Submitted:
22 March 2025
Posted:
24 March 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
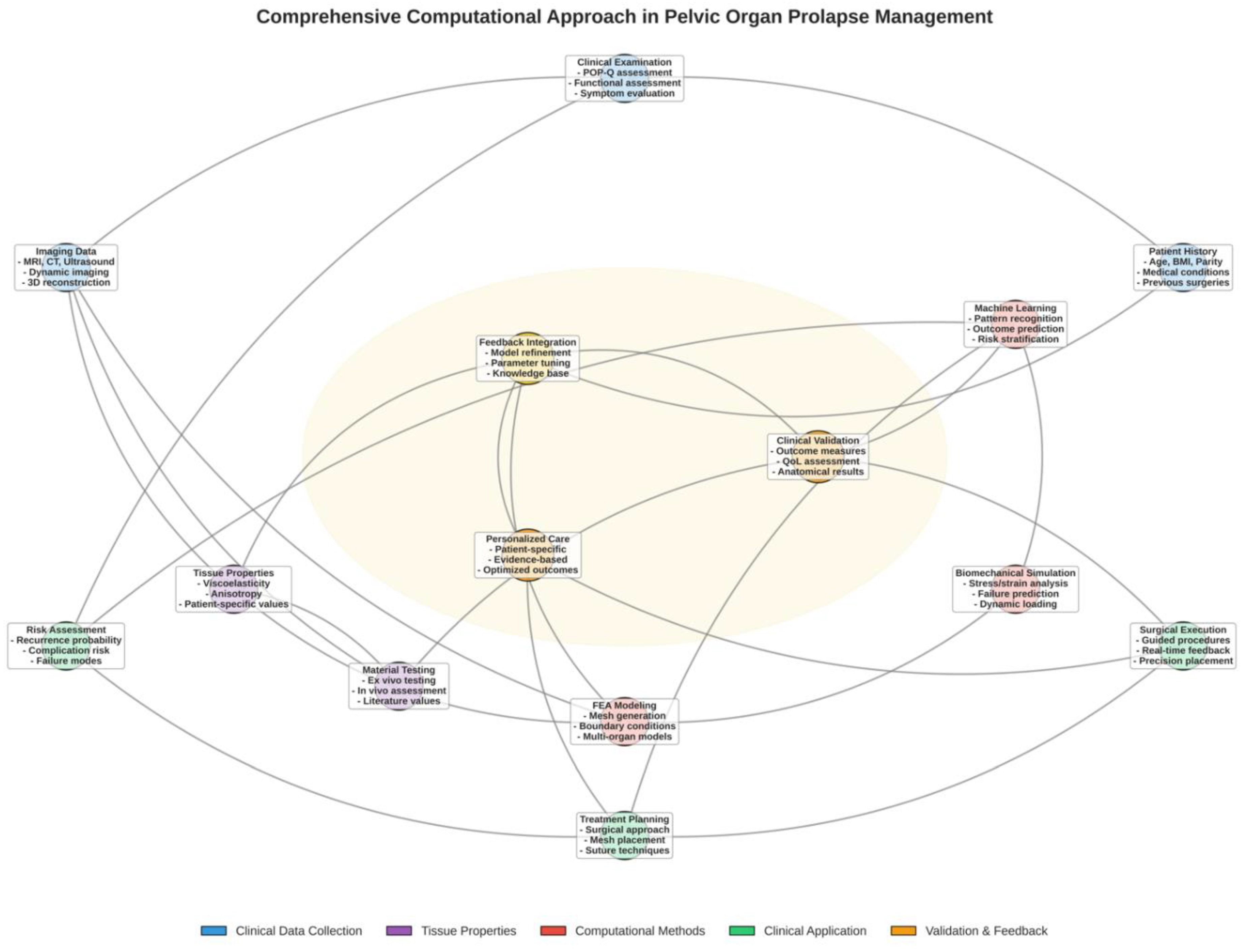
2. Materials and Methods
3. Results
3.1. Efficacy of Current Treatments and Limitations in Managing Pelvic Organ Prolapse
3.2. Rationale for Predictive Tools and the Role of Finite Element Analysis
3.3. Status Quo of Computational Modeling in Pelvic Organ Prolapse
3.3.1. Multi-Compartment Approaches and the Importance of Global Repair
3.3.2. Tissue-Specific Material Characterization and Anatomic Fidelity
3.3.3. Evaluations of Surgical Technique and Mesh-Related Interventions
3.3.4. Patient-Specific Modeling, Validation, and Clinical Integration
3.4. Perspectives
4. Discussion
- Standardized Definitions of Clinical Success: Surgical success must incorporate functional endpoints, including patient-reported outcomes, not merely anatomical descriptors such as stage reduction. Harmonized definitions of recurrence and validated PROMs will facilitate more meaningful comparisons across studies and more nuanced validation of computational models.
- Comprehensive, High-Quality Data: Both clinicians and engineers need access to standardized imaging protocols (MRI-based or US 3D reconstructions) and thorough datasets that reflect different loading conditions, tissue compositions, and patient demographics. Machine-learning–or FEA-based systems can only generate reliable predictions when trained on consistent, representative clinical data.
- Adaptive Computational Frameworks: Robust models should evolve over time by incorporating new postoperative data, surgical outcomes, and possibly multi-omic information. Such an iterative “learning” system can continuously refine mesh tension settings, suture placements, or even surgical techniques as more outcome data accrue.
- Collaborative, Multidisciplinary Efforts: Engineers, data scientists, imaging experts, and clinicians must jointly design and validate computational tools. This includes aligning goals on clinically meaningful endpoints, establishing clear workflows for model deployment, and ensuring outputs are interpretable for surgeons.
5. Conclusions
Author Contributions
Funding and Acknowledgments
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| POP | Pelvic Organ Prolapse |
| QoL | Quality of Life |
| PFMT | Pelvic Floor Muscle Training |
| FEA | Finite Element Analysis |
| MRI | Magnetic Resonance Imaging |
| US | Ultra Sound |
| PROMs | Patient-Reported Outcome Measures |
| POP-Q | Pelvic Organ Prolapse Quantification |
| 2D | Two-Dimensional |
| 3D | Three-Dimensional |
| ML | Machine Learning |
| VR | Virtual Reality |
| AR | Augmented Reality |
References
- Barber, M.D. Pelvic organ prolapse. BMJ 2016, i3853. [Google Scholar] [CrossRef] [PubMed]
- Hendrix, S.L.; Clark, A.; Nygaard, I.; Aragaki, A.; Barnabei, V.; McTiernan, A. Pelvic organ prolapse in the women’s health initiative: Gravity and gravidity. Am J Obstet Gynecol. 2002, 186, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.M.; Matthews, C.A.; Conover, M.M.; Pate, V.; Jonsson Funk, M. Lifetime Risk of Stress Urinary Incontinence or Pelvic Organ Prolapse Surgery. Obstet Gynecol. 2014, 123, 1201–1206. [Google Scholar] [CrossRef]
- Maher, C.; Feiner, B.; Baessler, K.; Schmid, C. Surgical management of pelvic organ prolapse in women. In: The Cochrane Collaboration, editor. Cochrane Database of Systematic Reviews [Internet]. Chichester, UK: John Wiley & Sons, Ltd; 2013 [cited 2025 Feb 17]. p. CD004014.pub5. Available from: https://doi.wiley.com/10.1002/14651858.CD004014.
- Smith, F.J.; Holman, C.D.J.; Moorin, R.E.; Tsokos, N. Lifetime Risk of Undergoing Surgery for Pelvic Organ Prolapse: Obstet Gynecol. 2010, 116, 1096–1100. [CrossRef]
- Danford, J.M.; Osborn, D.J.; Reynolds, W.S.; Biller, D.H.; Dmochowski, R.R. Postoperative pain outcomes after transvaginal mesh revision. Int Urogynecology J. 2015, 26, 65–69. [Google Scholar] [CrossRef]
- Duarte Thibault, M.; Chen, L.; Huebner, M.; DeLancey, J.O.; Swenson, C.W. A comparison of MRI-based pelvic floor support measures between young and old women with prolapse. Int Urogynecology J. 2023, 34, 2081–2088. [Google Scholar] [CrossRef]
- Silva, M.E.T.; Brandão, S.; Parente, M.P.L.; Mascarenhas, T.; Natal Jorge, R.M. Biomechanical properties of the pelvic floor muscles of continent and incontinent women using an inverse finite element analysis. Comput Methods Biomech Biomed Engin. 2017, 20, 842–852. [Google Scholar] [CrossRef]
- Espiño-Albela, A.; Castaño-García, C.; Díaz-Mohedo, E.; Ibáñez-Vera, A.J. Effects of Pelvic-Floor Muscle Training in Patients with Pelvic Organ Prolapse Approached with Surgery vs. Conservative Treatment: A Systematic Review. J Pers Med. 2022, 12, 806. [Google Scholar] [CrossRef]
- Hoff Brækken, I.; Majida, M.; Engh, M.E.; Bø, K. Morphological changes after pelvic floor muscle training measured by 3-dimensional ultrasonography: a randomized controlled trial. Obstet Gynecol. 2010, 115 Pt 1, 317–324. [Google Scholar] [CrossRef]
- Bø, K.; Hilde, G.; Stær-Jensen, J.; Siafarikas, F.; Tennfjord, M.K.; Engh, M.E. Postpartum pelvic floor muscle training and pelvic organ prolapse—a randomized trial of primiparous women. Am J Obstet Gynecol. 2015, 212, e1–e38. [Google Scholar] [CrossRef]
- Dumoulin, C.; Cacciari, L.P.; Hay-Smith, E.J.C. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women. Cochrane Incontinence Group, editor. Cochrane Database Syst Rev [Internet]. 2018 Oct 4 [cited 2025 Feb 17];2018(10). Available from: http://doi.wiley.com/10.1002/14651858.CD005654. [CrossRef]
- Abdulaziz, M.M.; Stothers, L.; Lazare, D.; Macnab, A. An integrative review and severity classification of complications related to pessary use in the treatment of female pelvic organ prolapse. Can Urol Assoc, J. 2015, 9, 400. [Google Scholar] [CrossRef]
- De Tayrac, R.; Letouzey, V. Basic science and clinical aspects of mesh infection in pelvic floor reconstructive surgery. Int Urogynecology, J. 2011, 22, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Mock, S.; Reynolds, W.S.; Dmochowski, R.R. Trans-Vaginal Mesh Revision: A Comprehensive Review on Etiologies and Management Strategies with Emphasis on Postoperative Pain Outcomes. LUTS Low Urin Tract Symptoms. 2014, 6, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Nygaard, I.; Brubaker, L.; Zyczynski, H.M.; Cundiff, G.; Richter, H.; Gantz, M.; et al. Long-term outcomes following abdominal sacrocolpopexy for pelvic organ prolapse. JAMA. 2013, 309, 2016–2024. [Google Scholar] [CrossRef] [PubMed]
- Vergeldt, T.F.M.; Weemhoff, M.; IntHout, J.; Kluivers, K.B. Risk factors for pelvic organ prolapse and its recurrence: a systematic review. Int Urogynecology, J. 2015, 26, 1559–1573. [Google Scholar] [CrossRef]
- Bump, R.C.; Mattiasson, A.; Bø, K.; Brubaker, L.P.; DeLancey, J.O.; Klarskov, P.; et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996, 175, 10–17. [Google Scholar] [CrossRef]
- Ruseckaite, R.; Jayasinghe, R.; Bavor, C.; Dean, J.; Daly, O.; Ahern, S. Evaluation and acceptability of patient-reported outcome measures in women following pelvic organ prolapse procedures. BMC Health Serv Res. 2023, 23, 624. [Google Scholar] [CrossRef]
- Conrad, S.J.; Bernard, S.; Gross, D.P.; McLean, L. Patient-Reported Outcome Measures for Pelvic Organ Prolapse: A Systematic Review Using the COnsensus-Based Standards for the Selection of Health Measurement Instruments (COSMIN) Checklist. Bjog. 2025, 132, 105–117. [Google Scholar] [CrossRef]
- Dieter, A.A.; Halder, G.E.; Pennycuff, J.F.; Singh, R.; El-Nashar, S.A.; Lipetskaia, L.; et al. Patient-Reported Outcome Measures for Use in Women With Pelvic Organ Prolapse: A Systematic Review. Obstet Gynecol. 2023, 141, 1098–1114. [Google Scholar] [CrossRef]
- Chapple, C.R.; Cruz, F.; Deffieux, X.; Milani, A.L.; Arlandis, S.; Artibani, W.; et al. Consensus Statement of the European Urology Association and the European Urogynaecological Association on the Use of Implanted Materials for Treating Pelvic Organ Prolapse and Stress Urinary Incontinence. Eur Urol. 2017, 72, 424–431. [Google Scholar] [CrossRef]
- Health C for D and, R. FDA’s Activities: Urogynecologic Surgical Mesh. FDA [Internet]. 2024 Aug 9 [cited 2025 Feb 17]; Available from: https://www.fda.
- Aboseif, C.; Liu, P. Pelvic Organ Prolapse. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 [cited 2025 Feb 17]. Available from: http://www.ncbi.nlm.nih. 5632. [Google Scholar]
- Jelovsek, J.E.; Chagin, K.; Lukacz, E.S.; Nolen, T.L.; Shepherd, J.P.; Barber, M.D.; et al. Models for Predicting Recurrence, Complications, and Health Status in Women After Pelvic Organ Prolapse Surgery. Obstet Gynecol. 2018, 132, 298–309. [Google Scholar] [CrossRef]
- Jelovsek, J.E.; Maher, C.; Barber, M.D. Pelvic organ prolapse. Lancet Lond Engl. 2007, 369, 1027–1038. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Chen, L.; Fenner, D.E.; Ashton-Miller, J.A.; DeLancey, J.O.L. A multi-compartment 3-D finite element model of rectocele and its interaction with cystocele. J Biomech. 2015, 48, 1580–1586. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ashton-Miller, J.A.; DeLancey, J.O.L. A 3D finite element model of anterior vaginal wall support to evaluate mechanisms underlying cystocele formation. J Biomech. 2009, 42, 1371–1377. [Google Scholar] [CrossRef]
- Yang, M.; Chen, C.; Wang, Z.; Long, J.; Huang, R.; Qi, W.; et al. Finite element analysis of female pelvic organ prolapse mechanism: current landscape and future opportunities. Front Med. 2024, 11, 1342645. [Google Scholar] [CrossRef]
- Xue, X.; Wang, H.; Xie, J.; Gao, Z.; Shen, J.; Yao, T. Two-dimensional biomechanical finite element modeling of the pelvic floor and prolapse. Biomech Model Mechanobiol. 2023, 22, 1425–1446. [Google Scholar] [CrossRef]
- Chen, Z.W.; Joli, P.; Feng, Z.Q.; Rahim, M.; Pirró, N.; Bellemare, M.E. Female patient-specific finite element modeling of pelvic organ prolapse (POP). J Biomech. 2015, 48, 238–245. [Google Scholar] [CrossRef]
- Liu, X.; Rong, Q.; Liu, Y.; Wang, J.; Xie, B.; Ren, S. Relationship between high intra-abdominal pressure and compliance of the pelvic floor support system in women without pelvic organ prolapse: A finite element analysis. Front Med. 2022, 9, 820016. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, N.; Wang, B.; Yang, J.; Liu, H.; Zhang, X.; et al. Creation of the biomechanical finite element model of female pelvic floor supporting structure based on thin-sectional high-resolution anatomical images. J Biomech. 2023, 146, 111399. [Google Scholar] [CrossRef]
- Jean Dit Gautier, E.; Mayeur, O.; Lepage, J.; Brieu, M.; Cosson, M.; Rubod, C. Pregnancy impact on uterosacral ligament and pelvic muscles using a 3D numerical and finite element model: preliminary results. Int Urogynecology, J. 2018, 29, 425–430. [Google Scholar] [CrossRef]
- Jeanditgautier, E.; Mayeur, O.; Brieu, M.; Lamblin, G.; Rubod, C.; Cosson, M. Mobility and stress analysis of different surgical simulations during a sacral colpopexy, using a finite element model of the pelvic system. Int Urogynecology, J. 2016, 27, 951–957. [Google Scholar] [CrossRef]
- Silva, M.E.T.; Bessa, J.N.M.; Parente, M.P.L.; Mascarenhas, T.; Natal Jorge, R.M.; Fernandes, A.A. Effect of mesh anchoring technique in uterine prolapse repair surgery: A finite element analysis. J Biomech. 2021, 127, 110649. [Google Scholar] [CrossRef] [PubMed]
- Lallemant, M.; Shimojyo, A.A.; Mayeur, O.; Ramanah, R.; Rubod, C.; Kerbage, Y.; et al. Mobility analysis of a posterior sacrospinous fixation using a finite element model of the pelvic system. Garcia Aznar, J.M.; editor. PLOS ONE. 2024, 19, e0299012. [Google Scholar] [CrossRef]
- Lamblin, G.; Mayeur, O.; Giraudet, G.; Jean Dit Gautier, E.; Chene, G.; Brieu, M.; et al. Pathophysiological aspects of cystocele with a 3D finite elements model. Arch Gynecol Obstet. 2016, 294, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Moura, R.; Oliveira, D.A.; Parente, M.P.L.; Kimmich, N.; Hynčík, L.; Hympánová, L.H.; et al. Patient-specific surrogate model to predict pelvic floor dynamics during vaginal delivery. J Mech Behav Biomed Mater. 2024, 160, 106736. [Google Scholar] [CrossRef]
- Lallemant, M.; Vega, A.; Chambert, J.; Jacquet, E.; Ramanah, R. Biomechanical interests of supra-cervical hysterectomy with sacrocolpopexy: first study using finite element modeling. Int Urogynecology, J. 2021, 32, 1599–1602. [Google Scholar] [CrossRef]
- Ahmed, H.M.; Salem, N.M.; Al-Atabany, W.; Al-Atabany, W. Finite Element Modelling of Corneal Biomechanics Assessment Using Ultrasonic Elastography [Internet]. In Review; 2022 [cited 2025 Feb 17]. Available from: https://www.researchsquare. 2053. [Google Scholar]
- Islam, M.T.; Righetti, R. A Novel Finite Element Model to Assess the Effect of Solid Stress Inside Tumors on Elastographic Normal Strains and Fluid Pressure. J Eng Sci Med Diagn Ther. 2019, 2, 031006. [Google Scholar] [CrossRef]
- Galbusera, F.; Cina, A.; Panico, M.; Albano, D.; Messina, C. Image-based biomechanical models of the musculoskeletal system. Eur Radiol Exp. 2020, 4, 49. [Google Scholar] [CrossRef]
- Trad, Z.; Barkaoui, A.; Chafra, M. ; Tavares JMRS. Finite Element Analysis Applications in Biomechanical Studies of the Knee Joint. In: Trad, Z.; Barkaoui, A.; Chafra, M.; Tavares JMRS, editors. FEM Analysis of the Human Knee Joint: A Review [Internet]. Cham: Springer International Publishing; 2018 [cited 2025 Feb 17]. p. 35–60. Available from. [CrossRef]
- Mononen, M.E.; Liukkonen, M.K.; Turunen, M.J. Machine Learning Model Trained with Finite Element Modeling Can Predict the Risk of Osteoarthritis: Data from the Osteoarthritis Initiative. Appl Sci. 2024, 14, 9538. [Google Scholar] [CrossRef]
- Nimmal Haribabu, G.; Basu, B. Implementing Machine Learning approaches for accelerated prediction of bone strain in acetabulum of a hip joint. J Mech Behav Biomed Mater. 2024, 153, 106495. [Google Scholar] [CrossRef]
- Goodbrake, C.; Motiwale, S.; Sacks, M.S. A neural network finite element method for contact mechanics. Comput Methods Appl Mech Eng. 2024, 419, 116671. [Google Scholar] [CrossRef]
- Shi, J.; Lin, F.; Rao, W. Learning-Based Finite Element Methods Modeling for Complex Mechanical Systems [Internet]. arXiv; 2024 [cited 2025 Feb 18]. Available from: http://arxiv.org/abs/2409.00160. arXiv:10.48550/arXiv.2409.00160.
- Courtecuisse, H.; Dequidt, J. Augmented reality biomechanical simulations for pelvic conditions diagnoses. In: Biomechanics of the Female Reproductive System: Breast and Pelvic Organs [Internet]. Elsevier; 2023 [cited 2025 Feb 17]. p. 455–79. Available from: https://linkinghub.elsevier. 9780. [Google Scholar]
- Motiwale, S.; Zhang, W.; Feldmeier, R.; Sacks, M.S. A neural network finite element approach for high speed cardiac mechanics simulations. Comput Methods Appl Mech Eng. 2024, 427, 117060. [Google Scholar] [CrossRef]
- Martin, L.; Jain, P.; Ferguson, Z.; Gholamalizadeh, T.; Moshfeghifar, F.; Erleben, K.; et al. A systematic comparison between FEBio and PolyFEM for biomechanical systems. Comput Methods Programs Biomed. 2024, 244, 107938. [Google Scholar] [CrossRef] [PubMed]
- Chatzistergos, P.; Behforootan, S.; Naemi, R.; Chockalingam, N. Finite Element Modeling. In: Foot and Ankle Biomechanics [Internet]. Elsevier; 2023 [cited 2025 Feb 17]. p. 365–86. Available from: https://linkinghub.elsevier. 9780. [Google Scholar]
- Zhu, J.; Su, Y.; Liu, Z.; Liu, B.; Sun, Y.; Gao, W.; et al. Real-time biomechanical modelling of the liver using LightGBM model. Int J Med Robot. 2022, 18, e2433. [Google Scholar] [CrossRef] [PubMed]
- Salman, S.; Babaoglu, B.; Kumbasar, S.; Bestel, M.; Ketenci Gencer, F.; Tuna, G.; et al. Comparison of Unilateral and Bilateral Sacrospinous Ligament Fixation Using Minimally Invasive Anchorage. Geburtshilfe Frauenheilkd. 2019, 79, 976–982. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




