1. Introduction
The various types of head and neck squamous cell carcinoma (HNSCC) represent the sixth to eighth most common cancer worldwide [
1,
2,
3] and the eighth to tenth most common cancer in Germany [
4]. Most cases of head and neck cancer in Germany are associated with exposure to carcinogens such as smoking and alcohol consumption [
5], but there is a growing number of oropharyngeal squamous cell carcinoma cases associated with oncogenic human-papilloma-virus subtypes [
6,
7,
8,
9]. Occupational hazards such as asbestos and polycyclic aromatic hydrocarbons make up most of the remaining cases [
10,
11].
There is currently no established screening program for the early detection of head and neck cancer in the general population [
12]. Despite advancements in the treatment of head and neck cancers, including minimally invasive operative techniques and particle-based radiation therapy, the rate of recurrence and residual disease remains high [
13,
14]. The recurrence rate is particularly high in the first two years after the end of primary treatment [
13,
14]. Structured disease follow-up is essential for the early diagnosis of recurrences and allows effective salvage treatment. The interval between the diagnosis of the primary tumor and the recurrence significantly affects the patient’s prognosis, making early diagnosis crucial [
15]. Despite this, there are no uniform standards in early follow-up of head and neck tumors [
16]. The only consensus in the guidelines is the implementation of regular early follow-up examinations [
17,
18,
19,
20,
21]. Early diagnosis is of special significance for immune therapy, which significantly improved outcomes in palliative treatment, but should begin before patients are symptomatic, as treatment effect is typically delayed. A large retrospective study has shown that 39% of HNSCC patients are asymptomatic at the time of recurrence diagnosis, making structured early follow-up exams essential [
17]. This must be balanced with the financial impact of travel expenses, particularly in rural areas [
22].
In our study, we examine the impact of implementing a structured initial restaging between three and six months after conclusion of initial treatment.
2. Materials and Methods
Study population
Our retrospective study is based on a population of patients with a first diagnosis of HNSCC who were treated with curative intent between 2010 and 2019 in the ENT department of the University Medicine of Greifswald, Germany. Of the 532 patients included in the study, 501 (94.17%) were men and 31 (5.83%) were women. Only patients with histologically confirmed head and neck squamous cell carcinoma (HNSCC) of the oral cavity, oropharynx, larynx, and hypopharynx capable of curative treatment were included. Exclusion criteria were a noncurative treatment intention, patients who could not receive a full curative treatment dose due to comorbidities or patient preference and persistent disease appearing before three months after the end of treatment. Furthermore, we excluded HNSCC of other locations and salivary gland cancer.
Intervention
Before 2017 patients were seen for outpatient visits including a clinical interview and examination, flexible endoscopy, and sonography every three months for the first two years. Further imaging and/or panendoscopy was performed on demand.
Starting in 2017 we offered patients at least one standardized post-treatment restaging at 3-6 months after the conclusion of cancer treatment. This included CT and/or MRI imaging and panendoscopy unless the primary site could be fully examined and was completely unremarkable. Patients following radiation with measurable nodal disease were and hard to examine laryngeal cancer were offered PET-imaging in accordance with the German insurance reimbursement.
Groups were therefore divided into the SF = standard follow-up = control group and AF = adapted follow-up = intervention group
Patient interviews included questions on general wellbeing, weight loss, fever, night sweat, dysphagia, bleeding or other side effects and persistent nicotine and alcohol use.
Case files were stratified by gender, age, primary tumor location, classification according to TNM classification of the Union for International Cancer Control (UICC), tumor stage, primary therapy performed, completion of primary therapy, time of recurrence (determined from initial diagnosis), recurrence location, recurrence therapy, recurrence-free interval, and the method of disease follow-up. A follow-up interval of least five years was observed for all patients if possible. Furthermore, nicotine/alcohol abuse (nicotine in pack years, alcohol abuse above the safe amount for women of 12g pure ethanol and men of over 24g pure ethanol per day as defined by the Federal Center for Health Education in Germany (BZgA - Bundeszentrale für Gesundheitliche Aufklärung).
Statistics and Data Analysis
Data analysis and graphs were created in R 4.4.3, (R Foundation for Statistical Computing, Vienna, Austria) and GraphPad Prism 10 (GraphPad Software, San Diego, CA, USA)
3. Results
We included a total of 532 patients in the study, comprising of 401 patients in the control group (SF) and 131 in the intervention group (AF). Specifics for both groups are found in
Table 1. An additional 125 patients were excluded during screening for the following reasons: uncommon tumors such as salivary gland and nasopharyngeal cancers, palliative intent, premature treatment discontinuation, metastatic disease, death before follow-up and loss to follow-up.
There was a difference in age distributions (p= 0.023), the AF being slightly older. Age in decades is compared to better illustrate group differences. Tumor extension is compared by TNM, though we did not evaluate affixes for simplification. T and N are included, M is not listed as patients with distant metastases were excluded. UICC Tumor stages were stratified, stage 4 being the most common in both groups. There were more stage 1 cases in the AF group (18% SF and 29% AF) and less stage 4 cases (49% SF and 37% AF). Alcohol and nicotine abuse was comparable in the SF and AF groups with a slight reduction in the AF group. Secondary cancers were observed about twice as often in the AF group (SF 13% vs. AF 27%). Despite the AF group being more recent, the average follow-up time was longer (SF 207 weeks (90, 316); AF 263 weeks (156, 304). This was even more pronounced in the patients experiencing recurrence (SF 118 weeks (76, 222); AF 213 weeks (81, 293) though the smaller numbers meant the result was not statistically significant. Treatment types were shifted towards lower intensity treatment in the AF group compared to SF with more surgery alone (44% to 29%) and less surgery and chemoradiation (19% to 29%). Recurrences were more often treated with curative intent in the AF group (57%) than the SF group (34%). Only six patients with oro- or hypopharyngeal cancer were HPV positive in the AF cohort, though it is unclear if the initial years were sufficiently screened. Inclusion or exclusion did not significantly change results and was therefore not listed separately.
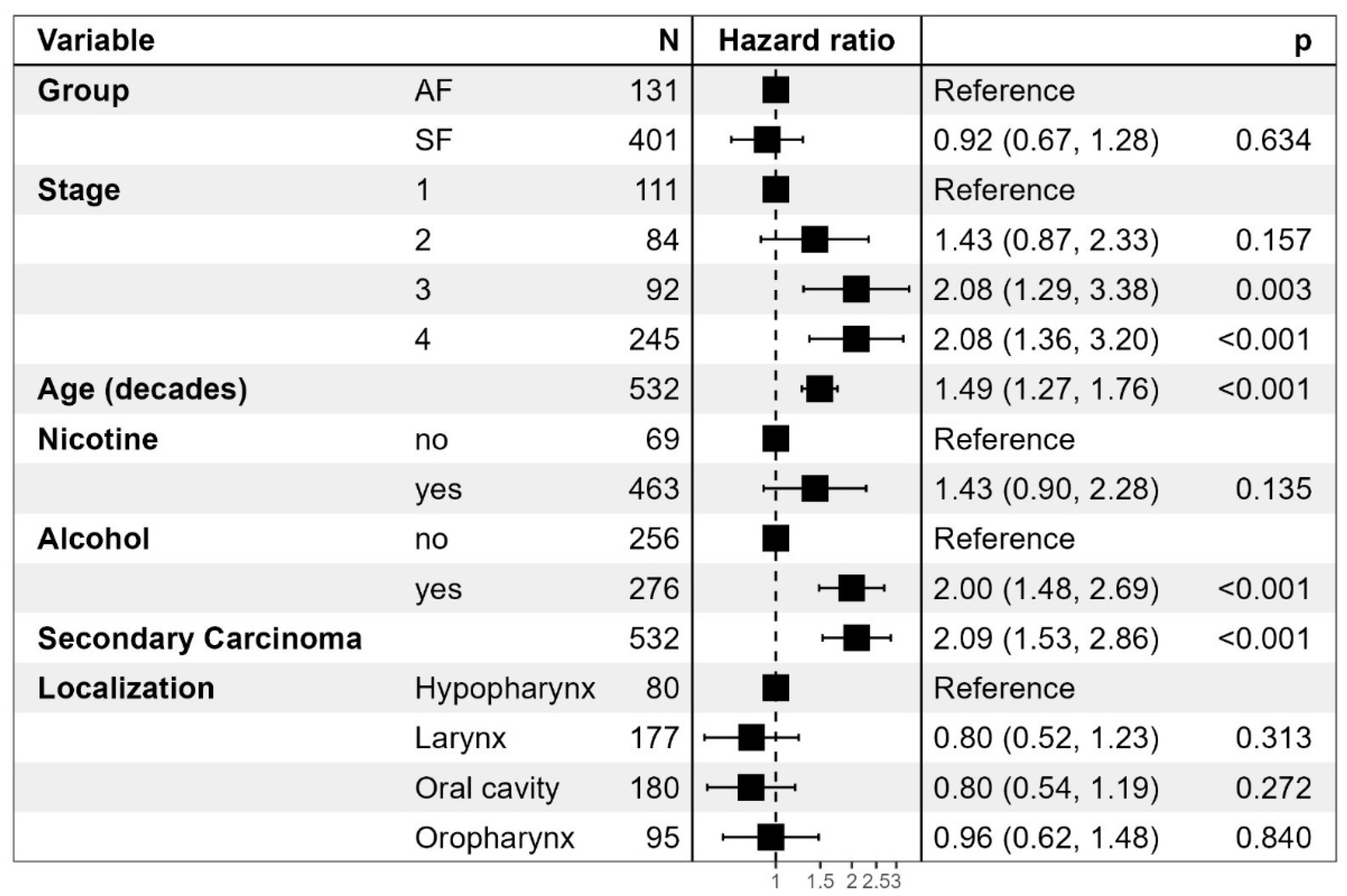
Overall mortality does not differ significantly as seen in
Table 2. There is a clear negative correlation between tumor stage and mortality. A higher age was also associated with an increased risk of death, as were nicotine and alcohol consumption. Secondary cancers conferred the highest risk of death with a hazard ratio of 2.09 (1.53 – 2.06) in both the SF group and the AF groups.
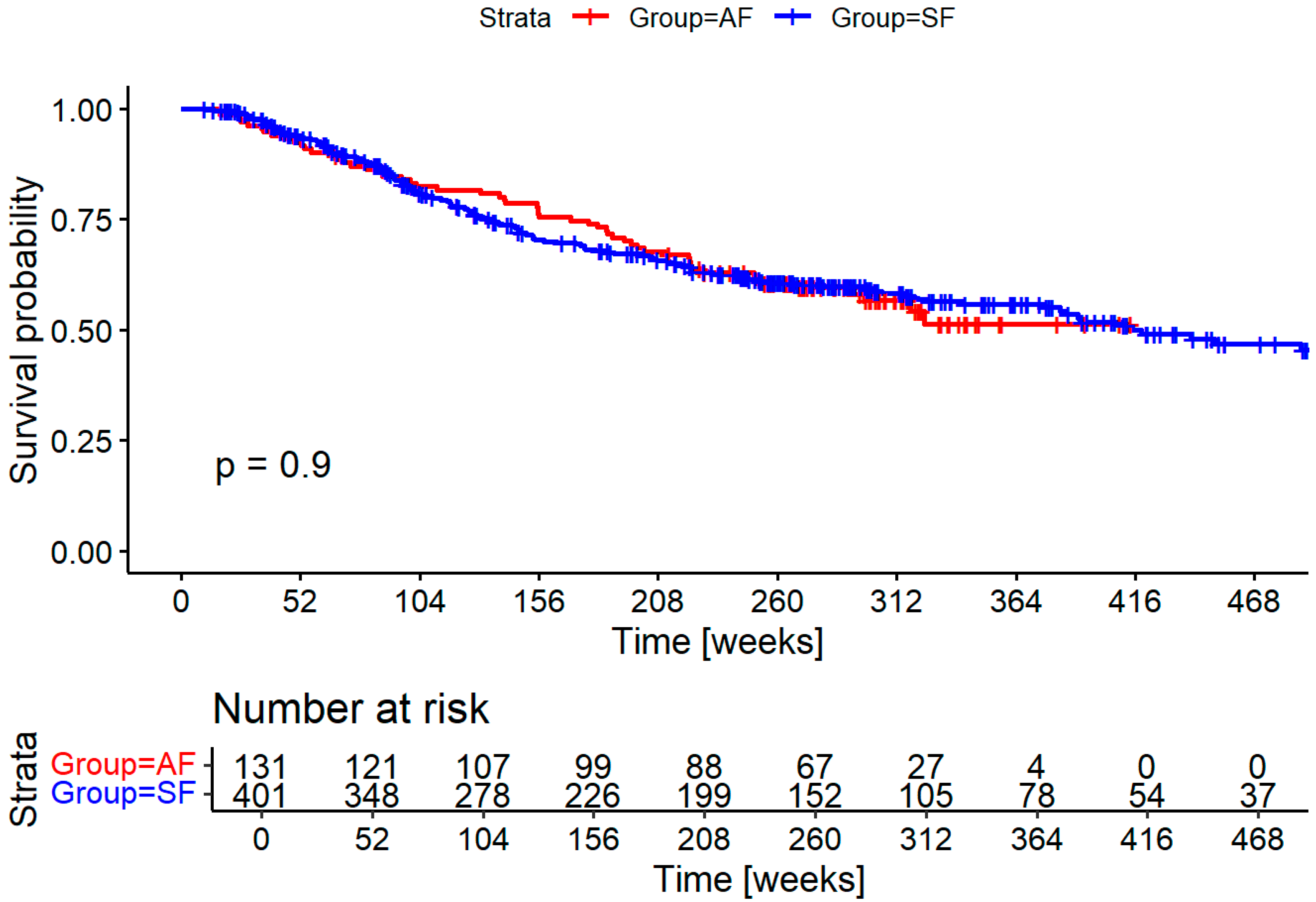
Survival did not significantly differ between groups as shown in
Figure 1. Cause of death could not be established with enough certainty in many cases to differentiate between deaths caused by the HNSCC and death caused by secondary cancers and other diseases.
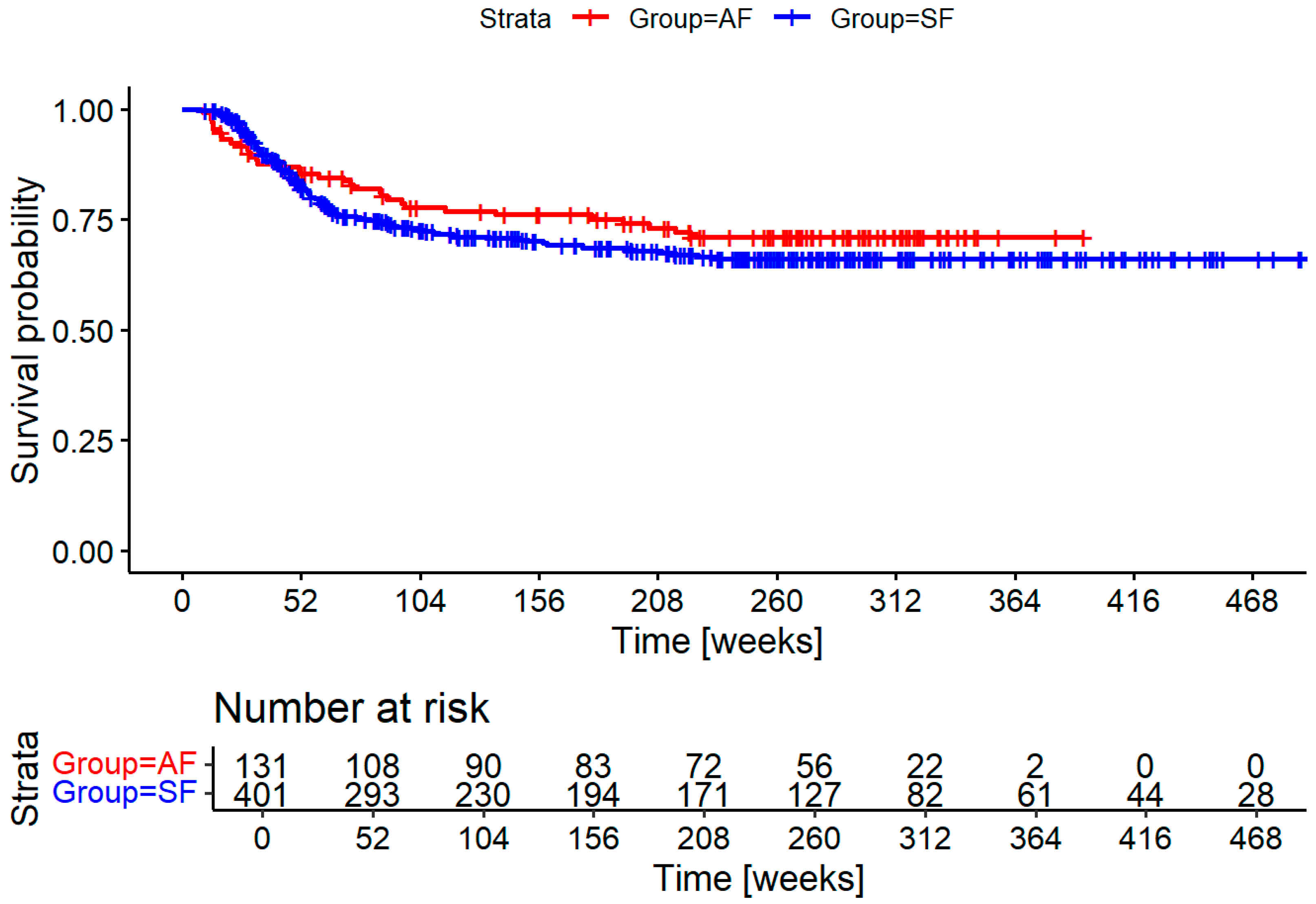
Figure 2 shows the recurrence free survival for both groups. There is a trend toward earlier diagnosis, and overall reduction in recurrences. Both differences are not statistically significant.
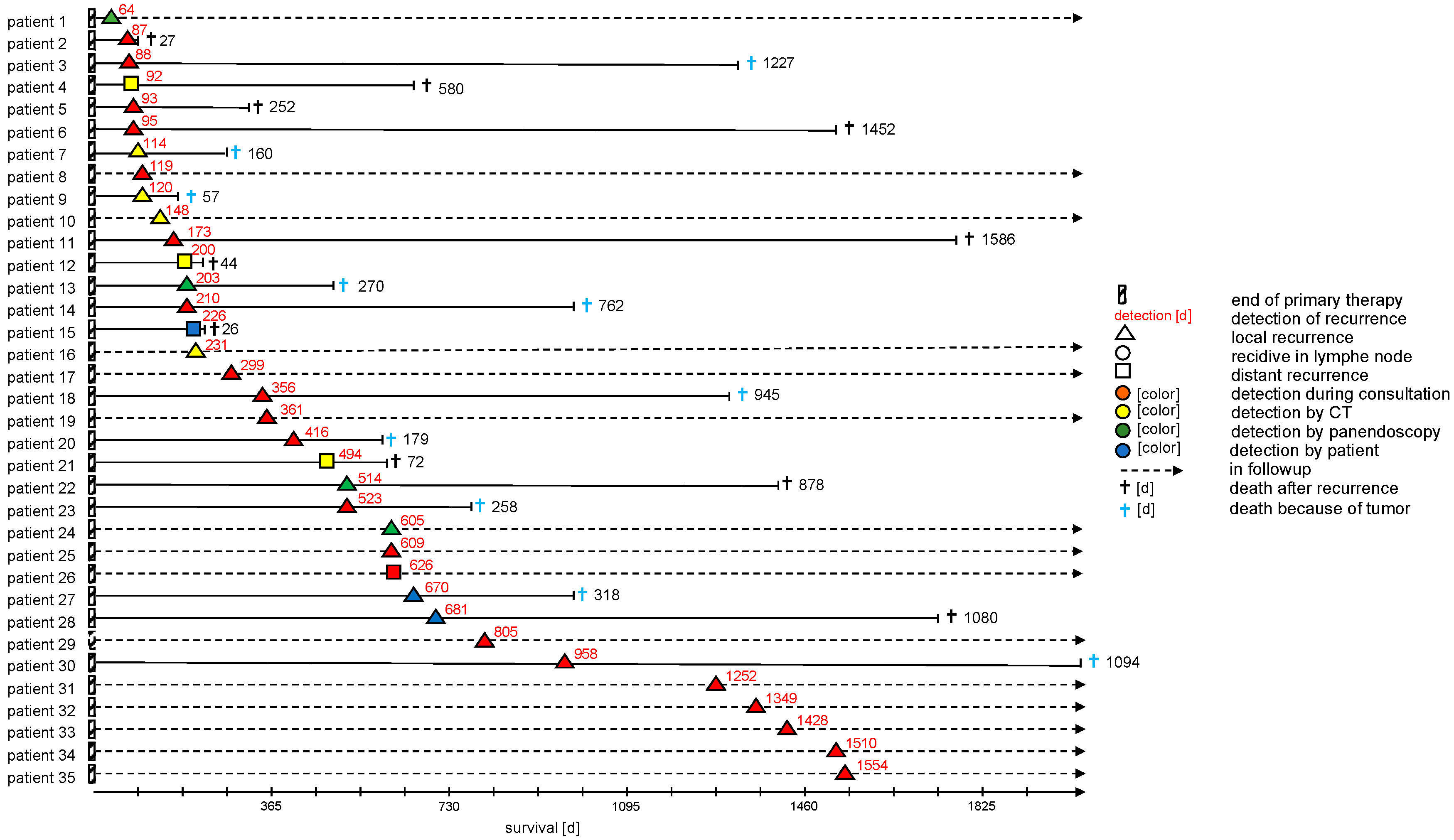
Figure 3 more closely describes the recurrence patients in the AF cohort including the location of the recurrence and the exam that lead to diagnosis. Most recurrences were identified by examination, followed by imaging and panendoscopy. Of note, only very few patients (9%) in that cohort were diagnosed after self-presentation while the vast majority of cases was found during follow-up exams.
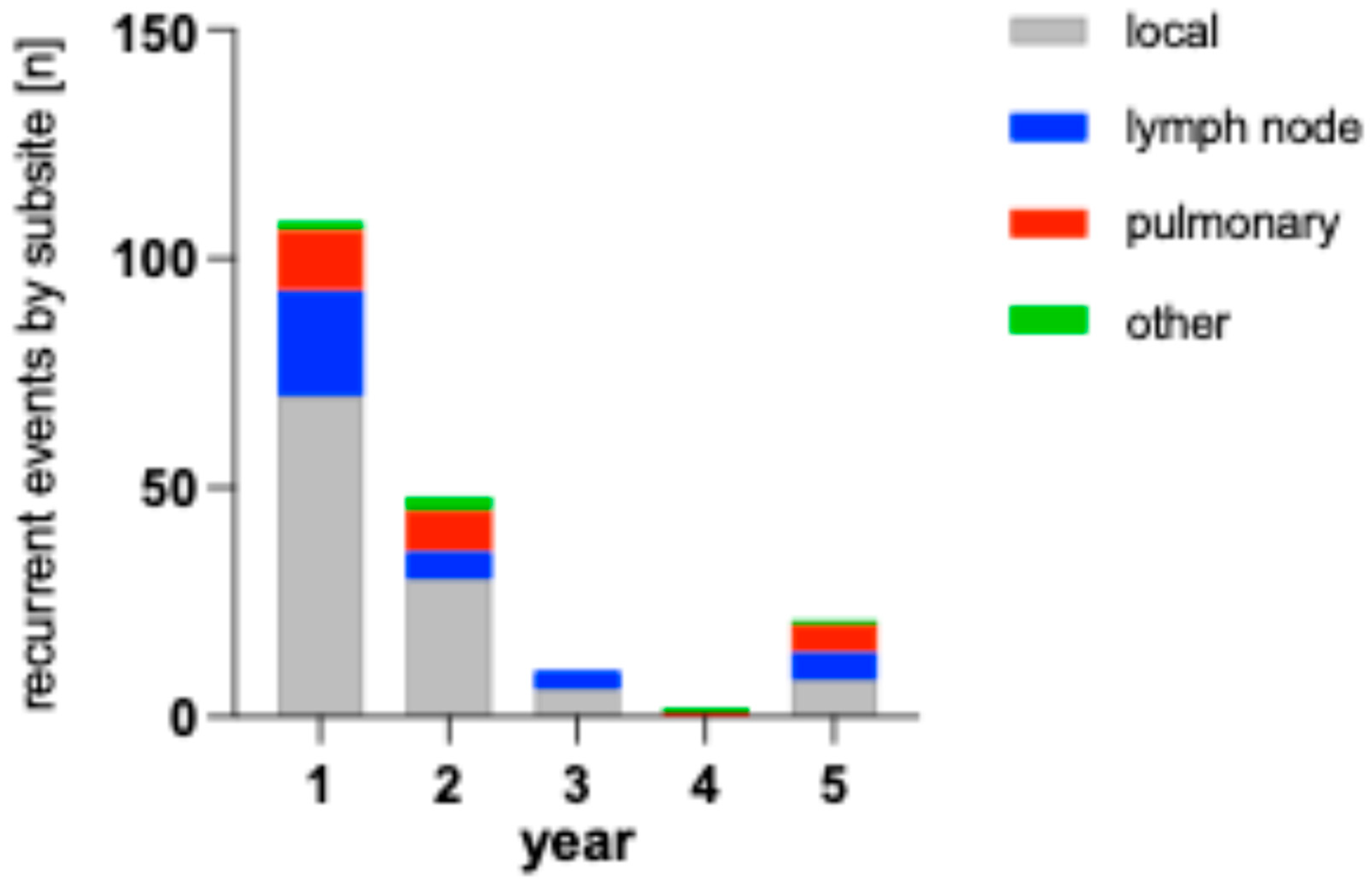
The bar chart in
Figure 4 shows the number of recurrences in both cohorts and their localization. As subsites are listed separately, single patients might have multiple events in this visualization.
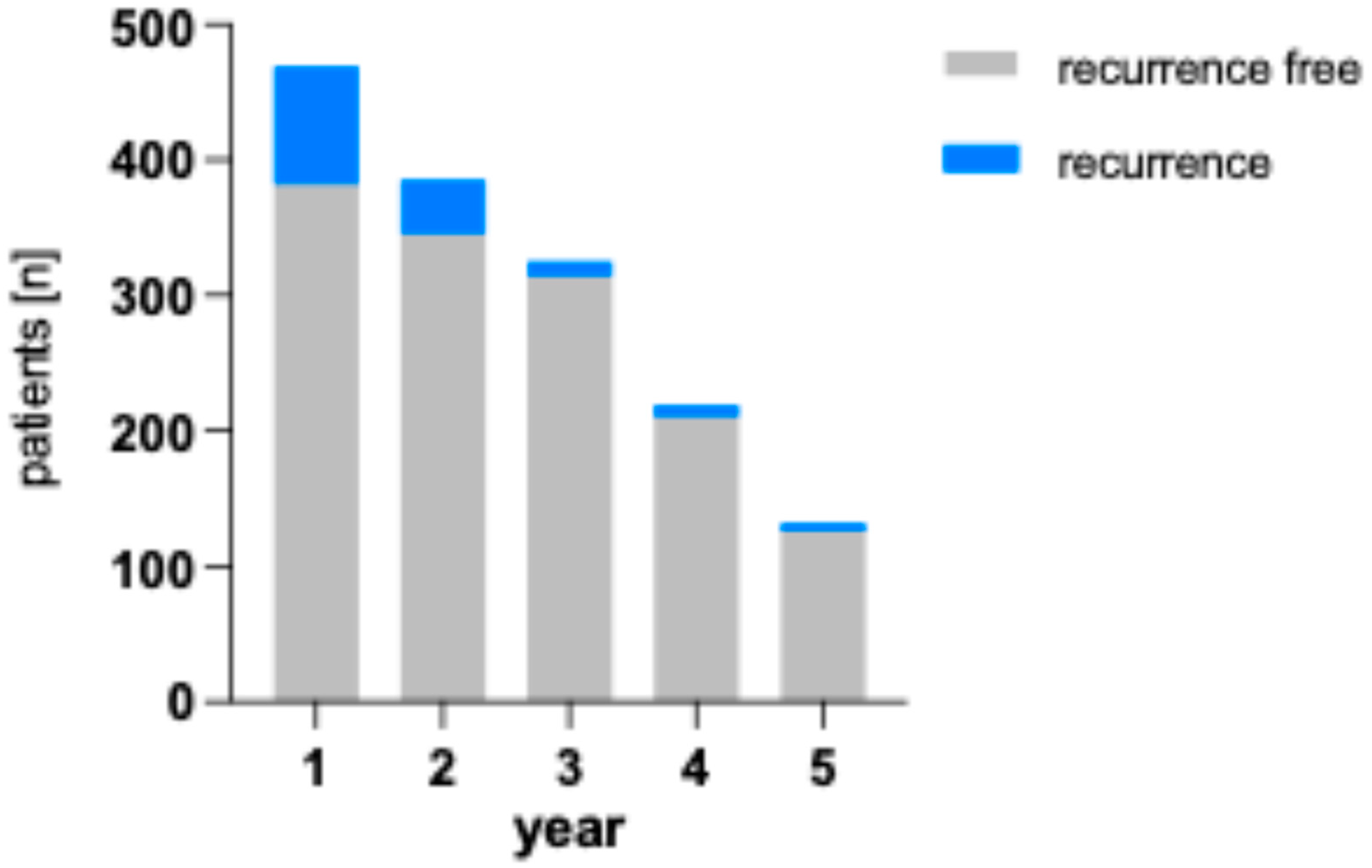
Figure 5 shows the number of recurrences per year by patient compared to healthy patients remaining in follow-up. The percentage of patients with recurrences are 18.6 % (1 in 5) in the first year, 10.4 % (1 in 10) in the second year, 3.4 % (1 in 30) in the third year, 4.1 % (1 in 24) in the fourth year and 4.5 % (1 in 22) in the fifth year.
4. Discussion
Introducing a standardized initial staging after the conclusion of cancer treatment was well received by our patients, as demonstrated by the high participation of 86% in an elective exam. Unfortunately, our initial hypothesis of significantly decreasing time to diagnosis for recurrences could not be shown in our cohort, though we see a trend evident when observing the graph in
Figure 2. The intervention coincided with certification with the German Cancer Society (DKG – Deutsche Krebsgesellschaft) and was one of several measures in the standardization of treatment processes and procedures. This explains why follow-up time in the intervention group was significantly longer despite being the more recent cohort. Additionally, access to most of the death records was available through registry information starting in the intervention cohort further skewing results.
The longer observation found significantly more secondary cancer in the intervention group. Secondary cancer was the highest predictor of mortality that matched similar observations through the SEER database [
23,
24]. It is likely that this is not due to any change in secondary cancer rates, but another sign of a more stringent observation of the intervention group. This also explains why mortality was similar between groups despite there being less recurrences, and these more likely having curative treatment strategies.
Most head and neck squamous cell carcinomas (HNSCC) exhibit recurrences within the first two years post-treatment and follow-up examinations are rightfully focused during this timeframe [
25,
26]. The NCCN guidelines stipulate that patients be examined every one to three months during the first year, with a reduction to every six to twelve months thereafter. The German guidelines such as the most recent version for oro- and hypopharyngeal cancer suggest visits every three months for the first two years and every half year until five years have passed [
27]. Use of guidelines in this timeframe is part of the DKG certification process that has been shown to improve outcomes on a population level in Germany [
28] .
Imaging is of most use to patients at higher risks of recurrence and is of most value within the first six months post-treatment [
29]. While regular imaging within the first six months post-treatment is recommended, studies suggest that beyond this period, imaging should be reserved for cases with suspicious clinical findings, emphasizing the need for evidence-based guidelines to inform clinical decisions in follow-up care [
30,
31]. Patients with extra nodal extension and advanced disease have the highest risk for distant metastases that are mostly found in the lung [
32]. This suggests that these patients are most likely to benefit from additional imaging toward the end of the first year after treatment, possibly via PET-CT[
33].
When choosing an imaging modality, there is strong evidence for the use of PET-CT in follow-up. The high negative predictive value around 95% is especially useful to rule out active disease in residual nodal findings after radiation therapy [
34]. Unfortunately, the high negative predictive value is contrasted by the low positive predictive value of 58.6 for primary sites and 52.1 for the neck [
33]. This requires the correlation of the findings with other modalities.
Neck ultrasound, especially when using advanced algorithms, can significantly improve the positive predictive value to 87% when differentiating neck nodes [
35].
Clinical exams including ultrasound and endoscopy remain the mainstay of follow-up exams as suggested in the German guidelines [
27]. Advanced endoscopy and modalities such as NBI (narrow band imaging) might further improve detection of early recurrences especially in the postoperative setting [
36]. The role of panendoscopy remains unclear as previously found by Muenscher et al. [
37]. That study was somewhat marred by the late onset of panendoscopy after one year when a large part of recurrences have already been diagnosed.
For our practice, we found the following conclusion from the data for early follow-up:
- -
an initial exam should be performed 3 months after completion of treatment, including evaluation of symptoms, flexible videoendoscopy, and ultrasound. We strongly suggest panendoscopy if there is any uncertainty in evaluating the primary site
- -
stage I-II disease should receive at least one initial imaging modality such as magnetic resonance imaging or cervical CT, although T1a laryngeal cancer might not need additional imaging.
- -
stage III and above should receive imaging at 3-6 months that includes the thorax and upper abdomen to detect lung and liver metastases and be offered additional imaging at least within the first two years.
- -
Stage IV disease should receive imaging at 3-6 months that includes the thorax and upper abdomen to detect lung and liver metastases, and it is strongly suggested that at least one additional imaging be provided towards the one-year mark to detect delayed asymptomatic distant metastases. Later imaging should be considered depending on comorbidities and patient compliance.
We find little conclusive evidence from our data to suggest the use of standardized screening for distant metastases besides the thorax and upper abdomen. Besides skin involvement, we only found three patients with bone metastases and two patients with liver metastases. Three of four had multiple metastases in other areas as well. All but one were covered by imaging the neck and thorax to upper abdomen.
After the initial follow-up phase, the evidence on the frequency of exams is much less clear. The low likelihood of detection must be weighed against the financial toxicity of repeated visits. This is especially true for more rural, low-income areas. Our area includes islands that often necessitate a drive of several hours to clinics and additional travel for outpatient CT or MRI exams.
Measures such as patient-reported outcome measures might play a significantly more important role in this phase of follow-up [
38]. This must be weighed against the low rate of self-presentation we found in our data as seen in
Figure 3. Further research into ways of better recognize high-risk disease might focus resources on these patients. This could be achieved through more advanced pathological risk stratification such as the use of RNA-based subtyping [
39], but more significantly by including ctDNA-based surveillance. Studies such as LIONESS have successfully used ctDNA as a means of predicting recurrence even late during treatment, thought reimbursement for these methods depends on larger studies in the future [
40].
All the aforementioned sub stratification does not help with second primary disease. Our data finds multiple late recurrences with aggressive presentation that were classified as recurrences, though their presentation makes a second primary likely. Previous data suggests that many of these might be second primary tumors [
41]. This is also relevant for distant metastases, that might be second primary cancers in up to a third of cases [
42].
5. Conclusions
Our cohort confirms the focus on structured initial cancer follow-up in HNSCC. Though we do not achieve significance, we find that an initial multimodal exam after treatment conclusion was well tolerated and showed a trend toward earlier diagnosis.
We confirm previous publications that find a high rate of secondary cancers significantly affecting OS in this patient collective. Late follow-up after more than two years remains a challenging topic that might be improved by ctDNA and other methods of risk stratification in the future, though second primaries might be more common that currently thought.
Author Contributions
Conceptualization, M.B. and B.L., data curation, P.D. and M.B., writing M.B. and P.D., review and editing F.I., C-J. B. and M.B., statistical analysis B.L., visualization P.D., B.L. and M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of the Greifswald University Medicine (protocol code BB 126/20, in May of 2020).
Informed Consent Statement
Only patients who agreed to be listed in the national cancer registry were included though no additional informed consent was obtained to avoid selection bias after explicit consent by the Ethics Committee.
Data Availability Statement
Primary data is not available to protect patient anonymity.
Acknowledgments
Thanks our colleagues and cancer center staff for their support.
Conflicts of Interest
The authors declare no conflicts of interest pertaining to the contents of this article.
Abbreviations
The following abbreviations are used in this manuscript:
| MDPI |
Multidisciplinary Digital Publishing Institute |
| DOAJ |
Directory of open access journals |
| HNSCC |
Head and Neck Squamous Cell Carcinoma |
| SF |
Standard follow-up – the control group |
| AF |
Adapted follow-up – the intervention group |
| UICC |
Union for International Cancer Control |
| BZgA |
Bundeszentrale für Gesundheitliche Aufklärung |
| DGK |
Deutsche Krebsgesellschaft |
| SEER |
Surveillance, Epidemiology, and End Results |
| NCCN |
National Comprehensive Cancer Network |
| PET-CT |
Positron Emission Tomography - Computer Tomography |
| CT |
Computer Tomography |
| MRI |
Magnetic Resonance Imaging |
| ctDNA |
Circulating tumor DNA |
References
- Abgral, R.; Querellou, S.; Potard, G.; Roux, P.Y.L.; Duc-Pennec, A.L.; Marianovski, R.; Pradier, O.; Bizais, Y.; Kraeber-Bodere, F.; Salaun, P.Y. Does 18F-FDG PET/CT Improve the Detection of Posttreatment Recurrence of Head and Neck Squamous Cell Carcinoma in Patients Negative for Disease on Clinical Follow-Up? J. Nucl. Med. 2009, 50, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Sahu, N.; Grandis, J.R. New Advances in Molecular Approaches to Head and Neck Squamous Cell Carcinoma. Anticancer Drugs 2011, 22, 656–664. [Google Scholar] [CrossRef]
- Rettig, E.M.; D’Souza, G. Epidemiology of Head and Neck Cancer. Surg Oncol Clin N Am 2015, 24, 379–396. [Google Scholar] [CrossRef]
- Robert-Koch-Institut Krebs in Deutschland Für 2017/2018. 2021.
- Hashim, D.; Genden, E.; Posner, M.; Hashibe, M.; Boffetta, P. Head and Neck Cancer Prevention: From Primary Prevention to Impact of Clinicians on Reducing Burden. Ann Oncol 2019, 30, 744–756. [Google Scholar] [CrossRef]
- D’Souza, G.; Kreimer, A.R.; Viscidi, R.; Pawlita, M.; Fakhry, C.; Koch, W.M.; Westra, W.H.; Gillison, M.L. Case-Control Study of Human Papillomavirus and Oropharyngeal Cancer. N Engl J Med 2007, 356, 1944–1956. [Google Scholar] [CrossRef]
- Gillison, M.L.; Shah, K.V. Human Papillomavirus-Associated Head and Neck Squamous Cell Carcinoma: Mounting Evidence for an Etiologic Role for Human Papillomavirus in a Subset of Head and Neck Cancers. Curr Opin Oncol 2001, 13, 183–188. [Google Scholar] [CrossRef]
- Tran, N.; Rose, B.R.; O’Brien, C.J. Role of Human Papillomavirus in the Etiology of Head and Neck Cancer. Head Neck 2007, 29, 64–70. [Google Scholar] [CrossRef]
- Kreimer, A.R.; Clifford, G.M.; Boyle, P.; Franceschi, S. Human Papillomavirus Types in Head and Neck Squamous Cell Carcinomas Worldwide: A Systematic Review. Cancer Epidemiol Biomarkers Prev 2005, 14, 467–475. [Google Scholar] [CrossRef]
- Langevin, S.M.; O’Sullivan, M.H.; Valerio, J.L.; Pawlita, M.; Applebaum, K.M.; Eliot, M.; McClean, M.D.; Kelsey, K.T. Occupational Asbestos Exposure Is Associated with Pharyngeal Squamous Cell Carcinoma in Men from the Greater Boston Area. Occup Environ Med 2013, 70, 858–863. [Google Scholar] [CrossRef]
- Harth, V.; Schafer, M.; Abel, J.; Maintz, L.; Neuhaus, T.; Besuden, M.; Primke, R.; Wilkesmann, A.; Thier, R.; Vetter, H.; et al. Head and Neck Squamous-Cell Cancer and Its Association with Polymorphic Enzymes of Xenobiotic Metabolism and Repair. J Toxicol Environ Health A 2008, 71, 887–897. [Google Scholar] [CrossRef]
- Brocklehurst, P.; Kujan, O.; O’Malley, L.A.; Ogden, G.; Shepherd, S.; Glenny, A.M. Screening Programmes for the Early Detection and Prevention of Oral Cancer. Cochrane Database Syst Rev 2013, 2013, CD004150. [Google Scholar] [CrossRef]
- Chang, J.H.; Wu, C.C.; Yuan, K.S.; Wu, A.T.H.; Wu, S.Y. Locoregionally Recurrent Head and Neck Squamous Cell Carcinoma: Incidence, Survival, Prognostic Factors, and Treatment Outcomes. Oncotarget 2017, 8, 55600–55612. [Google Scholar] [CrossRef]
- Leemans, C.R.; Tiwari, R.; Nauta, J.J.P.; Waal, I.V.D.; Snow, G.B. Recurrence at the Primary Site in Head and Neck Cancer and the Significance of Neck Lymph Node Metastases as a Prognostic Factor. Cancer 1994, 73, 187–190. [Google Scholar] [CrossRef]
- Stell, P.M. Time to Recurrence of Squamous Cell Carcinoma of the Head and Neck. Head Neck 1991, 13, 277–281. [Google Scholar] [CrossRef]
- Flynn, C.J.; Khaouam, N.; Gardner, S.; Higgins, K.; Enepekides, D.; Balogh, J.; MacKenzie, R.; Singh, S.; Davidson, J.; Poon, I. The Value of Periodic Follow-up in the Detection of Recurrences after Radical Treatment in Locally Advanced Head and Neck Cancer. Clin. Oncol. 2010, 22, 868–873. [Google Scholar] [CrossRef]
- Boysen, M.; Lövdal, O.; Winther, F.; Tausjö, J. The Value of Follow-up in Patients Treated for Squamous Cell Carcinoma of the Head and Neck. European Journal of Cancer 1992, 28, 426–430. [Google Scholar] [CrossRef]
- Leitlinienprogramm-Onkologie S3-Leitlinie Diagnostik, Therapie Und Nachsorge Des Larynxkarzinoms. 2019.
- Leitlinienprogramm-Onkologie S3-Leitlinie Mundhöhlenkarzinom. 2021.
- Koyfman, S.A.; Ismaila, N.; Crook, D.; D’Cruz, A.; Rodriguez, C.P.; Sher, D.J.; Silbermins, D.; Sturgis, E.M.; Tsue, T.T.; Weiss, J.; et al. Management of the Neck in Squamous Cell Carcinoma of the Oral Cavity and Oropharynx: ASCO Clinical Practice Guideline. J Clin Oncol 2019, 37, 1753–1774. [Google Scholar] [CrossRef] [PubMed]
- Nekhlyudov, L.; Lacchetti, C.; Davis, N.B.; Garvey, T.Q.; Goldstein, D.P.; Nunnink, J.C.; Ninfea, J.I.R.; Salner, A.L.; Salz, T.; Siu, L.L. Head and Neck Cancer Survivorship Care Guideline: American Society of Clinical Oncology Clinical Practice Guideline Endorsement of the American Cancer Society Guideline. J Clin Oncol 2017, 35, 1606–1621. [Google Scholar] [CrossRef]
- Planey, A.M.; Spees, L.P.; Biddell, C.B.; Waters, A.; Jones, E.P.; Hecht, H.K.; Rosenstein, D.; Wheeler, S.B. The Intersection of Travel Burdens and Financial Hardship in Cancer Care: A Scoping Review. JNCI Cancer Spectr. 2024, 8, pkae093. [Google Scholar] [CrossRef] [PubMed]
- Baxi, S.S.; Pinheiro, L.C.; Patil, S.M.; Pfister, D.G.; Oeffinger, K.C.; Elkin, E.B. Causes of Death in Long-term Survivors of Head and Neck Cancer. Cancer 2014, 120, 1507–1513. [Google Scholar] [CrossRef]
- Coca-Pelaz, A.; Rodrigo, J.P.; Suárez, C.; Nixon, I.J.; Mäkitie, A.; Sanabria, A.; Quer, M.; Strojan, P.; Bradford, C.R.; Kowalski, L.P.; et al. The Risk of Second Primary Tumors in Head and Neck Cancer: A Systematic Review. Head Neck 2020, 42, 456–466. [Google Scholar] [CrossRef]
- Beswick, D.M.; Gooding, W.E.; Johnson, J.T.; Branstetter, B.F. Temporal Patterns of Head and Neck Squamous Cell Carcinoma Recurrence with Positron-emission Tomography/Computed Tomography Monitoring. Laryngoscope 2012, 122, 1512–1517. [Google Scholar] [CrossRef] [PubMed]
- Haring, C.T.; Kana, L.A.; Dermody, S.M.; Brummel, C.; McHugh, J.B.; Casper, K.A.; Chinn, S.B.; Malloy, K.M.; Mierzwa, M.; Prince, M.E.P.; et al. Patterns of Recurrence in Head and Neck Squamous Cell Carcinoma to Inform Personalized Surveillance Protocols. Cancer 2023, 129, 2817–2827. [Google Scholar] [CrossRef] [PubMed]
- Leitlinienprogramm-Onkologie S3-Leitlinie Diagnostik, Therapie, Prävention Und Nachsorge Des Oro- Und Hypopharynxkarzinoms Leitlinie (Langversion). 2024.
- Schmitt, J.; Klinkhammer-Schalke, M.; Bierbaum, V.; Gerken, M.; Bobeth, C.; Rößler, M.; Dröge, P.; Ruhnke, T.; Günster, C.; Tol, K.K.; et al. Initial Cancer Treatment in Certified Versus Non-Certified Hospitals. Dtsch. Ärzteblatt Int. 2023, 120, 647–654. [Google Scholar] [CrossRef]
- Daga, D.; Mishra, A.; Sharma, S.S.; Rai, A.K.; Valsareddy, S.K.; Singh, U.; Chattopadhyay, U.; Prakash, G. Safe Delivery of Surgical Care in Head and Neck Cancer Patients During COVID-19—An Audit of Pattern of Presentation and Treatment Strategies in an Oncology Centre in the Northern India. Indian J. Surg. Oncol. 2021, 12, 250–256. [Google Scholar] [CrossRef]
- Kwiecien, C.; Workman, A.D.; Wilensky, J.; Lerner, D.K.; Rathi, V.K.; Douglas, J.E.; Kohanski, M.A.; Kuan, E.C.; Palmer, J.N.; Adappa, N.D. Longer-term Surveillance Imaging and Endoscopy Critical for Majority of Patients in Detection of Sinonasal Malignancy Recurrence. Int. Forum Allergy Rhinol. 2024, 14, 1739–1745. [Google Scholar] [CrossRef]
- Roman, B.R.; Patel, S.G.; Wang, M.B.; Pou, A.M.; Holsinger, F.C.; Myssiorek, D.; Goldenberg, D.; Swisher-McClure, S.; Lin, A.; Shah, J.P.; et al. Guideline Familiarity Predicts Variation in Self-Reported Use of Routine Surveillance PET/CT by Physicians Who Treat Head and Neck Cancer. J. Natl. Compr. Cancer Netw. 2015, 13, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, A.M.; Petruzzelli, G.J.; Clark, J.; Emami, B. Patterns of Spread in Recurrent Head and Neck Squamous Cell Carcinoma. Otolaryngol.—Head Neck Surg. 2001, 125, 393–396. [Google Scholar] [CrossRef]
- Gupta, T.; Master, Z.; Kannan, S.; Agarwal, J.P.; Ghsoh-Laskar, S.; Rangarajan, V.; Murthy, V.; Budrukkar, A. Diagnostic Performance of Post-Treatment FDG PET or FDG PET/CT Imaging in Head and Neck Cancer: A Systematic Review and Meta-Analysis. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 2083. [Google Scholar] [CrossRef]
- Mehanna, H.; Wong, W.-L.; McConkey, C.C.; Rahman, J.K.; Robinson, M.; Hartley, A.G.J.; Nutting, C.; Powell, N.; Al-Booz, H.; Robinson, M.; et al. PET-CT Surveillance versus Neck Dissection in Advanced Head and Neck Cancer. N. Engl. J. Med. 2016, 374, 1444–1454. [Google Scholar] [CrossRef] [PubMed]
- Wakonig, K.M.; Dommerich, S.; Fischer, T.; Arens, P.; Hamm, B.; Olze, H.; Lerchbaumer, M.H. The Diagnostic Performance of Multiparametric Ultrasound in the Qualitative Assessment of Inconclusive Cervical Lymph Nodes. Cancers 2023, 15, 5035. [Google Scholar] [CrossRef]
- Davaris, N.; Voigt-Zimmermann, S.; Kropf, S.; Arens, C. Flexible Transnasal Endoscopy with White Light or Narrow Band Imaging for the Diagnosis of Laryngeal Malignancy: Diagnostic Value, Observer Variability and Influence of Previous Laryngeal Surgery. Eur. Arch. Oto-Rhino-Laryngol. 2018, 276, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Muenscher, A.; Sehner, S.; Taleh, J.; Tribius, S.; Dalchow, C.; Moeckelmann, N.; Gulati, A.; Clauditz, T.; Knecht, R. Significance of Panendoscopy and CT in the Follow-up and Management of Squamous Cell Carcinoma of the Head and Neck: A Retrospective Clinical Assessment. J. Clin. Oncol. 2013, 31, e17003. [Google Scholar] [CrossRef]
- Hoe, S.V.; Hermans, R. Post-Treatment Surveillance Imaging in Head and Neck Cancer: A Systematic Review. Insights Imaging 2024, 15, 32. [Google Scholar] [CrossRef]
- Hess, J.; Unger, K.; Maihoefer, C.; Schüttrumpf, L.; Wintergerst, L.; Heider, T.; Weber, P.; Marschner, S.; Braselmann, H.; Samaga, D.; et al. A Five-MicroRNA Signature Predicts Survival and Disease Control of Patients with Head and Neck Cancer Negative for HPV Infection. Clin. Cancer Res. 2019, 25, 1505–1516. [Google Scholar] [CrossRef]
- Flach, S.; Howarth, K.; Hackinger, S.; Pipinikas, C.; Ellis, P.; McLay, K.; Marsico, G.; Walz, C.; Reichel, C.A.; Gires, O.; et al. Liquid Biopsy for Minimal Residual Disease Detection in Head and Neck Squamous Cell Carcinoma (LIONESS): A Personalized Cell-Free Tumor DNA Analysis for Patients with HNSCC. J. Clin. Oncol. 2022, 40, 6017. [Google Scholar] [CrossRef]
- Roest, R.H. de; Mes, S.W.; Poell, J.B.; Brink, A.; Wiel, M.A. van de; Bloemena, E.; Thai, E.; Poli, T.; Leemans, C.R.; Brakenhoff, R.H. Molecular Characterization of Locally Relapsed Head and Neck Cancer after Concomitant Chemoradiotherapy. Clin. Cancer Res. 2019, 25, 7256–7265. [Google Scholar] [CrossRef] [PubMed]
- Kjems, J.; Lilja-Fischer, J.K.; Friborg, J.; Tramm, T.; Overgaard, J. Separating Distant Recurrences from Second Primaries in Head and Neck Squamous Cell Carcinomas—A DAHANCA Group Analysis on Paired Tumor Samples. Head Neck 2024, 46, 2532–2539. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).