1. Introduction
Consumers increasingly demand highly effective cosmetic products. Aging-related skin concerns further necessitate the regular use of dermocosmetics tailored to specific skin needs. Moreover, recent advancements in cosmetic science have highlighted the importance of incorporating novel, sustainable ingredients derived from natural sources. Our research group has identified several such compounds from natural origins with potential applications in dermocosmetic formulations [
1,
2,
3]. The diversity of microalgae offers opportunities to obtain several kinds of compounds with high potential for application in dermocosmetics. For instance, pigments were well-described due to their antioxidant potential [
4,
5,
6]. Furthermore, lipophilic and hydrophilic compounds with high value-added, such as structural polysaccharides (PS) extracted from microalgae biomass and extracellular polymeric substances, have revealed practical applications due to the rheological properties, as well as to many biological activities, for instance, protecting cells from oxidative damage [
7,
8,
9].
A few species from the
Ankistrodesmus genus (Chlorophyceae) were successfully cultivated for biodiesel production due to the lipid content [
10,
11,
12]. However, it should be noted that some nutrient and environmental conditions, such as pH, CO
2 level, temperature, and light intensity, are the main factors behind changes in biomass composition during photosynthesis. For instance, carbohydrates and lipids are the central competing pathways for the biosynthesis of storage products in microalgae species. Thus, carbohydrates are an important raw material in fermentative biofuel production [
13]. Additionally, phototrophic, mixotrophic, and heterotrophic were tested as mode cultivations and monoculture or mix-culture of
Ankistrodesmus sp. with
Chlorella sp. [
14]. Moreover, the presence of extracellular polymeric substances from
Ankistrodesmus falcatus var.
acicularis [
9].
A. braunii has been successfully cultivated in tubular photobioreactors [
15], and has been probed to produce carbohydrates for dermocosmetic applications [
16]. This species of microalgae seems to be a proper candidate to extract compounds to be incorporated into innovative formulations to enhance cosmetic attributes. Thus, we aimed to cultivate
A. braunii to extract polysaccharides to be incorporated into a dermocosmetic prototype. In vitro assay of the antioxidant activity was conducted, and the in vivo cutaneous biocompatibility was performed to investigate the safety of a prototype gel containing the
A. braunii polysaccharides.
2. Materials and Methods
2.1. A. braunii Cultivation, Biomass Composition, and Polysaccharide Extraction
Ankistrodesmus braunii UTEX 245 sourced from the University of Texas Culture Collection (UTEX), was maintained at 25±1°C in agar medium. A small aliquot of this culture was used to inoculate 10 units of 10 mL glass tubes, each containing 5 mL Bold medium [
17] securely sealed. Fluorescent lamps provided light at the intensity of 60 µmol photons m
-2.s
-1. A rotatory shaker (Multitron, Inforts Ht, Switzerland), at 25±1°C with the inoculum tubes, was maintained at 110 rpm for 25 days. Subsequently, a portion of this biomass was exposed for 30 minutes to artificial UVC radiation in Petri dishes. Thus, 10% of these two different biomasses were used to inoculate
A. braunii in 500 mL Erlenmeyer flasks containing approximately 200 mL Bold medium at the same conditions in the batch mode. An airlift system was employed to ensure circulation of the culture, maintained by an air pump with a flow rate of 40±1 L.h
-1. The culture pH was maintained at 7.0±0.2 and continuous light intensity was regulated at 60 µmol photons m
-2.s
-1. Cultivation temperature was fixed at 25±1°C. Batch mode with 20 mM NaNO
3 was used for the cultivation experiments. Biomass was harvested at the maximum biomass concentration (Xm) by centrifugation. Dried biomass was lyophilized and then frozen for further analysis.
The total amounts of lipids (%), proteins (%), and carbohydrates (%) were determined in the dried biomass from each cultivation, as already described [
18,
19]. A ratio of freeze-dried biomass/water (1:45) was maintained for 120 min at 90 °C and under constant agitation at 400 rpm. The mixture was filtered using 0.45μm pore size. The filtrate was mixed in a separator funnel with absolute ethanol (1:3) and left in a cold chamber (approx. 4 °C). After the complete precipitation of PS, as white crystals, the mixture was centrifuged. The supernatant was stored for later recovery of the ethanol and the PS solid sample was redissolved in sterile water. After filtration, it was recrystallized using the same initial proportion of ethanol. The suspension was centrifuged, and the crystals were placed in an oven with air circulation at 55°C for 12h [
20].
2.2. In Vitro Antioxidant Activity
Antioxidant activity was assessed in vitro via the free radical neutralization assay using the stable free radical DPPH• (2,2-diphenyl-1-picrylhydrazyl) [
21]. Concisely, 10 μL of each extract and PS was incorporated into a 990 μL methanol solution of DPPH•. This mixture was incubated for 30 min at room temperature, followed by absorbance readings at 517 nm against a corresponding blank. The in vitro antioxidant activity, SA (%), was calculated for each extract and PS.
where: SA % is the scavenging activity of the free radical; A
DPPH is the absorption of DPPH against the blank, and A
sample is the absorption of the extract or the control against the blank. Butylated hydroxytoluene (BHT) was used as the reference substrance. All experiments were performed in triplicate.
2.3. Prototype Formulation
Ammonium acryloyldimethyltaurate/VP copolymer was used as the gelling agent. Two 1.0% w/w ammonium acryloyldimethyltaurate/VP copolymer dermocosmetics were prepared: 1) blank gel, and 2) gel with 5.0% (w/w) PS from the A. braunii.
2.4. In Vivo Trial - Cutaneous Biocompatibility
The study was conducted according to the Declaration of Helsinki, and approved by the Ethics Committee of Universidade Lusófona (protocol code 01/2016).
The evaluation of cutaneous biocompatibility was performed on 14 healthy male and female subjects, with an average age of 31.5±12.6 years. All participants provided oral and written informed consent prior to the study.
The assessment was carried out on the inner forearm, with each participant randomly assigned to a control site (untreated) and two treated sites: one for the blank sample and one for the 5.0% A. braunii PS gel. Randomized parameters included the selection of either the left or right forearm and either the upper or lower section of the forearm. A prerequisite washout period of one day was enforced, during which the participants refrained from applying any cosmetic products with moisturizers to the designated area.
Environment conditions were controlled at 21±2 °C with relative humidity between 40–60%. Baseline measurements of superficial skin (SK) hydration and transepidermal water loss (TEWL) were conducted using the Corneometer
® CM825 and the Tewameter
® TM 300 (CK Electronics GmbH, Köln, Germany). The test formulations were applied under occlusion for 24 hours using Finn Chambers
® epicutaneous patches (Epitest Ltd., Oy, Finland). SK hydration and TEWL were assessed again two hours after removing the patches. Results were expressed as ratios of post-application to baseline values to account for individual variability [
22,
23].
2.5. Statistical Analyses
All the measurements were made in triplicate. The overall results were evaluated by ANOVA. The means were considered statistically significant when p ˂ 0.05 (confidence level of 95 %). Tukey’s Test was used to compare the means using Minitab® 19 software.
3. Results
3.1. A. braunii Cultivation, Biomass and Polysaccharide Extraction
The composition of the biomass was characterized by a predominance of proteins, comprising 61% of the total mass, followed by lipids at 32%, and carbohydrates making up 6%. The extraction methodology detailed in this study successfully yielded white crystalline substances, achieving an extraction efficiency of approximately 4%.
3.2. In Vitro Antioxidant Activity
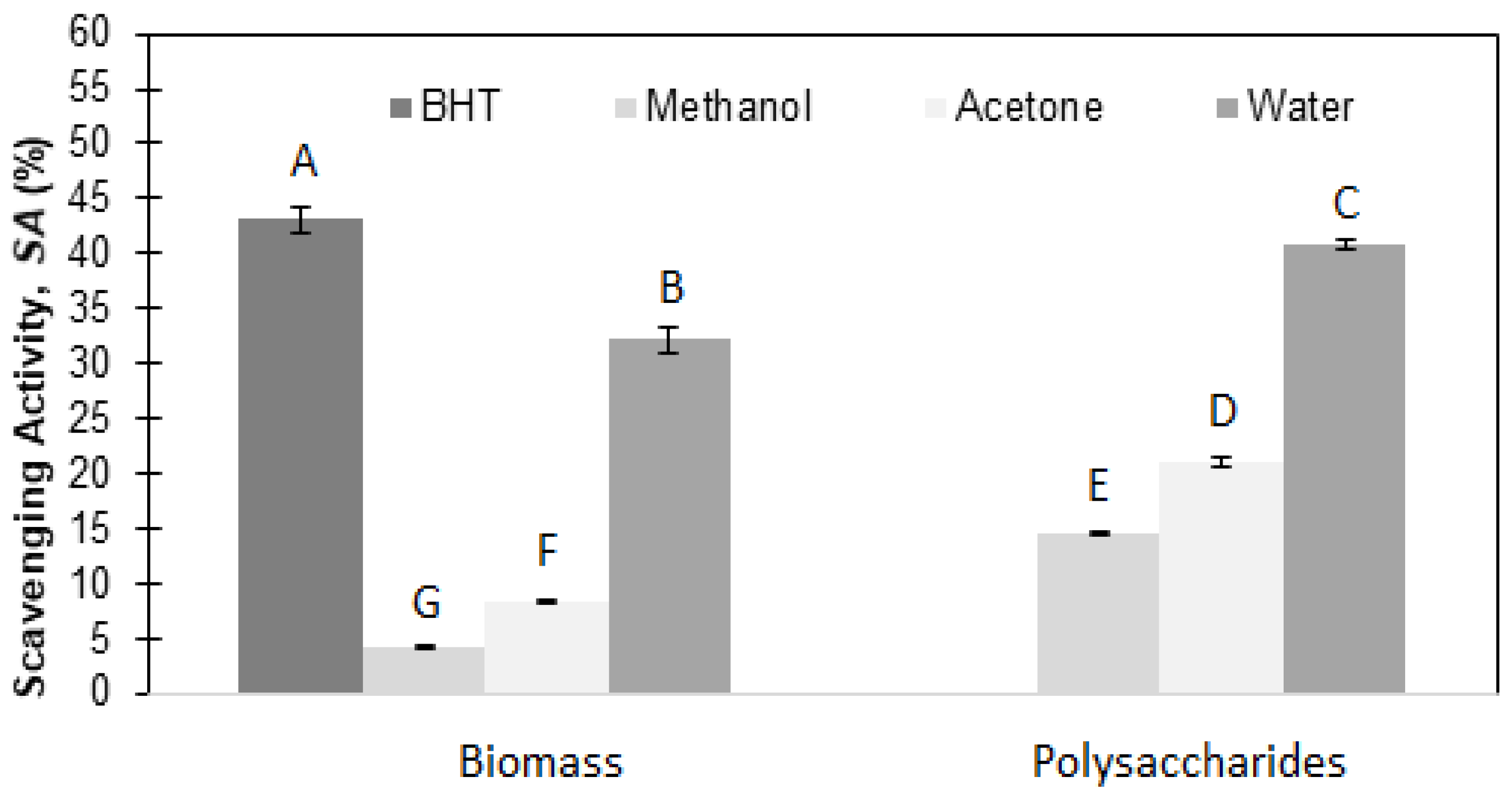
Three distinct extraction solvents—methanol, acetone, and water—were utilized to evaluate the radical scavenging activity (SA, %) in both biomass and polysaccharides from
A. braunii. As illustrated in
Figure 1, the aqueous extracts exhibited the highest SA values, followed by acetone extracts, and lastly, methanol extracts. Notably, the SA values for the polysaccharides consistently exceeded those obtained for the whole biomass, though they were lower than the control using BHT.
Considering the A. braunii aqueous PS superior performance through the DPPH• test, this sample was incorporated in a gel formulation prototype, and its safety profile was established by the cutaneous biocompatibility assay.
3.3. Cutaneous Biocompatibility of A. braunii Gel
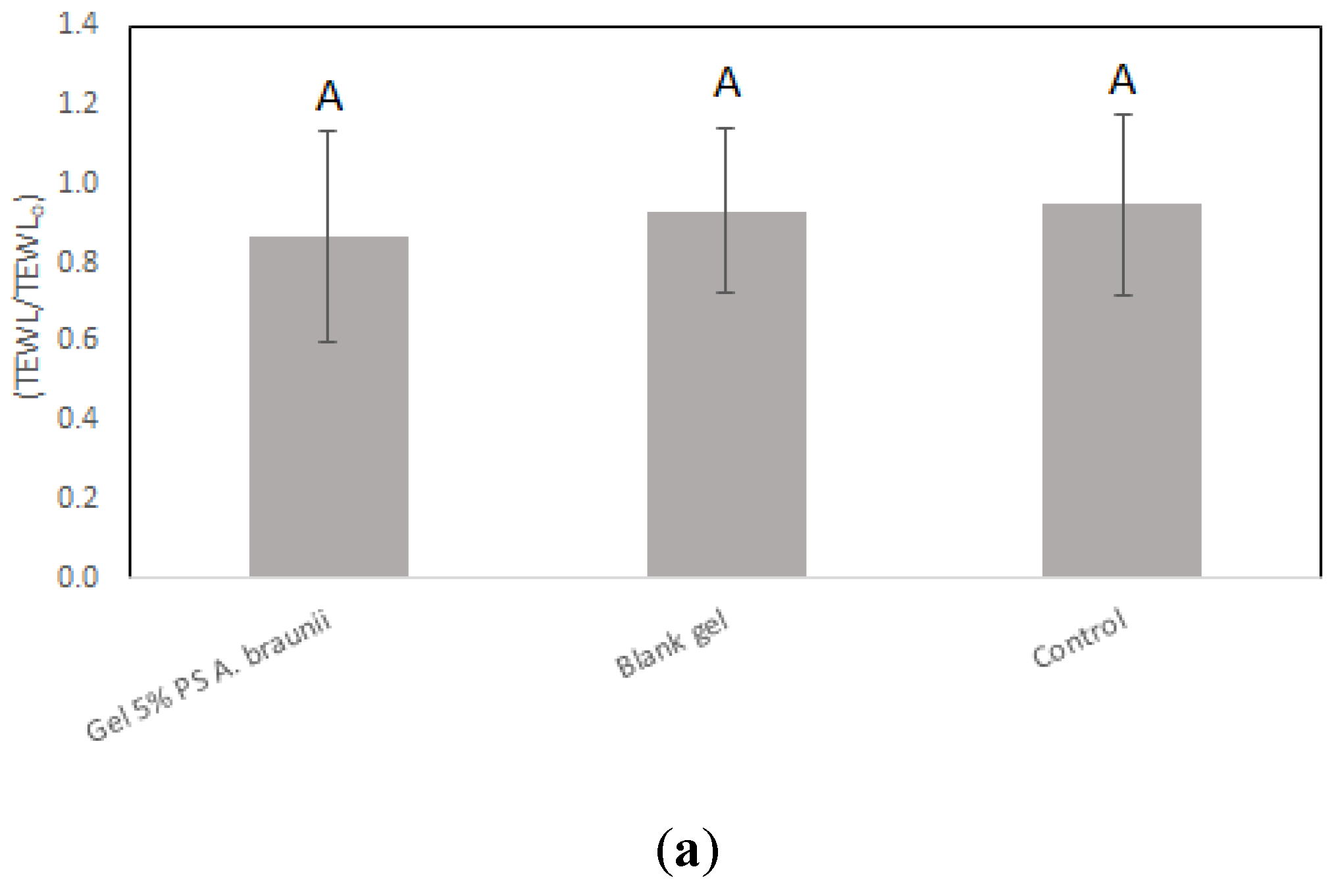
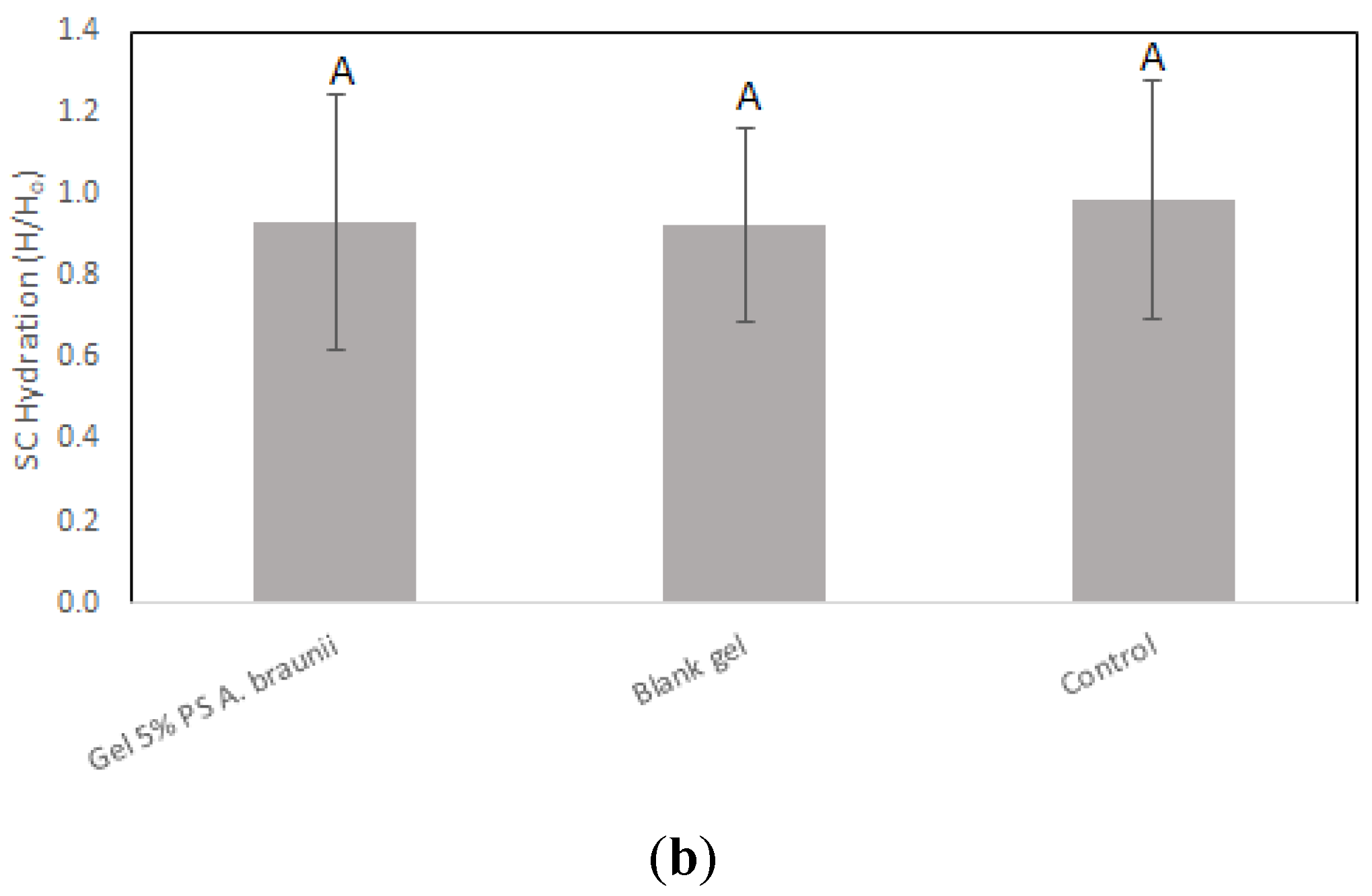
Assessment of cutaneous biocompatibility for the
A. braunii gel was rigorously performed, and the results affirm the gel's suitability for topical application. Upon application of both the blank gel and the gel containing 5.0% polysaccharides from
A. braunii, there was a sustained maintenance of stratum corneum hydration across all subjects, showcasing the gels' adeptness in preserving skin moisture. Notably, the TEWL measurements depicted in
Figure 2(a) further substantiate that neither the blank gel nor the polysaccharide-enriched gel adversely affected the skin's barrier function, as there was no significant increase in water loss compared to the untreated control. This uniformity in hydration and barrier function preservation across different formulations underscores the potential of
A. braunii polysaccharides in skin care products.
4. Discussion
Microalgae cultivation in closed system photobioreactors offers more controlled environmental conditions for microalgae development compared to cultivation in open systems, such as open ponds [
18]. Polysaccharides from some microalgae species are well described in the literature. However, there are no studies detailing the PS composition of
Ankistrodesmus sp. [
24]. Polar compounds such as PS can be extracted from natural resources using the proper solvent or a mixture of them. Many factors influence extraction yields, such as time, pH, type of solvent, and sample chemical composition, among others [
25]. Since most microalgae species contains high level of polar compounds, such as polysaccharides and proteins, this justifies why we reached the highest antioxidant activity using water as a solvent.
Moreover, a study revealed that the role of carotenoids, as antioxidants, is not the unique responsible for the antioxidant activity of microalgae samples. The presence of phenolic compounds can also increase the antioxidant activity values in some microalgae extracts [
26,
27], and here, we demonstrated the antioxidant activity of the water-soluble PS from microalgae [
28]. This protocol is particularly crucial for pinpointing cosmetic ingredients that offer antioxidant benefits. The insights gained from these results critically support the development of dermocosmetic products aimed at enhancing skin defense mechanisms against oxidative stress, thereby ensuring cellular health and integrity. Furthermore, the higher antioxidant activity may be quite relevant when considering incorporating this extract in dermocosmetics, namely for antiaging products, since this strategy may help slow the skin aging process and signs by including compounds that may act as damage defenders.
In vivo skin biocompatibility assays were conducted using non-invasive bioengineering approaches. This methodological approach is crucial for identifying dermocosmetic products that are compatible with human skin, highlighting the importance of such studies in ensuring product safety. These tests not only confirm the mildness of formulations but also their suitability for prolonged contact with skin, thereby supporting the potential of new ingredients for everyday cosmetic use. Superficial skin hydration and TEWL are biological variables that depend not only on the skin condition but also on the aging context [
29,
30]. Statistical analysis revealed no significant differences in SK hydration and TEWL between control sites and those treated with
A. braunii-derived PS gels, affirming the dermatological compatibility of the samples. The consistent preservation of SK hydration and TEWL post-application, mirroring control site values, underscores the efficacy of the compounds in maintaining skin homeostasis. These results indicate that
A. braunii polysaccharides, when applied topically, effectively support the skin's barrier functions without disrupting normal skin physiology, which is crucial for protecting against pathogens and preventing moisture loss [
31,
32].
Furthermore, the lack of adverse effects on SK hydration and TEWL parameters strengthens the safety profile of these compounds, making them ideal candidates for inclusion in dermocosmetic formulations. This not only ensures that the final products are safe for extended use but also contributes to the sustainability of the formulations, using natural ingredients that are effective without being invasive to the skin [
33].
Therefore, the collected data provide a solid foundation for recommending these microalgae extracts in the development of new skin care products, particularly those aimed at hydration and barrier protection [
34].
5. Conclusions
Herein, the Ankistrodesmus braunii species was cultivated in a closed photobioreactor system and polysaccharides were extracted and incorporated into a dermocosmetic prototype. The in vitro antioxidant scavenging activity indicated that the aqueous PS showed the highest SA values, even compared to biomass, unveiling the relevance of the water-soluble compounds. Consequently, a gel containing 5.0% w/w PS from A. braunii was prepared, and the cutaneous biocompatibility of this sample was demonstrated through in vivo skin superficial hydration and transepidermal water loss experiments performed in participants.
Overall, there has been an increasing interest in using microalgae as sustainable products to be included in dermocosmetics due to the valuable properties that arise from the multiple compounds that can be extracted from these species. Nonetheless, much is still to be done due to the vast variety of microalgae strains. This investigation encourages the development of dermocosmetics derived from microalgae, considering, for example, the safe profile of the polysaccharides from A. braunii, and their antioxidant potential.
Author Contributions
Conceptualization, A.L.M.-J. and A.R.B.; methodology, A.L.M.-J.; D.M.R.; J.C.M.d.C. and C.R.; validation, M.V.R.V. and P.R.; formal analysis, A.L.M.-J. and A.R.B.; investigation, A.L.M.-J.; D.M.R. and P.R.; resources, A.L.M.-J.; C.R. and A.R.B.; data curation, C.R.; writing—original draft preparation, A.L.M.-J.; A.R.B. and J.C.M.C.; writing—review and editing, A.L.M.-J., L.G.d.S.C and A.R.B.; visualization, A.R.B.; supervision, C.R. and A.R.B.; project administration, A.R.B.; funding acquisition, A.L.M.-J; D.M.R.; C.R. and A.R.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, grant number 303862/2022-0); Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, grant numbers 2015/11194-6, 2016/22000-0, 2022/09905-5, and 2024/01920-0); and FCT (Foundation for Science and Technology), I.P. through national funds under DOI 10.54499/UIDP/04567/2020, DOI 10.54499/UIDB/04567/2020 and DOI 10.54499/EXPL/BTM-MAT/0112/2021 projects attributed to CBIOS.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Universidade Lusófona’s Ethics Committee of School of Health Sciences and Technologies (protocol code 1/2016, 7 December 2016).
Informed Consent Statement
Written informed consent has been obtained from the participants of this investigation.
Data Availability Statement
Dataset available on request from the authors.
Acknowledgments
A.L.M.-J. and D.M.R. are grateful to FAPESP. A.R.B. is thankful to the CNPq, for the Research Productivity Scholarship, and to FAPESP.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Oliveira, A.C.; Morocho-Jácome, A.L.; Castro Lima, C.R.; Marques, G.A.; Bispo, M.O.; Barros, A.B.; Costa, J.G.; Santos de Almeida, T.; Rosado, C.; Carvalho, J.C.M.; et al. Cosmetics Applications. In Microalgae; Galanakis, C.M., Ed.; Academic Press: India, 2020; pp. 313–338. [Google Scholar]
- Morocho-Jácome, A.L.; Cezare-Gomes, E.A.; Carvalho, J.C.M.; Sauce, R.; Rosado, C.; Velasco, M.V.R.; Baby, A.R. UV-Screening from Microalgae. In Handbook of Microalgae-Based Processes and Products; Jacob-Lopes, E., Maroneze, M.M., Queiroz, M.I., Zepka, L.Q., Eds.; Elsevier: Amsterdam, 2020; pp. 647–657. [Google Scholar]
- Morocho-Jácome, A.L.; Freire, T.B.; de Oliveira, A.C.; de Almeida, T.S.; Rosado, C.; Velasco, M.V.R.; Baby, A.R. In Vivo SPF from Multifunctional Sunscreen Systems Developed with Natural Compounds—A Review. J Cosmet Dermatol 2020. [Google Scholar] [CrossRef] [PubMed]
- Cezare-Gomes, E.A.; Mejia-da-Silva, L. del C.; Pérez-Mora, L.S.; Matsudo, M.C.; Ferreira-Camargo, L.S.; Singh, A.K.; de Carvalho, J.C.M. Potential of Microalgae Carotenoids for Industrial Application.
- Morocho-Jácome, A.L.; Ruscinc, N.; Martinez, R.M.; de Carvalho, J.C.M.; Santos de Almeida, T.; Rosado, C.; Costa, J.G.; Velasco, M.V.R.; Baby, A.R. (Bio)Technological Aspects of Microalgae Pigments for Cosmetics. Appl Microbiol Biotechnol 2020. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, C.; Ghosh, T.; George, B.; Pancha, I.; Maurya, R.; Chokshi, K.; Ghosh, A.; Mishra, S. Microalgal Carotenoids: Potential Nutraceutical Compounds with Chemotaxonomic Importance. Algal Res 2016, 15, 24–31. [Google Scholar] [CrossRef]
- Levasseur, W.; Perré, P.; Pozzobon, V. A Review of High Value-Added Molecules Production by Microalgae in Light of the Classification. Biotechnol Adv 2020, 41, 107545. [Google Scholar] [CrossRef]
- De Jesus Raposo, M.F.; De Morais, A.M.B.; De Morais, R.M.S.C. Marine Polysaccharides from Algae with Potential Biomedical Applications. Mar Drugs 2015, 13, 2967–3028. [Google Scholar] [CrossRef]
- Wang, Q.; Shen, Q.; Wang, J.; Zhang, Y.; Zhang, Z.; Lei, Z.; Shimizu, K.; Lee, D. Fast Cultivation and Harvesting of Oil-Producing Microalgae Ankistrodesmus Falcatus Var. Acicularis Fed with Anaerobic Digestion Liquor via Biogranulation in Addition to Nutrients Removal. Science of the Total Environment 2020, 741, 140183. [Google Scholar] [CrossRef]
- Mansa, R.F.; Sipaut, C.S.; Yasir, S.; Dayou, J.; Joannes, C. Comparative Studies of Cell Growth, Total Lipid and Methyl Palmitate of Ankistrodesmus Sp. in Phototrophic, Mixotrophic and Heterotrophic Cultures for Biodiesel Production. International Journal of Renewable Energy Research 2018, 8, 438–450. [Google Scholar]
- Cobos, M.; Paredes, J.D.; Maddox, J.D.; Vargas-Arana, G.; Flores, L.; Aguilar, C.P.; Marapara, J.L.; Castro, J.C. Isolation and Characterization of Native Microalgae from the Peruvian Amazon with Potential for Biodiesel Production. Energies (Basel) 2017, 10, 224–240. [Google Scholar] [CrossRef]
- Yee, W. Microalgae from the Selenastraceae as Emerging Candidates for Biodiesel Production: A Mini Review. World J Microbiol Biotechnol 2016, 32, 64. [Google Scholar] [CrossRef]
- George, B.; Pancha, I.; Desai, C.; Chokshi, K.; Paliwal, C.; Ghosh, T.; Mishra, S. Effects of Different Media Composition, Light Intensity and Photoperiod on Morphology and Physiology of Freshwater Microalgae Ankistrodesmus Falcatus - a Potential Strain for Bio-Fuel Production. Bioresour Technol 2014, 171, 367–374. [Google Scholar] [CrossRef]
- Mansa, R.F.; Sipaut, C.S.; Yasir, S.; Dayou, J.; Joannes, C. Comparative Studies of Cell Growth, Total Lipid and Methyl Palmitate of Ankistrodesmus Sp. in Phototrophic, Mixotrophic and Heterotrophic Cultures for Biodiesel Production. International Journal of Renewable Energy Research 2018, 8, 438–450. [Google Scholar]
- Bresaola, M.D.; Morocho-Jácome, A.L.; Matsudo, M.C.; Carvalho, J.C.M. Semi-Continuous Process as a Promising Technique in Ankistrodesmus Braunii Cultivation in Photobioreactor. J Appl Phycol 2019, 31, 2197–2205. [Google Scholar] [CrossRef]
- Morocho-Jácome, A.L.; Dapievi Bresaola, M.; Carvalho, J.C.M.; Nicolai, M.; Rosado, C.; Baby, A.R. Carbohydrates in Ankistrodesmus Braunii Biomass Cultivated in Tubular Photobioreactors. Biomedical and Biopharmaceutical Research 2017, 2, 242–248. [Google Scholar] [CrossRef]
- UTEX Culture Collection of Algae UTEX.
- Bresaola, M.D.; Morocho-Jácome, A.L.; Matsudo, M.C.; Carvalho, J.C.M. Semi-Continuous Process as a Promising Technique in Ankistrodesmus Braunii Cultivation in Photobioreactor. J Appl Phycol 2019, 31, 2197–2205. [Google Scholar] [CrossRef]
- Morocho-Jácome, A.L.; Dapievi Bresaola, M.; Carvalho, J.C.M.; Nicolai, M.; Rosado, C.; Baby, A.R. Carbohydrates in Ankistrodesmus Braunii Biomass Cultivated in Tubular Photobioreactors. Biomedical and Biopharmaceutical Research 2017, 2, 242–248. [Google Scholar] [CrossRef]
- Chaiklahan, R.; Chirasuwan, N.; Triratana, P.; Loha, V.; Tia, S.; Bunnag, B. Polysaccharide Extraction from Spirulina Sp. and Its Antioxidant Capacity. Int J Biol Macromol 2013, 58, 73–78. [Google Scholar] [CrossRef]
- Falé, P.L.; Borges, C.; Madeira, P.J.A.; Ascensão, L.; Araújo, M.E.M.; Florêncio, M.H.; Serralheiro, M.L.M. Rosmarinic Acid, Scutellarein 4′-Methyl Ether 7-O-Glucuronide and (16S)-Coleon E Are the Main Compounds Responsible for the Antiacetylcholinesterase and Antioxidant Activity in Herbal Tea of Plectranthus Barbatus (Falso Boldo). Food Chem 2009, 114, 798–805. [Google Scholar] [CrossRef]
- Peres, D.A.; de Oliveira, C.A.; da Costa, M.S.; Tokunaga, V.K.; Mota, J.P.; Rosado, C.; Consiglieri, V.O.; Kaneko, T.M.; Velasco, M.V.R.; Baby, A.R. Rutin Increases Critical Wavelength of Systems Containing a Single UV Filter and with Good Skin Compatibility. Skin Research and Technology 2016, 22. [Google Scholar] [CrossRef]
- Oliveira, C.A. de; Peres, D.D.A.; Graziola, F.; Chacra, N.A.B.; Araújo, G.L.B. de; Flórido, A.C.; Mota, J.; Rosado, C.; Velasco, M.V.R.; Rodrigues, L.M.; et al. Cutaneous Biocompatible Rutin-Loaded Gelatin-Based Nanoparticles Increase the SPF of the Association of UVA and UVB Filters. European Journal of Pharmaceutical Sciences 2016, 81, 1–9. [Google Scholar] [CrossRef]
- Delattre, C.; Pierre, G.; Laroche, C.; Michaud, P. Production, Extraction and Characterization of Microalgal and Cyanobacterial Exopolysaccharides. Biotechnol Adv 2016. [CrossRef]
- Rijo, P.; Falé, P.L.; Serralheiro, M.L.; Simões, M.F.; Gomes, A.; Reis, C. Optimization of Medicinal Plant Extraction Methods and Their Encapsulation through Extrusion Technology. Measurement 2014, 58, 249–255. [Google Scholar] [CrossRef]
- Goiris, K.; Muylaert, K.; Fraeye, I.; Foubert, I.; De Brabanter, J.; De Cooman, L. Antioxidant Potential of Microalgae in Relation to Their Phenolic and Carotenoid Content. J Appl Phycol 2012, 24, 1477–1486. [Google Scholar] [CrossRef]
- Banskota, A.H.; Sperker, S.; Stefanova, R.; McGinn, P.J.; O’Leary, S.J.B. Antioxidant Properties and Lipid Composition of Selected Microalgae. J Appl Phycol 2019, 31, 309–318. [Google Scholar] [CrossRef]
- Marcati, A.; Ursu, A.V.; Laroche, C.; Soanen, N.; Marchal, L.; Jubeau, S.; Djelveh, G.; Michaud, P. Extraction and Fractionation of Polysaccharides and B-Phycoerythrin from the Microalga Porphyridium Cruentum by Membrane Technology. Algal Res 2014, 5, 258–263. [Google Scholar] [CrossRef]
- Berardesca, E.; Loden, M.; Serup, J.; Masson, P.; Rodrigues, L.M. The Revised EEMCO Guidance for the in Vivo Measurement of Water in the Skin. Skin Research and Technology 2018, 24, 351–358. [Google Scholar] [CrossRef]
- Sun, Q.; Stantchev, R.I.; Wang, J.; Parrott, E.P.J.; Cottenden, A.; Chiu, T.W.; Ahuja, A.T.; Pickwell-MacPherson, E. In Vivo Estimation of Water Diffusivity in Occluded Human Skin Using Terahertz Reflection Spectroscopy. J Biophotonics 2019, 12, 2–12. [Google Scholar] [CrossRef]
- Yao, Y.; Xu, B. Skin Health Promoting Effects of Natural Polysaccharides and Their Potential Application in the Cosmetic Industry. Polysaccharides 2022, 3, 818–830. [Google Scholar] [CrossRef]
- Miguel, S.P.; Ribeiro, M.P.; Otero, A.; Coutinho, P. Application of Microalgae and Microalgal Bioactive Compounds in Skin Regeneration. Algal Res 2021, 58. [Google Scholar] [CrossRef]
- Choi, H.Y.; Lee, Y.J.; Kim, C.M.; Lee, Y.-M. Revolutionizing Cosmetic Ingredients: Harnessing the Power of Antioxidants, Probiotics, Plant Extracts, and Peptides in Personal and Skin Care Products. Cosmetics 2024, 11, 157. [Google Scholar] [CrossRef]
- Albuquerque, P.B.S.; de Oliveira, W.F.; dos Santos Silva, P.M.; dos Santos Correia, M.T.; Kennedy, J.F.; Coelho, L.C.B.B. Skincare Application of Medicinal Plant Polysaccharides — A Review. Carbohydr Polym 2022, 277. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).