1. Introduction
Postpartum infections persist as a significant challenge in obstetrics, even though advancements in hygiene standards and enhanced diagnosis and treatment tools have led to a decrease in maternal mortality from infections in developed countries[
1]. During parturition, the uterus can get contaminated with bacteria, leading to postpartum uterine infections such as pruritis, metritis, and endometritis[
2]. The incidence of uterine infections exhibits significant variation among different research. Uterine infection is characterized by the attachment of infectious microorganisms to the lining of the uterus, their growth and spread within the tissue, and the production of bacterial toxins, all of which contribute to the development of uterine disease[
3].
Previous research has demonstrated the recovery of bacteria from the uterine tissues of infected cows, mainly identified as Escherichia coli (E. coli), Staphylococcus aureus (S. aureus), and Trueperella pyogenes (T. pyogenes) [
2,
4].E. coli is the most prevalent gram-negative bacterium among all mentioned microorganisms, leading to uterus infection through LPS. It is also used to design many inflammatory models in animals and cells[
5,
6]
Potassium permanganate (KMnO
4) is a potent oxidizing agent with strong antiseptic properties. It has been traditionally used for uterine lavage in cows, especially in cases of postpartum infections like metritis and endometritis [
7]. While it is effective as a disinfectant, KMnO
4 is also known to cause tissue irritation and oxidative stress, leading to ulcerative injury and apoptosis [
8]. Despite these adverse effects, it remains in use in various regions due to its accessibility and antimicrobial properties [
9,
10]. However, the exact molecular damage caused by KMnO
4 in uterine tissues has not been fully explored, particularly in comparison to the well-established inflammatory responses induced by LPS.
RNA molecules longer than 200 nucleotides in length that do not code for proteins but play critical roles in gene expression at both transcriptional and post-transcriptional levels are called long noncoding RNAs (lncRNAs) [
11,
12]. Research has demonstrated that lncRNAs regulate numerous biological processes, including inflammation, oxidative stress, cell differentiation, apoptosis, and reproductive functions [
13] [
14]. In the female reproductive system, specific lncRNAs have been shown to modulate critical processes, such as folliculogenesis, spermatogenesis, and embryo implantation [
15,
16]. For example, NEAT1 has been linked to corpus luteum formation and fertility [
17], while lncRNA-H19 has been implicated in regulating endometriosis [
18,
19]. These findings highlight the crucial regulatory roles that lncRNAs play in uterine biology and disease.
Given that LPS and KMnO4 are both associated with oxidative stress and inflammatory responses in uterine tissues, understanding their transcriptional effects is essential. This study aims to compare the transcriptional profiles of rat uterine tissues exposed to KMnO4 and LPS, focusing on the expression of lncRNAs and mRNAs involved in oxidative stress, toxicity, and inflammation. For the first time, we hypothesize that KMnO4 induces molecular damage similar to that caused by LPS, with both agents activating overlapping pathways related to oxidative stress and inflammation. Additionally, the study seeks to identify potential interactions between lncRNAs and their target mRNAs, paving the way for future research into the regulatory roles of lncRNAs in uterine health.
2. Material and Methods
2.1. Animal Model
The Animal Experiment Centre of Huazhong Agricultural University (Wuhan, P.R. China) provided eighteen adult female Sprague Dawley (SD) rats weighing between 190 and 200g. The rats were housed under standard conditions with a 12-hour light/dark cycle, fed a standard diet, and provided with water ad libitum for an acclimatization period of 7 days. After acclimatization, the rats were randomly assigned to three groups (n=6 per group): a control group, a potassium permanganate (KMnO4) group, and a lipopolysaccharide (LPS) group. The KMnO4 used in the experiment was purchased locally, and a 0.05% solution was prepared by dissolving the powder in distilled water. For dosing, each rat's body weight was used to calculate the appropriate dose of KMnO4 and LPS. A dose of 10 mg/kg body weight was administered to the KMnO4 group. Given that the rats weighed approximately 200g, the dose per rat was calculated as follows:
Dose per rat = Body weight (kg) × Dose (mg/kg) = 0.2kg × 10mg/kg = 2mg
This 2mg of KMnO4 was diluted in distilled water and delivered as an intrauterine injection with a total volume of 20 µL. The LPS group received an intrauterine injection of LPS (50 µL of a 1 mg/mL solution), equivalent to 50 µg of LPS per rat. The control group received an intrauterine instillation of 50 µL of normal saline. Specimens from all groups were collected 48 hours after injection for histopathological analysis to confirm the induction of tissue damage and inflammation, validating the success of the experimental model.
2.2. Histopathology and Wet and Dry Weight (W/D)
Uterine tissue in the fixative solution was removed and rinsed with water for 30 min, dehydrated in a vacuum tissue dehydrator: 30%, 50% and 75% ethanol for 2h, 85%, 90% and 95% for 1.5h, Anhydrous ethanol was dehydrated twice for 1h each time, xylene was transparent twice for 20 min each, and molten paraffin was soaked twice for 1h each. Embedding, remove the uterine tissue from dehydration and put it into the embedding frame, pour in the molten paraffin, remove and trim the wax block after the wax solidifies, and slice it with a thickness of 5 μm. The slices are transparent, rehydrated, and washed with tap water. Hematoxylin & Eosin staining were observed under electron microscopy. In order to determine the wet and dry weight of rat uterine tissues, the tissues were surgically removed and then rinsed with PBS three times. Subsequently, they were placed in an incubator at a temperature of 80℃ for 24h. Following a 24h period, we determined the uterus tissues' wet-to-dry ratio (W/D).
2.3. Myeloperoxidase (MPO), Malondialdehyde (MDA), and Superoxide dismutase (SOD) Assays
Cut an appropriate amount of uterine tissue, weigh it, grind it under liquid nitrogen until crushed, and add isotonic sodium chloride solution according to mass: volume = 1:9 to obtain 10% tissue homogenate, centrifuge at 4°C, 13,000× g for 10 min, collect the supernatant, and measure the MPO, MDA content and activities of SOD (Nanjing Jiancheng Bioengineering Institute, China). The testing steps are strictly following the kit instructions.
2.4. RNA Extraction and cdna Library Construction
After retrieving the cryopreservation tube containing the uterine tissues, place it on ice for 5 min. Next, carefully excise approximately 30 mg of uterine tissue using surgical scissors. Subsequently, the excised tissue is placed into a centrifuge tube with a volume capacity of 2 mL. Add 1 mL of Trizol reagent to the tube containing the uterine tissue. The extraction of total RNA from the control, LPS and KMnO4-treated groups, with two samples per group, was performed using RNAiso Plus (Takara, Dalian, China) following the guidelines provided by the manufacturer. The utilization of total RNA serves as the initial material for the construction of a complementary DNA (cDNA) library. RNA purification, reverse transcription-based cDNA synthesis, adaptor ligation and end repair, PCR amplification, and library construction are the main processes in this procedure. The library is sequenced using the Illumina HiSeq 4000 platform, made in San Diego, California, by Illumina Inc., after quality inspection and preparation.
2.5. Quality Control Data of Sequencing
After the process of base recognition, the initial image file (BCL) received by sequencing was transformed into raw data in the FASTQ format. The initial dataset was evaluated comprehensively to determine its suitability for bioinformatics analysis. The primary components of the quality analysis encompassed the evaluation of sequencing quality and the investigation of base composition—the acquisition of clean data involved eliminating linker and low-quality sequences from the raw data. The evaluation of data cleanliness was subsequently conducted using FASTQC v0.10.1.
2.6. The Alignment of a Reference Genome and the Mapping of Read Distribution Across the Entire Genome
Clean readings were obtained using Trimmomatic (v3.0) after the data was filtered and ribosomal RNA was eliminated using Bowtie2 (v2.2.5). HISAT2 (v2.1.0) was used to align the paired-end clean reads to the reference genome, and Bowtie2 (v2.2.5) was used to create the reference genome index. Using a reference-based methodology, the Scripture (beta2) and Cufflinks (v2.1.1) software produced the assembled transcripts for every sample [
20]. Furthermore, each sample's aligned reads were created using a reference-based method with StringTie (v1.3.1) [
21].
2.7. Analyzing the Differential Expression
HTSeq (v0.11.2) was used for quantification of genes and transcripts. To find differential expressions in transcriptomic or genomic data, DESeq2 (v1.18.1) was utilized. The thresholds for identifying significant differential expression in abiotic replication are an absolute value of the logarithm base 2 of the fold change <1, and it has been determined that transcripts or genes with a P< 0.05 indicate differential expression in biological replication.
2.8. LncRNA-Gene Interaction Predictions
The mRNA and lncRNA transcripts were screened, followed by quantitative analysis using the StringTie-eB program. This analysis determined transcript abundance, namely the FPKM values, for each sample transcript. In the previous study, the authors employed Cuffdiff (version 2.1.1) to compute the Fragments Per Kilobase of transcript per Million mapped reads (FPKM) for both long non-coding RNAs (lncRNAs) and coding genes across all samples [
22]. Transcripts with a p-adjust value of less than 0.05 were classified as differentially expressed.
2.9. KEGG and GO-Based Functional Annotation and Enrichment Analysis
The GOseq R package conducted GO enrichment scores on differentially expressed genes and lncRNA target genes. Significantly enriched GO keywords were those with adjusted P values below 0.05 [
23]. The KEGG database is valuable for comprehending biological systems' underlying functions and applications. It offers insights into several aspects, including cells, animals, and ecosystems, by leveraging molecular-level data, particularly about large-scale molecules. The genome sequencing and other high-throughput experimental methods used to collect the dataset used in this investigation are described in the following source:
http://www.genome.jp/kegg/. Using the KOBAS software, the enrichment analysis of lncRNA target genes or differentially expressed genes was carried out. [
24].
2.10. RT-qPCR Validation
The RNA samples extracted from uterine tissues, including KMnO
4-treated, LPS-treated and untreated tissues, were analyzed using reverse transcription-quantitative polymerase chain reaction (RT-qPCR) for lncRNA and mRNA sequencing. Subsequently, complementary DNA (cDNA) was synthesized using a reverse transcriptase reagent following the guidelines provided in the instruction manual. The SYBR Green Plus Reagent Kit with the Light Cycler 96 equipment from Roche, Basel, Switzerland, was used to conduct a quantitative polymerase chain reaction (qPCR). The experiment adhered to the manufacturer's recommendations, using GAPDH as the internal reference gene. The primer sequences employed in the investigation are shown in
Table 1.
2.11. Statistical Analysis
This study will use statistical tools to analyze and interpret the acquired data. The statistical studies were conducted utilizing the GraphPad Prism 9 software. A one-way analysis of variance (ANOVA) was used to assess if there were any significant variations in the levels of long non-coding RNAs (lncRNAs) and messenger RNAs (mRNAs) among the groups that were treated with LPS and KMnO4 as well as the control groups. Statistically significant differences were seen when the p-values were below the thresholds of 0.05 and 0.01, respectively.
4. Discussion
Uterine bacterial infections are frequently observed in multiparous cows, causing notable uterine destruction, decreased productivity, and lowered fertility One of the key pathogens in these infections is the endotoxin Escherichia coli (E. coli), which is also linked to inflammatory disorders such as endometritis and acute lung injury [
6,
27]. Although inflammation is a protective response aimed at fighting infections, excessive or prolonged inflammation can lead to tissue damage, organ dysfunction, and even life-threatening conditions [
28,
29]. Clinically, antibiotics are the primary treatment for uterine infections, but their overuse comes with significant drawbacks, including negative effects on reproductive health and suppression of immunity in dairy cows [
30]. Additionally, the global rise of antibiotic resistance has become a critical concern [
31,
32].
An alternative treatment, KMnO
4, has long been used due to its strong oxidizing properties that make it effective in treating bacterial and fungal infections [
33]. However, while KMnO4 is useful in wound disinfection, its oxidizing potential can also cause significant damage to healthy tissues. In cattle, excessive KMnO
4 exposure has been associated with ulcers and other tissue injuries. This dual-edged nature of KMnO4 underscores the need to understand its biological effects more thoroughly, especially in sensitive tissues like the uterus.
In this study, we utilized a rat model to compare the effects of KMnO
4 and LPS on uterine tissues, focusing on inflammation and oxidative stress at the molecular level. LPS is a well-established inducer of inflammation and oxidative damage, commonly used as a model for reproductive disorders [
34]. We sought to investigate the transcriptional changes, specifically in lncRNAs, which play a crucial role in gene regulation and could potentially serve as therapeutic targets.
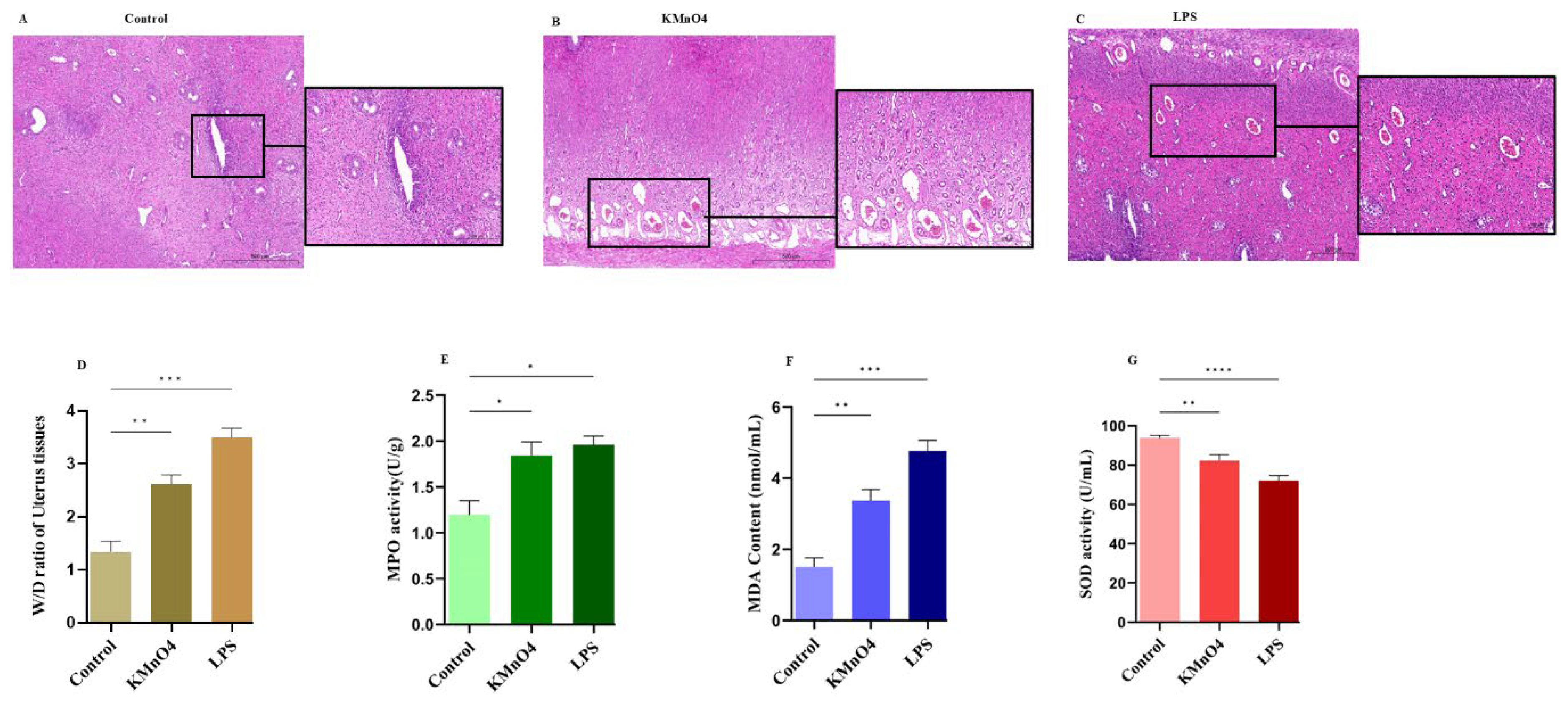
Histopathology was employed in the present study to ascertain the presence of oxidative stress inside the uterine tissues of rats. Necrosis, edema, and leukocyte infiltration were observed through direct observation. Furthermore, the results of wet and dry weight of uterus tissues also show obvious change. Moreover, biochemical assays measuring MDA, SOD, and MPO further confirm the successful establishment of the animal model.
Following the extraction of total uterine RNA, high-throughput sequencing was conducted on the Illumina platform to elucidate the profiles of long non-coding RNA (lncRNA) and gene expression in KMnO4 treated, comparing LPS group in rat uterine tissues.
LncRNAs are a class of RNA molecules with therapeutic capabilities due to their ability to induce alterations in DNA transcription through methylation and acetylation processes [
35]. This work examined the mRNA and lncRNA expression in tissues subjected to oxidative stress. A total of 1649 differentially expressed messenger RNAs (mRNAs) in KMnO
4 treated,1383 in LPS treated group were identified by screening and 1125 in KMnO
4 treated, 989 in LPS treated groups expressed lncRNAs. The function enrichment analysis revealed that a considerable proportion of the genes that exhibited differential expression were implicated in several biological processes, including reactive oxygen species, signal transduction involved in gene expression regulation, Wnt signaling pathway, regulation of macrophage activation, and various biochemical metabolic processes. The enrichment analysis of the KEGG pathway revealed that the differentially expressed genes primarily engaged in signal pathways associated with the MAPK signaling pathway, cGMP-PKG pathway, Wnt pathway, Necroptosis, and cAMP signaling pathways. Besides genes exhibiting variable expression, most lncRNAs had an ambiguous functional role. The hypothesis in this study posited that lncRNA has a regulatory role in gene expression, as mentioned earlier. Consequently, the function of lncRNA was predicted by considering its associated coding genes, as indicated by previous research [
36].
This study estimated target genes of differentially expressed lncRNA using cis and trans methods. Subsequently, GO and KEGG studies were conducted on the identified target genes to explore the potential functional role of the lncRNA[
37,
38]. Through the utilization of enrichment analysis of GO and KEGG pathways, as well as coding-noncoding co-expression network analysis, we have determined that the differentially expressed target genes primarily exhibit associations with Inflammation and apoptotic signaling pathways.
The lncRNAs TCONS_00030719, TCOS_00078464 and TCONS_00082446 exhibited differential expressions in the Control vs KMnO
4 group. They were enriched in various signaling pathways. The signaling pathways strongly correlate with oxidative damage and Inflammation. The identified gene Slc7a7 targets TCONS_00030719, TCONS_00030709, TCONS_00030721, TCONS_00030713. The gene Slc7a7 was found to be implicated in the regulation of lifespan, as well as in the cAMP signaling route and calcium signaling pathway, according to the KEGG pathway enrichment analysis. Prior research has established a strong association between the Slc7a7 gene and human longevity and hypertension. This gene plays a crucial role in regulating the immune response by activating transcription factors, which influence gene expression in immune cells [
39,
40].
Furthermore, in Control vs LPS, the target lncRNAs predicted for LGR6 include TCONS_00024057,TCONS_00024055,TCONS_00024054,TCONS_00024052,TCONS_00024053,TCONS_00024056. GPRs that are broadly expressed in a variety of cells, including neutrophils, monocytes, cochlear hair cells, and astrocytes, also contain different types such as leucine-rich repeat-containing G protein-coupled receptor 6 (LGR6)[
41,
42,
43]. According to the literature, it has been documented that LGR6 can modulate many inflammatory pathways and contribute to generating reactive oxygen species in the context of oxidative stress [
44,
45]. According to recent research, in a rat model of subarachnoid hemorrhage, Maresin 1 activates LGR6 to reduce neuroinflammation via the CREB/JMJD3/IRF4 pathway[
46]. Maresin 1 reduces diabetic kidney disease by activating the cAMP-SOD2-ROS pathway mediated by LGR6 in another study[
45].
In summary, this study provides new insights into the molecular mechanisms underlying KMnO4 and LPS-induced uterine damage, particularly the role of lncRNAs in regulating inflammation and oxidative stress. Our findings reveal that lncRNAs, through their interactions with key genes like Slc7a7 and LGR6, may act as important regulators of the immune response in reproductive tissues. Understanding these regulatory networks could pave the way for the development of targeted molecular therapies to treat uterine.
Figure 1.
Histopathological analysis of uterine tissues. (A-C) Histopathological changes demonstrated by H&E staining: (A) Control group, (B) KMnO4 group, and (C) LPS group. (D) Wet-to-dry ratio of uterine tissues in different groups. (E) Myeloperoxidase (MPO) assay results. (F) Malondialdehyde (MDA) activity. (G) Superoxide dismutase (SOD) activities.
Figure 1.
Histopathological analysis of uterine tissues. (A-C) Histopathological changes demonstrated by H&E staining: (A) Control group, (B) KMnO4 group, and (C) LPS group. (D) Wet-to-dry ratio of uterine tissues in different groups. (E) Myeloperoxidase (MPO) assay results. (F) Malondialdehyde (MDA) activity. (G) Superoxide dismutase (SOD) activities.
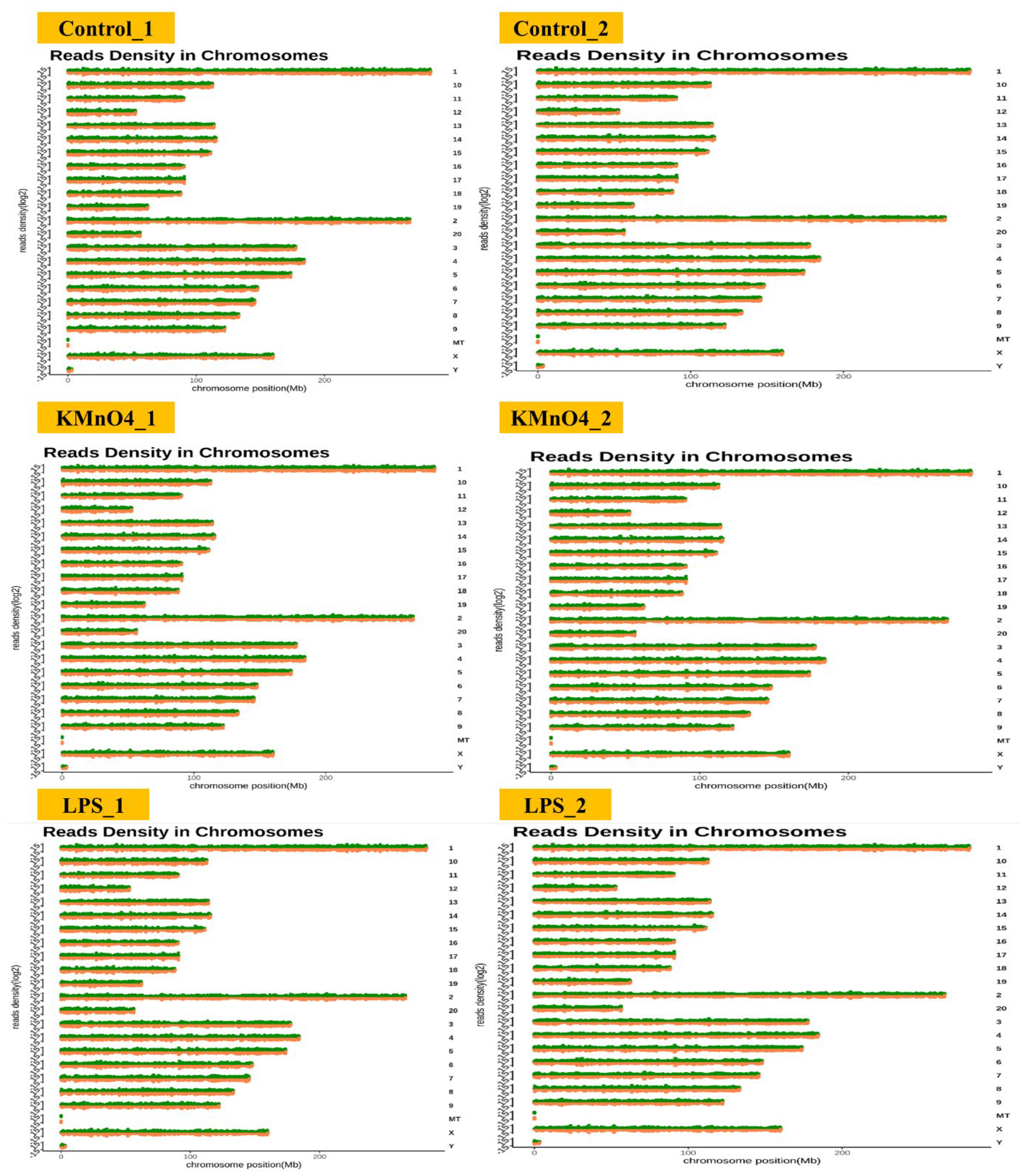
Figure 2.
Chromosomal Distribution of Differentially Expressed Transcripts. This figure represents the chromosomal distribution of differentially expressed transcripts identified across all experimental groups.
Figure 2.
Chromosomal Distribution of Differentially Expressed Transcripts. This figure represents the chromosomal distribution of differentially expressed transcripts identified across all experimental groups.
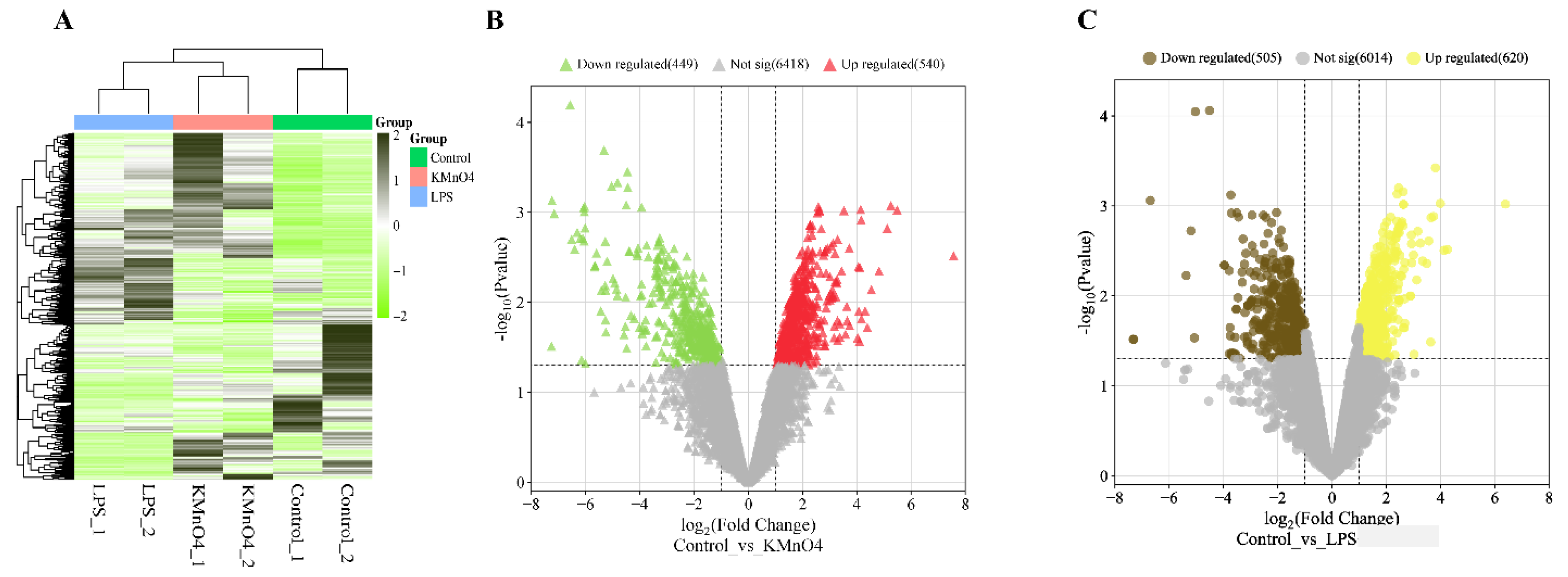
Figure 3.
Analysis of Differentially Expressed Genes in KMnO4-Treated, LPS-Treated, and Normal Uterine Tissues. (A) Heatmap displaying hierarchical clustering of differentially expressed genes across the experimental groups based on FPKM values. Log10(FPKM+1) transformation is applied for clustering. Genes with higher expression levels are represented in brown, while genes with lower expression are shown in yellow, indicating distinct expression profiles among the groups. (B) Volcano Plot of Differentially Expressed Genes in Control vs. KMnO4 Group illustrating the distribution of differentially expressed genes between the control and KMnO4-treated groups. Genes with significantly higher expression in the KMnO4 group are shown in brown, and genes with lower expression are shown in yellow. (C) Volcano Plot of Differentially Expressed Genes in LPS-Treated Group representing the differentially expressed genes in the LPS-treated group compared to controls. Red dots indicate genes with significantly higher expression, while blue dots represent genes with lower expression, emphasizing the transcriptional shifts induced by LPS treatment.
Figure 3.
Analysis of Differentially Expressed Genes in KMnO4-Treated, LPS-Treated, and Normal Uterine Tissues. (A) Heatmap displaying hierarchical clustering of differentially expressed genes across the experimental groups based on FPKM values. Log10(FPKM+1) transformation is applied for clustering. Genes with higher expression levels are represented in brown, while genes with lower expression are shown in yellow, indicating distinct expression profiles among the groups. (B) Volcano Plot of Differentially Expressed Genes in Control vs. KMnO4 Group illustrating the distribution of differentially expressed genes between the control and KMnO4-treated groups. Genes with significantly higher expression in the KMnO4 group are shown in brown, and genes with lower expression are shown in yellow. (C) Volcano Plot of Differentially Expressed Genes in LPS-Treated Group representing the differentially expressed genes in the LPS-treated group compared to controls. Red dots indicate genes with significantly higher expression, while blue dots represent genes with lower expression, emphasizing the transcriptional shifts induced by LPS treatment.
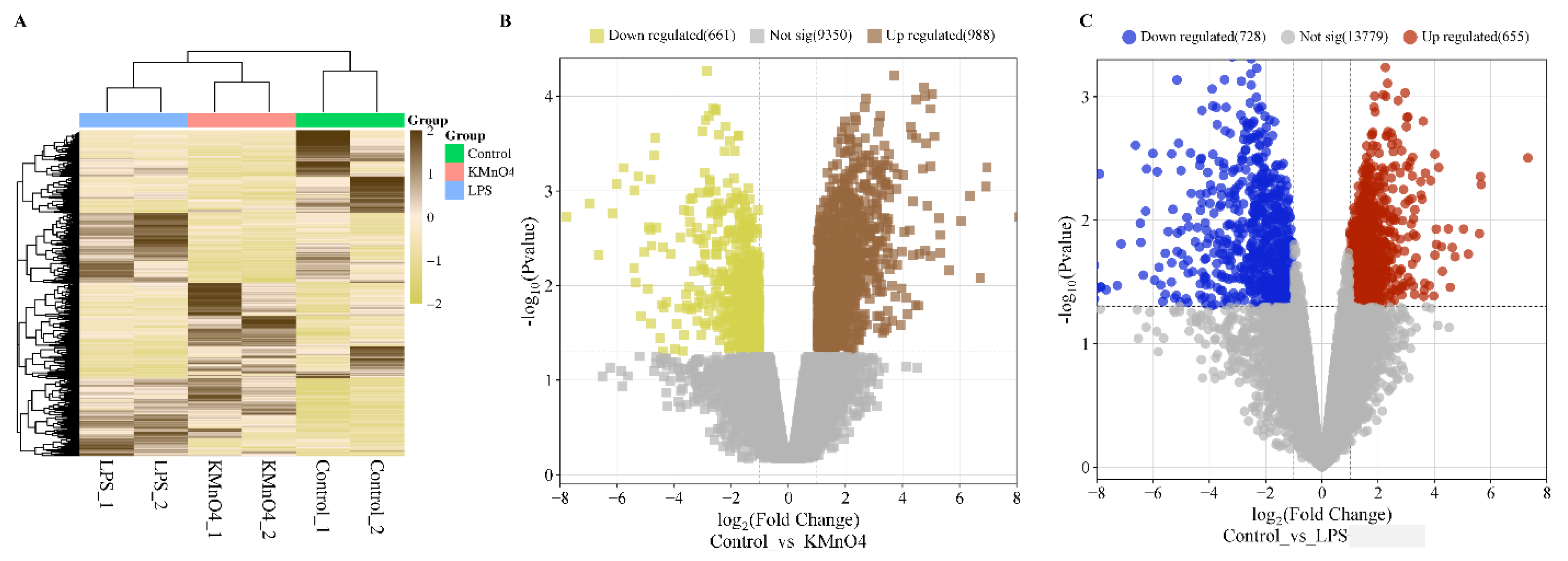
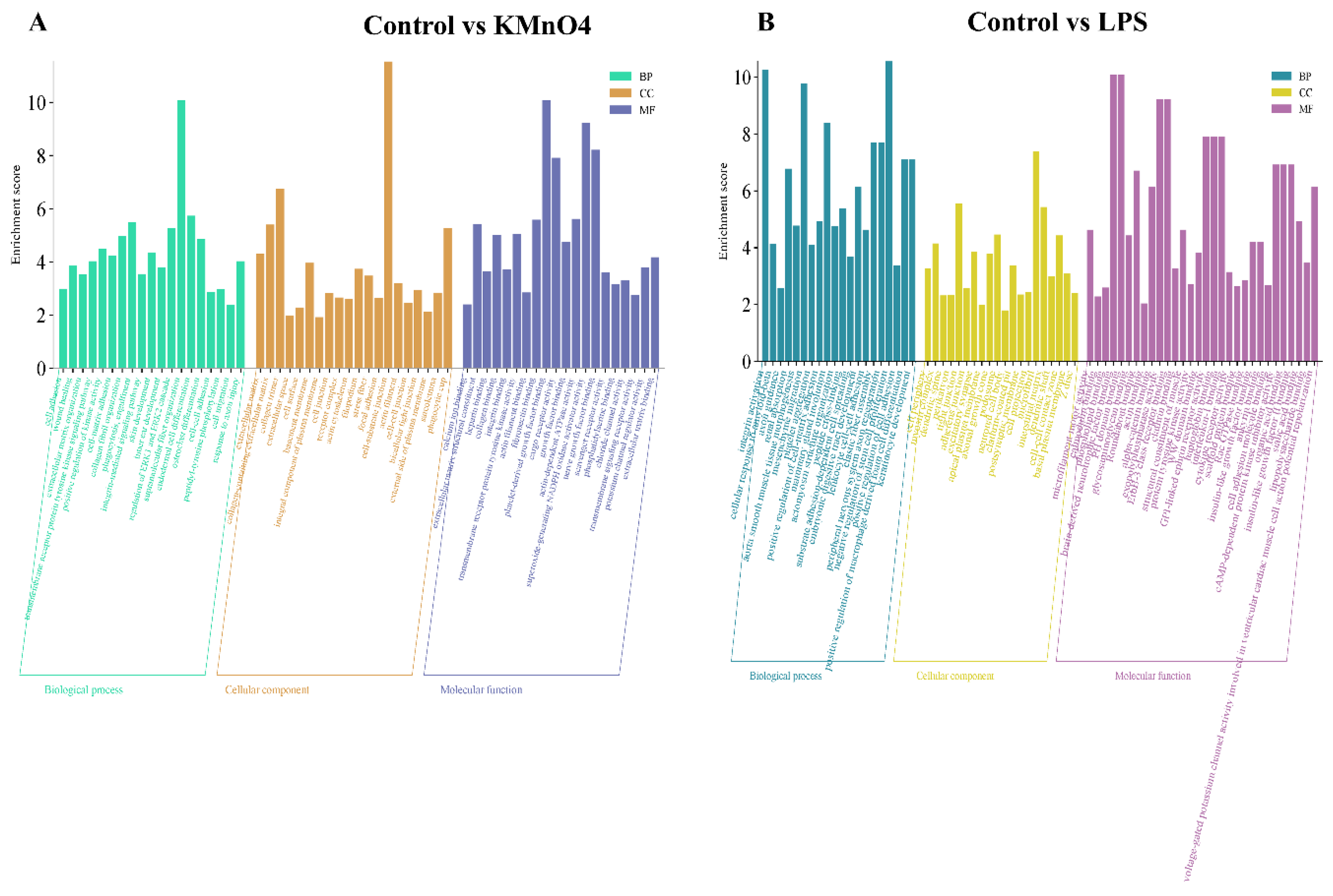
Figure 4.
GO Functional Analysis of Differentially Expressed mRNA Enrichment. (A) GO Enrichment Analysis of Control vs KMnO4 Group for differentially expressed. The analysis highlights significantly enriched biological processes, cellular components, and molecular functions, indicating the primary functional categories impacted by KMnO4 exposure. (B) GO enrichment analysis for differentially expressed mRNAs between the control and LPS-treated groups. The enriched GO terms reflect the key biological processes, cellular components, and molecular functions modulated by LPS treatment.
Figure 4.
GO Functional Analysis of Differentially Expressed mRNA Enrichment. (A) GO Enrichment Analysis of Control vs KMnO4 Group for differentially expressed. The analysis highlights significantly enriched biological processes, cellular components, and molecular functions, indicating the primary functional categories impacted by KMnO4 exposure. (B) GO enrichment analysis for differentially expressed mRNAs between the control and LPS-treated groups. The enriched GO terms reflect the key biological processes, cellular components, and molecular functions modulated by LPS treatment.
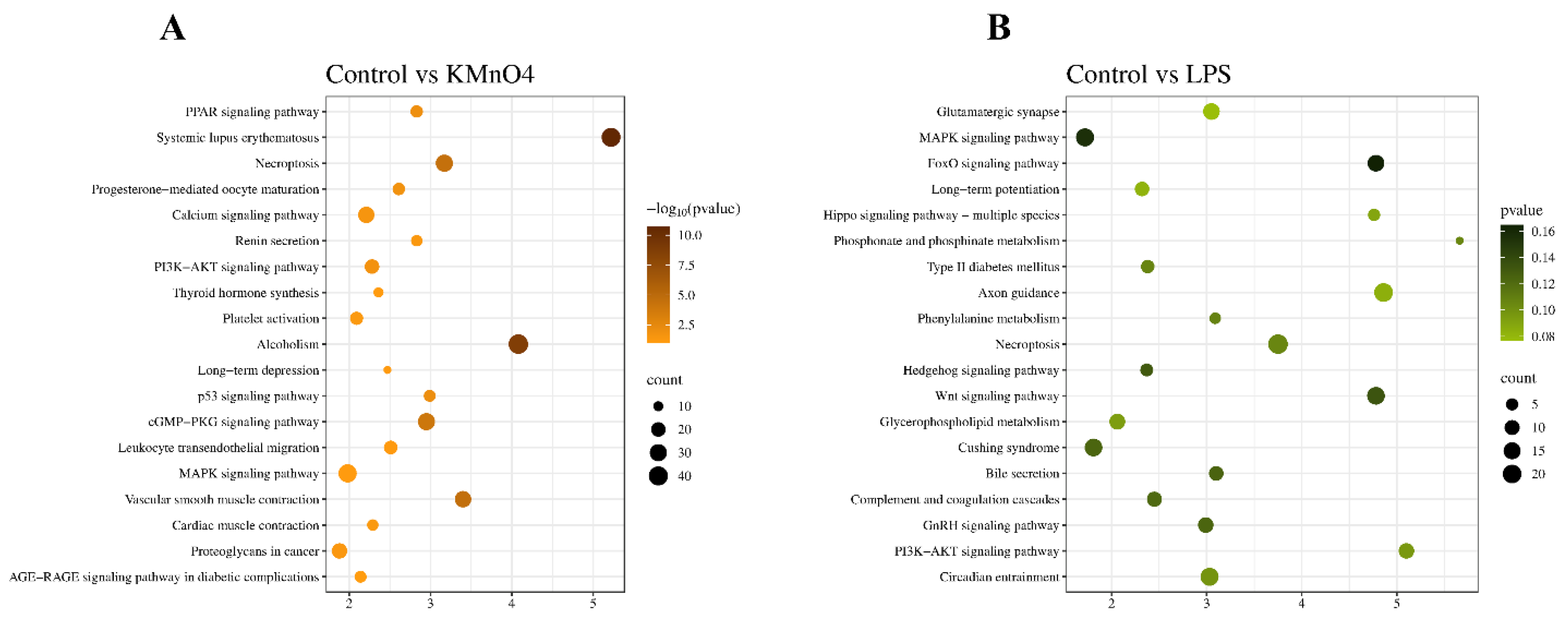
Figure 5.
KEGG Signaling Pathway Enrichment Analysis of Differentially Expressed mRNA. (A) Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis of differentially expressed mRNAs between the control and KMnO4-treated groups. The pathways enriched in the KMnO4 group reveal the signaling cascades and biological systems affected by KMnO4-induced oxidative stress and toxicity. (B) KEGG pathway enrichment analysis of differentially expressed mRNAs between the control and LPS-treated groups. This analysis uncovers the signaling pathways activated by LPS treatment, providing insight into the inflammatory and immune responses triggered in the uterine tissue.
Figure 5.
KEGG Signaling Pathway Enrichment Analysis of Differentially Expressed mRNA. (A) Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis of differentially expressed mRNAs between the control and KMnO4-treated groups. The pathways enriched in the KMnO4 group reveal the signaling cascades and biological systems affected by KMnO4-induced oxidative stress and toxicity. (B) KEGG pathway enrichment analysis of differentially expressed mRNAs between the control and LPS-treated groups. This analysis uncovers the signaling pathways activated by LPS treatment, providing insight into the inflammatory and immune responses triggered in the uterine tissue.
Figure 6.
mRNAs and lncRNAs with differential expressions. (A) LncRNAs with differential expressions grouped together. Based on FPKMs, hierarchical clustering clusters using log10(FPKM+1). Genes with higher expressions are indicated by dark green, and those with lower expressions are indicated light green. (B) A volcano plot of transcripts with differential expressions of mRNA. Genes with higher expressions indicated Red, with lower expressions indicated green. (C) A volcano plot of transcripts with differential expressions of lncRNA. Genes with higher expressions are indicated by yellow, and those with lower expressions are indicated by brown.
Figure 6.
mRNAs and lncRNAs with differential expressions. (A) LncRNAs with differential expressions grouped together. Based on FPKMs, hierarchical clustering clusters using log10(FPKM+1). Genes with higher expressions are indicated by dark green, and those with lower expressions are indicated light green. (B) A volcano plot of transcripts with differential expressions of mRNA. Genes with higher expressions indicated Red, with lower expressions indicated green. (C) A volcano plot of transcripts with differential expressions of lncRNA. Genes with higher expressions are indicated by yellow, and those with lower expressions are indicated by brown.
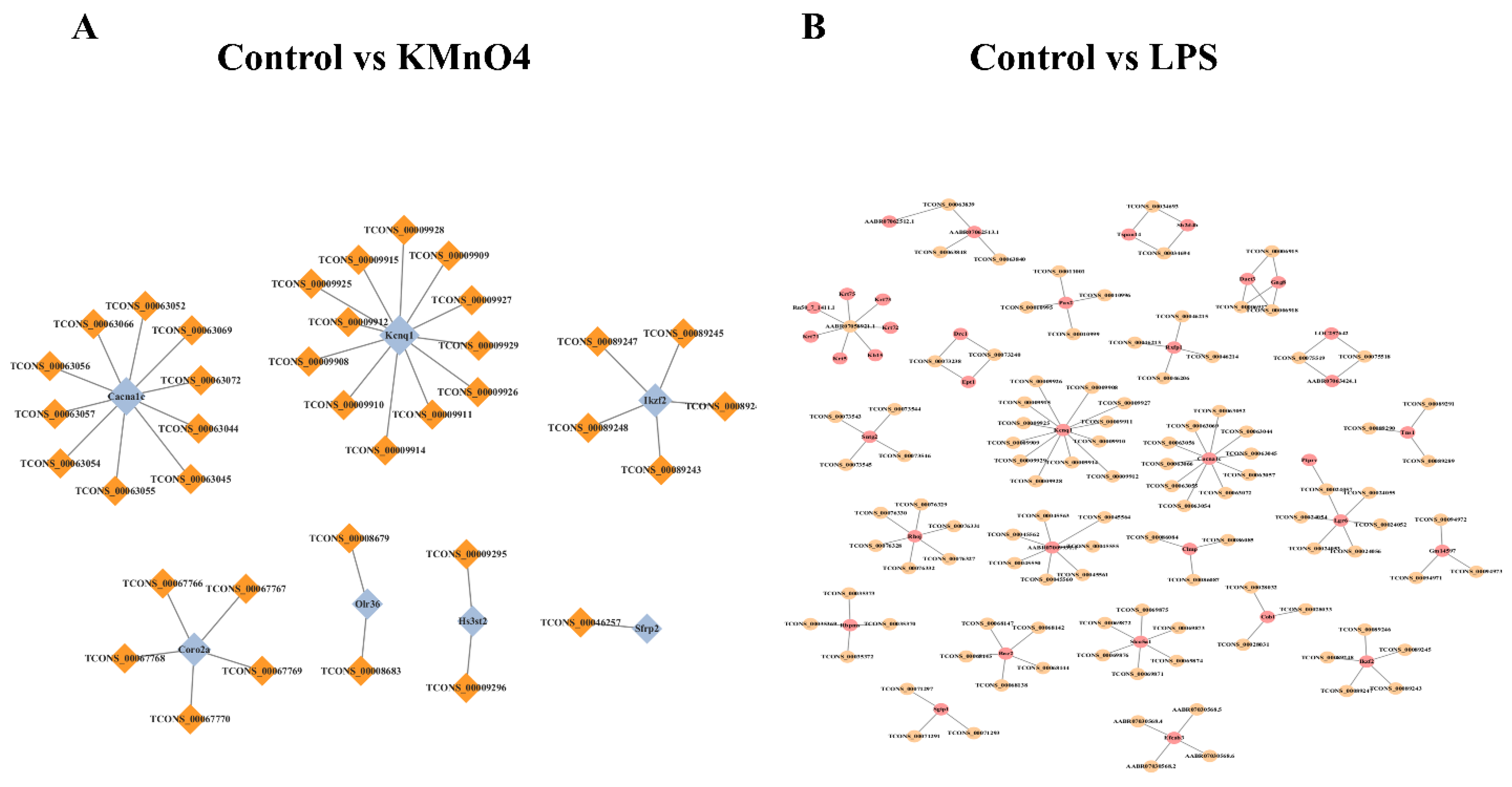
Figure 7.
Prediction and Functional Analysis of lncRNA Target Genes and mRNAs. (A & B) The network diagrams illustrate the interactions between predicted lncRNAs and their target mRNAs in the control group compared to the LPS-treated group (A) and the KMnO4-treated group (B). The visualization highlights key regulatory relationships between lncRNAs and mRNAs, emphasizing the differential gene regulation under treatment conditions.
Figure 7.
Prediction and Functional Analysis of lncRNA Target Genes and mRNAs. (A & B) The network diagrams illustrate the interactions between predicted lncRNAs and their target mRNAs in the control group compared to the LPS-treated group (A) and the KMnO4-treated group (B). The visualization highlights key regulatory relationships between lncRNAs and mRNAs, emphasizing the differential gene regulation under treatment conditions.
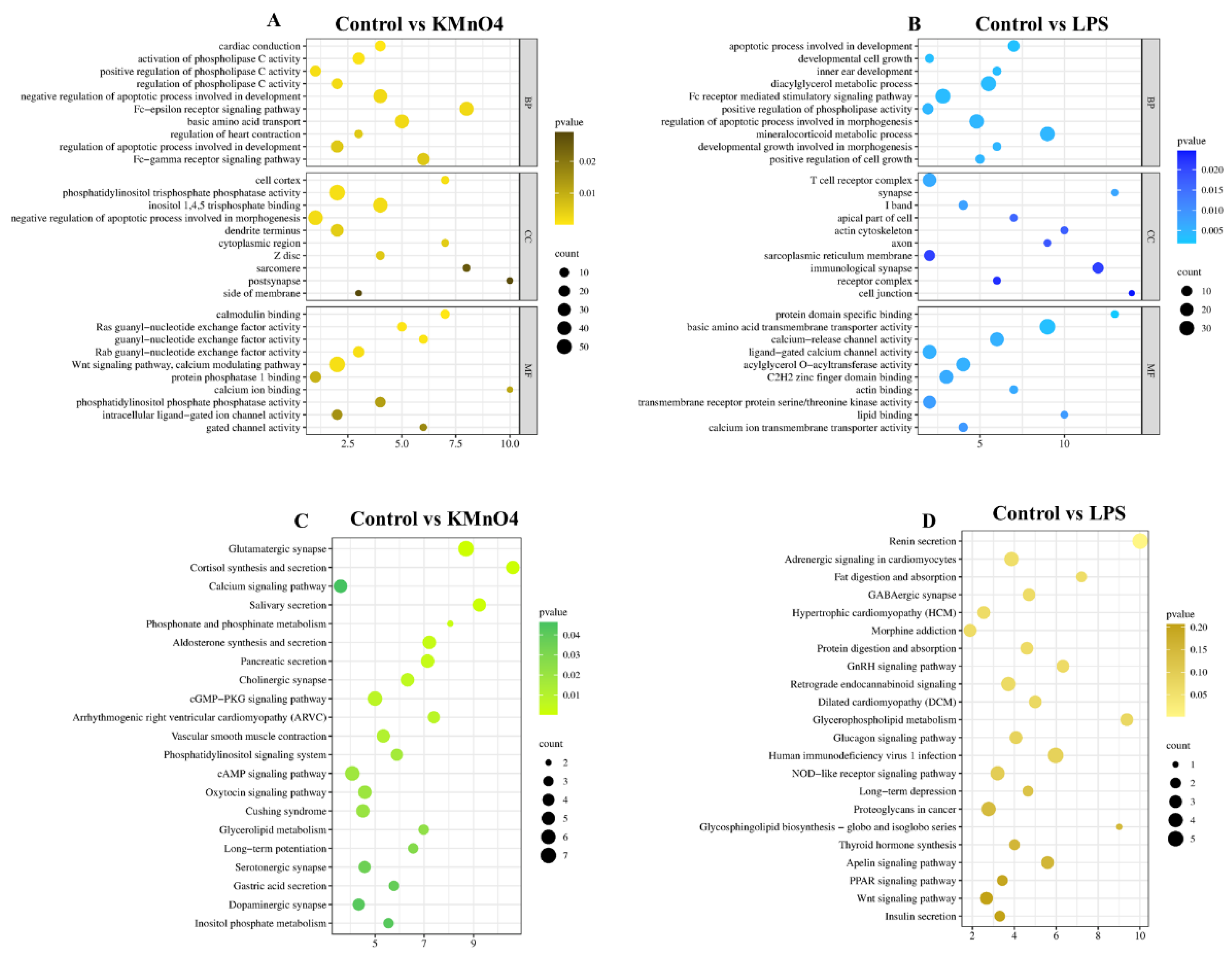
Figure 8.
Analysis of lncRNA Target Genes via GO and KEGG Enrichment. (A & B) These panels present the GO functional enrichment analyses of lncRNA target genes. The comparisons of control vs. KMnO4 (A) and control vs. LPS (B) reveal biological processes, cellular components, and molecular functions.(C & D) The bubble bar plots depict the most enriched KEGG pathways in the comparison of control vs. KMnO4 (C) and control vs. LPS (D). Bubble size represents the gene count, while color intensity indicates the significance of enrichment.
Figure 8.
Analysis of lncRNA Target Genes via GO and KEGG Enrichment. (A & B) These panels present the GO functional enrichment analyses of lncRNA target genes. The comparisons of control vs. KMnO4 (A) and control vs. LPS (B) reveal biological processes, cellular components, and molecular functions.(C & D) The bubble bar plots depict the most enriched KEGG pathways in the comparison of control vs. KMnO4 (C) and control vs. LPS (D). Bubble size represents the gene count, while color intensity indicates the significance of enrichment.
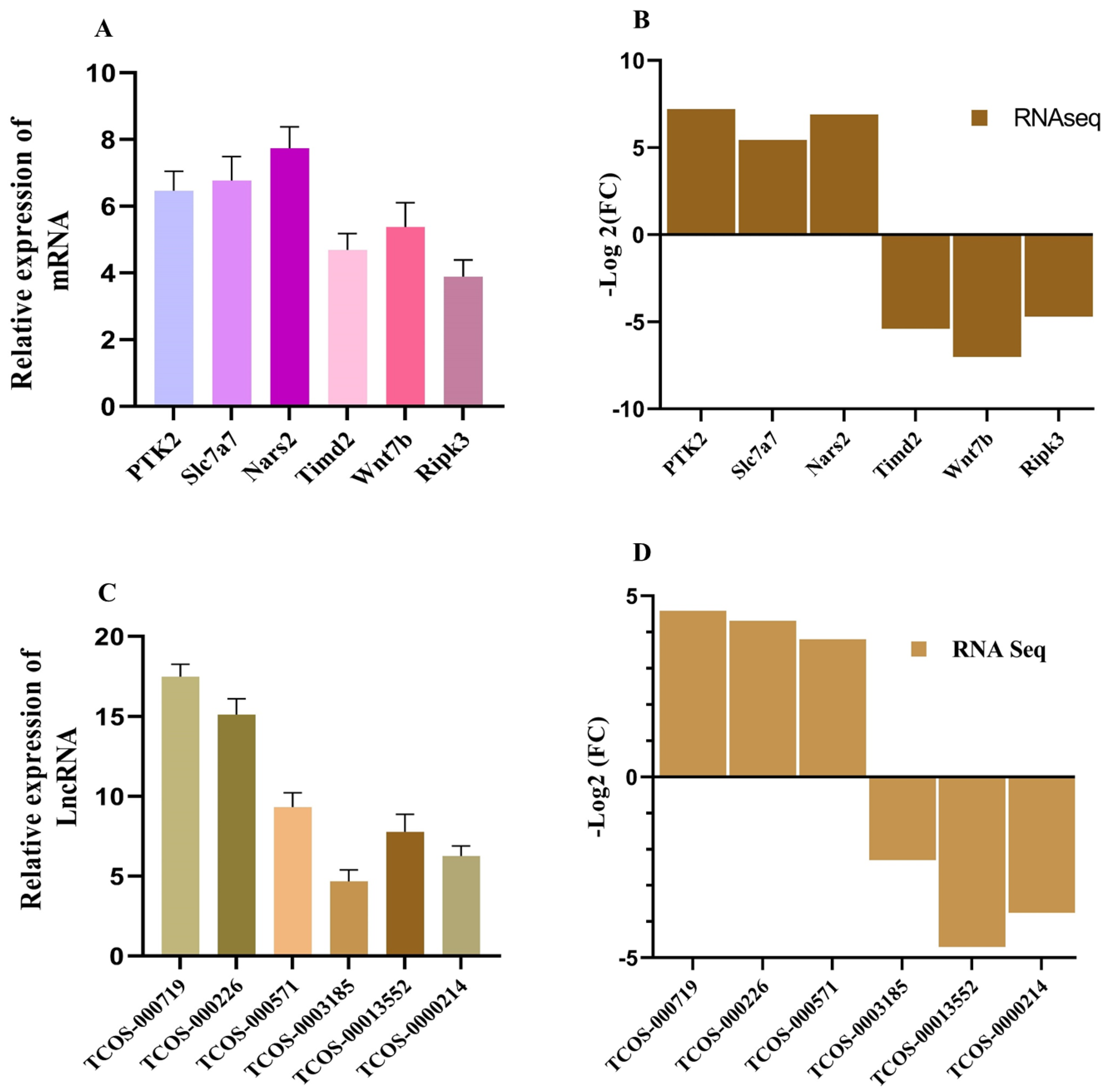
Figure 9.
RT-qPCR Validation of RNA-Seq Results. (A & B) Bar plots comparing the expression levels of up-regulated (A) and down-regulated (B) mRNAs, as determined by RNA-Seq and validated through RT-qPCR. The consistent trends between qPCR and RNA-Seq results confirm the reliability of the transcriptomic data. (C & D) Bar plots showing the differential expression of selected lncRNAs, comparing RNA-Seq data with RT-qPCR validation. Up-regulated (C) and down-regulated (D) lncRNAs exhibit consistent expression patterns across both methods.
Figure 9.
RT-qPCR Validation of RNA-Seq Results. (A & B) Bar plots comparing the expression levels of up-regulated (A) and down-regulated (B) mRNAs, as determined by RNA-Seq and validated through RT-qPCR. The consistent trends between qPCR and RNA-Seq results confirm the reliability of the transcriptomic data. (C & D) Bar plots showing the differential expression of selected lncRNAs, comparing RNA-Seq data with RT-qPCR validation. Up-regulated (C) and down-regulated (D) lncRNAs exhibit consistent expression patterns across both methods.
Table 1.
qPCR Primers for LncRNAs and mRNAs.
Table 1.
qPCR Primers for LncRNAs and mRNAs.
| Name |
Product Size (bp) |
Primer Sequence(5’-3’) |
| PTK2 |
246 |
Forward: CGTGTGGATGTTTGGTGTGT
Reverse: TGCACCTTCTCTTCCTCCAG |
| Slc7a7 |
190 |
Forward: CCTGTTCTTCCCCATCGTCT
Reverse: TGTGGTAGACGCTACGATCC |
| Nars2 |
235 |
Forward: GTGGATCCGGTCAGTCAGAT
Reverse: AAATGCCTTGGCTTCACAGG |
| Timd2 |
223 |
Forward: ACTGAAGCAATCCCTCCACA
Reverse: CTTCAATTCTGGCCTGCTCC |
| Wnt7b |
161 |
Forward: TTCACAACAATGAGGCAGGC
Reverse: AGCTGCGTTGTACTTCTCCT |
| RIPK3 |
182 |
Forward: TCCACATTTCAGGGAGGGTC
Reverse: ACCACCTCAGCTTCTCTTCC |
| TCOS_000719 |
216 |
Forward: AACAAGGAACAAGGCCACAC
Reverse: GGCTTTCCCAGGTCTTAGGT |
| TCOS_000226 |
234 |
Forward: CTGTCTGAAGGGCACATGTG
Reverse: TCCAGGATCTCAGCCACAAA |
| TCOS_000571 |
197 |
Forward: GCTGGATGTTGAGAGCACAG
Reverse: TTCTAGCTCCCCACTCCTCT |
| TCOS_0003185 |
250 |
Forward: ACTGTGTGTGCTGGGTATGA
Reverse: TGGCAAGTTTCGACTGTGTG |
| TCOS_13552 |
207 |
Forward: ACAACACTACCAGGGGACAG
Reverse: GCAGAGGCCCATAGTCTAGG |
| TCOS_000214 |
201 |
Forward: GTTCCCAAGGCTTGACCCAA
Reverse: AAGAATTGCCTGGTGTGTCCT |
Table 2.
Summary of Reads.
Table 2.
Summary of Reads.
| Sample Name |
Clean Reads |
Clean GC (%) |
Mapped Reads(%) |
Effective rate(%) |
| Control_1 |
57300644 |
50.09 |
93.54 |
87.53 |
| Control_2 |
51742860 |
50.69 |
91.38 |
85.51 |
| KMnO4_1 |
57128986 |
49.36 |
94.43 |
88.36 |
| KMnO4_2 |
51422446 |
50.56 |
93.09 |
88.63 |
| LPS |
65824496 |
49.65 |
92.68 |
85.74 |
| LPS |
78704690 |
49.41 |
92.91 |
87.48 |
Table 3.
KEGG pathways and their Genes.
Table 3.
KEGG pathways and their Genes.
| KEGG Pathways |
Enrichment |
Genes |
| MAPK signaling pathway |
1.98 |
Rac3;Cacnla;Cacnalc;Mef2c;Dusp8 |
| cGMP-PKG signaling pathway |
2.95 |
Wos3;Kcnj8;Pde3a;Mylk;Kcnmal;Mef2c;Pde5a;
Kcnmb1 |
| Necroptosis |
3.17 |
RIPK1;RIPK3;MLKL;H2afx |
| FOXO signalling pathway |
4.78 |
Cdkn2b;Tgfb2;Pck1;Fbxo32;PlKI;Ccdn2;Ccnb1;Irs2;Slc2a4 |
| GnRH pathway |
2.99 |
Plcb1;Cacnalc;Plcb4;Adcy3;Calml3;Camk2g;Adcy9 |
| Wnt pathway |
4 |
SfrD2;Plcbl;Rspo3;Camk2g;Nfatc4;Rac3;Sfrp4;Ccdn2;Plcb4 |
| PI3K-AKT signaling pathway |
5.1 |
PI3K;PTEN;AKT;FOXO;CytokineR |
| P53 signaling pathway |
2.99 |
CHK2;P53;Fas;Caspase8 |
| TNF signaling pathway |
2.21 |
TNFR1;TRADD;TRAF;TAK1;TAB1/2;MKK;IL18R |
| JAK-STAT signaling pathway |
2.38 |
JAK;STAT;IL13ra;IL6r;IL11r;IL4r; |