Introduction
Tooth loss has been a longstanding issue throughout human history, prompting the exploration of dental implant solutions. In ancient times, diverse materials, such as ivory, bone, metals, and precious stones, were employed to create replicas mimicking natural teeth.
Notably, the esteemed surgeon Ambrosius Paré utilized freshly extracted noncarious teeth as substitutes. The inception of modern dental implantology emerged in the 19th century and was marked by advancements, including porcelain and gutta-percha implants.
gold-plated tin capsules with gutta-percha, and endosseous implants constructed from iridio-platinum cylinders. In subsequent decades, there was limited progress until the late 1930s and 1940s, when the Strock brothers experimented with Vitallium screw implants and introduced the endodontic implant. The period from 1950--1970 witnessed notable developments, encompassing innovations such as blade vents by Linkow and ceramic bone screws by Sandhaus. After 1970, increased attention was devoted to comprehending the factors influencing the clinical success or failure of dental implants. However, challenges arose owing to the absence of standardized evaluation criteria, and studies have focused predominantly on a single implant type. An exception to this was Cranin et al.'s statistical evaluation of clinically applied endosteal implants, which employed stringent success criteria.
Histological examinations were conducted in conjunction with clinical evaluations to investigate the characteristics of the dental implants. The findings revealed the close adaptation of bone and attachment of the gingival epithelium, as well as the occurrence of fibrous encapsulation and epithelial downgrowth. These investigations highlighted the critical role of oral mucosa penetration in oral implantology and emphasized the importance of maintaining gingival health for long-term implant functionality. Consequently, the development of dental implants has focused on achieving direct implant‒bone contact without intervening in connective tissue. In the 1970s, Brånemark introduced threaded screw implants made from highly corrosion-resistant titanium with a tenacious titanium oxide (TiO2) passivation film on the surface. Substantial advancements have since been made in dental implantology, rendering dental implants indispensable in clinical dentistry. However, challenges lie ahead owing to the growing elderly population and medical conditions, such as diabetes, osteoporosis, obesity, and medication use, which can impede bone regeneration around implants and jeopardize their high success rates. Therefore, ongoing research endeavors are imperative to develop dental implants that actively stimulate bone formation and enhance long-term outcomes.
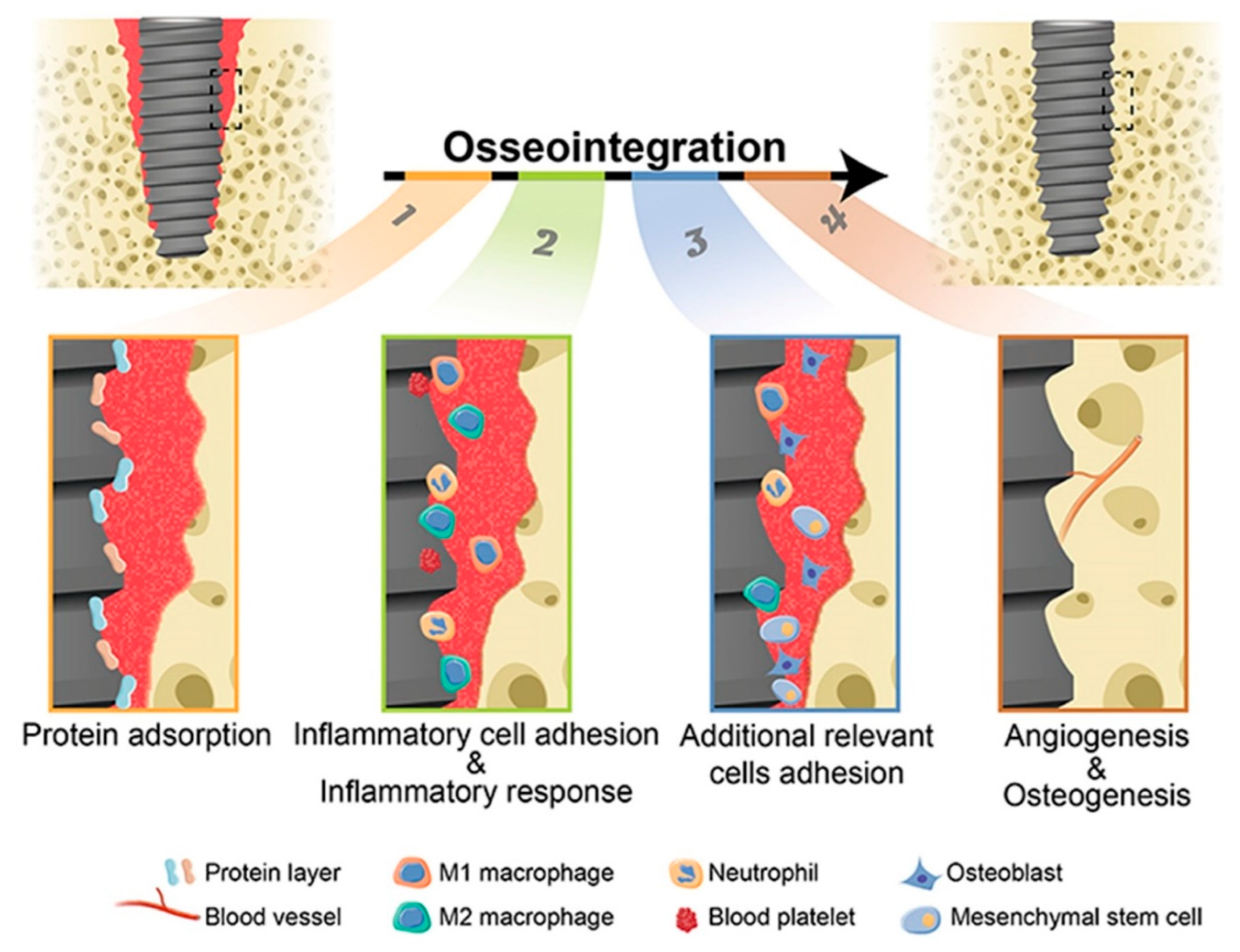
Osseointegration, which refers to the successful incorporation of dental implants into the jawbone, is pivotal in ensuring their enduring efficacy. This intricate process involves a series of complex healing mechanisms, commencing with the activation of inflammatory and bone cells at the interface between the implant and bone, followed by subsequent bone regeneration and mineralization. The attainment of optimal bone mineralization is paramount, as it establishes a robust bone-to-implant connection, thereby fostering biomechanical stability. Conversely, compromised bone quality poses a notable risk factor for implant failure, underscoring the imperative for continuous scholarly exploration to increase osseointegration. This necessitates advancements in dental implant design, surface characteristics, and placement techniques to optimize the establishment of a robust bone-to-implant interface.
Recent advancements in the field of dental implant design have focused on the utilization of threaded cylindrical or conical shapes, which serve to increase the surface area available for integration with bone tissue, thereby promoting sustained stability over extended periods. Additionally, researchers have explored the introduction of macroporosity on implant surfaces through diverse methodologies, with the aim of facilitating osseointegration. Notably, commercially pure titanium, distinguished by its exceptional biocompatibility and mechanical properties, remains the preferred biomaterial for dental implants. The incorporation of alloying elements, such as zirconium, into titanium implants has been investigated as a means to increase their mechanical strength, thereby enabling the implementation of implants with reduced dimensions. However, it is important to note that the adoption of zirconia as an implant material is motivated primarily by aesthetic considerations rather than its impact on bone biology. Collectively, these design innovations strive to optimize the functional attributes and long-term effectiveness of dental implants (
Figure 1) [
1].
In the evolving field of implant technology, advanced implant materials and surface engineering have led to significant advancements in the efficacy of dental and orthopedic implants. This research emphasizes the pivotal role of nanotechnology in fostering osseointegration by improving cellular interactions and facilitating accelerated healing processes. Furthermore, these findings highlight the critical importance of bioactive coatings and surface modifications, which establish conducive environments for bone growth and tissue integration, thereby enabling implants to operate as natural extensions of the human body. The emergence of biocompatible materials, such as zirconia and polymer-based implants, presents promising alternatives that combine mechanical strength with aesthetic benefits, thereby minimizing complications and enhancing patient outcomes. Collectively, these innovations signify a transformative shift in implant technology, with the goal of delivering safer and more effective solutions for patients [
2,
3,
4].
This comprehensive review examines recent advancements in implant dentistry, including cutting-edge biomaterials, innovative coatings, tissue engineering strategies, and revolutionary technologies for assessment. It analyzes advanced imaging modalities and biomimetic approaches in implant design. By synthesizing the literature, this scholarly review provides an overview of notable advancements in implant dentistry, contributing to academic understanding and guiding future research for improved patient care.
Revolutionary Technologies in Implant Dentistry
Magnetic Resonance Imaging (MRI) in Dental Implant Assessment and Optical Coherence Tomography (OCT) for Soft Tissue Evaluation:
In implant dentistry, magnetic resonance imaging (MRI) may serve as an alternative imaging technique. While prior clinical studies suggest that MRI data could inform implant design, they have not considered guided implant surgery. Advanced 3 Tesla (3T) MRI scanners, which utilize specialized coils and 3D isotropic resolution sequences (such as 3D T1-weighted black bone sequences), significantly enhance dental imaging by improving the resolution and signal-to-noise ratio, reducing the acquisition time, and minimizing artifacts. Studies have shown good agreement between cone beam computed tomography (CBCT) and magnetic resonance imaging (MRI) in assessing bone dimensions. To integrate MRI data effectively into computer-assisted surgical planning, it is essential to evaluate the creation of virtual 3D models of craniofacial bone in an STL format on the basis of MRI. Ultimately, hybrid models combining MRI-derived 3D jaw models with precise digital data of tooth surfaces can facilitate CAD/CAM manufacturing of surgical guides, highlighting the increasing importance of MRI in implant design [
5].
MRI has become a vital component of contemporary medical diagnosis. It is a vital tool because of its noninvasiveness, absence of ionizing radiation, and remarkable soft tissue contrast. Without the use of contrast agents, MRI is quite good at differentiating between different soft tissues. This ability supports treatment planning, accurate tumor location, and early illness diagnosis. Furthermore, MRI offers high-resolution images in three different planes (sagittal, axial, and coronal), enabling medical professionals to thoroughly examine human anatomy. Since MRI does not use ionizing radiation, it is safe for both pregnant women and children. It offers precise pictures for evaluating pediatric cancers, brain development, and congenital abnormalities. Additionally, MRI may be used to visualize blood arteries without intrusive treatments because of its ability to detect fluids and blood flow (a process known as MRangiography). Furthermore, as diffusion-weighted imaging (DWI) shows, it enables quantitative measurement of the fluid volume within lesions. Finally, by monitoring changes in blood flow during cognitive tasks, functional MRI provides information on a patient's brain activity in addition to their morphology [
6].
One noninvasive imaging technique that is becoming increasingly popular in dentistry is optical coherence tomography (OCT). With micrometric resolution, it can provide cross-sectional pictures of both soft and hard tissues. Similar to the fundamental idea of medical ultrasound imaging, OCT images are produced by measuring the intensity and time delay of the near-infrared light that is reflected or backscattered from the tissue structure via low coherence interferometry. The fast capture speed of OCT allows it to produce near real-time or video rate in situ pictures of tissues [
7]. OCT has quickly moved from the bench to the bedside and is being used extensively in dentistry and other healthcare fields. The main characteristics of OCT that make it a desirable diagnostic tool are its ability to measure the flow and spatial structural arrangement of the tissues, its use of a harmless light source, its fiber-optic-based systems that make it portable and pliable, and its ability to render images with a resolution of 0.5 μm. A number of variables affect how well a dental implant is placed. Biological factors that may be precisely evaluated via OCT devices include peri-implant mucositis, fit at the implant–abutment interface, and the proximity of the implant to the neurovascular bundle. Moreover, problems with implant-supported prostheses are frequently caused by an incorrect fit at the implant-abutment contact and leftover cement in submucosal locations. These problems may be efficiently assessed via optical coherence tomography (OCT), which makes it possible to find cement residues during cementation. OCT is also helpful in evaluating peri-implant mucositis, which allows for early detection and treatment to reduce the chance of implant failure [
8,
9].
Advanced imaging modalities: 3D printing and cone beam CT:
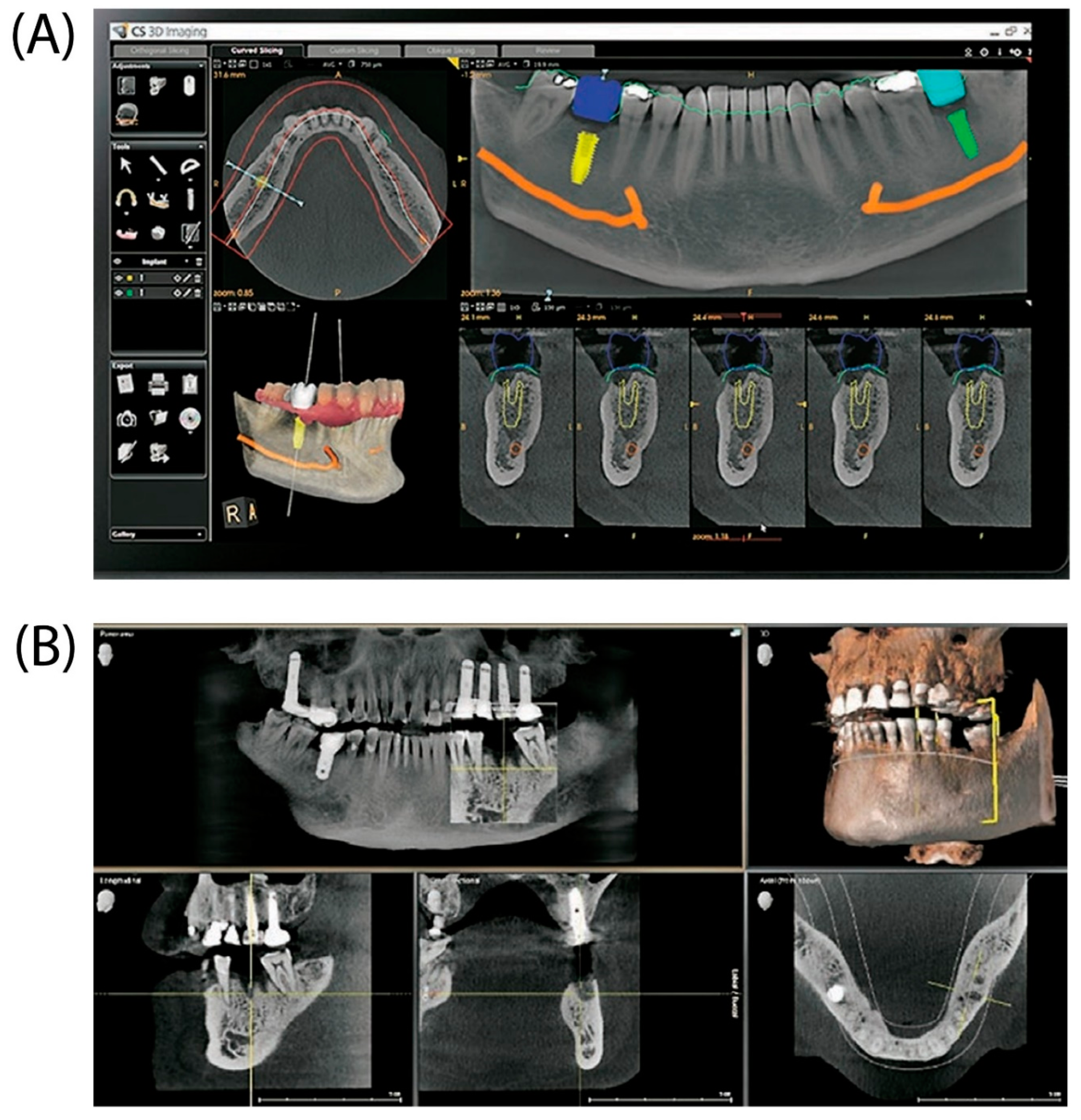
Dental implants are the major approach for replacing lost teeth because of their longevity, aesthetics, and functioning. Effective dental implant insertion requires detailed treatment planning and precision surgical execution. Three-dimensional (3D) imaging and virtual patient simulations have become effective tools for improving dental implant planning and placement accuracy (
Figure 2). For excellent results and long-term patient stability, dental implant planning and surgical implantation must be performed precisely. Clinicians may evaluate a patient's oral and maxillofacial anatomy in detail and identify any possible difficulties or issues throughout the treatment with precise planning. Clinicians can create an appropriate treatment plan that reduces the risk of complications by examining the amount and quality of accessible bone, the location of neighboring teeth, and proximity to important structures such as the sinuses and nerves. Considering the patient's functional needs and aesthetic preferences, precise planning also makes it easier to choose the ideal implant size, shape, and angulation.
Furthermore, osseointegration—the process by which an implant is fused with the surrounding bone—requires the exact surgical placement of dental implants. Implant failure, bone loss, and soft tissue issues are just a few of the mechanical and biological issues that can result from misalignment or incorrect implant placement.
The overall longevity and dependability of an implant are strongly influenced by its correct placement, which is essential for maximizing load distribution and enabling suitable operation [
10].
CBCT is frequently employed because of its advantages over traditional 2D radiology procedures. CBCT has several benefits, including the ability to generate 2D structures, 3D presentations of head and neck structures, reduced magnification error, lower radiation exposure, simpler access to scans via the DICOM standard, and fewer artifacts. The advantages listed above make implant planning and placement easier and more precise. CBCT scans the teeth and creates a computerized representation of the bone, teeth, and soft tissue. The main benefit of this approach is that it prevents nerve damage by aligning the nerve's course and selecting an appropriate implant length. Nerve damage can cause permanent or partial paralysis of the lips and chin. Choosing the appropriate implant size improves treatment success and implant longevity. CBCT allows accurate measurements for selecting the appropriate implant width and height [
11].
Figure 2.
(A) Illustration of a dental implant planning module featuring various visualization tools and implant placement options. (B) Images showing sagittal, coronal, and axial cross-sections with a comprehensive 3D reconstruction of the dental jaw structure [
11].
Figure 2.
(A) Illustration of a dental implant planning module featuring various visualization tools and implant placement options. (B) Images showing sagittal, coronal, and axial cross-sections with a comprehensive 3D reconstruction of the dental jaw structure [
11].
Biomimetic Approaches to Implant Design
In recent years, dental implants have become increasingly popular for replacing missing teeth, aiming to restore the normal function of the stomatognathic system. This is achieved through appropriate mechanical properties, stability, and effective bone integration and regeneration. To better meet patients’ needs and improve their quality of life, various medical techniques have evolved over time.
The development of modern implant dentistry is largely attributed to André Schroeder (1918–2004), a professor of operational dentistry and endodontics at the University of Bern, Switzerland, and Per-Ingvar Brånemark (1929–2014), a professor of anatomy at the University of Gothenburg, Sweden. In 1957, while researching bone regeneration and healing, Professor Brånemark discovered that titanium could bond permanently with bone without rejection, a phenomenon he termed "osseointegration."
A new era in dentistry was ushered in by osseointegration, which also made it possible to design biological acceptance standards for implants on the basis of the study of bone biology. Gosta Larsson, a 34-year-old man with a cleft palate, jaw abnormalities, and missing teeth in his lower jaw, was the first patient to receive titanium dental implants. He had four titanium implants (fixtures) placed into his jaw in 1965, and a fixed prosthesis was used to reconstruct it. Until Mr. Larsson's death, the dental implants were in place for almost 40 years.
On the basis of their chemical composition, dental implant materials can be roughly divided into three categories: metals, ceramics, and polymers. Furthermore, on the basis of their biological reactions, these biomaterials can be categorized into three main groups: biotolerant, bioinert, and bioactive. None of the materials used in dentistry are completely acceptable from a biological perspective, as the different levels of biocompatibility demonstrate. In this context, artificial structures must be selected to guarantee correct operation while minimizing adverse biological responses. [
12,
13].
As a biomaterial for dental implant manufacturing, polyether-ether-ketone (PEEK) is a promising polymeric biomaterial for dental implants, offering strength, wear resistance, and biocompatibility. While it promotes better adhesion and viability of osteoblasts than titanium does, its osteoconductivity is lower. Surface modifications can increase the hydrophilicity and osteointegration of these materials, making them comparable to titanium implants[
14].
Several techniques have recently been used to alter the chemical and topographical characteristics of conventional implant surfaces to improve their ability to adhere to bone cells (Wirth et al., 2017). According to Al-Nawas and Wagner (2017), titanium implant modifications improve load transmission from the implant to the bone by stimulating bone tissue and shortening the time it takes for osseointegration. The area accessible for bone binding increases with increasing surface roughness, which increases stability (Mello et al., 2016; Prasad et al., 2017). However, the chemical and morphological properties of the implant significantly affect the biological response of the surrounding tissues (Kasemo, 2002; Chaturvedi, 2009; Wennerberg and Albrektsson, 2010). Both early osseointegration and long-term remodeling depend on surface topography for osteoblast development (Bruschi et al., 2015; Smeets et al., 2016). Machined surfaces with macroirregularities, such as pores and grooves, are characteristic of early implant designs (Barfeie et al., 2015; Smeets et al., 2016). According to Coelho et al. (2015), effective implantation depends on appropriate drill-hole preparation and microgeometry. However, they noted that because of density necrosis and subsequent remodeling, implant stability tends to decline during the first several weeks of bone healing. Efforts to improve surface quality are still motivated by the need for initial stability and ideal bone remodeling [
15,
16].
Biomaterial Innovations in Implant Dentistry
Biocompatible Materials for Dental Implants:
The advancement of biomaterials in implant dentistry has significantly impacted the success rate and longevity of dental implants. Biocompatibility is a fundamental consideration when selecting materials for dental implants, as it ensures that these materials can integrate effectively with the surrounding tissues without causing adverse reactions. Among the various materials explored for dental implant applications, titanium and zirconia have emerged as two of the most widely used and studied materials owing to their unique properties that cater to both functional and aesthetic demands.
1. Titanium and Titanium Alloys
Titanium is a popular material for dental implants because of its excellent biocompatibility, mechanical strength, and capacity for osseointegration. The biocompatibility of titanium is largely due to the formation of a stable titanium dioxide (TiO2) oxide layer, which prevents corrosion and promotes bone integration. This property, known as osseointegration, is crucial for implant stability and long-term success [
17]. Titanium alloys, such as Ti-6Al-4 V, have been developed to enhance mechanical properties such as tensile strength and resistance to fracture under functional loading conditions [
18].
However, titanium implants are not without challenges. Corrosion, particularly in the oral environment, is a significant concern. The presence of acidic conditions, fluoride, and bacteria can lead to breakdown of the TiO2 layer, exposing the implant to further degradation [
19]. Additionally, the release of alloying elements such as aluminum and vanadium has raised concerns about their cytotoxicity and allergic reactions, prompting research into alternative compositions [
18].
To improve the surface characteristics of titanium implants, various modifications have been explored, including surface roughening, acid etching, and coating with materials such as hydroxyapatite. These modifications aim to increase bone‒implant contact, reduce bacterial colonization, and improve overall osseointegration [
17,
19]. Despite these challenges, titanium remains a preferred material for dental implants because of its long-term success rates and favorable mechanical properties.
2. Zirconia-Based Implants: Properties and Clinical Applications
Zirconia-based implants have gained considerable attention as alternatives to titanium, particularly for patients seeking metal-free options or those with allergies to metals. Zirconia (zirconium dioxide) is a ceramic material that offers several advantages, including excellent biocompatibility, corrosion resistance, and aesthetic appeal due to its tooth-like color [
20,
21]. The biocompatibility of zirconia is attributed to its stable ZrO2 layer, which prevents ion release and reduces inflammatory responses, making it suitable for patients prone to hypersensitivity or peri-implantitis [
22].
Zirconia implants also demonstrate favorable mechanical properties, such as high flexural strength, fracture toughness, and resistance to wear and corrosion [
20,
21]. The use of yttria-stabilized tetragonal zirconia polycrystals (Y-TZP) further enhances the mechanical stability of zirconia implants by preventing phase transformation, which could lead to structural failure [
20]. However, zirconia is susceptible to low-temperature degradation, also known as aging, which can affect its mechanical properties over time. Surface modification techniques, such as grit blasting, acid etching, and laser treatment, have been employed to improve the osseointegration capacity of zirconia implants by increasing surface roughness and bioactivity [
21].
Clinical studies have shown that zirconia implants have comparable success rates to titanium implants in terms of osseointegration and long-term stability. Additionally, the lower affinity of zirconia for bacterial plaque and reduced inflammatory infiltration make it a favorable option for reducing the risk of peri-implant diseases [
20,
21]. While more long-term clinical data are needed to fully validate the use of zirconia implants, current evidence suggests that they are a viable alternative to titanium, particularly in aesthetically demanding cases or for patients with metal sensitivities.
Novel Coatings for Dental Implant Surfaces
Dental implant technology has advanced significantly over recent years, driven by innovations in surface coatings that increase osseointegration, reduce the risk of infections, and improve the long-term success of implants. This review aims to explore two prominent coatings for dental implant surfaces—hydroxyapatite (HA) and bioactive glass (BG) coatings—on the basis of information from recent studies, with a focus on their characteristics, mechanisms of action, and clinical implications.
1. Hydroxyapatite coatings
Hydroxyapatite (HA), a calcium phosphate compound with a chemical structure similar to the mineral component of bone, is well known for its biocompatibility and osteoconductive properties. HA coatings are widely used to promote osseointegration by enhancing the direct bonding between the implant and bone tissue. The sol‒gel method has been highlighted as an effective deposition technique for producing uniform and homogeneous HA coatings on titanium and zirconia implants, improving their bioactivity and corrosion resistance [
23,
24].
Recent developments in HA coatings include the use of graphene oxide (GO) to reinforce HA, forming HA‒GO nanocomposites. These coatings, produced via electrophoretic deposition, improve the roughness and hydrophilicity of titanium surfaces, significantly enhancing the adhesion, proliferation, and osteogenic differentiation of bone marrow stem cells [
3]. Additionally, sol-gel-derived HA coatings on zirconia implants have demonstrated enhanced osseointegration, comparable to that of titanium implants, due to improved stability and nanoporous structure, which facilitate cell attachment and proliferation [
25].
Despite their benefits, pure HA coatings have limitations such as low abrasive resistance and fracture toughness, which can lead to mechanical failure under load-bearing conditions. To address these issues, researchers have explored incorporating other elements, such as zirconium and strontium, into HA to enhance its mechanical properties, bioactivity, and antimicrobial effects [
26].
2. Bioactive Glass Coatings
Bioactive glass (BG) is another promising material for dental implant coatings and is recognized for its ability to bond with both bone and soft tissues. BG, such as the commonly used 45S5 Bioglass®, forms a hydroxycarbonate apatite layer upon contact with physiological fluids, which mimics natural bone minerals and promotes bonding with surrounding tissue [
27]. BG coatings on implants accelerate bone formation, reduce healing time, and exhibit inherent antibacterial properties, which help mitigate the risk of peri-implantitis, a leading cause of implant failure [
28].
Recent studies have focused on multielement doping of BG, incorporating ions such as silver, cobalt, and zirconium to further enhance its bioactive and antimicrobial properties. For example, zirconium-doped BG has been shown to improve both the mechanical strength and bioactivity of the coating, whereas silver and cobalt ions provide additional antibacterial effects, reducing bacterial colonization on the implant surface [
27,
28]. These multielement-doped coatings are synthesized via techniques such as sol‒gel and plasma spraying, which allow for controlled composition and improved adhesion strength [
29].
However, challenges remain in optimizing the mechanical properties of BG coatings to ensure that they do not compromise the structural integrity of the implant. The brittleness and low fracture toughness of BG limit its application to nonload-bearing implants. Advances in coating techniques, such as thermal spraying and sol‒gel methods, are being explored to produce more resilient BG coatings with improved bonding strength and reduced dissolution rates [
29].
Tissue Engineering Approaches in Implant Dentistry
Substantial progress has been made in the field of implant dentistry, particularly in the use of tissue engineering approaches aimed at restoring and regenerating oral tissues. Growth factors and stem cells have emerged as key components in enhancing implant success rates, especially in challenging bone and soft tissue conditions. This review focuses on the integration of growth factors and stem cells, drawing on current research and practical applications to advance regenerative implant therapy.
1. Growth Factors in Regenerative Implant Therapy
Growth factors play crucial roles in enhancing the regeneration of bone and soft tissues, thereby providing a suitable environment for implant placement and osseointegration. Growth factors such as bone morphogenetic proteins (BMPs), transforming growth factor-beta (TGF-β), and vascular endothelial growth factor (VEGF) are instrumental in stimulating the cellular activities essential for bone and tissue healing. Studies have demonstrated the effectiveness of BMPs in enhancing osteogenic differentiation, which is critical for implant stability and success [
30].
The use of autologous platelet concentrates, such as platelet-rich plasma (PRP) and platelet-rich fibrin (PRF), has shown promise in improving wound healing and accelerating tissue regeneration around implants. The combination of growth factors, such as PRP, has been used effectively with scaffolds and bone grafts to improve the density and quality of bone at the implant site [
31]. However, challenges remain, such as variability in clinical outcomes, high production costs, and the need for optimized delivery systems [
32].
2. Stem cells in regenerative implant therapy
Stem cells, particularly mesenchymal stem cells (MSCs), have demonstrated significant potential in the field of implant dentistry because of their ability to differentiate into bone-forming cells such as osteoblasts. MSCs derived from bone marrow (BM-MSCs) and dental tissues have been shown to enhance osseointegration when used in conjunction with growth factors and biomaterial scaffolds [
33,
34]. For example, BM-MSCs combined with PRP have been studied in animal models and found to potentially improve the quality of bone‒implant contact (BIC) and bone density within the threads (BDWT), although further studies are needed to establish their definitive impact on implant success [
31].
Dental stem cells, including dental pulp stem cells (DPSCs) and periodontal ligament stem cells (PDLSCs), have been explored for their regenerative capabilities. These cells are appealing because of their ease of harvest and ability to regenerate oral tissues. Research suggests that DPSCs, when combined with BMPs, can support new bone formation around implants, providing an alternative to traditional bone grafting methods [
32].
Moreover, the role of extracellular vesicles (EVs) released by MSCs has also gained attention as a paracrine mechanism supporting bone regeneration and immune modulation. These EVs contain bioactive molecules such as cytokines and growth factors, which help regulate inflammation and promote tissue healing [
32,
33].
Recent findings from a systematic review further support the use of MSCs in augmenting dental implant osseointegration. Studies have shown that when MSCs are used with graft materials and scaffolds, they significantly promote osseointegration and new bone formation both in vitro and in vivo. The use of biofunctionalized biomaterial surfaces enhanced osteogenic differentiation and bone-implant integration, highlighting the importance of biomaterial surface modifications in improving implant stability and success. The majority of the reviewed studies utilized bone marrow-derived MSCs, which demonstrated significant potential in enhancing bone density and the BIC when used with bioactive scaffolds. However, there is notable heterogeneity in the types of scaffolds used, which include platelet-rich plasma (PRP), tricalcium phosphate, and hydroxyapatite, emphasizing the need for standardization in scaffold selection to maximize therapeutic outcomes [
34].
Challenges and Future Directions in Regenerative Implantology
The challenges in using growth factors and stem cells in regenerative implant therapy include the scalability of stem cell therapies, ethical concerns, and the ability to control cell differentiation. The complexities associated with optimizing the combination of stem cells, growth factors, and biomaterial scaffolds also require further research [
33,
34]. Additionally, the aging process affects MSC functionality, which may impair the success of implants in older patients. Strategies to increase MSC activity and osteogenesis in aged individuals are being actively investigated [
33].
Future advancements may include the development of biomaterials that serve as carriers for growth factors and stem cells, improving their stability and targeted delivery. Gene editing technologies, such as CRISPR-Cas9, hold potential for enhancing stem cell regenerative abilities, while personalized approaches could cater to patient-specific conditions, improving the predictability of outcomes [
33,
35]. Additionally, modifying implant surfaces to enhance the attachment and differentiation of MSCs could play a crucial role in improving osseointegration and implant longevity [
34].
Advanced Implant Materials and Surface Engineering
Nanomaterials for Enhanced Osseointegration:
The success of orthopedic and dental implants depends on their initial stability. Although titanium implants are widely used, there are risks associated with their bioinert nature and oxidation susceptibility. The oxide layer on titanium surfaces can cause thrombosis, which can lead to microbial growth in the surrounding tissue and potentially complicate matter. In addition, inflammation can occur around surgical sites during the procedure, possibly as a result of external heat or pressure. This can prevent normal bone formation around the surgical area, which can result in insufficient bonding between the implant and the bone[
36,
37].
Owing to decreased bone density and poor bone tissue microstructure caused by age-related illnesses, including diabetes and osteoporosis, which result in weak bone tissue, the risk of implant failure increases dramatically in elderly individuals.
In an effort to reduce surgical failure, recent research has focused on improving implant surface quality via surface functionalization. Improving the biofunctionality of implant materials is necessary to address these problems, which has led to the widespread use of different surface functionalization methods. A potent method for adjusting the physicochemical characteristics of implant surfaces that allows for targeted bioactivity while reducing side effects is surface functionalization. Although growth factors and inducers have been used to stimulate osteogenesis, there are certain drawbacks to their use, including their complexity, high cost of manufacture, and vulnerability to degradation in vivo[
38,
39].
Recent developments in surface functionalization have made use of a variety of materials, such as polymers and inorganics, to provide substrates with certain properties. Nanomaterial (NM)-based coatings are among those materials that have significant benefits, such as mechanical reinforcement, high specific surface area, unique cell‒matrix interactions, and programmable micron-/nanometer-sized multiporous topography. These characteristics improve mechanical qualities and control bone cell behaviors. Achieving long-lasting and stable coating layers is essential for granting bioactive characteristics in vivo, including osteoconductivity and osteogenicity [
40].
Among the notable members of the NM family, carbon nanomaterials (CNMs) have transformed a number of disciplines, including biology, medicine, electronics, physics, optics, and mechanics. Owing to their remarkable properties, CNMs have attracted much interest in the biological field. For example, fullerenes and carbon nanotubes (CNTs) have been thoroughly researched for use in medicine and therapy. In addition, advances in production and modification techniques have led to the current surge in popularity of graphene and nanocrystalline diamond (ND), and cellular activities such as adhesion, migration, proliferation, and differentiation into distinct lineages such as myogenesis, neuritogenesis, and osteogenesis can all be influenced by CNMs. Owing to their ability to induce osteogenesis and provide mechanical reinforcement, graphene, CNTs, NDs, carbon nanofibers (CNFs), fullerene, and carbon nanodots (CNDs) are potential materials for surface functionalization of implants [
41,
42].
Bioactive Coatings and Surface Modifications
Biomimetic dental implants represent a cutting-edge development for modifying implant surfaces. Alterations in coating thickness and roughness can influence the chemical inertness, cell adhesion, and antibacterial properties of dental implant surfaces. Various surface coatings and mechanical alterations have been explored to enhance osseointegration and reduce peri-implantitis. These modifications increase surface energy, promote cell proliferation and growth factor production, and ultimately improve the osseointegration process.
There are several reasons for dental implant failure, including issues related to the implant itself, the clinician, and the patient. Foreign-body responses and infections can accelerate alveolar bone depletion. Peri-implantitis, characterized by bacterial infections and microbial plaque accumulation, is a major cause of implant failure, often resulting in alveolar bone loss. The increased use of surface-coating technologies—including various combinations—has been shown to improve the biocompatibility, bioactivity, and antibacterial properties of dental implants.
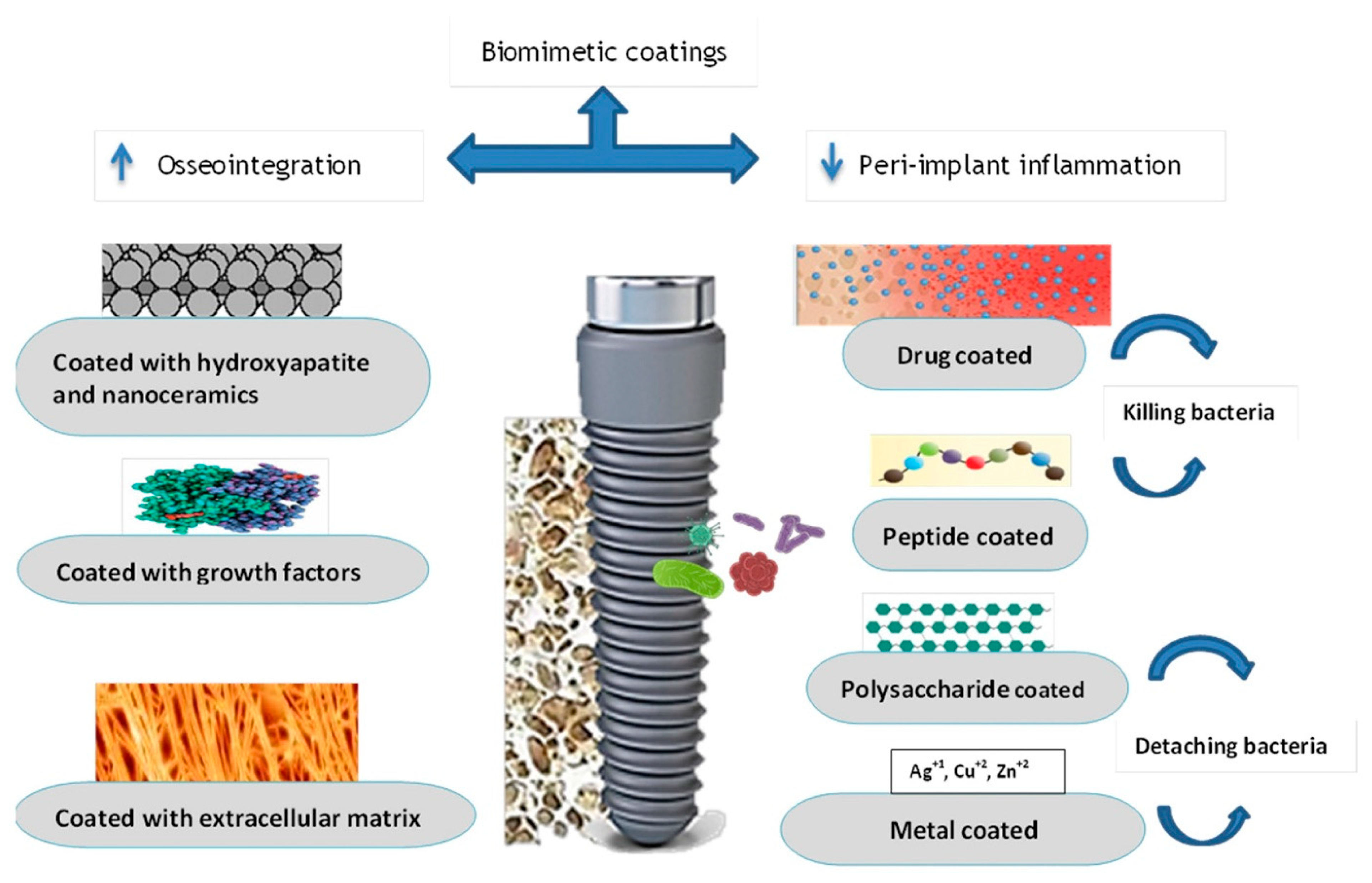
Biomimetic Dental Implants
A notable development in implant surface modification is the use of biomimetic dental implants (
Figure 3) [
43]. To reduce the risk of infectious disease transmission, biomimetic agents should be able to do the following:
Promotion of proper cell differentiation for new bone formation.
They can be synthesized or manufactured without requiring extraction from allografts.
It is resorbable in response to osteogenic activity.
Prevent implant loss due to coating delamination.
A lack of host immunological responses.
The material remains chemically stable until implant insertion.
Be cost-effective.
Coating to Improve Osseointegration
While advancements in surface topography have led to enhanced osseointegration, ongoing research continues to explore a wide range of inorganic and organic coatings to further improve the tissue integration of dental implants (
Figure 4). By coating strong biometals with bioactive materials, both the mechanical performance and the bone-bonding potential can be optimized. These bioactive coatings improve features such as chemical inertness, cell adhesion, and antibacterial properties, with variations in roughness influencing their effectiveness. The following sections predominantly address recent progress in the development of dental implants equipped with bioactive coatings.
1. Hydroxyapatite layer
Among the various methods, one of the most popular approaches is coating implants with hydroxyapatite (HA). HA is a biologically stable type of calcium phosphate that mineralizes to reinforce the organic matrix without inducing inflammation or immunogenic reactions. It consists of naturally occurring ions from physiological environments and exhibits excellent osteoconductive and osteointegration properties. Various ion-substituted hybrid coatings have paved the way for implant designs with diverse biological functionalities. These coatings have been shown to significantly improve cell attachment and promote bone formation by enhancing interface contact and bone-to-implant integration. Recently, advancements in implant surface technology have introduced nanohydroxyapatite, which can be combined with collagen, bioglass, or titanium dioxide to create a composite material that closely resembles the natural bone environment [
43,
44,
45].
2. Growth factors
During the initial phase of osseointegration, platelets and macrophages produce numerous growth factors that facilitate subsequent phases. Coating materials containing transforming growth factor (TGF), platelet-derived growth factor (PDGF), and fibroblast growth factor (FGF) have been used to accelerate this process. Vascular endothelial growth factor (VEGF), a signaling protein involved in vasculogenesis and angiogenesis, enhances alkaline phosphatase (ALP) activity and gene and protein expression related to vasculogenesis, as well as promoting osteoblast proliferation in vitro. In vivo studies have demonstrated that implant coating with VEGF significantly enhances osteoblast and endothelial cell activation. Bone morphogenetic proteins (BMPs), such as BMP-2, BMP-4, and BMP-7, are crucial for cartilage and bone development, regulating osteogenic cells and promoting mesenchymal stem cell (MSC) differentiation [
43,
46].
3. Extracellular matrix
Another strategy to improve the compatibility of dental implants is controlling cell‒matrix adhesion by allowing extracellular matrix (ECM) proteins to accumulate on implant surfaces. During the proliferative phase of osseointegration, fibroblasts are stimulated by growth factors to create ECM proteins such as hyaluronan, collagen, chondroitin sulfate, fibronectin, and elastin. These ECM proteins, by rearranging intracellular microfilaments and microtubules, play a vital role in bone healing. Through interactions with fibronectin arginine–glycine–aspartate motifs and cell surface integrins, ECM proteins direct osteoprogenitor cell migration to the implant surface, promoting cell adhesion and spreading. [
43].
4. Antimicrobial Coatings
Infections are frequently experienced after implant rehabilitation, leading to increased costs, patient dissatisfaction, and implant failure. Biofilms on the implant surface exacerbate the problem by shielding microbes from immune responses. Moreover, overuse of antibiotics may lead to resistant bacterial strains. The development of specialized implants with functional coatings that either directly target bacteria within biofilms or inhibit bacterial attachment and biofilm formation is a focus of ongoing research [
43].
5. Drug-Coated Dental Implants
Various bactericidal and bacteriostatic agents have been incorporated into implant surfaces to prevent biofilm formation. For example, the application of tetracycline to implant surfaces effectively eliminates bacteria, promoting cell growth and bone healing. Similarly, vancomycin-coated titanium implants inhibit Streptococcus aureus colonization, expediting bone repair. However, extensive vancomycin use has raised concerns about vancomycin-intermediate and vancomycin-resistant
S. aureus (VISA) strains. Bisphosphonates such as alendronate, etidronate, tiludronate, and zoledronate stimulate osteoblasts, enhance bone production, and inhibit osteoclastic activity and bone resorption [
43].
6. Polysaccharide Antibacterial Coatings
Natura chitosan, derived from chitin deacetylation, is a cationic polymer renowned for its antibacterial effects on implant surfaces. Triethoxysilylpropyl succinic anhydride (TESPSA) acts as a coupling agent, forming stable bonds with chitosan and enhancing its adhesion to titanium surfaces. The incorporation of silica–chitosan hybrids into titanium implants has demonstrated effective antibacterial properties. Polyelectrolyte multilayers containing hyaluronic acid and chitosan also have antibacterial effects on
S. aureus. Ag-conjugated chitosan nanoparticles on titanium surfaces inhibited the growth of
S. mutans and
P. gingivalis, reducing biofilm formation and bacterial adhesion. Furthermore, chitosan coatings containing the tetracycline digluconate have shown antimicrobial activity against Actinobacillus, Actinomycetemcomitans, and
S. epidermidis [
43].
Biocompatible Alternatives: Zirconia and Polymer-based Implants:
Titanium is widely preferred for its mechanical strength and resistance to deformation, particularly in dental applications where systematic reviews have underscored the reliability of titanium abutments. However, aesthetic concerns may arise with titanium, as it can lead to a grayish hue around the implant margin, impacting treatment aesthetics and patient satisfaction. Achieving a balance between successful restoration and meeting aesthetic expectations often involves exploring alternative materials [
47].
Zirconia
Zirconia, a polycrystalline ceramic, offers enhanced aesthetics and durability by mitigating the grayish effect on the mucosa. However, surface defects may develop and undergo plastic deformation under masticatory forces, which underscores the need for alternative biomaterials.
Bio-HPP
High-performance polymers (Bio-HPPs), derived from polyetheretherketone (PEEK), are promising substitutes for metallic dental materials and serve as denture superstructures on dental implants. Bio-HPP presents several clinical advantages over titanium alloys, including reduced hypersensitivity, radiolucency, minimal interference with MRI, absence of metallic color, and adaptability to surface treatments. Its feasibility, elasticity, and aesthetic appeal have contributed to its growing popularity in implant-supported restorations. However, similar to titanium and zirconia, Bio-HPP may still trigger peri-implant mucositis in the presence of bacterial challenges. If untreated, peri-implant mucositis can progress to peri-implantitis, potentially resulting in implant loss [
48].
Zirconia-based materials are increasingly used in dental implants because of their biological compatibility and chemical stability, reducing the risk of harmful substance release into surrounding tissues. Zirconia implants deter bacterial attachment and biofilm buildup, thereby decreasing the likelihood of inflammatory responses. Studies have shown comparable rates of osseointegration between zirconia and titanium implants over a 12-week period. The biocompatibility of zirconia, combined with its resistance to corrosion and wear, high flexural strength, modulus of elasticity, and aesthetic advantages, has led to its growing adoption as a dental implant material [
49,
50].
Conclusion
Over the course of this comprehensive review, we have examined the multifaceted advancements reshaping the landscape of implant dentistry. From the integration of novel imaging modalities—such as MRI, OCT, and CBCT—that facilitate more accurate diagnostics and treatment planning to the implementation of computer-aided design and manufacturing technologies that streamline surgical procedures, the field is evolving toward ever-greater precision and predictability. Concurrently, the refinement of biomaterials—including titanium alloys, zirconia implants, polymer-based alternatives such as PEEK, and innovative coatings such as hydroxyapatite and bioactive glass—serves to increase osseointegration, improve biocompatibility, and ultimately prolong implant longevity. Emerging tissue engineering approaches highlight the promise of growth factors, stem cells, and bioactive scaffolds to promote bone regeneration and accelerate healing, thus providing a more patient-centric approach to care.
As implant dentistry moves forward, future research will likely focus on personalizing treatments, optimizing surface modifications for improved cellular responses, and leveraging nanotechnology to achieve seamless integration at the tissue–implant interface. The synergy of multidisciplinary insights—from materials science and bioengineering to clinical best practices—will continue to usher in implants that not only restore oral function but also meet stringent aesthetic and biological demands. In this ongoing pursuit of excellence, the ultimate goal remains clear: to offer patients implants that are safer, more reliable, and better attuned to the complex interplay of biological and mechanical factors underlying oral health and rehabilitation.
Authors' contributions: All the authors conceived and designed the review paper, contributed significantly to the drafting and refinement of the manuscript, and provided substantial intellectual input throughout the analysis and critical review process. Additionally, all the authors actively participated in the acquisition of relevant literature and materials, ensuring the accuracy and completeness of the content. Furthermore, all the authors were involved in the critical review and revision of the manuscript, providing valuable insights and feedback. Finally, all the authors have given their final approval of the version to be published and take full responsibility for the integrity and accuracy of the work.
Statements and declarations
Financial declaration: No external funding was received for this review research.
Competing Interests: The authors declare that they have no conflicts of interest.
Ethics approval: Not applicable.
Consent to participate: Not applicable.
Consent for publication: Not applicable.
Availability of Data and Material: Not applicable
Declaration Regarding the Use of AI-Assisted Readability Enhancement: I hereby affirm that the utilization of AI-assisted tools in the refinement of the manuscript was strictly limited to enhancing its readability. At no point were AI technologies employed to supplant essential authorial responsibilities, including the generation of scientific, pedagogic, or medical insights; the formulation of scientific conclusions; or the issuance of clinical recommendations.The implementation of AI for readability enhancement was rigorously supervised under the discerning eye of human oversight and control.
List of Abbreviations
| Abbreviation |
Full Form |
| ALP |
Alkaline Phosphatase |
| BMP(s) |
Bone Morphogenetic Protein(s) |
| BDWT |
Bone Density Within the Threads |
| BIC |
Bone-Implant Contact |
| BG |
Bioactive Glass |
| BM-MSCs |
Bone Marrow-Derived Mesenchymal Stem Cells |
| CBCT |
Cone Beam Computed Tomography |
| CNDs |
Carbon Nanodots |
| CNFs |
Carbon Nanofibers |
| CNTs |
Carbon Nanotubes |
| DICOM |
Digital Imaging and Communications in Medicine |
| DPSCs |
Dental Pulp Stem Cells |
| ECM |
Extracellular Matrix |
| FGF |
Fibroblast Growth Factor |
| GO |
Graphene Oxide |
| HA |
Hydroxyapatite |
| MRI |
Magnetic Resonance Imaging |
| MSCs |
Mesenchymal Stem Cells |
| ND |
Nanocrystalline Diamond |
| OCT |
Optical Coherence Tomography |
| PDGF |
Platelet-Derived Growth Factor |
| PDLSCs |
Periodontal Ligament Stem Cells |
| PEEK |
Polyether-Ether-Ketone |
| PRF |
Platelet-Rich Fibrin |
| PRP |
Platelet-Rich Plasma |
| TGF-β |
Transforming Growth Factor-Beta |
| TiO2 |
Titanium Dioxide |
| VISA |
Vancomycin-Intermediate Staphylococcus aureus
|
| VEGF |
Vascular Endothelial Growth Factor |
| Y-TZP |
Yttria-Stabilized Tetragonal Zirconia Polycrystal |
References
- Alghamdi HS, Jansen JA. The development and future of dental implants. Dent Mater J. 2020 Mar 31;39(2):167-172. doi: 10.4012/dmj.2019-140. Epub 2020 Jan 22.PMID: 31969548.
- Alamoudi A. Nanoengineering and Surface Modifications of Dental Implants. Cureus. 2024 Jan 2;16(1):e51526. doi: 10.7759/cureus.51526. PMID: 38304686; PMCID: PMC10833059.
- Zhu, G., Wang, G., & Li, J. J. (2021). Advances in implant surface modifications to improve osseointegration. Materials Advances, 2(21), 6901-6927. [CrossRef]
- Zhang Y, Gulati K, Li Z, Di P, Liu Y. Dental Implant Nano-Engineering: Advances, Limitations and Future Directions. Nanomaterials (Basel). 2021 Sep 24;11(10):2489. doi: 10.3390/nano11102489. PMID: 34684930; PMCID: PMC8538755.
- Yeung, A. W. K., AlHadidi, A., Vyas, R., Bornstein, M. M., Watanabe, H., & Tanaka, R. (2024). Nonionizing diagnostic imaging modalities for visualizing health and pathology of periodontal and peri-implant tissues. Periodontology 2000, 95(1), 87–101. [CrossRef]
- Spagnuolo, G., & Soltani, P. (2024). Magnetic Resonance Imaging in Digital Dentistry: The Start of a New Era. Prosthesis, 6(4), 798–802. [CrossRef]
- Putra, R. H., Yoda, N., Astuti, E. R., & Sasaki, K. (2021). Potential Imaging Capability of Optical Coherence Tomography as Dental Optical Probe: A Mini-Review. Applied Sciences, 11(22), 11025. [CrossRef]
- Ali, S., Gilani, S. B. S., Shabbir, J., Almulhim, K. S., Bugshan, A., & Farooq, I. (2021). Optical coherence tomography’s current clinical medical and dental applications: a review. F1000Research, 10, 310. [CrossRef]
- Janjua, O. S., Jeelani, W., Khan, M. I., Qureshi, S. M., Shaikh, M. S., Zafar, M. S., & Khurshid, Z. (2023c). Use of Optical Coherence Tomography in Dentistry. International Journal of Dentistry, 2023, 1–10. [CrossRef]
- Saini, R. S., Bavabeedu, S. S., Quadri, S. A., Gurumurthy, V., Kanji, M. A., Kuruniyan, M. S., Binduhayyim, R. I. H., Avetisyan, A., & Heboyan, A. (2024). Impact of 3D imaging techniques and virtual patients on the accuracy of planning and surgical placement of dental implants: A systematic review. Digital Health, 10. [CrossRef]
- Jurić, B., & Matijaš, T. (2023). Uloga CBCT-a u području dentalne implantologije. RadiološKi Vjesnik, 47(1), 16–27. [CrossRef]
- Blum, I. R. (2024). Implant Dentistry: A journey from the beginnings to what has become an established discipline. Primary Dental Journal, 13(3), 2–3. [CrossRef]
- Cruz, M. B., Silva, N., Marques, J. F., Mata, A., Silva, F. S., & Caramês, J. (2022). Biomimetic Implant Surfaces and Their Role in Biological Integration—A Concise Review. Biomimetics, 7(2), 74. [CrossRef]
- Sotova, C., Yanushevich, O., Kriheli, N., Grigoriev, S., Evdokimov, V., Kramar, O., Nozdrina, M., Peretyagin, N., Undritsova, N., Popelyshkin, E., & Peretyagin, P. (2023). Dental Implants: Modern Materials and Methods of Their Surface Modification. Materials, 16(23), 7383. [CrossRef]
- Pabst, A., Asran, A., Lüers, S., Laub, M., Holfeld, C., Palarie, V., Thiem, D. G. E., Becker, P., Hartmann, A., Heimes, D., Al-Nawas, B., & Kämmerer, P. W. (2022). Osseointegration of a New, Ultrahydrophilic and Nanostructured Dental Implant Surface: A Comparative In Vivo Study. Biomedicines, 10(5), 943. [CrossRef]
- Al-Zubaidi, S. M., Madfa, A. A., Mufadhal, A. A., Aldawla, M. A., Hameed, O. S., & Yue, X. (2020). Improvements in Clinical Durability From Functional Biomimetic Metallic Dental Implants. Frontiers in Materials, 7. [CrossRef]
- Mechanical, chemical and biological aspects of titanium and titanium alloys in implant dentistry. (2018, February 1). PMID: 29460522.
- Nicholson, J. W. (2020). Titanium Alloys for Dental Implants: A review. Prosthesis, 2(2), 100–116. [CrossRef]
- Chaturvedi, T. P. (2009). An overview of the corrosion aspect of dental implants (titanium and its alloys). Indian Journal of Dental Research, 20(1), 91. [CrossRef]
- Lin, H., Yin, C., & Mo, A. (2021). Zirconia based dental biomaterials: structure, mechanical properties, biocompatibility, surface modification, and applications as implant. Frontiers in Dental Medicine, 2. [CrossRef]
- Qu, Y., & Liu, L. (2021). Zirconia Materials for Dental Implants: A Literature review. Frontiers in Dental Medicine, 2. [CrossRef]
- Chopra, D., Jayasree, A., Guo, T., Gulati, K., & Ivanovski, S. (2021). Advancing dental implants: Bioactive and therapeutic modifications of zirconia. Bioactive Materials, 13, 161–178. [CrossRef]
- Jaafar, A., Hecker, C., Árki, P., & Joseph, Y. (2020). Sol-Gel derived hydroxyapatite coatings for titanium implants: A review. Bioengineering, 7(4), 127. [CrossRef]
- Kim, J., Kang, I., Cheon, K., Lee, S., Park, S., Kim, H., & Han, C. (2021). Stable sol–gel hydroxyapatite coating on zirconia dental implant for improved osseointegration. Journal of Materials Science Materials in Medicine, 32(7). [CrossRef]
- Baheti, W., Lv, S., Mila, N., Ma, L., ·Amantai, D., Sun, H., & He, H. (2023). Graphene/hydroxyapatite coating deposit on titanium alloys for implant application. Journal of Applied Biomaterials & Functional Materials, 21, 228080002211481. [CrossRef]
- Arcos, D., & Vallet-Regí, M. (2020). Substituted hydroxyapatite coatings of bone implants. Journal of Materials Chemistry B, 8(9), 1781–1800. [CrossRef]
- Hammami, I., Gavinho, S. R., Pádua, A. S., Sá-Nogueira, I., Silva, J. C., Borges, J. P., Valente, M. A., & Graça, M. P. F. (2023). Bioactive Glass Modified with Zirconium Incorporation for Dental Implant Applications: Fabrication, Structural, Electrical, and Biological Analysis. International Journal of Molecular Sciences, 24(13), 10571. [CrossRef]
- Lung, C. Y. K., Abdalla, M. M., Chu, C. H., Yin, I., Got, S., & Matinlinna, J. P. (2021). A Multi-Element-Doped porous bioactive glass coating for implant applications. Materials, 14(4), 961. [CrossRef]
- Sergi, R., Bellucci, D., & Cannillo, V. (2020). A comprehensive review of bioactive glass coatings: state of the art, challenges and future perspectives. Coatings, 10(8), 757. [CrossRef]
- Fiorillo, L., Cervino, G., Galindo-Moreno, P., Herford, A. S., Spagnuolo, G., & Cicciù, M. (2021). Growth factors in oral tissue Engineering: New perspectives and current therapeutic options. BioMed Research International, 2021, 1–11. [CrossRef]
- Stramandinoli-Zanicotti, R., Sassi, L., Rebelatto, C., Boldrine-Leite, L., Brofman, P., & Carvalho, A. (2020). The effect of bone marrow-derived stem cells associated with platelet-rich plasma on the osseointegration of immediately placed implants. Journal of Clinical and Experimental Dentistry, e8–e13. [CrossRef]
- He, P., Zhang, Q., Motiwala, F. I., Shanti, R. M., Chang, B. M., & Le, A. D. (2021). Potential application of dental stem cells in regenerative reconstruction of oral and maxillofacial tissues: a narrative review. Frontiers of Oral and Maxillofacial Medicine, 4, 14. [CrossRef]
- Ma, Y., Wang, S., Wang, H., Chen, X., Shuai, Y., Wang, H., Mao, Y., & He, F. (2023). Mesenchymal stem cells and dental implant osseointegration during aging: from mechanisms to therapy. Stem Cell Research & Therapy, 14(1). [CrossRef]
- Sayed, M. E., Mugri, M. H., Almasri, M. A., Al-Ahmari, M. M., Bhandi, S., Madapusi, T. B., Varadarajan, S., Raj, A. T., Reda, R., Testarelli, L., & Patil, S. (2021). Role of stem cells in Augmenting dental implant osseointegration: a Systematic review. Coatings, 11(9), 1035. [CrossRef]
- Bijukumar, D. R., McGeehan, C., & Mathew, M. T. (2018). Regenerative Medicine Strategies in Biomedical implants. Current Osteoporosis Reports, 16(3), 236–245. [CrossRef]
- Bandyopadhyay, A., Mitra, I., Goodman, S. B., Kumar, M., & Bose, S. (2022). Improving biocompatibility for next generation of metallic implants. Progress in Materials Science, 133, 101053. [CrossRef]
- Marin, E., & Lanzutti, A. (2023). Biomedical Applications of Titanium Alloys: A Comprehensive Review. Materials, 17(1), 114. [CrossRef]
- Accioni, F., Vázquez, J., Merinero, M., Begines, B., & Alcudia, A. (2022). Latest Trends in Surface Modification for Dental Implantology: Innovative Developments and Analytical Applications. Pharmaceutics, 14(2), 455. [CrossRef]
- Cooper, L. F., & Shirazi, S. (2021). Osseointegration—the biological reality of successful dental implant therapy: a narrative review. Frontiers of Oral and Maxillofacial Medicine, 4, 39. [CrossRef]
- Wieszczycka, K., Staszak, K., Woźniak-Budych, M. J., Litowczenko, J., Maciejewska, B. M., & Jurga, S. (2021). Surface functionalization – The way for advanced applications of smart materials. Coordination Chemistry Reviews, 436, 213846. [CrossRef]
- Ahlawat, J., Asil, S. M., Barroso, G. G., Nurunnabi, M., & Narayan, M. (2020). Application of carbon nano onions in the biomedical field: recent advances and challenges. Biomaterials Science, 9(3), 626–644. [CrossRef]
- Kang MS, Lee JH, Hong SW, Lee JH, Han D-W. Nanocomposites for Enhanced Osseointegration of Dental and Orthopedic Implants Revisited: Surface Functionalization by Carbon Nanomaterial Coatings. Journal of Composites Science. 2021; 5(1):23. [CrossRef]
- Abdulghafor, M. A., Mahmood, M. K., Tassery, H., Tardivo, D., Falguiere, A., & Lan, R. (2023). Biomimetic coatings in implant dentistry: A quick update. Journal of Functional Biomaterials, 15(1), 15.[https://www.mdpi.com/2079-4983/15/1/15.
- Mahmud, S., Rahman, M., Kamruzzaman, M., Khatun, H., Ali, M. O., & Haque, M. M. (2023). Recent developments in hydroxyapatite coating on magnesium alloys for clinical applications. The results in Engineering, 17, 101002. [CrossRef]
- Mysore, T. H. M., Patil, A. Y., Hegde, C., Sudeept, M., Kumar, R., Soudagar, M. E. M., & Fattah, I. (2024). Apatite insights: From synthesis to biomedical applications. European Polymer Journal, 112842. [CrossRef]
- Liu, M., Liu, Y., & Luo, F. (2023). The role and mechanism of platelet-rich fibrin in alveolar bone regeneration. Biomedicine & Pharmacotherapy, 168, 115795. [CrossRef]
- Khaohoen, A., Sornsuwan, T., Chaijareenont, P., Poovarodom, P., Rungsiyakull, C., & Rungsiyakull, P. (2023). Biomaterials and Clinical Application of Dental Implants in Relation to Bone Density—A Narrative Review. Journal of Clinical Medicine, 12(21), 6924. [CrossRef]
- Reda, R., Zanza, A., Galli, M., De Biase, A., Testarelli, L., & Di Nardo, D. (2022). Applications and Clinical Behavior of BioHPP in Prosthetic Dentistry: A Short Review. Journal of Composites Science, 6(3), 90. [CrossRef]
- Lo Giudice, R., Sindoni, A., Tribst, J. P. M., Dal Piva, A. M. D. O., Lo Giudice, G., Bellezza, U., ... & Famà, F. (2022). Evaluation of zirconia and high performance polymer abutment surface roughness and stress concentration for implant-supported fixed dental prostheses. Coatings, 12(2), 238.[https://www.mdpi.com/2079-6412/12/2/238].
- Özcan, M., Volpato, C. A. M., Hian, L., Karahan, B. D., & Cesar, P. F. (2022). Graphene for zirconia and titanium composites in dental implants: significance and predictions. Current Oral Health Reports, 9(3), 66-74.[https://link.springer.com/article/10.1007/s40496-022-00310-3.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).