Submitted:
30 December 2024
Posted:
31 December 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Physiology of Sleep
2.1. Sleep Phases
2.2. Physiological Changes During Sleep
2.2.1. Electroencephalography, Electrooculography and Electromyography
2.2.2. Hear Rate and Heart Rate Variability
2.2.3. Body Movement
2.2.4. Respiration
2.2.5. Body Temperature
2.2.6. Blood Pressure
2.3. Altered Sleep Physiology in Neurological Diseases
3. Actual State of Technological Evolution
3.1. Basic Sleep Monitoring Devices
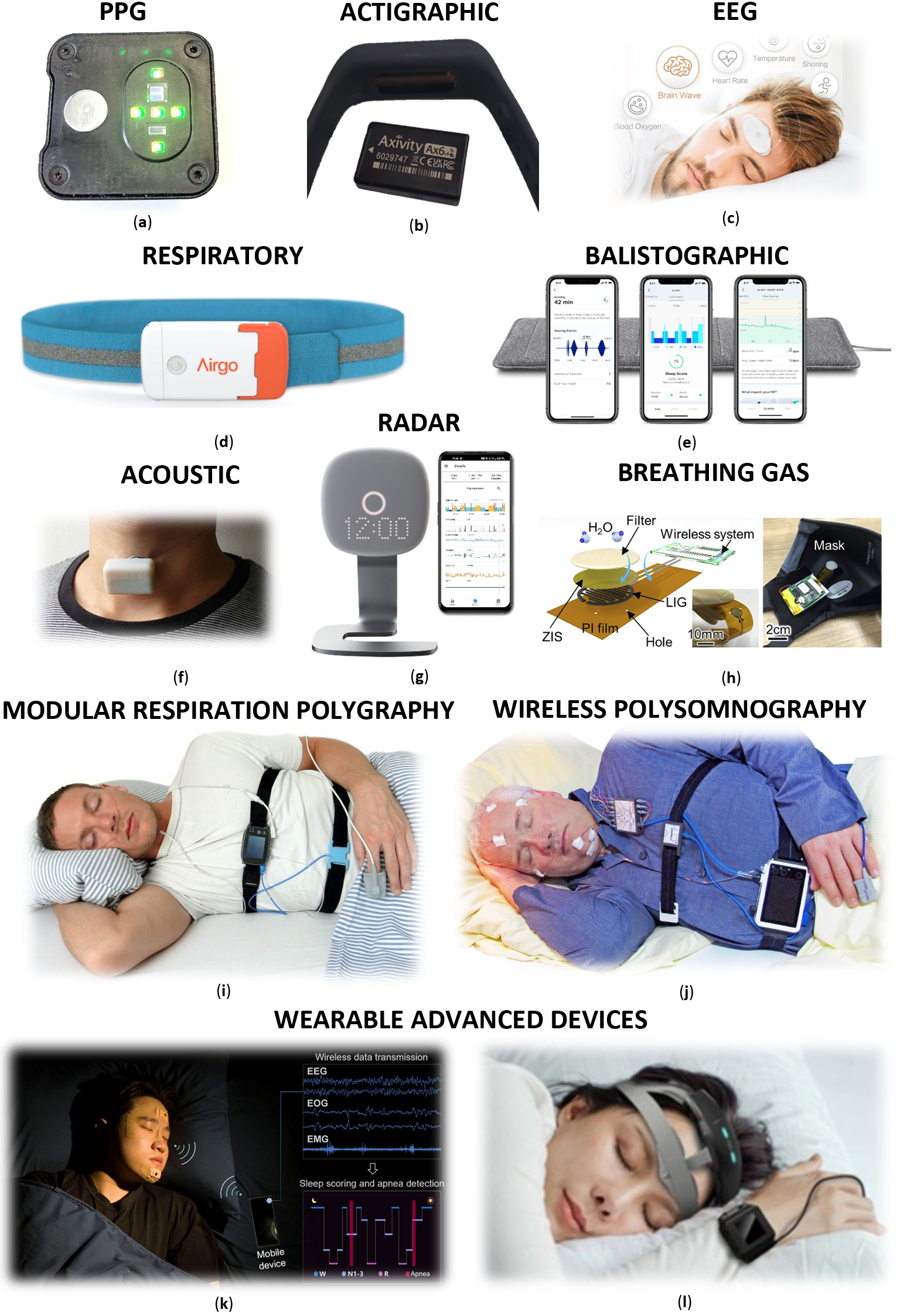
3.1.1. PPG-Based Devices
3.1.2. Actigraphic Devices
3.1.3. EEG-Based Devices
3.1.4. Respiratory-Based Devices
3.1.5. Ballistographic Sensors
3.1.6. Acoustic-Based Devices
3.1.7. Radar Systems Devices
3.1.8. Breath Gas Monitoring Devices
| Type | Application | Sensing Element | Key Parameters | Ref. |
|---|---|---|---|---|
| PPG | HR, SpO2, sleep stage | PPG (2× LED + PD), temperature, 3D IMU in ring |
[Oura Ring], 106 subjects, ML using 5-fold cross-validation, Sleep/wake accuracy 94% from accelerometric model and 96% from ANS and circadian features, 4-stage detection 57% resp. 79%, ARM Cortex MCU, Bluetooth |
[132] |
| PPG | HR, SpO2, sleep stage, movement | PPG, accelerometer, gyroscope on wristband |
[Xiaomi Band 9], Ambient light sensor, Bluetooth, 45 subjects, Sleep stage accuracy 78%, Sensitivity 89%, Specificity 35%, κ = 0.22 | [120] |
| PPG | HR, SpO2, sleep stage, movement, ECG, OSA | PPG, IMU, temperature in smartwatch | [Apple watch], Temperature, Ambient light sensor, Bluetooth, Processor S10 SiP, Memory 64 GB, GPS, Sleep stage agreement 53% (κ 1 = 0.2), Sensitivity 50.5-86.1%, Precision 72.7-87.8% |
[123,136,139] |
| PPG | HR, SpO2, movement, OSA, ODI |
PPG, accelerometer in ring |
[O2 Ring], 190 subjects, ODI sensitivity 87.30% and specificity 78.70%, SVMs model for OSA with sensitivity 97% and specificity 50%, Sampling rate 150 Hz, BLE, Recording time 16 hours, HR accuracy ±2 bpm, SpO2 accuracy ±3% | [124,138] |
| PPG | HR, SpO2, RR, HRV, skin temperature, stress, sleep stages | PPG (3× green + red + IR LED, 4× PD) |
[WHOOP 4.0], Sleep stage agreement 65% (κ = 0.52) | [139,140] |
| PPG | HR, SpO2, ECG, EDA, sleep patterns, stress, OSA, movement | PPG, BT, 3D accelerometer in smartwatch |
[Fitbit Sense 2], NFC, Ambient light sensor, Wi-fi, GPS + Glonass, Bluetooth, OSA sensitivity 88%, OSA specificity 52%, TST and SE overestimation 10%, Sleep stage sensitivity 61.7-78%, Precision 72.8-73.2% | [119,141] |
| PPG | HR, SpO2, RR, sleep stages, central and obstructive apnoea/hypopnoea |
3D accelerometer and PPG in glove | [UpNEA], MAX-30101 PPG, MAX-21105 IMU, PPG sampling rate 100 Hz, Accelerometer sampling rate 50 Hz, BLE, Tachycardia/bradycardia/atrial fibrillation/premature ventricular contraction detection, Accuracy 75.1% for apnoea/hypopnoea detection, Central vs obstructive accuracy about 83.2% |
[118] |
| PPG | HR, SpO2, RR, OSA | In-Ear PPG | 16-bit, MSP430F1611 microcontroller | [142] |
| PPG | HR, SpO2, RR, head position, apnoea | PPG (red + IR LED), 3D accelerometer on the nasal septum |
[MORFEA], MAX-30102 PPG, LSM6DSM accelerometer, Sampling rate 50 Hz, Modulation of PPG by breath, PSD 2 and PWA 3 method, Sensitivity 89% and precision 93% of apnoea detection, Bluetooth, Recording time 9h |
[143] |
| Actigraphic | Movements, light exposure |
3D MEMS accelerometer, light sensor |
[MotionWatch 8], Sleep patterns, PLMS detection, Circadian rhythm disorders, Memory 4 MBits, Recording time 3 months, Weight 9.1 g without strap |
[148] |
| Actigraphic | Movements, ambient and body temperature, light |
2D accelerometer, RGB - IR light, temperature |
[ActTrust 1], Sleep patterns and activity, Circadian rhythm disorders, Memory 4 MB, Recording time 3 months, Weight 38 g | [149] |
| Actigraphic | Movements, ambient and body temperature, light exposure |
3D accelerometer, RGB - IR light, temperature | [ActTrust 2], Sleep patterns and activity, Circadian rhythm disorders, Accelerometer sampling rate 25 Hz, Memory 8 MB, Resolution 12-Bit, Digital time display, Recording time 3 months, Weight 35 g | [149] |
| Actigraphic | Movements, ambient and body temperature, light exposure |
3D accelerometer, RGB - IR light, temperature, melanopic, Off-wrist capacitive sensor |
[Act Lumus], Sleep patterns and activity, Circadian rhythm disorders, Accelerometer sampling rate 25 Hz, Memory 8 MB, Resolution 12-Bit, Bluetooth, Recording time 1 month, Weight 31 g |
[150] |
| Actigraphic | Movements, light exposure |
Accelerometer, light sensor | [ActiGraph wGT3X-BT], Sleep patterns and physical activity, Weight 19 g, Sampling rate 30 – 100 Hz, Memory 4 GB, Recording time 25 days, BLE | [151] |
| Actigraphic | Movements, OSA, | IMU, temperature | [SleepActa], RTC, Sampling rate 100 Hz, 78 subjects, Dormi algorithms (Waso, TST, SE, SRI 4), CE Class I medical device, MCC 5 0.4 for mild AHI and MCC 0.3 for severe AHI | [101,153] |
| Actigraphic | HR, activity | 3D accelerometer, PPG | [Somno-Art], Sleep classification, Insomnia, OSA, Narcolepsy detection, AI algorithms for automatic sleep analysis, Bluetooth, Accelerometer sampling rate 250 Hz, Sleep/wake accuracy 87.8%, Sleep stages accuracy 68.5%, Recording time 40 hours | [155] |
| EEG |
EEG, HR, breathing, body movement and position | 4-channel EEG, PPG, 3D IMU, respiration in the headband | [Muse S], Sleep tracking & evaluation | [159] |
| EEG | EEG, HR, SpO2, movement, breathing temperature | 5-channel EEG, PPG, respiration, accelerometer on the headband | [Dreem 3S], Sleep tracking, AI quality evaluation & disturbance diagnosis | [160] |
| EEG | EEG, HR, SpO2, CBT, body movement and position, snoring | 1-channel EEG, PPG, 6-axis IMU sensor, sound, pressure sensor on the forehead |
[UmindSleep], Sleep tracking, Forehead temperature, AI evaluation & disorder diagnosis | [102,162] |
| EEG | EEG, sleep tracking | Highly elastic memory foam, flexible electrodes | Sleep tracking | [163] |
| EEG | EEG, EOG, chin EMG | 10× EEG, 1× EOG on the forehead, EMG electrodes |
Sleep tracking | [164] |
| Respiratory | RR, tidal volume, minute ventilation, body position |
Resistance-based sensor, IMU | Sleep disorder screening, Sleep staging, Respiratory pattern detection, Posture and activity detection, Bluetooth, Class IIa certified medical device, |
[103] |
| Respiratory | RR, breathing rhythm and depth | Textile RIP integrated into a suit, 3D accelerometer |
Smart signal processing algorithm, Sampling rate 10 Hz, Wireless communication, Peak power consumption 140 mW, Radio transmission range 20 m, | [168] |
| Respiratory | Respiratory effort, body position | IMU sensor | MCU CC2650 with ARM Cortex-M3, 16-bit resolution IMU, Wireless communication | [169] |
| Respiratory | Sleep apnoea | Strain gauge sensor | Sampling rate 10 Hz, Bluetooth, CNN 6, Accuracy 0.7609, Sensitivity of 0.78, Specificity of 0.72 | [170] |
| Respiratory | RR, HR, HRV, ECG, skin temperature, | ECG, thermometer, accelerometer |
Adhesive chest patch, Single use and fully disposable, Sleep staging, Wireless communication, Class IIa certified medical device |
[171] |
| Respiratory | RR, RE, HR, SpO2, sleep apnoea | PPG, ECG, SCG | PPG sampling rate 200 Hz, ECG sampling rate 120 Hz SCG sampling rate 500 Hz, Sleep staging, Bluetooth, Recording 10-hour, Sensitivity 100%, Precision 95% |
[172] |
| Respiratory | Sleep apnoea | BioZ 7 sensor | BioZ sampling rate 1024 Hz, ECG sampling rate 512 Hz, Stimulation signal 8 kHz – 160 kHz, Accuracy 72.8%, Sensitivity of 58.4%, Specificity of 76.2% | [178] |
| AFE 8 | RR, ECG, EEG | ADS129xR | 8-channels, 24-bit, Sampling rate 250 Hz – 32 kHz, CMRR 9 −115 dB, Internal oscillator | [173] |
| AFE | Respiration, ECG |
AFE4960 | 2 channels, 22-bit, Single ADC, SPI and I2C interface, Sine wave or square wave excitation |
[174] |
| AFE | Respiration, ECG, optical HR | AFE4500 | 4 input channels, 22-bit, single ADC, SPI and I2C interface |
[175] |
| AFE | Respiration, ECG |
ADAS1000 | 5 acquisition channels and one driven lead, SPI/QSPI interface, AC and DC lead-off detection | [176] |
| AFE | Respiration, ECG |
MAX30001 | High input impedance (>1 GΩ), SPI, 32-word ECG, 8-word BioZ, FIFO 10, EMI 11 filtering, ESD 12 protection, DC leads-off detection |
[177] |
| BCG | RR, HR, HRV, sleep, movement, snoring, stress | Dynamic ferro-electret under the mattress | [Emfit QS] Sleep monitoring, Stress level, Sleep quality and classification |
[180] |
| BCG | HR, RR |
Two pressure pads on mattress | [NAPS], BCG evaluation in sleep, 40 subjects | [181] |
| BCG | HR, RR | Set of oil pressure sensors in mattress | 16-bit, Sampling rate 100 Hz, KSVM 13 model, 42 subjects/3 nights, Apnoea precision rate 90.46% and recall rate 88.89%, |
[182] |
| BCG | HRV, RR variability | Hydraulic transducers under the mattress | Sleep quality and sleep related disorders, SVM and KNN classification methods, Sampling rate 100 Hz, Sleep stages detection accuracy 85%, κ = 0.74 | [183] |
| BCG | HR, HRV, RR, stroke volume | Murata SCA11H sensors (IMU) under the mattress |
Sleep management, Random Forest algorithm, Sleep phase classification, Wi-Fi | [184] |
| BCG | HR, RR | 300 × 580 mm electromechanical film sewn into a fitted sheet |
Quantification of sleep quality, restlessness, Neyman-Pearson detection test, Sequential detection algorithm, 16-bit, Sampling rate 250 Hz, 94% and 95.2% accuracy in sleep and restlessness state identification | [185] |
| BCG | RR, OSA events | Micromovement sensor in mattress | Apnoea Phase, Respiratory effort phase and arousal phase, 38 subjects, BP neural network, Accuracy 94.6%, Recall 93.1% |
[186] |
| BCG | HR, RR, sleep quality | 700 × 30 mm piezoelectric film sensor beneath the mattress |
Sampling rate 140 Hz, 32 subjects, AMPD 14 algorithm, Correlation coefficient 0.95, MAE 1.78 bpm for HR, Correlation coefficient 0.98, MAE 0.25 rpm for RR | [187] |
| BCG | HR, RR, sleep apnoea syndrome |
4 pressure sensors in mattress | Sleep apnoea syndrome severity, 136 subjects, Resolution 16-bit, Wavelet decomposition, Physio ICSS based algorithm, Accuracy 94.12% |
[188] |
| BCG | HR, HRV, RR, RRV, respiratory depth, movement | Murata SCA11H sensors (IMU) under the mattress |
Sleep stage detection, 20 subjects, Sampling rate 1kHz, Correlation coefficient 0.97 for HR, 0.67 for HF HRV, 0.54 for LF HRV, 0.54 for RR, 0.49 for RRV, Wi-Fi | [189] |
| BCG | HR, RR, apnoea and hypopnoea | 4 × 1 array PVDF film-based sensor under silicon pad on mattress |
26 apnoea patients + 6 healthy subjects, NI-DAQ 6221 (National Instruments, Austin, TX, USA), Sampling rate 250 Hz, PCA 15 method, Correlation coefficient for AHI 0.94, Apnoea detection with 72.9% sensitivity, 90.6% specificity and 85.5% accuracy | [190] |
| BCG | HR, RR, snoring, sleep stages classification |
MEMS ISM330 DLC 3D accelerometer and pressure sensor array on mattress |
STM32F411 ARM processor, Accuracy for HR 1.5 bpm, for RR 0.7 rpm, Snoring recognition 97.2%, Sleep stage prediction 79.7% | [191] |
| BCG | Sleep stages, HR, HRV, RR | Murata SCA11H + Apple watch 8 + actigraphy device |
6 subjects, Nonlinear methods, LSTM model, 73% agreement to PSG | [121] |
| BCG | HR, apnoea, snoring |
Pneumatic and sound sensor under mattress | [Withings Sleep Analyzer], Medical-grade apnoea detection, Sleep cycles detection, Bluetooth, Wi-Fi |
[104] |
| Acoustic | OSA, respiratory sounds, apnoea, AHI | Smartphone | DNN architecture, 3× CNN layers, Adam optimizer, Mel-frequency analysis, Sampling rate 256 Hz, 103 subjects, Sensitivity 0.79 and specificity 0.80 for moderate OSA, Sensitivity 0.78 and specificity 0.93 for severe OSA |
[195] |
| Acoustic | OSA, respiratory sounds | Smartphone iPhone 7 | Calibrated by oesophageal pressure manometry, ML algorithm, 13 subjects, Prediction of ΔPes 16 with MAE 17 6.75 cm H2O, r = 0.83 | [196] |
| Acoustic | OSA, snoring, apnoea, AHI |
Smartphone | FFT analysis, 10 kHz Sampling rate, 50 subjects, Snoring time correlation r = 0.93, AHI correlation r = 0.94, OSA sensitivity 0.7, OSA specificity 0.94 |
[197] |
| Acoustic | OSA, snoring | Tracheal sound and suprasternal pressure sensor PneaVoX |
Sensitivity 99.4%, Specificity 93.6% | [198] |
| Acoustic | Apnoea | Tracheal sensor AcuPebble SA100 |
63 subjects, OSA accuracy 89.77%, Central vs obstructive apnoea accuracy 82.54% |
[199] |
| Acoustic | Apnoea, hypopnoea | Tracheal sensor WADD | WADD and SOMNO automated software, 20 healthy and 10 apnoea diagnosed subjects, Apnoea detection sensitivity 88.6% and specificity 99.6% | [105] |
| Acoustic | OSA, snoring | Wireless headset Plantronics M165 near nose |
Sampling frequency 11 kHz, Mel-scale based features, 8 subjects, Snore detection accuracy 96.1%, Abnormal detection result accuracy 93.1% | [200] |
| Acoustic | Apnoea | Body worn audio amplifier | MSP430 microcontroller, SPP, Orthogonal Matching Pursuit algorithm, Accuracy 80%, Bluetooth, Streaming 8 kb/s |
[201] |
| Radar | RR, restless time | Vayyar FMCW 18 radar over bed | 6.014 GHz, 14 transmitting and 13 receiving antennas, 13 different sleeping postures, Distance 2.3m, RR accuracy 86 - 90% |
[202] |
| Radar | HR, RR, sleep analysis |
FMCW radar over bed | 24 GHz, 250 MHz bandwidth, FFT based on cepstral and autocorrelation analyses, 11 subjects, HR correlation 86%, RR correlation 91% |
[203] |
| Radar | RR, sleep stages, restlessness | Radar sleep monitor in form of alarm clock | [Somnofy] 23.8 GHz, Environment monitoring (sound, light, pressure, air quality, humidity, temperature), Night reports, Sleep assessment, Alerts |
[106] |
| Radar | RR, sleep scoring | Radar sleep monitor in form of alarm clock | [Somnofy] 23.8 GHz, FFT, 37 subjects, RR with MAE 0.18, Accuracy of sleep detection 0.97, Accuracy of wake detection 0.72 |
[204] |
| Radar | RR, inhale/exhale duration, NREM/REM stage detection | Radar-based IoT system on bedside wall | 2 × 4 linearly polarized antenna array on PCB, Distance 40-100 cm, FIR filter (VMD 19, CEEMDAN 20, LOES 21 algorithm), AMPD algorithm, Sampling rate 100 Hz, RR accuracy 97%, Inhale duration accuracy 93%, Inhale duration accuracy 92%, Wi-Fi |
[205] |
| Radar | RR, movement, sleep stage detection | Radar on bedside table | [SleepScore Max tracker] Automated sleeping scoring | [206] |
| Radar | RR, movement, sleep stage detection | Radar on bedside table | [S+ ResMed] 10.5 GHz, Emitting power 1 mW, Distance 1.5 m, Environment monitoring (room temperature, light, and sounds), 27 subjects, Sleep detection accuracy 93.8%, Wake detection accuracy 73.1% |
[207] |
| Radar | Body movements, respiration, apnoea | Wi-Fi based system | [WiFi-Sleep], Sleep stage analysis, PLMS, Accuracy for sleep classification 81.8%, Future developments - detecting chronic insomnia |
[208] |
| Breath gas | Sleep monitoring, apnoea, hypopnoea, breathing |
Platinum thermal sensor on patch | Response time 0.07 s, Sensitivity 1.4‰ °C−1, Sampling rate 32 Hz, 16-bits resolution, 10th order Butterworth 3Hz low-pass filter, RR filter 0.2–0.5 Hz Bluetooth | [227] |
| Breath gas | Sleep monitoring, respiration | Pressure, temperature sensors in facemask | Recognizing 8 breath patterns, 3D carbon nanofiber mats, discrimination between oral and nasal breathing, human body's physiology analysis |
[228] |
| Breath gas | Respiration, apnoea, RR, NREM/REM stage detection | Humidity sensor in facemask | Highly stable, Wireless monitoring Real time sleep apnoea, ZnIn2S4 nanolayer, High sensitivity and stability, Operating time 150 hours |
[107] |
| Breath gas | Respiration, apnoea, breathing |
Easy-to-process paper humidity sensor on patch | Low-cost, Flexible, High sensitivity 5.45 kΩ/% RH, Repeatability 85.7%, Sampling rate 18 Hz, Battery 3.7 V |
[230] |
| Breath gas | Respiration, sleep breathing patterns, humidity level, temperature |
Humidity, temperature, accelerometer, barometer, gyroscope, IMU sensors in facemask |
[NiteAura], Breathing conditions during sleep, Help with sleep-disordered breathing and set appropriate conditions to achieve deeper sleep | [231] |
3.2. Advanced Sleep Monitoring Devices
3.2.1. Limited Respiratory Polygraphy
3.2.2. Modular Systems
3.2.3. Wireless PSG Devices
3.2.4. Wearable Devices
| Type | Application | Sensing Element | Key Parameters | Ref. |
|---|---|---|---|---|
| PG | Flow, snoring, SpO2, HR, activity, light, thoracic & abdominal effort, body position |
Pressure, thermistor, light, SpO2 sensor, accelerometer, chest/abdominal pressure pad, microphone | [Samoa] & [SleepDoc Porti ®9], Bluetooth, Recording time 100 hours, Weight 135 g, Battery 3.6 V |
[237,266] |
| PG | Flow, thoracic effort, snoring, SpO2, HR, body position, PAP1 |
Pressure, RIP, SpO2 sensor, accelerometer | [Alice NightOne], Sleep-wake determination, Memory 4 GB, Weight 84 g without battery and sensors, Bluetooth | [238] |
| PG | Flow, thoracic effort, snoring, SpO2, HR |
Pressure, RIP, SpO2 sensor | [ApneaLinkTM Air], Memory 15 MB, Recording time 8 hours, Weight 66 g |
[240] |
| PG | Flow, thoracic & abdominal effort, snoring, SpO2, HR, body position | Pressure, thermistor, RIP, SpO2 sensor, microphone, accelerometer |
[ApneaTrak Legacy], USB connection, Recording time 24 hours, Weight 143.5 g |
[241] |
| PG | Flow, thoracic effort, snoring, SpO2, HR, PPG, body position, activity, PAP |
Pressure, RIP, SpO2 sensor, accelerometer | [SOMNOtouch RESP eco], USB,Additional sensor for abdominal effort/bruxism, Analysis of Cheyne-Stokes, | [242] |
| Modular PG | Flow, snoring, SpO2, HR, thoracic & abdominal effort, PPG wave, body position, movement, PAP, extension to include EEG, EOG, ECG, chin EMG and leg EMG | Accelerometer, thermistor, Pressure, RIP, SpO2 sensor, EEG, EOG, EMG attachable electrodes | [SOMNOtouchTM RESP], Scalable to PSG, Memory 512 MB, Sampling rate 4 - 512 Hz, Built-in chest effort sensor and sensor for body position, BP monitoring, , Weight 64 g |
[108,244] |
| Modular PG | Flow, snoring, SpO2, HR, thoracic & abdominal effort, PPG wave, body position, activity, PAP, ExG | Pressure sensor, RIP, SpO2 sensor, accelerometer, microphone, 2 bipolar attachable ExG electrodes |
[Nox T3sTM], Scalable to PSG, BLE, Memory 4 GB, Recording time 24 hours, BodySleep technology by Nox, Weight 86 g |
[167,245] |
| Modular PG | Flow, snoring and sound, SpO2, HR, activity, thoracic & abdominal effort, PPG wave, body position, extension to include EEG, EOG, ECG, chin EMG and leg EMG, ExG |
Pressure, RIP, SpO2 sensor, accelerometer, 1× Bipolar ExG electrodes, microphone, EEG, EOG, EMG attachable electrodes | [Embletta® MPR], Scalable to PSG, Sampling rate 8 kHz, Resolution 24-bit, Recording time 24 hours, Attachable ST/ST+ proxy for PSG Weight 153 g |
[246] |
| Wireless PSG | EEG, EOG, ECG, chin EMG, flow, SpO2, HR, PPG wave, thoracic & abdominal effort, snoring, movement, body position, leg EMG, PAP, Ambient light | Electrodes for EEG, EOG, chin EMG, EMG of limbs, and ECG, thermistor, pressure, RIP, SpO2, light sensor, microphone, accelerometer |
[SOMNO HD], Up to 70 channels, Sampling rate 4 kHz/channel, Bluetooth real-time data transmission, 6 wireless sensors available, Normal recording time 20 hours, Online recording 12 hours, Weight 190 g |
[109,244] |
| Wireless PSG | EEG, EOG, ECG, chin EMG, flow, thoracic & abdominal effort, sound, SpO2, HR, PPG wave, body position, activity, leg EMG, PAP |
Electrodes for EEG, EOG, chin EMG, EMG of limbs, and ECG, thermistor, RIP, pressure, SpO2 sensor, 3D accelerometer, microphone |
[Nox A1sTM], Memory 4 GB, Recording time 30 hours, Wireless PPG, Integrated snoring sensor, Built-in accelerometer, BLE, Ergonomic cable design, Weight 120 g |
[250,251] |
| Wearable patch-based PSG | EOG, EEG, chin EMG, ECG, forehead SpO2, snoring, leg movements, airflow, respiratory effort, position, activity | EEG, EOG, ECG, EMG, bioimpedance, pressure sensor, sound, 3D accelerometer |
[Onera STS], Sleep stages classification, Sleep-disordered breathing, PLMS, PSG with 15 channels, Acquisition 1-night | [255] |
| Wearable patch-based | EEG, EOG, EMG, HRV, HR, SpO2, snoring, head position, movement, ambient light, PAT |
3 frontal electrodes, 3D accelerometer, PPG, microphone, 2-channel EEG |
[Somfit], Sleep stages classification, OSA, Insomnia and circadian rhythm disorders, AHI and ODI index, DL2 on a CNN architecture, EEG (24-Bit, 0.5 – 30 Hz), Agreement of 76.14% across all sleep stages, 7 days of recording, BLE | [258] |
| Wearable patch-based PSG | EEG, EOG, EMG, ECG, HRV, HR, SpO2, movement, ambient light, PAT, airflow, effort, position, RR, snoring | 3 frontal electrodes, PPG, 3D accelerometer, microphone, inductive belts, nasal pressure cannula |
[Somfit Pro], Sleep stages classification, Agreement of 76.14%, 2× EEG (24-Bit, 0.5 – 30 Hz), Breathing disorders, DL on CNN, Recording time 8 hours, BLE | [258] |
| Wearable patch-based | EEG, EOG, chin EMG, SpO2, CO2 monitoring, movement | Nanomembrane electrodes | Sleep stages tracking, OSA detection, Evaluation by CNN, Bluetooth, OSA detection accuracy - 88.5% | [110] |
| Wearable patch-based | EEG, EOG, EMG, ECG, respiration |
Biphasic liquid metal composite electrodes | Sleep stage classification, Bruxism, Customizable digital printed bio-stickers, Light and flexible design |
[260] |
| Wearable headband | EEG, EOG, EMG, ECG, HR, head position and movement snoring | 3× frontal electrodes, optical sensor, microphone, accelerometer |
[SleepProfiler], Optional ECG and EMG electrodes, Memory 8 GB, Recording time 30 hours, 8 channels, Bluetooth, 3.7 V battery 650 mAh, Weight 71 g | [261] |
| Wearable headband PSG | EEG, EOG, EMG, ECG, SpO2, HR, head position and movement snoring, thoracic & abdominal effort | 3× frontal electrodes, RIP, PPG, optical sensor, microphone, accelerometer, nasal pressure cannula |
[SleepProfiler PSG2], Optional ECG and EMG electrodes, Recording time 26 hours, Bluetooth, 3.7 V battery 650 mAh | [267] |
| Wearable headband PSG | EEG, EMG, EOG, SpO2, HR, head position, snoring and ambient sound | Frontopolar EEG and chin EMG electrodes, PPG, 3D accelerometer, audio sensor, |
[WPSG-I], Automated sleep staging with good accuracy compared to PSG | [111] |
| Wearable headband | EEG, EMG, EOG, SpO2, HR, breathing rhythm, head motion | Gold-plated brass electrodes and dry-sensing electrodes, PPG, accelerometer, gyroscope |
[FRENZ Brainband], Determining sleep and concentration, AI, Accuracy of automatic sleep scoring 88%, Real-time, Supporting sleep quality with sounds |
[263,264] |
3.3. Application in Neurological Disorders
4. Discussion and Future Directions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Haghi, M.; Madrid, N.M.; Seepold, R. In-Home, Smart Sleep Monitoring System for Cardiorespiratory Estimation and Sleep Apnea Detection: Proof of Concept. IEEE Sens J 2024, 24, 13364–13377. [Google Scholar] [CrossRef]
- Marschall, S. Sleep Statistics and Facts. Available online: https://www.ncoa.org/adviser/sleep/sleep-statistics/ (accessed on 10 December 2024).
- Vasta, C. Sleep Statistics 2023 - Facts About Sleep - Sleep Care Online. Available online: https://www.sleepcareonline.com/articles/sleep-statistics-2023/ (accessed on 10 December 2024).
- Wade, A.; Zisapel, N.; Lemoine, P. Prolonged-Release Melatonin for the Treatment of Insomnia: Targeting Quality of Sleep and Morning Alertness. Aging health 2008, 4, 11–21. [Google Scholar] [CrossRef]
- Příhodová, I.; Dostálová, S.; et al. Spánková Medicína v Kazuistikách; 1st ed.; Mladá fronta, 2016; ISBN 978-80-204-4024-2.
- Ramar, K.; Malhotra, R.K.; Carden, K.A.; Martin, J.L.; Abbasi-Feinberg, F.; Aurora, R.N.; Kapur, V.K.; Olson, E.J.; Rosen, C.L.; Rowley, J.A.; et al. Sleep Is Essential to Health: An American Academy of Sleep Medicine Position Statement. Journal of Clinical Sleep Medicine 2021, 17, 2115–2119. [Google Scholar] [CrossRef] [PubMed]
- Koninklijke Philips Electronics, N.V. Alice 6 LDx Diagnostic Sleep System | Philips Healthcare. Available online: https://www.philips.ie/healthcare/product/HC1063315/alice-6-ldx-diagnostic-sleep-system (accessed on 9 December 2024).
- Nihon Kohden Corporation. Polysomnography | Nihon Kohden India. Available online: https://in.nihonkohden.com/en/products/neurology/sleep-diagnostics/polysomnography (accessed on 9 December 2024).
- Compumedics Limited. Grael 4K PSG:EEG – Compumedics. Available online: https://www.compumedics.com.au/en/products/grael-4k-psg-eeg/ (accessed on 9 December 2024).
- Vitazkova, D.; Zavodnik, T.; Gasparek, K.; Cernaj, L.; Kosnacová, H.; Vavrinsky, E.; Micjan, M.; Banovcin, P.; Kocan, I. Advanced Photoplethysmographic Multisensor Used in Polysomnographic Examinations. In Proceedings of the ELITECH´23; Kozakova, A., Ed.; Vydavateľstvo Spektrum STU: Bratislava, 2023; p. 6.
- Rundo, J.V.; Downey, R. Polysomnography. Handb Clin Neurol 2019, 160, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Romero-Corral, A.; Caples, S.M.; Lopez-Jimenez, F.; Somers, V.K. Interactions Between Obesity and Obstructive Sleep Apnea: Implications for Treatment. Chest 2010, 137, 711. [Google Scholar] [CrossRef]
- Drewes, A.M.; Nielsen, K.D.; Taagholt, S.J.; Svendsen, L.; Bjerregård, K.; Nielsson, L.; Kristensen, L. Ambulatory Polysomnography Using a New Programmable Amplifier System With On-Line Digitization of Data: Technical and Clinical Findings. Sleep 1996, 19, 347–354. [Google Scholar] [CrossRef]
- Bruyneel, M.; Ninane, V. Unattended Home-Based Polysomnography for Sleep Disordered Breathing: Current Concepts and Perspectives. Sleep Med Rev 2014, 18, 341–347. [Google Scholar] [CrossRef]
- Fry, J.M.; DiPhillipo, M.A.; Curran, K.; Goldberg, R.; Baran, A.S. Full Polysomnography in the Home. Sleep 1998, 21, 635–642. [Google Scholar] [CrossRef]
- Kourtis, L.C.; Regele, O.B.; Wright, J.M.; Jones, G.B. Digital Biomarkers for Alzheimer’s Disease: The Mobile/Wearable Devices Opportunity. NPJ Digit Med 2019, 2, 9. [Google Scholar] [CrossRef]
- Singh, H.; Sankari, A. Sleep and Neurodegenerative Disorders. StatPearls 2024. [Google Scholar]
- Rothman, S.M.; Mattson, M.P. Sleep Disturbances in Alzheimer’s and Parkinson’s Diseases. Neuromolecular Med 2012, 14, 194. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Tvedt, J.; Åkerstedt, T.; Cadar, D.; Wang, H.X. Impact of Sleep Duration and Sleep Disturbances on the Incidence of Dementia and Alzheimer’s Disease: A 10-Year Follow-up Study. Psychiatry Res 2024, 333, 115760. [Google Scholar] [CrossRef] [PubMed]
- Postuma, R.B.; Berg, D.; Stern, M.; Poewe, W.; Olanow, C.W.; Oertel, W.; Obeso, J.; Marek, K.; Litvan, I.; Lang, A.E.; et al. MDS Clinical Diagnostic Criteria for Parkinson’s Disease. Movement Disorders 2015, 30, 1591–1601. [Google Scholar] [CrossRef]
- Stefani, A.; Högl, B. Sleep in Parkinson’s Disease. Neuropsychopharmacology 2020, 45, 121–128. [Google Scholar] [CrossRef]
- Ju, Y.E.S.; McLeland, J.S.; Toedebusch, C.D.; Xiong, C.; Fagan, A.M.; Duntley, S.P.; Morris, J.C.; Holtzman, D.M. Sleep Quality and Preclinical Alzheimer Disease. JAMA Neurol 2013, 70, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Khosroazad, S.; Abedi, A.; Hayes, M.J. Sleep Signal Analysis for Early Detection of Alzheimer’s Disease and Related Dementia (ADRD). IEEE J Biomed Health Inform 2023, 27, 2264–2275. [Google Scholar] [CrossRef] [PubMed]
- Chokroverty, S. Overview of Sleep & Sleep Disorders. Indian J Med Res 2010, 131, 126–140. [Google Scholar] [PubMed]
- Patel, A.K.; Reddy, V.; Shumway, K.R.; Araujo, J.F. Physiology, Sleep Stages. StatPearls 2024. [Google Scholar]
- Binjabr, M.A.; Alalawi, I.S.; Alzahrani, R.A.; Albalawi, O.S.; Hamzah, R.H.; Ibrahim, Y.S.; Buali, F.; Husni, M.; BaHammam, A.S.; Vitiello, M. V.; et al. The Worldwide Prevalence of Sleep Problems Among Medical Students by Problem, Country, and COVID-19 Status: A Systematic Review, Meta-Analysis, and Meta-Regression of 109 Studies Involving 59427 Participants. Curr Sleep Med Rep 2023, 9, 161–179. [Google Scholar] [CrossRef]
- Barbeau, D.Y.; Weiss, M.D. Sleep Disturbances in Newborns. Children 2017, 4, 90. [Google Scholar] [CrossRef]
- Carskadon, M.A.; Dement, W.C. Normal Human Sleep: An Overview. Principles and Practice of Sleep Medicine: Fifth Edition 2010, 16–26. [CrossRef]
- Šonka, K.; Boháč, J.; Donič, V.; Tomori, Z. Apnoe a Další Poruchy Dýchání ve Spánku; 1.; Grada Publishing: Praha, 2004; ISBN 80-247-0430-7.
- Malhotra, R.K. AASM Scoring Manual 3: A Step Forward for Advancing Sleep Care for Patients with Obstructive Sleep Apnea. Journal of Clinical Sleep Medicine 2024, 20, 835–836. [Google Scholar] [CrossRef] [PubMed]
- van Gorp, H.; van Gilst, M.M.; Overeem, S.; Dujardin, S.; Pijpers, A.; van Wetten, B.; Fonseca, P.; van Sloun, R.J.G. Single-Channel EOG Sleep Staging on a Heterogeneous Cohort of Subjects with Sleep Disorders. Physiol Meas 2024, 45, 055007. [Google Scholar] [CrossRef] [PubMed]
- Klobucnikova, K. Základy fyziológie a porúch spánku; 1.; Vydavateľstvo UK: Bratislava, 2021; Volume 115, ISBN 978-80-223-5338-0. [Google Scholar]
- Borzova, C. Nespavost a jiné poruchy spánku; Grada: Praha, 2009; ISBN 9788024729787. [Google Scholar]
- Akre, K. REM Sleep Physiology. Available online: https://www.britannica.com/science/rapid-eye-movement-sleep (accessed on 9 December 2024).
- Tao, M.H.; Drake, C.L.; Lin, C.H. Association of Sleep Duration, Chronotype, Social Jetlag, and Sleep Disturbance with Phenotypic Age Acceleration: A Cross-Sectional Analysis. Sleep Health 2024, 10, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Sun, C.; Long, M.; Chen, C.; Chen, W. EOGNET: A Novel Deep Learning Model for Sleep Stage Classification Based on Single-Channel EOG Signal. Front Neurosci 2021, 15, 573194. [Google Scholar] [CrossRef]
- Maiti, S.; Sharma, S.K.; Bapi, R.S. Enhancing Healthcare with EOG: A Novel Approach to Sleep Stage Classification. 2023.
- Cruz, A.Â.S.; Wanner, S.P.; Stieler, E.; Romão, J.; Esteves, A.M.; de Araújo Andrade, H.; Lôbo, I.L.B.; Amaral, A.S.; Rabelo, P.C.R.; de Mello, M.T.; et al. Cardiac Autonomic Nervous Activity during Different Sleep Stages in Individuals with Spinal Cord Injury: The Influence of Physical Training. Sleep Med 2024, 117, 25–32. [Google Scholar] [CrossRef]
- Kim, H.; Jung, H.R.; Kim, J. Bin; Kim, D.-J. Autonomic Dysfunction in Sleep Disorders: From Neurobiological Basis to Potential Therapeutic Approaches. Journal of Clinical Neurology 2022, 18, 140. [Google Scholar] [CrossRef]
- Tobaldini, E.; Nobili, L.; Strada, S.; Casali, K.R.; Braghiroli, A.; Montano, N. Heart Rate Variability in Normal and Pathological Sleep. Front Physiol 2013, 4. [Google Scholar] [CrossRef]
- Ma, Y.; Mullington, J.M.; Wayne, P.M.; Yeh, G.Y. Heart Rate Variability during Sleep Onset in Patients with Insomnia with or without Comorbid Sleep Apnea. Sleep Med 2024, 122, 92–98. [Google Scholar] [CrossRef]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front Public Health 2017, 5. [Google Scholar] [CrossRef]
- Biczuk, B.; Żurek, S.; Jurga, S.; Turska, E.; Guzik, P.; Piskorski, J. Sleep Stage Classification Through HRV, Complexity Measures, and Heart Rate Asymmetry Using Generalized Estimating Equations Models. Entropy 2024, 26, 1100. [Google Scholar] [CrossRef]
- Dijk, D.J. Regulation and Functional Correlates of Slow Wave Sleep. J Clin Sleep Med 2009, 5, S6. [Google Scholar] [CrossRef]
- Trinder, J.; Kleiman, J.; Carrington, M.; Smith, S.; Breen, S.; Tan, N.; Kim, Y. Autonomic Activity during Human Sleep as a Function of Time and Sleep Stage. J Sleep Res 2001, 10, 253–264. [Google Scholar] [CrossRef]
- Skarpsno, E.S.; Mork, P.J.; Nilsen, T.I.L.; Holtermann, A. Sleep Positions and Nocturnal Body Movements Based on Free-Living Accelerometer Recordings: Association with Demographics, Lifestyle, and Insomnia Symptoms. Nat Sci Sleep 2017, Volume 9, 267–275. [Google Scholar] [CrossRef]
- Ibrahim, A.; Ferri, R.; Cesari, M.; Frauscher, B.; Heidbreder, A.; Bergmann, M.; Högl, B.; Stefani, A. Large Muscle Group Movements during Sleep in Healthy People: Normative Values and Correlation to Sleep Features. Sleep 2023, 46. [Google Scholar] [CrossRef] [PubMed]
- Gori, S.; Ficca, G.; Giganti, F.; Nasso, I. Di; Murri, L.; Salzarulo, P. Body Movements during Night Sleep in Healthy Elderly Subjects and Their Relationships with Sleep Stages. Brain Res Bull 2004, 63, 393–397. [Google Scholar] [CrossRef]
- Hoque, E.; Dickerson, R.F.; Stankovic, J.A. Monitoring Body Positions and Movements during Sleep Using WISPs. In Proceedings of the Wireless Health 2010; ACM, December 2010; pp. 44–53.
- Sapra, A.; Malik, A.; Bhandari, P. Vital Sign Assessment. StatPearls 2023. [Google Scholar]
- Gutierrez, G.; Williams, J.; Alrehaili, G.A.; McLean, A.; Pirouz, R.; Amdur, R.; Jain, V.; Ahari, J.; Bawa, A.; Kimbro, S. Respiratory Rate Variability in Sleeping Adults without Obstructive Sleep Apnea. Physiol Rep 2016, 4, e12949. [Google Scholar] [CrossRef]
- Suni, E.; Singh, A. Sleep-Related Breathing Disorders. Available online: https://www.sleepfoundation.org/sleep-related-breathing-disorders (accessed on 11 December 2024).
- Benjafield, A. V; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.M.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pépin, J.L.D.; et al. Estimation of the Global Prevalence and Burden of Obstructive Sleep Apnoea: A Literature-Based Analysis. Lancet Respir Med 2019, 7, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhou, B.; Li, Z.; Lu, Q.; Li, S.; Pu, Z.; Luo, F. Gender Differences of Clinical and Polysomnographic Findings with Obstructive Sleep Apnea Syndrome. Scientific Reports 2021, 11, 1–6. [Google Scholar] [CrossRef]
- Roberts, E.G.; Raphelson, J.R.; Orr, J.E.; LaBuzetta, J.N.; Malhotra, A. The Pathogenesis of Central and Complex Sleep Apnea. Curr Neurol Neurosci Rep 2022, 22, 405–412. [Google Scholar] [CrossRef]
- Berry, R.B.; Quan, S.F.; Abreu, A.R.; Bibbs, M.L.; DelRosso, L.; Harding, S.M.; Mao, M.M.; Plante, D.T.; Pressman, M.R. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications (Version 2.6); American Academy of Sleep Medicine, 2020.
- Advanced Sleep Medicine Services, Inc. » At What Severity Will Insurance Cover CPAP for Sleep Apnea? Available online: https://www.sleepdr.com/the-sleep-blog/at-what-severity-will-insurance-cover-cpap-for-sleep-apnea/ (accessed on 11 December 2024).
- Vasey, C.; McBride, J.; Penta, K. Circadian Rhythm Dysregulation and Restoration: The Role of Melatonin. Nutrients 2021, 13, 3480. [Google Scholar] [CrossRef] [PubMed]
- Kräuchi, K. The Thermophysiological Cascade Leading to Sleep Initiation in Relation to Phase of Entrainment. Sleep Med Rev 2007, 11, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Bazil, C.W.; Malow, B.; Sammaritano, M.R. Epilepsy and Sleep: The Clinical Spectrum.; Elsevier Science: Amsterdam, 2002; ISBN 978-0-444-50479-1. [Google Scholar]
- Chokroverty, S. Physiological Changes of Sleep. Sleep Disorders Medicine: Basic Science, Technical Considerations and Clinical Aspects: Fourth Edition 2017, 153–194. [CrossRef]
- Oshima-Saeki, C.; Taniho, Y.; Arita, H.; Fujimoto, E. Lower-Limb Warming Improves Sleep Quality in Elderly People Living in Nursing Homes. Sleep Science 2017, 10, 87–91. [Google Scholar] [CrossRef]
- Ko, Y.; Lee, J.Y. Effects of Feet Warming Using Bed Socks on Sleep Quality and Thermoregulatory Responses in a Cool Environment. J Physiol Anthropol 2018, 37, 13. [Google Scholar] [CrossRef] [PubMed]
- von Gaudecker, J.R. A Feasibility Randomized Controlled Crossover Trial of Home-Based Warm Footbath to Improve Sleep in the Chronic Phase of Traumatic Brain Injury. Journal of Neuroscience Nursing 2017, 49, 386. [Google Scholar] [CrossRef]
- Harding, E.C.; Yu, X.; Miao, A.; Andrews, N.; Ma, Y.; Ye, Z.; Lignos, L.; Miracca, G.; Ba, W.; Yustos, R.; et al. A Neuronal Hub Binding Sleep Initiation and Body Cooling in Response to a Warm External Stimulus. Current Biology 2018, 28, 2263–2273.e4. [Google Scholar] [CrossRef]
- Calhoun, D.A.; Harding, S.M. Sleep and Hypertension. Chest 2010, 138, 434–443. [Google Scholar] [CrossRef]
- Yilmaz, M.; Kayancicek, H.; Cekici, Y. Heart Rate Variability: Highlights from Hidden Signals. J Integr Cardiol 2018, 4. [Google Scholar] [CrossRef]
- Anghel, L.; Ciubară, A.; Nechita, A.; Nechita, L.; Manole, C.; Baroiu, L.; Ciubară, A.B.; Mușat, C.L. Sleep Disorders Associated with Neurodegenerative Diseases. Diagnostics 2023, 13, 2898. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, R.; Sanford, L.D.; Yang, L.; Zhou, J.; Tan, L.; Li, T.; Zhang, J.; Wing, Y.-K.; Shi, J.; et al. Sleep in Parkinson’s Disease: A Systematic Review and Meta-Analysis of Polysomnographic Findings. Sleep Med Rev 2020, 51, 101281. [Google Scholar] [CrossRef]
- Masi, G.; Amprimo, G.; Priano, L.; Ferraris, C. New Perspectives in Nonintrusive Sleep Monitoring for Neurodegenerative Diseases—A Narrative Review. Electronics (Basel) 2023, 12, 1098. [Google Scholar] [CrossRef]
- Gros, P.; Mery, V.P.; Lafontaine, A.-L.; Robinson, A.; Benedetti, A.; Kimoff, R.J.; Kaminska, M. Diagnosis of Obstructive Sleep Apnea in Parkinson’s Disease Patients: Is Unattended Portable Monitoring a Suitable Tool? Parkinsons Dis 2015, 2015, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.M.S. Sleep Disturbances in Parkinson’s Disease: The Contribution of Dopamine in REM Sleep Regulation. Sleep Med Rev 2013, 17, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ren, R.; Yang, L.; Zhang, H.; Shi, Y.; Vitiello, M. V.; Sanford, L.D.; Tang, X. Patterns of Polysomnography Parameters in 27 Neuropsychiatric Diseases: An Umbrella Review. Psychol Med 2023, 53, 4675–4695. [Google Scholar] [CrossRef]
- Ibrahim, A.; Cesari, M.; Heidbreder, A.; Defrancesco, M.; Brandauer, E.; Seppi, K.; Kiechl, S.; Högl, B.; Stefani, A. Sleep Features and Long-Term Incident Neurodegeneration: A Polysomnographic Study 2023.
- Westwood, A.J.; Beiser, A.; Jain, N.; Himali, J.J.; DeCarli, C.; Auerbach, S.H.; Pase, M.P.; Seshadri, S. Prolonged Sleep Duration as a Marker of Early Neurodegeneration Predicting Incident Dementia. Neurology 2017, 88, 1172–1179. [Google Scholar] [CrossRef]
- Clark, C.E.; Gold, J.; Rigby, B.R. Sleep Duration in Middle-Aged Years of Life Predicts the Age of Diagnosis of Parkinson’s Disease. Sleep Med X 2024, 8, 100123. [Google Scholar] [CrossRef]
- Chen, L.-K. From Sleep Tracking to Early Parkinson’ s Disease Diagnosis: A Promising New Frontier. JMA J 2024, 7, 562–563. [Google Scholar] [CrossRef]
- Yong, M.-H.; Fook-Chong, S.; Pavanni, R.; Lim, L.-L.; Tan, E.-K. Case Control Polysomnographic Studies of Sleep Disorders in Parkinson’s Disease. PLoS One 2011, 6, e22511. [Google Scholar] [CrossRef]
- Bliwise, D.L.; Karroum, E.G.; Greer, S.A.; Factor, S.A.; Trotti, L.M. Restless Legs Symptoms and Periodic Leg Movements in Sleep Among Patients with Parkinson’s Disease. J Parkinsons Dis 2022, 12, 1339–1344. [Google Scholar] [CrossRef]
- Boeve, B.F. REM Sleep Behavior Disorder. Ann N Y Acad Sci 2010, 1184, 15–54. [Google Scholar] [CrossRef]
- Marion, M.H.; Qurashi, M.; Marshall, G.; Foster, O. Is REM Sleep Behaviour Disorder (RBD) a Risk Factor of Dementia in Idiopathic Parkinson’s Disease? J Neurol 2008, 255, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Ferri, R.; Fulda, S.; Cosentino, F.I.I.; Pizza, F.; Plazzi, G. A Preliminary Quantitative Analysis of REM Sleep Chin EMG in Parkinson’s Disease with or without REM Sleep Behavior Disorder. Sleep Med 2012, 13, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Miglis, M.G.; Adler, C.H.; Antelmi, E.; Arnaldi, D.; Baldelli, L.; Boeve, B.F.; Cesari, M.; Dall’Antonia, I.; Diederich, N.J.; Doppler, K.; et al. Biomarkers of Conversion to α-Synucleinopathy in Isolated Rapid-Eye-Movement Sleep Behaviour Disorder. Lancet Neurol 2021, 20, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Modarres, M.H.; Elliott, J.E.; Weymann, K.B.; Pleshakov, D.; Bliwise, D.L.; Lim, M.M. Validation of Visually Identified Muscle Potentials during Human Sleep Using High Frequency/Low Frequency Spectral Power Ratios. Sensors 2021, 22, 55. [Google Scholar] [CrossRef] [PubMed]
- Winer, J.R. The Role of Actigraphy in Detecting and Characterizing the Early Phases of Alzheimer’s Disease. Sleep 2024, 47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ren, R.; Yang, L.; Zhang, H.; Shi, Y.; Okhravi, H.R.; Vitiello, M. V; Sanford, L.D.; Tang, X. Sleep in Alzheimer’s Disease: A Systematic Review and Meta-Analysis of Polysomnographic Findings. Transl Psychiatry 2022, 12, 136. [Google Scholar] [CrossRef]
- Chauhan, A.K.; Mallick, B.N. Association between Autophagy and Rapid Eye Movement Sleep Loss-Associated Neurodegenerative and Patho-Physio-Behavioral Changes. Sleep Med 2019, 63, 29–37. [Google Scholar] [CrossRef]
- Ju, Y.-E.S.; Ooms, S.J.; Sutphen, C.; Macauley, S.L.; Zangrilli, M.A.; Jerome, G.; Fagan, A.M.; Mignot, E.; Zempel, J.M.; Claassen, J.A.H.R.; et al. Slow Wave Sleep Disruption Increases Cerebrospinal Fluid Amyloid-β Levels. Brain 2017, 140, 2104–2111. [Google Scholar] [CrossRef]
- Bhandari, T. Decreased Deep Sleep Linked to Early Signs of Alzheimer’s Disease: Toxic Brain Protein Tau Elevated in Older People Who Sleep Poorly 2019.
- Pulver, R.L.; Kronberg, E.; Medenblik, L.M.; Kheyfets, V.O.; Ramos, A.R.; Holtzman, D.M.; Morris, J.C.; Toedebusch, C.D.; Sillau, S.H.; Bettcher, B.M.; et al. Mapping Sleep’s Oscillatory Events as a Biomarker of Alzheimer’s Disease. Alzheimer’s & Dementia 2024, 20, 301–315. [Google Scholar] [CrossRef]
- Roh, J.H.; Huang, Y.; Bero, A.W.; Kasten, T.; Stewart, F.R.; Bateman, R.J.; Holtzman, D.M. Disruption of the Sleep-Wake Cycle and Diurnal Fluctuation of β-Amyloid in Mice with Alzheimer’s Disease Pathology. Sci Transl Med 2012, 4. [Google Scholar] [CrossRef]
- Costandi, M. Disrupted Sleep May Predict Alzheimer’s. Nature 2012. [Google Scholar] [CrossRef]
- Gaeta, A.M.; Quijada-López, M.; Barbé, F.; Vaca, R.; Pujol, M.; Minguez, O.; Sánchez-de-la-Torre, M.; Muñoz-Barrutia, A.; Piñol-Ripoll, G. Predicting Alzheimer’s Disease CSF Core Biomarkers: A Multimodal Machine Learning Approach. Front Aging Neurosci 2024, 16. [Google Scholar] [CrossRef] [PubMed]
- Tao, P.; Svetnik, V.; Bliwise, D.L.; Zammit, G.; Lines, C.; Herring, W.J. Comparison of Polysomnography in People with Alzheimer’s Disease and Insomnia versus Non-Demented Elderly People with Insomnia. Sleep Med 2023, 101, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Lv, Q.; Xie, W.; Gong, S.; Zhuang, S.; Liu, J.; Mao, C.; Liu, C. Circadian Disruption and Sleep Disorders in Neurodegeneration. Transl Neurodegener 2023, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Arnao, V.; Cinturino, A.; Mastrilli, S.; Buttà, C.; Maida, C.; Tuttolomondo, A.; Aridon, P.; D’Amelio, M. Impaired Circadian Heart Rate Variability in Parkinson’s Disease: A Time-Domain Analysis in Ambulatory Setting. BMC Neurol 2020, 20, 152. [Google Scholar] [CrossRef]
- Devos, D.; Kroumova, M.; Bordet, R.; Vodougnon, H.; Guieu, J.D.; Libersa, C.; Destee, A. Heart Rate Variability and Parkinson’s Disease Severity. J Neural Transm (Vienna) 2003, 110, 997–1011. [Google Scholar] [CrossRef]
- Sattar, P.; Baldazzi, G.; Mandas, N.; Casaglia, E.; Figorilli, M.; Puligheddu, M.; Pani, D. Parkinson and REM Sleep Behaviour Disorder: HRV Difference During Polysomnography. In Proceedings of the Proceedings of the 16th International Joint Conference on Biomedical Engineering Systems and Technologies; SCITEPRESS - Science and Technology Publications, 2023; pp. 366–370.
- Kong, S.D.X.; Hoyos, C.M.; Phillips, C.L.; McKinnon, A.C.; Lin, P.; Duffy, S.L.; Mowszowski, L.; LaMonica, H.M.; Grunstein, R.R.; Naismith, S.L.; et al. Altered Heart Rate Variability during Sleep in Mild Cognitive Impairment. Sleep 2021, 44. [Google Scholar] [CrossRef]
- Kong, S.D.X.; Gordon, C.J.; Hoyos, C.M.; Wassing, R.; D’Rozario, A.; Mowszowski, L.; Ireland, C.; Palmer, J.R.; Grunstein, R.R.; Shine, J.M.; et al. Heart Rate Variability during Slow Wave Sleep Is Linked to Functional Connectivity in the Central Autonomic Network. Brain Commun 2023, 5. [Google Scholar] [CrossRef]
- SleepActa. Dormi | SleepActa. Available online: https://sleepacta.com/en/tecnologia/dormi/ (accessed on 9 December 2024).
- EEG Smart UMindSleep - Professional Wearable Sleep Tracker & Monitor. Available online: http://www.eegsmart.com/en/UMindSleep.html (accessed on 27 December 2024).
- MyAir, Inc. Products — AirgoTM. Available online: https://www.myairgo.com/products (accessed on 9 December 2024).
- Withings. Sleep Analyzer | Withings Europe. Available online: https://www.withings.com/eu/en/sleep-analyzer (accessed on 9 December 2024).
- Rodriguez-Villegas, E.; Chen, G.; Radcliffe, J.; Duncan, J. A Pilot Study of a Wearable Apnoea Detection Device. BMJ Open 2014, 4, e005299. [Google Scholar] [CrossRef]
- Vitalthing, AS. Somnofy | Vitalthings. Available online: https://vitalthings.com/en/products/somnofy/ (accessed on 14 December 2024).
- Honda, S.; Hara, H.; Arie, T.; Akita, S.; Takei, K. A Wearable, Flexible Sensor for Real-Time, Home Monitoring of Sleep Apnea. iScience 2022, 25, 104163. [Google Scholar] [CrossRef]
- SOMNOmedics, AG. SOMNOtouchTM RESP Polygraphy - When Size Matters! Available online: https://somnomedics.de/en/solutions/sleep_diagnostics/polygraphy-devices/somnotouch-resp/ (accessed on 16 December 2024).
- SOMNOmedics, AG. SOMNO HD - Home/Ambulatory PSG - The Future of Sleep Diagnostics. Available online: https://somnomedics.de/en/solutions/sleep_diagnostics/home_ambulatory_psg/somno-hd-home-ambulatory-psg/ (accessed on 16 December 2024).
- Kwon, S.; Kim, H.S.; Kwon, K.; Kim, H.; Kim, Y.S.; Lee, S.H.; Kwon, Y.T.; Jeong, J.W.; Trotti, L.M.; Duarte, A.; et al. At-Home Wireless Sleep Monitoring Patches for the Clinical Assessment of Sleep Quality and Sleep Apnea. Sci Adv 2023, 9. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, M.; Tian, M.; Liu, Q.; Zhou, X.; Liu, H.; Li, R.; Li, Z.; Dong, H.; Jia, L.; et al. Exploring the Potential of a New Wearable Sleep Monitoring Device for Clinical Application. Biomed Signal Process Control 2025, 99, 106856. [Google Scholar] [CrossRef]
- Scardulla, F.; Cosoli, G.; Spinsante, S.; Poli, A.; Iadarola, G.; Pernice, R.; Busacca, A.; Pasta, S.; Scalise, L.; D’Acquisto, L. Photoplethysmograhic Sensors, Potential and Limitations: Is It Time for Regulation? A Comprehensive Review. Measurement 2023, 218, 113150. [Google Scholar] [CrossRef]
- Ghamari, M. A Review on Wearable Photoplethysmography Sensors and Their Potential Future Applications in Health Care. Int J Biosens Bioelectron 2018, 4. [Google Scholar] [CrossRef]
- Ryals, S.; Chiang, A.; Schutte-Rodin, S.; Chandrakantan, A.; Verma, N.; Holfinger, S.; Abbasi-Feinberg, F.; Bandyopadhyay, A.; Baron, K.; Bhargava, S.; et al. Photoplethysmography—New Applications for an Old Technology: A Sleep Technology Review. Journal of Clinical Sleep Medicine 2023, 19, 189–195. [Google Scholar] [CrossRef]
- Přibil, J.; Přibilová, A.; Frollo, I. Comparative Measurement of the PPG Signal on Different Human Body Positions by Sensors Working in Reflection and Transmission Modes. In Proceedings of the 7th International Electronic Conference on Sensors and Applications; MDPI, December 2020; p. 69.
- Adams, T.; Wagner, S.; Baldinger, M.; Zellhuber, I.; Weber, M.; Nass, D.; Surges, R. Accurate Detection of Heart Rate Using In-Ear Photoplethysmography in a Clinical Setting. Front Digit Health 2022, 4. [Google Scholar] [CrossRef]
- Almarshad, M.A.; Islam, M.S.; Al-Ahmadi, S.; BaHammam, A.S. Diagnostic Features and Potential Applications of PPG Signal in Healthcare: A Systematic Review. Healthcare 2022, 10, 547. [Google Scholar] [CrossRef]
- Lazazzera, R.; Laguna, P.; Gil, E.; Carrault, G. Proposal for a Home Sleep Monitoring Platform Employing a Smart Glove. Sensors 2021, 21, 7976. [Google Scholar] [CrossRef]
- Google Inc. Fitbit Sense 2 Smartwatch. Available online: https://store.google.com/gb/product/fitbit_sense_2?hl=en-GB (accessed on 24 December 2024).
- Concheiro-Moscoso, P.; Groba, B.; Alvarez-Estevez, D.; del Carmen Miranda-Duro, M.; Pousada, T.; Nieto-Riveiro, L.; Mejuto-Muiño, F.J.; Pereira, J. Quality of Sleep Data Validation From the Xiaomi Mi Band 5 Against Polysomnography: Comparison Study. J Med Internet Res 2023, 25, e42073. [Google Scholar] [CrossRef]
- Jaworski, D.; Park, E.J. Nonlinear Heart Rate Variability Analysis for Sleep Stage Classification Using Integration of Ballistocardiogram and Apple Watch. Nat Sci Sleep 2024, 16, 1075–1090. [Google Scholar] [CrossRef]
- Xiaomi Inc. Xiaomi Smart Band 9. Available online: https://www.mi.com/global/product/xiaomi-smart-band-9/specs (accessed on 24 December 2024).
- Apple Inc. Apple Watch Series 10 - Technical Specifications. Available online: https://www.apple.com/apple-watch-series-10/specs/#footnote-1 (accessed on 24 December 2024).
- Wellue. Smart Ring Pulse Oximeter: O2Ring. Available online: https://getwellue.com/pages/o2ring-oxygen-monitor?currency=EUR (accessed on 28 December 2024).
- Oura. OuraRing Available online: https://ouraring.com/.
- Ringconn. A Journey to Health, Hidden in a Ring Available online: https://ringconn.com/?sscid=b1k8_16pr1b&.
- Kim, K.B.; Baek, H.J. Photoplethysmography in Wearable Devices: A Comprehensive Review of Technological Advances, Current Challenges, and Future Directions. Electronics (Basel) 2023, 12, 2923. [Google Scholar] [CrossRef]
- Vulcan, R.S.; André, S.; Bruyneel, M. Photoplethysmography in Normal and Pathological Sleep. Sensors (Basel) 2021, 21. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, L.; Violante, M.; Groppo, S. A Novel Algorithm for Detecting the Drowsiness Onset in Real-Time. IEEE Access 2022, 10, 42601–42606. [Google Scholar] [CrossRef]
- Romem, A.; Romem, A.; Koldobskiy, D.; Scharf, S.M. Diagnosis of Obstructive Sleep Apnea Using Pulse Oximeter Derived Photoplethysmographic Signals. Journal of Clinical Sleep Medicine 2014, 10, 285–290. [Google Scholar] [CrossRef]
- Li, Y.; Gao, H.; Ma, Y. Evaluation of Pulse Oximeter Derived Photoplethysmographic Signals for Obstructive Sleep Apnea Diagnosis. Medicine 2017, 96, e6755. [Google Scholar] [CrossRef]
- Altini, M.; Kinnunen, H. The Promise of Sleep: A Multi-Sensor Approach for Accurate Sleep Stage Detection Using the Oura Ring. Sensors 2021, 21, 4302. [Google Scholar] [CrossRef]
- Rosenberg, R.S.; Hout, S. Van The American Academy of Sleep Medicine Inter-Scorer Reliability Program: Sleep Stage Scoring. Journal of Clinical Sleep Medicine 2013, 09, 81–87. [Google Scholar] [CrossRef]
- Sridhar, N.; Shoeb, A.; Stephens, P.; Kharbouch, A.; Shimol, D. Ben; Burkart, J.; Ghoreyshi, A.; Myers, L. Deep Learning for Automated Sleep Staging Using Instantaneous Heart Rate. NPJ Digit Med 2020, 3, 106. [Google Scholar] [CrossRef]
- Tamura, T.; Maeda, Y.; Sekine, M.; Yoshida, M. Wearable Photoplethysmographic Sensors—Past and Present. Electronics (Basel) 2014, 3, 282–302. [Google Scholar] [CrossRef]
- Robbins, R.; Weaver, M.D.; Sullivan, J.P.; Quan, S.F.; Gilmore, K.; Shaw, S.; Benz, A.; Qadri, S.; Barger, L.K.; Czeisler, C.A.; et al. Accuracy of Three Commercial Wearable Devices for Sleep Tracking in Healthy Adults. Sensors 2024, 24, 6532. [Google Scholar] [CrossRef]
- Lolli, L. A Comment on “Validity and Reliability of the Oura Ring Generation 3 (Gen3) with Oura Sleep Staging Algorithm 2.0 (OSSA 2.0) When Compared to Multi-Night Ambulatory Polysomnography: A Validation Study of 96 Participants and 421,045 Epochs. Sleep Med 2024, 117, 216. [CrossRef] [PubMed]
- Tisyakorn, J.; Saiphoklang, N.; Sapankaew, T.; Thapa, K.; Anutariya, C.; Sujarae, A.; Tepwimonpetkun, C. Screening Moderate to Severe Obstructive Sleep Apnea with Wearable Device. Sleep and Breathing 2025, 29, 61. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.J.; Sargent, C.; Roach, G.D. A Validation of Six Wearable Devices for Estimating Sleep, Heart Rate and Heart Rate Variability in Healthy Adults. Sensors 2022, 22, 6317. [Google Scholar] [CrossRef] [PubMed]
- Capodilupo, J. Former Chief Technology Officer on Improved Accuracy & Design with WHOOP 4.0 Available online: https://www.whoop.com/us/en/thelocker/chief-technology-officer-whoop-4-0-accuracy.
- Park, J.-E.; Ahn, E.K.; Yoon, K.; Kim, J. Performance of Fitbit Devices as Tools for Assessing Sleep Patterns and Associated Factors. Journal of Sleep Medicine 2024, 21, 59–64. [Google Scholar] [CrossRef]
- Venema, B.; Schiefer, J.; Blazek, V.; Blanik, N.; Leonhardt, S. Evaluating Innovative In-Ear Pulse Oximetry for Unobtrusive Cardiovascular and Pulmonary Monitoring During Sleep. IEEE J Transl Eng Health Med 2013, 1, 2700208. [Google Scholar] [CrossRef]
- Manoni, A.; Loreti, F.; Radicioni, V.; Pellegrino, D.; Torre, L. Della; Gumiero, A.; Halicki, D.; Palange, P.; Irrera, F. A New Wearable System for Home Sleep Apnea Testing, Screening, and Classification. Sensors 2020, 20, 7014. [Google Scholar] [CrossRef]
- Perez-Pozuelo, I.; Posa, M.; Spathis, D.; Westgate, K.; Wareham, N.; Mascolo, C.; Brage, S.; Palotti, J. Detecting Sleep Outside the Clinic Using Wearable Heart Rate Devices. Scientific Reports 2022, 12, 1–13. [Google Scholar] [CrossRef]
- Imtiaz, S.A. A Systematic Review of Sensing Technologies for Wearable Sleep Staging. Sensors 2021, 21, 1562. [Google Scholar] [CrossRef]
- Nam, Y.; Kim, Y.; Lee, J. Sleep Monitoring Based on a Tri-Axial Accelerometer and a Pressure Sensor. Sensors 2016, 16, 750. [Google Scholar] [CrossRef]
- Nevsimalova, S.; Sonka, K. Poruchy spánku a bdění; 3rd ed.; Zdravotnické nakladatelství GALÉN, 2020; ISBN 9788074924781.
- CamNtech Ltd. PLMS Software - Actigraphy for Periodic Limb Movements in Sleep. Available online: https://www.camntech.com/plms-sw/ (accessed on 9 December 2024).
- Neurocare Group ActTrust Actigraph - Sleep Hygiene Assessment Tool for Patient Care. Available online: https://www.neurocaregroup.com/technology/actigraphy-devices (accessed on 9 December 2024).
- Condor Instruments. Melanopic EDI Logger | Melanopic Light Sensor - Condor Instruments. Available online: https://condorinst.com/en/actlumus-actigraph/ (accessed on 16 December 2024).
- ActiGraph, LLC. WGT3X-BT - ActiGraph Wearable Devices. Available online: https://theactigraph.com/actigraph-wgt3x-bt (accessed on 9 December 2024).
- Hayano, J.; Adachi, M.; Sasaki, F.; Yuda, E. Quantitative Detection of Sleep Apnea in Adults Using Inertial Measurement Unit Embedded in Wristwatch Wearable Devices. Scientific Reports 2024, 14, 1–9. [Google Scholar] [CrossRef]
- Benedetti, D.; Olcese, U.; Bruno, S.; Barsotti, M.; Tassoni, M.M.; Bonanni, E.; Siciliano, G.; Faraguna, U. Obstructive Sleep Apnoea Syndrome Screening Through Wrist-Worn Smartbands: A Machine-Learning Approach. Nat Sci Sleep 2022, 14, 941–956. [Google Scholar] [CrossRef] [PubMed]
- ActiGraph, LLC. ActiGraph LEAP - The Next Generation Multisensor Wearable for Patient-Centered Clinical Research. Available online: https://theactigraph.com/actigraph-leap (accessed on 9 December 2024).
- Somno Art. Somno-Art: Advanced Sleep Measurement Wearable Device. Available online: https://www.somno-art.com/ (accessed on 16 December 2024).
- Thiesse, L.; Staner, L.; Bourgin, P.; Roth, T.; Fuchs, G.; Kirscher, D.; Schaffhauser, J.Y.; Saoud, J.B.; Viola, A.U. Validation of Somno-Art Software, a Novel Approach of Sleep Staging, Compared with Polysomnography in Disturbed Sleep Profiles. SLEEP Advances 2022, 3. [Google Scholar] [CrossRef] [PubMed]
- Patterson, M.R.; Nunes, A.A.S.; Gerstel, D.; Pilkar, R.; Guthrie, T.; Neishabouri, A.; Guo, C.C. 40 Years of Actigraphy in Sleep Medicine and Current State of the Art Algorithms. npj Digital Medicine 2023 6:1 2023, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, P.; Ross, M.; Cerny, A.; Anderer, P.; van Meulen, F.; Janssen, H.; Pijpers, A.; Dujardin, S.; van Hirtum, P.; van Gilst, M.; et al. A Computationally Efficient Algorithm for Wearable Sleep Staging in Clinical Populations. Scientific Reports 2023, 13, 1–14. [Google Scholar] [CrossRef]
- InteraXon Inc. Muse S | MuseTM EEG-Powered Meditation & Sleep Headband. Available online: https://eu.choosemuse.com/products/muse-s-gen-2 (accessed on 9 December 2024).
- Beacon Biosignals, Inc. The Dreem Headband | Beacon Biosignals. Available online: https://beacon.bio/dreem-headband/ (accessed on 9 December 2024).
- American Academe of Sleep Medicine FDA Clears AI-Assisted Sleep Monitoring Device Dreem 3S. Available online: https://aasm.org/fda-clears-ai-assisted-sleep-monitoring-device-dreem-3s/ (accessed on 9 December 2024).
- Chen, X.; Jin, X.; Zhang, J.; Ho, K.W.; Wei, Y.; Cheng, H. Validation of a Wearable Forehead Sleep Recorder against Polysomnography in Sleep Staging and Desaturation Events in a Clinical Sample. Journal of Clinical Sleep Medicine 2023, 19, 711–718. [Google Scholar] [CrossRef]
- Nakamura, T.; Alqurashi, Y.D.; Morrell, M.J.; Mandic, D.P. Hearables: Automatic Overnight Sleep Monitoring with Standardized In-Ear EEG Sensor. IEEE Trans Biomed Eng 2020, 67, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Myllymaa, S.; Muraja-Murro, A.; Westeren-Punnonen, S.; Hukkanen, T.; Lappalainen, R.; Mervaala, E.; Töyräs, J.; Sipilä, K.; Myllymaa, K. Assessment of the Suitability of Using a Forehead EEG Electrode Set and Chin EMG Electrodes for Sleep Staging in Polysomnography. J Sleep Res 2016, 25, 636–645. [Google Scholar] [CrossRef]
- Chouraki, A.; Tournant, J.; Arnal, P.; Pépin, J.L.; Bailly, S. Objective Multi-Night Sleep Monitoring at Home: Variability of Sleep Parameters between Nights and Implications for the Reliability of Sleep Assessment in Clinical Trials. Sleep 2023, 46. [Google Scholar] [CrossRef]
- Braghiroli, A.; Kuller, D.; Godio, M.; Rossato, F.; Sacco, C.; Morrone, E. Validation Study of Airgo, an Innovative Device to Screen Sleep Respiratory Disorders. Front Med (Lausanne) 2022, 9, 938542. [Google Scholar] [CrossRef]
- Xu, L.; Han, F.; Keenan, B.T.; Kneeland-Szanto, E.; Yan, H.; Dong, X.; Chang, Y.; Zhao, L.; Zhang, X.; Lixs, J.; et al. Validation of the Nox-T3 Portable Monitor for Diagnosis of Obstructive Sleep Apnea in Chinese Adults. Journal of Clinical Sleep Medicine 2017, 13, 675–683. [Google Scholar] [CrossRef]
- Wu, D.; Wang, L.; Zhang, Y.T.; Huang, B.Y.; Wang, B.; Lin, S.J.; Xu, X.W. A Wearable Respiration Monitoring System Based on Digital Respiratory Inductive Plethysmography. Proceedings of the 31st Annual International Conference of the IEEE Engineering in Medicine and Biology Society: Engineering the Future of Biomedicine, EMBC 2009 2009, 4844–4847. [CrossRef]
- Hernandez, J.E.; Cretu, E. A Wireless, Real-Time Respiratory Effort and Body Position Monitoring System for Sleep. Biomed Signal Process Control 2020, 61, 102023. [Google Scholar] [CrossRef]
- Kristiansen, S.; Nikolaidis, K.; Plagemann, T.; Goebel, V.; Traaen, G.M.; Øverland, B.; Akerøy, L.; Hunt, T.E.; Loennechen, J.P.; Steinshamn, S.L.; et al. A Clinical Evaluation of a Low-Cost Strain Gauge Respiration Belt and Machine Learning to Detect Sleep Apnea. Smart Health 2023, 27, 100373. [Google Scholar] [CrossRef]
- Selvaraj, N. Screening of Sleep Architecture Using a Disposable Patch Sensor. 2017 IEEE EMBS International Conference on Biomedical and Health Informatics, BHI 2017 2017, 145–148. [CrossRef]
- Zavanelli, N.; Kim, H.; Kim, J.; Herbert, R.; Mahmood, M.; Kim, Y.S.; Kwon, S.; Bolus, N.B.; Torstrick, F.B.; Lee, C.S.D.; et al. At-Home Wireless Monitoring of Acute Hemodynamic Disturbances to Detect Sleep Apnea and Sleep Stages via a Soft Sternal Patch. Sci Adv 2021, 7. [Google Scholar] [CrossRef] [PubMed]
- Texas Instruments. ADS129x Low-Power, 8-Channel, 24-Bit Analog Front-End for Biopotential Measurements Datasheet (Rev. K) | Enhanced Reader (accessed on 16 December 2024).
- Texas Instruments. AFE4960 Data Sheet, Product Information and Support | TI.Com. Available online: https://www.ti.com/product/AFE4960 (accessed on 16 December 2024).
- Texas Instruments. AFE4500 Analog Front End (AFE) - TI | Mouser. Available online: https://www.mouser.sk/new/texas-instruments/ti-afe4500-afe/ (accessed on 16 December 2024).
- Analog DevicesAnalog Devices. ADAS1000 Datasheet and Product Info | Analog Devices. Available online: https://www.analog.com/en/products/adas1000.html#product-overview (accessed on 16 December 2024).
- Analog Devices. MAX30001 Datasheet and Product Info | Analog Devices. Available online: https://www.analog.com/en/products/max30001.html#product-overview (accessed on 16 December 2024).
- van Steenkiste, T.; Groenendaal, W.; Dreesen, P.; Lee, S.; Klerkx, S.; De Francisco, R.; Deschrijver, D.; Dhaene, T. Portable Detection of Apnea and Hypopnea Events Using Bio-Impedance of the Chest and Deep Learning. IEEE J Biomed Health Inform 2020, 24, 2589–2598. [Google Scholar] [CrossRef]
- Gambi, E.; Senigagliesi, L.; Creato, E.; Ricciuti, M. “In Bed” BCG Signal Analysis. Lecture Notes in Electrical Engineering 2021, 725, 27–38. [Google Scholar] [CrossRef]
- Emfit Ltd. Heart Rate Variability (HRV) during Sleep - Emfit Ltd. Available online: https://emfit.com/heart-rate-variability-hrv-during-sleep/ (accessed on 9 December 2024).
- Mack, D.C.; Patrie, J.T.; Suratt, P.M.; Felder, R.A.; Alwan, M. Development and Preliminary Validation of Heart Rate and Breathing Rate Detection Using a Passive, Ballistocardiography-Based Sleep Monitoring System. IEEE Transactions on Information Technology in Biomedicine 2009, 13, 111–120. [Google Scholar] [CrossRef]
- Zhao, W.; Ni, H.; Zhou, X.; Song, Y.; Wang, T. Identifying Sleep Apnea Syndrome Using Heart Rate and Breathing Effort Variation Analysis Based on Ballistocardiography. Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS 2015, 2015-November, 4536–4539. [CrossRef]
- Yi, R.; Enayati, M.; Keller, J.M.; Popescu, M.; Skubic, M. Non-Invasive in-Home Sleep Stage Classification Using a Ballistocardiography Bed Sensor. 2019 IEEE EMBS International Conference on Biomedical and Health Informatics, BHI 2019 - Proceedings 2019. [CrossRef]
- Silva, B.; Marinheiro, R.N. Non-Invasive Monitoring with Ballistocardiographic Sensors for Sleep Management. 2021 Telecoms Conference, ConfTELE 2021 2021. [CrossRef]
- Alivar, A.; Carlson, C.; Suliman, A.; Warren, S.; Prakash, P.; Thompson, D.; Natarajan, B. Motion Detection in Bed-Based Ballistocardiogram to Quantify Sleep Quality. Proceedings - IEEE Global Communications Conference, GLOBECOM 2017, 2018-January, 1–6. [CrossRef]
- Liu, F.; Zhou, X.; Wang, Z.; Wang, T.; Ni, H.; Yang, J. Identifying Obstructive Sleep Apnea by Exploiting Fine-Grained BCG Features Based on Event Phase Segmentation. Proceedings - 2016 IEEE 16th International Conference on Bioinformatics and Bioengineering, BIBE 2016 2016, 293–300. [CrossRef]
- Li, Y.X.; Huang, J.L.; Yao, X.Y.; Mu, S.Q.; Zong, S.X.; Shen, Y.F. A Ballistocardiogram Dataset with Reference Sensor Signals in Long-Term Natural Sleep Environments. Scientific Data 2024, 11, 1–9. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, X.; Zhao, W.; Liu, F.; Ni, H.; Yu, Z. Assessing the Severity of Sleep Apnea Syndrome Based on Ballistocardiogram. PLoS One 2017, 12, e0175351. [Google Scholar] [CrossRef]
- Nurmi, S.; Saaresranta, T.; Koivisto, T.; Meriheinä, U.; Palva, L. Validation of an Accelerometer Based BCG Method for Sleep Analysis. Aalto University publication series SCIENCE + TECHNOLOGY 2016, 12.
- Hwang, S.H.; Lee, H.J.; Yoon, H.N.; Jung, D.W.; Lee, Y.J.G.; Lee, Y.J.; Jeong, D.U.; Park, K.S. Unconstrained Sleep Apnea Monitoring Using Polyvinylidene Fluoride Film-Based Sensor. IEEE Trans Biomed Eng 2014, 61, 2125–2134. [Google Scholar] [CrossRef]
- He, C.; Tan, J.; Jian, X.; Zhong, G.; Cheng, L.; Lin, J. A Smart Flexible Vital Signs and Sleep Monitoring Belt Based on MEMS Triaxial Accelerometer and Pressure Sensor. IEEE Internet Things J 2022, 9, 14126–14136. [Google Scholar] [CrossRef]
- Jaworski, D.J.; Roshan, Y.M.; Tae, C.G.; Park, E.J. Detection of Sleep and Wake States Based on the Combined Use of Actigraphy and Ballistocardiography. Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS 2019, 6701–6704. [CrossRef]
- Balali, P.; Rabineau, J.; Hossein, A.; Tordeur, C.; Debeir, O.; van de Borne, P. Investigating Cardiorespiratory Interaction Using Ballistocardiography and Seismocardiography—A Narrative Review. Sensors 2022, 22, 9565. [Google Scholar] [CrossRef] [PubMed]
- Sadek, I.; Biswas, J.; Abdulrazak, B. Ballistocardiogram Signal Processing: A Review. Health Inf Sci Syst 2019, 7, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Romero, H.E.; Ma, N.; Brown, G.J.; Hill, E.A. Acoustic Screening for Obstructive Sleep Apnea in Home Environments Based on Deep Neural Networks. IEEE J Biomed Health Inform 2022, 26, 2941–2950. [Google Scholar] [CrossRef]
- Markandeya, M.N.; Abeyratne, U.R.; Hukins, C. Overnight Airway Obstruction Severity Prediction Centered on Acoustic Properties of Smart Phone: Validation with Esophageal Pressure. Physiol Meas 2020, 41. [Google Scholar] [CrossRef] [PubMed]
- Nakano, H.; Hirayama, K.; Sadamitsu, Y.; Toshimitsu, A.; Fujita, H.; Shin, S.; Tanigawa, T. Monitoring Sound To Quantify Snoring and Sleep Apnea Severity Using a Smartphone: Proof of Concept. Journal of Clinical Sleep Medicine 2014, 10, 73–78. [Google Scholar] [CrossRef]
- Penzel, T.; Sabil, A.K. The Use of Tracheal Sounds for the Diagnosis of Sleep Apnoea. Breathe (Sheff) 2017, 13, e37–e45. [Google Scholar] [CrossRef]
- Sanchez Gomez, J.; Pramono, R.X.A.; Imtiaz, S.A.; Rodriguez-Villegas, E.; Valido Morales, A. Validation of a Wearable Medical Device for Automatic Diagnosis of OSA against Standard PSG. J Clin Med 2024, 13, 571. [Google Scholar] [CrossRef]
- Fang, Y.; Liu, D.; Jiang, Z.; Wang, H. Monitoring of Sleep Breathing States Based on Audio Sensor Utilizing Mel-Scale Features in Home Healthcare. J Healthc Eng 2023, 2023, 6197564. [Google Scholar] [CrossRef]
- Werthammer, J.; Krasner, J.; DiBenedetto, J.; Stark, A.R. Apnea Monitoring by Acoustic Detection of Airflow. Pediatrics 1983, 71, 53–55. [Google Scholar] [CrossRef]
- Resuli, N.; Skubic, M.; Myungki, J. Noninvasive Respiration Monitoring of Different Sleeping Postures Using an RF Sensor. Proceedings - 2021 IEEE International Conference on Bioinformatics and Biomedicine, BIBM 2021 2021, 1485–1490. [CrossRef]
- Turppa, E.; Kortelainen, J.M.; Antropov, O.; Kiuru, T. Vital Sign Monitoring Using FMCW Radar in Various Sleeping Scenarios. Sensors 2020, 20, 6505. [Google Scholar] [CrossRef]
- Toften, S.; Kjellstadli, J.T.; Thu, O.K.F.; Ellingsen, O.-J. Noncontact Longitudinal Respiratory Rate Measurements in Healthy Adults Using Radar-Based Sleep Monitor (Somnofy): Validation Study. JMIR Biomed Eng 2022, 7, e36618. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Wen, L.; Li, Y.; Lu, J.; Zhang, Z.; Yuan, C.; Gu, C. Remote Respiratory Variables Tracking with Biomedical Radar-Based IoT System during Sleep. IEEE Internet Things J 2024, 11, 19937–19948. [Google Scholar] [CrossRef]
- SlepScore Labs. Sleep Tracker: How The SleepScore Max Works | SleepScore. Available online: https://www.sleepscore.com/blog/about-sleepscore-max-technology/ (accessed on 9 December 2024).
- Schade, M.M.; Bauer, C.E.; Murray, B.R.; Gahan, L.; Doheny, E.P.; Kilroy, H.; Zaffaroni, A.; Montgomery-Downs, H.E. Sleep Validity of a Non-Contact Bedside Movement and Respiration-Sensing Device. Journal of Clinical Sleep Medicine 2019, 15, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Wang, Y.; Niu, K.; Zeng, Y.; Gu, T.; Wang, L.; Guan, C.; Zhang, D. WiFi-Sleep: Sleep Stage Monitoring Using Commodity Wi-Fi Devices. IEEE Internet Things J 2021, 8, 13900–13913. [Google Scholar] [CrossRef]
- Walid, B.; Ma, J.; Ma, M.; Qi, A.; Luo, Y.; Qi, Y. Recent Advances in Radar-Based Sleep Monitoring — A Review. In Proceedings of the 2021 IEEE Intl Conf on Dependable, Autonomic and Secure Computing, Intl Conf on Pervasive Intelligence and Computing, Intl Conf on Cloud and Big Data Computing, Intl Conf on Cyber Science and Technology Congress (DASC/PiCom/CBDCom/CyberSciTech); IEEE, October 2021; pp. 759–766.
- Tran, V.P.; Al-Jumaily, A.A.; Islam, S.M.S. Doppler Radar-Based Non-Contact Health Monitoring for Obstructive Sleep Apnea Diagnosis: A Comprehensive Review. Big Data and Cognitive Computing 2019, 3, 3. [Google Scholar] [CrossRef]
- Boiko, A.; Martínez Madrid, N.; Seepold, R. Contactless Technologies, Sensors, and Systems for Cardiac and Respiratory Measurement during Sleep: A Systematic Review. Sensors 2023, 23, 5038. [Google Scholar] [CrossRef]
- Vitazkova, D.; Foltan, E.; Kosnacova, H.; Micjan, M.; Donoval, M.; Kuzma, A.; Kopani, M.; Vavrinsky, E. Advances in Respiratory Monitoring: A Comprehensive Review of Wearable and Remote Technologies. Biosensors (Basel) 2024, 14, 90. [Google Scholar] [CrossRef]
- Dinh, T.; Nguyen, T.; Phan, H.-P.; Nguyen, N.-T.; Dao, D.V.; Bell, J. Stretchable Respiration Sensors: Advanced Designs and Multifunctional Platforms for Wearable Physiological Monitoring. Biosens Bioelectron 2020, 166, 112460. [Google Scholar] [CrossRef]
- Hussain, T.; Ullah, S.; Fernández-García, R.; Gil, I. Wearable Sensors for Respiration Monitoring: A Review. Sensors 2023, 23, 7518. [Google Scholar] [CrossRef]
- Emam, S.; Nasrollahpour, M.; Colarusso, B.; Cai, X.; Grant, S.; Kulkarni, P.; Ekenseair, A.; Gharagouzloo, C.; Ferris, C.F.; Sun, N.-X. Detection of Presymptomatic Alzheimer’s Disease through Breath Biomarkers. Alzheimers Dement (Amst) 2020, 12, e12088. [Google Scholar]
- Tisch, U.; Schlesinger, I.; Ionescu, R.; Nassar, M.; Axelrod, N.; Robertman, D.; Tessler, Y.; Azar, F.; Marmur, A.; Aharon-Peretz, J.; et al. Detection of Alzheimer‘S and Parkinson‘S Disease From Exhaled Breath Using Nanomaterial-Based Sensors. Nanomedicine 2013, 8, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Xiu, K.; Wang, Z.; Hu, N.; Zhao, L.; Zhu, H.; Kong, F.; Xiao, J.; Cheng, L.; Bi, X. Multifunctional Wearable Humidity and Pressure Sensors Based on Biocompatible Graphene/Bacterial Cellulose Bioaerogel for Wireless Monitoring and Early Warning of Sleep Apnea Syndrome. Nano Energy 2023, 108, 108215. [Google Scholar] [CrossRef]
- Yang, W.; Li, N.; Zhao, S.; Yuan, Z.; Wang, J.; Du, X.; Wang, B.; Cao, R.; Li, X.; Xu, W.; et al. A Breathable and Screen-Printed Pressure Sensor Based on Nanofiber Membranes for Electronic Skins. Adv Mater Technol 2018, 3. [Google Scholar] [CrossRef]
- Kano, S.; Jarulertwathana, N.; Mohd-Noor, S.; Hyun, J.K.; Asahara, R.; Mekaru, H. Respiratory Monitoring by Ultrafast Humidity Sensors with Nanomaterials: A Review. Sensors 2022, 22, 1251. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Meng, J.; Zhao, X.; Zhang, W.; Fan, Y.; Shi, B.; Li, Z. A Wearable Self-Powered Multi-Parameter Respiration Sensor. Adv Mater Technol 2023, 8. [Google Scholar] [CrossRef]
- Zheng, H.; Zi, Y.; He, X.; Guo, H.; Lai, Y.-C.; Wang, J.; Zhang, S.L.; Wu, C.; Cheng, G.; Wang, Z.L. Concurrent Harvesting of Ambient Energy by Hybrid Nanogenerators for Wearable Self-Powered Systems and Active Remote Sensing. ACS Appl Mater Interfaces 2018, 10, 14708–14715. [Google Scholar] [CrossRef]
- Shin, J.; Jeong, B.; Kim, J.; Nam, V.B.; Yoon, Y.; Jung, J.; Hong, S.; Lee, H.; Eom, H.; Yeo, J.; et al. Sensitive Wearable Temperature Sensor with Seamless Monolithic Integration. Advanced Materials 2020, 32. [Google Scholar] [CrossRef]
- Kim, D.; Bae, J.; Lee, J.; Ahn, J.; Hwang, W.; Ko, J.; Kim, I. Porous Nanofiber Membrane: Rational Platform for Highly Sensitive Thermochromic Sensor. Adv Funct Mater 2022, 32. [Google Scholar] [CrossRef]
- Xue, H.; Yang, Q.; Wang, D.; Luo, W.; Wang, W.; Lin, M.; Liang, D.; Luo, Q. A Wearable Pyroelectric Nanogenerator and Self-Powered Breathing Sensor. Nano Energy 2017, 38, 147–154. [Google Scholar] [CrossRef]
- Roy, K.; Ghosh, S.K.; Sultana, A.; Garain, S.; Xie, M.; Bowen, C.R.; Henkel, K.; Schmeiβer, D.; Mandal, D. A Self-Powered Wearable Pressure Sensor and Pyroelectric Breathing Sensor Based on GO Interfaced PVDF Nanofibers. ACS Appl Nano Mater 2019, 2, 2013–2025. [Google Scholar] [CrossRef]
- Basra, A.; Mukhopadhayay, B.; Kar, S. Temperature Sensor Based Ultra Low Cost Respiration Monitoring System. In Proceedings of the 2017 9th International Conference on Communication Systems and Networks (COMSNETS); IEEE, December 2017; pp. 530–535.
- Cao, L.; Zhang, Z.; Li, J.; Wang, Z.; Ren, Y.; Wang, Q.; Huang, D.; Li, Z. A Low-Cost Flexible Perforated Respiratory Sensor Based on Platinum for Continuous Respiratory Monitoring. Micromachines (Basel) 2022, 13, 1743. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Zhao, Y.; Luo, N.; Chen, D.; Chen, M. Flexible Pressure and Temperature Dual-Mode Sensor Based on Buckling Carbon Nanofibers for Respiration Pattern Recognition. Sci Rep 2022, 12, 17434. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Lee, J.; Park, M.K.; Ko, S.H. Recent Developments in Wearable Breath Sensors for Healthcare Monitoring. Commun Mater 2024, 5, 41. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, S.; Zou, P.; Li, R.; Fan, Y. Paper-Based Humidity Sensor for Respiratory Monitoring. Materials 2022, 15, 6447. [Google Scholar] [CrossRef]
- iNDIEGOGO. NiteAura: Sleep Breath Care Mask Available online: https://www.indiegogo.com/projects/niteaura-sleep-breath-care-mask#/.
- Cleveland Medical Devices Inc. CMS & AASM Definitions for Type I, Type II, Type III Sleep Testing - CleveMed. Available online: https://clevemed.com/cms-aasm-guidelines-for-sleep-monitors-type-i-type-ii-type-iii/ (accessed on 16 December 2024).
- Lateef, A.; Q. Al-Neami, A.; T. Al-Aridhi, D. Review of Wireless Polysomnography System. University of Thi-Qar Journal for Engineering Sciences 2023, 13, 66–75. [Google Scholar] [CrossRef]
- Escourrou, P.; Grote, L.; Penzel, T.; Mcnicholas, W.T.; Verbraecken, J.; Tkacova, R.; Riha, R.L.; Hedner, J. The Diagnostic Method Has a Strong Influence on Classification of Obstructive Sleep Apnea. J Sleep Res 2015, 24, 730–738. [Google Scholar] [CrossRef]
- Riha, R.L. Diagnostic Approaches to Respiratory Sleep Disorders. J Thorac Dis 2015, 7, 1373–1384. [Google Scholar] [CrossRef]
- Riha, R.L.; Celmina, M.; Cooper, B.; Hamutcu-Ersu, R.; Kaditis, A.; Morley, A.; Pataka, A.; Penzel, T.; Roberti, L.; Ruehland, W.; et al. ERS Technical Standards for Using Type III Devices (Limited Channel Studies) in the Diagnosis of Sleep Disordered Breathing in Adults and Children. European Respiratory Journal 2023, 61. [Google Scholar] [CrossRef]
- Löwenstein Medical. Samoa - Polygraphy of the Future. Available online: https://loewensteinmedical.com/en/sleep-diagnostics/samoa/ (accessed on 16 December 2024).
- Vitazkova, D.; Vysehradsky, R.; Bellová, V.; Bullo, P.; Gavliakova, S. Telemedicína v Nastavení Neinvazívnej Pretlakovej Ventilácie. Studia pneumologica et phthiseologica 2023, 1, 21–28. [Google Scholar]
- Koninklijke Philips, N.V. Alice NightOne Home Sleep Testing System. Available online: https://www.airohealthcare.com/wp-content/uploads/2019/07/Philips_Alice_NightOne_Data_Sheet.pdf (accessed on 16 December 2024).
- ResMed. ResMed Product Support | Find Support for ApneaLinkTM Air. Available online: https://support.resmed.com/en-ap/diagnostics-and-titration/apnealink-air (accessed on 16 December 2024).
- Cadwell. Home Sleep Testing (HSAT) Devices | Cadwell ApneaTrak. Available online: https://www.cadwell.com/apneatrak/ (accessed on 16 December 2024).
- SOMNOmedics, AG. SOMNOtouchTM RESP Eco - Sleep Screening. Available online: https://somnomedics.de/en/solutions/sleep_diagnostics/sleep-screening/ (accessed on 23 December 2024).
- Küchler, G.; Fietze, I.; Partinen, M.; Jennum, P.J.; Hein, H.; Warmuth, R. Diagnostic Utility of a Nasal/Oral Cannula with Linearized Pressure Flow in Comparison to AASM Recommended Combination of Thermal and Nasal Pressure Sensor. 2017, PA2297. [CrossRef]
- Gehring, J.; Gesche, H.; Drewniok, G.; Küchler, G.; Patzak, A. Nocturnal Blood Pressure Fluctuations Measured by Using Pulse Transit Time in Patients with Severe Obstructive Sleep Apnea Syndrome. Sleep and Breathing 2018, 22, 337–343. [Google Scholar] [CrossRef]
- NOX Medical. Nox T3 - Nox Medical. Available online: https://noxmedical.com/support/nox-academy/nox-t3/ (accessed on 9 December 2024).
- Stowood Embletta MPR Polygraphy | Stowood. Available online: https://stowood.com/product/embla-embletta-mpr-pg/ (accessed on 16 December 2024).
- Birrer, V.; Elgendi, M.; Lambercy, O.; Menon, C. Evaluating Reliability in Wearable Devices for Sleep Staging. npj Digital Medicine 2024, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Emerging Technology. Cut the Cord | Sleep Review. Available online: https://sleepreviewmag.com/sleep-treatments/therapy-devices/emerging-technology/cut-the-cord/ (accessed on 16 December 2024).
- Neurosoft. Attended Polysomnography with Portable Sleep Monitor Neurosoft. Available online: https://neurosoft.com/en/catalog/psg/neuron-spectrum-am-psg_1#overview (accessed on 16 December 2024).
- NOX Medical. One Company. All Sleep Diagnostics. Available online: https://webinars.noxmedical.com/all_sleep_diagnostics_noxa1s?utm_campaign=Purchasing%20Guideline&utm_source=JCSM%20-%20AASM&utm_medium=Leaderboard%20Ad&utm_term=A1s (accessed on 16 December 2024).
- Yoon, D.W.; Hong, I.H.; Baik, I.; Shin, H.W. Evaluation of the Feasibility and Preference of Nox-A1 Type 2 Ambulatory Device for Unattended Home Sleep Test: A Randomized Crossover Study. Sleep Biol Rhythms 2019, 17, 297–304. [Google Scholar] [CrossRef]
- Kwon, S.; Kim, H.; Yeo, W.H. Recent Advances in Wearable Sensors and Portable Electronics for Sleep Monitoring. iScience 2021, 24. [Google Scholar] [CrossRef] [PubMed]
- Campbell, A.J.; Neill, A.M. Home Set-up Polysomnography in the Assessment of Suspected Obstructive Sleep Apnea. J Sleep Res 2011, 20, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Mykijtijn, I.J.; Sajkov, D.; Neill, A.M.; Douglas McEvoy, R. Portable Computerized Polysomnography in Attended and Unattended Settings. Chest 1999, 115, 114–122. [Google Scholar] [CrossRef]
- Onera. Sleep Diagnostics and Monitoring, Redefined. The First Self-Applied, No-Wire Polysomnography Solution. Available online: https://www.onerahealth.com/ (accessed on 16 December 2024).
- de Vries, J.; Rossi, A.; De Francisco, R.; Adnane, S.; Schneider, H.; Andries, D. 402 A Wireless Patch-Based Polysomnography System for Sleep Studies: Effect of the 2016 AASM Rules on AHI in Normal Individuals. Sleep 2021, 44, A160–A160. [Google Scholar] [CrossRef]
- Raschellà, F.; Knoops-Borm, M.; Sekeri, M.; Andries, D.; Oloo, M.; Moudab, I.; Musaka, S.; Mueller, S.; Stockhoff, M.; Tijssen, M.; et al. Clinical Validation of a Wireless Patch-Based Polysomnography System: A Pilot Study. medRxiv 2022, 2022.08.04.22278354. [CrossRef]
- Compumedics. Somfit - A Breakthrough for Home Sleep Testing. Available online: www.compumedics.com.au/wp-content/uploads/2021/11/AJ859-0-Somfit-brochure.pdf (accessed on 16 December 2024).
- McMahon, M.; Goldin, J.; Kealy, E.S.; Wicks, D.J.; Zilberg, E.; Freeman, W.; Aliahmad, B. Performance Investigation of Somfit Sleep Staging Algorithm. Nat Sci Sleep 2024, 16, 1027. [Google Scholar] [CrossRef]
- Reis Carneiro, M.; Majidi, C.; Tavakoli, M. Multi-Electrode Printed Bioelectronic Patches for Long-Term Electrophysiological Monitoring. Adv Funct Mater 2022, 32, 2205956. [Google Scholar] [CrossRef]
- Monitoring, A.B. Sleep Profiler PSG2 Available online: https://www.advancedbrainmonitoring.com/products/sleep-profiler-psg2.
- Levendowski, D.; Lee-Iannotti, J.; Shprecher, D.; Guevarra, C.; Timm, P.; Angel, E.; Mazeika, G.; St. Louis, E. P076 Sleep Staging Agreement Between Polysomnography and Sleep Profiler in Isolated REM Sleep Behavior Disorder. Sleep Adv 2021, 2, A45. [Google Scholar] [CrossRef]
- Frenz Brainband. Introducing Frenz Brainband - Clinically Proven, Fall Asleep Faster Available online: https://frenzband.com/.
- Hemsworth, M. The FRENZ Brainband Tracks and Stimulates the Brain in Different Ways Available online: https://www.trendhunter.com/trends/frenz-brainband.
- Nguyen, A.; Pogoncheff, G.; Dong, B.X.; Bui, N.; Truong, H.; Pham, N.; Nguyen, L.; Nguyen-Huu, H.; Bui-Diem, K.; Vu-Tran-Thien, Q.; et al. A Comprehensive Study on the Efficacy of a Wearable Sleep Aid Device Featuring Closed-Loop Real-Time Acoustic Stimulation. Sci Rep 2023, 13, 17515. [Google Scholar] [CrossRef]
- Dr. Fenyves und Gut Deutschland GmbH SleepDoc Porti 9. Available online: https://www.fg-deutschland.de/web/p9_e.html (accessed on 16 December 2024).
- Advanced Brain Monitoring, Inc. Sleep Profiler and PSG2 LE X8 Hardware Technical Manual. Available online: https://advancedbrainmonitoring.app.box.com/s/xlvegjpx1kr0wj2ggj7w851u6ba49u2o (accessed on 16 December 2024).
- SOMNOmedics, AG. SOMNOwatch® Eco ACTIGRAPHY Available online: https://somnomedics.de/enus/somnomedics-diagnostic-devices/sleep-diagnostics/actigraphy/somnowatch-eco/.
- Beacon Biosignals, Inc. The Dreem Headband: Clinically-Validated, Scalable Sleep Monitoring for Clinical Trials Available online: https://beacon.bio/products-services.
- den Bulcke, L. Van; Peeters, A.-M.; Heremans, E.; Davidoff, H.; Borzée, P.; Vos, M. De; Emsell, L.; den Stock, J. Van; Roo, M. De; Tournoy, J.; et al. Acoustic Stimulation as a Promising Technique to Enhance Slow-Wave Sleep in Alzheimer’s Disease: Results of a Pilot Study. J Clin Sleep Med 2023, 19, 2107–2112. [Google Scholar] [CrossRef] [PubMed]
- González, D.A.; Wang, D.; Pollet, E.; Velarde, A.; Horn, S.; Coss, P.; Vaou, O.; Wang, J.; Li, C.; Seshadri, S.; et al. Performance of the Dreem 2 EEG Headband, Relative to Polysomnography, for Assessing Sleep in Parkinson’s Disease. Sleep Health 2024, 10, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Levendowski, D.J.; Ferini-Strambi, L.; Gamaldo, C.; Cetel, M.; Rosenberg, R.; Westbrook, P.R. The Accuracy, Night-to-Night Variability, and Stability of Frontopolar Sleep Electroencephalography Biomarkers. Journal of Clinical Sleep Medicine 2017, 13, 791–803. [Google Scholar] [CrossRef] [PubMed]
- Levendowski, D.J.; Neylan, T.C.; Walsh, C.M.; Tsuang, D.; Salat, D.; Hamilton, J.M.; Lee-Iannotti, J.K.; Berka, C.; Mazeika, G.; Boeve, B.F.; et al. Proof-of-Concept for Characterization of Neurodegenerative Disorders Utilizing Two Non-REM Sleep Biomarkers. Front Neurol 2023, 14. [Google Scholar] [CrossRef]
- NOX Medical. Nox A1sTM PSG System Available online: https://noxmedical.com/products/nox-a1-psg-system/.
- Malhotra, A.; Younes, M.; Kuna, S.T.; Benca, R.; Kushida, C.A.; Walsh, J.; Hanlon, A.; Staley, B.; Pack, A.I.; Pien, G.W. Performance of an Automated Polysomnography Scoring System Versus Computer-Assisted Manual Scoring. Sleep 2013, 36, 573–582. [Google Scholar] [CrossRef]
- Biswal, S.; Sun, H.; Goparaju, B.; Brandon Westover, M.; Sun, J.; Bianchi, M.T. Expert-Level Sleep Scoring with Deep Neural Networks. Journal of the American Medical Informatics Association 2018, 25, 1643–1650. [Google Scholar] [CrossRef]
- Stenwig, H.; Soler, A.; Furuki, J.; Suzuki, Y.; Abe, T.; Molinas, M. Automatic Sleep Stage Classification with Optimized Selection of EEG Channels. Proceedings - 21st IEEE International Conference on Machine Learning and Applications, ICMLA 2022 2022, 1708–1715. [CrossRef]
- Shambroom, J.R.; Fábregas, S.E.; Johnstone, J. Validation of an Automated Wireless System to Monitor Sleep in Healthy Adults. J Sleep Res 2012, 21, 221–230. [Google Scholar] [CrossRef]
- Magliaro, C.; Vasquez, V.; Granella, P. NAP - Twin on a Chip Brains for Monitoring Individual Sleep Habits. Available online: https://www.horizoneic-nap.eu/home (accessed on 28 December 2024).
- Lujan, M.R.; Perez-Pozuelo, I.; Grandner, M.A. Past, Present, and Future of Multisensory Wearable Technology to Monitor Sleep and Circadian Rhythms. Front Digit Health 2021, 3, 721919. [Google Scholar] [CrossRef]
- Mohamed, M.; Mohamed, N.; Kim, J.G. Advancements in Wearable EEG Technology for Improved Home-Based Sleep Monitoring and Assessment: A Review. Biosensors (Basel) 2023, 13, 1019. [Google Scholar] [CrossRef]
- Grandner, M.A.; Lujan, M.R.; Ghani, S.B. Sleep-Tracking Technology in Scientific Research: Looking to the Future. Sleep 2021, 44. [Google Scholar] [CrossRef]
- Tran, N.T.; Tran, H.N.; Mai, A.T. A Wearable Device for At-Home Obstructive Sleep Apnea Assessment: State-of-the-Art and Research Challenges. Front Neurol 2023, 14, 1123227. [Google Scholar] [CrossRef] [PubMed]
- De Fazio, R.; Mattei, V.; Al-Naami, B.; De Vittorio, M.; Visconti, P. Methodologies and Wearable Devices to Monitor Biophysical Parameters Related to Sleep Dysfunctions: An Overview. Micromachines 2022, 13, 1335. [Google Scholar] [CrossRef] [PubMed]
- de Zambotti, M.; Goldstein, C.; Cook, J.; Menghini, L.; Altini, M.; Cheng, P.; Robillard, R. State of the Science and Recommendations for Using Wearable Technology in Sleep and Circadian Research. Sleep 2024, 47. [Google Scholar] [CrossRef]
- Cay, G.; Ravichandran, V.; Sadhu, S.; Zisk, A.H.; Salisbury, A.L.; Solanki, D.; Mankodiya, K. Recent Advancement in Sleep Technologies: A Literature Review on Clinical Standards, Sensors, Apps, and AI Methods. IEEE Access 2022, 10, 104737–104756. [Google Scholar] [CrossRef]
- Peake, J.M.; Kerr, G.; Sullivan, J.P. A Critical Review of Consumer Wearables, Mobile Applications, and Equipment for Providing Biofeedback, Monitoring Stress, and Sleep in Physically Active Populations. Front Physiol 2018, 9, 329783. [Google Scholar] [CrossRef]
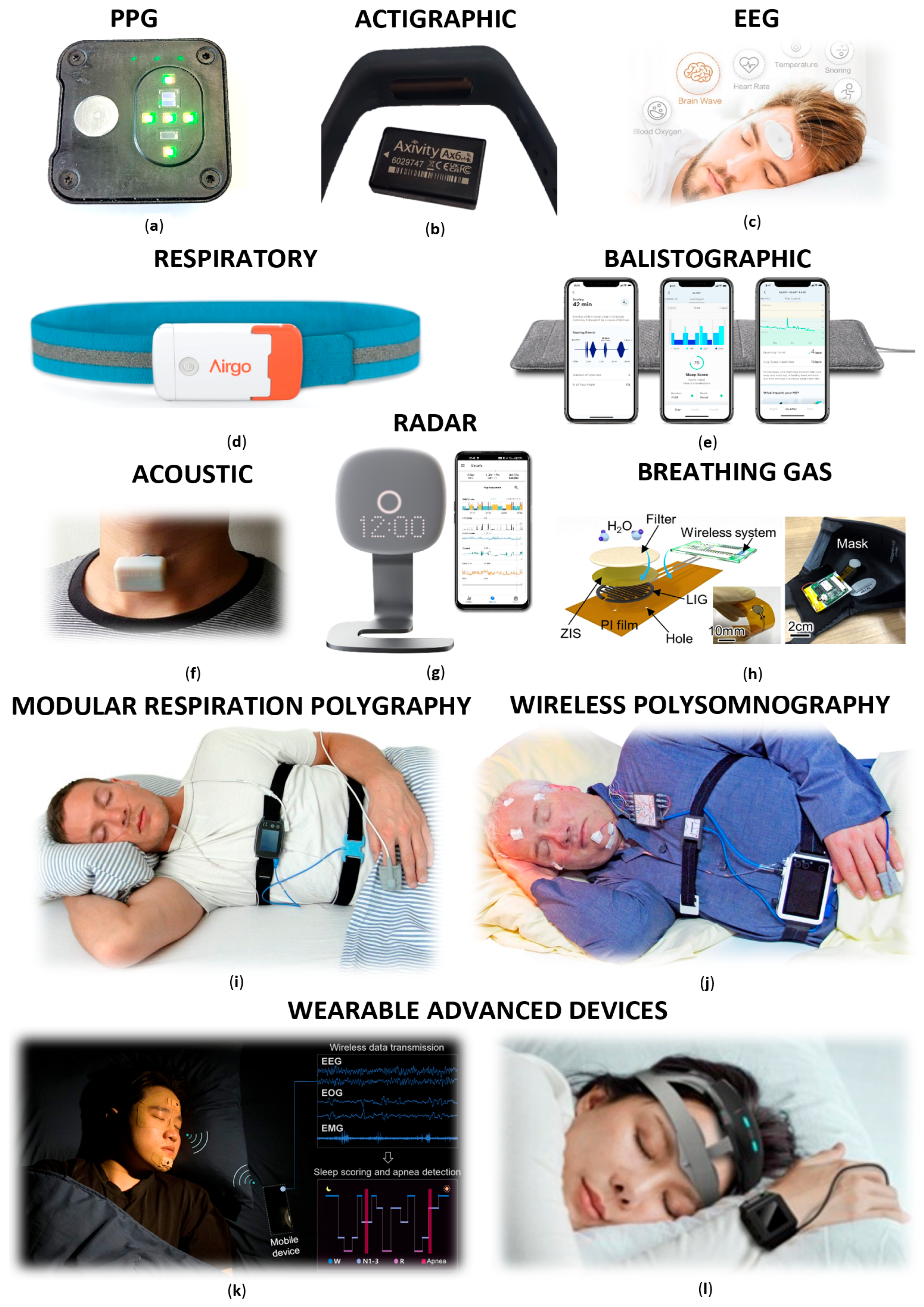
| Sleep parameter | Duration 1 |
|---|---|
| Sleep onset latency | ≤ 30 min |
| NREM 1 | 3 – 5% |
| NREM 2 | 45 – 55% |
| NREM 3 | 10 – 20% |
| REM | 20 – 25% |
| REM sleep latency | 60 - 100 min |
| Wakefulness after sleep onset | 1 – 5% |
| Sleep efficiency | > 85% |
| Classification of severity of sleep-related breathing disorders | AHI 1 |
|---|---|
| Without sleep – related breathing disorders | < 5 |
| Mild severity | 5 ≥ AHI < 15 |
| Moderate severity | 15 ≥ AHI <30 |
| Severe severity | ≥ 30 |
| NREM 1 | NREM 2 | NREM 3 | REM | |
|---|---|---|---|---|
| EEG | Characterized by LAMF 1 activity with predominant theta waves (4 – 7 Hz). Alpha activity dissipates, and typical vertex sharp waves lasting up to 0.5 s are visible. | Typical theta waves (4 – 7 Hz) with low to medium amplitude. Presence of sleep spindles (short bursts of 11 – 16 Hz) and K-complexes (sharp delta waves lasting 1 s), which play key roles in sleep maintenance and memory consolidation. Phase duration about 25 minutes and lengthens with each cycle, comprising about 45% of TST 2. | Characterized by slow delta waves (0.5 – 3.5 Hz) with high amplitudes of at least 75 μV in frontal leads. Delta waves constitute more than 20% of the duration of an EEG epoch. | Desynchronized EEG activity with sawtooth waves of 2 – 4 Hz and moderate amplitude, appearing in small clusters in frontal leads. Dream activity occurs with an emotional undertone. REM sleep consolidates memory traces and strengthens memory. |
| HR | Slight decrease compared to wakefulness. | Decrease of 5 – 8% compared to wakefulness. | Decrease of 5 – 8% compared to wakefulness. | Irregular. |
| HRV | Overall HRV increases, LF component decreases, LF/HF ratio decreases, HF component increases. | Overall HRV increases, LF component decreases, LF/HF ratio decreases, HF component increases. |
HRV peaks, LF components are at their lowest, LF/HF ratio decreases, HF components are at its highest. | Significant decrease in HRV, LF component increases, LF/HF ratio increases, HF component decreases. |
| RR | Slower rate compared to wakefulness, regular. | Slower rate compared to wakefulness, regular. | Slow and regular. | Rate equal or higher than wakefulness, breathing becomes shallow. |
| BP | Decrease compared to wakefulness, less pronounced than in NREM 2 and NREM 3. | Decrease of 5 – 14% compared to wakefulness. | More significant decrease than in NREM 2, 5 – 14% lower than wakefulness. | Increase of approximately 5% compared to NREM sleep. |
| CBT | Decrease compared to wakefulness. | Decrease compared to wakefulness. | Largest temperature drop. | Increase compared to NREM sleep. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





