1. Introduction
The high rate of reported chlamydial infections among young adults indicates the need for additional efforts in the prevention, control and diagnosis of chlamydial infection. Despite all national programs, strategies and guidelines, chlamydial infection is still the leading infection worldwide. According to reports from the CDC (Center for Disease Prevention and Control ), four million cases of chlamydial infection were reported in the United States in 2018, making it very common bacterial sexually transmitted disease [
1,
2].
In Europe, the global rate of transmitted chlamydial infections remains high, with clear heterogeneity between countries and reported cases. In the period from 2009 to 2018, there was a slight increase of 5.30%, but probably as a result of the use of more sensitive diagnostic tests, more accurate reporting of health facilities and a greater response to screening examinations in recent years compared to 2009, and not as a consequence of an increase in overall prevalence. In terms of gender and age, the same pattern as in previous years has remained largely the same, young women aged 20-24 are still the most vulnerable, while men of the same age are slightly less affected [
3,
4,
5,
6,
7,
8]. In Serbia, in 2019, chlamydial infection is still the leading sexually transmitted disease with 776 diagnosed cases, which gives an incidence of 11.11/100.00 inhabitants. In conclusion, according to the data obtained though epidemiological surveillance in the last ten years there has been a slight decline in chlamydial infection in the Republic of Serbia.
Chlamydial infection in a high percentage (70.00% - 90.00%) is accompanied by an asymptomatic nature, which is actually the biggest problem reflected in the late diagnosis and treatment of the infection. Untreated infection can lead to serious complications in the reproductive tract of women, such as pelvic inflammatory diseases, ectopic pregnancy, fallopian tube infertility, adnexitis, salpingitis, endometritis [
9,
10,
11,
12,
13]. Given its specific developmental cycle, asymptomatic nature, complications and consequences of these complications, it is necessary to take a serious approach from all sides, in order to keep the infection under control and preserve the reproductive health of young women most affected by the infection. Furthermore, to improve the reporting of diagnosed cases by health professionals from both health sectors (private and public), provide conditions for electronic reporting to all relevant levels and institutions for timely and complete reporting.
An infectious agent such as C. trachomatis is not at all easy to detect due to its biological and clinical characteristics, asymptomatic nature and specific developmental cycle, ability to avoid immune response, so it is very important to choose a test that will overcome all these challenges, and detect infection. Today, there is a large number of tests to detect chlamydial infection. The only test that can detect viable chlamydia is cell culture [
14]. However, this method is rarely used today due to the complexity of the procedure itself and low sensitivity. Commercial tests for the detection of chlamydial antigens use polyclonal antibodies to detect chlamydial lipopolysaccharide or monoclonal antibodies to detect the major outer membrane protein, such as the direct immunofluorescence (DIF) test. This method shows a high specificity of 98% and it is performed quickly, but on the other hand it is subjective and with low sensitivity (60.00% -75.00%) in relation to nucleic acid amplification tests and as such it is not recommended for routine testing for chlamydial infection [
15,
16]. Rapid immunochromatographic tests are of great help in terms of testing speed, cost, availability, high specificity (97.00% - 100.00%), but unfortunately due to very low sensitivity (35.00% - 60.00%) it cannot be recommended for the diagnosis of acute chlamydia infection [
17,
18,
19,
20,
21,
22]. Serological methods are indirect tests that register anti-MOMP (main outer membrane protein) IgA and IgG antibodies, i.e. the immune response to a given antigen, and these tests are not recommended for the diagnosis of acute chlamydial infection [
23,
24], but can be very useful in persistent infections and pathology of fallopian tubes [
25,
26,
27]. According to the recommendations of the American and European Centers for Disease Control, the only tests recommended for the diagnosis of chlamydial infection are nucleic acid amplification tests. These tests are far better in overall performance compared to all other tests with or without cultivation in diagnosis
C. trachomatis. They are suitable for detecting chlamydial infection in vaginal and endocervical swabs in women, urethral swabs in men, and first morning voided urine specimens from both male and female individuals [
28]. In addition to high sensitivity and specificity, these tests provide additional convenience in terms of sampling, which the patient can do himself. Samples of vaginal swabs taken by the patient in terms of sensitivity and specificity are equivalent to those collected by the clinician [
28,
29,
30]. Recent studies have also shown equivalence between urethral swabs and urine samples taken by patients themselves and urethral swab samples taken by a clinician [
31,
32].
The recommendations are clear, but unfortunately some countries are still unable to provide the conditions to perform nucleic acid amplification tests, concerning laboratory space, staff or equipment, and generally use other diagnostic methods which are not recommended for diagnosing acute chlamydial infection. Since the direct immunofluorescence test, rapid immunochromatographic test and serological tests, as individual, did not show satisfactory diagnostic efficiency, the main goal of our study was to improve diagnostic efficiency by combining individual tests.
2. Materials and Methods
Study Population
The study population involved 225 sexually active persons, of both sex, who were successively screened for genital chlamydial infection in Institute of Public Health Kragujevac. They were all Caucasian race and age from 18 to 50 years. The study omitted persons: (I) under the age of 18, (II) who had any disease, condition or other element that could significantly influence the result of the evaluation (pregnancy, menstrual cycle, recent use of antibiotics or topical preparations within the previous 72 hours, concurrent induction with other pathogens, etc.), (III) who were already involved in another clinical trial or the ones who refused to be engaged in the study and (IV) who had any other situation that could significantly inhibit their engagement in the study. The Ethical Committee of the Institute of Public Health Kragujevac approved the study. In line with the Declaration of Helsinki, all patients signed the Ethical Committee approved informed consent. All patients were informed about their examination, in every way.
Sampling and Data Collection
The standard laboratory protocols were used when processing the samples. We collected two swabs from every participant (cervical for females and urethral for males). We used the first swab for bacteriological and mycological examination, direct immunofluorescence (DIF) and rapid immunochromatographic (RT) tests for qualitative determination of anti-chlamydial antigens. We froze the second swab at -20 °C for subsequent determination of specific sequences of Chlamydia genome by RT-PCR test. We took a peripheral blood sample (3mL) from all patients and collected it in polystyrene tubes, centrifuged at 400g and then we aliquoted and stored the serum samples at -20 °C for the further analysis. We used the serum samples to quantitate the serum levels of IgA and IgG antibodies to MOMP antigen of C. trachomatis.
Screening Methods
Direct Immunofluorescence Test (DIF) for Qualitative Detection of Chlamydial Antigen
The Chlamydia Cell IF test is an available rapid direct immunofluorescence commercial test for the qualitative detection of chlamydial antigen in the samples of a patient (Cellabs Pty Lty, Brookvale, Australia). All samples were tested in accordance with the instructions of the manufacturer: murine monoclonal antibodies which contains fluorescein specifically binds to MOMP, a Chlamidia trachomatis antigen in the sample and this reaction emits bright green fluorescence. The fluorescence quality is good, because the MOMP is evenly distributed across the chlamydial membrane.
Rapid Immunochromatogaphic Test (RT) for Qualitative Detection of Chlamydia Antigen
Chlamydia test card is an available rapid chromatographic commercial immunoassay for the qualitative detection of chlamydial antigen in the samples of the patients (Ulti Med Products GmbH, Ahrensburg, Germany). All samples were tested in accordance with the instructions of the manufacturer: in this test polyclonal antibodies are used to detect chlamydial lipopolysaccharide. These antibodies are labeled with an enzyme that reacts with substrate and after binding to a specific antigen it releases a color that can be easily detected.
Determination of the Serum Level of the Antibodies to the Chlamydial MOMP Antigen
We used the serum samples to quantitate the serum levels of IgA and IgG antibodies specific forMOMP antigens of Chlamydia trachomatis. We determined the tested antibodies with a commercially available enzyme-linked immunosorbent assay (ELISA) kit in accordance with the instructions of the manufacturer (Euroimmun, Lubeck, Germany). The manufacturer suggested the cut-off values: RU/mlL ≥ 22 for IgGand S/Co ≥ 1.1 for IgA. Sample obtained after specific preparation is added to a microtitration plate whose wells are coated with purified MOMP antigen of Chlamidia trachomatis. After that, antibody quantification is performed by measuring the color intensity of the sample in a spectophotometer using a 450nm filter.
Diagnostic Method
Real-Time Polymerase Chain Reaction (RT-PCR)
C. trachomatis Real-TM PCR kit is a common, available nucleic acid amplification test for commercial usage, for qualitative detection of C. trachomatis DNA in the clinical materials by means of real-time hybridization fluorescence detection. We performed the test on a Sa Cycler-96 thermocycler in accordance with the instructions of the manufacturer (Sacace Biotechnologies, Como, Italy). Detection of Chlamydia trachomatis by polymerase chain reaction is based on highlighting a specific part of pathogen’s genome using specific primers.
Diagnostic Criteria
We tested all participants by all screening and diagnostic methods. The primary (independent) variable we obtained by RT-PCR assay, whereas secondary (dependent) variables are the results we obtained by DIF, RT and ELISA. The results which were obtained by screening tests were read by a researcher ignorant of the results we obtained from RT-PCR assay. We compared the diagnostic accuracy of the screening tests with the results obtained by the RT-PCR method representing the recommended diagnostic method (gold standard regarded as the best test in reasonable conditions) for the detection of acute chlamydial infection.
Statistical Analysis
We presented the variables as frequencies of individual parameters (categories), and we evaluated the statistical significance of differences by chi squared test, ORA (Overall Agreement) and Fisher exact test using free on-line calculator (
http://www.physics.csbsju.edu/stats/). We used MEDCALC statistical software for diagnostic test evaluation. Statistical difference of p < 0.05 was regarded as significant.
Formulas for calculating diagnostic efficiency parameters are presented in the abbreviation section
Table 1.
a – actually positive; b – false negative; c – false positive; d – actually negative.
List of Abbreviations:
DIF- direct immunofluorescence
RT- rapid lateral immunochromatographic test
IgA and IgG- antibodies (immunoglobulins)
PCR- polymerase chain reaction
ORA-overall agreement;
Kappa-statistic index;
χ2 –statistic test
3. Results
Through meticulous examination of individual test diagnostic efficiency, we sought to enhance chlamydial infection detection by combining tests, evaluating the clinical accuracy of each combination, and determining their superiority over individual tests while considering economic feasibility. We used two approaches to combine individual tests and performed all possible combinations in each group.
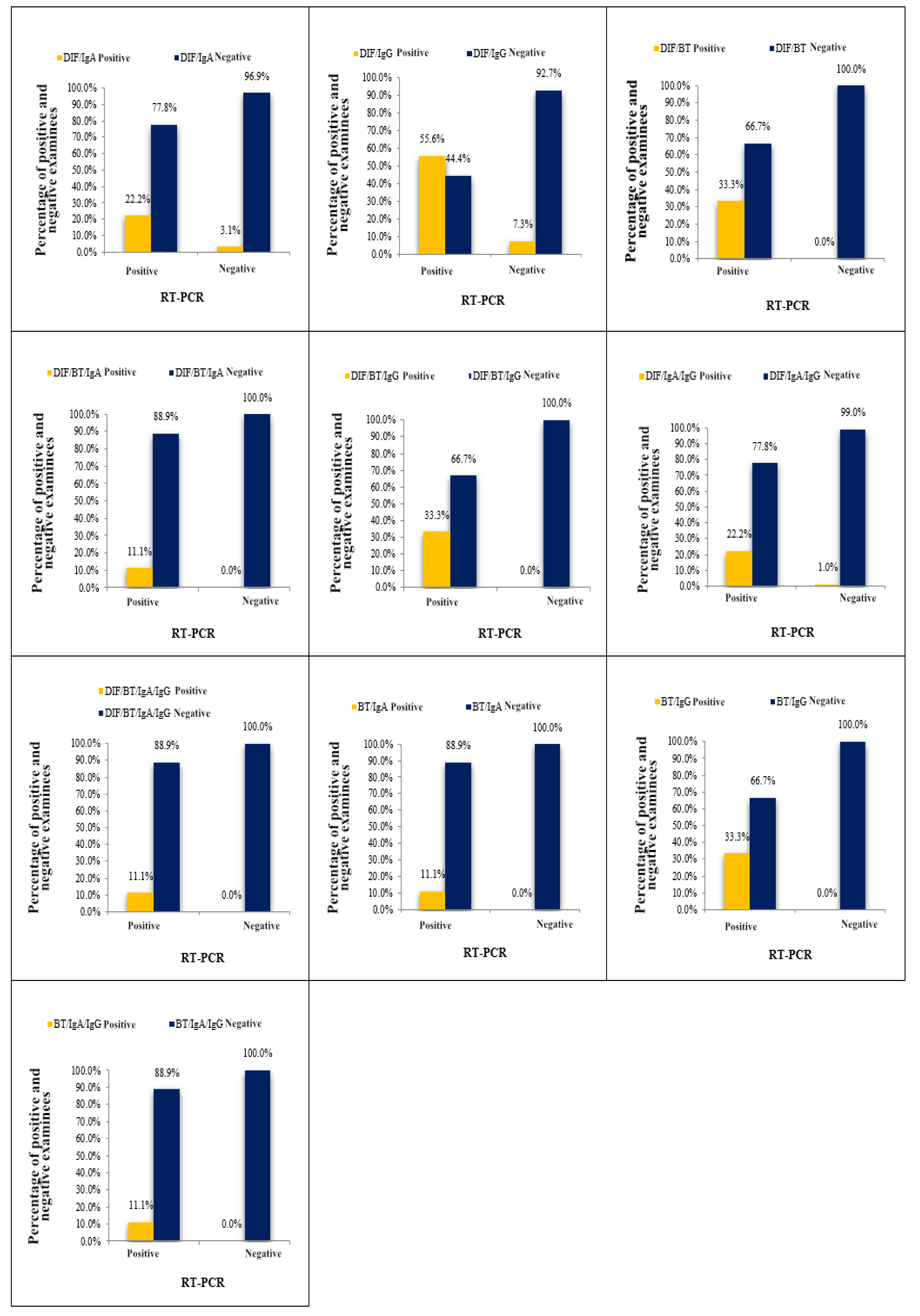
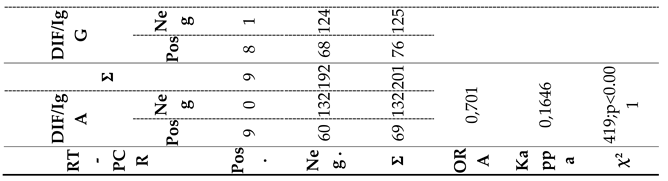
3.1. Concordance Between RT-PCR Assay and Multi-Test Positivity Approach for Result Comparison (Obtained by a Combination of Tests - Two or More Tests Positive Approach)
Using the "two or more tests positive" approach, the subjects' samples were considered positive for chlamydial infection when all the tests comprising the combination yielded positive results.
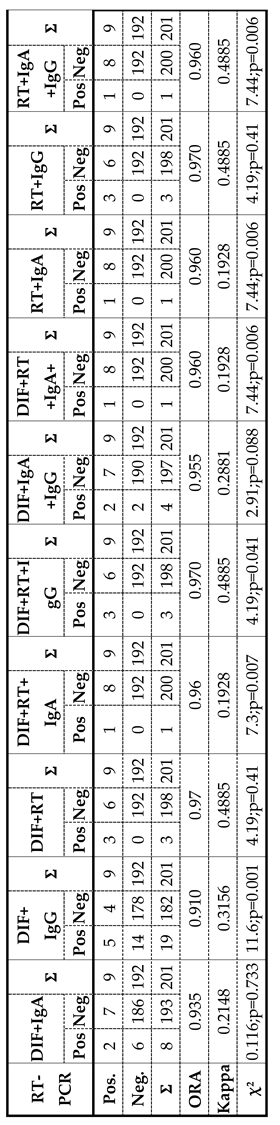
Table 2. and
Figure 1. display the outcomes of
C. trachomatis detection by a combination of “two or more tests positive” combination and RT-PCR assay. The agreement between the results of eleven combinations and the results obtained by RT-PCR assay was evaluated. Among the eleven analyzed combinations, the values of the ORA (representing statistical agreement) ranged from 91.00% - 97.00%, while the values of the
kappa index were 0.19-0.48. Notably, three combinations (DIF+RT; RT+IgG; DIF+RT+IgG;) displayed superior Performance and showed identical values of diagnostic parameter values. Due to significant variations among the tested combinations, we focused our analysis on those demonstrating the most favorable diagnostic parameters. Upon comparing the results obtained using three top-performing combinations and RT-PCR method, we noted that of the nine samples identified as positive on the basis of RT-PCR assay, the three first-rate combinations confirmed the diagnosis in three samples, while the remaining six samples were declared negative, resulting in a disagreement rate of 66.6% (false negative results). Conversely, there were no false positive results, as both diagnostic methods agreed on the negative results. Consequently, a total agreement of 97.00% was achieved, corresponding to a total disagreement of 3.00%. The results obtained from the three first-rate combinations and the RT-PCR method demonstrated a high percentage of total agreement, supported by a kappa index of 0.48 (
Table 2).
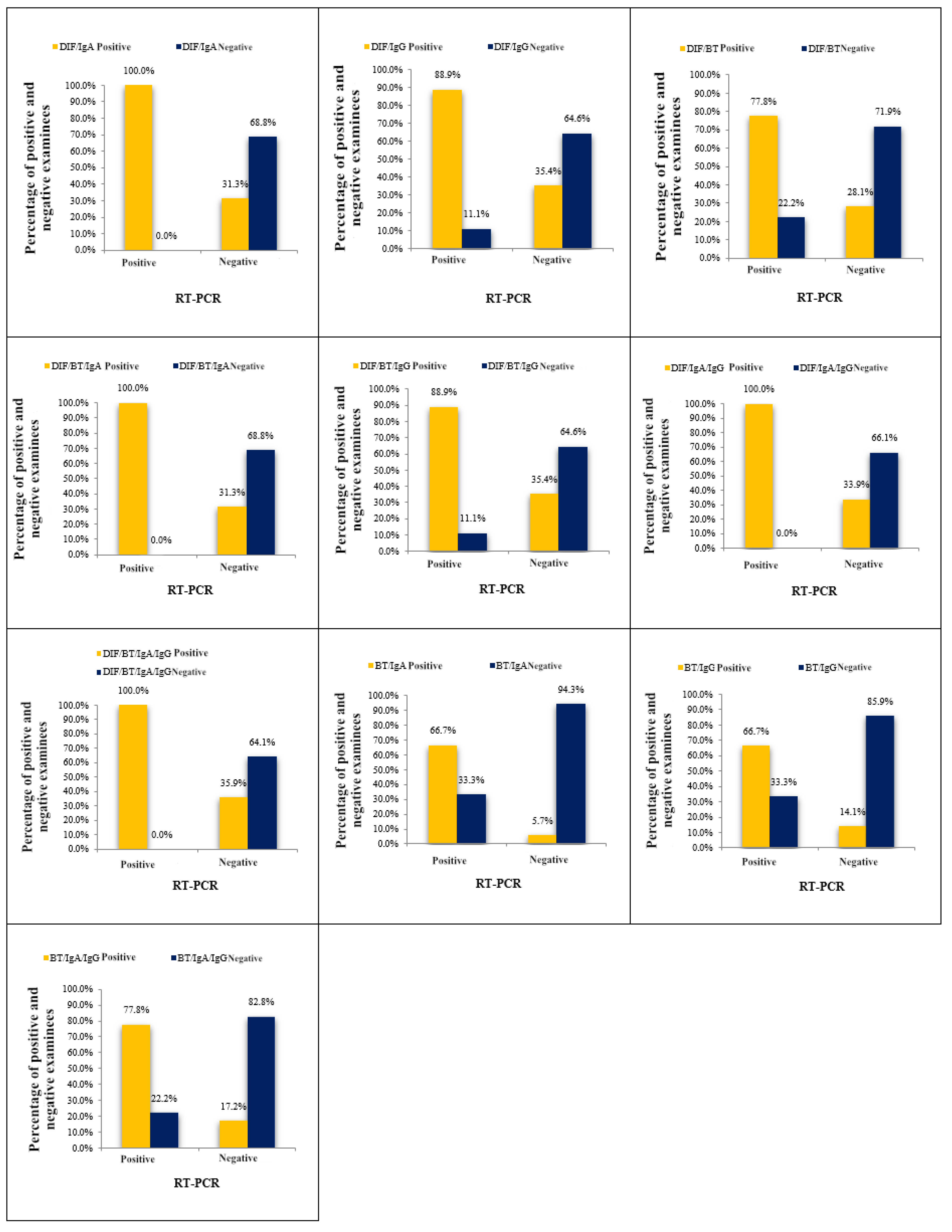
3.2. Concordance Between RT-PCR Assay and Multi-Test Positivity Approach for Result Comparison - Any Test Positive Approach
Ultimately, the cumulative agreement between the results obtained by the RT/IgA combination and the RT-PCR method reached 93.00%, corresponding to a total disagreement of 7.00%. The high percentage of total agreement was further supported by the kappa index of 0.42. These findings underscore the efficacy of the RT/IgA combination and its concordance with the RT-PCR method for chlamydial infection diagnosis.
Using the "any test positive" approach, subjects' samples were considered positive for chlamydial infection if any test within the combination yielded a positive result. In this study, we evaluated eleven combinations and assessed their agreement with a gold standard.
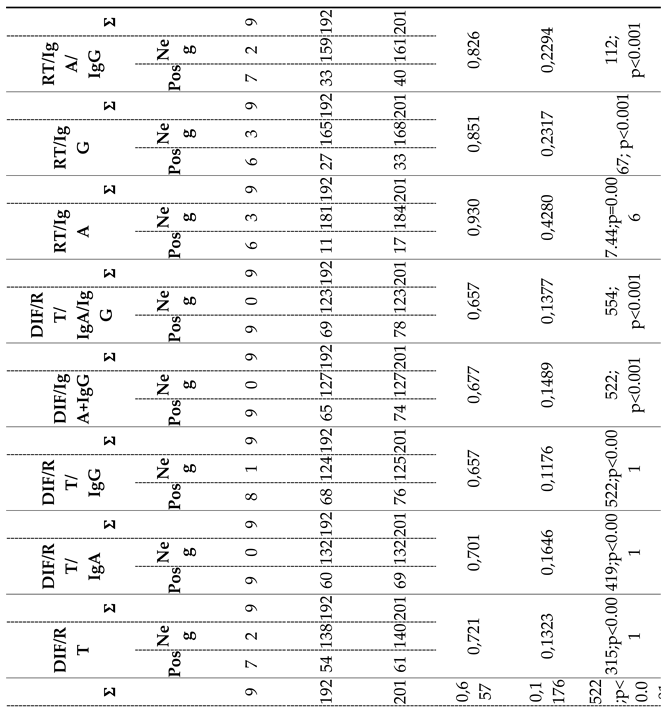
Table 3. and Supplementary
Figure 2. display the results of these combinations. The observed agreement (ORA) values ranged from 65.70% to 93.00%, and the kappa index values varied from 0.11 to 0.42.
Based on the diagnostic parameter values, the combination of RT/IgA was identified as the most effective among the eleven combinations tested. Among all subjects tested for chlamydial infection using both the RT/IgA combination and the RT-PCR method, the RT-PCR method confirmed infection in nine subjects, while the RT/IgA combination identified infection in six subjects, with three subjects classified as negative. This resulted in a disagreement rate of 33.30% (false negative results). Conversely, out of the 192 samples classified as negative by the RT-PCR assay, only eleven were categorized as positive using the RT/IgA combination, leading to a low disagreement rate of 5.70% (false positive findings).
3.3. Diagnostic Accuracy of the Tests
Following a comprehensive evaluation of the agreement outcomes between the RT-PCR assay, utilized as the gold standard in our study, and the results obtained through two distinct approaches, namely "two or more tests positive" and "any test positive" combinations, we proceeded to assess the diagnostic accuracy of both test combinations.
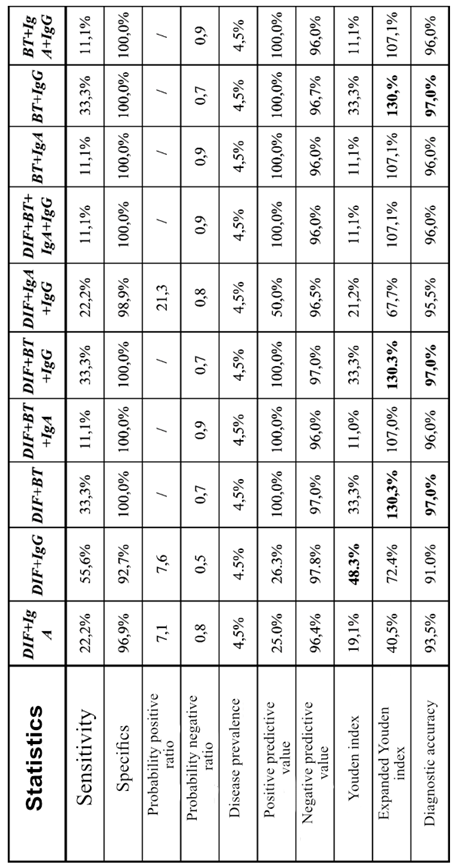
3.4. Diagnostic Accuracy of a Combination of Tests - "Two or More Tests Positive" Approach
Test combinations of the "two or more tests positive" approach
Table 4. gives high values of specificity (92.70% - 100.00%), but substantially low sensitivity (11.10% - 55.60%). The combination of DIF+IgG has the highest percentage of sensitivity of 55.60% with a high specificity of 92.70%, According to the values of the Juden index, the combination of DIF+IgG (48.30%) still stands out, showing the best-balanced relation between sensitivity and specificity. However, if we take into account PPV (Positive predictive value) and NPV (Negative predictive value) and calculate the extended Juden index, the situation changes. Namely, the combinations DIF+RT, DIF+RT+ IgG and RT+IgG, although with low sensitivity, but highest values of specificity, PPV and NPV shows the highest values of the extended Juden index of 130.30% and highest values of total diagnostic accuracy of 97.00% Table 4.
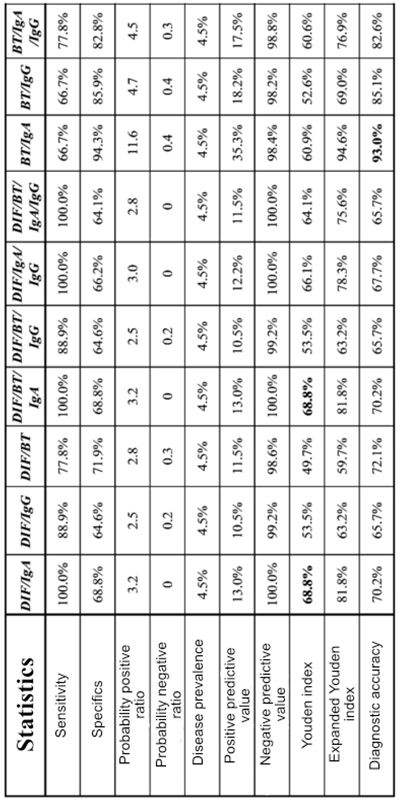
3.5. Diagnostic Accuracy of a Combination of Tests - "Any Test Positive" Approach
Table 5. shows parameters of the diagnostic accuracy of the combination of tests using "any test positive" approach. In this way, we improved the values of sensitivity (66.70% - 100%) with a slight decrease in specificity (64.10% - 94.30%). Combinations of DIF/IgA, DIF/RT/IgA, DIF/IgA/IgG and DIF/RT/IgA/IgG show a superior sensitivity of 100% with a satisfactory specificity of over 60%. According to the Juden index (68.80%), DIF/IgA, and DIF/RT/IgA combinations show the best balance of sensitivity and specificity. In comparison with "two or more tests positive" approach the PPV value of all analyzed test combinations dropped drastically (10.50%-35.30%) while NPV maintained high values (98.20% - 100%). Based on the results of the extended Juden index, taking into account PPV and NPV, the RT/IgA combination shows the highest values of 94.60%, as well as the highest values of total diagnostic accuracy of 93.00%.
This section may be divided by subheadings. It should provide a concise and precise description of the experimental results, their interpretation, as well as the experimental conclusions that can be drawn.
4. Discussion
In summary, based on the results of agreement tests and diagnostic efficiency parameters, we singled out combinations of tests "two or more tests positive" (DIF+RT; DIF+IgG; RT+IgG) and "any test positive" (BT/IgA; DIF/IgA) which demonstrated the best values of diagnostic parameters (
Table 5.). If possible, better results are achieved by a combination of different tests, which can raise the diagnostic efficiency of the tests to a higher level. Combinations of DIF+RT and RT+IgG do not improve diagnostic efficiency when compared to a rapid test that individually had the best parameters. When the combination of DIF+IgG is compared with DIF and IgG individual tests, the diagnostic efficiency is higher (ORA, kappa, specificity and PPV increase, but the sensitivity decreases), while compared to RT (sen: 33.30%; spec: 100.00%) shows a better-balanced relation between sensitivity (55.60%) and specificity (92.70%). The combination of RT/IgA in addition to high values of ORA and kappa shows a well-balanced ratio of sensitivity and specificity with a high specificity of 94.30%. On the other hand, the combination of DIF/IgA shows a high sensitivity of as much as 100.00% with a well-balanced relationship between sensitivity and specificity. (
Table 5.) In conclusion, a test that is highly sensitive, with not so high specificity, is suitable for a screening test. Based on that, our recommendation is that in cases when it is impossible to do the PCR method, to use the combination of DIF/IgA precisely because of the superior sensitivity of 100.00%. So, with this combination of tests, we will register all positive findings, i.e., we will not have false negative results, which is the basic role of the screening test. However, the lower specificity (68.80%) of this test combination means that this test combination will be false positive in 31.20% of individuals without chlamydial infection. In situations that require high specificity, the recommended combination is RT/IgA, which as a highly specific test has few false positive results, while the combinations of DIF+RT and BT + IgG, although showing a specificity of 100%, have low sensitivity (33.30%), due to which is why we prefer the RT/IgA combination.
According to World Health Organization report, the number of sexually transmitted bacterial infections worldwide is constantly on the rise, where the
Chlamydia trachomatis represents one of the leading pathogens. It is estimated that chlamydia sexually infects over 100 million people each year [
33]. If there is no spontaneous resolution of the infection, which occurs in a certain number of women, the infection spreads to the upper reproductive tract leading to persistent infection which could lead to serious consequences to the reproductive tract, including pelvic inflammatory disease, tubal factor infertility, as well as ectopic pregnancy [
34,
35,
36]. Given the asymptomatic nature of chlamydial infection, it is very important to choose a reliable test from the vast number of available tests.
In nations equipped with the necessary economic, spatial, and personnel resources for conducting nucleic acid amplification tests, clear recommendations are established. However, in countries lacking these prerequisites and operating under lower standards, alternative diagnostic methods, not endorsed for diagnosing acute chlamydial infections, are being utilized. Considering that neither of our studies recommends neither one from the analyzed tests due to low diagnostic efficiency, we tried to improve the diagnostic efficiency with the combination of tests, in relation to the individual tests. We have made two groups of test combinations: “two or more tests positive” and “any test positive". Combining “two or more tests positive” the best diagnostic efficiency, with low sensitivity shows combinations of DIF/BT, DIF/RT/IgG and RT/IgG. When compared with the fast test which one had individually the best parameters, combinations of DIF/RT, DIF /RT/IgG and RT/IgG did not improve diagnostic efficiency of the fast test which has been individually proven as the best .With these test combinations we preserved the high values of specifics, but along with the great decline of sensitivity. In this way, with this strict criterion, we have only increased diagnostics costs, but not diagnostic efficiency. Similar to that, these tests combinations cannot be recommended for acute chlamydial infections diagnosis. On the other side, by combining tests “any test positive" we improved the sensitivity with the slight decline of specifics. In this test combination the best diagnostic efficiency have shown RT/IgA combinations, and then RT/IgA/IgG which have shown the highest values of diagnostic accuracy in relation to the other combinations. Although these combinations, in relation to the fast test, had better balanced sensitivity and specificity, they did not improve diagnostic efficiency. Similarly, combinations of DIF/IgA and DIF/RT/IgA have got the best balanced sensitivity and specificity, but neither one of these combinations in a comparing to the individual tests did not improve diagnostic efficiency. Finally , analysis of the results from combining tests use has shown that in cases when it was not possible to perform tests of nucleic acid amplifications, it could be possible to use RT/IgA combination, both due to high ORA and kappa values, as well as due to well-balanced relation of sensitivity and specificity, with high specificity of 94.30%. On the other side, in situations which require high sensitivity, DIF/IgA combination is recommended, which in relation to the all others shows the best balance sensitivity and specificity with sensitivity of 100%. Similar results to ours also provided a study in which it was shown that combination of the results of different amplifications nucleic acid tests could, with preserved specificity, improve sensitivity detection of chlamydial infections with the note that use of individual tests for chlamydial infections diagnosis should be limited, especially with young woman [
37]. In contrast to this, but also to our results, are results of the study in which the amplifications nucleic acid tests combination by a strict criterion (both positive test result) showed low sensitivity and specificity [
38]. Some authors suggest that the results of a few imperfect tests could be used in combination in order to define the imperfect gold standard according to which the new test could be compared to [
39,
40,
41]. Furthermore, in one study with the help of combination “two or more tests positive” the gold standard has been defined, which served for comparison of new diagnostic tests [
42]. Further assumptions are that the use of three tests which are conditionally independent and grounded on the different clinical methods, i.e. antigen detection, cell culture and DNA amplification have less probability to make the same type errors than if combination consists of two amplifications tests [
43,
44].
5. Conclusions
Combining tests:” Two or more positive tests” or “Any test positive” did not improve the diagnostic efficiency compared to a single “Rapid test”, but our results also shows that combination Rapid test and immunoglobuline G class testing has the best diagnostic accuracy of 97%, expanded Youden index of 130%, specificity of 100% and it can be used for Chlamidia trachomatis detection in cases when diagnostic PCR test is not available.
Author Contributions
Conceptualization, J. T. P. and D. B.; methodology, J. T. P and D. B.; software, D. M.; validation, M. Š. and D. B.; formal analysis, M. Š.; investigation, J. T. P., V. N. and J. Č.; resources, J. T. P., V. N. and J. Č.; data curation, D. M.; writing—original draft preparation, J. T. P. and M. Š.; writing—review and editing, D. B. and M. Š.; visualization, P. S. and S. S.; supervision, D. B., M. Š. and A. N.; project administration, D.B.; funding acquisition, M. Š. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of NAME OF INSTITUTE (protocol code 4125 and date of approval 6th of July 2017.).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Acknowledgments
This study was supported by grant No III41010 from the Ministry of Education, Science and Technological Development of the Republic of Serbia.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
CDC Center for Disease Prevention and Control
DIF direct immunofluorescence test.
MOMP main outer membrane protein
RT rapid immunochromatographic test
RT-PCR Real-time polymerase chain reaction
ELISA enzyme-linked immunosorbent assay
ORA Overall Agreement
IgA and IgG- antibodies (immunoglobulins)
PPV Positive predictive value
NPV Negative predictive value
References
- Kreisel, K.M.; Spicknall, I.H.; Gargano, J.W.; Lewis, F.M.; Lewis, R.M.; Markowitz, L.E.; Roberts, H.; Satcher Johnson, A.; Song, R.; St. Cyr, S.B.; Weston, E.J.; Torrone, E.A.; Weinstock, H.S. Sexually transmitted infections among US women and men: Prevalence and incidence estimates. Sex Transm Dis 2021, 48, 208–214. [Google Scholar] [PubMed]
- CDC. Sexually Transmitted Disease Surveillance. Atlanta, GA: Department of Health and Human Services 2021, pp 21-41.
- European Centre for Disease Prevention and Control. Introduction to the Annual Epidemiological Report. In: ECDC. Annual epidemiological report for 2020 [Internet].
- European Centre for Disease Prevention Control. Chlamydia control in Europe - a survey of Member States Stockholm 2014.
- Leichliter, J.S.; Haderxhanaj, L.T.; Obafemi, O.A. Increasing sexually transmitted infections among adolescents in the USA. Lancet Child Adolesc Health 2021, 5, 609–611. [Google Scholar] [PubMed]
- Redmond, S.M.; Alexander-Kisslig, K.; Woodhall, S.C.; van den Broek, I.V.; van Bergen, J.; Ward, H.; et al. Genital chlamydia prevalence in Europe and non-European high income countries: systematic review and meta-analysis. PLoS One 2015, 10, e0115753. [Google Scholar]
- Lanjouw, E.; Ouburg, S.; de Vries, H.J.; Stary, A.; Radcliffe, K.; Unemo, M. European guidelines on the management of Chlamydia trachomatis infections. Int J STD AIDS 2015, 27, 333–348. [Google Scholar]
- Pereira, V.C.; Silva, S.N.; Carvalho, V.K.S.; et al. Strategies for the implementation of clinical practice guidelines in public health: An overview of systematic reviews. Health Res Policy Syst 2022, 20, 13. [Google Scholar]
- Piepert, J.F. Clinical practice. Genital chlamydial infections. N Engl J Med 2003, 349, pp 2424–30. [Google Scholar] [CrossRef]
- Haggerty, C.L.; Gottlieb, S.L.; Taylor, B.D.; Low, N.; Xu, F.; Ness, R.B. Risk of seqvelae after Chlamydia trachomatis genital infection in women. J Infect Dis 2010, 15, 134–155. [Google Scholar]
- Paavonen, J.; Eggert- Kruse, W. Chlamydia trachomatis impact on human reproduction. Hum Reprod Update 1999, 5, 433–447. [Google Scholar]
- Stamm, W.E.; Guinan, M.E.; Johnoson, C.; Starcher, T.; Holmes, K.K.; Mc Cormack, W.M. Effect of treatment regimens for Neisseria gonorrhoeae on simultaneous infection with Chlamydia trachomatis. New Engl J Med 1984, 310, 545–549. [Google Scholar]
- Rees, E. The treatment of pelvic inflammatory disease. Am J Obstet Gynecol 1980, 138, p 10427. [Google Scholar]
- Ripa, K.T.; Mardh, P.A. Cultivation of Chlamydia trachomatis in cycloheximide-treated mccoy cells. J Clin Microbiol 1977, 6, 328–331. [Google Scholar] [CrossRef] [PubMed]
- CDC. Screening Tests To Detect Chlamydia trachomatis and Neisseria gonorrhoeae infections Recommendations and Reports. MMWR 2002, 51, 15. [Google Scholar]
- Friedek, D.; Ekiel, A.; Martirosian, G. Chlamydia trachomatis: etiopathogenesis and diagnosis of infection. Pregl Epidemiol 2005, 59, 723–730. [Google Scholar]
- Schachter, J. Point-of-care tests using enzyme detection to diagnose Chlamydia trachomatis infection do not work. But when they fail in clinical trials, they reappear under different names. Sex Transm Infect 2016, 92, 406–407. [Google Scholar] [CrossRef]
- Abbai-Shaik, N.S.; Reddy, T.; Govender, S.; Ramiec, G. Poor Performance of the Chlamydia Rapid Test Device for the detection of Asymptomatic Infections in South African Men: A Pilot Study. Sex Transm Infect. J Sex Transm Dis 2016, 2016, 8695146. [Google Scholar] [CrossRef]
- Van Dommelen, L.; Van Tiel, F.H.; Ouburg, S.; et al. Alarmingly poor performance in Chlamydia trachomatis point-of-care testing. Sex Transm Infect 2010, 86, 355–359. [Google Scholar] [CrossRef]
- Van der Helm, J.J.; Sabajo, L.O.; Grunberg, A.W.; Morre, S.A.; Speksnijder, A.G.; de Vries, H.J. Point-of-care test for detection of urogenital Chlamydia in women shows low sensitivity. A performance evaluation study in two clinics in Suriname. PLoS One 2012, 7, e32122. [Google Scholar]
- Hislop, J.; Quayyum, Z.; Flett, G.; Boachie, C.; Fraser, C.; Mowatt, G. Systematic review of the clinical effectiveness and cost-effectiveness of rapid point-of-care tests for the detection of genital Chlamydia infection in women and men Review. Health Technol Assess 2010, 14, 1–97. [Google Scholar]
- Skidmore S Poorly performing point-of-care tests for chlamydia: What can be done? Sex Transm Infect 2010, 86, 330. [CrossRef]
- CDC. Recommendations for the Laboratory –Based Detection of Chlamydia trachomatis and Neisseria gonorrhoeae. Recommendations and Reports 2014.
- Puolakkainen, M. Laboratory diagnosis of persistent human chlamydial infection. Front Cell Infect Microbiol 2013, 3, 99. [Google Scholar] [CrossRef]
- Gijsen, A.P.; Land, J.A.; Goossens, V.J.; Slobbe, M.E.; Bruggeman, C.A. Chlamydia antibody testing in screening for tubal factor subfertility: the significance of IgG antibody decline over time. Hum Reprod 2002, 17, 699–703. [Google Scholar] [PubMed]
- den Hartog, J.E.; Morre´, S.A.; Land, J.A. Chlamydia trachomatis-associated tubal factor subfertility: immunogenetic aspects and serological screening. Hum Reprod Update 2006, 12, 719–730. [Google Scholar] [PubMed]
- Komoda, T. Kinetic study of antibodies (IgG, IgA) to Chlamydia trachomatis: Importance of IgA antibody in screening test for, C. trachomatis infection by peptid –based enzyme immunosorbent assay. Jpn J Infect Dis 2007, 60, 347–351. [Google Scholar]
- Workowski, K.A. Sexually Transmitted Infections Treatment Guidelines, Recommendations and Reports 2021, 70, 1–187. 70.
- Masek, B.J.; Arora, N.; Quinn, N.; et al. Performance of three nucleic acid amplification tests for detection of Chlamydia trachomatis and Neisseria gonorrhoeae by use of self-collected vaginal swabs obtained via an Internet-based screening program. J Clin Microbiol 2009, 47, 1663–1667. [Google Scholar]
- Knox, J.; Tabrizi, S.N.; Miller, P.; et al. Evaluation of self-collected samples in contrast to practitioner-collected samples for detection of Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis by polymerase chain reaction among women living in remote areas. Sex Transm Dis 2002, 29, 647–654. [Google Scholar]
- Dize, L.; Barnes, P., Jr.; Barnes, M.; et al. Performance of self-collected penile-meatal swabs compared to clinician-collected urethral swabs for the detection of Chlamydia trachomatis, Neisseria gonorrhoeae, Trichomonas vaginalis, and Mycoplasma genitalium by nucleic acid amplification assays. Diagn Microbiol Infect Dis 2016, 86, 131–135. [Google Scholar] [CrossRef]
- Berry, L. ; Stanley B Comparison of self-collected meatal swabs with urine specimens for the diagnosis of Chlamydia trachomatis and Neisseria gonorrhoeae in men. J Med Microbiol 2017, 66, 134–136. [Google Scholar]
- Nenoff, P.; Manos, A.; Ehrhard, I.; et al. Non-viral sexuallytransmitted infections - еpidemiology, clinicalmanifestations, diagnostics and therapy: Part 2: Chlamydia and mycoplasma. Hautarzt 2017, 68, 50–58. [Google Scholar] [CrossRef]
- LJubin-Sternak, S. ; Meštrovic T Chlamydia trachomatis and genital Mycoplasmas: pathogens with an impacton human reproductive health. J Pathog 2014, 2014, 183167. [Google Scholar]
- Morre Sa, van den Brule aJ, rozendaal l, et al Thenatural course of asymptomatic Chlamydiatrachomatis infections: 45% clearance and nodevelopment of clinical Pid after one-year follow-up. Int J STd aidS 2002, 13, 12–18.
- Chernesky Ma The laboratory diagnosis of Chlamydia trachomatis infections. Can J infect dis Med Microbiol 2005, 16, 39–44. [CrossRef] [PubMed]
- Martin, D.H.; Nsuami, M.; Schachter, J.; Hook, E.W., 3rd; Ferrero, D.; Quinn, T.C.; Gaydos, C. Use of multiple nucleic acid amplification tests to define the infected-patient"gold standard" in clinical trials of new diagnostic tests for Chlamydia trachomatis infections. Clin Microbiol 2004, 42, 4749–4758. [Google Scholar] [CrossRef] [PubMed]
- Hadgu, A.; Dendukuri, N. ; Wang L Evaluation of screening tests for detecting Chlamydia trachomatis: bias associated with the patient-infected-status algorithm. Epidemiology 2012, 23, 72–82. [Google Scholar] [CrossRef]
- Shrier, L.A.; Dean, D.; Klein, E.; Harter, K. ; Rice PA Limitations of screening tests for the detection of Chlamydia trachomatis in asymptomatic adolescent and young adult women. Am J Obstet Gynecol 2004, 190, 654–662. [Google Scholar] [CrossRef]
- Alonzo, T. A. and Pepe, M. S Assessing the accuracy of a new diagnostic test when a gold standard does not exist. Technical Report 156, Department of Biostatistics, University of Washington 1998.
- Torrance-Rynard, V. and Walter, S Efects of dependent errors in the assessment of diagnostic test performance. Statistics in Medicine 1997, 16, 2157–2175. [Google Scholar] [CrossRef]
- Todd, A.A.; Margaret, S. Pepe Using combination of reference tests to assess the accuracy of a new diagnostic test. Statist. Med 1999, 18, 2987–3003. [Google Scholar]
- David, H. Martin, Malanda Nsuami, Julius Schachter, et al Use of multiple Nucleic Acid Amplification Tests To Define the infeted –Patient “Gold Standard” in Clinical Trials of New Diagnostic Tests For Chlamydia trachomatis Infections. J Clin Microbiol 2004, 42, 4749–4758. [Google Scholar]
- Jelena Tošić Pajić. Diagnostic performance andeconomic aspects of tests for the detection of Chlamydia-e trachomatis [PhD thesis]. University Of Kragujevac. Faculty Of Medical Sciences Kragujevac, Serbia 2019.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).