Submitted:
10 February 2025
Posted:
11 February 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Menon, U.; Gentry-Maharaj, A.; Hallett, R.; Ryan, A.; Burnell, M.; Sharma, A.; Lewis, S.; Davies, S.; Philpott, S.; Lopes, A.; et al. Sensitivity and specificity of multimodal and ultrasound screening for ovarian cancer, and stage distribution of detected cancers: results of the prevalence screen of the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS). Lancet Oncol 2009, 10, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Ekerhovd, E.; Wienerroith, H.; Staudach, A.; Granberg, S. Preoperative assessment of unilocular adnexal cysts by transvaginal ultrasonography: a comparison between ultrasonographic morphologic imaging and histopathologic diagnosis. Am J Obstet Gynecol 2001, 184, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Einhorn, N.; Sjovall, K.; Knapp, R.C.; Hall, P.; Scully, R.E.; Bast, R.C., Jr.; Zurawski, V.R., Jr. Prospective evaluation of serum CA 125 levels for early detection of ovarian cancer. Obstet Gynecol 1992, 80, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, I.J.; Skates, S.J.; MacDonald, N.; Menon, U.; Rosenthal, A.N.; Davies, A.P.; Woolas, R.; Jeyarajah, A.R.; Sibley, K.; Lowe, D.G.; et al. Screening for ovarian cancer: a pilot randomised controlled trial. Lancet 1999, 353, 1207–1210. [Google Scholar] [CrossRef]
- Collins, W.P.; Bourne, T.H.; Campbell, S. Screening strategies for ovarian cancer. Curr Opin Obstet Gynecol 1998, 10, 33–39. [Google Scholar] [CrossRef]
- Goldstein, S.R.; Subramanyam, B.; Snyder, J.R.; Beller, U.; Raghavendra, B.N.; Beckman, E.M. The postmenopausal cystic adnexal mass: the potential role of ultrasound in conservative management. Obstet Gynecol 1989, 73, 8–10. [Google Scholar] [CrossRef]
- Sassone, A.M.; Timor-Tritsch, I.E.; Artner, A.; Westhoff, C.; Warren, W.B. Transvaginal sonographic characterization of ovarian disease: evaluation of a new scoring system to predict ovarian malignancy. Obstet Gynecol 1991, 78, 70–76. [Google Scholar]
- Ueland, F.R.; DePriest, P.D.; Pavlik, E.J.; Kryscio, R.J.; van Nagell, J.R., Jr. Preoperative differentiation of malignant from benign ovarian tumors: the efficacy of morphology indexing and Doppler flow sonography. Gynecol Oncol 2003, 91, 46–50. [Google Scholar] [CrossRef]
- Granberg, S.; Wikland, M.; Jansson, I. Macroscopic characterization of ovarian tumors and the relation to the histological diagnosis: criteria to be used for ultrasound evaluation. Gynecol Oncol 1989, 35, 139–144. [Google Scholar] [CrossRef]
- Timmerman, D.; Ameye, L.; Fischerova, D.; Epstein, E.; Melis, G.B.; Guerriero, S.; Van Holsbeke, C.; Savelli, L.; Fruscio, R.; Lissoni, A.A.; et al. Simple ultrasound rules to distinguish between benign and malignant adnexal masses before surgery: prospective validation by IOTA group. BMJ 2010, 341, c6839. [Google Scholar] [CrossRef]
- Tan, P.L.; Willatt, J.M.; Lindsell, D. The ability of ultrasound to detect gynaecological neoplasms and their ultrasound morphological features. Australas Radiol 2007, 51, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Kurjak, A.; Prka, M.; Arenas, J.M.; Sparac, V.; Merce, L.T.; Corusic, A.; Ivancic-Kosuta, M. Three-dimensional ultrasonography and power Doppler in ovarian cancer screening of asymptomatic peri- and postmenopausal women. Croat Med J 2005, 46, 757–764. [Google Scholar] [PubMed]
- Timor-Tritsch, I.E.; Goldstein, S.R. The complexity of a "complex mass" and the simplicity of a "simple cyst". J Ultrasound Med 2005, 24, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Van Calster, B.; Timmerman, D.; Valentin, L.; McIndoe, A.; Ghaem-Maghami, S.; Testa, A.C.; Vergote, I.; Bourne, T. Triaging women with ovarian masses for surgery: observational diagnostic study to compare RCOG guidelines with an International Ovarian Tumour Analysis (IOTA) group protocol. BJOG 2012, 119, 662–671. [Google Scholar] [CrossRef]
- Kaijser, J.; Bourne, T.; Valentin, L.; Sayasneh, A.; Van Holsbeke, C.; Vergote, I.; Testa, A.C.; Franchi, D.; Van Calster, B.; Timmerman, D. Improving strategies for diagnosing ovarian cancer: a summary of the International Ovarian Tumor Analysis (IOTA) studies. Ultrasound Obstet Gynecol 2013, 41, 9–20. [Google Scholar] [CrossRef]
- Van Calster, B.; Van Hoorde, K.; Valentin, L.; Testa, A.C.; Fischerova, D.; Van Holsbeke, C.; Savelli, L.; Franchi, D.; Epstein, E.; Kaijser, J.; et al. Evaluating the risk of ovarian cancer before surgery using the ADNEX model to differentiate between benign, borderline, early and advanced stage invasive, and secondary metastatic tumours: prospective multicentre diagnostic study. BMJ 2014, 349, g5920. [Google Scholar] [CrossRef]
- Sayasneh, A.; Ferrara, L.; De Cock, B.; Saso, S.; Al-Memar, M.; Johnson, S.; Kaijser, J.; Carvalho, J.; Husicka, R.; Smith, A.; et al. Evaluating the risk of ovarian cancer before surgery using the ADNEX model: a multicentre external validation study. Br J Cancer 2016, 115, 542–548. [Google Scholar] [CrossRef]
- Meys, E.M.J.; Jeelof, L.S.; Achten, N.M.J.; Slangen, B.F.M.; Lambrechts, S.; Kruitwagen, R.; Van Gorp, T. Estimating risk of malignancy in adnexal masses: external validation of the ADNEX model and comparison with other frequently used ultrasound methods. Ultrasound Obstet Gynecol 2017, 49, 784–792. [Google Scholar] [CrossRef]
- Padilla, L.A.; Radosevich, D.M.; Milad, M.P. Accuracy of the pelvic examination in detecting adnexal masses. Obstet Gynecol 2000, 96, 593–598. [Google Scholar] [CrossRef]
- Hartge, P.; Hayes, R.; Reding, D.; Sherman, M.E.; Prorok, P.; Schiffman, M.; Buys, S. Complex ovarian cysts in postmenopausal women are not associated with ovarian cancer risk factors: preliminary data from the prostate, lung, colon, and ovarian cancer screening trial. Am J Obstet Gynecol 2000, 183, 1232–1237. [Google Scholar] [CrossRef]
- Bailey, C.L.; Ueland, F.R.; Land, G.L.; DePriest, P.D.; Gallion, H.H.; Kryscio, R.J.; van Nagell, J.R., Jr. The malignant potential of small cystic ovarian tumors in women over 50 years of age. Gynecol Oncol 1998, 69, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Curtin, J.P. Management of the adnexal mass. Gynecol Oncol 1994, 55, S42–46. [Google Scholar] [CrossRef] [PubMed]
- Reimer, T.; Gerber, B.; Muller, H.; Jeschke, U.; Krause, A.; Friese, K. Differential diagnosis of peri- and postmenopausal ovarian cysts. Maturitas 1999, 31, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Owens, O.J.; Stewart, C.; Leake, R.E.; McNicol, A.M. A comparison of biochemical and immunohistochemical assessment of EGFR expression in ovarian cancer. Anticancer Res 1992, 12, 1455–1458. [Google Scholar]
- Scambia, G.; Benedetti-Panici, P.; Ferrandina, G.; Distefano, M.; Salerno, G.; Romanini, M.E.; Fagotti, A.; Mancuso, S. Epidermal growth factor, oestrogen and progesterone receptor expression in primary ovarian cancer: correlation with clinical outcome and response to chemotherapy. Br J Cancer 1995, 72, 361–366. [Google Scholar] [CrossRef]
- Koonings, P.P.; Campbell, K.; Mishell, D.R., Jr.; Grimes, D.A. Relative frequency of primary ovarian neoplasms: a 10-year review. Obstet Gynecol 1989, 74, 921–926. [Google Scholar] [CrossRef]
- Modesitt, S.C.; Pavlik, E.J.; Ueland, F.R.; DePriest, P.D.; Kryscio, R.J.; van Nagell, J.R., Jr. Risk of malignancy in unilocular ovarian cystic tumors less than 10 centimeters in diameter. Obstet Gynecol 2003, 102, 594–599. [Google Scholar] [CrossRef]
- Osmers, R.G.; Osmers, M.; von Maydell, B.; Wagner, B.; Kuhn, W. Evaluation of ovarian tumors in postmenopausal women by transvaginal sonography. Eur J Obstet Gynecol Reprod Biol 1998, 77, 81–88. [Google Scholar] [CrossRef]
- Auslender, R.; Atlas, I.; Lissak, A.; Bornstein, J.; Atad, J.; Abramovici, H. Follow-up of small, postmenopausal ovarian cysts using vaginal ultrasound and CA-125 antigen. J Clin Ultrasound 1996, 24, 175–178. [Google Scholar] [CrossRef]
- Di Legge, A.; Testa, A.C.; Ameye, L.; Van Calster, B.; Lissoni, A.A.; Leone, F.P.; Savelli, L.; Franchi, D.; Czekierdowski, A.; Trio, D.; et al. Lesion size affects diagnostic performance of IOTA logistic regression models, IOTA simple rules and risk of malignancy index in discriminating between benign and malignant adnexal masses. Ultrasound Obstet Gynecol 2012, 40, 345–354. [Google Scholar] [CrossRef]
- Landolfo, C.; Bourne, T.; Froyman, W.; Van Calster, B.; Ceusters, J.; Testa, A.C.; Wynants, L.; Sladkevicius, P.; Van Holsbeke, C.; Domali, E.; et al. Benign descriptors and ADNEX in two-step strategy to estimate risk of malignancy in ovarian tumors: retrospective validation in IOTA5 multicenter cohort. Ultrasound Obstet Gynecol 2023, 61, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Osmers, R.G.; Osmers, M.; von Maydell, B.; Wagner, B.; Kuhn, W. Preoperative evaluation of ovarian tumors in the premenopause by transvaginosonography. Am J Obstet Gynecol 1996, 175, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.N.; Tan, X.; Xiong, W.; Dong, X.; Liu, J.; Wang, Z.L.; Chen, H.X. Natural history and malignant potential of simple ovarian cysts in postmenopausal women: a systematic review and meta-analysis. Menopause 2023. [Google Scholar] [CrossRef] [PubMed]
- Bast, R.C., Jr.; Klug, T.L.; St John, E.; Jenison, E.; Niloff, J.M.; Lazarus, H.; Berkowitz, R.S.; Leavitt, T.; Griffiths, C.T.; Parker, L.; et al. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N Engl J Med 1983, 309, 883–887. [Google Scholar] [CrossRef]
- Jacobs, I.; Bast, R.C., Jr. The CA 125 tumour-associated antigen: a review of the literature. Hum Reprod 1989, 4, 1–12. [Google Scholar] [CrossRef]
- Jacobs, I.; Oram, D.; Fairbanks, J.; Turner, J.; Frost, C.; Grudzinskas, J.G. A risk of malignancy index incorporating CA 125, ultrasound and menopausal status for the accurate preoperative diagnosis of ovarian cancer. Br J Obstet Gynaecol 1990, 97, 922–929. [Google Scholar] [CrossRef]
- Conway, C.; Zalud, I.; Dilena, M.; Maulik, D.; Schulman, H.; Haley, J.; Simonelli, K. Simple cyst in the postmenopausal patient: detection and management. J Ultrasound Med 1998, 17, 369–372. [Google Scholar] [CrossRef]
- Timmerman, D.; Moerman, P.; Vergote, I. Meigs' syndrome with elevated serum CA 125 levels: two case reports and review of the literature. Gynecol Oncol 1995, 59, 405–408. [Google Scholar] [CrossRef]
- Kaijser, J.; Van Gorp, T.; Smet, M.E.; Van Holsbeke, C.; Sayasneh, A.; Epstein, E.; Bourne, T.; Vergote, I.; Van Calster, B.; Timmerman, D. Are serum HE4 or ROMA scores useful to experienced examiners for improving characterization of adnexal masses after transvaginal ultrasonography? Ultrasound Obstet Gynecol 2014, 43, 89–97. [Google Scholar] [CrossRef]
- Montagnana, M.; Danese, E.; Ruzzenente, O.; Bresciani, V.; Nuzzo, T.; Gelati, M.; Salvagno, G.L.; Franchi, M.; Lippi, G.; Guidi, G.C. The ROMA (Risk of Ovarian Malignancy Algorithm) for estimating the risk of epithelial ovarian cancer in women presenting with pelvic mass: is it really useful? Clin Chem Lab Med 2011, 49, 521–525. [Google Scholar] [CrossRef]
- Kaijser, J.; Van Gorp, T.; Van Hoorde, K.; Van Holsbeke, C.; Sayasneh, A.; Vergote, I.; Bourne, T.; Timmerman, D.; Van Calster, B. A comparison between an ultrasound based prediction model (LR2) and the risk of ovarian malignancy algorithm (ROMA) to assess the risk of malignancy in women with an adnexal mass. Gynecol Oncol 2013, 129, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Valentin, L.; Jurkovic, D.; Van Calster, B.; Testa, A.; Van Holsbeke, C.; Bourne, T.; Vergote, I.; Van Huffel, S.; Timmerman, D. Adding a single CA 125 measurement to ultrasound imaging performed by an experienced examiner does not improve preoperative discrimination between benign and malignant adnexal masses. Ultrasound Obstet Gynecol 2009, 34, 345–354. [Google Scholar] [CrossRef] [PubMed]
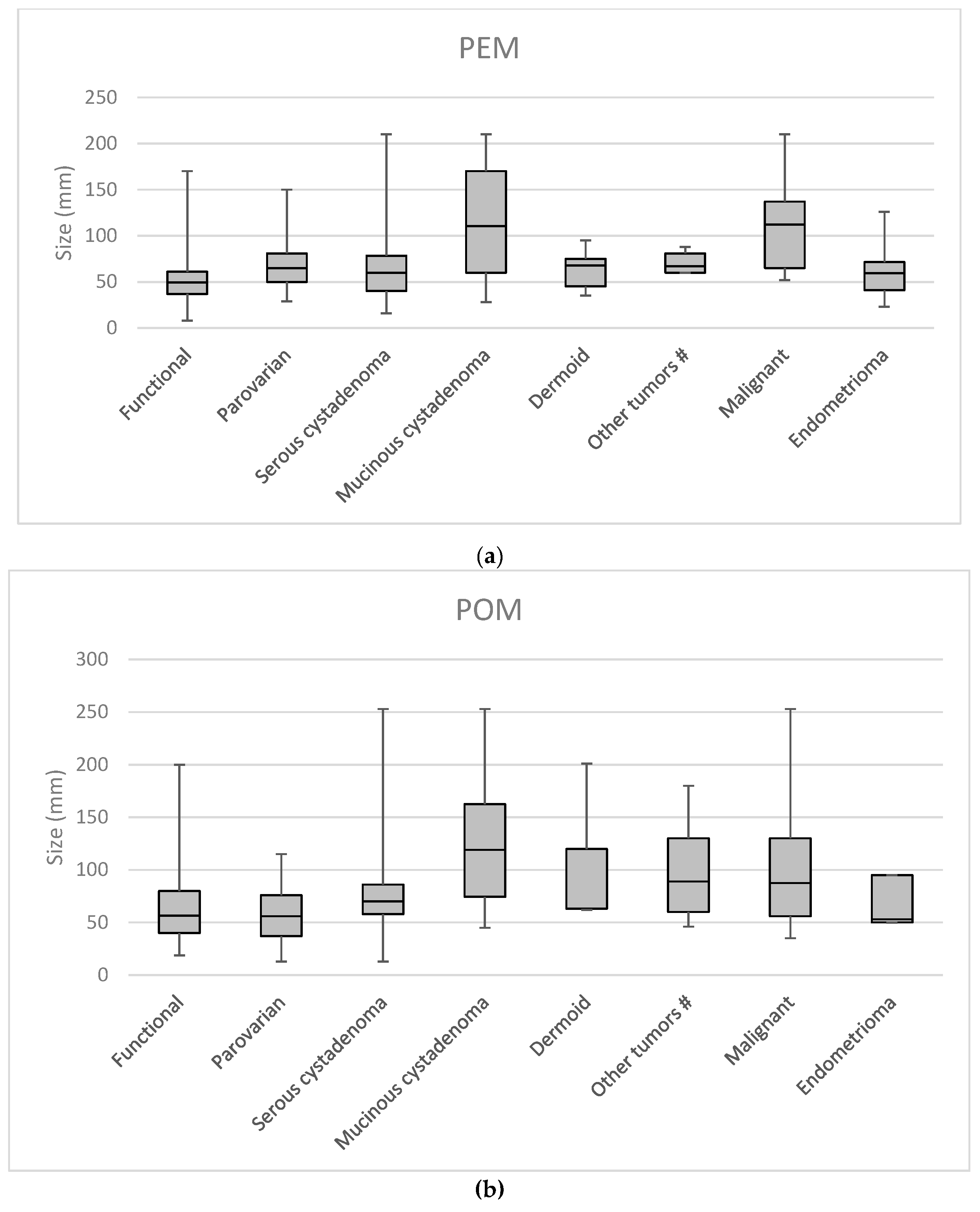
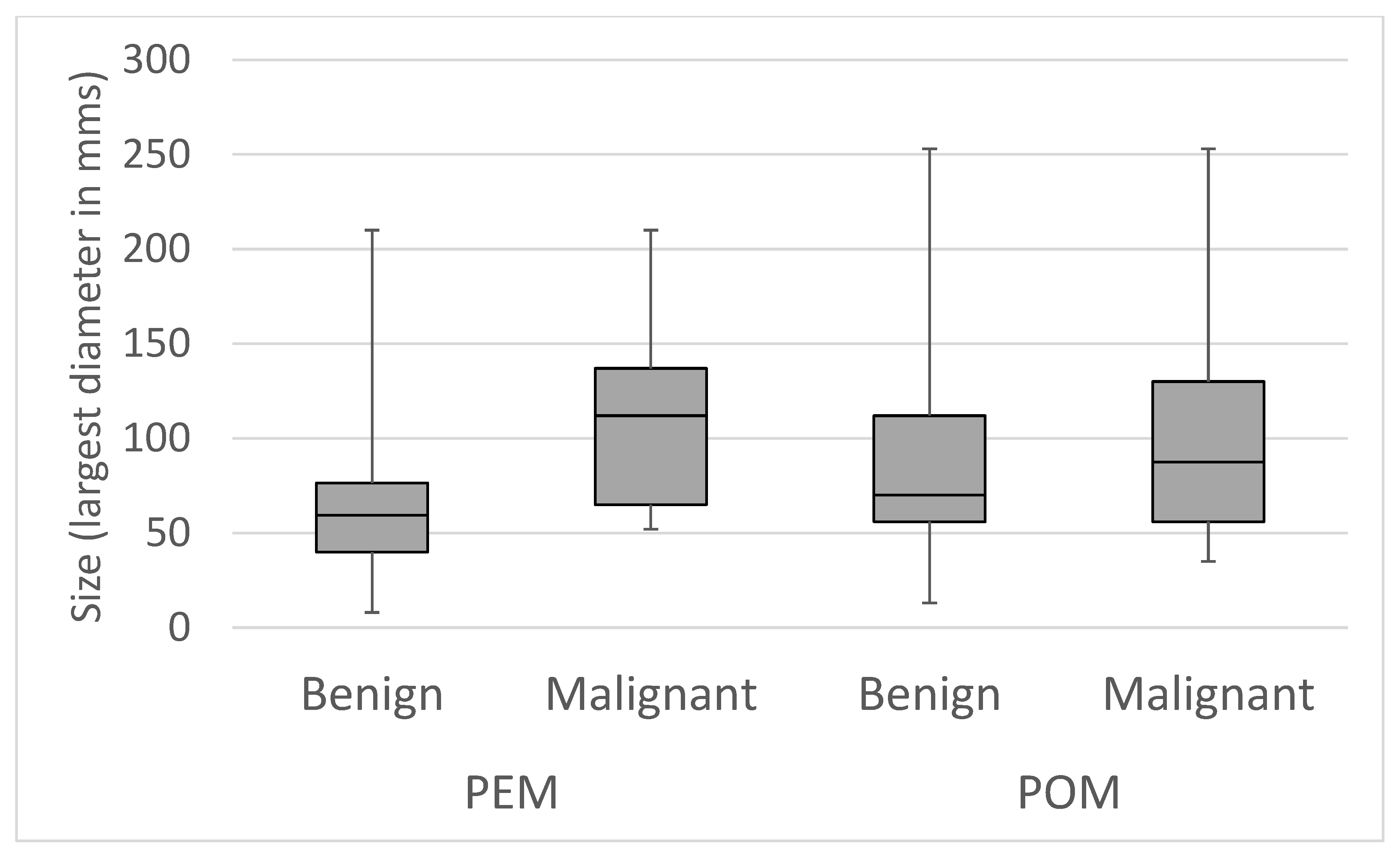
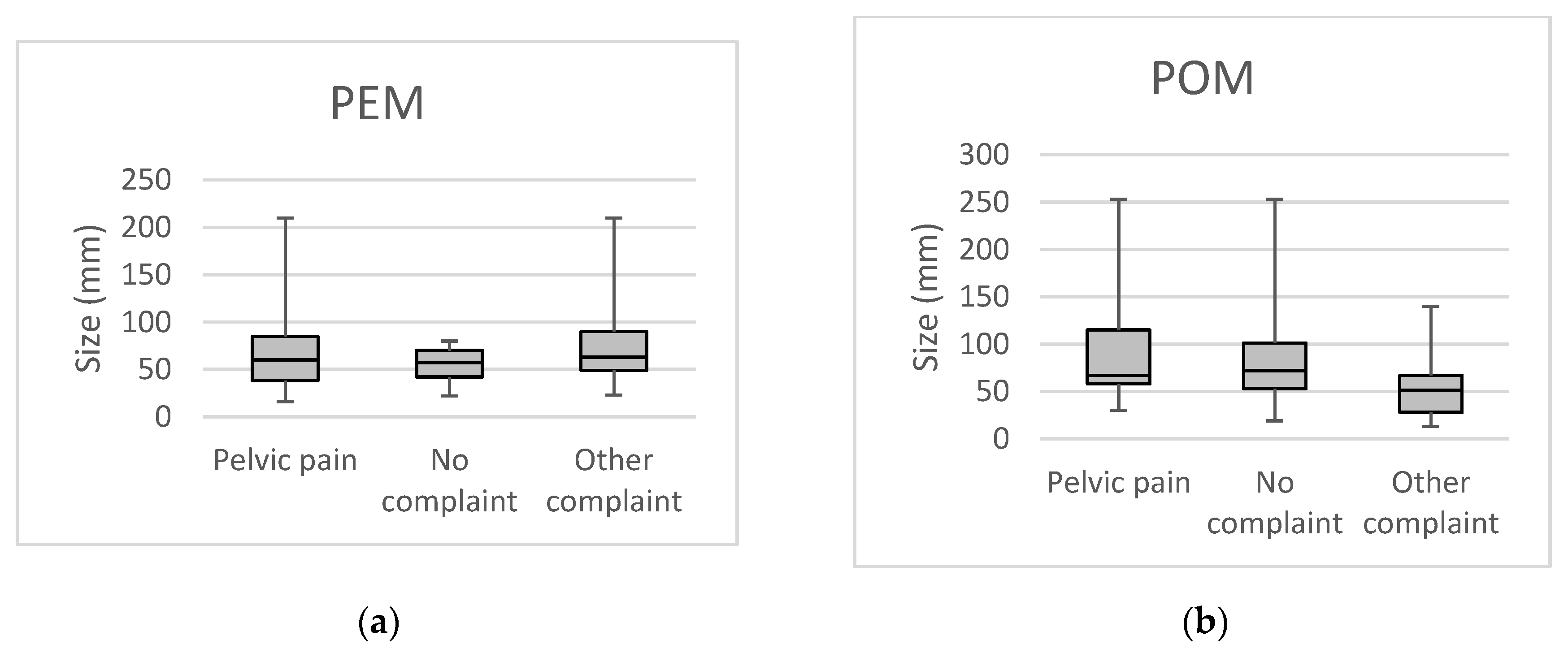
| PEM simplex | PEM complex | POM simplex | POM complex | |||||
|---|---|---|---|---|---|---|---|---|
| <5cm | ≥5cm | <5cm | ≥5cm | <5cm | ≥5cm | <5cm | ≥5cm | |
| Functional cysts | ||||||||
| Corp. lut. cyst. hem.* | 8 (25.00%) |
5 (12.20%) |
6 (20.69%) |
12 (12.90%) |
2 (13.33%) |
5 (4.17%) |
||
| Follicular cyst | 5 (15.63%) |
6 (14.63%) |
6 (20.69%) |
2 (2.15%) | 1 (11.11%) |
2 (1.67%) |
||
| Parovarian cysts | 1 (3.13%) |
11 (26.83%) |
3 (10.34%) |
3 (3.23%) |
2 (22.22%) |
5 (12.50%) |
3 (20.00%) |
5 (4.17%) |
| Benign | ||||||||
| Cystadenoma serosum | 16 (50.00%) |
16 (39.02%) |
3 (10.34%) |
21 (22.58%) |
5 (55.56%) |
27 (67.50%) |
4 (26.67%) |
34 (28.33%) |
| Cystadenoma mucinosum | 1 (3.13%) |
2 (4.88%) |
1 (3.45%) |
10 (10.75%) |
4 (10.00%) |
2 (13.33%) |
18 (15.00%) |
|
| Dermoid | 4 (13.79%) |
10 (10.75%) |
5 (4.17%) |
|||||
| Endometriosis | 1 (3.13%) |
1 (2.44%) |
6 (20.69%) |
12 (12.90%) |
3 (2.50%) |
|||
| Fibroid | 3 (3.23%) |
2 (5.00%) |
1 (6.67%) |
7 (5.83%) |
||||
| Hydrosalpinx | 1 (11.11%) |
1 (2.50%) |
2 (1.67%) |
|||||
| Struma ovarii | 1 (1.08%) |
1 (0.83%) |
||||||
| Brenner tu. | 3 (3.23%) |
2 (1.67%) |
||||||
| Malignant | 16 (17.20%) |
1 (2.50%) |
3 (20.00%) |
36 (30.00%) |
||||
| Risk factors | PEM simplex | PEM complex | POM simplex | POM complex | Total (% of total cases) |
||||
|---|---|---|---|---|---|---|---|---|---|
| <5cm | ≥5cm | <5cm | ≥5cm | <5cm | ≥5cm | <5cm | ≥5cm | ||
| Parity | |||||||||
| Nulliparous | 2 (6%) |
10 (24%) |
6 (21%) |
14 (15%) |
1 (11%) |
1 (3%) |
3 (20%) |
20 (17%) |
57 (15.04%) |
| 1-2 children | 20 (63%) |
23 (56%) |
19 (66%) |
65 (70%) |
6 (67%) |
31 (78%) |
8 (53%) |
78 (65%) |
250 (65.96%) |
| 3-4 children | 10 (31%) |
8 (20%) |
3 (10%) |
13 (14%) |
2 (22%) |
7 (18%) |
4 (27%) |
20 (17%) |
67 (17.68%) |
| ≥5 children | 1 (3%) |
1 (1%) |
1 (3%) |
2 (2%) |
5 (1.32%) |
||||
| Family history of ovarian cancer | 2 (6%) |
1 (3%) |
1 (1%) |
4 (1.06%) |
|||||
| Previous pelvic surgery | |||||||||
| Hysterectomy | 5 (16%) |
4 (10%) |
3 (10%) |
17 (18%) |
3 (33%) |
2 (5%) |
4 (27%) |
33 (28%) |
71 (18.73%) |
| Adnexectomy | 1 (3%) |
1 (2%) |
1 (3%) |
8 (9%) |
1 (7%) |
2 (2%) |
14 (3.69%) |
||
| Laparotomy | 1 (3%) |
4 (10%) |
1 (3%) |
10 (11%) |
1 (3%) |
5 (4%) |
22 (5.80%) |
||
| Punction | 3 (7%) |
1 (3%) |
1 (1%) |
1 (3%) |
1 (7%) |
7 (1.85%) |
|||
| No previous surgery | 25 (78%) |
29 (71%) |
23 (79%) |
57 (61%) |
6 (67%) |
36 (90%) |
9 (60%) |
80 (67%) |
265 (69.92%) |
| Total (n) | 32 | 41 | 29 | 93 | 9 | 40 | 15 | 120 | 379 (100%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





