Submitted:
22 January 2025
Posted:
23 January 2025
You are already at the latest version
Abstract
Keywords:
Introduction
Materials and Methods
Study Design and Patient Enrolment
Patient Selection and Grouping
Training Session
Data Collection
Data Analysis
Results
Baseline Characteristics of Study Sample
Comparison of Duration of Application of Non-Invasive Ventilatory Support (Hours) and Hospital Stay Between the Groups
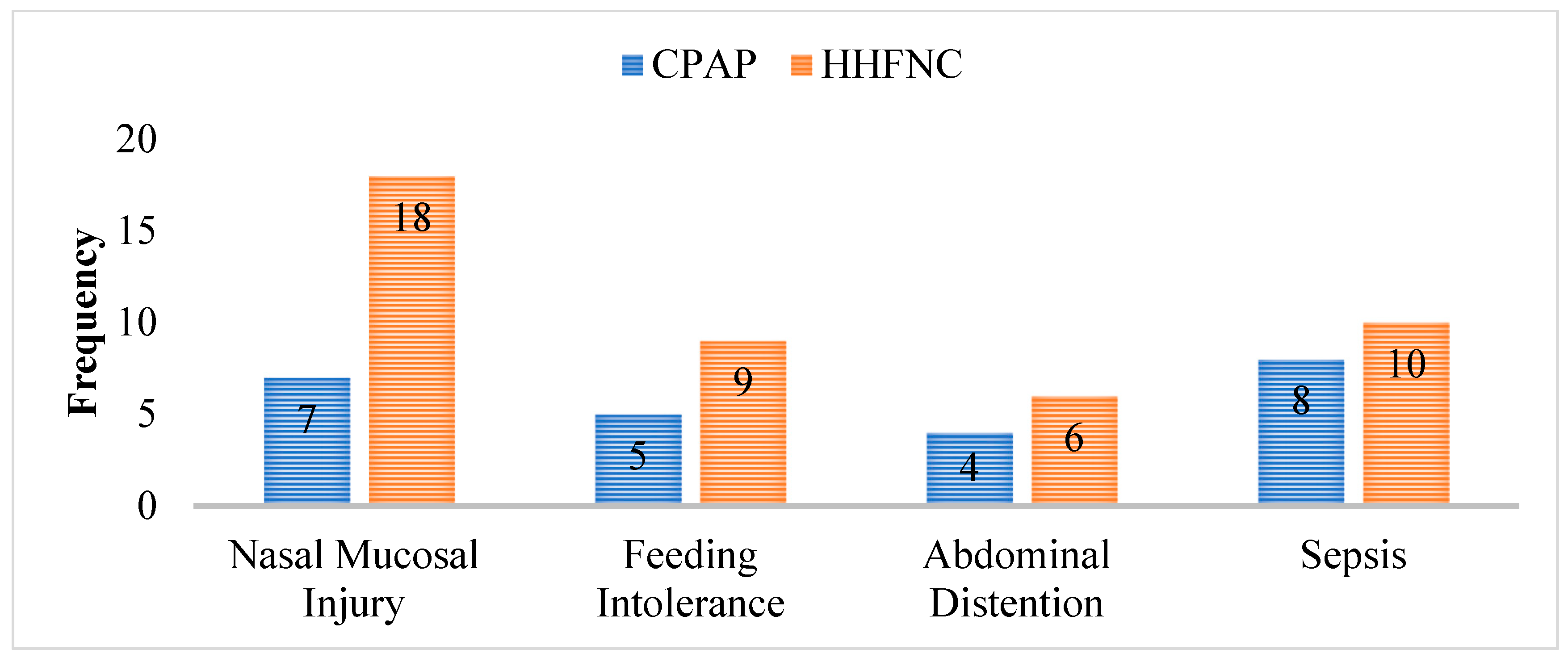
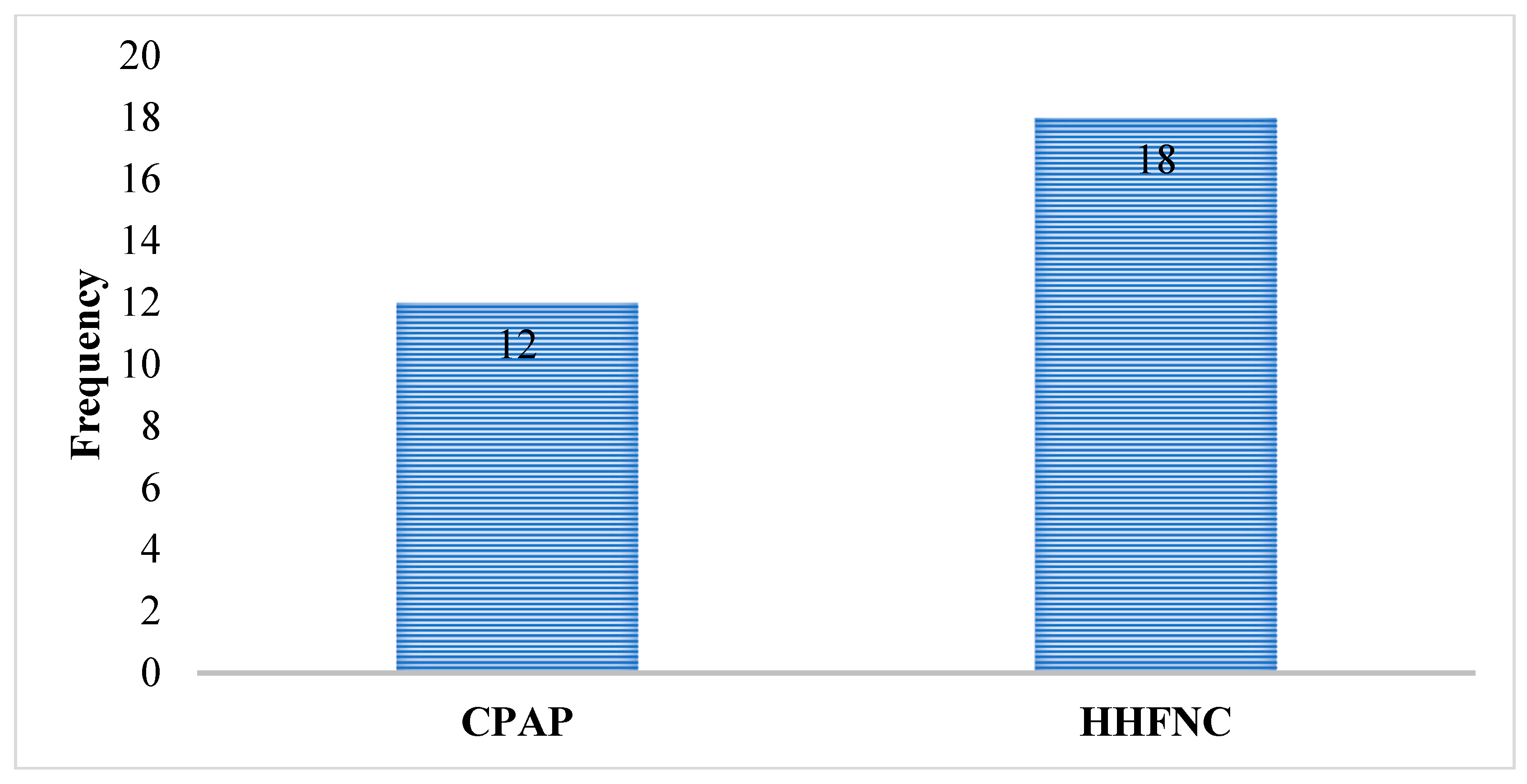
Comparison of Treatment Failure in Both Groups
Comparison of Duration of Neonatal Outcome Between the Groups
Discussion
Conclusions
Conflicts of Interest statement
Author’s contribution
Acknowledgments
Funding
Informed consent
Data Availability
Ethics Statement
References
- Long, M.E.; Mallampalli, R.K.; Horowitz, J.C.J.C.S. Pathogenesis of pneumonia and acute lung injury. 2022, 136, 747–769. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.S.; Subramanian, S.; Murki, S.; Rao, J.; Bai, M.; Penagaram, S. , et al. Predictors of mortality in neonatal pneumonia: an INCLEN childhood pneumonia study. 2021, 58, 1040–1045. [Google Scholar]
- Chisti, M.J.; Kawser, C.A.; Rahman, A.S.M.M.H.; Shahid, A.S.M.S.B.; Afroze, F.; Shahunja, K. , et al. Prevalence and outcome of anemia among children hospitalized for pneumonia and their risk of mortality in a developing country. 2022, 12, 10741. [Google Scholar] [PubMed]
- Nascimento-Carvalho CMJJdp. Community-acquired pneumonia among children: the latest evidence for an updated management. 2020, 96, 29–38.
- Malakian, A.; Aramesh, M.R.; Agahin, M.; Dehdashtian, M.J.B.p. Non-invasive duo positive airway pressure ventilation versus nasal continuous positive airway pressure in preterm infants with respiratory distress syndrome: a randomized controlled trial. 2021, 21, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kenner, C.; Boykova, M.V. Neonatal nursing care handbook: an evidence-based approach to conditions and procedures; Springer Publishing Company, 2021. [Google Scholar]
- Campbell, D.E. Continuing Care of the Infant After Transfer From Neonatal Intensive Care; Neonatology for Primary Care: American Academy of Pediatrics Itasca: IL, 2020. [Google Scholar]
- Sjöblom, A. Clinical and Physiological Consequences of Preoxygenation Using High-Flow Nasal Oxygen in Emergency Anaesthesia; Karolinska Institutet: Sweden, 2023. [Google Scholar]
- Çetinkaya, M.; Atasay, B.; Perk, Y.J.T.A.o.P.T.P.A. Turkish Neonatal Society guideline on the transfusion principles in newborns. 2018, 53 (Suppl 1), S101. [Google Scholar] [CrossRef]
- Owen, L.S.; Manley, B.J.; Davis, P.G.; Doyle, L.W.J.T.L. The evolution of modern respiratory care for preterm infants. 2017, 389, 1649–1659. [Google Scholar] [CrossRef] [PubMed]
- Dunn, M.S.; Kaempf, J.; de Klerk, A.; de Klerk, R.; Reilly, M.; Howard, D. , et al. Randomized trial comparing 3 approaches to the initial respiratory management of preterm neonates. 2011, 128, e1069–e1076. [Google Scholar]
- Murki, S.; Singh, J.; Khant, C.; Kumar Dash, S.; Oleti, T.P.; Joy, P. , et al. High-flow nasal cannula versus nasal continuous positive airway pressure for primary respiratory support in preterm infants with respiratory distress: a randomized controlled trial. 2018, 113, 235–241. [Google Scholar] [PubMed]
- Chang, C.-J.; Chiang, L.-L.; Chen, K.-Y.; Feng, P.-H.; Su, C.-L.; Hsu, H.-S.J.C.r.j. High-Flow Nasal Cannula versus Noninvasive Positive Pressure Ventilation in Patients with Heart Failure after Extubation: An Observational Cohort Study. 2020, 2020, 6736475. [Google Scholar] [CrossRef]
- Sreenan, C.; Lemke, R.P.; Hudson-Mason, A.; Osiovich, H.J.P. High-flow nasal cannulae in the management of apnea of prematurity: a comparison with conventional nasal continuous positive airway pressure. 2001, 107, 1081–1083. [Google Scholar] [CrossRef]
- Frat, J.-P.; Coudroy, R.; Marjanovic, N.; Thille, A.W.J. High-flow nasal oxygen therapy and noninvasive ventilation in the management of acute hypoxemic respiratory failure. 2017, 5((14)). [Google Scholar] [CrossRef]
- Dumpa, V.; Avulakunta, I.; Bhandari, V.J.E.R.o.R.M. Respiratory management in the premature neonate. 2023, 17, 155–170. [Google Scholar] [CrossRef]
- Lavizzari, A.; Colnaghi, M.; Ciuffini, F.; Veneroni, C.; Musumeci, S.; Cortinovis, I. , et al. Heated, humidified high-flow nasal cannula vs nasal continuous positive airway pressure for respiratory distress syndrome of prematurity: a randomized clinical noninferiority trial. 2016. [Google Scholar]
- Madar, J.; Roehr, C.C.; Ainsworth, S.; Ersdal, H.; Morley, C.; Ruediger, M. , et al. European Resuscitation Council Guidelines 2021: Newborn resuscitation and support of transition of infants at birth. 2021, 161, 291–326. [Google Scholar] [PubMed]
- De Paoli, A.; Morley, C.; Davis, P.J.A.o.D.i.C.-F.; Edition, N. Nasal CPAP for neonates: what do we know in 2003? 2003, 88, F168–F172. [Google Scholar] [CrossRef]
- Chan, S.Y. Development, Implementation, and Evaluation of a Clinical Practice Guideline for Care of Preterm Infants Receiving Non-Invasive Ventilation: A Before and After Study. The Chinese University of Hong Kong: Hong Kong, 2021. [Google Scholar]
- Shi, Y.; Muniraman, H.; Biniwale, M.; Ramanathan, R.J. A review on non-invasive respiratory support for management of respiratory distress in extremely preterm infants. 2020, 8, 270. [Google Scholar] [CrossRef]
- Abadesso, C.; Nunes, P.; Silvestre, C.; Matias, E.; Loureiro, H.; Almeida, H.J.P.R. Non-invasive ventilation in acute respiratory failure in children. 2012, 4, e16. [Google Scholar] [CrossRef]
- De Luca, D.; Tingay, D.G.; Van Kaam, A.H.; Courtney, S.E.; Kneyber, M.C.; Tissieres, P. , et al. Epidemiology of neonatal acute respiratory distress syndrome: prospective, multicenter, international cohort study. 2022, 23, 524–534. [Google Scholar]
- Silveyra, P.; Fuentes, N.; Rodriguez Bauza, D.E. Sex and gender differences in lung disease. Lung Inflammation in Health and Disease; Springer, 2021; Volume II, pp. 227–258. [Google Scholar]
- Sarkar, M.; Sinha, R.; Roychowdhoury, S.; Mukhopadhyay, S.; Ghosh, P.; Dutta, K. , et al. Comparative study between noninvasive continuous positive airway pressure and hot humidified high-flow nasal cannulae as a mode of respiratory support in infants with acute bronchiolitis in pediatric intensive care unit of a tertiary care hospital. 2018, 22, 85. [Google Scholar]
- Sharma, D.; Kaur, A.; Farahbakhsh, N.; Agarwal, S.J.T.J.o.M.-F.; Medicine, N. To compare nasal mask with binasal prongs in delivering continuous positive airway pressure for reducing need of invasive ventilation: randomized controlled trial. 2021, 34, 1896. [Google Scholar] [CrossRef]
- Shin, J.; Park, K.; Lee, E.H.; Choi, B.M. Humidified high flow nasal cannula versus nasal continuous positive airway pressure as an initial respiratory support in preterm infants with respiratory distress: a randomized, controlled non-inferiority trial. 2017, 32, 650. [Google Scholar] [CrossRef] [PubMed]
- Foglia, E.E.; Carper, B.; Gantz, M.; DeMauro, S.B.; Lakshminrusimha, S.; Walsh, M. , et al. Association between policy changes for oxygen saturation alarm settings and neonatal morbidity and mortality in infants born very preterm. 2019, 209, 17–22.e2. [Google Scholar]
- Sarker, S.K.; Choudhury, U.K.; Mohsin, M.; Mondal, S.K.; Begum, M.J.J.o.C.; Research, A.M. Diagnostic validity of ratio between differences of central venous to arterial CO2 and arterial to central venous O2 content in diagnosis of anaerobic metabolism among septic patients. 2021, 8, 34–38. [Google Scholar] [CrossRef]
- Cristea, A.I.; Ren, C.L.; Amin, R.; Eldredge, L.C.; Levin, J.C.; Majmudar, P.P. , et al. Outpatient respiratory management of infants, children, and adolescents with post-prematurity respiratory disease: an official American Thoracic Society clinical practice guideline. 2021, 204, e115–e133. [Google Scholar]
- Wu, C.; Chen, X.; Cai, Y.; Zhou, X.; Xu, S.; Huang, H. , et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. 2020, 180, 934–943. [Google Scholar]
- Friedman, M.L.; Nitu, M.E. Acute respiratory failure in children. 2018, 47, e268–e273. [Google Scholar] [CrossRef]
- Luo, J.; Duke, T.; Chisti, M.J.; Kepreotes, E.; Kalinowski, V.; Li, J.J. Efficacy of high-flow nasal cannula vs standard oxygen therapy or nasal continuous positive airway pressure in children with respiratory distress: a meta-analysis. 2019, 215, 199–208.e8. [Google Scholar] [CrossRef]
- Thota, U.R.; Palle, S.; Pandala, P.; Gangadhari, S.; Cherukuri, N.J.I. Outcome of High-flow Nasal Cannula Therapy in Children with Acute Respiratory Distress in A Tertiary Care Centre: A Prospective Cohort Study. 6 (S1), 13360–13367. [CrossRef]
- Jeengar, B. To compare the effectiveness of Heated Humidified High-Flow Nasal Cannula (HHHFNC) and Continuous Positive Airway Pressure (CPAP) in neonates with respiratory distress syndrome (RDS). 2024. [Google Scholar]
- Jose, D.; Parameswaran, N.J.I.J.o.P. Advances in management of respiratory failure in children. 2023, 90, 470–480. [Google Scholar] [CrossRef]
| Characteristics | Group A (n=128) | Group B (n=128) | p-value |
|---|---|---|---|
| Age (1-28 days) | 14.72±8.45 | 15.07±8.02 | 0.796 a |
| Gender | |||
| Male | 44 (40.0%) | 49 (44.5%) | 0.495 b |
| Female | 66 (60.0%) | 61 (55.5%) | |
| Gestational Age | 32.71±2.52 | 32.45±2.94 | 0.472 a |
| APGAR Score | 5.98+0.88 | 5.46+0.81 | 0.971 a |
| SPO2 | 69.69±18.2 | 70.43±17.38 | 0.735 a |
| Respiratory Rate | 52.29±11.23 | 55.75±11.66 | 0.028 a |
| HR Rate | 134.28±18.69 | 133.46±17/83 | 0.765 a |
| Arterial Ph | 6.26±1.37 | 6.09±1.41 | 0.363 a |
| PO2 | 63.31±12.1 | 63.02±11.5 | 0.941 a |
| PCO2 | 40.56±5.42 | 39.87±5.23 | 0.344 a |
| Duration of Application of Non-invasive Ventilatory Support (hours) | |||
|---|---|---|---|
| Study Group | Mean | Std. Deviation | p-value |
| CPAP (A) | 72.29 | 20.7 | 0.013a |
| HHFNC (B) | 65.20 | 15.9 | |
| Hospital stay in days | |||
| CPAP (A) | 24.25 | 6.07 | 0.000 a |
| HHFNC (B) | 20.14 | 3.50 | |
| Neonatal Outcome | Groups | Total | P-value | |
|---|---|---|---|---|
| CPAP | HHFNC | |||
| Discharged | 100(90.9%) | 94(85.5%) | 194(88.2%) | b 0.224 |
| Expired | 5(4.5%) | 4(3.6%) | 9(4.1%) | |
| Intubated | 5(4.5%) | 12(10.9%) | 17(7.7%) | |
| Total | 110 | 110 | 220 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





