Submitted:
04 December 2024
Posted:
06 December 2024
You are already at the latest version
Abstract
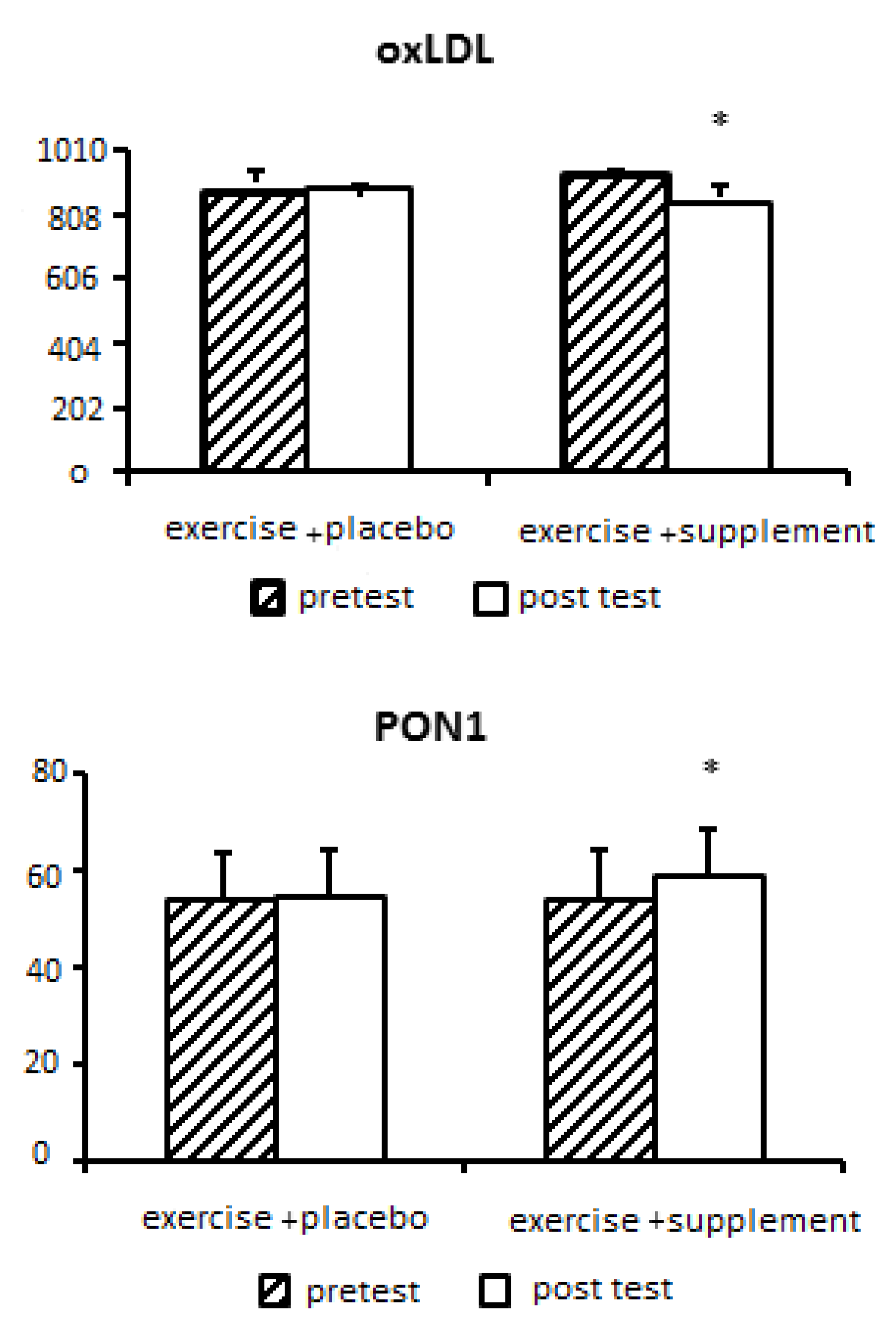
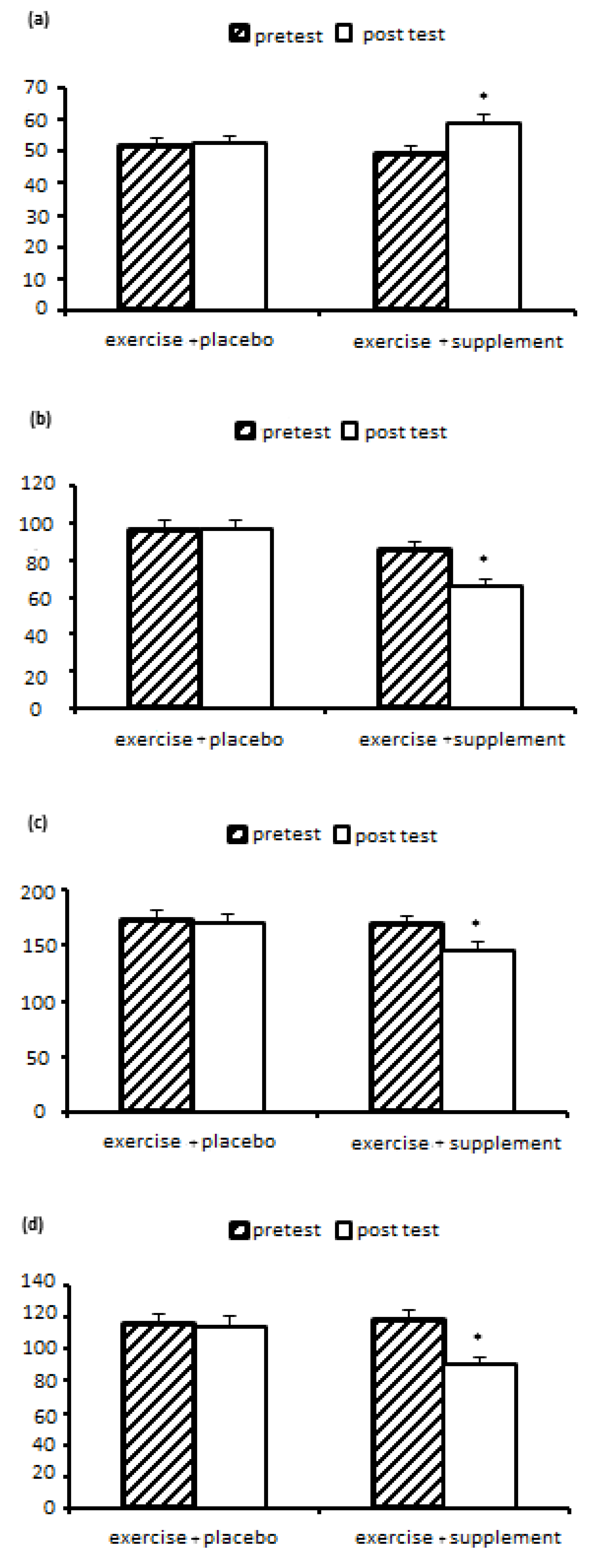
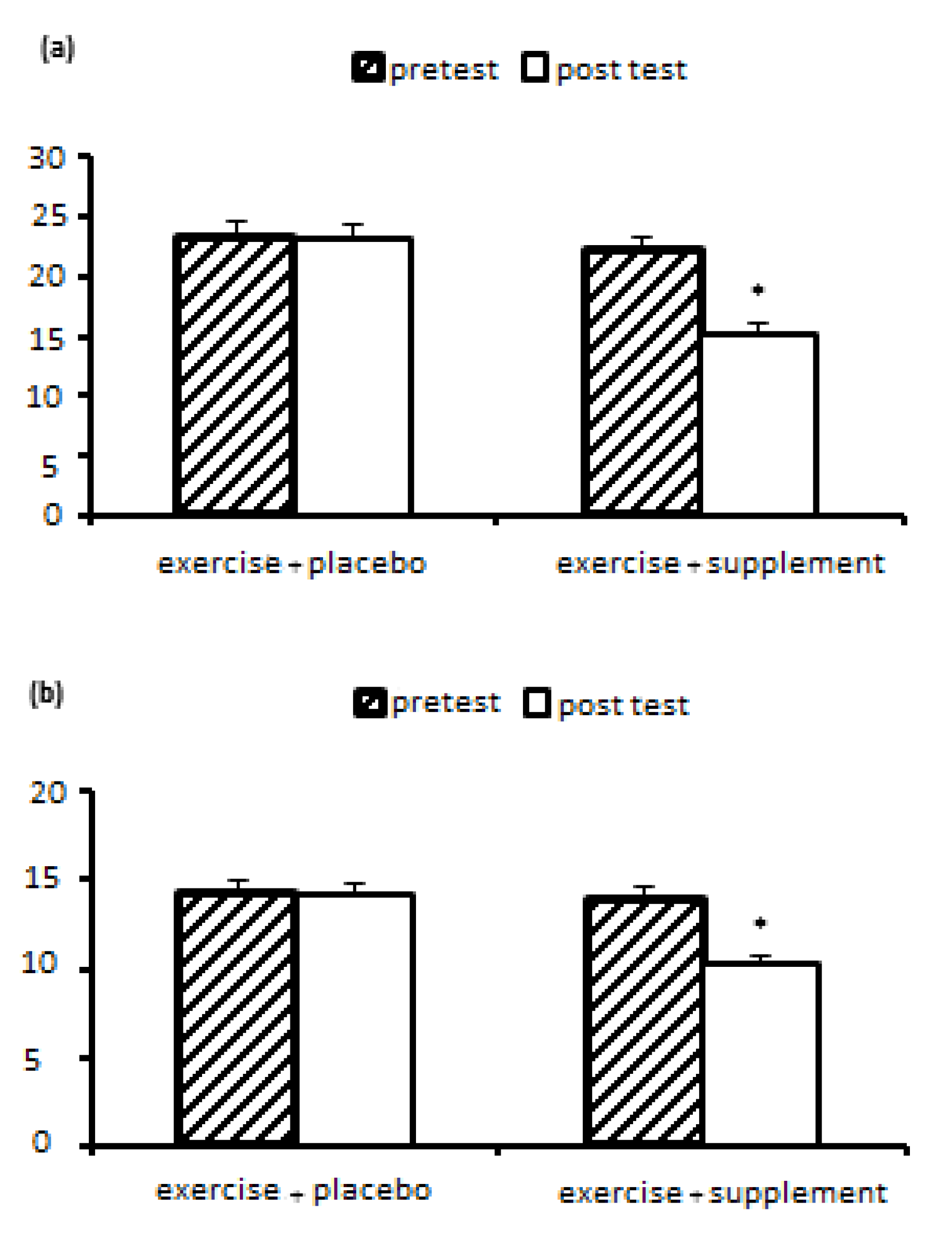
Nonalcoholic fatty liver disease (NAFLD) is a clinical pathological syndrome characterized by steatosis and fat accumulation in liver parenchymal cells without a history of excessive alcohol drinking in a patient. Currently, there is no definitive treatment for NAFLD, and its prevalence increases with age, obesity, and after menopause. Among the ways to treat it, we can mention regular sports exercises and the use of natural supplements. Therefore, the aim of this research is to investigate and compare the effect of aerobic-resistance training with royal jelly supplementation on changes in paraoxonase 1, oxidized LDL, liver function and lipid profile in postmenopausal women with non-alcoholic fatty liver disease. Methods: This semi-experimental study on 23 women with non-alcoholic fatty liver disease with average weight (71.34 ± 11.63 kg), age (48.54 ± 3.88 years), body mass index (27.63 ± 4.20 kg/m2) who were randomly Two groups of exercise+supplement (n=12) and exercise+placebo (n=11) were divided; done Both groups performed 8-station resistance exercises (8-12 repetitions in 2-4 sets) for 8 weeks, 3 sessions per week (for 35-40 minutes, from 10-15 RPE) and then From 10-15 minutes of active rest, they performed aerobic exercises with an intensity of 40-85% of the target heart rate, in two-minute intervals with 45 seconds of active rest. Royal jelly supplement (500 mg on training days, before each training session) was consumed. Blood sampling was done before and 48 hours after the last training session. Statistical analysis was performed using variance test with repeated measures (two groups x two stages of pre-test-post-test) in SPSS software with a significance level of p<0.05. Results: The results of the statistical analysis showed that the effect of eight weeks of exercise+supplement and exercise+placebo on PON1, oxLDL, lipid profiles (HDL, LDL, TC and TG) and liver enzymes (ALT, AST) in women with fatty liver non-alcoholic, there is a significant difference (P<0.05). The results showed a significant increase in PON1 (P=0.008) and HDL (P=0.005) in the exercise+supplement group compared to the exercise+placebo group. But a significant decrease for oxLDL (P=0.031), TC (P=0.045), TG (P=0.013), LDL (P=0.027), ALT (P=0.015) and AST (P=0.009) was observed in the exercise+supplement group compared to the exercise+placebo group. Conclusion: Based on the results, it can be concluded that aerobic-resistance exercises with the addition of royal jelly can probably be an efficient and recommended strategy to minimize the harmful effects of non-alcoholic fatty liver disease by affecting the activity of liver enzymes. paraoxonase 1, LDL oxidation and lipid profile. However, in order to obtain more accurate scientific evidence, it is necessary to investigate more doses and timing of royal jelly in future studies.
Keywords:
Introduction
Research Methodology
Exercise Protocol
Blood Sampling
Statistical Analysis
Discussion
Conclusions
References
- Malnick SD, Alin P, Somin M, Neuman MG. Fatty liver disease-alcoholic and non-alcoholic: similar but different. International Journal of Molecular Sciences. 2022 Dec 19;23(24):16226. [CrossRef] [PubMed]
- Noureddin M, Abdelmalek MF. Current Treatment Options, Including Diet, Exercise, and Medications: The Impact on Histology. Clinics in Liver Disease. 2023 May 1;27(2):397-412. [CrossRef] [PubMed]
- Ryu,S., Suh,B.S., Chang,Y., Kwon,M.J., Yun,K.E., Jung,H.S., Kim,C. W.,et al . (2015). Menopausal stages and non-alcoholic fatty liver disease in middle-aged women. European journal of obstetrics, gynecology, and reproductive biology, 190, 65–70.
- Fenton A. Weight, shape, and body composition changes at menopause. Journal of mid-life health. 2021 Jul 1;12(3):187-92. [CrossRef] [PubMed]
- TaheriChadorneshin,H., Abtahi-Eivary,S.H., Cheragh-Birjandi,S., Yaghoubi,A., & Ajam-Zibad,M. (2017). The effect of exercise training type on paraoxonase-1 and lipid profile in rats. Shiraz E-Medical Journal, 18(7).
- Zou,Y., Li,J., Lu,C., Wang,J., Ge,J., Huang,Y., Zhang,L., & Wang,Y. (2006). High-fat emulsion-induced rat model of nonalcoholic steatohepatitis. Life sciences, 79(11), 1100–11.
- Cuevas-Ramos, D., Mehta, R., & Aguilar-Salinas, C. A. (2019). Fibroblast Growth Factor 21 and Browning of White Adipose Tissue. Frontiers in physiology, 10, 37.
- Houben,T., Brandsma,E., Walenbergh,S., Hofker,M.H., & Shiri-Sverdlov, R. (2017). Oxidized LDL at the crossroads of immunity in non-alcoholic steatohepatitis. Biochimica et biophysica acta. Molecular and cell biology of lipids, 1862(4), 416–429.
- Mohanty S, Nayak N, Mukherjee T, Kumari S, Prabhakar PK, Pattnaik A. Obesity, herbal extracts, dietary supplements, mechanisms of action, medicinal plants, phytochemicals, clinical studies, management. Endocrine, metabolic & immune disorders drug targets. Endocr Metab Immune Disord Drug Targets. 2024 Sep 23.
- Omidi F, Hashemvarzi SA. The effect of eight weeks aerobic training with Royal Jelly consumption on some cardiovascular biomarkers during chronic high blood pressure induced by L-NAME in male rats. Sport Physiology. 2018 Jan 21;9(36):143-58.
- Etemad Z, Zohali S. The Effect of Aerobic Training and Royal Jelly Supplementation on Some Inflammatory Markers in Overweight Women. MEJDS 2021; 11 :21-21.
- Zhang,H., Chen,T., Ren,J., Xia,Y., Onuma,A., Wang,Y.,et al. (2021). Pre-operative exercise therapy triggers anti-inflammatory trained immunity of Kupffer cells through metabolic reprogramming. Nature metabolism, 3(6), 843–858.
- Miura,I., Komine,S., Okada,K., Wada,S., Warabi,E., Uchida,F.,et al. (2021). Prevention of non-alcoholic steatohepatitis by long-term exercise via the induction of phenotypic changes in Kupffer cells of hyperphagic obese mice. Physiological reports, 9(9), e14859.
- Bae, J. C., Suh, S., Park, S. E., Rhee, E. J., Park, C. Y., Oh, K. W., Park, S. W., Kim, S. W., Hur, K. Y., Kim, J. H., Lee, M. S., Lee, M. K., Kim, K. W., & Lee, W. Y. (2012). Regular exercise is associated with a reduction in the risk of NAFLD and decreased liver enzymes in individuals with NAFLD independent of obesity in Korean adults. PloS one, 7(10), e46819.
- Bhat,G., Baba,C.S., Pandey,A., Kumari,N., & Choudhuri,G.(2012).Life style modification improves insulin resistance and liver histology in patients with non-alcoholic fatty liver disease. World journal of hepatology,4(7),209–217.
- Hallsworth,K., Fattakhova,G., Hollingsworth,K.G., Thoma,C., Moore,S., Taylor,R.,et al. (2011). Resistance exercise reduces liver fat and its mediators in non-alcoholic fatty liver disease independent of weight loss. Gut, 60(9), 1278–1283.
- Ahmadi M, Abbassi Daloii A, Behbudi L. Comparison between the effects of eight weeks of aerobic and resistance training on paraoxonase-1, arylesterase activity and lipid profile in obese girls . SJKU 2016; 21 (4) :83-93.
- Rezvani, (2017). The effect of eight weeks of resistance training and low-calorie diet on body composition and relaxin of postmenopausal women. Journal of Physiology of Sport and Physical Activity, 10(2), 99-106.
- Ghasemi R, Abbassi Daloii A. The effect of 16 weeks of aerobic training on activity of serum paraoxonase, arylesterase, and lipid profile in postmenopausal women. EBNESINA 2017; 19 (3) :41-49.
- Banitalebi,E., Faramarzi,M., & Nasiri,S.(2018). Effect of a 10-week combined exercise training on new fatty liver markers in women with type 2 diabetes.SSU_Journals,26(3), 200-214.
- Fratini F, Cilia G, Mancini S, Felicioli A. Royal Jelly: An ancient remedy with remarkable antibacterial properties. Microbiological research. 2016 Nov 1;192:130-41. [CrossRef] [PubMed]
- Kwon,H. R., Han,K.A., Ku,Y.H., Ahn,H.J., Koo,B.K., Kim,H.C., et al. (2010). The effects of resistance training on muscle and body fat mass and muscle strength in type 2 diabetic women. Korean diabetes journal, 34(2), 101-110.
- Oršolić N, Jazvinšćak Jembrek M. Royal Jelly: Biological Action and Health Benefits. International Journal of Molecular Sciences. 2024 May 30;25(11):6023.
- Fatolahi, H., Azarbayjani, M. A., Peeri, M., & Homaee, H. M. (2017). The effect of exercise on paraoxonase-1 activity and lipid profile in obesity and insulin resistance conditions. Iranian Journal of Diabetes & Obesity (IJDO), 9.
- Otocka-Kmiecik, A., Orłowska-Majdak, M., Stawski, R., Szkudlarek, U., Kosielski, P., Padula, G., ... & Nowak, D. (2021). Repetitions of Strenuous Exercise Consistently Increase Paraoxonase 1 Concentration and Activity in Plasma of Average-Trained Men. Oxidative Medicine and Cellular Longevity, 2021.
- Bacchetti, T., Morresi, C., Ferretti, G., Larsson, A., Åkerfeldt, T., & Svensson, M. (2023). Effects of Seven Weeks of Combined Physical Training on High-Density Lipoprotein Functionality in Overweight/Obese Subjects. Metabolites, 13(10), 1068.
- Gharakhanlou, R., Afzalpour, M. E., Gaeini, A. A., & Rahnama, N. (2007). Effects of aerobic exercises on the serum paraoxonase 1/arylesterase activity and lipid profile in non-active healthy men. International Journal of Sports Science and Engineering, 1, 105-112.
- Nalcakan, G. R., Varol, S. R., Turgay, F., Nalcakan, M., Ozkol, M. Z., & Karamizrak, S. O. (2016). Effects of aerobic training on serum paraoxonase activity and its relationship with PON1-192 phenotypes in women. Journal of sport and health science, 5(4), 462-468.
- Vilahur, G.; Juan-Babot, O.; Peña, E.; Oñate, B.; Casaní, L.; Badimon, L. Molecular and cellular mechanisms involved in cardiac remodeling after acute myocardial infarction. J. Mol. Cell. Cardiol. 2011, 50, 522–533. [CrossRef]
- Kontush, A.; Chapman, M.J. Antiatherogenic function of HDL particle subpopulations: Focus on antioxidative activities. Curr. Opin. Lipidol. 2010, 21, 312–318. [CrossRef] [PubMed]
- Milaciu, M. V., Ciumărnean, L., Matei, D. M., Vesa, Ș. C., Sabin, O., Bocșan, I. C., ... & Acalovschi, M. (2021). Cytokines, paraoxonase-1, periostin and non-invasive liver fibrosis scores in patients with non-alcoholic fatty liver disease and persistently elevated aminotransferases: A pilot study. Experimental and Therapeutic Medicine, 21(5), 1-7.
- Karadeniz, A.; Simsek, N.; Karakus, E.; Yildirim, S.; Kara, A.; Can, I.; Kisa, F.; Emre, H.; Turkeli, M. Royaljelly modulates oxidative stress and apoptosis in liver and kidneys of rats treated with cisplatin. Oxid. Med. Cell. Longev. 2011, 2011.
- Park, J. H., Park, H., Lim, S. T., & Park, J. K. (2015). Effects of a 12-week healthy-life exercise program on oxidized low-density lipoprotein cholesterol and carotid intima-media thickness in obese elderly women. Journal of physical therapy science, 27(5), 1435-1439.
- Ghorbanian B, mamaghani H. Effect of eight weeks of rope training along with portulaca oleracea supplementation on serum levels of ox-LDL, Apo-A1, and Apo-B in overweight girls. Research in Medicine 2021; 45 (1) :15-22.
- Sadeghi, M., Mohammadi, A., & Khajehlandi, A. (2019). The Effect of Aerobic Exercise Training on Oxidized LDL Cholesterol (Ox-LDL) Levels and Some Cardiovascular Risk Factors in Women with Obesity. Jundishapur Journal of Health Sciences, 11(2).
- Chiang, S. H., Yang, K. M., Sheu, S. C., & Chen, C. W. (2021). The Bioactive Compound Contents and Potential Protective Effects of Royal Jelly Protein Hydrolysates against DNA Oxidative Damage and LDL Oxidation. Antioxidants, 10(4), 580.
- Cho, H. C., Kim, J. K., Lee, N. J., Kim, S. Y., & Yoon, N. K. (2014). Effects of combined exercise on cardiovascular risk factors and serum bdnf level in mid-aged women. Journal of exercise nutrition & biochemistry, 18(1), 61..
- Hojjati, Z., & Shahsavari, S. (2015). Acute Effects of Aerobic and Combined Exercise on Serum Lipid Profile in Women with Type II Diabetes..
- Petelin, A., Kenig, S., Kopinč, R., Deželak, M., Černelič Bizjak, M., & Jenko Pražnikar, Z. (2019). Effects of royal jelly administration on lipid profile, satiety, inflammation, and antioxidant capacity in asymptomatic overweight adults. Evidence-Based Complementary and Alternative Medicine, 2019.
- Barani F, Afzalpour M E, Ilbiegi S, Kazemi T, Mohammadi Fard M. The effect of resistance and combined exercise on serum levels of liver enzymes and fitness indicators in women with nonalcoholic fatty liver disease. J Birjand Univ Med Sci 2014; 21 (2) :188-202.
- Aslan, I., Kucuksayan, E., & Aslan, M. (2013). Effect of insulin analog initiation therapy on LDL/HDL subfraction profile and HDL associated enzymes in type 2 diabetic patients. Lipids in health and disease, 12(1), 1-11.
- Kanbur, M., Eraslan, G., Beyaz, L., Silici, S., Liman, B. C., Altınordulu, Ş., & Atasever, A. (2009). The effects of royal jelly on liver damage induced by paracetamol in mice. Experimental and Toxicologic Pathology, 61(2), 123-132.
- Bahari, H., Taheri, S., Rashidmayvan, M., Jamshidi, S., Jazinaki, M. S., & Pahlavani, N. (2023). The effect of Royal jelly on liver enzymes and glycemic indices: A systematic review and meta-analysis of randomized clinical trials. Complementary therapies in medicine, 77, 102974.
- Valizadeh, R., Nikbakht, M., Davodi, M., & Khodadoost, M. (2011). The effect of eight weeks elected aerobic exercise on the levels of (AST, ALT) enzymes of men patients with have fat liver. Procedia-Social and Behavioral Sciences, 15, 3362-3365.
- Xiong, Y., Peng, Q., Cao, C., Xu, Z., & Zhang, B. (2021). Effect of different exercise methods on non-alcoholic fatty liver disease: A meta-analysis and meta-regression. International journal of environmental research and public health, 18(6), 3242.
- Romero-Gómez M, Zelber- Sagi S, Trenell M. Treatment of NAFLD with diet, physical activity and exercise. Journal of Hepatology 2017; 67(4):829-846. [CrossRef] [PubMed]
- Curci, R., Bianco, A., Franco, I., Campanella, A., Mirizzi, A., Bonfiglio, C., & Osella, A. R. (2022). The Effect of Low Glycemic Index Mediterranean Diet and Combined Exercise Program on Metabolic-Associated Fatty Liver Disease: A Joint Modeling Approach. Journal of Clinical Medicine, 11(15), 4339.
- You M.M., Liu,Y.C., Chen,Y.F., Pan,Y.M., Miao,Z. N., Shi,Y.Z., et al. (2020). Royal jelly attenuates nonalcoholic fatty liver disease by inhibiting oxidative stress and regulating the expression of circadian genes in ovariectomized rats. Journal of food biochemistry, 44(3), e13138.
- Cemek, M., Yılmaz, F., Büyükokuroğlu, M. E., Büyükben, A., Aymelek, F., & Ayaz, A. (2012). Serum and liver tissue bio-element levels, and antioxidant enzyme activities in carbon tetrachloride-induced hepatotoxicity: protective effects of royal jelly. Journal of medicinal food, 15(8), 747-752.
| Week | Repetition | Sets | Active rest (stretching movements between each station) | Rest between sets | Color of the elastic band | RPE |
|---|---|---|---|---|---|---|
| 1 | 8 - 12 | 2 | 60 - 90 seconds | 90 seconds | Yellow | 10-11 |
| 2 | 8 - 12 | 2 | 60 - 90 seconds | 90 seconds | Yellow | 12-13 |
| 3 | 8 - 12 | 3 | 60 - 90 seconds | 90 seconds | Green | 12-13 |
| 4 | 8 - 12 | 3 | 60 - 90 seconds | 90 seconds | Green | 13-14 |
| 5 | 8 - 12 | 3 | 60 - 90 seconds | 90 seconds | Blue | 13-14 |
| 6 | 8 - 12 | 4 | 60 - 90 seconds | 90 seconds | Blue | 14-15 |
| 7 | 8 - 12 | 4 | 60 - 90 seconds | 90 seconds | Red | 14-15 |
| 8 | 8 - 12 | 4 | 60 - 90 seconds | 90 seconds | Red | 14-15 |
| Week | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Target maximum heart rate* (percentage) | 70 | 70 | 75 | 75 | 80 | 80 | 85 | 85 |
| Set and repeat | 5×2 | 6×2 | 7×2 | 8×2 | 9×2 | 10×2 | 11×2 | 2×12 |
| Active rest between sets (with 40% of target heart rate) | 45 seconds | 45 seconds | 45 seconds | 45 seconds | 45 seconds | 45 seconds | 45 seconds | 45 seconds |
| Variable | time effect | Time × group interaction effec | ||||
|---|---|---|---|---|---|---|
| F value | P value | Effect size (n2p) | F value | P value | Effect size (n2p) | |
| PON1(ng/mL) | 14/24 | 0/001 | 0/404 | 8/71 | 0/008 | 0/293 |
| oxLDL (ng/L) | 3/26 | 0/085 | 0/134 | 5/34 | 0/031 | 0/203 |
| Variable | time effect | Time × group interaction effect | ||||
|---|---|---|---|---|---|---|
| F value | P value | Effect size (n2p) | F value | P value | Effect size (n2p) | |
| HDL (mg/dL) | 14/06 | 0/001 | 0/401 | 10/01 | 0/005 | 0/323 |
| LDL (mg/dL) | 5/91 | 0/024 | 0/220 | 5/69 | 0/027 | 0/213 |
| TC (mg/dL) | 9/95 | 0/005 | 0/321 | 4/54 | 0/045 | 0/178 |
| TG (mg/dL) | 8/70 | 0/008 | 0/293 | 7/33 | 0/013 | 0/259 |
| Variable | time effect | Time × group interaction effect | ||||
|---|---|---|---|---|---|---|
| F value | P value | Effect size (n2p) | F value | P value | Effect size (n2p) | |
| AST (Units per liter) | 9/25 | 0/006 | 0/306 | 8/315 | 0/009 | 0/284 |
| ALT (Units per liter) | 7/69 | 0/011 | 0/268 | 6/98 | 0/015 | 0/249 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





