Submitted:
06 January 2025
Posted:
08 January 2025
You are already at the latest version
Abstract
Infiltration swales are a prospective key component of water-sensitive urban planning. The utilization of appropriate soil amendments is intended to facilitate their retention of pollutants from stormwater runoff of traffic areas. Little is known about the possibility of utilizing processed construction and demolition waste (CDW) as an amendment to improve pollutant retention. We conducted batch and field tests to investigate i) the leaching of metals from soil substrates containing CDW and ii) their retention potential for copper (Cu) and zinc (Zn) when charged with real traffic area runoff. To gain a comprehensive understanding of the chemical interactions, we iii) employed sequential extractions using an optimized protocol from treated and untreated soil substrates. In batch tests, the potential of vanadium leaching from technosols amended with brick-dominated CDW was apparent. When charged with traffic area runoff, the retentions of Cu and Zn in the technosols were comparable to those of the control soil without CDW. However, the simulation of high rainfall intensities reduced Cu and Zn retention in the technosols and the control. The results from the subsequent sequential extraction of Cu and Zn imply shifts in the chemical binding in the technosols compared to the control.
Keywords:
1. Introduction
2. Materials and Methods
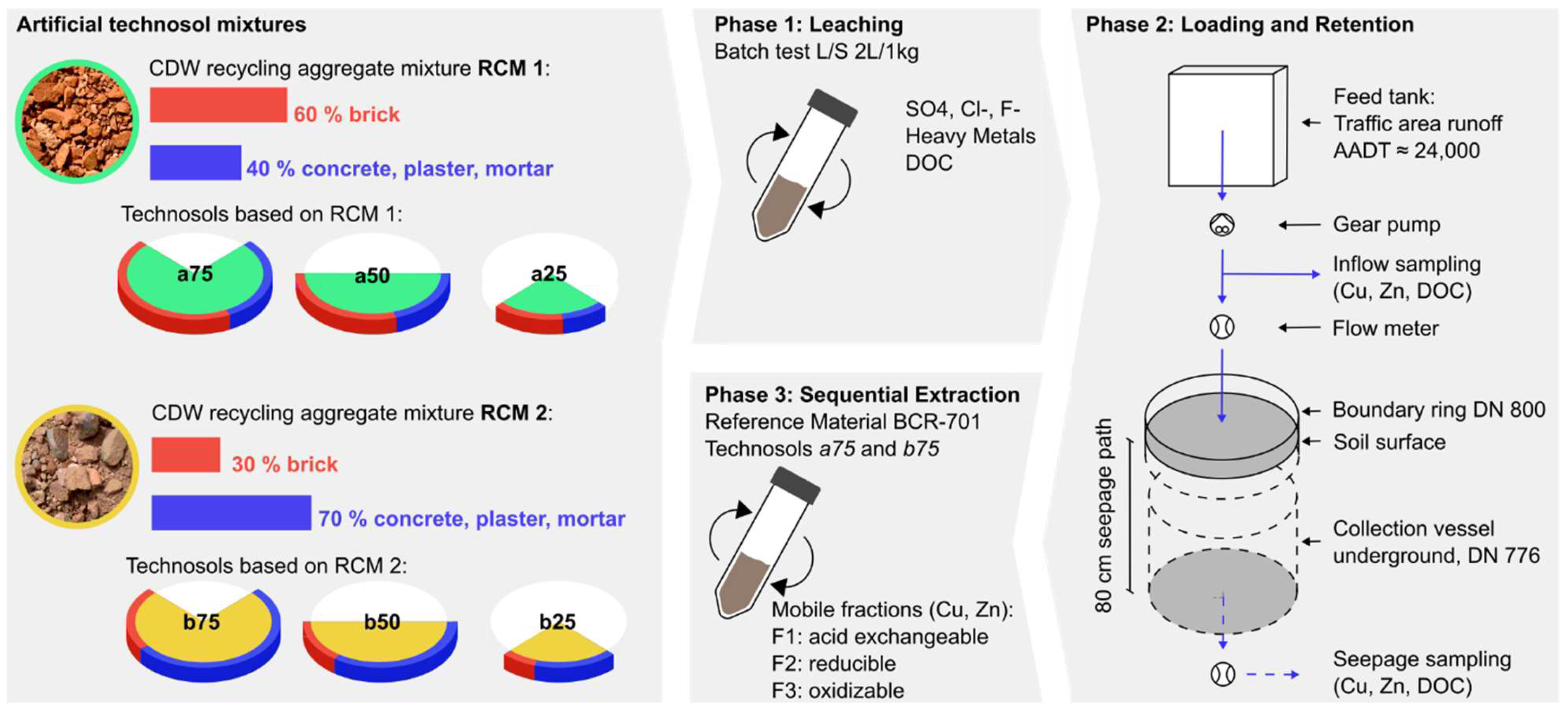
2.1. Technosols from CDW
2.2. Study Design
2.3. Data Processing
3. Results
3.1. Leaching Potential
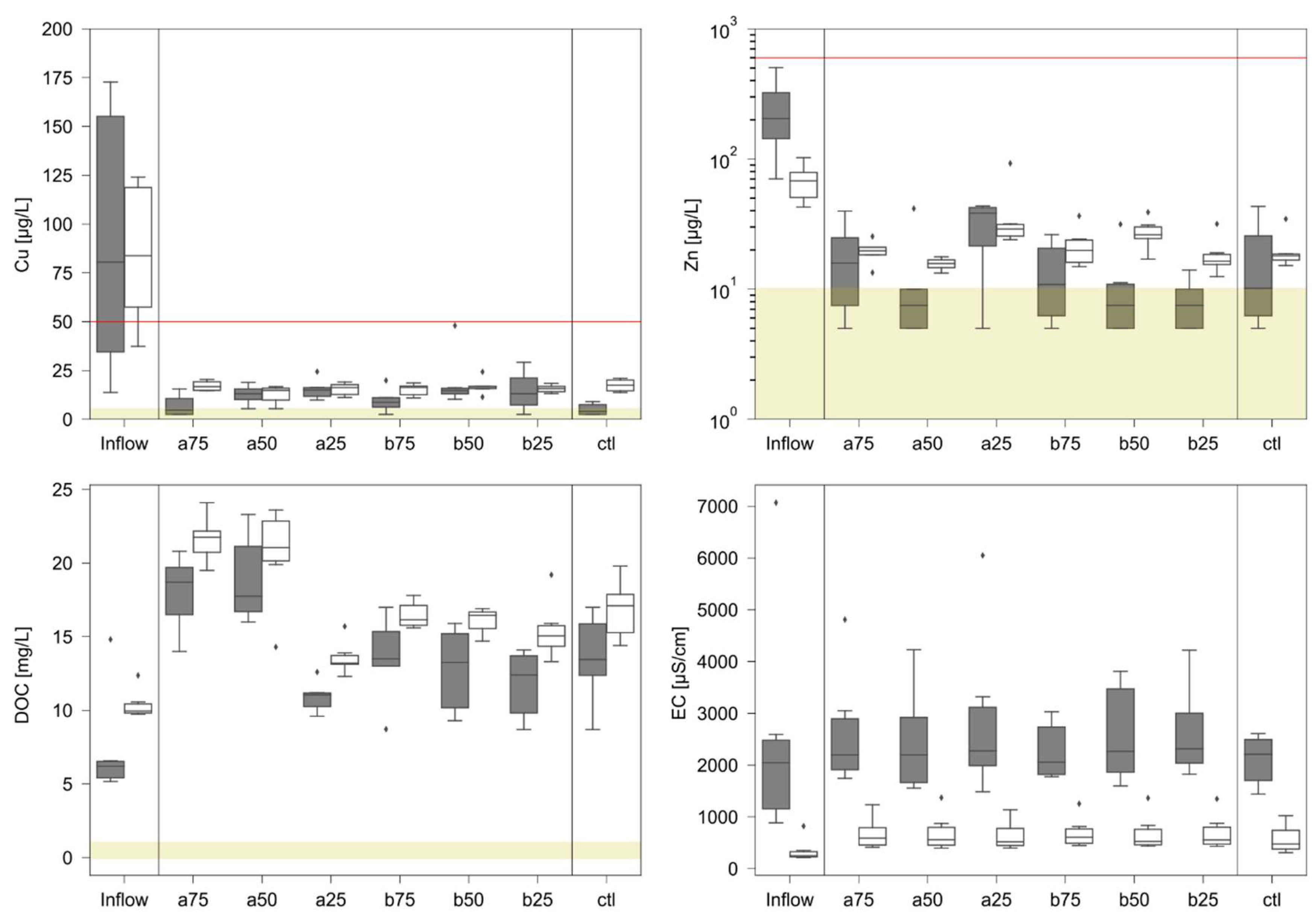
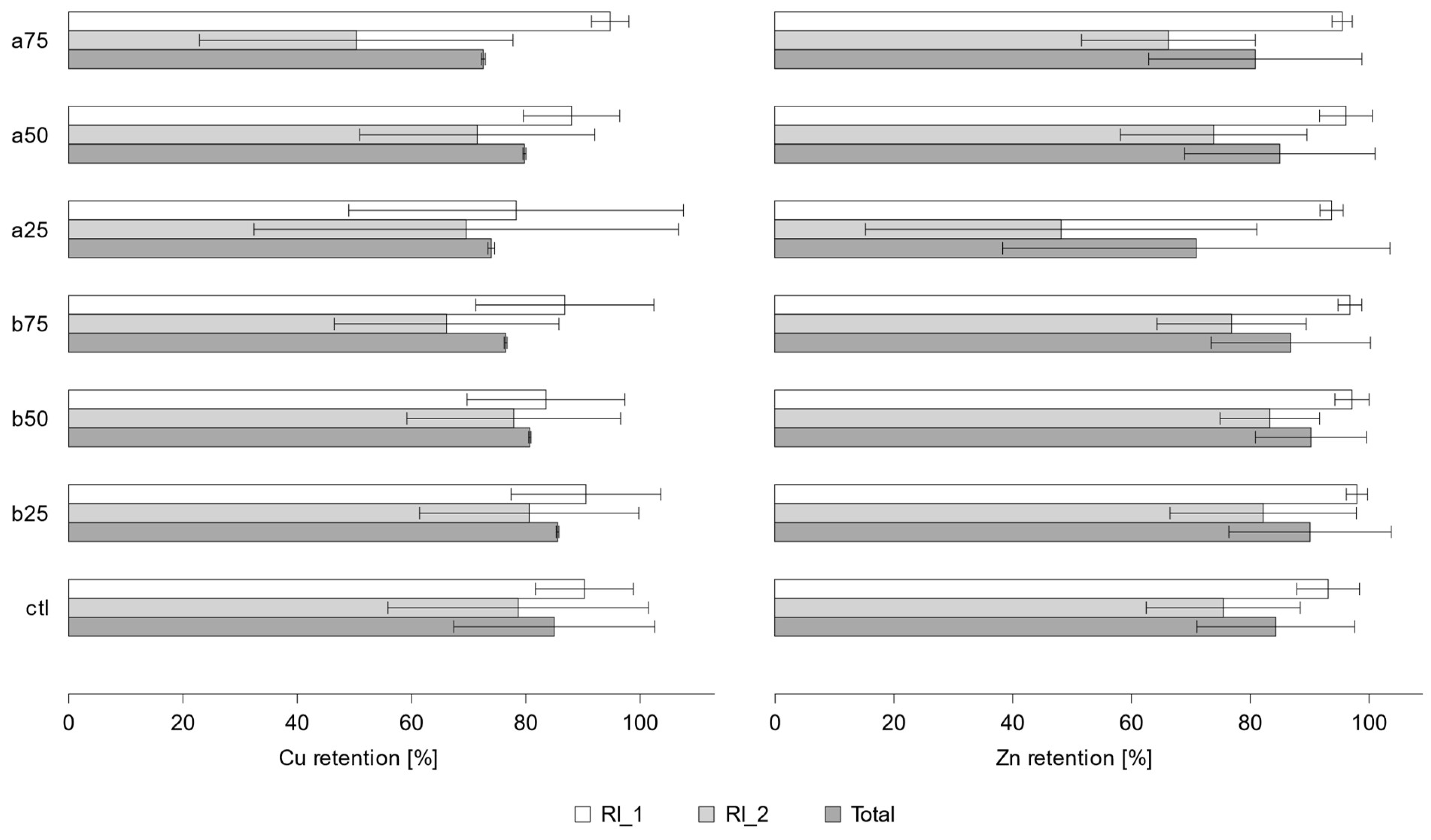
3.2. Retention and Water Quality
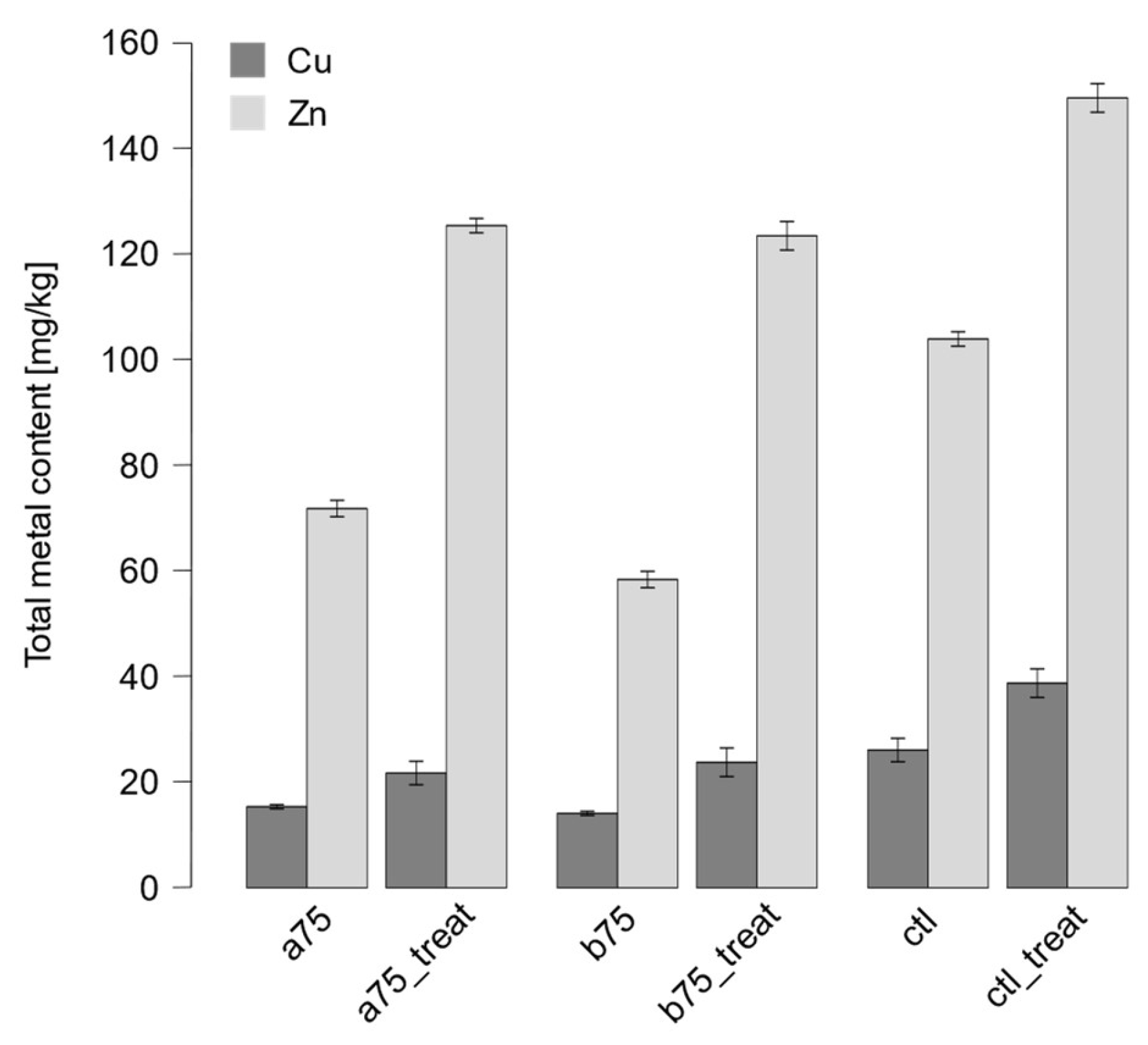
3.3. Sequential Extraction of Cu and Zn
4. Discussion
4.1. Risk of Substance Release
4.2. Retention and Binding
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AR | Aqua regia |
| BCR | Community Bureau of Reference, European Commission |
| CDW | Construction and demolition waste |
| RCM | Recycling mixture (in this study: crushed aggregates of mixed CDW) |
| RDS | Road-deposited sediments |
| SEP | Sequential extraction procedure |
| TAR | Traffic area runoff |
| TV_1 | Threshold values for seepage water according to BBodSchV |
| TV_2 | Threshold values for soil solution according to BBodSchV |
Appendix A
| Substance | Method | LOQ | Unit | BBodSchV [15] | ||
| TV_1 | TV_2 | |||||
| Batch tests | ||||||
| pH | DIN 38404-5: 2009-07 | |||||
| EC | DIN EN 27888: 1993-11 | 10 | µS/cm | |||
| Sb | AR | DIN EN ISO 17294-2: 2017-01 | 5 | µg/L | 5 | 10 |
| As | AR | DIN EN ISO 17294-2: 2017-01 | 5 | µg/L | 10 | 25 |
| Pb | AR | DIN EN ISO 17294-2: 2017-01 | 5 | µg/L | 10 | 85 |
| B | AR | DIN EN ISO 17294-2: 2017-01 | 50 | µg/L | 1000 | 1000 |
| Cd | AR | DIN EN ISO 17294-2: 2017-01 | 0.5 | µg/L | 3 | 7,5 |
| Cr | AR | DIN EN ISO 17294-2: 2017-01 | 5 | µg/L | 50 | 50 |
| Cr(VI) | AR | DIN ISO 15923-1: 2014-07 | 5 | µg/L | 8 | 8 |
| Co | AR | DIN EN ISO 17294-2: 2017-01 | 5 | µg/L | 10 | 125 |
| Cu | AR | DIN EN ISO 17294-2: 2017-01 | 5 | µg/L | 50 | 80 |
| Mo | AR | DIN EN ISO 17294-2: 2017-01 | 5 | µg/L | 35 | 70 |
| Ni | AR | DIN EN ISO 17294-2: 2017-01 | 5 | µg/L | 20 | 60 |
| Hg | AR | DIN EN ISO 12846: 2012-08 | 0.2 | µg/L | 1 | 1 |
| Se | AR | DIN EN ISO 17294-2: 2017-01 | 5 | µg/L | 10 | 10 |
| Tl | AR | DIN EN ISO 17294-2: 2017-01 | 0.5 | µg/L | ||
| V | AR | DIN EN ISO 17294-2: 2017-01 | 2 | µg/L | 4 | 70 |
| Zn | AR | DIN EN ISO 17294-2: 2017-01 | 50 | µg/L | 600 | 600 |
| Sn | AR | DIN EN ISO 17294-2: 2017-01 | 20 | µg/L | ||
| DOC | Filtration 0.45 µm | DIN 1484 | 1 | mg/l | ||
| Cl | DIN ISO 15923-1: 2014-07 | 2 | mg/l | |||
| SO4 | DIN ISO 15923-1: 2014-07 | 2 | mg/l | |||
| F | DIN 38405-4: 1985-07 | 0.2 | mg/l | 1,5 | ||
| Retention tests | ||||||
| pH | on-site | |||||
| EC | on-site | µS/cm | ||||
| O2 | on-site | mg/L | ||||
| Cu | AR | DIN EN ISO 17294-2: 2017-01 | µg/L | 50 | ||
| Zn | AR | DIN EN ISO 17294-2: 2017-01 | µg/L | 600 | ||
| Element | Rain intensity | Technosol | U Statistic | p-value |
|---|---|---|---|---|
| Cu | 121 L/(s·ha) | a75 | 21 | 0.732 |
| a50 | 33 | 0.019 | ||
| a25 | 36 | 0.005 | ||
| b75 | 28 | 0.142 | ||
| b50 | 36 | 0.005 | ||
| b25 | 30 | 0.073 | ||
| 221 L/(s·ha) | a75 | 18 | 1.000 | |
| a50 | 8 | 0.132 | ||
| a25 | 12 | 0.394 | ||
| b75 | 11 | 0.310 | ||
| b50 | 15 | 0.699 | ||
| b25 | 11.5 | 0.336 | ||
| Zn | 121 L/(s·ha) | a75 | 19 | 0.935 |
| a50 | 13 | 0.452 | ||
| a25 | 26.5 | 0.196 | ||
| b75 | 16.5 | 0.870 | ||
| b50 | 14.5 | 0.618 | ||
| b25 | 12 | 0.357 | ||
| 221 L/(s·ha) | a75 | 21.5 | 0.630 | |
| a50 | 6.5 | 0.078 | ||
| a25 | 31 | 0.041 | ||
| b75 | 19 | 0.937 | ||
| b50 | 28 | 0.132 | ||
| b25 | 14 | 0.589 |
| Mean background concentrations (n = 4) | Concentrations rain intensity RI_1 (n = 6) | concentrations rain intensity RI_2 (n = 6) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L/S | Cu [µg/L] | Zn [µg/L] | DOC [mg/L] | Cu [µg/L] | Zn [µg/L] | DOC [mg/L] | pH | EC [µS/cm] | Cu [µg/L] | Zn [µg/L] | DOC [mg/L] | pH | EC [µS/cm] | |
| inf | n.a. | n.a. | n.a. | n.a. | 73.4 ±61.5 |
247 ±169 |
7.4 ±3.9 |
7.8 ±0.1 | 2,588 ±2,128 | 84.7 ±34.6 |
68.1 ±20.5 |
10.4 ±0.9 | 7.8 ±0.1 | 351 ±218 |
| a75 | 0.51 | 6.5 ±2.6 | 6.5 ±2.6 | 35 ±14 | 7.0 ±5.1 |
17.5 ±13.8 |
15.4 ±6.7 |
7.7 ±0.1 |
2,648 ±1060 |
17.1 ±2.4 |
19.6 ±3.6 |
21.6 ±1.5 |
7.8 ±0.1 |
683 ±286 |
| a50 | 0.57 | 7.0 ±3,5 | 5 ±0 | 31 ±13 | 12.7 ±4.4 |
14.6 ±14.3 |
16.2 ±7.1 |
7.9 ±0.3 |
2,471 ±948 |
12.7 ±4.3 |
15.7 ±1.5 |
200.6 ±3.1 |
7.8 ±0.1 |
697 ±3389 |
| a25 | 0.55 | 6.5 ±2.6 | 5 ±0 | 38 ±18 | 15.3 ±4.7 |
24.8 ±18.8 |
9.4 ±3.9 |
7.5 ±0.1 |
2,896 ±1520 |
15.4 ±3.1 |
38.5 ±24.3 |
13.6 ±1.1 |
7.9 ±0.0 |
643 ±268 |
| b75 | 0.47 | 5 ±0 | 5 ±0 | 23 ±10 | 9.5 ±5.5 |
11.6 ±9.1 |
11.6 ±5.3 |
7.7 ±0.1 |
2,270 ±516 |
15.1 ±2.9 |
21.8 ±7.4 |
16.5 ±0.8 |
7.8 ±0.0 |
698 ±277 |
| b50 | 0.57 | 5 ±0 | 5 ±0 | 20 ±7 | 19.4 ±12.9 |
9.7 ±9.5 |
11.0 ±5.1 |
7.9 ±0.3 |
2,590 ±901 |
16.9 ±3.8 |
27.3 ±6.7 |
16.1 ±0.8 |
7.8 ±0.1 | 688 ±330 |
| b25 | 0.82 | 5 ±0 | 5 ±0 | 20 ±9 | 14.5 ±9.3 |
8.3 ±4.8 |
10.1 ±4.6 |
7.8 ±0.1 |
2,643 ±829 |
15.7 ±1.8 |
18.6 ±6.2 |
15.5 ±1.9 |
7.7 ±0.1 | 703 ±323 |
| ctl | 0.84 | 5 ±0 | 7.0 ±3.5 | 14 ±5 | 5.0 ±2.8 |
14.1 ±15.1 |
11.6 ±5.4 |
7.9 ±0.2 |
2,099 ±453 |
17.4 ±2.9 |
20.2 ±6.6 |
16.9 ±1.9 |
7.7 ±0.0 |
576 ±257 |
| Cu | Zn | ||||||
|---|---|---|---|---|---|---|---|
| Influent load | Effluent load | Retention | Influent load | Effluent load | Retention | ||
| a75 | RI_1 | 101 | 2.8 | 94.8 ±3.3 | 158 | 7.4 | 95.5 ±1.7 |
| RI_2 | 27.4 | 7.5 | 50.3 ±27.4 | 27.2 | 8.4 | 66.3 ±14.6 | |
| Total | 128 | 10.3 | 72.5±0.4 | 185 | 15.8 | 80.9±18.0 | |
| a50 | RI_1 | 110 | 6.1 | 88.0 ±8.4 | 225 | 5.6 | 96.2 ±4.4 |
| RI_2 | 46.0 | 6.0 | 71.5 ±20.6 | 40.2 | 7.3 | 73.9 ±15.7 | |
| Total | 156 | 12.0 | 79.8±0.3 | 265 | 12.9 | 85.0±16.0 | |
| a25 | RI_1 | 146 | 6.0 | 78.3 ±29.3 | 191 | 12.7 | 93.7 ±2.0 |
| RI_2 | 131 | 6.2 | 69.6 ±37.1 | 40.4 | 14.6 | 48.2 ±32.9 | |
| Total | 276 | 12.2 | 73.9±0.6 | 232 | 27.3 | 71.0±32.6 | |
| b75 | RI_1 | 80.9 | 4.0 | 86.8 ±15.6 | 172 | 5.3 | 96.8 ±2.0 |
| RI_2 | 31.5 | 5.9 | 66.1 ±19.7 | 39.4 | 8.5 | 76.9 ±12.6 | |
| Total | 112 | 9.9 | 76.5±0.3 | 211 | 13.8 | 86.9±13.4 | |
| b50 | RI_1 | 111 | 8.5 | 83.5 ±13.8 | 2134 | 4.5 | 97.2 ±2.9 |
| RI_2 | 54.5 | 6.7 | 77.9 ±18.7 | 77.8 | 10.6 | 83.3 ±8.4 | |
| Total | 166 | 15.2 | 80.7±0.2 | 292 | 15.1 | 90.3±9.3 | |
| b25 | RI_1 | 130 | 5.1 | 90.5 ±13.1 | 320 | 2.7 | 98.0 ±1.8 |
| RI_2 | 56.4 | 5.5 | 80.6 ±19.2 | 89.1 | 6.7 | 82.2 ±15.7 | |
| Total | 187 | 10.5 | 85.6±0.2 | 410 | 9.4 | 90.1±13.7 | |
| ctl | RI_1 | 75.7 | 2.1 | 90.2 ±8.5 | 150 | 7.3 | 93.2 ±5.3 |
| RI_2 | 105 | 7.3 | 78.7 ±22.8 | 48.9 | 9.1 | 75.5 ±13.0 | |
| Total | 181 | 9.4 | 73.9±17.6 | 199 | 16.3 | 84.3±13.3 | |
| Sample | S1 Acid exchangeable |
S2 Reducible |
S3 Oxidizable |
S4 Residue |
Sum S1-S4a |
Pseudo-total b | Recovery | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [mg/kg] | [%] | [mg/kg] | [%] | [mg/kg] | [%] | [mg/kg] | [%] | [mg/kg] | [mg/kg] | [%] | ||
| Cu | a75 | 0.13 ±0.06 |
0.82 ±0.3 |
0.87 ±0.48 |
5.45 ±2.9 |
4.66 ±0.26 |
29.2 ±0.9 |
10.3 ±0.42 |
64.5 ±3.8 |
16.1 ±0.69 |
8.28 | 193 |
| b75 | 0.1 ±0.0 |
0.68 ±0.1 |
0.28 ±0.03 |
1.91 ±0.6 |
4.33 ±1.28 |
29.6 ±3.4 |
9.94 ±1.59 |
67.9 ±2.7 |
14.7 ±2.04 |
7.57 | 194 | |
| ctl | 0.1 ±0.0 |
0.37 ±0.0 |
0.28 ±0.03 |
1.04 ±0.2 |
8.41 ±0.83 |
31.2 ±1.1 |
18.2 ±2.63 |
67.4 ±1.3 |
27.1 ±2.76 |
14.6 | 185 | |
| a75_treat | 0.2 ±0.0 |
0.89 ±0.1 |
0.1 ±0.0 |
0.45 ±0.0 |
8.05 ±0.34 |
35.9 ±1.5 |
14.1 ±1.57 |
62.8 ±1.6 |
22.5 ±1.61 |
17 | 132 | |
| b75_treat | 0.22 ±0.0 |
90 ±0.1 |
0.11 ±0.0 |
0.45 ±0.0 |
6.04 ±0.67 |
24.6 ±4.0 |
18.2 ±2.1 |
74.1 ±4.0 |
24.6 ±2.2 |
10.5 | 234 | |
| ctl_treat | 0.2 ±0.0 |
0.5 ±0.0 |
0.27 ±0.02 |
0.67 ±0.0 |
8.93 ±0.49 |
22.3 ±0.7 |
30.7 ±2.81 |
76.6 ±0.7 |
40.1 ±2.91 |
18 | 223 | |
| Zn | a75 | 7.65 ±1.16 |
10.3 ±1.0 |
19.1 ±0.88 |
25.8 ±1.6 |
7.21 ±0.76 |
9.7 ±1.3 |
40.3 ±4.43 |
54.2 ±3.1 |
74.3 ±1.23 |
61.9 | 120 |
| b75 | 5.73 ±0.50 |
9.49 ±2.0 |
11.4 ±3.08 |
18.9 ±1.5 |
5.37 ±1.79 |
8.89 ±1.2 |
37.9 ±8.7 |
62.8 ±2.2 |
60.4 ±4.33 |
49.6 | 122 | |
| ctl | 6.66 ±0.18 |
6.2 ±0.3 |
14.3 ±1.3 |
13.3 ±0.5 |
11.2 ±0.39 |
10.5 ±0.8 |
75.3 ±5.54 |
70.1 ±0.7 |
107 ±12.4 |
79.6 | 135 | |
| a75_treat | 22.4 ±1.16 |
17.3 ±0.8 |
26.5 ±0.62 |
20.4 ±1.0 |
11.6 ±0.07 |
8.98 ±0.2 |
69.2 ±4.09 |
53.3 ±1.8 |
131 ±7.18 |
108 | 120 | |
| b75_treat | 10.4 ±1.27 |
8.17 ±1.9 |
23.3 ±2.20 |
18.3 ±2.8 |
8.68 ±2.59 |
6.8 ±1.2 |
85.3 ±15.9 |
66.8 ±3.5 |
128 ±4.32 |
60.1 | 213 | |
| ctl_treat | 6.75 ±0.16 |
4.36 ±0.3 |
14.2 ±0.41 |
9.2 ±0.8 |
8.32 ±0.15 |
5.38 ±0.4 |
125 ±11.4 |
81.1 ±1.5 |
155 ±8.90 |
69.4 | 223 | |
| a Sum of means of S1-S4, b Values from single determination | ||||||||||||
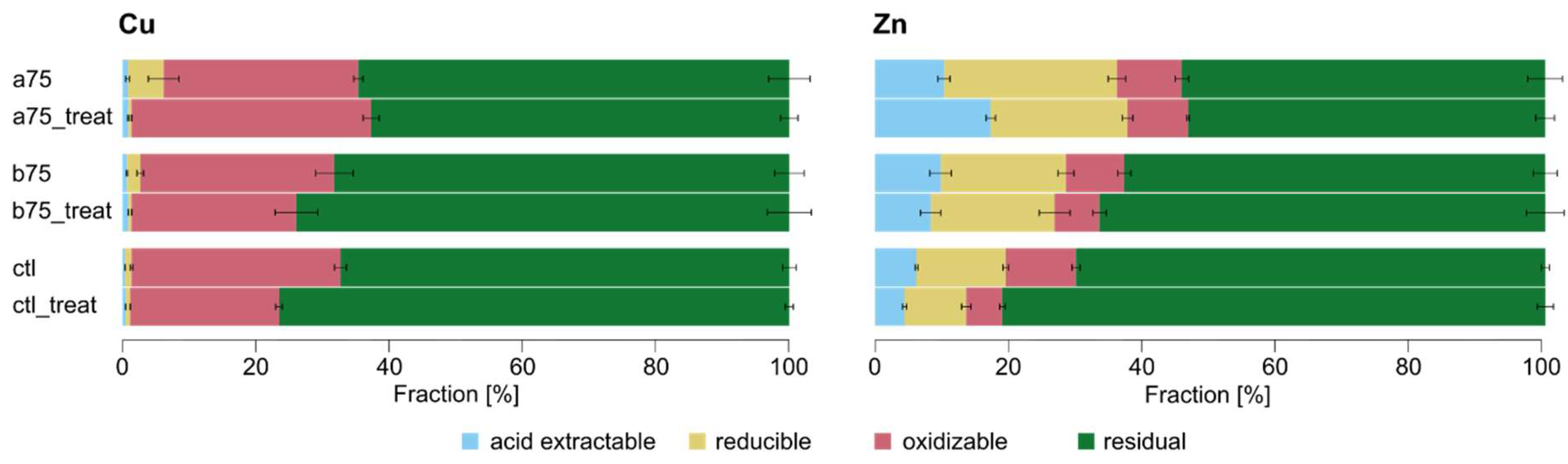
| Type | S1 Acid exchangeable [%] |
S2 Reducible [%] |
S3 oxidizable [%] |
S4 residual [%] |
total [mg/kg] | |
|---|---|---|---|---|---|---|
| Cu | ||||||
| a75 | Technosol | 0.8 ±0.3 | 5.4 ±2.3 | 29.2 ±0.7 | 64.6 ±3.1 | 15.9 ±0.4 |
| a75_treat | Technosol | 0.9 ±0.1 | 0.5 ±0.0 | 35.9 ±1.2 | 62.7 ±1.3 | 22.5 ±1.6 |
| b75 | Technosol | 0.7 ±0.1 | 2.0 ±0.5 | 29.1 ±2.8 | 68.2 ±2.2 | 14.6 ±2.3 |
| b75 _treat | Technosol | 0.9 ±0.0 | 0.5 ±0.0 | 24.7 ±3.2 | 73.9 ±3.3 | 24.6 ±1.4 |
| ctl | Soil | 0.4 ±0.0 | 1.0 ±0.2 | 31.3 ±0.9 | 67.3 ±1.0 | 27.0 ±2.8 |
| ctl_treat | Soil | 0.5 ±0.0 | 0.7 ±0.0 | 22.3 ±0.5 | 76.5 ±0.6 | 40.1 ±2.8 |
| [46] | RDS | 7.0 ±1.4 | 37.2 ±2.6 | 20.5 ±2.2 | 35.3 ±3.1 | 409.0 |
| [49] | RDS | 6.0 | 43.6 | 25.5 | 24.9 | 84.2 |
| [37] | RDS | 1.9 | 8.2 | 10.3 | 79.6 | 670.0 |
| [48] | RDS | 4.9 ±1.2 | 23.6 ±3.1 | 26.2 ±3.2 | 45.4 ±3.9 | 163.0 |
| [53] | RDS | 7.3 | 44.6 | 26.8 | 21.3 | 207.0 |
| [19] | Carbonate sand | 2.6 ±0.1 | 1.6 ±0.2 | 16.7 ±0.4 | 79.1 ±6.6 | 137 ±8 |
| Zn | ||||||
| a75 | Technosol | 10.3 ±0.9 | 25.8 ±1.3 | 9.7 ±1.0 | 54.2 ±2.6 | 74.3 ±4.2 |
| a75_treat | Technosol | 17.3 ±0.7 | 20.4±0.8 | 9.0 ±0.2 | 53.3 ±1.4 | 129.7 ±3.3 |
| b75 | Technosol | 9.8 ±1.6 | 18.7 ±1.2 | 8.7 ±1.0 | 62.8 ±1.8 | 60.4 ±11.1 |
| b75 _treat | Technosol | 8.3 ±1.5 | 18.5 ±2.3 | 6.7 ±1.0 | 66.5 ±2.8 | 128 ±15.0 |
| ctl | Soil | 6.2 ±0.2 | 13.3 ±0.4 | 10.5 ±0.6 | 70.0 ±0.6 | 107.5 ±5.6 |
| ctl_treat | Soil | 4.4 ±0.3 | 9.2 ±0.7 | 5.4 ±0.4 | 81.0 ±1.2 | 155 ±9.2 |
| [46] | RDS | 27.2 ±2.5 | 42.1 ±2.0 | 9.5 ±0.6 | 21.2 ±1.6 | 671 |
| [49] | RDS | 25.1 | 55.1 | 9.6 | 10.2 | 443 |
| [37] | RDS | 28.8 | 28.9 | 14.1 | 28.3 | 640 |
| [48] | RDS | 32.7 ±2.2 | 36.6 ±1.5 | 8.3 ±1.3 | 22.4 ±2.1 | 471 |
| [53] | RDS | 26.7 | 42.5 | 13.2 | 17.6 | 344 |
| [54] | RDS | 33.2 | 29.7 | 20.9 | 16.2 | 113.0 |
| [19] | Carbonate sand | 14.4 ±0.1 | 10.4 ±0.7 | 6.7 ±0.9 | 68.5 ±4.6 | 813 ±37 |
References
- Huber, M.; Welker, A.; Helmreich, B. Critical review of heavy metal pollution of traffic area runoff: Occurrence, influencing factors, and partitioning. Sci Total Environ 2016, 541, 895–919. [Google Scholar] [CrossRef] [PubMed]
- Werkenthin, M.; Kluge, B.; Wessolek, G. Metals in European roadside soils and soil solution – A review. Environmental Pollution 2014, 189, 98–110. [Google Scholar] [CrossRef]
- Barrett, M.; Katz, L.; Taylor, S. Removal of Dissolved Heavy Metals in Highway Runoff. Transportation Research Record 2014, 2436, 131–138. [Google Scholar] [CrossRef]
- Gavrić, S.; Leonhardt, G.; Österlund, H.; Marsalek, J.; Viklander, M. Metal enrichment of soils in three urban drainage grass swales used for seasonal snow storage. Science of The Total Environment 2021, 760, 144136. [Google Scholar] [CrossRef]
- DWA. Arbeitsblatt DWA-A 138-1 - Anlagen zur Versickerung von Niederschlagswasser - Teil 1: Planung, Bau, Betrieb. 2024. [Google Scholar]
- Zhang, X.; Guo, S.; Liu, J.; Zhang, Z.; Song, K.; Tan, C.; Li, H. A Study on the Removal of Copper (II) from Aqueous Solution Using Lime Sand Bricks. Applied Sciences 2019, 9, 670. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, P.; Yang, L.; Huang, T. Adsorption characteristics of construction waste for heavy metals from urban stormwater runoff. Chinese Journal of Chemical Engineering 2015, 23, 1542–1550. [Google Scholar] [CrossRef]
- Caro, D.; Lodato, C.; Damgaard, A.; Cristóbal, J.; Foster, G.; Flachenecker, F.; Tonini, D. Environmental and socio-economic effects of construction and demolition waste recycling in the European Union. Science of The Total Environment 2024, 908, 168295. [Google Scholar] [CrossRef] [PubMed]
- Soto-Paz, J.; Arroyo, O.; Torres-Guevara, L.E.; Parra-Orobio, B.A.; Casallas-Ojeda, M. The circular economy in the construction and demolition waste management: A comparative analysis in emerging and developed countries. Journal of Building Engineering 2023, 78, 107724. [Google Scholar] [CrossRef]
- Deeb, M.; Groffman, P.M.; Blouin, M.; Egendorf, S.P.; Vergnes, A.; Vasenev, V.; Cao, D.L.; Walsh, D.; Morin, T.; Séré, G. Using constructed soils for green infrastructure – challenges and limitations. SOIL 2020, 6, 413–434. [Google Scholar] [CrossRef]
- Minixhofer, P.; Scharf, B.; Hafner, S.; Weiss, O.; Henöckl, C.; Greiner, M.; Room, T.; Stangl, R. Towards the Circular Soil Concept: Optimization of Engineered Soils for Green Infrastructure Application. Sustainability 2022, 14, 905. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, Y. The contents and release behavior of heavy metals in construction and demolition waste used in freeway construction. Environmental Science and Pollution Research 2020, 27, 1078–1086. [Google Scholar] [CrossRef]
- Butera, S.; Christensen, T.H.; Astrup, T.F. Composition and leaching of construction and demolition waste: Inorganic elements and organic compounds. Journal of Hazardous Materials 2014, 276, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Knoll, S.; Mindermann, S.; Porter, L.; Pauleit, S.; Duthweiler, S.; Prügl, J.; Helmreich, B. The potential of processed mineral construction and demolition waste to increase the water capacity of urban tree substrates - A pilot scale study in Munich. Sustainable Cities and Society 2024, 113, 105661. [Google Scholar] [CrossRef]
- German Federal Office of Justice. BBodSchV Bundes-Bodenschutz- und Altlastenverordnung. 2021. [Google Scholar]
- DIN. DIN 19529: Leaching of solid materials - Batch test for the examination of the leaching behaviour of inorganic and organic substances at a liquid to solid ratio of 2 l/kg. 2015, 19529, 27.
- Rommel, S.H.; Helmreich, B. Influence of Temperature and De-Icing Salt on the Sedimentation of Particulate Matter in Traffic Area Runoff. Water 2018, 10, 1738. [Google Scholar] [CrossRef]
- Rauret, G.; López-Sánchez, J.; Lück, D.; Yli-Halla, M.; Muntau, H.; Quevauviller, P. The certification of the extractable contents (mass fractions) of Cd, Cr, Cu, Ni, Pb and Zn in freshwater sediment following a sequential extraction procedure : BCR-701; Publications Office, 2001. [Google Scholar]
- Rommel, S.H.; Stinshoff, P.; Helmreich, B. Sequential extraction of heavy metals from sorptive filter media and sediments trapped in stormwater quality improvement devices for road runoff. Science of The Total Environment 2021, 782, 146875. [Google Scholar] [CrossRef]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: fundamental algorithms for scientific computing in Python. Nature Methods 2020, 17, 261–272. [Google Scholar] [CrossRef]
- McKinney, W.; et al. Data structures for statistical computing in python. In Proceedings of the 9th Python in Science Conference 2010; Volume 445, pp. 51–56.
- Hunter, J.D. Matplotlib: A 2D Graphics Environment. Computing in Science & Engineering 2007, 9, 90–95. [Google Scholar] [CrossRef]
- Waskom, M.L. seaborn: statistical data visualization. Journal of Open Source Software 2021, 9. [Google Scholar] [CrossRef]
- Vollpracht, A.; Weiler, L. Recycling of Slightly Contaminated Demolition Waste—Part 1: Inorganic Constituents. In Proceedings of the 3rd RILEM Spring Convention and Conference (RSCC 2020), Cham, 2021//, 2021; pp. 87–101. [Google Scholar]
- Galvín, A.P.; Ayuso, J.; Agrela, F.; Barbudo, A.; Jiménez, J.R. Analysis of leaching procedures for environmental risk assessment of recycled aggregate use in unpaved roads. Construction and Building Materials 2013, 40, 1207–1214. [Google Scholar] [CrossRef]
- Alonso-Santurde, R.; Coz, A.; Quijorna, N.; Viguri, J.R.; Andrés, A. Valorization of Foundry Sand in Clay Bricks at Industrial Scale. Journal of Industrial Ecology 2010, 14, 217–230. [Google Scholar] [CrossRef]
- Susset, B.; Maier, U.; Finkel, M.; Grathwohl, P. Weiterentwicklung von Kriterien zur Beurteilung des schadlosen und ordnungsgemäßen Einsatzes mineralischer Ersatzbaustoffe und Prüfung alternativer Wertevorschläge. 2018. [Google Scholar]
- Bary, B.; Sellier, A. Coupled moisture—carbon dioxide–calcium transfer model for carbonation of concrete. Cement and Concrete Research 2004, 34, 1859–1872. [Google Scholar] [CrossRef]
- Garrabrants, A.C.; Sanchez, F.; Kosson, D.S. Changes in constituent equilibrium leaching and pore water characteristics of a Portland cement mortar as a result of carbonation. Waste Management 2004, 24, 19–36. [Google Scholar] [CrossRef] [PubMed]
- Van Gerven, T.; Cornelis, G.; Vandoren, E.; Vandecasteele, C.; Garrabrants, A.C.; Sanchez, F.; Kosson, D.S. Effects of progressive carbonation on heavy metal leaching from cement-bound waste. AIChE Journal 2006, 52, 826–837. [Google Scholar] [CrossRef]
- Van Gerven, T.; Van Baelen, D.; Dutré, V.; Vandecasteele, C. Influence of carbonation and carbonation methods on leaching of metals from mortars. Cement and Concrete Research 2004, 34, 149–156. [Google Scholar] [CrossRef]
- Engelsen, C.J.; van der Sloot, H.A.; Wibetoe, G.; Petkovic, G.; Stoltenberg-Hansson, E.; Lund, W. Release of major elements from recycled concrete aggregates and geochemical modelling. Cement and Concrete Research 2009, 39, 446–459. [Google Scholar] [CrossRef]
- Müllauer, W.; Beddoe, R.E.; Heinz, D. Effect of carbonation, chloride and external sulphates on the leaching behaviour of major and trace elements from concrete. Cement and Concrete Composites 2012, 34, 618–626. [Google Scholar] [CrossRef]
- Reijonen, I.; Metzler, M.; Hartikainen, H. Impact of soil pH and organic matter on the chemical bioavailability of vanadium species: The underlying basis for risk assessment. Environmental Pollution 2016, 210, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Helmreich, B.; Hilliges, R.; Schriewer, A.; Horn, H. Runoff pollutants of a highly trafficked urban road – Correlation analysis and seasonal influences. Chemosphere 2010, 80, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Hilliges, R.; Endres, M.; Tiffert, A.; Brenner, E.; Marks, T. Characterization of road runoff with regard to seasonal variations, particle size distribution and the correlation of fine particles and pollutants. Water Science and Technology 2016, 75, 1169–1176. [Google Scholar] [CrossRef]
- Pérez, G.; López-Mesas, M.; Valiente, M. Assessment of Heavy Metals Remobilization by Fractionation: Comparison of Leaching Tests Applied to Roadside Sediments. Environmental Science & Technology 2008, 42, 2309–2315. [Google Scholar] [CrossRef]
- Amelung, W.; Blume, H.-P.; Fleige, H.; Horn, R.; Kandeler, E.; Kögel-Knabner, I.; Kretzschmar, R.; Stahr, K.; Wilke, B.-M. Scheffer/Schachtschabel - Lehrbuch der Bodenkunde, 17 ed.; Springer-Verlag GmbH Springer Nature: Berlin, 2018; p. 767. [Google Scholar]
- Boivin, P.; Saadé, M.; Pfeiffer, H.R.; Hammecker, C.; Degoumois, Y. DEPURATION OF HIGHWAY RUNOFF WATER INTO GRASS-COVERED EMBANKMENTS. Environmental Technology 2008, 29, 709–720. [Google Scholar] [CrossRef]
- Kocher, B.; Wessolek, G.; Stoffregen, H. Water and heavy metal transport in roadside soils. Pedosphere 2005, 15, 746–753. [Google Scholar]
- Kluge, B.; Wessolek, G. Heavy metal pattern and solute concentration in soils along the oldest highway of the world – the AVUS Autobahn. Environmental Monitoring and Assessment 2012, 184, 6469–6481. [Google Scholar] [CrossRef]
- Turer, D.G.; Maynard, B.J. Heavy metal contamination in highway soils. Comparison of Corpus Christi, Texas and Cincinnati, Ohio shows organic matter is key to mobility. Clean Technologies and Environmental Policy 2003, 4, 235–245. [Google Scholar] [CrossRef]
- Hatt, B.E.; Fletcher, T.D.; Deletic, A. Hydrologic and pollutant removal performance of stormwater biofiltration systems at the field scale. Journal of Hydrology 2009, 365, 310–321. [Google Scholar] [CrossRef]
- Davidson, C.M.; Duncan, A.L.; Littlejohn, D.; Ure, A.M.; Garden, L.M. A critical evaluation of the three-stage BCR sequential extraction procedure to assess the potential mobility and toxicity of heavy metals in industrially-contaminated land. Analytica Chimica Acta 1998, 363, 45–55. [Google Scholar] [CrossRef]
- Long, Y.-Y.; Hu, L.-F.; Fang, C.-R.; Wu, Y.-Y.; Shen, D.-S. An evaluation of the modified BCR sequential extraction procedure to assess the potential mobility of copper and zinc in MSW. Microchemical Journal 2009, 91, 1–5. [Google Scholar] [CrossRef]
- Sutherland, R.A.; Tack, F.M.G.; Ziegler, A.D. Road-deposited sediments in an urban environment: A first look at sequentially extracted element loads in grain size fractions. Journal of Hazardous Materials 2012, 225-226, 54–62. [Google Scholar] [CrossRef]
- Bacon, J.R.; Davidson, C.M. Is there a future for sequential chemical extraction? Analyst 2008, 133, 25–46. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, R.A.; Tack, F.M.G.; Tolosa, C.A.; Verloo, M.G. Operationally Defined Metal Fractions in Road Deposited Sediment, Honolulu, Hawaii. Journal of Environmental Quality 2000, 29, 1431–1439. [Google Scholar] [CrossRef]
- Kartal, Ş.; Aydın, Z.; Tokalıoğlu, Ş. Fractionation of metals in street sediment samples by using the BCR sequential extraction procedure and multivariate statistical elucidation of the data. Journal of Hazardous Materials 2006, 132, 80–89. [Google Scholar] [CrossRef]
- Pallewatta, S.; Weerasooriyagedara, M.; Bordoloi, S.; Sarmah, A.K.; Vithanage, M. Reprocessed construction and demolition waste as an adsorbent: An appraisal. Science of The Total Environment 2023, 882, 163340. [Google Scholar] [CrossRef]
- Kretzschmar, R.; Sticher, H. Transport of humic-coated iron oxide colloids in a sandy soil: Influence of Ca2+ and trace metals. Environmental Science and Technology 1997, 31, 3497–3504. [Google Scholar] [CrossRef]
- Arabyarmohammadi, H.; Salarirad, M.M.; Behnamfard, A. Characterization and utilization of clay-based construction and demolition wastes as adsorbents for zinc (II) removal from aqueous solutions: an equilibrium and kinetic study. Environmental Progress & Sustainable Energy 2014, 33, 777–789. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, H. Concentrations and chemical forms of potentially toxic metals in road-deposited sediments from different zones of Hangzhou, China. Journal of Environmental Sciences 2009, 21, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Tokalıoğlu, Ş.; Kartal, Ş. Multivariate analysis of the data and speciation of heavy metals in street dust samples from the Organized Industrial District in Kayseri (Turkey). Atmospheric Environment 2006, 40, 2797–2805. [Google Scholar] [CrossRef]
| Sample | CDW | Material ratios [% v/v] | Grain size distribution [% w/w] | kf [m/s] ± SD |
CaCO3 [% w/w] |
pH before treatment with TAR |
pH after treatment with TAR |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bricks | Concrete + mortar + plaster |
GWC | TS + SS |
Clay | Silt | Sand | Gravel | ||||||
| a75 | RCM1: 75 % | 41 | 27 | 10 | 22 | 9.6 | 13.8 | 40.7 | 35.9 | 1.8x10-3 ± 1.7x10-4 | 45.3 | 7.5 | 8.0 |
| a50 | RCM1: 50 % | 28 | 18 | 10 | 44 | 9.5 | 12.0 | 43.2 | 35.3 | 1.0x10-3 ± 7.6x10-5 | 40.7 | 7.5 | n.a. |
| a25 | RCM1: 25 % |
14 | 9 | 10 | 67 | 9.1 | 12.2 | 43.4 | 35.2 | 1.1x10-3 ± 8.1 x10-5 | 29.3 | 7.5 | n.a. |
| b75 | RCM2: 75 % | 20 | 48 | 10 | 22 | 8.9 | 12.4 | 42.1 | 36.6 | 2.2x10-4 ± 1.4x10-5 | 53.7 | 7.8 | 8.2 |
| b50 | RCM2: 50 % | 14 | 32 | 10 | 44 | 9.5 | 12.2 | 41.3 | 36.9 | 2.8x10-4 ± 1.6x10-5 | 46.7 | 7.8 | n.a. |
| b25 | RCM2: 25% | 7 | 16 | 10 | 67 | 10.5 | 11.3 | 42.9 | 35.3 | 3.1x10-4 ± 1.7x10-5 | 34.6 | 7.7 | n.a. |
| ctl | - | - | - | 10 | 90 | 9.3 | 11.4 | 43.7 | 35.7 | 9.6x10-4 ± 5.6x10-5 | 22.3 | 7.8 | 7.6 |
| Step | Fraction | Solution | Process description |
|---|---|---|---|
| S1 | Acid extractable | 40 mL acetic acid (0.11 mol/L) | Addition of the solution to the samples in centrifuge tubes;Overnight extraction (16 ± 2 h) in an end-over-end shaker at 30 ± 10 rpm and 21 ± 2°C;Centrifugation at 4000 g for 20 min to separate the extract from the technosol;Pipetting off the supernatant, filtering with 0.45 µm, and stabilizing with 50 µl of 65% HNO₃;Washing the residue with 20 mL of ultrapure water, shaking for 15 min, centrifuging for 20 min, pipetting off, and discarding the supernatant |
| S2 | Reducible | 40 mL hydroxylammonium chloride (0.5 mol/L) | Addition of the solution from a 1-L mixture (containing 25 ml of 2 mol/L HNO₃) to the residue;Continuation of the procedure as described in S1 |
| S3 | Oxidizable | 2x 10 mL hydrogen peroxide (8.8 mol/L);50 mL ammonium acetate (1 mol/L) | Addition of 10 ml H₂O₂ to the residue, covering the tubes, and reaction at 21 ± 2°C for 1 h with occasional manual shaking;Placement of the tubes in a water bath (85 ± 5°C), reduction of the volume to <3 mL (occasional manual shaking);Addition of 10 mL H₂O₂ (tubes in the water bath) and reaction until the volume is reduced to 1 mL (occasional manual shaking);Addition of 50 ml NH₄OAc (pH 2) and continuation of the procedure as described in S1 |
| S4 | Residue | HNO₃: HCl = 3:1 | Digestion of the residue with AR |
| Technosols | Soil | CDW mixtures | |||||||
|---|---|---|---|---|---|---|---|---|---|
| a75 | a50 | a25 | b75 | b50 | b25 | ctl | RCM1 | RCM2 | |
| pH [-] | 8.7 ±0.1 |
8.8 ±0.0 |
8.7 ±0.0 |
8.8 ±0.1 |
8.7 ±0.1 |
8.7 ±0.0 |
8.5 ±0.0 |
10.5 ±0.5 |
10.3 ±0.3 |
| EC [µS/cm] | 278 ±17.6 |
215 ±9.5 |
205 ±5.4 |
269 ±39.3 |
217 ±19.1 |
206 ±4.0 |
212 ±2.9 |
552 ±28.1 |
503 ±65.3 |
| Fluoride [mg/L] | 0.6 ±0.2 |
0.5 ±0.0 |
0.5 ±0.0 |
0.4 ±0.0 |
0.5 ±0.0 |
0.5 ±0.0 |
0.5 ±0.0 |
0.6 ±0.0 |
0.6 ±0.1 |
| Chloride [mg/L] | 1.6 ±0.9 |
< LOQ | < LOQ | < LOQ | 2.0 ±0.8 |
2.2 ±0.9 |
< LOQ | 3.7 ±1.9 |
1.4 ±0.6 |
| SO4 [mg/L] | 52.3 ±6.0 |
26.7 ±2.4 |
11.2 ±1.4 |
44.0 ±10.4 |
19.0 ±0.8 |
9.6 ±1.4 |
1.4 ±0.5 |
167 ±41.9 |
170 ±8.2 |
| Sb [µg/L] | < LOQ | 3.3 ±1.2 |
4.2 ±1.2 |
3.3 ±1.2 |
< LOQ | < LOQ | 3.3 ±1.2 |
< LOQ | < LOQ |
| As [µg/L] | 8.3 ±0.5 |
7.0 ±0.8 |
< LOQ | 4.8 ±1.6 |
5.7 ±0.9 |
< LOQ | < LOQ | < LOQ | < LOQ |
| Pb [µg/L] | < LOQ | 8.0 ±2.2 |
4.3 ±2.6 |
3.3 ±1.2 |
4.3 ±2.6 |
5.8 ±2.7 |
< LOQ | < LOQ | < LOQ |
| B [µg/L] | 150 ±8.2 |
96.7 ±17.0 |
70.0 ±0.0 |
100 ±8.2 |
103 ±26.2 |
73.3 ±4.7 |
< LOQ | 193 ±17.0 |
143 ±60.2 |
| Cd [µg/L] | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ |
| Cr [µg/L] | < LOQ | 4.3 ±2.6 |
< LOQ | < LOQ | < LOQ | < LOQ | < LOQ | 23.3 ±2.5 |
37.3 ±10.8 |
| Cr(VI) [µg/L] | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ |
25.0 ±2.9 |
38.7 ±10.9 |
| Co [µg/L] | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ |
| Cu [µg/L] | < LOQ | 3.3 ±1.2 |
3.3 ±1.2 |
< LOQ | < LOQ | 3.3 ±1.2 |
< LOQ | < LOQ | < LOQ |
| Mo [µg/L] | 8.0 ±0.8 |
5.3 ±0.5 |
6.0 ±0.0 |
6.3 ±0.5 |
5.3 ±0.5 |
4.8 ±1.6 |
4.5 ±1.5 |
9.8 ±5.3 |
2.5 ±0.0 |
| Ni [µg/L] | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ |
| Hg [µg/L] | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ |
| Se [µg/L] | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ |
| Tl [µg/L] | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ |
| V [µg/L] | 43.3 ±4.7 |
33.3 ±4.7 |
16.7 ±4.7 |
26.7 ±4.7 |
23.3 ±4.7 |
20.0 ±0.0 |
5.0 ±0.0 |
166.7 ±47.1 |
66.7 ±9.4 |
| Zn [µg/L] | < LOQ | 25.0 ±0.0 |
25.0 ±0.0 |
25.0 ±0.0 |
25.0 ±0.0 |
25.0 ±0.0 |
25.0 ±0.0 |
25.0 ±0.0 |
25.0 ±0.0 |
| Sn [µg/L] | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ |
| DOC [mg/L] | 5.7 ±0.5 |
6.3 ±0.5 |
6.7 ±0.5 |
5.7 ±0.5 |
5.7 ±0.5 |
7.3 ±1.2 |
6.0 ±0.8 |
n.a. | n.a. |
| Metal | Substrate | S1 Acid exchangeable |
S2 Reducible |
S3 Oxidizable |
S4 Residue |
Sum S1-S4 |
|---|---|---|---|---|---|---|
| Cu | a75 | 1.1 | -11.8 | 52.2 | 58.6 | 100 |
| b75 | 1.2 | -1.7 | 17.2 | 83.3 | 100 | |
| ctl | 0.8 | -0.1 | 3.9 | 95.5 | 100 | |
| Zn | a75 | 26.5 | 13.3 | 8.0 | 52.2 | 100 |
| b75 | 7.0 | 17.7 | 4.9 | 70.3 | 100 | |
| ctl | 0.2 | -0.1 | -6.2 | 106.1 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





