Submitted:
31 January 2025
Posted:
05 February 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Hydrodistillation of Essential Oil
2.3. Gas Chromatography/Mass Spectrometry
2.4. Data Analysis
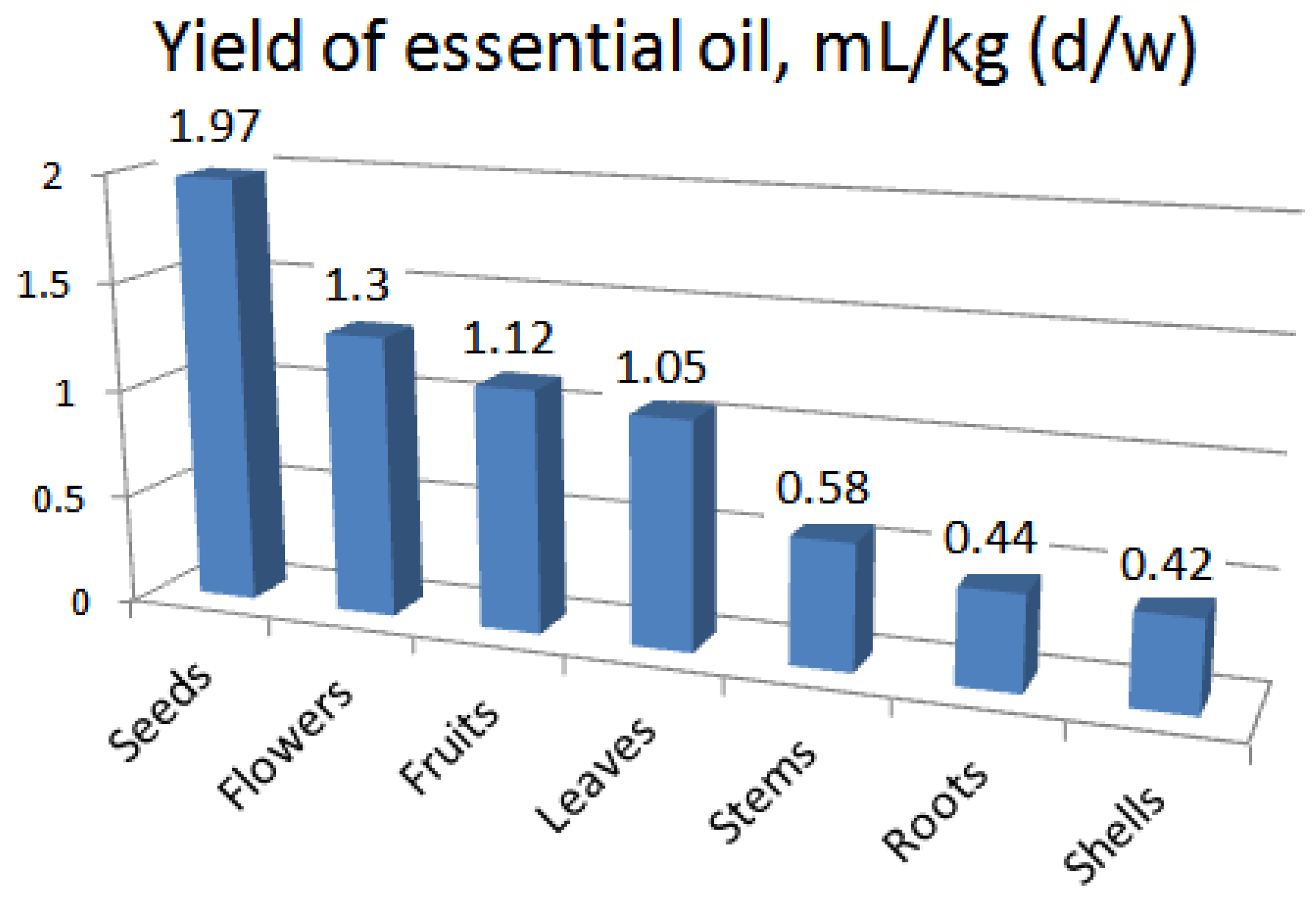
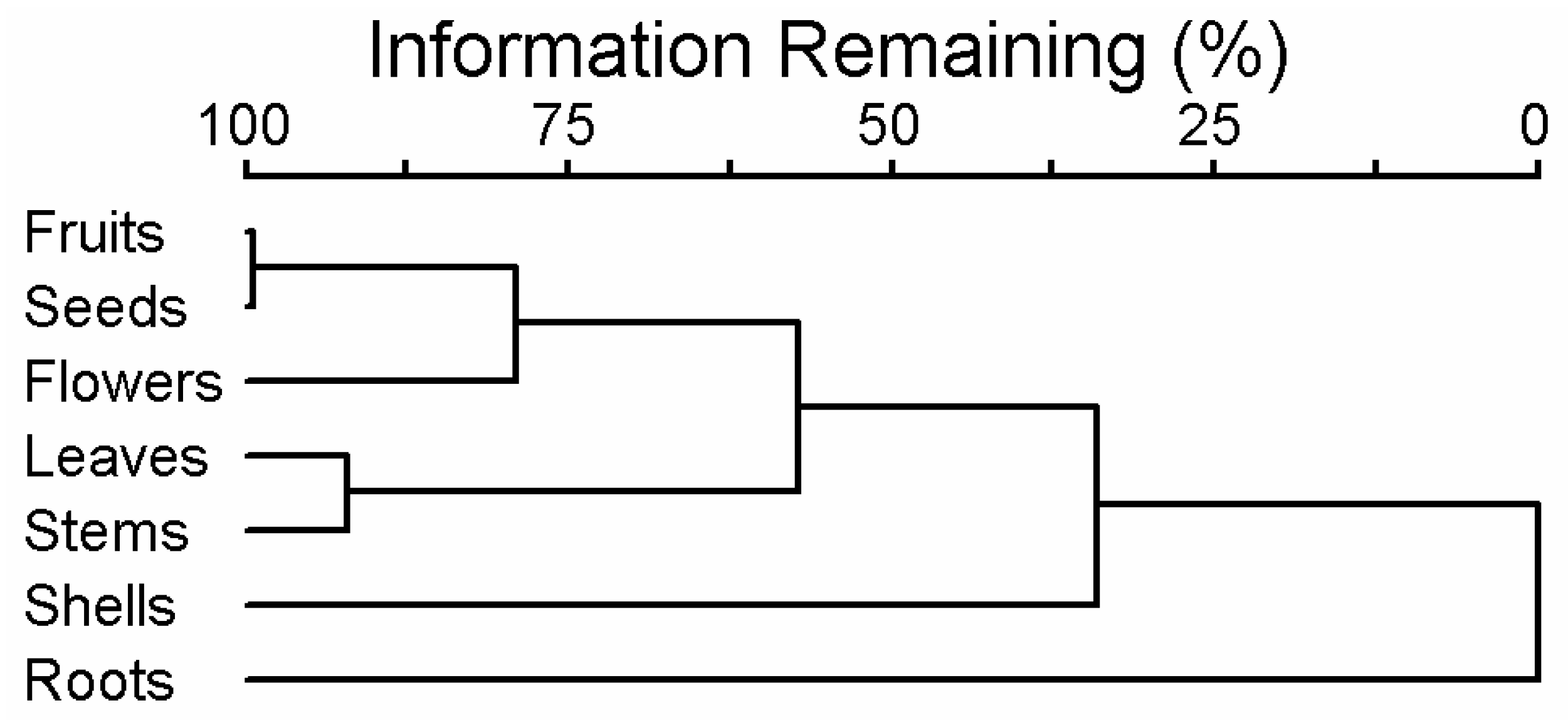
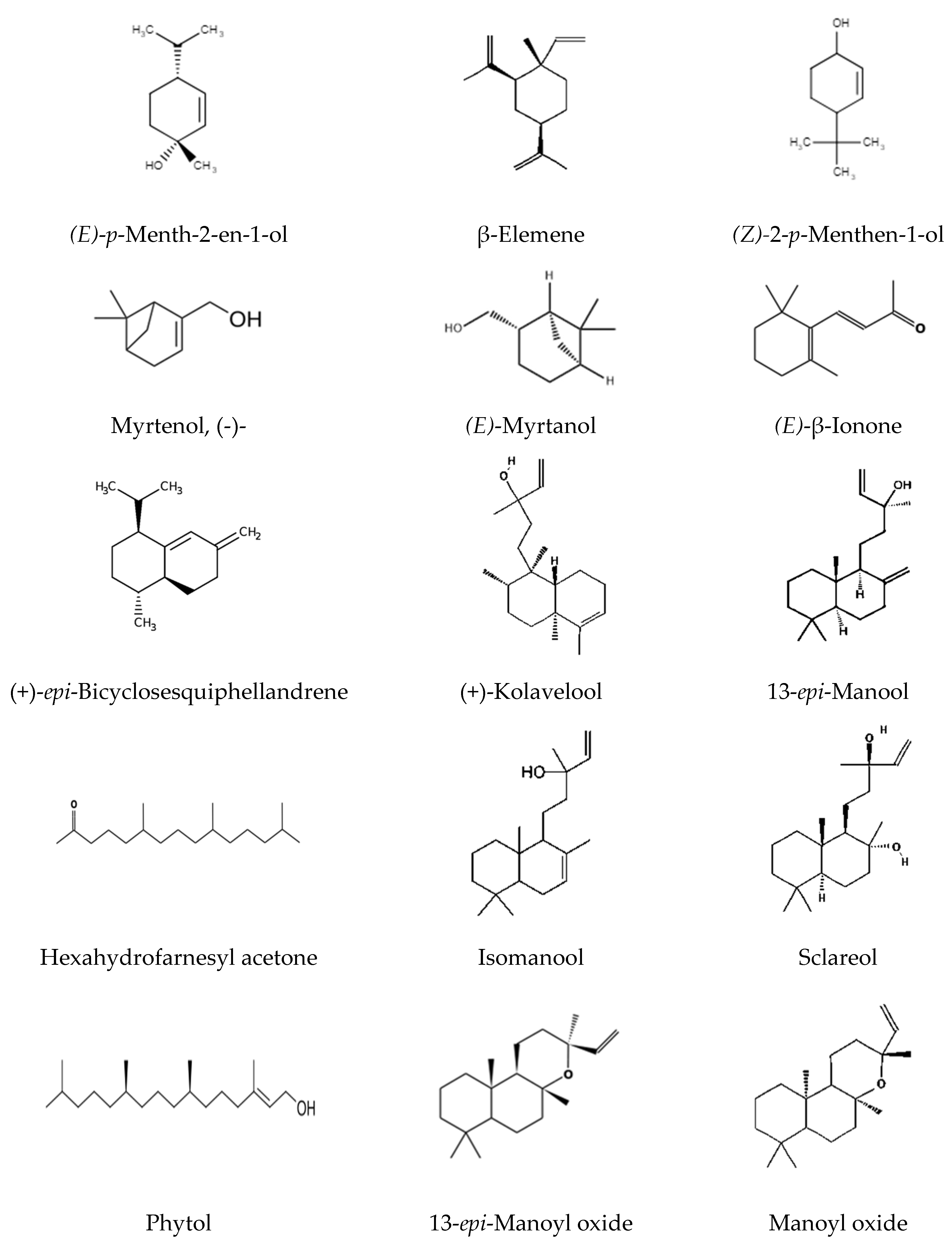
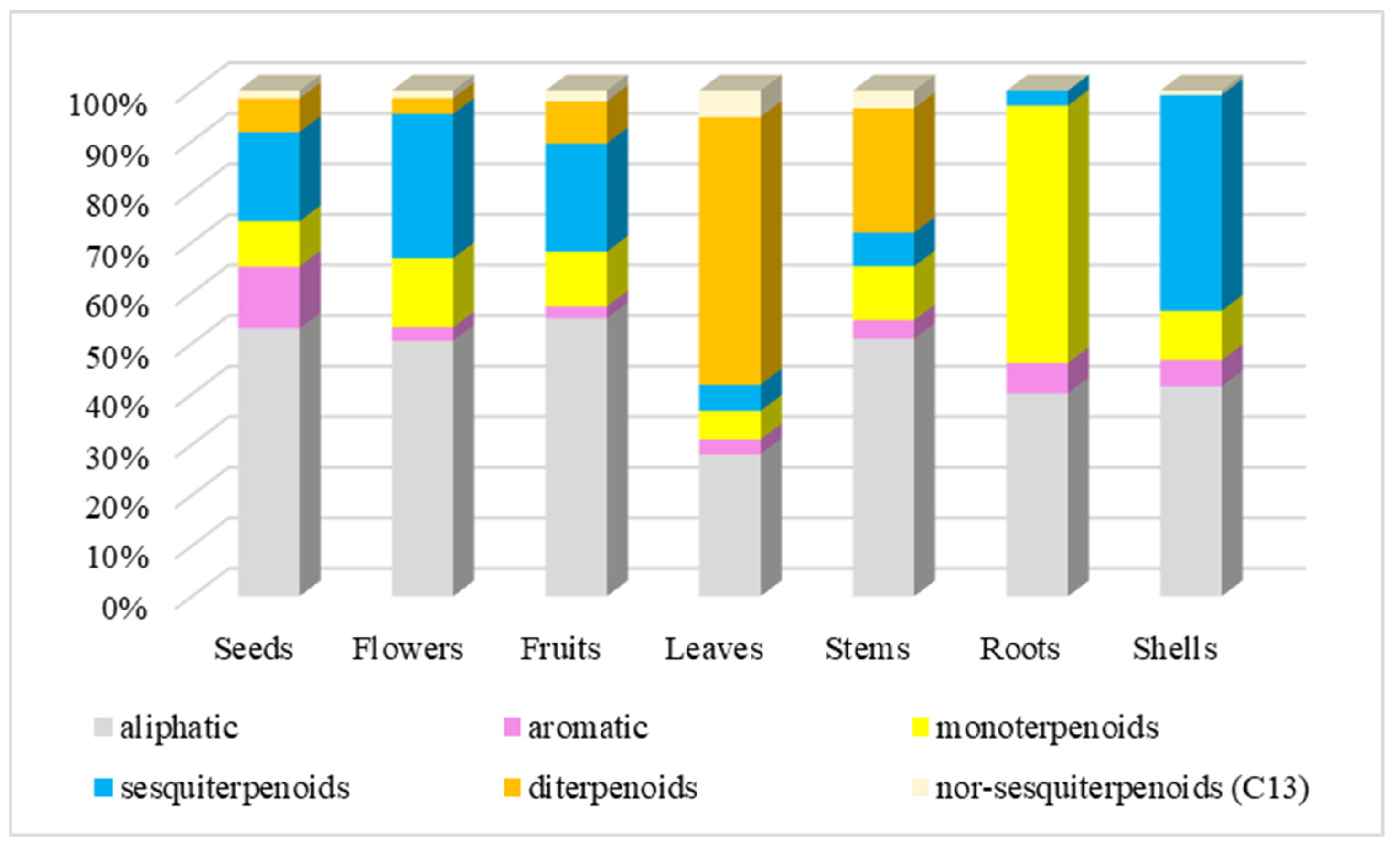
3. Results
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gilia Capitata. Calscape. California Native Plant Society. 2024.
- Grant, V. Genetic and Taxonomic Studies in Gilia: VI. Interspecific Relationships in the Leafy-Stemmed Gilias. aliso 1954, 3, 35–49. [Google Scholar] [CrossRef]
- Grant, V.; Grant, A. Genetic and Taxonomic Studies in Gilia: X. Conspectus of the Subgenus Gilia. Aliso: A Journal of Systematic and Evolutionary Botany 1956, 3, 297–300. [Google Scholar] [CrossRef]
- Jaramillo, Z.; Leigh, J. Revision of the Genus Gilia of Utah. Journal of Undergraduate Research 2019, 105. [Google Scholar]
- Baskin, Je.M.; Baskin, C.C. Propagation Protocol for Production of Container (Plug) Gilia Capitata Sims Plants 2002.
- Grant, V. Genetic and Taxonomic Studies in Gilia: I. Gilia Capitata. aliso 1950, 239–316. [Google Scholar] [CrossRef]
- Porter, J.M. Gilia Capitata Subsp. Capitata, in Jepson Flora Project (Eds.). Jepson eFlora 2023 2023.
- Gilia Capitata Sims. Kew Plants of the World.
- Nagy, E.S.; Rice, K.J. Local Adaptation in Two Subspecies of an Annual Plant: Implications for Migration and Gene Flow. Evolution 1997, 51, 1079. [Google Scholar] [CrossRef]
- Kruckeberg, A.R. INTRASPECIFIC VARIABILITY IN THE RESPONSE OF CERTAIN NATIVE PLANT SPECIES TO SERPENTINE SOIL. American J of Botany 1951, 38, 408–419. [Google Scholar] [CrossRef]
- Brown, H.S. Differential Chiasma Frequencies in Self-Pollinating and Cross-Pollinating Species of the Genus Gilia. Aliso: A Journal of Systematic and Floristic Botany 1961, 67–81.
- Grant, V. Seed Germination in Gilia Capitata and Its Relatives. Madroño 1949, 87–93.
- Keeley, J.E.; Keeley, S.C. Role of Fire in the Germination of Chaparral Herbs and Suffrutescents. Madroño 1987, 240–249.
- Hayes, Je. Top 10 Oregon Native Plants for Pollinators: Week 8.
- Grant, V.; Grant, A. Flower Pollination in the Phlox Family. Columbia Univ. Press, N.Y. 1965.
- Nagy, E.S. Selection for Native Characters in Hybrids Between Two Locally Adapted Plant Subspecies. Evolution 1997, 51, 1469. [Google Scholar] [CrossRef]
- Cseke, L.J.; Kaufman, P.B.; Kirakosyan, A. The Biology of Essential Oils in the Pollination of Flowers. Natural Product Communications 2007, 2, 1934578X0700201225. [Google Scholar] [CrossRef]
- European Pharmacopoeia; 11th ed.; Council of Europe: Strasbourg, 2022.
- Raal, A.; Ilina, T.; Kovalyova, A.; Koshovyi, O. Volatile Compounds in Distillates and Hexane Extracts from the Flowers of Philadelphus Coronarius and Jasminum Officinale. ScienceRise: Pharmaceutical Science 2024, 37–46. [CrossRef]
- Hrytsyk, Y.; Koshovyi, O.; Lepiku, M.; Jakštas, V.; Žvikas, V.; Matus, T.; Melnyk, M.; Grytsyk, L.; Raal, A. Phytochemical and Pharmacological Research in Galenic Remedies of Solidago Canadensis L. Herb. Phyton 2024, 93, 2303–2315. [Google Scholar] [CrossRef]
- McCune, B.; Mefford, M.J. PC-ORD. Multivariate Analysis of Ecological Data. 2018.
- Raal, A.; Gontova, T.; Ivask, A.; Orav, A.; Koshovyi, O. Yield, Composition, and Chemotypes of Essential Oils from Origanum Vulgare L. Aerial Parts Cultivated in Different European Countries. Agronomy 2024, 14, 3046. [Google Scholar] [CrossRef]
- Raal, A.; Gontova, T.; Palmeos, M.; Orav, A.; Sayakova, G.; Koshovyi, O. Comparative Analysis of Content and Composition of Essential Oils of Thymus Vulgaris L. from Different Regions of Europe. PEAS 2024, 73, 332. [Google Scholar] [CrossRef]
- Raal, A.; Kokitko, V.; Odyntsova, V.; Orav, A.; Koshovyi, O. Comparative Analysis of the Essential Oil of the Underground Organs of Valeriana Spp. from Different Countries. Phyton 2024, 93, 1365–1382. [Google Scholar] [CrossRef]
- Raal, A.; Ilina, T.; Kovaleva, A.; Orav, A.; Karileet, M.; Džaniašvili, M.; Koliadzhyn, T.; Grytsyk, A.; Koshovyi, O. Variation in the Composition of the Essential Oil of Commercial Artemisia Absinthium L. Herb Samples from Different Countries. ScienceRise: Pharmaceutical Science 2024, 19–28. [CrossRef]
- Raal, A.; Komarov, R.; Orav, A.; Kapp, K.; Grytsyk, A.; Koshovyi, O. Chemical Composition of Essential Oil of Common Juniper (Juniperus Communis L.) Branches from Estonia. SR: PS 2022, 66–73. [CrossRef]
- Filipowicz, N.; Kamiński, M.; Kurlenda, J.; Asztemborska, M.; Ochocka, J.R. Antibacterial and Antifungal Activity of Juniper Berry Oil and Its Selected Components. Phytotherapy Research 2003, 17, 227–231. [Google Scholar] [CrossRef]
- Razavi, S.M.; Nejad-Ebrahimi, S. Phytochemical Analysis and Allelopathic Activity of Essential Oils of Ecballium Elaterium A. Richard Growing in Iran. Natural Product Research 2010, 24, 1704–1709. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wang, S.; Shi, J.; Sun, Z.; Lei, Z.; Yin, Z.; Qian, Z.; Tang, H.; Xie, H. Genotypic and Environmental Effects on the Volatile Chemotype of Valeriana Jatamansi Jones. Front. Plant Sci. 2018, 9, 1003. [Google Scholar] [CrossRef]
- Avoseh, O.N.; Mtunzi, F.M.; Ogunwande, I.A.; Ascrizzi, R.; Guido, F. Albizia Lebbeck and Albizia Zygia Volatile Oils Exhibit Anti-Nociceptive and Anti-Inflammatory Properties in Pain Models. Journal of Ethnopharmacology 2021, 268, 113676. [Google Scholar] [CrossRef]
- Ganesan, T.; Subban, M.; Christopher Leslee, D.B.; Kuppannan, S.B.; Seedevi, P. Structural Characterization of N-Hexadecanoic Acid from the Leaves of Ipomoea Eriocarpa and Its Antioxidant and Antibacterial Activities. Biomass Conv. Bioref. 2024, 14, 14547–14558. [Google Scholar] [CrossRef]
- Johannes, E.; Litaay, M.; Syahribulan, S. The Bioactivity of Hexadecanoic Acid Compound Isolated from Hydroid Aglaophenia Cupressina Lamoureoux as Antibacterial Agent against Salmonella Typhi. Int. J. Biol. Med. Res. 2016, 5469–5472. [Google Scholar]
- Sánchez-Sevilla, J.F.; Cruz-Rus, E.; Valpuesta, V.; Botella, M.A.; Amaya, I. Deciphering Gamma-Decalactone Biosynthesis in Strawberry Fruit Using a Combination of Genetic Mapping, RNA-Seq and eQTL Analyses. BMC Genomics 2014, 15, 218. [Google Scholar] [CrossRef]
- Chambers, A.H.; Evans, S.A.; Folta, K.M. Methyl Anthranilate and γ-Decalactone Inhibit Strawberry Pathogen Growth and Achene Germination. J. Agric. Food Chem. 2013, 61, 12625–12633. [Google Scholar] [CrossRef]
- Gong, X.; Sun, C.; Abame, M.A.; Shi, W.; Xie, Y.; Xu, W.; Zhu, F.; Zhang, Y.; Shen, J.; Aisa, H.A. Synthesis of CBD and Its Derivatives Bearing Various C4′-Side Chains with a Late-Stage Diversification Method. J. Org. Chem. 2020, 85, 2704–2715. [Google Scholar] [CrossRef] [PubMed]
- Jou, Y.-J.; Hua, C.-H.; Lin, C.-S.; Wang, C.-Y.; Wan, L.; Lin, Y.-J.; Huang, S.-H.; Lin, C.-W. Anticancer Activity of γ-Bisabolene in Human Neuroblastoma Cells via Induction of P53-Mediated Mitochondrial Apoptosis. Molecules 2016, 21, 601. [Google Scholar] [CrossRef] [PubMed]
- Yeo, S.K.; Ali, A.Y.; Hayward, O.A.; Turnham, D.; Jackson, T.; Bowen, I.D.; Clarkson, R. β-Bisabolene, a Sesquiterpene from the Essential Oil Extract of Opoponax ( Commiphora Guidottii ), Exhibits Cytotoxicity in Breast Cancer Cell Lines. Phytotherapy Research 2016, 30, 418–425. [Google Scholar] [CrossRef]
- Dahham, S.; Tabana, Y.; Iqbal, M.; Ahamed, M.; Ezzat, M.; Majid, A.; Majid, A. The Anticancer, Antioxidant and Antimicrobial Properties of the Sesquiterpene β-Caryophyllene from the Essential Oil of Aquilaria Crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Tundis, R.; Menichini, F.; Saab, A.M.; Statti, G.A.; Menichini, F. Cytotoxic Activity of Essential Oils from Labiatae and Lauraceae Families against in Vitro Human Tumor Models. Anticancer Res 2007, 27, 3293–3299. [Google Scholar]
- Yoshihiro, I.; Toshiko, H.; Akiko, S.; Kazuma, H.; Hajime, H.; Shigeki, K. Biphasic Effects of Geranylgeraniol, Terpenone and Phytolon Thegrowth of Staphylococcus Aureus. 2005, 1770–1774.
- Sabudak, T.; Ozturk, M.; Goren, A.C.; Kolak, U.; Topcu, G. Fatty Acids and Other Lipid Composition of Five Trifolium Species with Antioxidant Activity. Pharmaceutical Biology 2009, 47, 137–141. [Google Scholar] [CrossRef]
- McGinty, D.; Letizia, C.S.; Api, A.M. Fragrance Material Review on Phytol. Food and Chemical Toxicology 2010, 48, S59–S63. [Google Scholar] [CrossRef]
- Demetzos, C.; Dimas, K.S. Labdane-Type Diterpenes: Chemistry and Biological Activity. In Studies in Natural Products Chemistry; Elsevier, 2001; Vol. 25, pp. 235–292 ISBN 978-0-08-044001-9.
- Tran, Q.T.N.; Wong, W.S.F.; Chai, C.L.L. Labdane Diterpenoids as Potential Anti-Inflammatory Agents. Pharmacological Research 2017, 124, 43–63. [Google Scholar] [CrossRef]
- Villamizar, Jo.E.; Juncosa, Jo.; Pittelaud, Je.; Hernández, M.; Canudas, N.; Tropper, E.; Salazar, F.; Fuentes, Ju. Facile Access to Labdane-Type Diterpenes: Synthesis of Coronarin C, Zerumin B, Labda-8(17), 13(14)-Dien-15,16-Olide and Derivatives from (+)-Manool. Journal of chemical research, 2007, 6, 342-346. 2007, 342–346.
- Cyr, A.; Wilderman, P.R.; Determan, M.; Peters, R.J. A Modular Approach for Facile Biosynthesis of Labdane-Related Diterpenes. J Am Chem Soc 2007, 129, 6684–6685. [Google Scholar] [CrossRef]
- Zhou, J.; Xie, X.; Tang, H.; Peng, C.; Peng, F. The Bioactivities of Sclareol: A Mini Review. Front. Pharmacol. 2022, 13, 1014105. [Google Scholar] [CrossRef]
- Jameel, S.; Bhat, K.A. Sclareol: Isolation, Structural Modification, Biosynthesis, and Pharmacological Evaluation – A Review. Pharm Chem J 2024, 57, 1568–1579. [Google Scholar] [CrossRef]
- Park, J.; Lee, K.; Jung, E.; Kang, S.; Kim, Y.J. Sclareol Isolated from Salvia Officinalis Improves Facial Wrinkles via an Antiphotoaging Mechanism. J of Cosmetic Dermatology 2016, 15, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Li, C.; Zhang, L.; Lin, Ji.; Jiang, N.; Wang, Qa.; Xu, Qi.; Zheng, H.; Gu, L.; Jia, Yi.; et al. Mechanism of Antifungal Activity and Therapeutic Action of β-Ionone on Aspergillus Fumigatus Keratitis via Suppressing LOX1 and JNK/P38 MAPK Activation. International Immunopharmacology 2022, 108992. [Google Scholar] [CrossRef]
- Kang, C.-H.; Jayasooriya, R.G.P.T.; Choi, Y.H.; Moon, S.-K.; Kim, W.-J.; Kim, G.-Y. β-Ionone Attenuates LPS-Induced pro-Inflammatory Mediators Such as NO, PGE2 and TNF-α in BV2 Microglial Cells via Suppression of the NF-κB and MAPK Pathway. Toxicology in Vitro 2013, 27, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Caboni, P.; Ntalli, N.G.; Aissani, N.; Cavoski, I.; Angioni, A. Nematicidal Activity of ( E, E )-2,4-Decadienal and ( E )-2-Decenal from Ailanthus Altissima against Meloidogyne Javanica. J. Agric. Food Chem. 2012, 60, 1146–1151. [Google Scholar] [CrossRef]
- Mirek, J.; Walkowiak-Nowicka, K.; Słocińska, M. The Effect of (E,E)-2,4-Decadienal, (E)-2-Decenal, 2-Undecanone and Furfural on Reproduction of Tenebrio Molitor. In Proceedings of the 1st International Electronic Conference on Entomology; MDPI: Sciforum.net, July 1 2021; p. 10540.
- Feng, Ye.; An, Qi.; Zhao, Zh.; Wu, M.; Yang, Ch.; Liang, W.Y.; Xu, Xu.; Jiang, T.; Zhang, Gu. Beta-Elemene: A Phytochemical with Promise as a Drug Candidate for Tumor Therapy and Adjuvant Tumor Therapy. Biomedicine & Pharmacotherapy 2024, 116266.
- Chen, X.; Huang, C.; Li, K.; Liu, J.; Zheng, Y.; Feng, Y.; Kai, G. Recent Advances in Biosynthesis and Pharmacology of β-Elemene. Phytochem Rev 2023, 22, 169–186. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, R.; Xu, L.; Xie, S.; Dong, J.; Jing, Y. β-Elemene Piperazine Derivatives Induce Apoptosis in Human Leukemia Cells through Downregulation of c-FLIP and Generation of ROS. PLoS ONE 2011, 6, e15843. [Google Scholar] [CrossRef]
- Ricciardelli, A.; Casillo, A.; Papa, R.; Monti, D.M.; Imbimbo, P.; Vrenna, G.; Artini, M.; Selan, L.; Corsaro, M.M.; Tutino, M.L.; et al. Pentadecanal Inspired Molecules as New Anti-Biofilm Agents against Staphylococcus Epidermidis. Biofouling 2018, 34, 1110–1120. [Google Scholar] [CrossRef]
- Casillo, A.; Papa, R.; Ricciardelli, A.; Sannino, F.; Ziaco, M.; Tilotta, M.; Selan, L.; Marino, G.; Corsaro, M.M.; Tutino, M.L.; et al. Anti-Biofilm Activity of a Long-Chain Fatty Aldehyde from Antarctic Pseudoalteromonas Haloplanktis TAC125 against Staphylococcus Epidermidis Biofilm. Front. Cell. Infect. Microbiol. 2017, 7. [Google Scholar] [CrossRef]
- Labbozzetta, M.; Poma, P.; Tutone, M.; McCubrey, J.A.; Sajeva, M.; Notarbartolo, M. Phytol and Heptacosane Are Possible Tools to Overcome Multidrug Resistance in an In Vitro Model of Acute Myeloid Leukemia. Pharmaceuticals 2022, 15, 356. [Google Scholar] [CrossRef]
- Mrabti, H.N.; Jaouadi, I.; Zeouk, I.; Ghchime, R.; El Menyiy, N.; Omari, N.E.; Balahbib, A.; Al-Mijalli, S.H.; Abdallah, E.M.; El-Shazly, M.; et al. Biological and Pharmacological Properties of Myrtenol: A Review. CPD 2023, 29, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Barbhuiya, P.A.; Pathak, M.P. Myrtenol: A Promising Terpene with Potent Pharmacological Properties. Pharmacological Research - Natural Products 2024, 4, 100067. [Google Scholar] [CrossRef]
- Yang, J.-Y.; Lee, H.-S. Verbenone Structural Analogues Isolated from Artemesia Aucheri as Natural Acaricides against Dermatophagoides Spp. and Tyrophagus Putrescentiae. J. Agric. Food Chem. 2013, 61, 12292–12296. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-Y.; Lee, H.-W.; Lee, H.-S. Growth Inhibitory Activities of Myrtanol and Structural Analogues from Thymus Tosevii against Intestinal Bacteria. Food Sci Biotechnol 2015, 24, 169–174. [Google Scholar] [CrossRef]
- Cha, D.H.; Roh, G.H.; Hesler, S.P.; Wallingford, A.; Stockton, D.G.; Park, S.K.; Loeb, G.M. 2-Pentylfuran: A Novel Repellent of Drosophila Suzukii. Pest Management Science 2021, 77, 1757–1764. [Google Scholar] [CrossRef]
- Kladi, M.; Vagias, C.; Furnari, G.; Moreau, D.; Roussakis, C.; Roussis, V. Cytotoxic Cuparene Sesquiterpenes from Laurencia Microcladia. Tetrahedron Letters 2005, 46, 5723–5726. [Google Scholar] [CrossRef]
- Pajaro-Castro, N.; Flechas, M.C.; Ocazionez, R.; Stashenko, E.; Olivero-Verbel, J. Potential Interaction of Components from Essential Oils with Dengue Virus Proteins. Boletin Latinoamericano y del Caribe de plantas Medicinales y Aromaticas 2015, 14, 141–155. [Google Scholar]
- Pan, S.-P.; Pirker, T.; Kunert, O.; Kretschmer, N.; Hummelbrunner, S.; Latkolik, S.L.; Rappai, J.; Dirsch, V.M.; Bochkov, V.; Bauer, R. C13 Megastigmane Derivatives From Epipremnum Pinnatum: β-Damascenone Inhibits the Expression of Pro-Inflammatory Cytokines and Leukocyte Adhesion Molecules as Well as NF-κB Signaling. Front. Pharmacol. 2019, 10, 1351. [Google Scholar] [CrossRef]
- Uddin, A.N.; Labuda, I.; Burns, F.J. A Novel Mechanism of Filaggrin Induction and Sunburn Prevention by β-Damascenone in Skh-1 Mice. Toxicology and Applied Pharmacology 2012, 265, 335–341. [Google Scholar] [CrossRef]
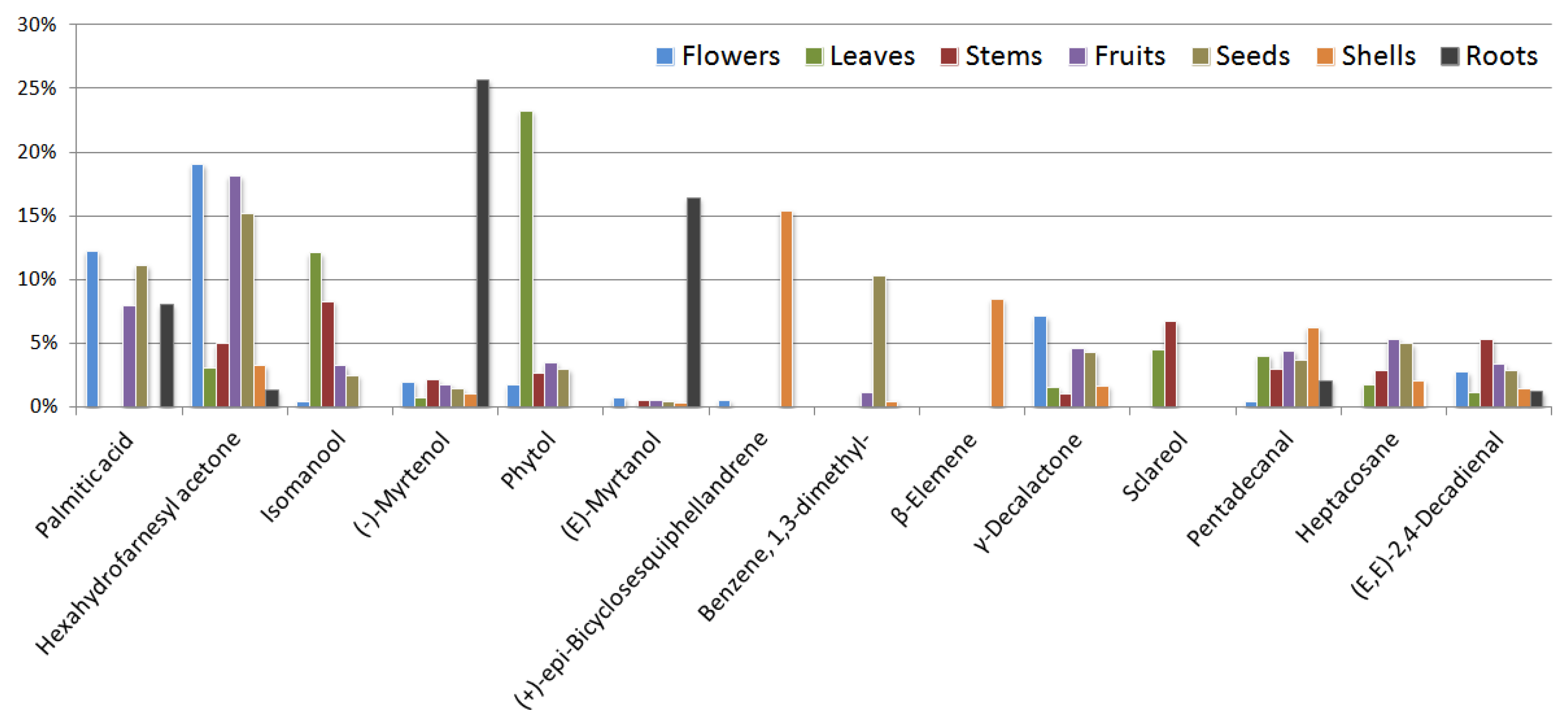
| Compound | RI | Library NIST23 | Content (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Flowers | Leaves | Stems | Fruits | Shells | Seeds | Roots | |||
| Hexanal | 799 | 798 | 3.35 | 1.42 | 3.20 | nd | 4.49 | 0.20 | 2.31 |
| (E)-2-Hexenal | 848 | 848 | 0.88 | 2.09 | 0.43 | nd | nd | nd | nd |
| 1-Hexanol | 864 | 864 | 2.69 | 0.28 | nd | 0.19 | 0.93 | 0.04 | 0.08 |
| 1,3-d’Dimethyl-benzene, | 866 | 866 | nd | nd | nd | 1.11 | 0.44 | 10.27 | nd |
| (E,Z)-4-Ethylidenecyclohexene | 876 | 877 | 0.36 | nd | 0.07 | nd | nd | nd | nd |
| 2-Heptanone | 890 | 889 | 0.29 | 0.07 | 0.13 | nd | 0.74 | nd | 0.08 |
| Nonane | 900 | 900 | 0.28 | nd | 0.19 | nd | nd | nd | nd |
| Heptanal | 902 | 901 | 0.26 | 0.17 | nd | nd | 0.50 | nd | 0.28 |
| (E)-2-Heptenal | 954 | 954 | 0.17 | 0.23 | 3.23 | nd | 0.46 | nd | 0.15 |
| Benzaldehyde | 958 | 958 | 0.28 | 0.41 | 0.99 | nd | 0.41 | nd | 1.03 |
| 1-Heptanol | 968 | 968 | 0.05 | nd | nd | nd | 0.14 | nd | nd |
| 1-Octen-3-ol | 978 | 978 | 0.14 | nd | 1.65 | 0.21 | 0.46 | 0.19 | 0.22 |
| 2,3-Octanedione | 983 | 983 | 0.26 | nd | 0.34 | nd | nd | nd | 0.14 |
| 6-Methyl-5-hepten-2-one | 986 | 986 | 0.11 | nd | 0.10 | nd | nd | nd | 0.09 |
| 2-Pentylfuran | 991 | 991 | 2.90 | 0.76 | 1.37 | nd | 1.20 | nd | 2.89 |
| (Z)-2-(2-Pentenyl)furan | 1001 | 1001 | 0.18 | nd | 0.21 | nd | nd | nd | 0.20 |
| Octanal | 1003 | 1002 | 0.37 | 0.53 | 0.30 | nd | nd | nd | 0.26 |
| (E,E)-2,4-Heptadienal, | 1010 | 1010 | 0.17 | 0.34 | 0.74 | nd | nd | nd | nd |
| α-Terpinene | 1016 | 1016 | 0.40 | nd | nd | nd | nd | nd | nd |
| p-Cymene | 1023 | 1023 | 0.12 | nd | nd | nd | nd | nd | nd |
| 2-Ethyl-1-hexanol | 1028 | 1027 | nd | nd | nd | nd | nd | nd | 0.49 |
| β-Phellandrene | 1028 | 1028 | 0.67 | nd | nd | nd | nd | nd | nd |
| 3-Octen-2-one | 1038 | 1038 | 0.13 | nd | 1.22 | nd | nd | nd | 0.09 |
| Benzeneacetaldehyde | 1042 | 1042 | 0.65 | 1.27 | 0.98 | 0.39 | 2.45 | 0.31 | 1.43 |
| (E)-2-Octenal | 1057 | 1056 | 0.80 | nd | 2.11 | nd | 0.62 | nd | 1.04 |
| γ-Terpinene | 1057 | 1057 | 0.47 | nd | nd | nd | nd | nd | nd |
| Acetophenone | 1065 | 1064 | nd | nd | nd | nd | 0.12 | nd | 0.10 |
| (Z)-2-Octen-1-ol | 1066 | 1067 | nd | nd | 0.51 | nd | nd | nd | nd |
| 1-Octanol | 1069 | 1069 | 2.89 | 2.02 | nd | 2.53 | 0.88 | 2.26 | 0.37 |
| (E)-β-Terpinolene | 1087 | 1087 | 0.16 | nd | nd | nd | nd | nd | nd |
| 3,5-Octadien-2-one | 1092 | 1093 | 0.25 | nd | 0.49 | nd | nd | nd | 0.12 |
| Linalool | 1098 | 1098 | 0.34 | 0.50 | 0.28 | nd | 0.58 | 0.29 | nd |
| Nonanal | 1103 | 1102 | 2.17 | 1.25 | 1.49 | 1.96 | 1.18 | 1.78 | 1.38 |
| (Z)-2-p-Menthen-1-ol | 1119 | 1120 | 3.62 | 1.43 | 3.82 | 3.28 | 1.98 | 2.70 | nd |
| (E)-p-2-Menthen-1-ol | 1138 | 1138 | 2.07 | 0.81 | 2.14 | 2.36 | 1.32 | 1.91 | nd |
| (E)-Verbenol | 1143 | 1143 | 0.20 | nd | nd | nd | nd | nd | nd |
| (R,S)-5-Ethyl-6-methyl-3E-hepten-2-one | 1145 | 1145 | nd | nd | 0.41 | nd | nd | nd | 0.19 |
| (E,Z)-2,6-Nonadienal | 1152 | 1152 | 0.27 | 0.35 | 0.47 | nd | nd | nd | 0.18 |
| (E)-2-Nonenal | 1158 | 1158 | 0.94 | 0.44 | 0.92 | 0.46 | 0.57 | 0.42 | 0.95 |
| α-Phellandren-8-ol | 1165 | 1165 | 0.08 | 0.03 | nd | 0.03 | 0.09 | nd | 0.05 |
| 1-Nonanol | 1170 | 1170 | nd | 0.15 | 0.56 | 0.32 | 0.70 | 0.29 | 0.22 |
| L-α-Terpineol | 1189 | 1189 | 0.39 | nd | nd | nd | nd | nd | nd |
| (-)-Myrtenol | 1195 | 1195 | 1.94 | 0.67 | 2.12 | 1.73 | 0.97 | 1.44 | 25.72 |
| Decanal | 1204 | 1204 | 1.06 | 1.16 | 2.21 | 1.34 | nd | 1.03 | 1.89 |
| (E)-Piperitol | 1206 | 1206 | 1.21 | nd | 1.05 | 1.63 | 1.39 | 1.31 | nd |
| (E,E)-2,4-Nonadienal | 1212 | 1212 | nd | nd | 0.74 | 0.25 | nd | 0.23 | 0.39 |
| Bicyclo [3.3.0]octan-2-one, 7-methylene-6(or 8)-methyl- | 1220 | 1220 | nd | nd | nd | nd | nd | nd | 2.20 |
| Carvone | 1244 | 1243 | nd | nd | nd | nd | nd | nd | 1.47 |
| p-Mentha-1(7),8(10)-dien-9-ol | 1245 | 1246 | nd | nd | nd | nd | nd | nd | 1.19 |
| Geraniol | 1253 | 1254 | nd | nd | nd | nd | nd | nd | 1.01 |
| (E)-Myrtanol | 1258 | 1258 | 0.70 | nd | 0.47 | 0.54 | 0.34 | 0.43 | 16.44 |
| 1-Decanol | 1270 | 1271 | 0.46 | 0.19 | nd | 0.64 | 0.88 | 0.53 | nd |
| Nonanoic acid | 1271 | 1272 | 1.04 | nd | nd | nd | nd | nd | nd |
| (E)-Bornyl acetate | 1285 | 1285 | nd | nd | nd | nd | 0.73 | nd | nd |
| Thymol | 1289 | 1290 | nd | nd | nd | nd | nd | nd | 0.55 |
| (E,Z)-2,4-Decadienal | 1292 | 1292 | 0.77 | nd | 1.61 | 0.87 | nd | 0.71 | 0.32 |
| (E)-Undec-4-enal | 1298 | 1296 | nd | nd | 0.33 | nd | nd | nd | nd |
| Undecanal | 1306 | 1305 | 0.19 | 0.25 | 0.33 | 0.43 | nd | 0.39 | 0.32 |
| 2-Methoxy-4-vinylphenol | 1312 | 1312 | 0.41 | 0.51 | nd | nd | 0.53 | nd | nd |
| (E,E)-2,4-Decadienal | 1315 | 1315 | 2.71 | 1.10 | 5.29 | 3.36 | 1.43 | 2.85 | 1.18 |
| Eugenol | 1357 | 1357 | nd | 0.97 | nd | nd | nd | nd | nd |
| Dihydro-5-pentyl-2(3H)-furanone | 1362 | 1362 | nd | nd | nd | nd | nd | nd | 0.25 |
| 2-Undecenal | 1363 | 1363 | nd | nd | 3.89 | 1.26 | 0.83 | 1.12 | nd |
| n-Decanoic acid | 1373 | 1372 | nd | nd | nd | nd | nd | nd | 1.32 |
| 2-Butyl-2-octenal | 1372 | 1373 | 0.34 | nd | nd | 0.32 | nd | 0.26 | nd |
| (E)-β-Damascenone | 1385 | 1385 | 0.27 | nd | nd | 0.58 | 0.72 | 0.45 | nd |
| β-Elemene | 1393 | 1393 | nd | nd | nd | nd | 8.46 | nd | nd |
| 6,10-Dimethyl-2-undecanone, | 1404 | 1404 | nd | nd | 0.25 | nd | nd | nd | nd |
| Dodecanal | 1408 | 1408 | 0.29 | 0.26 | 0.28 | 0.36 | nd | 0.30 | 0.50 |
| 7-epi-α-Cedrene | 1416 | 1417 | nd | nd | nd | nd | nd | nd | 0.56 |
| Copaene | 1421 | 1420 | 1.28 | nd | nd | nd | 2.87 | nd | nd |
| Caryophyllene | 1421 | 1421 | 1.34 | 0.54 | nd | nd | 2.77 | nd | nd |
| (E)-Geranylacetone | 1453 | 1453 | 0.54 | 0.85 | 0.52 | 0.72 | nd | 0.63 | 0.86 |
| Humulene | 1455 | 1456 | nd | nd | nd | nd | 1.52 | nd | nd |
| γ-Decalactone | 1469 | 1469 | 7.09 | 1.50 | 1.04 | 4.62 | 1.66 | 4.23 | nd |
| 1-Dodecanol | 1474 | 1474 | nd | 0.20 | 0.13 | nd | 0.43 | nd | 0.94 |
| (+)-epi-Bicyclosesquiphellandrene | 1484 | 1484 | 0.48 | nd | nd | nd | 15.41 | nd | nd |
| (E)-β-Ionone | 1488 | 1488 | 1.14 | 4.54 | 3.24 | 1.30 | nd | 0.97 | nd |
| β-Selinene | 1488 | 1489 | nd | nd | nd | nd | 0.41 | nd | nd |
| α-(3-Methylbutylidene)- benzeneacetaldehyde | 1492 | 1492 | nd | nd | 0.21 | nd | nd | nd | nd |
| (+)-Cuparene | 1510 | 1508 | nd | nd | nd | nd | nd | nd | 0.29 |
| α-Farnesene | 1510 | 1510 | 1.01 | nd | nd | nd | nd | nd | nd |
| Tridecanal | 1511 | 1511 | nd | 0.53 | 0.34 | 0.53 | nd | 0.44 | 0.29 |
| (Z)-γ-Bisabolene | 1517 | 1518 | 0.57 | nd | nd | nd | nd | nd | nd |
| n-Tridecan-1-ol | 1575 | 1575 | nd | nd | nd | nd | nd | nd | 0.25 |
| Caryophyllene oxide | 1587 | 1587 | 0.89 | nd | nd | nd | nd | nd | nd |
| 2,2,4-Trimethyl-1,3-pentanediol diisobutyrate | 1600 | 1599 | 0.38 | nd | nd | nd | nd | nd | 0.92 |
| Tetradecanal | 1613 | 1613 | 0.24 | 0.76 | 0.54 | 1.03 | 1.55 | 0.88 | 0.50 |
| Benzophenone | 1629 | 1629 | nd | 0.20 | 0.14 | nd | nd | nd | nd |
| τ-Muurolol | 1644 | 1646 | 0.62 | nd | nd | nd | nd | nd | nd |
| α-Cadinol | 1658 | 1658 | 0.41 | nd | nd | nd | nd | nd | nd |
| 1-Tetradecanol | 1677 | 1677 | nd | nd | 0.49 | 1.01 | 1.81 | 0.97 | 1.52 |
| Pentadecanal | 1715 | 1715 | 0.37 | 4.01 | 2.99 | 4.38 | 6.25 | 3.65 | 2.06 |
| 1-Tetradecene | 1736 | 1736 | nd | nd | 0.65 | 0.54 | nd | 0.50 | nd |
| Myristic acid | 2765 | 1764 | nd | nd | nd | nd | nd | nd | 0.85 |
| n-Pentadecanol | 1779 | 1778 | nd | nd | nd | 0.43 | 1.63 | 0.44 | 0.38 |
| Ambrial | 1804 | 1804 | nd | nd | 0.48 | nd | nd | nd | nd |
| Farnesyl acetaldehyde | 1845 | 1845 | nd | nd | 0.25 | nd | nd | nd | nd |
| Hexahydrofarnesyl acetone | 1851 | 1851 | 19.09 | 3.10 | 4.94 | 18.16 | 3.24 | 15.21 | 1.27 |
| Di-2-methylpropyl phthalate | 1878 | 1878 | 1.58 | 0.64 | 0.72 | 0.55 | 0.81 | 0.42 | 2.61 |
| 1-Hexadecanol | 1889 | 1889 | nd | 1.05 | 0.78 | 1.47 | 0.83 | 1.55 | 0.74 |
| Manoyl oxide | 1896 | 1897 | nd | 2.28 | 2.96 | 0.62 | nd | 0.51 | nd |
| Roughanic acid | 1904 | 1904 | nd | nd | 0.70 | nd | nd | nd | nd |
| Farnesyl acetone | 1928 | 1928 | 0.50 | nd | nd | nd | nd | nd | nd |
| Cembrene A | 1969 | 1969 | 0.82 | nd | nd | nd | nd | nd | nd |
| Palmitic acid | 1976 | 1974 | 12.24 | nd | nd | 7.95 | nd | 11.13 | 8.05 |
| 13-epi-Manoyl oxide | 2018 | 2018 | nd | 1.15 | 0.51 | nd | nd | nd | nd |
| Epi-13-Manool | 2061 | 2061 | nd | 1.01 | 0.77 | nd | nd | nd | nd |
| Kolavelool | 2070 | 2070 | nd | 1.67 | 0.97 | nd | nd | nd | nd |
| Isomanool | 2093 | 2094 | 0.39 | 12.07 | 8.29 | 3.23 | nd | 2.48 | nd |
| γ-Palmitolactone | 2103 | 2104 | nd | nd | 0.41 | nd | nd | nd | nd |
| Phytol | 2116 | 2114 | 1.72 | 23.26 | 2.63 | 3.51 | nd | 2.97 | nd |
| Sclareol | 2225 | 2227 | nd | 4.45 | 6.70 | nd | nd | nd | nd |
| Nonadecane | 2300 | 2300 | 0.29 | 0.53 | 0.17 | 1.95 | nd | 1.71 | nd |
| Pentacosane | 2500 | 2500 | nd | 0.99 | 0.64 | 3.34 | nd | 3.10 | nd |
| Bis(2-ethylhexyl) phthalate | 2551 | 2550 | nd | nd | 0.38 | nd | nd | nd | nd |
| Heptacosane | 2700 | 2701 | nd | 1.74 | 2.84 | 5.34 | 2.07 | 4.95 | nd |
| Octacosane | 2800 | 2800 | nd | nd | 0.52 | 1.47 | 1.51 | 1.47 | nd |
| TOTAL | 93.74 | 86.73 | 92.87 | 88.30 | 81.31 | 89.92 | 91.18 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





