1. Introduction
Passion fruit, a member of the Passiflora family, is widely cultivated in tropical and subtropical regions, also known as "Brazilian fruit", "passion fruit," and "egg fruit" [
1]. Passion fruit is favored by many consumers due to its delicious flavor and rich nutritional content, as well as its anti-inflammatory, antibacterial, blood pressure-lowering, and antioxidant physiological functions [
2,
3]. Studies have shown that passion fruits are classified by their skin color into purple, red, and yellow varieties, and that the extracts from purple-skinned passion fruits exhibit superior activity and physicochemical properties compared to those from red- and yellow-skinned varieties [
4]. Currently, purple passion fruit dominates the market and is more economical than the other two types. Therefore, this study focuses on purple passion fruit.
However, as passion fruit's edible portion is primarily the pulp, the peel is often discarded, resulting in substantial waste of biological resources and significant environmental pollution [
5]. Passion fruit peels are rich in dietary fiber, polyphenols, flavonoids, and other bioactive compounds [
6,
7]. Notably, dietary fiber is resistant to human intestinal enzyme digestion of carbohydrates, which is significant for adjusting the body's dietary structure, preventing obesity, and improving quality of life [
8]. Based on water solubility, total dietary fiber (TDF) is categorized into soluble dietary fiber (SDF) and insoluble dietary fiber (IDF) [
9], SDF possesses good viscosity and gel-forming properties, enabling it to absorb water, expand, and delay gastric emptying, thereby aiding metabolism; IDF consists of fibers that are undissolved or poorly hydrated, increasing fecal volume and weight, hastening the transit of food through the digestive tract, and promoting bowel movements. The functions of SDF include lowering cholesterol, regulating blood sugar, modulating intestinal bacteria, and reducing the risk of hypertension [
10]. IDF primarily promotes intestinal peristalsis and the excretion of bile acids, thereby helping to prevent cardiovascular diseases like atherosclerosis and coronary heart disease [
11].
Research on passion fruit peels has primarily focused on their biological activities and functions. Reports indicate that used passion fruit peels can serve as a heterogeneous catalyst in biodiesel production from palm oil [
12]. Studies indicate that adding passion fruit pericarp in suitable amounts can enhance antioxidant, anti-inflammatory, and short-chain fatty acid levels in broiler chickens, thereby benefiting poultry health [
13]. A study discovered that a passion fruit peel extract, rich in anti-inflammatory and antioxidant properties, effectively alleviated pulmonary fibrosis in mice, suggesting its potential as a promising antifibrotic agent [
14]. Regrettably, research on SDF in passion fruit peels is scarce, with virtually no studies conducted on purple passion fruit peels.
In this study, we employed six methods to extract SDF from the pericarp of purple passion fruit, and we analyzed and compared the extracted SDF for composition, physicochemical properties, antioxidant capacity, monosaccharide content, and structural characterization of the soluble fibers.
2. Materials and Methods
2.1. Materials and Reagents
Purple-skinned passion fruit was obtained from Beiliu City, Guangxi, China. Folinol reagent, ethanol, sulfuric acid, hydrochloric acid, sodium carbonate, sodium nitrite, ABTS, glacial acetic acid, anhydrous sodium acetate, ferric chloride, and trichloroacetic acid were purchased from Sinopharm Chemical Reagent, China. Bovine serum protein V was purchased from Solepol Technology Company, Beijing, China. Gallic acid, anhydrous sodium carbonate and potassium persulfate were purchased from Komeo Chemical Reagent Company, Tianjin, China. Cellulase was purchased from Shandong Loncote Enzyme Preparation Company, Shandong, China. Sodium hydroxide, ferrous sulfate, phenol was purchased from Jinshan Chemical Reagent Company, Chengdu, China. Rutin was provided by Yuanye Biotechnology Company, Shanghai, China. Kaumas Brilliant Blue was obtained from Blue Season Technology Development Company, Shanghai, China. All the above chemical reagents were of analytical grade.
2.2. Material Preparation
Before extraction, the fresh passion fruit pericarp was washed, had its pith removed, was drained, and then dried at 60°C in a constant-temperature oven until a constant weight was achieved. The dried pericarp was pulverized, and the resulting powder was sifted through a 60-mesh sieve before being set aside.
2.3. Extraction of SDF from Passion Fruit Pericarp
Weigh out 30 g of pre-treated passion fruit pericarp powder and mix well. Following the specific conditions for each extraction method, perform hot extraction and filtration, then collect the filtrate. Concentrate the filtrate using a rotary evaporator, then add 3-4 times the mass of the concentrate of a 95% ethanol solution. Place it in a 5°C environment for 12 hours, then let it stand overnight. Load the mixture into centrifuge tubes and centrifuge at 4500 r/min for 10 minutes. Collect the precipitate, add an appropriate amount of deionized water, and wash the precipitate three times with a 95% ethanol solution at a 60°C water bath. After centrifugation at 4500 r/min for 10 minutes, collect the precipitate, wash it three times with a 95% ethanol solution, add an appropriate amount of deionized water, and heat to dissolve in a 60°C water bath. Load into a flat dish or small beaker, then cool and pre-freeze at -5°C. Wait until the solution is frozen into a solid ice cube, and finally lyophilize for 48-72 hours to obtain the SDF.
2.3.1. Enzyme-Assisted Extraction Method (EAE)
The method of Dong et al [
15] was referred to with slight modification. Add deionized water with a liquid to material ratio of 1:26 g/mL, and then add 1.16 % cellulase, stir well, and place it in a constant temperature water bath at 54 ℃ for 4 h. The solution was then extracted from the cellulase and then mixed with the cellulase.
2.3.2. Ultrasonic-Assisted Extraction Method (UAE)
Refer to the method of Moczkowska et al [
16] with slight modification. Add the deionized water with a liquid to material ratio of 1:49 g/mL, oscillate and shake well, then cover and put into an ultrasonic cleaner with an ultrasonic temperature of 69 ℃, an ultrasonic time of 15 min, and an ultrasonic power of 105 W.
2.3.3. Acid-Assisted Extraction Method (AAE-1)
Refer to the method of Jia et al [
17] with slight modification. Add the hydrochloric acid solution with a liquid-to-feed ratio of 1:25 g/mL concentration of 0.5 mol/L, shake well with shaking, and then heat the extract for 75 min in a 75 ℃ water bath.
2.3.4. Alkali-Assisted Extraction Method (AAE-2)
Refer to the method of Jiang et al [
18] with slight modification. Add the sodium hydroxide solution with a liquid/feed ratio of 1:30 g/mL and a concentration of 35 mg/mL, and then heat the extract for 5 h at 70 ℃ in a water bath after shaking.
2.3.5. Microwave-Assisted Extraction Method (MAE)
Refer to the method of Homa et al [
19] with slight modification. Add the deionized water with the liquid to material ratio of 1:50 g/mL, shake well, and then heat the extract in a 70 ℃ water bath for 90 min. the microwave extraction power was 500 W, and the extraction time was 60 s. The extraction time was 60 s. The extraction power was 500 W, and the extraction time was 60 s. The extraction time was 60 min.
2.3.6. Hot Water-Assisted Extraction Method (HWE)
Refer to the method of Wang et al [
20] with slight modification. Add the deionized water with the liquid to material ratio of 1:25 g/mL, shake well, and then heat the extract for 4 h in a 70 ℃ water bath.
2.4. Determination of SDF Components
2.4.1. Extraction Rate
Weigh 1 g of passion fruit pericarp powder in a 50 mL centrifuge tube, and set up three parallel experimental groups for each extraction method. Operate according to the six extraction methods mentioned above, after re-solubilization, the centrifugal tube was used as a container and lyophilized until completely dry, the SDF of passion fruit peel was obtained, and the formula was calculated as follows:
where m1 represents the mass of the centrifuge tube after constant weight , m2 represents the mass of the centrifuge tube recorded after freeze-drying, and m0 represents the mass of the weighed passion fruit peel powder.
2.4.2. Determination of Total Sugars
The method of Yue et al [
21] was referred to with slight modifications. The SDF extracted from each of the six methods was weighed 0.1 g. The determination of the water content of SDF was carried out first. SDF was hydrolyzed by adding 50 mL of distilled water and 15 mL of H
2SO
4, and after fully dissolved, it was refluxed, cooled, and pumped by a condensation reflux device, and the filtrate was collected and fixed with water to 100 mL for the calculation of total sugar content,using glucose as the standard:
where V1 represents the volume of the solution fixed after hydrolysis and reflux (mL), V2 represents the volume of the solution absorbed during the determination (mL), m1 represents the total sugar content in SDF (μg), m2 represents the mass of SDF (g), and ω represents the water content of SDF (%).
2.4.3. Determination of Reducing Sugars
Refer to the method of Lv et al [
22] with slight modification. Weigh the SDF extracted by the six methods each 0.5 g, respectively, add 50 mL of water placed in a 30 ℃ water bath heated to dissolve, dissolved and cooled to 50 mL, as a solution to be measured to calculate the content of reducing sugar, using glucose as the standard:
where C represents the amount of sugar found from the standard curve (mg), VT is the volume of the extract (mL), m represents the mass of SDF (mg), VS is the volume of the sample at the time of determination (mL).
2.4.4. Polyphenol Determination
Referring to the method of K.et al [
23] with slight modifications. Accurately weigh 0.01 g of passion fruit peel SDF extracted by each method, heat and dissolve, cool and volume to 10 mL, as the solution to be measured. Each take 1 mL of the solution to be measured in a 10 mL test tube, add 1.0 mL of forintol reagent, shake well; after 2 min, then add 1.5 mL of 10 % sodium carbonate solution, respectively, add distilled water to stabilize the volume, oscillation and shaking, and leave it at 25 ℃ for 1.5 h. Determine the absorbance value of the soluble fibers at 765 nm by using a spectrophotometer and calculate the polyphenol content as follows,using gallic acid as the standard:
where TPC represents the content of polyphenols in SDF(mg GAE/g dw), C represents the mass concentration of polyphenols in hydrolysate (mg/mL), V represents the volume of reaction liquid (mL), N represents the dilution ratio of reaction liquid, m represents the mass of SDF (g).
2.4.5. Flavonoid Determination
Refer to the method of Da et al [
24] with slight modification. Take passion fruit peel powder about 1 g, add 50 mL of ethanol solution cold immersion 30 min, boiling water bath 98-100 ℃ reflux extraction and filtration, collect the filtrate for use. Take 1.0 mL of the solution to be tested, add water to 2.0 mL, add sodium nitrite, aluminum nitrate, sodium hydroxide solution, respectively, 4 mL each, at 510 nm wavelength to determine the absorbance, the formula is as follows,using rutin as the standard:
where w represents the value of total flavonoid content in the specimen (mg/g), ρ represents the mass concentration of total flavonoids obtained in the standard curve (mg/L), ρ0 represents the mass concentration of total flavonoids in the blank to be measured solution on the standard curve (mg/L), V1 represents the value of the volume of the extracted solution added to and calibrated in the specimen (mL), V3 represents the value of the final volume of the finalized specimen (mL), m represents mass of the specimen (g), V2 represents the value of the volume of the extract solution dispensed (mL).
2.4.6. Protein Determination
Referring to the method of K. et al [
25] with slight modification. Weigh 100 mg of SDF of passion fruit pericarp extracted by each method, add distilled water and dissolve to 100 mL, determine the absorbance value of the blank reagent as a control, and calculate the content of protein in the sample, using bovine serum protein as the standard:
where m' represents mass of protein (μg) from which the standard curve was prepared, V represents total volume of sample extract (mL), Vs represents volume of sample extract taken during the determination (mL), m represents mass of sample (g).
2.4.7. Molecular Weight (Mw) Determination
Referring to the method of Fu et al [
26] with slight modification. The molecular weight distribution of the samples was analyzed by molecular exclusion chromatography-multiangle laser light scattering-refractive index detector ( SEC-MALLS-RI ). The weight and number-average molecular weight (Mw and Mn) and polydispersity index (Mw/Mn) of various fractions in 0.1 M NaNO
3 aqueous solution containing 0.02% NaN
3 were measured on a DAWN HELEOS-II laser photometer (Wyatt Technology, CA, USA) equipped with Three tandem columns (300×8 mm, Shodex Ohpak SB-805, 804 and 803; Showa Denko K.K., Tokyo, Japan) which was held at 45℃ using a model column heater by Sanshu Biotech. Co., LTD (Shanghai, China). The flow rate is 0.4 mL/min. A differential refractive index detector (Optilab T-rEX, Wyatt Technology Co., USA) was simultaneously connected to give the concentration of fractions and the dn/dc value.
2.5. Determination of Physicochemical Properties
2.5.1. Water/Oil-Holding Capacity (WHC/OHC)
Referring to the method of He et al [
27] with slight modifications. A 0.100 g sample of passion fruit peel SDF was placed in a 20 mL centrifuge tube with 10 mL of distilled water or food-grade rapeseed oil, mixed well, and then left at room temperature for 24 h. The sample was centrifuged at 4500 r/min for 15 min, and the supernatant was removed and weighed:
where M1 represents weight of the sample after water or oil absorption (g), M represents weight of the sample itself (g).
2.5.2. Swelling Capacity (SC)
Referring to the method of Xu et al. [
28], with slight modifications. Weigh 0.100 g of SDF sample and place it in a test tube. Add 10 mL of distilled water and let it stand at room temperature for 24 hours. Read the free expansion volume of the sample in the test tube and calculate the expansion volume per gram of fiber (mL/g):
where V1 represents the volume of the sample after swelling (mL), V represents the initial volume of the sample (mL), M representshe dry weight of the sample (g).
2.5.3. Cation Exchange Capacity (CEC)
Referring to the method of Wei et al. [
29] with slight modification. Accurately weigh 1.00 g of the sample in a conical flask, add 30 mL of deionized water, mix thoroughly, add phenolphthalein indicator and titrate with 0.1 mol/L NaOH solution as titrant. The volume of NaOH consumed was recorded:
where c represents sample concentration, v represents volume of NaOH consumed, m represents sample mass.
2.6. Antioxidant Activity
2.6.1. DPPH Free Radical Scavenging Capacity
The referenced method [
30] was used with slight modifications. Briefly, 100 μL of sample (50 % ethanol solution) and 100 μL of 0.2 mM DPPH solution (absorbance value in the range of 0.7-0.9) were mixed in a 96-well plate and allowed to stand for 30 min at room temperature in the dark, then the absorbance value was measured using an enzyme meter at 510 nm. The DPPH radical scavenging rate was calculated according to the formula:
where A0 represents absorbance value of 40 µL H
2O + 160 µL DPPH, A1 represents absorbance value of 40 µL sample solution + 160 µL DPPH, A2 represents absorbance value of 40 µL sample solution + 160 µL anhydrous ethanol.
2.6.2. ABTS Free Radical Scavenging Capacity
The referenced method [
31] with slight modifications. Prepare 7.4 mM ABTS diammonium salt and 2.6 mM potassium persulfate solution separately, mix 1:1 (v/v) as required and react in the dark for 12 h. Dilute with distilled water until the absorption value of this working solution at 734 nm is about 0.7 to spare. 100 μL of the sample solution and 100 μL of ABTS working solution were mixed in a 96-well plate, and the absorbance value was measured at 734 nm after 30 min of standing reaction:
where A1 represents absorbance value of 40 µL sample + 160 µL ABTS working solution, A0 represents absorbance value of 40 µL distilled water + 160 µL ABTS working solution.
2.6.3. Ferrous Ion Reduction Capacity
Methods referenced [
32] with slight modifications. Prepare 0.3 M acetate buffer, 10 mM TPTZ solution, 20 mM FeCl
3 solution, mixed in the ratio of 10:1:1 and set aside. 40 μL of sample solution and 200 μL of FRAP working solution were mixed in a 96-well plate and reacted at 37 °C for 10 min, then the absorbance values were measured at 593 nm using a spectrophotometer. Iron sulfate was used as the standard.
2.7. Determination of Monosaccharides
Measure the monosaccharide composition of SDF using ion chromatography. First, place SDF in a solution of 1 mL 2M trichloroacetic acid at 121 ℃ for 2 hours, blow dry the solution with nitrogen gas, then add methanol for cleaning and drying, repeat 2-3 times, dissolve in sterile water solution for later use. The ion chromatography column is Dionex ™ CarboPacTM PA20 (150 x 3.0 mm, 10 µ m), mobile phases A (sterile water), B (0.1M sodium hydroxide), and C (0.1M sodium hydroxide, 0.2M sodium acetate), with a flow rate of 0.5mL/min. The column temperature is 30 ℃. Using fucose, rhamnose, arabinose, galactose, glucose, xylose, mannose, fructose, ribose, galacturonic acid, glucuronic acid, mannuronic acid, and glucuronic acid as monosaccharide standards.
2.8. Determination of Scanning Electron Microscope (SEM)
A scanning electron microscope (HITACHI Regulus 8100, Hitachi, Tokyo, Japan) was used to observe the particle and microstructure of SDF at an accelerated voltage of 10 kV. The sample was coated on a conductive carbon strip, sputtered with a layer of gold, and the scanning electron microscope images were collected at different magnifications.
2.9. Determination of Fourier Transform Infrared Spectroscopy (FT-IR)
FT-IR spectra of SDF were determined using a spectrometer (Nicolet iZ-10, Thermo Nicolet, USA). The SDF samples were mixed with KBr powder and then pressed into 1 mm pellets for FT-IR measurement in the range of 4000 to 400 cm-1 by Sanshu Biotech. Co., LTD (Shanghai, China).
2.10. Statistical Analysis
The results were expressed as mean ± standard deviation (n ≥ 3). One-way analysis of variance (ANOVA) and Duncan’s multiple range tests in SPSS 27 software (IBM, Chicago,IL, USA) were used to analyze the significant differences (p < 0.05). GraphPad Prism 9.5.1 (GraphPad Software, San Diego, CA, USA) and Excel 2019 software were used for data analysis and graph processing.
3. Results
3.1. Analysis of the Composition of SDF
Table 1 shows the molecular weight and main chemical components of passion fruit peel. Regarding the TDF extraction rate, EAE showed the highest rate at 83.6%, significantly different from other methods, while HWE exhibited the lowest at 67.79%, also significantly different from the others. The SDF extraction rate was highest for AAE-1 at 17.05%, significantly different from other methods, and lowest for HWE at 6.36%, also significantly different from the others. This indicates that the use of HWE in combination with other extraction methods significantly enhances the extraction rates of SDF and TDF compared to HWE alone, aligning with previous research [
33]. In terms of appearance, SDF fibers exhibited varying colors: AAE-1 fibers were brick-red, AAE-2 fibers were tan with a rough, water-soluble texture, EAE fibers were dark purple with a fine feel, HWE fibers were light brown, MAE fibers were plum-red, and UAE fibers were whitish brown.
Among the six methods, AAE-1 and AAE-2 yielded SDF with higher water content than the others, possibly due to the loose, rough, and porous structure of the SDF, which readily absorbs water at room temperature [
34]. In terms of protein content, AAE-1's SDF had a significantly lower protein content than other methods, suggesting that acid extraction was most effective at purifying SDF, likely because the acid concentration altered protein structure and solubility, resulting in deformation or hydrolysis [
35]. MAE had a significantly higher total sugar content than other methods, with no significant difference between acid extraction and hot water immersion, possibly due to MAE's high extraction efficiency and speed, which facilitates better sugar retention and extraction, and MAE can also break down polysaccharide chains, leading to a relative increase in total sugar content [
36]. EAE achieved the highest extraction of reducing sugars, likely because the enzymes can target specific glycosidic bonds and efficiently convert polysaccharides or complex carbohydrates into reducing sugars [
33]. In contrast, AAE-1 and AAE-2 had lower yields of reducing sugars, attributed to excessively high acid-base concentrations. Zhang et al. [
37] showed that both acid and alkaline methods can disrupt glycosidic bonds or degrade cellulose. Similarly, MAE yielded the highest content of polyphenols and flavonoids, and could reach higher temperatures faster than other methods; it is hypothesized that microwave energy increases solvent and plant material temperatures, promoting polyphenol and flavonoid release [
38]. This finding is consistent with previous research [
39].
The biological activity of SDF generally depends on the molecular weight, thus the determination of molecular weight is an essential index for the characterization of the functional properties of SDF [
40]. The weight-average molecular weight reflects the degree of polymerization of the fiber and the interaction between the molecules [
41]. From
Table 1, it can be seen that the Mw of UAE is the lowest, indicating that the cross-linked structure of UAE SDF is less and the interaction with oil molecules is weak, which is consistent with the results of the determination of oil holding capacity. On the contrary, HWE has higher Mw and Mn, which indicates that the SDF extracted by this method not only has more large molecular weight components, but also has an advantage in quantity, which is consistent with the research results of Zou et al [
42]. A larger Mw/Mn index indicates a wider molecular weight distribution [
43]. The results show that MAE extraction yields the highest Mw/Mn, which may be due to its relatively low corresponding Mn value.
In summary, of the six extraction methods, AAE-1 achieved the highest SDF extraction rate and water content and was most effective at purifying the sample; EAE preserved the most reducing sugars and yielded the highest total dietary fiber (TDF) content; AAE-2 effectively lowered flavonoid and polyphenol content; MAE had the highest total sugar content in the SDF of passion fruit peels and a high number of molecules with different molecular weights; HWE obtained the SDF with a higher number of components and number of large molecular weights; and UAE was used to obtain the smallest molecular weights in the SDF. Furthermore, the SDF products' color from passion fruit pericarp differed significantly across extraction methods.
3.2. Analysis of Physicochemical Properties of SDF
3.2.1. Analysis of WHC/OHC
Hydrophilic groups in SDF exhibit excellent water retention, increasing fecal volume, enhancing defecation speed, reducing rectal pressure, thereby preventing constipation. Additionally, SDF's water retention capacity helps organize food, preventing dehydration and shrinkage [
44].
Table 2 shows that all methods except HWE improve SDF's water holding capacity, among which AAE-2 provided the highest water holding capacity at 7.82 g/g. In a single process, AAE-2 fibers had a significantly higher water holding capacity (WHC) than other extractions, thus, AAE-1 and AAE-2 are suitable for extracting SDF from passion fruit peels with enhanced water holding capacity. This may be due to the suitable pH environment breaking chemical bonds in the fibers, releasing more water-holding groups. This could be because a suitable pH environment facilitates the breaking of chemical bonds, releasing additional water-holding groups and enhancing WHC [
45]. Conversely, SEM results indicate that AAE-1 and AAE-2 possess a fluffy, porous microstructure, allowing for greater water retention and significantly enhancing WHC.
OHC refers to the ability of fibers to retain oil after being mixed with it. SDF can absorb intestinal fat, thereby reducing fat absorption, aiding in weight control, and preventing obesity; additionally, SDF's fat-wrapping ability can decrease fat-bile contact, lessen cholesterol absorption from food, and preserve intestinal health [
46].
Table 2 indicates that SDF from passion fruit peel extracted by EAE, AAE-1, and AAE-2 exhibited high OHC, with the enzyme method showing the highest oil holding capacity at 5.86 g/g, followed by the acid and alkali methods, which did not significantly differ. UAE had the lowest oil holding capacity among the fibers. This outcome contrasts with previous findings [
47], and it is hypothesized that the higher OHC of EAE might result from different enzyme selections, possibly targeting specific enzymes to disrupt plant cell walls and enhance fiber properties.
3.2.2. Analysis of SC
SC refers to the phenomenon of fibers increasing in volume upon absorbing solvent molecules. In food processing, SDF's SC can enhance food texture and taste and boost water retention; in pharmaceuticals, SC influences drug bioavailability [
48].
Table 2 shows that AAE-2's SDF had the highest SC at 4.24 mL/g among the six methods, significantly outperforming other methods. It is hypothesized that the alkaline environment dissociates cellulose's hydroxyl groups, weakening inter-chain bonds and increasing fiber swelling, consistent with prior research [
49]. However, AAE-1 had the lowest SDF swelling force, possibly due to acidic conditions. Despite being extractable, acid treatment can damage functional groups and lower polysaccharide molecular weight, affecting SC [
50].
3.2.3. Analysis of CEC
Dietary fibers have side-chain functional groups like carboxyl and hydroxyl, which are weakly acidic and can reversibly bind with cations, notably organic cations. This can facilitate or inhibit certain chemical reactions, potentially benefiting human health [
51]. For instance, a higher intake of dietary fiber can bind heavy metals, mitigate their toxicity, and reduce blood pressure [
52].
Table 2 shows that AAE-1 (1.17 mL/g) and AAE-2 (1.14 mL/g) yielded SDF with the highest cation exchange capacity (CEC), significantly different from other methods. This could be attributed to the high temperature and prolonged treatment time in the extraction process, which likely opened the plant cell wall structure, exposed functional groups, and thus increased CEC [
17].
3.3. Analysis of Antioxidant Activity
Evaluating the antioxidant activity of SDF is highly significant. Antioxidants help eliminate free radicals in the body and reduce oxidative stress damage to cells, potentially preventing chronic diseases such as diabetes, cancer, and cardiovascular diseases [
53]. This study assessed the DPPH and ABTS free radical scavenging abilities, as well as iron ion reducing capacity, to evaluate the antioxidant efficiency of SDF obtained through different extraction methods.
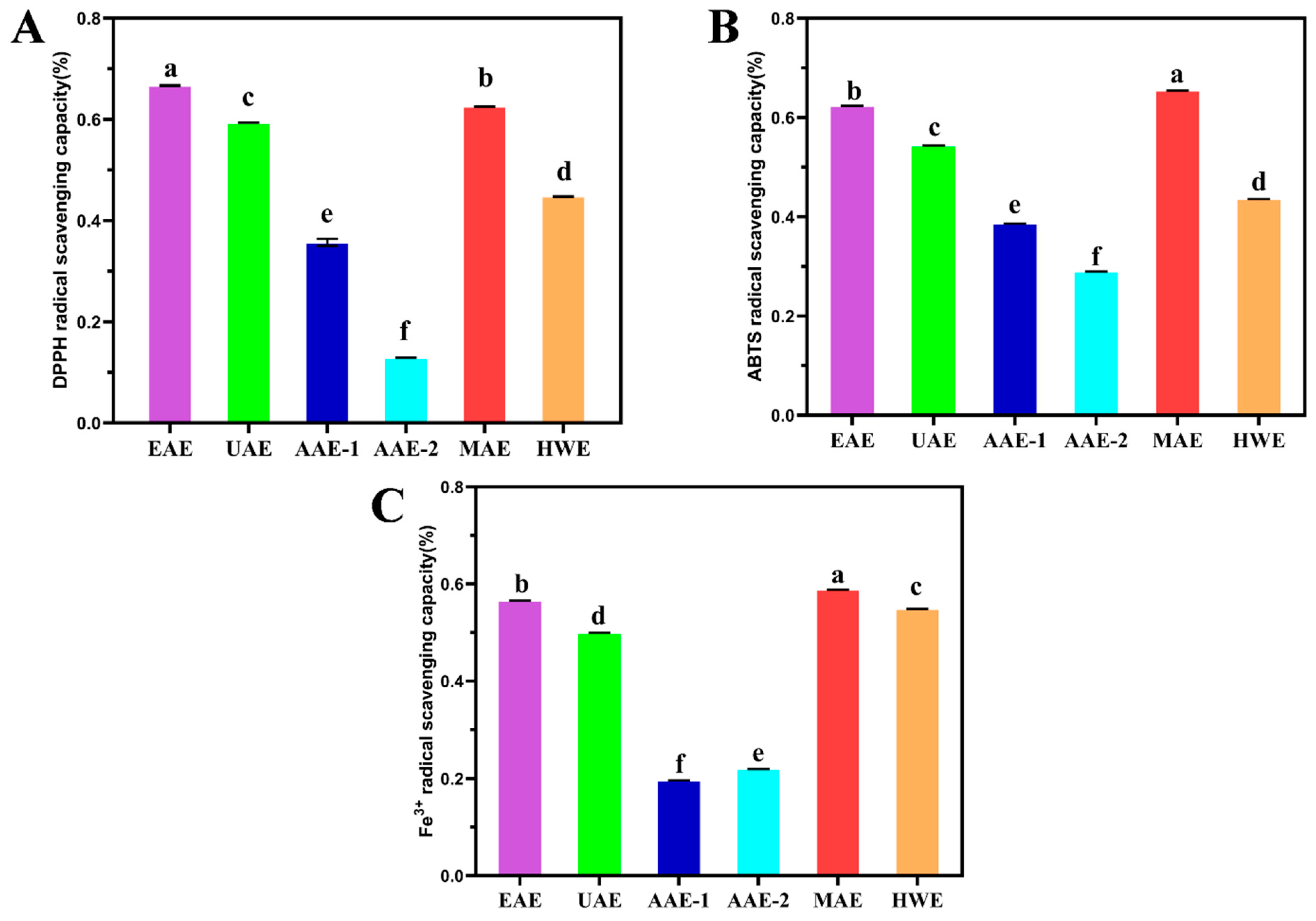
3.3.1. DPPH Radical Scavenging Activity
The DPPH is a highly stable nitrogen-centered free radical, and is widely used to assess antioxidant capacities in extracts, compounds, and isolates. It reflects antioxidant content and activity in samples indirectly by measuring residual DPPH after interaction [
54].
Figure 1(A) shows that SDF extracted by six methods all exhibit DPPH free radical scavenging ability, among which EAE-extracted SDF demonstrates the highest DPPH scavenging ability, reaching 66.67%, significantly different from other methods. This is likely due to EAE’s reputation as a gentle, efficient, and eco-friendly method that enhances extraction efficiency, thus boosting antioxidant content and activity [
55,
56]. Concurrently, DPPH scavenging ability is positively correlated with polysaccharide concentration [
54,
57,
58], which aligns with the high total and reducing sugar content observed in EAE-extracted SDF in this study.
3.3.2. ABTS Radical Scavenging Activity
The ABTS (2,2′-azino-di-(3-ethylbenzthiazoline sulfonic acid)) is a widely used chemical in antioxidant assays. This assay is extensively applied to measure antioxidant capacities in various samples, including food, pharmaceuticals, nutraceuticals, and cosmetics [
59].
Figure 1(B) shows that SDF extracted by the six methods all possess ABTS free radical scavenging ability, with the SDF extracted by MAE exhibiting the highest ABTS scavenging ability, reaching 65.46%, significantly different from other methods. Studies have shown that the content of polyphenols and flavonoids in SDF is related to the ABTS free radical scavenging ability [
60]. This aligns with the findings of this study. The high ABTS scavenging ability of MAE may stem from its high polyphenol and flavonoid content.
3.3.3. Fe3+ Reducing Power
The ferric reducing antioxidant power ( FRAP ) method judges the antioxidant activity by the dynamic reaction of metal iron ions between the oxidized state and the reduced state, that is, by measuring the reduction degree of the blue-purple complex formed by the reaction of Fe
3+ with 2,4,6-tripyridyltriazine(TPTZ) under the action of antioxidants, it can play a quantitative evaluation role [
61]. From
Figure 1(C), it can be seen that SDF extracted by six methods all have iron ion reduction ability. Among them, SDF obtained by MAE has the highest iron ion reduction ability, reaching 58.83 %, which is significantly different from other extraction methods. AAE-2 has the lowest iron ion reduction ability, only 19.63 %, which is also significantly different from other extraction methods. This may be related to the content of polyphenol compounds. The phenolic hydroxyl groups in polyphenols can complex with iron ions, thereby enhancing the reduction ability [
62]. Therefore, in order to obtain SDF from passion fruit peel with better iron ion reduction ability, MAE extraction can be used, or the content of polyphenols can be increased.
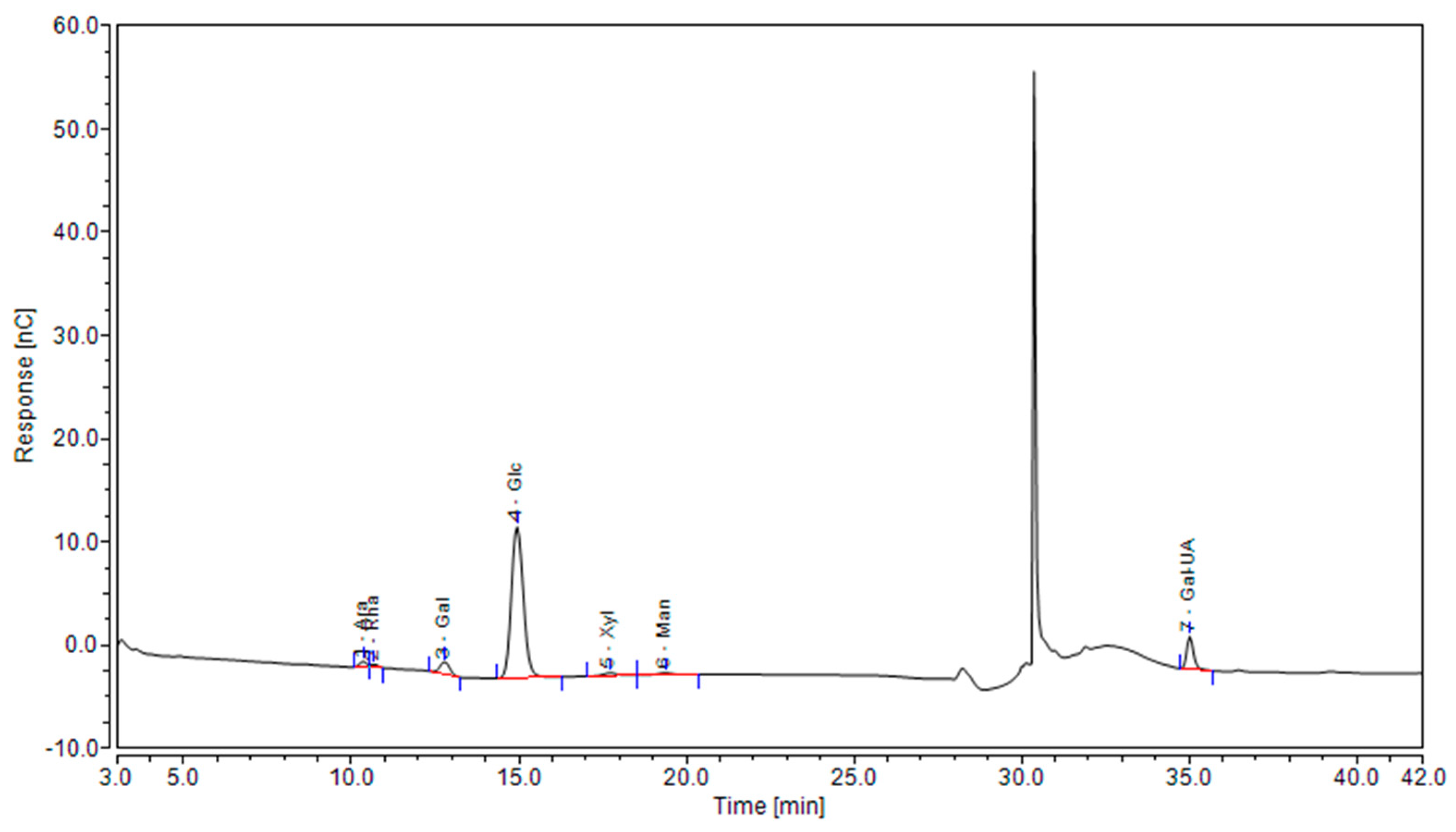
3.4. Analysis of Monosaccharide Composition
According to the ion chromatogram of
Figure 2, there are seven kinds of monosaccharides in the SDF of passion fruit peel, which are arabinose ( Ara ), rhamnose ( Rha ), galactose ( Gal ), glucose ( Glu ), xylose ( Xyl ), mannose ( Man ) and galacturonic acid ( Gal-UA ). These components are basically the same as the monosaccharide composition of pomegranate peel SDF extracted by Xiong et al [
63]. The peak values of Glu and Gal-UA were more significant, indicating that the SDF of passion fruit peel was mainly composed of Glu and Gal-UA, which was consistent with the results of high Glu content in oat glucan ( OG ) and high Gal-UA content in apple pectin ( AP ) studied by Zou et al [
64].
It can be clearly seen in
Table 2 that different extraction methods have little effect on the sugar composition of SDF, but affect the content of monosaccharide units. Each SDF has its own prominent monosaccharide substances, which is consistent with the previous research results [
65,
66]. For example, studies have shown that pectin must be characterized by high levels of Gal-UA. Pectin helps gastrointestinal motility, moisturizing, regulating blood lipids, and can also be used as a thickener and gelling agent in the food industry [
19]. It can be seen from
Table 3 that among the SDF obtained by different extraction methods, the Gal-UA of AAE-2 and HWE accounted for the highest proportion in their monosaccharide composition(17.2899 µg/mg、35.6795 µg/mg), so it can be said that the SDF obtained by the two methods contained pectin. AAE-1 obtained the highest Xyl content(3.2187 µg/mg), which may be due to the strong acid environment that breaks the glycosidic bond of pentosan, the main component of hemicellulose, and releases xylose monomers [
19]. Among the SDF obtained by EAE, the content of Ara ( 1.6722μg / mg ) and Rha ( 0.8411μg / mg ) was the lowest, but both Ara and Rha had the function of reducing blood glucose and enhancing immunity, which was of great significance to the health of the body [
67].
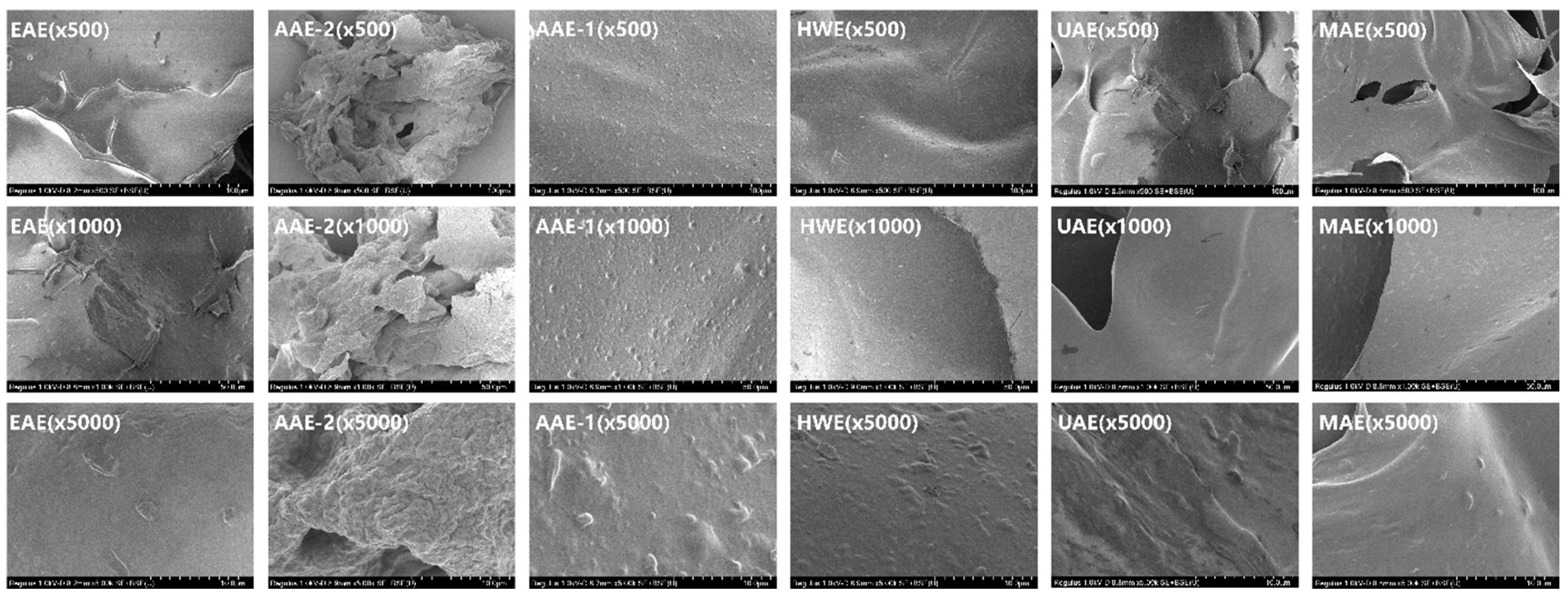
3.5. Analysis of SEM
Figure 3 reveals the microstructure of SDF, with EAE exhibiting a wrinkled, strip-like structure, possibly resulting from enzymatic alterations and glycosidic bond cleavage [
68]. The AAE-2 structure is rough and cotton-like, consistent with prior studies [
69], and this may result from alkaline treatment disrupting the cellulose molecular structure, creating a loose, cotton-like formation. However, prolonged alkali extraction intensifies cellulose degradation, increasing roughness. The AAE-1 structure is rough and resembles a mountain landscape. This could be due to dissolved molecules re-aggregating after fiber structure disruption, though uneven recombination may also contribute to the mountain-like surface. In summary, the SDF structures of EAE, AAE-2, and AAE-1 are rough, loose, and porous, and their large surface area significantly enhances water, oil retention, and swelling abilities, which coincides with the results of the above physicochemical properties.
SDF from HWE exhibits a smooth microstructure but has a low yield, aligning with Li et al.'s research [
33]. The smooth surface likely results from HWE's lack of chemical reagents, with fibers mildly softened thermally without damage or change. The SDF surface from UAE is smoother than EAE's porous structure [
65], possibly due to the shorter ultrasound duration and lower power. He et al. [
66] reported that a low aggregation degree and loose structure might relate to a high neutral polysaccharide content. In this study, glucose, a neutral polysaccharide, comprised over half of the total monosaccharide composition in MAE, AAE-1, and EAE. Thus, the irregular lumps on MAE's surface, AAE-1's mountain-like roughness, and EAE's wrinkled strip structure may result from these factors.
In summary, different extraction methods will cause different changes in the structure of SDF, which is the same as the current research results [
63].
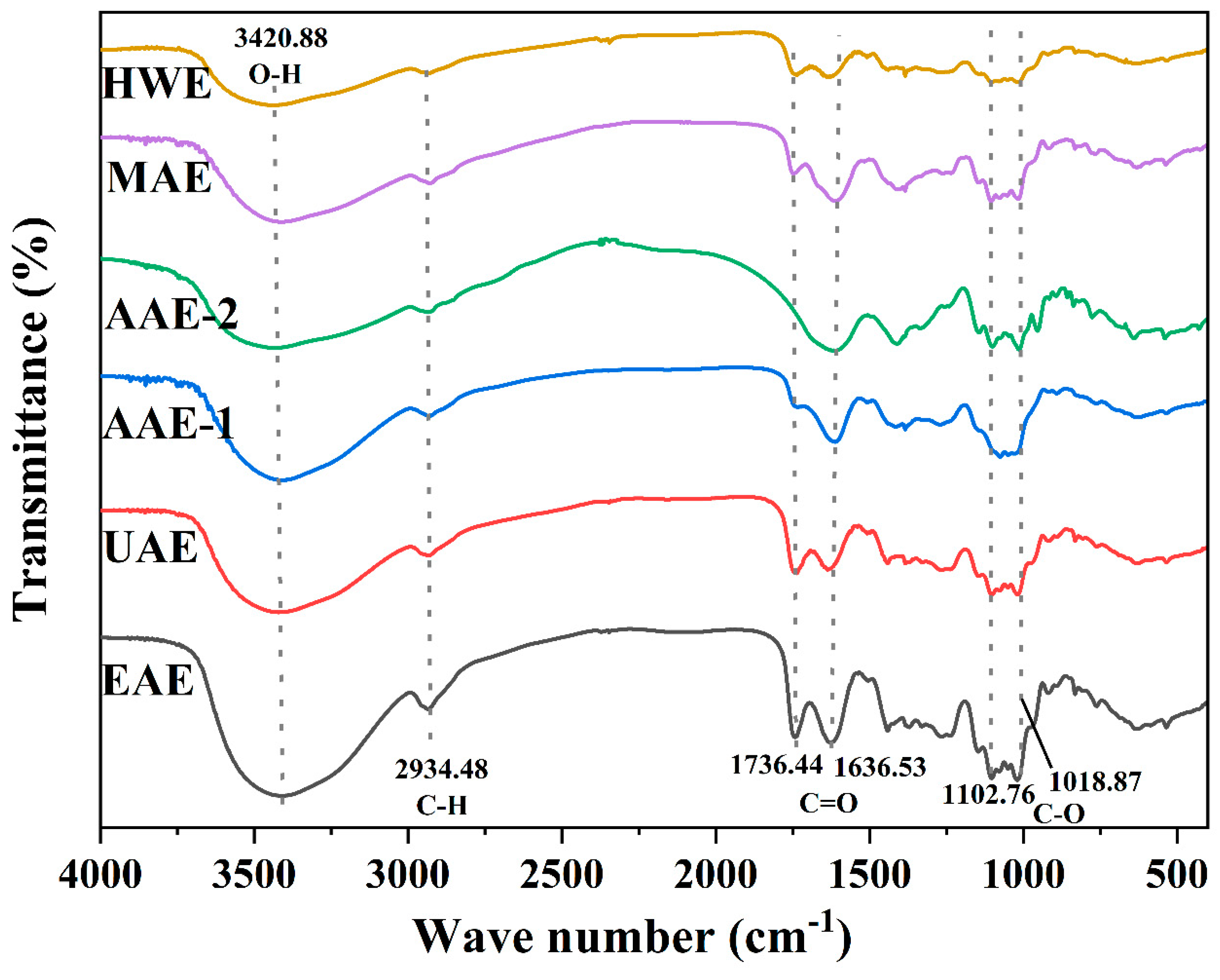
3.6. Analysis of FT-IR Spectra
The SDF spectrograms of passion fruit peels extracted by different methods were similar as shown in
Figure 4, but the absorption intensities of the characteristic peaks were diverse. A strong and broad absorption peak was observed at 3200-3600 cm
-1 for the six SDF samples, which was induced by the O-H stretching vibration, and this could be sourced from hemicellulose and pectin [
33]. The absorption peak in the range of 2500-2950 cm
-1 may be due to the C-H bond stretching vibration of the methyl and methylene groups [
70]. There were absorption peaks caused by C=O stretching vibration in the range of 1600-1800 cm-1 , in which no absorption peak was observed at 1736.44 cm-1 for AAE-2, and the absorption peaks of AAE-1 were also weaker here, which may be due to the hydrolysis of stretching of ester carbonyls of hemicellulose and pectin by acid and alkali solutions [
43], and at the mean while, this corresponds to the same reasons for the low reducing sugar yields of the two methods we determined above. There are absorption peaks in the region of 1050-1150 cm-1. As SDF is a kind of polysaccharide and the C-O bond is an important part of the polysaccharide skeleton, thus the absorption peaks in this region are most likely to be attributed to the stretching vibration of C-O [
71]. Also the stretching vibrations of the C-O-C and C-O-H bonds may produce absorption peaks in this range, which are characteristic peaks of pyranose [
72].
4. Conclusions
This study compared the impact of six extraction methods on the dietary fiber quality in purple passion fruit peel. The results revealed that SDF from various methods shared the same monosaccharide composition. However, the methods significantly varied in their impact on chemical composition and molecular weight, physicochemical properties, antioxidant activity, and structural features. For instance, AAE-1 and AAE-2 possess high water content and holding capacity attributed to their fluffy, rough surface structure, but low reducing sugar production and insignificant C=O bond stretching vibrations caused by the stimulation of acidic and alkaline environments; both the EAE and MAE methods exhibit strong antioxidant capacity. The HWE and UAE structural surfaces are smooth. Overall, different methods differentially affect the composition and activity of soluble fiber in passion fruit peel, with each method being suitable for specific scenarios. The findings provide a theoretical foundation for the development and utilization of passion fruit peel.
Author Contributions
Investigation, Conceptualization, Methodology, Writing—review & editing, Funding acquisition, Y.S.; Writing—original draft & editing, Data curation, Y.S.; Investigation, Methodology, Data curation, Y.L. and Y.M.; Software, Formal analysis, C.L. ; Validation, Formal analysis, N.N.; Investigation, Conceptualization, Writing—review & editing, X.S.; Supervision, Writing—review & editing, J.Y.
Funding
This research was funded by the Natural Science Foundation of Guizhou Province (ZK[2023]451); Zunyi Technology and Big Data Bureau, Moutai Institute Joint Science and Technology Research and Development Project (ZSKHHZ[2021] No. 308); Research Foundation for Scientific Scholars of Moutai Institute (mygccrc[2022]091); National Natural Science Foundation of China(32202854); Guizhou Provincial Basic Research Program (Natural Science) (No. QKHJC-ZK-2022-129).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- de Araújo Esteves Duarte, Isabella; Dragan, M.; Karla, B.T.; Livia, D.L.D.O.; Maria, C.A. de Araújo Esteves Duarte Isabella; Dragan, M.; Karla, B.T.; Livia, D.L.D.O.; Maria, C.A. Brazilian passion fruit as a new healthy food: from its composition to health properties and mechanisms of action. Food Funct 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Zeraik, M.; Pereira, C. Passion fruit: a functional food? Revista Brasileira De Farmacognosia 2010, 20, 459–471. [Google Scholar] [CrossRef]
- Dhawan, K.; Dhawan, S.; Sharma, A. Passiflora: A review update. J Ethnopharmacol 2004, 94, 1–23. [Google Scholar] [CrossRef]
- Nerdy, N.; Ritarwan, K. Hepatoprotective Activity and Nephroprotective Activity of Peel Extract from Three Varieties of the Passion Fruit (Passiflora Sp.) in the Albino Rat. Open Access Macedonian Journal of Medical Sciences 2019, 7, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Thokchom, R.; Mandal, G. Production Preference and Importance of Passion Fruit (Passiflora Edulis): A Review. Journal of Agricultural Engineering and Food Technology 2020. [Google Scholar]
- Wasagu, R.L.M.; Aa, A.A.S.; Ug, K.S.T.; Zaharadeen, A. Comparative Chemical Analysis, Phytochemical Screening and Antimicrobial Activities of the Rinds, Seeds and Juice of (Passiflora edulis var. flavicarpa) Passion Fruit. Journal of Natural Sciences Research 2016. [Google Scholar]
- Sihombing, J.R.; Dharma, A.; Chaidir, Z.; Almahdy; Munaf, E. Phytochemical screening and antioxidant activities of 31 fruit peel extract from Sumatera, Indonesia. Journal of Chemical and Pharmaceutical Research 2015. [Google Scholar]
- Efimtseva, E.A.; Chelpanova, T.I. [Dietary fiber as modulators of gastrointestinal hormonal peptide secretion]. Vopr Pitan 2021, 90, 20–35. [Google Scholar] [CrossRef] [PubMed]
- Waddell, I.S.; Orfila, C. Dietary fiber in the prevention of obesity and obesity-related chronic diseases: From epidemiological evidence to potential molecular mechanisms. Crit Rev Food Sci 2023, 63, 8752–8767. [Google Scholar] [CrossRef]
- Guan, Z.W.; Yu, E.Z.; Feng, Q. Soluble Dietary Fiber, One of the Most Important Nutrients for the Gut Microbiota. Molecules 2021, 26. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Zhang, X.; Zhao, L.; Zou, J.; Qiu, R.; Liu, X.; Hu, Z.; Wang, K. Insoluble dietary fiber from wheat bran retards starch digestion by reducing the activity of alpha-amylase. Food Chem 2023, 426, 136624. [Google Scholar] [CrossRef] [PubMed]
- Tarigan, J.B.; Singh, K.; Sinuraya, J.S.; Supeno, M.; Sembiring, H.; Tarigan, K.; Rambe, S.M.; Karo-Karo, J.A.; Sitepu, E.K. Waste Passion Fruit Peel as a Heterogeneous Catalyst for Room-Temperature Biodiesel Production. Acs Omega 2022, 7, 7885–7892. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.; Huang, L.L.; Luo, H.L.; Huang, Y.C.; Huang, X.Y.; Chen, G.; Gui, J.; Liu, Z.L.; Yang, L.; Liu, X.Z. Passion fruit peel and its zymolyte enhance gut function in Sanhuang broilers by improving antioxidation and short-chain fatty acids and decreasing inflammatory cytokines. Poultry Sci 2023, 102, 102672. [Google Scholar] [CrossRef] [PubMed]
- Chilakapati, S.R.; Serasanambati, M.; Manikonda, P.K.; Chilakapati, D.R.; Watson, R.R. Passion fruit peel extract attenuates bleomycin-induced pulmonary fibrosis in mice. Can J Physiol Pharm 2014, 92, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Wang, D.; Hu, R.; Long, Y.; Lv, L. Chemical composition, structural and functional properties of soluble dietary fiber obtained from coffee peel using different extraction methods. Food Res Int 2020, 136, 109497. [Google Scholar] [CrossRef]
- Moczkowska, M.; Karp, S.; Niu, Y.; Kurek, M.A. Enzymatic, enzymatic-ultrasonic and alkaline extraction of soluble dietary fibre from flaxseed - A physicochemical approach. Food Hydrocolloid 2019, 90, 105–112. [Google Scholar] [CrossRef]
- Jia, Y.; Gao, X.; Xue, Z.; Wang, Y.; Lu, Y.; Zhang, M.; Panichayupakaranant, P.; Chen, H. Characterization, antioxidant activities, and inhibition on α-glucosidase activity of corn silk polysaccharides obtained by different extraction methods. Int J Biol Macromol 2020, 163, 1640–1648. [Google Scholar] [CrossRef]
- Jiang, G.; Ramachandraiah, K.; Wu, Z.; Ameer, K. The Influence of Different Extraction Methods on the Structure, Rheological, Thermal and Functional Properties of Soluble Dietary Fiber from Sanchi (Panax notoginseng) Flower. Foods 2022, 11, 1995. [Google Scholar] [CrossRef] [PubMed]
- Bagherian, H.; Ashtiani, F.Z.; Fouladitajar, A.; Mohtashamy, M. Comparisons between conventional, microwave- and ultrasound-assisted methods for extraction of pectin from grapefruit. Chemical Engineering & Processing Process Intensification 2011, 50, 1237–1243. [Google Scholar]
- Wang, M.; Zhang, C.; Xu, Y.; Ma, M.; Yao, T.; Sui, Z. Impact of Six Extraction Methods on Molecular Composition and Antioxidant Activity of Polysaccharides from Young Hulless Barley Leaves. Foods 2023, 12. [Google Scholar] [CrossRef]
- Yue, F.; Zhang, J.; Xu, J.; Niu, T.; Lü, X.; Liu, M. Effects of monosaccharide composition on quantitative analysis of total sugar content by phenol-sulfuric acid method. Front Nutr 2022, 9, 963318. [Google Scholar] [CrossRef]
- Lv, X.; Wang, P.; Wang, T.; Zhao, J.; Zhang, Y. Development and validation of an improved 3-methyl-2-benzothiazolinone hydrazone method for quantitative determination of reducing sugar ends in chitooligosaccharides. Food Chem 2021, 343, 128532. [Google Scholar] [CrossRef]
- Klarić, D.A.; Mornar, A.; Kovačić, J.; Jeličić, M.L.; Brusač, E.; Brletić, I.; Klarić, I. Polyphenol content and antioxidant activity of phytoestrogen containing food and dietary supplements: DPPH free radical scavenging activity by HPLC. Acta Pharmaceut 2022, 72, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Da, S.L.; Pezzini, B.R.; Soares, L. Spectrophotometric determination of the total flavonoid content in Ocimum basilicum L. (Lamiaceae) leaves. Pharmacogn Mag 2015, 11, 96–101. [Google Scholar] [CrossRef]
- Kielkopf, C.L.; Bauer, W.; Urbatsch, I.L. Methods for Measuring the Concentrations of Proteins. Cold Spring Harb Protoc 2020, 2020, 102277. [Google Scholar] [CrossRef]
- Fu, Y.; Li, F.; Ding, Y.; Li, H.; Xiang, X.; Ye, Q.; Zhang, J.; Zhao, L.; Qin, W.; Gan, R.; et al. Polysaccharides from loquat (Eriobotrya japonica) leaves: Impacts of extraction methods on their physicochemical characteristics and biological activities. Int J Biol Macromol 2020, 146, 508–517. [Google Scholar] [CrossRef]
- He, X.; Wang, B.; Zhao, B.; Meng, Y.; Chen, J.; Yang, F. Effect of Hydrothermal Treatment on the Structure and Functional Properties of Quinoa Protein Isolate. Foods 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, X.; Sun, M.; Li, D.; Hua, M.; Miao, X.; Su, Y.; Chi, Y.; Wang, J.; Niu, H. Optimization of Mixed Fermentation Conditions of Dietary Fiber from Soybean Residue and the Effect on Structure, Properties and Potential Biological Activity of Dietary Fiber from Soybean Residue. Molecules 2023, 28. [Google Scholar] [CrossRef]
- Wei, C.; Ge, Y.; Liu, D.; Zhao, S.; Wei, M.; Jiliu, J.; Hu, X.; Quan, Z.; Wu, Y.; Su, Y. , et al. Effects of High-Temperature, High-Pressure, and Ultrasonic Treatment on the Physicochemical Properties and Structure of Soluble Dietary Fibers of Millet Bran. Front Nutr 2021, 8, 820715. [Google Scholar] [CrossRef]
- Mtetwa, M.D.; Qian, L.S.; Zhu, H.A.; Cui, F.J.; Zan, X.Y.; Sun, W.J.; Wu, D.; Yang, Y. Ultrasound-assisted extraction and antioxidant activity of polysaccharides from Acanthus ilicifolius. J Food Meas Charact 2020, 14. [Google Scholar] [CrossRef]
- Yang, L.; Zhao, T.; Wei, H.; Zhang, M.; Zou, Y.; Mao, G.; Wu, X. Carboxymethylation of polysaccharides from Auricularia auricula and their antioxidant activities in vitro. Int J Biol Macromol 2011, 49, 1124–1130. [Google Scholar] [CrossRef]
- Yang, W.; Wu, J.; Liu, W.; Ai, Z.; Cheng, Y.; Wei, Z.; Zhang, H.; Ma, H.; Cui, F.; Zhou, C. , et al. Structural characterization, antioxidant and hypolipidemic activity of Grifola frondosa polysaccharides in novel submerged cultivation. Food Biosci 2021, 42, 101187. [Google Scholar] [CrossRef]
- Li, P.; Li, C.; Fu, X.; Huang, Q.; Chen, Q. Physicochemical, functional and biological properties of soluble dietary fibers obtained from Rosa roxburghii Tratt pomace using different extraction methods. Process Biochem 2023, 128, 40–48. [Google Scholar] [CrossRef]
- Zheng, H.; Sun, Y.; Zeng, Y.; Zheng, T.; Jia, F.; Xu, P.; Xu, Y.; Cao, Y.; He, K.; Yang, Y. Effects of Four Extraction Methods on Structure and In Vitro Fermentation Characteristics of Soluble Dietary Fiber from Rape Bee Pollen. Molecules 2023, 28, 4800. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, Z.; Deng, Y.; Chen, G. Effect of extraction method on the structure and bioactivity of polysaccharides from activated sludge. Water Res 2024, 253, 121196. [Google Scholar] [CrossRef]
- Peng, F.; Ren, X.; Du, B.; Chen, L.; Yu, Z.; Yang, Y. Structure, Physicochemical Property, and Functional Activity of Dietary Fiber Obtained from Pear Fruit Pomace (Pyrus ussuriensis Maxim) via Different Extraction Methods. Foods 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.F.; Lv, G.Y.; Song, T.T.; Xu, Z.W.; Wang, M.Y. Effects of different extraction methods on the structural and biological properties of Hericium coralloides polysaccharides. Food Chem 2024, 445, 138752. [Google Scholar] [CrossRef] [PubMed]
- Azaroual, L.; Liazid, A.; Mansouri, F.E.; Brigui, J.; Ruíz-Rodriguez, A.; Barbero, G.F.; Palma, M. Optimization of the Microwave-Assisted Extraction of Simple Phenolic Compounds from Grape Skins and Seeds. Agronomy 2021, 11, 1527. [Google Scholar] [CrossRef]
- Mansouri, F.E.; Silva, J.; Cacciola, F.; Asraoui, F.; Tayeq, H.; Ben, A.Y.; Lovillo, M.P.; Chouaibi, N.; Brigui, J. Evaluation of Different Extraction Methods on the Phenolic Profile and the Antioxidant Potential of Ceratonia siliqua L. Pods Extracts. Molecules 2022, 27. [Google Scholar] [CrossRef]
- Peng, F.; Ren, X.; Du, B.; Chen, L.; Yu, Z.; Yang, Y. Structure, Physicochemical Property, and Functional Activity of Dietary Fiber Obtained from Pear Fruit Pomace (Pyrus ussuriensis Maxim) via Different Extraction Methods. Foods 2022, 11, 2161. [Google Scholar] [CrossRef]
- Shen, M.; Weihao, W.; Cao, L. Soluble dietary fibers from black soybean hulls: Physical and enzymatic modification, structure, physical properties, and cholesterol binding capacity. J Food Sci 2020, 85, 1668–1674. [Google Scholar] [CrossRef]
- Zou, X.; Xiao, J.; Chi, J.; Zhang, M.; Zhang, R.; Jia, X.; Mei, D.; Dong, L.; Yi, Y.; Huang, F. Physicochemical properties and prebiotic activities of polysaccharides from Zizyphus jujube based on different extraction techniques. Int J Biol Macromol 2022, 223, 663–672. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, J.; Yang, X.; Yang, X.; Zhang, X.; Wu, F. Extraction ofHeracleum dissectum soluble dietary fiber by different methods: Structure and antioxidant properties. J Food Sci 2024, 89, 3400–3411. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wu, Y.; Jiang, X.; Gan, D.; Fan, J.; Sun, Y.; Liu, W.; Li, X. Dietary fiber extraction from citrus peel pomace: Yield optimization and evaluation of its functionality, rheological behavior, and microstructure properties. J Food Sci 2023, 88. [Google Scholar] [CrossRef]
- A, S.W.; A, Y.F.; B, Y.X.; A, B.Z.; A, J.P.; A, L.Z.; A, L.Y.; A, K.L.; B, S.W.; A, Q.Z. The effects of different extraction methods on physicochemical, functional and physiological properties of soluble and insoluble dietary fiber from Rubus chingii Hu. fruits. J Funct Foods 2022. [Google Scholar]
- Jurevičiūtė, I.; Keršienė, M.; Bašinskienė, L.; Leskauskaitė, D.; Jasutienė, I. Characterization of Berry Pomace Powders as Dietary Fiber-Rich Food Ingredients with Functional Properties. Foods 2022, 11, 716. [Google Scholar] [CrossRef]
- Shen, M.; Weihao, W.; Cao, L. Soluble dietary fibers from black soybean hulls: Physical and enzymatic modification, structure, physical properties, and cholesterol binding capacity. J Food Sci 2020. [Google Scholar] [CrossRef] [PubMed]
- Ozyurt; Vasfiye; Hazal; Tles; Semih. EFFECT OF FOOD PROCESSING ON THE PHYSICOCHEMICAL PROPERTIES OF DIETARY FIBRE. Acta Scientiarum Polonorum. Technologia Alimentaria 2016, 15, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Khan, G.M.; Khan, N.M.; Khan, Z.U.; Ali, F.; Jan, A.K.; Muhammad, N.; Elahi, R. Effect of extraction methods on structural, physiochemical and functional properties of dietary fiber from defatted walnut flour. Food Sci Biotechnol 2018, 27, 1015–1022. [Google Scholar] [CrossRef]
- Geng, X.; Guo, D.; Wu, B.; Wang, W.; Zhang, D.; Hou, S.; Bau, T.; Lei, J.; Xu, L.; Cheng, Y. , et al. Effects of different extraction methods on the physico-chemical characteristics and biological activities of polysaccharides from Clitocybe squamulosa. Int J Biol Macromol 2024, 259, 129234. [Google Scholar] [CrossRef] [PubMed]
- Daou, C.; Zhang, H. Functional and physiological properties of total, soluble, and insoluble dietary fibres derived from defatted rice bran. J Food Sci Tech Mys 2014, 51, 3878–3885. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Huang, C.; Ou, S. In vitro binding capacities of three dietary fibers and their mixture for four toxic elements, cholesterol, and bile acid. J Hazard Mater 2011, 186, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Kasote, D.M.; Katyare, S.S.; Hegde, M.V.; Bae, H. Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int J Biol Sci 2015, 11, 982–991. [Google Scholar] [CrossRef]
- Huang, Y.; Xie, W.; Tang, T.; Chen, H.; Zhou, X. Structural characteristics, antioxidant and hypoglycemic activities of polysaccharides from Mori Fructus based on different extraction methods. Front Nutr 2023, 10. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Dong, X.; Tong, J. Optimization of enzyme-assisted extraction of polysaccharides from alfalfa and its antioxidant activity. Int J Biol Macromol 2013, 62, 387–396. [Google Scholar] [CrossRef]
- Zhang, J.; Jia, S.; Liu, Y.; Wu, S.; Ran, J. Optimization of enzyme-assisted extraction of the Lycium barbarum polysaccharides using response surface methodology. Carbohyd Polym 2011, 86, 1089–1092. [Google Scholar] [CrossRef]
- Geng, X.; Guo, D.; Wu, B.; Wang, W.; Zhang, D.; Hou, S.; Bau, T.; Lei, J.; Xu, L.; Cheng, Y. , et al. Effects of different extraction methods on the physico-chemical characteristics and biological activities of polysaccharides from Clitocybe squamulosa. Int J Biol Macromol 2024, 259, 129234. [Google Scholar] [CrossRef]
- Wu, Y.T.; Huo, Y.F.; Xu, L.; Xu, Y.Y.; Wang, X.L.; Zhou, T. Purification, characterization and antioxidant activity of polysaccharides from Porphyra haitanensis. Int J Biol Macromol 2020, 165, 2116–2125. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Fang, C.; Ran, C.; Tan, Y.; Yu, Q.; Kan, J. Comparison of different extraction methods for polysaccharides from bamboo shoots (Chimonobambusa quadrangularis) processing by-products. Int J Biol Macromol 2019, 130, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Hua, M.; Sun, Y.; Shao, Z.; Lu, J.; Lu, Y.; Liu, Z. Functional soluble dietary fiber from ginseng residue: Polysaccharide characterization, structure, antioxidant, and enzyme inhibitory activity. J Food Biochem 2020, 44, e13524. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhang, P.; Warner, R.D.; Fang, Z. In vitro and cellular antioxidant activities of 3-deoxyanthocyanidin colourants. Food Biosci 2021. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, J.; Wang, X.; Guo, M.; Cheng, C.; Zhang, Y. Effects of three biological combined with chemical methods on the microstructure, physicochemical properties and antioxidant activity of millet bran dietary fibre. Food Chem 2023, 411, 135503. [Google Scholar] [CrossRef]
- Xiong, M.; Feng, M.; Chen, Y.; Li, S.; Fang, Z.; Wang, L.; Lin, D.; Zhang, Q.; Liu, Y.; Luo, Y. , et al. Comparison on structure, properties and functions of pomegranate peel soluble dietary fiber extracted by different methods. Food Chemistry: X 2023, 19, 100827. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Xu, X.; Chao, Z.; Jiang, X.; Zheng, L.; Jiang, B. Properties of plant-derived soluble dietary fibers for fiber-enriched foods: A comparative evaluation. Int J Biol Macromol 2022, 223, 1196–1207. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.; Zhang, X.; Liu, C.; Zhang, W.; Han, X.; Zhao, H. Effects of extraction methods on the structure and functional properties of soluble dietary fiber from blue honeysuckle (Lonicera caerulea L.) berry. Food Chem 2024, 431, 137135. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Yan, X.; Liang, J.; Li, S.; He, H.; Xiong, Q.; Lai, X.; Hou, S.; Huang, S. Comparison of different extraction methods for polysaccharides from Dendrobium officinale stem. Carbohyd Polym 2018, 198, 101–108. [Google Scholar] [CrossRef]
- Tan, X.; Cheng, X.; Ma, B.; Cui, F.; Wang, D.; Shen, R.; Li, X.; Li, J. Characterization and Function Analysis of Soluble Dietary Fiber Obtained from Radish Pomace by Different Extraction Methods. Molecules 2024, 29. [Google Scholar] [CrossRef]
- Xiong, F.; Li, X.; Zheng, L.; Hu, N.; Cui, M.; Li, H. Characterization and antioxidant activities of polysaccharides from Passiflora edulis Sims peel under different degradation methods. Carbohyd Polym 2019, 218, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhou, S.; Li, Y.; Tian, J.; Zhang, C. Structure, physicochemical properties and effects on nutrients digestion of modified soluble dietary fiber extracted from sweet potato residue. Food Res Int 2021, 150, 110761. [Google Scholar] [CrossRef]
- Song, Y.; Sun, G.; Wang, D.; Chen, J.; Lv, J.; Jiang, S.; Zhang, G.; Yu, S.; Zheng, H. Optimization of Composite Enzymatic Extraction, Structural Characterization and Biological Activity of Soluble Dietary Fiber from Akebia trifoliata Peel. Molecules 2024, 29, 2085. [Google Scholar] [CrossRef]
- Gu, Q.; Gao, X.; Zhou, Q.; Li, Y.; Li, G.; Li, P. Characterization of soluble dietary fiber from citrus peels (Citrus unshiu), and its antioxidant capacity and beneficial regulating effect on gut microbiota. Int J Biol Macromol 2023, 246, 125715. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Xie, W.; Tang, T.; Chen, H.; Zhou, X. Structural characteristics, antioxidant and hypoglycemic activities of polysaccharides from Mori Fructus based on different extraction methods. Front Nutr 2023, 10. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).