Submitted:
19 December 2024
Posted:
20 December 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Nomenclature and Synthesis
1.2. Photocatalytic Properties and Applications
1.3. Pesticides and Pharmaceuticals as Emerging Pollutants
1.4. Bibliometric Study
2. Results and Discussion
2.1. Publication Patterns and Characteristics
2.2. Author Performance
2.3. Journals Performance and WoS Categories
2.4. Countries and Institutions Performance
2.5. Keywords Analysis
2.5.1. Trending Keywords
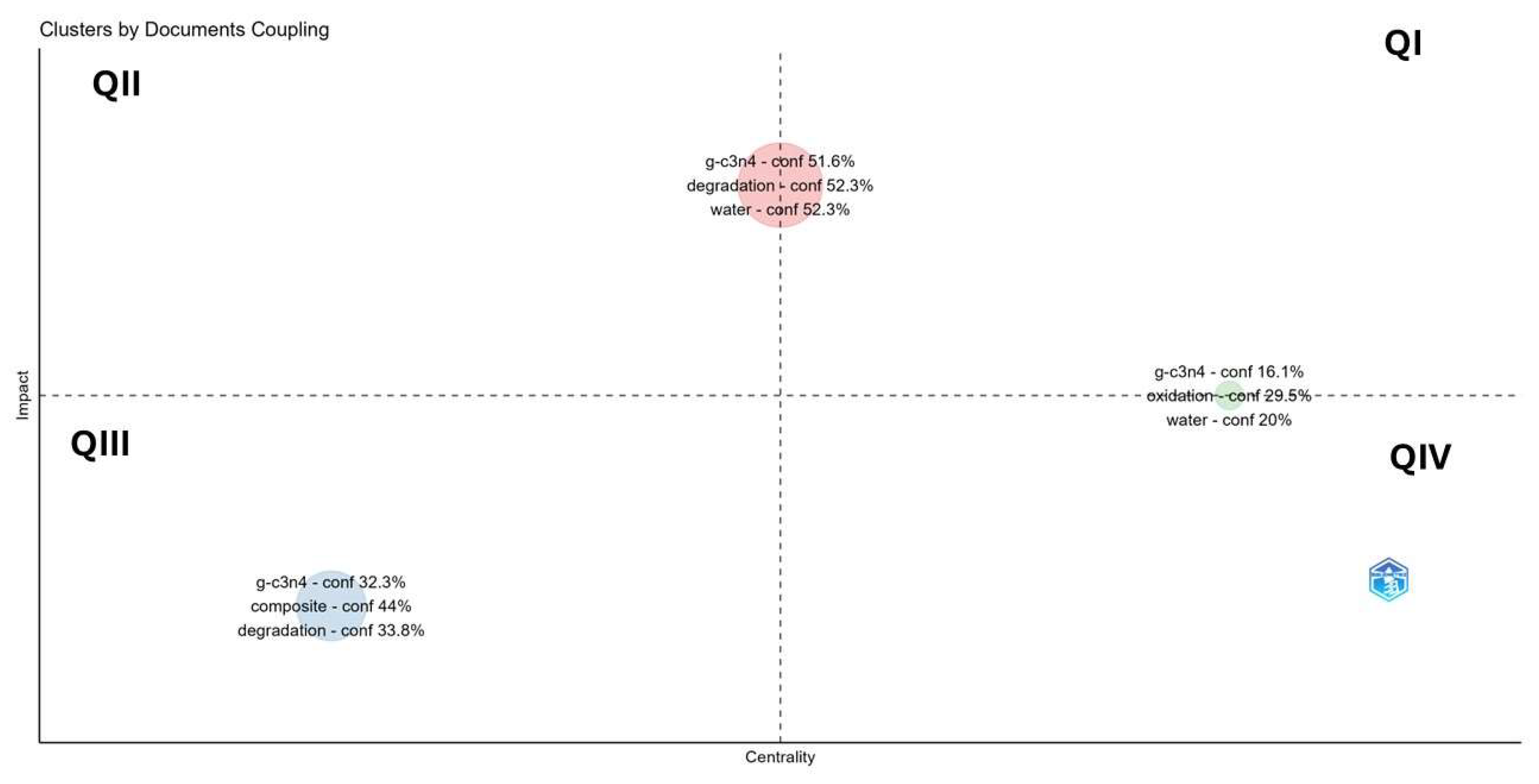
2.5.2. Impact and Centrality of Critical Documents
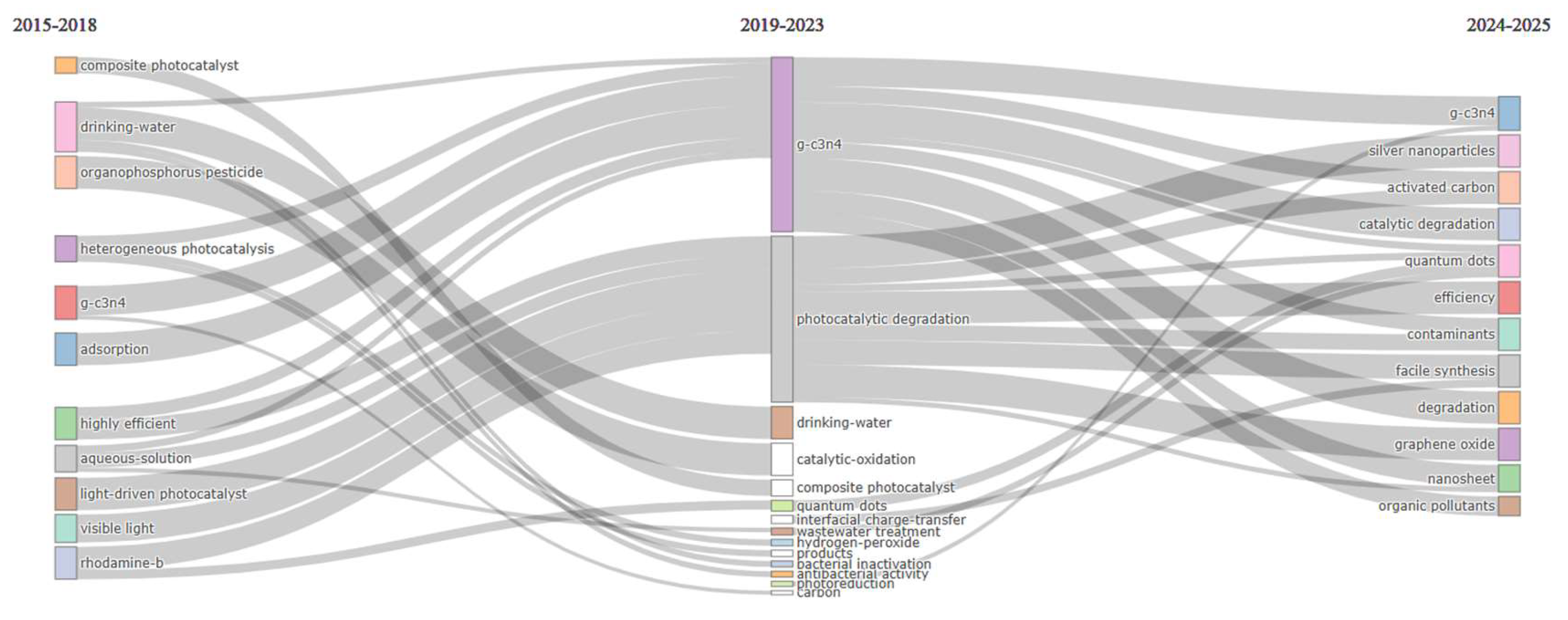
2.5.3. Thematic Evolution Analysis
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dong, G.; Zhang, Y.; Pan, Q.; Qiu, J. A fantastic graphitic carbon nitride (g-C3N4) material: electronic structure, photocatalytic and photoelectronic properties. J. Photochem. Photobiol. C: Photochem. Rev.; 2014, 20, 33–50. [Google Scholar] [CrossRef]
- Shariatinia, Z. Graphitic carbon nitride: Applications. In Handbook of Carbon-Based Nanomaterials. Thomas, S.; Sarathchandran, C.; Ilangovan, S. A.; Moreno-Piraján, J. C. (Eds.). Elsevier. Amsterdam. 2021. pp. 591-628.
- Savateev, O.; Antonietti, M.; Wang, S. Carbon nitrides. Structure, properties and applications in science and technology. Walter de Gruyter GmbH, Berlin. 2023.
- Liebig, J. Uber Einige Stickstoff – Verbindungen. Ann. Pharm.; 1834, 10, 1–47. [Google Scholar] [CrossRef]
- Zhu, J. J.; Wei, Y.C.; Chen, W.K.; Zhao, Z.; Thomas, A. Graphitic carbon nitride as a metal-free catalyst for no decomposition. Chem. Commun.; 2010, 46, 6965–6967. [Google Scholar] [CrossRef]
- Wang, X.C.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater.; 2009, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Blechert, S.; Antonietti, M. Polymeric graphitic carbon nitride for heterogeneous photocatalysis. ACS Catal.; 2012, 2, 1596–1606. [Google Scholar] [CrossRef]
- Liu, A.Y.; Cohen, M.L. Prediction of new low compressibility solids. Science, 1989, 245, 841–842. [Google Scholar] [CrossRef] [PubMed]
- Asadzadeh-Khaneghah, S.; Habibi-Yangjeh, A. g-C3N4/carbon dot-based nanocomposites serve as efficacious photocatalysts for environmental purification and energy generation: a review. J. Clean. Prod.; 2020, 276, 124319. [Google Scholar] [CrossRef]
- Ong, W.J.; Tan, L.L.; Ng, Y.H.; Yong, S.T.; Chai, S.P. Graphitic carbon nitride (g-C3N4)-based photocatalysts for artificial photosynthesis and environmental remediation: are we a step closer to achieving sustainability? Chem. Rev.; 2016, 116, 7159–7329. [Google Scholar] [CrossRef]
- Miller, T.S.; Jorge, A.B.; Suter, T.M.; Sella, A.; Corà, F.; McMillan, P.F. Carbon nitrides: synthesis and characterization of a new class of functional materials. Phys. Chem. Chem. Phys.; 2017, 19, 15613–15638. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Xiao, P.; Li, H.; Carabineiro, S. A. Graphitic carbon nitride: synthesis, properties, and applications in catalysis. ACS Appl. Mater. Interfaces, 2014, 6, 16449–16465. [Google Scholar] [CrossRef] [PubMed]
- Teter, D.M.; Hemley, R.J. Low-compressibility carbon nitrides. Science, 1996, 271, 53–55. [Google Scholar] [CrossRef]
- Inagaki, M.; Tsumura, T.; Kinumoto, T.; Toyoda, M. Graphitic carbon nitrides (g-C3N4) with comparative discussion to carbon materials. Carbon, 2019, 141, 580–607. [Google Scholar] [CrossRef]
- Zhu, B.; Zhang, L.; Cheng, B.; Yu, J. First-principle calculation study of tri-s-triazine-based g-C3N4: a review Appl. Catal. B: Environ.; 2018, 224, 983–999. [Google Scholar] [CrossRef]
- Mamba, G.; Mishra, A.K. Graphitic carbon nitride (g-C3N4) nanocomposites: a new and exciting generation of visible light driven photocatalysts for environmental pollution remediation. Appl. Catal. B: Environ.; 2016, 198, 347–377. [Google Scholar] [CrossRef]
- Reddy, K.R.; Reddy, C.V.; Nadagouda, M.N.; Shetti, N.P.; Jaesool, S.; Aminabhavi, T.M. Polymeric graphitic carbon nitride (g-C3N4)-based semiconducting nanostructured materials: synthesis methods, properties and photocatalytic applications. J. Environ. Manag.; 2019, 238, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, S.; Liu, Y.; Alharbi, N.S.; Rabah, S.O.; Wang, S.; Wang, X. Synthesis and fabrication of g-C3N4-based materials and their application in elimination of pollutants. Sci. Total Environ.; 2020, 731, 139054. [Google Scholar] [CrossRef] [PubMed]
- Ismael, M. A review on graphitic carbon nitride (g-C3N4) based nanocomposites: synthesis, categories, and their application in photocatalysis. J. Alloy. Compd.; 2020, 846, 156446. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. A critical review on graphitic carbon nitride (g-C3N4)-based materials: Preparation, modification and environmental application. Coord. Chem. Rev.; 2022, 453, 214338. [Google Scholar] [CrossRef]
- Kiskan, B.; Zhang, J.; Wang, X.; Antonietti, M.; Yagci, Y. Mesoporous graphitic carbon nitride as a heterogeneous visible light photoinitiator for radical polymerization. ACS Macro Lett.; 2012, 1, 546–549. [Google Scholar] [CrossRef]
- Li, H.; Wang, L.; Liu, Y.; Lei, J.; Zhang, J. Mesoporous graphitic carbon nitride materials: synthesis and modifications. Res. Chem. Intermed.; 2016, 42, 3979–3998. [Google Scholar] [CrossRef]
- Yu, J.; Lei, J.; Wang, L.; Guillard, C.; Zhang, J.; Liu, Y.; Anpo, M. g-C3N4 quantum dots-modified mesoporous TiO2-SiO2 for enhanced photocatalysis. Res. Chem. Intermed.; 2019, 45, 4237–4247. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Li, H.; Chen, C. Fabrication of g-C3N4/TiO2 composite photocatalyst with extended absorption wavelength range and enhanced photocatalytic performance. J. Photochem. Photobiol, A: Chem.; 2016, 317, 151–160. [Google Scholar] [CrossRef]
- Sudhaik, A.; Raizada, P.; Shandilya, P.; Jeong, D.Y.; Lim, J.H.; Singh, P. Review on fabrication of graphitic carbon nitride based efficient nanocomposites for photodegradation of aqueous phase organic pollutants. J. Ind. Eng. Chem.; 2018, 67, 28–51. [Google Scholar] [CrossRef]
- Jiang, W.; Luo, W.; Wang, J.; Zhang, M.; Zhu, Y. Enhancement of catalytic activity and oxidative ability for graphitic carbon nitride. J. Photochem. Photobiol. C: Photochem. Rev.; 2016, 28, 87–115. [Google Scholar] [CrossRef]
- Jiang, L.; Yuan, X.; Pan, Y.; Liang, J.; Zeng, G.; Wu, Z.; Wang, H. Doping of graphitic carbon nitride for photocatalysis: a review. Appl. Catal. B: Environ.; 2017, 217, 388–406. [Google Scholar] [CrossRef]
- Wen, J.; Xie, J.; Chen, X.; Li, X. A review on g-C3N4-based photocatalysts. Appl. Surf. Sci.; 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Hasija, V.; Raizada, P.; Sudhaik, A.; Sharma, K.; Kumar, A.; Singh, P.; Jonnalagadda, S.B.; Thakur, V. K. (2019). Recent advances in noble metal free doped graphitic carbon nitride based nanohybrids for photocatalysis of organic contaminants in water: a review. Appl. Mat. Today, 2019, 15, 494–524. [Google Scholar] [CrossRef]
- Zhang, S.; Gu, P.; Ma, R.; Luo, C.; Wen, T.; Zhao, G.; Cheng, W.; Wang, X. Recent developments in fabrication and structure regulation of visible-light-driven g-C3N4-based photocatalysts towards water purification: a critical review Catal. Today, 2019, 335, 65–77. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, M.; Cheng, B.; Shao, Y. Recent advances in g-C3N4-based heterojunction photocatalysts. J. Mat. Sci. Technol.; 2020, 56, 1–17. [Google Scholar] [CrossRef]
- Chai, B.; Peng, T.; Mao, J.; Li, K.; Zan, L. Graphitic carbon nitride (g-C3N4)-Pt-TiO2 nanocomposite as an efficient photocatalyst for hydrogen production under visible light irradiation. Phys. Chem. Chem. Phys.; 2012, 14, 16745–16752. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, S.; Yu, H.; Quan, X. g-C3N4/TiO2 hybrid photocatalyst with wide absorption wavelength range and effective photogenerated charge separation. Sep. Purif. Technol.; 2012, 99, 50–54. [Google Scholar] [CrossRef]
- Hayat, A.; Al-Sehemi, A.G.; El-Nasser, K.S.; Taha, T.A.; Al-Ghamdi, A.A.; Syed, J.A. S.; Amin, M.A.; Ali, T.; Bashir, T.; Palamanit, A.; Nawawi, W.I. Graphitic carbon nitride (g–C3N4)–based semiconductor as a beneficial candidate in photocatalysis diversity. Int. J. Hydrog. Energy, 2022, 47, 5142–5191. [Google Scholar] [CrossRef]
- Hayat, A.; Syed, J.A.S.; Al-Sehemi, A.G.; El-Nasser, K.S.; Taha, T.A.; Al-Ghamdi, A.A.; Amin, M.A.; Ajmak, Z.; Iqbal, W.; Palamanit, A.; Medina, D.I.; Nawawi, W.I.; Sohail, M. State of the art advancement in rational design of g-C3N4 photocatalyst for efficient solar fuel transformation, environmental decontamination and future perspectives. Int. J. Hydrog. Energy, 2022, 47, 10837–10867. [Google Scholar] [CrossRef]
- Wudil, Y.S.; Ahmad, U.F.; Gondal, M.A.; Al-Osta, M.A.; Almohammedi, A.; Sa’id, R.S.; Hrahshehh, F.; Haruna, K.; Mohamed, M.J.S. Tuning of graphitic carbon nitride (g-C3N4) for photocatalysis: A critical review. Arab. J. Chem.; 2023, 16, 104542. [Google Scholar] [CrossRef]
- Wan, Z.; Zhang, G.; Wu, X.; Yin, S. Novel visible-light-driven Z-scheme Bi12GeO20/g-C3N4 photocatalyst: oxygen-induced pathway of organic pollutants degradation and proton assisted electron transfer mechanism of Cr (VI) reduction. Appl. Catal. B: Environ.; 2017, 207, 17–26. [Google Scholar] [CrossRef]
- Zhang, S.; Li, J.; Zeng, M.; Zhao, G.; Xu, J.; Hu, W.; Wang, X. In situ synthesis of water-soluble magnetic graphitic carbon nitride photocatalyst and its synergistic catalytic performance. ACS Appl. Mater Interfaces, 2013, 5, 12735–12743. [Google Scholar] [CrossRef] [PubMed]
- Ejeta, S.Y.; Imae, T. Photodegradation of pollutant pesticide by oxidized graphitic carbon nitride catalysts J. Photochem. Photobiol, A: Chem.; 2021, 404, 112955.
- Zhang, J.; Chen, X.; Takanabe, K.; Maed,a K. ; Domen, K.; Epping, J.D.; Fu, X.; Antonietti, M.; Wang, X. Synthesis of a carbon nitride structure for visible-light catalysis by copolymerization. Angew. Chem. Int. Ed. Engl.; 2010, 49, 441–444. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, P.; Oliva-Teles, L.; Guimarães, L.; Carvalho, A.P. Occurrence of pharmaceutical and pesticide transformation products in freshwater: update on environmental levels, toxicological information and future challenges. Rev. Environ. Contam. Toxicol.; 2022, 260, 14. [Google Scholar] [CrossRef]
- Pérez-Lucas, G.; Vela, N.; El Aatik, A.; Navarro, S. Environmental risk of groundwater pollution by pesticide leaching through the soil profile. In Pesticides—Use and misuse and their impact in the environment; Larramendy, M., Soloneski, S., Eds.; Eds.; IntechOpen: London, UK, 2019; pp. 45–71. [Google Scholar]
- EEA. European Waters—Assessment of Status and Pressures 2018; EEA Report No 7/2018; European Environment Agency: Copenhagen, Denmark, 2018; https://www.eea.europa.eu/publications/state-of-water/ (Accessed on 6 October 2024). [Google Scholar]
- Mohaupt, V.; Volker, J.; Altenburger, R.; Birk, S.; Kirst, I.; Kuhnel, D.; Kuster, E.; Semeradova, S.; Šubelj, G.; Whalley, C. Pesticides in European Rivers, Lakes and Groundwaters—Data Assessment. European Topic Centre on Inland, Coastal and Marine Waters; Technical Report 1/2020; European Environment Agency: Magdeburg, Germany, 2020. [Google Scholar]
- Silva, J.A. Wastewater treatment and reuse for sustainable water resources management: a systematic literature review. Sustainability, 1094. [Google Scholar]
- SDWF. Pesticides and water pollution. Safe Drinking Water Foundation. https://www.safewater.org/fact-sheets-1/2017/1/23/pesticides. 2017 (Accessed on 7 October, 2024).
- Mukherjee, P.; Banerjee, G.; Saha, N.; Mazumdar, A. Overview on the emergence of pesticide contamination and treatment methodologies. Water Air Soil Pollut.; 2024, 235, 587. [Google Scholar] [CrossRef]
- Hernández-Tenorio, R.; González-Juárez, E.; Guzmán-Mar, J.L.; Hinojosa-Reyes, L.; Hernández-Ramírez, A. Review of occurrence of pharmaceuticals worldwide for estimating concentration ranges in aquatic environments at the end of the last decade. J. Hazard. Mater. Adv.; 2022, 8, 100172. [Google Scholar]
- de Oliveira, M.; Frihling, B.E.F.; Velasques, J.; Magalhães Filho, F.J.C.; Cavalheri, P. S.; Migliolo, L. Pharmaceuticals residues and xenobiotics contaminants: occurrence, analytical techniques and sustainable alternatives for wastewater treatment. Sci. Total Environ.; 2020. 705.
- Kanakaraju, D.; Glass, B.D.; Oelgemöller, M. Advanced oxidation process-mediated removal of pharmaceuticals from water: A review. J. Environ. Manag.; 2018, 219, 189–207. [Google Scholar] [CrossRef]
- Kaur, R.; Kaur, H. Solar driven photocatalysis - an efficient method for removal of pesticides from water and wastewater. Biointerface Res. Appl. Chem.; 2021, 11, 9071–9084. [Google Scholar]
- Rasooly, S.S.; Anwer, M.; Tsnim, G. A review on treatment methods for pesticide contaminated water. IOSR J. Environ. Sci. Toxicol. Food Technol.; 2022, 16, 24. [Google Scholar]
- Pérez-Lucas, G.; El Aatik, A.; Aliste, M.; Hernández, V.; Fenoll, J.; Navarro, S. Reclamation of aqueous waste solutions polluted with pharmaceutical and pesticide residues by biological-photocatalytic (solar) coupling in situ for agricultural reuse. Chem. Eng. J.; 2022, 448, 137616. [Google Scholar] [CrossRef]
- Soto-Verjel, J.; Maturana, A.Y.; Villamizar, S.E. Advanced catalytic oxidation coupled to biological systems to treat pesticide contaminated water: A review on technological trends and future challenges. Water Sci. Technol.; 2022, 85, 1263–1294. [Google Scholar] [CrossRef] [PubMed]
- Rengifo-Herrera, J.A.; Pulgarin, C. Why five decades of massive research on heterogeneous photocatalysis, especially on TiO2, has not yet driven to water disinfection and detoxification applications? Critical review of drawbacks and challenges. Chem. Eng. J.; 2023, 477, 146875. [Google Scholar] [CrossRef]
- von Gunten, U. Oxidation processes and me. Water Res. 2024, 253, 121148. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment - A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Han, H.; Wang, Y.; Liu, S.; Zhao, J.; Meng, X.; Li, Z. Recent advances of photocatalytic application in water treatment: A review. Nanomaterials, 2021, 11, 1804. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, A.; Alalm, M.G.; Awad, H.M.; Islam, M.; Qyyum, M.A.; Al-Muhtaseb, A.A. H.; Osman, A.I.; Lee, M. Solar photooxidation of recalcitrant industrial wastewater: a review. Environ. Chem. Lett.; 2022, 20, 1839–1862. [Google Scholar] [CrossRef]
- Pritchard, A. Statistical bibliography or bibliometrics. J. Doc.; 1969, 25, 348–9. [Google Scholar]
- Donthu, N.; Kumar, S.; Mukherjee, D.; Pandey, N.; Lim, W.M. How to conduct a bibliometric analysis: An overview and guidelines. J. Bus. Res.; 2021, 133, 285–296. [Google Scholar] [CrossRef]
- Andreo-Martínez, P.; Ortiz-Martínez, V.M.; García-Martínez, N.; de los Ríos, A.P.; Hernández-Fernández, F.J.; Quesada-Medina, J. Production of biodiesel under supercritical conditions: State of the art and bibliometric analysis. Appl. Energy, 2020, 264, 114753. [Google Scholar] [CrossRef]
- Price, D.J. ; 1986. Little Science, Big Science... And beyond. Columbia University Press, New York.
- Veiga-del-Baño, J. M.; Cámara, M. Á.; Oliva, J.; Hernández-Cegarra, A. T.; Andreo-Martínez, P.; Motas, M. Mapping of emerging contaminants in coastal waters research: A bibliometric analysis of research output during 1986-2022. Mar. Pollut. Bull, 2023, 194, 115366. [Google Scholar] [CrossRef]
- Veiga-del-Baño, J.M.; Andreo-Martínez, P.; Pérez-Lucas, G.; Navarro, S. Overview of the evolution and trends of the QuEChERS sample preparation procedure. Rev. Environ. Contam. Toxicol., 2024, 262, 22. [Google Scholar] [CrossRef]
- Lotka, A.J. The frequency distribution of scientific productivity. JWasA, 1926, 16, 317–323. [Google Scholar]
- Zhi, W.; Ji, G. Constructed wetlands, 1991–2011: a review of research development, current trends, and future directions. Sci. Total Environ.; 2012, 441, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Crane, D. Social structure in a group of scientists: a test of the “invisible college” hypothesis. Am. Sociol. Rev.; 1969, 34, 335–352. [Google Scholar] [CrossRef]
- Elango, B.; Rajendran, P. Authorship trends and collaboration pattern in the marine sciences literature: a scientometric study. Int. J. Inf. Dissem. Technol.; 2012, 2, 166. [Google Scholar]
- Bradford, S.C. Documentation. Crosby Lockwood, London, UK. 1948.
- Nash-Stewart, C.E.; Kruesi, L.M.; Del Mar, C.B. Does Bradford’s law of scattering predict the size of the literature in cochrane reviews? J. Med. Libr. Assoc.
- Balakrishnan, A.; Chinthala, M.; Polagani, R.K.; Vo, D.V.N. Removal of tetracycline from wastewater using g-C3N4 based photocatalysts: a review. Environ. Res.; 2023, 216, 114660. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Ouyang, X.; Deng, Y.; Wang, J.; Tang, L. A novel g-C3N4/g-C3N4− x homojunction with efficient interfacial charge transfer for photocatalytic degradation of atrazine and tetracycline. J. Hazard. Mat.; 2023, 441, 129845. [Google Scholar] [CrossRef]
- Habibi-Yangjeh, A.; Akhundi, A. Novel ternary g-C3N4/Fe3O4/Ag2CrO4 nanocomposites: magnetically separable and visible-light-driven photocatalysts for degradation of water pollutants. J. Mol. Catal. A: Chem.; 2016, 415, 122–130. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M.; Mishra, S.R. Photocatalytic performance of g-C3N4 based nanocomposites for effective degradation/removal of dyes from water and wastewater. Mat. Res. Bull.; 2021, 143, 111417. [Google Scholar] [CrossRef]
- Annadurai, T.; Khedulkar, A. P.; Lin, J. Y.; Adorna Jr, J.; Yu, W. J.; Pandit, B.; et al. S-scheme N-doped carbon dots anchored g-C3N4/Fe2O3 shell/core composite for photoelectrocatalytic trimethoprim degradation and water splitting. Appl Catal. B: Environ.; 2023, 320, 121928. [Google Scholar]
- Xie, Z.; Feng, Y.; Wang, F.; Chen, D.; Zhang, Q.; Zeng, Y.; et al. Construction of carbon dots modified MoO3/g-C3N4 Z-scheme photocatalyst with enhanced visible-light photocatalytic activity for the degradation of tetracycline. Appl. Catal. B: Environ.; 2018, 229, 96–104. [Google Scholar] [CrossRef]
- Yang, X.; Ye, Y.; Sun, J.; Li, Z.; Ping, J.; Sun, X. Recent advances in g-C3N4-based photocatalysts for pollutant degradation and bacterial disinfection: design strategies, mechanisms, and applications. Small, 2022, 18, 2105089. [Google Scholar] [CrossRef] [PubMed]
- Dolai, S.; Vanluchene, A.; Stavárek, P.; Dzik, P.; Fajgar, R.; Soukup, K.; Klusoň, P. Graphitic carbon nitride thin films for light-induced photocatalysis in a slit geometry microreactor. J. Environ. Chem. Eng.; 2022, 10, 108790. [Google Scholar] [CrossRef]
- Ragupathi, V.; Panigrahi, P.; Subramaniam, N.G. Scalable fabrication of graphitic-carbon nitride thin film for optoelectronic application. Mater. Today: Proc.; 2023, 80, 2115–2118. [Google Scholar] [CrossRef]
- Dragović, A.; Zrnić, N.; Dragović, B.; Dulebenets, M.A. A comprehensive review of Maritime Bibliometric Studies (2014-2024). Ocean Eng.; 2024, 311, 118917. [Google Scholar] [CrossRef]
- Li, X.; Xiong, J.; Gao, X.; Huang, J.; Feng, Z.; Chen, Z.; Zhu, Y. Recent advances in 3D g-C3N4 composite photocatalysts for photocatalytic water splitting, degradation of pollutants and CO2 reduction. J AlloysCompd.; 2019, 802, 196–209. [Google Scholar] [CrossRef]
- Basumatary, F.; Sarkar, A.; Mushahary, N.; Das, B.; Saikia, P.; Selvaraj, M.; Basumatary, S. Graphitic carbon nitride composites as advanced versatile materials for adsorption and photocatalytic degradation of emerging pollutants from wastewater. Proc. Saf. Environ. Prot.; 2024, 191, 2416–2468. [Google Scholar] [CrossRef]
- Ahmad, I. Comparative study of metal (Al, Mg, Ni, Cu and Ag) doped ZnO/g-C3N4 composites: efficient photocatalysts for the degradation of organic pollutants. Sep. Purif. Technol.; 2020, 251, 117372. [Google Scholar] [CrossRef]
- Liu, X.; Ma, R.; Zhuang, L.; Hu, B.; Chen, J.; Liu, X.; Wang, X. Recent developments of doped g-C3N4 photocatalysts for the degradation of organic pollutants. Crit. Rev. Environ. Sci. Technol.; 2021, 51, 751–790. [Google Scholar] [CrossRef]
- Mishra, S. R.; Gadore, V.; Ahmaruzzaman, M. Sustainability-driven photocatalysis: oxygen-doped gC3N4 for organic contaminant degradation. RSC Sustain.; 2024, 2, 91–100. [Google Scholar] [CrossRef]
- Das, S.; Chowdhury, A. Recent advancements of g-C3N4-based magnetic photocatalysts towards the degradation of organic pollutants: a review. Nanotechnology, 2021, 33, 072004. [Google Scholar] [CrossRef]
- Chong MN, Jin B, Chow CWK, Saint C. Recent developments in photocatalytic water treatment technology: a review. Water Res.; 2010, 44, 2997–3027.
- Yang, X.; Ye, Y.; Sun, J.; Li, Z.; Ping, J.; Sun, X. Recent advances in g-C3N4-based photocatalysts for pollutant degradation and bacterial disinfection: design strategies, mechanisms, and applications. Small, 2022, 18, 2105089. [Google Scholar] [CrossRef]
- Taghilou, S.; Nakhjirgan, P.; Esrafili, A.; Dehghanifard, E.; Kermani, M.; Kakavandi, B.; Pelalak, R. Performance, progress, and mechanism of g-C3N4-based photocatalysts in the degradation of pesticides: A systematic review. Chemosphere, 2024, 368, 143667. [Google Scholar] [CrossRef]
- Wang, T.; Nie, C.; Ao, Z.; Wang, S.; An, T. Recent progress in g-C3N4 quantum dots: Synthesis, properties and applications in photocatalytic degradation of organic pollutants. J. Mat. Chem. A, 2020, 8, 485–502. [Google Scholar] [CrossRef]
- Wen, J.; Zhou, L.; Tang, Q.; Xiao, X.; Sun, S. Photocatalytic degradation of organic pollutants by carbon quantum dots functionalized g-C3N4: A review. Ecotoxicol. Environ. Saf.; 2023, 262, 115133. [Google Scholar] [CrossRef]
- Patial, S.; Sudhaik, A.; Chandel, N.; Ahamad, T.; Raizada, P.; Singh, P.; et al. A review on carbon quantum dots modified g-C3N4-based photocatalysts and potential application in wastewater treatment. Applied Sciences, 2022, 12, 11286. [Google Scholar] [CrossRef]
- Zhao, G. Q.; Zou, J.; Hu, J.; Long, X.; Jiao, F. P. A critical review on graphitic carbon nitride (g-C3N4)-based composites for environmental remediation. Sep. Purif. Technol.; 2021, 279, 119769. [Google Scholar] [CrossRef]
- Umapathi, R.; Raju, C. V.; Ghoreishian, S. M.; Rani, G. M.; Kumar, K.; Oh, M. H.; et al. Recent advances in the use of graphitic carbon nitride-based composites for the electrochemical detection of hazardous contaminants. Coord. Chem. Rev.; 2022, 470, 214708. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, C.; Liu, R.; Sun, Y.; Ren, B.; Tong, Y.; Tao, Y. Advancing antibiotic detection and degradation: recent innovations in graphitic carbon nitride (g-C3N4) applications. J. Environ. Sci. 2024. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Chen, Z.; Zhao, W.; Liu, C.; Qian, X.; Zhang, M.; et al. Recent advances in photodegradation of antibiotic residues in water. Chem. Eng. J.; 2021, 405, 126806. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Pal, A. A Brief Review on the Latest Developments on Pharmaceutical Compound Degradation Using g-C3N4-Based Composite Catalysts. Catalysts, 2023, 13, 925. [Google Scholar] [CrossRef]
- Wu, Jun.; Ding, Xingchen.; Zhu, Xiashi. Preparation of organic compound/g-C3N4 composites and their applications in photocatalysis. Mater. Chem. Front.
- Chen, L.; Maigbay, M. A.; Li, M.; Qiu, X. Synthesis and modification strategies of g-C3N4 nanosheets for photocatalytic applications. Adv. Powder Mater.; 2024, 3, 100150. [Google Scholar] [CrossRef]
- Guo, C.; Lei, J.; Geng, W.; Lin, J.; Meng, S.; Ye, S.; et al. Efficient pollutant removal using tetrahydrofuran functionalized carbon nitride nanosheets with enhanced photocatalytic performance. Appl. Surf. Sci.; 2024, 649, 159155. [Google Scholar] [CrossRef]
- Persson, O.; Danell, R.; Schneider, J.W. How to use Bibexcel for various types of bibliometric analysis. Celebrating scholarly communication studies: A Festschrift for Olle Persson at his 60th Birthday, 2009, 5, 9–24. [Google Scholar]
- Aria, M.; Cuccurullo, C. Bibliometrix: An R-tool for comprehensive science mapping analysis. Informetrics, 2017, 11, 959–975. [Google Scholar] [CrossRef]
- Clarivate_Analytics. Journal Impact Factor, Journal Citation Reports. 2024. https://jcr.clarivate.com/jcr/home?app=jcr&referrer=target%3Dhttps:%2F%2Fjcr.clarivate.com%2Fjcr%2Fhome&Init=Yes&authCode=null&SrcApp=IC2LS. (Accessed on ). 10 October.
- Andreo-Martínez, P.; Ortiz-Martínez, V. M.; García-Martínez, N.; de Los Ríos, A. P.; Hernández-Fernández, F. J.; Quesada-Medina, J. Production of biodiesel under supercritical conditions: State of the art and bibliometric analysis. Appl. Energy, 2020, 264, 114753. [Google Scholar] [CrossRef]
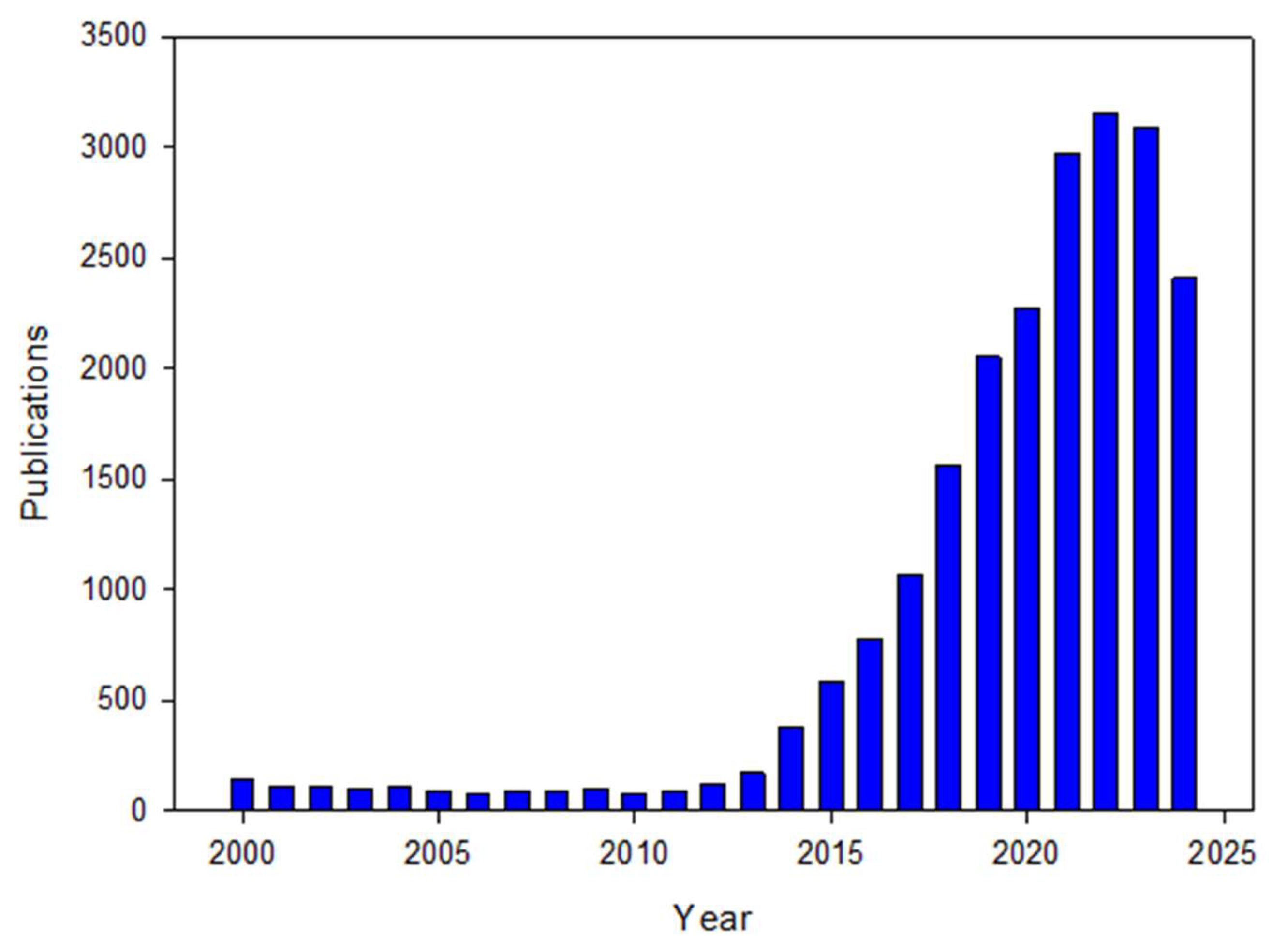
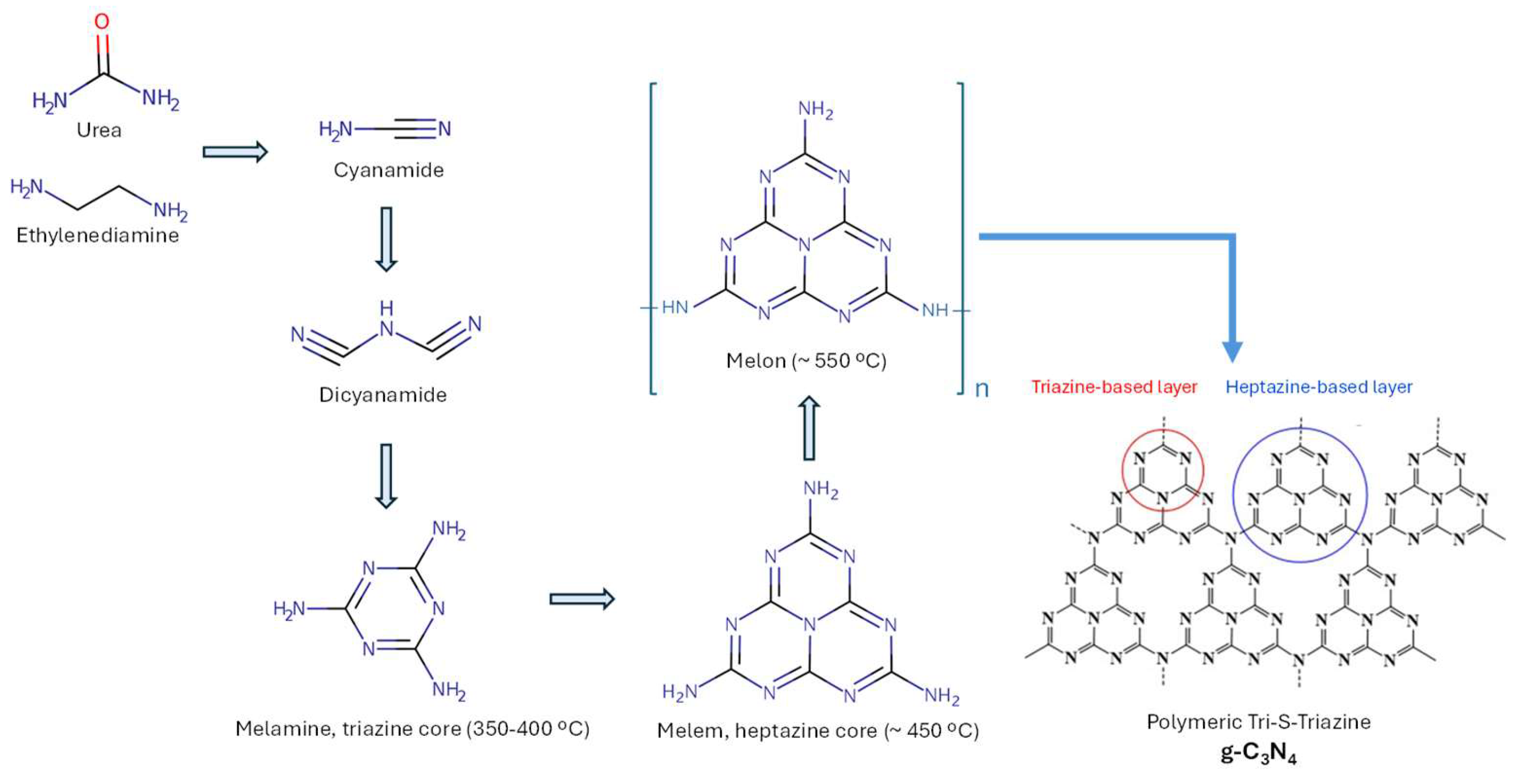
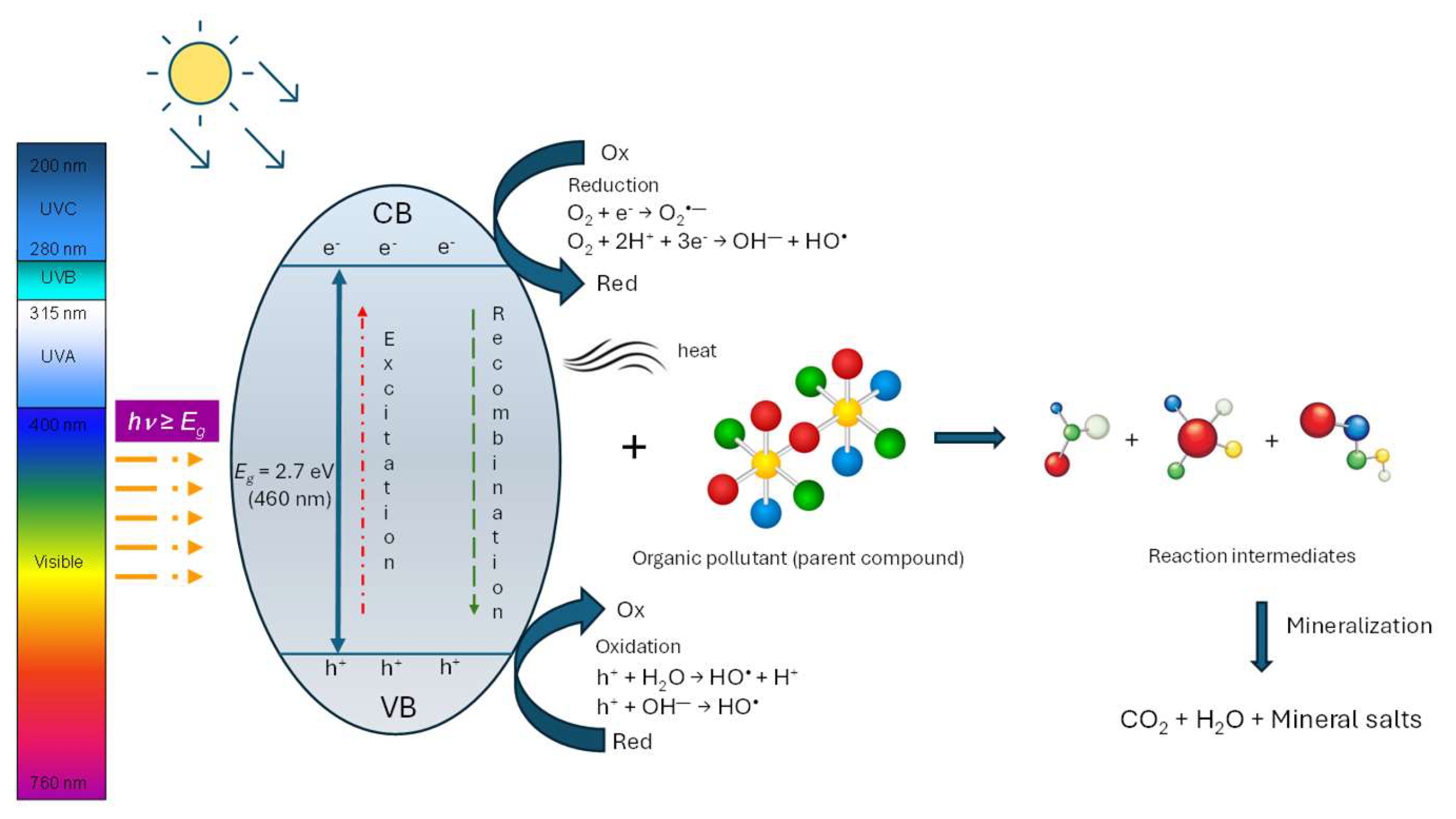
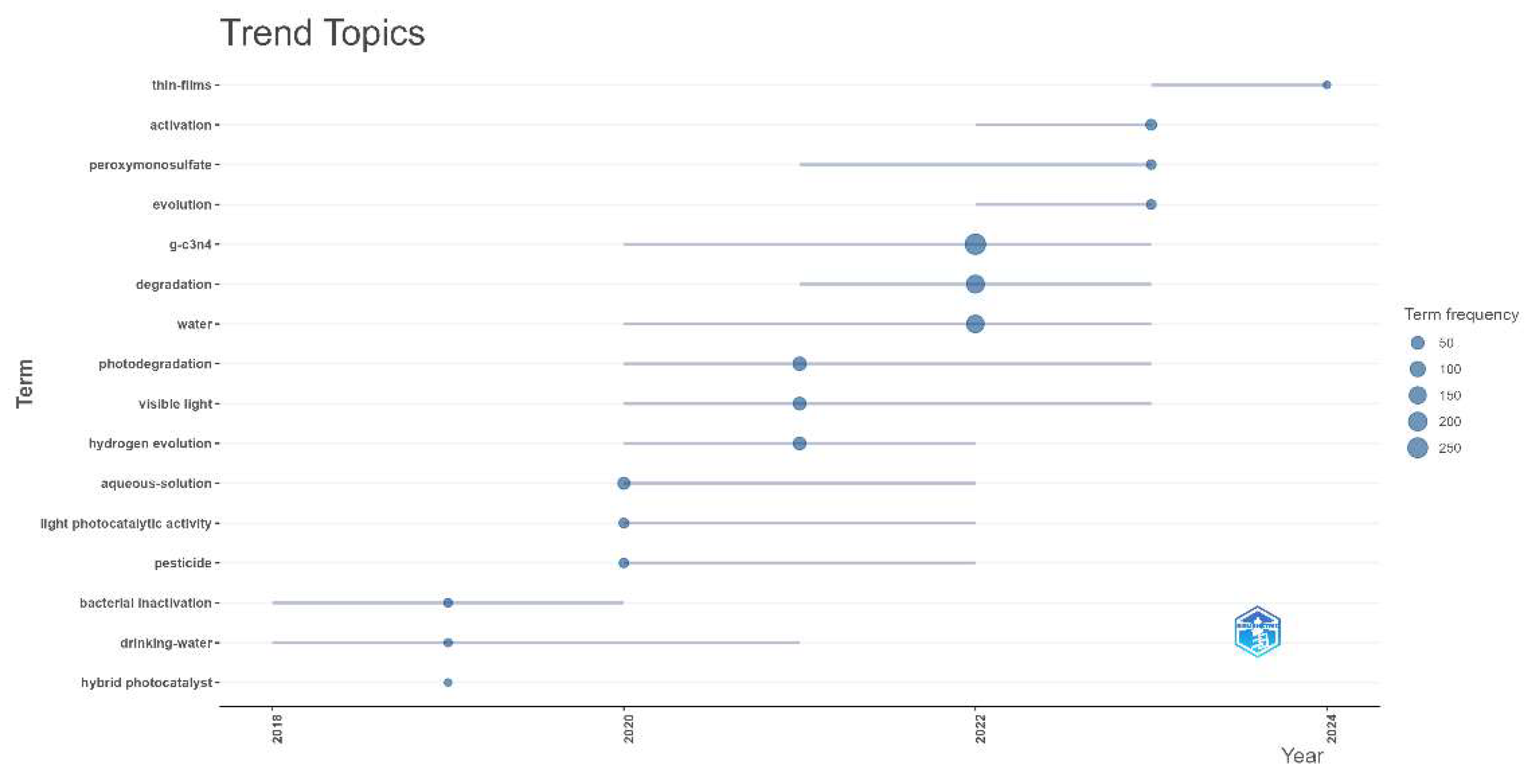
| PY | TP | TP AAI (%) | AU | AU AAI (%) | AU/TP | NR | NR AAI (%) | NR/TP | PG | PG AAI (%) | PG/TP |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2015 | 3 | 0.0 | 19 | 0.0 | 6.3 | 260 | 0.0 | 86.7 | 38 | 0.0 | 12.7 |
| 2016 | 4 | 33.3 | 23 | 21.1 | 5.8 | 185 | -28.8 | 46.3 | 42 | 10.5 | 10.5 |
| 2017 | 7 | 75.0 | 38 | 65.2 | 5.4 | 352 | 90.3 | 50.3 | 76 | 81.0 | 10.9 |
| 2018 | 21 | 200.0 | 144 | 278.9 | 6.9 | 1486 | 322.2 | 70.8 | 279 | 267.1 | 13.3 |
| 2019 | 31 | 47.6 | 205 | 42.4 | 6.6 | 2062 | 38.8 | 66.5 | 343 | 22.9 | 11.1 |
| 2020 | 82 | 164.5 | 505 | 146.3 | 6.2 | 5011 | 143.0 | 61.1 | 1014 | 195.6 | 12.4 |
| 2021 | 119 | 45.1 | 735 | 45.5 | 6.2 | 8919 | 78.0 | 74.9 | 1605 | 58.3 | 13.5 |
| 2022 | 143 | 20.2 | 965 | 31.3 | 6.7 | 10032 | 12.5 | 70.2 | 1896 | 18.1 | 13.3 |
| 2023 | 167 | 16.8 | 1093 | 13.3 | 6.5 | 11291 | 12.5 | 67.6 | 2258 | 19.1 | 13.5 |
| 2024 | 52 | -68.9 | 317 | -71.0 | 6.1 | 4499 | -60.2 | 86.5 | 1419 | -37.2 | 27.3 |
| Average | 53.4 | 57.3 | 6.3 | 60.8 | 68.1 | 63.6 | 13.8 | ||||
| Total | 629 | 4044 | 44097 | 8970 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





