Submitted:
24 February 2025
Posted:
25 February 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
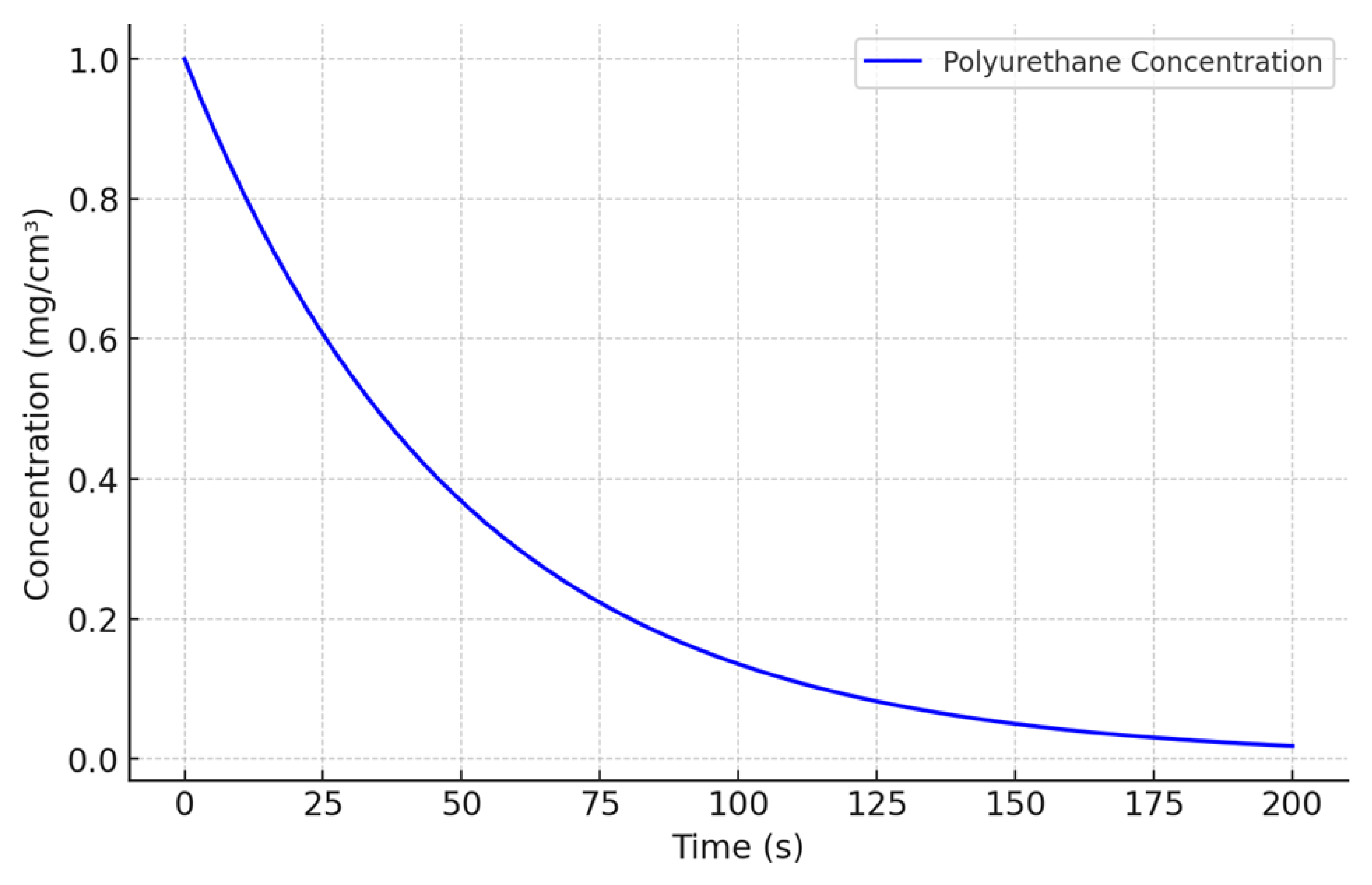
3.1. Assumptions and Basic Equation
- M(t) represents the remaining mass of DNA/RNA within the PU matrix at time t.
- krel is the release rate constant, which depends on environmental factors such as pH and temperature.
- C(t) represents the concentration of PU at time t.
- C0 is the initial concentration of PU.
- k is the degradation rate constant, which depends on the specific biological conditions.
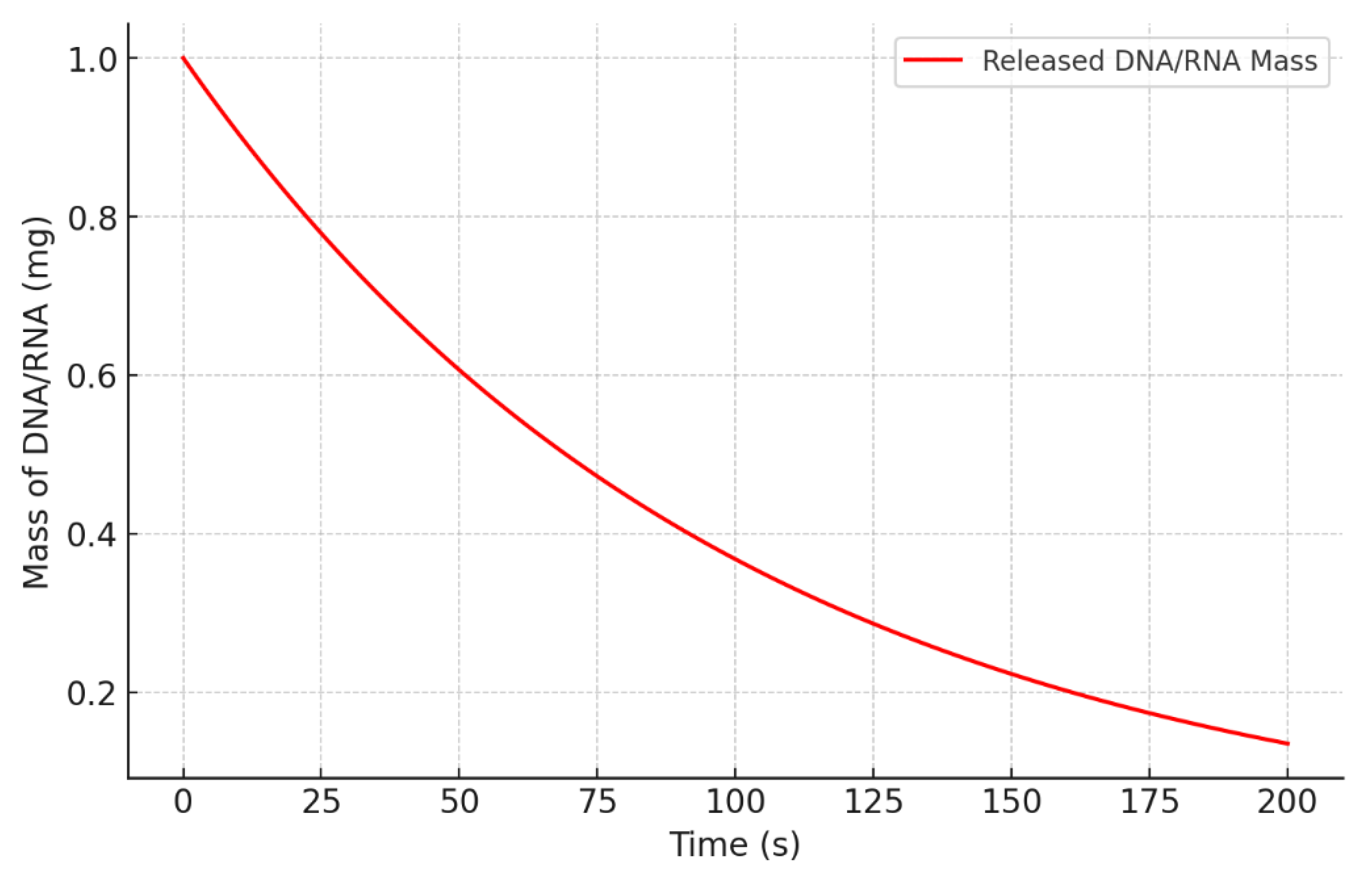
3.2. Higuchi Model for Diffusion
- Mt is the mass of DNA/RNA released at time t.
- M0 is the initial mass of encapsulated DNA/RNA.
- D is the diffusion coefficient, which characterizes the diffusion rate through the PU matrix.
- L is the length of the PU matrix.
3.3. Modeling the Interaction of PU Vectors with Cellular Membranes
3.3.1. Assumptions
- The electric charge of the PU.
- The type of cellular receptors present on the membrane.
- The dynamics of the lipid bilayer of the cellular membrane.
3.3.2. Modeling PU Adsorption on the Cellular Membrane
- θ represents the fraction of the membrane surface covered by PU nanoparticles.
- K is the adsorption constant, which depends on the nanoparticle and membrane affinity.
- C is the concentration of nanoparticles in the extracellular environment.
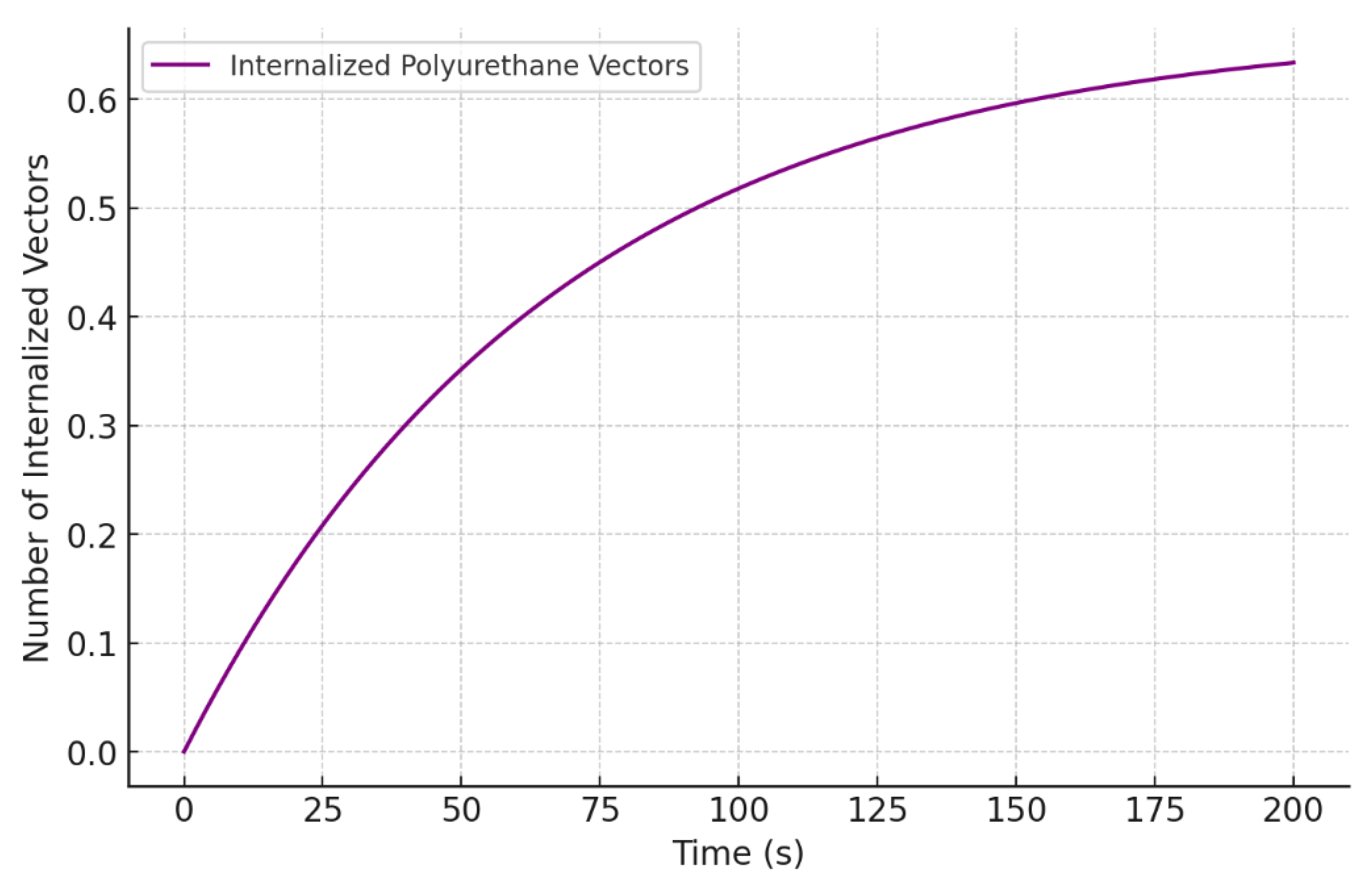
3.3.3. Modeling Internalization via Endocytosis
- N(t) is the number of internalized PU vectors at time t.
- is the rate of internalization, which depends on the efficiency of endocytosis.
- is the maximum number of available binding sites on the cellular membrane.
- is the rate of degradation of the PU vectors within the cell.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AuNPs | gold nanoparticles |
| CPPs | cell-penetrating peptides |
| DNA | deoxyribonucleic acid |
| FDA | U.S. Food and Drug Administration |
| MDPI | Multidisciplinary Digital Publishing Institute |
| MNPs | magnetic nanoparticles |
| PEI | polyethylenimine |
| PLL | poly(L-lysine) |
| PU | polyurethane |
| RNA | ribonucleic acid |
| VEGF | vascular endothelial growth factor |
References
- Roden, D.M.; Wilke, R.A.; Kroemer, H.K.; Stein, C.M. Pharmacogenomics: The Genetics of Variable Drug Responses. Circulation 2011, 123, 1661–1670. [CrossRef]
- Strianese, O.; Rizzo, F.; Ciccarelli, M.; Galasso, G.; D’Agostino, Y.; Salvati, A.; Del Giudice, C.; Tesorio, P.; Rusciano, M.R. Precision and Personalized Medicine: How Genomic Approach Improves the Management of Cardiovascular and Neurodegenerative Disease. Genes 2020, 11, 747. [CrossRef]
- Hippman, C.; Nislow, C. Pharmacogenomic Testing: Clinical Evidence and Implementation Challenges. JPM 2019, 9, 40. [CrossRef]
- Bishop, J.R.; Ellingrod, V.L. Precision Pharmacotherapy Enables Precision Medicine. Pharmacotherapy 2017, 37, 985–987. [CrossRef]
- Lesko, L.J.; Schmidt, S. Clinical Implementation of Genetic Testing in Medicine: A US Regulatory Science Perspective. Brit J Clinical Pharma 2014, 77, 606–611. [CrossRef]
- Gottlieb, A.; Stein, G.Y.; Ruppin, E.; Sharan, R. PREDICT: A Method for Inferring Novel Drug Indications with Application to Personalized Medicine. Molecular Systems Biology 2011, 7, 496. [CrossRef]
- Stegemann, S.; Ternik, R.L.; Onder, G.; Khan, M.A.; Van Riet-Nales, D.A. Defining Patient Centric Pharmaceutical Drug Product Design. AAPS J 2016, 18, 1047–1055. [CrossRef]
- Sussman, C.; Liberatore, R.A.; Drozdz, M.M. Delivery of DNA-Based Therapeutics for Treatment of Chronic Diseases. Pharmaceutics 2024, 16, 535. [CrossRef]
- Verma, A.; Awasthi, A. Developing Non-Viral or Viral Vectors for Efficient and Targeted Delivery of Genetic Material, Such as DNA or RNA, for Gene Therapy Applications. Pharmaspire 2023, 15, 243–256. [CrossRef]
- Donkuru, M.; Badea, I.; Wettig, S.; Verrall, R.; Elsabahy, M.; Foldvari, M. Advancing Nonviral Gene Delivery: Lipid- and Surfactant-Based Nanoparticle Design Strategies. Nanomedicine 2010, 5, 1103–1127. [CrossRef]
- Nugroho, R.A.; Saputra, O.A.; Pradita, A.L.; Fajar, A.B.N.; Wibowo, F.R. Lignin-based Polyurethane Composites Enhanced with Hydroxyapatite for Controlled Drug Delivery and Potential Cellular Scaffold Applications. Polymer Composites 2024, 45, 15159–15172. [CrossRef]
- Shi, B.; Zheng, M.; Tao, W.; Chung, R.; Jin, D.; Ghaffari, D.; Farokhzad, O.C. Challenges in DNA Delivery and Recent Advances in Multifunctional Polymeric DNA Delivery Systems. Biomacromolecules 2017, 18, 2231–2246. [CrossRef]
- Ding, M.; Li, J.; Tan, H.; Fu, Q. Self-Assembly of Biodegradable Polyurethanes for Controlled Delivery Applications. Soft Matter 2012, 8, 5414. [CrossRef]
- Cheng, J.; Tang, X.; Zhao, J.; Shi, T.; Zhao, P.; Lin, C. Multifunctional Cationic Polyurethanes Designed for Non-Viral Cancer Gene Therapy. Acta Biomaterialia 2016, 30, 155–167. [CrossRef]
- Parlea, L.; Puri, A.; Kasprzak, W.; Bindewald, E.; Zakrevsky, P.; Satterwhite, E.; Joseph, K.; Afonin, K.A.; Shapiro, B.A. Cellular Delivery of RNA Nanoparticles. ACS Comb. Sci. 2016, 18, 527–547. [CrossRef]
- Song, W.; Muhammad, S.; Dang, S.; Ou, X.; Fang, X.; Zhang, Y.; Huang, L.; Guo, B.; Du, X. The State-of-Art Polyurethane Nanoparticles for Drug Delivery Applications. Front. Chem. 2024, 12, 1378324. [CrossRef]
- Zhou, L.; Yu, L.; Ding, M.; Li, J.; Tan, H.; Wang, Z.; Fu, Q. Synthesis and Characterization of pH-Sensitive Biodegradable Polyurethane for Potential Drug Delivery Applications. Macromolecules 2011, 44, 857–864. [CrossRef]
- Gaur, N.; Dharwadkar, R.; Thomas, J. Personalized Therapy Using Deep Learning Advances. In Deep Learning for Targeted Treatments; Malviya, R., Ghinea, G., Dhanaraj, R.K., Balusamy, B., Sundram, S., Eds.; Wiley, 2022; pp. 171–197 ISBN 978-1-119-85732-7. [CrossRef]
- Yang, T.; Chin, W.; Cherng, J.; Shau, M. Synthesis of Novel Biodegradable Cationic Polymer: N , N -Diethylethylenediamine Polyurethane as a Gene Carrier. Biomacromolecules 2004, 5, 1926–1932. [CrossRef]
- Park, S.-Y.; Yun, Y.H.; Park, B.-J.; Seo, H.-I.; Chung, I. Fabrication and Biological Activities of Plasmid DNA Gene Carrier Nanoparticles Based on Biodegradable L-Tyrosine Polyurethane. Pharmaceuticals 2021, 15, 17. [CrossRef]
- Woo, J.; Bae, S.-H.; Kim, B.; Park, J.S.; Jung, S.; Lee, M.; Kim, Y.-H.; Choi, D. Cardiac Usage of Reducible Poly(Oligo-D-Arginine) As a Gene Carrier for Vascular Endothelial Growth Factor Expression. PLoS ONE 2015, 10, e0144491. [CrossRef]
- Jones, C.H.; Chen, C.-K.; Ravikrishnan, A.; Rane, S.; Pfeifer, B.A. Overcoming Nonviral Gene Delivery Barriers: Perspective and Future. Mol. Pharmaceutics 2013, 10, 4082–4098. [CrossRef]
- Dunbar, C.E.; High, K.A.; Joung, J.K.; Kohn, D.B.; Ozawa, K.; Sadelain, M. Gene Therapy Comes of Age. Science 2018, 359, eaan4672. [CrossRef]
- Pabari, R.M.; Tambuwala, M.M.; Lajczak-McGinley, N.; Aljabali, A.; Kirby, B.P.; Keely, S.; Ramtoola, Z. Novel Polyurethane Based Particulate Formulations of Infliximab Reduce Inflammation in DSS Induced Murine Model of Colitis – A Preliminary Study. International Journal of Pharmaceutics 2021, 604, 120717. [CrossRef]
- Zhang, M.; Hemp, S.T.; Zhang, M.; Allen, M.H.; Carmean, R.N.; Moore, R.B.; Long, T.E. Water-Dispersible Cationic Polyurethanes Containing Pendant Trialkylphosphoniums. Polym. Chem. 2014, 5, 3795–3803. [CrossRef]
- Omrani, I.; Babanejad, N.; Shendi, H.K.; Nabid, M.R. Preparation and Evaluation of a Novel Sunflower Oil-based Waterborne Polyurethane Nanoparticles for Sustained Delivery of Hydrophobic Drug. Euro J Lipid Sci & Tech 2017, 119, 1600283. [CrossRef]
- Shoaib, M.; Bahadur, A.; Saeed, A.; Rahman, M.S.U.; Naseer, M.M. Biocompatible, pH-Responsive, and Biodegradable Polyurethanes as Smart Anti-Cancer Drug Delivery Carriers. Reactive and Functional Polymers 2018, 127, 153–160. [CrossRef]
- Joseph, T.; Kar Mahapatra, D.; Esmaeili, A.; Piszczyk, Ł.; Hasanin, M.; Kattali, M.; Haponiuk, J.; Thomas, S. Nanoparticles: Taking a Unique Position in Medicine. Nanomaterials 2023, 13, 574. [CrossRef]
- Bouchemal, K.; Briançon, S.; Perrier, E.; Fessi, H.; Bonnet, I.; Zydowicz, N. Synthesis and Characterization of Polyurethane and Poly(Ether Urethane) Nanocapsules Using a New Technique of Interfacial Polycondensation Combined to Spontaneous Emulsification. International Journal of Pharmaceutics 2004, 269, 89–100. [CrossRef]
- Bouchemal, K.; Briançon, S.; Couenne, F.; Fessi, H.; Tayakout, M. Stability Studies on Colloidal Suspensions of Polyurethane Nanocapsules. j nanosci nanotechnol 2006, 6, 3187–3192. [CrossRef]
- Fiorio, R.; Pistor, V.; Zattera, A.J.; Petzhold, C.L. Influence of Synthesis Temperature on Thermal Properties of Thermoplastic Polyurethane Prepared by Torque Rheometer. Polymer Engineering & Sci 2012, 52, 1678–1684. [CrossRef]
- Mouren, A.; Avérous, L. Sustainable Cycloaliphatic Polyurethanes: From Synthesis to Applications. Chem. Soc. Rev. 2023, 52, 277–317. [CrossRef]
- Thang, N.H.; Chien, T.B.; Cuong, D.X. Polymer-Based Hydrogels Applied in Drug Delivery: An Overview. Gels 2023, 9, 523. [CrossRef]
- Subjakova, V.; Oravczova, V.; Hianik, T. Polymer Nanoparticles and Nanomotors Modified by DNA/RNA Aptamers and Antibodies in Targeted Therapy of Cancer. Polymers 2021, 13, 341. [CrossRef]
- Pannier, A.K.; Shea, L.D. Controlled Release Systems for DNA Delivery. Molecular Therapy 2004, 10, 19–26. [CrossRef]
- Song, N.; Ding, M.; Pan, Z.; Li, J.; Zhou, L.; Tan, H.; Fu, Q. Construction of Targeting-Clickable and Tumor-Cleavable Polyurethane Nanomicelles for Multifunctional Intracellular Drug Delivery. Biomacromolecules 2013, 14, 4407–4419. [CrossRef]
- Khosroushahi, A.Y.; Naderi-Manesh, H.; Yeganeh, H.; Barar, J.; Omidi, Y. Novel Water-Soluble Polyurethane Nanomicelles for Cancer Chemotherapy: Physicochemical Characterization and Cellular Activities. J Nanobiotechnol 2012, 10, 2. [CrossRef]
- Lee, S.W.L.; Paoletti, C.; Campisi, M.; Osaki, T.; Adriani, G.; Kamm, R.D.; Mattu, C.; Chiono, V. MicroRNA Delivery through Nanoparticles. Journal of Controlled Release 2019, 313, 80–95. [CrossRef]
- Zhang, L.; Wang, D.; Fischer, H.; Fan, P.; Widdicombe, J.H.; Kan, Y.W.; Dong, J. Efficient Expression of CFTR Function with Adeno-Associated Virus Vectors That Carry Shortened CFTR Genes. Proc. Natl. Acad. Sci. U.S.A. 1998, 95, 10158–10163. [CrossRef]
- Fujii, S.; Yamasaki, S.; Hanada, K.; Ueda, S.; Kawamura, M.; Shimizu, K. Cancer Immunotherapy Using Artificial Adjuvant Vector Cells to Deliver NY-ESO-1 Antigen to Dendritic Cells in Situ. Cancer Science 2022, 113, 864–874. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




