Submitted:
16 March 2025
Posted:
17 March 2025
You are already at the latest version
Abstract
Keywords:
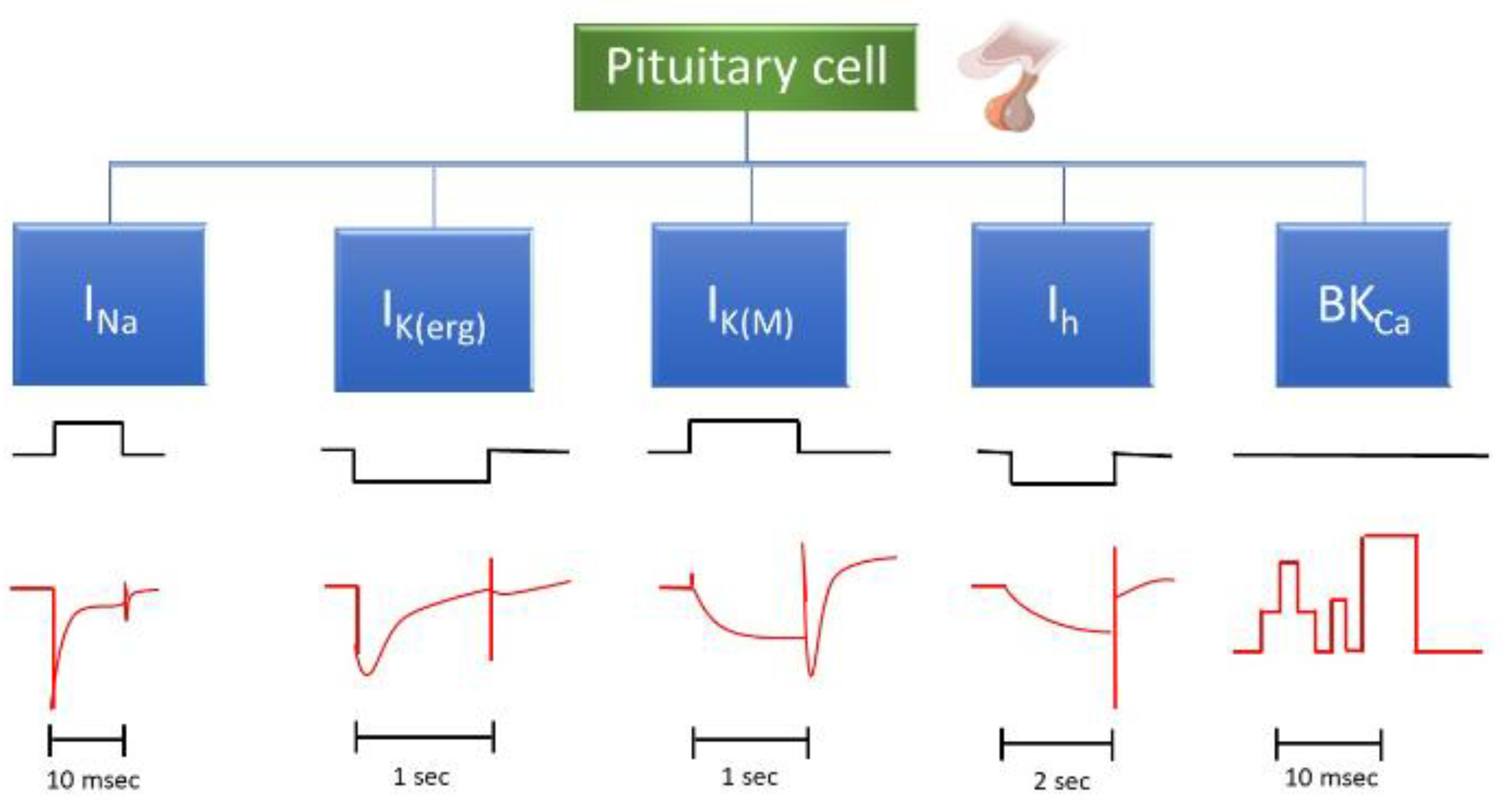
1. Introduction
A. Voltage-Gated Na+ Current (INa)
- 1. GV-58 ((2R)-2-[(6-{[(5-methylthiophen-2-yl)methyl]amino}-9-propyl-9H-purin-2-yl)amino]butan-1-ol)
- 2.
- Esaxerenone (ESAX, Minnebro®, CS-3150, XL-550, (4S)-4-(5,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)-2-fluoro-N-(1-methyl-1H-pyrazol-4-yl)benzamide)
B. erg-Mediated K+ Current (IK(erg))
- 1
- Risperidone (Risperdal®, 3-[2-[4-(6-fluoro-1,2-benzisoxazol-3-yl)piperidin-1-yl]ethyl]-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one)
- 2
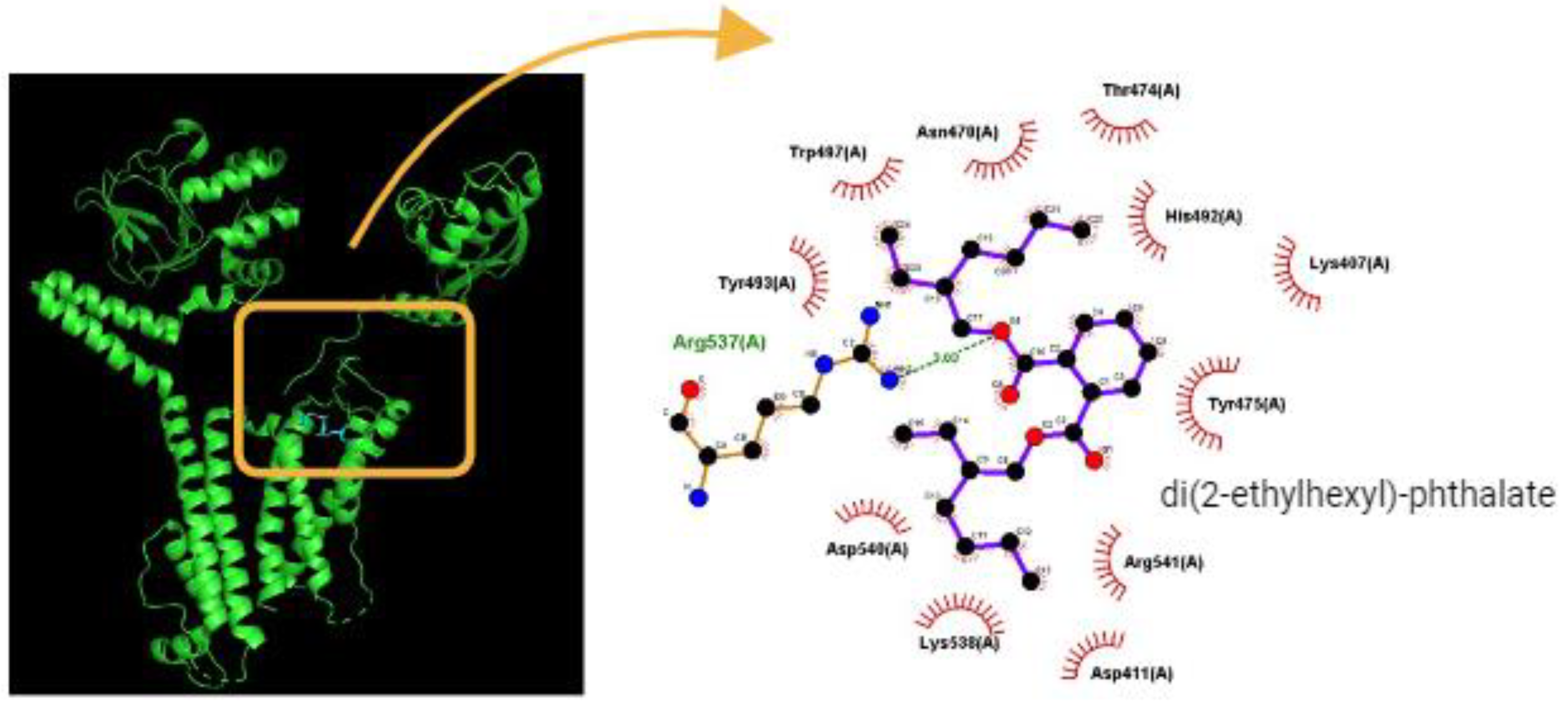
- Di(2-ethylhexyl)-phthalate (DEHP)
C. M-Type K+ Current (IK(M))
- 1. Solifenacin (SOL, Vesicare®, (R)-1-phenyl-3-(1-piperidin-4-ylpropyl)oxy-1,1-diphenyl-4-ylbutan-1-amine)
- 2.
- Kynurenic acid (KYNA, 4-hydroxyquinoline-2-carboxylic acid)
D. Hyperpolarization-Activated Cation Current (Ih)
- 1. Carisbamate (CRS, RW1-333369, Vimpat®, (S)-2-Oxo-1-pyrrolidineacetamide)
- 2
- Cannabidiol (CBD, 2-[(1R,6R)-3-methyl-6-prop-1-en-2-ylcyclohex-2-en-1-yl]-5-pentylbenzene-1,3-diol)
E. Large-conductance Ca2+-activated K+ (BKCa) channel
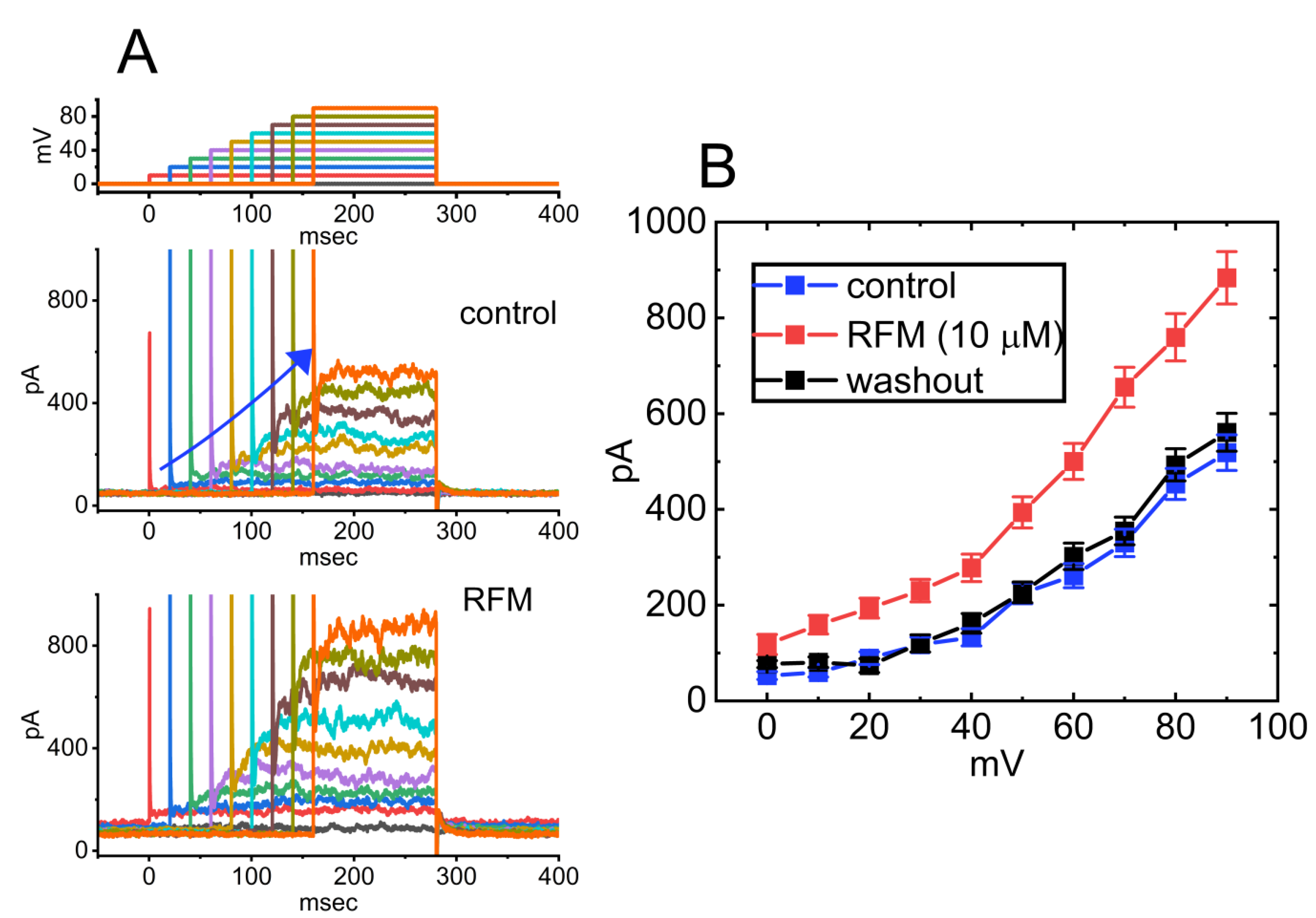
- 1. Rufinamide (RFM, Banzel®, Inovelon®, ethyl 1-(2,6-difluorophenyl)-1H-1,2,3-triazole-4-carboxylate)
- 2 QO-40 ((5-(chloromethyl)-3-(naphthalen-1-yl)-2-(trifluoromethyl)pyrazolo [1,5-a]pyrimidin-7(4 H)-one)
2. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AP | action potential |
| BKCa channel | large-conductance Ca2+-activated K+ channel |
| HERG channel | human erg K+ channel |
| Ih | hyperpolarization-activated cationic current |
| IK(Ca) | Ca2+-activated K+ current |
| IK(erg) | erg-mediated K+ current |
| IK(M) | M-type K+ current |
| INaINa(P) | voltage-gated Na+ currentpersistent Na+ current |
| Kerg channel | erg-mediated KV channel |
| NaV channel | voltage-gated Na+ channel |
| PitNet | pituitary neuroendocrine tumor |
References
- Abbott, G.W. KCNQs: Ligand- and Voltage-Gated Potassium Channels. Front Physiol 2020, 11, 583. [Google Scholar] [CrossRef] [PubMed]
- Abrams, P.; Andersson, K.E. Muscarinic receptor antagonists for overactive bladder. BJU Int 2007, 100, 987–1006. [Google Scholar] [CrossRef]
- Anderson, D.; Yu, T.W.; Hinçal, F. Effect of some phthalate esters in human cells in the comet assay. Teratog Carcinog Mutagen 1999, 19, 275–280. [Google Scholar] [CrossRef]
- Aragay, A.M.; Katz, A.; Simon, M.I. The G alpha q and G alpha 11 proteins couple the thyrotropin-releasing hormone receptor to phospholipase C in GH3 rat pituitary cells. J Biol Chem 1992, 267, 24983–24988. [Google Scholar] [CrossRef] [PubMed]
- Arai, K.; Tsuruoka, H.; Homma, T. CS-3150, a novel non-steroidal mineralocorticoid receptor antagonist, prevents hypertension and cardiorenal injury in Dahl salt-sensitive hypertensive rats. Eur J Pharmacol 2015, 769, 266–273. [Google Scholar] [CrossRef]
- Armeni, E.; Grossman, A. The Spectrum of Familial Pituitary Neuroendocrine Tumors. Endocr Pathol 2023, 34, 57–78. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, S. Rufinamide. Neurotherapeutics 2007, 4, 155–162. [Google Scholar] [CrossRef]
- Barg, S.; Galvanovskis, J.; Göpel, S.O.; Rorsman, P.; Eliasson, L. Tight coupling between electrical activity and exocytosis in mouse glucagon-secreting alpha-cells. Diabetes 2000, 49, 1500–1510. [Google Scholar] [CrossRef]
- Bauer, C.K.; Schwarz, J.R. Ether-à-go-go K+ channels: effective modulators of neuronal excitability. J Physiol 2018, 596, 769–783. [Google Scholar] [CrossRef]
- Besag, F.M.C.; Vasey, M.J.; Chin, R.F.M. Current and emerging pharmacotherapy for the treatment of Lennox-Gastaut syndrome. Expert Opin Pharmacother 2023, 24, 1249–1268. [Google Scholar] [CrossRef]
- Bhat, A.A.; Gupta, G.; Afzal, O.; Kazmi, I.; Al-Abbasi, F.A.; Alfawaz Altamimi, A.S.; Almalki, W.H.; Alzarea, S.I.; Singh, S.K.; Dua, K. Neuropharmacological effect of risperidone: From chemistry to medicine. Chem Biol Interact 2023, 369, 110296. [Google Scholar] [CrossRef] [PubMed]
- Black, B.J.; Atmaramani, R.; Pancrazio, J.J. Spontaneous and evoked activity from murine ventral horn cultures on microelectrode arrays. Front Cell Neurosci 2017, 11, 304. [Google Scholar] [CrossRef] [PubMed]
- Breier, A.F.; Malhotra, A.K.; Su, T.P.; Pinals, D.A.; Elman, I.; Adler, C.M.; Lafargue, R.T.; Clifton, A.; Pickar, D. Clozapine and risperidone in chronic schizophrenia: effects on symptoms, parkinsonian side effects, and neuroendocrine response. Am J Psychiatry 1999, 156, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Britch, S.C.; Babalonis, S.; Walsh, S.L. Cannabidiol: pharmacology and therapeutic targets. Psychopharmacology (Berl) 2021, 238, 9–28. [Google Scholar] [CrossRef]
- Brown, D.A.; Passmore, G.M. Neural KCNQ (Kv7) channels. Br J Pharmacol 2009, 156, 1185–1195. [Google Scholar] [CrossRef]
- Carboni, E.; Rolando, M.T.; Silvagni, A.; Di Chiara, G. Increase of dialysate dopamine in the bed nucleus of stria terminalis by clozapine and related neuroleptics. Neuropsychopharmacology 2000, 22, 140–147. [Google Scholar] [CrossRef]
- Catterall, W.A.; Lenaeus, M.J.; Gamal El-Din, T.M. Structure and pharmacology of voltage-gated sodium and calcium channels. Annu Rev Pharmacol Toxicol 2020, 60, 133–154. [Google Scholar] [CrossRef]
- Chan, H.Y.; Lin, W.W.; Lin, S.K.; Hwang, T.J.; Su, T.P.; Chiang, S.C.; Hwu, H.G. Efficacy and safety of aripiprazole in the acute treatment of schizophrenia in Chinese patients with risperidone as an active control: a randomized trial. J Clin Psychiatry 2007, 68, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.T.; Gao, Z.H.; Li, S.W.; Liu, P.Y.; Lo, Y.C.; Wu, S.N. Characterization in dual activation by oxaliplatin, a platinum-based chemotherapeutic agent of hyperpolarization-activated cation and electroporation-induced currents. Int J Mol Sci 2020, 21, 396. [Google Scholar] [CrossRef]
- Chang, W.T.; Wu, S.N. Activation of voltage-gated sodium current and inhibition of erg-mediated potassium current caused by telmisartan, an antagonist of angiotensin II type-1 receptor, in HL-1 atrial cardiomyocytes. Clin Exp Pharmacol Physiol 2018, 45, 797–807. [Google Scholar] [CrossRef]
- Chang, W.T.; Wu, S.N. Characterization of direct perturbations on voltage-gated sodium current by esaxerenone, a nonsteroidal mineralocorticoid receptor blocker. Biomedicines 2021, 9, 549. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.T.; Wu, S.N. Effective activation of BKCa channels by QO-40 (5-(chloromethyl)-3-(Naphthalen-1-yl)-2-(Trifluoromethyl)Pyrazolo [1,5-a]pyrimidin-7(4H)-one), known to be an opener of KCNQ2/Q3 channels. Pharmaceuticals (Basel) 2021, 14, 388. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, S.; Das, U.; Jabbar, S.; Gangisetty, O.; Rousseau, B.; Hanft, S.; Sarkar, D.K. Developmental pluripotency-associated 4 increases aggressiveness of pituitary neuroendocrine tumors by enhancing cell stemness. Neuro Oncol 2025, 27, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.S.; So, E.C.; Wu, S.N. Modulating hyperpolarization-activated cation currents through small molecule perturbations: magnitude and gating Control. Biomedicines 2023, 11, 2177. [Google Scholar] [CrossRef]
- Chen, L.; Cho, H.Y.; Chuang, T.H.; Ke, T.L.; Wu, S.N. The effectiveness of isoplumbagin and plumbagin in regulating amplitude, gating kinetics, and voltage-dependent hysteresis of erg-mediated K+ currents. Biomedicines 2022, 10, 780. [Google Scholar] [CrossRef]
- Chen, Y.; Li, D.; Li, N.; Loh, P.; Guo, Y.; Hu, X.; Zhang, J.; Dou, B.; Wang, L.; Yang, C.; Guo, T.; Chen, S.; Liu, Z.; Chen, B.; Chen, Z. Role of nerve signal transduction and neuroimmune crosstalk in mediating the analgesic effects of acupuncture for neuropathic pain. Front Neurol 2023, 14, 1093849. [Google Scholar] [CrossRef]
- Cho, H.Y.; Chen, P.C.; Chuang, T.H.; Yu, M.C.; Wu, S.N. Activation of voltage-gated Na+ current by GV-58, a known activator of CaV channels. Biomedicines 2022, 10, 721. [Google Scholar] [CrossRef]
- Cho, H.Y.; Chuang, T.H.; Wu, S.N. Effective perturbations on the amplitude and hysteresis of erg-mediated potassium current caused by 1-octylnonyl 8-[(2-hydroxyethyl)[6-oxo-6(undecyloxy)hexyl]amino]-octanoate (SM-102), a cationic lipid. Biomedicines 2021, 9, 1367. [Google Scholar] [CrossRef]
- Chojnacki, C.; Gąsiorowska, A.; Popławski, T.; Konrad, P.; Chojnacki, M.; Fila, M.; Blasiak, J. Beneficial Effect of Increased Tryptophan Intake on Its Metabolism and Mental State of the Elderly. Nutrients 2023, 15, 847. [Google Scholar] [CrossRef]
- Chuang, T.H.; Cho, H.Y.; Wu, S.N. Effective Accentuation of Voltage-Gated Sodium Current Caused by Apocynin (4'-Hydroxy-3'-methoxyacetophenone), a Known NADPH-Oxidase Inhibitor. Biomedicines 2021, 9, 1146. [Google Scholar] [CrossRef]
- Crunelli V, David F, Morais TP, Lorincz ML. HCN channels and absence seizures. Neurobiol Dis 2023, 181, 106107. [Google Scholar] [CrossRef]
- De Alcubierre, D.; Ferrari, D.; Mauro, G.; Isidori, A.M.; Tomlinson, J.W.; Pofi, R. Glucocorticoids and cognitive function: a walkthrough in endogenous and exogenous alterations. J Endocrinol Invest 2023, 46, 1961–1982. [Google Scholar] [CrossRef] [PubMed]
- Demeter, I.; Nagy, K.; Gellért, L.; Vécsei, L.; Fülöp, F.; Toldi, J. A novel kynurenic acid analog (SZR104) inhibits pentylenetetrazole-induced epileptiform seizures. An electrophysiological study : special issue related to kynurenine. J Neural Transm (Vienna) 2012, 119, 151–154. [Google Scholar] [CrossRef]
- Deng, J.; Liao, X.; Cao, H. Neuroendocrine tumors in a patient with multiple endocrine neoplasia type 1 syndrome: A case report and review of the literature. Medicine (Baltimore) 2023, 102, e34350. [Google Scholar] [CrossRef]
- DiFrancesco, D. The role of the funny current in pacemaker activity. Circ Res 2010, 106, 434–446. [Google Scholar] [CrossRef] [PubMed]
- Elkommos, S.; Mula, M. Current and future pharmacotherapy options for drug-resistant epilepsy. Expert Opin Pharmacother 2022, 23, 2023–2034. [Google Scholar] [CrossRef]
- Fernandes, M.J.; Carneiro, J.E.; Amorim, R.P.; Araujo, M.G.; Nehlig, A. Neuroprotective agents and modulation of temporal lobe epilepsy. Front Biosci (Elite Ed) 2015, 7, 79–93. [Google Scholar] [CrossRef]
- Fortunati, N.; Guaraldi, F.; Zunino, V.; Penner, F.; D'Angelo, V.; Zenga, F.; Pecori Giraldi, F.; Catalano, M.G.; Arvat, E. Effects of environmental pollutants on signaling pathways in rat pituitary GH3 adenoma cells. Environ Res 2017, 158, 660–668. [Google Scholar] [CrossRef]
- Fraguas-Sánchez, A.I.; Torres-Suárez, A.I. Medical use of cannabinoids. Drugs 2018, 78, 1665–1703. [Google Scholar] [CrossRef]
- Gandía, L.; Lara, B.; Imperial, J.S.; Villarroya, M.; Albillos, A.; Maroto, R.; García, A.G.; Olivera, B.M. Analogies and differences between omega-conotoxins MVIIC and MVIID: binding sites and functions in bovine chromaffin cells. Pflugers Arch 1997, 435, 55–64. [Google Scholar] [CrossRef]
- Gaudenzi, C.; Mifsud, K.R.; Reul, J.M.H.M. Insights into isoform-specific mineralocorticoid receptor action in the hippocampus. J Endocrinol 2023, 258, e220293. [Google Scholar] [CrossRef] [PubMed]
- Ghisari, M.; Bonefeld-Jorgensen, E.C. Effects of plasticizers and their mixtures on estrogen receptor and thyroid hormone functions. Toxicol Lett 2009, 189, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Ghovanloo, M.R.; Ruben, P.C. Cannabidiol and sodium channel pharmacology: General overview, mechanism, and clinical implications. Neuroscientist 2022, 28, 318–334. [Google Scholar] [CrossRef]
- Gillum, N.; Karabekian, Z.; Swift, L.M.; Brown, R.P.; Kay, M.W.; Sarvazyan, N. Clinically relevant concentrations of di (2-ethylhexyl) phthalate (DEHP) uncouple cardiac syncytium. Toxicol Appl Pharmacol 2009, 236, 25–38. [Google Scholar] [CrossRef]
- Halperin, R.; Tirosh, A. Progress report on multiple endocrine neoplasia type 1. Fam Cancer 2025, 24, 15. [Google Scholar] [CrossRef] [PubMed]
- Hamill, O.P.; Marty, A.; Neher, E.; Sakmann, B.; Sigworth, F.J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch 1981, 391, 85–100. [Google Scholar] [CrossRef]
- Hamilton, A.; Zhang, Q.; Gao, R.; Hill, T.G.; Salehi, A.; Knudsen, J.G.; Draper, M.B.; Johnson, P.R.V.; Rorsman, P.; Tarasov, A.I. Nicotinic Signaling Stimulates Glucagon Secretion in Mouse and Human Pancreatic α-Cells. Diabetes 2025, 74, 53–64. [Google Scholar] [CrossRef]
- Harnod, T.; Yang, Y.C.; Chiu, L.T.; Wang, J.H.; Lin, S.Z.; Ding, D.C. Use of bladder antimuscarinics is associated with an increased risk of dementia: a retrospective population-based case-control study. Sci Rep 2021, 11, 4827. [Google Scholar] [CrossRef] [PubMed]
- Haug, T.M.; Hafting, T.; Sand, O. Inhibition of BK channels contributes to the second phase of the response to TRH in clonal rat anterior pituitary cells. Acta Physiol Scand 2004, 180, 347–357. [Google Scholar] [CrossRef]
- He, C.; Chen, F.; Li, B.; Hu, Z. Neurophysiology of HCN channels: from cellular functions to multiple regulations. Prog Neurobiol 2014, 112, 1–23. [Google Scholar] [CrossRef]
- He, F.Z.; McLeod, H.L.; Zhang, W. Current pharmacogenomic studies on hERG potassium channels. Trends Mol Med 2013, 19, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Hinkle PM, Tashjian AH Jr. Thyrotropin-releasing hormone regulates the number of its own receptors in the GH3 strain of pituitary cells in culture. Biochemistry 1975, 14, 3845–3851. [Google Scholar] [CrossRef]
- Hu, C.H.; Wu, S.N.; So, E.C. Tyrosine kinase inhibitors, ionic currents, and cardiac arrhythmia. Front Oncol 2023, 13, 1218821. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.H.; So, E.C.; Liu, Y.C.; Wu, S.N. Glucocorticoids stimulate the activity of large-conductance Ca2+-activated K+ channels in pituitary GH3 and AtT-20 cells via a non-genomic mechanism. Steroids 2006, 71, 129–140. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, M.; Chen, X.; Zhang, R.; Le, A.; Hong, M.; Zhang, Y.; Jia, L.; Zang, W.; Jiang, C.; Wang, J.; Fan, X.; Wang, J. Tryptophan metabolism in central nervous system diseases: Pathophysiology and potential therapeutic strategies. Aging Dis 2023, 14, 858–878. [Google Scholar] [CrossRef] [PubMed]
- Hung, T.Y.; Wu, S.N.; Huang, C.W. The Modulation of Ubiquinone, a Lipid Antioxidant, on Neuronal Voltage-Gated Sodium Current. Nutrients 2022, 14, 3393. [Google Scholar] [CrossRef]
- Hung, T.Y.; Wu, S.N.; Huang, C.W. Concerted suppressive effects of carisbamate, an anti-epileptic alkyl-carbamate drug, on voltage-gated Na+ and hyperpolarization-activated cation currents. Front Cell Neurosci 2023, 17, 1159067. [Google Scholar] [CrossRef]
- Irisawa, H.; Brown, H.F.; Giles, W. Cardiac pacemaking in the sinoatrial node. Physiol Rev 1993, 73, 197–227. [Google Scholar] [CrossRef]
- Jaeger, R.J.; Rubin, R.J. Migration of a phthalate ester plasticizer from polyvinyl chloride blood bags into stored human blood and its localization in human tissues. N Engl J Med 1972, 287, 1114–1118. [Google Scholar] [CrossRef]
- Jeong, H.J.; Min, S.; Baek, J.; Kim, J.; Chung, J.; Jeong, K. Real-time reaction monitoring of azide-alkyne cycloadditions using benchtop NMR-based signal amplification by reversible exchange (SABRE). ACS Meas Sci Au 2023, 3, 134–142. [Google Scholar] [CrossRef]
- Jia, C.; Qi, J.; Zhang, F.; Mi, Y.; Zhang, X.; Chen, X.; Liu, L.; Du, X.; Zhang, H. Activation of KCNQ2/3 potassium channels by novel pyrazolo[1,5-a]pyrimidin-7(4H)-one derivatives. Pharmacology 2011, 87, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Jorratt, P.; Ricny, J.; Leibold, C.; Ovsepian, S.V. Endogenous modulators of NMDA receptor control dendritic field expansion of cortical neurons. Mol Neurobiol 2023, 60, 1440–1452. [Google Scholar] [CrossRef]
- Jurewicz, J.; Hanke, W. Exposure to phthalates: reproductive outcome and children health. A review of epidemiological studies. Int J Occup Med Environ Health 2011, 24, 115–141. [Google Scholar] [CrossRef] [PubMed]
- Kario, K.; Nishizawa, M.; Kato, M.; Ishii, H.; Uchiyama, K.; Nagai, M.; Takahashi, N.; Asakura, T.; Shiraiwa, T.; Yoshida, T.; Kaneshiro, M.; Taguchi, T.; Shiosakai, K.; Sugimoto, K. Nighttime home blood pressure lowering effect of esaxerenone in patients with uncontrolled nocturnal hypertension: the EARLY-NH study. Hypertens Res 2023, 46, 1782–1794. [Google Scholar] [CrossRef]
- Kelly, M.J.; Wagner, E.J. Canonical transient receptor potential channels and hypothalamic control of homeostatic functions. J Neuroendocrinol. 2024, 36, e13392. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Ishizaka, M.; Kazusaka, A.; Fujita, S. Di-(2-ethylhexyl) phthalate suppresses tamoxifen-induced apoptosis in GH3 pituitary cells. Arch Toxicol 2007, 81, 27–33. [Google Scholar] [CrossRef]
- Klaessens, S.; Stroobant, V.; De Plaen, E.; Van den Eynde, B.J. Systemic tryptophan homeostasis. Front Mol Biosci 2022, 9, 897929. [Google Scholar] [CrossRef]
- Knaus HG, McManus OB, Lee SH, Schmalhofer WA, Garcia-Calvo M, Helms LM, Sanchez M, Giangiacomo K, Reuben JP, Smith AB 3rd, et al. Tremorgenic indole alkaloids potently inhibit smooth muscle high-conductance calcium-activated potassium channels. Biochemistry 1994, 33, 5819–5828. [Google Scholar] [CrossRef]
- Kreder, K.J. Solifenacin. Urol Clin North Am 2006, 33, 483–490. [Google Scholar] [CrossRef]
- Krithivasan, R.; Miller, G.Z.; Belliveau, M.; Gearhart, J.; Krishnamoorthi, V.; Lee, S.; Kannan, K. Analysis of ortho-phthalates and other plasticizers in select organic and conventional foods in the United States. J Expo Sci Environ Epidemiol 2023, 33, 778–786. [Google Scholar] [CrossRef]
- Kuo, P.C.; Yang, C.J.; Lee, Y.C.; Chen, P.C.; Liu, Y.C.; Wu, S.N. The comprehensive electrophysiological study of curcuminoids on delayed-rectifier K+ currents in insulin-secreting cells. Eur J Pharmacol 2018, 819, 233–241. [Google Scholar] [CrossRef]
- Kushmerick, C.; Romano-Silva, M.A.; Gomez, M.V.; Prado, M.A. Muscarinic regulation of Ca2+ oscillation frequency in GH3 cells. Brain Res 1999, 851, 39–45. [Google Scholar] [CrossRef]
- Lai, M.C.; Wu, S.N.; Huang, C.W. Rufinamide, a triazole-derived antiepileptic drug, stimulates Ca2+-activated K+ currents while inhibiting voltage-gated Na+ currents. Int J Mol Sci 2022, 23, 13677. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Lee, M.L.; Shih, C.C.; Liou, H.H. Carisbamate (RWJ-333369) inhibits glutamate transmission in the granule cell of the dentate gyrus. Neuropharmacology 2011, 61, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Choi, J.S.; Choi, B.H.; Hahn, S.J. Inhibition of cloned hERG potassium channels by risperidone and paliperidone. Naunyn Schmiedebergs Arch Pharmacol 2017, 390, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Leng, L.; Zhang, Y. Effects of an estrogen receptor antagonist on proliferation, prolactin secretion and growth factor expression in the MMQ pituitary prolactinoma cell line. J Clin Neurosci 2011, 18, 1694–1698. [Google Scholar] [CrossRef]
- Lerma, E.; White, W.B.; Bakris, G. Effectiveness of nonsteroidal mineralocorticoid receptor antagonists in patients with diabetic kidney disease. Postgrad Med 2023, 135, 224–233. [Google Scholar] [CrossRef]
- Leucht, S.; Schneider-Thoma, J.; Burschinski, A.; Peter, N.; Wang, D.; Dong, S.; Huhn, M.; Nikolakopoulou, A.; Salanti, G.; Davis, J.M. Long-term efficacy of antipsychotic drugs in initially acutely ill adults with schizophrenia: systematic review and network meta-analysis. World Psychiatry 2023, 22, 315–324. [Google Scholar] [CrossRef]
- Li, S.; Dai, J.; Zhang, L.; Zhang, J.; Zhang, Z.; Chen, B. An association of elevated serum prolactin with phthalate exposure in adult men. Biomed Environ Sci 2011, 24, 31–39. [Google Scholar] [CrossRef]
- Li, Y.; Patel, M.; Baroudi, J.; Wu, M.; Gatti, S.; Liang, M.; Wipf, P.; Badawi, Y.; Meriney, S.D. A cross-sectional study of ageing at the mouse neuromuscular junction and effects of an experimental therapeutic approach for dynapenia. J Physiol 2023, 601, 4135–4150. [Google Scholar] [CrossRef]
- Liang, M.; Tarr, T.B.; Bravo-Altamirano, K.; Valdomir, G.; Rensch, G.; Swanson, L.; DeStefino, N.R.; Mazzarisi, C.M.; Olszewski, R.A.; Wilson, G.M.; Meriney, S.D.; Wipf, P. Synthesis and biological evaluation of a selective N- and p/q-type calcium channel agonist. ACS Med Chem Lett 2012, 3, 985–990. [Google Scholar] [CrossRef]
- Lin, D.S.; Lin, F.J.; Lin, Y.S.; Lee, J.K.; Lin, Y.H. The effects of mineralocorticoid receptor antagonists on cardiovascular outcomes in patients with end-stage renal disease and heart failure. Eur J Heart Fail 2023, 25, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.H.; Lin, J.F.; Yu, M.C.; Wu, S.N.; Wu, C.L.; Cho, H.Y. Characterization in potent modulation on voltage-gated Na+ current exerted by deltamethrin, a pyrethroid insecticide. Int J Mol Sci 2022, 23, 14733. [Google Scholar] [CrossRef]
- Liu, C.; Jinlong, Q.I.; Zhang, H.; Jia, Q. Pharmacokinetic study of QO-58, A new potassium channel opener. Chin Pharmacol Bull 2014, 30, 574–577. [Google Scholar]
- Liu, Y.C.; So, E.C.; Wu, S.N. Cannabidiol modulates M-type K+ and hyperpolarization-activated cation currents. Biomedicines 2023, 11, 2651. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.K.; Wu, S.N.; Lee, C.T.; Li, H.F.; Chiang, H.T. Characterization of action potential waveform-evoked L-type calcium currents in pituitary GH3 cells. Pflugers Arch 2001, 442, 547–557. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Smith, S.J.; Drummond, P.D. Modulation of the hypothalamic-pituitary-adrenal (HPA) axis by plants and phytonutrients: a systematic review of human trials. Nutr Neurosci 2022, 25, 1704–1730. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Zheng, J.; Cao, Y.; Bresnahan, R.; Martin-McGill, K.J. Carisbamate add-on therapy for drug-resistant focal epilepsy. Cochrane Database Syst Rev 2021, 12, CD012121. [Google Scholar] [CrossRef]
- Majdinasab, N.; Orakifar, N.; Kouti, L.; Shamsaei, G.; Seyedtabib, M.; Jafari, M. Solifenacin versus posterior tibial nerve stimulation for overactive bladder in patients with multiple sclerosis. Front Neurosci 2023, 17, 1107886. [Google Scholar] [CrossRef]
- Maljevic, S.; Wuttke, T.V.; Lerche, H. Nervous system KV7 disorders: breakdown of a subthreshold brake. J Physiol 2008, 586, 1791–1801. [Google Scholar] [CrossRef]
- Männikkö, R.; Pandey, S.; Larsson, H.P.; Elinder, F. Hysteresis in the voltage dependence of HCN channels: conversion between two modes affects pacemaker properties. J Gen Physiol 2005, 125, 305–326. [Google Scholar] [CrossRef]
- Maria, S.A.; Kumar, A.; Wilfred, P.M.; Shanthi, M.; Peedicayil, J. Inhibition of contractility of isolated caprine detrusor by the calcium channel blocker cilnidipine and reversal by calcium channel openers. Curr Ther Res Clin Exp 2023, 99, 100717. [Google Scholar] [CrossRef]
- Martínez, A.; García-Gutiérrez, P.; Zubillaga, R.A.; Garza, J.; Vargas, R. Main interactions of dopamine and risperidone with the dopamine D2 receptor. Phys Chem Chem Phys 2021, 23, 14224–14230. [Google Scholar] [CrossRef]
- Masutomi, N.; Shibutani, M.; Takagi, H.; Uneyama, C.; Lee, K.Y.; Hirose, M. Alteration of pituitary hormone-immunoreactive cell populations in rat offspring after maternal dietary exposure to endocrine-active chemicals. Arch Toxicol 2004, 78, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Mok, M.H.; Fricker, A.C.; Weil, A.; Kew, J.N. Electrophysiological characterisation of the actions of kynurenic acid at ligand-gated ion channels. Neuropharmacology 2009, 57, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Moroni, F.; Cozzi, A.; Sili, M.; Mannaioni, G. Kynurenic acid: a metabolite with multiple actions and multiple targets in brain and periphery. J Neural Transm (Vienna) 2012, 119, 133–139. [Google Scholar] [CrossRef]
- Mourão, A.A.; de Mello, A.B.S.; Dos Santos Moreira, M.C.; Rodrigues, K.L.; Lopes, P.R.; Xavier, C.H.; Gomes, R.M.; Freiria-Oliveira, A.H.; Blanch, G.T.; Colombari, E.; Pedrino, G.R. Median preoptic nucleus excitatory neurotransmitters in the maintenance of hypertensive state. Brain Res Bull 2018, 142, 207–215. [Google Scholar] [CrossRef]
- Munkhjargal, U.; Fukuda, D.; Ganbaatar, B.; Suto, K.; Matsuura, T.; Ise, T.; Kusunose, K.; Yamaguchi, K.; Yagi, S.; Yamada, H.; Soeki, T.; Wakatsuki, T.; Sata, M. A selective mineralocorticoid receptor blocker, esaxerenone, attenuates vascular dysfunction in diabetic C57BL/6 mice. J Atheroscler Thromb 2023, 30, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Nagy, K.; Plangár, I.; Tuka, B.; Gellért, L.; Varga, D.; Demeter, I.; Farkas, T.; Kis, Z.; Marosi, M.; Zádori, D.; Klivényi, P.; Fülöp, F.; Szatmári, I.; Vécsei, L.; Toldi, J. Synthesis and biological effects of some kynurenic acid analogs. Bioorg Med Chem 2011, 19, 7590–7596. [Google Scholar] [CrossRef]
- Ohashi, N.; Uta, D.; Ohashi, M.; Baba, H. Analgesic effect of ivabradine against inflammatory pain mediated by hyperpolarization-activated cyclic nucleotide-gated cation channels expressed on primary afferent terminals in the spinal dorsal horn. Pain 2022, 163, 1356–1369. [Google Scholar] [CrossRef]
- Ojala, K.S.; Kaufhold, C.J.; Davey, M.R.; Yang, D.; Liang, M.; Wipf, P.; Badawi, Y.; Meriney, S.D. Potentiation of neuromuscular transmission by a small molecule calcium channel gating modifier improves motor function in a severe spinal muscular atrophy mouse model. Hum Mol Genet 2023, 32, 1901–1911. [Google Scholar] [CrossRef] [PubMed]
- Panebianco, M.; Prabhakar, H.; Marson, A.G. Rufinamide add-on therapy for refractory epilepsy. Cochrane Database Syst Rev 2018, 4, CD011772. [Google Scholar] [CrossRef] [PubMed]
- Parsons, M.; Robinson, D.; Cardozo, L. Darifenacin in the treatment of overactive bladder. Int J Clin Pract 2005, 59, 831–838. [Google Scholar] [CrossRef]
- Paul, E.R.; Schwieler, L.; Erhardt, S.; Boda, S.; Trepci, A.; Kämpe, R.; Asratian, A.; Holm, L.; Yngve, A.; Dantzer, R.; Heilig, M.; Hamilton, J.P.; Samuelsson, M. Peripheral and central kynurenine pathway abnormalities in major depression. Brain Behav Immun 2022, 101, 136–145. [Google Scholar] [CrossRef]
- Pearce, P.T.; McNally, M.; Funder, J.W. Nuclear localization of type 1 aldosterone binding sites in steroid-unexposed GH3 cells. Clin Exp Pharmacol Physiol 1986, 13, 647–654. [Google Scholar] [CrossRef]
- Pieterman, C.R.C.; Valk, G.D. Update on the clinical management of multiple endocrine neoplasia type 1. Clin Endocrinol (Oxf) 2022, 97, 409–423. [Google Scholar] [CrossRef] [PubMed]
- Pong, A.W.; Ross, J.; Tyrlikova, I.; Giermek, A.J.; Kohli, M.P.; Khan, Y.A.; Salgado, R.D.; Klein, P. Epilepsy: expert opinion on emerging drugs in phase 2/3 clinical trials. Expert Opin Emerg Drugs 2022, 27, 75–90. [Google Scholar] [CrossRef]
- Raman, G.; Tunnicliffe, D.; Lai, E.; Bennett, T.; Caldwell, P. Safety and tolerability of solifenacin in children and adolescents with overactive bladder- a systematic review. J Pediatr Urol 2023, 19, 19.e1–19.e13. [Google Scholar] [CrossRef]
- Rezvani, A.H.; Lawrence, A.J.; Arolfo, M.P.; Levin, E.D.; Overstreet, D.H. Novel medication targets for the treatment of alcoholism: preclinical studies. Recent Pat CNS Drug Discov 2012, 7, 151–162. [Google Scholar] [CrossRef]
- Rodrigues, T.C.M.L.; de Moura, J.P.; Dos Santos, A.M.F.; Monteiro, A.F.M.; Lopes, S.M.; Scotti, M.T.; Scotti, L. Epileptic targets and drugs: A mini-review. Curr Drug Targets 2023, 24, 212–224. [Google Scholar] [CrossRef]
- Sakakibara, K.; Feng, G.G.; Li, J.; Akahori, T.; Yasuda, Y.; Nakamura, E.; Hatakeyama, N.; Fujiwara, Y.; Kinoshita, H. Kynurenine causes vasodilation and hypotension induced by activation of KCNQ-encoded voltage-dependent K+ channels. J Pharmacol Sci. 2015, 129, 31–37. [Google Scholar] [CrossRef]
- Sankar, R.; Chez, M.; Pina-Garza, J.E.; Dixon-Salazar, T.; Flamini, J.R.; Hyslop, A.; McGoldrick, P.; Millichap, J.J.; Resnick, T.; Rho, J.M.; Wolf, S. Proposed anti-seizure medication combinations with rufinamide in the treatment of Lennox-Gastaut syndrome: Narrative review and expert opinion. Seizure 2023, 110, 42–57. [Google Scholar] [CrossRef] [PubMed]
- Sankaranarayanan, S.; Simasko, S.M. Characterization of an M-like current modulated by thyrotropin-releasing hormone in normal rat lactotrophs. J Neurosci 1996, 16, 1668–1678. [Google Scholar] [CrossRef]
- Secondo, A.; De Mizio, M.; Zirpoli, L.; Santillo, M.; Mondola, P. The Cu-Zn superoxide dismutase (SOD1) inhibits ERK phosphorylation by muscarinic receptor modulation in rat pituitary GH3 cells. Biochem Biophys Res Commun 2008, 376, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Sills, G.J. Pharmacological diversity amongst approved and emerging antiseizure medications for the treatment of developmental and epileptic encephalopathies. Ther Adv Neurol Disord 2023, 16, 17562864231191000. [Google Scholar] [CrossRef] [PubMed]
- Simasko, S.M.; Sankaranarayanan, S. Characterization of a hyperpolarization-activated cation current in rat pituitary cells. Am J Physiol 1997, 272, E405–14. [Google Scholar] [CrossRef]
- Singh, M.; Sapkota, K.; Sakimura, K.; Kano, M.; Cowell, R.M.; Overstreet-Wadiche, L.; Hablitz, J.J.; Nakazawa, K. Maturation of GABAergic synaptic transmission from neocortical parvalbumin interneurons involves N-methyl-D-aspartate receptor recruitment of Cav2.1 channels. Neuroscience 2023, 513, 38–53. [Google Scholar] [CrossRef]
- Sinvani, L.; Afroz-Hossain, A.; Muran, A.; Strunk, A.; Williams, M.S.; Qiu, M.; Zeltser, R.; Makaryus, A.N.; Wolf-Klein, G.; Pekmezaris, R. Electrocardiogram monitoring practices for hospitalized adults receiving antipsychotics: A retrospective cohort study. J Psychiatr Pract 2022, 28, 108–116. [Google Scholar] [CrossRef]
- So, E.C.; Foo, N.P.; Ko, S.Y.; Wu, S.N. Bisoprolol, known to be a selective β₁-receptor antagonist, differentially but directly suppresses IK(M) and IK(erg) in pituitary cells and hippocampal neurons. Int J Mol Sci 2019, 20, 657. [Google Scholar] [CrossRef]
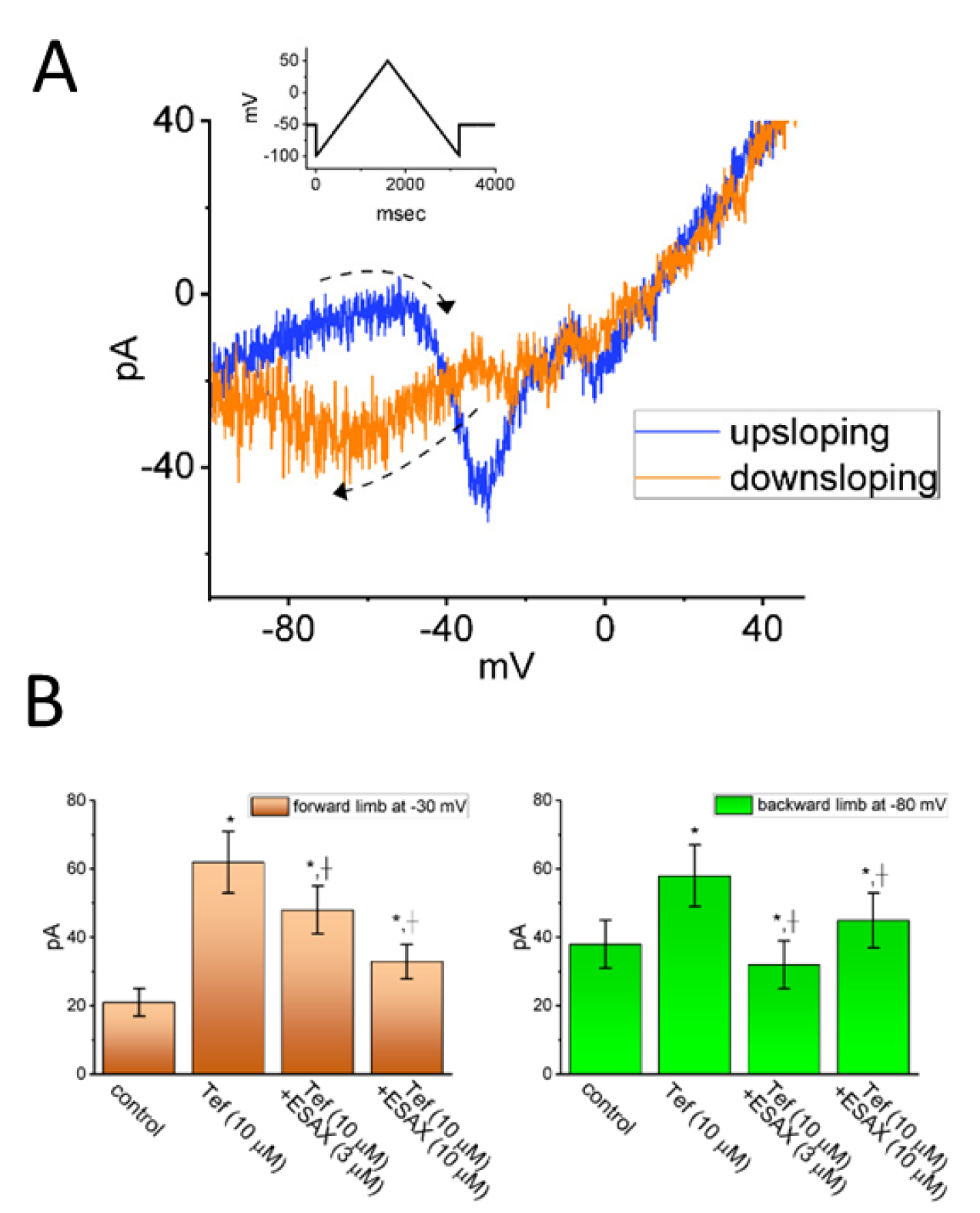
- So, E.C.; Wu, S.N.; Lo, Y.C.; Su, K. Differential regulation of tefluthrin and telmisartan on the gating charges of INa activation and inactivation as well as on resurgent and persistent INa in a pituitary cell line (GH3). Toxicol Lett 2018, 285, 104–112. [Google Scholar] [CrossRef]
- Stojilkovic, S.S. Pituitary cell type-specific electrical activity, calcium signaling and secretion. Biol Res 2006, 39, 403–423. [Google Scholar] [CrossRef] [PubMed]
- Stojilkovic SS, Tabak J, Bertram. Ion channels and signaling in the pituitary gland. Endoc Rev 2010, 31, 845–915. [Google Scholar] [CrossRef] [PubMed]
- Stojkovic, M.; Radmanovic, B.; Jovanovic, M.; Janjic, V.; Muric, N.; Ristic, D.I. Risperidone induced hyperprolactinemia: From basic to clinical studies. Front Psychiatry 2022, 13, 874705. [Google Scholar] [CrossRef]
- Suter, M.R.; Kirschmann, G.; Laedermann, C.J.; Abriel, H.; Decosterd, I. Rufinamide attenuates mechanical allodynia in a model of neuropathic pain in the mouse and stabilizes voltage-gated sodium channel inactivated state. Anesthesiology 2013, 118, 160–172. [Google Scholar] [CrossRef]
- Tarr, T.B.; Lacomis, D.; Reddel, S.W.; Liang, M.; Valdomir, G.; Frasso, M.; Wipf, P.; Meriney, S.D. Complete reversal of Lambert-Eaton myasthenic syndrome synaptic impairment by the combined use of a K+ channel blocker and a Ca2+ channel agonist. J Physiol 2014, 592, 3687–3696. [Google Scholar] [CrossRef]
- Tarr, T.B.; Valdomir, G.; Liang, M.; Wipf, P.; Meriney, S.D. New calcium channel agonists as potential therapeutics in Lambert-Eaton myasthenic syndrome and other neuromuscular diseases. Ann N Y Acad Sci 2012, 1275, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Teng, B.C.; Song, Y.; Zhang, F.; Ma, T.Y.; Qi, J.L.; Zhang, H.L.; Li, G.; Wang, K. Activation of neuronal Kv7/KCNQ/M-channels by the opener QO58-lysine and its anti-nociceptive effects on inflammatory pain in rodents. Acta Pharmacol Sin 2016, 37, 1054–1062. [Google Scholar] [CrossRef]
- Ullah, S.; Ahmad, S.; Guo, X.; Ullah, S.; Ullah, S.; Nabi, G.; Wanghe, K. A review of the endocrine disrupting effects of micro and nano plastic and their associated chemicals in mammals. Front Endocrinol (Lausanne) 2023, 13, 1084236. [Google Scholar] [CrossRef]
- Vandenberg JI, Perry MD, Perrin MJ, Mann SA, Ke Y, Hill AP. HERG K+ channels: structure, function, and clinical significance. Physiol Rev 2012, 92, 1393–1478. [Google Scholar] [CrossRef]
- Vécsei, L.; Horváth, Z.; Tuka, B. Old and new neuroendocrine molecules: somatostatin, cyseamine, pantethine and kynurenine. Ideggyogy Sz 2014, 67, 107–112. [Google Scholar]
- Viudez-Martínez, A.; García-Gutiérrez, M.S.; Manzanares, J. Cannabidiol regulates the expression of hypothalamus-pituitary-adrenal axis-related genes in response to acute restraint stress. J Psychopharmacol 2018, 32, 1379–1384. [Google Scholar] [CrossRef]
- Vohora, D.; Saraogi, P.; Yazdani, M.A.; Bhowmik, M.; Khanam, R.; Pillai, K.K. Recent advances in adjunctive therapy for epilepsy: focus on sodium channel blockers as third-generation antiepileptic drugs. Drugs Today (Barc) 2010, 46, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Wahl-Schott C, Fenske S, Biel M. HCN channels: new roles in sinoatrial node function. Curr Opin Pharmacol 2014, 15, 83–90. [Google Scholar] [CrossRef]
- Weiss, B. Endocrine disruptors as a threat to neurological function. J Neurol Sci 2011, 305, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Welk, B.; McClure, J.A. The impact of anticholinergic use for overactive bladder on cognitive changes in adults with normal cognition, mild cognitive impairment, or dementia. Eur Urol Open Sci 2022, 46, 22–29. [Google Scholar] [CrossRef]
- Wier, H.A.; Cerna, A.; So, T.Y. Rufinamide for pediatric patients with Lennox-Gastaut syndrome: a comprehensive overview. Paediatr Drugs 2011, 13, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; White, H.V.; Boehm, B.A.; Meriney, C.J.; Kerrigan, K.; Frasso, M.; Liang, M.; Gotway, E.M.; Wilcox, M.R.; Johnson, J.W.; Wipf, P.; Meriney, S.D. New Cav2 calcium channel gating modifiers with agonist activity and therapeutic potential to treat neuromuscular disease. Neuropharmacology 2018, 131, 176–189. [Google Scholar] [CrossRef]
- Wu, S.N.; Chiang, H.T.; Shen, A.Y.; Lo, Y.K. Differential effects of quercetin, a natural polyphenolic flavonoid, on L-type calcium current in pituitary tumor (GH3) cells and neuronal NG108-15 cells. J Cell Physiol 2003, 195, 298–308. [Google Scholar] [CrossRef]
- Wu, S.N.; Jan, C.R.; Li, H.F.; Chiang, H.T. Characterization of inhibition by risperidone of the inwardly rectifying K+ current in pituitary GH3 cells. Neuropsychopharmacology 2000, 23, 676–689. [Google Scholar] [CrossRef]
- Wu, S.N.; Li, H.F. Characterization of riluzole-induced stimulation of large-conductance calcium-activated potassium channels in rat pituitary GH3 cells. J Investig Med 1999, 47, 484–495. [Google Scholar]
- Wu, S.N.; Lo, Y.K.; Li, H.F.; Shen, A.Y. Functional coupling of voltage-dependent L-type Ca2+ current to Ca2+-activated K+ current in pituitary GH3 cells. Chin J Physiol 2001, 44, 161–167. [Google Scholar]
- Wu, S.N.; Yang, W.H.; Yeh, C.C.; Huang, H.C. The inhibition by di(2-ethylhexyl)-phthalate of erg-mediated K⁺ current in pituitary tumor (GH₃) cells. Arch Toxicol 2012, 86, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.N.; Yu, M.C. Inhibition of Voltage-Gated Na+ Currents Exerted by KB-R7943 (2-[2-[4-(4-nitrobenzyloxy)phenyl]ethyl]isothiourea), an Inhibitor of Na+-Ca2+ Exchanging Process. Int J Mol Sci 2023, 24, 1805. [Google Scholar] [CrossRef] [PubMed]
- Wulfsen, I.; Hauber, H.P.; Schiemann, D.; Bauer, C.K.; Schwarz, J.R. Expression of mRNA for voltage-dependent and inward-rectifying K channels in GH3/B6 cells and rat pituitary. J Neuroendocrinol 2000, 12, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Wyant GA, Yu W, Doulamis IP, Nomoto RS, Saeed MY, Duignan T, McCully JD, Kaelin WG Jr. Mitochondrial remodeling and ischemic protection by G protein-coupled receptor 35 agonists. Science 2022, 377, 621–629. [Google Scholar] [CrossRef]
- Xiao, Y.F.; Chandler, N.; Dobrzynski, H.; Richardson, E.S.; Tenbroek, E.M.; Wilhelm, J.J.; Sharma, V.; Varghese, A.; Boyett, M.R.; Iaizzo, P.A.; Sigg, D.C. Hysteresis in human HCN4 channels: a crucial feature potentially affecting sinoatrial node pacemaking. Sheng Li Xue Bao 2010, 62, 1–13. [Google Scholar]
- Yeh, P.S.; Wu, S.J.; Hung, T.Y.; Huang, Y.M.; Hsu, C.W.; Sze, C.I.; Hsieh, Y.J.; Huang, C.W.; Wu, S.N. Evidence for the Inhibition by temozolomide, an imidazotetrazine family alkylator, of intermediate-conductance Ca2+-activated K+ channels in glioma cells. Cell Physiol Biochem 2016, 38, 1727–1742. [Google Scholar] [CrossRef]
- Yoshida, Y.; Fujiwara, M.; Kinoshita, M.; Sada, K.; Miyamoto, S.; Ozeki, Y.; Iwamoto, M.; Mori, Y.; Nagai, S.; Matsuda, N.; Noguchi, T.; Okamoto, M.; Gotoh, K.; Masaki, T.; Shibata, H. Effects of esaxerenone on blood pressure, urinary albumin excretion, serum levels of NT-proBNP, and quality of life in patients with primary aldosteronism. Hypertens Res 2024, 47, 157–167. [Google Scholar] [CrossRef]
- Yu, C.; Deng, X.J.; Xu, D. Gene mutations in comorbidity of epilepsy and arrhythmia. J Neurol 2023, 270, 1229–1248. [Google Scholar] [CrossRef]
- Yue C, Yaari Y. KCNQ/M channels control spike afterdepolarization and burst generation in hippocampal neurons. J Neurosci 2004, 24, 4614–4624. [Google Scholar] [CrossRef]
- Zaborska, K.E.; Jordan, K.L.; Thorson, A.S.; Dadi, P.K.; Schaub, C.M.; Nakhe, A.Y.; Dickerson, M.T.; Lynch, J.C.; Weiss, A.J.; Dobson, J.R.; Jacobson, D.A. Liraglutide increases islet Ca2+ oscillation frequency and insulin secretion by activating hyperpolarization-activated cyclic nucleotide-gated channels. Diabetes Obes Metab 2022, 24, 1741–1752. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Mi, Y.; Qi, J.L.; Li, J.W.; Si, M.; Guan, B.C.; Du, X.N.; An, H.L.; Zhang, H.L. Modulation of K(v)7 potassium channels by a novel opener pyrazolo[1,5-a]pyrimidin-7(4H)-one compound QO-58. Br J Pharmacol. 2013, 168, 1030–1042. [Google Scholar] [CrossRef] [PubMed]
- Zhang Y, Li D, Darwish Y, Fu X, Trussell LO, Huang H. KCNQ channels enable reliable presynaptic spiking and synaptic transmission at high frequency. J Neurosci 2022, 42, 3305–3315. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Y.; Tao, Y.; Guo, X.; Cui, Y.; Li, Z. Phthalates (PAEs) and reproductive toxicity: Hypothalamic-pituitary-gonadal (HPG) axis aspects. J Hazard Mater 2023, 459, 132182. [Google Scholar] [CrossRef]
- Zhou, X.; Li, A.; Mi, X.; Li, Y.; Ding, Z.; An, M.; Chen, Y.; Li, W.; Tao, X.; Chen, X.; Li, Y. Hyperexcited limbic neurons represent sexual satiety and reduce mating motivation. Science 2023, 379, 820–825. [Google Scholar] [CrossRef]
- Mishra, P.; Narayanan, R. The enigmatic HCN channels: A cellular neurophysiology perspective. Proteins. 2025, 93, 72–92. [Google Scholar] [CrossRef]
- Chen, L.; Chehade, H.D.; Chu, H.Y. Motor cortical neuronal hyperexcitability associated with α-synuclein aggregation. NPJ Parkinsons Dis 2025, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Cui, W.; Zhu, D.; Gao, N.; Zhu, Y. Common tools for pituitary adenomas research: cell lines and primary cells. Pituitary 2020, 23, 182–188. [Google Scholar] [CrossRef]
- Villa, C.; Birtolo, M.F.; Perez-Rivas, L.G.; Righi, A.; Assie, G.; Baussart, B.; Asioli, S. Grading and staging for pituitary neuroendocrine tumors. Brain Pathol 2025, 35, e13299. [Google Scholar] [CrossRef]
- Cui, R.; Duan, H.; Hu, W.; Li, C.; Zhong, S.; Liang, L.; Chen, S.; Hu, H.; He, Z.; Wang, Z.; Guo, X.; Chen, Z.; Xu, C.; Zhu, Y.; Chen, Y.; Sai, K.; Yang, Q.; Guo, C.; Mou, Y.; Jiang, X. Establishment of Human Pituitary Neuroendocrine Tumor Derived Organoid and Its Pilot Application for Drug Screening. J Clin Endocrinol Metab 2025, 110, e827–e840. [Google Scholar] [CrossRef]
- Calvanese, F.; Jannelli, G.; Feuvret, L.; Vasiljevic, A.; Manet, R.; Sergeant, C.; Raverot, G.; Jouanneau, E. Aggressive Pituitary Tumors and Pituitary Carcinomas: definition, management and overview for clinical practice. Neuro-Oncology Advances 2025, vdae114. [Google Scholar] [CrossRef]
- Perrier, N.D. From Initial Description by Wermer to Present-Day MEN1, What have We Learned? World J Surg 2018, 42, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Gedvilaite-Vaicechauskiene, G.; Kriauciuniene, L.; Tamasauskas, A.; Rovite, V.; Mandrika, I.; Wu, S.N.; Huang, C.W.; Poskiene, L.; Liutkeviciene, R. Pituitary Adenoma: SSTR2 rs2236750, SSTR5 rs34037914, and AIP rs267606574 Genetic Variants, Serum Levels, and Ki-67 Labeling Index Associations. Medicina (Kaunas) 2024, 60, 1252. [Google Scholar] [CrossRef]
- Cui, R.; Duan, H.; Hu, W.; Li, C.; Zhong, S.; Liang, L.; Chen, S.; Hu, H.; He, Z.; Wang, Z.; Guo, X.; Chen, Z.; Xu, C.; Zhu, Y.; Chen, Y.; Sai, K.; Yang, Q.; Guo, C.; Mou, Y.; Jiang, X. Establishment of Human Pituitary Neuroendocrine Tumor Derived Organoid and Its Pilot Application for Drug Screening. J Clin Endocrinol Metab 2025, 110, e827–e840. [Google Scholar] [CrossRef] [PubMed]
| Ionic current | Chemical or drug | Abbreviated name | Chemical structure* | Reference |
| INa | GV-58 | NA | 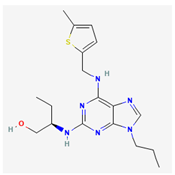 |
Cho et al., 2022 |
| Esaxerenone | ESAX | 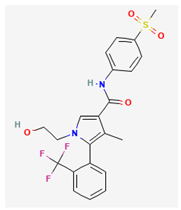 |
Chang and Wu, 2021a | |
| IK(erg) | Risperidone | NA | 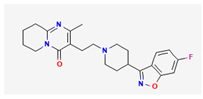 |
Lee et al., 2017, Wu et al., 2000 |
| Di(2-ethylhexyl)-phthalate | DEHP | 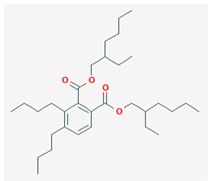 |
Wu et al., 2012 | |
| IK(M) | Solifenacin | SOL | 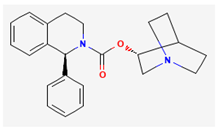 |
Cho et al., 2021 |
| Kynurenic acid | KYNA | 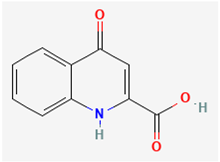 |
Sakakibara et al., 2015, Lo et al., 2021 | |
| Ih | Carisbamate | CRS | 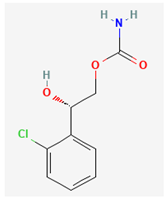 |
Chen et al., 2023, Hung et al., 2023 |
| Cannabidiol | CBD | 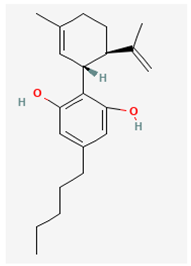 |
Liu et al., 2023 | |
| BKCa channel | Rufinamide | RFM | 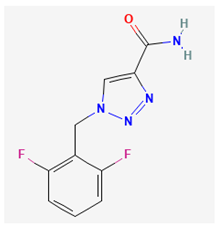 |
Lai et al., 2022 |
| QO-40 | NA | 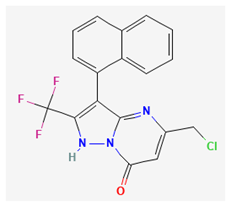 |
Chang and Wu, 2021b |
| Cell line | ATCC* website | BCRC* website | ScienCellTM website |
| AtT-20 | https://www.atcc.org/products/ccl-89 (CCL-89) | https://catalog.bcrc.firdi.org.tw/BcrcContent?bid=60244&rowid=1 | |
| GH3 | https://www.atcc.org/products/ccl-82.1 (CCL-82.1) | https://catalog.bcrc.firdi.org.tw/BcrcContent?bid=60015&rowid=1 | |
| GH4C1 | https://www.atcc.org/products/ccl-82.2 (CCL-82.2) | NA | |
| MMQ | https://www.atcc.org/products/crl-10609 (CRL-10609) | NA | |
| R1220 | https://sciencellonline.com/rat-pituitary-cel |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





