1. Introduction
The kidneys play a crucial role in the body’s homeostasis, namely in the regulation of water and electrolytes. This is possible due to their ability to filter blood and form urine with different concentrations of water and waste products. In addition, the kidneys perform endocrine functions, by producing renin, calcitriol and erythropoietin (EPO), which are responsible for regulating blood pressure, bone metabolism and producing red blood cells, respectively [
1,
2].
Advancing age is associated with changes in the structure and function of the kidneys, which may be exacerbated when associated with risk factors for renal diseases [
3]. Certain pathologies, such as diabetes and arterial hypertension, promote the renal aging process, predisposing the elderly to chronic kidney disease (CKD), a condition that is becoming increasingly prevalent. CKD is associated with several complications, such as anemia, mainly caused by decreased red blood cells production and survival and/or iron deficiency. CKD-related anemia is usually associated with more severe disease stages and with an increased risk of cardiovascular events and mortality [
4].
Since CKD is considered a global public health problem and CKD-related anemia is a significant complication associated with disease progression, early diagnosis is essential to implement interventions or therapies to slow disease progression, improve asthenia, cognitive and cardiac functions and, consequently, enhance the patients’ quality of life [
5]. This is particularly challenging in the elderly patients, as the signs and symptoms of CKD may overlap with physiological modifications that accompany aging. Thus, it is vital to understand the renal aging process and its progression to CKD, as well as the pathophysiology of anemia in the elderly patient with CKD.
2. Aging Kidney
Renal aging is a physiological, not pathological, process that occurs with advancing age [
6]. Although limited, healthy renal aging still allows for the maintenance of homeostatic balance under healthy conditions. However, the association of aging with risk factors for renal disturbances or chronic diseases, such as arterial hypertension, diabetes and obesity, can exacerbate these age-related changes [
3].
It is important to recognize a normal physiological aging process in order to adopt an appropriate clinical approach [
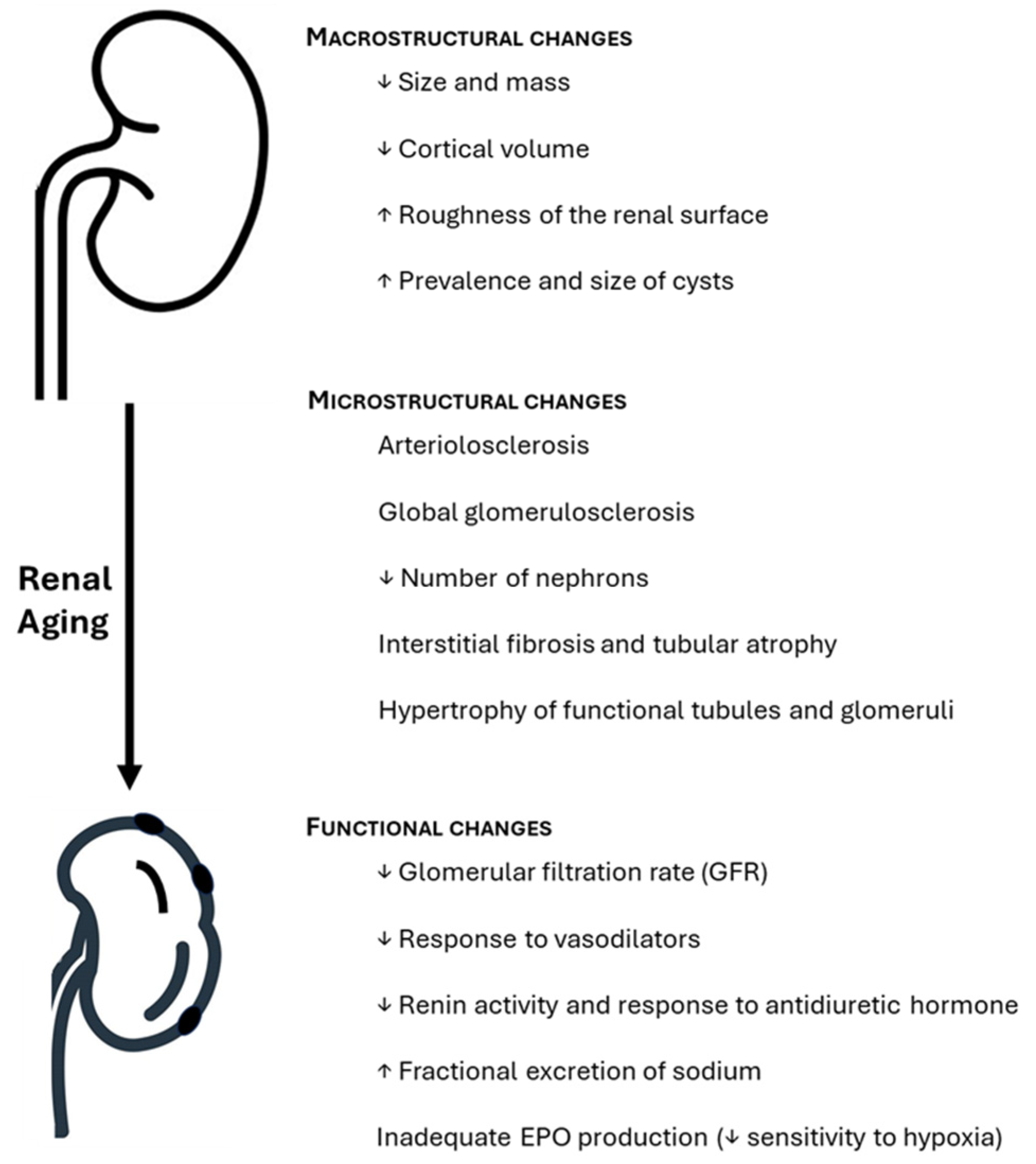
7]. The changes that the kidneys undergo with age are divided into two categories: anatomical changes (subdivided into microstructural and macrostructural) and functional changes (
Figure 1). Macrostructural changes are assessed through imaging studies such as computed axial tomography (TAC), while microstructural changes are based on analysis of renal biopsies [
8,
9].
2.1. Anatomical Changes
2.1.1. Macrostructural Changes
From the age of 50, loss of renal mass and volume is documented, with renal cortical atrophy and increased renal medullary volume, consequently leading to a decline in renal parenchyma volume [
10]. In the renal medulla, the interstitial tissue increases with the onset of signs of fibrosis and consequent atrophy of the renal pyramids, especially after the age of 70. The renal surface also undergoes changes, becoming more granular and scared. Of note, it is important to differentiate the granularity of the renal surface resulting from healthy renal aging from scars resulting from pyelonephritis.
The amount of sinus fat increases slightly with age and may occasionally account for up to 17% of the post-mortem kidney weight. Also, renal parenchymal cysts become more frequent, larger and denser with age (
Figure 1). The age-related increased prevalence of cysts is associated with overweight, with the presence of hypertension and albuminuria, and is more commonly observed in males [
9,
11].
2.1.2. Microstructural Changes
Nephrosclerosis
Nephrosclerosis is commonly associated with CKD, but may be present in healthy renal aging, with its prevalence progressively increasing with age. Arteriosclerosis and arteriolosclerosis refer to the hardening and thickening of the walls of arteries and arterioles, respectively, due to increased fibrous tissue and deposition of hyaline material in the innermost layer of the vessels, the intima. Arteriosclerosis can lead to ischemia of the nephrons, resulting in glomerulosclerosis and tubular atrophy.
Nephron Hypertrophy
Hypertrophy of the remaining nephrons is a compensatory mechanism in response to the decrease in functional glomeruli alongside with and increase in sclerotic and hyaline glomeruli. Hypertrophy causes the glomeruli to move apart and the glomerular density in the renal cortex to decrease. In healthy aging, hypertrophy is also observed in the tubules, which increase in volume. This leads to a decreased glomerular density in the renal cortes and increased tubular volume, resulting in a larger cortical area per glomerulus [
3,
13].
Number of Nephrons
It is described that the average number of nephrons with non-sclerotic glomeruli decreases with healthy aging, which associates glomerulosclerosis and nephrosclerosis [
3,
14]. Sclerotic glomeruli can be completely reabsorbed or undergo changes, such as atrophy, so they are not easily detected in tissue samples [
15,
16]. Thus, the actual loss of glomeruli with aging becomes difficult to quantify [
17]. There are also other factors, not related to aging, that influence the loss of nephrons, such as the number of nephrons in the newborn and its birth weight [
3,
6,
9,
18]. A low birth weight is indicative of a low number of nephrons, which is compensated by an increase in the single nephron glomerular filtration rate (snGFR) and an increase in glomerular size, allowing for stable overall glomerular filtration rate (GFR) [
3,
19].
Glomerular Filtration Rate per Nephron (snGFR)
GFR is considered one of the most important assessments of kidney function [
3]. However, individual variability in nephron number, due to acquired or hereditary causes, difficults its function assessment at the nephron level [
20]. The progressive loss of functional glomeruli due to glomerulosclerosis triggers a compensatory response resulting in increased snGFR and glomerular capillary hydraulic pressure in an attempt to preserve total GFR. This compensation, however, leads to increased tension in the glomerular capillary walls, causing hypertension, hyperfiltration and further damage to the remaining nephrons.
2.2. Functional Changes
2.2.1. Physiological Decline in GFR
With healthy aging, GFR declines progressively, beginning around the age of 30–40 years at a rate of approximately 0.8 mL/min/1.73 m2/year [
21]. This decline in GFR may be caused by decreased glomerular lobulation and glomerulosclerosis and, consequently, by a reduction in the surface area available for filtration. In addition, there are other changes, such as reduced cardiac output and increased renal arteriolar resistance, which can also reduce filtration at the kidneys level. The permeability of the glomerular filtration barrier has also been studied. Although there are reports of a higher prevalence of proteinuria in individuals over 65, only a minority of healthy patients over 80 years present clinical proteinuria. Some studies suggest that the permeability of the glomerular filtration barrier in humans is only minimally altered with aging [
6,
22].
2.2.2. Hormonal Changes
Nitric oxide is a vasodilator that plays a crucial role in maintaining renal blood flow and regulating blood pressure. Nitric oxide production in the kidneys tends to decrease with aging. This reduction contributes to decreased renal plasma flow and sodium retention, and heightened the risk of kidney damage, such as glomerulosclerosis [
7]. One of the main changes observed is compromised endothelial vasodilation, caused by diminishing responses to vasodilators, such as nitric oxide, and increasing sensitivity to vasoconstrictors, such as angiotensin II.
The renal response in the elderly is reduced, reflected in lower plasma renin levels, which lead to reduced angiotensin II and aldosterone levels. This regulation is extremely important due to its ability to regulate vasoconstriction of the glomerular capillaries, but also to maintain renal homeostasis, through the induction of aldosterone and antidiuretic hormone (ADH) secretion and, consequently, to regulate the reabsorption of water and sodium in the renal tubules [
23]. As plasma concentrations of renin and aldosterone are reduced, fractional excretion of sodium increase, and urinary concentrating capacity declines in the elderly, due to the compromised response to ADH [
24].
Erythropoietin (EPO) is produced almost exclusively by the kidneys and is responsible for regulating the production of red blood cells. In the elderly, a decrease in renal sensitivity to hypoxia, in the erythropoiesis efficacy and in the concentration of hemoglobin (Hb) are observed. Despite an increased production of EPO, as a compensatory mechanism associated with age, increased erythrocyte turnover or increased resistance to EPO also occur. Thus, although EPO may be increased, the response appears to be disproportional, leading to gradually lower Hb levels with aging, still within normal reference ranges [
25,
26].
3. Chronic Kidney Disease
3.1. Definition and Classification
Chronic kidney disease (CKD) is a global public health problem, defined as the presence of kidney damage or loss of kidney function for 3 or more months. Its prevalence is rising worldwide and is highly associated with an increased risk of cardiovascular morbidity and mortality, premature death, and decreased quality of life [
27,
28].
According to Kidney Disease Improving Global Outcomes (KDIGO), this pathology is classified according to the cause, GFR, and albuminuria categories, allowing patients with CKD to be categorized according to the disease severity and risk [
29,
30]. These two parameters complement each other, being prognostic indicators [
27].
Albuminuria is divided into 3 categories and can be calculated by the albumin excretion rate (AER) in 24-hour urine or by the albumin-creatinine ratio (ACR) in type II urine, preferably. ACR has advantages over AER due to the errors associated with collecting 24-hour urine; ACR also allows occasional urine collection when the first urine sample in the morning is not possible. The use of creatinine reflects a reference due to its constant excretion over 24 hours, providing an estimate with less associated error [
30,
31,
32].
Regarding GFR, it can be determined in two ways: the measured glomerular filtration rate (mGFR), where the clearance (urinary and plasma) of exogenous filtration markers are measured, and the estimated glomerular filtration rate (eGFR), via equations, based on serum concentrations of endogenous filtration markers like creatinine [
33]. While both have associated errors, eGFR using creatinine concentration is widely used since this biomarker is routinely available in basic metabolic panels. Cystatin C is added to creatinine based GFR estimates if a more accurate measurement is required. On the other hand, mGFR is reserved for scenarios requiring maximum accuracy to eliminate residual error associated with the equations.
According to eGFR, CKD is classified in 5 stages [
30]. However, the GFR value alone may be insufficient for diagnosis, especially in earlier stages where patients are frequently asymptomatic. Therefore, CKD is diagnosed if, for 3 or more months, the GFR is less than 60 mL/min/1.73 m
2 or if greater GFR is accompanied by the presence of one or more markers of kidney injury (
Table 1) [
27,
30,
34].
Despite its often-silent early progression, signs or symptoms such as foamy urine, changes in urination frequency, asthenia, nausea, loss of appetite, and weight loss may be observed. In more advanced stages of the disease, other symptoms may appear, such as difficulty concentrating, paresthesia, edema, dyspnea, vomiting, insomnia, and halitosis (ammonia odor) [
34].
3.2. Etiology and Risk Factors
CKD can be caused by primary kidney diseases. However, it is mainly caused by other diseases, such as diabetes, hypertension, systemic lupus erythematosus, human immunodeficiency virus (HIV) infection, sickle cell anemia, chronic kidney infections, glomerular diseases such as glomerulonephritis, polycystic kidney disease, among others [
34,
35].
CKD risk factors are divided into those that are modifiable and those that are non-modifiable (
Table 2). Modifiable risk factors have a high clinical importance, as they are those where interventions can be made to modulate CKD risk. When a potentially modifiable factor is identified, it should be corrected, as it will have an impact on the patient’s quality of life and the progression of CKD [
36,
37].
The RENA study assessed the prevalence of CKD in Portugal in a sample population with an average age of 56.7 years. The overall prevalence of CKD was 20.9% and this was higher in individuals with diabetes mellitus than in those without (31.4% vs 19.8%). Furthermore, regarding anthropometry, it was found that individuals with normal weight, pre-obesity, and obesity of all classes presented CKD prevalences of 18.0%, 45.8% and 36%, respectively [
38].
3.3. Prevalence in the Elderly
The prevalence of CKD is notably high among the elderly, which is mainly due to the increased frequency of risk factors for CKD, such as obesity, diabetes and hypertension, coumpounded by age-related renal function decline [
39]. The third National Health and Nutrition Examination Survey (NHANES III) found that the prevalence of CKD was 8.5%, 12.6% and 39.4% in individuals aged 20-39 years, 40-59 years and 60 years or older, respectively [
40].
Epidemiological studies have shown that decreased eGFR and increased albuminuria are common in the elderly [
28,
41]. However, it is important to understand whether these changes are consequences of healthy aging or whether they result from pathology [
28,
42].
3.4. Calculation of GFR in the Elderly
The increasing prevalence of CKD in older populations raises questions about the accuracy of eGFR measurements in this demographicThere are several factors that affect creatinine metabolism, namely age, gender, ethnicity, daily protein intake, malnutrition and medication [
43]. With regard to age, functional and anatomical changes in the kidneys, as well as loss of muscle mass, will alter creatinine metabolism and, consequently, underestimate the decline in eGFR. These changes should be taken into account, as eGFR calculated for elderly individuals may be less accurate due to these factors [
23,
43].
To date, more than 50 equations have been proposed using creatinine to calculate eGFR. The first equation that was used worldwide was the Cockcroft-Gault (CG) equation [
44], which included age, gender, weight and serum creatinine as variables. However, it has some limitations, namely the fact that the group of individuals used for its development included few male individuals and it was created to estimate clearance creatinine rather than GFR [
45].
In 1999, Levey, et al. proposed a new equation, the Modification of Diet in Renal Disease (MDRD) [
46]. This equation is more complex than the CG equation and has undergone subsequent modifications. It has advantages over the CG equation, namely the fact that it adds the ethnicity variable. However, it excludes individuals over the age of 70 years. Given that serum creatinine differs with age and between age groups, this equation tends to underestimate eGFR and, consequently, overestimate the population prevalence of CKD [
45].
In 2009 a new equation was developed by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) [
47], which included patients over 65 years of age as well as diabetics. This is currently recommended by KDIGO and the most widely used globally, having undergone a change in 2021 to remove ethnicity as a variable and maintain age, gender and creatinine. However, it still has limitations, namely the overestimation of eGFR in adults aged 18-30, as well as some differences in relation to ethnicity. This equation tends to slightly overestimate GFR in individuals of non-black ethnicity and underestimate eGFR in individuals of black ethnicity. On the other hand, the CKD-EPI equation that uses both markers (creatinine and cystatin C) has proven to improve accuracy for both ethnic groups, with smaller differences between these groups [
45].
An equation was also later proposed by the Berlin Initiative Study (BIS) [
48] which is limited to individuals over 70 years of age and which aimed to overcome the limitations of the CKD-EPI equation with regard to age and ethnicity [
45].
Currently, the 2024 KDIGO guidelines recommend, in general cases, the use of the 2021 “CKD-EPI creatinine” [
30,
49] as the first choice, but the use of the “CKD-EPI creatinine-cystatin C” equation was also recommended as a confirmatory test, when necessary (
Table 3) [
50]. This guideline states that when abnormalities in creatinine metabolism are suspected, as is the case in the elderly, the initial test should include cystatin C. Furthermore, since the publication of KDIGO 2012, equations were developed by the European Kidney Function Consortium (EKFC) which obtained good results when compared with the CKD-EPI 2009 and 2021 equations [
30,
51].
3.5. Anemia – a Complication of CKD
CKD is associated with several complications, with different prevalence and severity, depending on the stage of the disease. However, these are interrelated and contribute to increased morbidity and mortality, and reduced quality of life. The main complications of CKD include hypertension, cardiovascular complications, CKD-associated mineral and bone disorders, water and salt retention, metabolic acidosis, electrolyte disturbances, dyslipidemia, nutritional problems, and anemia [
54,
55].
A 2023 study conducted in the United States between 2016 and 2019 collected data from approximately 5 million individuals and assessed the relationship between anemia and eGFR. It was observed that the lower the eGFR, the higher the prevalence of anemia. Severe anemia was present in 1.3%, 3.1%, 7.5%, 17.4%, and 29.7% of men with eGFRs of 60–74, 45–59, 30–44, 15–29, and less than 15 mL/min/1.73 m2, respectively. In women, severe anemia was present in 1.9%, 3.9%, 8.6%, 19.4%, and 37.6%, respectively, in the same eGFR categories mentioned above [
56].
Another population-based study involving more than 3000 individuals aged over 49 years (mean age 65 years) concluded that persistently elevated serum creatinine levels due to decreased renal function was associated with a higher prevalence of anemia in both genders. Based on World Health Organization (WHO) criteria, anemia was diagnosed in 1.6% of individuals with serum creatinine <125 μmol/L, in 8% with serum creatinine between 150 and 174 μmol/L, and in 40% of patients with serum creatinine ≥200 μmol/L [
57].
According to the KDIGO 2024 Clinical Practice Guidelines [
30], adults with CKD with stage G3 and A1 diabetes and non-diabetics had a prevalence of anemia of 14.9% and 11.5%, respectively. This prevalence increases to 60.7% and 57.4%, respectively, in CKD stages G5 and A3, demonstrating the important increase in the prevalence of anemia with worsening CKD.
4. Anemia in the Elderly
4.1. Definition
Anemia is higly prevalent in the elderly and often undervalued [
58,
59]. Although Hb levels naturally decline with age, anemia is not considered a normal finding in aged individuals.
The optimal Hb concentration values appropriate to physiological needs vary according to age, sex, altitude of residence, and specific conditions such as pregnancy. The WHO defines anemia according to Hb concentration: < 13 g/dL in men and < 12 g/dL in women [
60,
61]. However, the reference population used for this determination did not include individuals aged65 and older, raising the question of whether this criteria applies to the elderly. This WHO definition also did not differentiate reference values between pre- and post-menopausal women [
60,
61,
62]. New studies have been carried out to assess the prevalence of anemia in the elderly population, proposing new definitions of anemia for these individuals [
63,
64,
65].
4.2. Adverse Health Effects
Although anemia in the elderly is predominantly mild, it is associated with several adverse effects, including decreased physical performance, frailty, reduced muscle strength (and, consequently, increased risk of falls), decreased cognitive performance, dementia, increased risk and length of hospitalization. A well described inverse relationship exists between Hb concentration and muscle strength, physical performance, disability, and mortality [
61,
66].
Anemia in the elderly is associated with a series of signs and symptoms associated with the frailty of the elderly, including weight loss, impaired mobility, generalized weakness, lack of balance and greater vulnerability to stress, collectively defining it as a geriatric syndrome [
59,
67].
4.3. Prevalence
As mentioned, the prevalence of anemia varies according to several factors, such as age, gender and ethnicity.
Regarding the association between anemia and age, the InCHANTI study [
58] found that a gradual decline in both Hb concentration and renal function with age in both genders, with the increased prevalence of anemia becoming progressively more evident with worsening renal function. Another study also carried out in Italy [
66], involving approximately 8,000 elderly individuals, confirmed this trend. It was observed that, among those aged 85 to 89, the prevalence of mild, moderate and severe anemia was 22.1%, 4.2% and 0.3%, respectively, and that in individuals aged 90 or over, the prevalence values were 31.5%, 9.0% and 1.3%, respectively. Based on these data, the study concluded that there is a marked increase in the prevalence of the various degrees of anemia severity at more advanced ages. This study also found that the prevalence of moderate anemia in hospitalized cases (13.5%) was significantly higher than that observed in non-hospitalized cases (1.6%) [
66].
Other studies have also corroborated this trend, with more pronounced increases in older age groups [
58,
68,
69]. A study from New Zealand population [
70] observed a prevalence of anemia in individuals over 65 years of age of 48%, in which 68% of these had mild to moderate anemia.
Regarding the prevalence of anemia according to gender, it is recognized that anemia in the elderly affects more men than women. According to the NHANES III, the prevalence of anemia is higher in women up to the age of 75. From the age of 75 to 84, there is a difference of approximately 5 percentage points between the two genders, with men having a higher prevalence; after the age of 85, the relationship reverses again, with women having a higher prevalence [
60,
71,
72]. These data were also confirmed by the InCHANTI study [
58]. As men age, they experience a decline in androgen levels, reducing their stimulating action on erythropoiesis [
73,
74]. The reduction in the prevalence of anemia in postmenopausal women may be related to the absence of menstruation. Due to these physiological changes between the sexes, at advanced ages, the reference values for the diagnosis of anemia may not adequately reflect the biological concept of normality for elderly people [
71].
The prevalence of anemia also varies with ethnicity, with black individuals having a prevalence of anemia 2 to 3 times higher than that of Caucasians. A study conducted in the United States in elderly individuals (≥65 years old) found that the prevalence of anemia in non-Hispanic black men and women was 27.5% and 28.0%, respectively, and in non-Hispanic white men and women of similar ages, 9.2% and 8.7%, respectively. For Mexican-American men and women, the prevalence of anemia was 11.5% and 9.3%, respectively. However, the definition of anemia for different ethnicities is slightly different; a study from NHANES III showed that WHO-defined anemia is a strong predictor of mortality and mobility impairment among white and Mexican-American individuals, but that these criteria do not apply to black individuals [
68,
71].
4.4. Etiology
The etiology of anemia in the elderly can be divided into 3 main categories: nutritional deficiencies (mainly due to deficiency in iron, folic acid and vitamin B12); chronic diseases (such asCKD, inflammatory or infectious pathologies and tumors); and unknown causes [
66,
68,
75,
76].
In a study carried out in individuals aged ≥ 65 years, the etiology of anemia was distributed as follows: 56.6% due to nutritional deficiency, 21.1% due to hematologic neoplasia, 20.8% due to anemia of chronic diseases and 9.7% due to anemia of unknown cause [
77].
The NHANES III study also examined the etiology of anemia in the elderly. For the diagnosis of nutritional deficiency anemia, the following were considered: the values of iron, vitamin B12 and folate. Anemia due to chronic diseases was diagnosed when serum iron was decreased without evidence of iron deficiency. Anemia due to CKD was considered when eGFR < 30 mL/min and anemia due to unknown causes resulted from the exclusion of the remaining ones. It was reported that anemia due to nutritional deficiency, chronic diseases and due to unknown causes had prevalences of 34.3%, 32.2% and 33.6%, respectively [
68].
The categorization of the etiology of anemia in the elderly has limitations, namely the fact that many cases considered unexplained are simply the exclusion of the other two categories. In fact, anemia in the elderly is often multifactorial (
Figure 2) and derived from comorbidities that are common in older individuals (approximately 40% of individuals aged >80 years have 4 or more comorbidities) [
78,
79,
80]. A study carried out on more than 19 thousand individuals aged≥64 years old, in Austria, concluded that multifactorial anemia was frequently observed. For example, the coexistence of renal failure (decreased eGFR) and increased inflammatory markers had a prevalence of 28.1% [
79]. Determining the cause of anemia is extremely important to ensure that the most appropriate treatment is applied. However, the existence of comorbidities, as well as the use of multiple drugs, make diagnosis difficult in elderly patients.
4.4.1. Nutritional Deficiency Anemia
The main cause of nutritional deficiency anemia in the elderly is iron deficiency, typically presenting with microcytosis. Diagnosis is essential to treat symptoms and identify the cause of iron deficiency, which is often related to pathologies of the gastrointestinal tract, such as gastritis, peptic ulcer, gastrointestinal polyps, cancer and inflammatory bowel disease [
81]. Diagnostic tests include the evaluation of several parameters, such as serum ferritin, transferrin, serum iron, total iron binding capacity and soluble transferrin receptor (sTfR), alongside with measures of hypochromia (mean blood cell volume, MCH) microcytosis (mean cellular volume, MCV [
81,
82].
Although dietary supplementation has reduced folate deficiency, it still occurs due to malnutrition and alcoholism [
83,
84]. Vitamin B12 deficiency is mainly due to cobalamin malabsorption syndrome, to malabsorption in cases of atrophic gastritis, or therapeutic use of antacids. Unlike iron deficiency, anemia due to vitamin B12 and/or folate deficiency is macrocytic [
81].
4.4.2. Anemia Resulting From Chronic Diseases
Anemia of chronic diseases, which includes CKD, arises from acute or chronic infections, oncological diseases and chronic inflammatory diseases. This anemia is typically normocytic and normochromic, with mild to moderate reductions in Hb concentration. Hepcidin plays a crucial role in its pathophysiology, by inhibiting iron absorption on enterocytes, as well as iron release by macrophages, reducing the overall iron available for erythropoiesis. Diagnosis can be complicated by coexisting conditions, such as iron deficiency and thalassemia, which can results in microcytic anemia, but evaluation of iron metabolism parameters, such as transferrin, sTfR and serum ferritin, can aid in diagnosis [
81,
85]. However, the diagnosis can undergo evaluation of inflammatory markers, such as C-reactive protein, red blood cells sedimentation rate, interleukin-6 (IL-6) and hepcidin [
81,
86].
4.4.3. Anemia of Unknown Cases
Anemia of unknown cause in the elderly is usually mild, normocytic and hypoproliferative. It is diagnosed when nutritional deficiencies, CKD or inflammatory conditions are excluded. This type of anemia may be associated with several age-related physiological mechanisms, such as declining renal function, androgen deficiency, chronic low-grade inflammation associated with aging (inflammaging), myelodysplastic syndromes and impaired response to EPO [
80,
87,
88]. These patients commonly have lower EPO levels than in cases of iron deficiency, which suggests the presence of a subclinical pro-inflammatory state [
88,
89].
5. CKD Anemia
5.1. Multifactorial Nature
CKD anemia is mainly due to decreased production of erythropoietin by the kidneys, leadind to impaired erythropoiesis, as well as shortened erythrocyte survival [
4]. This tupe ofanemia is predominantly normochromic, normocytic and hypoproliferative, usually occurring in more advanced disease stages. In cases of iron deficiency, also common in CKD, microcytic anemia may also be common, further accentuating the decline in Hb concentration [
4,
90]. Other causes that can exacerbate CKD anemia include blood loss, inflammation, and other nutritional deficiencies in addition to iron deficiency (
Figure 3) [
91,
92].
5.2. Normal Erythropoiesis Versus Pathophysiology of CKD Anemia
Erythropoiesis is a complex physiological process regulated by EPO. This hormone produced by peritubular interstitial cells in the cortex and outer layer of the renal medulla, stimulates erythroid cells in the bone marrow to proliferate and differentiate. In its absence, progenitor cells undergo apoptosis [
93,
94].
EPO synthesis is regulated by hypoxia inducible factor (HIF), a transcription factor that promotes erythropoiesis, not only by promoting EPO production by the kidneys, but also by improving iron absorption and utilization [
95]. Under normoxic conditions, the presence of oxygen allows the activation of the prolyl-hydroxylase domain (PHD) enzyme. PHD hydroxylates proline residues of HIF-1α. The von Hippel-Lindau protein (pVHL) then recognizes the hydroxylated HIF-1α, binds to it and promotes its ubiquitination. The ubiquitinated HIF-1α is directed to the proteasome, where it is degraded. Thus, without the formation of the HIF complex, there is a blockage of the transcription of genes regulated by it, including the EPO gene. Under hypoxic conditions, PHD activity is inhibited, HIF is not hydroxylated, allowing HIF complex to enter the nucleus and binds to the hypoxia response element (HRE), activating EPO gene transcription, while suppressing the hepcidin gene.
The major extrarenal source of EPO is the liver. However, the mechanism of hepatic EPO gene expression is different from the kidney, since it is much less sensitive to hypoxic stimuli [
96,
97,
98]. In patients with CKD, reduced renal blood flow disrupts oxygen delivery to the kidney. Thus, the renal tissue needs to adapt to a lower oxygen consumption, considering the new normal tissue oxygen gradient. Consequently, PHD remains active, preventing the formation of the HIF heterodimer and the EPO gene is not activated. Furthermore, since CKD is an inflammatory condition, there is production of inflammatory cytokines, including IL-1α, IL-1β, TGF-β and TNF-α, which inhibit EPO production.
Iron homeostasis is ensured by a process that regulates the absorption and secretion of iron, to prevent anemia or hemochromatosis. Since the body only absorbs a very small fraction of daily iron from diet,, most of the iron used in erythropoiesis comes from recycling of iron from senescent erythrocytes that have been phagocytosed by specialized reticuloendothelial macrophages. Thus, when serum iron levels are decreased, iron absorption in the gastrointestinal tract increases, but only within certain limits (increase up to 20%), since its absorption is mediated by receptors; in this case, the mobilization of iron from macrophages also increases. On the contrary, when serum iron levels are increased, iron absorption from the gastrointestinal tract as well as iron mobilization from macrophages decreases [
95]. Since iron is an essential component of the heme group present in Hb, iron deficiency results in decreased Hb synthesis and consequently in hypochromia and microcytosis of erythrocytes. Low Hb levels induce hypoxia, promoting the stimulation of EPO production by the kidney peritubular cells and increasing erythropoiesis. However, if iron deficiency persists, deficient erythropoiesis persists [
4,
95].
Hepcidin is a peptide produced by hepatocytes and freely filtered by the glomeruli, that can be measured in urine. The function of this peptide is to bind to ferroportin (an iron exporting protein), forming a hepcidin-ferroportin complex. This complex is internalized and subsequently degraded in the lysosomes. Consequently, the release of iron into the bloodstream decreases, as does the release of iron by macrophages. When hepcidin levels are low, ferroportin develops a higher than normal activity, causing ingested iron in the diet to be more absorbed in the intestine [
99,
100]. When dysregulated, this mechanism can result in hemochromatosis [
101]. When hepcidin is increased, ferroportin activity is suppressed, leading to anemia, common in cases of inflammation, kidney failure and chronic diseases [
95]. In fact, under inflammatory conditions and chronic diseases, such as CKD, there is an increased production of cytokines, such as IL-1, IL-6, TNF-α and interferon-γ that stimulate the production of hepcidin, reduce the production of EPO and erythropoiesis [
4,
98,
102].
The mechanisms that lead to anemia in CKD are therefore multifactorial. As shown in
Figure 3, the pathophysiology of CKD involves the progressive reduction in EPO production, the reduced bone marrow response to EPO stimuli (due to uremic toxins and erythropoiesis-suppressing cytokines), and the decreased erythrocyte half-life. In addition, the increased production of hepcidin due to systemic inflammation resulting from CKD, leads to ineffective use of iron stores, resulting in absolute or functional iron deficiency, which can be severe due to iron malabsorption. Blood loss, in cases of hematuria or repeated blood sampling for analytical purposes, may further aggravate CKD anemia.
5.3. Diagnosis
In patients with CKD, it is common to develop progressive and debilitating anemia which, if left untreated, can lead to dependence on blood transfusions [
103]. According to NHANES [
104], Hb concentration remains normal during the early stages of renal failure, but inevitably decreases with the decline in eGFR and worsening of CKD. The clinical manifestations associated with anemia in CKD are common to any other anemia of different causes, so the observed symptoms of fatigue, thoracalgia, tachycardia, dyspnea and changes in sleep pattern are nonspecific for CKD anemia [
4]. It is very important to determine other causes of anemia before starting any treatment, as some of these causes can be corrected, such as iron, vitamin B12 or folate deficiencies, hypothyroidism, hemolysis, blood loss, bone marrow disorders and hematological neoplasms [
103,
104].
According to the KDIGO Clinical Practice Guideline for Anemia in Chronic Kidney Disease [
105], assessment of anemia in patients with CKD, regardless of age and disease stage, should include: hemogram (erythrocyte count, Hb concentration, hematocrit, hematimetric indices (mean corpuscular volume (MCV), mean hemoglobin concentration (MHC) and mean corpuscular hemoglobin concentration (MCHC)), leukocyte and platelet counts); reticulocyte count and reticulocyte maturity indices; serum iron, ferritin and transferrin saturation (TSAT); serum levels of folate and vitamin B12.
The Hb concentration is the most recommended parameter for diagnosing anemia. The reticulocyte count helps assess bone marrow activity, while red cells indices (such as MCV, MCH, MCHC and red blood cell distribution width (RDW) provide insights into anemia type. CKD anemia is often normochromic, normocytic and hypoproliferative, as in other chronic diseases. Cases of hereditary disorders of Hb formation, such as thalassemia or sickle cell anemia, generally present microcytosis, as is the case in cases of iron deficiency anemia. Hypochromia is also common in this type of anemia. Anemia due to vitamin B12 and folate deficiency, on the other hand, is generally macrocytic, as occurs in hematopoietic disorders, resulting from the action of toxins, or associated with hypothyroidism, liver disease, severe hemorrhages and in the case of myelodysplasia, in which macrocytosis is associated with leukopenia or thrombocytopenia [
105]. As regards to MCH and MCHC, hypochromia is indicative of prolonged iron deficiency or hemoglobinopathy, such as thalassemia or sickle cell anemia. On the contrary, hyperchromia may be related to hemolytic uremic syndrome. Additionally, performing a peripheral blood smear allows for manual examination of cellular morphology and identification of potential abnormalities [
105,
106].
As mentioned, the reticulocyte count allows the assessment of bone marrow response to anemia. Decreased reticulocyte counts indicative of low erythropoietic activity in the bone marrow (hypoproliferative erythropoiesis), which may be the result of low levels of EPO in circulation, or due to bone marrow suppression by cytokines or other components resulting from the inflammatory process. Reticulocytes may be elevated in cases of bleeding and hemolysis [
105].
The study of iron metabolism is used to assess iron storage and iron availability for erythropoiesis, by assessing ferritin and transferrin saturation(TSAT), respectively [
103]. However, it is considered that these two parameters do not allow an accurate assessment of iron storage in the bone marrow [
103,
105].
Decreased ferritin (≤ 100 ng/mL) and TSAT (≤ 20%) levels are indicative of iron deficiency, suggesting a deficiency in iron availability for erythropoiesis. In chronic diseases, such as CKD, the levels of iron stored in bone marrow macrophages may be normal, but unable to be released due to the action of hepcidin. Ferritin is an inflammatory marker, so its interpretation should be performed with caution in patients with CKD, since inflammation is present in these patients [
105].
6. Conclusions
Renal aging is a natural physiological process that results in a progressive decline in kidney function. Distinguishing healthy renal aging from pathological conditions is essential for appropriate clinical management. CKD is considered a global public health problem and its prevalence has increased over the years, significantly affecting the quality of life of patients, particularly the elderly. Early diagnosis of CKD is extremely important, especially to prevent or delay the progression of the disease to more severe stages, which are often accompanied by an increased prevalence and severity of complications, such as anemia and cardiovascular diseases.
CKD anemia, which is multifactorial in nature, results from factors such as EPO deficiency, chronic inflammation (causing increased hepcidin production), iron deficiency (absolute or functional) and reduced erythropoietic activity in the bone marrow. This condition worsens patient morbidity and mortality, and may increase the risk and length of hospitalization, negatively impacting quality of life. Understanding the pathophysiology of CKD anemia is crucial for early diagnosis and treatment, which is essential to improving the patient’s quality of life and, if possible, slowing the progression of the disease. The proposal of new classes of treatment agents must also take in consideration the multiple mechanisms involved in anemia of CKD.
Author Contributions
Conceptualization, A.S.-S., L.B.; writing—original draft preparation, S.S.; writing—review and editing, S.S., I.L., M.S.-F., A.S.-S., L.B.; supervision, A.S.-S., L.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the FCT through the project UIDP/04378/2020 and UIDB/04378/2020 of the Research Unit on Applied Molecular Biosciences—UCIBIO and the project LA/P/0140/2020 of the Associate Laboratory Institute for Health and Bioeconomy—i4HB.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Morsch, C.; Veronese, F.J.V. Doença Renal Crônica: Definição e Complicações. Clinical and Biomedical Research 2011, 31. [Google Scholar]
- Diseases NIoHNNIoDaDaK. Your Kidneys & How They Work. 2018.
- Hommos, M.S.; Glassock, R.J.; Rule, A.D. Structural and Functional Changes in Human Kidneys with Healthy Aging. J Am Soc Nephrol. 2017, 28, 2838–2844. [Google Scholar] [CrossRef]
- Farinha, A.; Robalo Nunes, A.; Mairos, J.; Fonseca, C. [Anemia in Chronic Kidney Disease: The State of the Art]. Acta Med Port. 2022, 35, 758–764. [Google Scholar] [CrossRef]
- Shiferaw, W.S.; Akalu, T.Y.; Aynalem, Y.A. Risk Factors for Anemia in Patients with Chronic Renal Failure: A Systematic Review and Meta-Analysis. Ethiop J Health Sci. 2020, 30, 829–842. [Google Scholar] [PubMed]
- Sobamowo, H.; Prabhakar, S.S. The Kidney in Aging: Physiological Changes and Pathological Implications. Prog Mol Biol Transl Sci. 2017, 146, 303–340. [Google Scholar] [PubMed]
- Núñez, J.-F.M. The normal ageing kidney – morphology and physiology. Reviews in Clinical Gerontology 2008, 18, 175–197. [Google Scholar] [CrossRef]
- Lorenz, E.C.; Vrtiska, T.J.; Lieske, J.C.; Dillon, J.J.; Stegall, M.D.; Li, X.; et al. Prevalence of renal artery and kidney abnormalities by computed tomography among healthy adults. Clin J Am Soc Nephrol. 2010, 5, 431–438. [Google Scholar] [CrossRef]
- Denic, A.; Glassock, R.J.; Rule, A.D. Structural and Functional Changes With the Aging Kidney. Adv Chronic Kidney Dis. 2016, 23, 19–28. [Google Scholar] [CrossRef]
- Wang, X.; Vrtiska, T.J.; Avula, R.T.; Walters, L.R.; Chakkera, H.A.; Kremers, W.K.; et al. Age, kidney function, and risk factors associate differently with cortical and medullary volumes of the kidney. Kidney Int. 2014, 85, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Rule, A.D.; Sasiwimonphan, K.; Lieske, J.C.; Keddis, M.T.; Torres, V.E.; Vrtiska, T.J. Characteristics of renal cystic and solid lesions based on contrast-enhanced computed tomography of potential kidney donors. Am J Kidney Dis. 2012, 59, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Rule, A.D.; Amer, H.; Cornell, L.D.; Taler, S.J.; Cosio, F.G.; Kremers, W.K.; et al. The association between age and nephrosclerosis on renal biopsy among healthy adults. Ann Intern Med. 2010, 152, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Elsherbiny, H.E.; Alexander, M.P.; Kremers, W.K.; Park, W.D.; Poggio, E.D.; Prieto, M.; et al. Nephron hypertrophy and glomerulosclerosis and their association with kidney function and risk factors among living kidney donors. Clin J Am Soc Nephrol. 2014, 9, 1892–1902. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.C.; Busque, S.; Workeneh, B.; Ho, B.; Derby, G.; Blouch, K.L.; et al. Effects of aging on glomerular function and number in living kidney donors. Kidney Int. 2010, 78, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Nyengaard, J.R.; Bendtsen, T.F. Glomerular number and size in relation to age, kidney weight, and body surface in normal man. The Anatomical Record. 1992, 232, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Denic, A.; Lieske, J.C.; Chakkera, H.A.; Poggio, E.D.; Alexander, M.P.; Singh, P.; et al. The Substantial Loss of Nephrons in Healthy Human Kidneys with Aging. J Am Soc Nephrol. 2017, 28, 313–320. [Google Scholar] [CrossRef]
- Noronha, I.L.; Santa-Catharina, G.P.; Andrade, L.; Coelho, V.A.; Jacob-Filho, W.; Elias, R.M. Glomerular filtration in the aging population. Front Med (Lausanne) 2022, 9, 769329. [Google Scholar] [CrossRef]
- Bertram, J.F.; Douglas-Denton, R.N.; Diouf, B.; Hughson, M.D.; Hoy, W.E. Human nephron number: Implications for health and disease. Pediatr Nephrol. 2011, 26, 1529–1533. [Google Scholar] [CrossRef]
- Luyckx, V.A.; Brenner, B.M. Birth weight, malnutrition and kidney-associated outcomes--a global concern. Nat Rev Nephrol. 2015, 11, 135–149. [Google Scholar] [CrossRef]
- Denic, A.; Mathew, J.; Lerman, L.O.; Lieske, J.C.; Larson, J.J.; Alexander, M.P.; et al. Single-Nephron Glomerular Filtration Rate in Healthy Adults. New England Journal of Medicine 2017, 376, 2349–2357. [Google Scholar] [CrossRef]
- Lindeman, R.D.; Tobin, J.; Shock, N.W. Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc. 1985, 33, 278–285. [Google Scholar] [CrossRef]
- Rodríguez-Puyol, D. The aging kidney. Kidney Int. 1998, 54, 2247–2265. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.J.; Rakheja, D.; Yu, X.; Saxena, R.; Vaziri, N.D.; Silva, F.G. The aging kidney. Kidney Int. 2008, 74, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Musso, C.G.; Oreopoulos, D.G. Aging and physiological changes of the kidneys including changes in glomerular filtration rate. Nephron Physiol. 2011, 119 (Suppl. 1), 1–5. [Google Scholar] [CrossRef]
- Musso, C.G.; Musso, C.A.; Joseph, H.; De Miguel, R.; Rendo, P.; Gonzalez, E.; et al. Plasma erythropoietin levels in the oldest old. Int Urol Nephrol. 2004, 36, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Stauder, R.; Valent, P.; Theurl, I. Anemia at older age: Etiologies, clinical implications, and management. Blood. 2018, 131, 505–514. [Google Scholar] [CrossRef]
- Santos-Silva, A.; Costa, E.; Alves, R. Chronic Kidney Disease. In Biomarkers of Cardiometabolic Risk, Inflammation and Disease; Palavra, F., Reis, F., Marado, D., Sena, A., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 95–111. [Google Scholar]
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Callaghan, C.A.; Lasserson, D.S.; et al. Global Prevalence of Chronic Kidney Disease - A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0158765. [Google Scholar] [CrossRef] [PubMed]
- Ammirati, A.L. Chronic Kidney Disease. Rev Assoc Med Bras (1992) 2020, (Suppl. 1), s03–s09. [Google Scholar] [CrossRef] [PubMed]
- Stevens, P.E.; Ahmed, S.B.; Carrero, J.J.; Foster, B.; Francis, A.; Hall, R.K.; et al. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney International. 2024, 105, S117–S314. [Google Scholar]
- Keane, W.F.; Eknoyan, G. Proteinuria, albuminuria, risk, assessment, detection, elimination (PARADE): A position paper of the National Kidney Foundation. American Journal of Kidney Diseases 1999, 33, 1004–1010. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.G.; Bruns, D.E.; Hortin, G.L.; Sandberg, S.; Aakre, K.M.; McQueen, M.J.; et al. Current issues in measurement and reporting of urinary albumin excretion. Clin Chem. 2009, 55, 24–38. [Google Scholar] [CrossRef]
- Levey, A.S.; Coresh, J.; Tighiouart, H.; Greene, T.; Inker, L.A. Measured and estimated glomerular filtration rate: Current status and future directions. Nat Rev Nephrol. 2020, 16, 51–64. [Google Scholar] [CrossRef]
- Foundation, N.K. Chronic Kidney Disease (CKD). 2023.
- Clinic, M. Chronic Kdiney Disease. Diseases & Conditions 2024.
- Fund, A.K. Risk factors for kidney disease. 2024.
- Prevention) CCfDCa. Risk Factors for Chronic Kidney Disease. Chronic Kidney Disease 2024.
- Vinhas, J.; Aires, I.; Batista, C.; Branco, P.; Brandão, J.; Nogueira, R.; et al. RENA Study: Cross-Sectional Study to Evaluate CKD Prevalence in Portugal. Nephron 2020, 144, 479–487. [Google Scholar] [CrossRef]
- Mallappallil, M.; Friedman, E.A.; Delano, B.G.; McFarlane, S.I.; Salifu, M.O. Chronic kidney disease in the elderly: Evaluation and management. Clin Pract (Lond) 2014, 11, 525–535. [Google Scholar] [CrossRef]
- Coresh, J.; Astor, B.C.; Greene, T.; Eknoyan, G.; Levey, A.S. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003, 41, 1–12. [Google Scholar] [CrossRef]
- Health. NIo. CKD in the General Population 2022.
- Kovesdy, C.P. Epidemiology of chronic kidney disease: An update 2022. Kidney Int. Suppl. (2011) 2022, 12, 7–11. [Google Scholar] [CrossRef]
- Raman, M.; Middleton, R.J.; Kalra, P.A.; Green, D. Estimating renal function in old people: An in-depth review. Int Urol Nephrol. 2017, 49, 1979–1988. [Google Scholar] [CrossRef] [PubMed]
- Cockcroft, D.W.; Gault, M.H. Prediction of creatinine clearance from serum creatinine. Nephron 1976, 16, 31–41. [Google Scholar] [CrossRef]
- Delanaye, P.; Cavalier, E.; Pottel, H.; Stehlé, T. New and old GFR equations: A European perspective. Clin Kidney J. 2023, 16, 1375–1383. [Google Scholar] [CrossRef]
- Levey, A.S.; Bosch, J.P.; Lewis, J.B.; Greene, T.; Rogers, N.; Roth, D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999, 130, 461–470. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd.; Feldman, H.I.; et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Schaeffner, E.S.; Ebert, N.; Delanaye, P.; Frei, U.; Gaedeke, J.; Jakob, O.; et al. Two novel equations to estimate kidney function in persons aged 70 years or older. Ann Intern Med. 2012, 157, 471–481. [Google Scholar] [CrossRef]
- Foundation, NK. eGFR Calculator. 2024.
- Foundation, NK. NKF and ASN Release New Way to Diagnose Kidney Diseases. 2021.
- Lee, S.; Lee, G.H.; Kim, H.; Yang, H.S.; Hur, M. Application of the European Kidney Function Consortium Equation to Estimate Glomerular Filtration Rate: A Comparison Study of the CKiD and CKD-EPI Equations Using the Korea National Health and Nutrition Examination Survey (KNHANES 2008-2021). Medicina (Kaunas) 2024, 60, 612. [Google Scholar] [CrossRef]
- Foundation, NK. CKD-EPI Creatinine Equation (2021). 2021.
- Foundation, NK. CKD-EPI Creatinine-Cystatin Equation (2021). 2021.
- Fox, C.S.; Matsushita, K.; Woodward, M.; Bilo, H.J.; Chalmers, J.; Heerspink, H.J.; et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: A meta-analysis. Lancet 2012, 380, 1662–1673. [Google Scholar] [CrossRef]
- Bello, A.K.; Alrukhaimi, M.; Ashuntantang, G.E.; Basnet, S.; Rotter, R.C.; Douthat, W.G.; et al. Complications of chronic kidney disease: Current state, knowledge gaps, and strategy for action. Kidney Int. Suppl. (2011) 2017, 7, 122–129. [Google Scholar] [CrossRef]
- Farrington, D.K.; Sang, Y.; Grams, M.E.; Ballew, S.H.; Dunning, S.; Stempniewicz, N.; et al. Anemia Prevalence, Type, and Associated Risks in a Cohort of 5.0 Million Insured Patients in the United States by Level of Kidney Function. Am J Kidney Dis. 2023, 81, 201–209.e1. [Google Scholar] [CrossRef]
- Cumming, R.G.; Mitchell, P.; Craig, J.C.; Knight, J.F. Renal impairment and anaemia in a population-based study of older people. Intern Med J. 2004, 34, 20–23. [Google Scholar] [CrossRef]
- Ble, A.; Fink, J.C.; Woodman, R.C.; Klausner, M.A.; Windham, B.G.; Guralnik, J.M.; et al. Renal function, erythropoietin, and anemia of older persons: The InCHIANTI study. Arch Intern Med. 2005, 165, 2222–2227. [Google Scholar] [CrossRef]
- Price, E.A.; Schrier, S.L. Anemia in the elderly: Introduction. Semin Hematol. 2008, 45, 207–209. [Google Scholar] [CrossRef] [PubMed]
- Steensma, D.P.; Tefferi, A. Anemia in the elderly: How should we define it, when does it matter, and what can be done? Mayo Clin Proc. 2007, 82, 958–966. [Google Scholar] [CrossRef]
- WHO. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Gonzalez, L.M.B.; Seidl, E.M.F. Aging from the perspective of elderly men. Paidéia (Ribeirão Preto) 2011, 21, 345–352. [Google Scholar] [CrossRef]
- Plan and operation of the Third National Health and Nutrition Examination Survey, 1988–1994. Series 1: Programs and collection procedures. Vital Health Stat 1 1994, 1–407.
- Beutler, E.; Felitti, V.J.; Koziol, J.A.; Ho, N.J.; Gelbart, T. Penetrance of 845G→A (C282Y) HFE hereditary haemochromatosis mutation in the USA. Lancet 2002, 359, 211–218. [Google Scholar] [CrossRef]
- Beutler, E.; Waalen, J. The definition of anemia: What is the lower limit of normal of the blood hemoglobin concentration? Blood 2006, 107, 1747–1750. [Google Scholar] [CrossRef]
- Tettamanti, M.; Lucca, U.; Gandini, F.; Recchia, A.; Mosconi, P.; Apolone, G.; et al. Prevalence, incidence and types of mild anemia in the elderly: The "Health and Anemia" population-based study. Haematologica. 2010, 95, 1849–1856. [Google Scholar] [CrossRef]
- Chaves, P.H.; Carlson, M.C.; Ferrucci, L.; Guralnik, J.M.; Semba, R.; Fried, L.P. Association between mild anemia and executive function impairment in community-dwelling older women: The Women’s Health and Aging Study II. J Am Geriatr Soc. 2006, 54, 1429–1435. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Eisenstaedt, R.S.; Ferrucci, L.; Klein, H.G.; Woodman, R.C. Prevalence of anemia in persons 65 years and older in the United States: Evidence for a high rate of unexplained anemia. Blood 2004, 104, 2263–2268. [Google Scholar] [CrossRef]
- Endres, H.G.; Wedding, U.; Pittrow, D.; Thiem, U.; Trampisch, H.J.; Diehm, C. Prevalence of anemia in elderly patients in primary care: Impact on 5-year mortality risk and differences between men and women. Curr Med Res Opin. 2009, 25, 1143–1158. [Google Scholar] [CrossRef]
- Nathavitharana, R.L.; Murray, J.A.; D’Sousa, N.; Sheehan, T.; Frampton, C.M.; Baker, B.W. Anaemia is highly prevalent among unselected internal medicine inpatients and is associated with increased mortality, earlier readmission and more prolonged hospital stay: An observational retrospective cohort study. Intern Med J. 2012, 42, 683–691. [Google Scholar] [CrossRef]
- Robinson, B.E. Epidemiology of chronic kidney disease and anemia. J Am Med Dir Assoc. 2006, 7, S3–S6, quiz S17-21. [Google Scholar] [CrossRef] [PubMed]
- Geisel, T.; Martin, J.; Schulze, B.; Schaefer, R.; Bach, M.; Virgin, G.; et al. An etiologic profile of anemia in 405 geriatric patients. Anemia. 2014, 2014, 932486. [Google Scholar] [CrossRef]
- Kaufman, J.M.; Vermeulen, A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev. 2005, 26, 833–876. [Google Scholar] [CrossRef]
- Al-Sharefi, A.; Mohammed, A.; Abdalaziz, A.; Jayasena, C.N. Androgens and Anemia: Current Trends and Future Prospects. Front Endocrinol (Lausanne). 2019, 10, 754. [Google Scholar] [CrossRef]
- Price, E.A.; Mehra, R.; Holmes, T.H.; Schrier, S.L. Anemia in older persons: Etiology and evaluation. Blood Cells Mol Dis. 2011, 46, 159–165. [Google Scholar] [CrossRef]
- Shavelle, R.M.; MacKenzie, R.; Paculdo, D.R. Anemia and mortality in older persons: Does the type of anemia affect survival? Int J Hematol. 2012, 95, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Akman, T.; Arıca, D.; Hatipoğlu, B.; Arslanoğlu, E.; Koca, E.; Karakuş, S.; et al. A Cross Sectional Analysis of Etiology of Anemia Among Elderly Patients. Osmangazi Tıp Dergisi. 2023, 45, 844–852. [Google Scholar] [CrossRef]
- Barnett, K.; Mercer, S.W.; Norbury, M.; Watt, G.; Wyke, S.; Guthrie, B. Epidemiology of multimorbidity and implications for health care, research, and medical education: A cross-sectional study. Lancet. 2012, 380, 37–43. [Google Scholar] [CrossRef]
- Bach, V.; Schruckmayer, G.; Sam, I.; Kemmler, G.; Stauder, R. Prevalence and possible causes of anemia in the elderly: A cross-sectional analysis of a large European university hospital cohort. Clin Interv Aging. 2014, 9, 1187–1196. [Google Scholar] [PubMed]
- Girelli, D.; Marchi, G.; Camaschella, C. Anemia in the Elderly. Hemasphere 2018, 2, e40. [Google Scholar] [CrossRef]
- Halawi, R.; Moukhadder, H.; Taher, A. Anemia in the elderly: A consequence of aging? Expert Rev Hematol. 2017, 10, 327–335. [Google Scholar] [CrossRef]
- Lopez, A.; Cacoub, P.; Macdougall, I.C.; Peyrin-Biroulet, L. Iron deficiency anaemia. Lancet 2016, 387, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Loikas, S.; Koskinen, P.; Irjala, K.; Löppönen, M.; Isoaho, R.; Kivelä, S.L.; et al. Vitamin B12 deficiency in the aged: A population-based study. Age Ageing 2007, 36, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Andrès, E.; Federici, L.; Serraj, K.; Kaltenbach, G. Update of nutrient-deficiency anemia in elderly patients. Eur J Intern Med. 2008, 19, 488–493. [Google Scholar] [CrossRef]
- Goodnough, L.T. Iron deficiency syndromes and iron-restricted erythropoiesis (CME). Transfusion. 2012, 52, 1584–1592. [Google Scholar] [CrossRef]
- Goodnough, L.T.; Schrier, S.L. Evaluation and management of anemia in the elderly. Am J Hematol. 2014, 89, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Makipour, S.; Kanapuru, B.; Ershler, W.B. Unexplained anemia in the elderly. Semin Hematol. 2008, 45, 250–254. [Google Scholar] [CrossRef]
- Gowanlock, Z.; Sriram, S.; Martin, A.; Xenocostas, A.; Lazo-Langner, A. Erythropoietin Levels in Elderly Patients with Anemia of Unknown Etiology. PLoS ONE 2016, 11, e0157279. [Google Scholar] [CrossRef]
- Sriram, S.; Xenocostas, A.; Lazo-Langner, A. Erythropoietin in anemia of unknown etiology: A systematic review and meta-analysis. Hematology. 2016, 21, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Dmitrieva, O.; de Lusignan, S.; Macdougall, I.C.; Gallagher, H.; Tomson, C.; Harris, K.; et al. Association of anaemia in primary care patients with chronic kidney disease: Cross sectional study of quality improvement in chronic kidney disease (QICKD) trial data. BMC Nephrol. 2013, 14, 24. [Google Scholar] [CrossRef]
- Babitt, J.L.; Lin, H.Y. Mechanisms of anemia in CKD. J Am Soc Nephrol. 2012, 23, 1631–1634. [Google Scholar] [CrossRef] [PubMed]
- Ku, E.; Del Vecchio, L.; Eckardt, K.U.; Haase, V.H.; Johansen, K.L.; Nangaku, M.; et al. Novel anemia therapies in chronic kidney disease: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2023, 104, 655–680. [Google Scholar] [CrossRef] [PubMed]
- Kunadharaju, R.; Silberstein, P. Erythropoiesis☆. Reference Module in Biomedical Sciences; Elsevier, 2015. [Google Scholar]
- Macdougall, I.C. Chapter 13 - Development of Recombinant Erythropoietin and Erythropoietin Analogs. In: Singh AK, Williams GH, editors. Textbook of Nephro-Endocrinology (Second Edition); Academic Press: 2018; pp. 217–232.
- Singh, A.K. Chapter 12 - Erythropoiesis: The Roles of Erythropoietin and Iron. In Textbook of Nephro-Endocrinology (Second Edition); Singh, A.K., Williams, G.H., Eds.; Academic Press, 2018; pp. 207–215. [Google Scholar]
- Haase, V.H. Hypoxic regulation of erythropoiesis and iron metabolism. Am J Physiol Renal Physiol. 2010, 299, F1–F13. [Google Scholar] [CrossRef]
- Haase, V.H. Regulation of erythropoiesis by hypoxia-inducible factors. Blood Reviews. 2013, 27, 41–53. [Google Scholar] [CrossRef]
- Zadrazil, J.; Horak, P. Pathophysiology of anemia in chronic kidney diseases: A review. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2015, 159, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Ganz, T. Regulation of iron metabolism by hepcidin. Annu Rev Nutr. 2006, 26, 323–342. [Google Scholar] [CrossRef] [PubMed]
- Tomosugi, N.; Kawabata, H.; Wakatabe, R.; Higuchi, M.; Yamaya, H.; Umehara, H.; et al. Detection of serum hepcidin in renal failure and inflammation by using ProteinChip System. Blood. 2006, 108, 1381–1387. [Google Scholar] [CrossRef]
- Pietrangelo, A. Hemochromatosis: An endocrine liver disease. Hepatology. 2007, 46, 1291–1301. [Google Scholar] [CrossRef] [PubMed]
- Antunes, S.A.; Canziani, M.E. Hepcidin: An important iron metabolism regulator in chronic kidney disease. J Bras Nefrol. 2016, 38, 351–355. [Google Scholar] [CrossRef]
- Macdougall, I.C. Anaemia in CKD—Treatment standard. Nephrology Dialysis Transplantation. 2023, 39, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Astor, B.C.; Muntner, P.; Levin, A.; Eustace, J.A.; Coresh, J. Association of kidney function with anemia: The Third National Health and Nutrition Examination Survey (1988-1994). Arch Intern Med. 2002, 162, 1401–1408. [Google Scholar] [CrossRef]
- Chapter 1: Diagnosis and evaluation of anemia in CKD. Kidney Int Suppl (2011) 2012, 2, 288–291. [CrossRef] [PubMed]
- CPR 1.2.: Evaluation of Anemia in CKD. Am. J. Am J. Kidney Dis. 2006, 47, S28–S32.
Figure 1.
Anatomo-functional changes in the renal aging process. EPO, erythropoietin.
Figure 1.
Anatomo-functional changes in the renal aging process. EPO, erythropoietin.
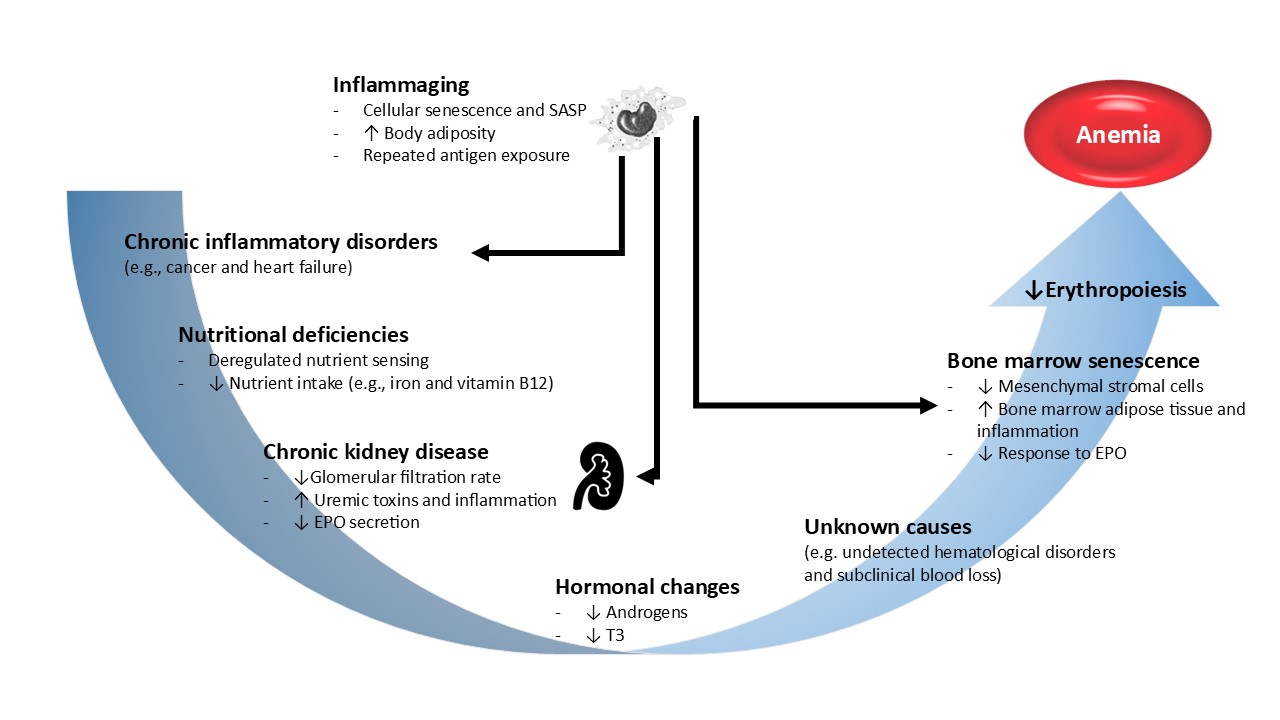
Figure 2.
Etiology of anemia in the elderly. The etiology of anemia in the elderly is frequently multifactorial. Inflammaging is a low-grade chronic inflammation state, that develops with advancing age and is a significant risk factor for multiple diseases that are highly prevalent in older individuals, such as type 2 diabetes, atherosclerosis and cancer. Inflammaging and/or chronic inflammatory disorders increase the synthesis of erythropoiesis inhibiting cytokines such as IFN-gamma and TNF-alpha. Decreased nutritional intake in the elderly may lead to deficiencies in nutrients that are vital for red blood cell production, namely iron, folate and vitamin B12. The physiological decline in renal function with age, together with aged-related diseases, such as diabetes and arterial hypertension, predispose the elderly patients to chronic kidney disease (CKD) development. In turn, CKD is characterized by reduced in EPO synthesis impairing the EPO-mediated prevention of apoptosis of erythroid progenitors in bone marrow. Other important hormonal changes such as a decrease in androgen levels may also contribute to decreased erythropoiesis. Finally, the aged bone marrow, with increased adipose tissue and an inflammatory ambient, becomes less responsive to EPO stimulation, exacerbating anemia in the elderly. EPO, erythropoietin; SASP, senescence-associated secretory phenotype.
Figure 2.
Etiology of anemia in the elderly. The etiology of anemia in the elderly is frequently multifactorial. Inflammaging is a low-grade chronic inflammation state, that develops with advancing age and is a significant risk factor for multiple diseases that are highly prevalent in older individuals, such as type 2 diabetes, atherosclerosis and cancer. Inflammaging and/or chronic inflammatory disorders increase the synthesis of erythropoiesis inhibiting cytokines such as IFN-gamma and TNF-alpha. Decreased nutritional intake in the elderly may lead to deficiencies in nutrients that are vital for red blood cell production, namely iron, folate and vitamin B12. The physiological decline in renal function with age, together with aged-related diseases, such as diabetes and arterial hypertension, predispose the elderly patients to chronic kidney disease (CKD) development. In turn, CKD is characterized by reduced in EPO synthesis impairing the EPO-mediated prevention of apoptosis of erythroid progenitors in bone marrow. Other important hormonal changes such as a decrease in androgen levels may also contribute to decreased erythropoiesis. Finally, the aged bone marrow, with increased adipose tissue and an inflammatory ambient, becomes less responsive to EPO stimulation, exacerbating anemia in the elderly. EPO, erythropoietin; SASP, senescence-associated secretory phenotype.
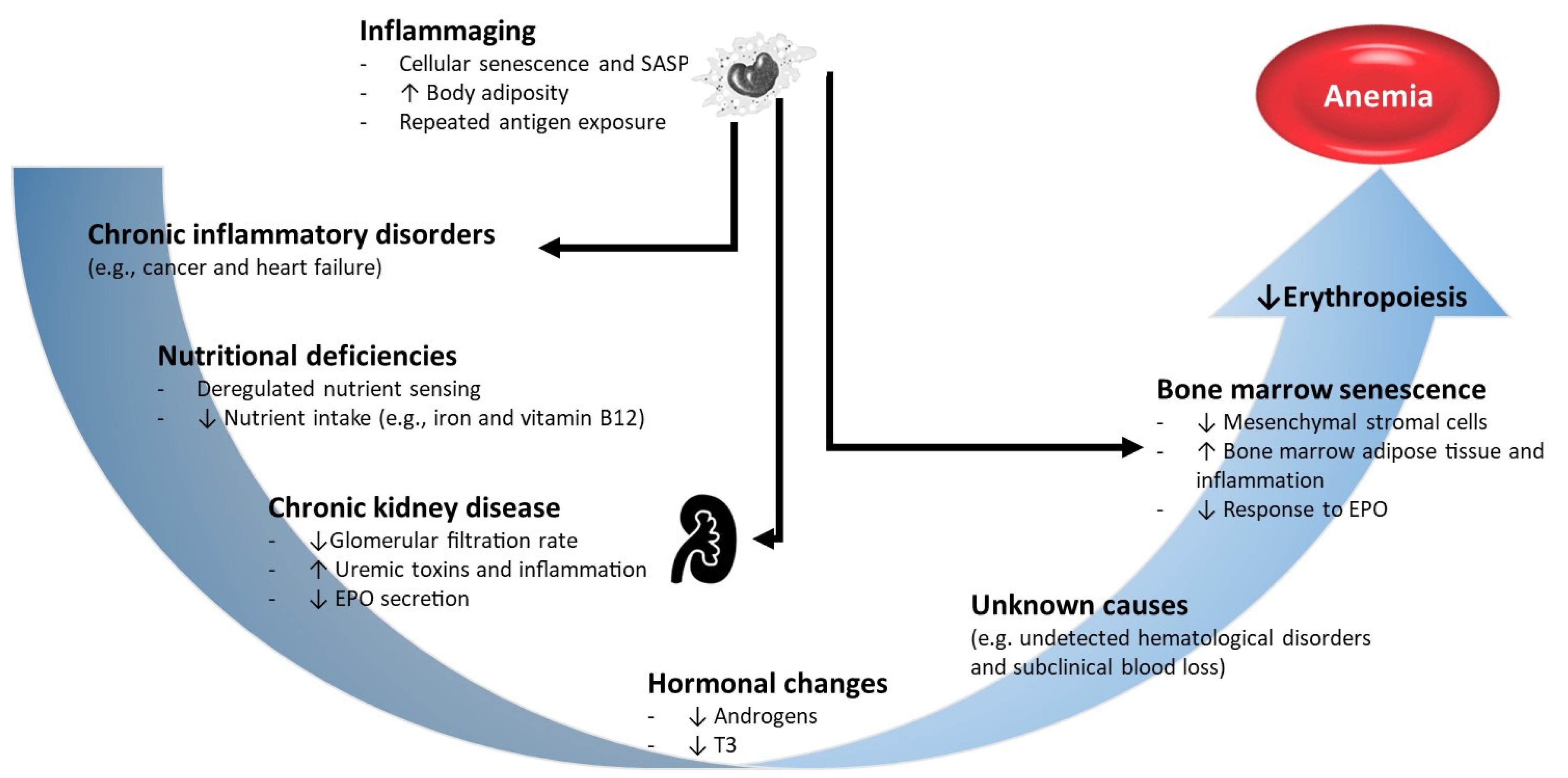
Figure 3.
Mechanisms involved in anemia of chronic kidney disease (CKD). CKD is a long-standing progressive condition characterized by the deterioration of renal function, including decreased glomerular filtration rate (GFR) and compromised erythropoietin (EPO) production. CKD-related anemia is mainly attributable to inappropriate erythropoietin synthesis, particularly in advanced stages of the disease. Inflammation also plays an important role in promoting anemia, as CKD pathophysiology involves a final common activation of inflammatory pathways, irrespective of etiology. CKD and its associated risk factors (e.g. obesity) cause high levels of intereukin-6 (IL) that promote hepatic synthesis of hepcidin, a major peptide that regulates systemic iron metabolism. By interacting with its receptor ferroportin, a transmembrane iron-export protein, hepcidin inhibits iron absorption from enterocytes and its mobilization from macrophages of the reticuloendothelial system and from hepatic stores. Decreased renal hepcidin clearance further augments its circulating levels, aggravating iron availability for erythropoiesis. Inflammatory cytokines – such as IFN-γ and TNF-α – as well as uremic toxins (e.g. polyamines) inhibit bone marrow erythropoiesis. Although the exact mechanisms of uremic inhibition remain unknown, it is accepted that accumulation of uremic toxins in blood impairs erythropoietin synthesis. In addition, the uremic and pro-inflammatory systemic environment damages red blood cells (RBC), shortening their lifespan and further contributing to anemia. The uremic syndrome also leads to neurological symptoms (anorexia, nausea and vomiting) that may compromise adequate nutrient intake, limiting the availability of vital elements for erythropoiesis. Finally, secondary hyperparathyroidism is a common complication of CKD, and excessive levels of parathyroid hormone (a “uremic toxin”) hampers normal erythropoiesis by downregulating the erythropoietin receptors, and causes a disturbed calcium metabolism in the peripheral RBC, promoting a hemolytic effect on these cells. AA, amino acids; EPO, erythropoietin; FA, fatty acids; FPN, ferroportin; HPT, hyperparathyroidism; IFN, interferon; IL, interleukin; PTH, parathyroid hormone; TNF, tumor necrosis factor.
Figure 3.
Mechanisms involved in anemia of chronic kidney disease (CKD). CKD is a long-standing progressive condition characterized by the deterioration of renal function, including decreased glomerular filtration rate (GFR) and compromised erythropoietin (EPO) production. CKD-related anemia is mainly attributable to inappropriate erythropoietin synthesis, particularly in advanced stages of the disease. Inflammation also plays an important role in promoting anemia, as CKD pathophysiology involves a final common activation of inflammatory pathways, irrespective of etiology. CKD and its associated risk factors (e.g. obesity) cause high levels of intereukin-6 (IL) that promote hepatic synthesis of hepcidin, a major peptide that regulates systemic iron metabolism. By interacting with its receptor ferroportin, a transmembrane iron-export protein, hepcidin inhibits iron absorption from enterocytes and its mobilization from macrophages of the reticuloendothelial system and from hepatic stores. Decreased renal hepcidin clearance further augments its circulating levels, aggravating iron availability for erythropoiesis. Inflammatory cytokines – such as IFN-γ and TNF-α – as well as uremic toxins (e.g. polyamines) inhibit bone marrow erythropoiesis. Although the exact mechanisms of uremic inhibition remain unknown, it is accepted that accumulation of uremic toxins in blood impairs erythropoietin synthesis. In addition, the uremic and pro-inflammatory systemic environment damages red blood cells (RBC), shortening their lifespan and further contributing to anemia. The uremic syndrome also leads to neurological symptoms (anorexia, nausea and vomiting) that may compromise adequate nutrient intake, limiting the availability of vital elements for erythropoiesis. Finally, secondary hyperparathyroidism is a common complication of CKD, and excessive levels of parathyroid hormone (a “uremic toxin”) hampers normal erythropoiesis by downregulating the erythropoietin receptors, and causes a disturbed calcium metabolism in the peripheral RBC, promoting a hemolytic effect on these cells. AA, amino acids; EPO, erythropoietin; FA, fatty acids; FPN, ferroportin; HPT, hyperparathyroidism; IFN, interferon; IL, interleukin; PTH, parathyroid hormone; TNF, tumor necrosis factor.
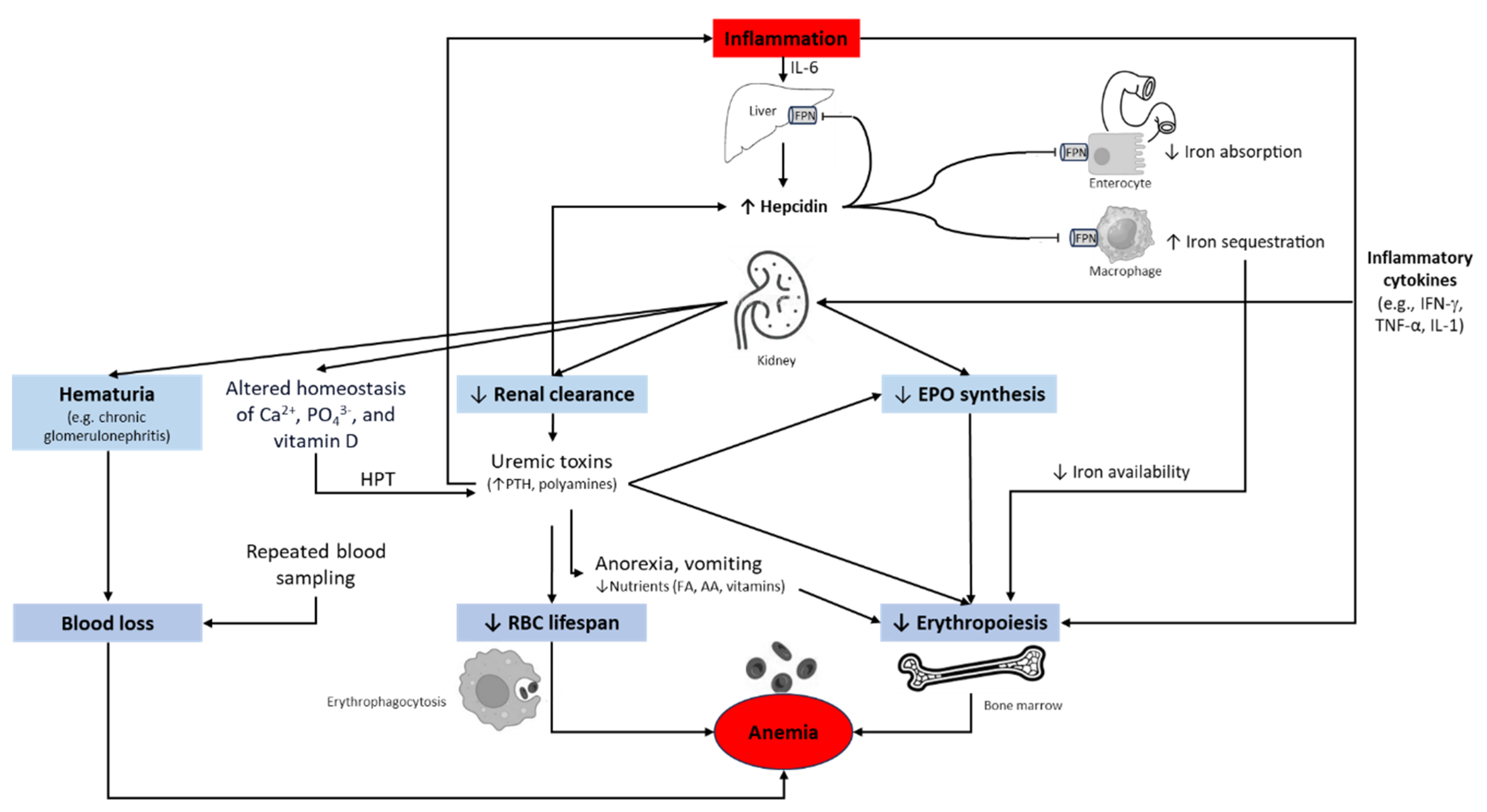
Table 1.
Findings evidencing kidney injury.
Table 1.
Findings evidencing kidney injury.
Albuminuria (AER ≥ 30 mg/24h or ACR ≥ 30 mg/g) Modifications in urinary sediment Changes in electrolytes or changes caused by tubular damage Anatomical or structural anomalies detected by imaging Pathological anomalies detected by histology History of kidney transplant |
Table 2.
Risk factors for chronic kidney disease (CKD).
Table 2.
Risk factors for chronic kidney disease (CKD).
| Non-modifiable risk factors |
Common modifiable risk factors |
Less common modifiable risk factors |
|
Diabetes HTA Obesity Dyslipidemia Metabolic acidosis High protein diet Smoking |
|
Table 3.
CKD-EPI Creatinine and CKD-EPI Creatinine-Cystatin C equations for calculating glomerular filtration rate.
Table 3.
CKD-EPI Creatinine and CKD-EPI Creatinine-Cystatin C equations for calculating glomerular filtration rate.
| CKD-EPI Creatinine Equation (2021) |
|
|
CKD-EPI Creatinine-Cystatin CEquation (2021)
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).