1. Introduction
Placenta accreta spectrum disorder (PASD) is an abnormal attachment of the placenta to the uterine wall.[
1] Globally, PASD occurs in approximately 1:1000 deliveries, having increased from 0.04% to 0.9% over the last 20 years.[
2] There is no overall incidence of PASD in Indonesia, but there has been an increase in incidence at Dr. Soetomo Regional General Hospital Surabaya from 0% in 2014 to 2% in 2016 and is predicted to continue to increase.[
3] Research by Desmalia, et al[
4] reported that the prevalence of accreta among all pregnant women in 2018–2021 was 0.08% (2 out of 2,424 pregnant women); 0.21% (4 out of 1,910 pregnant women); 1.72% (23 out of 1,336 pregnant women) and 2.8% (36 out of 1,285 pregnant women) at Dr. Mohammad Hoesin General Hospital Palembang, respectively.[
4] In a study at Dr. Zainoel Abidin Hospital Banda Aceh, the incidence of PASD in 2019–2020 was 2.3%.[
5] This disorder is a contributor to maternal mortality due to postpartum hemorrhage that occurs.
PASD increases maternal morbidity up to 18-fold and is most commonly associated with massive postpartum hemorrhage. The mortality rate from PASD is approximately 7% but increases to 30% if not diagnosed antenatally due to attempts to separate the placenta after delivery.[
6]
The pathogenesis of PASD is also influenced by the inflammatory process. Therefore, the examination of biomarkers related to the inflammatory process, namely Matrix Metalloproteinase (MMP) and CXC Motif Chemokine Receptor 2 (CXCR2), is expected to lead researchers to a bright spot regarding the pathogenesis of PASD. Cell movement across the extracellular matrix (ECM), as well as the modification and breakdown of ECM by MMPs, are crucial components of the wound healing process. Matrix Metalloproteinases are a collection of enzymes that include zinc (Zn) and require calcium to function. They play a role in breaking down the extracellular matrix (ECM). MMP-2 has a role in wound healing by accelerating cell migration, while MMP-9 is secreted by keratinocytes at the leading edge of the wound to promote cell migration and the development of new epithelial tissue. Both MMP-2 and MMP-9 are present in the damaged epithelium.[
7] Matrix metalloproteinases play a vital role as enzymes in the initial stages of pregnancy by facilitating trophoblast cell penetration and invasion. This is supported by the research of Chen, et al.[
8] who reported a higher positive level of MMP-9 expression in the PASD group compared with the control group. Research by El-Hussieny, et al.[
9] also reported MMP-2 staining in the cytoplasm of trophoblast villus and extravillous placental tissue of PASD patients was higher than normal placenta. The biomarker CXCR2 is involved in trophoblast invasion, neovascularisation, and vascular remodelling processes..[
10] Women with preeclampsia exhibit less CXCR2 expression in their placental tissue compared to those with normal pregnancies.[
11] In PASD, the levels of immunostaining expression are likely elevated compared to a standard pregnancy due to the overexpression of CXCR2. Study by Ma, et al.[
12] also reported that CXCR2 as a CXCL1 receptor promotes endothelial cell proliferation, migration, and angiogenesis and plays a role in decidual angiogenesis during the first trimester of pregnancy.
This study is expected to analyze the role of CXCR2, MMP-2, and MMP-9 in the pathogenesis of PASD.
2. Materials and Methods
2.1. Study Population
This case-control study involves fifty-one people, comprising 17 PASD patients and 34 healthy controls. The minimum sample size was determined using the sample size estimate formula for analytical research with numerical data in order to ascertain the mean difference between two independent groups. Using a confidence level of 95% (α = 0.05, zα = 1.96), a power level of 90% (β = 0.1, zβ = 1.28), and a total proportion of 35% from a previous study by Chen et al.[
8], a minimum sample size of 15 was obtained. Recruitment of study subjects was carried out using the consecutive sampling method. With a case-control ratio of 1:2, a minimum of 51 subjects were required, consisting of 17 patients with PASD and 34 patients without PASD. The study population consisted of pregnant women who had previously undergone a caesarean section with and without PASD and visited Dr. Mohammad Hoesin Hospital Palembang between June and December 2023. The inclusion criteria for the case group included pregnant women with PASD. The control group consisted of expectant mothers with history of caesarean section without PASD and were not yet in labor. The exclusion criteria included pregnant women with multiple fetus, preeclampsia, and those who refused to participate in the study.
The minimum sample size was determined using the sample size estimate procedure for analytical research with numerical data in order to ascertain the mean difference between two independent groups. The minimum sample size was determined using a confidence level of 95% (α = 0.05, zα = 1.96), a power level of 90% (β = 0.1, zβ = 1.28), and a total proportion of 35% from a prior study conducted by Chen et al.[
8] The recruitment of study subjects was conducted using the sequential sampling technique. To maintain a case-control ratio of 1:2, a minimum of 51 individuals were needed, including 17 patients with PASD and 34 patients without PASD. The study population consisted of pregnant women who had previously undergone a caesarean section and visited Dr. Mohammad Hoesin Hospital Palembang between June and December 2023, both with and without PASD. The case group consisted of pregnant women with PASD who met the inclusion criteria. The control group comprised pregnant women who had previously undergone a caesarean section but did not have PASD and were not in labor. The exclusion criteria encompassed pregnant ladies with multiple gestation, preeclampsia, and individuals who declined to partake in the study.
2.2. Data Collection
Basic subject characteristics were collected from electronic medical records and medical history taking. The blood sample along with placental tissue were obtained during the third trimester of pregnancy. PASD was detected using 2-dimensional ultrasonography using GE® Voluson™ E-6 (General Electric, Austria) and Samsung® RS80A (Samsung Medison, Seoul, Korea). A total of 20 milliliters of blood samples were taken into serum separator tubes (SSTs) for 25-OHD, zinc, and calcium ion measurements. Serum 25-OHD, calcium ion, and zinc levels were measured using Chemiluminescent Microparticle Immunoassay (CMIA), Ion Selective Electrode (ISE), and Inductively Coupled Plasma-Mass Spectrometry (ICP-MS) respectively.
The expression levels of MMP-2, MMP-9, and CXCR2 were assessed by immunohistochemical analysis.. Five small tissue pieces of approximately 1 × 1 × 1 cm in size were cut from the newborn placenta at the central zone (avoiding blood vessels and/or calcium deposits), i.e. at the midpoint between the cord insertion point and the edge of the placental disc. The tissues were fixed in 10% buffered formalin (pH 7.0) and sent to the anatomical pathology laboratory to be processed into paraffin blocks. Tissues were then sectioned at approximately 4 μm. The excised tissues were left at 40 C for 12 hours before fixation with anti-CXCR2, anti-MMP-2, and anti-MMP-9 antibodies. The examination was conducted at the Anatomical Pathology Laboratory of Barokah Palembang and the Department of Anatomical Pathology of Dr. Mohammad Hoesin Hospital Palembang. Further analysis of the expression of MMP-2, MMP-9, and CXCR2 in placental and uterine tissue were done using a Nikon Eclipse 80i microscope and ImageJ image-processing software. The results were interpreted by an anatomical pathologist (KM). Each region was assigned a score of 3 for strong (deep brown) staining, 2 for moderate (light brown) staining, 1 for weak (bluish purple) staining, and 0 for no staining (purple) intensity. The immunostaining density value ranged from 4 points for 81–100% to 3 points for 51–80%, 2 points for 11–50%, 1 point for 1–10%, and 0 points for 0% of cells that exhibited positive staining. The immunohistochemical score was determined by multiplying the value of immunostaining intensity with the value of immunostaining density. In preparation for assessing the expression of each sample with the evaluated antibodies, each sample was subjected to five visual field examinations..
Prior to their enrollment in the study, each participating women provided written informed consent. The study received ethical approval from the Ethics Committee of the Faculty of Medicine, University of Sriwijaya (DP.04.03/D.XVIII.6.11/ETIKRSMH/14/2023).
2.3. Statistical Analysis
All data were analyzed using STATA version 15 (StataCorp LLC, UK). Univariate analysis was on the basic characteristics of the subjects, which included maternal age, parity, gestational age, and comorbidities. Data were presented descriptively with frequency and percent for categorical data (nominal and ordinal) and mean and standard deviation for numerical data (interval and ratio). Bivariate analysis of the association of each variable with the incidence of PASD was performed through bivariate tests using Pearson chi-square test with Fisher's exact alternative for categorical data and independent T-test with Mann-Whitney alternative for numerical data. Correlation tests with Pearson correlation test and Spearman rho test were performed to assess the correlation of calcium ion levels with CXCR2, MMP-2, and MMP-9.
3. Results
3.1. Characteristics of Research Subjects
A case-control diagnostic study was undertaken at the Obstetrics and Gynecology Department of Dr. Mohammad Hoesin General Hospital Palembang. A total of 51 pregnant women met the inclusion criteria as research subjects. There were 17 pregnant women in the case group and 34 pregnant women in the control group (Figure 52). The study subjects had an average age of 31.82 ± 4.68 years, the majority aged 20 – 35 years (72.5%) with a BMI of 26.83 ± 3.27 kg/m2. Most of the study subjects were multiparous pregnant women (66.7%) with fullterm gestational age (52.9%) and did not have did not have comorbidity such as Diabetes Mellitus (DM) or hypertension (96.1%). The gestational age in the case group was significantly younger than the control group (p=0.005). Meanwhile, the general characteristics of the study subjects such as maternal age, BMI, parity, and comorbidities between the two groups were not significantly different. The general characteristics of the study subjects in each group are presented in
Table 1.
The PASD group had a higher proportion of subjects aged 20 – 35 years (76.5%), multiparous (76.5%), and preterm (76.5%) than the non-PASD group. The non-PASD group had a higher proportion of subjects with obesity I (70.5%), obesity II (23.5%), at full-term (67.6%), and no comorbidities (97.1%) than the PASD group.
3.2. Expression of CXCR2, MMP-2 and MMP-9 in Placental and Uterine Tissue
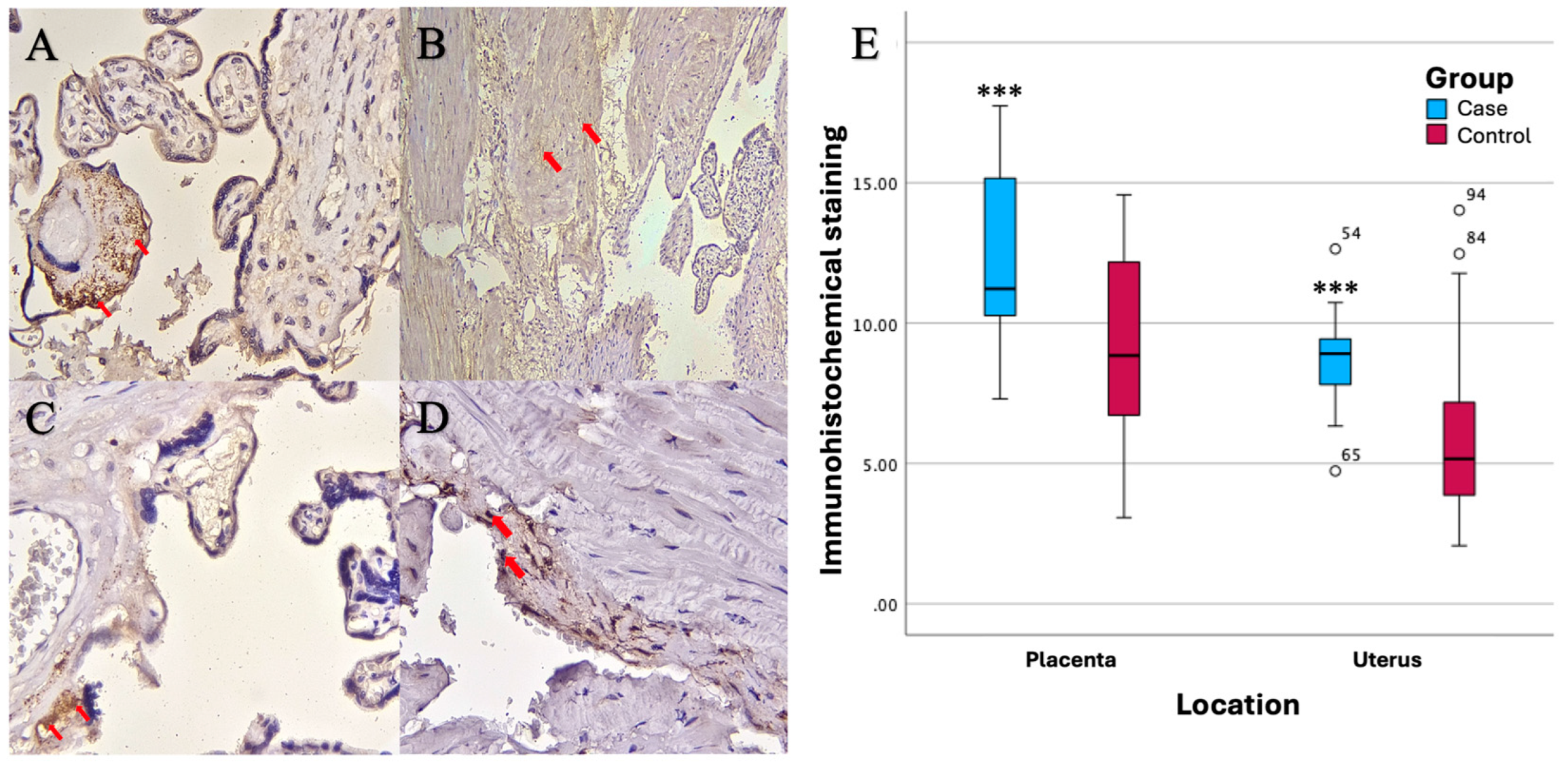
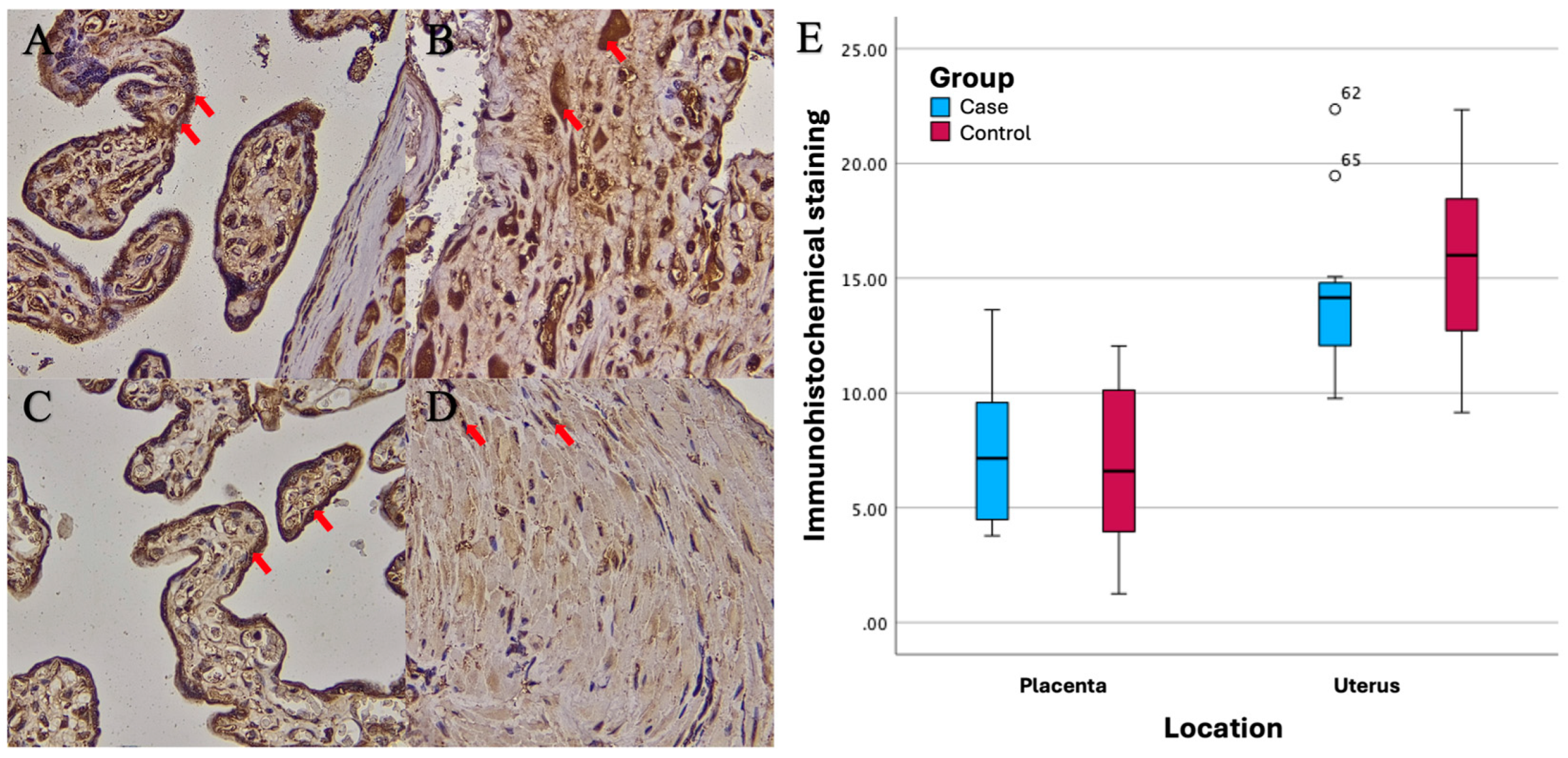
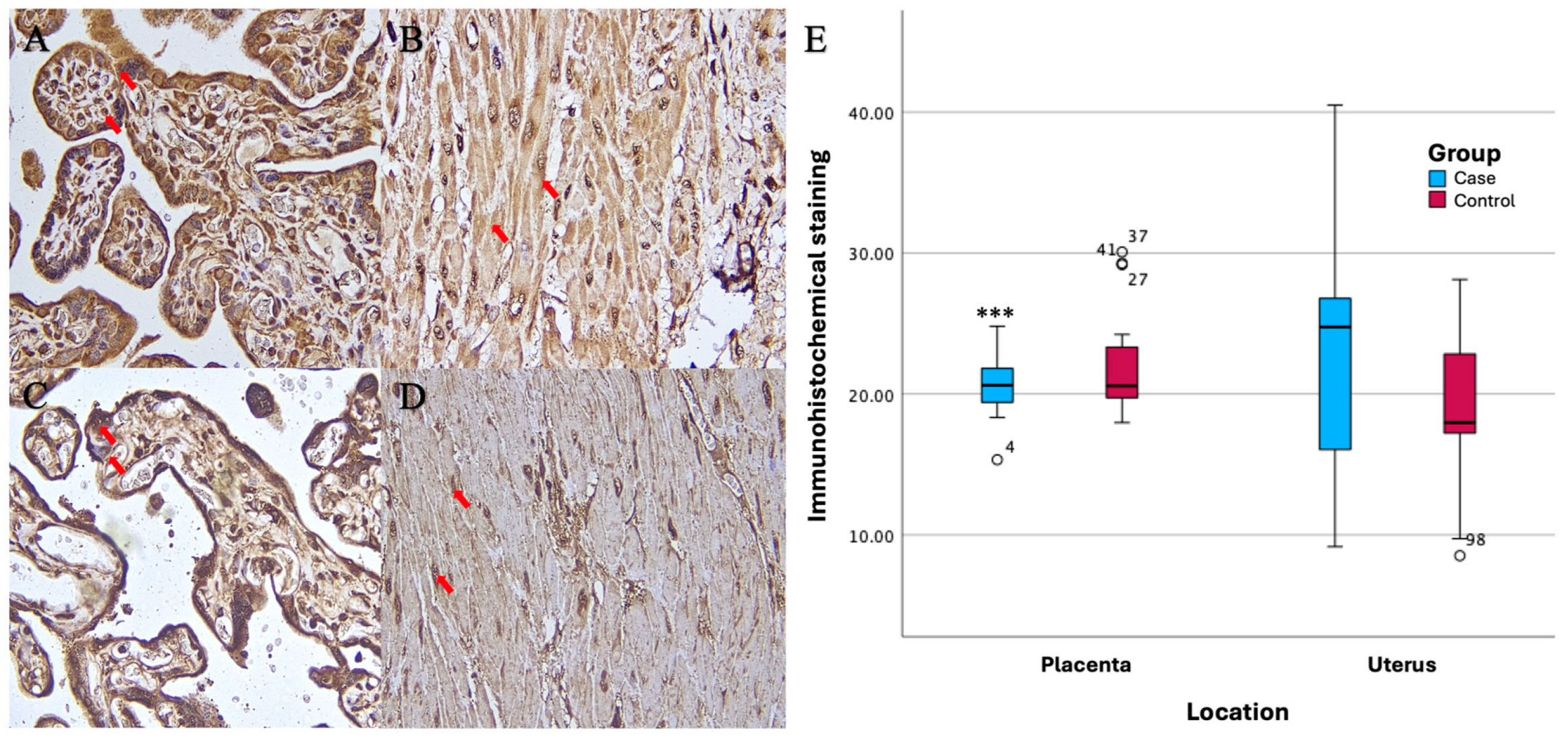
Based on the results of imuhohistochemical (IHC) examination, there were significant differences in immunostaining expression levels in several tissues in the PASD group compared with the non-PASD group. The expression of CXCR2, MMP-2, and MMP-9 was characterized by brown staining in the cytoplasm and cell membrane, while purple staining showed hematoxylin and eosin-stained nuclei. The histology picture of the tissue before IHC staining is shown in
Figure 1, while the results of the IHC examination are shown in
Figure 2,
Figure 3 and
Figure 4.
Immunohistochemical staining with anti-CXCR2 antibody showed different expression levels between PASD and non-PASD groups in placental and uterine tissues. In the PASD group, both placenta and uterus had CXCR2 protein expression. However, there was no expression of CXCR2 protein in the placenta or uterus in the non-PASD group (
Figure 2).
Different expression levels between PASD and non-PASD groups in placental and uterine tissues were obtained in immunohistochemical stains with anti-MMP-2 antibody. In the PASD group, both placenta and uterus were found to have MMP-2 protein expression. However, there was no MMP-2 protein expression in the placenta or uterus in the non-PASD group (
Figure 3).
Immunohistochemical staining with anti-MMP-9 antibody showed different expression levels between PASD and non-PASD groups in placental and uterine tissues. In the PASD group, both placenta and uterus had MMP-9 protein expression. However, there was no MMP-9 protein expression in the placenta or uterus in the non-PASD group (
Figure 4).
There were significant differences in the immunostaining expression levels (percentage of positive cells) of CXCR2 in placental tissue and CXCR2 and MMP-9 in uterine tissue in the PASD group compared with the non-PASD group (
Table 2).
There were significant differences in the immunostaining expression levels (percentage of positive cells) of CXCR2 in placental tissue and CXCR2 and MMP-9 in uterine tissue in the PASD group compared with the non-PASD group (
Table 3).
3.3. Correlation of 25-OHD, Zinc, and Calcium Ion Levels with CXCR2, MMP-2, and MMP-9 Expression
In this study, there was no correlation of 25-OHD, zinc, and calcium ion levels with the expression of CXCR2, MMP-2, and MMP-9 in all groups, the PASD group, and the non-PASD group.
Table 4.
Correlation of 25-OHD levels with CXCR2, MMP-2, and MMP-9 expression
Table 4.
Correlation of 25-OHD levels with CXCR2, MMP-2, and MMP-9 expression
| 25-OHD levels |
p value* |
r |
PASD (n=17) |
Non-PASD (n=34) |
| p value* |
r |
p value* |
r |
| Placental CXCR2 |
0.733 |
0.048 |
0.693 |
0.103 |
0.370 |
-0.158 |
| Uterine CXCR2 |
0.716 |
-0.052 |
0.327 |
-0.252 |
0.778 |
0.050 |
| Placental MMP-2 |
0.061 |
0.264 |
0.119 |
0.392 |
0.301 |
0.182 |
| Uterine MMP-2 |
0.705 |
0.054 |
0.534 |
-0.162 |
0.411 |
0.145 |
| Placental MMP-9 |
0.498 |
0.097 |
0.929 |
0.023 |
0.305 |
0.181 |
| Uterine MMP-9 |
0.458 |
0.106 |
0.103 |
0.408 |
0.717 |
-0.064 |
Spearman correlation test showed no correlation between 25-OHD levels and the expression of CXCR2, MMP-2, and MMP-9 in all groups, PASD group, and non-PASD group (p>0.05).
Table 5.
Correlation of 25-OHD levels with CXCR2, MMP-2, and MMP-9 expression
Table 5.
Correlation of 25-OHD levels with CXCR2, MMP-2, and MMP-9 expression
| Zinc levels |
p value |
r |
PASD (n=17) |
Non-PASD (n=34) |
| p value |
r |
p value |
r |
| Placental CXCR2 |
0.590a
|
-0.077 |
0.993a
|
-0.002 |
0.496a
|
-0.120 |
| Uterine CXCR2 |
0.806a
|
0.035 |
0.666a
|
-0.112 |
0.547a
|
0.106 |
| Placental MMP-2 |
0.564a
|
0.082 |
0.636a
|
0.123 |
0.761a
|
0.054 |
| Uterine MMP-2 |
0.491a
|
-0.098 |
0.276a
|
-0.279 |
0.758a
|
0.054 |
| Placental MMP-9 |
0.365b
|
-0.129 |
0.453b
|
-0.195 |
0.487b
|
-0.123 |
| Uterine MMP-9 |
0.785b
|
0.039 |
0.704b
|
-0.099 |
0.627b
|
0.086 |
Correlation test showed no correlation between zinc levels and the expression of CXCR2, MMP-2, and MMP-9 in all groups, PASD group, and non-PASD group (p>0.05).
Table 6.
Correlation of 25-OHD levels with CXCR2, MMP-2, and MMP-9 expression
Table 6.
Correlation of 25-OHD levels with CXCR2, MMP-2, and MMP-9 expression
| Calcium ion content |
p value* |
r |
PASD (n=17) |
Non-PASD (n=34) |
| p value* |
r |
p value* |
r |
| Placental CXCR2 |
0.495 |
-0.097 |
0.8886 |
-0.037 |
0.983 |
-0.003 |
| Uterine CXCR2 |
0.794 |
0.037 |
0.618 |
-0.130 |
0.234 |
0.210 |
| Placental MMP-2 |
0.342 |
0.135 |
0.485 |
0.182 |
0.828 |
0.039 |
| Uterine MMP-2 |
0.750 |
-0.045 |
0.989 |
-0.004 |
0.499 |
-0.120 |
| Placental MMP-9 |
0.393 |
-0.122 |
0.874 |
0.042 |
0.471 |
-0.128 |
| Uterine MMP-9 |
0.827 |
0.031 |
0.349 |
0.242 |
0.426 |
-0.141 |
Spearman correlation test showed no correlation between calcium ion levels and the expression of CXCR2, MMP-2, and MMP-9 in all groups, PASD group, and non-PASD group (p>0.05).
4. Discussion
4.1. Characteristics of Research Subjects
A diagnostic study with a case-control design was conducted to analyze the relationship of uterine surgery history, short delivery interval, serum 25-OHD level, serum zinc level, serum calcium ion level, and expression of CXCR2, MMP-2, and MMP-9 to the incidence of PASD. The study was conducted at the Obstetrics and Gynecology Department of Dr. Mohammad Hoesin Hospital Palembang involving 51 pregnant women with a history of previous cesarean section consisting of a case group (17 pregnant women with PASD) and a control group (34 pregnant women without PASD). The study subjects had an average age of 31.82 ± 4.68 years, mostly aged 20 - 35 years (72.5%) with a BMI of 26.83 ± 3.27 kg/m2. Most of the study subjects were multiparous pregnant women (66.7%) with fullterm gestational age (52.9%) and did not have DM or hypertension (96.1%). There was no significant difference in the general characteristics of the study subjects between the two groups.
Matching the general characteristics of subjects in the case and control groups is important to prevent the influence of confounding variables that could potentially affect the results of the study. Research by Liang, et al.[
13] reported that BMI with an Odds Ratio (OR) of 1.04 and 95% Confidence Interval (CI) of 1.02–1.06, parity (OR=1.60; 95%CI: 1.42–1.79), and the number of previous cesarean deliveries (OR=2.57; 95%CI: 2.02–3.26) were independent risk factors for the incidence of PASD in the group of pregnant women with a history of previous cesarean section. Research by Zhang, et al.[
14] reported that maternal age >35 years increased the risk of PASD (OR = 1.26; 95%CI 1.14–1.40). Research by Kilicci, et al.[
15] also reported that pregnant women with diabetes and hypertension had a risk of PASD of 3.83 and 29.7 times, respectively. In this study, there were no significant differences in the variables of maternal age, BMI, parity, number of previous cesarean deliveries, and comorbidities between the case and control groups, so these four variables were not confounding variables that could potentially affect the results of the study.
The gestational age in the case group of 35 (30–39) weeks was significantly lower than the control group of 37.5 (34–40) weeks (p=0.005). This is in accordance with the results of the research of Shi, et al.[
16] who reported lower gestational age in the case group (37.9 ± 1.9 weeks) compared with the control group (36.1 ± 5.3 weeks, p<0.001). This is because the management of PASD with termination of pregnancy by elective cesarean section was carried out at a lower gestational age of 34–36 weeks.[
17]
4.2. Expression of CXCR2, MMP-2 and MMP-9 in Placental and Uterine Tissue
Based on the results of immunohistochemical (IHC) examination, there were significant differences in immunostaining expression (percentage of positive cells) and IHC score of CXCR2 in placental tissue and CXCR2 and MMP-9 in uterine tissue in the PASD group compared with the non-PASD group. No previous research has been conducted to evaluate the expression of CXCR2 in the uterine and placental tissues of patients with PASD. Wu et al.[
11] examined the level of CXCR2 expression in the placental tissue of 38 patients with preeclampsia, comparing it to 21 normal pregnancies. The expression of CXCR2 was reduced in the placental tissue of patients with preeclampsia compared to those with a normal pregnancy. This was evident from the lower intensity of immunostaining observed in the cytoplasm of villous syncytiotrophoblast cells and decidual cells. CXCR2 overexpression in PASD is likely to result in higher levels of immunostaining expression compared with control (normal pregnancy). CXCR2 expression has a role in increasing trophoblast invasion, vascular remodeling, and neovascularization.[
10] Ma's study,[
12] et al also reported that CXCR2 as a CXCL1 receptor promotes endothelial cell proliferation, migration, and angiogenesis and plays a role in decidual angiogenesis during the first trimester of pregnancy. Continued study of these biomarkers may bring researchers to light regarding the pathogenesis of PASD.
The currently existing explanation concerning the cause of PASD is that a malfunction in the interface between the endometrium and myometrium results in the inability of the uterine scar area to undergo normal decidualization, hence permitting deeper infiltration of trophoblasts. Therefore, cesarean section wound healing is believed to be a contributing factor in the occurrence of PASD. Cell migration on the extracellular matrix (ECM), together with the modification and destruction of ECM by MMPs, are crucial components of the wound healing process. Matrix metalloproteinases are a class of enzymes that include zinc (Zn) and require calcium for their activity. They play a role in breaking down the ECM. MMP-2 has a function in wound healing by speeding up the movement of cells, whereas MMP-9 is produced by keratinocytes at the forefront of the wound to enhance cell movement and the process of re-epithelialization. MMP-2 and MMP-9 are detected in the damaged epithelium.[
7]
Matrix Metalloproteinase is considered a crucial enzyme involved in the penetration of trophoblast cells during the initial stages of pregnancy. Additionally, it seems to have a role in the invasion of trophoblast cells. Research by El-Hussieny, et al.[
9] on 26 PASD and 31 non-PASD reported MMP-2 staining in the cytoplasm of trophoblast villus and extravillous placental tissue of PASD patients was significantly higher than normal placenta. Most PASD cases showed moderate (30.8%) or strong (42.3%) staining, while most control cases showed negative (32.3%) or weak (38.7%) staining. Research by Chen, et al[
8] examined MMP-9 expression in 10 PASD patients and 10 non-PASD patients. The positive level of MMP-9 expression in the PASD group (60%) was significantly higher than the control group (10%). MMP-9 was mainly localized in the cytoplasm of trophoblasts with positive brown granulators or punctata. Placenta accreta is characterized by a reduction in E-cadherin levels and an increase in MMP-9 levels. Trophoblasts are believed to increase trophoblast invasion in accreta.[
8]
The IHC score was calculated from the staining intensity and the percentage of positive cells stained. In this study, there was a significant difference in the CXCR2 IHC score in placental tissue and CXCR2 and MMP-9 in uterine tissue in the PASD group compared with the non-PASD group. However, due to the small sample size, the results of this study should be interpreted with caution. Further studies with larger sample sizes are needed to assess the expression of CXCR2, MMP-2, and MMP-9 in uterine and placental tissues of PASD patients.
Trophoblast cell implantation and invasion are closely related to the expression of MMPs. Gelatinases, namely MMP-2 and MMP-9, are key in the invasion process. MMP-2 and MMP-9 are expressed differently in trophoblast cells. MMP-2 is the main regulator of trophoblast invasion in early pregnancy. MMP-2 is localized at the base of the placenta during early pregnancy and is dominant over trophoblast MMP-9 at 6–8 weeks gestation. MMP-2 is more abundantly secreted in early pregnancy until week 9. MMP-2 is then continuously expressed throughout pregnancy, but its activity is reduced in the placenta at term gestation. MMP-9 is mainly expressed by trophoblast cells after week 9.[
18,
19]
Overexpressed laminin subunit gamma 2 (LAMC2) promotes excessive trophoblast invasion via phosphatidyl inositol-3 kinase (PI3K)/AKT serine/threonine kinase (Akt)/MMP-2/9 pathway in PASD pathogenesis.[
20] However, the increase in MMP-9 expression is independent of the increase of MMP-2.[
21] The expression of MMP-2 is constant and most proinflammatory stimuli do not produce a rise in MMP-2 expression because the MMP-2 gene does not include binding sites for proinflammatory transcription factors like activator protein 1. The activation of MMP-2 is crucial for its role in promoting angiogenesis and invasion. However, the activation of MMP-9 occurs through the release of the MMP-9 prodomain by serine proteases or other MMPs. MMP-9 can be activated through alternative pathways in response to oxidative stress that affects cysteine activation. Stress-activated mitogen-activated protein kinase (MAPK) pathway plays a role in the increased expression of MMP-9 in human trophoblast cells. Tumor Necrosis Factor (TNF)-α might activate two different pathways leading to MMP-9 expression namely extracellular signal regulated kinase (Erk)-1/2 pathway which then initiates nuclear factor-kappaB (NF-κB) activation as well as stress-activated protein kinase (SAPK)/c-Jun N-terminal kinase (JNK) pathway which activates AP-1.[
22] In addition, an in vivo study by Costanzo, et al.[
21] reported an increase in MMP-2 levels five hours after injury and reached the highest levels on day 7 and then rapidly decreased. On the other hand, MMP-9 levels remained elevated even after 2 weeks from injury. This is thought to be the reason MMP-2 in this study did not increase along with the increase of MMP-9.[
23]
4.3. Correlation of 25-OHD, Zinc, and Calcium Ion Levels with CXCR2, MMP-2, and MMP-9 Expression
In this study, there was no correlation of 25-OHD, zinc, and calcium ion levels with CXCR2, MMP-2, and MMP-9 expression in all subjects, PASD group, and non-PASD group. Not many previous studies have reported the correlation of 25-OHD, zinc, and calcium ion levels with CXCR2, MMP-2, and MMP-9. CXC chemokine receptor 2 (CXCR2), also known as interleukin-8 receptor, is activated by IL-8. Intracellular calcium ions are reported to increase in response to IL-8.[
24] CXCR2 also mediates intracellular calcium mobilization and neutrophil migration and infiltration as a result of disruption of the PDZ motif-containing CXCR2 complex inhibits intracellular calcium mobilization, chemotaxis, and transepithelial neutrophil migration.[
25] CXCR2 expression in rat placenta has also been reported to be associated and may serve as a potential biomarker for zinc deficiency during pregnancy.[
26] CXCR2 is thought to be negatively correlated with 25-OHD levels. Dauletbaev et al.[
27] reported that high concentrations of 25-OHD and 1,25(OH) D
23 moderately downregulate IL-8 in hyperinflammatory macrophages.
The catalytic region of MMP has two zinc ion binding sites (Zn
2+) and one calcium ion binding site (Ca
2+). MMP activity requires activation of Zn
2+ and Ca
2+.[
28] Changes in extracellular zinc and calcium ion levels regulate the activation and dynamics of MMP-2 and MMP-9 in trophoblast cells, [
29] and thus affect cellular behavior including trophoblast cell invasion in PASD. Study by Cruz et al.[
30] reported the regulation of MMP-2 and MMP-9 activity by calcium ion concentration. Study by Zong et al.[
31] also reported increased expression of MMP-2 and MMP-9 in rat placental tissue after zinc administration. Through in vitro observation, zinc was shown to promote trophoblast cell invasion and migration through increased signal transducer and activator of transcription 3 (STAT3)-MMP-2/9 activity. Therefore, increased levels of zinc and calcium ions are thought to be positively correlated with the expression of MMP-2 and MMP-9 in PASD. On the other hand, vitamin D levels are thought to be negatively correlated with MMP-2 and MMP-9 expression. Ma et al.[
32] reported decreased expression of MMP-2 and MMP-9 in rat placental tissue and human trophoblast cells supplemented with vitamin D. Vitamin D administration was also reported to inhibit trophoblast migration and invasion.
4.4. Strength and Limitation of the Study
This is the first study to simultaneously assess clinical, laboratory and immunohistochemical parameters to predict the incidence of PASD. In addition, the study population included pregnant women with a history of cesarean delivery, which is known to be a major risk factor for PASD. No previous studies have reported the diagnostic value of the PAI score in a population of pregnant women without placenta previa. In addition, few studies have been conducted on PAI scores prospectively. This study is also the first study to describe the expression of CXCR2 in placental tissue as well as the expression of CXCR2, MMP-2 and MMP-9 in uterine tissue of PASD patients. This study also measured the diagnostic value of serum 25-OHD, serum zinc levels, and serum calcium ion levels. The modified PAI score scoring system consisting of zinc, lacunae, and myometrial thickness parameters in the smallest sagittal section of this study can be further developed to be used in clinical practice to help confirm the diagnosis of PASD.
This study was a case-control study conducted at only one hospital (single center). In addition, only patients with histopathologically confirmed diagnoses were included in the PASD group, resulting in a small sample size. Furthermore, the findings of this study are relevant in women who have previously undergone cesarean delivery, as all PASD events in our sample were observed in women with that specific risk factor. Pregnant women without a history of cesarean delivery but with other predisposing factors such as a history of uterine surgery were not included in this study. This study also did not examine other biomarkers that may play a role in PASD. Extensive multicenter studies with larger sample sizes are needed to generalize the results and to determine whether combining various other clinical and laboratory parameters can improve accuracy in identifying PASD.
5. Conclusions
The expression level of CXCR2 immunostaining in placental and uterine tissues of patients with PASD was significantly different from patients without PASD. The expression level of MMP-2 immunostaining in placental and uterine tissues of patients with PASD was not significantly different from patients without PASD. The MMP-9 immunostaining expression level of PASD patients was significantly different from that of patients without PASD in uterine tissue, but not in placental tissue.
Author Contributions
Conceptualization, P.M., K.M., P.M.L., and I.A.L.; methodology P.M., K.M., P.M.L., and I.A.L.; software, I.A.L. and B.S.; validation, K.M., P.M.L., and I.A.L.; formal analysis, P.M. and I.A.L.; investigation, P.M.; resources, P.M.; data curation, P.M.; writing-original draft preparation, P.M., C.K., H.A., and B.S.; writing-review and editing, K.M., P.M.L., and I.A.L.; visualization, P.M. and B.S.; supervision, K.M., P.M.L., and I.A.L.; project administration, P.M.. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Faculty of Medicine, University of Sriwijaya (DP.04.03/D.XVIII.6.11/ETIKRSMH/14/2023).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank all the pregnant women who participated in this study; may you and your babies always be healthy. We also thank all the medical staffs at Dr. Mohammad Hoesin General Hospital Palembang who were involved in this study. Last, but not least, we also thank the Prodia Laboratory, the Anatomical Pathology Laboratory of Barokah Palembang, and the Department of Anatomical Pathology of Dr. Mohammad Hoesin Hospital Palembang. May this study bring us a new understanding of the pathogenesis of placenta accreta.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Cahill AG, Beigi R, Heine RP, Silver RM, Wax JR. Placenta Accreta Spectrum. Am J Obstet Gynecol 2018;219:B2–16. [CrossRef]
- Garmi G, Salim R. Epidemiology, Etiology, Diagnosis, and Management of Placenta Accreta. Obstet Gynecol Int 2012;2012:1–7. [CrossRef]
- Aryananda RA. Resurgence of placenta accreta in Indonesia. Majalah Obstetri & Ginekologi 2018;26:98–9. [CrossRef]
- Desmalia A, Bernolian N, Martadiansyah A, Theodorus T, Dewi C, Mirani P, et al. The Diagnostic Methods of Placenta Accreta Spectrum Disorders. Majalah Kedokteran Sriwijaya 2022;54:1–7.
- Yeni CM, Andayani H, Aulia, Indirayani I, Razali R. The Association betweeen Cesarean Section and Placenta Accreta: Indonesian Journal of Obstetrics and Gynecology 2022;10:127–32. [CrossRef]
- Fonseca A, Ayres de Campos D. Maternal morbidity and mortality due to placenta accreta spectrum disorders. Best Pract Res Clin Obstet Gynaecol 2021;72:84–91. [CrossRef]
- Caley MP, Martins VLC, O’Toole EA. Metalloproteinases and Wound Healing. Adv Wound Care (New Rochelle) 2015;4:225–34. [CrossRef]
- Chen Y, Wang L, Bao J, Sha X, Cui L, Huang Q, et al. Persistent hypoxia induced autophagy leading to invasiveness of trophoblasts in placenta accreta. J Matern Fetal Neonatal Med 2021;34:1297–303. [CrossRef]
- El-Hussieny M, Mohammed EM, Zenhom NM, Refaie MM, Okasha AM, Tawab MA El. Possible Role of TGF-β1, MMP-2, E-CAD, β-Catenin and Antioxidants in Pathogenesis of Placenta Accreta. Fetal Pediatr Pathol 2021;40:222–32. [CrossRef]
- Yang G, Rosen DG, Liu G, Yang F, Guo X, Xiao X, et al. CXCR2 promotes ovarian cancer growth through dysregulated cell cycle, diminished apoptosis, and enhanced angiogenesis. Clinical Cancer Research 2010;16:3875–86. [CrossRef]
- Wu D, Hong H, Huang X, Huang L, He Z, Fang Q, et al. CXCR2 is decreased in preeclamptic placentas and promotes human trophoblast invasion through the Akt signaling pathway. Placenta 2016;43:17–25. [CrossRef]
- Ma C, Liu G, Liu W, Xu W, Li H, Piao S, et al. CXCL1 stimulates decidual angiogenesis via the VEGF-A pathway during the first trimester of pregnancy. Mol Cell Biochem 2021;476:2989–98. [CrossRef]
- Liang Y, Zhang L, Bi S, Chen J, Zeng S, Huang L, et al. Risk Factors and Pregnancy Outcome in Women with a History of Cesarean Section Complicated by Placenta Accreta. Maternal-Fetal Medicine 2022;4:179–85. [CrossRef]
- Zhang D, Yang S, Hou Y, Su Y, Shi H, Gu W. Risk factors , outcome and management survey of placenta accreta in 153 cases : a five-year experience from a hospital of Shanghai , China 2017.
- Kılıçcı Ç, Eken MK, İlhan G, Çöğendez E, Şanverdi İ, Keskin M, et al. Evaluation of Risk Factors, Incidence, Perinatal and Maternal Outcome of Placenta Previa Cases with and without Placenta Accreta Spectrum. Duzce Medical Journal 2018;19:75–80.
- Shi XM, Wang Y, Zhang Y, Wei Y, Chen L, Zhao YY. Effect of Primary Elective Cesarean Delivery on Placenta Accreta: A Case-Control Study. Chin Med J (Engl) 2018;131:672–6. [CrossRef]
- American College of Obstetricians and Gynecologists. Obstetric Care Consensus No. 7: Placenta Accreta Spectrum. Obstetrics and Gynecology 2018;132:E259–75. [CrossRef]
- Nissi R, Talvensaari-Mattila A, Kotila V, Niinimäki M, Järvelä I, Turpeenniemi-Hujanen T. Circulating matrix metalloproteinase MMP-9 and MMP-2/TIMP-2 complex are associated with spontaneous early pregnancy failure. Reprod Biol Endocrinol 2013;11:2. [CrossRef]
- Jain C V., Jessmon P, Barrak CT, Bolnick AD, Kilburn BA, Hertz M, et al. Trophoblast survival signaling during human placentation requires HSP70 activation of MMP2-mediated HBEGF shedding. Cell Death & Differentiation 2017 24:10 2017;24:1772–83. [CrossRef]
- Wang R, Liu W, Zhao J, Liu L, Li S, Duan Y, et al. Overexpressed LAMC2 promotes trophoblast over-invasion through the PI3K/Akt/MMP2/9 pathway in placenta accreta spectrum. J Obstet Gynaecol Res 2023;49:548–59. [CrossRef]
- Costanzo RM, Perrino LA. Peak in matrix metaloproteinases-2 levels observed during recovery from olfactory nerve injury. Neuroreport 2008;19:327–31. [CrossRef]
- Cohen M, Meisser A, Haenggeli L, Bischof P. Involvement of MAPK pathway in TNF-α-induced MMP-9 expression in human trophoblastic cells. Mol Hum Reprod 2006;12:225–32. [CrossRef]
- Nikolov A, Popovski N. Role of Gelatinases MMP-2 and MMP-9 in Healthy and Complicated Pregnancy and Their Future Potential as Preeclampsia Biomarkers. Diagnostics 2021;11. [CrossRef]
- Werry TD, Christie MI, Dainty IA, Wilkinson GF, Willars GB. Ca2+ signalling by recombinant human CXCR2 chemokine receptors is potentiated by P2Y nucleotide receptors in HEK cells. Br J Pharmacol 2002;135:1199. [CrossRef]
- Wu Y, Wang S, Farooq SM, Castelvetere MP, Hou Y, Gao JL, et al. A Chemokine Receptor CXCR2 Macromolecular Complex Regulates Neutrophil Functions in Inflammatory Diseases. Journal of Biological Chemistry 2012;287:5744–55. [CrossRef]
- Xu W, Wu H, Shang L. Identification of novel candidate indicators for assessing zinc status during pregnancy in mice from microarray data. BMC Pharmacol Toxicol 2019;20:1–10. [CrossRef]
- Dauletbaev N, Herscovitch K, Das M, Chen H, Bernier J, Matouk E, et al. Down-regulation of IL-8 by high-dose vitamin D is specific to hyperinflammatory macrophages and involves mechanisms beyond up-regulation of DUSP1. Br J Pharmacol 2015;172:4757–71. [CrossRef]
- Bahabayi A, Yang N, Xu T, Xue Y, Ma L, Gu X, et al. Expression of Matrix Metalloproteinase-2,-7,-9 in Serum during Pregnancy in Patients with Pre-Eclampsia: A Prospective Study. Int J Environ Res Public Health 2022;19. [CrossRef]
- Tobwala S, Srivastava DK, Tobwala S, Srivastava DK. Cooperative binding of calcium ions modulates the tertiary structure and catalytic activity of Matrix-Metalloproteinase-9. Adv Enzyme Res 2013;1:17–29. [CrossRef]
- Meraz-Cruz N, Ortega A, Estrada-Gutierrez G, Flores A, Espejel A, Hernandez-Guerrero C, et al. Identification of a calcium-dependent matrix metalloproteinase complex in rat chorioallantoid membranes during labour. Mol Hum Reprod 2006;12:633–41. [CrossRef]
- Zong L, Wei X, Gou W, Huang P, Lv Y. Zinc improves learning and memory abilities of fetal growth restriction rats and promotes trophoblast cell invasion and migration via enhancing STAT3-MMP-2/9 axis activity 2017;8:115190–201.
- Ma L, Chen YH, Liu ZB, Gao L, Wang B, Fu L, et al. Supplementation with high-dose cholecalciferol throughout pregnancy induces fetal growth restriction through inhibiting placental proliferation and trophoblast epithelial-mesenchymal transition. J Nutr Biochem 2021;91:108601. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).