Submitted:
21 December 2024
Posted:
23 December 2024
You are already at the latest version
Abstract
Keywords:
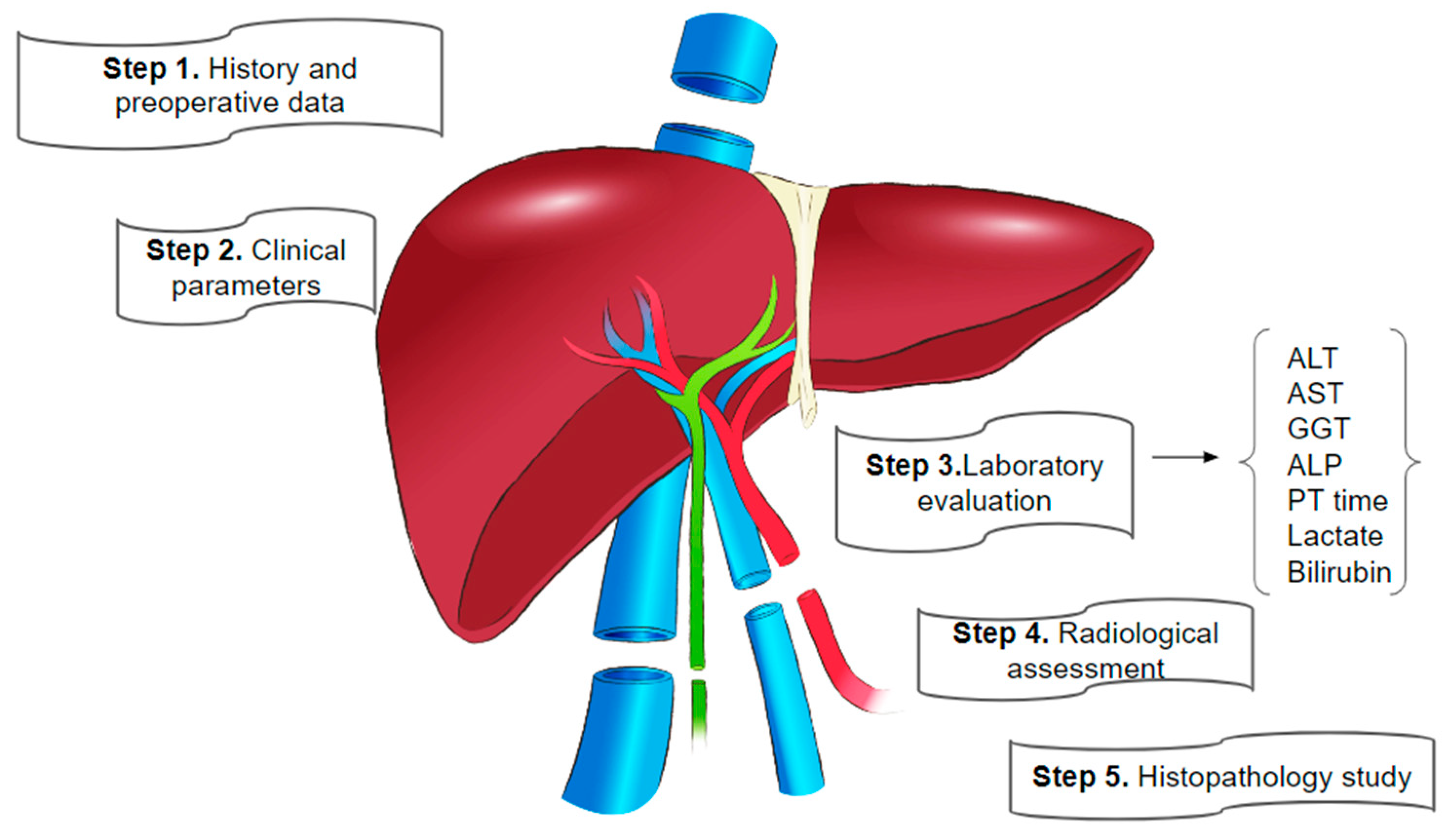
1. Introduction
2. Pre-Donation Assessment Phase
2.1. Pre-Donation Testing: General Overview
2.2. Serological and Biochemical Tests
2.2.1. Biochemical (Static) Tests (STF)
2.2.2. Liver Function Tests (LFTs)
2.3. Imaging Techniques
2.4. Role of Transient Elastography
2.5. Liver Biopsy
2.6. Additional Examinations
3. Donor Qualities and Donor-Recipient Matching
3.1. Donor Types and Graft-Related Considerations
3.2. Living Donor Liver Transplantation (LDLT) versus Deceased Donor Liver Transplantation (DDLT)
3.3. Donor-Recipient Matching and the Expansion of the Donor Pool
3.4. Donor Risk Scoring Systems: Donor Risk Index (DRI) and Model for End-Stage Liver Disease (MELD)
3.5. L-GrAFT Score for Postoperative Graft Function
3.6. e-GLR Score Predicts Early Graft Loss in Adult Live-Donor Liver Transplantation
4. Organ Procurement and Transplantation Stage
4.1. Ex-Vivo Methods for Graft Quality Assessment
4.1.1. Static Cold Storage (SCS)
4.1.2. Normothermic Perfusion (NMP)
4.1.3. Hypothermic Machine Perfusion (HMP)
5. Post-Transplantation Assessment
5.1. Immediate Post-Operative Testing
5.2. Dynamic Test as Point-of-Care Examination in Liver Transplantation
5.3. Post -Transplant Imaging
5.4. Biomarkers and Genomic Testing
6. Emerging Technologies- Auxillary and Novel Tests
6.1. Artificial Intelligence (AI) and Machine Learning
6.2. Regenerative Medicine: Bioengineered Organs as Alternatives
7. Conclusions and Future Directions
References
- Lucey, M.R.; Furuya, K.N.; Foley, D.P. Liver Transplantation. N Engl J Med 2023, 389, 1888–1900. [Google Scholar] [CrossRef]
- Bruinsma, B.G.; Wu, W.; Ozer, S.; Farmer, A.; Markmann, J.F.; Yeh, H.; Uygun, K. Warm Ischemic Injury Is Reflected in the Release of Injury Markers during Cold Preservation of the Human Liver. PLoS One 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Higashi, H.; Yagi, H.; Kuroda, K.; Tajima, K.; Kojima, H.; Nishi, K.; Morisaku, T.; Hirukawa, K.; Fukuda, K.; Matsubara, K.; et al. Transplantation of Bioengineered Liver Capable of Extended Function in a Preclinical Liver Failure Model. American Journal of Transplantation 2022, 22, 731–744. [Google Scholar] [CrossRef]
- Devarbhavi, H.; Asrani, S.K.; Arab, J.P.; Nartey, Y.A.; Pose, E.; Kamath, P.S. Global Burden of Liver Disease: 2023 Update. J Hepatol 2023, 79, 516–537. [Google Scholar] [CrossRef] [PubMed]
- Dutkowski, P.; Schlegel, A.; Slankamenac, K.; Oberkofler, C.E.; Adam, R.; Burroughs, A.K.; Schadde, E.; Müllhaupt, B.; Clavien, P.-A. The Use of Fatty Liver Grafts in Modern Allocation Systems. Ann Surg 2012, 256, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Burra, P.; Burroughs, A.; Graziadei, I.; Pirenne, J.; Valdecasas, J.C.; Muiesan, P.; Samuel, D.; Forns, X. EASL Clinical Practice Guidelines: Liver Transplantation. J Hepatol 2016, 64, 433–485. [Google Scholar] [CrossRef]
- Cherkassky, L. Does the United States Do It Better? A Comparative Analysis of Liver Allocation Protocols in the United Kingdom and the United States. Cambridge Quarterly of Healthcare Ethics 2011, 20, 418–433. [Google Scholar] [CrossRef] [PubMed]
- Koffron, A.; Stein, J.A. Liver Transplantation: Indications, Pretransplant Evaluation, Surgery, and Posttransplant Complications. Med Clin North Am 2008, 92, 861–888. [Google Scholar] [CrossRef]
- (170) Graft Assessment during and Post-Liver Transplantation - YouTube. Available online: https://www.youtube.com/watch?v=eUl-9nQ7FjA (accessed on 9 December 2024).
- Busuttil, R.W.; Klintmalm, G.B.G. Transplantation of the Liver: Third Edition; 2015; pp. 2015–1538. [Google Scholar] [CrossRef]
- Puri, P.; Kumar, A.; Qaleem, M. Donor Evaluation Protocol for Live and Deceased Donors. J Clin Exp Hepatol 2024, 14. [Google Scholar] [CrossRef]
- Kaltenbach, M.G.; Harhay, M.O.; Abt, P.L.; Goldberg, D.S. Trends in Deceased Donor Liver Enzymes Prior to Transplant: The Impact on Graft Selection and Outcomes. American Journal of Transplantation 2020, 20, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Huaman, M.A.; Vilchez, V.; Mei, X.; Shah, M.B.; Daily, M.F.; Berger, J.; Gedaly, R. Decreased Graft Survival in Liver Transplant Recipients of Donors with Positive Blood Cultures: A Review of the United Network for Organ Sharing Dataset. Transpl Int 2017, 30, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Piratvisuth, T.; Tredger, J.M.; Hayllar, K.A.; Williams, R. Contribution of True Cold and Rewarming Ischemia Times to Factors Determining Outcome after Orthotopic Liver Transplantation. Liver Transpl Surg 1995, 1, 296–301. [Google Scholar] [CrossRef] [PubMed]
- (170) Liver Function Tests-III:: Test for Detoxification Reaction (Hippuric Acid Test, MEGX Test) - YouTube. Available online: https://www.youtube.com/watch?v=dhB78yYnAws (accessed on 9 December 2024).
- Jochum, C.; Beste, M.; Penndorf, V.; Farahani, M.S.; Testa, G.; Nadalin, S.; Malaga, M.; Broelsch, C.E.; Gerken, G. Quantitative Liver Function Tests in Donors and Recipients of Living Donor Liver Transplantation. Liver Transpl 2006, 12, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Caimano, M.; Bianco, G.; Coppola, A.; Marrone, G.; Agnes, S.; Lai, Q.; Spoletini, G. Indocyanine Green Clearance Tests to Assess Liver Transplantation Outcomes: A Systematic Review. Int J Surg 2024, 110, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Levesque, E.; Martin, E.; Dudau, D.; Lim, C.; Dhonneur, G.; Azoulay, D. Current Use and Perspective of Indocyanine Green Clearance in Liver Diseases. Anaesth Crit Care Pain Med 2016, 35, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Hori, T.; Iida, T.; Yagi, S.; Taniguchi, K.; Yamamoto, C.; Mizuno, S.; Yamagiwa, K.; Isaji, S.; Uemoto, S. K(ICG) Value, a Reliable Real-Time Estimator of Graft Function, Accurately Predicts Outcomes in Adult Living-Donor Liver Transplantation. Liver Transpl 2006, 12, 605–613. [Google Scholar] [CrossRef] [PubMed]
- De Gasperi, A.; Mazza, E.; Prosperi, M. Indocyanine Green Kinetics to Assess Liver Function: Ready for a Clinical Dynamic Assessment in Major Liver Surgery? World J Hepatol 2016, 8, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Mobley, C.M.; Zarrinpar, A. Portable Device for the Analysis of Liver Function: A Boon to Liver Surgery and Critical Care. Expert Rev Med Devices 2016, 13, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Piekarski, J.; Goldberg, H.I.; Royal, S.A.; Axel, L.; Moss, A.A. Difference between Liver and Spleen CT Numbers in the Normal Adult: Its Usefulness in Predicting the Presence of Diffuse Liver Disease. Radiology 1980, 137, 727–729. [Google Scholar] [CrossRef]
- Vohra, S.; Goyal, N.; Gupta, S. Preoperative CT Evaluation of Potential Donors in Living Donor Liver Transplantation. Indian J Radiol Imaging 2014, 24, 350–359. [Google Scholar] [CrossRef]
- Chen, Y. Sen; Cheng, Y.F.; De Villa, V.H.; Wang, C.C.; Lin, C.C.; Huang, T.L.; Jawan, B.; Chen, C.L. Evaluation of Living Liver Donors. Transplantation 2003, 75. [Google Scholar] [CrossRef]
- (172) IMAGING IN LIVER TRANSPLANTATION l Criteria l Donor and Recipient Imaging l Pre and Post Op Imaging - YouTube. Available online: https://www.youtube.com/watch?v=GtvZltSjS1Y&t=27s (accessed on 10 December 2024).
- Lupsor-Platon, M.; Feier, D.; Stefănescu, H.; Tamas, A.; Botan, E.; Sparchez, Z.; Maniu, A.; Badea, R. Diagnostic Accuracy of Controlled Attenuation Parameter Measured by Transient Elastography for the Non-Invasive Assessment of Liver Steatosis: A Prospective Study. Journal of Gastrointestinal and Liver Diseases 2015, 24, 35–42. [Google Scholar] [CrossRef]
- Sasso, M.; Miette, V.; Sandrin, L.; Beaugrand, M. The Controlled Attenuation Parameter (CAP): A Novel Tool for the Non-Invasive Evaluation of Steatosis Using Fibroscan. Clin Res Hepatol Gastroenterol 2012, 36, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.Y.; Wang, J.H.; Guo, Y.; Wang, X.; Wu, X.D.; Xu, C.S.; Zhao, Y.; Zang, Y.J. Transient Elastography for Assessment of Fibrosis and Steatosis of Liver Grafts from Brain-Death Donors. Clin Res Hepatol Gastroenterol 2020, 44, 155–161. [Google Scholar] [CrossRef]
- Cunha, G.M.; Fan, B.; Navin, P.J.; Olivié, D.; Venkatesh, S.K.; Ehman, R.L.; Sirlin, C.B.; Tang, A. Interpretation, Reporting, and Clinical Applications of Liver MR Elastography. Radiology 2024, 310. [Google Scholar] [CrossRef]
- Barr, R.G.; Ferraioli, G.; Palmeri, M.L.; Goodman, Z.D.; Garcia-Tsao, G.; Rubin, J.; Garra, B.; Myers, R.P.; Wilson, S.R.; Rubens, D.; et al. Elastography Assessment of Liver Fibrosis: Society of Radiologists in Ultrasound Consensus Conference Statement. Radiology 2015, 276, 845–861. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.N.; Fowler, K.J.; Ozturk, A.; Potu, C.K.; Louie, A.L.; Montes, V.; Henderson, W.C.; Wang, K.; Andre, M.P.; Samir, A.E.; et al. Liver Fibrosis Imaging: A Clinical Review of Ultrasound and Magnetic Resonance Elastography. J Magn Reson Imaging 2020, 51, 25–42. [Google Scholar] [CrossRef]
- Navin, P.J.; Olson, M.C.; Knudsen, J.M.; Venkatesh, S.K. Elastography in the Evaluation of Liver Allograft. Abdominal Radiology 2021, 46, 96–110. [Google Scholar] [CrossRef] [PubMed]
- Park, C.C.; Nguyen, P.; Hernandez, C.; Bettencourt, R.; Ramirez, K.; Fortney, L.; Hooker, J.; Sy, E.; Savides, M.T.; Alquiraish, M.H.; et al. Magnetic Resonance Elastography vs. Transient Elastography in Detection of Fibrosis and Noninvasive Measurement of Steatosis in Patients With Biopsy-Proven Nonalcoholic Fatty Liver Disease. Gastroenterology 2017, 152, 598–607.e2. [Google Scholar] [CrossRef] [PubMed]
- Idilman, I.S.; Yildiz, A.E.; Karaosmanoglu, A.D.; Ozmen, M.N.; Akata, D.; Karcaaltincaba, M. Proton Density Fat Fraction: Magnetic Resonance Imaging Applications beyond the Liver. Diagn Interv Radiol 2022, 28, 83–91. [Google Scholar] [CrossRef]
- Caussy, C.; Reeder, S.B.; Sirlin, C.B.; Loomba, R. Non-Invasive, Quantitative Assessment of Liver Fat by MRI-PDFF as an Endpoint in NASH Trials. Hepatology 2018, 68, 763. [Google Scholar] [CrossRef] [PubMed]
- Rey, J.W.; Wirges, U.; Dienes, H.P.; Fries, J.W.U. Hepatic Steatosis in Organ Donors: Disparity between Surgery and Histology? Transplant Proc 2009, 41, 2557–2560. [Google Scholar] [CrossRef] [PubMed]
- Frankel, W.L.; Tranovich, J.G.; Salter, L.; Bumgardner, G.; Baker, P. The Optimal Number of Donor Biopsy Sites to Evaluate Liver Histology for Transplantation. Liver Transplantation 2002, 8, 1044–1050. [Google Scholar] [CrossRef]
- Liver Disease. Available online: https://www.transfusionguidelines.org/dsg/wb/guidelines/liver-disease (accessed on 10 December 2024).
- Trilianos, P.; Tsangaris, A.; Tawadros, A.; Deshpande, V. The Reliability of Fibro-Test in Staging Orthotopic Liver Transplant Recipients with Recurrent Hepatitis C. http://www.xiahepublishing.com/ 2020, 8, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Hacking, C., & W.Y. (2016) Hepascore. Radiopaedia 2016. 2016.
- García Ureña, M.A.; Ruiz-Delgado, F.C.; Moreno González, E.; Jiménez Romero, C.; García García, I.; Loinzaz Segurola, C.; González-Pinto, I.; Gómez Sanz, R. Hepatic Steatosis in Liver Transplant Donors: Common Feature of Donor Population? World J Surg 1998, 22, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Piella, G.; Farré, N.; Esono, D.; Cordobés, M.Á.; Vázquez-Corral, J.; Bilbao, I.; Gómez-Gavara, C. LiverColor: An Artificial Intelligence Platform for Liver Graft Assessment. Diagnostics (Basel) 2024, 14. [Google Scholar] [CrossRef] [PubMed]
- Yersiz, H.; Lee, C.; Kaldas, F.M.; Hong, J.C.; Rana, A.; Schnickel, G.T.; Wertheim, J.A.; Zarrinpar, A.; Agopian, V.G.; Gornbein, J.; et al. Assessment of Hepatic Steatosis by Transplant Surgeon and Expert Pathologist: A Prospective, Double-Blind Evaluation of 201 Donor Livers. Liver Transpl 2013, 19, 437–449. [Google Scholar] [CrossRef]
- Dutkowski, P.; Schlegel, A.; Slankamenac, K.; Oberkofler, C.E.; Adam, R.; Burroughs, A.K.; Schadde, E.; Müllhaupt, B.; Clavien, P.-A. The Use of Fatty Liver Grafts in Modern Allocation Systems: Risk Assessment by the Balance of Risk (BAR) Score. Ann Surg 2012, 256, 861–869. [Google Scholar] [CrossRef]
- Burra, P.; Burroughs, A.; Graziadei, I.; Pirenne, J.; Valdecasas, J.C.; Muiesan, P.; Samuel, D.; Forns, X. EASL Clinical Practice Guidelines: Liver Transplantation. J Hepatol 2016, 64, 433–485. [Google Scholar] [CrossRef]
- Martínez, J.A.; Pacheco, S.; Bachler, J.P.; Jarufe, N.; Briceño, E.; Guerra, J.F.; Benítez, C.; Wolff, R.; Barrera, F.; Arrese, M. Accuracy of the BAR Score in the Prediction of Survival after Liver Transplantation. Ann Hepatol 2019, 18, 386–392. [Google Scholar] [CrossRef] [PubMed]
- NHS Blood and Transplant Liver Transplant Surgery. 2015.
- Miller, C.M.; Durand, F.; Heimbach, J.K.; Kim-Schluger, L.; Lee, S.G.; Lerut, J.; Lo, C.M.; Quintini, C.; Pomfret, E.A. The International Liver Transplant Society Guideline on Living Liver Donation. Transplantation 2016, 100, 1238–1243. [Google Scholar] [CrossRef]
- Stulin, I.D. The Diagnosis of Brain Death. N Engl J Med 2001, 344, 87. [Google Scholar] [CrossRef]
- Thuong, M.; Ruiz, A.; Evrard, P.; Kuiper, M.; Boffa, C.; Akhtar, M.Z.; Neuberger, J.; Ploeg, R. New Classification of Donation after Circulatory Death Donors Definitions and Terminology. Transpl Int 2016, 29, 749–759. [Google Scholar] [CrossRef]
- Categories of Non-Heart-Beating Donors - PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/7482956/ (accessed on 11 December 2024).
- Croome, K.P.; Taner, C.B. The Changing Landscapes in DCD Liver Transplantation. Curr Transplant Rep 2020, 7, 194–204. [Google Scholar] [CrossRef] [PubMed]
- van Beekum, C.J.; Vilz, T.O.; Glowka, T.R.; von Websky, M.W.; Kalff, J.C.; Manekeller, S. Normothermic Machine Perfusion (NMP) of the Liver - Current Status and Future Perspectives. Ann Transplant 2021, 26. [Google Scholar] [CrossRef]
- Miller, C.M. Ethical Dimensions of Living Donation: Experience with Living Liver Donation. Transplant Rev (Orlando) 2008, 22, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Broelsch, C.E.; Malagó, M.; Testa, G.; Gamazo, C.V. Living Donor Liver Transplantation in Adults: Outcome in Europe. Liver Transpl 2000, 6, s64–s65. [Google Scholar] [CrossRef] [PubMed]
- Valentín-Gamazo, C.; Malagó, M.; Karliova, M.; Lutz, J.T.; Frilling, A.; Nadalin, S.; Testa, G.; Ruehm, S.G.; Erim, Y.; Paul, A.; et al. Experience after the Evaluation of 700 Potential Donors for Living Donor Liver Transplantation in a Single Center. Liver Transpl 2004, 10, 1087–1096. [Google Scholar] [CrossRef]
- Molinari, M.; Matz, J.; Decoutere, S.; El-Tawil, K.; Abu-Wasel, B.; Keough, V. Live Liver Donors’ Risk Thresholds: Risking a Life to Save a Life. HPB (Oxford) 2014, 16, 560–574. [Google Scholar] [CrossRef] [PubMed]
- Kotloff, R.M.; Blosser, S.; Fulda, G.J.; Malinoski, D.; Ahya, V.N.; Angel, L.; Byrnes, M.C.; DeVita, M.A.; Grissom, T.E.; Halpern, S.D.; et al. Management of the Potential Organ Donor in the ICU: Society of Critical Care Medicine/American College of Chest Physicians/Association of Organ Procurement Organizations Consensus Statement. Crit Care Med 2015, 43, 1291–1325. [Google Scholar] [CrossRef] [PubMed]
- Laing, R.W.; Scalera, I.; Isaac, J.; Mergental, H.; Mirza, D.F.; Hodson, J.; Wilkin, R.J.W.; Perera, M.T.P.R.; Muiesan, P. Liver Transplantation Using Grafts From Donors After Circulatory Death: A Propensity Score-Matched Study From a Single Center. Am J Transplant 2016, 16, 1795–1804. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, J.; Wu, W.; Huang, D.; Zheng, H.; Xu, Z.; Li, X.; Wang, N.; Qin, J.; Zhu, Z.; et al. Incidence of Ischemia Reperfusion Injury Related Biliary Complications in Liver Transplantation: Effect of Different Types of Donors. Transplant Proc 2022, 54, 1865–1873. [Google Scholar] [CrossRef] [PubMed]
- Gilbo, N.; Jochmans, I.; Sainz-Barriga, M.; Nevens, F.; Van Der Merwe, S.; Laleman, W.; Verslype, C.; Cassiman, D.; Verbeke, L.; Van Malenstein, H.; et al. Age Matching of Elderly Liver Grafts with Elderly Recipients Does Not Have a Synergistic Effect on Long-Term Outcomes When Both Are Carefully Selected. Transplant Direct 2019, 5. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Nobori, S.; Harada, S.; Sugimoto, R.; Yoshikawa, M.; Ushigome, H.; Yoshimura, N. Impact of Donor and Recipient Age on Outcomes After Living Donor Liver Transplant. Transplant Proc 2022, 54, 438–442. [Google Scholar] [CrossRef]
- Caso Maestro, O.; Justo Alonso, I.; Marcacuzco Quinto, A.; Manrique Municio, A.; Calvo Pulido, J.; García-Sesma, A.; Jiménez-Romero, C. Expanding Donor Age in Liver Transplantation Using Liver Grafts from Nonagenarian Donors. Clin Transplant 2022, 36, e14684. [Google Scholar] [CrossRef] [PubMed]
- Reyes, J.; Perkins, J.; Kling, C.; Montenovo, M. Size Mismatch in Deceased Donor Liver Transplantation and Its Impact on Graft Survival. Clin Transplant 2019, 33, e13662. [Google Scholar] [CrossRef] [PubMed]
- Addeo, P.; Bachellier, P.; Noblet, V. Combination of Donor Anthropometrics With Recipient Imaging to Improve Matching in Liver Transplantation. Liver Transplantation 2022, 28, 512–513. [Google Scholar] [CrossRef]
- Kostakis, I.D.; Raptis, D.A.; Davidson, B.R.; Iype, S.; Nasralla, D.; Imber, C.; Sharma, D.; Pissanou, T.; Pollok, J.M. Donor-Recipient Body Surface Area Mismatch and the Outcome of Liver Transplantation in the UK. Progress in Transplantation 2023, 33, 61–68. [Google Scholar] [CrossRef]
- Rustgi, V.K.; Marino, G.; Halpern, M.T.; Johnson, L.B.; Umana, W.O.; Tolleris, C. Role of Gender and Race Mismatch and Graft Failure in Patients Undergoing Liver Transplantation. Liver Transplantation 2002, 8, 514–518. [Google Scholar] [CrossRef]
- Germani, G.; Zeni, N.; Zanetto, A.; Adam, R.; Karam, V.; Belli, L.S.; O’Grady, J.; Mirza, D.; Klempnauer, J.; Cherqui, D.; et al. Influence of Donor and Recipient Gender on Liver Transplantation Outcomes in Europe. Liver International 2020, 40, 1961–1971. [Google Scholar] [CrossRef] [PubMed]
- Lauterio, A.; Di Sandro, S.; De Carlis, R.; Ferla, F.; Pinotti, E.; De Carlis, L. Every Liver Graft Should Be Evaluated for Transplantation. Transplantation 2018, 102, e456–e457. [Google Scholar] [CrossRef] [PubMed]
- Tisone, G.; Manzia, T.M.; Zazza, S.; De Liguori Carino, N.; Ciceroni, C.; De Luca, I.; Toti, L.; Casciani, C.U. Marginal Donors in Liver Transplantation. Transplant Proc 2004, 36, 525–526. [Google Scholar] [CrossRef]
- Goldaracena, N.; Cullen, J.M.; Kim, D.S.; Ekser, B.; Halazun, K.J. Expanding the Donor Pool for Liver Transplantation with Marginal Donors. International Journal of Surgery 2020, 82, 30–35. [Google Scholar] [CrossRef] [PubMed]
- De Carlis, R.; Lauterio, A.; Ferla, F.; Di Sandro, S.; Sguinzi, R.; De Carlis, L. Hypothermic Machine Perfusion of Liver Grafts Can Safely Extend Cold Ischemia for Up to 20 Hours in Cases of Necessity. Transplantation 2017, 101, e223–e224. [Google Scholar] [CrossRef] [PubMed]
- Halazun, K.J.; Quillin, R.C.; Rosenblatt, R.; Bongu, A.; Griesemer, A.D.; Kato, T.; Smith, C.; Michelassi, F.; Guarrera, J. V.; Samstein, B.; et al. Expanding the Margins: High Volume Utilization of Marginal Liver Grafts Among >2000 Liver Transplants at a Single Institution. Ann Surg 2017, 266, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Giretti, G.; Barbier, L.; Bucur, P.; Marques, F.; Perarnau, J.M.; Ferrandière, M.; Tellier, A.C.; Kerouredan, V.; Altieri, M.; Causse, X.; et al. Recipient Selection for Optimal Utilization of Discarded Grafts in Liver Transplantation. Transplantation 2018, 102, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Cillo, U.; Burra, P.; Mazzaferro, V.; Belli, L.; Pinna, A.D.; Spada, M.; Nanni Costa, A.; Toniutto, P.; Avolio, A.; Cescon, M.; et al. A Multistep, Consensus-Based Approach to Organ Allocation in Liver Transplantation: Toward a “Blended Principle Model. ” American Journal of Transplantation 2015, 15, 2552–2561. [Google Scholar] [CrossRef]
- Accardo, C.; Vella, I.; Pagano, D.; di Francesco, F.; Li Petri, S.; Calamia, S.; Bonsignore, P.; Tropea, A.; Gruttadauria, S. Donor-Recipient Matching in Adult Liver Transplantation: Current Status and Advances. Biosci Trends 2023, 17, 203–210. [Google Scholar] [CrossRef]
- Berthiaume, F.; Barbe, L.; Mokuno, Y.; MacDonald, A.D.; Jindal, R.; Yarmush, M.L. Steatosis Reversibly Increases Hepatocyte Sensitivity to Hypoxia-Reoxygenation Injury. Journal of Surgical Research 2009, 152, 54–60. [Google Scholar] [CrossRef]
- Ting, P. sheng; Hamilton, J.P.; Gurakar, A.; Urrunaga, N.H.; Ma, M.; Glorioso, J.; King, E.; Toman, L.P.; Wesson, R.; Garonzik-Wang, J.; et al. Hepatitis C-Positive Donor Liver Transplantation for Hepatitis C Seronegative Recipients. Transplant Infectious Disease 2019, 21. [Google Scholar] [CrossRef] [PubMed]
- Kwong, A.J.; Wall, A.; Melcher, M.; Wang, U.; Ahmed, A.; Subramanian, A.; Kwo, P.Y. Liver Transplantation for Hepatitis C Virus (HCV) Non-Viremic Recipients with HCV Viremic Donors. American Journal of Transplantation 2019, 19, 1380–1387. [Google Scholar] [CrossRef] [PubMed]
- Ballarin, R.; Cucchetti, A.; Spaggiari, M.; Montalti, R.; Di Benedetto, F.; Nadalin, S.; Troisi, R.I.; Valmasoni, M.; Longo, C.; De Ruvo, N.; et al. Long-Term Follow-up and Outcome of Liver Transplantation from Anti-Hepatitis C Virus-Positive Donors: A European Multicentric Case-Control Study. Transplantation 2011, 91, 1265–1272. [Google Scholar] [CrossRef]
- Saab, S.; Ghobrial, R.M.; Ibrahim, A.B.; Kunder, G.; Durazo, F.; Han, S.; Farmer, D.G.; Yersiz, H.; Goldstein, L.I.; Busuttil, R.W. Hepatitis C Positive Grafts May Be Used in Orthotopic Liver Transplantation: A Matched Analysis. American Journal of Transplantation 2003, 3, 1167–1172. [Google Scholar] [CrossRef]
- Mario Angelico∙ Alessandra Nardi∙ Tania Marianelli∙ Lucio Caccamo ∙ Renato Romagnoli ∙ Giuseppe Tisone∙ Antonio D. Pinna ∙ Alfonso W. Avolio∙ Stefano Fagiuoli ∙ Patrizia Burra∙ Mario Strazzabosco ∙ Alessandro Nanni Costa Hepatitis B-Core Antibody Positive Donors in Liver Transplantation and Their Impact on Graft Survival: Evidence from the Liver Match Cohort Study. J Hepatol 2013.
- Ming Lei, L.-N.Y.J.-Y.Y.T.-F.W.B.L.W.-T.W.H.W.M.-Q.X.Z.-Y.C.Y.-G.W.L.M.Y.L.Y.J.W.T.L.B.W.W.W.H.X.M.C.Z.W.Y. Safety of Hepatitis B Virus Core Antibody-Positive Grafts in Liver Transplantation: A Single-Center Experience in China. World J Gastroenterol 2018.
- G.M. Abouna Organ Shortage Crisis: Problems and Possible Solutions. Transplantation proceedings 2008.
- Mateus Silva Feijó, M.R.G.-V.V.S.F.A.F.F.S.C.G.F.F.J.L.G.D.P.B. de C.N.T.G.C.R.U.A.W. Impact of Donor Positive Blood Culture in Deceased Donor Liver Transplantation. Transplant Proc 2020.
- Raquel Martinez-Reviejo, S.T.A.C.H.N.K.O.M.J.R. Solid Organ Transplantation from Donors with Recent or Current SARS-CoV-2 Infection: A Systematic Review. Anaesth Crit Care Pain Med 2022.
- Christine M Durand, D.S.J.S. Realizing HOPE: The Ethics of Organ Transplantation From HIV-Positive Donors. Ann Intern Med 2016.
- C J E Watson, R.R.K.A.W.D.C.G.B.A.R.C.H.B.C.C.D.C.J.A.B. How Safe Is It to Transplant Organs from Deceased Donors with Primary Intracranial Malignancy? An Analysis of UK Registry Data. American Journal of Transplantation 2010.
- H Myron Kauffman, W.S.C.M.A.M.Y.C.D.W.H. Deceased Donors with a Past History of Malignancy: An Organ Procurement and Transplantation Network/United Network for Organ Sharing Update. Transplantation 2007.
- Seckler, F.; Turco, C.; Mohkam, K.; Addeo, P.; Robin, F.; Cauchy, F.; Maulat, C.; Brustia, R.; Paquette, B.; Faitot, F.; et al. Liver Transplantation Using Allografts with Recent Liver Blunt Trauma: A Nationwide Audit from the French CRISTAL Biomedicine Agency Registry. Transplantation 2023, 107, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Zarrinpar, A., L.C., N.E., Y.H., A.V.G., K.F.M., F.D.G., & B.R.W. A Rapid, Reproducible, Noninvasive Predictor of Liver Graft Survival. Journal of Surgical Research 2015.
- Mataya, L., A.A., T.J.R., & R.L.F. Decision Making in Liver Transplantation-Limited Application of the Liver Donor Risk Index. Liver Transplantation. Liver Transplantation 2014.
- Avegail Flores, S.K.A. The Donor Risk Index: A Decade of Experience. Liver Transplantation 2017.
- Cardoso, F.S.; Bagulho, L.; Coelho, J.S.; Lamelas, J.; Mateus, É.; Mendes, M.; Glória, H.; Ribeiro, V.; Mega, R.; Pena, A.; et al. The Impact of Donor Risk Index, Recipients’ and Operative Characteristics on Post Liver Transplant One-Year Graft Failure: A Cohort Analysis. GE Port J Gastroenterol 2023, 30, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Vatche G. Agopian, M.M.P.H.-L.M.D.M.M.W.D.M.V.X.M.F.M.K.M.A.Z.M.P.H.Y.M.D.G.F.M.J.R.H.M.R.W.B.M.P. Evaluation of Early Allograft Function Using the Liver Graft Assessment Following Transplantation Risk Score Model . JAMA Surg 2018.
- Agopian, V.G.; Markovic, D.; Klintmalm, G.B.; Saracino, G.; Chapman, W.C.; Vachharajani, N.; Florman, S.S.; Tabrizian, P.; Haydel, B.; Nasralla, D.; et al. Multicenter Validation of the Liver Graft Assessment Following Transplantation (L-GrAFT) Score for Assessment of Early Allograft Dysfunction. J Hepatol 2021, 74, 881–892. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, T.; Luo, T.; He, S.; Huang, C.; Jia, Z.; Zhan, L.; Wang, D.; Zhu, X.; Guo, Z.; et al. Prediction of Graft Survival Post-Liver Transplantation by L-GrAFT Risk Score Model, EASE Score, MEAF Scoring, and EAD. Front Surg 2021, 8. [Google Scholar] [CrossRef] [PubMed]
- Pamecha, V.; Patil, N.S.; Gattu, T.; Kumar, G.; Pattnaik, B.; Mohapatra, N.; Sindwani, G.; Choudhury, A. E-GLR Score Predicts Early Graft Loss in Adult Live-Donor Liver Transplantation. Annals of Surgery Open 2023, 4, e332. [Google Scholar] [CrossRef]
- Morioka, D.; Egawa, H.; Kasahara, M.; Ito, T.; Haga, H.; Takada, Y.; Shimada, H.; Tanaka, K. Outcomes of Adult-to-Adult Living Donor Liver Transplantation: A Single Institution’s Experience with 335 Consecutive Cases. Ann Surg 2007, 245, 315–325. [Google Scholar] [CrossRef]
- Kaido, T.; Egawa, H.; Tsuji, H.; Ashihara, E.; Maekawa, T.; Uemoto, S. In-Hospital Mortality in Adult Recipients of Living Donor Liver Transplantation: Experience of 576 Consecutive Cases at a Single Center. Liver Transplantation 2009, 15, 1420–1425. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Hardy, M.A.; Halazun, K.J.; Woodland, D.C.; Ratner, L.E.; Samstein, B.; Guarrera, J. V.; Brown, R.S.; Emond, J.C. Survival Outcomes Following Liver Transplantation (SOFT) Score: A Novel Method to Predict Patient Survival Following Liver Transplantation. American Journal of Transplantation 2008, 8, 2537–2546. [Google Scholar] [CrossRef]
- Halldorson, J.B.; Bakthavatsalam, R.; Fix, O.; Reyes, J.D.; Perkins, J.D. D-MELD, a Simple Predictor of Post Liver Transplant Mortality for Optimization of Donor/Recipient Matching. American Journal of Transplantation 2009, 9, 318–326. [Google Scholar] [CrossRef]
- Philipp Dutkowski, C.E.O.K.S.M.A.P.E.S.B.M.A.G.P.A.C. Are There Better Guidelines for Allocation in Liver Transplantation? A Novel Score Targeting Justice and Utility in the Model for End-Stage Liver Disease Era. Annals of Surgery 2011.
- Braat, A.E.; Blok, J.J.; Putter, H.; Adam, R.; Burroughs, A.K.; Rahmel, A.O.; Porte, R.J.; Rogiers, X.; Ringers, J. The Eurotransplant Donor Risk Index in Liver Transplantation: ET-DRI. American Journal of Transplantation 2012, 12, 2789–2796. [Google Scholar] [CrossRef]
- Cillo, U.; Burra, P.; Mazzaferro, V.; Belli, L.; Pinna, A.D.; Spada, M.; Nanni Costa, A.; Toniutto, P.; Avolio, A.; Cescon, M.; et al. A Multistep, Consensus-Based Approach to Organ Allocation in Liver Transplantation: Toward a “Blended Principle Model. ” American Journal of Transplantation 2015, 15, 2552–2561. [Google Scholar] [CrossRef]
- Karangwa, S.A.; Dutkowski, P.; Fontes, P.; Friend, P.J.; Guarrera, J. V.; Markmann, J.F.; Mergental, H.; Minor, T.; Quintini, C.; Selzner, M.; et al. Machine Perfusion of Donor Livers for Transplantation: A Proposal for Standardized Nomenclature and Reporting Guidelines. American Journal of Transplantation 2016, 16, 2932–2942. [Google Scholar] [CrossRef] [PubMed]
- Huang, V.; Karimian, N.; Detelich, D.; Raigani, S.; Geerts, S.; Beijert, I.; Fontan, F.M.; Aburawi, M.M.; Ozer, S.; Banik, P.; et al. Split-Liver Ex Situ Machine Perfusion: A Novel Technique for Studying Organ Preservation and Therapeutic Interventions. J Clin Med 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.J.E.; Kosmoliaptsis, V.; Pley, C.; Randle, L.; Fear, C.; Crick, K.; Gimson, A.E.; Allison, M.; Upponi, S.; Brais, R.; et al. Observations on the Ex Situ Perfusion of Livers for Transplantation. American Journal of Transplantation 2018, 18, 2005–2020. [Google Scholar] [CrossRef] [PubMed]
- David Nasralla, C.C.C.H.M.M.Z.A.A.J.B.C.D.L.C.V.C.S.J. et al. A Randomized Trial of Normothermic Preservation in Liver Transplantation. Nature 2018.
- Guarrera, J. V.; Henry, S.D.; Samstein, B.; Odeh-Ramadan, R.; Kinkhabwala, M.; Goldstein, M.J.; Ratner, L.E.; Renz, J.F.; Lee, H.T.; Brown, R.S.; et al. Hypothermic Machine Preservation in Human Liver Transplantation: The First Clinical Series. American Journal of Transplantation 2010, 10, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Matteo Ravaioli, L.M.A.A.G.F.V.D.P.G.G.F.O.V.C.P.C.M.B.F.V.A.D.G.S.A.S.; et al. Hypothermic Oxygenated Perfusion Versus Static Cold Storage for Expanded Criteria Donors in Liver and Kidney Transplantation: Protocol for a Single-Center Randomized Controlled Trial. JMIR Res Protoc 2020.
- Dutkowski, P.; Polak, W.G.; Muiesan, P.; Schlegel, A.; Verhoeven, C.J.; Scalera, I.; Deoliveira, M.L.; Kron, P.; Clavien, P.A. First Comparison of Hypothermic Oxygenated Perfusion versus Static Cold Storage of Human Donation after Cardiac Death Liver Transplants. Ann Surg 2015, 262, 764–771. [Google Scholar] [CrossRef]
- Horné, F.; Drefs, M.; Schirren, M.J.; Koch, D.T.; Cepele, G.; Jacobi, S.J.; Payani, E.; Börner, N.; Werner, J.; Guba, M.O.; et al. Hypothermic Oxygenated Machine Perfusion (HOPE) Prior to Liver Transplantation Mitigates Post-Reperfusion Syndrome and Perioperative Electrolyte Shifts. J Clin Med 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Mergental, H.; Laing, R.W.; Kirkham, A.J.; Perera, M.T.P.R.; Boteon, Y.L.; Attard, J.; Barton, D.; Curbishley, S.; Wilkhu, M.; Neil, D.A.H.; et al. Transplantation of Discarded Livers Following Viability Testing with Normothermic Machine Perfusion. Nat Commun 2020, 11. [Google Scholar] [CrossRef]
- Schlegel, A.; Muller, X.; Mueller, M.; Stepanova, A.; Kron, P.; de Rougemont, O.; Muiesan, P.; Clavien, P.A.; Galkin, A.; Meierhofer, D.; et al. Hypothermic Oxygenated Perfusion Protects from Mitochondrial Injury before Liver Transplantation. EBioMedicine 2020, 60. [Google Scholar] [CrossRef] [PubMed]
- Karangwa, S.; Panayotova, G.; Dutkowski, P.; Porte, R.J.; Guarrera, J. V.; Schlegel, A. Hypothermic Machine Perfusion in Liver Transplantation. International Journal of Surgery 2020, 82, 44–51. [Google Scholar] [CrossRef]
- Hashimoto, M.; Watanabe, G. Hepatic Parenchymal Cell Volume and the Indocyanine Green Tolerance Test. Journal of Surgical Research 2000, 92, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Berenguer, M.; de Martin, E.; Hessheimer, A.J.; Levitsky, J.; Maluf, D.G.; Mas, V.R.; Selzner, N.; Hernàndez-Èvole, H.; Lutu, A.; Wahid, N.; et al. European Society for Organ Transplantation Consensus Statement on Biomarkers in Liver Transplantation. Transplant International 2023, 36. [Google Scholar] [CrossRef] [PubMed]
- Finkenstedt, A.; Auer, C.; Glodny, B.; Posch, U.; Steitzer, H.; Lanzer, G.; Pratschke, J.; Biebl, M.; Steurer, M.; Graziadei, I.; et al. Patatin-like Phospholipase Domain-Containing Protein 3 Rs738409-g in Recipients of Liver Transplants Is a Risk Factor for Graft Steatosis. Clinical Gastroenterology and Hepatology 2013, 11, 1667–1672. [Google Scholar] [CrossRef]
- Mowry, C.J.; Alonso, C.; Iruarrizaga-Lejarreta, M.; Ortiz, P.; Levitsky, J.; Rinella, M. Utility of Metabolomic Biomarkers to Identify Nonalcoholic Fatty Liver Disease in Liver Transplant Recipients. Transplant Direct 2021, 7, E784. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Zhou, Y.; Yuan, J.; Lv, T.; Yang, J.; Shi, Y.; Yang, J. Mitochondrial MiR-23b-5p Is a New Biomarker of Warm Ischaemic Injury in Donor Livers and a Candidate for Graft Evaluation: Experimental Studies. Int J Surg 2023, 109, 1880–1892. [Google Scholar] [CrossRef] [PubMed]
- Weemhoff, J.L.; Woolbright, B.L.; Jenkins, R.E.; McGill, M.R.; Sharpe, M.R.; Olson, J.C.; Antoine, D.J.; Curry, S.C.; Jaeschke, H. Plasma Biomarkers to Study Mechanisms of Liver Injury in Patients with Hypoxic Hepatitis. Liver International 2017, 37, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Philip J Starkey Lewis, J.D.V.P.K.J.S.D.G.N.C.D.J.A.N.S.F.N.D.D.J.W.E.M.C.J.P.N.J.M.C.E.G.B.K.P. Circulating MicroRNAs as Potential Markers of Human Drug-Induced Liver Injury. Hepatology 2011.
- Christoph Roderburg, F.B.D.V.C.A.K.J.J.M.V.J.G.A.T.S.C.K.K.K.H.W.Z.M.L.C.T.F.T.T.L. Elevated MiR-122 Serum Levels Are an Independent Marker of Liver Injury in Inflammatory Diseases. Liver International 2015.
- Resch, T.; Hackl, H.; Esser, H.; Günther, J.; Schwelberger, H.; Ritschl, P.V.; Ebner, S.; Maglione, M.; Mellitzer, V.; Biebl, M.; et al. Expression of MICA in Zero Hour Biopsies Predicts Graft Survival After Liver Transplantation. Front Immunol 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Accardo, C.; Vella, I.; Pagano, D.; di Francesco, F.; Li Petri, S.; Calamia, S.; Bonsignore, P.; Tropea, A.; Gruttadauria, S. Donor-Recipient Matching in Adult Liver Transplantation: Current Status and Advances. Biosci Trends 2023, 17, 203–210. [Google Scholar] [CrossRef]
- Briceño, J.; Cruz-Ramírez, M.; Prieto, M.; Navasa, M.; De Urbina, J.O.; Orti, R.; Gómez-Bravo, M.Á.; Otero, A.; Varo, E.; Tomé, S.; et al. Use of Artificial Intelligence as an Innovative Donor-Recipient Matching Model for Liver Transplantation: Results from a Multicenter Spanish Study. J Hepatol 2014, 61, 1020–1028. [Google Scholar] [CrossRef] [PubMed]
- Briceño, J.; Calleja, R.; Hervás, C. Artificial Intelligence and Liver Transplantation: Looking for the Best Donor-Recipient Pairing. Hepatobiliary and Pancreatic Diseases International 2022, 21, 347–353. [Google Scholar] [CrossRef]
- Guijo-Rubio, D.; Briceño, J.; Gutiérrez, P.A.; Ayllón, M.D.; Ciria, R.; Hervás-Martínez, C. Statistical Methods versus Machine Learning Techniques for Donor-Recipient Matching in Liver Transplantation. PLoS One 2021, 16. [Google Scholar] [CrossRef] [PubMed]
- Medved, D.; Ohlsson, M.; Höglund, P.; Andersson, B.; Nugues, P.; Nilsson, J. Improving Prediction of Heart Transplantation Outcome Using Deep Learning Techniques. Sci Rep 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Lau, L.; Kankanige, Y.; Rubinstein, B.; Jones, R.; Christophi, C.; Muralidharan, V.; Bailey, J. Machine-Learning Algorithms Predict Graft Failure after Liver Transplantation. Transplantation 2017, 101, e125–e132. [Google Scholar] [CrossRef] [PubMed]
- Nitski, O.; Azhie, A.; Qazi-Arisar, F.A.; Wang, X.; Ma, S.; Lilly, L.; Watt, K.D.; Levitsky, J.; Asrani, S.K.; Lee, D.S.; et al. Long-Term Mortality Risk Stratification of Liver Transplant Recipients: Real-Time Application of Deep Learning Algorithms on Longitudinal Data. Lancet Digit Health 2021, 3, e295–e305. [Google Scholar] [CrossRef]
- Watt, K.D.S.; Pedersen, R.A.; Kremers, W.K.; Heimbach, J.K.; Charlton, M.R. Evolution of Causes and Risk Factors for Mortality Post-Liver Transplant: Results of the NIDDK Long-Term Follow-up Study. American Journal of Transplantation 2010, 10, 1420–1427. [Google Scholar] [CrossRef] [PubMed]
- Maria Trinidad Serrano, A.G.-G.J.A.Y.B.L.C.C.V.J.J.A. Outcome of Liver Transplantation Using Donors Older than 60 Year of Age. Clin Transplant 2010.
- Levitsky, J.; Goldberg, D.; Smith, A.R.; Mansfield, S.A.; Gillespie, B.W.; Merion, R.M.; Lok, A.S.F.; Levy, G.; Kulik, L.; Abecassis, M.; et al. Acute Rejection Increases Risk of Graft Failure and Death in Recent Liver Transplant Recipients. Clinical Gastroenterology and Hepatology 2017, 15, 584–593.e2. [Google Scholar] [CrossRef]
- Piella, G.; Farré, N.; Esono, D.; Cordobés, M.Á.; Vázquez-Corral, J.; Bilbao, I.; Gómez-Gavara, C. LiverColor: An Artificial Intelligence Platform for Liver Graft Assessment. Diagnostics 2024, 14. [Google Scholar] [CrossRef] [PubMed]
- Higashi, H.; Yagi, H.; Kuroda, K.; Tajima, K.; Kojima, H.; Nishi, K.; Morisaku, T.; Hirukawa, K.; Fukuda, K.; Matsubara, K.; et al. Transplantation of Bioengineered Liver Capable of Extended Function in a Preclinical Liver Failure Model. American Journal of Transplantation 2022, 22, 731–744. [Google Scholar] [CrossRef]
- I J Fox, J.R.C.S.S.K.T.C.G.N.R.C.P.I.W.K.D.B.V.S.S.C.S. Treatment of the Crigler-Najjar Syndrome Type I with Hepatocyte Transplantation. New England Journal of Medicine 1998.
- Nishikawa, T.; Bell, A.; Brooks, J.M.; Setoyama, K.; Melis, M.; Han, B.; Fukumitsu, K.; Handa, K.; Tian, J.; Kaestner, K.H.; et al. Resetting the Transcription Factor Network Reverses Terminal Chronic Hepatic Failure. Journal of Clinical Investigation 2015, 125, 1533–1544. [Google Scholar] [CrossRef]
- Shimoda, H.; Yagi, H.; Higashi, H.; Tajima, K.; Kuroda, K.; Abe, Y.; Kitago, M.; Shinoda, M.; Kitagawa, Y. Decellularized Liver Scaffolds Promote Liver Regeneration after Partial Hepatectomy. Sci Rep 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Koui, Y.; Kido, T.; Ito, T.; Oyama, H.; Chen, S.W.; Katou, Y.; Shirahige, K.; Miyajima, A. An In Vitro Human Liver Model by IPSC-Derived Parenchymal and Non-Parenchymal Cells. Stem Cell Reports 2017, 9, 490–498. [Google Scholar] [CrossRef]
- Piella, G.; Farré, N.; Esono, D.; Cordobés, M.Á.; Vázquez-Corral, J.; Bilbao, I.; Gómez-Gavara, C. LiverColor: An Artificial Intelligence Platform for Liver Graft Assessment. Diagnostics 2024, 14. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Hassan-Ally, M.; Casas-Ferreira, A.M.; Suvitaival, T.; Ma, Y.; Vilca-Melendez, H.; Rela, M.; Heaton, N.; Wayel, J.; Legido-Quigley, C. Deregulation of the Purine Pathway in Pre-Transplant Liver Biopsies Is Associated with Graft Function and Survival after Transplantation. J Clin Med 2020, 9. [Google Scholar] [CrossRef] [PubMed]
|
Factors |
Reference |
Place and year of publication |
Findings |
Current consensus |
|---|---|---|---|---|
| Age | Gilbo N. et al.[61] | Belgium, 2019 | Older donor grafts can be safely utilized in older recipients if other risk factors are minimized. |
No donor age limit is set due to improved outcomes with older donors. |
| Nakamura T. et al.[62] | Japan, 2022 | Liver grafts from elderly donors show slower recovery patterns during the initial phase, but eventually lead to satisfactory outcomes. | ||
| Maestro O. C. et al.[63] | Spain, 2022 | The outcomes from grafts of nonagenarian donors are similar to those from octogenarian donors. | ||
| Liver graft Size | Reyes J. et al. [64] | U. S., 2019 | In deceased donors, the ratio of donor to recipient body surface area is an important predictor of graft survival. |
The maximum implantable graft volume in cirrhotic patients is the sum of the recipient liver volume and the right upper abdominal cavity’s dimensions. This volume also correlates with portal hypertension severity. |
| Addeo P. et al. [65] | France, 2022 | Integrating donor anthropometrics with recipient imaging can enhance donor-recipient matching processes and help prevent complications. | ||
| Kostakis I. D. et al. [66] | U. K., 2023 | A mismatch in size is linked to higher rates of portal vein thrombosis within the first three months in data obtained from 85% deceased brain-dead donors (DBD) and 15% from deceased circulatory-dead donors (DCD). | ||
| Gender | Rustgi V. K. et al. [67] | U. S., 2022 | Patients with a gender mismatch have a 6.9% higher risk of graft failure. |
The impact of gender mismatch on post-transplant outcomes remains debated, requiring larger, well-calibrated studies to clarify its potential effects in liver transplantation. |
| Germani G. et al. [68] | Italy, 2020 | In male recipients, a mismatch in donor-recipient gender, along with the use of obese donors for female recipients, is associated with lower survival rates after liver transplantation. |
| Donor Factor | Implications | Potential for Use |
|---|---|---|
|
Hepatic Steatosis |
Decreased mitochondrial membrane potential. Enhanced cell damage in cold ischemia and ischemic reperfusion injury. Graft rejection or dysfunction[77]. | Severe steatosis graft is generally rejected. Grafts with moderate steatosis should be considered in combination with other risk factors, such as advanced donor age and prolonged CIT [11]. |
|
HCV positive donors |
Fibrosis. Directly acting antivirals have changed the landscape of the management of HCV-infected patients[78]. |
If no significant donor fibrosis, good outcomes can be achieved from HCV antibody-positive allografts. Such organs should not be automatically rejected [78,79]. Numerous reports of successful outcomes to an HCV antibody-positive recipient exist. [80,81] |
| Donors withHbcAb | Suboptimal graft quality, poor outcomes. Risk of reactivation and uncontrolled replication due to immunosuppression[82]. |
With nucleoside analogs and hepatitis B immunoglobulin, there are encouraging results with such donors. HBcAb-positive status- should not be the only reason to discard a donor liver[83]. |
| Donors with Bacteraemia and Infections | Lower graft survival[13] Caution needs to be exercised with septic donors. Some transplant centers have been using DCD from bacteremic donors with good outcomes.[84] |
The incidence of infection transmission is low. Source of sepsis should be remote from the liver, the donors should be under appropriate and sensitive antibiotic cover, ideally for 24–48 h, and organs from donors with multi-drug-resistant sepsis must be avoided [85]. |
|
COVID-19 Donors |
Given the COVID-19 pandemic, testing for COVID-19 in the donor is standard practice. Bloodstream-related transmission remains questionable. | No evidence-based guidelines exist. Transplantation of organs other than the lungs seems to be a safe practice with a low risk of transmission. Consider donors with low viral replication (Ct > 30) at procurement[86]. |
| Donors with HIV infections-to recipients living with HIV. | Transmission of drug resistance and HIV superinfection in recipients. Data on such donors is scarce[11]. |
Banned in America until 2013,after the passage of the HIV Organ Policy Equity Act, allowing donations from HIV-positive donors to an HIV-positive recipient. Favourable outcomes in kidney transplants[87]. |
|
Donors with Malignancy |
Metastatic malignancy- excluded due to risk of tumor transmission. Exceptions of this are donors with nonmelanoma skin cancer and low-grade primary CNS tumors (grade I or II) [88]. | Reports show a very low risk of transmission of donor-derived malignancies by the donor organ. The potential risks and benefits should be weighed against the risks of waiting time and the urgency of the transplant. The risk stratification is not absolute[89]. |
|
Donors with Blunt liver Trauma |
Poor graft function Specific liver trauma management during transplantation [90] |
The French registry data reported 142 LTs from donors with recent liver trauma. The one-year overall and graft survival rates were 85% and 81%, respectively, while the 5-year rates were 77% and 72%. This suggests that donors with recent liver trauma may be safe and acceptable [90]. |
| Score system | Reference | Place and year of publication | Factors | Limitation |
|---|---|---|---|---|
| P-SOFT (the Preallocation scoretopredict Survival Out-comes Following Liver Transplant Score) and SOFT Score |
Rana A. et al. [101] | U. S., 2008 | Donor age BMI History of prior transplant Albumin levels Need for Dialysis ICU admissions MELD score Life support Encephalopathy Portal vein thrombosis Ascites |
Subjective variables and complexity limit clinical use in pretransplant decisions. |
| D-MELD | Halldorson J. B. et al.[102] | U. S., 2009 | The product of donor age and preoperative MELD score |
Weak predictive power. |
| BAR | Dutkowski P. et al.[103] | Switzerland, 2011 | Donor age Recipient age CIT Retransplantation need Life support need MELD score |
Does not account for graft steatosis and suboptimal function. Inaccurate in predicting transplant survival. |
| ET-DRI (Eurotransplant-Donor-Risk-Index) | Braat A. E. et al. [104] | Eurotransplant region, 2012 | Donor age Cause of death DCD Split liver grafts Organ location CIT Rescue allocation GGT levels |
Limited effectiveness in predicting early outcomes after liver transplantation. |
| ISO (Italian Score for Organ allocation) | Cillo U. et al. [105] | Italy, 2015 | MELD score Urgency HCC |
Needs prospective validation to confirm superiority over MELD score. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).