Submitted:
30 March 2025
Posted:
31 March 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Sources
2.2. Study Population
2.3. Clinical Outcomes and Follow-Up
2.4. Statistical Analysis
2.5. Sensitivity and Subgroup Analysis
3. Results
3.1. Cohort Chacateristics
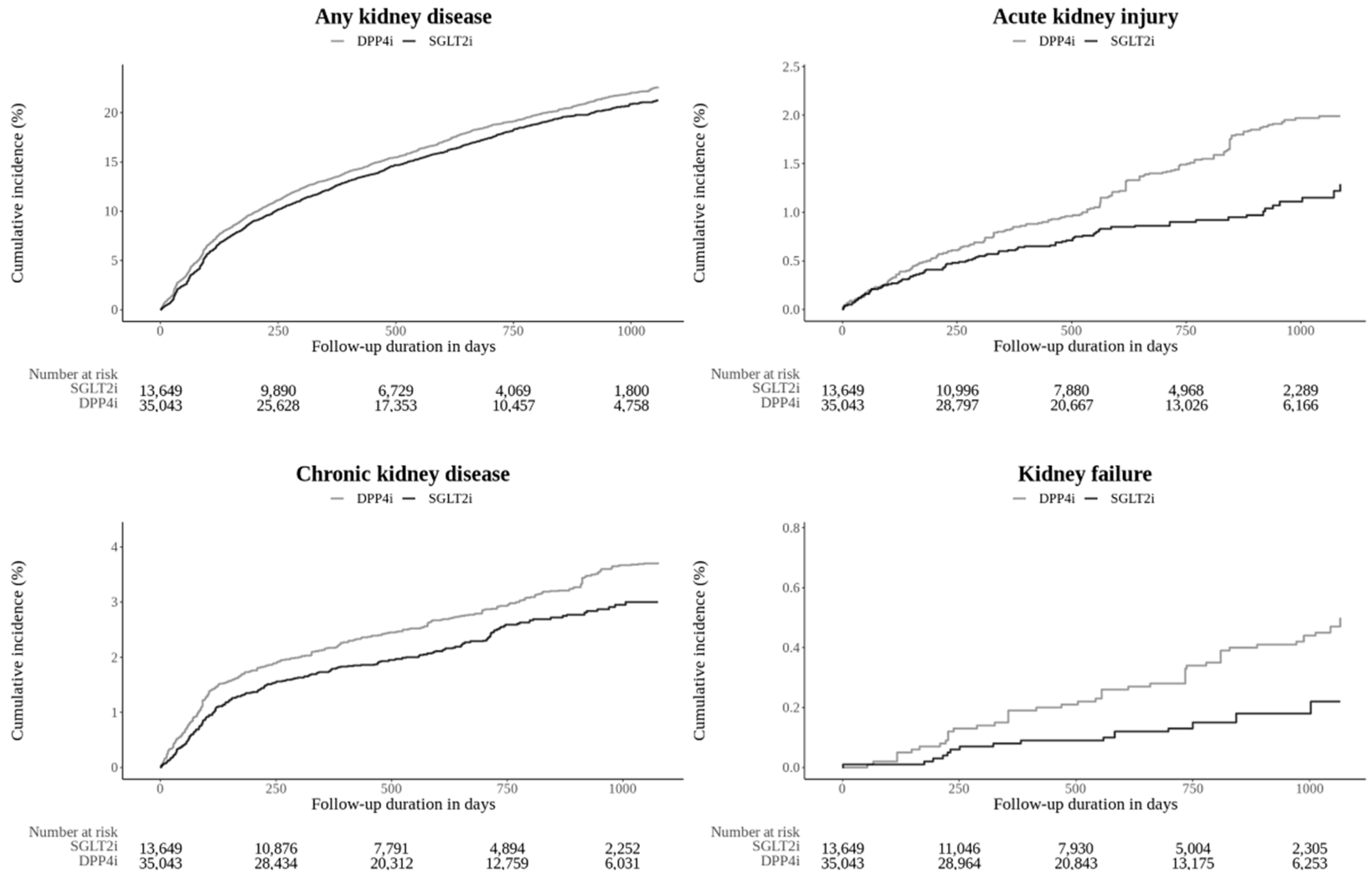
3.2. Outcome Assessment
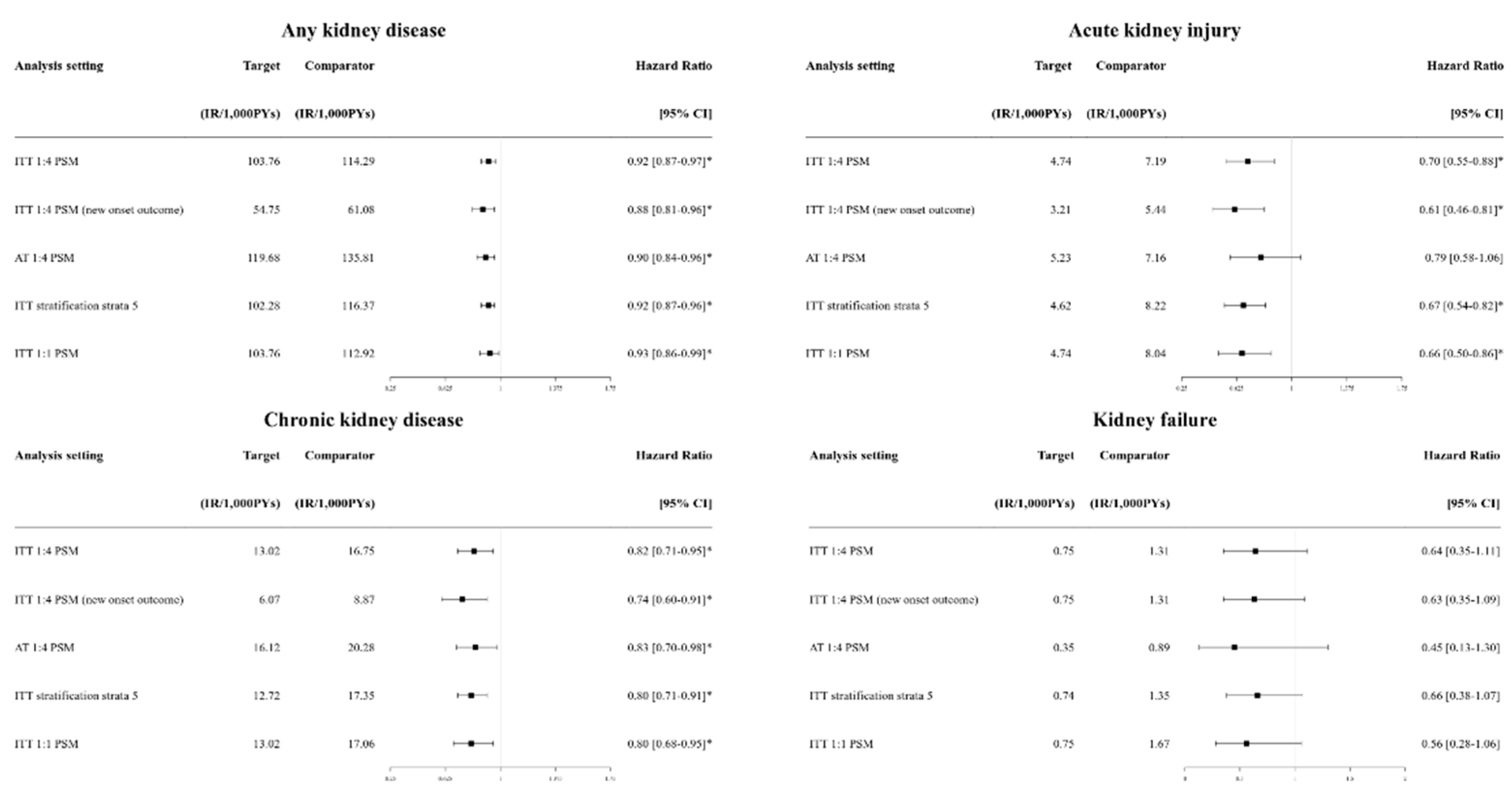
3.3. Sensitivity Analysis
3.4. Subgroup Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SGLT2i | Sodium-glucose co-transporter 2 inhibitors |
| DPP4i | Dipeptidyl peptidase 4 inhibitors |
| T2DM | Type 2 diabetes mellitus |
| AKI | Acute kidney injury |
| CKD | Chronic kidney disease |
| ESRD | End-stage renal disease |
| CRRT | Continuous renal replacement therapy |
| AMI | Acute myocardial infarction |
| HHF | Hospitalization with heart failure |
| PS | Propensity score |
| PY | Person-year |
| HR | Hazard ratio |
| CI | Confidence interval |
References
- Zinman, B; Wanner, C; Lachin, J.M; et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. New England Journal of Medicine 2015, 373, 2117-2128.
- Wiviott, S.D; Raz, I; Bonaca, M.P; et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. New England Journal of Medicine 2019, 380, 347-357.
- Perkovic, V; Jardine, M.J; Neal, B; et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. New England Journal of Medicine 2019, 380, 2295-2306.
- Heerspink, H.J; Stefánsson, B.V; Correa-Rotter, R; et al. Dapagliflozin in patients with chronic kidney disease. New England Journal of Medicine 2020, 383, 1436-1446.
- Heerspink, H.J; Karasik, A; Thuresson, M; et al. Kidney outcomes associated with use of SGLT2 inhibitors in real-world clinical practice (CVD-REAL 3): a multinational observational cohort study. The Lancet Diabetes & Endocrinology 2020, 8, 27-35.
- Berger, M.L; Sox, H; Willke, R.J; et al. Good practices for real-world data studies of treatment and/or comparative effectiveness: recommendations from the joint ISPOR-ISPE Special Task Force on real-world evidence in health care decision making. Value in Health 2017, 20, 1003-1008.
- Kim, J.-W; Kim, C; Kim, K.-H; et al. Scalable Infrastructure Supporting Reproducible Nationwide Healthcare Data Analysis toward FAIR Stewardship. Scientific Data 2023, 10, 674. [CrossRef]
- Tian, Y; Schuemie, M.J; Suchard, M.A. Evaluating large-scale propensity score performance through real-world and synthetic data experiments. International Journal of Epidemiology 2018, 47, 2005-2014.
- Schuemie, M; Reps, J; Black, A; et al. Health-Analytics Data to Evidence Suite (HADES): Open-Source Software for Observational Research. Stud Health Technol Inform 2024, 310, 966-970. [CrossRef]
- Vandenbroucke, J.P; Elm, E.V; Altman, D.G; et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and Elaboration. Annals of Internal Medicine 2007, 147, W-163-W-194. [CrossRef]
- Berger, M.L; Sox, H; Willke, R.J; et al. Good practices for real-world data studies of treatment and/or comparative effectiveness: recommendations from the joint ISPOR-ISPE Special Task Force on real-world evidence in health care decision making. Value in Health 2017, 20, 1003-1008.
- Bailey, C.J; Day, C; Bellary, S. Renal protection with SGLT2 inhibitors: effects in acute and chronic kidney disease. Current Diabetes Reports 2022, 22, 39-52.
- Nespoux, J; Vallon, V. Renal effects of SGLT2 inhibitors: an update. Current Opinion in Nephrology and Hypertension 2020, 29, 190.
- Rampersad, C; Kraut, E; Whitlock, R.H; et al. Acute kidney injury events in patients with type 2 diabetes using SGLT2 inhibitors versus other glucose-lowering drugs: a retrospective cohort study. American Journal of Kidney Diseases 2020, 76, 471-479. e1.
- Idris, I; Zhang, R; Mamza, J.B; et al. Significant reduction in chronic kidney disease progression with sodium-glucose cotransporter-2 inhibitors compared to dipeptidyl peptidase-4 inhibitors in adults with type 2 diabetes in a UK clinical setting. Diabetes, Obesity and Metabolism 2022, 24, 2138-2147.
- Au, P.C; Tan, K.C; Cheung, B.M; Wong, I.C; Li, H.-L; Cheung, C.-L. Association between SGLT2 inhibitors vs DPP4 inhibitors and renal outcomes among patients with type 2 diabetes. The Journal of Clinical Endocrinology & Metabolism 2022, 107, e2962-e2970.
- Arshad, M; Hoda, F; Siddiqui, N.A; Najmi, A.K; Ahmad, M. Genito Urinary Infection and Urinary Tract Infection in Patients with Type 2 Diabetes Mellitus Receiving SGLT2 Inhibitors: Evidence from a Systematic Literature Review of Landmark Randomized Clinical Trial. Drug Research 2024.
- Alkabbani, W; Zongo, A; Minhas-Sandhu, J.K; et al. Sodium-glucose Cotransporter-2 inhibitors and urinary tract infections: a propensity score–matched population-based cohort study. Canadian Journal of Diabetes 2022, 46, 392-403. e13.
- Neuen, B.L; Oshima, M; Agarwal, R; et al. Sodium-glucose cotransporter 2 inhibitors and risk of hyperkalemia in people with type 2 diabetes: a meta-analysis of individual participant data from randomized, controlled trials. Circulation 2022, 145, 1460-1470.
- Umpierrez, G.E; Davis, G.M; ElSayed, N.A; et al. Hyperglycemic crises in adults with diabetes: a consensus report. Diabetes Care 2024, 47, 1257-1275.
- Ekanayake, P; Hupfeld, C; Mudaliar, S. Sodium-glucose cotransporter type 2 (SGLT-2) inhibitors and ketogenesis: the good and the bad. Current Diabetes Reports 2020, 20, 1-10.
- Rong, X; Zhu, Y; Wen, B; et al. Risk of hypovolemia associated with sodium–glucose cotransporter-2 inhibitors treatment: A meta-analysis of randomized controlled trials. Frontiers in Cardiovascular Medicine 2022, 9, 973129.
- D’Andrea, E; Wexler, D.J; Kim, S.C; Paik, J.M; Alt, E; Patorno, E. Comparing effectiveness and safety of SGLT2 inhibitors vs DPP-4 inhibitors in patients with type 2 diabetes and varying baseline HbA1c levels. JAMA Internal Medicine 2023, 183, 242-254.
- Ogawa, W; Sakaguchi, K. Euglycemic diabetic ketoacidosis induced by SGLT2 inhibitors: possible mechanism and contributing factors. Journal of Diabetes Investigation 2016, 7, 135.
- Adil, M; Khan, R.A; Kalam, A; et al. Effect of anti-diabetic drugs on bone metabolism: Evidence from preclinical and clinical studies. Pharmacological Reports 2017, 69, 1328-1340.
- Wang, X; Zhang, F; Zhang, Y; et al. Effect of SGLT2 inhibitors on fractures, BMD, and bone metabolism markers in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Osteoporosis International 2023, 34, 2013-2025.
- Wang, X; Zhang, F; Zhang, Y; et al. Effect of SGLT2 inhibitors on fractures, BMD, and bone metabolism markers in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Osteoporosis International 2023, 34, 2013-2025.
- Filion, K.B; Lix, L.M; Oriana, H; et al. Sodium glucose cotransporter 2 inhibitors and risk of major adverse cardiovascular events: multi-database retrospective cohort study. BMJ 2020, 370.
- Mascolo, A; Scavone, C; Scisciola, L; Chiodini, P; Capuano, A; Paolisso, G. SGLT-2 inhibitors reduce the risk of cerebrovascular/cardiovascular outcomes and mortality: a systematic review and meta-analysis of retrospective cohort studies. Pharmacological Research 2021, 172, 105836.
- Zhang, L; Wang, Y; Schuemie, M.J; Blei, D.M; Hripcsak, G. Adjusting for indirectly measured confounding using large-scale propensity score. Journal of Biomedical Informatics 2022, 134, 104204. [CrossRef]
| Characteristics | Before PS Matching | After PS Matching | ||||
|---|---|---|---|---|---|---|
| SGLT2i (n = 16,736) |
DPP4i (n = 67,463) |
Std. Diff | SGLT2i(n = 13,649) | DPP4i (n = 35,043) |
Std. Diff | |
| Female, n (%) | 6544 (39.1) | 29279 (43.4) | -0.09 | 5,473 (40.1) | 14,087 (40.2) | 0.00 |
| Age group, n (%) | ||||||
| < 40 | 1,523 (9.1) | 2,361 (3.5) | 0.19 | 915 (6.7) | 2,348 (6.7) | 0.04 |
| 40-59 | 8,853 (52.9) | 25,906 (38.4) | 0.12 | 6,920 (50.7) | 17,802 (50.8) | 0.01 |
| 60-74 | 5,372 (32.1) | 28,065 (41.6) | 0.09 | 4,886 (35.8) | 12,370 (35.3) | 0.01 |
| ≥ 75 | 988 (5.9) | 11,131 (16.5) | -0.17 | 928 (6.8) | 2,523 (7.2) | -0.03 |
| Medical history, n (%) | ||||||
| Hyperlipidemia | 14,410 (86.1) | 56,265 (83.4) | 0.07 | 11,738 (86.0) | 30,242 (86.3) | -0.01 |
| Hypertensive disorder | 10,996 (65.7) | 42,975 (63.7) | 0.04 | 8,885 (65.1) | 22,848 (65.2) | 0.00 |
| Cerebrovascular disease | 1,004 (6.0) | 5,060 (7.5) | -0.06 | 846 (6.2) | 2,208 (6.3) | 0.00 |
| Heart disease | 4,720 (28.2) | 13,763 (20.4) | 0.18 | 3,535 (25.9) | 9,251 (26.4) | -0.01 |
| Atrial fibrillation | 435 (2.6) | 1,214 (1.8) | 0.05 | 328 (2.4) | 841 (2.4) | 0.00 |
| Heart failure | 1,807 (10.8) | 4,588 (6.8) | 0.14 | 1,283 (9.4) | 3,364 (9.6) | -0.01 |
| Ischemic heart disease | 3,029 (18.1) | 8,096 (12.0) | 0.17 | 2,238 (16.4) | 5,852 (16.7) | -0.01 |
| Peripheral vascular disease | 2,544 (15.2) | 13,155 (19.5) | -0.11 | 2,211 (16.2) | 5,817 (16.6) | -0.01 |
| Osteoporosis | 1,439 (8.6) | 9,512 (14.1) | -0.18 | 1,297 (9.5) | 3,434 (9.8) | -0.01 |
| Medication use, n (%) | ||||||
| ACE inhibitor / ARB | 6,661 (39.8) | 23,477 (34.8) | 0.10 | 5,255 (38.5) | 13,386 (38.2) | 0.00 |
| Antithrombotic agents | 7,950 (47.5) | 32,113 (47.6) | 0.00 | 6,442 (47.2) | 16,540 (47.2) | 0.00 |
| Calcium channel blockers | 5,322 (31.8) | 21,319 (31.6) | 0.00 | 4,313 (31.6) | 11,039 (31.5) | 0.00 |
| Diuretics | 3,381 (20.2) | 13,358 (19.8) | 0.01 | 2,689 (19.7) | 7,044 (20.1) | -0.01 |
| Insulins | 1,205 (7.2) | 5,195 (7.7) | -0.02 | 983 (7.2) | 2,523 (7.2) | 0.00 |
| Lipid modifying agents | 8,853 (52.9) | 32,653 (48.4) | 0.09 | 7,111 (52.1) | 18,292 (52.2) | 0.00 |
| Outcomes | SGLT2i (n = 13,649) |
DPP4i (n = 35,043) |
HR [95% CI] | ||
|---|---|---|---|---|---|
| Events, n | IR | Events, n | IR | ||
| Renal outcomes | |||||
| Any kidney outcomes | 950 | 54.75 | 2,722 | 61.08 | 0.88 [0.81-0.96]* |
| Acute kidney injury | 72 | 3.21 | 320 | 5.44 | 0.61 [0.46-0.81]* |
| Chronic kidney disease | 134 | 6.07 | 511 | 8.87 | 0.74 [0.60-0.91]* |
| Dialysis | 25 | 1.11 | 105 | 1.77 | 0.64 [0.39-1.01] |
| Kidney failure | 17 | 0.75 | 78 | 1.31 | 0.63 [0.35-1.09] |
| Kidney transplantation | 0 | 0.00 | <5 | <0.08 | 0.20 [NA-2.06] |
| Safety outcomes | |||||
| Urinary tract infection | 901 | 49.41 | 2,408 | 50.70 | 0.97 [0.89-1.06] |
| Genital infection | 686 | 837 | 34.44 | 15.46 | 2.38 [2.12-2.68]* |
| Diabetic ketoacidosis | 21 | 0.93 | 35 | 0.59 | 1.27 [0.68-2.30] |
| Hyperkalemia | 58 | 2.58 | 306 | 5.19 | 0.49 [0.36-0.67]* |
| Hypokalemia | 73 | 265 | 3.25 | 4.49 | 0.82 [0.61-1.09] |
| Hypovolemia | 214 | 627 | 9.83 | 11.01 | 0.92 [0.77-1.09] |
| Hypoglycemia | 46 | 134 | 2.04 | 2.26 | 0.97 [0.65-1.42] |
| Bone fracture | 610 | 1,714 | 30.54 | 32.95 | 0.91 [0.82-1.02] |
| Renal outcomes | SGLT2i with cv risk (n = 12,980) |
DPP4i with cv risk (n = 33,362) | HR [95% CI] | SGLT2i with renal risk (n = 2,678) | DPP4i with renal risk (n = 7,202) | HR [95% CI] |
|---|---|---|---|---|---|---|
| Event, (IR) | Event, (IR) | Event, (IR) | Event, (IR) | |||
| Any kidney outcomes |
904 (55.48) | 2,602 (62.36) | 0.90 [0.83-0.98]* |
- | - | - |
| Acute kidney injury | 66 (3.10) | 309 (5.54) | 0.53 [0.39-0.72]* |
15 (3.83) | 104 (9.67) | 0.40 [0.21-0.70]* |
| Chronic kidney disease |
139 (6.65) | 499 (9.15) | 0.81 [0.66-0.998]* |
37 (10.29) | 177 (18.2) | 0.68 [0.45-0.98]* |
| Dialysis | 25 (1.17) | 98 (1.74) | 0.72 [0.44-1.14] |
7 (1.72) | 44 (3.91) | 0.46 [0.17-1.05] |
| Kidney failure | 17 (0.79) | 65 (1.15) | 0.75 [0.40-1.32] |
0 (0.00) | < 5 (< 0.44) | 0.17 [NA-2.70] |
| Kidney transplantation | 0 (0.00) | < 5 (<0.09) | 0.25 [NA-5.13] |
0 (0.00) | < 5 (< 0.44) | 0.17 [NA-2.70] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





