Submitted:
09 December 2024
Posted:
09 December 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Bio-Generated Silica (Biosilica)
2.3. Fabrication of Silicone Rubber Sheets Filled with Silica
2.4. Characterizations of Silica Nanoparticles and Silicone Rubber
2.4.1. X-Ray Diffraction (XRD)
2.4.2. Attenuated Total Reflection Fourier Transform Infrared Spectroscopy (ATR-FTIR)
2.4.3. Scanning Electron Microscopy (SEM)
2.4.4. Specific Surface Area
2.4.5. Thermogravimetric Analysis (TGA)
2.4.6. Tensile and Tear Properties
2.4.7. Crosslinking Density
2.4.8. Dynamic Mechanical Properties of Uncured Silicone Compound and Cured Silicone Rubber
3. Results and Discussion
3.1. Structures and Properties of Biosilica and Zeosil 175
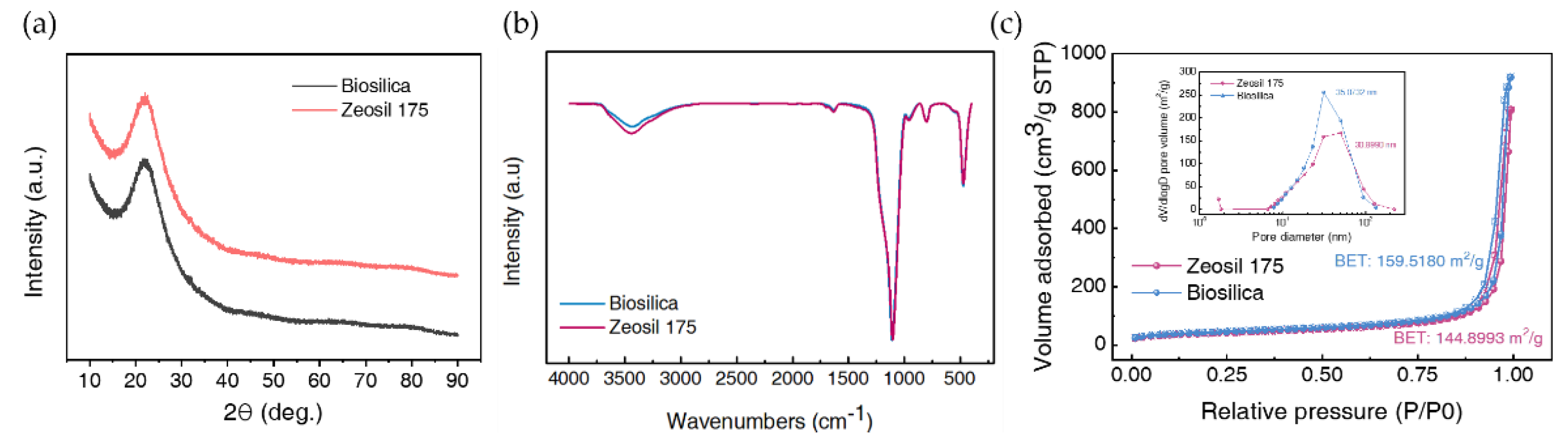
3.1.1. XRD
3.1.2. FT-IR
3.1.3. BET
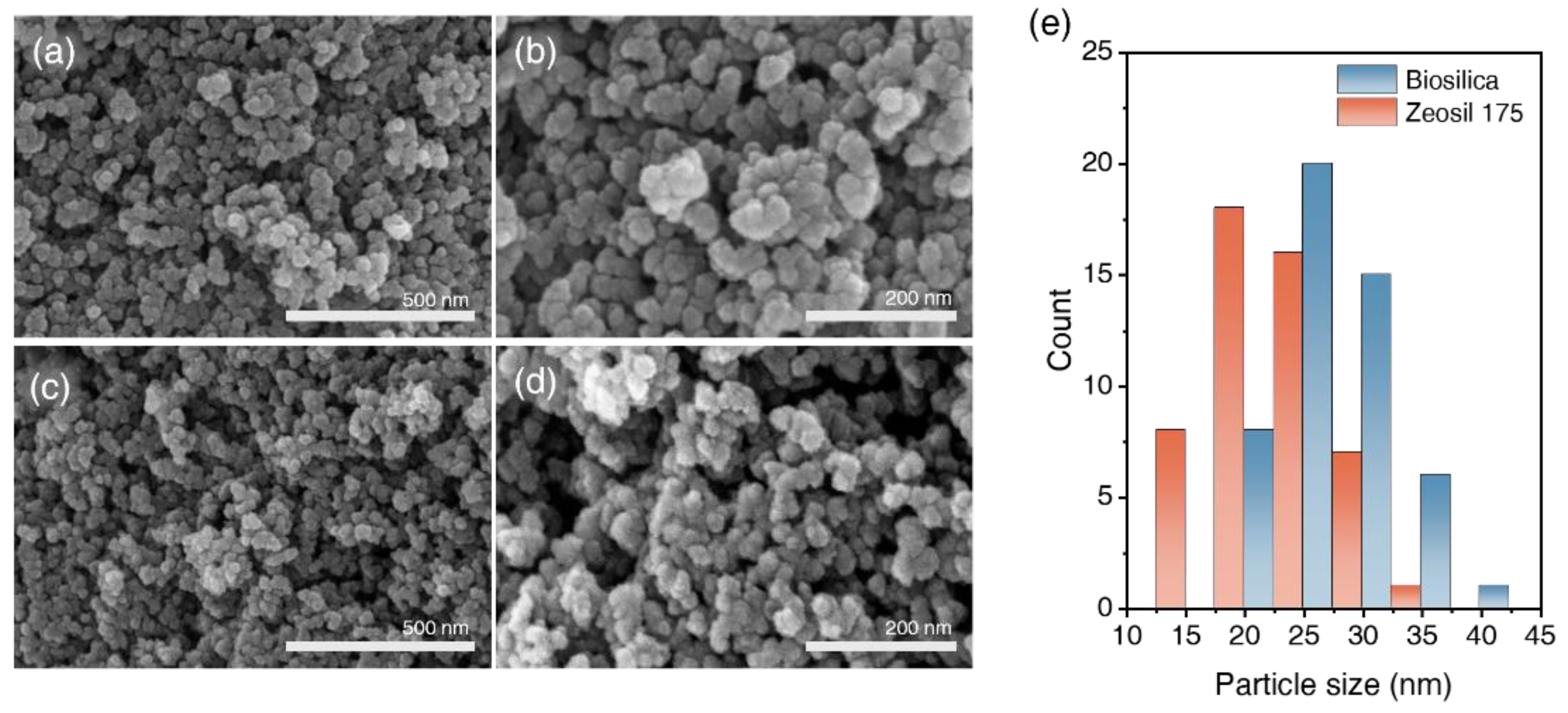
3.1.4. The Morphology of Biosilica and Zeosil 175
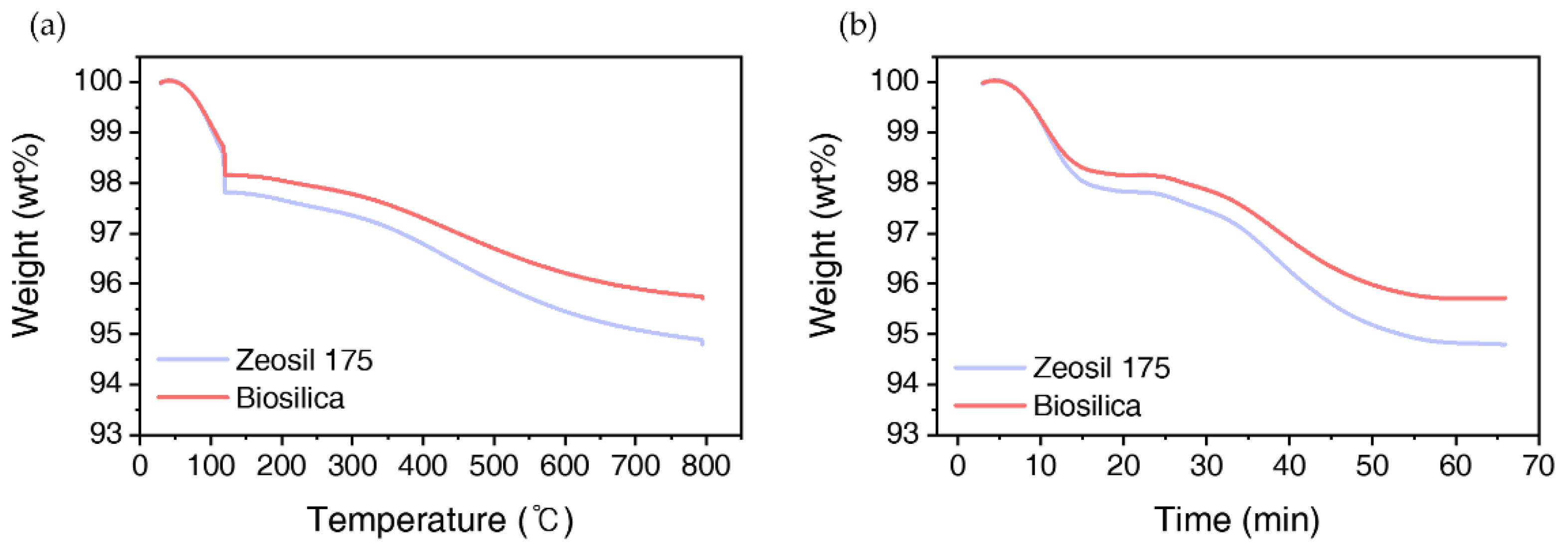
3.1.5. Thermogravimetric Analysis
3.2. Results of Silicone Rubber Filled with Silica
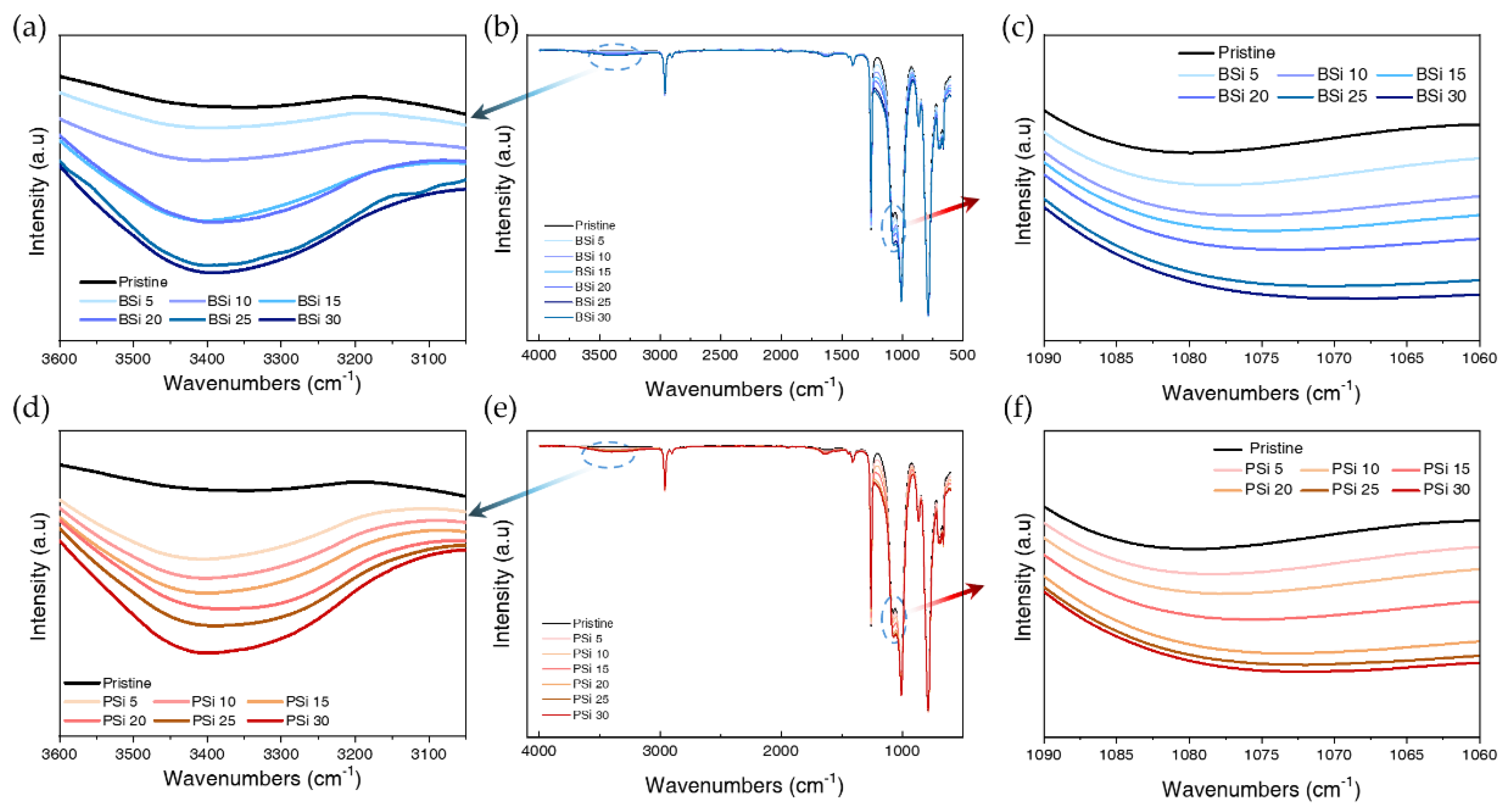
3.2.1. FT-IR
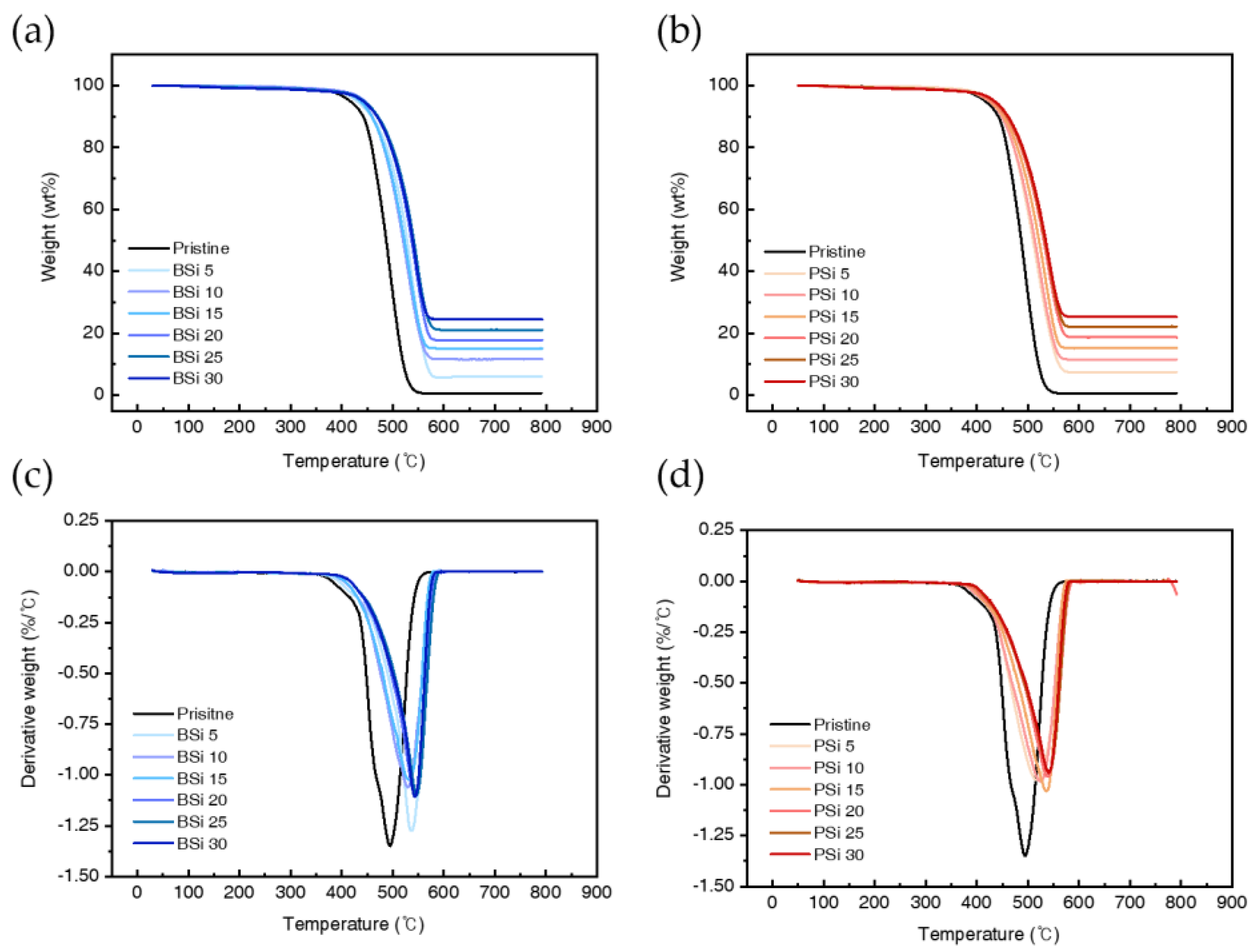
3.2.2. Thermogravimetric Analysis of PSi and BSi
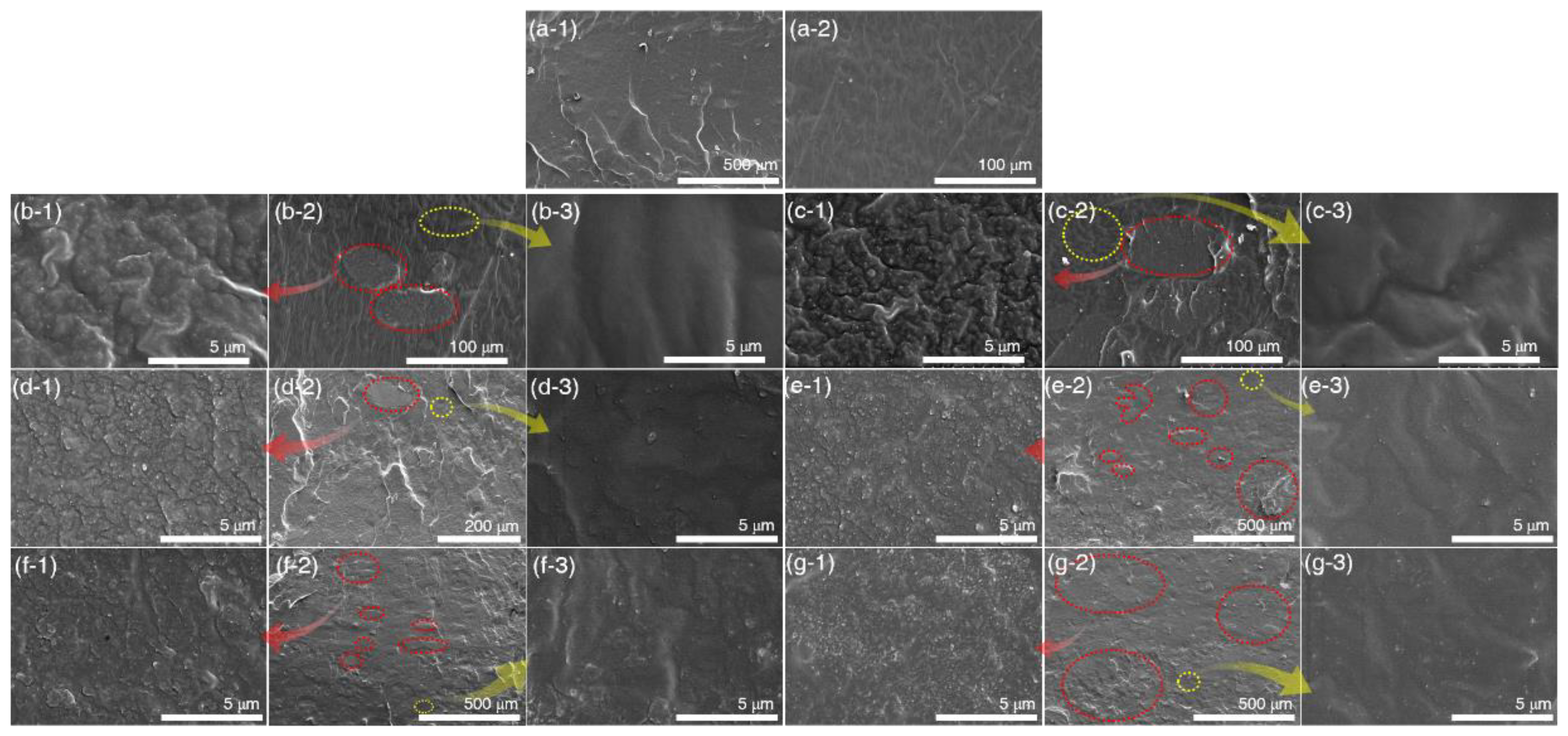
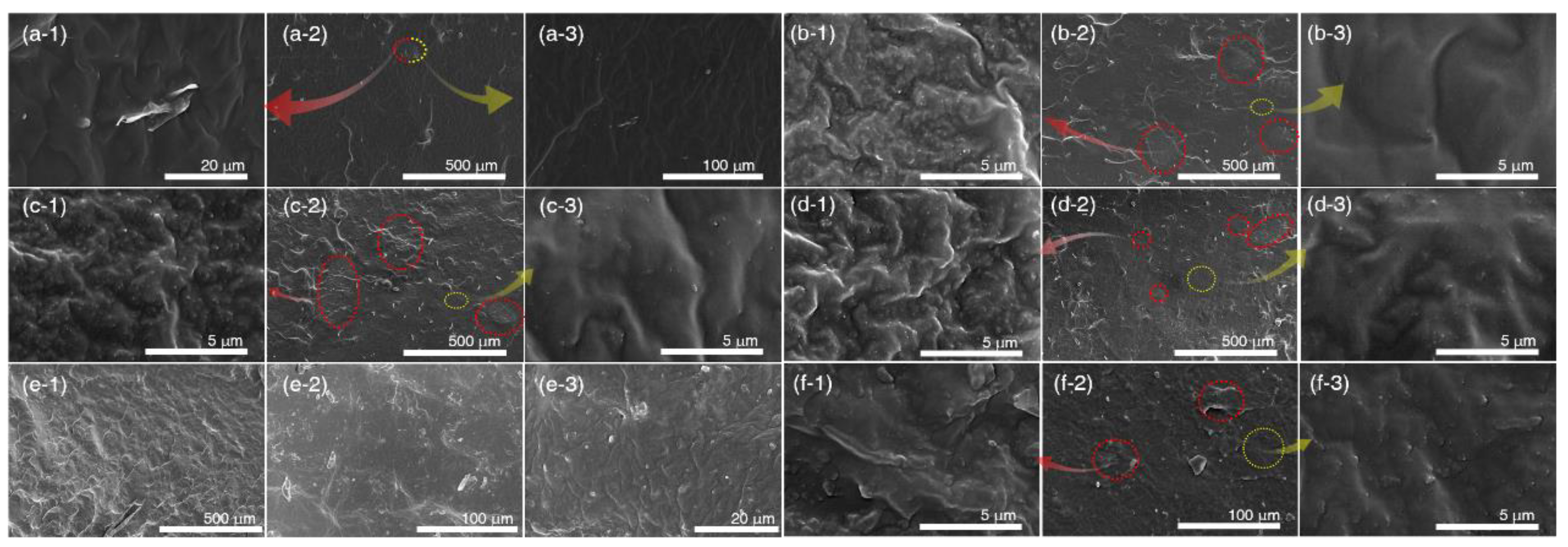
3.2.3. Morphology of Silicone Rubber
3.2.4. Chemo-Mechanical Properties
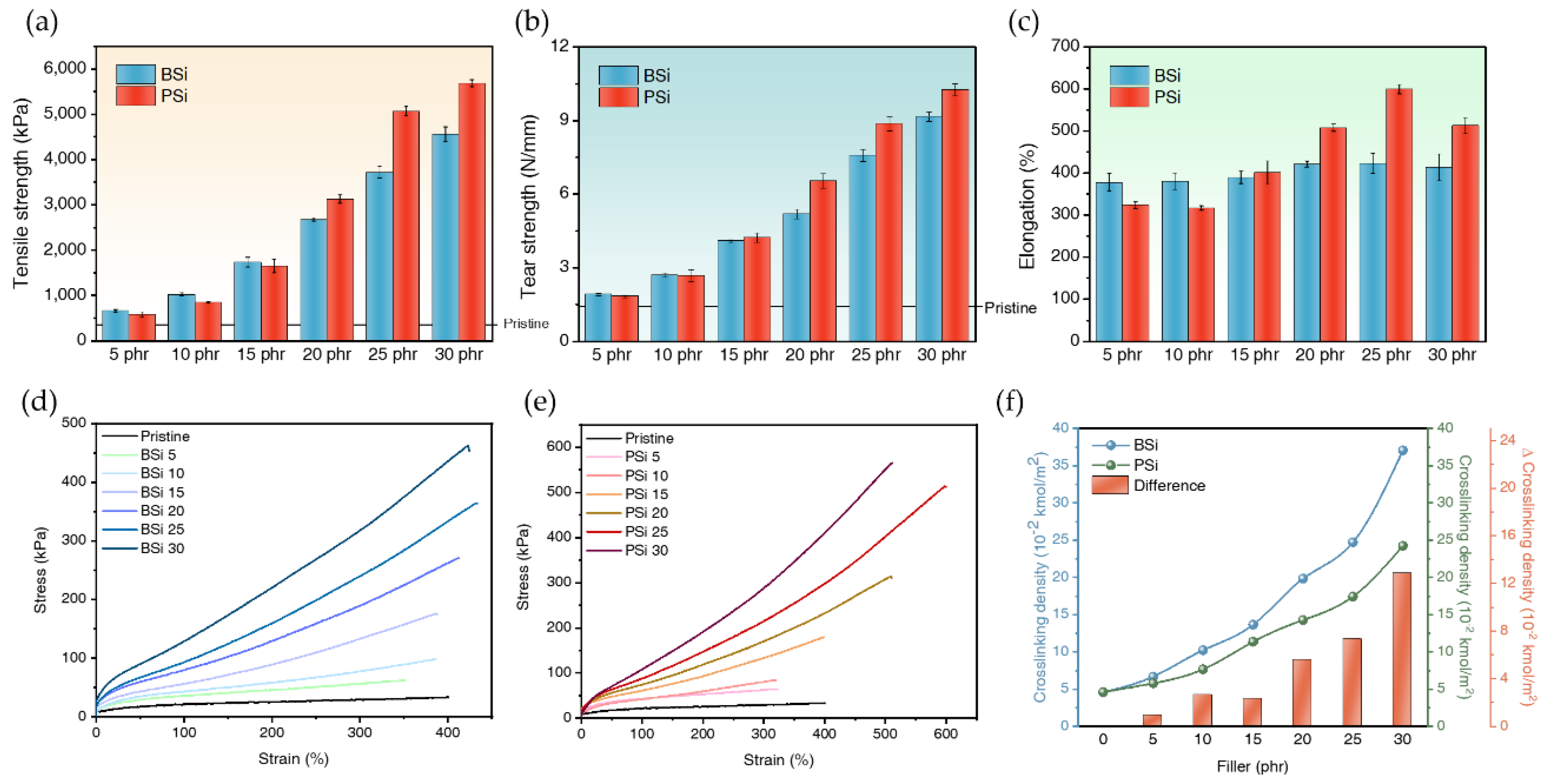
3.2.4.1. Tensile Strength
3.2.4.2. Tear Strength
3.2.4.3. Elongation at Break
3.2.5. Crosslinking Density
3.2.6. Analysis of Cured Silicone Rubber
3.2.7. Viscoelastic Characteristics of Uncured Silicone Compound
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Satbaev, B.; Yefremova, S.; Zharmenov, A.; Kablanbekov, A.; Yermishin, S.; Shalabaev, N.; Satbaev, A.; Khen, V. Rice Husk Research: From Environmental Pollutant to a Promising Source of Organo-Mineral Raw Materials. Materials 2021, 14, 4119. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R. A Review Study on Precipitated Silica and Activated Carbon from Rice Husk. J. Chem. Eng. Process Technol. 2013, 04. [Google Scholar] [CrossRef]
- Sharma, N.K.; Williams, W.S.; Zangvil, A. Formation and Structure of Silicon Carbide Whiskers from Rice Hulls. J. Am. Ceram. Soc. 1984, 67, 715–720. [Google Scholar] [CrossRef]
- Bakar, R.A.; Yahya, R.; Gan, S.N. Production of High Purity Amorphous Silica from Rice Husk. Procedia Chem. 2016, 19, 189–195. [Google Scholar] [CrossRef]
- Fernandes, I.J.; Calheiro, D.; Sánchez, F.A.L.; Camacho, A.L.D.; Rocha, T.L.A. de C.; Moraes, C.A.M.; Sousa, V.C. de. Characterization of Silica Produced from Rice Husk Ash: Comparison of Purification and Processing Methods. Mater. Res. 2017, 20, 512–518. [Google Scholar] [CrossRef]
- Ma, X.; Zhou, B.; Gao, W.; Qu, Y.; Wang, L.; Wang, Z.; Zhu, Y. A Recyclable Method for Production of Pure Silica from Rice Hull Ash. Powder Technol. 2012, 217, 497–501. [Google Scholar] [CrossRef]
- Riveros, H.; Garza, C. Rice Husks as a Source of High Purity Silica. J. Cryst. Growth 1986, 75, 126–131. [Google Scholar] [CrossRef]
- Liou, T.-H.; Yang, C.-C. Synthesis and Surface Characteristics of Nanosilica Produced from Alkali-Extracted Rice Husk Ash. Mater. Sci. Eng. B 2011, 176, 521–529. [Google Scholar] [CrossRef]
- Yalçin, N.; Sevinç, V. Studies on Silica Obtained from Rice Husk. Ceram. Int. 2001, 27, 219–224. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, D.; Zheng, C.; Yu, K.; Zhang, X.; Ma, W. Bioinspired High-Performance Silicone Elastomers by Catalyst-Free Dopamine Cross-Linking. Ind. Eng. Chem. Res. 2024, 63, 1853–1863. [Google Scholar] [CrossRef]
- Eduok, U.; Faye, O.; Szpunar, J. Recent Developments and Applications of Protective Silicone Coatings: A Review of PDMS Functional Materials. Prog. Org. Coat. 2017, 111, 124–163. [Google Scholar] [CrossRef]
- Mirzadeh, H.; Khorasani, M.T. Physical, Mechanical, and Biocompatibility Evaluation of Three Different Types of Silicone Rubber. J. Appl. Polym. Sci. 2003, 88, 2522–2529. [Google Scholar] [CrossRef]
- Yilgör, E.; Yilgör, I. Silicone Containing Copolymers: Synthesis, Properties and Applications. Prog. Polym. Sci. 2014, 39, 1165–1195. [Google Scholar] [CrossRef]
- Song, J.S.; Lee, S.; Cha, G.C.; Jung, S.H.; Choi, S.Y.; Kim, K.H.; Mun, M.S. Surface Modification of Silicone Rubber by Ion Beam Assisted Deposition (IBAD) for Improved Biocompatibility. J. Appl. Polym. Sci. 2005, 96, 1095–1101. [Google Scholar] [CrossRef]
- Shit, S.C.; Shah, P. A Review on Silicone Rubber. Natl. Acad. Sci. Lett. 2013, 36, 355–365. [Google Scholar] [CrossRef]
- Mazurek, P.; Vudayagiri, S.; Ladegaard Skov, A. How to Tailor Flexible Silicone Elastomers with Mechanical Integrity: A Tutorial Review. Chem. Soc. Rev. 2019, 48, 1448–1464. [Google Scholar] [CrossRef]
- Cochrane, H.; Lin, C.S. The Influence of Fumed Silica Properties on the Processing, Curing, and Reinforcement Properties of Silicone Rubber. Rubber Chem. Technol. 1993, 66, 48–60. [Google Scholar] [CrossRef]
- Boonstra, B.B.; Cochrane, H.; Dánnenberg, E.M. Reinforcement of Silicone Rubber by Particulate Silica. Rubber Chem. Technol. 1975, 48, 558–576. [Google Scholar] [CrossRef]
- Gomes, C.M.; Cheung, N.; Gomes, G.M.; Sousa, A.K.; Peruzzi, A.P. Improvement of Water Resistance in Magnesia Cements with Renewable Source Silica. Constr. Build. Mater. 2021, 272, 121650. [Google Scholar] [CrossRef]
- Eissa, M.M.; Botros, S.H.; Diab, M.; Shafik, E.S.; Rozik, N.N. Rice Husk Fibers and Their Extracted Silica as Promising Bio-Based Fillers for EPDM/NBR Rubber Blend Vulcanizates. Clean Technol. Environ. Policy 2023, 25, 3203–3218. [Google Scholar] [CrossRef]
- Choophun, N.; Chaiammart, N.; Sukthavon, K.; Veranitisagul, C.; Laobuthee, A.; Watthanaphanit, A.; Panomsuwan, G. Natural Rubber Composites Reinforced with Green Silica from Rice Husk: Effect of Filler Loading on Mechanical Properties. J. Compos. Sci. 2022, 6, 369. [Google Scholar] [CrossRef]
- Sethuramalingam, V.C.; Prabagaran, S.; Ganesan, K. Studies on Influence of Silica Filler and Rice Husk Ash on the Mechanical Properties of Vulcanized Hybrid Rubber Composite. Mater. Today Proc. 2021, 37, 2207–2213. [Google Scholar] [CrossRef]
- Jiang, Z.; Fu, Z.; Ning, K. Study on Properties of Precipitated and Fumed Silica Reinforced Polydimethylsiloxane Silicone Rubber. In 2023 IEEE 4th International Conference on Electrical Materials and Power Equipment (ICEMPE); 2023; pp 1–4. [CrossRef]
- Azmi, M.A.; Mahzan, S.; Ahmad, S.; Salleh, S.M.; Rahman, H.A.; Choiron, M.A.; Ismail, A.; Taib, H. Vibration Exposure of Polydimethylsiloxane (PDMS) Reinforced Silica (SiO2): Comparison of Different Source of Silica (SiO2) as Filler. IOP Conf. Ser. Mater. Sci. Eng. 2019, 494, 012069. [Google Scholar] [CrossRef]
- Kalapathy, U.; Proctor, A.; Shultz, J. A Simple Method for Production of Pure Silica from Rice Hull Ash. Bioresour. Technol. 2000, 73, 257–262. [Google Scholar] [CrossRef]
- Mueller, R.; Kammler, H.K.; Wegner, K.; Pratsinis, S.E. OH Surface Density of SiO2 and TiO2 by Thermogravimetric Analysis. Langmuir 2003, 19, 160–165. [Google Scholar] [CrossRef]
- Wisser, F.M.; Abele, M.; Gasthauer, M.; Müller, K.; Moszner, N.; Kickelbick, G. Detection of Surface Silanol Groups on Pristine and Functionalized Silica Mixed Oxides and Zirconia. J. Colloid Interface Sci. 2012, 374, 77–82. [Google Scholar] [CrossRef]
- Shim, S.E.; Isayev, A.I. Ultrasonic Devulcanization of Precipitated Silica-Filled Silicone Rubber. Rubber Chem. Technol. 2001, 74, 303–316. [Google Scholar] [CrossRef]
- Marzocca, A.J.; Rodríguez Garraza, A.L.; Mansilla, M.A. Evaluation of the Polymer–Solvent Interaction Parameter χ for the System Cured Polybutadiene Rubber and Toluene. Polym. Test. 2010, 29, 119–126. [Google Scholar] [CrossRef]
- Scott, R.L.; Magat, M. The Thermodynamics of High-Polymer Solutions: I. The Free Energy of Mixing of Solvents and Polymers of Heterogeneous Distribution. J. Chem. Phys. 1945, 13, 172–177. [Google Scholar] [CrossRef]
- Lu, H.; Feng, S. Supramolecular Silicone Elastomers with Healable and Hydrophobic Properties Crosslinked by “Salt-Forming Vulcanization. ” J. Polym. Sci. Part Polym. Chem. 2017, 55, 903–911. [Google Scholar] [CrossRef]
- Fanse, S.; Bao, Q.; Zou, Y.; Wang, Y.; Burgess, D.J. Impact of Polymer Crosslinking on Release Mechanisms from Long-Acting Levonorgestrel Intrauterine Systems. Int. J. Pharm. 2022, 612, 121383. [Google Scholar] [CrossRef] [PubMed]
- Jyoti, A.; Singh, R.K.; Kumar, N.; Aman, A.K.; Kar, M. ‘Synthesis and Properties of Amorphous Nanosilica from Rice Husk and Its Composites. Mater. Sci. Eng. B 2021, 263, 114871. [Google Scholar] [CrossRef]
- Biswas, R.K.; Khan, P.; Mukherjee, S.; Mukhopadhyay, A.K.; Ghosh, J.; Muraleedharan, K. Study of Short Range Structure of Amorphous Silica from PDF Using Ag Radiation in Laboratory XRD System, RAMAN and NEXAFS. J. Non-Cryst. Solids 2018, 488, 1–9. [Google Scholar] [CrossRef]
- Xu, T.; Jia, Z.; Luo, Y.; Jia, D.; Peng, Z. Interfacial Interaction between the Epoxidized Natural Rubber and Silica in Natural Rubber/Silica Composites. Appl. Surf. Sci. 2015, 328, 306–313. [Google Scholar] [CrossRef]
- Lee, J.H.; Kwon, J.H.; Lee, J.-W.; Lee, H.; Chang, J.H.; Sang, B.-I. Preparation of High Purity Silica Originated from Rice Husks by Chemically Removing Metallic Impurities. J. Ind. Eng. Chem. 2017, 50, 79–85. [Google Scholar] [CrossRef]
- Shui, Y.; Huang, L.; Wei, C.; Sun, G.; Chen, J.; Lu, A.; Sun, L.; Liu, D. How the Silica Determines Properties of Filled Silicone Rubber by the Formation of Filler Networking and Bound Rubber. Compos. Sci. Technol. 2021, 215, 109024. [Google Scholar] [CrossRef]
- Curthoys, G.; Davydov, V.Y.; Kiselev, A.V.; Kiselev, S.A.; Kuznetsov, B.V. Hydrogen Bonding in Adsorption on Silica. J. Colloid Interface Sci. 1974, 48, 58–72. [Google Scholar] [CrossRef]
- Ansarifar, A.L.B. Reinforcement of Silicone Rubber with Precipitated Amorphous White Silica Nanofiller – Effect of Silica Aggregates on the Rubber Properties. J. Rubb. Res. 2006, 9, 140–158. [Google Scholar]
- Kralevich, M.L.; Koenig, J.L. FTIR Analysis of Silica-Filled Natural Rubber. Rubber Chem. Technol. 1998, 71, 300–309. [Google Scholar] [CrossRef]
- Tarrío-Saavedra, J.; López-Beceiro, J.; Naya, S.; Artiaga, R. Effect of Silica Content on Thermal Stability of Fumed Silica/Epoxy Composites. Polym. Degrad. Stab. 2008, 93, 2133–2137. [Google Scholar] [CrossRef]
- Liu, D.; Song, L.; Song, H.; Chen, J.; Tian, Q.; Chen, L.; Sun, L.; Lu, A.; Huang, C.; Sun, G. Correlation between Mechanical Properties and Microscopic Structures of an Optimized Silica Fraction in Silicone Rubber. Compos. Sci. Technol. 2018, 165, 373–379. [Google Scholar] [CrossRef]
- Bernal-Ortega, P.; Anyszka, R.; Morishita, Y.; di Ronza, R.; Blume, A. Determination of the Crosslink Density of Silica-Filled Styrene Butadiene Rubber Compounds by Different Analytical Methods. Polym. Bull. 2024, 81, 995–1018. [Google Scholar] [CrossRef]
- Tong, Y.; Liu, H.; Chen, A.; Guan, H.; Kong, J.; Liu, S.; He, C. Effect of Surface Chemistry and Morphology of Silica on the Thermal and Mechanical Properties of Silicone Elastomers. J. Appl. Polym. Sci. 2018, 135, 46646. [Google Scholar] [CrossRef]
- Bendjaouahdou, C.; Bensaad, S. Properties of Polypropylene/(Natural Rubber)/Organomontmorillonite Nanocomposites Prepared by Melt Blending. J. Vinyl Addit. Technol. 2011, 17, 48–57. [Google Scholar] [CrossRef]
- Lolage, M.; Parida, P.; Gupta, A.; Rautaray, D. Synergistic Effects of Silica and Nanoclay on Curing Characteristics, Processing Behaviour and Mechanical Properties of Solution Styrene Butadiene Rubber (SBR)–Based Tire Tread Compounds. Emergent Mater. 2022, 5, 957–966. [Google Scholar] [CrossRef]
- Lipińska, M.; Soszka, K. Viscoelastic Behavior, Curing and Reinforcement Mechanism of Various Silica and POSS Filled Methyl-Vinyl Polysiloxane MVQ Rubber. Silicon 2019, 11, 2293–2305. [Google Scholar] [CrossRef]

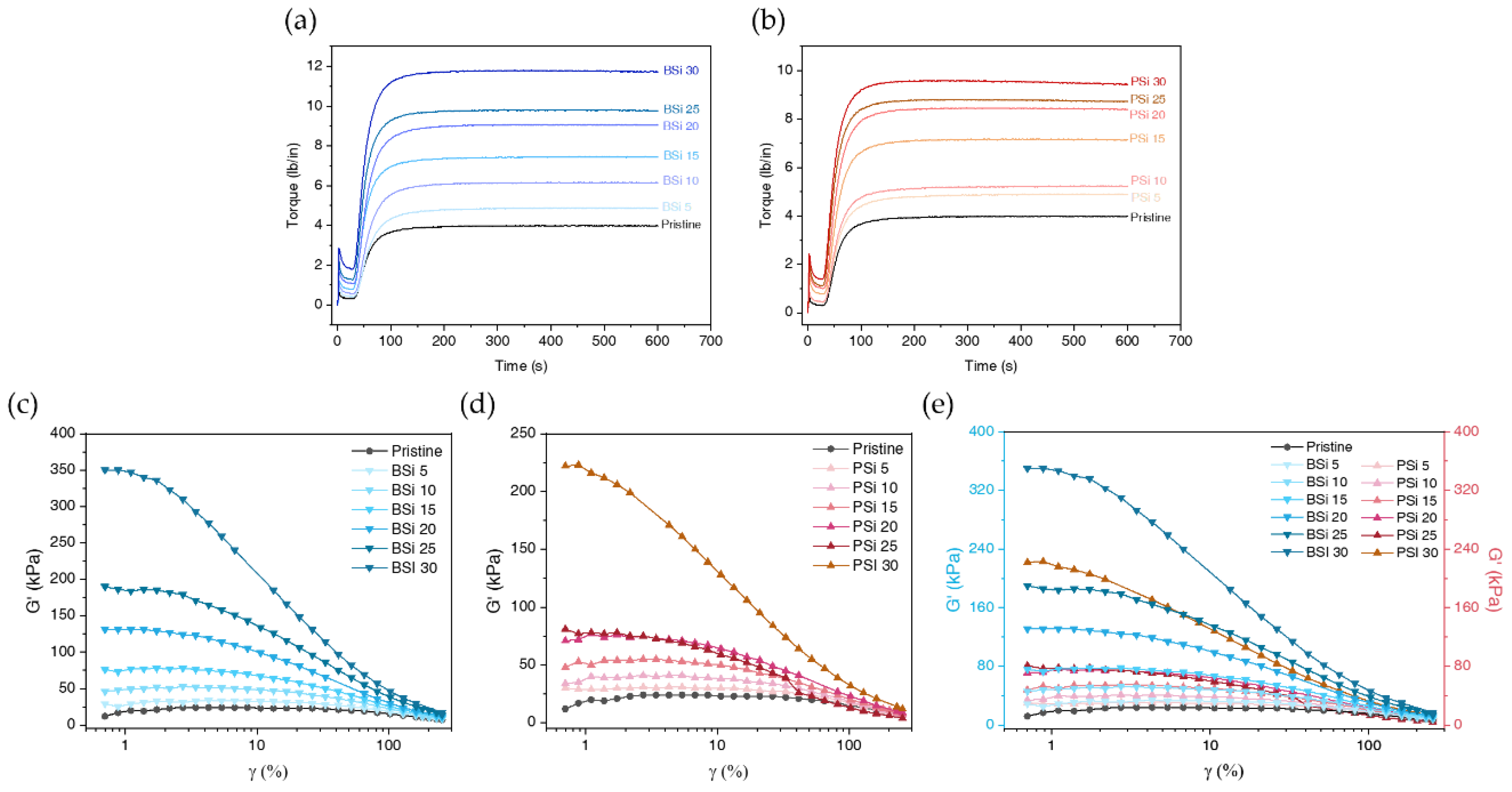
| BSi | PSi | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| phr | 5 | 10 | 15 | 20 | 25 | 30 | 5 | 10 | 15 | 20 | 25 | 30 | |
| PDMS gum | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | |
| Catalyst | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| Biosilica | 5 | 10 | 15 | 20 | 25 | 30 | |||||||
| Zeosil 175 | 5 | 10 | 15 | 20 | 25 | 30 | |||||||
| Sample | Modulus E100 (kPa) |
Modulus E300 (kPa) |
Tensile strength (kPa) |
Elongation at break (%) |
|---|---|---|---|---|
| Pristine | 21.38 | 29.32 | 33.73 | 400.40 |
| BSi 5 | 35.99 | 56.29 | 61.98 | 351.40 |
| BSi 10 | 43.25 | 77.57 | 98.65 | 386.10 |
| BSi 15 | 56.39 | 133.08 | 175.74 | 388.10 |
| BSi 20 | 79.73 | 189.46 | 270.57 | 412.10 |
| BSi 25 | 93.07 | 239.87 | 364.32 | 433.25 |
| BSi 30 | 129.06 | 317.93 | 462.68 | 424.20 |
| PSi 5 | 42.95 | 68.76 | 64.63 | 323.05 |
| PSi 10 | 41.68 | 80.51 | 83.94 | 319.80 |
| PSi 15 | 61.19 | 133.4 | 179.36 | 398.95 |
| PSi 20 | 74.33 | 170.24 | 313.91 | 510.35 |
| PSi 25 | 87.77 | 214.77 | 513.38 | 599.55 |
| PSi 30 | 108.07 | 288.22 | 565.45 | 510.70 |
| Sample | ts2 (s) |
tc90 (s) |
ML (lb/in) |
MH (lb/in) |
MH-ML (lb/in) |
|---|---|---|---|---|---|
| Pristine | 54 | 97 | 0.31 | 4.01 | 3.7 |
| BSi 5 | 54 | 109 | 0.41 | 4.9 | 4.49 |
| BSi 10 | 49 | 104 | 0.56 | 6.18 | 5.62 |
| BSi 15 | 42 | 90 | 0.79 | 7.46 | 6.67 |
| BSi 20 | 43 | 96 | 1.07 | 9.09 | 8.01 |
| BSi 25 | 40 | 85 | 1.27 | 9.82 | 8.54 |
| BSi 30 | 39 | 84 | 1.81 | 11.8 | 9.99 |
| PSi 5 | 51 | 104 | 0.43 | 4.91 | 4.47 |
| PSi 10 | 49 | 99 | 0.45 | 5.24 | 4.79 |
| PSi 15 | 45 | 93 | 0.78 | 7.18 | 6.4 |
| PSi 20 | 43 | 90 | 1.01 | 8.46 | 7.46 |
| PSi 25 | 40 | 82 | 1.11 | 8.82 | 7.71 |
| PSi 30 | 39 | 81 | 1.38 | 9.6 | 8.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





