Introduction
Following the isolation of the non-sulfated glycosaminoglycan hyaluronic acid (HA) from the vitreous humor of the bovine eye (with hyaloid meaning vitreous) in 1934, veterinary medicine saw the first HA application in orthopedics, marked by the successful intra-articular HA injection in racehorses for relief of traumatic arthritis [
1,
2]. The 1970s saw the introduction of HA, physiologically synthesized by type B synoviocytes, for treating joint pain in humans [
2]. HA, thanks to its excellent viscoelastic properties at concentrations as low as 0.1%, along with high moisture retention and biocompatibility, and hygroscopic properties, acts as a potent lubricant, shock absorber, joint structure stabilizer, and helps maintain water balance- and flow resistance-regulator [
3]. The foundation of the viscosupplementation concept, developed by Balazs and Denlinger in their seminal 1993 paper, was the hypothesis that injecting exogenous HA intra-articularly into osteoarthritic diarthrodial joints, such as the knee, restores synovial fluid rheology and possibly promotes the synthesis of higher molecular weight, more functional endogenous HA, thereby improving mobility, pain, and joint function [
4].
Limiting HA viscosupplementation to a purely mechanical or rheological concept may be reductive. Chondrocytes, the unique resident cell of joint cartilage, act as mechanosensors and osmosensors and express the networks of proteins and glycoproteins that form the extracellular matrix of joint cartilage [
2,
3]. As osteoarthritis (OA) progresses, chondrocytes lose their ability to maintain cartilage homeostasis as a result of a decline in mitotic and synthetic activity [
5]. Mainly mediated by interaction with the CD44 cell surface receptor (CD44 = cluster of differentiation protein 44) and CD44-mediated signaling, HA is likely to have a role in the survival and apoptotic pathways of chondrocytes [
2,
3]. At least
in vitro, HA has a direct effect on chondrocyte survival by inhibiting nitric oxide-induced apoptosis and dedifferentiation, with a restored expression of aggrecan and Type-II collagen [
6].
A therapeutic strategy for OA that
transcends a purely rheological and symptomatic approach
through a regenerative medicine perspective could involve integrating the strong rheological properties a
long with the beneficial
, though modest
, effects of HA on chondrocyte and cartilage homeostasis with additional ingredients primarily
aimed
at protecting and revitalizing chondrocytes, synoviocytes, and cartilage, all within the same medical device for intra-articular administration. As repeatedly demonstrated in single-agent studies and confirmed in combination with HA, intra-articular natural-origin, trout-derived polynucleotides, also known by the acronym PN HPT™ (Polynucleotides High Purification Technology), contribute further viscosupplementation thanks to their high hydrophilic and viscoelastic properties and reduce pain more effectively and more rapidly than HA [
7,
8,
9,
10,
11]. In that sense, the PN HPT™/HA combination seems ideal. However, the primary contribution of PN HPT™ to the synergistic combination with HA isw to provide nitrogen bases and precursors for nucleosides and nucleotides to chondrocytes and other mesenchymal cells, thus supporting the resilience and vitality of these cells [
7,
8,
9,
10,
11].
The illustrated prospective multicentric six-month multi-center study aimed to confirm the value and efficacy, repeatedly established in methodologically sound studies, of a single intra-articular injection of a proprietary PN HPT™/HA-based medical device in improving the subjective and objective manifestations of knee osteoarthritis [
7,
8,
9,
10,
11].
Materials and Methods
Open-label, real-life clinical data collection in unselected adult patients of both genders with unilateral or bilateral symptomatic knee osteoarthritis. Enrolled patients should have displayed pain, muscle weakness, joint instability, brief morning stiffness, crepitus, and functional limitations for at least two months [
12]. Only based on the investigator’s judgment, the enrolment strategy aimed to mirror the real-life, everyday orthopedic ambulatory practice. After baseline evaluation, all patients received a single unilateral or bilateral 4-mL intra-articular injection of the proprietary PN HPT™/HA combination (CONDROTIDE HA medical device, Mastelli Srl, Sanremo, Italy). Efficacy and safety assessments, performed at baseline before a single PN HPT™/HA injection and after three and six months of follow-up, included the Lequesne index, Numeric Pain Rating Scale (NPRS) focused on pain intensity, the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), overall safety, and treatment-emergent adverse events.
Primary Endpoints
The primary endpoints of the clinical data collection were the variations in disability and pain, evaluated by the investigator using the Lequesne index (
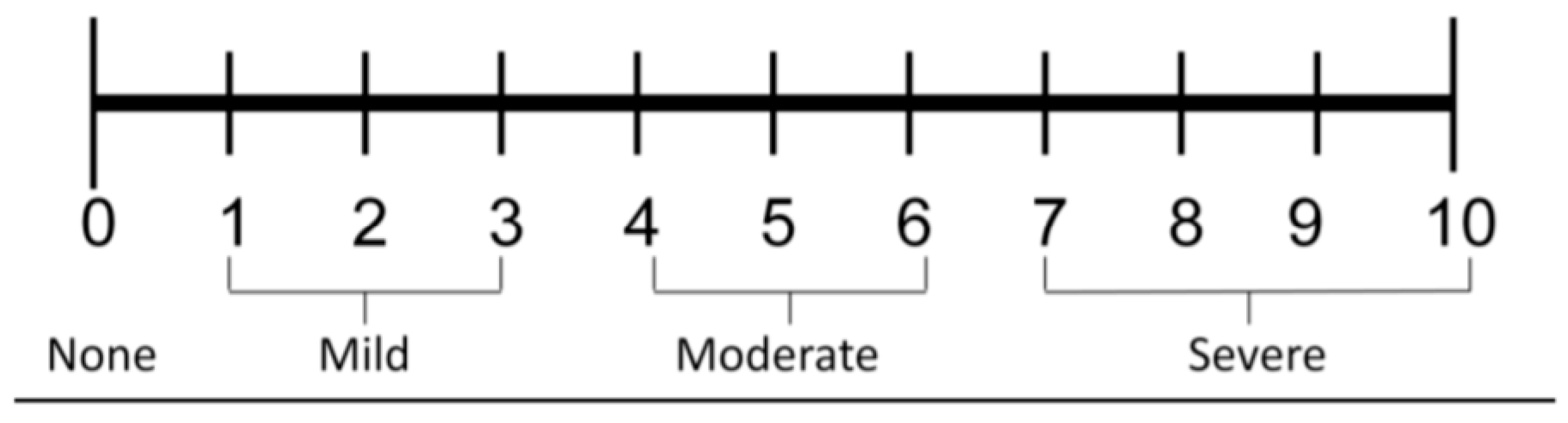
Table 1) and the Numeric Pain Rating Scale (NPRS) (
Figure 1) after three and six months (T1 and T2 visits, respectively), compared to the pre-injection baseline assessment (T0) [
13,
14].
The ten-question Lequesne algofunctional index includes three sections: “Pain or discomfort’”(L1), “Maximum distance walked” (L2), and “Difficulties in performing activities of daily living” (L3) [
13]. The Numeric Pain Rating Scale (NPRS) is a unidimensional eleven-score (0 = no pain to 10 = extreme or worst possible pain) self-assessment scale for pain intensity or other pain quality if requested: actual or experienced in the previous 24 hours [
14].
Secondary Endpoint
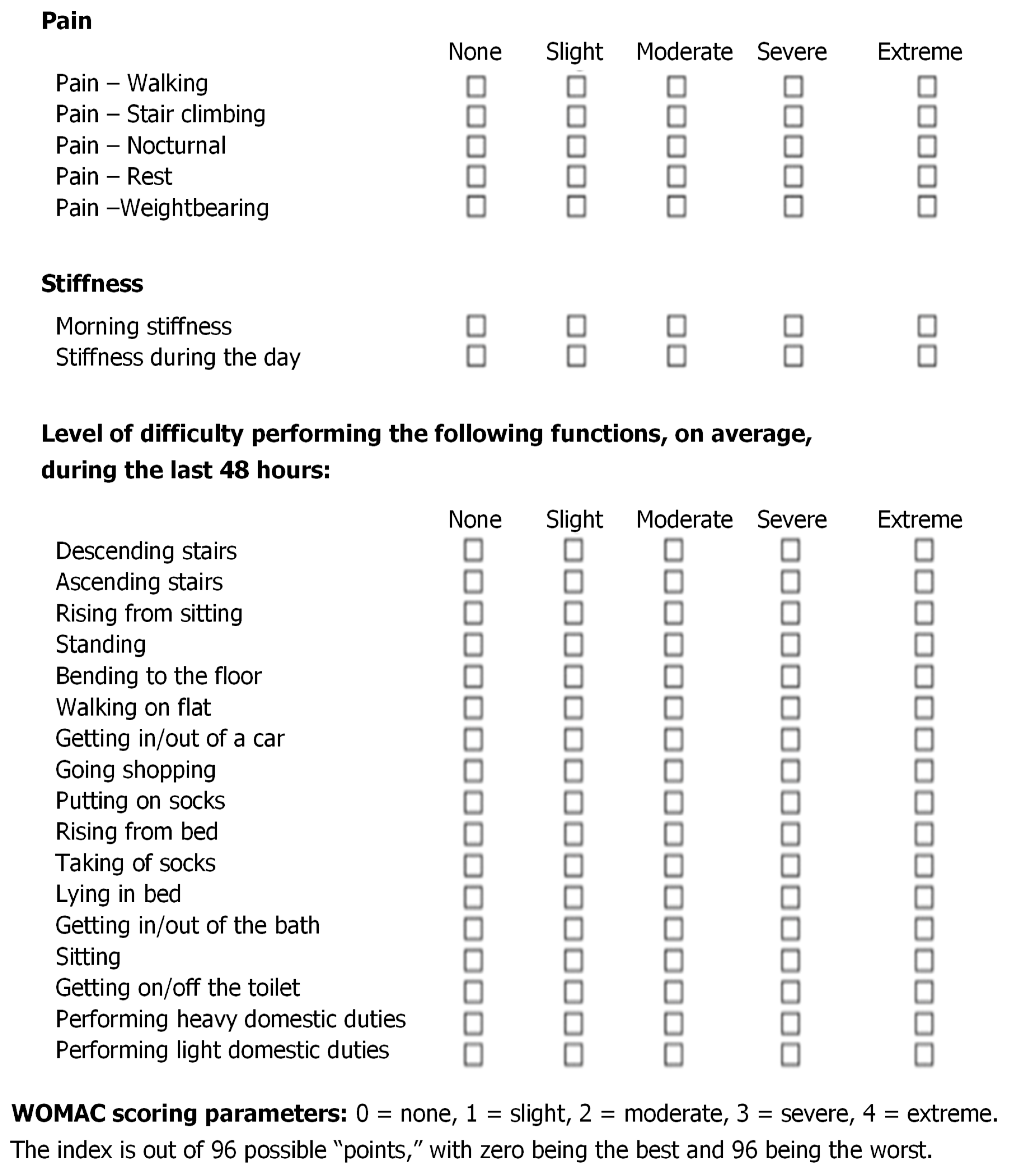
The secondary endpoint of the real-life clinical data collection was the variation, evaluated by the investigator after three and six months of follow-up, of the three-dimensional McMaster University OA (WOMAC) index—pain (five questions), stiffness (two questions), and physical function (seventeen questions) [
15]. Three ordinal subscales, with points (scores) ranging from 0 to 4, compose the Likert version of the WOMAC index. Each subscale is summated to maximum points of 20, 8, and 68, respectively. (
Figure 2).
Independently of each other, the investigators and the cohort patients assessed the overall clinical evolution of the symptomatic knee osteoarthritis as “Worsened”, “No change”, “Slightly improved”, “Improved”, and “Optimal results”.
Severity, on average, during the last 48 hours of:
Sample Size and Statistical Analysis
The sample size was estimated using the G*Power statistical program version 3.14 based on the worst-case hypothesis and considering two effect sizes. Based on available outcomes from published studies in knee osteoarthritis, the sample size calculation assumed a conservative 35% long-term improvement in the Lequesne index score after the PN HPT™ intra-articular injection. The minimum statistical power of detecting a significant two-tailed divergence compared to the expected Lequesne index score curve was set at 0.90 [statistical power: one less the ß-risk of false-negative type II errors]. Under those two assumptions, the estimated statistical power would have been greater than 0.91 with a study sample of 48 patients [
16].
For both the primary and secondary efficacy endpoints, inferential statistics compared the evolution of the mean Lequesne index, NPRS, and WOMAC scores at baseline and T1 and T2 with the Kruskal-Wallis test (one-way ANOVA on ranks), the non-parametric equivalent of the independent-sample analysis of variance for within-subject variations. Statistical analysis assessed whether the Lequesne index, NPRS, and WOMAC score curves diverged from the expected evolution (null hypothesis efficacy, +35%) [
16]. All statistical tests were two-sided with a 5% significance level; statistical program: StatPlus release v7.
Results
Demographic and Baseline Characteristics
Fifty-four (54) patients of both genders were consecutively enrolled, with no specific selection criteria to mirror the real-life ambulatory practice. Twelve (12) patients received bilateral treatment for a total of sixty-six (66) joints treated with CONDROTIDE HA.
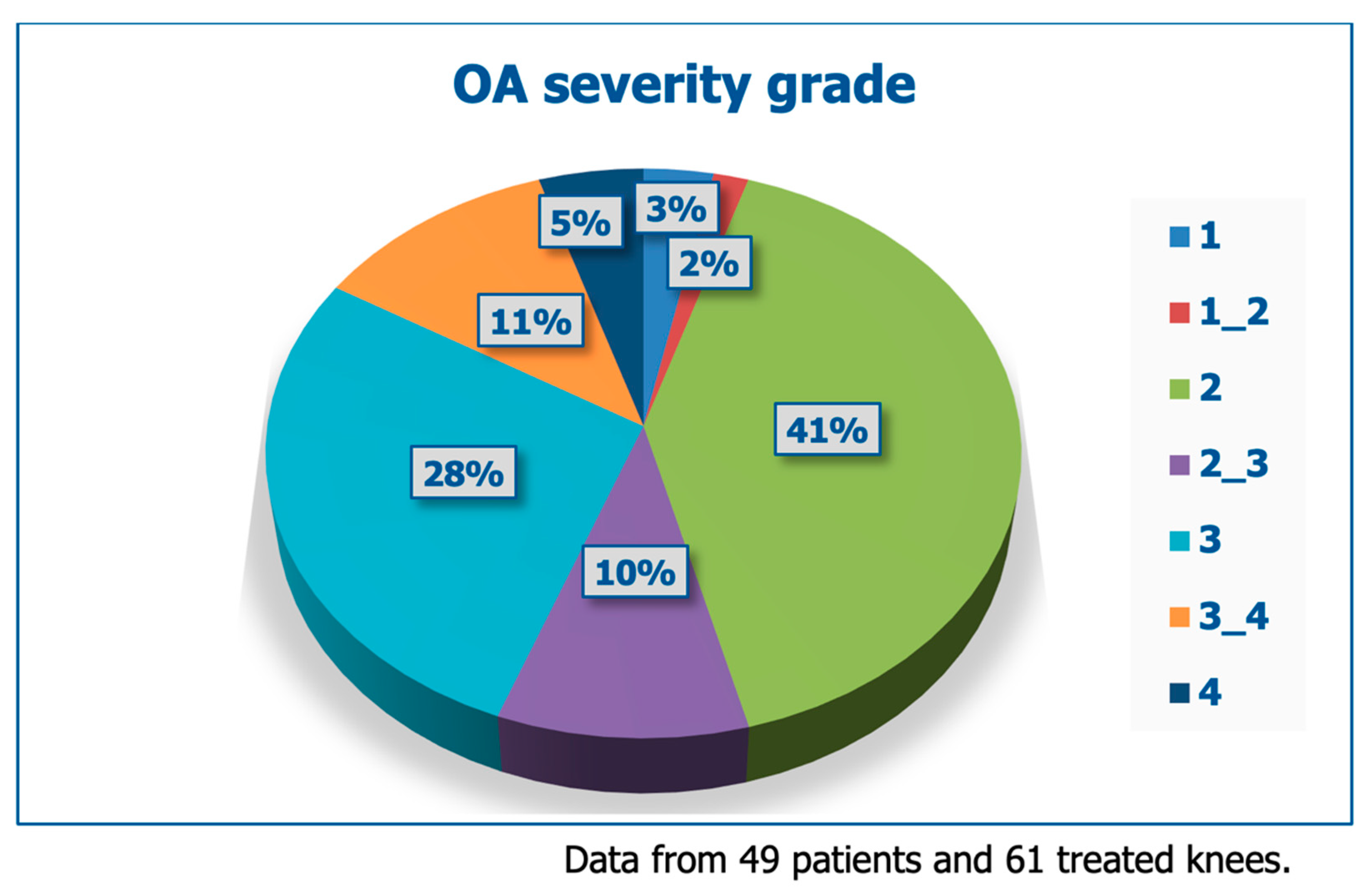
Table 2 and
Figure 3 illustrate the demographic data and baseline clinical severity of treated patients; beyond pain, most patients experienced problems with motion.
Primary Endpoints
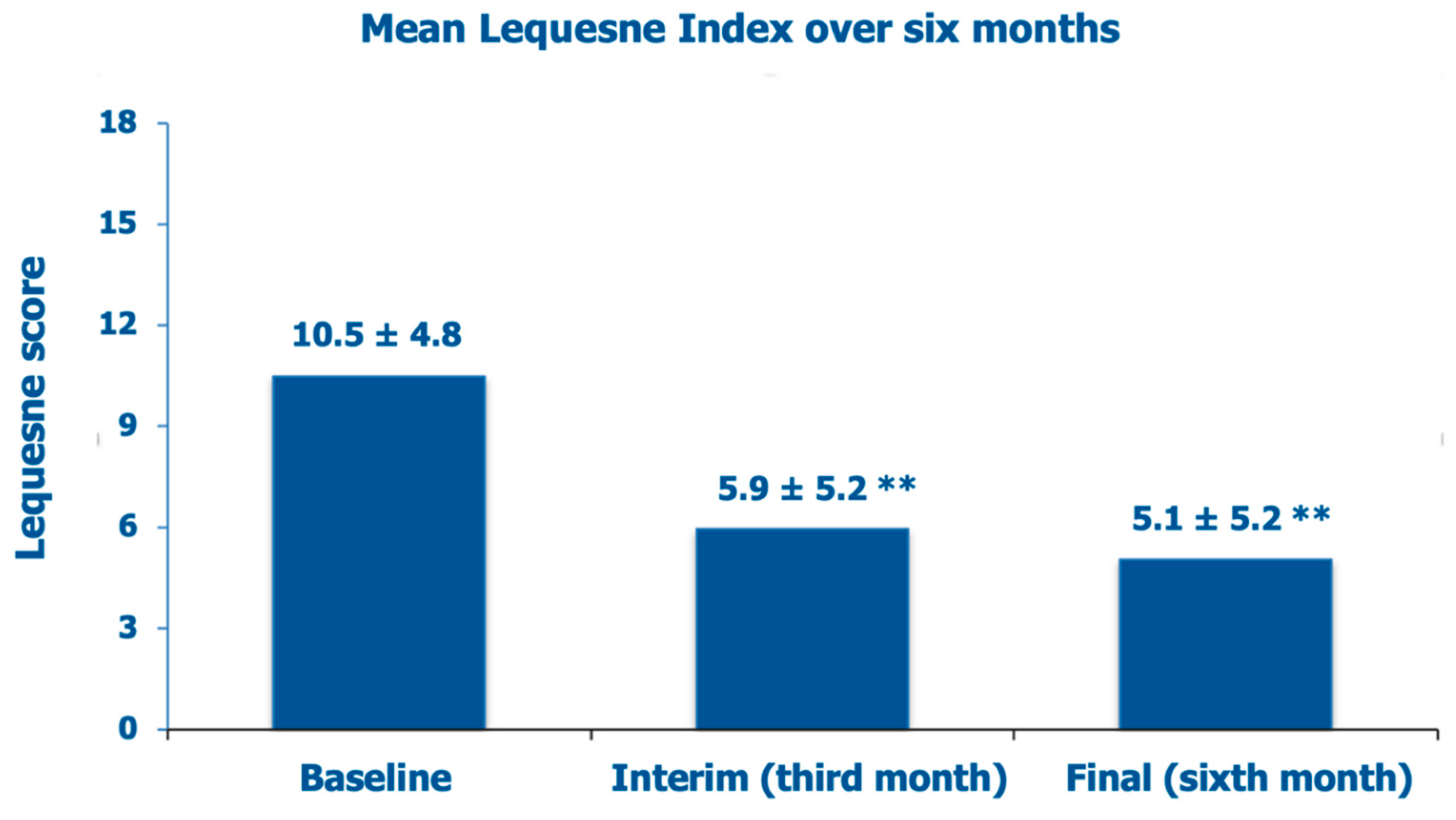
The mean baseline Lequesne index score was 10.5 ± 4.8 (borderline score between “severe” and “very severe” symptoms and disability, with pretty frequent pain, stiffness, and difficulties in performing daily activities), improving to “moderate” clinical severity at the interim assessment after three months (–43.8% vs. baseline), with only occasional pain and stiffness but little troubles to perform daily activities (
Figure 4). At the final visit after six months of follow-up, the Lequesne index had improved further within the “moderate” clinical severity class (–51.4% vs. baseline).
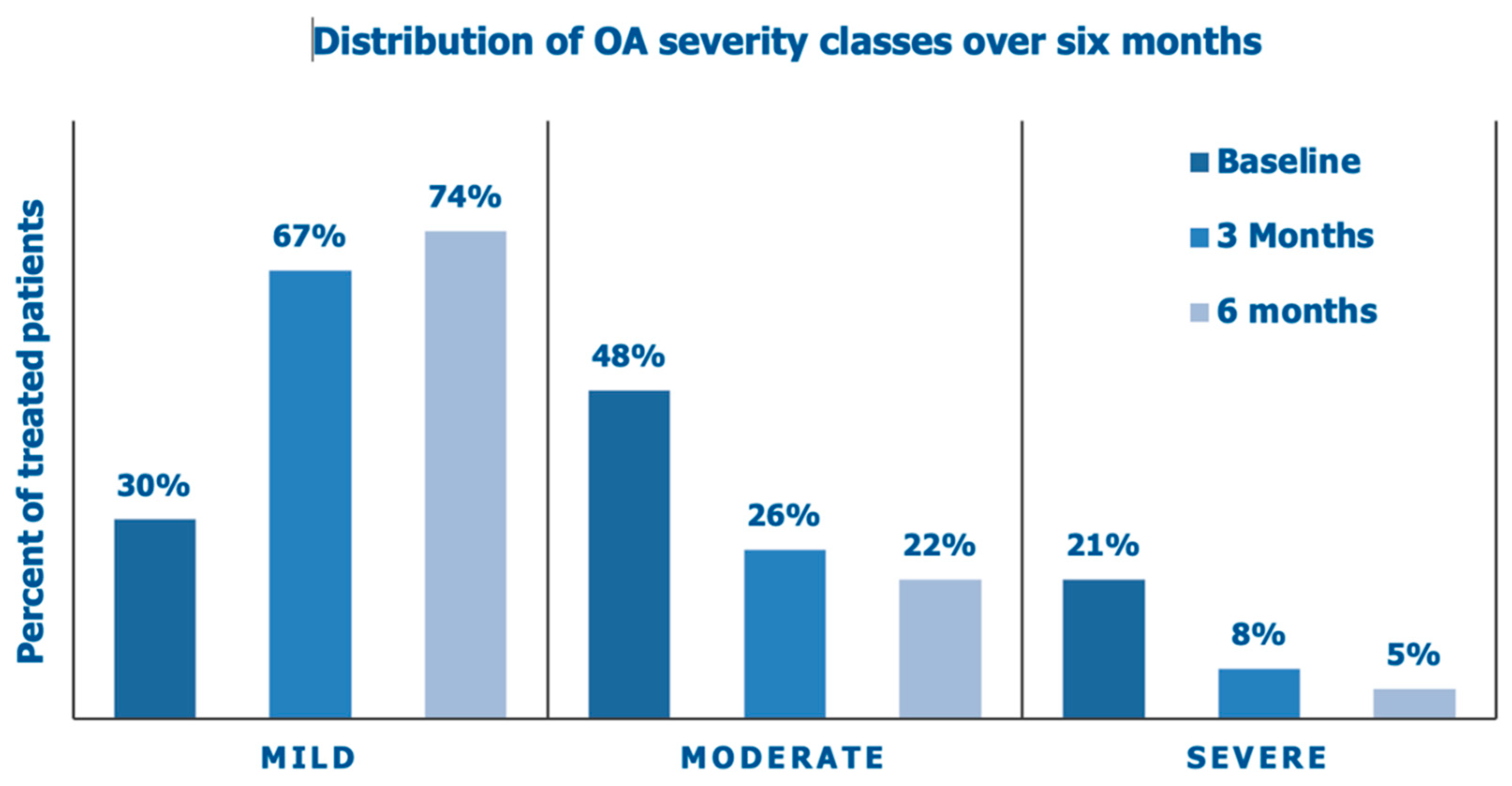
Figure 5 illustrates the improving distribution of the Lesquesne severity categories over the six months of the prospective data collection and the steady decrease of OA patients with a moderate and high-grade Lequesne index.
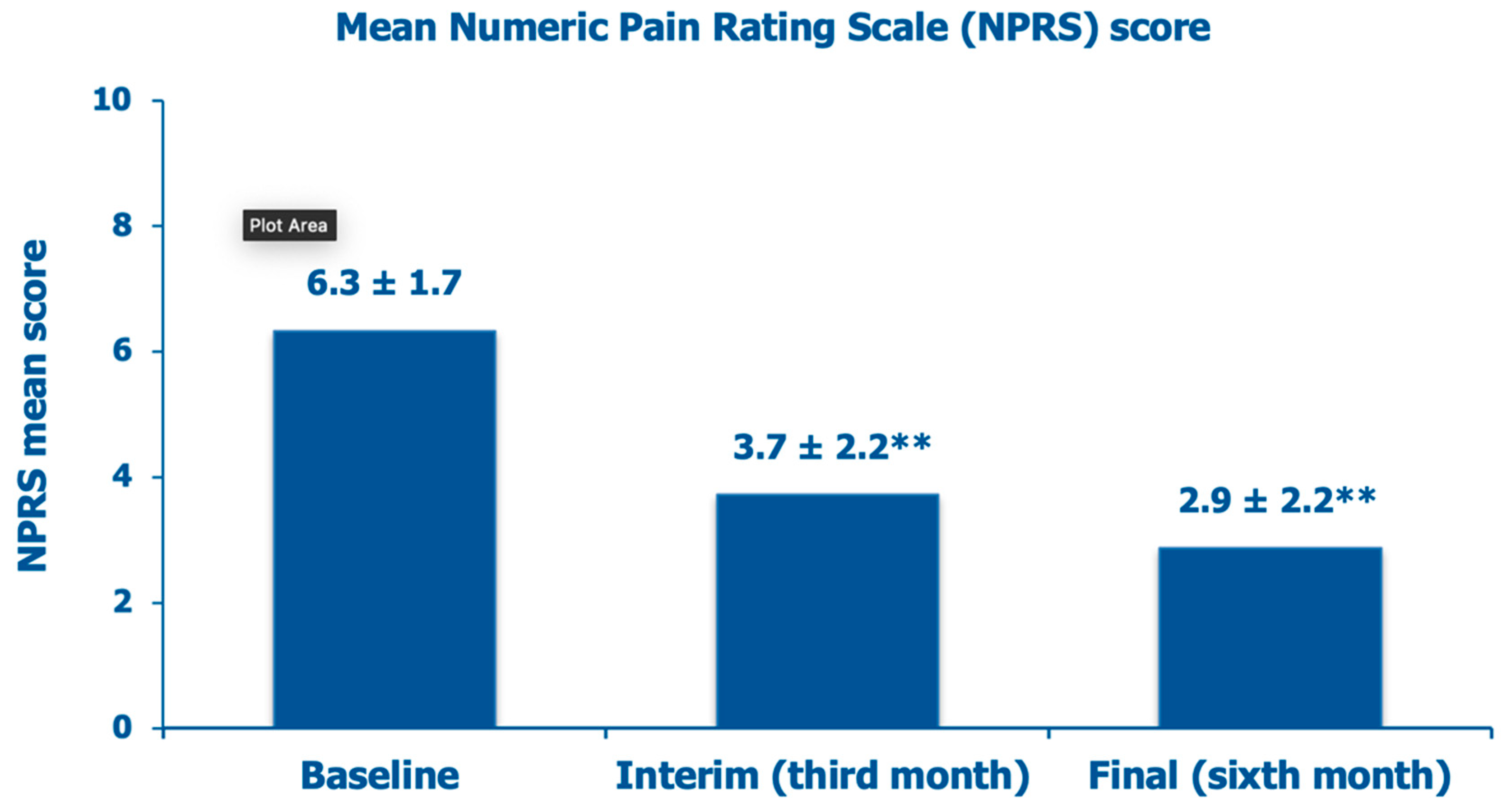
The mean NPRS score, which was 6.3 ± 1.7 at baseline, a borderline score level between moderate (score range, 4-6) and severe knee pain (score range, 7-10), decreased by 42.2% after three months and by 54.7% at the final follow-up assessment (
Figure 6).
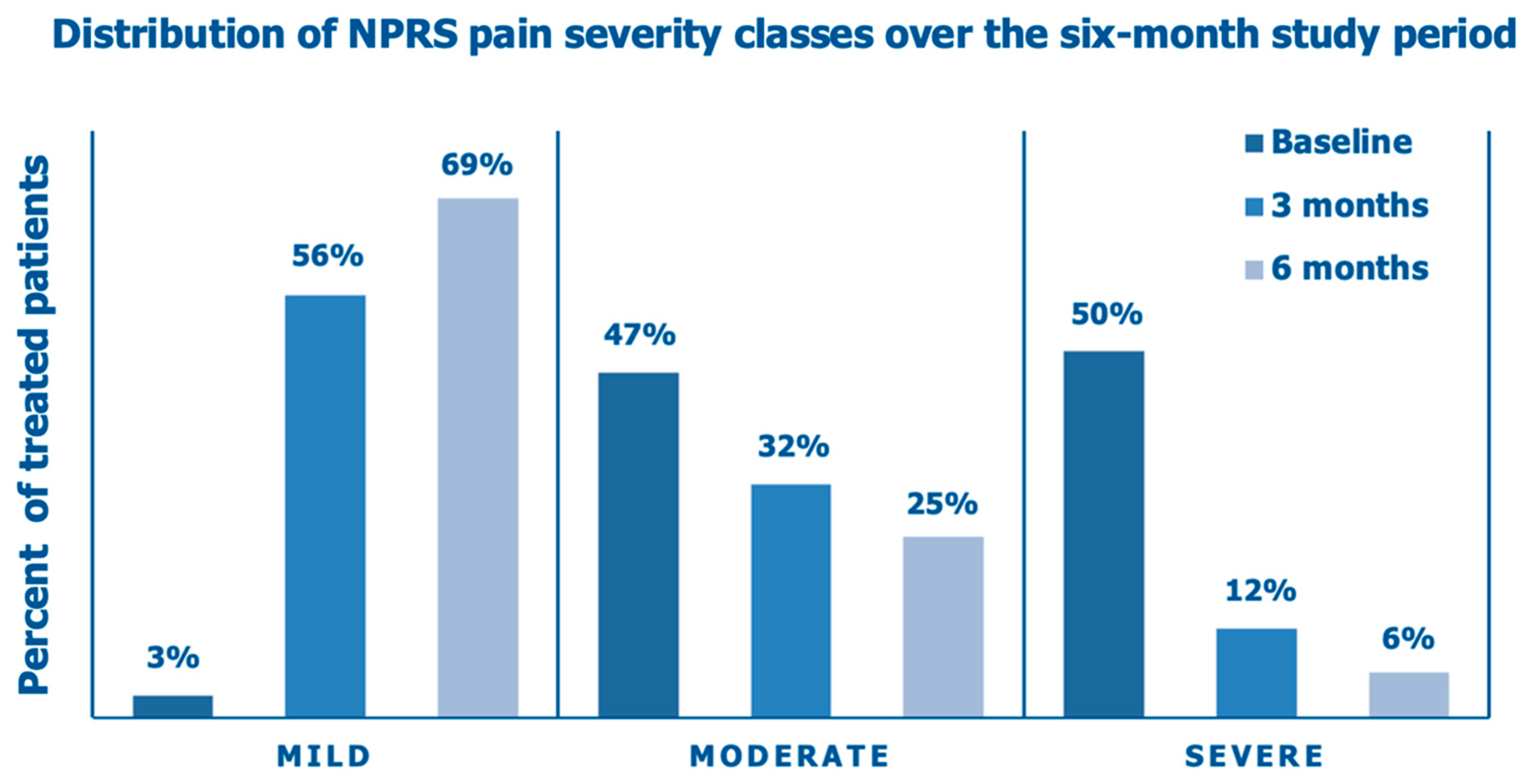
Figure 7 illustrates the improving distribution of the NPRS pain categories over the six months of the prospective data collection following the intra-articular PN HPT™/HA injection, with a steady shift from an overwhelming majority of the enrolled OA patients (97%) with moderate/severe baseline pain, stiffness, and interference with daily activities to a substantial majority of the same patients (69%) with mild pain, occasional discomfort, stiffness, and little interference with daily activities (NPRS score range, 0-3) at the end of the follow-up period.
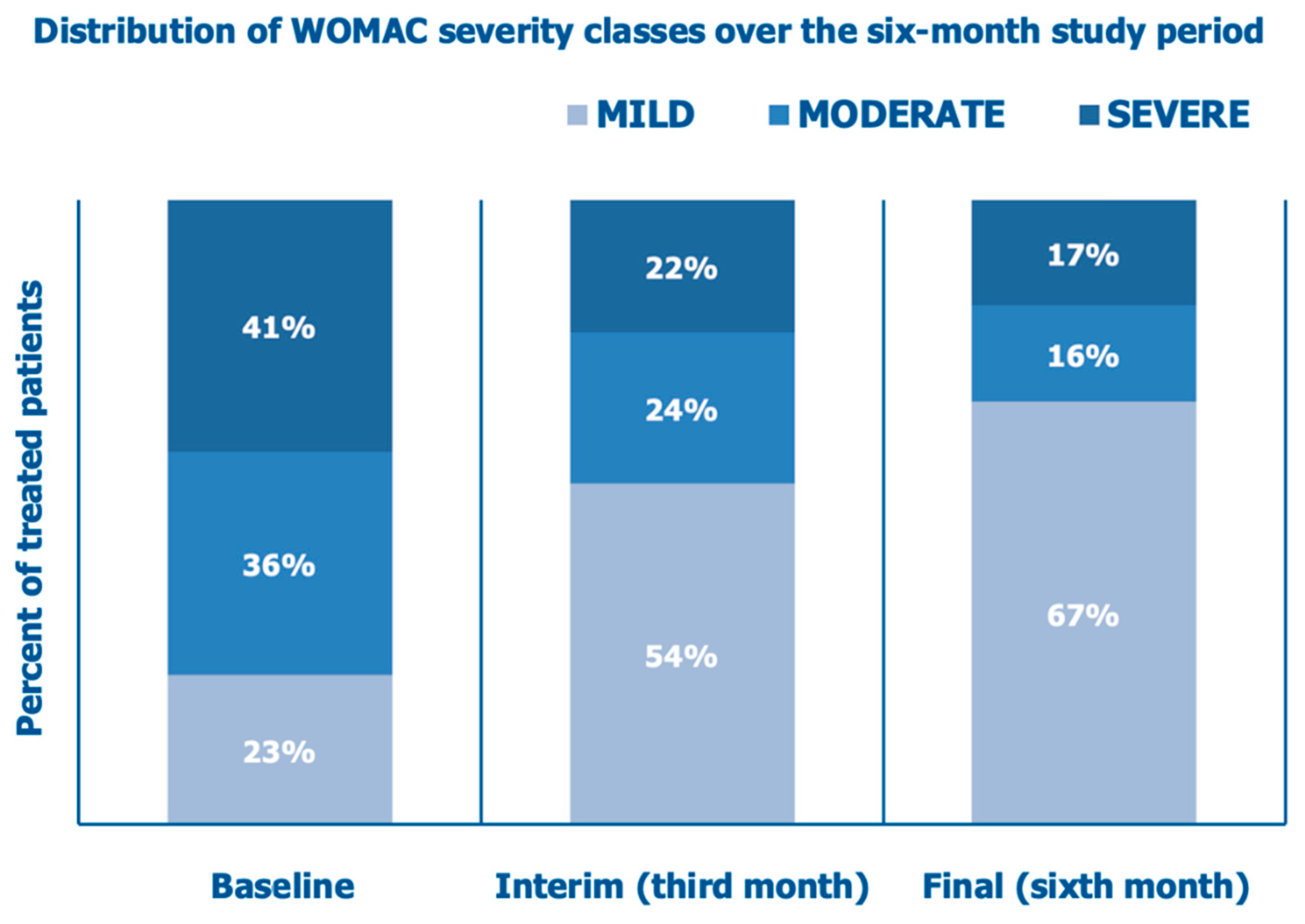
Secondary Endpoint (WOMAC Score)
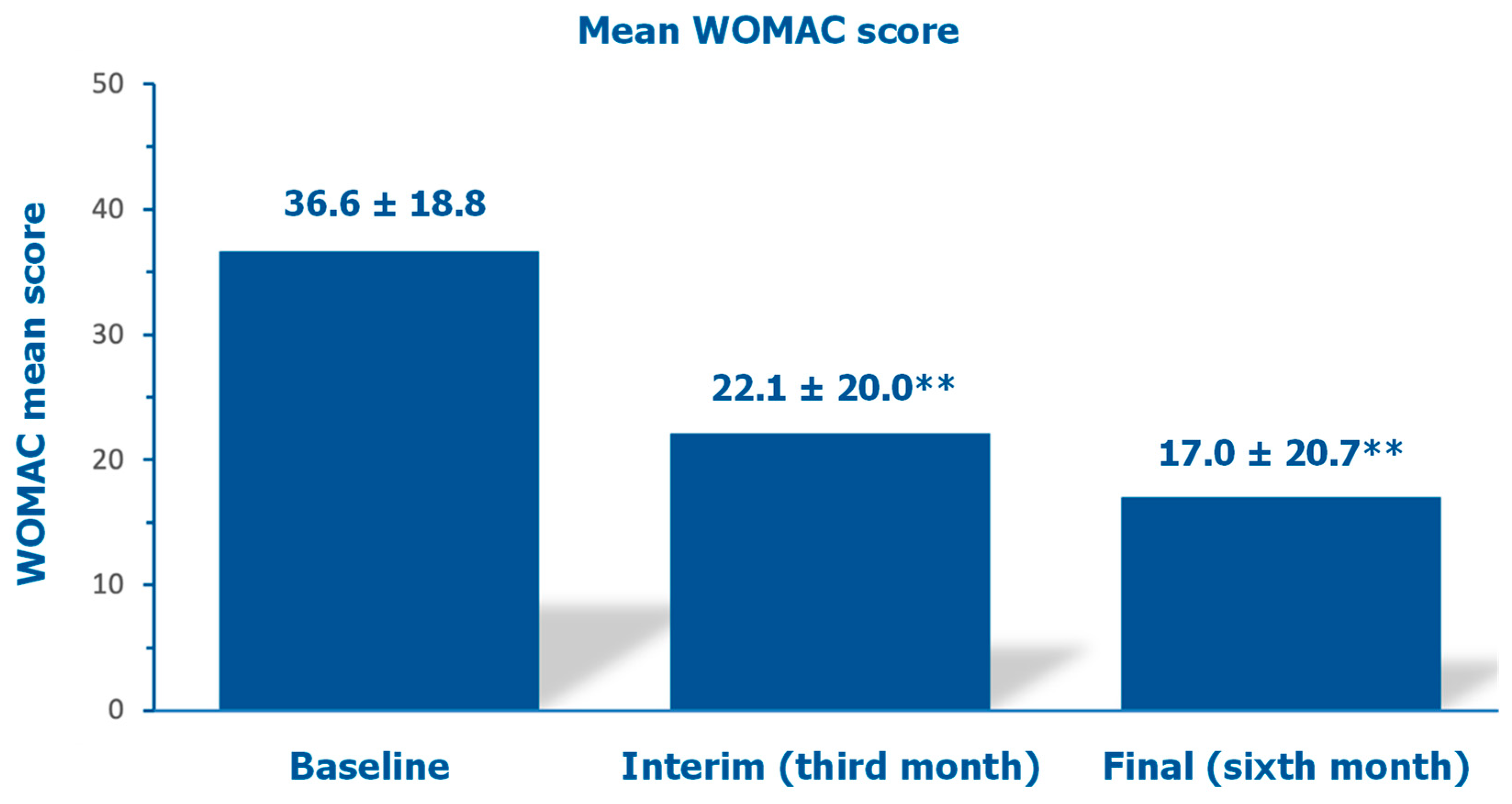
The mean investigator-assessed total WOMAC score, 36.6 ±18.8 at baseline, decreased by 39.6% after three months and by 53.6% at the final follow-up assessment (
Figure 8).
Figure 9 illustrates how the percent of cohort patients with severely or moderately symptomatic diseases and a handicap in performing daily activities (WOMAC scores >40 and between 21 and 40, respectively) progressively abated from 77% at baseline to 46% at the interim assessment and 33% at the final follow-up assessment after six months.
Table 3 illustrates how investigators and cohort patients independently assessed the overall symptomatic evolution of knee osteoarthritis. There was no side effect.
Discussion
The debate surrounding the actual value of HA in knee OA rages on even in recent evidence-based guidelines, supporting the likely benefits of combining HA with other intra-articular ingredients in the same medical device [
17]. Beyond improving pain and mobility, many proposed strategies seek to restore the damaged cartilage or at least vicariate its function, enhance the congruence of joint surfaces, and prevent further deterioration of chondral morphology and function [
18,
19]. However, all proposed drugs, surgery, and other methods have never primarily focused on restoring the physiological microenvironment of cartilage. Consequently, the biochemical and functional characteristics of the resulting fibrocartilage repair tissue inevitably differ from those of the physiological hyaline cartilage [
20,
21,
22].
The disease burden associated with knee OA in developed countries suggests the benefits of aiming to restore the physiological microenvironment of cartilage as a primary therapeutic goal. In Italy, a survey conducted in the Chianti area in Tuscany among 1,006 individuals over 65 years old highlighted that 22.4% lamented daily knee OA symptoms, often with severe disability and impressive societal costs, compounded by a further 7.2% lamenting both knee and hip OA symptoms [
23].
Even as a single ingredient of medical devices for intra-articular OA treatment, natural-origin, highly purified PN HPT™ improve the fluid dynamics of synovial fluid with an intrinsic viscosupplementation efficacy. Furthermore, PN HPT™ tend to re-establish a proper physiological joint microenvironment, as evidenced in biopsies of atrophic cartilage after exposure to PN HPT™, leading to the production of Type-II collagen, aggrecan, and extracellular matrix (ECM) at levels comparable to normal controls [
24]. Immunohistochemistry reveals that the
in vitro ECM deposition by atrophic cartilage exposed to 1% or 2% PN HPT™ is abundant in Type-II collagen and similar to hyaline cartilage. Conversely, although new ECM production occurs following exposure to 1% or 2% HA, it is rich in Type-X collagen fibers and resembles fibrous scar cartilage [
24]. Under environmental conditions that mimic the articular microenvironment of healthy hyaline cartilage, the optical density of fibers deposed after exposure to PN HPT™ matches the ground substance surrounding them, rendering those new fibers invisible within the ECM. Additionally, synoviocyte vitality is significantly higher after exposure to PN HPT™ compared to HA [
24].
Considering all these factors, several previous studies have shown that the rationale for combining PN HPT™ and HA in the same medical device for intra-articular injection is compelling [
7,
8,
9,
10,
11]. A recent study investigating the efficacy of PN HPT™/HA pericapsular injections to control the degenerative signs and related symptoms of mandibular condyle osteoarthritis (temporomandibular joint osteoarthritis) has further confirmed the rationale [
25]. Microinvasive cosmetic plastic surgery has also reaped the benefits of the peculiar properties of PN HPT™ and the PN HPT™/HA combination [
26,
27]. The study has some limitations due to its non-selective data collection design, such as lacking a control group and relying solely on validated yet inherently subjective rating scales. However, it can be viewed as a reasonably predictive photograph of real-life ambulatory practice in knee osteoarthritis. The PN HPT™ combination acts rapidly, with most symptomatic and mobility benefits, which seem pretty impressive, reaped by the third week. Of course, the study can only pretend to confirm previous observations—what it does. The lack of a control group is a severe bias that prevents quantitative outcomes from being weighed against positive and negative controls.
Conclusions
The illustrated data collection confirms previous findings regarding the real-life benefits—beyond mere viscosupplementation—of combining PN HPT™ and HA in a single medical device for intra-articular injection in knee osteoarthritis. Even a single injection provides meaningful and long-term benefits for several months.
Author Contributions
The real-life patients included a paragraph in their informed consent form for ambulatory treatment that permitted the authors to use their data retrospectively and anonymously for research purposes without restrictions. All authors contributed to the open-label data collection, reviewed the manuscript drafts, and agreed to its submission. The authors are accountable for the clinical and editorial accuracy and integrity of the manuscript submitted to the Journal of Clinical Medicine. They confirm having adhered to the ethical policies of the journal as outlined in the journal’s author guidelines.
Funding
No author received funds or other benefits for their research or manuscript; they have no paid or unpaid relationships with industry manufacturers, publishers, or other companies in any way connected to the manuscript submission.
Data Availability Statement
According to current regulations, the Corresponding Author has archived all datasets (clinical data and iconographic documentation). All datasets are available upon reasonable request after being converted to an anonymous form.
Conflicts of Interest
The authors declare no conflict of interest. No author received funds or other benefits for their research or manuscript; they have no paid or unpaid relationships with industry manufacturers, publishers, or other companies in any way connected to the manuscript submission.
References
- Butler, J.; Rydell, N.W.; Balazs, E.A. Hyaluronic acid in synovial fluid. VI. Effect of intra-articular injection of hyaluronic acid on the clinical symptoms of arthritis in track horses. Acta Vet Scand 1970, 11, 139–155.
- Gupta, R.C.; Lall R.; Srivastava A.; Sinha A. Hyaluronic Acid: molecular mechanisms and therapeutic mechanisms and therapeutic trajectory. Front Vet Sci 2019, 6, 192.
- Walvekar, P.; Lulinski, P.; Kumar, P.; Aminabhavi, T.M.; Choonara, Y.E. A review of hyaluronic acid-based therapeutics for the treatment and management of arthritis. Int J Biol Macromol 2024, 264(Pt 2), 130645. [CrossRef]
- Balazs, E.A.; Denlinger, J.L. Viscosupplementation: a new concept in the treatment of osteoarthritis. J Rheumatol 1993, 39, 3–9.
- Martin, J.A.; Buckwalter, J.A. Aging, articular cartilage chondrocyte senescence and osteoarthritis. Biogerontology 2002, 3, 257–264. [CrossRef]
- Peng, H.; Zhou, J-l.; Liu, S-q.; Hu, Q-j.; Ming J-h,.; Qiu, B. Hyaluronic acid inhibits nitric oxide-induced apoptosis and dedifferentiation of articular chondrocytes in vitro. Inflamm Res 2010, 59, 519–530.
- Vanelli, R.; Costa, P.; Rossi S.M.P.; Benazzo, F. Efficacy of intra-articular polynucleotides in the treatment of knee osteoarthritis: a randomized, double-blind clinical trial. Knee Surg Sports Traumatol Arthrosc 2010, 18, 901–907. [CrossRef]
- Giarratana, L.S.; Marelli B.M.; Crapanzano, C.; De Martinis, S.E.; Gala, L.; Ferraro, M.; et al. A randomized, double-blind clinical trial on the treatment of knee osteoarthritis: the efficacy of polynucleotides compared to standard hyaluronan viscosupplementation. Knee 2014, 21, 661–668. [CrossRef]
- Stagni, C.; Rocchi, M.; Mazzotta, A; et al. Randomised, double-blind comparison of a fixed co-formulation of intra-articular polynucleotides and hyaluronic acid versus hyaluronic acid alone in the treatment of knee osteoarthritis: two-year follow-up. BMC Musculoskelet Disord 2021, 22, 773. [CrossRef]
- Guelfi, M.; Fabbrini, R.; Guelfi, M.G. Intra-articular treatment of knee and ankle osteoarthritis with polynucleotides: prospective case record cohort vs historical controls. J Biol Regul Homeost Agents 2020, 34, 1949–1953. [CrossRef]
- Migliore, A.; Graziano, E.; Martín, L.S.M.; Sorbino, A.; Raichi. M.; Bon, G. Three-year management of hip osteoarthritis with intra-articular polynucleotides: a real-life cohort retrospective study. J Biol Regul Homeost Agents 2021, 35, 1189–1194.
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759.
- Lequesne M.G. The algofunctional indices for hip and knee osteoarthritis. J Rheumatol 1997, 24, 779–781.
- Jensen, M.P; Karoly, P. Self-report scales and procedures for assessing pain in adults. In Handbook of pain assessment, 3rd ed.; Turk, D.C., Melzack, R., Eds.; Guilford Press: New York, USA, 2011; 19–44.
- Bellamy, N.; Buchanan, W.W.; Goldsmith, C.H.; Campbell, J.; Stitt L.W. Validation study of WOMAC: a health status instrument for measuring clinically important patient-relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 1988; 15, 1833–1840.
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang A-G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav Res Methods 2009, 41, 1149–1160. [CrossRef]
- Bannuru, R.R.; Osani, M.C.; Vaysbrot, E.E.; Arden, N.K.; Bennell, K.; Bierma-Zeinstra, S.M.A.; et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr Cartil 2019, 27, 1578–1589. [CrossRef]
- Lotto, M.L.; Wright, E.J,.; Appleby, D.; Zelicof. S.B.; Lemos, M.J.; Lubowitz, J.H. Ex vivo comparison of mechanical versus thermal chondroplasty: assessment of tissue effect at the surgical endpoint. Arthroscopy 2008, 24, 410–415.
- Tuan, R.S. A second-generation autologous chondrocyte implantation approach to the treatment of focal articular cartilage defects. Arthritis Res Ther 2007, 9, 109. [CrossRef]
- Nagle, J.A. Knee joint preservation with autologous cartilage implantation. AORN J 2007, 86, 550–562. [CrossRef]
- Riegger-Krugh, C.L.; McCarty, E.C.; Robinson, M.S.; Wegzyn, D.A. Autologous chondrocyte implantation: current surgery and rehabilitation. Med Sci Sports Exerc 2008; 40, 206–214.
- Gorensek, M.; Jaksimović, C.; Kregar-Velikonja, N.; Gorensek, M.; Knezevic, M.; Jeras, M.; et al. Nucleus pulposus repair with cultured autologous elastic cartilage-derived chondrocytes. Cell Mol Biol Lett 2004, 9, 363–373.
- Cecchi, F.; Mannoni, A.; Molino-Lova, R.; Ceppatelli, S.; Benvenuti, E.; Bandinelli, S.; et al. Hip and knee pain epidemiology in a community-based sample of Italian persons aged 65 and older. Osteoarthr Cartil 2008, 16, 1039–1046.
- Gennero, L.; Denysenko, T.; Calisti, G.F.; et al. Protective effects of polynucleotides on cartilage degradation in experimental cultures. Mastelli internal clinical research report, R&D Code: DF02.1, Issued in Sanremo (Italy) on 17/01/2025. Available upon request. [CrossRef]
- Cenzato, N.; Crispino, R.; Russillo. A.; Del Fabbro. M.; Tartaglia. G.M. Clinical effectiveness of polynucleotide TMJ injection compared with physiotherapy: a 3-month randomised clinical trial. Br J Oral Maxillofac Surg 2024, 62, 807–812. [CrossRef]
- Cavallini M, Bartoletti E, Maioli E on behalf of The Polynucleotides HPT™ Priming Board. Consensus report on the use of PN-HPT™ (polynucleotides highly purified technology) in aesthetic medicine. J Cosmet Dermatol 2021, 20, 922–928. [CrossRef]
- Cavallini M, Bartoletti E, Maioli E on behalf of The Polynucleotides HPT™ Priming Board. Value and benefits of the Polynucleotides HPT™ Dermal Priming Paradigm. A consensus on practice guidance for aesthetic medicine practitioners and future research. Clin Exp Dermatol Ther 2024, 9, 224. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).