1. Introduction
Essential oils (EO) and their bioactive compounds have significant potential in ruminant nutrition due to their effects on various microorganisms. Their antimicrobial properties are attributed to the presence of terpenoids and phenolic compounds [
1,
2,
3]. Studies shows different mechanisms of action in the rumen as such as changes in the volatile fatty acid (VFA) profile, nutrients digestibility and, reduction in methane (CH
4) and ammonia production [
4,
5,
6].
Copaiba oil is extracted from the tree copaibeira in Brazil (
Copaifera spp.), which is a native tree from tropical regions in Latin America and West Africa that produces an oil-resin obtained from the drilling of the trunk [
7]. Its composition varies according to the species, commonly presenting about 80% of its active compounds such as sesquiterpenes and 20% as diterpenes. The β-caryophyllene is the sequiterpene present in the highest concentration, representing approximately 50% of the composition in the EO [
8].
The combination of sesquiterpenes and diterpenes present in copaiba oil has shown antimicrobial activity observed on several pathogenic microorganisms, mainly gram-positive bacteria such as
Staphylococcus spp. and Streptococcus spp. [
8,
9].
This antimicrobial activity in the copaiba oil might potentially be used as an additive in ruminant diets. However, this potential as a nutritional additive, especially for the manipulation of the rumen environment, is still unknown. Therefore, this study evaluated the effect of the copaiba oil or monensin, as nutritional additives for beef cattle, on intake, digestibility, and in situ ruminal degradability and variables (pH, N-NH3, and VFAs).
2. Materials and Methods
The experiment was conducted at the Animal Metabolism Laboratory of the School of Veterinary Medicine and Animal Science of the Universidade Federal do Mato Grosso do Sul (UFMS) in Campo Grande, MS, Brazil. The study was approved by the UFMS Ethics and Animal Use Committee (CEUA) under protocol No. 639/2014.
2.1. Animals, Management, and Treatments
Five rumen cannulated crossbred steers (1/2 Nellore + 1/2 Holstein) with initial body weight (BW) of 279 ± 22 kg were assigned to a 5 x 5 Latin square design. All steers were housed individually in covered pens with feeders and water troughs.
Animals were fed with chopped hay from
Megathyrsus maximus (Syn.
Panicum maximum Jacq.) cv. Massai and protein/energy supplementation (
Table 1). The concentrate supplement contained ground corn (850 g kg
-1), soybean meal (75 g kg
-1), urea (25 g kg
-1), and mineral premix (50 g kg
-1). The experimental treatments consisted of addition of copaiba oil-resin (COP) or monensin sodium ionophore to the concentrate supplement as follow: Control (0 g of COP), COP1.25 (1.25 g of COP), COP2.50 (2.50 of COP), and COP3.75 (3.75 g of COP) per kg of DM of the total diet, respectively). Furthermore, monensin (40 mg of sodium monensin per kg
-1 DM of the total diet) was also evaluated as positive control. The additives were weighed daily and used based on the previous day’s feed intake. The inclusion of the additives was right before feed providing.
The roughage was supplied
ad libitum twice daily at 7:00 a.m. and 5:00 p.m. The amount of roughage offered was adjusted daily to maintain refusals of approximately 150 g kg
-1. The concentrate supplement was supplied in the amount of 15 g kg
-1 BW once a day at 7:00 a.m. in a separate feeder. The diet met the nutritional requirements of growing cattle with an expected average daily gain of 1 kg d
-1 [
10].
The experiment lasted for 105 days and was divided into five experimental periods of 21 days. The animals were adapted to the treatments for 14 days and samples were collected in the last seven days/period. At the beginning of each experimental period, the animals were weighed after 14 hours of solids fasting to adjust the amount of concentrate supplement offered.
2.2. Nutrient Intake and Apparent Digestibility
The DM and nutrient intake were evaluated from days 15 to 19 of each experimental period. At the same period total feces were collected from each animal immediately after defecation. The feces samples were weighed and homogenized, and 100 g kg-1 were stored at -20 ° C for further analysis.
Nutrient’s intake (amount offered - refusal) and apparent digestibility coefficients ((ingested nutrient - excreted nutrient)/ingested nutrient) of dry matter (DM), organic matter (OM), crude protein (CP), neutral detergent fiber corrected by ash and protein content (apNDF), acid detergent fiber corrected by ash and protein content (apADF), ether extract (EE), and non-fiber carbohydrates (NFC) were estimated.
2.3. In Situ Degradability Assessment
Ruminal disappearances of DM and NDF were performed between days 15 to 19 in each experimental period using 50 μm porosity nylon bags (7 x 14 cm). These bags were weighed empty and received 5 g of hay (grounded in 2 mm sieve). These bags were introduced in the rumen 7 hours (before feeding) and removed after the incubation times (3, 6, 12, 24, 48, 72, 96, and 120 hours). The procedures of ruminal disappearance were performed as described in Cortada Neto et al. [
11].
The DM soluble fraction (“a”), the potentially insoluble degradable fraction (“b”), the degradation rate (“c”), and the effective degradability (ED) of the DM and NDF were calculated according to Ørskov and McDonald [
12], based on the equation: ED = a + (bxc)/(c + k), where “k” corresponds to the estimated ruminal solids passage rate, considered in the present study as 0.05 h
-1 as suggested by Huntington and Givens [
13]. The potential degradation of DM and NDF was considered to be the one in which degradation stabilized over the incubation times.
2.4. Ruminal Fluid Sampling
Ruminal fluid samples were collected to determine the VFA’s profile, pH, and N-NH3 on the last two days of each experimental period. During this period, samples were taken at four-hour intervals to obtain representative samples of the zero-hour (before morning feeding), 2, 4, 6, 8, 10, 12, 14, 16, 18, 20, and 22 hours after the morning feeding. Ruminal fluid was collected manually (via cannula) in aliquots of approximately 100 mL, at different rumen sites, and filtered through a cotton diaper. The rumen pH was measured immediately after sampling (B474; Micronal, São Paulo, SP, Brazil). For VFA profile, 4 mL of ruminal fluid was acidified with 1 mL of metaphosphoric acid (25%) and stored at -20 °C for further analysis. The N-NH3 was determined in 50 mL aliquots of ruminal fluid acidified with 1 mL of H2SO4 (50%) and stored at -20 °C.
2.5. Chemical Analysis
The chemical composition evaluations of the diets, refusals, and feces were evaluated according to the AOAC [
14]: DM (method 967.03), crude protein (CP, method 981.10), ether extract (EE, method 920.29), and ash (method 942.05). The NDF and ADF contents were analyzed using the Tecnal TE-149® fiber determinants (Tecnal, Piracicaba, SP, Brazil) through 5 x 5 cm non-woven bags (NWF) and 100 μm porosity, with 0.5 g of sample per bag. These determinations were according to the methodology of Van Soest [
15], using thermostable amylase (Termamyl 120 L Novozymes A/S, Bagsvaerd, Denmark). The NDF and ADF contents were subsequently corrected for the presence of ash and protein (apNDF and apADF, respectively). The same procedure was performed to evaluate the NDF content of the material resulting from the in situ ruminal degradation. However, this analysis did not include the use of thermostable amylase, and correction for ash and protein was not performed.
The non-fiber carbohydrate (NFC) content of forages was calculated as proposed by Sniffen et al. [
16], using the equation: NFC = 100 - (CP + ash + apNDF + EE). The concentrate supplement content was calculated as proposed by Hall [
17], using the equation: NFC = 100 - ((CP - CP derived from urea + inclusion of urea) + MM + apNDF + EE).
The N-NH
3 content was obtained in the supernatant of ruminal fluid samples after thawing by 2N KOH distillation according to an adaptation of the method described by Fenner [
18], conducted by Ribeiro et al. [
19]. The concentration of VFA’s was determined by gas chromatography (Shimadzu GC-2010, Kyoto, Japan) according to the methodology described by Erwin et al. [
20].
2.6. Statistical Analyses
Statistical analyses were performed using the GLIMMIX procedure in the SAS statistical software (SAS University Edition, SAS Institute Inc., Cary, NC, USA), with the Satterthwaite approximation, to determine denominator degrees of freedom for fixed effects. Nutrient intake and apparent digestibility data were analyzed using a 5 x 5 Latin square design. The statistical model used was: Yijk = μ + Ti + Pj + Ak + eijk; Where: Yijk = observation of treatment effect i in the period j, of animal k, μ = overall average, Ti = treatment effect i, in which i = 1 (control), 2 (COP1.25), 3 (COP2.50), 4 (COP3.75), and 5 (Monensin); Pj = period effect j (j = 5 periods); Ak = animal effect k (k = 5 animals), and eijk = random error associated with each observation.
For the in-situ degradation of DM and NDF and the ruminal pH and N-NH3, a Latin square design with subdivided plots was considered, where the plots were the treatments, and the subplots were incubation times of rumen samples or the different ruminal fluid sampling times. The model included effects from treatment, incubation time, animal, period, and treatment x time for the in-situ DM and NDF degradation rates. For the pH and N-NH3 ruminal variables, the statistical model included effects from treatment, sampling times, animal, period, and treatment x time. The statistical model used was: Yijkl = μ + Ti + Hj + Ak + Pl + (TH) ij + eijkl; Where: Yijkl = observation of the treatment effect i for incubation hours (degradation rate) or collection time (ruminal variables) j in animal k; μ = overall average; Ti = effect of treatment where i = 1 (control), 2 (COP1.25), 3 (COP2.50), 4 (COP3.75), and 5 (Monensin); Hj = effect of incubation times for disappearance (j = 1, …, 8) or sampling time for ruminal variables (j = 1, …, 13); Ak = animal effect (k = 1, …, 5), Pl = effect of period (1 = 1, …, 5); THij = interaction between treatment i and time j; and eijkl = random error associated with each observation.
The covariance structure that presented the lowest Akaike information criterion in each analysis for the variables repeated overtime was chosen. The covariance structures used were toepliz (pH) and first-order autoregressive structure (N-NH3, DM and NDF degradation, and VFA’s). When significant differences were observed (P ≤ 0.05) in the F-test (ANOVA), means were compared using the Tukey’s test; differences were considered significant with P ≤ 0.05.
3. Results
3.1. Nutrient’s Intake
The COP (1.25, 2.50, and 3.75 g kg
-1) did not affect the intake of hay DM (kg day and g kg
-1 BW) when compared to the control (
Table 2). Overall, the sodium monensin reduced (P ≤ 0.05) the intake of hay DM (kg day
-1 and g kg
-1 of BW) and consequently decreased the intake of nutrients. However, monesin did not affect the total CP, total EE, and total NFC (kg day
-1;
Table 2).
3.2. Ruminal Variables
The COP or sodium monensin did not affect (P > 0.05) the digestibility coefficients of DM, OM, CP, apNDF, EE, and NFC (
Table 3).
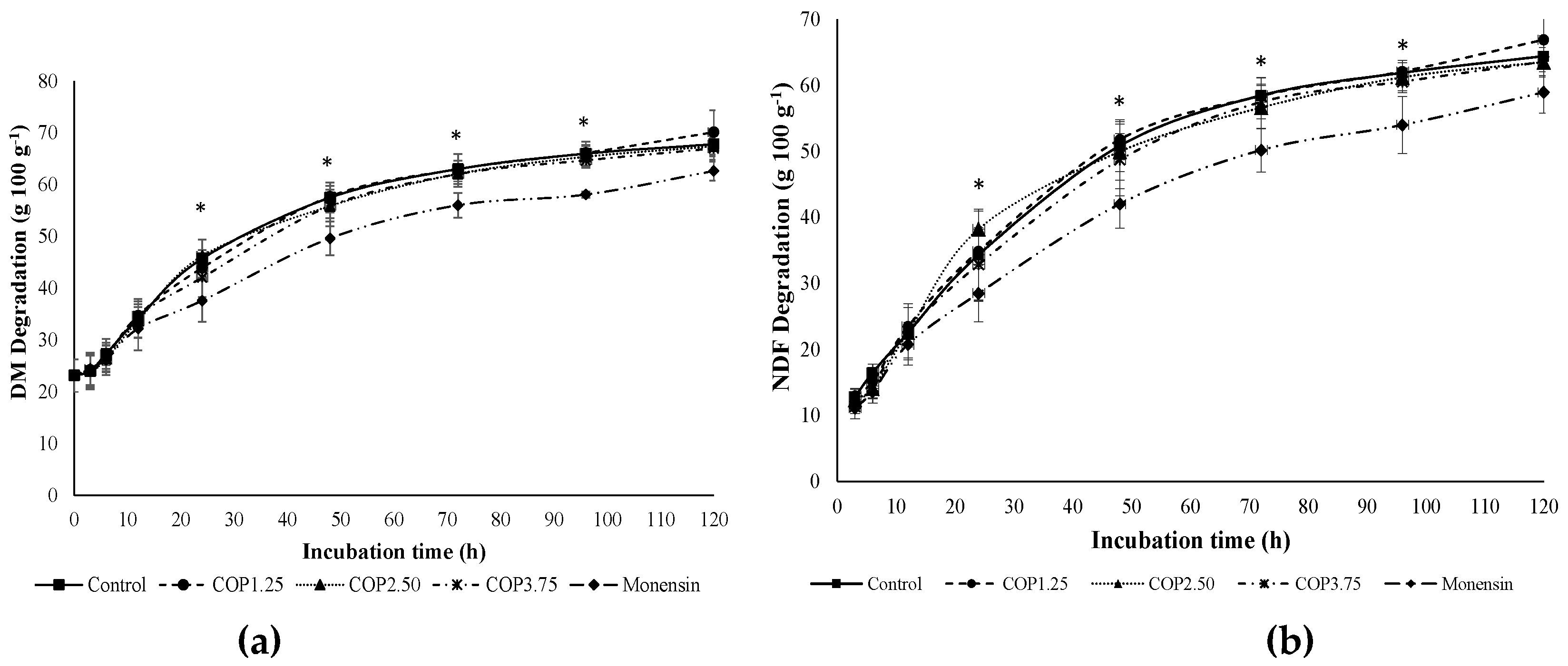
The COP did not affect DM and NDF degradation; however, the sodium monensin reduced (P ≤ 0.05) the degradation of hay DM and NDF at 24, 48, 72, and 96 hours after incubation compared to the control (
Figure 1).
The COP did not affect (P > 0.05) the estimated ruminal degradation parameters of DM and NDF compared to the control (
Table 4). However, monensin inclusion decreased (P ≤ 0.05) fractions b and c, and effective degradability (ED) of DM compared to the control (
Table 4). The monensin did not affect (P > 0.05) fraction c, however, decreased the degradation (P ≤ 0.05) of fraction b and the ED of NDF fraction compared to the control (
Table 4).
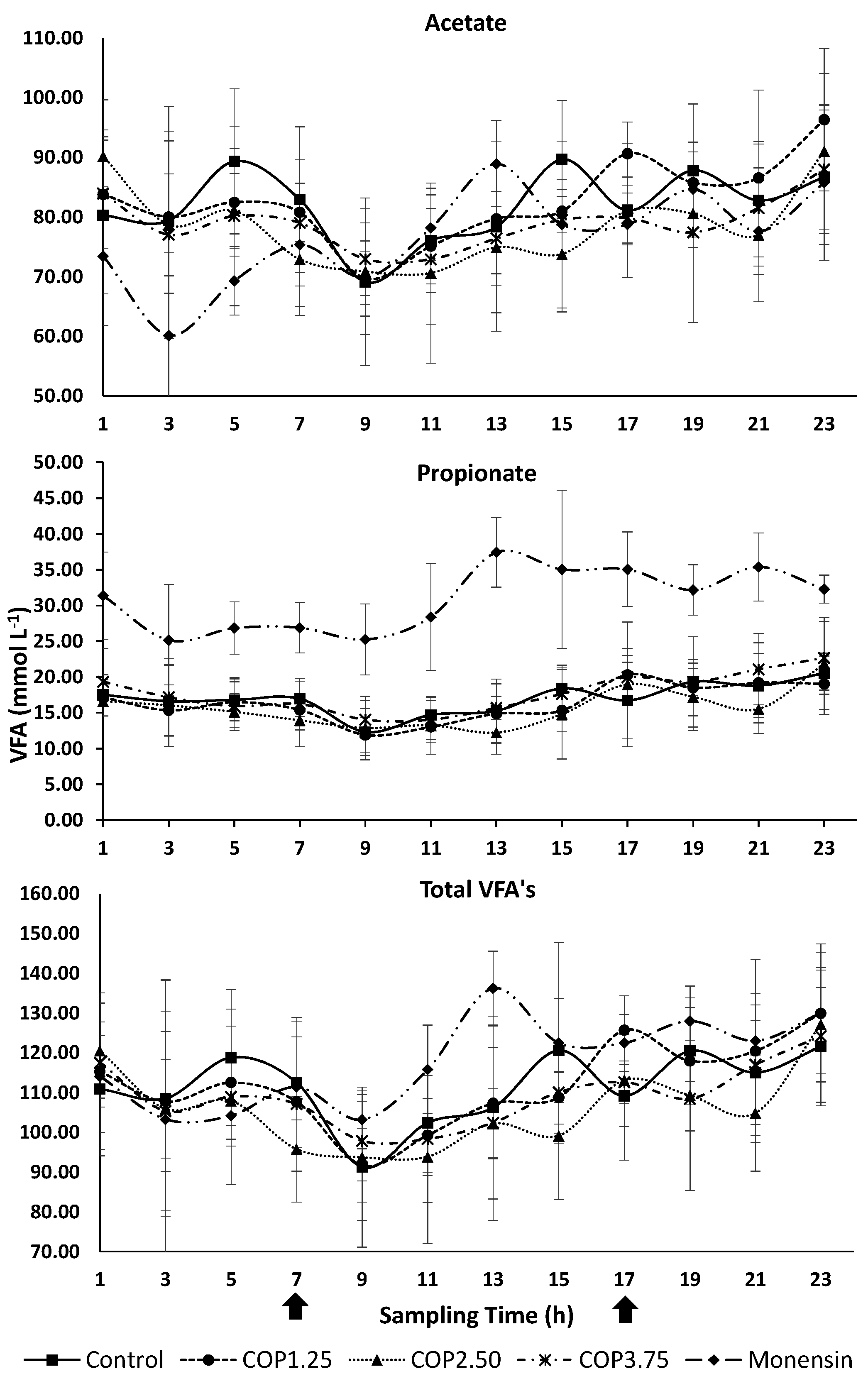
The COP did not affect (P > 0.05) the production of VFAs in mmol L
-1 or mmol 100 mmol
-1 compared to the control (
Table 5). However, the monensin increased (<0.0001) propionate production. These increases in propionate production (mmol L
-1) when monesin was provided resulted in lower (P ≤ 0.05) acetate and butyrate concentrations (mmol L
-1), higher concentration of propionate, and consequently lowest acetate:propionate ratio (
Table 5). Interactions (P ≤ 0.05) between sampling times and concentrations (mmol L-1) of acetate, propionate, and total VFA’s were observed. The VFA concentrations were higher in the monensin treatment between the morning and afternoon feedings (
Figure 2).
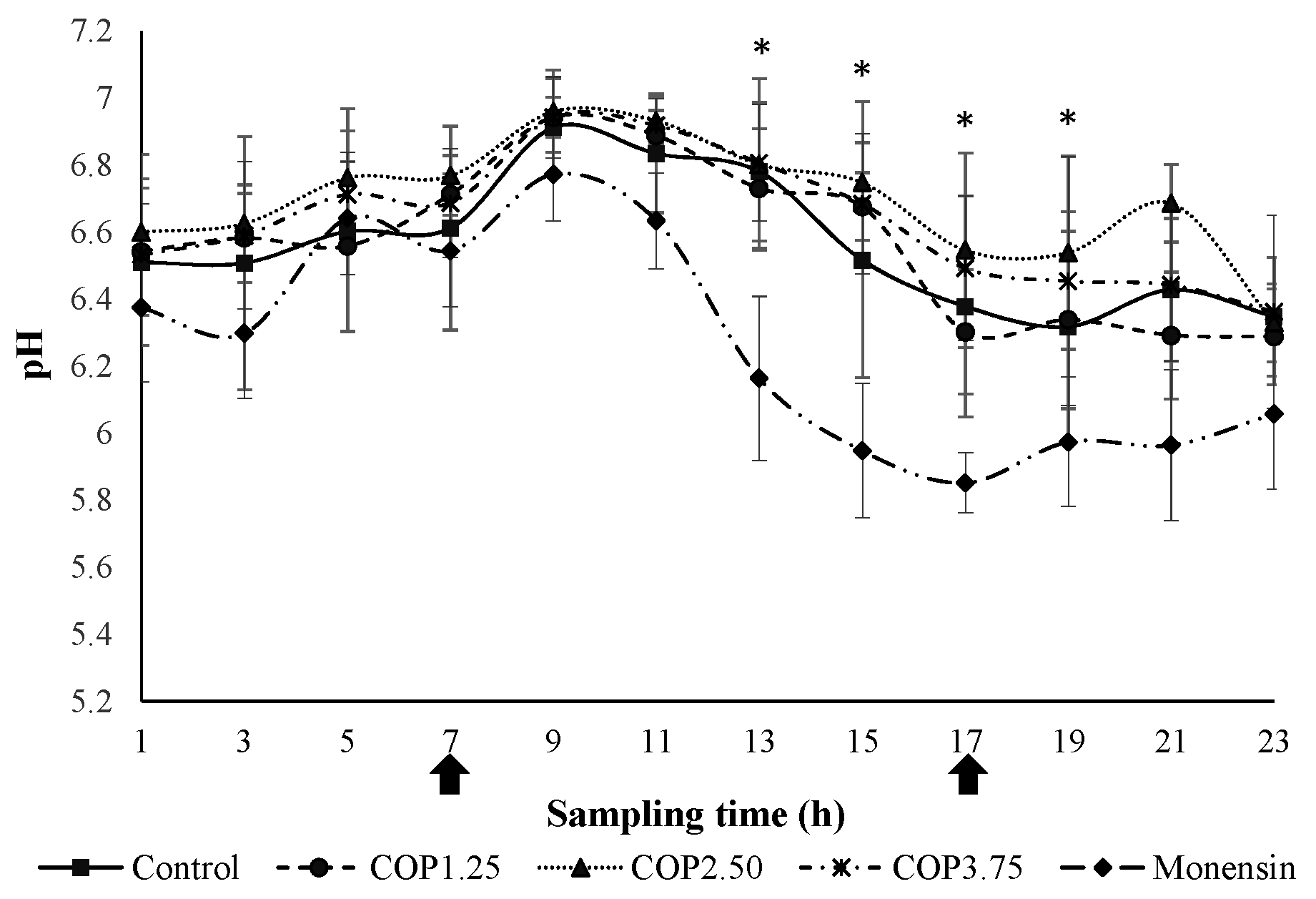
The rumen pH were lower (P ≤ 0.05) when monensin were included between 13 and 21 hours compared to the control (
Figure 3). In all treatments, the highest pH values were observed between 9:00 and 11:00 a.m. (two to four hours after feeding), with gradual reduction after this time range (
Figure 3).
The COP did not affect (P > 0.05) the N-NH
3 concentration in the ruminal fluid compared to the control (
Figure 4). The concentration of rumen N-NH
3 in the monensin treatment was higher (P ≤ 0.05) than in the COP1.25 and COP2.50 (
Figure 4). The highest (P ≤ 0.05) N-NH
3 concentrations in all treatments were observed between 11:00 a.m. and 3:00 p.m. (four to eight hours after feeding,
Figure 4).
4. Discussion
In the current study, the lack of EO in changes on nutrient intake corroborates results from other studies evaluating the addition of EO to the diet of ruminants [
21,
22,
23]. However, the use of EO as nutritional additives has shown different results on feed intake, which may be associated with high dose variability and the composition of evaluated compounds [
24].
The reduction in DM intake with monensin inclusion observed in the present study is well documented in the literature [
25]. This effect may occur due to an increase of ruminal propionic acid concentration, which reflects in increased energy efficiency, allowing nutritional requirements to be met with lower levels of feed intake [
26]. The magnitude of the reduction in feed intake promoted by monensin is associated with forage digestion, the animal’s ability to store undigested feed, and its energy requirements [
27].
The absence of effects in nutrient apparent digestibility when COP was provided is corroborated by similar results found in other study using different levels of COP [
28]. The addition of EO commonly reduces ruminal degradation of rapidly fermentable substrates, such as starch and protein, providing changes in their digestion site; however, it does not affect the apparent digestibility in the total digestive tract [
29].
Monensin can increase dietary digestibility by increasing the DM retention time in the rumen as the result of lower voluntary intake, stimulating rumination, improving the ruminal environment, and allowing increased digestibility [
30]. However, monensin did not affect nutrient digestibility in this study.
The COP did not change the ruminal degradation of hay DM and NDF. The supplementation to non-lactating Holstein cows of 2 mL of allicin, zingiberene, or citral, which are the main active components of garlic, ginger, and lemongrass oils, respectively, resulted in increases of DM and NDF ruminal degradability between 48 and 72 h of incubation [
31]. Studies indicate that some EO would mainly reduce the degradation of rapidly degradable substrates, such as starch and protein, due to the inhibition of proteolytic and amylolytic bacteria activity [
30,
32]. In our study, the observation of this result was not possible due to the high roughage: concentrate ratio (approximately 50:50), low quality of the forage used (low protein content and high NDF content), and low rate of supplement intake by the animals.
The use of ionophores in diets can lead to a reduction in the fiber fraction degradability due to its effect on cellulolytic bacteria, and consequently, reduce DM ruminal degradability [
11]. This result supports the observations of reduction in DM and NDF degradation parameters obtained in the present study when sodium monensin was provided to the steers.
The EO has shown potential to change rumen VFA production [
33]. In fact, EO may change rumen bacteria profile and this reflects in different patterns of rumen fermentation. Ruminal pH remained above 5.8 in all treatments, a value considered normal for the proper functioning of the rumen [
34]; however, the ruminal pH when monensin was provided remained below 6.2 in mostly sampling times, which may impair fiber digestion due to the reduced activity of cellulolytic microorganisms that are sensitive to pH reductions below this limit [
35]; this fact may have contributed to lower the DM, and NDF degradability observed in the monensin treatment. The most substantial decrease in rumen pH in the monensin treatment may have been due to lower apNDF, CP intake from lower hay intake. The lower consumption of forage and consequently of fiber results in shorter consumption and rumination times, and consequently reduces animal saliva production, which is mostly responsible for buffering the rumen environment [
36].
The change in VFA production caused by monensin, reflecting in lower acetate:propionate ratio, higher propionic acid production, and lower acetate and butyrate proportions is an effect commonly described in the literature and justified by its action against gram-positive bacteria [
37]. Gram-positive bacteria that produce acetate, butyrate, and H
2 are inhibited by ionophores, and gram-negative bacteria that produce propionate find better conditions to reproduce in the presence of ionophores [
38]. Observed interactions may be associated with a slower consumption rate of the diet containing monensin.
The effect of essential oils seems to be pH-dependent, and an increase in antimicrobial activity has been shown with a reduction in pH values. Only the undissociated form of molecules can interact with the lipid bilayer of the bacterial cell membrane, increasing the undissociated form with the decrease in pH, and thus, increasing its hydrophobicity and allowing greater interaction with the bacterial cell membrane [
1].
In the present study, the low-quality hay DM intake corresponded on average to 493 g kg
-1 of the total feed intake by the animals in the treatments with the COP, maintaining the rumen pH always above 6.3, which may have limited their antimicrobial activity. Studies have shown the antimicrobial activity of COP, especially on gram-positive and antifungal microorganisms [
8]. However, under the conditions of the present study, this effect could not be observed. Although the components of COP have the potential to control or limit the growth of several pathogenic and non-pathogenic microorganism species, it did not have an ionospheric effect on the rumen environment, despite the wide range of daily doses. However, the absence of an effect may be due to a rumen environment not conducive to its action, with pH values close to neutrality, and therefore, this should be observed in subsequent evaluations.
5. Conclusions
The addition of COP to the concentrate did not affect the intake, digestibility, ruminal degradability, and ruminal variables in beef cattle. The addition of sodium monensin reduced DM intake, ruminal degradations of DM and NDF, increases propionate production and, reduces acetate: propionate ratio.
Author Contributions
Conceptualization, G.F.; methodology, G.F and F.F.; formal analysis, L.I. and M.V.; investigation, A.B and R.R; writing—original draft preparation, A.B. and A.M.; writing—review and editing, J.S. and G.F.; supervision, G.F.; project administration, G.F.; funding acquisition, G.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), grant number 564435/2010-4 and Fundação de Apoio ao Desenvolvimento do Ensino, Ciência e Tecnologia do Estado de Mato Grosso do Sul (FUNDECT) grat number TO 014/12.
Institutional Review Board Statement
The study was approved by the ethics committee of the Universidade Federal do Mato Grosso do Sul in Campo Grande under protocol No. 639/2014.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Calsamiglia, S.; Busquet, M.; Cardozo, P.W.; Castillejos, L.; Ferret, A. Invited Review: Essential Oils as Modifiers of Rumen Microbial Fermentation. J Dairy Sci 2007, 90. [Google Scholar] [CrossRef] [PubMed]
- Patra, A.K. Effects of Essential Oils on Rumen Fermentation, Microbial Ecology and Ruminant Production. Asian J Anim Vet Adv 2011, 6. [Google Scholar] [CrossRef]
- Rofiq, M.N. The Use of Plant Essential Oils as Feed Additives for Ruminants. Indonesian Bulletin of Animal and Veterinary Sciences 2016, 26. [Google Scholar] [CrossRef]
- LUO, Z.; LIU, T.; CAIRANG, D.; CHENG, S.; HU, J.; SHI, B.; ZHU, H.; CHEN, H.; ZHANG, T.; YI, X. Oregano Essential Oil as a Natural Plant Additive Affects Growth Performance and Serum Antibody Levels by Regulating the Rumen Microbiota of Calves. Animals 2024, 14. [Google Scholar] [CrossRef] [PubMed]
- EZAR, A.; SARAH, D.; ARDIAN, A.; ANAS, M.; APRIANTO, M.; HANIM, C.; KURNIAWATI, A.; MUHLISIN, M.; YUSIATI, L. M. Effect of nutmeg essential oil (Myristica fragrans Houtt.) on methane production, rumen fermentation, and nutrient digestibility in vitro. Scientific Reports 2024, 14. [Google Scholar] [CrossRef]
- KARA, K.; PIRCI, G. Immunity, rumen metagenomics, ruminal variables, and growth performance of calves fed milk with sage (Salvia officinalis) essential oil. Tropical Animal Health and Production 2024, 56. [Google Scholar] [CrossRef]
- Leandro, L.M.; De Sousa Vargas, F.; Barbosa, P.C.S.; Neves, J.K.O.; Da Silva, J.A.; Da Veiga-Junior, V.F. Chemistry and Biological Activities of Terpenoids from Copaiba (Copaifera Spp.) Oleoresins. Molecules 2012, 17. [Google Scholar] [CrossRef]
- Tobouti, P.L.; de Andrade Martins, T.C.; Pereira, T.J.; Mussi, M.C.M. Antimicrobial Activity of Copaiba Oil: A Review and a Call for Further Research. Biomedicine & Pharmacotherapy 2017, 94. [Google Scholar] [CrossRef]
- Alencar, É.N.; Xavier-Júnior, F.H.; Morais, A.R. V.; Dantas, T.R.F.; Dantas-Santos, N.; Verissimo, L.M.; Rehder, V.L.G.; Chaves, G.M.; Oliveira, A.G.; Egito, E.S.T. Chemical Characterization and Antimicrobial Activity Evaluation of Natural Oil Nanostructured Emulsions. J Nanosci Nanotechnol 2015, 15. [Google Scholar] [CrossRef]
- National Research Council Nutrient Requirements of Beef Cattle; National Academies Press: Washington, D.C., 2000. ISBN 978-0-309-06934-2.
- Cortada, I. M.; Vedovatto, M.; D’Oliveira, M. C.; Bento, A. L. L.; Gaspar, A. O.; Franco, G. L. Effects of antibiotic growth promoters and concentrate on intake, digestibility, degradability, and ruminal variables in beef steers. Arquivo Brasileiro de Medicina Veterinária e Zootecnia, 74. [CrossRef]
- Ørskov, E.R.; McDonald, I. The Estimation of Protein Degradability in the Rumen from Incubation Measurements Weighted According to Rate of Passage. J Agric Sci 1979, 92. [Google Scholar] [CrossRef]
- Huntington, J.A.; Givens, D.I. The in Situ Technique for Studying Rumen Degradation of Feeds: A Review of the Procedure; 1995.
-
Association of Official Analytical Chemists Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1990.
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J Dairy Sci 1991, 74. [Google Scholar] [CrossRef] [PubMed]
- Sniffen, C.J.; O’Connor, J.D.; Van Soest, P.J.; Fox, D.G.; Russell, J.B. A Net Carbohydrate and Protein System for Evaluating Cattle Diets: II. Carbohydrate and Protein Availability. J Anim Sci 1992, 70. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.B. Calculation of Non-Structural Carbohydrate Content of Feeds That Contain Non-Protein Nitrogen; Gainesville, 2000.
- Fenner, H. Method for Determining Total Volatile Bases in Rumen Fluid by Steam Distillation. J Dairy Sci 1965, 48. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, S.S.; Vasconcelos, J.T.; Morais, M.G.; Ítavo, C.B.C.F.; Franco, G.L. Effects of Ruminal Infusion of a Slow-Release Polymer-Coated Urea or Conventional Urea on Apparent Nutrient Digestibility, in Situ Degradability, and Rumen Parameters in Cattle Fed Low-Quality Hay. Anim Feed Sci Technol 2011, 164. [Google Scholar] [CrossRef]
- Erwin, E.S.; Marco, G.J.; Emery, E.M. Volatile Fatty Acid Analyses of Blood and Rumen Fluid by Gas Chromatography. J Dairy Sci 1961, 44. [Google Scholar] [CrossRef]
- Tomkins, N.W.; Denman, S.E.; Pilajun, R.; Wanapat, M.; McSweeney, C.S.; Elliott, R. Manipulating Rumen Fermentation and Methanogenesis Using an Essential Oil and Monensin in Beef Cattle Fed a Tropical Grass Hay. Anim Feed Sci Technol 2015, 200. [Google Scholar] [CrossRef]
- Vendramini, T.H.A.; Takiya, C.S.; Silva, T.H.; Zanferari, F.; Rentas, M.F.; Bertoni, J.C.; Consentini, C.E.C.; Gardinal, R.; Acedo, T.S.; Rennó, F.P. Effects of a Blend of Essential Oils, Chitosan or Monensin on Nutrient Intake and Digestibility of Lactating Dairy Cows. Anim Feed Sci Technol 2016, 214. [Google Scholar] [CrossRef]
- Silvestre, T., Martins, L. F., Cueva, S. F., Wasson, D. E., Stepanchenko, N., Räisänen, S. E., Somai, S., Hile, M.L. & Hristov, A. N. (2023). Lactational performance, rumen fermentation, nutrient use efficiency, enteric methane emissions, and manure greenhouse gas-emitting potential in dairy cows fed a blend of essential oils. Journal of dairy science, 106(11), 7661-7674. [CrossRef]
- Cobellis, G.; Acuti, G.; Forte, C.; Menghini, L.; De Vincenzi, S.; Orrù, M.; Valiani, A.; Pacetti, D.; Trabalza-Marinucci, M. Use of Rosmarinus Officinalis in Sheep Diet Formulations: Effects on Ruminal Fermentation, Microbial Numbers and in Situ Degradability. Small Ruminant Research 2015, 126. [Google Scholar] [CrossRef]
- Ahvanooei, M. R.; Norouzian, M. A.; Piray, A. H.; Vahmani, P.; Ghaffari, M. H. Effects of monensin supplementation on rumen fermentation, methane emissions, nitrogen balance, and metabolic responses of dairy cows: A systematic review and dose-response meta-analysis. Journal of Dairy Science 2024, 107. [Google Scholar] [CrossRef]
- Russell, J.B.; Strobel, H.J. Effect of Ionophores on Ruminal Fermentation. Appl Environ Microbiol 1989, 55. [Google Scholar] [CrossRef]
- Bell, N.L.; Anderson, R.C.; Callaway, T.R.; Franco, M.O.; Sawyer, J.E.; Wickersham, T.A. Effect of Monensin Inclusion on Intake, Digestion, and Ruminal Fermentation Parameters by Bos Taurus Indicus and Bos Taurus Taurus Steers Consuming Bermudagrass Hay. J Anim Sci 2017, 95. [Google Scholar] [CrossRef] [PubMed]
- Teobaldo, R. W.; De Paula, N. F.; Zervoudakis, J. T.; Fonseca, M. A.; Cabral, L. S.; Martello, H. F.; Rocha, J.K.L.; Ribeiro, I.J.; Mundim, A. T. Inclusion of a blend of copaiba, cashew nut shell and castor oil in the protein-energy supplement for grazing beef cattle improves rumen fermentation, nutrient intake and fibre digestibility. Animal Production Science 2020, 60. [Google Scholar] [CrossRef]
- Cobellis, G.; Trabalza-Marinucci, M.; Yu, Z. Critical Evaluation of Essential Oils as Rumen Modifiers in Ruminant Nutrition: A Review. Science of The Total Environment 2016, 545–546. [Google Scholar] [CrossRef] [PubMed]
- Ellis, W.C.; Horn, G.W.; Delaney, D.; Pond, K.R. Effects of Ionophores on Grazed Forage Utilization and Their Economic Value for Cattle on Wheat Pasture. In Proceedings of the National wheat pasture symposium, Oklahoma Agricultural Experiment Station; 1983; pp. 343–345. [Google Scholar]
- Suksombat, W.; Nanon, A.; Meeprom, C.; Lounglawan, P. Feed Degradability, Rumen Fermentation and Blood Metabolites in Response to Essential Oil Addition to Fistulated Non-lactating Dairy Cow Diets. Animal Science Journal 2017, 88. [Google Scholar] [CrossRef] [PubMed]
- Hart, K.J.; Yáñez-Ruiz, D.R.; Duval, S.M.; McEwan, N.R.; Newbold, C.J. Plant Extracts to Manipulate Rumen Fermentation. Anim Feed Sci Technol 2008, 147. [Google Scholar] [CrossRef]
- Silva, G. G.; Takiya, C. S.; Del Valle, T. A.; de Jesus, E. F.; Grigoletto, N. T.; Nakadonari, B.; Cortinhas, C.S. Acedo, T.S.; Rennó, F.P. Nutrient digestibility, ruminal fermentation, and milk yield in dairy cows fed a blend of essential oils and amylase. Journal of dairy science 2018, 101. [Google Scholar] [CrossRef]
- Kolver, E.S.; de Veth, M.J. Prediction of Ruminal PH from Pasture-Based Diets. J Dairy Sci 2002, 85. [Google Scholar] [CrossRef]
- Grant, R.H.; Mertens, D.R. Influence of Buffer PH and Raw Corn Starch Addition on In Vitro Fiber Digestion Kinetics. J Dairy Sci 1992, 75. [Google Scholar] [CrossRef]
- Chibisa, G.E.; Beauchemin, K.A.; Penner, G.B. Relative Contribution of Ruminal Buffering Systems to PH Regulation in Feedlot Cattle Fed Either Low- or High-Forage Diets. Animal 2016, 10. [Google Scholar] [CrossRef]
- Anassori, E.; Dalir-Naghadeh, B.; Pirmohammadi, R.; Taghizadeh, A.; Asri-Rezaei, S.; Maham, M.; Farahmand-Azar, S.; Farhoomand, P. Garlic: A Potential Alternative for Monensin as a Rumen Modifier. Livest Sci 2011, 142. [Google Scholar] [CrossRef]
- Bergen, W.G.; Bates, D.B. Ionophores: Their Effect on Production Efficiency and Mode of Action. J Anim Sci 1984, 58. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).