] cycling, as well as the formation and degradation of organic matter [
31]. Studies have shown that polymers could improve soil structure [
32], and inhibit soil microbial growth by limiting soil nutrient uptake or reducing the number of nitrogen-fixing bacteria [
33], thus increasing the water stability of soil aggregates, ANS, nitrate nitrogen, ammonium nitrogen, and other nutrients [
34,
35]. The results of this study showed that the contents of soil MWD, GMD, TN, ANS, ammonium nitrogen, nitrate nitrogen, and available potassium of the PN100 and PN80 treatments increased compared with those of the N100 treatment. This indicates that the combined application of polymer and nitrogen fertilizer could improve the availability of soil nutrients by the slow releasing of fertilizer nutrients. Soil enzymes play a crucial role in soil nutrient cycling. Soil protease and urease activities mediate the transformation of soil nitrogen [
36], and nitric acid and nitrite reductase are involved in the denitrification [
37]. It was found that the combined application of PPM and nitrogen fertilizer (PN100 and PN80) significantly increased the activities of protease and nitrite reductase related to nitrogen cycling compared with the N100 treatment. Besides, the RDA (
Figure 4a) found that compared with the N100 treatment, the decrease in the relative abundance of soil bacteria (
Subgroup-6, Rokubacteriales, Ralstonia) in the PN80 treatment was due to their significantly negative correlation with soil protease and nitrite reductase activities, and the changes in these soil enzymes and microorganisms were mainly affected by the soil physicochemical properties of different treatments. Therefore, polymer PPM could improve the soil microenvironment and affect the activities of enzymes involved in the soil nitrogen transformation (urease, nitrate reductase, protease), thereby stimulating the mineralization and hydrolysis of soil organic nitrogen.
4.3. Effect of Combined Application of Polymer and Nitrogen Fertilizer on Soil Metabolic Pathways
It was found that the DAMs of the polymer treatments were mainly enriched in the Carbon metabolism and Biosynthesis of amino acid pathways. Soil metabolites, as important nitrogen sources for soil microorganisms, are closely related to the soil microbial community composition and metabolic pathways [
38]. The network analysis (
Figure 6) found that bacteria
Polyangium (
Proteobacteria) was significantly negatively correlated with MBN and metabolites (Stachyose, Epinephrine) in the PN100 treatment. This may be due to the fact that the addition of polymer PPM regulates the absorption of metabolites (stachyose, epinephrine) by soil microorganisms, thus increasing the content of SOC and MBN [
39]. It was also found that the bacteria AKYH767 (Bacteroidetes) and Saccharomonospora (Actinobacteria) of the PN80 treatment were positively correlated with TN and SOC, while the fungi Mortierella and Geopora were negatively correlated with TN and SOC. Studies have shown that
AKYH767 (
Bacteroidetes) and
Saccharomonospora (
Actinobacteria) play an important role in the decomposition of soil organic matter [
40], indicating that polymer addition combined with nitrogen reduction (PN80) could increase soil TN and SOC content by regulating the relative abundances of certain bacteria. Furthermore, the bacterial (
MND1, EV818SWSAP88, 67-14), fungal (
Coprinellus), and nematode (
Achromadora, Heterocephalobellus, and
Panagrolaimus) mainly participated in the transformation of nitrogenous substances by regulating the secretion of Dihydroxyacetone phosphate (Figure 8), a key metabolite in the Biosynthesis of amino acid pathway. Therefore, although the application of polymer PPM to soil can regulate the relative abundance of specific biocommunities and form a more complex soil biological network, the combination with nitrogen fertilizer has a more significant effect on soil biocommunity.
5. Conclusions
The combined application of polymer PPM and nitrogen fertilizer regulated the species abundance of specific biocommunities and biocommunity structure. Under nitrogen reduction, there were still significant changes in soil biocommunity after polymer application. The combined application of polymer and nitrogen fertilizer significantly increased the diversity and species abundance of soil bacterial and omnivorous nematode communities compared with the N100 treatment. Among them, the relative abundances of soil bacteria (KD4-96, RB41 (Acidobacteria)), herbivorous nematodes (Helicotylenchus and Boleodorus), and omnivorous nematode Aporcelaimellus increased significantly. However, there was no change in the structure of the soil fungal community. The combined application of polymer and nitrogen fertilizer further regulated the soil physicochemical properties by regulating the relative abundance of soil bacteria and fungi. For example, soil nutrient content (total nitrogen, alkali hydrolyzable nitrogen, ammonium nitrogen, nitrate nitrogen, available potassium, organic carbon, microbial biomass carbon and nitrogen) and protease and nitrite reductase activities showed an increasing trend after the application of polymer, which further facilitated the nitrification of soil nitrogen. On the whole, the polymer PPM regulated the soil microbial community structure, increased the abundance of specific species, regulated carbon metabolism, amino acid biosynthesis, and nucleotide metabolism pathways, and optimized the relationship between soil microbial and nematode communities and soil properties. These regulated the soil nitrogen transformation and reduced soil nitrogen loss. This study provides a scientific basis for nitrogen reduction and nitrogen fertilizer use efficiency improvement in drip-irrigated wheat fields in arid areas.
Author Contributions
Conceptualization, Y.S. , C.W. and S.Z.; Data curation, C.S. ; Methodology, Y.S. , C.W. , D.H. and H.H.; Writing—original draft, Y.S. and C.W.; Writing—review and editing, Y.S., C.W, H.F.; Conceptualization, Investigation, Supervision, Review & editing, Funding acquisition, K.W.. All authors have read and agreed to the published version of the manuscript.
Funding
Supported by the Special Fund for Key Science & Technology Program in Xinjiang Province of China (No. 2022B02053), the wheat earmarked fund for XJARS No.XJARA-01-14, Guizhou Province Science and Technology Plan Project (No.Qiankehezhongyindi [2023] 004), the Guiding Science and Technology Plan Project of Xinjiang production and Construction Corps (No.2023ZD063), the Program of Shihezi University (No.CGZH202204, CXFZ202207), Eighth Division Shihezi Science and Technology Plan Project (2024BX03).
Data Availability Statement
The data presented in this study are available at reasonable request to the corresponding author.
References
- Feng, Z., Liu, C., Li, X., Xiao, M., Wang, Y., Li, L., & Li, X. (2024). The effect of phosphorus content on wheat root-associated prokaryotic community depends on growth stage and variety. Soil Science Society of America Journal, 88(2), 224-238.
- Kan, Z. R., Xu, Y., Virk, A. L., Liu, M., Pei, X., Li, Y., Yang, H., & Chen, C. (2024). Organic fertilizer substitution benefits microbial richness and wheat yield under warming. The Science of the total environment, 945, 174007. [CrossRef]
- Zhou, J., Jiang, X., Zhou, B., Zhao, B., Ma, M., Guan, D.,& Qin, J. (2016). Thirty four years of nitrogen fertilization decreases fungal diversity and alters fungal community composition in black soil in northeast China. Soil Biology and Biochemistry, 95, 135-143.
- Bebber, D. P., & Richards, V. R. (2022). A meta-analysis of the effect of organic and mineral fertilizers on soil microbial diversity. Applied Soil Ecology, 175, 104450.
- Xun, W., Zhao, J., Xue, C., Zhang, G., Ran, W., Wang, B., Shen, Q., & Zhang, R. (2016). Significant alteration of soil bacterial communities and organic carbon decomposition by different long-term fertilization management conditions of extremely low-productivity arable soil in South China. Environmental microbiology, 18(6), 1907–1917.
- Liu, X., Cheng, Y., Zhang, Y., Li, Y., Wang, F., & Shen, C. (2024). Soil microbiome response to reduced nitrogen supply in an over-fertilized wheat-maize system. Agronomy, 14(11), 2631.
- Chen, L. J., Feng, Q., Wei, Y. P., Li, C. S., Zhao, Y., Li, H. Y., & Zhang, B. G. (2017). Effects of saline water irrigation and fertilization regimes on soil microbial metabolic activity. Journal of Soils and Sediments, 17, 376-383.
- Zhang, Y., Li, T., Wu, H., Bei, S., Zhang, J., & Li, X. (2019). Effect of different fertilization practices on soil microbial community in a wheat–maize rotation system. Sustainability, 11(15), 4088.
- Zhou, X., Yang, X., Feng, S., Zhang, J., Wu, J., Liu, J., ... & Shen, T. (2023). Optimization of controlled-release urea application based on the winter wheat yield. European Journal of Agronomy, 151, 126987.
- Xiao, Y., Peng, F., Zhang, Y., Wang, J., Zhuge, Y., Zhang, S., & Gao, H. (2019). Effect of bag-controlled release fertilizer on nitrogen loss, greenhouse gas emissions, and nitrogen applied amount in peach production. Journal of Cleaner Production, 234, 258-274. [CrossRef]
- Hong, D., Chang, D., Shao, C., Cui, W., Lu, X., Dong, W., & Liu, Y. (2024). Effects of polymer conditioner and nitrogen fertilizer application on nitrogen absorption and utilization of drip-irrigated wheat in arid areas. Agronomy, 14(2), 232.
- Awad, Y. M., Lee, S. S., Ok, Y. S., & Kuzyakov, Y. (2017). Effects of biochar and polyacrylamide on decomposition of soil organic matter and 14 C-labeled alfalfa residues. Journal of Soils and Sediments, 17, 611-620.
- Yuan, Y., Gai, S., Tang, C., Jin, Y., Cheng, K., Antonietti, M., & Yang, F. (2022). Artificial humic acid improves maize growth and soil phosphorus utilization efficiency. Applied Soil Ecology, 179, 104587.
- Tian, X., Wang, K., Liu, Y., Fan, H., Wang, J., & An, M. (2020). Effects of polymer materials on soil physicochemical properties and bacterial community structure under drip irrigation. Applied Soil Ecology, 150, 103456.
- Liu, X. , Liu, H., Zhang, Y., Chen, G., Li, Z., & Zhang, M. (2023). Straw return drives soil microbial community assemblage to change metabolic processes for soil quality amendment in a rice-wheat rotation system. Soil Biology and Biochemistry, 185, 109131.
- Li, B. B. , Roley, S. S., Duncan, D. S., Guo, J., Quensen, J. F., Yu, H. Q., & Tiedje, J. M. (2021). Long-term excess nitrogen fertilizer increases sensitivity of soil microbial community to seasonal change revealed by ecological network and metagenome analyses. Soil Biology and Biochemistry, 160, 108349.
- Wei, Q. , Yin, Y., Tong, Q., Gong, Z., & Shi, Y. (2024). Multi-omics analysis of excessive nitrogen fertilizer application: Assessing environmental damage and solutions in potato farming. Ecotoxicology and Environmental Safety, 284, 116916.
- Liu, X. , Liu, H., Zhang, Y., Chen, G., Li, Z., & Zhang, M. (2023). Straw return drives soil microbial community assemblage to change metabolic processes for soil quality amendment in a rice-wheat rotation system. Soil Biology and Biochemistry, 185, 109131. [CrossRef]
- Juan, H. , Zhen, L., & Jian, Z. (2012). Improvement of indophenol blue colorimetric method on activity of urease in soil. Journal of Civil, Architectural & Environmental Engineering, 34, 102-107.
- Watanabe, K., & Hayano, K. (1995). Seasonal variation of soil protease activities and their relation to proteolytic bacteria and Bacillus spp in paddy field soil. Soil Biology and Biochemistry, 27(2), 197-203.
- Frankeberger, W. T., & Johanson, J. B. (1983). Method of measuring invertase activity in soils. Plant and soil, 74, 301-311.
- Li, B. B. , Roley, S. S., Duncan, D. S., Guo, J., Quensen, J. F., Yu, H. Q., & Tiedje, J. M. (2021). Long-term excess nitrogen fertilizer increases sensitivity of soil microbial community to seasonal change revealed by ecological network and metagenome analyses. Soil Biology and Biochemistry, 160, 108349.
- Wei, Q. , Yin, Y., Tong, Q., Gong, Z., & Shi, Y. (2024). Multi-omics analysis of excessive nitrogen fertilizer application: Assessing environmental damage and solutions in potato farming. Ecotoxicology and Environmental Safety, 284, 116916.
- Wang, C. , Liu D., Bai E. (2018). Decreasing soil microbial diversity is associated with decreasing microbial biomass under nitrogen addition. Soil Biology and Biochemistry, 120:126-133.
- AM, A. E. G., & Morsy, A. S. M. (2017). Integrated impact of organic and inorganic fertilizers on growth, yield of maize (Zea mays L.) and soil properties under Upper Egypt conditions. Journal of Plant Production, 8(11), 1103-1112.
- Li, X., He, J. Z., Liu, Y. R., & Zheng, Y. M. (2013). Effects of super absorbent polymers on soil microbial properties and Chinese cabbage (Brassica chinensis) growth. Journal of Soils and Sediments, 13, 711-719.
- Sojka, R. E., Entry, J. A., & Fuhrmann, J. J. (2006). The influence of high application rates of polyacrylamide on microbial metabolic potential in an agricultural soil. Applied Soil Ecology, 32(2), 243-252.
- Vestergård, M. (2004). Nematode assemblages in the rhizosphere of spring barley (Hordeum vulgare L.) depended on fertilisation and plant growth phase. Pedobiologia, 48(3), 257-265. [CrossRef]
- Koch, H. , & Sessitsch, A. (2024). The microbial-driven nitrogen cycle and its relevance for plant nutrition. Journal of experimental botany, 75(18), 5547–5556.
- Liu, H., Liu, J., Yu, J., Xu, S., & Shi, J. (2013). Effect of soil amendment of polyacrylate on soil microbial biomass nitrogen and soil enzyme activity. Soil Fertil. Sci. China, 1, 25-31.
- Wu, H. , Cui, H., Fu, C., Li, R., Qi, F., Liu, Z., Yang, G., Xiao, K., & Qiao, M. (2024). Unveiling the crucial role of soil microorganisms in carbon cycling: A review. The Science of the total environment, 909, 168627.
- Zhang, X., Chen, C., & Sulitan. (2017). On appropriate applied amount of polyacrylamide (PAM) for saline soil improvement. Soils, 49(6), 1216-1220.
- Wang, J.; et al. (2022). Feasibility of agricultural utilization of river sludge as a planting substrate following treatment with polyacrylamide. Journal of Cleaner Production 367, 132964.
- Wang, J., Liu, M., Han, K., Zhao, H., Zhang, H., Ma, Q., & Wu, L. (2022). Feasibility of agricultural utilization of river sludge as a planting substrate following treatment with polyacrylamide. Journal of Cleaner Production, 367, 132964. [CrossRef]
- Wu, Y. , Li, F., Zheng, H., Hong, M., Hu, Y., Zhao, B., & De, H. (2019). Effects of three types of soil amendments on yield and soil nitrogen balance of maize-wheat rotation system in the Hetao Irrigation Area, China. Journal of Arid Land, 11, 904-915.
- Zumstein, M. T. , Helbling, D. E. (2019). Biotransformation of antibiotics: Exploring the activity of extracellular and intracellular enzymes derived from wastewater microbial communities. Water Research, 155, 115–123.
- Ni L. X., Xu J. J., Chu X. L., Li S. Y., Wang P. F., Li Y. P., Li Y., Zhu L., Wang C. (2016). Correlation among soil enzyme activities, root enzyme activities, and contaminant removal in twostage in situ conxtructed wetlands purifying domestic wastewater. Bulletin of Environmental Contamination and Toxicology, 97, 131–137. [CrossRef]
- HUANG, J. ,Lu, Y. (2022). Decomposition of soil polymeric organic matter by Bacteroidetes and Clostridia: progress and perspectives. Microbiology China, 49(3), 1147-1157.
- Wang, H. , Li, J., Chen, H., Liu, H., & Nie, M. (2022). Enzymic moderations of bacterial and fungal communities on short-and long-term warming impacts on soil organic carbon. Science of The Total Environment, 804, 150197.
- Podosokorskaya, O. A. , Bonch-Osmolovskaya, E. A., Novikov, A. A., Kolganova, T. V., & Kublanov, I. V. (2013). Ornatilinea apprima gen. nov., sp. nov., a cellulolytic representative of the class Anaerolineae. International Journal of Systematic and Evolutionary Microbiology, 63(Pt_1), 86-92. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).