1. Introduction
Cardiovascular disease (CVD) is a significant global health concern, consistently impacting human life and overall well-being. It is the leading cause of mortality worldwide, affecting millions of individuals of all ages and demographics. According to the World Health Organization (WHO), CVD is responsible for a staggering 9.6 million deaths annually in men and 8.9 million in women, collectively constituting nearly one-third of all deaths attributed to diseases. In the Philippines, the primary causes of death in 2021 were coronary heart disease (CHD), other cerebrovascular diseases or stroke, and various forms of cancer. Notably, there were over half a million reported cases of CHD in 2021, making up approximately 19% of the total deaths in the country (Bray et al., 2021; Corpuz, 2023; Daniels et al., 2014; Nguyen and Cheng-Lai, 2013; Timmis et al., 2020; Timmis et al., 2022).
Conventional treatments for heart failure involve a combination of medications such as Angiotensin converting enzyme inhibitors (ACEIs), beta-blockers, diuretics, corticosteroid receptor antagonists, sodium-glucose cotransporter two inhibitors, and others. These drugs aim to reduce the heart’s workload, enhance efficiency, alleviate symptoms, and target specific aspects of heart failure. Despite the extensive pharmaceutical options, heart failure remains a significant medical challenge, with persistently high rates of illness and mortality suggesting that current treatments, while valuable, often fall short in completely managing the condition (Chia et al., 2016; Krum et al., 2011).
Amaranthus viridis, commonly referred to as “Spinach Tagalog” or “Kolitis,” is a locally available vegetable in the Philippines and widely distributed in Asia. Abundant in bioactive compounds like flavonoids, alkaloids, saponins, and polyphenols, this plant has been linked to various pharmacological activities. Significantly, the bioactive compounds present in Amaranthus viridis demonstrates noteworthy properties including antioxidants, anti-inflammatory, antithrombotic, antiarrhytmic, and anti-hypertensive effect which collectively contribute to cardiovascular health (Bachheti et al., 2022; Pirdhankar et al., 2023; Saravanan and Ponmurugan, 2012; Shah et al., 2019).
The zebrafish (Danio rerio) is a versatile model organism in cardiovascular research. Native to Southeast Asia, this small tropical freshwater fish is particularly valuable for studying cardiac function and disease. A key feature is the transparency of zebrafish embryos, enabling non-invasive, real-time visualization of internal organs, including the developing heart. This transparency facilitates detailed observations of cardiac morphogenesis and function during various developmental stages, as early as 24 hours post-fertilization (Irion and Nüsslein-Volhard, 2022; Shen and Zuo, 2020; Tavares and Lopes, 2013).
Furthermore, zebrafish exhibit external development, and their embryos undergo rapid and synchronous growth. This characteristic makes them particularly suitable for studies involving developmental biology and the effects of various compounds on embryonic development, including potential therapeutic agents. The external development also allows for easy manipulation and observation, making zebrafish a cost-effective and time-efficient model for cardiovascular studies (Eimon and Ashkenazi, 2010). The findings from this research are expected to offer valuable insights that can contribute to further exploration and understanding within the field of cardiovascular health.
2. Material and Methods
2.1. Collection and Authentication of Plant Material
Approximately 2 kilograms of fresh young leaves from the actively growing tops of
Amaranthus viridis were collected at Barangay Tabunan, Cebu City as depicted in
Figure 1. Subsequently, a whole plant sample was transported to the Department of Biology at the University of San Carlos for botanical authentication by Mr. Val Salares. A voucher specimen was submitted to the University of San Carlos Herbarium.
2.2. Plant Extraction
The leaves were washed with distilled water to remove any external impurities and will be air-dried in the shade at 21-27°C for 2 weeks to remove excess moisture. The leaves were grounded to a fine powder using an Osterizer blender for 5 minutes to achieve 68-200 µm particle size. After grinding, straining was done to obtain a finely powdered particle using a 100 µm sieve strain (Alsaud and Farid, 2020). The finely powdered leaves were stored in Erlenmeyer flasks and covered with wooden cork to prevent contamination from moisture and other pollutants. Twenty grams of dried powder were macerated in 200 mL of ethanol solvent at room temperature for 72 hours. The extract was filtered using a Whatman filter paper number 1. Subsequently, the filtrate was transferred to a Heidolph rotary evaporator for evaporation, resulting in the collection of a solid concentrate (Gahlot et al., 2018). Following this, the extract was placed in amber vials and stored at a temperature of 4°C.
Preparations were made for various concentrations of 25, 50, 100, 200, and 400 µg/mL. The final crude extracts were resuspended to dimethyl sulfoxide (DMSO) and subjected to sonication to ensure homogeneity. They were stored in appropriately labeled amber vials for safety and future use (Lee and Yang, 2021).
2.3. Preparation of Egg Water
Throughout the experiment, the medium for zebrafish embryos was consisted of egg water. The egg water was prepared by thoroughly mixing 40 g of sea salt (Mars Fishcare Europe, UK) with 1 L of distilled water. Following this, 1.5 mL from the stock salts were dissolved in another 1 L of distilled water to attain a final concentration of 60 μg/ml (Westerfield, 2007).
2.4. Ethics Declaration
All protocols were subjected to approval by the Institutional Animal Care and Use Committee (IACUC) at the University of San Carlos. Furthermore, future experiments related to zebrafish were taken place at the Medical Biology Laboratory of the Department of Biology at the University of the Visayas.
2.5. Establishment of Zebrafish Aquarium and Husbandry
An aquarium with a divider in the center, measuring 30 cm in length, 20 cm in height, and 20 cm in width, was constructed using acrylic glass containing 5 liters of dechlorinated water. The tank was equipped with Precision aquarium air pumps (PR-7500 and PR-2500, China), Xinyou aquarium sponge filters (China), thermometers, and Zetlight control units (China). The water temperature in the tank was maintained between 24-29 ℃. Light conditions, following a 10-hour dark and 14-hour light cycle (10:14), were regulated using a timer. Tap water with a pH range of 6.5 to 8 was utilized for water quality. Monitoring and daily documentation of pH, total hardness, nitrate, nitrite, free chloride, and carbonate were carried out using 6in1 Aquarium freshwater test strips (China). To ensure water quality, a 25% water change was performed daily using AquaCare aquarium water conditioner (Seachem, Philippines), ParaGuard Parasite control (Seachem, Philippines), and anti-chlorine (SeaQuest). Weekly testing of ammonia levels was also conducted with Ammonia aquarium freshwater test kits (China). Zebrafish was housed at a density of 4-10 adult fish per liter and fed twice daily with dry feeds dispensed by a food timer (Warmtone, China), supplemented by one feeding of decapsulated brine shrimp. Routine cleaning, including the cleaning of sponge filters, was conducted weekly on the fish tanks. (Avdesh et al., 2012; Evidente et al., 2021).
2.6. Procurement of Zebrafish
A total of 420 at 72hpf zebrafish larvae were used for the entire study. To facilitate the breeding, 2 males and 2 females adult wild type zebrafish (> 6 months old with 3-4 cm in length) were purchased from a local breeder since a 1:1 ratio of male and female will give the highest production of embryos around 200 - 300 fertilized eggs and lesser aggression in the tank (Ruhl et al., 2009). The zebrafish was further identified by Mr. Dave Valles of the University of San Carlos. The identification of male and female zebrafish will be based on specific characteristics, as illustrated in
Figure 2. Upon acquisition, the differentiation between males and females were based on specific characteristics. Females were identified by their larger underbelly, while males were recognized for their slimmer and darker coloration compared to females. Subsequently, male and female zebrafish were separated and individually placed in containers.
For transportation, the zebrafish were carefully placed inside a container within a box and transported using an air-conditioned delivery car. Upon acquisition, a thorough inspection of the zebrafish was conducted to identify potential signs of sickness, with a focus on behaviors such as decreased movement, lethargy, low food intake, and anxiety. Daily records were diligently maintained to monitor their food intake. Additionally, the zebrafish’s anxiety behavior was observed as they initially dive to the bottom of a novel environment, followed by monitoring their exploration of the upper portion after six minutes, as indicated by Kirsten et al. (2018). The zebrafish were maintained according to standard protocols outlined by Alestrom et al. (2019) and Avdesh et al. (2012), with confirmation from the Institutional Animal Care and Use Committee (IACUC). During transportation, the protocol involved placing two adult zebrafish per 500 mL and maintaining a 1:1 ratio of air to water in the vessel. Additionally, adult zebrafish underwend a 24-hour period of fasting before transportation to reduce excretion and fouling in the transportation vessel.
2.7. Breeding and Embryo Isolation
The process of zebrafish breeding and isolation were conducted according to the protocol of outlined by the Zebrafish Information Network (ZFIN). Before spawning, a tank with a central divider housed a 1:2 ratio of male and female adult zebrafish. Separate breeding boxes, each holding less than 1 L of water, was arranged in a separate container filled with fresh tap water. In the evening, the zebrafish were introduced into the tank, and the following morning, when spawning occurs, fertilized eggs were collected on the enclosure floor. Zebrafish typically lay eggs within the first two hours after lights are turned on.
Post-spawning, the upper compartment, along with the adult fish, were transferred to a new tank or returned using a fishnet. The water containing settled eggs were siphoned and passed through a 0.10 mm strainer. The collected fertilized eggs were washed 3 times with egg water to remove debris. Subsequently, all fertilized eggs were transferred into each 1000 mL beakers filled with 100 mL egg water. The beakers were maintained at in a room temperature, providing an environment for the embryos to develop over a period of 72 hours. (Avdesh et al., 2012; Mason, 2016). Selection of embryos for analysis were based on symmetrical appearance in the one-cell stage or the presence of well-defined dark masses within a chorion, indicating fertility. Only embryos without morphological defects were chosen for further analysis.
2.8. Preliminary Mortality Test of A. viridis Extracts Against Zebrafish Larvae
Seventy zebrafish larvae at 72 hours post-fertilization were carefully distributed into a 96-well plate, with each well accommodating one larva. The larvae were subjected to 100 µL A. viridis leaf extracts at 25, 50, 100, 200, and 400 µg/mL concentration as a soaking drug, along with a vehicle control containing the highest concentration of DMSO used in the experimental treatments and a control group treated with egg water without any leaf extract. Each treatment concentration was replicated 10 times to ensure statistical reliability. The zebrafish larvae were left in a room temperature and were inspected after 24 hours. The concentration exhibiting the least number of dead larvae were recorded and then discarded. Death was judged by the coagulation of larvae, the absence of the heartbeat, or the lack of movement observed for 20 seconds. The extract associated with the zero-mortality rate or did not lead to any observable side effect under stereomicroscope was selected as the best extract for further investigation into its cardioprotective activity (Li et al., 2021).
2.9. Treatment Protocol for Assessing Cardioprotective Activity
A total of 240 zebrafish larvae (72 hpf) were used across 3 trials with 10 replicates per treatment. Each larva was placed in a well of a 96-well plate. The first treatment group received 100 µL of A. viridis extract at concentrations of 3.125, 6.25, 12.5, 25, and 50 µg/mL. The second group (positive control) received 100 µL of 200 µM eplerenone, the third group (negative control) received 100 µL of 200 µM verapamil, and the fourth group was exposed to egg water. After 4 hours of treatment, all groups were exposed to 100 µL of 200 µM verapamil for 30 minutes to induce heart failure. (Kossack et al., 2017; Maciag et al., 2022).
2.10. Heart Rate Assessment
The heart rate of each zebrafish larva per treatment were assessed. The zebrafish larvae have easily observable hearts, which can be seen through a stereomicroscope. A 15-second video of each specimen were recorded using a camera attached to the stereomicroscope. These videos were played in slow motion to accurately count the number of heartbeats. The heart rate was calculated using the formula (Heideman et al., 2005; Hoage et al., 2012):
2.11. Scoring on Cardiac Phenotypes
After the treatment, the zebrafish larvae were analyzed using a dissecting microscope. For imaging purposes, photographs were captured using a Nikon D750 camera. The acute heart failure (AHF) phenotypes were classified into 4 groups as shown in Figure 7. Category I end stage of AHF: heart with no contraction; Category II severe AHF: heart with contraction only in the atrium and no circulation; Category III mild AHF: heart with a distinct contraction in both cardiac chambers but morphological abnormalities, including edema, accumulated blood cells in front of the heart, stretched heart; Category IV normal heart. Larvae were tallied into the four AHF categories and the heart failure attenuation was determined by the percentage of each category (Haege et al., 2021; Hoyberghs et al., 2020; Maciag et al., 2022).
2.12. Disposal of Zebrafish Carcass
Disposal of the dead zebrafish followed the NIH guidelines (2013) by placing them into 1 part sodium hypochlorite and five parts water for 5 minutes. Then, the larvae were set into a tightly sealed bag and disposing of them in a yellow bin as clinical waste.
2.13. Qualitative Phytochemical Analysis
The qualitative phytochemicals analyses were involved in determining the primary group of bioactive compounds such as saponins, flavonoids, phenols, and alkaloids which are known to have cardioprotective properties. The most cardioprotective concentration were used for phytochemical analyses. All experiments were conducted in triplicate to ensure the reliability of results.
2.14. Statistical Analyses
The analysis began with descriptive statistics to summarize mortality rates at various concentrations. This involves calculating the mean mortality rate and standard deviation for each concentration of the verapamil and ethanolic leaf extract. Additionally, Kruskal-Wallis Test (Minitab 21.1.1) was applied to
describe the phenotypic differences in zebrafish hearts, followed by pairwise comparison. To compare the means of cardiac function indicators between the control group and the group treated with the candidate concentration extract, one-way analysis of variance (ANOVA), followed by Dunnett’s t-test will be utilized. A p-value of <0.05 were considered statistically significant.
3. Results
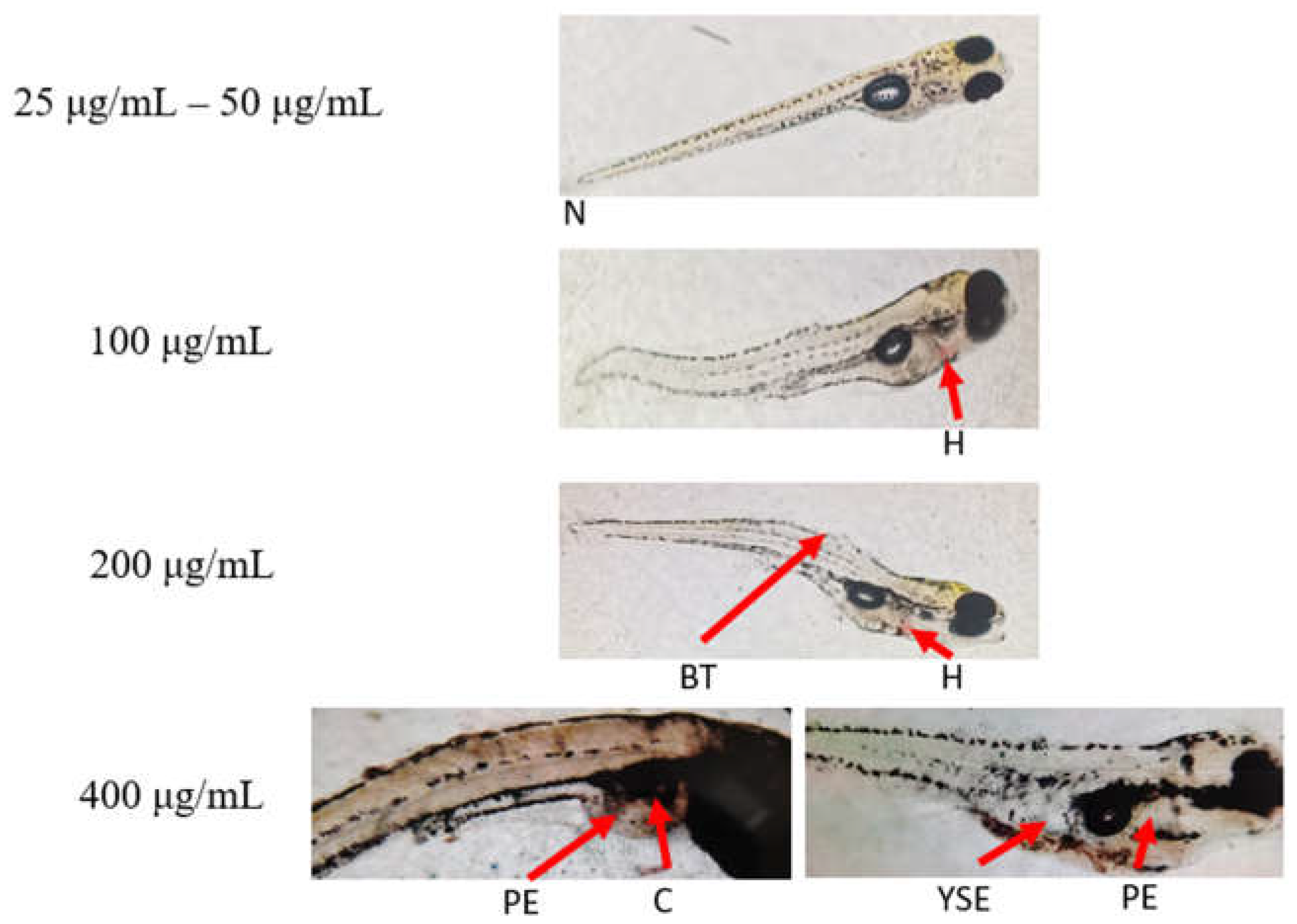
3.1. In Vivo Maximum Non-Toxic Concentration of the Ethanolic Leaf Extracts of A. viridis Against 72hpf Zebrafish
A. viridis leaf extract showed mortality and toxicity at concentrations starting from 50 μg/mL. At concentrations ranging from 100 μg/mL to 400 μg/mL, 100% mortality occurred with distinct morphological abnormalities such as hemorrhage, bent tails, pericardial edema, yolk sac edema, coagulation and a cloudy appearance. In contrast, all larvae exposed to 25 μg/mL remained alive, indicating this as the non-toxic concentration of A. viridis leaf extract.
3.2. Heart Rate Response of Verapamil-Induced Zebrafish Larvae Treated with A. viridis Leaf Ethanolic Extracts
As shown in
Figure 2, the heart rate of zebrafish larvae in egg water (control) was within the normal range of 120–180 bpm (De Luca et al., 2014), recorded at 151.57 ± 3.86 bpm. Verapamil treatment significantly reduced the heart rate to 71.60 ± 6.74 bpm, confirming heart failure (Li et al., 2024). However, eplerenone provided protection from heart damage, resulting in a moderate heart rate recovery to 126.37 ± 3.65 bpm. The cardioprotective effect of
A. viridis leaf extract was evident across all tested concentrations, from 50 μg/mL to 3.125 μg/mL, with heart rates of 91.47 ± 6.85 bpm, 118.20 ± 5.37 bpm, 121.20 ± 2.75 bpm, 120.30 ± 6.09 bpm, and 117.60 ± 6.16 bpm, respectively. These values indicate an improvement in heart rate, suggesting cardioprotective properties at each of these concentrations.
Figure 3.
Heart rate response of 72 hpf zebrafish treated with A. viridis leaf extract in verapamil-induced heart failure, expressed as mean ± SD. Means without ‘A’ differ significantly from the control (p < 0.001). ‘--’ indicates the normal heart rate range of 120–180 bpm.
Figure 3.
Heart rate response of 72 hpf zebrafish treated with A. viridis leaf extract in verapamil-induced heart failure, expressed as mean ± SD. Means without ‘A’ differ significantly from the control (p < 0.001). ‘--’ indicates the normal heart rate range of 120–180 bpm.
3.3. Cardiac Scoring of the Cardioprotective Response of A. viridis Leaf Ethanolic Extracts on Verpamil-Induced Heart Failure in Zebrafish Larvae
Based on the results, the verapamil-induced zebrafish larvae (negative control) had lower scores between 1 and 2, indicating heart failure in this group (Li et al., 2024). In contrast, the eplerenone-treated larvae (positive control) scored higher, between 3 and 4, suggesting protection from verapamil-induced damage. As shown in Figures 10 and 11, A. viridis demonstrated promising results, consistently scoring in the higher range across all concentrations (3.125, 6.25, 12.5, 25, and 50 μg/mL). Some larvae showed lower scores in the extract treatments, indicating individual variations in tolerance (Meyer et al., 2013). The Kruskal-Wallis test and pairwise comparisons revealed a highly significant difference between the negative control and the treatments (p < 0.001). The positive control did not differ significantly from any treatments. These results suggest that the treatments provide cardiac protection for the larvae, even at low concentrations, with the lowest effective concentration being 3.125 μg/mL.
Figure 4.
Cardiac scores of 72hpf zebrafish treated with A. viridis leaf extract in verapamil-induced heart failure model. Kruskal-Wallis test, followed by pairwise comparison with Dunn Bonferroni corrections were used in cardiac scoring for the cardiac phenotypes of the larvae. (** p ≤ 0.001); n = 80 larvae per treatment, divided into three independent experiments.
Figure 4.
Cardiac scores of 72hpf zebrafish treated with A. viridis leaf extract in verapamil-induced heart failure model. Kruskal-Wallis test, followed by pairwise comparison with Dunn Bonferroni corrections were used in cardiac scoring for the cardiac phenotypes of the larvae. (** p ≤ 0.001); n = 80 larvae per treatment, divided into three independent experiments.
Figure 5.
Cardiac scores of 72hpf zebrafish treated with A. viridis leaf extract in verapamil-induced heart failure model. Kruskal-Wallis test, followed by pairwise comparison with Dunn Bonferroni corrections were used in cardiac scoring for the cardiac phenotypes of the larvae. (** p ≤ 0.001); n = 80 larvae per treatment, divided into three independent experiments.
Figure 5.
Cardiac scores of 72hpf zebrafish treated with A. viridis leaf extract in verapamil-induced heart failure model. Kruskal-Wallis test, followed by pairwise comparison with Dunn Bonferroni corrections were used in cardiac scoring for the cardiac phenotypes of the larvae. (** p ≤ 0.001); n = 80 larvae per treatment, divided into three independent experiments.
3.4. Phytochemical Composition of A. viridis Crude Leaf Extracts
For the phytochemical screening, qualitative tests were conducted to detect the presence of alkaloids, flavonoids, phenols, and saponins in
A. viridis. As shown in
Table 1, the results revealed that
A. viridis contains flavonoids and saponins, while no alkaloids or phenols were detected.
Table 1.
Cardiac functions and phenotypes of 72hpf zebrafish in different treatments.
Table 1.
Cardiac functions and phenotypes of 72hpf zebrafish in different treatments.
| Treatments |
Concentration |
Cardiac functions and phenotypes |
| Egg water |
60 μg/mL |
Normal function and no visible abnormality |
| NC - Verapamil |
200 μM |
No contraction in the heart; Weak contraction in the atria; No circulation; Pericardial edema |
| PC - Eplerenone + Verapamil |
200 μM |
Some showed slow circulation, while most had no visible abnormalities |
| Leaf extract + Verapamil |
50 μg/mL |
Venous congestion; No contraction in the heart; Weak contraction in the atria; No circulation; |
| Leaf extract + Verapamil |
25 μg/mL |
Some showed slow circulation, while most had no visible abnormalities |
| Leaf extract + Verapamil |
12.5 μg/mL |
Some showed slow circulation, while most had no visible abnormalities |
| Leaf extract + Verapamil |
6.25 μg/mL |
Some showed slow circulation, while most had no visible abnormalities |
| Leaf extract + Verapamil |
3.125 μg/mL |
Some showed slow circulation, while most had no visible abnormalities |
Table 2.
Qualitative phytochemical composition of A. viridis. (‘+’ indicates presence and ‘-’ indicates absence.
Table 2.
Qualitative phytochemical composition of A. viridis. (‘+’ indicates presence and ‘-’ indicates absence.
| A. viridis |
| Alkaloids |
- |
| Flavonoids |
+ |
| Phenols |
- |
| Saponins |
+ |
3.5. In Vitro Free Radical Scavenging Activity of A. viridis Ethanolic Leaf Extracts
The DPPH radical scavenging activity of A. viridis ethanolic leaf extracts at various concentrations is presented in Figure 12. The extract exhibited the highest scavenging activity at 50 μg/mL, achieving an inhibition rate of 45.06% ± 10.90%. A concentration-dependent decline in scavenging activity was observed at lower concentrations, with inhibition rates of 34.95% ± 4.16% at 25 μg/mL, 24.36% ± 2.69% at 12.5 μg/mL, 9.92% ± 4.31% at 6.25 μg/mL, and 6.04% ± 4.66% at 3.125 μg/mL, respectively.
Figure 6.
Percentage inhibition of DPPH radical scavenging activity of various concentrations. Data were expressed as mean ± standard deviation. Means with different letters indicate a significant difference, p = <0.001.
Figure 6.
Percentage inhibition of DPPH radical scavenging activity of various concentrations. Data were expressed as mean ± standard deviation. Means with different letters indicate a significant difference, p = <0.001.
4. Discussion
Zebrafish (Danio rerio) is essential in cardiovascular research because their transparent embryos and larvae allow real-time observation of heart development and function (Echeazarra et al., 2021; Hou et al., 2022). Their hearts are similar to human hearts, making them ideal for studying cardiac diseases and therapies (Bowley et al., 2022; Brown et al., 2016). Additionally, zebrafish can regenerate cardiac tissue and are useful for examining the effects of various drugs on heart health (González-Rosa et al., 2017). Verapamil-induced heart failure in zebrafish is particularly valuable for research, as it mimics aspects of human heart failure and provides a platform for testing potential treatments and understanding disease mechanisms. These attributes make zebrafish a crucial model for advancing cardiovascular research and developing new treatments for heart disease (Li et al., 2022; Narumanchi et al., 2021; Zhu et al., 2018).
4.1. Toxicity of A. viridis Ethanolic Leaf Extracts on Zebrafish Larvae
The results demonstrated a direct proportional relationship between the concentration of A. viridis extract and mortality rates among zebrafish larvae. Specifically, higher concentrations of the extract were associated with increased mortality, while lower concentrations resulted in fewer to zero mortality. This observation is consistent with the findings of Abidin et al. (2020), who reported that higher concentrations of Piper sarmentosum reduce the survival of zebrafish larvae, particularly with prolonged exposure.
Moreover, Abidin et al. (2020) found that increased concentrations of bioactive compounds led to lower survival rates in zebrafish embryos. Alafiatayo et al. (2019) further supported this by noting that prolonged exposure to higher extract concentrations of Cucurma longa results in greater accumulation and increased toxicity in zebrafish.
Based on these findings, the maximum non-toxic concentration of A. viridis leaf extract for zebrafish larvae was determined to be 25 µg/mL. This concentration represents a tolerable level and serves as a suitable baseline for assessing potential cardioprotective effects while minimizing toxicity.
4.2. Cardioprotective Activity of the Ethanolic Leaf Extracts of A. viridis on Verapamil-Induced Zebrafish Larvae
This study demonstrated that A. viridis leaf extract exhibits cardioprotective activity at all tested concentrations on verapamil-induced heart failure in zebrafish larvae as shown through physio-morphological assessments. Verapamil is a calcium channel blocker used in humans to manage hypertension and certain heart conditions. In zebrafish larvae, verapamil induces heart damage by disrupting calcium ion influx into cardiac cells. Calcium is crucial for maintaining normal heart contractions and overall cardiac health, so this disruption impairs cardiac function and causes structural damage (Horng et al., 2024).
The damage is further exacerbated by oxidative stress, the downregulation of cardiomyocyte biomarkers, and the obstruction of protein synthesis (Chatterjee et al., 2010; Abbate, 2021). Phenolic compounds in plant extracts can interact with regulatory pathways, activating peroxisome proliferator-activated receptor gamma (PPARγ) and mitogen-activated protein kinase (MAPK). This activation helps to inhibit verapamil-induced cardiac injury (Dubińska-Magier et al., 2016).
Cardiac analysis revealed noticeable improvements in both heart rate and structure, even at very low concentrations. Similarly, Zhou et al. (2020) found that 100 μg/mL of timosaponin B-II extracted from Anemarrhena improved heart function due to its anti-inflammatory properties. Additionally, Chen et al. (2021) reported that 5 μg/mL of Gardenia jasminoides extract reduced inflammation in a zebrafish embryo model. These concentrations align with the significant levels used in morphological and molecular analyses in both studies. Based on the findings, the lowest effective concentration of A. viridis leaf extract for zebrafish larvae was determined to be 3.125 μg/mL.
4.3. Phytochemicals Composition of A. viridis Leaf Ethanolic Extracts
A. viridis was evaluated for its cardioprotective potential due to its bioactive compounds, which contribute to its medicinal properties, including antioxidant, anti-inflammatory, and anti-hypertensive activities. It is important to note that the phytochemical composition of A. viridis can vary due to environmental factors such as climate, seasonal changes, soil conditions, and geographical location (Kumar, 2017).
Phytochemical analysis is essential for identifying the specific bioactive compounds responsible for the observed cardioprotective effects. In this study, flavonoids and saponins were detected in the A. viridis leaf ethanolic extracts, which align with findings from Fouad et al. (2024) who reported the presence of flavonoids, phenols, and saponins in A. viridis extracts and highlighted their role in various therapeutic activities. Furthermore, Kumari et al., (2018) confirmed the presence of various phytochemicals in A. viridis leaf extract across different solvents, including aqueous, methanol, chloroform, and hexane. These phytochemicals included flavonoids, alkaloids, phenolics, steroids, terpenoids, saponins, cardiac glycosides, and tannins.
The cardioprotective activities of A. viridis leaf extract were notably linked to its flavonoid and saponin content. Flavonoids are well-documented for their antioxidant properties, which play a critical role in neutralizing reactive oxygen species (ROS) which helps preserve cardiac cell integrity and reduce inflammation within the heart. Additionally, flavonoids enhance endothelial function, promoting vascular relaxation and subsequently lowering blood pressure (Ullah et al., 2020). This protective mechanism is consistent with findings by Kumar et al. (2013), who observed that flavonoids from A. viridis significantly reduced oxidative stress and inflammation in rat models, thereby decreasing cardiotoxicity.
Saponins, another key component of A. viridis leaf extract, also contribute to cardioprotection through their anti-inflammatory and antihypertensive properties. They modulate immune responses and reduce the production of pro-inflammatory cytokines, thus alleviating inflammation in cardiovascular tissues. Saponins can improve vascular function and reduce blood vessel constriction, further aiding in blood pressure regulation. Saravanan et al. (2013) demonstrated that saponins from A. viridis mitigated inflammation and improved cardiac function in isoproterenol (ISO)-induced heart failure in rats.
The mechanisms underlying these effects involve several biochemical processes. Flavonoids reduce oxidative stress by scavenging ROS and inhibiting inflammatory pathways, which helps maintain cardiovascular health. Saponins contribute to this protective effect by reducing inflammation and improving vascular health (Li et al., 2020).
Supporting these observations, Krishna et al. (2023) reported that flavonoids and saponins from A. viridis alleviated verapamil-induced cardiotoxicity in rat models through their antioxidant and anti-inflammatory actions.
4.4. Free Radical Scavenging Activity of A. viridis Ethanolic Leaf Extract
The results demonstrated significant free radical scavenging activity of A. viridis ethanolic leaf extract in all concentrations tested, which contributes to its cardioprotective properties. This antioxidant potential plays a crucial role in preventing oxidative stress-induced cardiac damage and supports the traditional use of A. viridis in treating cardiovascular diseases (Valaei et al., 2021).
The antioxidant property of A. viridis can be primarily attributed to its phytochemical composition such as flavonoid and saponins. These compounds are known for their ability to chelate metal ions and inhibit lipid peroxidation, which are crucial mechanisms in preventing oxidative stress-induced cardiac damage. The presence of these bioactive compounds supports the extract’s observed cardioprotective effects in verapamil-induced heart failure (Kumari et al., 2018).
Furthermore, the ability of A. viridis leaf extract to scavenge free radicals correlates with its protective effects against verapamil-induced heart failure in zebrafish. This correlation is supported by research of Nowak et al., (2018), who demonstrated that natural antioxidants could effectively protect cardiac damage from oxidative stress.
The observed free radical scavenging activity also supports the traditional use of A. viridis in various cardiovascular conditions. As noted by Lalhminghlui and Jagetia (2023), natural antioxidants from plant sources often provide additional benefits beyond their primary antioxidant activity, including anti-inflammatory and membrane-stabilizing properties, which contribute to their overall therapeutic effect.
5. Conclusions
Cardiovascular disease (CVD) remains one of the most pressing health challenges worldwide, requiring immediate and effective management. With the incidence of CVDs continuing to rise, there is an increasing need for alternative treatments to complement existing therapies. Natural products, which are abundant in bioactive compounds, offer promising potential in addressing this issue. These bioactive compounds, derived from plants and other natural sources, have shown potential for therapeutic use, making them a valuable resource in the search for new, effective treatments for cardiovascular conditions.
The zebrafish larvae were able to tolerate concentration of up to 25 μg/mL of A. viridis ethanolic leaf extract, indicating that their tolerance to the extract is dose-dependent. Even at the lowest concentration of 3.125 μg/mL, the extract still exhibited cardioprotective activity, as shown through physio-morphological analyses in zebrafish larvae exposed to verapamil-induced heart damage.
6. Recommendation
Additional improvements for future research are advised to enrich the pharmacological application of plant extracts on a verapamil-induced zebrafish research model. It is encouraged to isolate and identify the specific cardioprotective compounds of the whole plant extracts. Since only the end-stage (72 hpf) is applied in both survival and phenotype screening, future studies can increase the time frame and include observations during 96, 120, and 144 hpf. It is also recommended to incorporate blood flow in addition to the heart rate of the treated zebrafish larvae. Lastly, quantitative analysis on the levels of Reactive Oxygen Species (ROS) could also be performed to measure the oxidative stress and inflammatory pathways.
Author Contributions
Writing—original draft, Leonel Paolo Rodriguez; Supervision, Norielyn Abalos.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Abdel-Alim, M.E.; Serag, M.S.; Moussa, H.R.; Elgendy, M.A.; Mohesien, M.T.; Salim, N.S. Phytochemical Screening and Antioxidant Potential of Lotus corniculatusand Amaranthus viridis. Egypt. J. Bot. 2023, 63, 665–681. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Hanif, S.; Iftkhar, T. Phytochemical Profiling with Antioxidant and Antimicrobial Screening of Amaranthus viridis L. Leaf and Seed Extracts. Open J. Med Microbiol. 2013, 03, 164–171. [Google Scholar] [CrossRef]
- Aronson, J. K. (Ed.). (2014). Meyler’s Side Effects of Drugs 15E: The International Encyclopedia of Adverse Drug Reactions and Interactions. Newnes.
- Bachheti, R.K.; Worku, L.A.; Gonfa, Y.H.; Zebeaman, M.; Deepti; Pandey, D.P.; Bachheti, A. Prevention and Treatment of Cardiovascular Diseases with Plant Phytochemicals: A Review. Evidence-Based Complement. Altern. Med. 2022, 2022, 1–21. [CrossRef]
- Bagher, P.; Segal, S.S. Regulation of blood flow in the microcirculation: role of conducted vasodilation. Acta Physiol. 2010, 202, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, K.; Chopra, C.; Bhardwaj, P.; Dhanjal, D.S.; Singh, R.; Najda, A.; Cruz-Martins, N.; Singh, S.; Sharma, R.; Kuča, K.; et al. Biogenic Metallic Nanoparticles from Seed Extracts: Characteristics, Properties, and Applications. J. Nanomater. 2022, 2022, 1–22. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Cao, B.; Varghese, C.; Mikkelsen, B.; Weiderpass, E.; Soerjomataram, I. Comparing cancer and cardiovascular disease trends in 20 middle- or high-income countries 2000–19: A pointer to national trajectories towards achieving Sustainable Development goal target 3.4. Cancer Treat. Rev. 2021, 100, 102290. [Google Scholar] [CrossRef]
- Brittijn, S.A.; Duivesteijn, S.J.; Belmamoune, M.; Bertens, L.F.; Bitter, W.; Debruijn, J.D.; Champagne, D.L.; Cuppen, E.; Flik, G.; Vandenbroucke-Grauls, C.M.; et al. Zebrafish development and regeneration: new tools for biomedical research. Int. J. Dev. Biol. 2009, 53, 835–850. [Google Scholar] [CrossRef]
- Brown, D.R.; Samsa, L.A.; Qian, L.; Liu, J. Advances in the Study of Heart Development and Disease Using Zebrafish. J. Cardiovasc. Dev. Dis. 2016, 3, 13. [Google Scholar] [CrossRef] [PubMed]
- Bucciarelli, V.; Caterino, A.L.; Bianco, F.; Caputi, C.G.; Salerni, S.; Sciomer, S.; Maffei, S.; Gallina, S. Depression and cardiovascular disease: The deep blue sea of women's heart. Trends Cardiovasc. Med. 2019, 30, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Chia, N. , Fulcher, J., & Keech, A. (2016). Beta-blocker, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker, nitrate-hydralazine, diuretics, aldosterone antagonist, ivabradine, devices and digoxin (BANDAID 2): an evidence-based mnemonic for the treatment of systolic heart failure. Internal medicine journal, 46(6), 653-662.
- Choy, K.W.; Murugan, D.; Mustafa, M.R. Natural products targeting ER stress pathway for the treatment of cardiovascular diseases. Pharmacol. Res. 2018, 132, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Corpuz, J. C. (2023). Cardiovascular disease in the Philippines: a new public health emergency? Journal of Public Health, fdad175.
- Daniels, M. , Donilon, T., & Bollyky, T. J. (2014). The emerging global health crisis: noncommunicable diseases in low-and middle-income countries. Council on Foreign Relations independent task force report, (72).
- Dlugos, C.A.; Rabin, R.A. Structural and Functional Effects of Developmental Exposure to Ethanol on the Zebrafish Heart. Alcohol. Clin. Exp. Res. 2010, 34, 1013–1021. [Google Scholar] [CrossRef]
- Dubey, A.; Ghosh, N.S.; Singh, R. Zebrafish as An Emerging Model: An Important Testing Platform for Biomedical Science. J. Pharm. Negat. Results 2022, 13, 1–7. [Google Scholar] [CrossRef]
- Dweck, M.R.; Williams, M.C.; Moss, A.J.; Newby, D.E.; Fayad, Z.A. Computed Tomography and Cardiac Magnetic Resonance in Ischemic Heart Disease. Circ. 2016, 68, 2201–2216. [Google Scholar] [CrossRef] [PubMed]
- Eimon, P.M.; Ashkenazi, A. The zebrafish as a model organism for the study of apoptosis. Apoptosis 2009, 15, 331–349. [Google Scholar] [CrossRef] [PubMed]
- Forbes, E.L.; Preston, C.D.; Lokman, P.M. Zebrafish (Danio rerio) and the egg size versus egg number trade off: effects of ration size on fecundity are not mediated by orthologues of the Fec gene. Reprod. Fertil. Dev. 2010, 22, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Fukuta, H.; Little, W.C. The Cardiac Cycle and the Physiologic Basis of Left Ventricular Contraction, Ejection, Relaxation, and Filling. Hear. Fail. Clin. 2008, 4, 1–11. [Google Scholar] [CrossRef]
- Gandhi, P., Samarth, R. M., & Peter, K. (2020). Bioactive Compounds of Amaranth (Genus Amaranthus). Bioactive Compounds in Underutilized Vegetables and Legumes, 1-37.
- Geetha, R.G.; Ramachandran, S. Recent Advances in the Anti-Inflammatory Activity of Plant-Derived Alkaloid Rhynchophylline in Neurological and Cardiovascular Diseases. Pharmaceutics 2021, 13, 1170. [Google Scholar] [CrossRef]
- Genge, C. E., Lin, E., Lee, L., Sheng, X., Rayani, K., Gunawan, M., & Tibbits, G. F. (2016). The zebrafish heart as a model of mammalian cardiac function. Reviews of Physiology, Biochemistry and Pharmacology, Vol. 171, 99-136.
- Giardoglou, P.; Beis, D. On Zebrafish Disease Models and Matters of the Heart. Biomedicines 2019, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- González-Rosa, J.M. Zebrafish Models of Cardiac Disease: From Fortuitous Mutants to Precision Medicine. Circ. Res. 2022, 130, 1803–1826. [Google Scholar] [CrossRef]
- Haege, E.R.; Huang, H.-C.; Huang, C.-C. Identification of Lactate as a Cardiac Protectant by Inhibiting Inflammation and Cardiac Hypertrophy Using a Zebrafish Acute Heart Failure Model. Pharmaceuticals 2021, 14, 261. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-C.; Monte, A.; Cook, J.M.; Kabir, M.S.; Peterson, K.P. Zebrafish Heart Failure Models for the Evaluation of Chemical Probes and Drugs. ASSAY Drug Dev. Technol. 2013, 11, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Hu, N. , Sedmera, D., Yost, H. J., & Clark, E. B. (2000). Structure and function of the developing zebrafish heart. The Anatomical Record: An Official Publication of the American Association of Anatomists, 260(2), 148-157.
- Iamonico, D.; Hussain, A.N.; Sindhu, A.; Kumar, V.N.S.A.; Shaheen, S.; Munir, M.; Fortini, P. Trying to Understand the Complicated Taxonomy in Amaranthus (Amaranthaceae): Insights on Seeds Micromorphology. Plants 2023, 12, 987. [Google Scholar] [CrossRef]
- Imam, M.U.; Zhang, S.; Ma, J.; Wang, H.; Wang, F. Antioxidants Mediate Both Iron Homeostasis and Oxidative Stress. Nutrients 2017, 9, 671. [Google Scholar] [CrossRef]
- Iqbal, M. J., Hanif, S., Mahmood, Z., Anwar, F., & Jamil, A. (2012). Antioxidant and antimicrobial activities of Chowlai (Amaranthus viridis L.) leaf and seed extracts. J. Med. Plants Res, 6(27), 4450-4455.
- Irion, U., & Nüsslein-Volhard, C. (2022). Developmental genetics with model organisms. Proceedings of the National Academy of Sciences, 119(30), e2122148119.
- Jewhurst, K.; McLaughlin, K.A. Beyond the Mammalian Heart: Fish and Amphibians as a Model for Cardiac Repair and Regeneration. J. Dev. Biol. 2015, 4, 1. [Google Scholar] [CrossRef]
- Jin, L.; Pan, Y.; Li, Q.; Li, J.; Wang, Z. Elabela gene therapy promotes angiogenesis after myocardial infarction. J. Cell. Mol. Med. 2021, 25, 8537–8545. [Google Scholar] [CrossRef] [PubMed]
- Jing, L. , Xi, L., Chen, Y., Chen, Z., Zhang, L. H., Fang, F., & Jiang, L. X. (2013). National survey of doctor-reported secondary preventive treatment for patients with acute coronary syndrome in China. Chinese medical journal, 126(18), 3451-3455.
- Jyotsna, F.; Ahmed, A.; Kumar, K.; Kaur, P.; Chaudhary, M.H.; Kumar, S.; Khan, E.; Khanam, B.; Shah, S.U.; Varrassi, G.; et al. Exploring the Complex Connection Between Diabetes and Cardiovascular Disease: Analyzing Approaches to Mitigate Cardiovascular Risk in Patients With Diabetes. Cureus 2023, 15, e43882. [Google Scholar] [CrossRef] [PubMed]
- Keßler, M.; Just, S.; Rottbauer, W. Ion Flux Dependent and Independent Functions of Ion Channels in the Vertebrate Heart: Lessons Learned from Zebrafish. Stem Cells Int. 2012, 2012, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kirshenblatt-Gimblett, B.; Fernandez, D.G. Culture Ingested: On the Indigenization of Phillipine Food. Gastronomica 2003, 3, 58–71. [Google Scholar] [CrossRef]
- Kossack, M.; Hein, S.; Juergensen, L.; Siragusa, M.; Benz, A.; Katus, H.A.; Most, P.; Hassel, D. Induction of cardiac dysfunction in developing and adult zebrafish by chronic isoproterenol stimulation. J. Mol. Cell. Cardiol. 2017, 108, 95–105. [Google Scholar] [CrossRef]
- Kris-Etherton, P. M. , Petersen, K. S., Velarde, G., Barnard, N. D., Miller, M., Ros, E.,& Freeman, A. M. (2020). Barriers, opportunities, and challenges in addressing disparities in diet-related cardiovascular disease in the United States. Journal of the American Heart Association, 9(7), e014433.
- Krum, H.; Massie, B.; Abraham, W.T.; Dickstein, K.; Kober, L.; McMurray, J.J.; Desai, A.; Gimpelewicz, C.; Kandra, A.; Reimund, B.; et al. Direct renin inhibition in addition to or as an alternative to angiotensin converting enzyme inhibition in patients with chronic systolic heart failure: rationale and design of the Aliskiren Trial to Minimize OutcomeS in Patients with HEart failuRE (ATMOSPHERE) study. Eur. J. Hear. Fail. 2011, 13, 107–114. [Google Scholar] [CrossRef]
- Anuradha; Kumari, M. ; Zinta, G.; Chauhan, R.; Kumar, A.; Singh, S.; Singh, S. Genetic resources and breeding approaches for improvement of amaranth (Amaranthus spp.) and quinoa (Chenopodium quinoa). Front. Nutr. 2023, 10, 1129723. [Google Scholar] [CrossRef]
- Lahera, V.; Goicoechea, M.; De Vinuesa, S.G.; Miana, M.; de las Heras, N.; Cachofeiro, V.; Luño, J. Endothelial dysfunction, oxidative stress and inflammation in atherosclerosis: beneficial effects of statins. Curr. Med. Chem. 2007, 14, 243–248. [Google Scholar] [CrossRef]
- Lee, R.T.; Walsh, K. The Future of Cardiovascular Regenerative Medicine. Circulation 2016, 133, 2618–2625. [Google Scholar] [CrossRef]
- Li, S. , Liu, H., Li, Y., Qin, X., Li, M., Shang, J., & Zhou, M. (2021). Shen-Yuan-Dan capsule attenuates verapamil-induced zebrafish heart failure and exerts antiapoptotic and anti-inflammatory effects via reactive oxygen species–induced NF-κB pathway. Frontiers in Pharmacology, 12, 626515.
- Li, Q.; Wang, P.; Chen, L.; Gao, H.; Wu, L. Acute toxicity and histopathological effects of naproxen in zebrafish (Danio rerio) early life stages. Environ. Sci. Pollut. Res. 2016, 23, 18832–18841. [Google Scholar] [CrossRef]
- Li, J.; Zhu, Y.; Zhao, X.; Zhao, L.; Wang, Y.; Yang, Z. Screening of anti-heart failure active compounds from fangjihuangqi decoction in verapamil-induced zebrafish model by anti-heart failure index approach. Front. Pharmacol. 2022, 13, 999950. [Google Scholar] [CrossRef]
- Low Wang, C. C. , Hess, C. N., Hiatt, W. R., & Goldfine, A. B. (2016). Clinical update: cardiovascular disease in diabetes mellitus: atherosclerotic cardiovascular disease and heart failure in type 2 diabetes mellitus–mechanisms, management, and clinical considerations. Circulation, 133(24), 2459-2502.
- Mahmood, A., Eqan, M., Pervez, S., Javed, R., Ullah, R., Islam, A., & Rafiq, M. (2021). Drugs Resistance in Heart Diseases. Biochemistry of Drug Resistance, 295-334.
- Meid, H. , & S Haddad, P. (2017). The antidiabetic potential of quercetin: underlying mechanisms. Current medicinal chemistry, 24(4), 355-364.
- Minamino, T. (2012). Cardioprotection From Ischemia/Reperfusion Injury–Basic and Translational Research–. Circulation Journal, 76(5), 1074-1082.
- Monteiro, L.M.; Vasques-Nóvoa, F.; Ferreira, L.; Pinto-Do-Ó, P.; Nascimento, D.S. Restoring heart function and electrical integrity: closing the circuit. npj Regen. Med. 2017, 2, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C. , & Cheng-Lai, A. (2013). The polypill: a potential global solution to cardiovascular disease. Cardiology in review, 21(1), 49-54.
- Nowbar, A. N. , Gitto, M., Howard, J. P., Francis, D. P., & Al-Lamee, R. (2019). Mortality from ischemic heart disease: Analysis of data from the World Health Organization and coronary artery disease risk factors From NCD Risk Factor Collaboration. Circulation: cardiovascular quality and outcomes, 12(6), e005375.
- Omondi, J. O. (2017). Phenotypic variation in morphology, yield and seed quality in selected accessions of leafy Amaranths (Doctoral dissertation, Maseno University).
- Pearson, T. A. , Mensah, G. A., Alexander, R. W., Anderson, J. L., Cannon III, R. O., Criqui, M. & Vinicor, F. (2003). Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. circulation, 107(3), 499-511.
- Peter, K.; Gandhi, P. Rediscovering the therapeutic potential of Amaranthus species: A review. Egypt. J. Basic Appl. Sci. 2017, 4, 196–205. [Google Scholar] [CrossRef]
- Pichaivel, M. , Dhandayuthapani, D., Porwal, O., Sharma, P. K., & Manickam, D. (2022). Phytochemical And Pharmacological Evaluation Of Hydroalcoholic Extract Of Amaranthus Viridis Linn. Journal of Pharmaceutical Negative Results, 2250-2269.
- Pirdhankar, M. S. , Sadamate, P. S., & Tasgaonkar, D. R. (2023). Cardioprotective Herbal Plants: A Review. International Journal for Research in Applied Science & Engineering Technology, 11(3), 1114-1118.
- Plein, S.; Greenwood, J.P.; Ridgway, J.P.; Cranny, G.; Ball, S.G.; Sivananthan, M.U. Assessment of non–ST-segment elevation acute coronary syndromes with cardiac magnetic resonance imaging. Circ. 2004, 44, 2173–2181. [Google Scholar] [CrossRef]
- Poon, K.L.; Brand, T. The zebrafish model system in cardiovascular research: A tiny fish with mighty prospects. Glob. Cardiol. Sci. Pr. 2013, 2013, 9–28. [Google Scholar] [CrossRef] [PubMed]
- Pruthvi, N. (2010). Phytochemical Studies And Evaluation Of Leaves Of Amaranthus Viridis (Amaranthaceae) For Antidiabetic Activity (Doctoral dissertation, Rajiv Gandhi University of Health Sciences (India).
- Raggi, P.; Genest, J.; Giles, J.T.; Rayner, K.J.; Dwivedi, G.; Beanlands, R.S.; Gupta, M. Role of inflammation in the pathogenesis of atherosclerosis and therapeutic interventions. Atherosclerosis 2018, 276, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Ribas, L.; Piferrer, F. The zebrafish (Danio rerio) as a model organism, with emphasis on applications for finfish aquaculture research. Rev. Aquac. 2013, 6, 209–240. [Google Scholar] [CrossRef]
- Rotariu, D.; Babes, E.E.; Tit, D.M.; Moisi, M.; Bustea, C.; Stoicescu, M.; Radu, A.-F.; Vesa, C.M.; Behl, T.; Bungau, A.F.; et al. Oxidative stress – Complex pathological issues concerning the hallmark of cardiovascular and metabolic disorders. Biomed. Pharmacother. 2022, 152, 113238. [Google Scholar] [CrossRef]
- Salvamani, S. , Gunasekaran, B., Shukor, M. Y., Shaharuddin, N. A., Sabullah, M. K., & Ahmad, S. A. (2016). Anti-HMG-CoA reductase, antioxidant, and anti-inflammatory activities of Amaranthus viridis leaf extract as a potential treatment for hypercholesterolemia. Evidence-Based Complementary and Alternative Medicine, 2016.
- Santoso, F.; Farhan, A.; Castillo, A.L.; Malhotra, N.; Saputra, F.; Kurnia, K.A.; Chen, K.H.-C.; Huang, J.-C.; Chen, J.-R.; Hsiao, C.-D. An Overview of Methods for Cardiac Rhythm Detection in Zebrafish. Biomedicines 2020, 8, 329. [Google Scholar] [CrossRef] [PubMed]
- Sapna, F.; Raveena, F.; Chandio, M.; Bai, K.; Sayyar, M.; Varrassi, G.; Khatri, M.; Kumar, S.; Mohamad, T. Advancements in Heart Failure Management: A Comprehensive Narrative Review of Emerging Therapies. Cureus 2023, 15, e46486. [Google Scholar] [CrossRef]
- Saravanan, G.; Ponmurugan, P. Amaranthus viridis Linn., a common spinach, modulates C-reactive protein, protein profile, ceruloplasmin and glycoprotein in experimental induced myocardial infarcted rats. J. Sci. Food Agric. 2012, 92, 2459–2464. [Google Scholar] [CrossRef] [PubMed]
- Sarker, U.; Oba, S. Nutraceuticals, antioxidant pigments, and phytochemicals in the leaves of Amaranthus spinosus and Amaranthus viridis weedy species. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Schwitter, J.; Wacker, C.M.; Wilke, N.; Al-Saadi, N.; Sauer, E.; Huettle, K.; Schönberg, S.O.; Luchner, A.; Strohm, O.; Ahlstrom, H.; et al. MR-IMPACT II: Magnetic Resonance Imaging for Myocardial Perfusion Assessment in Coronary artery disease Trial: perfusion-cardiac magnetic resonance vs. single-photon emission computed tomography for the detection of coronary artery disease: a comparative multicentre, multivendor trial. Eur. Hear. J. 2012, 34, 775–781. [Google Scholar] [CrossRef]
- Sedmera, D. , Reckova, M., DeAlmeida, A., Sedmerova, M., Biermann, M., Volejnik, J., & Thompson, R. P. (2003). Functional and morphological evidence for a ventricular conduction system in zebrafish and Xenopus hearts. American Journal of Physiology-Heart and Circulatory Physiology, 284(4), H1152-H1160.
- Shah, S.M.A.; Akram, M.; Riaz, M.; Munir, N.; Rasool, G. Cardioprotective Potential of Plant-Derived Molecules: A Scientific and Medicinal Approach. Dose-Response 2019, 17. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Zuo, Z. Zebrafish (Danio rerio) as an excellent vertebrate model for the development, reproductive, cardiovascular, and neural and ocular development toxicity study of hazardous chemicals. Environ. Sci. Pollut. Res. 2020, 27, 43599–43614. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.M.; Danaei, G.; Farzadfar, F.; Stevens, G.A.; Woodward, M.; Wormser, D.; Kaptoge, S.; Whitlock, G.; Qiao, Q.; Lewington, S.; et al. The Age-Specific Quantitative Effects of Metabolic Risk Factors on Cardiovascular Diseases and Diabetes: A Pooled Analysis. PLOS ONE 2013, 8, e65174. [Google Scholar] [CrossRef] [PubMed]
- Stajer, D.; Bervar, M.; Horvat, M. CARDIOGENIC SHOCK FOLLOWING A SINGLE THERAPEUTIC ORAL DOSE OF VERAPAMIL. Int. J. Clin. Pr. 2001, 55, 69–70. [Google Scholar] [CrossRef]
- Sogorski, A.; Spindler, S.; Wallner, C.; Dadras, M.; Wagner, J.; Behr, B.; Lehnhardt, M.; Kolbenschlag, J. Optimizing remote ischemic conditioning (RIC) of cutaneous microcirculation in humans: Number of cycles and duration of acute effects. J. Plast. Reconstr. Aesthetic Surg. 2020, 74, 819–827. [Google Scholar] [CrossRef]
- Srivastava, D. , & Baldwin, H. S. (2001). Molecular determinants of cardiac development. Moss and Adams’ Heart Disease in Infants, Children, and Adolescents, Hugh D. Allen, Howard P. Gutgesell, et al., 3-23.
- Stevens, C.M.; Rayani, K.; Genge, C.E.; Singh, G.; Liang, B.; Roller, J.M.; Li, C.; Li, A.Y.; Tieleman, D.P.; van Petegem, F.; et al. Characterization of Zebrafish Cardiac and Slow Skeletal Troponin C Paralogs by MD Simulation and ITC. Biophys. J. 2016, 111, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Sullivan-Brown, J.; E Bisher, M.; Burdine, R.D. Embedding, serial sectioning and staining of zebrafish embryos using JB-4 resin. Nat. Protoc. 2010, 6, 46–55. [Google Scholar] [CrossRef]
- Swathy, M.; Saruladha, K. A comparative study of classification and prediction of Cardio-Vascular Diseases (CVD) using Machine Learning and Deep Learning techniques. ICT Express 2021, 8, 109–116. [Google Scholar] [CrossRef]
- Tavares, B.; Lopes, S.S. The Importance of Zebrafish in Biomedical Research. Acta medica Port. 2013, 26, 583–592. [Google Scholar] [CrossRef]
- Timmis, A.; Townsend, N.; Gale, C.P.; Torbica, A.; Lettino, M.; Petersen, S.E.; Mossialos, E.A.; Maggioni, A.P.; Kazakiewicz, D.; May, H.T.; et al. European Society of Cardiology: Cardiovascular Disease Statistics 2019. Eur. Heart J. 2020, 41, 12–85. [Google Scholar] [CrossRef]
- Timmis, A.; Vardas, P.; Townsend, N.; Torbica, A.; Katus, H.; De Smedt, D.; Gale, C.P.; Maggioni, A.P.; Petersen, S.E.; Huculeci, R.; et al. European Society of Cardiology: cardiovascular disease statistics 2021. Eur. Hear. J. 2022, 43, 716–799. [Google Scholar] [CrossRef] [PubMed]
- Ward, E.; Jemal, A.; Cokkinides, V.; Singh, G.K.; Cardinez, C.; Ghafoor, A.; Thun, M. Cancer Disparities by Race/Ethnicity and Socioeconomic Status. CA: A Cancer J. Clin. 2004, 54, 78–93. [Google Scholar] [CrossRef] [PubMed]
- White, R.; Rose, K.; Zon, L. Zebrafish cancer: the state of the art and the path forward. Nat. Rev. Cancer 2013, 13, 624–636. [Google Scholar] [CrossRef]
- Wiciński, M.; Socha, M.; Walczak, M.; Wódkiewicz, E.; Malinowski, B.; Rewerski, S.; Górski, K.; Pawlak-Osińska, K. Beneficial Effects of Resveratrol Administration—Focus on Potential Biochemical Mechanisms in Cardiovascular Conditions. Nutrients 2018, 10, 1813. [Google Scholar] [CrossRef]
- Williams, J.S.; Walker, R.J.; Egede, L.E. Achieving Equity in an Evolving Healthcare System: Opportunities and Challenges. Am. J. Med Sci. 2016, 351, 33–43. [Google Scholar] [CrossRef]
- World Health Organization. (2007). Prevention of cardiovascular disease. Pocket guidelines for assessment and management of cardiovascular risk. Africa: Who/Ish cardiovascular risk prediction charts for the African region. World Health Organization.
- Zhu, X.-Y.; Wu, S.-Q.; Guo, S.-Y.; Yang, H.; Xia, B.; Li, P.; Li, C.-Q. A Zebrafish Heart Failure Model for Assessing Therapeutic Agents. Zebrafish 2018, 15, 243–253. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).