1. Introduction
Tasteless, colorless, odorless, and soluble in water, thallium is a chemical element that has been known to cause accidental intoxication as well as intentional poisoning. In western countries, thallium zinc sulfate was used in the 1950s as rat and ant poison [
1] but was banned firstly in American homes in 1965 and then commercially in 1975. However, thallium is still utilized in rodenticides and insecticides in several countries where, on account of its odorless and tasteless properties, it has led to both unintentional poisoning and, in some cases, illegal use. In medicine, thallium salts were previously employed in the treatment of dermatophytosis [
2]. Prior to the adoption of technetium-99m in nuclear medicine, thallium-201, a radioactive isotope, was the primary agent [
3] and continues to be utilized in neuroradiology [
4], radiotherapy [
5], scintigraphy [
6] and for stress tests assessing coronary artery disease [
7]. This kind of thallium is around 4,000 times less powerful than zinc sulfate.
In industry, thallium is a vital element commonly used in high technology industries where there is currently a growing demand for semiconductors. The occupational exposure limit for thallium is 0.1 milligrams (mg) per cubic meter for no longer than eight hours a day. Acutely hazardous levels are those reaching 15 mg/m
3 and over. Thallium can easily enter the body via skin absorption and inhalation. Cases of occupational thallium intoxication have been reported, mainly in Asian countries [
8,
9,
10].
Wastewater discharges from manufacturing plants or metal mining activities may result in elevated levels of thallium in receiving water. For this reason, it is a priority pollutant regulated by the American Environmental Protection Agency (US EPA) [
11,
12,
13,
14]. Thallium has been utilized as a pesticide in Africa, where it has led to food contamination. Chinese herbal medicines have also been compromised. Intoxication results from cumulative absorption via dermal, respiratory, and gastrointestinal pathways. Instances of inadvertent snorting by cocaine users [
15], unintentional injection by heroin users [
16,
17,
18], and dermal absorption through protective gloves have been documented [
9].
Thallium contamination has cumulative effects. The chronic exposure of populations to this pollutant has been associated with many pathologies. Thallium urinary levels have been associated with a reduction in cognitive performance in older US adults [
19], with Parkinson’s disease [
20] and also with a risk of gestational diabetes [
21]. Plasma thallium levels have been associated with decreased renal function [
22], central obesity [
23,
24], ischemic stroke [
25] and increased all-cause and cardiovascular mortality in the general population in China [
26]. Studies with DNA methylation probes in exposed populations indicated alterations that could be implicated in cancer progression and respiratory diseases [
27]. A longitudinal study showed that thallium exposure at low concentration leads to early damage of multiple organs in children [
28].
Thallium (Tl+), one of the most toxic heavy metals, shares an ionic ratio similar to potassium (K+), allowing it to replace K+ in enzymatic processes. Its primary target within cells is the mitochondria, where it disrupts intrinsic pathways, affects antiapoptotic and proapoptotic proteins, and activates oxidative stress mechanisms. Tl+ exposure leads to increased reactive oxygen species (ROS) and lipid peroxidation, causing cellular damage and triggering antioxidant responses. In humans, Tl+ is absorbed through skin and mucous membranes, distributing across organs like bones, kidneys, liver, and the central nervous system, with neurotoxic effects that are now recognized as a significant global health concern due to rising reports of Tl+ pollution [
29].
The establishment of surveillance protocols to monitor toxic substances like thallium is essential, particularly in high-risk environments and workplaces. According to the Environmental Protection Agency (EPA) and the Occupational Safety and Health Administration (OSHA), thallium poses significant health risks through occupational and environmental exposure and has been identified at multiple hazardous waste sites nationwide. OSHA has set workplace exposure limits (PEL-TWA) at 0.1 mg of soluble thallium compounds per cubic meter of air for an 8-hour workday, underscoring the need for strict control measures to limit skin and respiratory exposure [
30]. The NIOSH (National Institute for Occupational Safety and Health) has indicated the same limit value (REL-TWA = 0.1 mg/m3) for work up to 10 hours. The Association of American Industrial Hygienists (ACGIH) set a much lower limit in 2009 (TLV-TWA = 0.02 mg/m3), confirming the notations "skin" (skin passage), "stel" (short time exposure limit, limit valid even for a short period) and "ceiling" (limit not to be exceeded).
This brief review of the effects of thallium indicates how important it is for the clinician to consider this form of intoxication among the many possible etiological diagnoses of cases with neurological symptoms. In fact, even in Western countries, a person can be intoxicated by inadvertent exposure through alternative drugs, uncontrolled food products and contaminated water. Even intentional ingestion for suicidal purposes must be considered on account of the possibility of purchasing it online [
31]. The rarity of thallium poisoning and the non-specific clinical signs of presentation may cause a delay in treatment and admission. Because intoxication often appears to be analogous to other diseases, ill patients may go unnoticed. If left untreated, thallium poisoning can cause irreversible harm to the digestive and neurological systems, or in extreme situations, result in paralysis, coma, and death.
In this article we report on a case of unintentional thallium poisoning which had not been correctly identified in two previous hospital centers. Early treatment with Prussian Blue led to a rapid improvement. We completed the study through a scoping review to assess the relationship between delay in treatment and outcome.
2. Methods
Considering that there were no reviews in the literature that allowed us to study the relationship between delay in diagnosis, the treatment of intoxication and residual health problems, we conducted a rapid scoping review on this topic, following the PRISMA-Scr (Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews) checklist [
32]. We opted for a rapid review to have immediate reassurance for our idea of administering the drug before receiving toxicological confirmation of the diagnosis. We used the PCC framework, according to the JBI Scoping Review Methodology Group [
33].
A scoping review approach was selected to provide a broad overview of available evidence on Prussian Blue therapy for thallium intoxication. Given the rarity of documented cases and the fragmented nature of available data, a full systematic review was deemed impractical. A scoping review allows for the identification and mapping of key findings across diverse studies since it highlights treatment outcomes and gaps in the literature without requiring comprehensive quality assessments. This approach is particularly suited to emerging clinical topics where formal guidelines are absent and rapid evidence synthesis can inform clinical decision-making.
Cases of thallium intoxication or poisoning were included as "participants"; the "concept" was the relationship between the latency between exposure and treatment and the outcome; the "context" included case-report/series of intoxication, whatever their origin (accidental, voluntary, unknown). The overview of the existing evidence was conducted without a formal assessment of the methodological quality of the included studies. The rapid scoping search was conducted on a single database (PubMed) and included publications in English, German and Italian.
Cases or case series where treatment had included Prussian Blue were included; cases where other types of treatment had been used were excluded. The selection of the articles was carried out by two authors (N.M. and F.C.); a third author (P.M. S.) was called upon for doubtful cases.
3. Detailed Case Description
A 40-year-old female patient, working as a pharmacist in central Italy, came to our emergency room after a week’s progressive onset of paresthesia of the lower arms, with severe muscle pain, reduced strength, generalized asthenia, visual disturbances with conjunctival hyperemia, chest pain, abdominal pain, nausea and diarrhea. During the week, the extent and intensity of her symptoms had gradually worsened. The patient had taken ibuprofen, without gaining relief. Finally, being unable to walk unaided because of severe pain in the trunk and limbs, and subsequently in the arms, she was confined to a wheelchair. Previously she had been to two different peripheral hospital emergency rooms but had not been hospitalized. Eye drops had been prescribed for eye symptoms.
At clinical examination in the emergency department the patient was alert and lucid, eupneic, with rhythmic cardiac action and normal pulmonary findings. The abdomen was treatable; Murphy’s and Blumberg’s signs were negative. Severe arthralgias prevented walking and movement. There was no fever, but pharyngeal hyperemia, lymphadenopathy, and skin rashes were observed. The patient complained of paresthesia of the tingling type at the tips of the fingers and the tips of the feet, with a slight superficial hypoesthesia of the anterior surface of the legs. Discriminative tactile, thermal and painful sensitivity was normal. The patient did not present any deficits of the cranial nerves or global strength (although this was difficult to evaluate due to pain limitation); the index-nose test was well performed symmetrically, and she had normally evoked osteotendinous reflexes.
The patient was admitted to the Department of Emergency, Anesthesiology and Resuscitation Sciences of the Gemelli General Hospital. At anamnesis, she failed to report any chronic pathology, drug usage or food allergies. She denied taking any food supplements, alternative medicines, cosmetics or exotic food products. She lived in a country house and had not travelled outside Italy. She owned a cat, had a flower garden and a vegetable garden and no history of tick bites.
The patient’s background was further explored to assess potential chronic exposure sources, including any prior use of contaminated water, dietary habits that could increase thallium ingestion (such as home-grown produce), and possible occupational or environmental contacts. This investigation aimed to determine whether the acute presentation was compounded by underlying, low-level exposure over time that might have contributed to the severity of symptoms and delayed diagnosis.
Vital parameters were within limits: blood pressure 121/67 mmHg, heart rate 79 bpm, SpO2 98%, temperature 36.5 °C. The venous blood gas analysis showed pH 7.39, Lactates 0.7 mmol/L, potassium 3.7, and no electrolyte alterations. Chest X-ray, electrocardiogram, urine tests, and basic blood tests were negative. Blood tests for Leptospira, Borrelia, Rickettsia, Bartonella, Human immunodeficiency virus (HIV), Cytomegalovirus (CMV), Epstein Barr virus (EBV), Toxoplasma, Syphilis, Hepatitis C virus (HCV), Hepatitis B virus (HBV), Enterovirus, Adenovirus, and Chikungunya virus were all negative.
Considering that the patient lived in the countryside, the doctors investigated the source of water supply. The patient confirmed that the house was supplied with drinkable water from the public water supply, but also had well water that was not drinkable and used only for cleaning.
Having learned of the existence of a well with non-potable water, the doctors investigated further by asking if the water line of the well was separate from that of the municipal water supply with potable water. To their surprise, they learned that the line was the same and that the type of water introduced into the domestic pipes could be potable or not, depending on the positioning of a valve. The patient added that the partition valve was always operated by her husband and that he had been away on business for a week. She didn’t know how to position the valve correctly.
The information gathered prompted the doctors to request a water analysis from the relevant Regional Agency (ARPA) which, due to the urgency of the case, provided the report within a few days.
Toxicological analyses were carried out by the national reference laboratory, situated in another Region. Plasma and urinary samples for barium and thallium were obtained on the 2nd day after admission, but the results naturally arrived a few days later when the patient had already been discharged. However, based on the patient’s clinical history, the doctors decided to start treatment with Prussian Blue (Radiogardase, 3 g x 3 / die) before receiving the results of the tests. The low toxicity of Prussian Blue made the clinically guided "ex juvantibus" treatment advisable.
The Prussian Blue treatment, that was started on the first day of hospitalization, immediately resulted in pain relief and a general improvement in symptoms. Twelve hours after the start of Prussian Blue therapy, the pain was noticeably reduced. In the following days, there was a significant improvement in the patient’s clinical conditions: paresthesia, myalgias and joint pains disappeared, and tenderness in her knee joints diminished.
Table 1 reports the results of toxicological analyses conducted on the patient during hospitalization and over a period of several months after discharge.
On the fifth day, the patient was discharged with instructions to remain in telematic contact with the Poison Control Center of the Gemelli General Hospital and to repeat the urine and plasma tests on the dates indicated by the center.
Water quality was analyzed using inductively coupled plasma mass spectrometry (ICP-MS), according to the ISO 7294-2:2023 method. When water analyses became available, they indicated evident thallium contamination in the well water, with values off the scale. The laboratory provided the highest measurable value, 150 µg/L, which is 75 times higher than the maximum United States Environmental Protection Agency (US-EPA) safety value of 2 µg/L for drinkable water. The levels of other elements (barium, chromium, copper, vanadium, aluminum, cadmium, arsenic, lead, mercury, boron, nickel, selenium, manganese, and antimony) in the well water were within the safety levels.
Blood and urine were analyzed by dynamic cell reaction inductively coupled plasma mass spectrometry (DRC-ICP-MS). Toxicology tests indicated elevated urinary thallium levels from the second day of hospitalization, with increased urinary excretion during therapy (between the tenth and thirtieth day) and subsequent reduction, which nevertheless reached normality only after 120 days. Plasma thallium levels were very high on admission (267 times the upper reference limit) and showed a gradual reduction but failed to return to normal after 30 days of treatment. For this reason and given the absence of side effects from the treatment, the doctors decided to continue treatment with Prussian Blue, reducing the dose (1.5 g three times a day) on day 45. Plasma thallium levels were not normal even on the 120th day after the start of treatment.
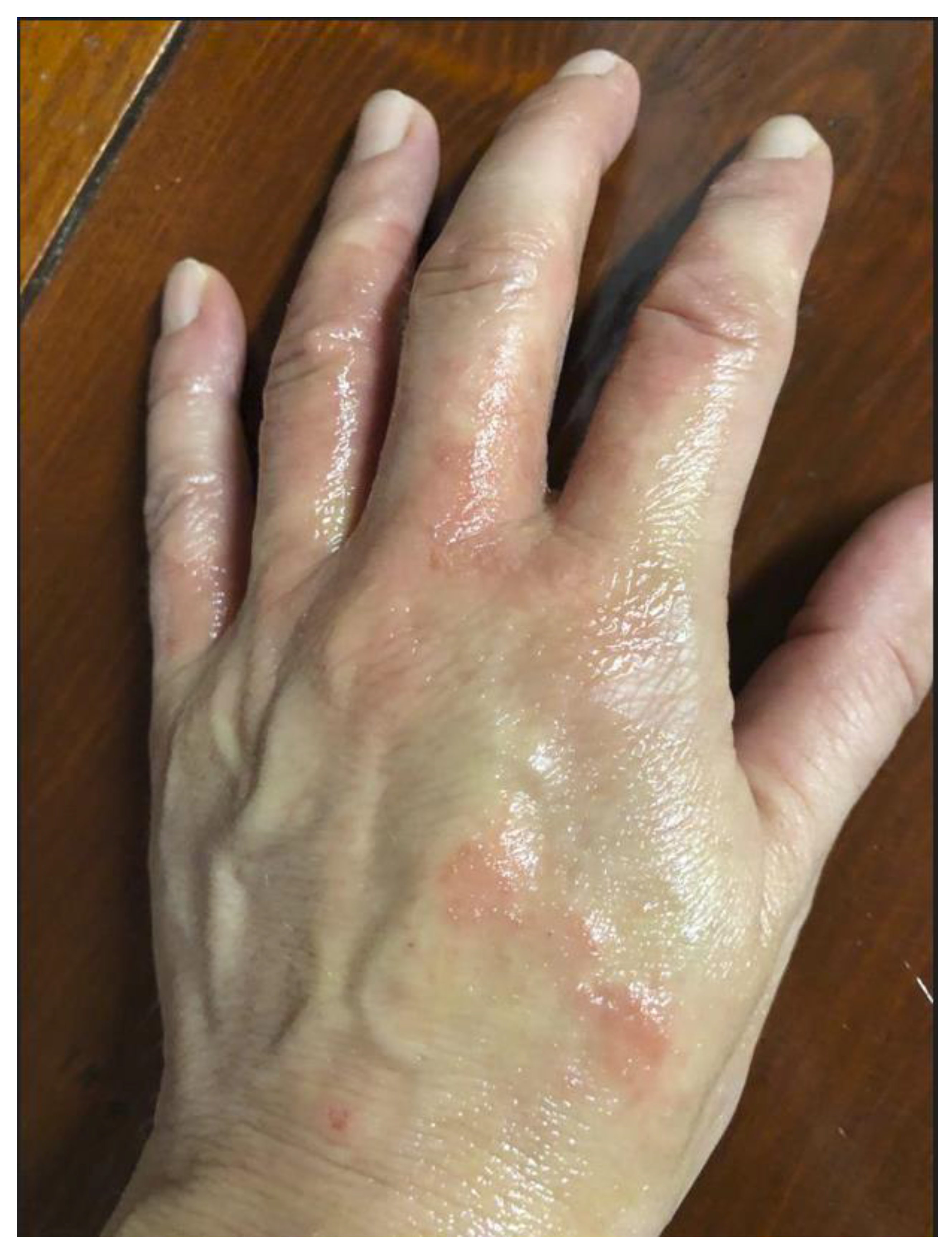
The first telematic clinical check-up enabled doctors to detect the appearance of a rash on one hand (
Figure 1). The hand appeared hyperemic, with signs of parakeratosis of the skin and nails. Moreover, there was the onset of alopecia. The patient reported the disappearance of pain and recovery of mobility, even if marked asthenia persisted. The alopecia had worsened at the 30-day check-up, while the general clinical picture showed a slow but gradual improvement. By day 120, hair loss had been resolved and the patient had returned to the pre-intoxication clinical and functional state.
4. Results of the Review
A PubMed search was performed using the keywords [thallium (poisoning or intoxication) AND (case-report or case series) AND (Prussian Blue) AND (case report or case series)]. The search provided 32 entries, of which the title and abstract were examined. Five entries that were not consistent with the research were excluded, while the remaining 27 articles were analyzed. From each work, the age and sex of the victim, the cause of poisoning, symptoms, latency between the onset of symptoms and treatment with Prussian Blue, any additional treatments and the patient’s conditions at the end of the follow-up were extracted.
Table 2 reports the result of the review.
Generally, patients with delayed treatment exhibited severe symptoms upon admission. Treatment with Prussian Blue significantly reduced thallium levels in plasma and urine; however, in cases with longer treatment latency, permanent neurological sequelae were invariably present. Complete recovery was observed only in cases where Prussian Blue was administered within the first few hours of exposure.
5. Discussion
5.1. Adverse Effects of Thallium
Thallium poisoning has a long history and, as we have seen, the occurrence of cases of poisoning cannot be ruled out even in the most advanced countries. The United States ceased domestic mining of thallium in 1981, but since then the growing demand in the semiconductor industry (more than 2 metric tons in 1987) has been met by imports from Belgium (54%), the Netherlands (16%), the Federal-Republic of Germany (14%), the United Kingdom (6%), and other sources (10%) [
62]. Production was estimated at 14.06 metric tons in the rest of the world [
63], and this has caused increasing ecological problems globally. Furthermore, thallium is widely distributed in the earth’s crust, and this multiplies the possibility that waters are polluted by this element, as was evident in the case reported above.
In 2019, the American Association of Poison Control Centers reported 49 single exposures in the US, resulting in one major outcome but no fatalities which are a rare but not impossible occurrence. Thallium poisoning is prevalent in developing countries; however, few data are available. In this rapid review, we found 101 patients treated with Prussian Blue, 7 of whom had fatal outcomes (
Table 2). Mortality rates for acute thallium toxicity have been reported to range from six to fifteen percent. A lethal dose for human beings ranges from 10 to 15 mg/kg, but mortality can still occur at lower dosages [
64,
65,
66].
The major hazard for the general population is exposure to polluted water. Water pollution is generally attributed to the interaction between groundwater and thallium-bearing pyrite ores. In China, a water source contaminated with thallium (with values up to 10 µg/L) caused significant public health problems [
67]. In Italy, high levels of pollution (up to 9000 µg/L) have been observed in wastewater from abandoned mining plants [
68,
69,
70,
71,
72] and resulting in contamination of the water drunk by the surrounding population [
73]. Exposure to low doses of thallium, far below the US-EPA maximum allowable level in drinking water (2 μg/L) [
74], is a threat to human health [
75]. Over 10 years ago, the US-EPA aimed to lower the maximum contaminant level of thallium in drinking water to 0.5 μg/L, but this measure has not yet been adopted in America or any European country.
Studies conducted on general population subjects with moderate thallium absorption (urinary levels less than 0.2 μg /L) reported a number of health problems, including impaired thyroid function [
76], metabolic changes (increased waist circumference and body mass index) [
77], hypertension [
78], impaired glomerular filtration [
79], and autism spectrum disorders [
80,
81], as well as osteoarthritis [
82]. Placenta transports about 50% of the metal from mother to fetus [
83] and maternal exposure is associated with low birth weight [
84] and preterm birth [
85]. Thallium is also associated with genetic and epigenetic changes [
86].
Thallium is similar in structure to potassium and is consequently processed in a comparable manner at cellular level [
87]. Thallium toxicity is associated with some primary toxicologic effects. Tissues exhibiting elevated potassium concentrations accumulate significant levels of thallium. This results in initial stimulation, subsequently leading to the inhibition of potassium-dependent processes. Inhibition of pyruvate kinase and succinate dehydrogenase disrupts the Krebs cycle and glucose metabolism, resulting in decreased ATP production, swelling, and vacuolization due to impairment of the sodium-potassium ATPase. Thallium’s strong capacity to form disulfide bonds interferes with the cross-linking of cysteine residues, leading to a decrease in keratin synthesis. Riboflavin sequestration caused by thallium and the inhibition of flavin adenine dinucleotide disrupts the electron transport chain, resulting in decreased ATP production [
88]. Ribosomes are adversely affected by thallium, particularly impacting protein synthesis through damage to the 60S ribosome. The reduction of ribosome synthesis and its biogenesis results in impairment of protein synthesis, blockage of cell cycle progression and apoptosis [
89]. Thallium at high doses induces degeneration of myelin in both the central and peripheral nervous systems [
29]. Low-dose exposure is associated with mitochondrial dysfunction, neurite shortening, loss of substrate adhesion, and an increase in cytoplasmic calcium [
90,
91].
The toxicokinetic phases of thallium are divided into three distinct stages: 1) the Intravascular Distribution Phase during which, in the first 4 hours following exposure, thallium is distributed to organs through the bloodstream; 2) the CNS Distribution Phase in which over a period of 4 to 48 hours thallium is distributed within the central nervous system; 3) the Elimination Phase that commences approximately 24 hours after exposure and is primarily facilitated through renal excretion and fecal elimination. This phase is gradual and may require up to 30 days for completion [
88]. Analysis of thallium accumulated in hair by laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) allows for the reconstruction of exposure for forensic purposes [
55].
Acute exposure symptoms include gastrointestinal manifestations, which may arise within 3-4 hours. These include abdominal pain, nausea, vomiting, diarrhea, or constipation, with rare instances of blood in vomitus or stools. Neurological symptoms typically present 2-5 days post-exposure and may include ascending peripheral neuropathies, distal motor weakness, ataxia, tremor, cranial nerve palsies, headache, seizures, insomnia, and coma, potentially leading to death. Ocular symptoms may involve diplopia, ptosis, seventh cranial nerve palsy, nystagmus, optic neuropathy, and lens opacities. Dermatological symptoms can present early as nonspecific scaling and acneiform or pustular eruptions, followed by alopecia due to disruption of cysteine disulfide bonds, and also as late manifestations such as Mees lines on nails, hypohidrosis, anhidrosis, and painful glossitis. Chronic exposure leads to continuation of the aforementioned effects. In chronic poisoning, neurological symptoms may persist despite a decrease in blood thallium levels. [
88].
5.2. Our Observations
Our clinical case matches those of other cases that have been documented in the literature, where the presence of a triad has been demonstrated, raising the possibility of thallium intoxication. This triad includes alopecia, signs of motor or sensory neurological impairment, and abdominal pain [
92]. However, alopecia appears, as we have seen, several days after the onset of the clinical picture which is initially characterized by paresthesia and sharp pain. Following thallium exposure, the presentation pattern typically includes vomiting, nausea, and abdominal pain in the first few hours after exposure; peripheral sensorimotor neuropathy after four or more days, and generalized alopecia weeks later.
Cases of accidental poisoning have been reported recently [
92], as well as cases of attempted suicide [
31] and criminal intoxication in different countries, including Italy [
52,
93,
94,
95]. A case of acute, nonintentional thallium poisoning was due to thallium-contaminated alternative medicine [
96]. However, reports of poisoning were more frequent in the past. Thallium poisoning during pregnancy may have fetal effects ranging from severe toxicity with residual sequelae to outwardly normal development [
97]. A recent case of criminal intoxication with long-term misdiagnosis and neurologic outcomes has also been observed [
98]. Another case of criminal intent was studied for three years in six different hospitals without reaching a diagnosis [
59]. Chronic intoxication is sometimes suspected with the onset of alopecia [
54]. However, since thallium is tasteless, odorless and water-soluble and can be absorbed through the skin, inhaled or ingested, thallium intoxication is frequently misdiagnosed, or the diagnosis is delayed. A retrospective analysis of cases with delayed admission showed significant symptoms associated with central nervous system damage and changes in magnetic resonance images and electroencephalograms [
56]. Cases of severe neurological impairment with coma have been shown to respond positively to treatment; but the rarity of these occurrences prevents the generalizability of these observations [
56].
Recently, an interesting report of a case of accidental thallium poisoning in a chemist who survived 10 years after intoxication has been published [
61]. The first symptom was of numbness in the feet; this rose up the legs causing terrific pain. Paralysis included the digestive system, thus making the body unable to absorb food and causing cachexia. Severe nerve damage was followed by alopecia. Initially, a neurologist diagnosed Guillain-Barré syndrome and prescribed immunoglobulin treatment and plasmapheresis. This incorrect treatment had no effect, whereas the correct diagnosis and treatment with Prussian Blue resulted in a very slow improvement. Thallium was used in the laboratory, but there had been no occupational exposure; the metal had probably been ingested through beverages.
Early recognition of the diagnosis is generally the key to successful treatment as was seen recently in reported cases of contaminated meat poisoning [
19]. Cases of delayed diagnosis have an unfavorable outcome, as in the one recently reported by Zou [
99]. A 41-year-old male was admitted with acute polyneuropathy and abdominal pain. The patient received treatment for suspected Guillain-Barré syndrome and subsequently for autoimmune encephalopathy. Over the following 42 days, he exhibited progressive muscle weakness, delirium, and alopecia, ultimately leading to a diagnosis of thallium toxicity. After treatment with a combination of Prussian Blue, activated charcoal, and continuous venous hemofiltration, the patient showed improvement; however, neuropsychiatric and neuromuscular sequelae persisted. This case demonstrates that delaying a diagnosis until symptoms have evolved can compromise the possibility of recovery and, probably, the life of the patient. The decision to proceed with the administration of Prussian Blue was taken before obtaining confirmation of water pollution and poisoning. This
ex juvantibus criterion should be followed in all cases in which the clinical picture is compatible with thallium poisoning because Prussian Blue is not very toxic and induces an improvement that can be the best guide for the clinician.
Even in a medically advanced country like Italy, and in a hospital that for decades has had a Poison Control Center of which the first author of this article is the Director, it is not easy to obtain a water dosage and a toxicological analysis within a period of time compatible with the survival of a thallium-poisoned patient. This is the main reason that leads us to stress the need to start immediate treatment of cases that could be caused by thallium intoxication, before the diagnosis of thallotoxicosis is confirmed. The rapid relief of painful symptoms and a pause in the progression of neurological damage are important indicators that enable us to calmly await the results of toxicological tests confirming the diagnosis. Conversely, in cases where the symptoms are due to a different cause, a few days of administration of a drug of low toxicity prevents the patient from running excessive risks.
Lowering blood levels as soon as possible is the main objective when treating acute thallium poisoning [
48]. Prussian Blue functions as a univalent cation exchanger that improves fecal clearance, blocks enterohepatic circulation of thallium, and preferentially binds unabsorbed thallium in the gut. Activated charcoal, which is often administered alongside Prussian Blue, improves fecal removal while simultaneously adsorbing thallium. Activated charcoal and Prussian Blue are both recognized therapies; however, Prussian Blue is the standard of care because of its greater safety and effectiveness [
99]. Nevertheless, these therapies cannot eliminate absorbed thallium that has reached the bloodstream. Prussian Blue cannot sequester thallium outside of the digestive tract, and thallium has a lengthy physiological half-life. Consequently, additional research has suggested combining Prussian Blue with blood purification procedures including continuous veno venous hemofiltration, hemoperfusion, and hemodialysate that use extracorporeal filtration devices to eliminate thallium from the bloodstream [
100,
101].
The recommendation to use Prussian Blue for emergency treatment of patients with symptoms such as those described above can encounter two main obstacles: firstly, the emergency physician must be aware of thallium poisoning and its treatment and suspect the disease, and secondly, it is essential that the drug be available. Drug availability can be a problem since the limited number of cases is an obvious impediment to hospital supply of the drug. The lack of the drug can be a serious problem, especially in countries where the probability of thallotoxicosis is higher [
53]. We therefore recommend that doctors working in peripheral hospitals contact larger hospitals with clinical toxicology services. The lack of knowledge about the effects of thallium and the clinical picture of intoxication can be effectively countered by referring to facilities such as the Poison Control Center of the Gemelli General Hospital that is able to provide advice in doubtful cases.
The case presented also raises the problem of hygiene in homes. Italian law prohibits the introduction of non-potable water into the domestic water supply, and a dwelling with the characteristics described in the case reported is deemed unfit for habitation. It is surprising that the person who was poisoned had a degree in a subject that requires knowledge of the toxic effects of thallium, and it is difficult to understand how one could live for years in such a high-risk situation. The slow rate at which urinary and plasma thallium levels decreased (the latter failed to reach normal limits even after 120 days of treatment), suggests that besides the acute exposure episode during which the patient drank contaminated water for the entire week of her husband’s absence, there could have been previous chronic low-level exposure due to contaminated water used for washing or watering vegetables.
5.3. Implications for Physicians and Policymakers
Given the severe outcomes associated with delayed diagnosis of thallium poisoning, it is essential to standardize protocols for the emergency management of suspected cases. Rapid access to Prussian Blue and hemoperfusion should be prioritized in emergency settings, particularly in regions with higher thallium contamination risks. Training and awareness programs for emergency physicians could enhance the recognition of early symptoms, especially priority indicators like peripheral neuropathy and abdominal pain; alopecia may have a delayed onset. Furthermore, initiating treatment with Prussian Blue as an ‘ex juvantibus’ approach, even before toxicological confirmation, is advisable due to its low toxicity and proven efficacy in reducing thallium levels. For policymakers, the implementation of surveillance and control measures as well as guidelines for clinical practice could significantly improve patient outcomes and reduce the incidence of long-term complications.
5.4. Study Limitations
This study has several limitations that should be considered when interpreting the findings. Firstly, the rarity of documented thallium poisoning cases limits the generalizability of the results, as the cases reviewed may not fully represent the variability in clinical presentation and treatment response across different populations. Additionally, the fragmented nature of available data restricts the depth of analysis and prevents the application of a fully systematic review approach, imposing the use of a scoping review. Although this methodology is suitable for mapping available evidence, it does not allow for a rigorous quality assessment of each study, and therefore potentially introduces bias. Lastly, the absence of long-term follow-up data in some cases limits insights into the chronic effects of thallium poisoning and the durability of Prussian Blue’s efficacy in preventing sequelae. Future research with larger sample sizes and more comprehensive follow-up would be valuable for confirming these preliminary findings and enhancing clinical guidance.
6. Conclusions
In conclusion, thallium poisoning, although rare, should be considered in cases presenting with paresthesia, progressive neurological deficits, and severe osteomuscular pain in the abdomen and thorax. Prompt treatment with Prussian Blue not only offers the potential for rapid symptom relief but also serves as a therapeutic confirmation of the diagnosis. It is vital to commence treatment early in order to prevent irreversible neurological damage and improve patient outcomes. Given the challenges in obtaining a prompt diagnosis, heightened awareness and standardized protocols are essential to enhance clinical response and minimize the risk of long-term complications.
Author Contributions
Conceptualization, P.M.S.; methodology, P.M.S., N.M. F.C. and M.P.; writing—original draft preparation, N.M.; writing—review and editing, N.M., F.C., M.R. and P.M.S. All authors have read and agreed to the published version of the manuscript
Funding
This research received no external funding
Institutional Review Board Statement
Not applicable
Informed Consent Statement
Written informed consent has been obtained from the patient to publish this paper.
Data Availability Statement
Personal data is unavailable due to privacy or ethical restrictions
Acknowledgments
We thank Ms. E. A. Wright who revised the language.
Conflicts of Interest
The authors declare no conflicts of interest
References
- Link, V.B.; Mohr, C.O. Rodenticides in bubonic-plague control. Bull World Health Organ. 1953;9(5):585-96.
- Bureau, Y.; Barriere, J. L’acétate de thallium dans le traitement des teignes scolaires; résultats obtenus à la clinique dermatologique de Nantes [Thallium acetate in the treatment of tinea in school children; results obtained at the Nantes dermatological clinic]. Bull Soc Fr Dermatol Syphiligr. 1957;64(1):105-6. French.
- Poudyal, B.; Shrestha, P.; Chowdhury, Y.S. Thallium-201. 2023 Jul 17. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan–.
- Shiga, H.; Wakabayashi, H.; Washiyama, K.; Noguchi, T.; Hiromasa, T.; Miyazono, S.; Kumai, M.; Ogawa, K.; Taki, J.; Kinuya, S.; Miwa, T. Thallium-201 Imaging in Intact Olfactory Sensory Neurons with Reduced Pre-Synaptic Inhibition In Vivo. Mol Neurobiol. 2020;57(12):4989-4999. [CrossRef]
- Rigby, A.; Blower, J.E.; Blower, P.J.; Terry, S.Y.A.; Abbate, V. Targeted Auger electron-emitter therapy: Radiochemical approaches for thallium-201 radiopharmaceuticals. Nucl Med Biol. 2021;98-99:1-7. [CrossRef]
- Jo, O.; Schlicht, S.; Slavin, J.; Di Bella, C.; Pang, G.; Powell, G.; Spelman, T.; Choong, P.F. The role of Thallium-201 scintigraphy and Tc-99m pentavalent dimercaptosuccinic acid in diagnosis and grading of chondrosarcoma. Eur J Radiol. 2020;125:108846. [CrossRef]
- Chen, G.B.; Wu, H.; He, X.J.; Huang, J.X.; Yu, D.; Xu, W.Y.; Yu, H. Adenosine stress thallium-201 myocardial perfusion imaging for detecting coronary artery disease at an early stage. J Xray Sci Technol. 2013;21(2):317-22. [CrossRef]
- Liu, C.H.; Huang, C.Y.; Huang, C.C. Occupational neurotoxic diseases in Taiwan. Saf Health Work. 2012;3(4):257-67. [CrossRef]
- Hirata, M.; Taoda, K.; Ono-Ogasawara, M.; Takaya, M.; Hisanaga, N. A probable case of chronic occupational thallium poisoning in a glass factory. Ind Health. 1998;36(3):300-3. [CrossRef]
- Hirata, M. [A study on occupational peripheral nerve disorders: a general view]. Sangyo Eiseigaku Zasshi. 2023;65(3):117-124. Japanese. [CrossRef]
- Tsai, K.P. Toxic effects of thallium (Tl+) on prokaryotic alga Microcystis aeruginosa: Short and long-term influences by potassium and humic acid. Chemosphere. 2024;346:140618. [CrossRef]
- Yu, H.Y.; Chang, C.; Li, F.; Wang, Q.; Chen, M.; Zhang, J. Thallium in flowering cabbage and lettuce: Potential health risks for local residents of the Pearl River Delta, South China. Environ Pollut. 2018;241:626-635.
- Schwalfenberg, G.; Rodushkin, I.; Genuis, S.J. Heavy metal contamination of prenatal vitamins. Toxicol Rep. 2018;5:390-395. [CrossRef]
- Liu, Y.; Wang, Q.; Zhuang, W.; Yuan, Y.; Yuan, Y.; Jiao, K.; Wang, M.; Chen, Q. Calculation of Thallium’s toxicity coefficient in the evaluation of potential ecological risk index: A case study. Chemosphere. 2018;194:562-569. [CrossRef]
- Insley, B.M.; Grufferman, S.; Ayliffe, H.E. Thallium poisoning in cocaine abusers. Am J Emerg Med. 1986;4(6):545-8. [CrossRef]
- Questel, F.; Dugarin, J.; Dally, S. Thallium-contaminated heroin. Ann Intern Med. 1996;124(6):616. [CrossRef]
- Molavi, N.; Ghaderi, A.; Banafshe, H.R. Determination of thallium in urine, blood, and hair in illicit opioid users in Iran. Hum Exp Toxicol. 2020;39(6):808-815. [CrossRef]
- Afshari, R., Mégarbane, B., Zavar, A. Thallium poisoning: one additional and unexpected risk of heroin abuse. Clin Toxicol (Phila). 2012;50(8):791-2. [CrossRef]
- Wang, X.; Xiao, P.; Wang, R.; Luo, C.; Zhang, Z.; Yu, S.; Wu, Q.; Li, Y.; Zhang, Y.; Zhang, H.; Zhao, X. Relationships between urinary metals concentrations and cognitive performance among U.S. older people in NHANES 2011-2014. Front Public Health. 2022;10:985127. [CrossRef]
- Bjorklund, G.; Stejskal, V.; Urbina, M.A.; Dadar, M.; Chirumbolo, S.; Mutter, J. Metals and Parkinson’s Disease: Mechanisms and Biochemical Processes. Curr Med Chem. 2018;25(19):2198-2214. [CrossRef]
- Zhang, Q.Q.; Li, J.H.; Wang, Y.D.; Li, X.N.; Wang, J.Q.; Zhou, M.Y.; Dong, M.R.; Chen, G.M.; Ye, Y.F.; Zhang, H.H.; Zhu, W.; Liu, T.; Zhang, B. Association between maternal thallium exposure and risk of gestational diabetes mellitus: Evidence from a birth cohort study. Chemosphere. 2021;270:128637. [CrossRef]
- Zhang, Z.; Zhang, M.; Qu, Y.; Zhao, F.; Ji, S.; Li, Z.; et al. Association of Thallium Exposure with Decreased Renal Function among Chinese Adults - China, 2017-2018. China CDC Wkly. 2024;6(34):867-871. [CrossRef]
- Zhang, Z.; Xiao, Y.; Long, P.; Yu, Y.; Liu, Y.; Liu, K.; et al. Associations between plasma metal/metalloid mixtures and the risk of central obesity: A prospective cohort study of Chinese adults. Ecotoxicol Environ Saf. 2024;270:115838. [CrossRef]
- Shen, T.; Zhong, L.; Ji, G.; Chen, B.; Liao, M.; Li, L.; Huang, H.; et al. Associations between metal(loid) exposure with overweight and obesity and abdominal obesity in the general population: A cross-sectional study in China. Chemosphere. 2024;350:140963. [CrossRef]
- Wen, Y.; Huang, S.; Zhang, Y.; Zhang, H.; Zhou, L.; Li, D.; et al. Associations of multiple plasma metals with the risk of ischemic stroke: A case-control study. Environ Int. 2019;125:125-134. [CrossRef]
- Shi, L.; Yuan, Y.; Xiao, Y.; Long, P.; Li, W.; Yu, Y.; et al. Associations of plasma metal concentrations with the risks of all-cause and cardiovascular disease mortality in Chinese adults. Environ Int. 2021;157:106808. [CrossRef]
- Chen, C.S.; Yuan, T.H.; Lu, T.P.; Lee, H.Y.; Chen, Y.H.; Lai, L.C.; et al. Exposure-associated DNA methylation among people exposed to multiple industrial pollutants. Clin Epigenetics. 2024;16(1):111. [CrossRef]
- Duan, W.; Wang, Y.; Li, Z.; Fu, G.; Mao, L.; Song, Y.; et al. Thallium exposure at low concentration leads to early damage on multiple organs in children: A case study followed-up for four years. Environ Pollut. 2020;258:113319. [CrossRef]
- Osorio-Rico, L.; Santamaria, A.; Galván-Arzate, S. Thallium Toxicity: General Issues, Neurological Symptoms, and Neurotoxic Mechanisms. Adv Neurobiol. 2017;18:345-353. [CrossRef]
- 13 November.
- Spadaro, A.; Lee, A.S.Y.; Pineda, H.; Ruck, B.; Calello, D.P.; Greller, H.A.; Nelson, L.S.; Parris, M.A. Attempted Self-Harm with Elemental Thallium Purchased Online: Case Report with Analytical Confirmation. J Med Toxicol. 2024;20(4):416-421. [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med. 2018;169(7):467-473. [CrossRef]
- .
- Aprea, M.C.; Bettinelli, M.; Perbellini, L.; Negri, S.; Iavicoli, I.; Lovreglio, P.; Perico, A.; Ricossa, M.C.; Salamon, F. [Societa’ italiana valori di riferimento - quarta lista dei valori di riferimento per elementi, composti organici e loro metaboliti - edizione 2017] Italian society of reference values - Fourth list of reference values for elements, organic compounds and their metabolites - 2017 edition.
- Pedersen, R.S.; Olesen, A.S.; Freund, L.G.; Solgaard, P.; Larsen, E. Thallium intoxication treated with long-term hemodialysis, forced diuresis and Prussian blue. Acta Med Scand. 1978;204(5):429-32. [CrossRef]
- Stevens, W.J. Thallium intoxication caused by a homoeopathic preparation. Toxicol Eur Res. 1978;1(5):317-20.
- Heath, A.; Ahlmén, J.; Branegård, B.; Lindstedt, S.; Wickström, I.; Andersen, O. Thallium poisoning--toxin elimination and therapy in three cases. J Toxicol Clin Toxicol. 1983;20(5):451-63. [CrossRef]
- Wainwright, A.P.; Kox, W.J.; House, I.M.; Henry, J.A.; Heaton, R.; Seed, W.A. Clinical features and therapy of acute thallium poisoning. Q J Med. 1988;69(259):939-44. [CrossRef]
- Niehues, R.; Horstkotte, D.; Klein, R.M.; Kühl, U.; Kutkuhn, B.; Hort, W.; Iffland, R.; Strauer, B.E. Wiederholte Ingestion potentiell letaler Thalliummengen in suizidaler Absicht [Repeated ingestion with suicidal intent of potentially lethal amounts of thallium]. Dtsch Med Wochenschr. 1995;120(12):403-8. German. [CrossRef]
- Malbrain, M.L.; Lambrecht, G.L.; Zandijk, E.; Demedts, P.A.; Neels, H.M.; Lambert, W.; De Leenheer, A.P.; Lins, R.L.; Daelemans, R. Treatment of severe thallium intoxication. J Toxicol Clin Toxicol. 1997;35(1):97-100. [CrossRef]
- Atsmon, J.; Taliansky, E.; Landau, M.; Neufeld, M.Y. Thallium poisoning in Israel. Am J Med Sci. 2000;320(5):327-30. [CrossRef]
- Pau, P.W. Management of thallium poisoning. Hong Kong Med J. 2000;6(3):316-8.
- Lu, C.I.; Huang, C.C.; Chang, Y.C.; Tsai, Y.T.; Kuo, H.C.; Chuang, Y.H.; Shih, T.S. Short-term thallium intoxication: dermatological findings correlated with thallium concentration. Arch Dermatol. 2007;143:93–98. [CrossRef]
- Pelclová, D.; Urban, P.; Ridzon, P.; Senholdová, Z.; Lukás, E.; Diblík, P.; Lacina, L. Two-year follow-up of two patients after severe thallium intoxication. Hum Exp Toxicol. 2009;28(5):263-72. [CrossRef]
- Sun, T.W.; Xu, Q.Y.; Zhang, X.J.; Wu, Q.; Liu, Z.S.; Kan, Q.C.; Sun, C.Y.; Wang, L. Management of thallium poisoning in patients with delayed hospital admission. Clin Toxicol (Phila). 2012;50(1):65-9. Erratum in: Clin Toxicol (Phila). 2012;50(2):160. https://doi.org/10.3109/15563650.2011.638926. [CrossRef]
- Riyaz, R.; Pandalai, S.L.; Schwartz, M.; Kazzi, Z.N. A fatal case of thallium toxicity: challenges in management. J Med Toxicol. 2013;9(1):75-8. [CrossRef]
- Zhang, H.T.; Qiao, B.P.; Liu, B.P.; Zhao, X.G. Study on the treatment of acute thallium poisoning. Am J Med Sci. 2014;347:377–381. [CrossRef]
- Huang, C.; Zhang, X.; Li, G.; Jiang, Y.; Wang, Q.; Tian, R. A case of severe thallium poisoning successfully treated with hemoperfusion and continuous veno-venous hemofiltration. Hum Exp Toxicol. 2014; 33(5):554-8. [CrossRef]
- Li, J.M.; Wang, W.; Lei, S.; Zhao, L.L.; Zhou, D.; Xiong, H. Misdiagnosis and long-term outcome of 13 patients with acute thallium poisoning in China. Clin Toxicol (Phila). 2014;52(3):181-6. [CrossRef]
- Sojáková, M.; Žigrai, M.; Karaman, A.; Plačková, S.; Klepancová, P.; Hrušovský, Š. Thallium intoxication. Case Report. Neuro Endocrinol Lett. 2015;36(4):311-5.
- Li, S.; Huang, W.; Duan, Y.; Xing, J.; Zhou, Y. Human fatality due to thallium poisoning: autopsy, microscopy, and mass spectrometry assays. J Forensic Sci. 2015;60(1):247-51. [CrossRef]
- Yumoto, T.; Tsukahara, K.; Naito, H.; Iida, A.; Nakao, A. A Successfully Treated Case of Criminal Thallium Poisoning. J Clin Diagn Res. 2017;11(4):OD01-OD02. [CrossRef]
- Almassri, I.; Sekkarie, M. Cases of thallium intoxication in Syria: A diagnostic and a therapeutic challenge. Avicenna J Med. 2018;8(3):78-81. [CrossRef]
- Yang, G.; Li, C.; Long, Y.; Sheng, L. Hair Loss: Evidence to Thallium Poisoning. Case Rep Emerg Med. 2018 ;2018:1313096. [CrossRef]
- Ash, R.D.; He, M. Details of a thallium poisoning case revealed by single hair analysis using laser ablation inductively coupled plasma mass spectrometry. Forensic Sci Int. 2018;292:224-231. [CrossRef]
- Lin, G.; Yuan, L.; Bai, L.; Liu, Y.; Wang, Y.; Qiu, Z. Successful treatment of a patient with severe thallium poisoning in a coma using Prussian blue and plasma exchange: A case report. Medicine (Baltimore). 2019;98(8):e14629. [CrossRef]
- Lin, G.; Yuan, L.; Peng, X.; Long, J.; Wang, C.; Bai, L.; Lu, X.; Dong, J.; Liu, Y.; Wang, Y.; Qiu, Z. Clinical characteristics and treatment of thallium poisoning in patients with delayed admission in China. Medicine (Baltimore). 2019;98(29):e16471. [CrossRef]
- Liu, H.; Liao, G. Long-term misdiagnosis and neurologic outcomes of thallium poisoning: A case report and literature review. Brain Behav. 2021;11(3):e02032. [CrossRef]
- Pragst, F.; Hartwig, S. Repeated poisoning of the life partner by thallium - a case of questionable Munchausen by adult proxy syndrome with ensuing attempted murder. Int J Legal Med. 2022;136(3):695-704. [CrossRef]
- Wang, T.T.; Wen, B.; Yu, X.N.; Ji, Z.G.; Sun, Y.Y.; Li, Y.; Zhu, S.L.; Cao, Y.L.; Wang, M.; Jian, X.D.; Wang, T. Early diagnosis, treatment, and outcomes of five patients with acute thallium poisoning. World J Clin Cases. 2021;9(19):5082-5091. [CrossRef]
- Graham, F. Daily briefing: The chemist who survived thallium poisoning. Nature. 2023. Epub ahead of print. [CrossRef]
- U.S. Bureau of Mines. 1988. Mineral commodity summaries. Washington, DC.
- 1 October.
- Galván-Arzate, S.; Santamaría, A. Thallium toxicity. Toxicol Lett. 1998;99:1–13. [CrossRef]
- Cvjetko, P.; Cvjetko, I.; Pavlica, M. Thallium toxicity in humans. Arh Hig Rada Toksikol. 2010;61:111–119. [CrossRef]
- Lennartson, A. Toxic thallium. Nat Chem. 2015;7:610. [CrossRef]
- Yu, Y.; Yu, Z.; Xiang, M.; Zhou, Z.; Hu, G.; Zhang, Y.; Ma, R.; Li, H. Screening and prioritization of chemical hazards for deriving human health ambient water quality criteria in China. J. Environ. Manag. 2019;245:223–229. [CrossRef]
- Biagioni, C.; D’Orazio, M.; Vezzoni, S.; Dini, A.; Orlandi, P. Mobilization of TI-Hg-As-Sb-(Ag,Cu)-Pb sulfosalt melts during low-grade metamorphism in the Alpi Apuane (Tuscany, Italy) Geology. 2013. [CrossRef]
- Biagioni, C.; D’Orazio, M.; Lepore, G.O.; d’Acapito, F.; Vezzoni, S. Thallium-rich rust scales in drinkable water distribution systems: A case study from northern Tuscany, Italy. Sci. Total Environ. 2017. [CrossRef]
- D’Orazio, M.; Biagioni, C.; Dini, A.; Vezzoni, S. Thallium-rich pyrite ores from the Apuan Alps, Tuscany, Italy: constraints for their origin and environmental concerns. Miner. Depos. 2017. [CrossRef]
- Ghezzi, L.; D’Orazio, M.; Doveri, M.; Lelli, M.; Petrini, R.; Giannecchini, R. Groundwater and potentially toxic elements in a dismissed mining area: Thallium contamination of drinking spring water in the Apuan Alps (Tuscany, Italy) J. Geochem. Explor. 2019. [CrossRef]
- Perotti, M.; Petrini, R.; D’Orazio, M.; Ghezzi, L.; Giannecchini, R.; Vezzoni, S. Thallium and Other Potentially Toxic Elements in the Baccatoio Stream Catchment (Northern Tuscany, Italy) Receiving Drainages from Abandoned Mines. Mine Water Environ. 2018;37:1–11. [CrossRef]
- Campanella, B.; Onor, M.; D’Ulivo, A.; Giannecchini, R.; D’Orazio, M.; Petrini, R.; Bramanti, E. Human exposure to thallium through tap water: A study from Valdicastello Carducci and Pietrasanta (northern Tuscany, Italy) Sci. Total Environ. 2016;1:548–549. [CrossRef]
- 12 October.
- Campanella, B.; Colombaioni, L.; Benedetti, E.; Di Ciaula, A.; Ghezzi, L.; Onor, M.; D’Orazio, M.; Giannecchini, R.; Petrini, R.; Bramanti, E. Toxicity of Thallium at Low Doses: A Review. Int J Environ Res Public Health. 2019;16(23):4732. [CrossRef]
- Yorita Christensen, K.L. Metals in blood and urine, and thyroid function among adults in the United States 2007–2008. Int. J. Hyg. Environ. Health. 2013;216:624–632. [CrossRef]
- Padilla, M.A.; Elobeid, M.; Ruden, D.M.; Allison, D.B. An examination of the association of selected toxic metals with total and central obesity indices: NHANES 99-02. Int. J. Environ. Res. Public Health. 2010;7:3332–3347. [CrossRef]
- Li, Y.; Pan, Y.; Wang, K.; Ding, Y.; Li, Z.; Lu, M.; Xu, D. Association of urinary thallium with hypertension in children and adolescents aged 8-17 years: NHANES 2005-2018. Environ Sci Pollut Res Int. 2023;30(46):102927-102935. [CrossRef]
- Weaver, V.M.; Vargas, G.G.; Silbergeld, E.K.; Rothenberg, S.J.; Fadrowski, J.J.; Rubio-Andrade, M.; Parsons, P.J.; Steuerwald, A.J.; Navas-Acien, A.; Guallar, E. Impact of urine concentration adjustment method on associations between urine metals and estimated glomerular filtration rates (eGFR) in adolescents. Environ. Res. 2014;132:226–232. [CrossRef]
- Adams, J.B.; Audhya, T.; McDonough-Means, S.; Rubin, R.A.; Quig, D.; Geis, E.; Gehn, E.; Loresto, M.; Mitchell, J.; Atwood, S.; et al. Toxicological status of children with autism vs. neurotypical children and the association with autism severity. Biol. Trace Elem. Res. 2013;151:171–180. [CrossRef]
- Adams J., Howsmon D.P., Kruger U., Geis E., Gehn E., Fimbres V., Pollard E., Mitchell J., Ingram J., Hellmers R., et al. Significant association of urinary toxic metals and autism-related symptoms-A nonlinear statistical analysis with cross validation. PLoS ONE. 2017. [CrossRef]
- Chen, L.; Zhao, Y.; Liu, F.; Chen, H.; Tan, T.; Yao, P.; Tang, Y. Biological aging mediates the associations between urinary metals and osteoarthritis among U.S. adults. BMC Med. 2022;20(1):207. [CrossRef]
- Baeyens, W.; Vrijens, J.; Gao, Y.; Croes, K.; Schoeters, G.; Den Hond, E.; Sioen, I.; Bruckers, L.; Nawrot, T.; Nelen, V.; et al. Trace metals in blood and urine of newborn/mother pairs, adolescents and adults of the Flemish population (2007–2011) Int. J. Hyg. Environ. Health. 2014;217:878–890. [CrossRef]
- Xia, W.; Du, X.; Zheng, T.; Zhang, B.; Li, Y.; Bassig, B.A.; Zhou, A.; Wang, Y.; Xiong, C.; Li, Z.; et al. A case–control study of prenatal thallium exposure and low birth weight in China. Environ. Health Perspect. 2016;124:164–169. [CrossRef]
- Jiang, Y.; Xia, W.; Zhang, B.; Pan, X.; Liu, W.; Jin, S.; Huo, W.; et al. Predictors of thallium exposure and its relation with preterm birth. Environ Pollut. 2018;233:971-976. [CrossRef]
- Sánchez-Chapul, L.; Santamaría, A.; Aschner, M.; Ke, T.; Tinkov, A.A.; Túnez, I.; Osorio-Rico, L.; Galván-Arzate, S.; Rangel-López, E. Thallium-induced DNA damage, genetic, and epigenetic alterations. Front Genet. 2023;14:1168713. [CrossRef]
- Marjanović Čermak, A.M.; Mustać, S.; Cvjetko, P.; Pavičić, I.; Kifer, D.; Bešić, E.; Domijan, A.M. Thallium Toxicity and its Interference with Potassium Pathways Tested on Various Cell Lines. Biol Trace Elem Res. 2024;202(11):5025-5035. [CrossRef]
- Kemnic, T.R.; Coleman, M. Thallium Toxicity. 2023 Jul 10. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan–.
- Chou, Y.T.; Lo, K.Y. Thallium(I) treatment induces nucleolar stress to stop protein synthesis and cell growth. Sci. Rep. 2019;9:6905. [CrossRef]
- Bramanti, E.; Onor, M.; Colombaioni, L. Neurotoxicity Induced by Low Thallium Doses in Living Hippocampal Neurons: Evidence of Early Onset Mitochondrial Dysfunction and Correlation with Ethanol Production. ACS Chem. Neurosci. 2019;10:451–459. [CrossRef]
- Colombaioni, L.; Onor, M.; Benedetti, E.; Bramanti, E. Thallium stimulates ethanol production in immortalized hippocampal neurons. PLoS ONE. 2017;12:e0188351. [CrossRef]
- Jimenez, O.; Cáceres, H.; Gimenez, L.; Soto, L.; Montenegro, M.; Rueda, J.A.A. Thallium poisoning: a case report. J Yeungnam Med Sci. 2023;40(3):311-314. [CrossRef]
- Di Candia, D.; Muccino, E.; Battistini, A.; Boracchi, M.; Gentile, G.; Zoja, R. Thallium toxicity due to adultered infusion with thallium sulfate in eight members belonging to the same family nucleus: Autopsy findings and ICP-MS analysis (inductively coupled plasma mass spectrometry) in a triple homicide. Leg Med (Tokyo). 2020;42:101661. [CrossRef]
- Ratti, F.; Facchini, A.; Beck, E.; Cazzaniga, S.; Francesconi, S.; Tedesco, C.; Terrani, A.; Ciceri, G.; Colombo, R.; Saini, M.; Petrolini, V.M.; Citerio, G. Correction to: "Familial venoms": a thallium intoxication cluster. Intensive Care Med. 2018;44(12):2321-2322. Erratum for: Intensive Care Med. 2018;44(12):2298-2299. https://doi.org/10.1007/s00134-018-5403-6. [CrossRef]
- Rossetto, I.; Franconi, F.; Felthous, A.R.; Carabellese, F.; Rivellini, G.; Carabellese, F. An unusual case of serial poisoning of family members. J Forensic Sci. 2021;66(5):2060-2066. [CrossRef]
- Senthilkumaran, S.; Balamurugan, N.; Jena, N.N.; Menezes, R.G.; Thirumalaikolundusubramanian, P. Acute Alopecia: Evidence to Thallium Poisoning. Int J Trichology. 2017;9(1):30-32. [CrossRef]
- Hoffman, R.S. Thallium poisoning during pregnancy: a case report and comprehensive literature review. J Toxicol Clin Toxicol. 2000;38(7):767-75. [CrossRef]
- Liu, H.; Liao, G. Long-term misdiagnosis and neurologic outcomes of thallium poisoning: A case report and literature review. Brain Behav. 2021;11(3):e02032. [CrossRef]
- Zou, H.; Zou, S. Advanced thallium toxicity. Pract Neurol. 2023;23(1):85-87. [CrossRef]
- Misra, U.K.; Kalita, J.; Yadav, R.K.; Ranjan, P. Thallium poisoning: emphasis on early diagnosis and response to haemodialysis. Postgrad Med J. 2003;79(928):103-5. doi: 10.1136/pmj.79.928.103. Zhao, G.; Ding, M.; Zhang, B.; Lv, W.; Yin, H.; Zhang, L.; Ying, Z.; Zhang, Q. Clinical manifestations and management of acute thallium poisoning. Eur Neurol. 2008;60:292–297. doi: 10.1159/000157883.
- Tian, Y.R.; Sun, L.L.; Wang, W.; Du, F.; Song, A.X.; Ni, C.Y.; Zhu, Q.; Wan, Q. A case of acute thallotoxicosis successfully treated with double-filtration plasmapheresis. Clin Neuropharmacol. 2005;28(6):292-4. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).