Submitted:
24 March 2025
Posted:
25 March 2025
You are already at the latest version
Abstract
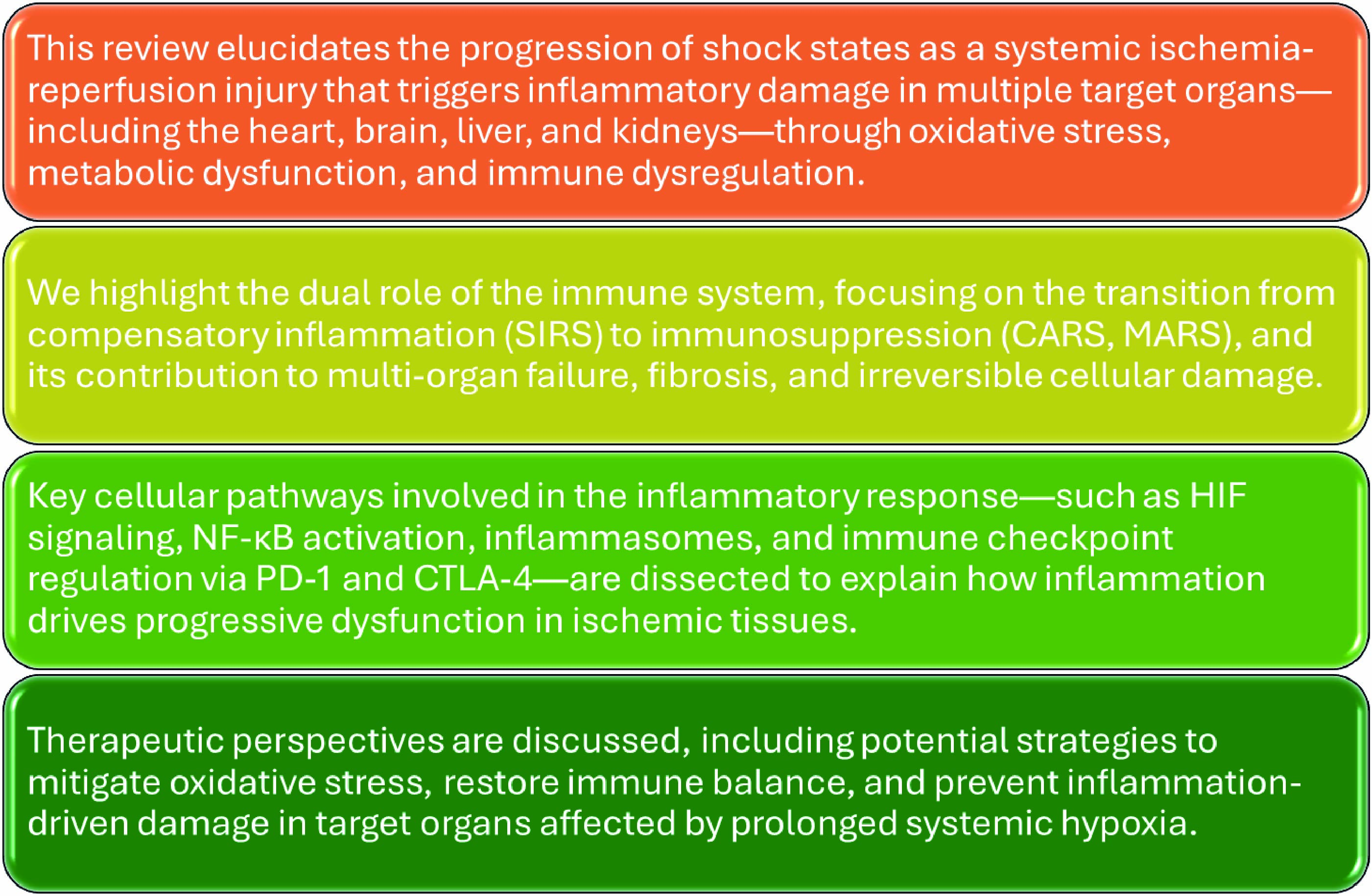
Keywords:
1. Introduction
2. Classification and Categorization of Shock State
| Hypovolemic Shock Categories | Volume Mechanism | Tissue Injury | Clinical Scenario |
|---|---|---|---|
| Hemorrhagic shock | Acute hemorrhage (critical) | No major soft tissue injury | Aortic dissection rupture |
| Traumatic hemorrhagic shock | Acute hemorrhage (critical) | With major soft tissue injury | Polytrauma |
| Pure hypovolemic shock | Reduction (critical) of circulating plasma volume (fluid loss) without hemorrhage | No major soft tissue injury | Persistent fever, diarrhea, or vomiting |
| Traumatic hypovolemic shock | Reduction (critical) of circulating plasma volume (fluid loss) without hemorrhage | With major soft tissue injury | Large surface burns or deep skin lesions |
3. Progression of Shock State
4. The Micro-Verse
5. Adaptative Micro-Verse System During Shock Progression
6. The Macro-Verse
7. Ischemia Phase and Immune System
8. IL-1 Signaling Pathway: Activation and Inhibition
9. IL-6 and TNF-α Pathways in Shock Progression
10. Integration of IL-1, IL-6, and TNF-α in Inflammatory Waves
11. Regulation and Pathophysiological Implications
12. CTLA-4 and PD-1: Immune Checkpoint Pathways
13. CTLA-4 and PD-1 Signaling Mechanisms
14. Integration Inflammatory/Anti-inflammatory Signaling
15. Oxidative Stress and Shock States
16. Conclusion
References
- Millham, F.H. A brief history of shock. Surgery 2010, 148, 1026–1037. [Google Scholar] [CrossRef]
- AConvertino, V.; Lye, K.R.; Koons, N.J.; Joyner, M.J. Physiological comparison of hemorrhagic shock and V˙O2max: A conceptual framework for defining the limitation of oxygen delivery. Exp. Biol. Med. 2019, 244, 690–701. [Google Scholar] [CrossRef]
- Kislitsina, O.N.; Rich, J.D.; Wilcox, J.E.; Pham, D.T.; Churyla, A.; Vorovich, E.B.; Ghafourian, K.; Yancy, C.W. Shock – Classification and Pathophysiological Principles of Therapeutics. Curr. Cardiol. Rev. 2019, 15, 102–113. [Google Scholar] [CrossRef]
- Ioannou, A.; Lucca, J.D.; Tsokos, G.C. Immunopathogenesis of ischemia/reperfusion-associated tissue damage. Clin. Immunol. 2011, 141, 3–14. [Google Scholar] [CrossRef]
- Lee, J.-M.; Grabb, M.C.; Zipfel, G.J.; Choi, D.W. Brain tissue responses to ischemia. The Journal of Clinical Investigation. 2000, 106, 723–731. [Google Scholar] [CrossRef]
- Kalogeris, T.; Baines, C.P.; Krenz, M.; Korthuis, R.J. Chapter Six - Cell Biology of Ischemia/Reperfusion Injury. In: Jeon KW, editor. International Review of Cell and Molecular Biology. 298: Academic Press; 2012. p. 229-317.
- Kalogeris, T.; Baines, C.P.; Krenz, M.; Korthuis, R.J. Ischemia/Reperfusion. Compr Physiol. 2016, 7, 113–170. [Google Scholar] [CrossRef]
- Doyle, K.P.; Simon, R.P.; Stenzel-Poore, M.P. Mechanisms of ischemic brain damage. Neuropharmacology 2008, 55, 310–318. [Google Scholar] [CrossRef]
- Amantea, D.; Nappi, G.; Bernardi, G.; Bagetta, G.; Corasaniti, M.T. Post-ischemic brain damage: pathophysiology and role of inflammatory mediators. FEBS J. 2008, 276, 13–26. [Google Scholar] [CrossRef]
- Olthof, P.B.; van Golen, R.F.; Meijer, B.; van Beek, A.A.; Bennink, R.J.; Verheij, J.; et al. Warm ischemia time-dependent variation in liver damage, inflammation, and function in hepatic ischemia/reperfusion injury. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2017, 1863, 375–385. [Google Scholar] [CrossRef]
- Cannistrà,, M. ; Ruggiero, M.; Zullo, A.; Gallelli, G.; Serafini, S.; Maria, M.; Naso, A.; Grande, R.; Serra, R.; Nardo, B. Hepatic ischemia reperfusion injury: A systematic review of literature and the role of current drugs and biomarkers. Int. J. Surg. 2016, 33, S57–S70. [Google Scholar] [CrossRef]
- van der Kaaij, N.P.; Kluin, J.; Haitsma, J.J.; Bakker, M.A.D.; Lambrecht, B.N.; Lachmann, B.; de Bruin, R.W.; Bogers, A.J. Ischemia of the lung causes extensive long-term pulmonary injury: an experimental study. Respir. Res. 2008, 9, 28–28. [Google Scholar] [CrossRef]
- Chen-Yoshikawa, T.F. Ischemia–Reperfusion Injury in Lung Transplantation. 2021, 10, 1333.
- Saikumar, P.; AVenkatachalam, M. Role of apoptosis in hypoxic/ischemic damage in the kidney. Semin. Nephrol. 2003, 23, 511–521. [Google Scholar] [CrossRef]
- Moens, A.; Claeys, M.; Timmermans, J.; Vrints, C. Myocardial ischemia/reperfusion-injury, a clinical view on a complex pathophysiological process. Int. J. Cardiol. 2005, 100, 179–190. [Google Scholar] [CrossRef]
- Gonzalez, L.M.; Moeser, A.J.; Blikslager, A.T. Animal models of ischemia-reperfusion-induced intestinal injury: progress and promise for translational research. Am. J. Gastrointest. Physiol. Liver Physiol. 2015, 308, G63–G75. [Google Scholar] [CrossRef]
- Hesketh, E.E.; Czopek, A.; Clay, M.; Borthwick, G.; Ferenbach, D.; Kluth, D.; et al. Renal ischaemia reperfusion injury: a mouse model of injury and regeneration. J Vis Exp. 2014(88).
- Xia, Z.; Li, H.; Irwin, M. Myocardial ischaemia reperfusion injury: the challenge of translating ischaemic and anaesthetic protection from animal models to humans. Br. J. Anaesth. 2016, 117, ii44–ii62. [Google Scholar] [CrossRef]
- Ma, R.; Xie, Q.; Li, Y.; Chen, Z.; Ren, M.; Chen, H.; Li, H.; Li, J.; Wang, J. Animal models of cerebral ischemia: A review. Biomed. Pharmacother. 2020, 131, 110686. [Google Scholar] [CrossRef]
- Wu, M.-Y.; Yiang, G.-T.; Liao, W.-T.; Tsai, A.P.Y.; Cheng, Y.-L.; Cheng, P.-W.; Li, C.-Y.; Li, C.J. Current Mechanistic Concepts in Ischemia and Reperfusion Injury. Cell. Physiol. Biochem. 2018, 46, 1650–1667. [Google Scholar] [CrossRef]
- Soares, R.O.S.; Losada, D.M.; Jordani, M.C.; Évora, P.; Castro-E-Silva, O. Ischemia/Reperfusion Injury Revisited: An Overview of the Latest Pharmacological Strategies. Int. J. Mol. Sci. 2019, 20, 5034. [Google Scholar] [CrossRef]
- Nour, M.; Scalzo, F.; Liebeskind, D.S. Ischemia-Reperfusion Injury in Stroke. Interventional Neurology. 2013, 1(3-4):185-99.
- Gillani, S.; Cao, J.; Suzuki, T.; Hak, D.J. The effect of ischemia reperfusion injury on skeletal muscle. Injury 2012, 43, 670–675. [Google Scholar] [CrossRef]
- Dorweiler, B.; Pruefer, D.; Andrasi, T.B.; Maksan, S.M.; Schmiedt, W.; Neufang, A.; et al. Ischemia-Reperfusion Injury. European Journal of Trauma and Emergency Surgery. 2007, 33, 600–612. [Google Scholar] [CrossRef]
- Rodríguez-Lara, S.Q.; Trujillo-Rangel, W.A.; Castillo-Romero, A.; Totsuka-Sutto, S.E.; Garcia-Cobián, T.A.; Cardona-Muñoz, E.G.; Miranda-Díaz, A.G.; Ramírez-Lizardo, E.J.; García-Benavides, L. Effect of Telmisartan in the Oxidative Stress Components Induced by Ischemia Reperfusion in Rats. Oxidative Med. Cell. Longev. 2019, 2019, 1–13. [Google Scholar] [CrossRef]
- Rodríguez-Lara, S.Q.; García-Benavides, L.; Miranda-Díaz, A.G. The Renin-Angiotensin-Aldosterone System as a Therapeutic Target in Late Injury Caused by Ischemia-Reperfusion. Int. J. Endocrinol. 2018, 2018, 1–18. [Google Scholar] [CrossRef]
- Chen, T.; Vunjak-Novakovic, G. In Vitro Models of Ischemia-Reperfusion Injury. Regenerative Engineering and Translational Medicine. 2018, 4, 142–153. [Google Scholar] [CrossRef]
- Shiva, N.; Sharma, N.; Kulkarni, Y.A.; Mulay, S.R.; Gaikwad, A.B. Renal ischemia/reperfusion injury: An insight on in vitro and in vivo models. Life Sci. 2020, 256, 117860. [Google Scholar] [CrossRef]
- Cowled, P.; Fitridge, R. Pathophysiology of Reperfusion Injury. In: Fitridge R, editor. Mechanisms of Vascular Disease: A Textbook for Vascular Specialists. Cham: Springer International Publishing; 2020. p. 415-40.
- Pefanis, A.; Ierino, F.L.; Murphy, J.M.; Cowan, P.J. Regulated necrosis in kidney ischemia-reperfusion injury. Kidney Int. 2019, 96, 291–301. [Google Scholar] [CrossRef]
- Hirao, H.; Nakamura, K.; Kupiec-Weglinski, J.W. Liver ischaemia–reperfusion injury: a new understanding of the role of innate immunity. Nat. Rev. Gastroenterol. Hepatol. 2021, 19, 239–256. [Google Scholar] [CrossRef]
- Cao, H.; Cheng, Y.; Gao, H.; Zhuang, J.; Zhang, W.; Bian, Q.; Wang, F.; Du, Y.; Li, Z.; Kong, D.; et al. In Vivo Tracking of Mesenchymal Stem Cell-Derived Extracellular Vesicles Improving Mitochondrial Function in Renal Ischemia–Reperfusion Injury. ACS Nano 2020, 14, 4014–4026. [Google Scholar] [CrossRef]
- Yan, H.-F.; Tuo, Q.-Z.; Yin, Q.-Z.; Lei, P. The pathological role of ferroptosis in ischemia/reperfusion-related injury. Zool. Res. 2020, 41, 220–230. [Google Scholar] [CrossRef]
- Mayer, A.R.; Dodd, A.B.; Ling, J.M.; Stephenson, D.D.; Rannou-Latella, J.G.; Vermillion, M.S.; Mehos, C.J.; Johnson, V.E.; Gigliotti, A.P.; Dodd, R.J.; et al. Survival Rates and Biomarkers in a Large Animal Model of Traumatic Brain Injury Combined With Two Different Levels of Blood Loss. Shock 2020, 55, 554–562. [Google Scholar] [CrossRef]
- Slaughter, A.L.; Nunns, G.R.; D’Alessandro, A.; Banerjee, A.; Hansen, K.C.; Moore, E.E.; et al. The Metabolopathy of Tissue Injury, Hemorrhagic Shock, and Resuscitation in a Rat Model. 2018, 49, 580–590.
- Sheppard, F.R.; Macko, A.R.; Glaser, J.J.; Vernon, P.J.; Burdette, A.J.; Paredes, R.M.; Koeller, C.A.; Pusateri, A.E.; Tadaki, D.K.; Cardin, S. Nonhuman Primate (Rhesus Macaque) Models of Severe Pressure-Targeted Hemorrhagic and Polytraumatic Hemorrhagic Shock. Shock 2018, 49, 174–186. [Google Scholar] [CrossRef]
- Nugent, W.H.; Cestero, R.F.; Ward, K.; Jubin, R.; Abuchowski, A.; Song, B.K. Effects of Sanguinate on Systemic and Microcirculatory Variables in a Model of Prolonged Hemorrhagic Shock. Shock 2019, 52, 108–115. [Google Scholar] [CrossRef]
- Ozcelebi, E.; Iskit, A.B.J.J.o.C.; Care, I. Experimental animal models of sepsis and septic shock. 2023, 14, 96–100.
- Ramos-Benitez, M.J.; Ruiz-Jimenez, C.; Rosado-Franco, J.J.; Ramos-Pérez, W.D.; Mendez, L.B.; Osuna, A.; Espino, A.M. Fh15 Blocks the Lipopolysaccharide-Induced Cytokine Storm While Modulating Peritoneal Macrophage Migration and CD38 Expression within Spleen Macrophages in a Mouse Model of Septic Shock. mSphere 2018, 3. [Google Scholar] [CrossRef]
- Wang, Y.; Polten, F.; Jäckle, F.; Korf-Klingebiel, M.; Kempf, T.; Bauersachs, J.; Freitag-Wolf, S.; Lichtinghagen, R.; Pich, A.; Wollert, K.C. A mouse model of cardiogenic shock. Cardiovasc. Res. 2021, 117, 2414–2415. [Google Scholar] [CrossRef]
- Rienzo, M.; Imbault, J.; El Boustani, Y.; Beurton, A.; Sampedrano, C.C.; Pasdois, P.; Pernot, M.; Bernus, O.; Haïssaguerre, M.; Couffinhal, T.; et al. A total closed chest sheep model of cardiogenic shock by percutaneous intracoronary ethanol injection. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Rodríguez-Lara, S.Q.; Cardona-Muñoz, E.G.; Ramírez-Lizardo, E.J.; Totsuka-Sutto, S.E.; Castillo-Romero, A.; García-Cobián, T.A.; García-Benavides, L. Alternative Interventions to Prevent Oxidative Damage following Ischemia/Reperfusion. Oxidative Med. Cell. Longev. 2016, 2016, 7190943. [Google Scholar] [CrossRef]
- Chatauret, N.; Badet, L.; Barrou, B.; Hauet, T. Ischemia-reperfusion: From cell biology to acute kidney injury. Progres En Urol. 2014, 24, S4–S12. [Google Scholar] [CrossRef]
- Yu, H.; Kalogeris, T.; Korthuis, R.J. Reactive species-induced microvascular dysfunction in ischemia/reperfusion. Free. Radic. Biol. Med. 2019, 135, 182–197. [Google Scholar] [CrossRef]
- Bertini, P.; Guarracino, F. Pathophysiology of cardiogenic shock. 2021, 27, 409–415.
- Kuo, K.; Palmer, L. Pathophysiology of hemorrhagic shock. J Vet Emerg Crit Care (San Antonio). 2022, 32(S1):22-31.
- Burgdorff, A.-M.; Bucher, M.; Schumann, J. Vasoplegia in patients with sepsis and septic shock: pathways and mechanisms. J. Int. Med Res. 2018, 46, 1303–1310. [Google Scholar] [CrossRef]
- Russell, J.A.; Rush, B.; Boyd, J. Pathophysiology of Septic Shock. Critical Care Clinics. 2018, 34, 43–61. [Google Scholar] [CrossRef]
- Lambden, S.; Creagh-Brown, B.C.; Hunt, J.; Summers, C.; Forni, L.G. Definitions and pathophysiology of vasoplegic shock. Crit. Care 2018, 22, 1–8. [Google Scholar] [CrossRef]
- Safiejko, K.; Smereka, J.; Pruc, M.; Ladny, J.R.; Jaguszewski, M.J.; Filipiak, K.J.; Yakubtsevich, R.; Szarpak, L. Efficacy and safety of hypertonic saline solutions fluid resuscitation on hypovolemic shock: A systematic review and meta-analysis of randomized controlled trials. Cardiol. J. 2022, 29, 966–977. [Google Scholar] [CrossRef]
- Pacagnella, R.C.; Borovac-Pinheiro, A. Assessing and managing hypovolemic shock in puerperal women. Best Pr. Res. Clin. Obstet. Gynaecol. 2019, 61, 89–105. [Google Scholar] [CrossRef]
- Han, S.-J.; Zhou, Z.-W.M.; Yang, C.M.; Wei, K.-P.B.; Ma, J.-Z.B.; Chu, Z.-F.M.; Gu, P.M. Hemorrhagic, hypovolemic shock resuscitated with Ringer’s solution using bicarbonate versus lactate: A CONSORT-randomized controlled study comparing patient outcomes and blood inflammatory factors. Medicine 2022, 101, e31671. [Google Scholar] [CrossRef]
- Thompson, K.; Venkatesh, B.; Finfer, S. Sepsis and septic shock: current approaches to management. Intern. Med. J. 2019, 49, 160–170. [Google Scholar] [CrossRef]
- Cecconi, M.; Evans, L.; Levy, M.; Rhodes, A. Sepsis and septic shock. The Lancet. 2018, 392, 75–87. [Google Scholar] [CrossRef]
- Gavelli, F.; Castello, L.M.; Avanzi, G.C. Management of sepsis and septic shock in the emergency department. Intern. Emerg. Med. 2021, 16, 1649–1661. [Google Scholar] [CrossRef]
- Standl, T.; Annecke, T.; Cascorbi, I.; Heller, A.R.; Sabashnikov, A.; Teske, W. The Nomenclature, Definition and Distinction of Types of Shock. Dtsch Arztebl Int. 2018, 115, 757–768. [Google Scholar] [CrossRef]
- Baker, S.Y.; Tarkowski, A.F.; Falk, J.L. Shock Overview. In: Shiber JR, Weingart SD, editors. Emergency Department Critical Care. Cham: Springer International Publishing; 2020. p. 1-20.
- Dell’Anna, A.M.; Torrini, F.; Antonelli, M. Shock: Definition and Recognition. In: Pinsky MR, Teboul J-L, Vincent J-L, editors. Hemodynamic Monitoring. Cham: Springer International Publishing; 2019. p. 7-20.
- Parks, J.; Vasileiou, G.; Parreco, J.; Pust, G.D.; Rattan, R.; Zakrison, T.; Namias, N.; Yeh, D.D. Validating the ATLS Shock Classification for Predicting Death, Transfusion, or Urgent Intervention. J. Surg. Res. 2019, 245, 163–167. [Google Scholar] [CrossRef]
- Bonanno, F. Time to Change ATLS Classifications of Hemorrhagic Shock. J. Emergencies, Trauma, Shock. 2024, 17, 252–254. [Google Scholar] [CrossRef]
- Bonanno, F.G. Management of Hemorrhagic Shock: Physiology Approach, Timing and Strategies. J. Clin. Med. 2022, 12, 260. [Google Scholar] [CrossRef]
- Bonanno, F.G. The need for a physiological classification of hemorrhagic shock. J. Emergencies, Trauma, Shock. 2020, 13, 177–182. [Google Scholar] [CrossRef]
- McIntyre, W.F.; Um, K.J.; Alhazzani, W.; Lengyel, A.P.; Hajjar, L.; Gordon, A.C.; et al. Association of Vasopressin Plus Catecholamine Vasopressors vs Catecholamines Alone With Atrial Fibrillation in Patients With Distributive Shock: A Systematic Review and Meta-analysis. JAMA. 2018, 319, 1889–1900. [Google Scholar] [CrossRef]
- Jeschke, M.G.; van Baar, M.E.; Choudhry, M.A.; Chung, K.K.; Gibran, N.S.; Logsetty, S. Burn injury. Nature Reviews Disease Primers. 2020, 6, 11. [Google Scholar] [CrossRef]
- Annane, D.; Ouanes-Besbes, L.; de Backer, D.; DU,, B. ; Gordon, A.C.; Hernández, G.; Olsen, K.M.; Osborn, T.M.; Peake, S.; Russell, J.A.; et al. A global perspective on vasoactive agents in shock. Intensiv. Care Med. 2018, 44, 833–846. [Google Scholar] [CrossRef]
- Hill, K.L.; Rustin, M.A.; Asche, M.A.; Bennett, C.E.; Patel, P.C.; Jentzer, J.C. Cardiogenic Shock Classification and Associated Mortality Risk. Mayo Clin. Proc. 2023, 98, 771–783. [Google Scholar] [CrossRef]
- Jentzer, J.C.; van Diepen, S.; Barsness, G.W.; Henry, T.D.; Menon, V.; Rihal, C.S.; Naidu, S.S.; Baran, D.A. Cardiogenic Shock Classification to Predict Mortality in the Cardiac Intensive Care Unit. Circ. 2019, 74, 2117–2128. [Google Scholar] [CrossRef]
- Sarma, D.; Jentzer, J.C. Cardiogenic Shock: Pathogenesis, Classification, and Management. Critical Care Clinics. 2024, 40, 37–56. [Google Scholar] [CrossRef]
- Ohbe, H.; Jo, T.; Yamana, H.; Matsui, H.; Fushimi, K.; Yasunaga, H. Early enteral nutrition for cardiogenic or obstructive shock requiring venoarterial extracorporeal membrane oxygenation: a nationwide inpatient database study. Intensiv. Care Med. 2018, 44, 1258–1265. [Google Scholar] [CrossRef]
- Stickles, S.P.; Carpenter, C.R.; Gekle, R.; Kraus, C.K.; Scoville, C.; Theodoro, D.; Tran, V.H.; Ubiñas, G.; Raio, C. The diagnostic accuracy of a point-of-care ultrasound protocol for shock etiology: A systematic review and meta-analysis. CJEM 2019, 21, 406–417. [Google Scholar] [CrossRef]
- Prasad, K.; Lee, P.J.I.J.o.A. Role of oxyradicals in the pathophysiology of hemorrhagic shock. 2002, 11, 113–128.
- Cecconi, M.; De Backer, D.; Antonelli, M.; Beale, R.; Bakker, J.; Hofer, C.; Jaeschke, R.; Mebazaa, A.; Pinsky, M.R.; Teboul, J.L.; et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensiv. Care Med. 2014, 40, 1795–1815. [Google Scholar] [CrossRef]
- Fox, S.; Vashisht, R.; Siuba, M.; Dugar, S. Evaluation and management of shock in patients with COVID-19. Clevel. Clin. J. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Spapen, H.D.; Jacobs, R.; Honoré, P.M.J.J.o.E.; Medicine, C.C. Sepsis-induced multi-organ dysfunction syndrome—a mechanistic approach. 2017. 2017, 1. [Google Scholar] [CrossRef]
- Gourd, N.M.; Nikitas, N. Multiple Organ Dysfunction Syndrome. 2020, 35, 1564–1575.
- Schmid-Schönbein, G.W.; Chang, M. The Autodigestion Hypothesis for Shock and Multi-organ Failure. Ann. Biomed. Eng. 2013, 42, 405–414. [Google Scholar] [CrossRef]
- Wang, D.; Wang, X.; Mu, J.; Kuang, Z.; Zhang, J.; Lu, X.; Wang, X.; Lin, F. Prognostic indicators and outcome in patients with acute liver failure, sepsis and with and without shock: a retrospective cohort study. Ann. Med. 2024, 57, 2438833. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Yin, Y.; Li, T.; Zhuang, S. The Value of Serum Procalcitonin, Thromboelastography Combined with Platelet Count in Predicting the Short-Term Progression of Septic Shock in the Intensive Care Unit. Int. J. Gen. Med. 2024, ume 17, 3361–3370. [Google Scholar] [CrossRef]
- Kumar, A.; Roberts, D.; Wood, K.E.; Light, B.; Parrillo, J.E.; Sharma, S.; Suppes, R.; Feinstein, D.; Zanotti, S.; Taiberg, L.; et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock*. Crit. Care Med. 2006, 34, 1589–1596. [Google Scholar] [CrossRef]
- Kvarstein, G.; Mirtaheri, P.; Tønnessen, T.I. Detection of organ ischemia during hemorrhagic shock. Acta Anaesthesiol. Scand. 2003, 47, 675–686. [Google Scholar] [CrossRef]
- EDouzinas, E.; Andrianakis, I.; Livaditi, O.; Paneris, P.; Tasoulis, M.; Pelekanou, A.; Betrosian, A.; Giamarellos-Bourboulis, E.J. The level of hypotension during hemorrhagic shock is a major determinant of the post-resuscitation systemic inflammatory response: an experimental study. BMC Physiol. 2008, 8, 15–15. [Google Scholar] [CrossRef]
- Barbosa Evora, P.R.; Celotto, A.C.; Sumarelli Albuquerque, A.A.; Martinez Évora, P. Circulatory Shock, Ischemia-Reperfusion Injury, Systemic Inflammatory Response Syndrome (SIRS), and Multiple Organ Failure. In: Barbosa Evora PR, Celotto AC, Sumarelli Albuquerque AA, Martinez Évora P, editors. Vasoplegic Endothelial Dysfunction: Circulatory Shock and Methylene Blue. Cham: Springer International Publishing; 2021. p. 29-34.
- Geevarghese, M.; Patel, K.; Gulati, A.; Ranjan, A.K. Role of adrenergic receptors in shock. Front. Physiol. 2023, 14, 1094591. [Google Scholar] [CrossRef]
- Gatica, S.; Aravena, D.; Echeverría, C.; Santibanez, J.F.; Riedel, C.A.; Simon, F. Effects of Adrenergic Receptor Stimulation on Human Hemostasis: A Systematic Review. In Advances in Molecular Pathology; Simon, F., Bernabeu, C., Eds.; Springer Nature Switzerland: Cham, 2023. [Google Scholar]
- Póvoa, P.; Carneiro, A.H. Adrenergic support in septic shock: a critical review. Hosp. Pr. 2010, 38, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Belletti, A.; Landoni, G.; Lomivorotov, V.V.; Oriani, A.; Ajello, S. Adrenergic Downregulation in Critical Care: Molecular Mechanisms and Therapeutic Evidence. Journal of Cardiothoracic and Vascular Anesthesia. 2020, 34, 1023–1041. [Google Scholar] [CrossRef]
- Brierre, S.; Deboisblanc, B.P.; Kumari, R. The Endocrine System during Sepsis. The American Journal of the Medical Sciences. 2004, 328, 238–247. [Google Scholar] [CrossRef]
- Diamond-Fox, S.; Gatehouse, A. The Endocrine System and Associated Disorders. Fundamentals of Applied Pathophysiology for Paramedics2024. p. 240-62.
- Heming, N.; Sivanandamoorthy, S.; Meng, P.; Annane, D. The Endocrine System in Sepsis. In: Wiersinga WJ, Seymour CW, editors. Handbook of Sepsis. Cham: Springer International Publishing; 2018. p. 61-79.
- Tsigos, C.; Kyrou, I.; Kassi, E.; Chrousos, G.P.J.E. Stress: endocrine physiology and pathophysiology. 2020.
- Baird, N.A.; Turnbull, D.W.; Johnson, E.A. Induction of the Heat Shock Pathway during Hypoxia Requires Regulation of Heat Shock Factor by Hypoxia-inducible Factor-1. J. Biol. Chem. 2006, 281, 38675–38681. [Google Scholar] [CrossRef] [PubMed]
- Ince, C.; Mik, E.G. Microcirculatory and mitochondrial hypoxia in sepsis, shock, and resuscitation. J. Appl. Physiol. 2016, 120, 226–235. [Google Scholar] [CrossRef]
- Hirota, K.J.C.; Targets, H.D.-D. Involvement of hypoxia-inducible factors in the dysregulation of oxygen homeostasis in sepsis. 2015, 15, 29–40.
- Apichartpiyakul, P.; Mani, R.; Arworn, S.; Rerkasem, K. Ischemia/Reperfusion: A Potential Cause of Tissue Necrosis. In: Téot L, Meaume S, Akita S, Del Marmol V, Probst S, editors. Skin Necrosis. Cham: Springer Nature Switzerland; 2024. p. 15-21.
- Zhou, M.; Jia, X.; Liu, H.; Xue, Y.; Wang, Y.; Li, Z.; Wu, Y.; Rui, Y. Bibliometric analysis of skeletal muscle ischemia/reperfusion (I/R) research from 1986 to 2022. Heliyon 2024, 10, e37492. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhou, R.; Wu, Y.; Kong, G.; Zeng, J.; Li, X.; Wang, B.; Gu, C.; Liao, F.; Qi, F.; et al. In vitro modeling of skeletal muscle ischemia-reperfusion injury based on sphere differentiation culture from human pluripotent stem cells. Exp. Cell Res. 2024, 439, 114111. [Google Scholar] [CrossRef]
- Blaisdell, F.W. The pathophysiology of skeletal muscle ischemia and the reperfusion syndrome: a review. Cardiovascular Surgery. 2002, 10, 620–630. [Google Scholar] [CrossRef]
- Vignaud, A.; Hourde, C.; Medja, F.; Agbulut, O.; Butler-Browne, G.; Ferry, A. Impaired Skeletal Muscle Repair after Ischemia-Reperfusion Injury in Mice. J. Biomed. Biotechnol. 2010, 2010, 1–10. [Google Scholar] [CrossRef]
- Khalaf, R.; Bateman, D.D.; Reyes, J.; Najafali, D.; Rampazzo, A.; Gharb, B.B. Systematic review of pathologic markers in skin ischemia with and without reperfusion injury in microsurgical reconstruction: Biomarker alterations precede histological structure changes. Microsurgery 2024, 44, e31141. [Google Scholar] [CrossRef]
- Berry, C.E.; Le, T.; An, N.; Griffin, M.; Januszyk, M.; Kendig, C.B.; Fazilat, A.Z.; Churukian, A.A.; Pan, P.M.; Wan, D.C. Pharmacological and cell-based treatments to increase local skin flap viability in animal models. J. Transl. Med. 2024, 22, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Dulgar, A.G.; Yaprak, G.K.; Kapukaya, R.; Yaprak, Ö.; Kesiktaş, E. Protective Effect of Hydrogen Sulfide against Ischemia–reperfusion Injury in Rat Skin Flaps. 2024, 32, 68–73.
- George, J.; Lu, Y.; Tsuchishima, M.; Tsutsumi, M. Cellular and molecular mechanisms of hepatic ischemia-reperfusion injury: The role of oxidative stress and therapeutic approaches. Redox Biol. 2024, 75, 103258. [Google Scholar] [CrossRef]
- Chullo, G.; Panisello-Rosello, A.; Marquez, N.; Colmenero, J.; Brunet, M.; Pera, M.; Rosello-Catafau, J.; Bataller, R.; García-Valdecasas, J.C.; Fundora, Y. Focusing on Ischemic Reperfusion Injury in the New Era of Dynamic Machine Perfusion in Liver Transplantation. Int. J. Mol. Sci. 2024, 25, 1117. [Google Scholar] [CrossRef]
- Hu, Y.; Tian, X.; Zhao, Y.; Wang, Z.; Lin, M.; Sun, R.; Wang, Y.; Wang, Z.; Li, G.; Zheng, S.; et al. Sirtuin 5 Alleviates Liver Ischemia/Reperfusion Injury by Regulating Mitochondrial Succinylation and Oxidative Stress. Antioxidants Redox Signal. 2024, 40, 616–631. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhang, D.; Dai, S.; Liu, F.; Zhang, W.; Shen, T. Desflurane attenuates renal ischemia-reperfusion injury by modulating ITGB1/CD9 and reducing oxidative stress in tubular epithelial cells. Redox Biology. 2025, 80:103490.
- Ikenoue, M.; Choijookhuu, N.; Yano, K.; Fidya; Takahashi, N. ; Ishizuka, T.; Shirouzu, S.; Yamaguma, Y.; Kai, K.; Higuchi, K.; et al. The crucial role of SETDB1 in structural and functional transformation of epithelial cells during regeneration after intestinal ischemia reperfusion injury. Histochem. 2024, 161, 325–336. [Google Scholar] [CrossRef]
- Shi, Y.; Jiang, B.; Zhao, J. Induction mechanisms of autophagy and endoplasmic reticulum stress in intestinal ischemia-reperfusion injury, inflammatory bowel disease, and colorectal cancer. Biomed. Pharmacother. 2023, 170, 115984. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Nie, H. Advances in lung ischemia/reperfusion injury: unraveling the role of innate immunity. Inflamm. Res. 2024, 73, 393–405. [Google Scholar] [CrossRef]
- Lu, P.; Qi, Y.; Li, X.; Zhang, C.; Chen, Z.; Shen, Z.; Liang, J.; Zhang, H.; Yuan, Y. PEDF and 34-mer peptide inhibit cardiac microvascular endothelial cell ferroptosis via Nrf2/HO-1 signalling in myocardial ischemia-reperfusion injury. J. Cell. Mol. Med. 2024, 28. [Google Scholar] [CrossRef]
- Yinzhi, D.; Jianhua, H.; Hesheng, L. The roles of liver sinusoidal endothelial cells in liver ischemia/reperfusion injury. J. Gastroenterol. Hepatol. 2023, 39, 224–230. [Google Scholar] [CrossRef]
- Li, P.; Wang, Z.; Zhao, T.; Cheng, X.; Zhang, Z.; Wang, J.; Wang, S.; Huang, R.; Hui, Z. Protective Effect of Compound Tongluo Decoction on Brain Vascular Endothelial Cells after Ischemia-Reperfusion by Inhibition of Ferroptosis Through Regulating Nrf2/ARE/SLC7A11 Signaling Pathway. Adv. Biol. 2023, 8, e2300416. [Google Scholar] [CrossRef]
- Xiang, Q.; Yi, X.; Zhu, X.-H.; Wei, X.; Jiang, D.-S. Regulated cell death in myocardial ischemia–reperfusion injury. Trends Endocrinol. Metab. 2023, 35, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Zhang, Z.; Chen, L.; Wang, X.; Zhong, Y.; Xie, D.; Liao, W. LncRNA 93358 Aggravates the Apoptosis of Myocardial Cells After Ischemia-Reperfusion by Mediating the PI3K/AKT/mTOR Pathway. J. Biochem. Mol. Toxicol. 2024, 38, e70085. [Google Scholar] [CrossRef]
- Wang, J.; Zhuang, H.; Jia, L.; He, X.; Zheng, S.; Ji, K.; Xie, K.; Ying, T.; Zhang, Y.; Li, C.; et al. Nuclear receptor subfamily 4 group A member 1 promotes myocardial ischemia/reperfusion injury through inducing mitochondrial fission factor-mediated mitochondrial fragmentation and inhibiting FUN14 domain containing 1-depedent mitophagy. Int. J. Biol. Sci. 2024, 20, 4458–4475. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Tao, Y.; Che, G.; Yun, Y.; Ren, M.; Liu, Y. WSB1, as an E3 ligase, restrains myocardial ischemia–reperfusion injury by activating β-catenin signaling via promoting GSK3β ubiquitination. Molecular Medicine. 2024, 30, 31. [Google Scholar] [CrossRef]
- Yang, W.; Lei, X.; Liu, F.; Sui, X.; Yang, Y.; Xiao, Z.; et al. Meldonium, as a potential neuroprotective agent, promotes neuronal survival by protecting mitochondria in cerebral ischemia–reperfusion injury. Journal of Translational Medicine. 2024, 22, 771. [Google Scholar] [CrossRef]
- Zhang, H.; Feng, Y.; Si, Y.; Lu, C.; Wang, J.; Wang, S.; Li, L.; Xie, W.; Yue, Z.; Yong, J.; et al. Shank3 ameliorates neuronal injury after cerebral ischemia/reperfusion via inhibiting oxidative stress and inflammation. Redox Biol. 2023, 69, 102983. [Google Scholar] [CrossRef] [PubMed]
- Balk, R.A. Systemic inflammatory response syndrome (SIRS). Virulence. 2014, 5, 20–26. [Google Scholar] [CrossRef]
- Kaukonen, K.-M.; Bailey, M.; Pilcher, D.; Cooper, D.J.; Bellomo, R. Systemic Inflammatory Response Syndrome Criteria in Defining Severe Sepsis. New Engl. J. Med. 2015, 372, 1629–1638. [Google Scholar] [CrossRef]
- Matsuda, N.; Hattori, Y. Systemic Inflammatory Response Syndrome (SIRS): Molecular Pathophysiology and Gene Therapy. J. Pharmacol. Sci. 2006, 101, 189–198. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, X.; Jiang, Q.; Jiao, F.; Du, Q.; Liu, J.; Luo, M.; Li, A.; Deng, L.; Xiong, Y. Systemic inflammatory response syndrome in patients with severe fever with thrombocytopenia syndrome: prevalence, characteristics, and impact on prognosis. BMC Infect. Dis. 2024, 24, 1–10. [Google Scholar] [CrossRef]
- Ashayeripanah, M.; Vega-Ramos, J.; Fernandez-Ruiz, D.; Valikhani, S.; Lun, A.T.; White, J.T.; Young, L.J.; Yaftiyan, A.; Zhan, Y.; Wakim, L.; et al. Systemic inflammatory response syndrome triggered by blood-borne pathogens induces prolonged dendritic cell paralysis and immunosuppression. Cell Rep. 2024, 43, 113754. [Google Scholar] [CrossRef]
- Radvinsky, D.S.; Yoon, R.S.; Schmitt, P.J.; Prestigiacomo, C.J.; Swan, K.G.; Liporace, F.A. Evolution and Development of the Advanced Trauma Life Support (ATLS) Protocol: A Historical Perspective. Orthopedics 2012, 35, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, A.; Branicki, F.; Abu-Zidan, F.M. Educational and Clinical Impact of Advanced Trauma Life Support (ATLS) Courses: A Systematic Review. World J. Surg. 2013, 38, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Parrino, C.R.; Fransman, R.B.; Varone, A.J.; Galvagno, S.M. Advanced Trauma Life Support. In: Faintuch J, Faintuch S, editors. Recent Strategies in High Risk Surgery. Cham: Springer Nature Switzerland; 2024. p. 171-94.
- Egi, M.; Ogura, H.; Yatabe, T.; Atagi, K.; Inoue, S.; Iba, T.; et al. The Japanese Clinical Practice Guidelines for Management of Sepsis and Septic Shock 2020 (J-SSCG 2020). Journal of Intensive Care. 2021, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Executive Summary: Surviving Sepsis Campaign: International Guidelines for the Management of Sepsis and Septic Shock 2021. Crit. Care Med. 2021, 49, 1974–1982. [Google Scholar] [CrossRef]
- Nazer, L.; Abusara, A.; Aloran, B.; Szakmany, T.; Nabulsi, H.; Petushkov, A.; Charpignon, M.-L.; Ahmed, T.; Cobanaj, M.; Elaibaid, M.; et al. Patient diversity and author representation in clinical studies supporting the Surviving Sepsis Campaign guidelines for management of sepsis and septic shock 2021: a systematic review of citations. BMC Infect. Dis. 2023, 23, 1–10. [Google Scholar] [CrossRef]
- Petrosoniak, A.; Hicks, C. Resuscitation Resequenced: A Rational Approach to Patients with Trauma in Shock. Emergency Medicine Clinics. 2018, 36, 41–60. [Google Scholar] [CrossRef]
- Buja, L.M.; Maximilian, L. Pathobiology of Myocardial Ischemia and Reperfusion Injury: Models, Modes, Molecular Mechanisms, Modulation, and Clinical Applications. Cardiol. Rev. 2022, 31, 252–264. [Google Scholar] [CrossRef]
- Jurcau, A.; Ardelean, I.A. Molecular pathophysiological mechanisms of ischemia/reperfusion injuries after recanalization therapy for acute ischemic stroke. J. Integr. Neurosci. 2021, 20, 727–744. [Google Scholar] [CrossRef]
- Nemeth, N.; Peto, K.; Magyar, Z.; Klarik, Z.; Varga, G.; Oltean, M.; Mantas, A.; Czigany, Z.; Tolba, R.H. Hemorheological and Microcirculatory Factors in Liver Ischemia-Reperfusion Injury—An Update on Pathophysiology, Molecular Mechanisms and Protective Strategies. Int. J. Mol. Sci. 2021, 22, 1864. [Google Scholar] [CrossRef]
- Goncharov, R.G.; Sharapov, M.G. Ischemia–Reperfusion Injury: Molecular Mechanisms of Pathogenesis and Methods of Their Correction. Molecular Biology. 2023, 57, 1143–1164. [Google Scholar] [CrossRef]
- Jurcau, A.; Simion, A. Neuroinflammation in Cerebral Ischemia and Ischemia/Reperfusion Injuries: From Pathophysiology to Therapeutic Strategies. Int. J. Mol. Sci. 2021, 23, 14. [Google Scholar] [CrossRef]
- Gheitasi, I.; Akbari, G.; Savari, F. Physiological and cellular mechanisms of ischemic preconditioning microRNAs-mediated in underlying of ischemia/reperfusion injury in different organs. Mol. Cell. Biochem. 2024, 480, 855–868. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Man, K. New Insights in Mechanisms and Therapeutics for Short- and Long-Term Impacts of Hepatic Ischemia Reperfusion Injury Post Liver Transplantation. Int. J. Mol. Sci. 2021, 22, 8210. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Chen, P.; Zhong, J.; Cheng, Y.; Chen, H.; He, Y.; et al. HIF-1α in myocardial ischemia-reperfusion injury (Review). Mol Med Rep. 2021, 23, 352. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Zhao, J.; Yang, E.; Wang, Y.; Wang, Q.; Wu, Y.; Tong, F.; Tan, Y.; Zhou, J.; Kang, C. Neuronal STAT3/HIF-1α/PTRF axis-mediated bioenergetic disturbance exacerbates cerebral ischemia-reperfusion injury via PLA2G4A. Theranostics 2022, 12, 3196–3216. [Google Scholar] [CrossRef]
- Ni, H.; Li, J.; Zheng, J.; Zhou, B. Cardamonin attenuates cerebral ischemia/reperfusion injury by activating the HIF-1α/VEGFA pathway. Phytotherapy Res. 2022, 36, 1736–1747. [Google Scholar] [CrossRef]
- Fang, Z.; Zhang, Y.; Zhao, X.; Jin, W.; Yu, L. The Role of PKC and HIF-1 and the Effect of Traditional Chinese Medicinal Compounds on Cerebral Ischemia-Reperfusion Injury. Evidence-Based Complement. Altern. Med. 2022, 2022, 1–12. [Google Scholar] [CrossRef]
- Han, X.; Jiang, Z.; Hou, Y.; Zhou, X.; Hu, B. Myocardial ischemia-reperfusion injury upregulates nucleostemin expression via HIF-1α and c-Jun pathways and alleviates apoptosis by promoting autophagy. Cell Death Discov. 2024, 10, 1–10. [Google Scholar] [CrossRef]
- Liu, C.; Pei, S.; Dai, H.; Liu, Z.; Ye, M.; Liu, H.; He, X.; Wu, S.; Qin, Y.; Lin, F. Downregulation of SIRT3 Aggravates Lung Ischemia Reperfusion Injury by Increasing Mitochondrial Fission and Oxidative Stress through HIF-1α-Dependent Mechanisms. Oxidative Med. Cell. Longev. 2022, 2022, 1–19. [Google Scholar] [CrossRef]
- Fedele AO, Whitelaw ML, Peet DJJMi. Regulation of gene expression by the hypoxia-inducible factors. 2002, 2, 229.
- Mole, D.R.; Blancher, C.; Copley, R.R.; Pollard, P.J.; Gleadle, J.M.; Ragoussis, J.; et al. Genome-wide Association of Hypoxia-inducible Factor (HIF)-1α and HIF-2α DNA Binding with Expression Profiling of Hypoxia-inducible Transcripts *. Journal of Biological Chemistry. 2009, 284, 16767–16775. [Google Scholar] [CrossRef] [PubMed]
- Dames, S.A.; Martinez-Yamout, M.; De Guzman, R.N.; Dyson, H.J.; Wright, P.E. Structural basis for Hif-1α/CBP recognition in the cellular hypoxic response. Proc. Natl. Acad. Sci. 2002, 99, 5271–5276. [Google Scholar] [CrossRef]
- Dayan, F.; Monticelli, M.; Pouysségur, J.; Pécou, E. Gene regulation in response to graded hypoxia: The non-redundant roles of the oxygen sensors and FIH in the HIF pathway. J. Theor. Biol. 2009, 259, 304–316. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-W.; Bae, S.-H.; Jeong, J.-W.; Kim, S.-H.; Kim, K.-W. Hypoxia-inducible factor (HIF-1)α: its protein stability and biological functions. Exp. Mol. Med. 2004, 36, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kierans, S.J.; Fagundes, R.R.; Malkov, M.I.; Sparkes, R.; Dillon, E.T.; Smolenski, A.; Faber, K.N.; Taylor, C.T. Hypoxia induces a glycolytic complex in intestinal epithelial cells independent of HIF-1-driven glycolytic gene expression. Proc. Natl. Acad. Sci. 2023, 120. [Google Scholar] [CrossRef]
- Bhattarai, D.; Xu, X.; Lee, K. Hypoxia-inducible factor-1 (HIF-1) inhibitors from the last decade (2007 to 2016): A “structure–activity relationship” perspective. 2018, 38, 1404–1442.
- Graham AM, Presnell JSJPo. Hypoxia Inducible Factor (HIF) transcription factor family expansion, diversification, divergence and selection in eukaryotes. 2017, 12, e0179545.
- Diseri, A.; Stravodimos, G.; Argyriou, A.; Spyroulias, G.A.; Leonidas, D.D.; Liakos, P. Expression, purification, and biophysical analysis of a part of the C-terminal domain of human hypoxia inducible factor-2α (HIF-2α). Biochemical and Biophysical Research Communications. 2024, 739:150965.
- Jiang, Y.; Cukic, B.; Adjeroh, D.A.; Skinner, H.D.; Lin, J.; Shen, Q.J.; Jiang, B.-H. An Algorithm for Identifying Novel Targets of Transcription Factor Families: Application to Hypoxia-inducible Factor 1 Targets. Cancer Informatics 2009, 7, CIN.S1054–89. [Google Scholar] [CrossRef]
- Stroka, D.M.; Burkhardt, T.; Desbaillets, I.; Wenger, R.H.; Neil, D.A.H.; Bauer, C.; Gassmann, M.; Candinas, D. HIF-1 is expressed in normoxic tissue and displays an organ-specific regulation under systemic hypoxia. FASEB J. 2001, 15, 2445–2453. [Google Scholar] [CrossRef]
- Kaluz, S.; Kaluzová,, M. ; Stanbridge, E.J. Regulation of gene expression by hypoxia: Integration of the HIF-transduced hypoxic signal at the hypoxia-responsive element. Clin. Chim. Acta 2008, 395, 6–13. [Google Scholar] [CrossRef]
- Talks, K.L.; Turley, H.; Gatter, K.C.; Maxwell, P.H.; Pugh, C.W.; Ratcliffe, P.J.; et al. The Expression and Distribution of the Hypoxia-Inducible Factors HIF-1α and HIF-2α in Normal Human Tissues, Cancers, and Tumor-Associated Macrophages. The American Journal of Pathology. 2000, 157, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Wiesener, M.S.; Jürgensen, J.S.; Rosenberger, C.; Scholze, C.; Hörstrup, J.H.; Warnecke, C.; et al. Widespread, hypoxia-inducible expression of HIF-2α in distinct cell populations of different organs. 2003, 17, 271–273.
- Bartoszewski, R.; Moszyńska, A.; Serocki, M.; Cabaj, A.; Polten, A.; Ochocka, R.; et al. Primary endothelial cell-specific regulation of hypoxia-inducible factor (HIF)-1 and HIF-2 and their target gene expression profiles during hypoxia. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2019, 33, 7929–7941. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yao, L.; Yang, J.; Wang, Z.; Du, G. PI3K/Akt and HIF-1 signaling pathway in hypoxia-ischemia (Review). Mol Med Rep. 2018, 18, 3547–3554. [Google Scholar] [CrossRef]
- Fábián, Z.; Taylor, C.T.; Nguyen, L.K. Understanding complexity in the HIF signaling pathway using systems biology and mathematical modeling. J. Mol. Med. 2016, 94, 377–390. [Google Scholar] [CrossRef]
- Taylor, C.T.; Scholz, C.C. The effect of HIF on metabolism and immunity. Nat. Rev. Nephrol. 2022, 18, 573–587. [Google Scholar] [CrossRef] [PubMed]
- Taylor Cormac, T. Mitochondria and cellular oxygen sensing in the HIF pathway. Biochemical Journal. 2007, 409, 19–26. [Google Scholar] [CrossRef]
- Pugh, C.W.; Ratcliffe, P.J. New horizons in hypoxia signaling pathways. [CrossRef]
- Liu, J.; Wei, Q.; Guo, C.; Dong, G.; Liu, Y.; Tang, C.; Dong, Z. Hypoxia, HIF, and Associated Signaling Networks in Chronic Kidney Disease. Int. J. Mol. Sci. 2017, 18, 950. [Google Scholar] [CrossRef]
- Ferreira, B.L.; Leite, G.G.F.; Brunialti, M.K.C.; Assuncao, M.; Azevedo, L.C.P.; Freitas, F.; Salomao, R. HIF-1α and Hypoxia Responsive Genes are Differentially Expressed in Leukocytes From Survivors and Non-Survivors Patients During Clinical Sepsis. Shock 2020, 56, 80–91. [Google Scholar] [CrossRef]
- Baloglu, E. Hypoxic Stress-Dependent Regulation of Na,K-ATPase in Ischemic Heart Disease. Int. J. Mol. Sci. 2023, 24, 7855. [Google Scholar] [CrossRef]
- Ramzan, R.; Cybulski, P.; Ruppert, V.; Weber, P.; Irqsusi, M.; Mirow, N.; et al. Does MRNA Upregulation of Cytochrome C Oxidase Subunit 4 Isoform 2 Sustain Atrial Fibrillation? 2021, 69(S 01):DGTHG-eP103.
- Indik, J.H.; Hilwig, R.W.; Zuercher, M.; Kern, K.B.; Berg, M.D.; Berg, R.A. Preshock Cardiopulmonary Resuscitation Worsens Outcome From Circulatory Phase Ventricular Fibrillation With Acute Coronary Artery Obstruction in Swine. 2009, 2, 179–184.
- Textoris, J.; Beaufils, N.; Quintana, G.; Ben Lassoued, A.; Zieleskiewicz, L.; Wiramus, S.; Blasco, V.; Lesavre, N.; Martin, C.; Gabert, J.; et al. Hypoxia-inducible factor (HIF1α) gene expression in human shock states. Crit. Care 2012, 16, 1–6. [Google Scholar] [CrossRef]
- Yang, H.; Du, L.; Zhang, Z. Potential biomarkers in septic shock besides lactate. Exp. Biol. Med. 2020, 245, 1066–1072. [Google Scholar] [CrossRef] [PubMed]
- Valenza, F.; Aletti, G.; Fossali, T.; Chevallard, G.; Sacconi, F.; Irace, M.; Gattinoni, L. Lactate as a marker of energy failure in critically ill patients: hypothesis. Crit. Care 2005, 9, 588–593. [Google Scholar] [CrossRef]
- Eickelberg, O.; Seebach, F.; Riordan, M.; Thulin, G.; Mann, A.; Reidy, K.H.; Van Why, S.K.; Kashgarian, M.; Siegel, N. Functional Activation of Heat Shock Factor and Hypoxia-Inducible Factor in the Kidney. J. Am. Soc. Nephrol. 2002, 13, 2094–2101. [Google Scholar] [CrossRef]
- Gorecki, G.; Cochior, D.; Moldovan, C.; Rusu, E. Molecular mechanisms in septic shock (Review). Exp. Ther. Med. 2021, 22, 1–5. [Google Scholar] [CrossRef]
- Hierholzer, C.; Billiar, T.R. Molecular mechanisms in the early phase of hemorrhagic shock. Langenbeck's Arch. Surg. 2001, 386, 302–308. [Google Scholar] [CrossRef]
- Kültz, D. MOLECULAR AND EVOLUTIONARY BASIS OF THE CELLULAR STRESS RESPONSE. Annu. Rev. Physiol. 2005, 67, 225–257. [Google Scholar] [CrossRef]
- Dufour-Gaume, F.; Frescaline, N.; Cardona, V.; Prat, N.J. Danger signals in traumatic hemorrhagic shock and new lines for clinical applications. Front. Physiol. 2023, 13, 999011. [Google Scholar] [CrossRef] [PubMed]
- Chalkias, A. Shear Stress and Endothelial Mechanotransduction in Trauma Patients with Hemorrhagic Shock: Hidden Coagulopathy Pathways and Novel Therapeutic Strategies. Int. J. Mol. Sci. 2023, 24, 17522. [Google Scholar] [CrossRef] [PubMed]
- Fecher, A.; Stimpson, A.; Ferrigno, L.; Pohlman, T.H. The Pathophysiology and Management of Hemorrhagic Shock in the Polytrauma Patient. J. Clin. Med. 2021, 10, 4793. [Google Scholar] [CrossRef]
- Herzum I, Renz HJCmc. Inflammatory markers in SIRS, sepsis and septic shock. 2008, 15, 581–587.
- Klein Klouwenberg, P.M.C.; Ong, D.S.Y.; Bonten, M.J.M.; Cremer, O.L. Classification of sepsis, severe sepsis and septic shock: the impact of minor variations in data capture and definition of SIRS criteria. Intensive Care Medicine. 2012, 38, 811–819. [Google Scholar] [CrossRef]
- Peng, Y.; Zhang, W.; Xu, Y.; Li, L.; Yu, W.; Zeng, J.; Ming, S.; Fang, Z.; Wang, Z.; Gao, X. Performance of SOFA, qSOFA and SIRS to predict septic shock after percutaneous nephrolithotomy. World J. Urol. 2020, 39, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Jentzer, J.C.; Lawler, P.R.; van Diepen, S.; Henry, T.D.; Menon, V.; Baran, D.A.; Džavík, V.; Barsness, G.W.; Holmes, D.R.; Kashani, K.B. Systemic Inflammatory Response Syndrome Is Associated With Increased Mortality Across the Spectrum of Shock Severity in Cardiac Intensive Care Patients. Circ. Cardiovasc. Qual. Outcomes 2020, 13. [Google Scholar] [CrossRef] [PubMed]
- Wulff, A.; Montag, S.; Marschollek, M.; Jack, T. Clinical Decision-Support Systems for Detection of Systemic Inflammatory Response Syndrome, Sepsis, and Septic Shock in Critically Ill Patients: A Systematic Review. Methods Inf. Med. 2019, 58, e43–e57. [Google Scholar] [CrossRef]
- Cavaillon, J.-M.; Giamarellos-Bourboulis, E.J. Immunosuppression is Inappropriately Qualifying the Immune Status of Septic and SIRS Patients. Shock 2019, 52, 307–317. [Google Scholar] [CrossRef]
- Toliver-Kinsky, T.; Kobayashi, M.; Suzuki, F.; Sherwood, E.R. 19 - The Systemic Inflammatory Response Syndrome. In: Herndon DN, editor. Total Burn Care (Fifth Edition): Elsevier; 2018. p. 205-20.e4.
- Zhang, Y.; Chen, Y.; Meng, Z. Immunomodulation for Severe COVID-19 Pneumonia: The State of the Art. Front. Immunol. 2020, 11, 577442. [Google Scholar] [CrossRef] [PubMed]
- Nomellini, V.; Kaplan, L.J.; Sims, C.A.; Caldwell, C.C. Chronic Critical Illness and Persistent Inflammation: What can we Learn from the Elderly, Injured, Septic, and Malnourished? Shock 2018, 49, 4–14. [Google Scholar] [CrossRef]
- Chakraborty, R.K.; Burns, B. Systemic Inflammatory Response Syndrome: StatPearls Publishing, Treasure Island (FL); 2023 2023.
- Zhang, B.; Xiao, Q.; Ma, Q.; Han, L. Clinical treatment for persistent inflammation, immunosuppression and catabolism syndrome in patients with severe acute pancreatitis (Review). Exp. Ther. Med. 2023, 26, 1–10. [Google Scholar] [CrossRef]
- Arlati, S. Pathophysiology of Acute Illness and Injury. In: Aseni P, De Carlis L, Mazzola A, Grande AM, editors. Operative Techniques and Recent Advances in Acute Care and Emergency Surgery. Cham: Springer International Publishing; 2019. p. 11-42.
- Mehta, Y.; Paul, R.; Ansari, A.S.; Banerjee, T.; Gunaydin, S.; Nassiri, A.A.; Pappalardo, F.; Premužić,, V. ; Sathe, P.; Singh, V.; et al. Extracorporeal blood purification strategies in sepsis and septic shock: An insight into recent advancements. World J. Crit. Care Med. 2023, 12, 71–88. [Google Scholar] [CrossRef]
- Amara, U.E.; Nashrah, U.; Balal, A.; Shaikh, N.; Chanda, A.H.; Al-Jalham, K.M.A. Urosepsis and Septic Shock: A Simple Infection Progressing to Complex One. In Applied Microbiology in Intensive Care Medicine; Shaikh, N., Chanda, A.H., Eds.; Springer Nature Singapore: Singapore, 2024; pp. 51–59. [Google Scholar]
- Vergadi, E.; Vaporidi, K.; Tsatsanis, C. Regulation of Endotoxin Tolerance and Compensatory Anti-inflammatory Response Syndrome by Non-coding RNAs. Front. Immunol. 2018, 9, 2705. [Google Scholar] [CrossRef]
- Sendler, M.; Brandt, C.v.D.; Glaubitz, J.; Wilden, A.; Golchert, J.; Weiss, F.U.; Homuth, G.; Chama, L.L.D.F.; Mishra, N.; Mahajan, U.M.; et al. NLRP3 Inflammasome Regulates Development of Systemic Inflammatory Response and Compensatory Anti-Inflammatory Response Syndromes in Mice With Acute Pancreatitis. Gastroenterology 2020, 158, 253–269.e14. [Google Scholar] [CrossRef] [PubMed]
- DeGasperi, A.; Bucci, L.; Wahlen, B.M. Multiple Organ Dysfunction Syndrome After Trauma: Update 2017. In: Aseni P, De Carlis L, Mazzola A, Grande AM, editors. Operative Techniques and Recent Advances in Acute Care and Emergency Surgery. Cham: Springer International Publishing; 2019. p. 727-32.
- Kell, D.B.; Pretorius, E. To What Extent Are the Terminal Stages of Sepsis, Septic Shock, Systemic Inflammatory Response Syndrome, and Multiple Organ Dysfunction Syndrome Actually Driven by a Prion/Amyloid Form of Fibrin? Seminars in thrombosis and hemostasis. 2018, 44, 224–238. [Google Scholar] [CrossRef]
- Asim, M.; Amin, F.; El-Menyar, A. Multiple organ dysfunction syndrome: Contemporary insights on the clinicopathological spectrum. Qatar Med J. 2020, 2020, 22. [Google Scholar] [CrossRef] [PubMed]
- Osuchowski, M.F.; Craciun, F.; Weixelbaumer, K.M.; Duffy, E.R.; Remick, D.G. Sepsis Chronically in MARS: Systemic Cytokine Responses Are Always Mixed Regardless of the Outcome, Magnitude, or Phase of Sepsis. J. Immunol. 2012, 189, 4648–4656. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Pattern Recognition Receptors and Inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef]
- Li, D.; Wu, M. Pattern recognition receptors in health and diseases. Signal Transduct. Target. Ther. 2021, 6, 1–24. [Google Scholar] [CrossRef]
- Newman, M.-A.; Sundelin, T.; Nielsen, J.T.; Erbs, G. MAMP (microbe-associated molecular pattern) triggered immunity in plants. Front. Plant Sci. 2013, 4, 139. [Google Scholar] [CrossRef] [PubMed]
- Fleshner, M. Stress-evoked sterile inflammation, danger associated molecular patterns (DAMPs), microbial associated molecular patterns (MAMPs) and the inflammasome. Brain, Behav. Immun. 2013, 27, 1–7. [Google Scholar] [CrossRef]
- Parker, J. ANNUAL PLANT REVIEWS VOLUME 34. 2009.
- Cicchinelli, S.; Pignataro, G.; Gemma, S.; Piccioni, A.; Picozzi, D.; Ojetti, V.; Franceschi, F.; Candelli, M. PAMPs and DAMPs in Sepsis: A Review of Their Molecular Features and Potential Clinical Implications. Int. J. Mol. Sci. 2024, 25, 962. [Google Scholar] [CrossRef]
- Alpkvist, H.; Ziegler, I.; Mölling, P.; Tina, E.; Sellvén, L.; Norrby-Teglund, A.; Cajander, S.; Strålin, K. Damage-associated molecular patterns in bacteraemic infection, including a comparative analysis with bacterial DNA, a pathogen-associated molecular pattern. Sci. Rep. 2024, 14, 1–10. [Google Scholar] [CrossRef]
- Alpkvist, H. Damage-associated molecular patterns and pathogen-associated molecular patterns in severe bacterial infections: Karolinska Institutet; 2024.
- Mahaling, B.; Low, S.W.Y.; Beck, M.; Kumar, D.; Ahmed, S.; Connor, T.B.; Ahmad, B.; Chaurasia, S.S. Damage-Associated Molecular Patterns (DAMPs) in Retinal Disorders. Int. J. Mol. Sci. 2022, 23, 2591. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Aziz, M.; Wang, P. Damage-Associated Molecular Patterns As Double-Edged Swords in Sepsis. Antioxidants Redox Signal. 2021, 35, 1308–1323. [Google Scholar] [CrossRef] [PubMed]
- Jentho, E.; Weis, S. DAMPs and Innate Immune Training. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef]
- Tavalaee, M.; Rahmani, M.; Drevet, J.R.; Nasr-Esfahani, M.H. The NLRP3 inflammasome: molecular activation and regulation in spermatogenesis and male infertility; a systematic review. Basic Clin. Androl. 2022, 32, 1–11. [Google Scholar] [CrossRef]
- Carroll, K.A.; Sawden, M.; Sharma, S. DAMPs, PAMPs, NLRs, RIGs, CLRs and TLRs – Understanding the Alphabet Soup in the Context of Bone Biology. Current Osteoporosis Reports. 2025, 23, 6. [Google Scholar] [CrossRef]
- Pantalone, D.; Bergamini, C.; Martellucci, J.; Alemanno, G.; Bruscino, A.; Maltinti, G.; Sheiterle, M.; Viligiardi, R.; Panconesi, R.; Guagni, T.; et al. The Role of DAMPS in Burns and Hemorrhagic Shock Immune Response: Pathophysiology and Clinical Issues. Review. Int. J. Mol. Sci. 2021, 22, 7020. [Google Scholar] [CrossRef]
- Moriyama, K.; Nishida, O. Targeting Cytokines, Pathogen-Associated Molecular Patterns, and Damage-Associated Molecular Patterns in Sepsis via Blood Purification. Int. J. Mol. Sci. 2021, 22, 8882. [Google Scholar] [CrossRef]
- Rosenzweig, J.M.; Lei, J.; Burd, I. Interleukin-1 Receptor Blockade in Perinatal Brain Injury. Front. Pediatr. 2014, 2, 108–108. [Google Scholar] [CrossRef] [PubMed]
- Nimma, S.; Gu, W.; Maruta, N.; Li, Y.; Pan, M.; Saikot, F.K.; Lim, B.Y.J.; McGuinness, H.Y.; Zaoti, Z.F.; Li, S.; et al. Structural Evolution of TIR-Domain Signalosomes. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef]
- Bhatt, A.; Mishra, B.P.; Gu, W.; Sorbello, M.; Xu, H.; Ve, T.; Kobe, B. Structural characterization of TIR-domain signalosomes through a combination of structural biology approaches. IUCrJ 2024, 11, 695–707. [Google Scholar] [CrossRef]
- Ferrao, R.; Li, J.; Bergamin, E.; Wu, H. Structural Insights into the Assembly of Large Oligomeric Signalosomes in the Toll-Like Receptor–Interleukin-1 Receptor Superfamily. Sci. Signal. 2012, 5, re3–re3. [Google Scholar] [CrossRef] [PubMed]
- Krumm, B.; Xiang, Y.; Deng, J. Structural biology of the IL-1 superfamily: Key cytokines in the regulation of immune and inflammatory responses. Protein Sci. 2014, 23, 526–538. [Google Scholar] [CrossRef]
- Fields, J.K.; Günther, S.; Sundberg, E.J. Structural Basis of IL-1 Family Cytokine Signaling. Front. Immunol. 2019, 10, 1412. [Google Scholar] [CrossRef]
- Boraschi, D. What Is IL-1 for? The Functions of Interleukin-1 Across Evolution. Front. Immunol. 2022, 13, 872155. [Google Scholar] [CrossRef] [PubMed]
- Evavold, C.L.; Kagan, J.C. Diverse Control Mechanisms of the Interleukin-1 Cytokine Family. Front. Cell Dev. Biol. 2022, 10, 910983. [Google Scholar] [CrossRef]
- Boersma, B.; Jiskoot, W.; Lowe, P.; Bourquin, C. The interleukin-1 cytokine family members: Role in cancer pathogenesis and potential therapeutic applications in cancer immunotherapy. Cytokine Growth Factor Rev. 2021, 62, 1–14. [Google Scholar] [CrossRef]
- Ding, Y.; Yi, J.; Wang, J.; Sun, Z. Interleukin-1 receptor antagonist: a promising cytokine against human squamous cell carcinomas. Heliyon 2023, 9, e14960. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Sowdhamini, R. A genome-wide search of Toll/Interleukin-1 receptor (TIR) domain-containing adapter molecule (TICAM) and their evolutionary divergence from other TIR domain containing proteins. Biol. Direct 2022, 17, 1–14. [Google Scholar] [CrossRef]
- Galozzi, P.; Bindoli, S.; Doria, A.; Sfriso, P. The revisited role of interleukin-1 alpha and beta in autoimmune and inflammatory disorders and in comorbidities. Autoimmun. Rev. 2021, 20, 102785. [Google Scholar] [CrossRef]
- Neri, M.; Fineschi, V.; Paolo, M.; Pomara, C.; Riezzo, I.; Turillazzi, E.; Cerretani, D. Cardiac Oxidative Stress and Inflammatory Cytokines Response after Myocardial Infarction. Curr. Vasc. Pharmacol. 2015, 13, 26–36. [Google Scholar] [CrossRef]
- Aimo, A.; Castiglione, V.; Borrelli, C.; Saccaro, L.F.; Franzini, M.; Masi, S.; Emdin, M.; Giannoni, A. Oxidative stress and inflammation in the evolution of heart failure: From pathophysiology to therapeutic strategies. Eur. J. Prev. Cardiol. 2019, 27, 494–510. [Google Scholar] [CrossRef] [PubMed]
- Southcombe, J.H.; Redman, C.W.G.; Sargent, I.L.; Granne, I. Interleukin-1 family cytokines and their regulatory proteins in normal pregnancy and pre-eclampsia. Clin. Exp. Immunol. 2015, 181, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.; Maier, O. Interrelation of Oxidative Stress and Inflammation in Neurodegenerative Disease: Role of TNF. Oxidative Med. Cell. Longev. 2015, 2015, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Boraschi, D.; Italiani, P.; Weil, S.; Martin, M.U. The family of the interleukin-1 receptors. 2018, 281, 197–232.
- Yazdi, A.S.; Ghoreschi, K. The Interleukin-1 Family. In: Ma X, editor. Regulation of Cytokine Gene Expression in Immunity and Diseases. Dordrecht: Springer Netherlands; 2016. p. 21-9.
- Schett, G.; Dayer, J.-M.; Manger, B. Interleukin-1 function and role in rheumatic disease. Nat. Rev. Rheumatol. 2015, 12, 14–24. [Google Scholar] [CrossRef]
- Wan, S.; Chen, Q.; Xiang, Y.; Sang, Y.; Tang, M.; Song, Y.; et al. Interleukin-1 increases cyclooxygenase-2 expression and prostaglandin E2 production in human granulosa-lutein cell via nuclear factor kappa B/P65 and extracellular signal-regulated kinase 1/2 signaling pathways. Molecular and Cellular Endocrinology. 2023, 566-567:111891.
- Netea, M.G.; van de Veerdonk, F.L.; van der Meer, J.W.; Dinarello, C.A.; Joosten, L.A. Inflammasome-Independent Regulation of IL-1-Family Cytokines. Annu. Rev. Immunol. 2015, 33, 49–77. [Google Scholar] [CrossRef]
- Cavalli, G.; Colafrancesco, S.; Emmi, G.; Imazio, M.; Lopalco, G.; Maggio, M.C.; Sota, J.; Dinarello, C.A. Interleukin 1α: a comprehensive review on the role of IL-1α in the pathogenesis and treatment of autoimmune and inflammatory diseases. Autoimmun. Rev. 2021, 20, 102763. [Google Scholar] [CrossRef]
- De Jesus, N.M.; Wang, L.; Lai, J.; Rigor, R.R.; Francis Stuart, S.D.; Bers, D.M.; et al. Antiarrhythmic effects of interleukin 1 inhibition after myocardial infarction. Heart Rhythm. 2017, 14, 727–736. [Google Scholar] [CrossRef]
- Bageghni, S.A.; Hemmings, K.E.; Yuldasheva, N.Y.; Maqbool, A.; Gamboa-Esteves, F.O.; Humphreys, N.E.; Jackson, M.S.; Denton, C.P.; Francis, S.; Porter, K.E.; et al. Fibroblast-specific deletion of IL-1 receptor-1 reduces adverse cardiac remodeling following myocardial infarction. J. Clin. Investig. 2019, 4. [Google Scholar] [CrossRef]
- Pascual-Figal, D.A.; Bayes-Genis, A.; Asensio-Lopez, M.C.; Hernández-Vicente, A.; Garrido-Bravo, I.; Pastor-Perez, F.; Díez, J.; Ibáñez, B.; Lax, A. The Interleukin-1 Axis and Risk of Death in Patients With Acutely Decompensated Heart Failure. Circ. 2019, 73, 1016–1025. [Google Scholar] [CrossRef]
- Bui, C.B.; Kolodziej, M.; Lamanna, E.; Elgass, K.; Sehgal, A.; Rudloff, I.; Schwenke, D.O.; Tsuchimochi, H.; Kroon, M.A.G.M.; Cho, S.X.; et al. Interleukin-1 Receptor Antagonist Protects Newborn Mice Against Pulmonary Hypertension. Front. Immunol. 2019, 10, 1480. [Google Scholar] [CrossRef]
- Royce, S.G.; Nold, M.F.; Bui, C.; Donovan, C.; Lam, M.; Lamanna, E.; Rudloff, I.; Bourke, J.E.; Nold-Petry, C.A. Airway Remodeling and Hyperreactivity in a Model of Bronchopulmonary Dysplasia and Their Modulation by IL-1 Receptor Antagonist. Am. J. Respir. Cell Mol. Biol. 2016, 55, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Borthwick, L.A. The IL-1 cytokine family and its role in inflammation and fibrosis in the lung. Semin. Immunopathol. 2016, 38, 517–534. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Nemeth, D.P.; McKim, D.B.; Zhu, L.; DiSabato, D.J.; Berdysz, O.; et al. Cell-Type-Specific Interleukin 1 Receptor 1 Signaling in the Brain Regulates Distinct Neuroimmune Activities. Immunity. 2019, 50, 317–333e6. [Google Scholar] [CrossRef]
- Murray, K.N.; Parry-Jones, A.R.; Allan, S.M. Interleukin-1 and acute brain injury. 2015, 9.
- Frangogiannis, N.G. Interleukin-1 in cardiac injury, repair, and remodeling: pathophysiologic and translational concepts. Discoveries 2015, 3, e41. [Google Scholar] [CrossRef]
- Bodnar, C.N.; Watson, J.B.; Higgins, E.K.; Quan, N.; Bachstetter, A.D. Inflammatory Regulation of CNS Barriers After Traumatic Brain Injury: A Tale Directed by Interleukin-1. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Rudemiller Nathan, P.; Patel Mehul, B.; Karlovich Norah, S.; Wu, M.; McDonough Alicia, A.; et al. Interleukin-1 Receptor Activation Potentiates Salt Reabsorption in Angiotensin II-Induced Hypertension via the NKCC2 Co-transporter in the Nephron. Cell Metabolism. 2016, 23, 360–368. [Google Scholar] [CrossRef]
- Ren, J.; Liu, K.; Wu, B.; Lu, X.; Sun, L.; Privratsky, J.R.; Xing, C.; Robson, M.J.; Mao, H.; Blakely, R.D.; et al. Divergent Actions of Renal Tubular and Endothelial Type 1 IL-1 Receptor Signaling in Toxin-Induced AKI. J. Am. Soc. Nephrol. 2023, 34, 1629–1646. [Google Scholar] [CrossRef]
- Schunk, S.J.; Triem, S.; Schmit, D.; Zewinger, S.; Sarakpi, T.; Becker, E.; Hütter, G.; Wrublewsky, S.; Küting, F.; Hohl, M.; et al. Interleukin-1α Is a Central Regulator of Leukocyte-Endothelial Adhesion in Myocardial Infarction and in Chronic Kidney Disease. Circulation 2021, 144, 893–908. [Google Scholar] [CrossRef]
- Winkler, A.; Sun, W.; De, S.; Jiao, A.; Sharif, M.N.; Symanowicz, P.T.; Athale, S.; Shin, J.H.; Wang, J.; Jacobson, B.A.; et al. The Interleukin-1 Receptor–Associated Kinase 4 Inhibitor PF-06650833 Blocks Inflammation in Preclinical Models of Rheumatic Disease and in Humans Enrolled in a Randomized Clinical Trial. Arthritis Rheumatol. 2021, 73, 2206–2218. [Google Scholar] [CrossRef]
- Basu, R.; Whitley, S.K.; Bhaumik, S.; Zindl, C.L.; Schoeb, T.R.; Benveniste, E.N.; Pear, W.S.; Hatton, R.D.; Weaver, C.T. IL-1 signaling modulates activation of STAT transcription factors to antagonize retinoic acid signaling and control the TH17 cell–iTreg cell balance. Nat. Immunol. 2015, 16, 286–295. [Google Scholar] [CrossRef]
- Zhou, Z.; Tian, Z.; Zhang, M.; Zhang, Y.; Ni, B.; Hao, F. Upregulated IL-1 Receptor-associated Kinase 1 (IRAK1) in Systemic Lupus Erythematosus: IRAK1 Inhibition Represses Th17 Differentiation with Therapeutic Potential. Immunol. Investig. 2018, 47, 468–483. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, R.; Lee, E.-J.; Jang, M.S.; Jeun, E.-J.; Hong, C.-P.; Kim, J.-H.; Park, A.; Yun, C.H.; Hong, S.-W.; Kim, Y.-M.; et al. Small intestinal eosinophils regulate Th17 cells by producing IL-1 receptor antagonist. J. Exp. Med. 2016, 213, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Ritvo, P.-G.; Klatzmann, D. Interleukin-1 in the Response of Follicular Helper and Follicular Regulatory T Cells. Front. Immunol. 2019, 10, 250. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Min, H.K.; Lee, S.H.; Shin, H.J.; Lee, W.Y.; Cho, Y.-G.; Kwok, S.-K.; Ju, J.H.; Cho, M.-L.; Park, S.-H. IL-1 receptor antagonist (IL-1Ra)-Fc ameliorate autoimmune arthritis by regulation of the Th17 cells/Treg balance and arthrogenic cytokine activation. Immunol. Lett. 2016, 172, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Sha, Y.; Markovic-Plese, S. Activated IL-1RI Signaling Pathway Induces Th17 Cell Differentiation via Interferon Regulatory Factor 4 Signaling in Patients with Relapsing-Remitting Multiple Sclerosis. Front. Immunol. 2016, 7, 543. [Google Scholar] [CrossRef]
- De Nardo, D.; Balka, K.R.; Cardona Gloria, Y.; Rao, V.R.; Latz, E.; Masters, S.L. Interleukin-1 receptor–associated kinase 4 (IRAK4) plays a dual role in myddosome formation and Toll-like receptor signaling. Journal of Biological Chemistry. 2018, 293, 15195–15207. [Google Scholar] [CrossRef]
- Kaneko, N.; Kurata, M.; Yamamoto, T.; Morikawa, S.; Masumoto, J. The role of interleukin-1 in general pathology. Inflamm. Regen. 2019, 39, 12. [Google Scholar] [CrossRef]
- Vollmer, S.; Strickson, S.; Zhang, T.; Gray, N.; Lee, K.L.; Rao, V.R.; Cohen, P. The mechanism of activation of IRAK1 and IRAK4 by interleukin-1 and Toll-like receptor agonists. Biochem. J. 2017, 474, 2027–2038. [Google Scholar] [CrossRef]
- Eeckhout, B.V.D.; Tavernier, J.; Gerlo, S. Interleukin-1 as Innate Mediator of T Cell Immunity. Front. Immunol. 2021, 11. [Google Scholar] [CrossRef]
- Liu, X.; Yamashita, T.; Chen, Q.; Belevych, N.; Mckim, D.B.; Tarr, A.J.; Coppola, V.; Nath, N.; Nemeth, D.P.; Syed, Z.W.; et al. Interleukin 1 Type 1 Receptor Restore: A Genetic Mouse Model for Studying Interleukin 1 Receptor-Mediated Effects in Specific Cell Types. J. Neurosci. 2015, 35, 2860–2870. [Google Scholar] [CrossRef]
- Jenei-Lanzl, Z.; Meurer, A.; Zaucke, F. Interleukin-1β signaling in osteoarthritis – chondrocytes in focus. Cellular Signalling. 2019, 53:212-23.
- Zhang, J.; Macartney, T.; Peggie, M.; Cohen, P. Interleukin-1 and TRAF6-dependent activation of TAK1 in the absence of TAB2 and TAB3. Biochem. J. 2017, 474, 2235–2248. [Google Scholar] [CrossRef] [PubMed]
- Ismail, H.M.; Yamamoto, K.; Vincent, T.L.; Nagase, H.; Troeberg, L.; Saklatvala, J. Interleukin-1 Acts via the JNK-2 Signaling Pathway to Induce Aggrecan Degradation by Human Chondrocytes. Arthritis Rheumatol. 2015, 67, 1826–1836. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, D.; Nagarkoti, S.; Kumar, A.; Dubey, M.; Singh, A.K.; Pathak, P.; Chandra, T.; Barthwal, M.K.; Dikshit, M. Oxidized LDL induced extracellular trap formation in human neutrophils via TLR-PKC-IRAK-MAPK and NADPH-oxidase activation. Free. Radic. Biol. Med. 2016, 93, 190–203. [Google Scholar] [CrossRef]
- Mozaffari Godarzi, S.; Valizade Gorji, A.; Gholizadeh, B.; Mard, S.A.; Mansouri, E. Antioxidant effect of p-coumaric acid on interleukin 1-β and tumor necrosis factor-α in rats with renal ischemic reperfusion. Nefrología. 2020, 40, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Berner, J.; Miebach, L.; Kordt, M.; Seebauer, C.; Schmidt, A.; Lalk, M.; Vollmar, B.; Metelmann, H.-R.; Bekeschus, S. Chronic oxidative stress adaptation in head and neck cancer cells generates slow-cyclers with decreased tumour growth in vivo. Br. J. Cancer 2023, 129, 869–883. [Google Scholar] [CrossRef]
- Batista, A.F.; Rody, T.; Forny-Germano, L.; Cerdeiro, S.; Bellio, M.; Ferreira, S.T.; Munoz, D.P.; De Felice, F.G. Interleukin-1β mediates alterations in mitochondrial fusion/fission proteins and memory impairment induced by amyloid-β oligomers. J. Neuroinflammation 2021, 18, 1–15. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, H.; Yu, M.; Schugar, R.C.; Qian, W.; Tang, F.; Liu, W.; Yang, H.; McDowell, R.E.; Zhao, J.; et al. IL-1 induces mitochondrial translocation of IRAK2 to suppress oxidative metabolism in adipocytes. Nat. Immunol. 2020, 21, 1219–1231. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, N.; Zhu, H.; Zhu, S.; Pan, S.; Xu, J.; Zhang, X.; Zhang, Y.; Wang, J. Circulating interleukin-1β promotes endoplasmic reticulum stress-induced myocytes apoptosis in diabetic cardiomyopathy via interleukin-1 receptor-associated kinase-2. Cardiovasc. Diabetol. 2015, 14, 1–9. [Google Scholar] [CrossRef]
- Tsutsui, H.; Cai, X.; Hayashi, S. Interleukin-1 Family Cytokines in Liver Diseases. Mediat. Inflamm. 2015, 2015, 630265. [Google Scholar] [CrossRef]
- West, A.P. Mitochondrial dysfunction as a trigger of innate immune responses and inflammation. Toxicology 2017, 391, 54–63. [Google Scholar] [CrossRef]
- Kandel-Kfir, M.; Almog, T.; Shaish, A.; Shlomai, G.; Anafi, L.; Avivi, C.; Barshack, I.; Grosskopf, I.; Harats, D.; Kamari, Y. Interleukin-1α deficiency attenuates endoplasmic reticulum stress-induced liver damage and CHOP expression in mice. J. Hepatol. 2015, 63, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Kalueff, A.V.; Song, C. N -methyl- d -aspartate receptor-mediated calcium overload and endoplasmic reticulum stress are involved in interleukin-1beta-induced neuronal apoptosis in rat hippocampus. J. Neuroimmunol. 2017, 307, 7–13. [Google Scholar] [CrossRef]
- Pan, L.; Hong, Z.; Yu, L.; Gao, Y.; Zhang, R.; Feng, H.; Su, L.; Wang, G. Shear stress induces human aortic endothelial cell apoptosis via interleukin-1 receptor-associated kinase 2-induced endoplasmic reticulum stress. Mol. Med. Rep. 2017, 16, 7205–7212. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Fang, D. Endoplasmic reticulum-associated degradation and beyond: The multitasking roles for HRD1 in immune regulation and autoimmunity. J. Autoimmun. 2020, 109, 102423–102423. [Google Scholar] [CrossRef]
- Brozzi, F.; Nardelli, T.R.; Lopes, M.; Millard, I.; Barthson, J.; Igoillo-Esteve, M.; Grieco, F.A.; Villate, O.; Oliveira, J.M.; Casimir, M.; et al. Cytokines induce endoplasmic reticulum stress in human, rat and mouse beta cells via different mechanisms. Diabetologia 2015, 58, 2307–2316. [Google Scholar] [CrossRef]
- Czarny, P.; Wigner, P.; Galecki, P.; Sliwinski, T. The interplay between inflammation, oxidative stress, DNA damage, DNA repair and mitochondrial dysfunction in depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2018, 80:309-21.
- Singer, J.W.; Fleischman, A.; Al-Fayoumi, S.; Mascarenhas, J.O.; Yu, Q.; Agarwal, A. Inhibition of interleukin-1 receptor-associated kinase 1 (IRAK1) as a therapeutic strategy. Oncotarget 2018, 9, 33416–33439. [Google Scholar] [CrossRef] [PubMed]
- Lorente-Sorolla, C.; Garcia-Gomez, A.; Català-Moll, F.; Toledano, V.; Ciudad, L.; Avendaño-Ortiz, J.; Maroun-Eid, C.; Martín-Quirós, A.; Martínez-Gallo, M.; Ruiz-Sanmartín, A.; et al. Inflammatory cytokines and organ dysfunction associate with the aberrant DNA methylome of monocytes in sepsis. Genome Med. 2019, 11, 1–18. [Google Scholar] [CrossRef]
- Murakami, I.; Matsushita, M.; Iwasaki, T.; Kuwamoto, S.; Kato, M.; Nagata, K.; Horie, Y.; Hayashi, K.; Imamura, T.; Morimoto, A.; et al. Interleukin-1 loop model for pathogenesis of Langerhans cell histiocytosis. Cell Commun. Signal. 2015, 13, 13–15. [Google Scholar] [CrossRef]
- Nixon, A.J.; Grol, M.W.; Lang, H.M.; Ruan, M.Z.C.; Stone, A.; Begum, L.; Chen, Y.; Dawson, B.; Gannon, F.; Plutizki, S.; et al. Disease-Modifying Osteoarthritis Treatment With Interleukin-1 Receptor Antagonist Gene Therapy in Small and Large Animal Models. Arthritis Rheumatol. 2018, 70, 1757–1768. [Google Scholar] [CrossRef]
- Palomo, J.; Dietrich, D.; Martin, P.; Palmer, G.; Gabay, C. The interleukin (IL)-1 cytokine family – Balance between agonists and antagonists in inflammatory diseases. Cytokine 2015, 76, 25–37. [Google Scholar] [CrossRef]
- Cavalli, G.; Dinarello, C.A. Treating rheumatological diseases and co-morbidities with interleukin-1 blocking therapies. Rheumatology 2015, 54, 2134–2144. [Google Scholar] [CrossRef] [PubMed]
- Lopalco, G.; Cantarini, L.; Vitale, A.; Iannone, F.; Anelli, M.G.; Andreozzi, L.; et al. Interleukin-1 as a Common Denominator from Autoinflammatory to Autoimmune Disorders: Premises, Perils, and Perspectives. 2015, 2015, 194864.
- Supino, D.; Minute, L.; Mariancini, A.; Riva, F.; Magrini, E.; Garlanda, C. Negative Regulation of the IL-1 System by IL-1R2 and IL-1R8: Relevance in Pathophysiology and Disease. Front. Immunol. 2022, 13, 804641. [Google Scholar] [CrossRef] [PubMed]
- Striz, I. Cytokines of the IL-1 family: recognized targets in chronic inflammation underrated in organ transplantations. Clin. Sci. 2017, 131, 2241–2256. [Google Scholar] [CrossRef]
- Broderick, L.; Hoffman, H.M. IL-1 and autoinflammatory disease: biology, pathogenesis and therapeutic targeting. Nat. Rev. Rheumatol. 2022, 18, 448–463. [Google Scholar] [CrossRef]
- Lo, W.-Y.; Peng, C.-T.; Wang, H.-J. MicroRNA-146a-5p Mediates High Glucose-Induced Endothelial Inflammation via Targeting Interleukin-1 Receptor-Associated Kinase 1 Expression. Front. Physiol. 2017, 8, 551. [Google Scholar] [CrossRef]
- Di Paolo, N.C. ; Shayakhmetov,DM Interleukin 1α and the inflammatory process. Nat. Immunol. 2016, 17, 906–913. [Google Scholar] [CrossRef]
- Hauptmann, J.; Johann, L.; Marini, F.; Kitic, M.; Colombo, E.; Mufazalov, I.A.; Krueger, M.; Karram, K.; Moos, S.; Wanke, F.; et al. Interleukin-1 promotes autoimmune neuroinflammation by suppressing endothelial heme oxygenase-1 at the blood–brain barrier. Acta Neuropathol. 2020, 140, 549–567. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Dinarello, C.A.; Molgora, M.; Garlanda, C. Interleukin-1 and Related Cytokines in the Regulation of Inflammation and Immunity. Immunity 2019, 50, 778–795. [Google Scholar] [CrossRef]
- Fahey, E.; Doyle, S.L. IL-1 Family Cytokine Regulation of Vascular Permeability and Angiogenesis. Front. Immunol. 2019, 10, 1426. [Google Scholar] [CrossRef]
- John, A.; Günes, C.; Bolenz, C.; Vidal-Y-Sy, S.; Bauer, A.T.; Schneider, S.W.; Gorzelanny, C. Bladder cancer-derived interleukin-1 converts the vascular endothelium into a pro-inflammatory and pro-coagulatory surface. BMC Cancer 2020, 20, 1–13. [Google Scholar] [CrossRef]
- Mailhot, B.; Christin, M.; Tessandier, N.; Sotoudeh, C.; Bretheau, F.; Turmel, R.; Pellerin, È.; Wang, F.; Bories, C.; Joly-Beauparlant, C.; et al. Neuronal interleukin-1 receptors mediate pain in chronic inflammatory diseases. J. Exp. Med. 2020, 217. [Google Scholar] [CrossRef] [PubMed]
- DiSabato, D.J.; Nemeth, D.P.; Liu, X.; Witcher, K.G.; O’Neil, S.M.; Oliver, B.; et al. Interleukin-1 receptor on hippocampal neurons drives social withdrawal and cognitive deficits after chronic social stress. Molecular Psychiatry. 2021, 26, 4770–4782. [Google Scholar] [CrossRef]
- Karpenko, M.N.; Vasilishina, A.A.; Gromova, E.A.; Muruzheva, Z.M.; Bernadotte, A. Interleukin-1β, interleukin-1 receptor antagonist, interleukin-6, interleukin-10, and tumor necrosis factor-α levels in CSF and serum in relation to the clinical diversity of Parkinson’s disease. Cellular Immunology. 2018, 327:77-82.
- Sun, M.; Brady, R.D.; Wright, D.K.; Kim, H.A.; Zhang, S.R.; Sobey, C.G.; Johnstone, M.R.; O'Brien, T.; Semple, B.D.; McDonald, S.J.; et al. Treatment with an interleukin-1 receptor antagonist mitigates neuroinflammation and brain damage after polytrauma. Brain, Behav. Immun. 2017, 66, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Van Tassell, B.W.; Raleigh, J.M.V.; Abbate, A. Targeting Interleukin-1 in Heart Failure and Inflammatory Heart Disease. Current Heart Failure Reports. 2015, 12, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Abbate, A.; Toldo, S.; Marchetti, C.; Kron, J.; Van Tassell, B.W.; Dinarello, C.A. Interleukin-1 and the Inflammasome as Therapeutic Targets in Cardiovascular Disease. Circ. Res. 2020, 126, 1260–1280. [Google Scholar] [CrossRef]
- Cavalli, G.; Foppoli, M.; Cabrini, L.; Dinarello, C.A.; Tresoldi, M.; Dagna, L. Interleukin-1 Receptor Blockade Rescues Myocarditis-Associated End-Stage Heart Failure. Front. Immunol. 2017, 8, 131. [Google Scholar] [CrossRef]
- Van Tassell, B.W.; Canada, J.; Carbone, S.; Trankle, C.; Buckley, L.; Erdle, C.O.; Abouzaki, N.A.; Dixon, D.; Kadariya, D.; Christopher, S.; et al. Interleukin-1 Blockade in Recently Decompensated Systolic Heart Failure. Circ. Hear. Fail. 2017, 10. [Google Scholar] [CrossRef]
- Herder, C.; Gala, T.d.L.H.; Carstensen-Kirberg, M.; Huth, C.; Zierer, A.; Wahl, S.; Sudduth-Klinger, J.; Kuulasmaa, K.; Peretz, D.; Ligthart, S.; et al. Circulating Levels of Interleukin 1-Receptor Antagonist and Risk of Cardiovascular Disease. Arter. Thromb. Vasc. Biol. 2017, 37, 1222–1227. [Google Scholar] [CrossRef]
- Meyer, N.J.; Reilly, J.P.; Anderson, B.J.; Palakshappa, J.A.; Jones, T.K.; Dunn, T.G.; Shashaty, M.G.S.; Feng, R.; Christie, J.D.; Opal, S.M. Mortality Benefit of Recombinant Human Interleukin-1 Receptor Antagonist for Sepsis Varies by Initial Interleukin-1 Receptor Antagonist Plasma Concentration*. Crit. Care Med. 2018, 46, 21–28. [Google Scholar] [CrossRef]
- Dinarello, C.A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 2017, 281, 8–27. [Google Scholar] [CrossRef]
- Shakoory, B.; Carcillo, J.A.; Chatham, W.W.; Amdur, R.L.; Zhao, H.; Dinarello, C.A.; et al. Interleukin-1 Receptor Blockade Is Associated With Reduced Mortality in Sepsis Patients With Features of Macrophage Activation Syndrome: Reanalysis of a Prior Phase III Trial*. 2016, 44, 275–281.
- Wohlfarth, P.; Agis, H.; Gualdoni, G.A.; Weber, J.; Staudinger, T.; Schellongowski, P.; Robak, O. Interleukin 1 Receptor Antagonist Anakinra, Intravenous Immunoglobulin, and Corticosteroids in the Management of Critically Ill Adult Patients With Hemophagocytic Lymphohistiocytosis. J. Intensiv. Care Med. 2017, 34, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, D.; Robinson, S.; Romero, D.L. Recent Advances in the Discovery of Small Molecule Inhibitors of Interleukin-1 Receptor-Associated Kinase 4 (IRAK4) as a Therapeutic Target for Inflammation and Oncology Disorders. J. Med. Chem. 2014, 58, 96–110. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Zhao, C.; Li, D.; Dai, R. Mechanism of interleukin-1 receptor antagonist protection against myocardial ischaemia/reperfusion-induced injury. Arch. Cardiovasc. Dis. 2018, 111, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Huang, J.; Dai, D.; Wang, X.; Gao, J.; Han, W.; Zhang, R. Recombinant human interleukin-1 receptor antagonist treatment protects rats from myocardial ischemia–reperfusion injury. Biomed. Pharmacother. 2018, 111, 1–5. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Liu, Y.; Li, S.-P.; Li, J.-J.; Zhang, Z.; Xiao, X.-C.; Ou, Y.; Wang, H.; Cai, J.-Z.; Yang, S. Hypoxia inducible factor 1α promotes interleukin-1 receptor antagonist expression during hepatic ischemia-reperfusion injury. World J. Gastroenterol. 2022, 28, 5573–5588. [Google Scholar] [CrossRef]
- Salmeron, K.E.; Maniskas, M.E.; Edwards, D.N.; Wong, R.; Rajkovic, I.; Trout, A.; Rahman, A.A.; Hamilton, S.; Fraser, J.F.; Pinteaux, E.; et al. Interleukin 1 alpha administration is neuroprotective and neuro-restorative following experimental ischemic stroke. J. Neuroinflammation 2019, 16, 1–14. [Google Scholar] [CrossRef]
- Italiani, P.; Manca, M.L.; Angelotti, F.; Melillo, D.; Pratesi, F.; Puxeddu, I.; Boraschi, D.; Migliorini, P. IL-1 family cytokines and soluble receptors in systemic lupus erythematosus. Arthritis Res. Ther. 2018, 20, 27. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, K.; Guo, M.; Yang, Y.; Zhang, H. Negative regulator IL-1 receptor 2 (IL-1R2) and its roles in immune regulation of autoimmune diseases. Int. Immunopharmacol. 2024, 136, 112400. [Google Scholar] [CrossRef]
- Højen, J.F.; Kristensen, M.L.V.; McKee, A.S.; Wade, M.T.; Azam, T.; Lunding, L.P.; de Graaf, D.M.; Swartzwelter, B.J.; Wegmann, M.; Tolstrup, M.; et al. IL-1R3 blockade broadly attenuates the functions of six members of the IL-1 family, revealing their contribution to models of disease. Nat. Immunol. 2019, 20, 1138–1149. [Google Scholar] [CrossRef]
- Morton, A.C.; Rothman, A.M.K.; Greenwood, J.P.; Gunn, J.; Chase, A.; Clarke, B.; Hall, A.S.; Fox, K.; Foley, C.; Banya, W.; et al. The effect of interleukin-1 receptor antagonist therapy on markers of inflammation in non-ST elevation acute coronary syndromes: the MRC-ILA Heart Study. Eur. Hear. J. 2014, 36, 377–384. [Google Scholar] [CrossRef]
- Buckley, L.F.; Abbate, A. Interleukin-1 blockade in cardiovascular diseases: a clinical update. Eur. Hear. J. 2018, 39, 2063–2069. [Google Scholar] [CrossRef] [PubMed]
- Luz-Crawford, P.; Djouad, F.; Toupet, K.; Bony, C.; Franquesa, M.; Hoogduijn, M.J.; Jorgensen, C.; Noël, D. Mesenchymal Stem Cell-Derived Interleukin 1 Receptor Antagonist Promotes Macrophage Polarization and Inhibits B Cell Differentiation. STEM CELLS 2015, 34, 483–492. [Google Scholar] [CrossRef]
- Franco, J.H.; Chen, X.; Pan, Z.K. Novel Treatments Targeting the Dysregulated Cell Signaling Pathway during Sepsis. J. Cell. Signal. 2021, 2, 228–234. [Google Scholar] [CrossRef]
- Alehashemi, S.; Goldbach-Mansky, R. Human Autoinflammatory Diseases Mediated by NLRP3-, Pyrin-, NLRP1-, and NLRC4-Inflammasome Dysregulation Updates on Diagnosis, Treatment, and the Respective Roles of IL-1 and IL-18. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Manchikalapati, R.; Schening, J.; Farias, A.J.; Sacco, K.A. CLINICAL UTILITY OF INTERLEUKIN-1 INHIBITORS IN PEDIATRIC SEPSIS. Shock 2023, 61, 340–345. [Google Scholar] [CrossRef] [PubMed]
- AGleeson, T.; Nordling, E.; Kaiser, C.; Lawrence, C.B.; Brough, D.; Green, J.P.; Allan, S.M. Looking into the IL-1 of the storm: are inflammasomes the link between immunothrombosis and hyperinflammation in cytokine storm syndromes? Discov. Immunol. 2022, 1, kyac005. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R.; Szabo, G. Interleukin-1 and inflammasomes in alcoholic liver disease/acute alcoholic hepatitis and nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology 2016, 64, 955–965. [Google Scholar] [CrossRef]
- Bachstetter, A.D.; Bodnar, C.N.; Morganti, J.M. Depression following a traumatic brain injury: uncovering cytokine dysregulation as a pathogenic mechanism. Neural Regen. Res. 2018, 13, 1693–1704. [Google Scholar] [CrossRef]
- Rose-John, S. Interleukin-6 Family Cytokines. 2018, 10(2).
- Unver, N.; McAllister, F. IL-6 family cytokines: Key inflammatory mediators as biomarkers and potential therapeutic targets. Cytokine Growth Factor Rev. 2018, 41, 10–17. [Google Scholar] [CrossRef]
- Hasegawa, H.; Mizoguchi, I.; Chiba, Y.; Ohashi, M.; Xu, M.; Yoshimoto, T. Expanding Diversity in Molecular Structures and Functions of the IL-6/IL-12 Heterodimeric Cytokine Family. 2016, 7.
- Baran, P.; Hansen, S.; Waetzig, G.H.; Akbarzadeh, M.; Lamertz, L.; Huber, H.J.; et al. The balance of interleukin (IL)-6, IL-6·soluble IL-6 receptor (sIL-6R), and IL-6·sIL-6R·sgp130 complexes allows simultaneous classic and trans-signaling. Journal of Biological Chemistry. 2018, 293, 6762–6775. [Google Scholar] [CrossRef]
- Kaur, S.; Bansal, Y.; Kumar, R.; Bansal, G. A panoramic review of IL-6: Structure, pathophysiological roles and inhibitors. Bioorganic & Medicinal Chemistry. 2020, 28, 115327. [Google Scholar]
- Kang, S.; Tanaka, T.; Kishimoto, T. Therapeutic uses of anti-interleukin-6 receptor antibody. Int. Immunol. 2014, 27, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Sims, N.A. Influences of the IL-6 cytokine family on bone structure and function. Cytokine 2021, 146, 155655. [Google Scholar] [CrossRef]
- Dawson, R.E.; Jenkins, B.J.; Saad, M.I. IL-6 family cytokines in respiratory health and disease. Cytokine 2021, 143, 155520. [Google Scholar] [CrossRef]
- Rose-John, S. Therapeutic targeting of IL-6 trans-signaling. Cytokine 2021, 144, 155577. [Google Scholar] [CrossRef] [PubMed]
- Manore, S.G.; Doheny, D.L.; Wong, G.L.; Lo, H.-W. IL-6/JAK/STAT3 Signaling in Breast Cancer Metastasis: Biology and Treatment. Front. Oncol. 2022, 12, 866014. [Google Scholar] [CrossRef]
- Huang, B.; Lang, X.; Li, X. The role of IL-6/JAK2/STAT3 signaling pathway in cancers. Front. Oncol. 2022, 12, 1023177. [Google Scholar] [CrossRef]
- Potere, N.; Batticciotto, A.; Vecchié,, A. ; Porreca, E.; Cappelli, A.; Abbate, A.; Dentali, F.; Bonaventura, A. The role of IL-6 and IL-6 blockade in COVID-19. Expert Rev. Clin. Immunol. 2021, 17, 601–618. [Google Scholar] [CrossRef]
- Chen, L.Y.; Biggs, C.M.; Jamal, S.; Stukas, S.; Wellington, C.L.; Sekhon, M.S. Soluble interleukin-6 receptor in the COVID-19 cytokine storm syndrome. Cell Rep. Med. 2021, 2, 100269. [Google Scholar] [CrossRef]
- Huseni, M.A.; Wang, L.; Klementowicz, J.E.; Yuen, K.; Breart, B.; Orr, C.; et al. CD8+ T cell-intrinsic IL-6 signaling promotes resistance to anti-PD-L1 immunotherapy. Cell Reports Medicine. 2023, 4(1).
- Kaewbandit, N.; Malla, A.; Boonyayothin, W.; Rattanapisit, K.; Phetphoung, T.; Pisuttinusart, N.; Strasser, R.; Saetung, R.; Tawinwung, S.; Phoolcharoen, W. Effect of plant produced Anti-hIL-6 receptor antibody blockade on pSTAT3 expression in human peripheral blood mononuclear cells. Sci. Rep. 2023, 13, 1–13. [Google Scholar] [CrossRef]
- Lin, Y.-K.; Yeh, C.-T.; Kuo, K.-T.; Fong, I.-H.; Yadav, V.K.; Kounis, N.G.; et al. Apolipoprotein (a)/Lipoprotein(a)-Induced Oxidative-Inflammatory α7-nAChR/p38 MAPK/IL-6/RhoA-GTP Signaling Axis and M1 Macrophage Polarization Modulate Inflammation-Associated Development of Coronary Artery Spasm. 2022, 2022, 9964689.
- Huang, Y.-P.; Chen, D.-R.; Lin, W.-J.; Lin, Y.-H.; Chen, J.-Y.; Kuo, Y.-H.; Chung, J.-G.; Hsia, T.-C.; Hsieh, W.-T. Ergosta-7,9(11),22-trien-3β-ol Attenuates Inflammatory Responses via Inhibiting MAPK/AP-1 Induced IL-6/JAK/STAT Pathways and Activating Nrf2/HO-1 Signaling in LPS-Stimulated Macrophage-like Cells. Antioxidants 2021, 10, 1430. [Google Scholar] [CrossRef] [PubMed]
- Rose-John, S.; Jenkins, B.J.; Garbers, C.; Moll, J.M.; Scheller, J. Targeting IL-6 trans-signalling: past, present and future prospects. Nat. Rev. Immunol. 2023, 23, 666–681. [Google Scholar] [CrossRef]
- Xu, J.; Lin, H.; Wu, G.; Zhu, M.; Li, M. IL-6/STAT3 Is a Promising Therapeutic Target for Hepatocellular Carcinoma. 2021, 11.
- Fiebelkow, J.; Guendel, A.; Guendel, B.; Mehwald, N.; Jetka, T.; Komorowski, M.; Waldherr, S.; Schaper, F.; Dittrich, A. The tyrosine phosphatase SHP2 increases robustness and information transfer within IL-6-induced JAK/STAT signalling. Cell Commun. Signal. 2021, 19, 1–19. [Google Scholar] [CrossRef]
- Kang, S.; Kishimoto, T. Interplay between interleukin-6 signaling and the vascular endothelium in cytokine storms. Exp. Mol. Med. 2021, 53, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Fodor, A.; Tiperciuc, B.; Login, C.; Orasan, O.H.; Lazar, A.L.; Buchman, C.; et al. Endothelial Dysfunction, Inflammation, and Oxidative Stress in COVID-19—Mechanisms and Therapeutic Targets. 2021, 2021, 8671713.
- Xu S-w, Ilyas I, Weng J-p. Endothelial dysfunction in COVID-19: an overview of evidence, biomarkers, mechanisms and potential therapies. Acta Pharmacologica Sinica. 2023, 44, 695–709.
- Youn, J.Y.; Zhang, Y.; Wu, Y.; Cannesson, M.; Cai, H. Therapeutic application of estrogen for COVID-19: Attenuation of SARS-CoV-2 spike protein and IL-6 stimulated, ACE2-dependent NOX2 activation, ROS production and MCP-1 upregulation in endothelial cells. Redox Biol. 2021, 46, 102099. [Google Scholar] [CrossRef]
- McElvaney, O.J.; Curley, G.F.; Rose-John, S.; McElvaney, N.G. Interleukin-6: obstacles to targeting a complex cytokine in critical illness. Lancet Respir. Med. 2021, 9, 643–654. [Google Scholar] [CrossRef]
- Aliyu, M.; Zohora, F.T.; Anka, A.U.; Ali, K.; Maleknia, S.; Saffarioun, M.; Azizi, G. Interleukin-6 cytokine: An overview of the immune regulation, immune dysregulation, and therapeutic approach. Int. Immunopharmacol. 2022, 111, 109130. [Google Scholar] [CrossRef]
- Shekhawat, J.; Gauba, K.; Gupta, S.; Purohit, P.; Mitra, P.; Garg, M.; Misra, S.; Sharma, P.; Banerjee, M. Interleukin-6 Perpetrator of the COVID-19 Cytokine Storm. Indian J. Clin. Biochem. 2021, 36, 440–450. [Google Scholar] [CrossRef]
- Kucka, K.; Wajant, H. Receptor Oligomerization and Its Relevance for Signaling by Receptors of the Tumor Necrosis Factor Receptor Superfamily. Front. Cell Dev. Biol. 2021, 8. [Google Scholar] [CrossRef]
- Belenguer, G.; Duart-Abadia, P.; Jordán-Pla, A.; Domingo-Muelas, A.; Blasco-Chamarro, L.; Ferrón, S.R.; Morante-Redolat, J.M.; Fariñas, I. Adult Neural Stem Cells Are Alerted by Systemic Inflammation through TNF-α Receptor Signaling. Cell Stem Cell 2021, 28, 285–299.e9. [Google Scholar] [CrossRef] [PubMed]
- Dostert, C.; Grusdat, M.; Letellier, E.; Brenner, D. The TNF Family of Ligands and Receptors: Communication Modules in the Immune System and Beyond. Physiol. Rev. 2019, 99, 115–160. [Google Scholar] [CrossRef] [PubMed]
- Croft, M.; Siegel, R.M. Beyond TNF: TNF superfamily cytokines as targets for the treatment of rheumatic diseases. Nat. Rev. Rheumatol. 2017, 13, 217–233. [Google Scholar] [CrossRef]
- Al-Lamki, R.S.; Mayadas, T.N. TNF receptors: signaling pathways and contribution to renal dysfunction. Kidney Int. 2015, 87, 281–296. [Google Scholar] [CrossRef] [PubMed]
- Kyriakis, J.M. Activation of the AP-1 transcription factor by inflammatory cytokines of the TNF family. . 1999, 7, 217–231. [Google Scholar]
- Chattopadhyay, S.; Veleeparambil, M.; Poddar, D.; Abdulkhalek, S.; Bandyopadhyay, S.K.; Fensterl, V.; Sen, G.C. EGFR kinase activity is required for TLR4 signaling and the septic shock response. Embo Rep. 2015, 16, 1535–1547. [Google Scholar] [CrossRef]
- Gatica-Andrades, M.; Vagenas, D.; Kling, J.; Nguyen, T.T.K.; Benham, H.; Thomas, R.; Körner, H.; Venkatesh, B.; Cohen, J.; Blumenthal, A. WNT ligands contribute to the immune response during septic shock and amplify endotoxemia-driven inflammation in mice. Blood Adv. 2017, 1, 1274–1286. [Google Scholar] [CrossRef]
- Krakauer, T. Staphylococcal Superantigens: Pyrogenic Toxins Induce Toxic Shock. Toxins 2019, 11, 178. [Google Scholar] [CrossRef]
- O’Shea, J.J.; Gadina, M.; Siegel, R.M. 9 - Cytokines and Cytokine Receptors. In: Rich RR, Fleisher TA, Shearer WT, Schroeder HW, Frew AJ, Weyand CM, editors. Clinical Immunology (Fifth Edition). London: Elsevier; 2019. p. 127-55.e1.
- Wu, L.; Xiong, X.; Wu, X.; Ye, Y.; Jian, Z.; Zhi, Z.; Gu, L. Targeting Oxidative Stress and Inflammation to Prevent Ischemia-Reperfusion Injury. Front. Mol. Neurosci. 2020, 13, 28. [Google Scholar] [CrossRef]
- Konishi, T.; Lentsch, A.B. Hepatic Ischemia/Reperfusion: Mechanisms of Tissue Injury, Repair, and Regeneration. Gene Expr. 2017, 17, 277–287. [Google Scholar] [CrossRef]
- Guo, Z.; Yu, S.; Chen, X.; Ye, R.; Zhu, W.; Liu, X. NLRP3 Is Involved in Ischemia/Reperfusion Injury. CNS Neurol. Disord. - Drug Targets 2016, 15, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Lendak, D.F.; Mihajlović,, D. M.; Novakov-Mikić,, A.S.; Mitić,, I.M.; Boban, J.M.; Brkić,, S.V. The role of TNF-α superfamily members in immunopathogenesis of sepsis. Cytokine 2018, 111, 125–130. [Google Scholar] [CrossRef]
- Polat, G.; Ugan, R.A.; Cadirci, E.; Halici, Z. Sepsis and Septic Shock: Current Treatment Strategies and New Approaches. Eurasian J. Med. 2017, 49, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Toro, J.-J.; Márquez-Coello, M.; García-Álvarez, J.-M.; Martín-Aspas, A.; Rivera-Fernández, R.; de Benito, A.S.; Girón-González, J.-A. Soluble membrane receptors, interleukin 6, procalcitonin and C reactive protein as prognostic markers in patients with severe sepsis and septic shock. PLOS ONE 2017, 12, e0175254. [Google Scholar] [CrossRef]
- Wang, S.-S.; Yan, C.-S.; Luo, J.-M. NLRC4 gene silencing-dependent blockade of NOD-like receptor pathway inhibits inflammation, reduces proliferation and increases apoptosis of dendritic cells in mice with septic shock. Aging 2021, 13, 1440–1457. [Google Scholar] [CrossRef]
- Pérez-Torres, I.; Aisa-Álvarez, A.; Casarez-Alvarado, S.; Borrayo, G.; Márquez-Velasco, R.; Guarner-Lans, V.; Manzano-Pech, L.; Cruz-Soto, R.; Gonzalez-Marcos, O.; Fuentevilla-Álvarez, G.; et al. Impact of Treatment with Antioxidants as an Adjuvant to Standard Therapy in Patients with Septic Shock: Analysis of the Correlation between Cytokine Storm and Oxidative Stress and Therapeutic Effects. Int. J. Mol. Sci. 2023, 24, 16610. [Google Scholar] [CrossRef] [PubMed]
- Hobai, I.A. CARDIOMYOCYTE REPROGRAMMING IN ANIMAL MODELS OF SEPTIC SHOCK. Shock 2022, 59, 200–213. [Google Scholar] [CrossRef]
- Chen, X.; Bi, M.; Yang, J.; Cai, J.; Zhang, H.; Zhu, Y.; et al. Cadmium exposure triggers oxidative stress, necroptosis, Th1/Th2 imbalance and promotes inflammation through the TNF-α/NF-κB pathway in swine small intestine. Journal of Hazardous Materials. 2022, 421:126704.
- Galeone, A.; Grano, M.; Brunetti, G. Tumor Necrosis Factor Family Members and Myocardial Ischemia-Reperfusion Injury: State of the Art and Therapeutic Implications. Int. J. Mol. Sci. 2023, 24, 4606. [Google Scholar] [CrossRef]
- Grishanova, A.Y.; Perepechaeva, M.L. Aryl Hydrocarbon Receptor in Oxidative Stress as a Double Agent and Its Biological and Therapeutic Significance. Int. J. Mol. Sci. 2022, 23, 6719. [Google Scholar] [CrossRef]
- Fakhri, S.; Abbaszadeh, F.; Moradi, S.Z.; Cao, H.; Khan, H.; Xiao, J. Effects of Polyphenols on Oxidative Stress, Inflammation, and Interconnected Pathways during Spinal Cord Injury. Oxidative Med. Cell. Longev. 2022, 2022, 1–34. [Google Scholar] [CrossRef]
- Tiegs, G.; Horst, A.K. TNF in the liver: targeting a central player in inflammation. Semin. Immunopathol. 2022, 44, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Robert, M.; Miossec, P. Reactivation of latent tuberculosis with TNF inhibitors: critical role of the beta 2 chain of the IL-12 receptor. Cell. Mol. Immunol. 2021, 18, 1644–1651. [Google Scholar] [CrossRef] [PubMed]
- Ward-Kavanagh Lindsay, K.; Lin Wai, W.; Šedý John, R.; Ware Carl, F. The TNF Receptor Superfamily in Co-stimulating and Co-inhibitory Responses. Immunity. 2016, 44, 1005–1019. [Google Scholar] [CrossRef]
- Yang, S.; Wang, J.; Brand, D.D.; Zheng, S.G. Role of TNF–TNF Receptor 2 Signal in Regulatory T Cells and Its Therapeutic Implications. Front. Immunol. 2018, 9, 784. [Google Scholar] [CrossRef]
- Akdis, M.; Aab, A.; Altunbulakli, C.; Azkur, K.; Costa, R.A.; Crameri, R.; et al. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor β, and TNF-α: Receptors, functions, and roles in diseases. Journal of Allergy and Clinical Immunology. 2016, 138, 984–1010. [Google Scholar] [CrossRef] [PubMed]
- Fajgenbaum, D.C.; June, C.H. Cytokine Storm. 2020, 383, 2255–2273.
- Humphries, F.; Yang, S.; Wang, B.; Moynagh, P.N. RIP kinases: key decision makers in cell death and innate immunity. Cell Death Differ. 2014, 22, 225–236. [Google Scholar] [CrossRef]
- Obál, I.; Klausz, G.; Mándi, Y.; Deli, M.; Siklós, L.; Engelhardt, J.I. Intraperitoneally administered IgG from patients with amyotrophic lateral sclerosis or from an immune-mediated goat model increase the levels of TNF-α, IL-6, and IL-10 in the spinal cord and serum of mice. Journal of Neuroinflammation. 2016, 13, 121. [Google Scholar] [CrossRef]
- Pratim Das, P.; Medhi, S. Role of inflammasomes and cytokines in immune dysfunction of liver cirrhosis. Cytokine. 2023, 170:156347.
- Dirchwolf, M.; Podhorzer, A.; Marino, M.; Shulman, C.; Cartier, M.; Zunino, M.; Paz, S.; Muñoz, A.; Bocassi, A.; Gimenez, J.; et al. Immune dysfunction in cirrhosis: Distinct cytokines phenotypes according to cirrhosis severity. Cytokine 2015, 77, 14–25. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, J.Y.; Yang, J.W.; Lee, K.H.; Effenberger, M.; Szpirt, W.; Kronbichler, A.; Shin, J.I. Immunopathogenesis and treatment of cytokine storm in COVID-19. Theranostics 2021, 11, 316–329. [Google Scholar] [CrossRef]
- Hobbs, K.J.; Bayless, R.; Sheats, M.K. A Comparative Review of Cytokines and Cytokine Targeting in Sepsis: From Humans to Horses. Cells 2024, 13, 1489. [Google Scholar] [CrossRef]
- Li, H.; Breedijk, A.; Dietrich, N.; Nitschke, K.; Jarczyk, J.; Nuhn, P.; Krämer, B.K.; Yard, B.A.; Leipe, J.; Hauske, S. Lipopolysaccharide Tolerance in Human Primary Monocytes and Polarized Macrophages. Int. J. Mol. Sci. 2023, 24, 12196. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.K.; Beckermann, K.E.; Johnson, D.B.; Das, S. Combining anti-cytotoxic T-lymphocyte antigen 4 (CTLA-4) and -programmed cell death protein 1 (PD-1) agents for cancer immunotherapy. Expert Opin. Biol. Ther. 2021, 21, 1623–1634. [Google Scholar] [CrossRef] [PubMed]
- Kolacinska, A.; Cebula-Obrzut, B.; Pakula, L.; Chalubinska-Fendler, J.; Morawiec-Sztandera, A.; Pawlowska, Z.; Zawlik, I.; Morawiec, Z.; Jesionek-Kupnicka, D.; Smolewski, P. Immune checkpoints: Cytotoxic T-lymphocyte antigen 4 and programmed cell death protein 1 in breast cancer surgery. Oncol. Lett. 2015, 10, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Zak, K.M.; Kitel, R.; Przetocka, S.; Golik, P.; Guzik, K.; Musielak, B.; Dömling, A.; Dubin, G.; Holak, T.A. Structure of the Complex of Human Programmed Death 1, PD-1, and Its Ligand PD-L1. Structure 2015, 23, 2341–2348. [Google Scholar] [CrossRef]
- Qin, S.; Xu, L.; Yi, M.; Yu, S.; Wu, K.; Luo, S. Novel immune checkpoint targets: moving beyond PD-1 and CTLA-4. Mol. Cancer 2019, 18, 155. [Google Scholar] [CrossRef]
- Pandey, P.; Khan, F.; Qari, H.A.; Upadhyay, T.K.; Alkhateeb, A.F.; Oves, M. Revolutionization in Cancer Therapeutics via Targeting Major Immune Checkpoints PD-1, PD-L1 and CTLA-4. Pharmaceuticals 2022, 15, 335. [Google Scholar] [CrossRef]
- Wojtukiewicz, M.Z.; Rek, M.M.; Karpowicz, K.; Górska, M.; Polityńska, B.; Wojtukiewicz, A.M.; et al. Inhibitors of immune checkpoints—PD-1, PD-L1, CTLA-4—new opportunities for cancer patients and a new challenge for internists and general practitioners. Cancer and Metastasis Reviews. 2021, 40, 949–982. [Google Scholar] [CrossRef]
- Quagliariello, V.; Passariello, M.; Rea, D.; Barbieri, A.; Iovine, M.; Bonelli, A.; et al. Evidences of CTLA-4 and PD-1 Blocking Agents-Induced Cardiotoxicity in Cellular and Preclinical Models. 2020, 10, 179.
- Rudick, C.P.; Cornell, D.L.; Agrawal, D.K. Single versus combined immunoregulatory approach using PD-1 and CTLA-4 modulators in controlling sepsis. Expert Rev. Clin. Immunol. 2017, 13, 907–919. [Google Scholar] [CrossRef]
- Maurea, N.; De Lorenzo, C.; Passariello, M.; Di Mauro, A.; Cipullo, C.; Bisceglia, I.; Paccone, A.; Iovine, M.; Bruzzese, F.; Palma, G.; et al. CTLA-4 and PD-1 blocking agents affects longitudinal and radial strain in preclinical models, increases systemic SDF-1, cardiac fibronectin, S-100 calgranulin, galectine-3 and NLRP-3/MyD-88 pathways. Eur. Hear. J. - Cardiovasc. Imaging 2023, 24. [Google Scholar] [CrossRef]
- Aghbash, P.S.; Eslami, N.; Shamekh, A.; Entezari-Maleki, T.; Baghi, H.B. SARS-CoV-2 infection: The role of PD-1/PD-L1 and CTLA-4 axis. Life Sciences. 2021, 270:119124.
- Shi, L.Z.; Goswami, S.; Fu, T.; Guan, B.; Chen, J.; Xiong, L.; et al. Blockade of CTLA-4 and PD-1 Enhances Adoptive T-cell Therapy Efficacy in an ICOS-Mediated Manner. Cancer Immunology Research. 2019, 7, 1803–1812. [Google Scholar] [CrossRef]
- Rudd, C.E.; Taylor, A.; Schneider, H. CD28 and CTLA-4 coreceptor expression and signal transduction. Immunol. Rev. 2009, 229, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.S.; Sansom, D.M. Confusing signals: Recent progress in CTLA-4 biology. Trends Immunol. 2015, 36, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.; Gharibi, T.; Marofi, F.; Babaloo, Z.; Baradaran, B. CTLA-4: From mechanism to autoimmune therapy. Int. Immunopharmacol. 2020, 80, 106221. [Google Scholar] [CrossRef] [PubMed]
- Van Coillie, S.; Wiernicki, B.; Xu, J. Molecular and Cellular Functions of CTLA-4. In: Xu J, editor. Regulation of Cancer Immune Checkpoints: Molecular and Cellular Mechanisms and Therapy. Singapore: Springer Singapore; 2020. p. 7-32.
- Parry, R.V.; Chemnitz, J.M.; Frauwirth, K.A.; Lanfranco, A.R.; Braunstein, I.; Kobayashi, S.V.; Linsley, P.S.; Thompson, C.B.; Riley, J.L. CTLA-4 and PD-1 Receptors Inhibit T-Cell Activation by Distinct Mechanisms. Mol. Cell. Biol. 2005, 25, 9543–9553. [Google Scholar] [CrossRef]
- Iwai, Y.; Hamanishi, J.; Chamoto, K.; Honjo, T. Cancer immunotherapies targeting the PD-1 signaling pathway. J. Biomed. Sci. 2017, 24, 1–11. [Google Scholar] [CrossRef]
- Chen, R.; Zhou, L. PD-1 signaling pathway in sepsis: Does it have a future? Clinical Immunology. 2021, 229:108742.
- Kuipers, H.; Muskens, F.; Willart, M.; Hijdra, D.; van Assema, F.B.J.; Coyle, A.J.; et al. Contribution of the PD-1 ligands/PD-1 signaling pathway to dendritic cell-mediated CD4+ T cell activation. 2006, 36, 2472–2482.
- Ai, L.; Xu, A.; Xu, J. Roles of PD-1/PD-L1 Pathway: Signaling, Cancer, and Beyond. In: Xu J, editor. Regulation of Cancer Immune Checkpoints: Molecular and Cellular Mechanisms and Therapy. Singapore: Springer Singapore; 2020. p. 33-59.
- Wu, Q.; Jiang, L.; Li, S.-C.; He, Q.-J.; Yang, B.; Cao, J. Small molecule inhibitors targeting the PD-1/PD-L1 signaling pathway. Acta Pharmacol. Sin. 2020, 42, 1–9. [Google Scholar] [CrossRef]
- Riella, L.V.; Paterson, A.M.; Sharpe, A.H.; Chandraker, A. Role of the PD-1 Pathway in the Immune Response. Am. J. Transplant. 2012, 12, 2575–2587. [Google Scholar] [CrossRef]
- Bai W, Liu Z-Q, He P-Y, Muhuyati. The role of IL-6, IL-10, TNF-α and PD-1 expression on CD4 T cells in atrial fibrillation. Heliyon. 2023, 9(8).
- Das, R.; Verma, R.; Sznol, M.; Boddupalli, C.S.; Gettinger, S.N.; Kluger, H.; Callahan, M.; Wolchok, J.D.; Halaban, R.; Dhodapkar, M.V.; et al. Combination Therapy with Anti–CTLA-4 and Anti–PD-1 Leads to Distinct Immunologic Changes In Vivo. J. Immunol. 2015, 194, 950–959. [Google Scholar] [CrossRef]
- Kuo, I.-Y.; Yang, Y.-E.; Yang, P.-S.; Tsai, Y.-J.; Tzeng, H.-T.; Cheng, H.-C.; Kuo, W.-T.; Su, W.-C.; Chang, C.-P.; Wang, Y.-C. Converged Rab37/IL-6 trafficking and STAT3/PD-1 transcription axes elicit an immunosuppressive lung tumor microenvironment. Theranostics 2021, 11, 7029–7044. [Google Scholar] [CrossRef]
- Hu, X.; Ren, J.; Xue, Q.; Luan, R.; Ding, D.; Tan, J.; et al. Anti-PD-1/PD-L1 and anti-CTLA-4 associated checkpoint inhibitor pneumonitis in non-small cell lung cancer: Occurrence, pathogenesis and risk factors (Review). Int J Oncol. 2023, 63, 122. [Google Scholar] [CrossRef]
- Quagliariello, V.; Passariello, M.; Di Mauro, A.; Cipullo, C.; Paccone, A.; Barbieri, A.; et al. Immune checkpoint inhibitor therapy increases systemic SDF-1, cardiac DAMPs Fibronectin-EDA, S100/Calgranulin, galectine-3, and NLRP3-MyD88-chemokine pathways. 2022, 9.
- Prauchner, C.A. Oxidative stress in sepsis: Pathophysiological implications justifying antioxidant co-therapy. Burns 2017, 43, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Bar-Or, D.; Carrick, M.M.; Mains, C.W.; Rael, L.T.; Slone, D.; Brody, E.N. Sepsis, oxidative stress, and hypoxia: Are there clues to better treatment? Redox Report. 2015, 20, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Gielis, J.F.; Beckers, P.A.J.; Briedé,, J. J.; Cos, P.; Van Schil, P.E. Oxidative and nitrosative stress during pulmonary ischemia-reperfusion injury: from the lab to the OR. Ann. Transl. Med. 2017, 5, 131–131. [Google Scholar] [CrossRef] [PubMed]
- Trujillo-Rangel, W.Á.; García-Valdés, L.; Villar, M.M.-D.; Castañeda-Arellano, R.; Totsuka-Sutto, S.E.; García-Benavides, L. Therapeutic Targets for Regulating Oxidative Damage Induced by Ischemia-Reperfusion Injury: A Study from a Pharmacological Perspective. Oxidative Med. Cell. Longev. 2022, 2022, 1–25. [Google Scholar] [CrossRef]
- Forceville, X.; Van Antwerpen, P.; Preiser, J.-C. Selenocompounds and Sepsis: Redox Bypass Hypothesis for Early Diagnosis and Treatment: Part A—Early Acute Phase of Sepsis: An Extraordinary Redox Situation (Leukocyte/Endothelium Interaction Leading to Endothelial Damage). Antioxidants Redox Signal. 2021, 35, 113–138. [Google Scholar] [CrossRef]
- Joffre, J.; Hellman, J. Oxidative Stress and Endothelial Dysfunction in Sepsis and Acute Inflammation. Antioxidants Redox Signal. 2021, 35, 1291–1307. [Google Scholar] [CrossRef]
- Navarro-Yepes, J.; Burns, M.; Anandhan, A.; Khalimonchuk, O.; del Razo, L.M.; Quintanilla-Vega, B.; et al. Oxidative Stress, Redox Signaling, and Autophagy: Cell Death Versus Survival. Antioxidants & Redox Signaling. 2014, 21, 66–85. [Google Scholar]
- Helan, M.; Malaska, J.; Tomandl, J.; Jarkovsky, J.; Helanova, K.; Benesova, K.; Sitina, M.; Dastych, M.; Ondrus, T.; Goldbergova, M.P.; et al. Kinetics of Biomarkers of Oxidative Stress in Septic Shock: A Pilot Study. Antioxidants 2022, 11, 640. [Google Scholar] [CrossRef]
- Burgoyne, J.R.; Mongue-Din, H.; Eaton, P.; Shah, A.M. Redox Signaling in Cardiac Physiology and Pathology. Circulation Research. 2012, 111, 1091–1106. [Google Scholar] [CrossRef]
- Holmström, K.M.; Finkel, T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell Biol. 2014, 15, 411–421. [Google Scholar] [CrossRef]
- Lennicke, C.; Cochemé,, H. M. Redox metabolism: ROS as specific molecular regulators of cell signaling and function. Mol. Cell 2021, 81, 3691–3707. [Google Scholar] [CrossRef]
- Zuo, J.; Zhang, Z.; Luo, M.; Zhou, L.; Nice, E.C.; Zhang, W.; Wang, C.; Huang, C. Redox signaling at the crossroads of human health and disease. Medcomm 2022, 3, e127. [Google Scholar] [CrossRef] [PubMed]
- Nakamori, Y.; Park, E.J.; Shimaoka, M. Immune Deregulation in Sepsis and Septic Shock: Reversing Immune Paralysis by Targeting PD-1/PD-L1 Pathway. 2021, 11.
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; et al. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: chronic diseases and aging. Archives of Toxicology. 2023, 97, 2499–2574. [Google Scholar]
- Chung, T.D.; Linville, R.M.; Guo, Z.; Ye, R.; Jha, R.; Grifno, G.N.; Searson, P.C. Effects of acute and chronic oxidative stress on the blood–brain barrier in 2D and 3D in vitro models. Fluids Barriers CNS 2022, 19, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Casiraghi, V.; Sorce, M.N.; Santangelo, S.; Invernizzi, S.; Bossolasco, P.; Lattuada, C.; Battaglia, C.; Venturin, M.; Silani, V.; Colombrita, C.; et al. Modeling of TDP-43 proteinopathy by chronic oxidative stress identifies rapamycin as beneficial in ALS patient-derived 2D and 3D iPSC models. Exp. Neurol. 2024, 383, 115057. [Google Scholar] [CrossRef]
- Thirupathi, A.; Wang, M.; Lin, J.K.; Fekete, G.; István, B.; Baker, J.S.; Gu, Y. Effect of Different Exercise Modalities on Oxidative Stress: A Systematic Review. BioMed Res. Int. 2021, 2021. [Google Scholar] [CrossRef] [PubMed]
- Leyane, T.S.; Jere, S.W.; Houreld, N.N. Oxidative Stress in Ageing and Chronic Degenerative Pathologies: Molecular Mechanisms Involved in Counteracting Oxidative Stress and Chronic Inflammation. Int. J. Mol. Sci. 2022, 23, 7273. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, H.; Li, C. Dietary Regulation of Oxidative Stress in Chronic Metabolic Diseases. Foods 2021, 10, 1854. [Google Scholar] [CrossRef]
- Morris, G.; Gevezova, M.; Sarafian, V.; Maes, M. Redox regulation of the immune response. Cellular & Molecular Immunology. 2022, 19, 1079–1101. [Google Scholar]
- Al-Shehri, S.S. Reactive oxygen and nitrogen species and innate immune response. Biochimie 2021, 181, 52–64. [Google Scholar] [CrossRef]
- Meuren, L.M.; Prestes, E.B.; Papa, M.P.; de Carvalho, L.R.P.; Mustafá,, Y. M.; da Costa, L.S.; Da Poian, A.T.; Bozza, M.T.; Arruda, L.B. Infection of Endothelial Cells by Dengue Virus Induces ROS Production by Different Sources Affecting Virus Replication, Cellular Activation, Death and Vascular Permeability. Front. Immunol. 2022, 13, 810376. [Google Scholar] [CrossRef] [PubMed]
- Andrés, C.M.C.; de la Lastra, J.M.P.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. The Role of Reactive Species on Innate Immunity. Vaccines 2022, 10, 1735. [Google Scholar] [CrossRef] [PubMed]
- Kotlyarov, S. Immune Function of Endothelial Cells: Evolutionary Aspects, Molecular Biology and Role in Atherogenesis. Int. J. Mol. Sci. 2022, 23, 9770. [Google Scholar] [CrossRef] [PubMed]
- Sul, O.-J.; Ra, S.W. Quercetin Prevents LPS-Induced Oxidative Stress and Inflammation by Modulating NOX2/ROS/NF-kB in Lung Epithelial Cells. Molecules 2021, 26, 6949. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Dai, K.; Zhu, M.; Liang, Z.; Pan, J.; Zhang, Z.; Xue, R.; Cao, G.; Hu, X.; et al. Bombyx mori Akirin hijacks a viral peptide vSP27 encoded by BmCPV circRNA and activates the ROS-NF-κB pathway against viral infection. Int. J. Biol. Macromol. 2021, 194, 223–232. [Google Scholar] [CrossRef]
- Thiruvengadam, R.; Venkidasamy, B.; Easwaran, M.; Chi, H.Y.; Thiruvengadam, M.; Kim, S.-H. Dynamic interplay of reactive oxygen and nitrogen species (ROS and RNS) in plant resilience: unveiling the signaling pathways and metabolic responses to biotic and abiotic stresses. Plant Cell Rep. 2024, 43, 1–24. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Y.; Liu, Y.; Yin, K.; Wang, D.; Li, B.; Yu, H.; Xing, M. ROS-Induced Hepatotoxicity under Cypermethrin: Involvement of the Crosstalk between Nrf2/Keap1 and NF-κB/iκB-α Pathways Regulated by Proteasome. Environ. Sci. Technol. 2021, 55, 6171–6183. [Google Scholar] [CrossRef] [PubMed]
- Akhter, N.; Wilson, A.; Thomas, R.; Al-Rashed, F.; Kochumon, S.; Al-Roub, A.; et al. ROS/TNF-α Crosstalk Triggers the Expression of IL-8 and MCP-1 in Human Monocytic THP-1 Cells via the NF-κB and ERK1/2 Mediated Signaling. 2021, 22, 10519.
- Korbecki, J.; Simińska, D.; Gąssowska-Dobrowolska, M.; Listos, J.; Gutowska, I.; Chlubek, D.; Baranowska-Bosiacka, I. Chronic and Cycling Hypoxia: Drivers of Cancer Chronic Inflammation through HIF-1 and NF-κB Activation: A Review of the Molecular Mechanisms. Int. J. Mol. Sci. 2021, 22, 10701 https://. [Google Scholar] [CrossRef]
- Murao, A.; Aziz, M.; Wang, H.; Brenner, M.; Wang, P. Release mechanisms of major DAMPs. Apoptosis 2021, 26, 152–162. [Google Scholar] [CrossRef]
- Kang, N.; Liu, X.; Haneef, K.; Liu, W. Old and new damage-associated molecular patterns (DAMPs) in autoimmune diseases. Rheumatol. Autoimmun. 2022, 2, 185–197. [Google Scholar] [CrossRef]
- Torp, M.-K.; Vaage, J.; Stensløkken, K.-O. Mitochondria-derived damage-associated molecular patterns and inflammation in the ischemic-reperfused heart. 2023, 237, e13920.
- Ferrusquía-Jiménez, N.I.; Chandrakasan, G.; Torres-Pacheco, I.; Rico-Garcia, E.; Feregrino-Perez, A.A.; Guevara-González, R.G. Extracellular DNA: A Relevant Plant Damage-Associated Molecular Pattern (DAMP) for Crop Protection Against Pests—A Review. J. Plant Growth Regul. 2020, 40, 451–463. [Google Scholar] [CrossRef]
- Lyu, Y.; Wang, T.; Huang, S.; Zhang, Z. Mitochondrial Damage-Associated Molecular Patterns and Metabolism in the Regulation of Innate Immunity. J. Innate Immun. 2023, 15, 665–679. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Dai, Z.; Wu, W.; Wang, Z.; Zhang, N.; Zhang, L.; Zeng, W.-J.; Liu, Z.; Cheng, Q. Regulatory mechanisms of immune checkpoints PD-L1 and CTLA-4 in cancer. J. Exp. Clin. Cancer Res. 2021, 40, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Kgokolo, M.C.M.; Anderson, K.; Siwele, S.C.; Steel, H.C.; Kwofie, L.L.I.; Sathekge, M.M.; Meyer, P.W.A.; Rapoport, B.L.; Anderson, R. Elevated Levels of Soluble CTLA-4, PD-1, PD-L1, LAG-3 and TIM-3 and Systemic Inflammatory Stress as Potential Contributors to Immune Suppression and Generalized Tumorigenesis in a Cohort of South African Xeroderma Pigmentosum Patients. Front. Oncol. 2022, 12, 819790. [Google Scholar] [CrossRef]
- Zabeti Touchaei, A.; Vahidi, S. MicroRNAs as regulators of immune checkpoints in cancer immunotherapy: targeting PD-1/PD-L1 and CTLA-4 pathways. Cancer Cell International. 2024, 24, 102. [Google Scholar] [CrossRef]
- He, M.; Wang, M.; Xu, T.; Zhang, M.; Dai, H.; Wang, C.; Ding, D.; Zhong, Z. Reactive oxygen species-powered cancer immunotherapy: Current status and challenges. J. Control. Release 2023, 356, 623–648. [Google Scholar] [CrossRef]
- Qiu, M.; Shu, H.; Li, L.; Shen, Y.; Tian, Y.; Ji, Y.; Sun, W.; Lu, Y.; Kong, X. Interleukin 10 Attenuates Angiotensin II-Induced Aortic Remodelling by Inhibiting Oxidative Stress-Induced Activation of the Vascular p38 and NF-κB Pathways. Oxidative Med. Cell. Longev. 2022, 2022, 1–15. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.; Tang, D. Aerobic Exercise Alleviates Inflammation, Oxidative Stress, and Apoptosis in Mice with Chronic Obstructive Pulmonary Disease. International Journal of Chronic Obstructive Pulmonary Disease. 2021, 16(null):1369-79.
- Bai, J.; Qian, B.; Cai, T.; Chen, Y.; Li, T.; Cheng, Y.; et al. Aloin Attenuates Oxidative Stress, Inflammation, and CCl4-Induced Liver Fibrosis in Mice: Possible Role of TGF-β/Smad Signaling. Journal of Agricultural and Food Chemistry. 2023, 71, 19475–19487. [Google Scholar] [CrossRef]
- Sabra, R.T.; Bekhit, A.A.; Sabra, N.T.; El-Moeze, N.A.A.; Fathy, M. Nebivolol ameliorates sepsis-evoked kidney dysfunction by targeting oxidative stress and TGF-β/Smad/p53 pathway. Sci. Rep. 2024, 14, 1–13. [Google Scholar] [CrossRef]
- Farideh, J.-H.; Kahin, S.; Mansoureh, K.; Faezeh, S.; Alireza, J.; Mojgan Noor, B.; et al. Curcumin Attenuates Oxidative Stress-Induced Effects on TGF-β Expression and NF-κB Signaling in Bovine Aortic Endothelial Cells. Acta Biochimica Iranica. 2023, 1(2).
- Wójcik, P.; Gęgotek, A.; Žarković,, N. ; Skrzydlewska, E. Oxidative Stress and Lipid Mediators Modulate Immune Cell Functions in Autoimmune Diseases. Int. J. Mol. Sci. 2021, 22, 723. [Google Scholar] [CrossRef]
- Shu, P.; Liang, H.; Zhang, J.; Lin, Y.; Chen, W.; Zhang, D. Reactive oxygen species formation and its effect on CD4+ T cell-mediated inflammation. Front. Immunol. 2023, 14, 1199233. [Google Scholar] [CrossRef] [PubMed]
- Kono, M. New insights into the metabolism of Th17 cells. Immunol. Med. 2022, 46, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Paquissi, F.C.; Abensur, H. The Th17/IL-17 Axis and Kidney Diseases, With Focus on Lupus Nephritis. Front. Med. 2021, 8. [Google Scholar] [CrossRef]
- Kim, M.E.; Kim, D.H.; Lee, J.S. FoxO Transcription Factors: Applicability as a Novel Immune Cell Regulators and Therapeutic Targets in Oxidative Stress-Related Diseases. Int. J. Mol. Sci. 2022, 23, 11877. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Chen, Q.; Xia, Y. Oxidative Stress Contributes to Inflammatory and Cellular Damage in Systemic Lupus Erythematosus: Cellular Markers and Molecular Mechanism. J. Inflamm. Res. 2023, ume 16, 453–465. [Google Scholar] [CrossRef]
- Das, A.; Chowdhury, O.; Gupta, P.; Das, N.; Mitra, A.; Ghosh, S.; et al. Arsenic-induced differential inflammatory responses in mouse thymus involves NF-κB/STAT-3 disruption, Treg bias and autophagy activation. Life Sciences. 2023, 314:121290.
- Yue, Y.; Ren, Y.; Lu, C.; Li, P.; Zhang, G. Epigenetic regulation of human FOXP3+ Tregs: from homeostasis maintenance to pathogen defense. Front. Immunol. 2024, 15, 1444533. [Google Scholar] [CrossRef]
- Mertowska, P.; Mertowski, S.; Podgajna, M.; Grywalska, E. The Importance of the Transcription Factor Foxp3 in the Development of Primary Immunodeficiencies. J. Clin. Med. 2022, 11, 947. [Google Scholar] [CrossRef]
- Kierans, S.J.; Taylor, C.T. Regulation of glycolysis by the hypoxia-inducible factor (HIF): implications for cellular physiology. J. Physiol. 2020, 599, 23–37. [Google Scholar] [CrossRef]
- Infantino, V.; Santarsiero, A.; Convertini, P.; Todisco, S.; Iacobazzi, V. Cancer Cell Metabolism in Hypoxia: Role of HIF-1 as Key Regulator and Therapeutic Target. Int. J. Mol. Sci. 2021, 22, 5703. [Google Scholar] [CrossRef]
- Lee, S.-H.; Golinska, M.; Griffiths, J.R. HIF-1-Independent Mechanisms Regulating Metabolic Adaptation in Hypoxic Cancer Cells. Cells 2021, 10, 2371. [Google Scholar] [CrossRef]
- Qiu, B.; Yuan, P.; Du, X.; Jin, H.; Du, J.; Huang, Y. Hypoxia inducible factor-1α is an important regulator of macrophage biology. Heliyon 2023, 9, e17167. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Wu, D.; Ma, Y.; Cao, Y.; Liu, Q.; Tang, M.; Pu, Y.; Zhang, T. Reactive oxygen species trigger NF-κB-mediated NLRP3 inflammasome activation involvement in low-dose CdTe QDs exposure-induced hepatotoxicity. Redox Biol. 2021, 47, 102157. [Google Scholar] [CrossRef]
- Dominic, A.; Le, N.-T.; Takahashi, M. Loop Between NLRP3 Inflammasome and Reactive Oxygen Species. Antioxidants Redox Signal. 2022, 36, 784–796. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yin, K.; Wang, D.; Wang, Y.; Lu, H.; Zhao, H.; Xing, M. Polystyrene microplastics-induced cardiotoxicity in chickens via the ROS-driven NF-κB-NLRP3-GSDMD and AMPK-PGC-1α axes. Sci. Total. Environ. 2022, 840, 156727. [Google Scholar] [CrossRef]
- He, J.; Liu, D.; Zhao, L.; Zhou, D.; Rong, J.; Zhang, L.; Xia, Z. Myocardial ischemia/reperfusion injury: Mechanisms of injury and implications for management (Review). Exp. Ther. Med. 2022, 23, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Algoet, M.; Janssens, S.; Himmelreich, U.; Gsell, W.; Pusovnik, M.; Eynde, J.V.D.; Oosterlinck, W. Myocardial ischemia-reperfusion injury and the influence of inflammation. Trends Cardiovasc. Med. 2022, 33, 357–366. [Google Scholar] [CrossRef]
- Zhang, Q.; Jia, M.; Wang, Y.; Wang, Q.; Wu, J. Cell Death Mechanisms in Cerebral Ischemia–Reperfusion Injury. Neurochem. Res. 2022, 47, 3525–3542. [Google Scholar] [CrossRef]
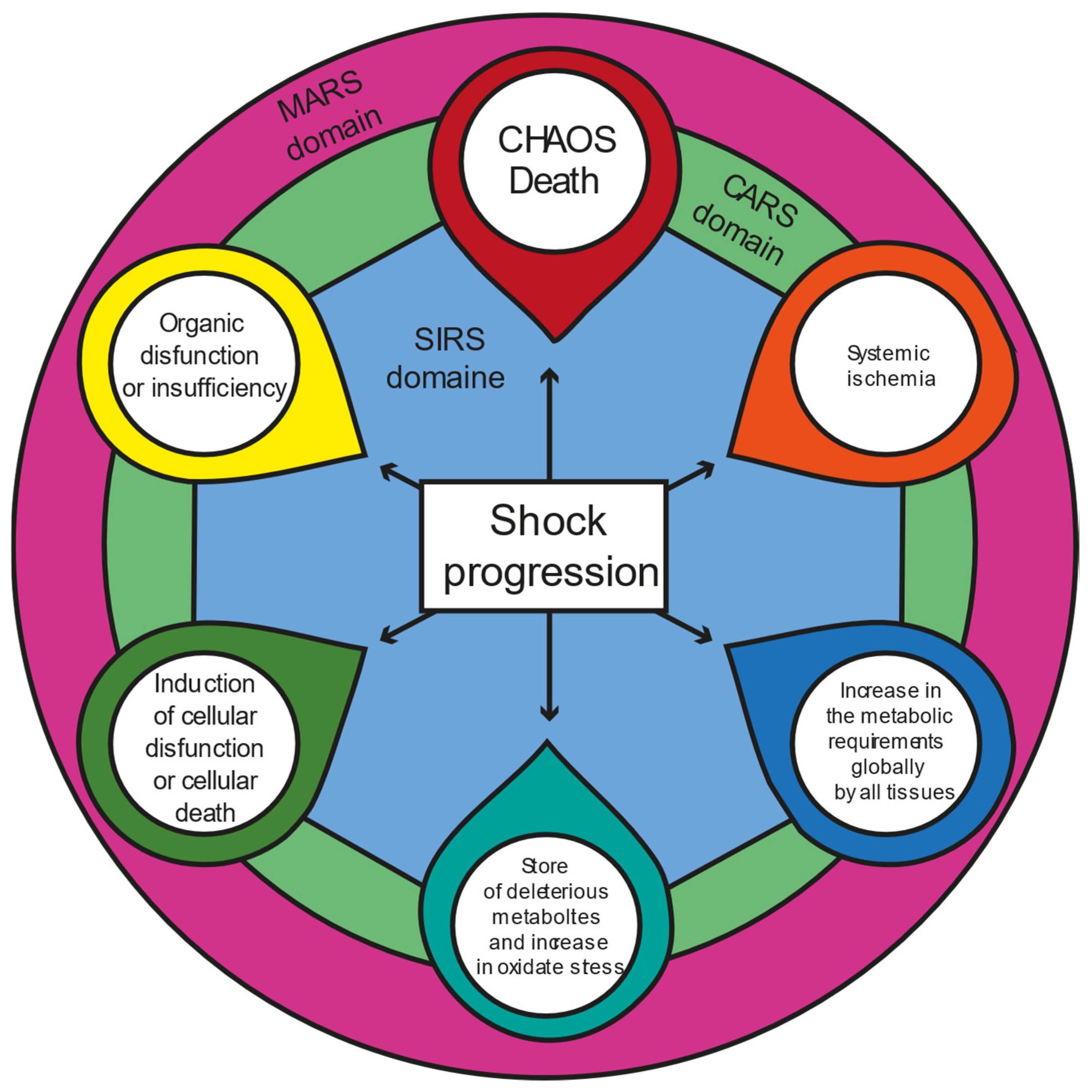
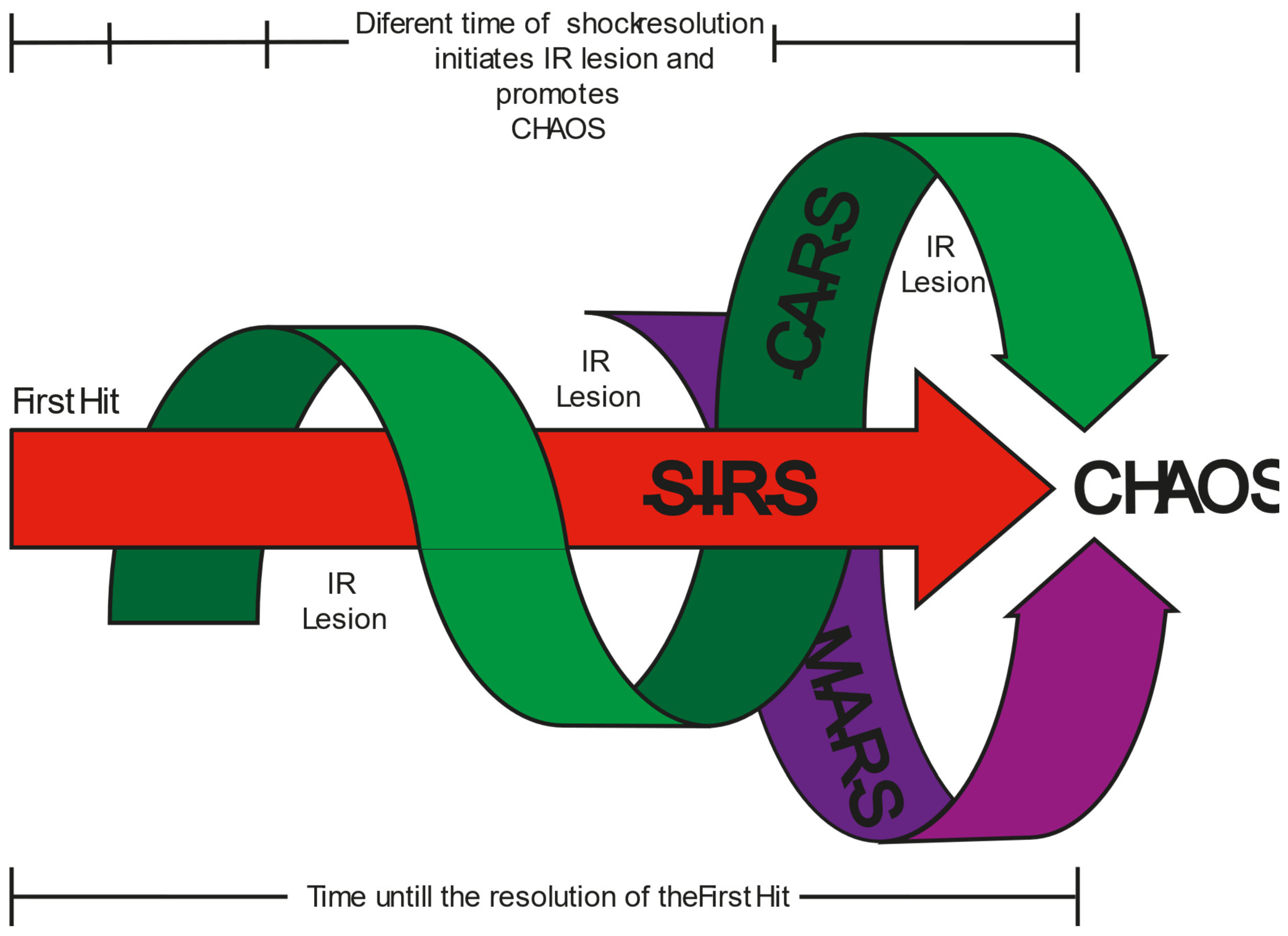
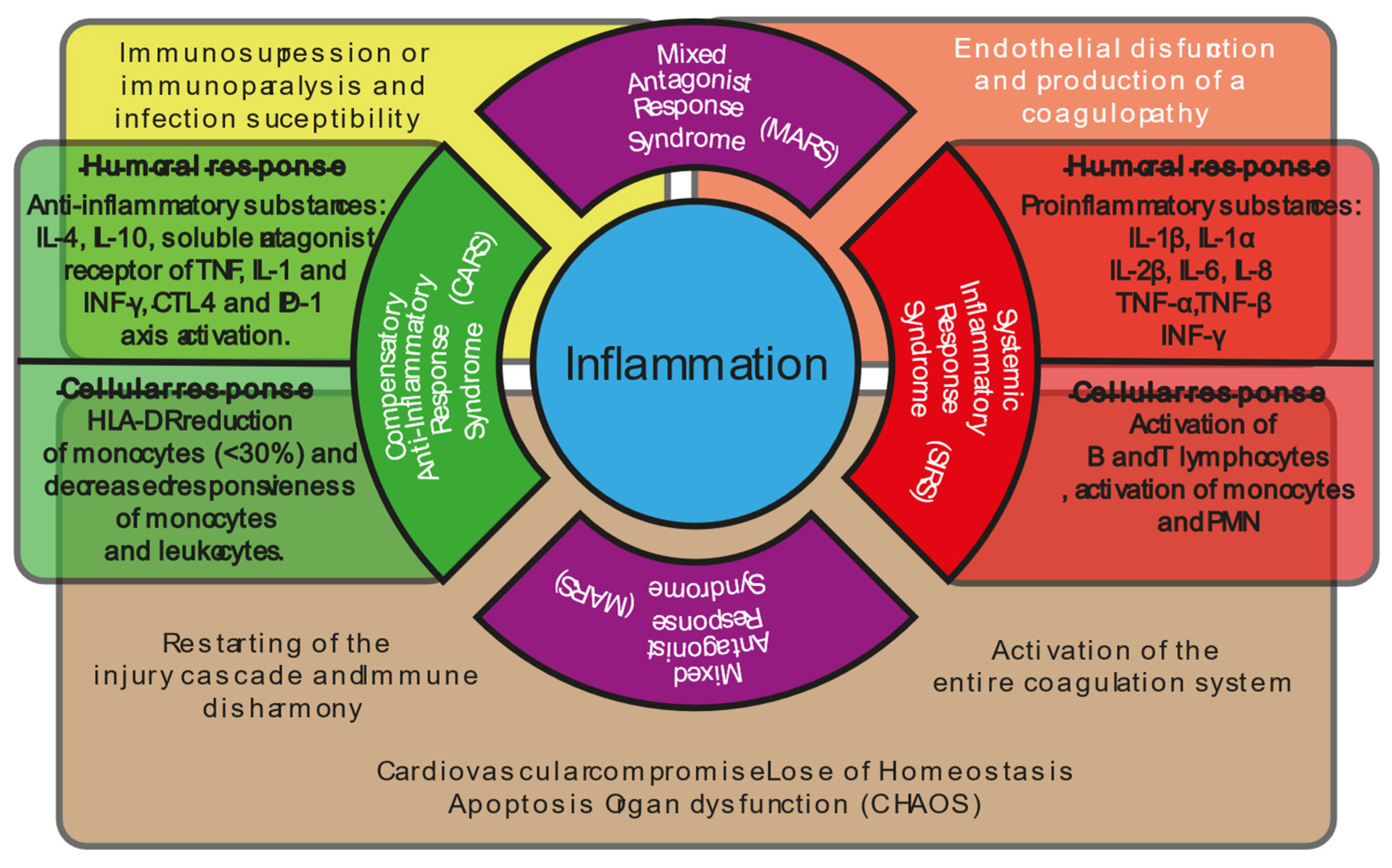

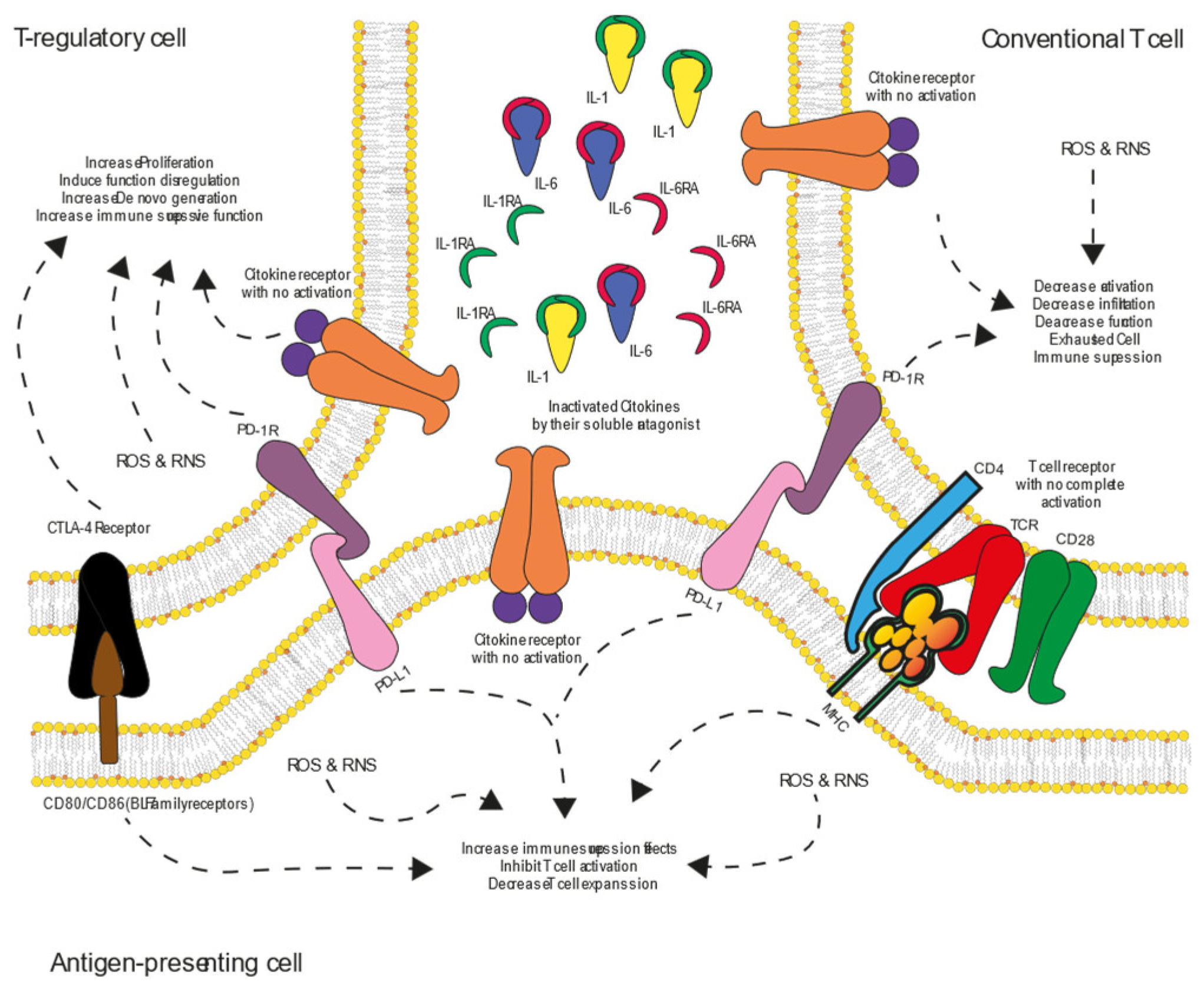
| Injury cascade | |||
|---|---|---|---|
| Stage | Description | Functions | |
| I | Local reaction in the site of the first hit (direct lesion or infection) activation of SIRS | The pro-inflammatory response is designed to limit the initial injury, prevent its spread (contention), and start tissue repair. | |
| II | Early compensatory anti-inflammatory response (CARS). | Anti-inflammatory response designed to maintain immune balance if the lesion is too extensive or shock progression. | |
| III | MARS | Overlaying of SIRS on CARS | It results in progressive endothelial dysfunction, increased microvascular permeability producing coagulopathy, and activation of the entire coagulation system. |
| Overlaying of CARS on SIRS | It results in immunosuppression or immunoparalysis, susceptibility to infection, or restarting the injury cascade. | ||
| IV | CHAOS | Immune disharmony, deregulation of SIRS and CARS (MODS, SOF and MOF) | |
| IMMUNE EXHAUSTION PATHWAYS | |||||
|---|---|---|---|---|---|
| PATHWAY | Expression | Main Inducers | Coupled Signaling Pathways | Cellular Effects | Effects of Overactivity/Inactivity |
|
CTLA-4 PATHWAY CTLA-4 COMPETES WITH CD28 FOR B7 LIGANDS (CD80/CD86) ON ANTIGEN-PRESENTING CELLS (APCS) |
Induced after initial TCR activation but rapidly internalized in effector T cells. Constitutively expressed in Tregs. | TCR activation, IL-2, TGF-β, Treg differentiation. | Negatively regulates TCR signaling and costimulatory pathways via CD28-B7 interaction | Prevents excessive T-cell activation, reduces inflammatory cytokine production, and maintains immune homeostasis | Overactivity leads to excessive suppression of T-cell activation, reducing inflammatory cytokine production necessary for proper immune response and tissue repair. This can impair clearance of pathogens and delay wound healing. Inactivity results in uncontrolled immune activation, increasing oxidative stress and tissue damage due to excessive pro-inflammatory cytokine release. |
|
PD-1 PATHWAY PD-1 INTERACTS WITH ITS LIGANDS PD-L1 AND PD-L2, WHICH ARE EXPRESSED ON APCS AND SOME NON-IMMUNE CELLS |
Induced in activated T cells, especially in response to chronic stimulation. Sustained expression in persistent infections. | Chronic TCR activation, IL-6, IL-10, TGF-β, hypoxia, IFN-γ. | Inhibits PI3K-Akt, Ras-MEK-ERK, and JAK-STAT signaling, reducing T-cell proliferation and cytokine production | Suppresses T-cell proliferation, decreases cytokine production, and induces T-cell exhaustion in chronic infections and cancer | Overactivity causes prolonged T-cell exhaustion, leading to reduced ability to control infections and impaired antioxidant defenses, increasing oxidative stress. This contributes to chronic inflammation and defective tissue regeneration. Inactivity results in excessive immune activation, enhancing reactive oxygen species (ROS) production, damaging tissues, and overwhelming reparative mechanisms. |
| Clinical Impact of Oxidative Stress in Shock States | |
|---|---|
| Mechanism | Clinical impact |
| Excessive ROS/RNS production | Multi-organ dysfunction (MODS), endothelial damage, coagulopathy |
| Uncontrolled NF-κB and inflammasome activation | Cytokine storm in septic shock |
| Loss of PD-1/CTLA-4 function | Persistent immune activation, tissue destruction |
| Treg/Th17 imbalance | Chronic inflammation, increased susceptibility to secondary infections |
| Reperfusion-induced ROS overload | Worsening of ischemia-reperfusion injury, increased mortality |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





