1. Introduction
Spinal arachnoid cysts (SACs) are rare lesions, accounting for 1-2% of all spinal tumors [
1]. The majority are in the extradural space (EDACs), while only 10% of all arachnoid cysts are intradural (IDACs) [
2] SACs can occur in both pediatric and adult patients, most commonly presenting between the fifth and sixth decades of life, with significant variation in their distribution among gender [
3]. In most cases, spinal cysts are considered idiopathic, as no underlying cause can be identified. However, SACs may sometimes develop secondary to an arachnoiditis phenomenon caused by spinal trauma, inflammation, infection, or as a result of medical and surgical interventions [
4]. These cysts are most frequently located at the thoracic spine although they can potentially develop at any level from extradural to intradural or intramedullary [
5,
6,
7]. Since they are space-occupying lesions with a compressive effect on the spinal column, SACs can cause neurological dysfunction and myelopathy, often requiring surgical intervention; therapeutic management becomes particularly challenging when arachnoiditis is involved. The rarity of these cases limits a comprehensive understanding of their underlying mechanisms, resulting in a lack of consensus on the optimal treatment strategy. This study aims to explore the effectiveness and outcomes of the neurosurgical management in patients with SACs treated at our Institution.
2. Materials and Methods
Adult patients who underwent surgical treatment at our Neurosurgery Department for SACs between January 2020 and December 2023 were included in this observational, single-center, retrospective study: clinical onset, imaging, surgical technique, and neurological long-term status were analyzed. The applied Inclusion and Exclusion criteria are listed in
Table 1
The study was conducted in accordance with the Declaration of Helsinki and Ethical approval was waived by our local ethics committee in view of the retrospective nature of the study and all the procedures being performed were part of the routine care.
Data Collection
Patient demographics, medical history (e.g., previous spinal trauma, infections, or surgeries), clinical onset, pre- and post-operative neurological evaluations (Karnofsky Performance Status -KPS- and American Spinal Injury Association Impairment Scale-AIS-scores), surgical details, and post-operative complications were obtained from medical records and outpatient reports. Radiological imaging, including mandatory pre- and post-operative MRIs, were collected to assess lesions’ reduction or resolution, recurrence, and the occurrence of surgical complications: the longitudinal and transverse diameters of the SAC were measured on T2-weighted sagittal MRI images. In all cases, the diagnosis was confirmed through histological examination after intraoperative biopsy.
3. Results
3.1. Patients overview
Our retrospective series, based on the application of inclusion and exclusion criteria, was composed of five patients. The age of the patients at the time of diagnosis ranged from 43 to 68 years, with an average age of 53.4 years. The group consisted of 3 male and 2 female patients. Etiology was distributed as follows: the most common (60%) was post-operative (3 patients had undergone prior spinal surgeries), 20% was post-traumatic (1 patient had suffered a burst fracture of L1 treated with lumbar arthrodesis) and another 20% was infectious (1 patient had a history of an epidural abscess). The most common symptoms described were paresthesia, gait disturbances, and back pain; in addition, neurogenic bowel or bladder (n=2), limb weakness (n=2), paraplegia (n=1), and sexual dysfunction (n=1) occurred (see
Table 2).
3.2. Radiological Data
In most cases, the lesion was located at the thoracic level (n=3); one case at the cervical spine and another one at the thoracolumbar junction. In all patients, the pathology involved more than one vertebral level, ranging from 2 to 4 segments. Among these cysts, 3 were intradural and 2 were intramedullary.
3.3. Surgical Details and Postoperative Outcomes
The majority of patients underwent surgical resection of the cystic formation combined with adhesiolysis (n=4), while in one patient, the procedure included opening the syrinx cavity following lysis of adherences. The symptoms that most frequently improved after surgery were limb weakness (n=3), gait ataxia (n=2), and neurogenic bladder (n=2). In contrast, only one patient each experienced improvement in paresthesia or dysesthesia and lower back pain. The average preoperative and postoperative KPS scores were 70% and 82%, respectively, reflecting an overall improvement in health status in all patients except one. The American Spinal Injury Association Impairment Scale (AIS) level remained unchanged from preoperative to postoperative in all cases except for two patients, whose AIS level improved (from B to C and from C preoperatively to D postoperatively). Surgical complications included cerebrospinal fluid fistulas (n=2), which were resolved surgically, and one infectious complication. Additionally, four patients out of five experienced disease recurrence, necessitating further surgical re-interventions (see
Table 2).
3.4. Illustrative Cases
3.4.1. Case 1
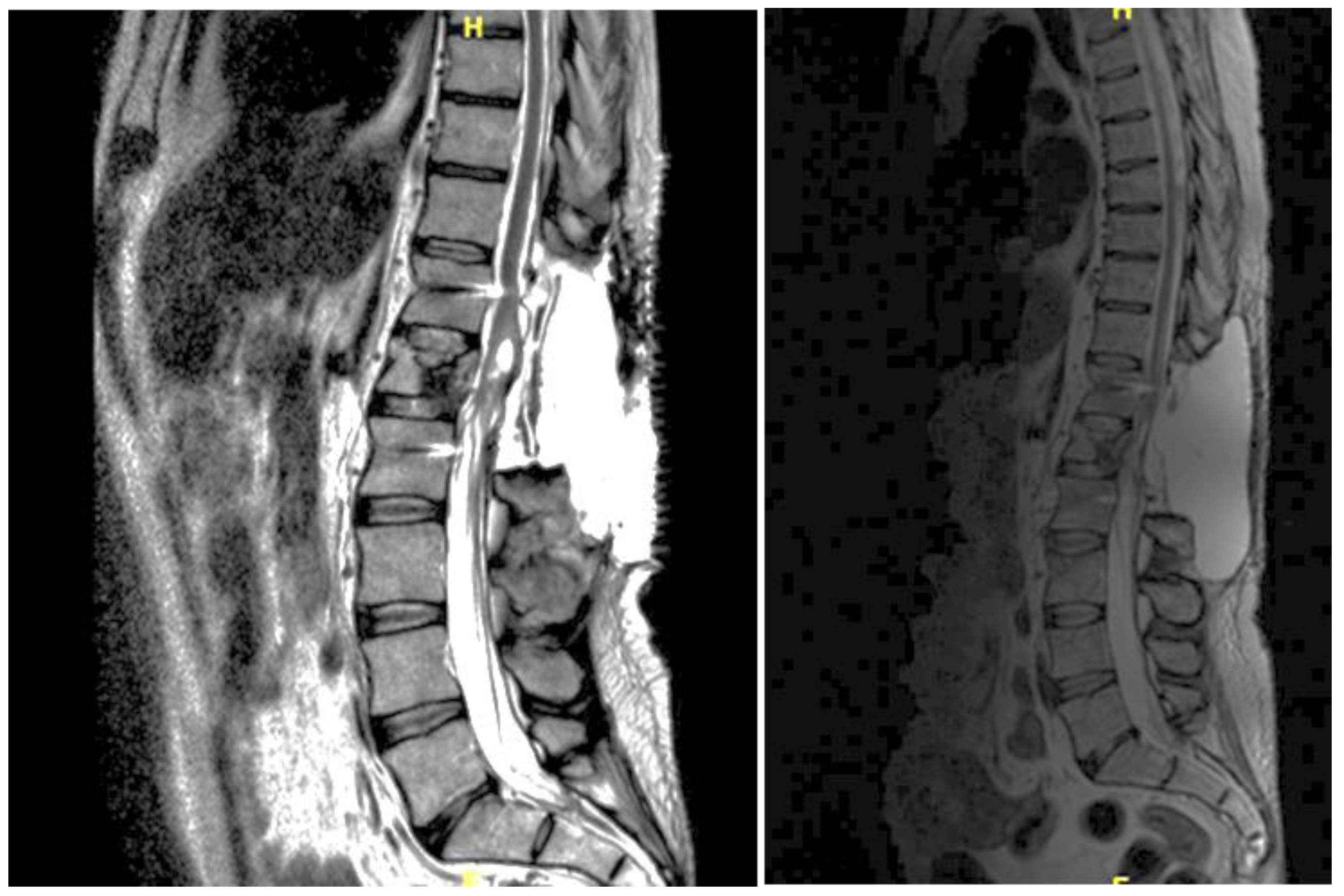
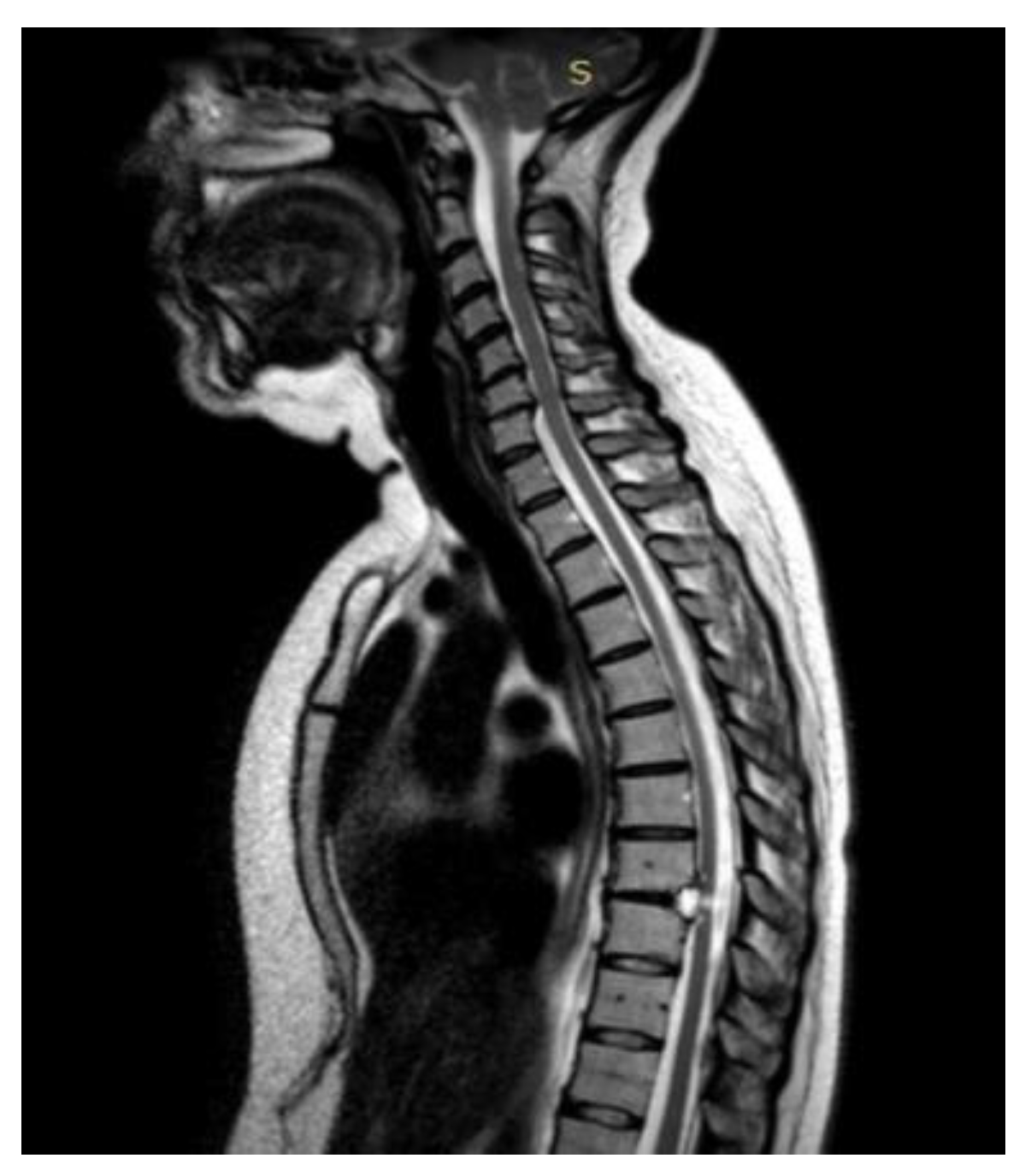
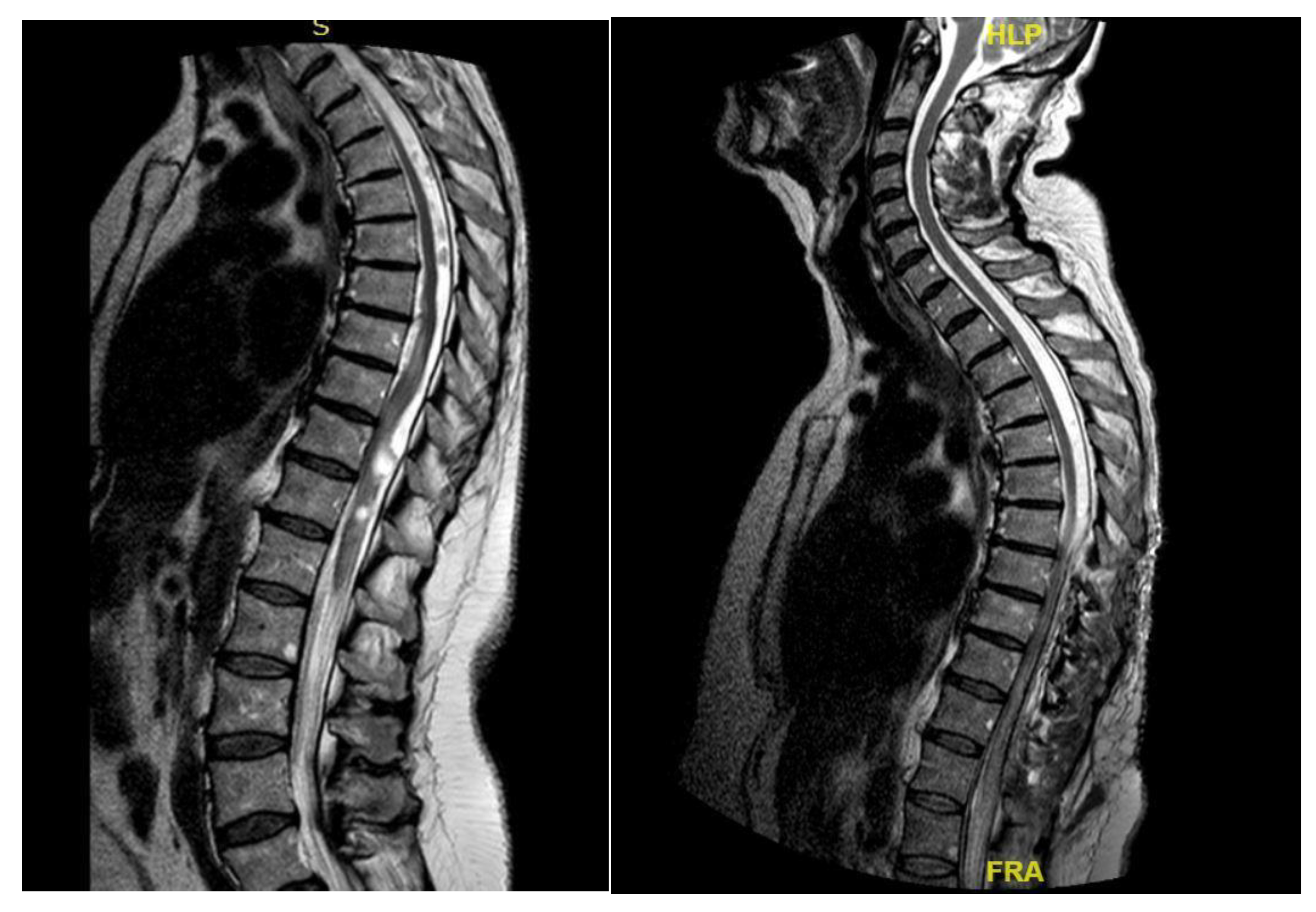
A 46-year-old man with a history of post traumatic burst fracture of L1 in a car accident, treated with lumbar fusion at D12-L2, developed seven months later lower limb weakness, sensory loss, and bladder and bowel dysfunction [
Figure 1]. MRI revealed syringomyelia at D12-L2 compressing the conus medullaris. Initial surgery involved syrinx decompression through myelotomy, leading to significant symptom improvement. However, three months later, his symptoms recurred with an increased syrinx size. A second surgery introduced a syringo-subarachnoid shunt and removed scar adhesions, with temporary improvement although a post operative cerebrospinal fluid fistula occured. He underwent a third surgery with a syringo-peritoneal shunt, resolving the syrinx. Post-operatively, a follow-up MRI showed a significant reduction in the syrinx size and improvement in myelopathy: the patient’s neurological status improved, with gradual recovery of bladder and bowel functions.
3.4.2. Case 2
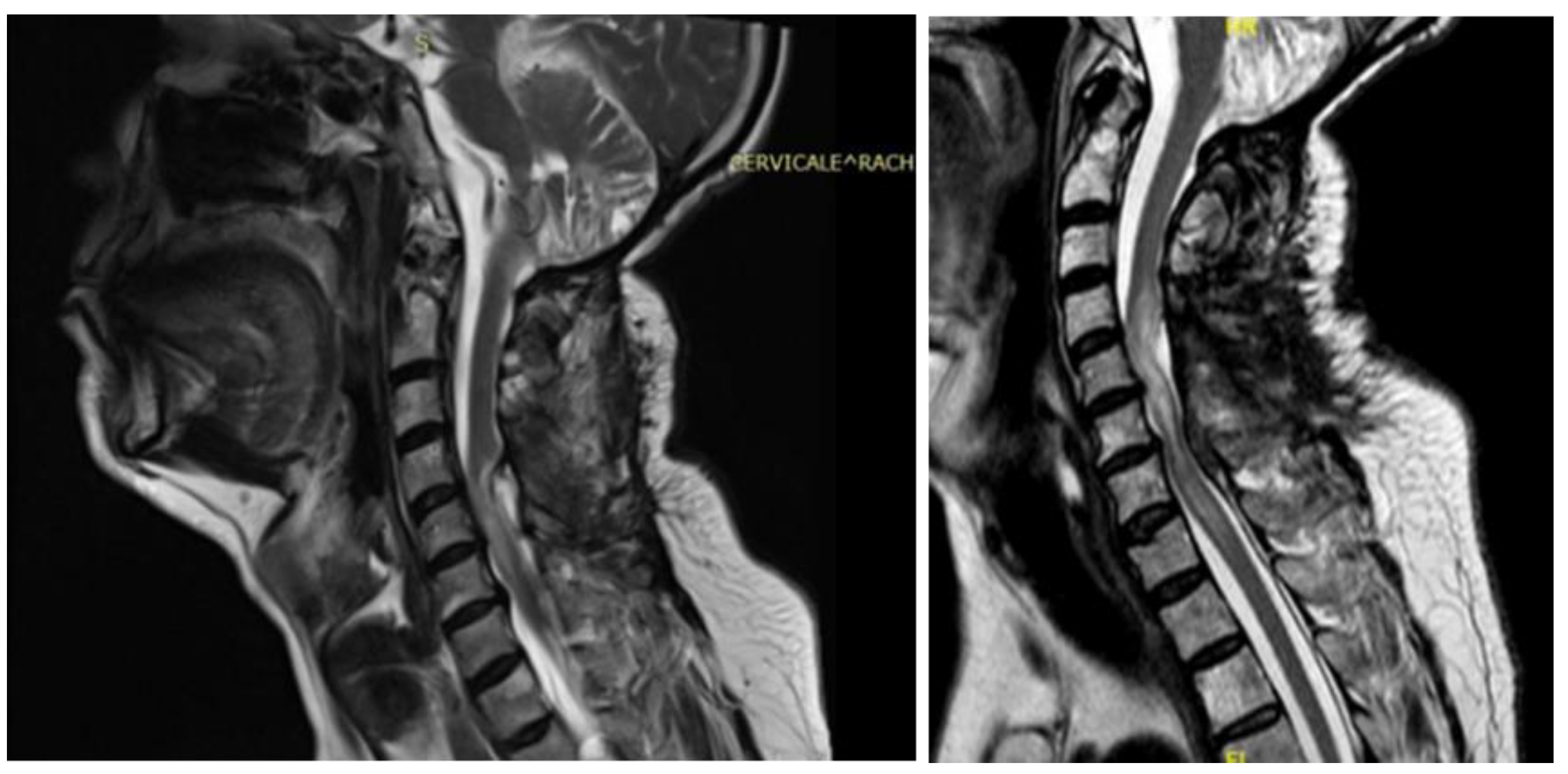
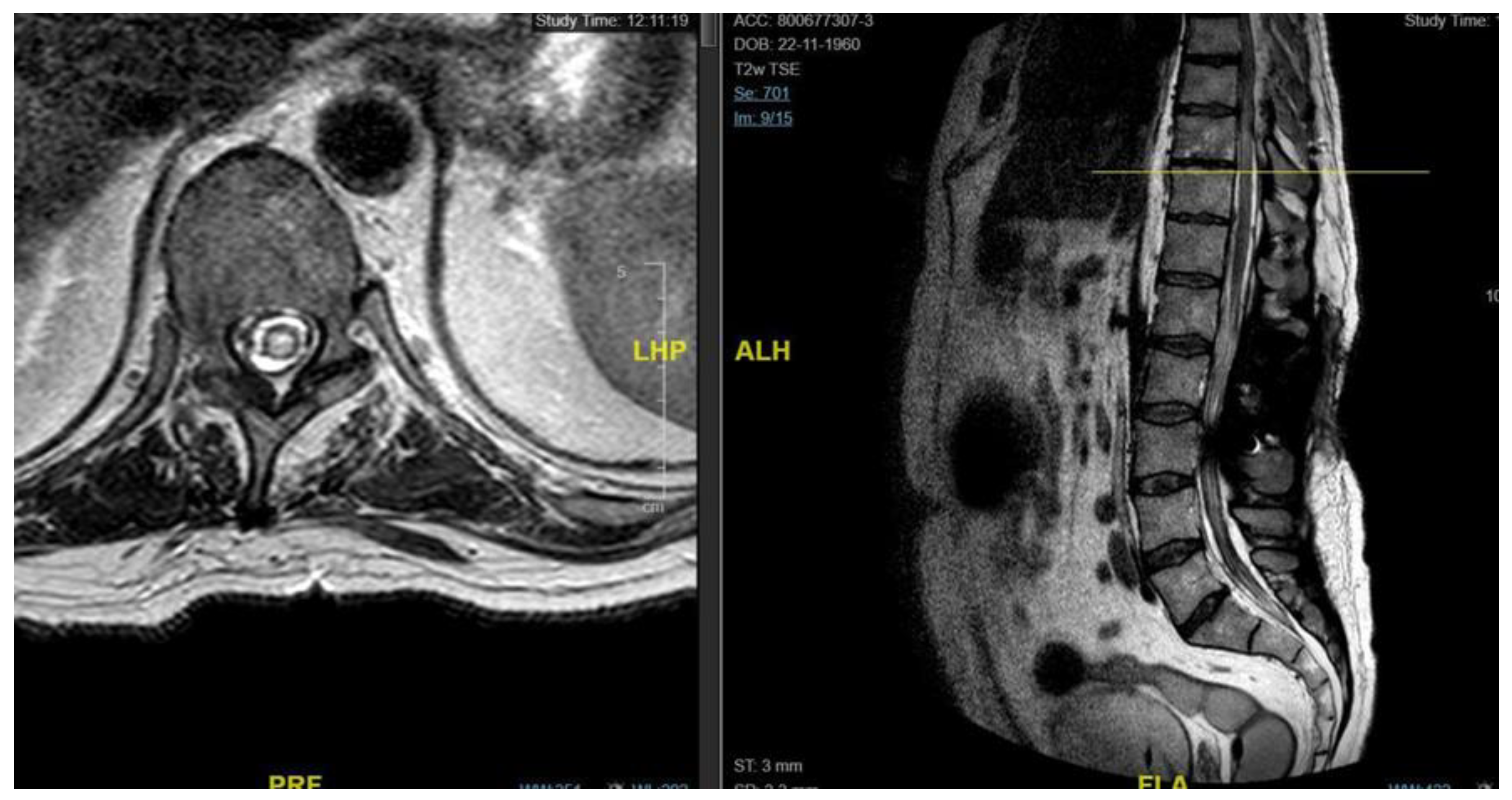
A 48-year-old man with a history of multiple neurosurgical interventions since his childhood presented with left upper limb weakness and gait worsening seven months after the removal of a cervical spinal catheter previously inserted (Torkildsen shunt). Over the next five months, his condition deteriorated, resulting in an inability to walk independently. Neurological examination revealed left upper limb weakness and spastic paraparesis in the lower limbs. The MRI of the cervico-dorsal spine showed myelopathy at C5-C6 level with a dorsal cystic lesion [
Figure 2]. Surgery was performed to remove the cyst and perform the lysis of arachnoid adherence. A left-sided hemilaminectomy (C5-C7) exposed and excised the cyst compressing the spinal cord. Postoperatively, the patient initially improved but later developed tetraparesis. The MRI performed two months later showed persistent cord swelling and myelopathy extending to D1-D2 [
Figure 3]. A second surgery included decompressive laminectomy (C6-C7) and removal of intradural adhesions. Despite these interventions, the patient’s tetraparesis remained stable with slight upper limb improvement. Follow-up MRI revealed ongoing signal changes from C3-C4 to D4-D5. A third surgery revealed intraoperatively new arachnoid adhesions and included an exploratory biopsy. Despite no further neurological decline, MRI continued to show persistent intramedullary signal abnormalities at three-year follow-up.
3.4.3. Case 3
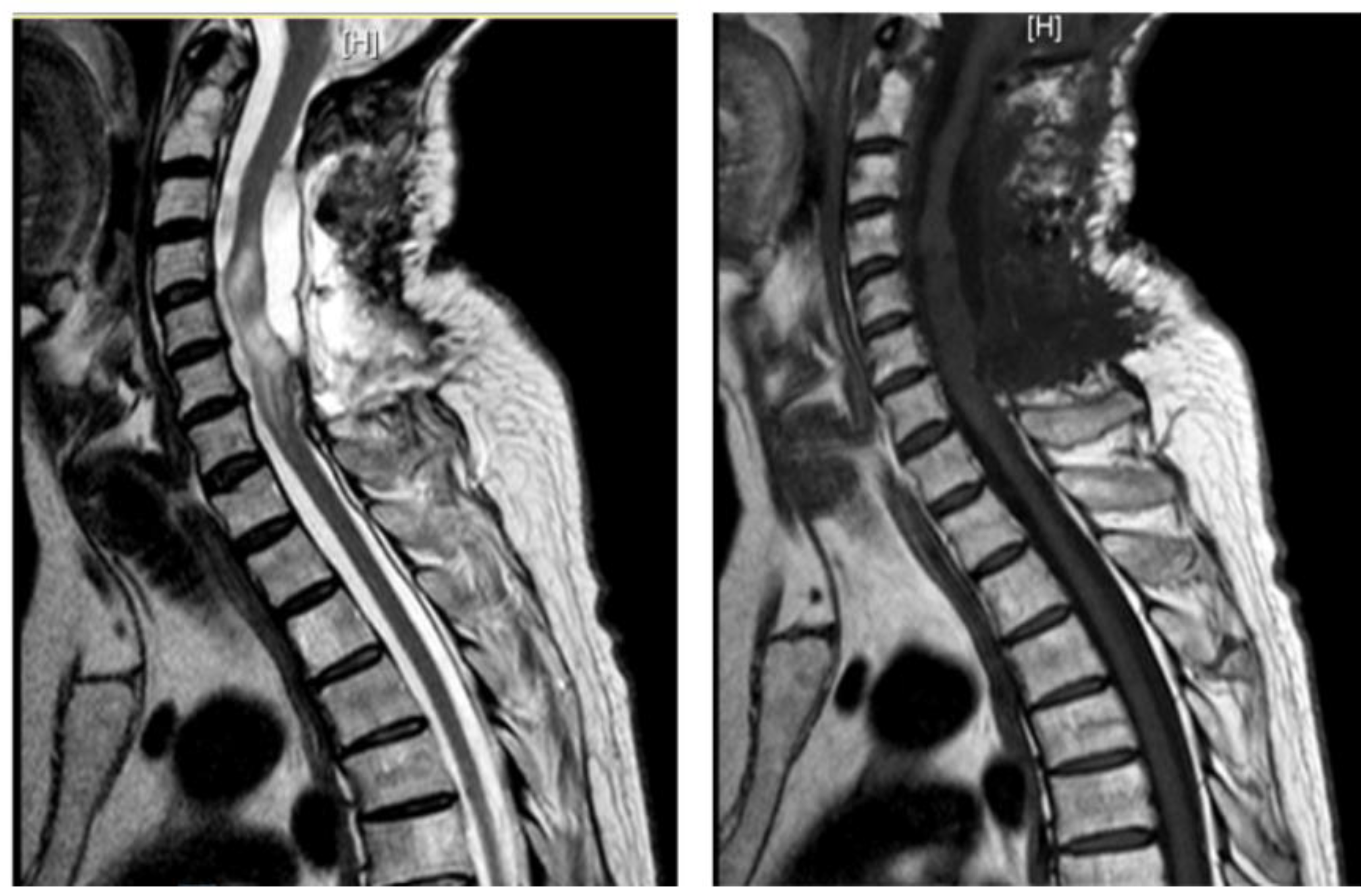
A 62-year-old man, 10 months after surgery for the removal of a spinal cavernoma, developed recurrent left-sided lumbosciatica, paresthesia, hypoesthesia, and an episode of acute urinary retention. Neurological examination revealed weakness in the left lower limb, mild hyperreflexia, and neurogenic bladder. MRI showed an intradural-extramedullary cyst from D3 to D8 and an extradural component from D9 to D11, compressing the spinal cord and causing myelopathy [
Figure 4]. The patient underwent a left hemilaminectomy (D6-D10) for cyst removal. Histology confirmed a spinal arachnoid cyst. Postoperatively, the patient remained neurologically stable, the post-operative MRI showed the absence of the anterior cystic collection at the dorsal spine, with only a slight reduction in the area of myelopathy and persistent central spinal cord signal alteration at the D9-10 level [
Figure 5]. Eight months later, he experienced worsening of symptoms, including low back pain and paraplegia. The performed MRI revealed a cystic mass from D4 to D8. He underwent a decompressive laminectomy (D5-D10) and cyst fenestration. Postoperative MRI showed resolution of the cyst but an extension of the myelopathic area. Despite these issues, the patient did not experience new neurological deficits over 3 years of follow-up.
3.4.4. Case 4
A 43-year-old woman, following a microdiscectomy at D8-D9 and 2 years of symptom relief, developed sensory deficits and gait difficulties. Neurological evaluation revealed right lower limb weakness and an ataxic gait. Pre-operative MRI identified an intradural cyst at D8-D9 [
Figure 6]. Surgical intervention involved a right hemilaminectomy (D7-D9) to remove the cyst and resolve arachnoid adhesions. Histology confirmed an arachnoid cyst. An initial post-operative improvement was followed by symptoms worsening, including new onset of syringomyelia.
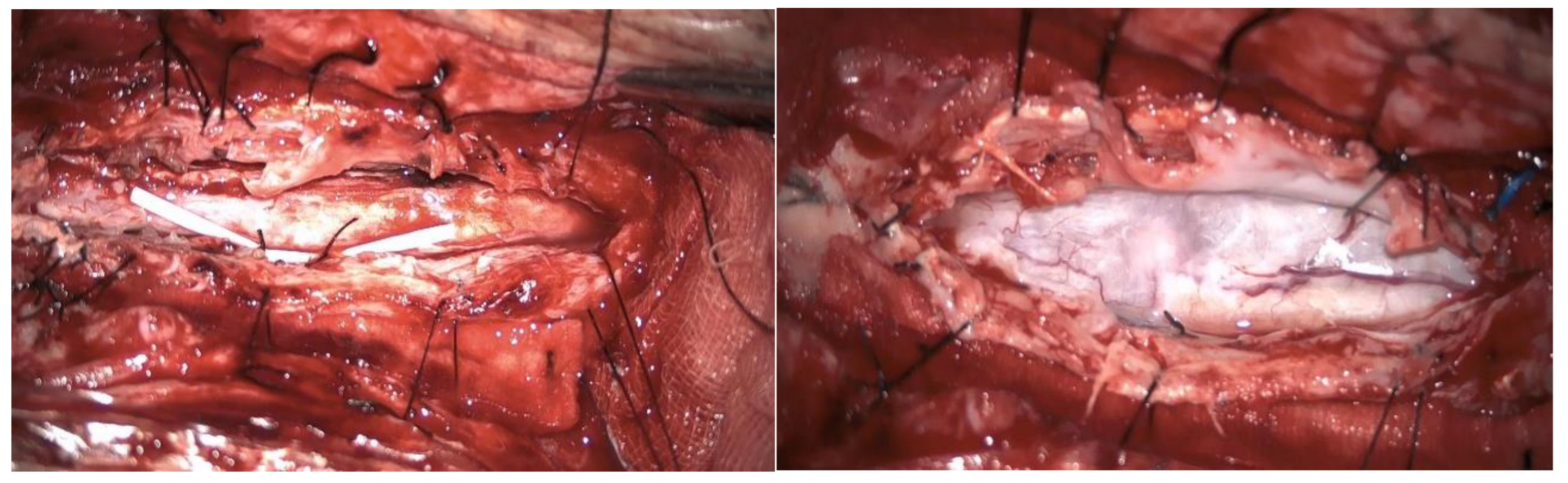
A second surgery addressed the syringomyelia with laminectomy (D7-D10) and adhesion lysis: postoperative MRI showed resolution of the cyst but persistent malacic changes. A cerebrospinal fluid fistula was treated surgically. After further deterioration and expansion of the syringomyelic cavity, a third surgery including reopening of the previous surgical incision and exposure of the laminectomy allowed the dissection of numerous scar septa both above and below the dural plane, freeing the spinal cord circumferentially. A myelotomy was performed to drain the syringomyelic cavity and establish communication with the subdural space, placing of a syringoperitoneal shunt and additional adhesion lysis [
Figure 7]. Post-operative MRI indicated reduced syringomyelic size [
Figure 8]. The patient remains clinically stable after 4 years of follow-up.
3.4.5. Case 5
A 68-year-old woman presented with progressively worsening bilateral lower limb hypoesthesia, leading to a neurosurgical evaluation about one year after symptom onset. Her medical history included an epidural abscess extending from D12 to L5, treated three years prior with targeted antibiotic therapy and CT-guided drainage.
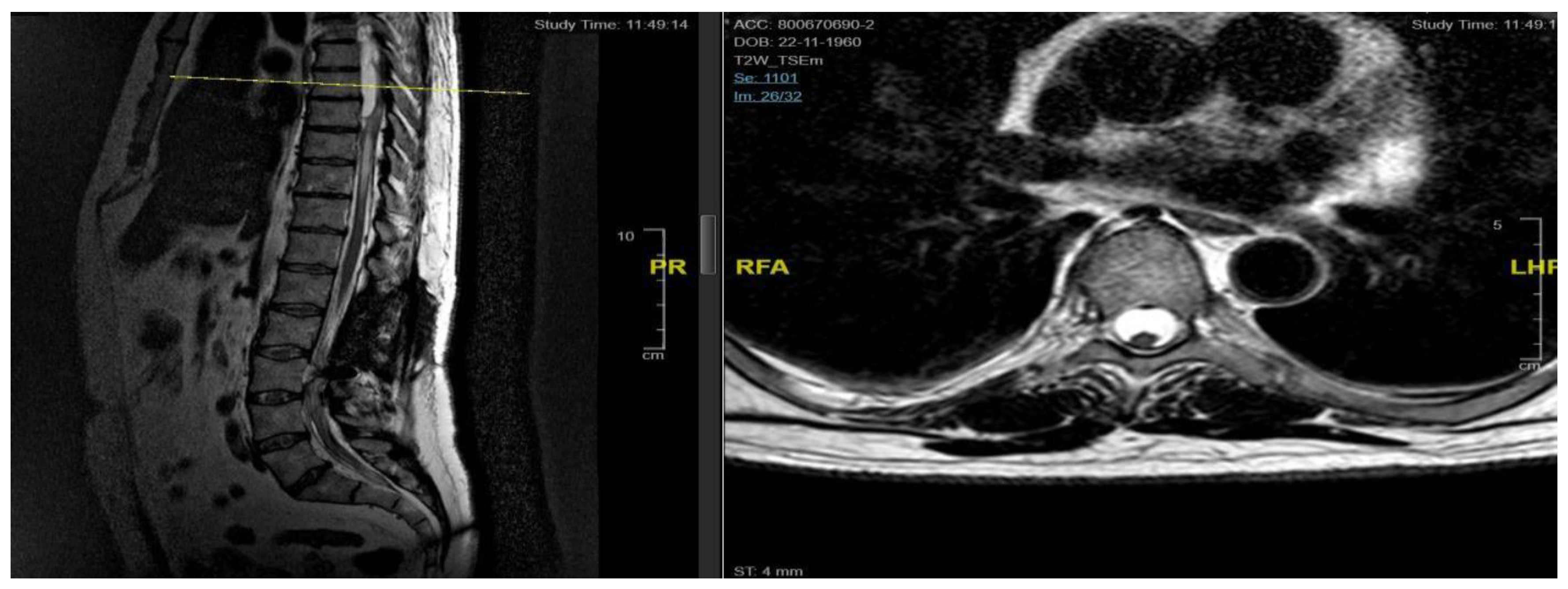
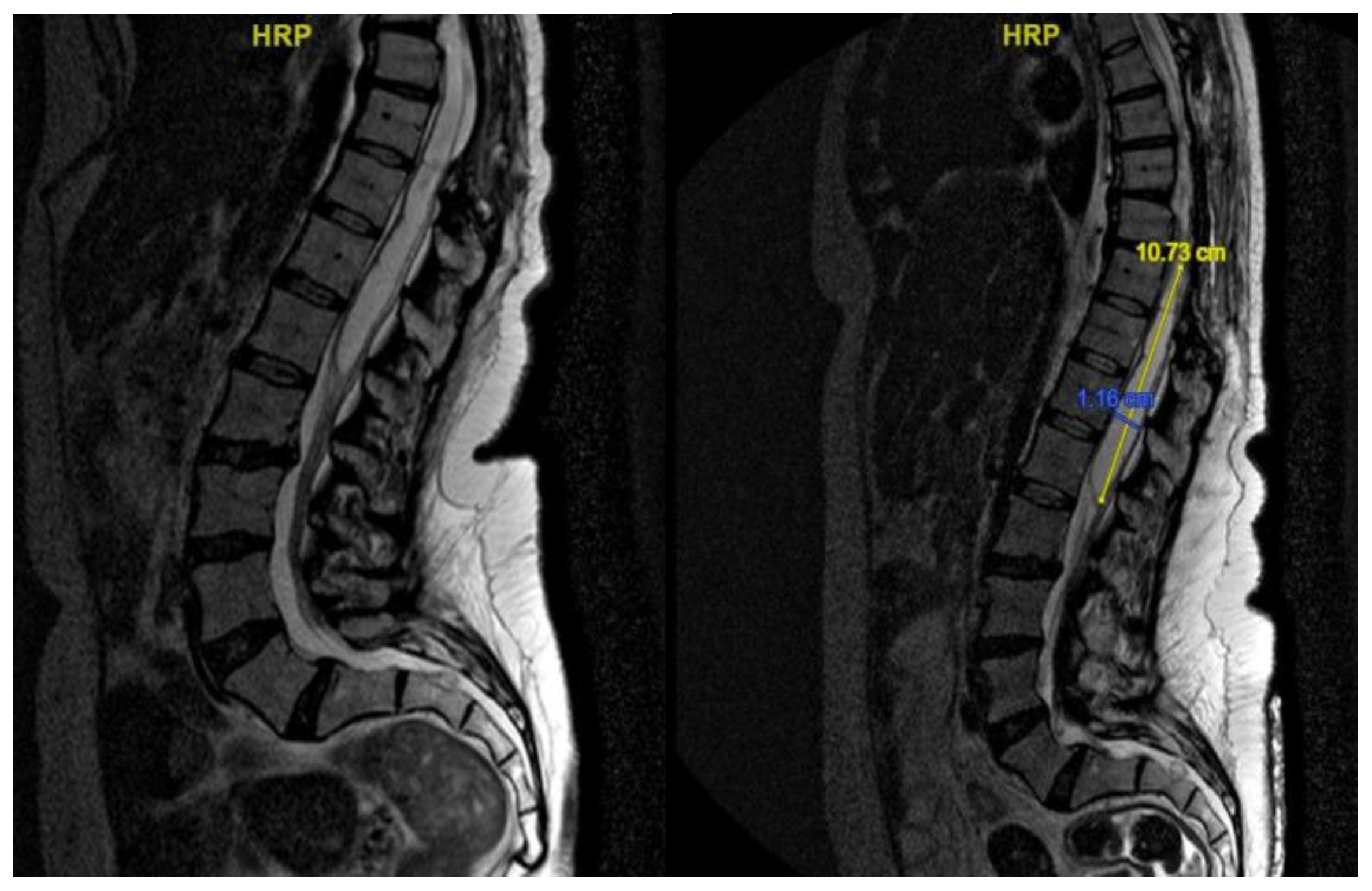
Neurological examination revealed ataxic gait and diffuse proprioceptive hypoesthesia in the lower limbs. MRI of the thoracolumbar spine with contrast identified intradural cystic collections at D8-D11, located dorsal to the spinal cord and causing signs of myelopathy [
Figure 9].
The patient underwent decompressive laminectomy using piezosurgery at the affected spinal levels to remove the cysts and release arachnoid adhesions. Intraoperative monitoring of sensory and motor evoked potentials was performed. After exposing the dural plane, a full laminectomy was carried out, revealing the cystic lesion enveloping the spinal cord and numerous septa anchoring to neural structures, obstructing normal cerebrospinal fluid flow. The cyst was fenestrated into the subarachnoid space, and the adherent perimedullary structures were dissected to restore cerebrospinal fluid circulation and initial pulsatility of the exposed neural structures. Post-operatively, the patient exhibited lower limb weakness and right lower limb dysesthesia. Post-operative MRI showed complete resolution of the cystic collections, improved spinal cord expansion, and stability of pre-existing myelopathy from D8 to the conus medullaris [
Figure 9]. Throughout the follow-up period, which was eventually interrupted by the patient, the neurological status remained stable.
4. Discussion
Spinal arachnoid cysts (SACs) account for 1-2% of all spinal tumors [
1]. Due to their rarity, most literature consists of case reports and small case series. While secondary SACs are estimated to be less than 10% of the total [
8], our study focused solely on secondary cysts to explore their relationship with arachnoiditis and assess clinical outcomes post-surgery.
SACs in adults typically appear between the fifth and sixth decades of life [
9,
10]. The analyzed cohort of patients were diagnosed between 43 and 68 years, with a mean age of 53.4 years and a typical clinical onset period, around the third or fourth decade; a male predominance was highlighted although there is a notable discrepancy in literature regarding gender prevalence [
3,
4,
5,
6,
7,
8,
9,
10].
Although extradurally located SACs are usually more common than intradural ones (10% of all SACs) [
2], our series only included intradural cysts. Two patients had syringomyelia; one case (20%) was associated with a spinal cyst, consistent with literature where 33-46% of SAC cases present with syringomyelia. The syringomyelia in one patient extended caudally from the cyst, aligning with Viswanathan et al.’s findings, while the other case had isolated syringomyelia likely due to post-traumatic arachnoiditis, matching literature indicating post-traumatic origins for syringomyelia in 4% of cases.
The most common location for SACs is the thoracic spine, with less frequent occurrences in the lumbar, lumbosacral, thoracolumbar, and sacral regions. Cervical involvement is rare [
10]. Our study described most cysts in the thoracic spine, with two cases in the dorso-lumbar and cervical regions. The higher frequency in the thoracic spine may be due to its length and narrow diameter, which exacerbate neurological symptoms from spinal cord compression [
11]. SACs typically span three to four vertebral segments cranio-caudally, and our study’s average was 3.5 levels, similar to Bassiouni et al. series [
12]. Large cysts, like one in our study extending over six segments, are not uncommon. Intradural cysts are predominantly dorsal (>75%), although they can also be ventral or intramedullary [
10,
11,
12,
13,
14]. Our cases included dorsal, ventral, and intramedullary cysts.
Clinical presentation of SACs depends on location. For dorsal cysts, back pain is the most frequent symptom, though only half of our patients with posterior cysts reported it. Other common symptoms include radicular pain, paresthesias, muscle weakness, or spastic paralysis [
10,
11]. In our study, sensory disturbances were present at onset, while transitory motor deficits developed in 40% of cases post-surgery. Cervical cysts typically present with progressive cervical myelopathy, consistent with our findings. Literature suggests ventral cysts often cause muscle weakness and myelopathy, while dorsal cysts are more likely associated with neuropathic pain and paresthesias [
11]. In our study, ventral cysts did not predominantly cause motor disturbances at onset, unlike dorsal cysts.
Syringomyelia, causing central spinal cord lesions, is linked to sensory loss, chronic pain, and motor deficits. When it affects the anterior horn, it can lead to arm weakness and absent deep tendon reflexes [
15]. In our series patients with syringomyelia, neither neuropathic pain nor upper limb motor changes were reported. One patient had bowel and bladder issues, likely due to the dorsal-lumbar syringomyelia.
Diagnosis was confirmed preoperatively with MRI, which still represents the gold standard. All cysts in our study were associated with myelopathy and edema, showing hypodensity on T1 and hyperdensity on T2, or syringomyelia, consistent with Klekamp et al.’s findings that secondary arachnoid cysts show the abovementioned characteristics due to the arachnoiditis affecting cerebrospinal fluid dynamics.
Surgical treatment involved posterior access via laminectomy for all cases. This approach was used even for cysts spanning more than three vertebral levels, with one case opting for hemilaminectomy, as described Lee et al.2013. Minimally invasive techniques, such as laminoplasty suggested by Eroglu et al., are increasingly being described by Authors: in our series we preliminary applied decompressive laminectomy through Piezosurgery in one case as recently published [
16]. Laminectomy length did not exceed five segments. Some authors recommend complete resection for cysts up to three segments and partial resection for larger cysts [
17]: in our experience performing complete resection on larger cysts was not associated to complications like post-operative kyphosis [
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20]. Wang Y. et al. [
20] propose complete resection as feasible for small dorsal cysts; in our series, complete resection was applied to all SACs, and recurrences occurred only in a ventral cyst, aligning with higher recurrence rates for anterior cysts. As some authors suggest [
22], for large cysts, microsurgical drainage or fenestration before resection could facilitate wall removal. Aside from one recurrent SAC case, recurrences in our study were associated with myelopathy from ongoing arachnoiditis-related scar formation. Despite meticulous arachnoid dissection and cyst resection, symptoms persisted, supporting literature that surgery alleviates symptoms short-term but does not cure the underlying disease which is progressive overtime [
17]. However, surgical treatment stabilized clinical and neurological status for all patietns except in one case. The trend of more rapid improvement in motor symptoms versus neuropathic pain, reported in other studies was confirmed [
11,
12,
13,
14,
15,
16,
17]. In cases with syringomyelia, not in line with other series, long-term management with microsurgical dissection and duraplasty was ineffective. One patient required a syringoperitoneal shunt after initial syringopleural shunt failure due to arachnoid adhesions. The efficacy of syringoperitoneal shunting combined with arachnoidolysis in reducing syringomyelia and stabilizing myelopathy supports findings by Mitsuyama et al. and Koyanagi et al. The effectiveness of surgery in achieving lasting clinical improvement depends on patient selection, thorough surgical technique and ongoing follow-up. Further research with larger patient cohorts and extended follow-up is needed to refine surgical strategies and optimize long-term outcomes for this challenging condition.
5. Conclusions
This study demonstrates that surgical resection combined with adhesiolysis can improve clinical outcomes in patients with spinal arachnoid cysts (SACs), potentially relieving neurological symptoms and stabilizing the overall clinical condition. However, careful management and patient selection is essential due to the high risk of complications and recurrences.
Author Contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used “Conceptualization, A.I. and D.A.; methodology, L.I, G.M.; validation, G.P, L.E, A.M, M.L, R.T, S.B, E.B., and M.I.; formal analysis, A.I.; investigation, G.M.; data curation, G.M.; writing—original draft preparation, A.I.; writing—review and editing, D.A.; visualization, M.I.; supervision, M.I. All authors have read and agreed to the published version of the manuscript.” Please turn to the CRediT taxonomy for the term explanation. Authorship must be limited to those who have contributed substantially to the work reported.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and Ethical review and approval were waived for this study being the procedures part of rountine standard care.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
We encourage all authors of articles published in MDPI journals to share their research data. In this section, please provide details regarding where data supporting reported results can be found, including links to publicly archived datasets analyzed or generated during the study. Where no new data were created, or where data is unavailable due to privacy or ethical restrictions, a statement is still required. Suggested Data Availability Statements are available in section “MDPI Research Data Policies” at
https://www.mdpi.com/ethics.
Acknowledgments
In this section, you can acknowledge any support given which is not covered by the author contribution or funding sections. This may include administrative and technical support, or donations in kind (e.g., materials used for experiments). Where GenAI has been used for purposes such as generating text, data, or graphics, or for study design, data collection, analysis, or interpretation of data, please add “During the preparation of this manuscript/study, the author(s) used [tool name, version information] for the purposes of [description of use]. The authors have reviewed and edited the output and take full responsibility for the content of this publication.”.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| Fig. |
figure |
| MRI |
Magnetic Resonance Imaging |
| CT |
computed tomography |
| Tab. |
table |
| d |
days |
| Y |
years |
| M |
male |
| W |
women |
| NRS |
numeric rating scale for pain |
References
- U. Eroglu et al., ‘Surgical Management of Spinal Arachnoid Cysts in Adults’, World Neurosurg, vol. 122, pp. e1146–e1152, Feb. 2019. [CrossRef]
- N. E. Epstein, ‘Review/Perspective On the Diagnosis and Surgical Management of Spinal Arachnoid Cysts’. [CrossRef]
- K. Naito, T. Yamagata, K. Ohata, and T. Takami, ‘Safety and Efficacy of Syringoperitoneal Shunting with a Programmable Shunt Valve for Syringomyelia Associated with Extensive Spinal Adhesive Arachnoiditis: Technical Note’, World Neurosurg, vol. 132, pp. 14–20, Dec. 2019. [CrossRef]
- B. Jones et al., ‘Surgical techniques used in the management of intra-arachnoid diverticula in dogs across four referral centres and their immediate outcome’, Journal of Small Animal Practice, vol. 63, no. 7, pp. 520–525, Jul. 2022. [CrossRef]
- F. S. Abou Fakhr, S. V. Kanaan, F. M. Youness, M. H. Hourani, and M. C. Haddad, ‘Thoracic spinal intradural arachnoid cyst: report of two cases and review of literature’, Eur Radiol, vol. 12, no. 4, pp. 877–882, Apr. 2002. [CrossRef]
- B. T. Andrews, P. R. Weinstein, M. L. Rosenblum, and N. M. Barbaro, ‘Intradural arachnoid cysts of the spinal canal associated with intramedullary cysts’, J Neurosurg, vol. 68, no. 4, pp. 544–549. 1988. [CrossRef]
- M. W. Nabors et al., ‘Updated assessment and current classification of spinal meningeal cysts’, J Neurosurg, vol. 68, no. 3, pp. 1988. [CrossRef]
- H. Fukumoto, K. Samura, T. Katsuta, K. Miki, K. Fukuda, and T. Inoue, ‘Extensive Multilocular Spinal Extradural Meningeal Cyst That Developed 16 Years After Traumatic Brachial Plexus Injury: A Case Report’, World Neurosurg, vol. 86, pp. 510.e5-510.e10, Feb. 2016. [CrossRef]
- M. D. Fam et al., ‘Spinal arachnoid cysts in adults: diagnosis and management. A single-center experience’, J Neurosurg Spine, vol. 29, no. 6, pp. 711–719, Sep. 2018. [CrossRef]
- R. Sadek and A. Nader-Sepahi, ‘Spinal Arachnoid Cysts: Presentation, management and pathophysiology’, Clin Neurol Neurosurg, vol. 180, pp. 87–96, May 2019. [CrossRef]
- M. Y. Wang, A. D. O. Levi, and B. A. Green, ‘Intradural spinal arachnoid cysts in adults’, Surg Neurol, vol. 60, no. 1, pp. 49–55, Jul. 2003. [CrossRef]
- H. Bassiouni, A. Hunold, S. Asgari, U. Hübschen, H. J. König, and D. Stolke, ‘Spinal intradural juxtamedullary cysts in the adult: Surgical management and outcome’, Neurosurgery, vol. 55, no. 6, pp. 1352–1359, Dec. 2004. [CrossRef]
- Fortuna, E. La Torre, and P. Ciappetta, ‘Arachnoid diverticula: a unitary approach to spinal cysts communicating with the subarachnoid space’, Acta Neurochir (Wien), vol. 39, no. 3–4, pp. 259–268, Sep. 1977. [CrossRef]
- E. Kendall, A. R. Valentine, and B. Keis, ‘Spinal arachnoid cysts: Clinical and radiological correlation with prognosis’, Neuroradiology, vol. 22, no. 5, pp. 225–234, Jan. 1982. [CrossRef]
- Leclerc, L. Matveeff, and E. Emery, ‘Syringomyelia and hydromyelia: Current understanding and neurosurgical management’, Rev Neurol (Paris), vol. 177, no. 5, pp. 498–507, May 2021. [CrossRef]
- Iacoangeli A, Alsagheir M, Aiudi D, Gladi M, Di Rienzo A, Esposito DP, Diab M, Naas H, Eldellaa A, Gigante A, Iacoangeli M, Alshafai NS, Luzardo G. Microendoscopic Tailored Spine Decompression as a Less-Invasive, Stability-Preserving Surgical Option to Instrumented Correction in Complex Spine Deformities: A Preliminary Multicenter Experience. World Neurosurg. 2024 Jun;186:e142-e150. [CrossRef] [PubMed]
- J. Klekamp, ‘A New Classification for Pathologies of Spinal Meninges - Part 2: Primary and Secondary Intradural Arachnoid Cysts’, Neurosurgery, vol. 81, no. 2, pp. 217–229, Aug. 2017. [CrossRef]
- Baig, Mirza; et al., ‘Surgical management and outcomes in spinal intradural arachnoid cysts: the experience from two tertiary neurosurgical centres’, Acta Neurochir (Wien), vol. 164, no. 5, pp. 1217–1228, May 2022. 20 May 1217; 22. [CrossRef]
- P. Kalsi et al., ‘Spinal arachnoid cysts: A case series & systematic review of the literature’, Brain and Spine, vol. 2, Jan. 2022. [CrossRef]
- Z. B. Moses, G. N. Friedman, D. L. Penn, I. H. Solomon, and J. H. Chi, “Intradural spinal arachnoid cyst resection: implications of duraplasty in a large case series,” J - Cerca con Google’. Accessed: Sep. 15, 2024.
- Y. bo Wang, D. hua Wang, and S. lin Deng, ‘Symptomatic secondary spinal arachnoid cysts: a systematic review’, Spine Journal, vol. 23, no. 8, pp. 1199–1211, Aug. 2023. [CrossRef]
- M. Y. Wang, A. D. O. Levi, and B. A. Green, ‘Intradural spinal arachnoid cysts in adults’, Surg Neurol, vol. 60, no. 1, pp. 49–55, Jul. 2003. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).