Submitted:
09 January 2025
Posted:
10 January 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body Mass Index |
| BWHS | Black Women’s Health Study |
| GD | Gestational diabetes |
| HbA1c | Hemoglobin A1c |
| IFG | Impaired fasting glucose |
| NHW | Non-Hispanic White |
| NSES | Neighborhood socioeconomic status |
| SUI | Stress urinary incontinence |
| T2D | Type 2 diabetes |
| UI | Urinary incontinence |
| US | United States |
References
- Grodstein, F.; Fretts, R.; Lifford, K.; Resnick, N.; Curhan, G. Association of age, race, and obstetric history with urinary symptoms among women in the Nurses' Health Study. Am. J. Obstet. Gynecol. 2003, 189, 428–434. [CrossRef]
- Minassian VA, Stewart WF, Wood GC. Urinary incontinence in women: variation in prevalence estimates and risk factors. Obstet Gynecol 2008;111:324-31.
- Milsom, I.; Gyhagen, M. Breaking news in the prediction of pelvic floor disorders. Best Pr. Res. Clin. Obstet. Gynaecol. 2019, 54, 41–48. [CrossRef]
- Patel, U.J.; Godecker, A.L.; Giles, D.L.M.; Brown, H.W.M. Updated Prevalence of Urinary Incontinence in Women: 2015–2018 National Population-Based Survey Data. Urogynecology 2022, 28, 181–187. [CrossRef]
- Abufaraj, M.; Xu, T.; Cao, C.; Siyam, A.; Isleem, U.; Massad, A.; Soria, F.; Shariat, S.F.; Sutcliffe, S.; Yang, L. Prevalence and trends in urinary incontinence among women in the United States, 2005–2018. 2021, 225, 166.e1–166.e12. [CrossRef]
- Landefeld, C.S.; Bowers, B.J.; Feld, A.D.; Hartmann, K.E.; Hoffman, E.; Ingber, M.J.; King, J.T.; McDougal, W.S.; Nelson, H.; Orav, E.J.; et al. National Institutes of Health State-of-the-Science Conference Statement: Prevention of Fecal and Urinary Incontinence in Adults. Ann. Intern. Med. 2008, 148, 449–458. [CrossRef]
- Bani-Issa, W.; Almomani, F.; Eldeirawi, K. Urinary incontinence among adult women with diabetes in Jordan: epidemiology, correlates and perceived impact on emotional and social well-being. J. Clin. Nurs. 2013, 23, 2451–2460. [CrossRef]
- Chong, E.C.; Khan, A.A.; Anger, J.T. The Financial Burden of Stress Urinary Incontinence Among Women in the United States. Curr. Urol. Rep. 2011, 12, 358–362. [CrossRef]
- Coyne, K.S.; Wein, A.; Nicholson, S.; Kvasz, M.; Chen, C.-I.; Milsom, I. Economic Burden of Urgency Urinary Incontinence in the United States: A Systematic Review. J. Manag. Care Pharm. 2014, 20, 130–140. [CrossRef]
- Coyne, K.S.; Sexton, C.C.; Clemens, J.; Thompson, C.L.; Chen, C.-I.; Bavendam, T.; Dmochowski, R. The Impact of OAB on Physical Activity in the United States: Results from OAB-POLL. Urology 2013, 82, 799–806. [CrossRef]
- Townsend, M.K.; Curhan, G.C.; Resnick, N.M.; Grodstein, F. The incidence of urinary incontinence across Asian, black, and white women in the United States. Am. J. Obstet. Gynecol. 2010, 202, 378.e1–378.e7. [CrossRef]
- Waetjen LE, Liao S, Johnson WO, et al. Factors associated with prevalent and incident urinary incontinence in a cohort of midlife women: a longitudinal analysis of data: study of women's health across the nation. Am J Epidemiol 2007;165:309-18.
- Danforth, K.N.; Townsend, M.K.; Lifford, K.; Curhan, G.C.; Resnick, N.M.; Grodstein, F. Risk factors for urinary incontinence among middle-aged women. Am. J. Obstet. Gynecol. 2006, 194, 339–345. [CrossRef]
- Phelan S, Kanaya AM, Subak LL, et al. Prevalence and risk factors for urinary incontinence in overweight and obese diabetic women: action for health in diabetes (look ahead) study. Diabetes Care 2009;32:1391-7.
- Matthews CA, Whitehead WE, Townsend MK, Grodstein F. Risk factors for urinary, fecal, or dual incontinence in the Nurses' Health Study. Obstet Gynecol 2013;122:539-45.
- Komesu, Y.M.; Schrader, R.M.; Ketai, L.H.; Rogers, R.G.; Dunivan, G.C. Epidemiology of mixed, stress, and urgency urinary incontinence in middle-aged/older women: the importance of incontinence history. Int. Urogynecology J. 2015, 27, 763–772. [CrossRef]
- Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care 2002;25:1862-8.
- Bellamy, L.; Casas, J.-P.; Hingorani, A.D.; Williams, D. Type 2 diabetes mellitus after gestational diabetes: A systematic review and meta-analysis. Lancet 2009, 373, 1773–1779. [CrossRef]
- Brown JS, Vittinghoff E, Lin F, Nyberg LM, Kusek JW, Kanaya AM. Prevalence and risk factors for urinary incontinence in women with type 2 diabetes and impaired fasting glucose: findings from the National Health and Nutrition Examination Survey (NHANES) 2001-2002. Diabetes Care 2006;29:1307-12.
- Danforth KN, Townsend MK, Curhan GC, Resnick NM, Grodstein F. Type 2 diabetes mellitus and risk of stress, urge and mixed urinary incontinence. J Urol 2009;181:193-7.
- Devore, E.E.; Minassian, V.A.; Grodstein, F. Factors associated with persistent urinary incontinence. Am. J. Obstet. Gynecol. 2013, 209, 145.e1–145.e6. [CrossRef]
- Bani-Issa, W.A.; Halabi, J.O.; Abdullah, A.R.; Hasan, H.A.; Raigangar, V.L. Prevalence and Risk Factors for Incontinence Among Emirati Women With Diabetes. J. Transcult. Nurs. 2013, 25, 42–50. [CrossRef]
- Izci, Y.; Topsever, P.; Filiz, T.M.; Çınar, N.D.; Uludağ, C.; Lagro-Janssen, T. The association between diabetes mellitus and urinary incontinence in adult women. Int. Urogynecology J. 2009, 20, 947–952. [CrossRef]
- Metzger, B.E.; Buchanan, T.A.; Coustan, D.R.; de Leiva, A.; Dunger, D.B.; Hadden, D.R.; Hod, M.; Kitzmiller, J.L.; Kjos, S.L.; Oats, J.N.; et al. Summary and Recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care 2007, 30, S251–S260. [CrossRef]
- Ashwal E, Hod M. Gestational diabetes mellitus: Where are we now? Clin Chim Acta 2015;451:14-20.
- American College of Obstetrics and Gynecologists. Practice Bulletin No. 137: Gestational diabetes mellitus. Obstet Gynecol 2013;122:406-16.
- Varner, M.W.; Rice, M.M.; Landon, M.B.; Casey, B.M.; Reddy, U.M.; Wapner, R.J.; Rouse, D.J.; Tita, A.T.N.; Thorp, J.M.; Chien, E.K.M.; et al. Pregnancies After the Diagnosis of Mild Gestational Diabetes Mellitus and Risk of Cardiometabolic Disorders. Obstet. Gynecol. 2017, 129, 273–280. [CrossRef]
- Shah, N.S.; Wang, M.C.; Freaney, P.M.; Perak, A.M.; Carnethon, M.R.; Kandula, N.R.; Gunderson, E.P.; Bullard, K.M.; Grobman, W.A.; O’brien, M.J.; et al. Trends in Gestational Diabetes at First Live Birth by Race and Ethnicity in the US, 2011-2019. JAMA 2021, 326, 660–669. [CrossRef]
- Gregory, E.C.; Ely, D.M. Trends and Characteristics in Gestational Diabetes: United States, 2016-2020.. 2022, 71, 1–15.
- Wang, Y.; Chen, L.; Horswell, R.; Xiao, K.; Besse, J.; Johnson, J.; Ryan, D.H.; Hu, G. Racial Differences in the Association Between Gestational Diabetes Mellitus and Risk of Type 2 Diabetes. J. Women's Heal. 2012, 21, 628–633. [CrossRef]
- Xiang, A.H.; Li, B.H.; Black, M.H.; Sacks, D.A.; Buchanan, T.A.; Jacobsen, S.J.; Lawrence, J.M. Racial and ethnic disparities in diabetes risk after gestational diabetes mellitus. Diabetologia 2011, 54, 3016–3021. [CrossRef]
- Vesentini, G.; Barbosa, A.M.P.; Damasceno, D.C.; Marini, G.; Piculo, F.; Matheus, S.M.M.; Hallur, R.L.S.; Nunes, S.K.; Catinelli, B.B.; Magalhães, C.G.; et al. Alterations in the structural characteristics of rectus abdominis muscles caused by diabetes and pregnancy: A comparative study of the rat model and women. PLOS ONE 2020, 15, e0231096. [CrossRef]
- Prudencio, C.B.; Nunes, S.K.; Pinheiro, F.A.; Filho, C.I.S.; Antônio, F.I.; Nava, G.T.d.A.; Rudge, M.V.C.; Barbosa, A.M.P.; Calderon, I.M.P.; Souza, F.P.; et al. Relaxin-2 during pregnancy according to glycemia, continence status, and pelvic floor muscle function. Int. Urogynecology J. 2022, 33, 3203–3211. [CrossRef]
- Kim, C.; McEwen, L.N.; Sarma, A.V.; Piette, J.D.; Herman, W.H. Stress Urinary Incontinence in Women with a History of Gestational Diabetes Mellitus. J. Women's Heal. 2008, 17, 783–792. [CrossRef]
- Chuang, C.; Lin, I.; Horng, H.; Hsiao, Y.; Shyu, I.; Chou, P. The impact of gestational diabetes mellitus on postpartum urinary incontinence: a longitudinal cohort study on singleton pregnancies. BJOG: Int. J. Obstet. Gynaecol. 2012, 119, 1334–1343. [CrossRef]
- Barbosa, A.M.P.; Dias, A.; Marini, G.; Calderon, I.M.P.; Witkin, S.; Rudge, M.V.C. Urinary incontinence and vaginal squeeze pressure two years post-cesarean delivery in primiparous women with previous gestational diabetes mellitus. 2011, 66, 1341–1345. [CrossRef]
- Sartorao Filho CI, Nunes SK, Magyori ABM, Calderon IMP, Barbosa AMP, Rudge MVC. The role of Gestational Diabetes Mellitus and pelvic floor 3D-ultrasound assessment during pregnancy predicting urinary incontinence: a prospective cohort study. BMC Pregnancy Childbirth 2023;23:637.
- American Diabetes A. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020;43:S14-S31.
- Rosenberg L, Adams-Campbell L, Palmer JR. The Black Women's Health Study: a follow-up study for causes and preventions of illness. J Am Med Womens Assoc (1972) 1995;50:56-8.
- Russell C, Palmer JR, Adams-Campbell LL, Rosenberg L. Follow-up of a large cohort of Black women. Am J Epidemiol 2001;154:845-53.
- Russell CW, Boggs DA, Palmer JR, Rosenberg L. Use of a web-based questionnaire in the Black Women's Health Study. Am J Epidemiol 2010;172:1286-91.
- Wise, L.A.; Rosenberg, L.; Radin, R.G.; Mattox, C.; Yang, E.B.; Palmer, J.R.; Seddon, J.M. A Prospective Study of Diabetes, Lifestyle Factors, and Glaucoma Among African-American Women. Ann. Epidemiology 2011, 21, 430–439. [CrossRef]
- Palmer JR, Castro-Webb N, Bertrand K, Bethea TN, Denis GV. Type II Diabetes and Incidence of Estrogen Receptor Negative Breast Cancer in African American Women. Cancer Res 2017;77:6462-9.
- Block, G.; Hartman, A.M.; Naughton, D. A Reduced Dietary Questionnaire: Development and Validation. Epidemiology 1990, 1, 58–64. [CrossRef]
- Boggs DA, Palmer JR, Spiegelman D, Stampfer MJ, Adams-Campbell LL, Rosenberg L. Dietary patterns and 14-y weight gain in African American women. Am J Clin Nutr 2011;94:86-94.
- Coogan PF, Cozier YC, Krishnan S, et al. Neighborhood socioeconomic status in relation to 10-year weight gain in the Black Women's Health Study. Obesity (Silver Spring) 2010;18:2064-5.
- US Census Bureau. Census 2000 basics. Washington, DC: US Government Printing Office; 2002.
- Villarroel, M.A.; Lucas, J.W. QuickStats: Percentage of Mothers with Gestational Diabetes,* by Maternal Age — National Vital Statistics System, United States, 2016 and 2021. Mmwr-Morbidity Mortal. Wkly. Rep. 2023, 72, 16–132. [CrossRef]
- Bloomgarden, Z.T. Gestational Diabetes Mellitus and Obesity. Diabetes Care 2010, 33, e60–e65. [CrossRef]
- Alvaro, R.; Araco, F.; Gravante, G.; Sorge, R.; Overton, J.; Vellone, E.; Venturini, G.; Piccione, E. Epidemiological aspects of urinary incontinence in a female population of an Italian region. Int. Urogynecology J. 2010, 21, 873–883. [CrossRef]
- Davidson, M.B.; Landsman, P.B.; Alexander, C.M. Lowering the Criterion for Impaired Fasting Glucose Will Not Provide Clinical Benefit. Diabetes Care 2003, 26, 3329–3330. [CrossRef]
- Benjamin SM, Valdez R, Geiss LS, Rolka DB, Narayan KM. Estimated number of adults with prediabetes in the US in 2000: opportunities for prevention. Diabetes Care 2003;26:645-9.
- Ying, Y.; Xu, L.; Huang, R.; Chen, T.; Wang, X.; Li, K.; Tang, L. Relationship Between Blood Glucose Level and Prevalence and Frequency of Stress Urinary Incontinence in Women. Urogynecology 2021, 28, 304–310. [CrossRef]
- Brown, J.S.; Nyberg, L.M.; Kusek, J.W.; Burgio, K.L.; Diokno, A.C.; Foldspang, A.; Fultz, N.H.; Herzog, A.; Hunskaar, S.; Milsom, I.; et al. Proceedings of the national institute of diabetes and digestive and kidney diseases international symposium on epidemiologic issues in urinary incontinence in women. Am. J. Obstet. Gynecol. 2003, 188, S77–S88. [CrossRef]
- Brown, J.S. Urinary Incontinence: An Important and Underrecognized Complication of Type 2 Diabetes Mellitus. J. Am. Geriatr. Soc. 2005, 53, 2028–2029. [CrossRef]
- Brown JS, Luft J. Women & diabetes. Urinary incontinence. Diabetes Self Manag 2005;22:48-50.
- Smith DB. Urinary incontinence and diabetes: a review. J Wound Ostomy Continence Nurs 2006;33:619-23.
- Brown, J.S.; Wessells, H.; Chancellor, M.B.; Howards, S.S.; Stamm, W.E.; Stapleton, A.E.; Steers, W.D.; Eeden, S.K.V.D.; McVary, K.T. Urologic Complications of Diabetes. Diabetes Care 2005, 28, 177–185. [CrossRef]
- Rizk, D.E.E.; Padmanabhan, R.K.; Tariq, S.; Shafiullah, M.; Ahmed, I. Ultra-structural morphological abnormalities of the urinary bladder in streptozotocin-induced diabetic female rats. Int. Urogynecology J. 2005, 17, 143–154. [CrossRef]
- Stevens LA, Sellers DJ, McKay NG, Chapple CR, Chess-Williams R. Muscarinic receptor function, density and G-protein coupling in the overactive diabetic rat bladder. Auton Autacoid Pharmacol 2006;26:303-9.
- Marini, G.; Piculo, F.; Vesentini, G.; Damasceno, D.C.; Delella, F.K.; Calderon, I.M.; Daneshgari, F.; Felisbino, S.L.; Barbosa, A.M.; Rudge, M.V. The influence of hyperglycemia on the remodeling of urethral connective tissue in pregnant rats. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 221, 81–88. [CrossRef]
- Rudge, M.V.C.; The Diamater Study Group; Souza, F.P.; Abbade, J.F.; Hallur, R.L.S.; Marcondes, J.P.C.; Piculo, F.; Marini, G.; Vesentini, G.; Thabane, L.; et al. Study protocol to investigate biomolecular muscle profile as predictors of long-term urinary incontinence in women with gestational diabetes mellitus. BMC Pregnancy Childbirth 2020, 20, 1–14. [CrossRef]
- Vesentini, G.; El Dib, R.; Righesso, L.A.R.; Piculo, F.; Marini, G.; Ferraz, G.A.R.; Calderon, I.d.M.P.; Barbosa, A.M.P.; Rudge, M.V.C. Pelvic floor and abdominal muscle cocontraction in women with and without pelvic floor dysfunction: a systematic review and meta-analysis. Clinics 2019, 74, e1319. [CrossRef]
- Sapsford, R.R.; Hodges, P.W. Contraction of the pelvic floor muscles during abdominal maneuvers. Arch. Phys. Med. Rehabilitation 2001, 82, 1081–1088. [CrossRef]
- Catinelli, B.B.; Rossignoli, P.S.; Floriano, J.F.; Carr, A.M.; de Oliveira, R.G.; dos Santos, N.J.; Úbeda, L.C.C.; Spadella, M.A.; Hallur, R.L.S.; Sobrevia, L.; et al. Reversal of diabetic-induced myopathy by swimming exercise in pregnant rats: a translational intervention study. Sci. Rep. 2022, 12, 1–12. [CrossRef]
- Richardson, D.K.; Kashyap, S.; Bajaj, M.; Cusi, K.; Mandarino, S.J.; Finlayson, J.; DeFronzo, R.A.; Jenkinson, C.P.; Mandarino, L.J. Lipid Infusion Decreases the Expression of Nuclear Encoded Mitochondrial Genes and Increases the Expression of Extracellular Matrix Genes in Human Skeletal Muscle. J. Biol. Chem. 2005, 280, 10290–10297. [CrossRef]
- Berria, R.; Wang, L.; Richardson, D.K.; Finlayson, J.; Belfort, R.; Pratipanawatr, T.; De Filippis, E.A.; Kashyap, S.; Mandarino, L.J. Increased collagen content in insulin-resistant skeletal muscle. Am. J. Physiol. Metab. 2006, 290, E560–E565. [CrossRef]
- Krakowiak, P.; Walker, C.K.; Tancredi, D.J.; Hertz-Picciotto, I. Maternal Recall Versus Medical Records of Metabolic Conditions from the Prenatal Period: A Validation Study. Matern. Child Heal. J. 2015, 19, 1925–1935. [CrossRef]
- Hosler, A.S.; Nayak, S.G.; Radigan, A.M. Agreement Between Self-Report and Birth Certificate for Gestational Diabetes Mellitus: New York State PRAMS. Matern. Child Heal. J. 2009, 14, 786–789. [CrossRef]
- Diokno AC, Normolle DP, Brown MB, Herzog AR. Urodynamic tests for female geriatric urinary incontinence. Urology 1990;36:431-9.
- Herzog, A.R.; Fultz, N.H. Prevalence and Incidence of Urinary Incontinence in Community-Dwelling Populations. J. Am. Geriatr. Soc. 1990, 38, 273–281. [CrossRef]
- Newburger EC, Curry A. Educational Attainment in the United States, March 1999. Current Population Reports: Washington, DC: U.S. Department of Commerce; 2000.
- Vimalananda VG, Palmer JR, Gerlovin H, et al. Night-shift work and incident diabetes among African-American women. Diabetologia 2015;58:699-706.
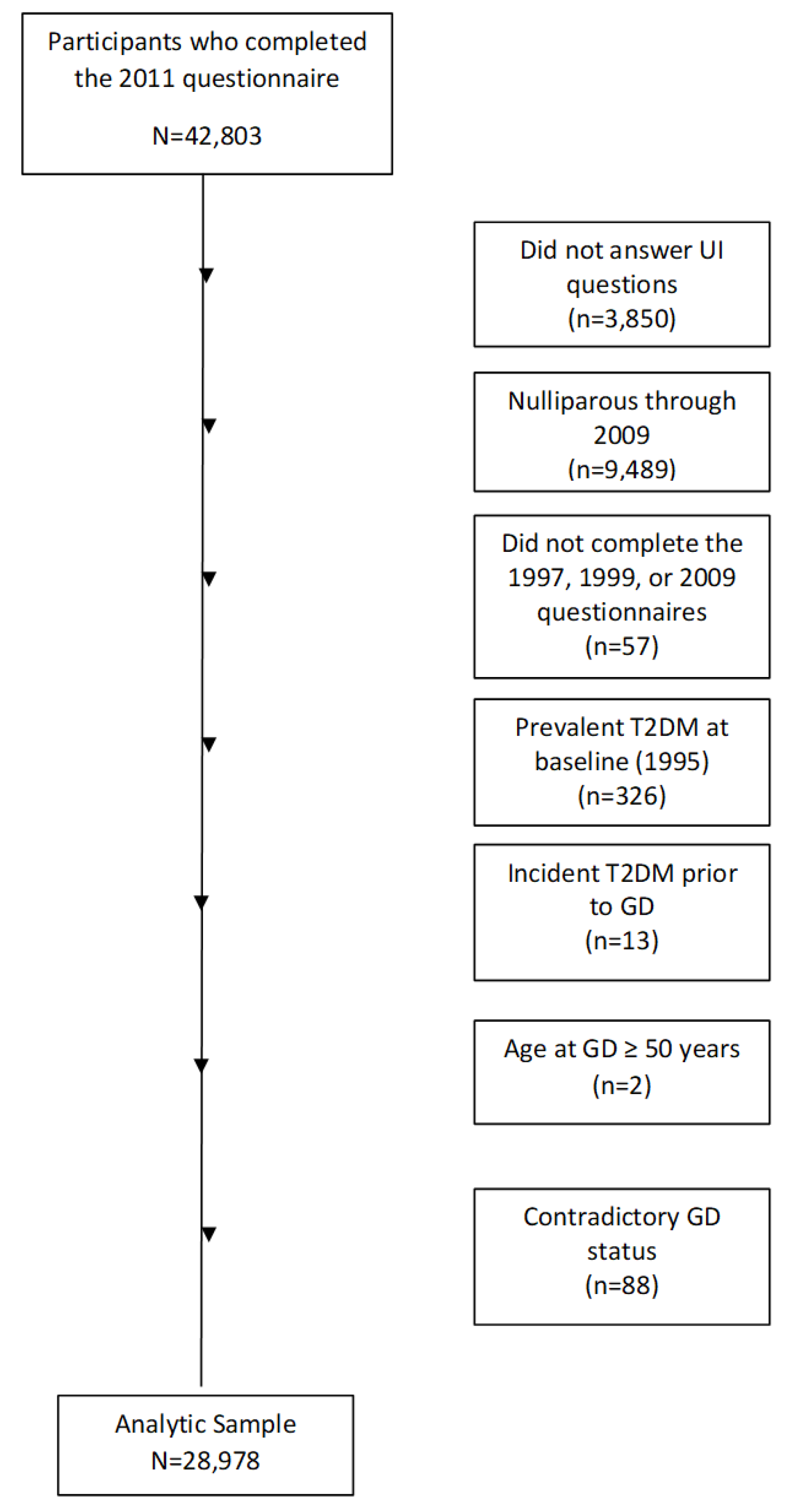
| Frequency of Urinary Incontinence | Type of Urinary Incontinence (any frequency)a | ||||||||
| Never (n=14045) | ≤ 1/month (n=8087) | 2-3 month (n=2523) | ≥ 1 week (n=4323) | Stress (n=4946) | Urgency (n=4886) | Mixed (n=3123) | Other (n=1680) | ||
| Age, years, mean (SD) | 24.2 (6.2) | 24.1 (6.2) | 23.8 (6.2) | 23.2 (6.0) | 24.3 (6.2) | 24.4 (6.3) | 23.8 (6.1) | 23.0 (6.0) | |
| Body Mass Index, kg/m2, mean (SD) | 27.3 (5.9) | 28.1 (6.2) | 29.2 (6.8) | 30.2 (7.0) | 27.3 (5.9) | 28.1 (6.0) | 29.0 (6.7) | 30.0 (7.0) | |
| Parity (%) | |||||||||
| 1 birth | 47 | 44 | 43 | 40 | 44 | 41 | 42 | 43 | |
| 2 birth | 30 | 32 | 32 | 31 | 33 | 32 | 31 | 29 | |
| 3+ birth | 23 | 24 | 25 | 29 | 23 | 27 | 27 | 27 | |
| Years of education (%) | |||||||||
| ≤12 | 19 | 17 | 19 | 20 | 17 | 18 | 20 | 16 | |
| 13-15 | 36 | 36 | 37 | 36 | 37 | 35 | 37 | 37 | |
| ≥16 | 44 | 47 | 44 | 44 | 46 | 47 | 43 | 47 | |
| Neighborhood Socioeconomic Status, quintiles (%) | |||||||||
| Q1 (low) | 18 | 18 | 19 | 18 | 17 | 18 | 20 | 19 | |
| Q5 (high) | 19 | 19 | 18 | 18 | 20 | 19 | 16 | 18 | |
| Western Diet, quintiles (%) | |||||||||
| Q1 (low) | 20 | 19 | 17 | 17 | 17 | 18 | 16 | 21 | |
| Q5 (high) | 17 | 19 | 18 | 21 | 19 | 20 | 21 | 17 | |
| Prudent diet, quintiles (%) | |||||||||
| Q1 (low) | 19 | 18 | 19 | 18 | 19 | 17 | 19 | 18 | |
| Q5 (high) | 19 | 18 | 18 | 18 | 17 | 20 | 16 | 19 | |
| Vigorous activity, hours/week (%) | |||||||||
| None | 35 | 34 | 36 | 42 | 34 | 38 | 38 | 35 | |
| < 5 | 49 | 52 | 49 | 45 | 52 | 48 | 47 | 51 | |
| ≥ 5 | 11 | 10 | 10 | 9 | 10 | 9 | 10 | 10 | |
| Cigarette smoking status, (%) | |||||||||
| Current | 13 | 13 | 15 | 17 | 13 | 15 | 16 | 13 | |
| past | 21 | 22 | 23 | 25 | 21 | 23 | 24 | 25 | |
| never | 66 | 65 | 62 | 58 | 65 | 62 | 60 | 62 | |
| Recent diuretic use (2011), (%) | |||||||||
| Yes | 10 | 11 | 13 | 15 | 9 | 14 | 14 | 12 | |
| Urinary Incontinence Frequency | Urinary Incontinence Type | |||||||||
| OR 95% CIa | OR (95% CI) a | |||||||||
| Ever Gestational Diabetesb,c | Never | ≤ 1/month | 2-3/ month | ≥ 1 week | Stress | Urge | Mixed | Otherd | ||
| n | Yes | 707 | 457 | 179 | 268 | 304 | 262 | 212 | 103 | |
| Total | 14,045 | 8,087 | 2,523 | 4,323 | 4,946 | 4,886 | 3,123 | 1,680 | ||
| Age-Adjusted | REF | 1.14 (1.01, 1.29) |
1.48 (1.25, 1.75) |
1.32 (1.14, 1.52) |
1.24 (1.08, 1.43) |
1.10 (0.95, 1.27) |
1.48 (1.25, 1.72) |
1.24 (1.00, 1.54) |
||
| Multivariable- Adjustede |
REF | 1.09 (0.97, 1.23) |
1.36 (1.15, 1.62) |
1.18 (1.02, 1.37) |
1.18 (1.03, 1.36) |
1.03 (0.88, 1.19) |
1.31 (1.12, 1.54) |
1.16 (0.94, 1.44) |
||
| Ever Type 2 Diabetesb,f | ||||||||||
| n | Yes | 1,883 | 1,244 | 466 | 921 | 717 | 916 | 669 | 265 | |
| Total | 14,045 | 8,087 | 2,523 | 4,323 | 4,946 | 4,886 | 3,123 | 1,680 | ||
| Age-Adjusted | REF | 1.17 (1.08, 1.26) |
1.44 (1.28, 1.61) |
1.68 (1.54, 1.83) |
1.09 (1.00, 1.20) |
1.46 (1.34, 1.60) |
1.68 (1.52, 1.85) |
1.20 (1.05, 1.38) |
||
| Multivariable- Adjustede |
REF | 1.07 (0.99, 1.16) |
1.15 (1.02, 1.30) |
1.16 (1.06, 1.27) |
1.02 (0.92, 1.12) |
1.16 (1.06, 1.28) |
1.23 (1.11, 1.36) |
0.99 (0.85, 1.14) |
||
| Urinary Incontinence Frequencya | Urinary Incontinence Typea | |||||||||
| OR 95% CI | OR (95% CI) | |||||||||
| No T2Db | Never | ≤ 1/month | 2-3 month | ≥ 1 week | Stress | Urge | Mixed | Otherc | ||
| Ever GDd | Yes | 447 | 281 | 103 | 151 | 194 | 160 | 109 | 63 | |
| Total | 12,162 | 6,843 | 2,057 | 3,402 | 4,229 | 3,970 | 2,454 | 1,415 | ||
| Age-Adjusted | REF | 1.13 (0.97, 1.32) |
1.41 (1.13, 1.75) |
1.27 (1.05, 1.54) |
1.26 (1.06, 1.50) |
1.12 (0.93, 1.35) |
1.28 (1.04, 1.59) |
1.23 (0.94, 1.61) |
||
| Multivariable-Adjustede | REF | 1.11 (0.95, 1.29) |
1.36 (1.09, 1.69) |
1.23 (1.01, 1.49) |
1.23 (1.03, 1.46) |
1.10 (0.91, 1.32) |
1.23 (0.99, 1.53) |
1.19 (0.91, 1.56) |
||
| T2Db | ||||||||||
| Ever GDd | Yes | 260 | 176 | 76 | 117 | 110 | 102 | 103 | 110 | |
| Total | 1,883 | 1,244 | 466 | 921 | 717 | 916 | 669 | 265 | ||
| Age-Adjusted | REF | 1.01 (0.82, 1.25) |
1.27 (0.96, 1.70) |
0.93 (0.73, 1.18) |
1.12 (0.87, 1.43) |
0.76 (0.59, 0.98) |
1.21 (0.94, 1.56) |
1.10 (0.76, 1.60) |
||
| Multivariable- Adjustede |
REF | 1.00 (0.80, 1.24) |
1.32 (0.98, 1.76) |
0.95 (0.74, 1.21) |
1.11 (0.86, 1.43) |
0.78 (0.61, 1.01) |
1.19 (0.92, 1.55) |
1.13 (0.77, 1.66) |
||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





