1. Introduction
Degenerative cervical myelopathy represents the most frequent cause of functional impairment from medullary origin in adult patients over 50 years of age, may result into major disability like tetraparesia and is the consequence of the degenerative changes in the cervical spine [
1]. The clinical presentation of DCM is variable [
4]. In order to harmonize the assessment of disability due to DCM, the modified-JOA (mJOA) is now commonly used [
2,
3,
4,
5].
MRI represents the gold standard imaging modality for the diagnosis of the disease. Different MRI signs have been reported with variable clinical significance [
5,
6,
7,
8,
9,
10,
11].
According to the literature, it seems that one MRI sign alone remains poorly correlated with the clinical presentation. The absence of clear correlation between the symptoms and MRI findings poses some difficulties to evaluate precisely the disease’s severity and can thus make the decision-making uncomfortable for the clinicians.
In order to help the practitioners to accurately assess the severity of the disease on MRI and therefore choose the best treatment option, the objective of the present study was to establish a multiparametric-weighted scoring system, easy to use in daily practice, based on the most significant MRI signs and correlated as strongly as possible with the clinical presentation (mJOA) – the SIMS for Severity on Imaging Myelopathy Score.
2. Materials and Methods
Study Design and Population
This is a single-center retrospective analysis of prospectively collected data including 99 operated patients with a diagnosis of cervical degenerative stenosis on MRI and who had a clinical evaluation with mJOA score established by at least one of the senior neurosurgeons of the Spine and Cord Unit of the Neurological Hospital of the Hospices Civils de Lyon (HCL), between January 2015 and March 2021, and who benefited from a cervical MRI with axial and sagittal slices, both within an interval of less than 1 year. The indication for surgery was left to the surgeon’s discretion and could be based on the patient’s clinical and functional assessment, radiological evolution or abnormalities found on evoked potentials.
Inclusion criteria were 1) Age ≥18 years; 2) mJOA score found in the patient’s medical record; 3) cervical MRI with T2-weighted sequences including axial and sagittal slices
The following patients were excluded from the study: 1) Age < 18 years; 2) History of surgery and/or trauma and/or infection and/or neoplasia and/or congenital deformity of the cervical spine; 3) Presence of severe and/or advanced neurological or systemic disease that could influence the clinical or electrophysiological evaluation.
An informed consent has been obtained from participants. The study was approved by Ethical Committee of French College of Neurosurgery (IRB00011687). All methods were carried out in accordance with relevant guidelines and regulations.
Selection of Variables
The selection of the parameters composing the SIMS was made on the basis of literature searches, from the PubMed platform, with the aim to retain initially the variables best correlated with clinical symptoms. These parameters had to be measurable on MRI images from the HCL visualization software (PACS) at the most stenotic level.
The measurement of the different variables for each patient was done jointly by 2 neurosurgeons specialized in spine surgery (CB and AM). The determination of the most stenotic level was made by consensus between the two practitioners.
Initially, 8 variables were selected on basis of literature data. Due to the lack of clear correlation with the clinical presentation in the statistical analysis for 2 criteria, it was decided to keep 6 criteria: Fujiwara Ratio ; T2-weighted intramedullary hyperintensity ; Cerebrospinal fluid cisterns ; Torg-Pavlov Ratio ; Local Kyphosis ; Number of stenotic level
Categorization for Each SIMS Criteria
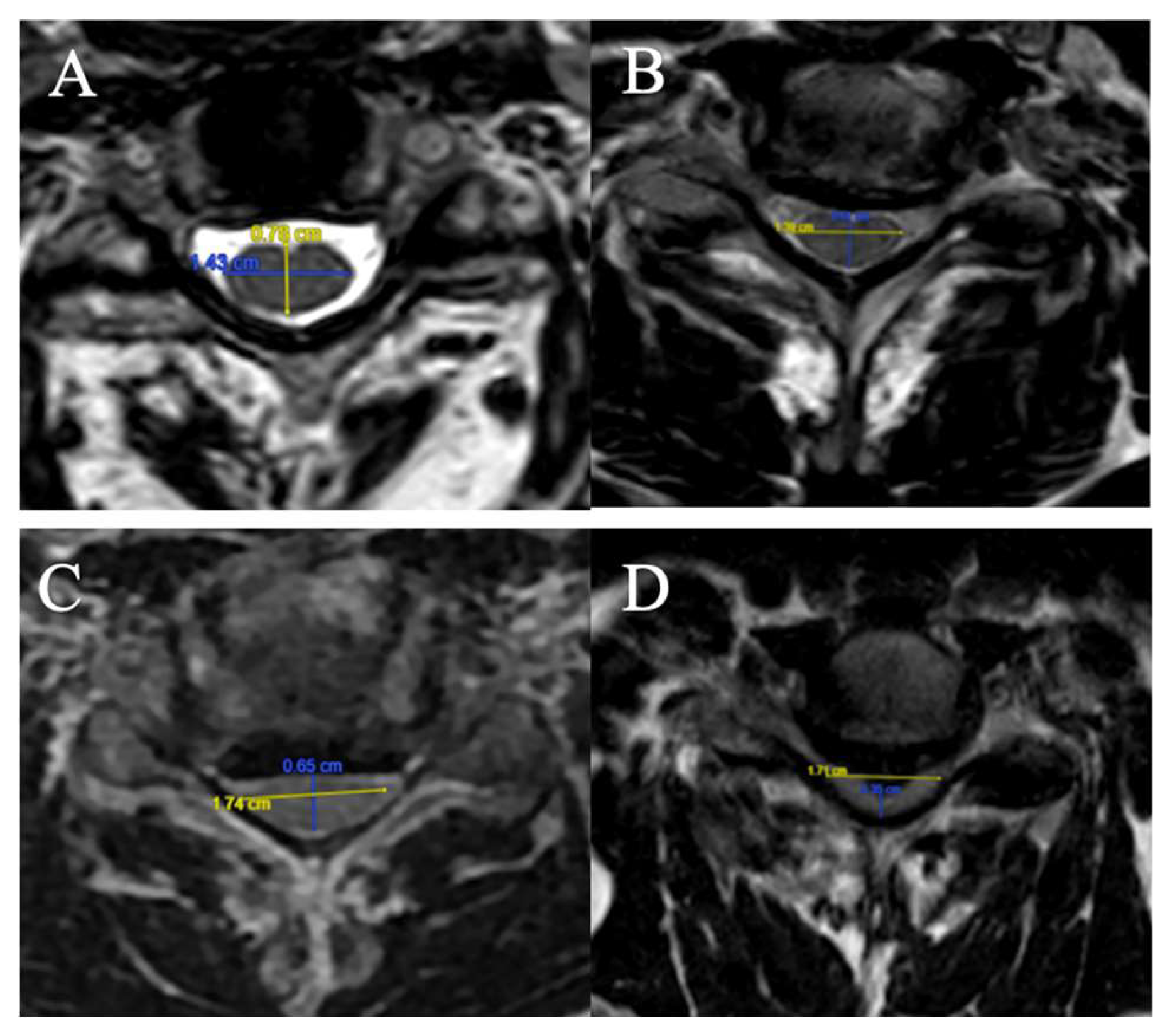
Fujiwara Ratio
The FR is defined as the ratio of the anteroposterior diameter of the spinal cord to the transverse diameter on an axial slice at maximum compression on T2-weighted sequences (
Figure 1).
4 grades have been selected:
(A) FR ≥ 0.5;
(B) 0.4 ≤ FR < 0.5;
(C) 0.3 ≤ FR < 0.4;
D) FR < 0.3.
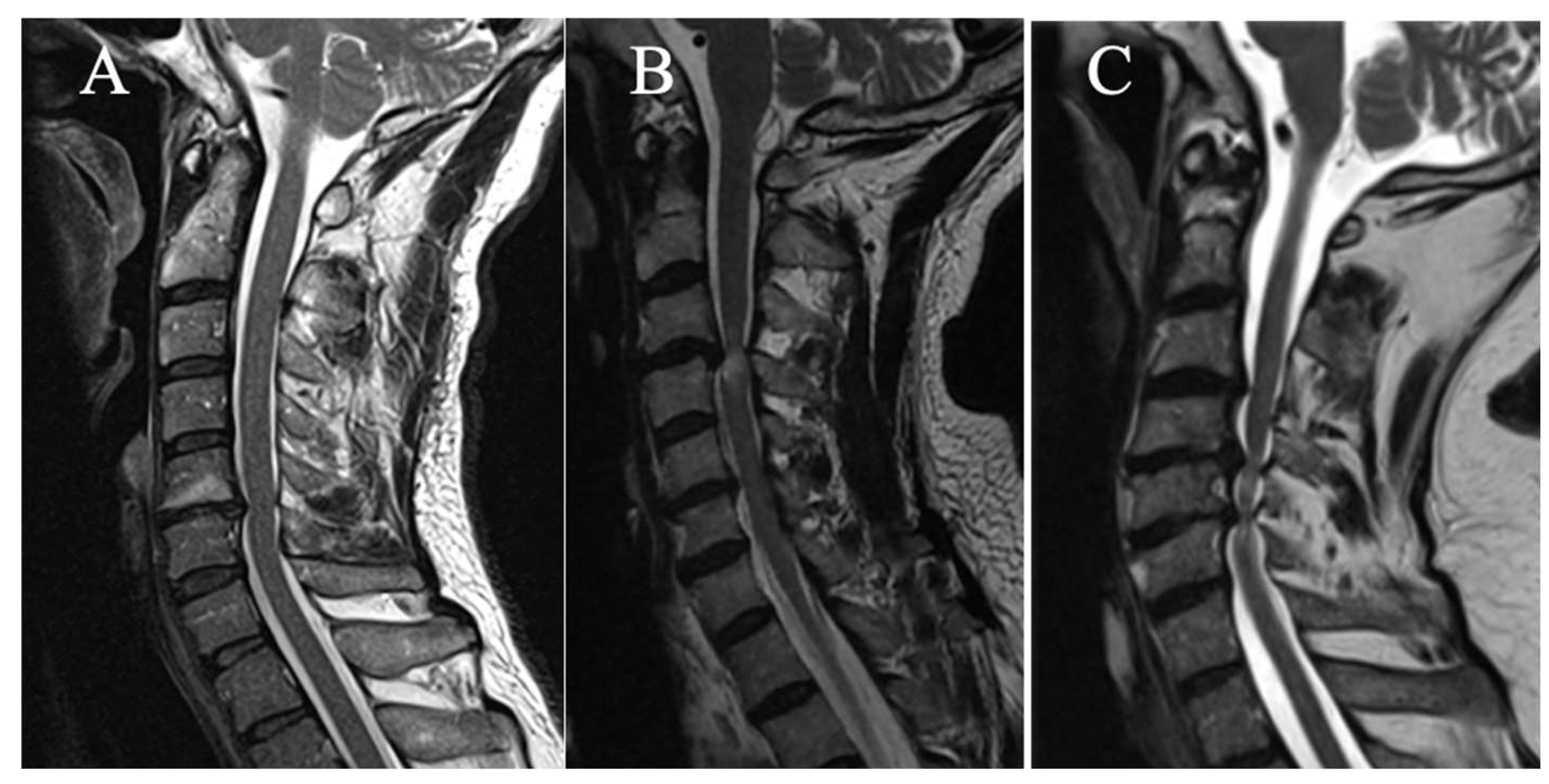
T2-Weight Intramedullary Hyperintensity
3 grades have been selected on sagittal slice (
Figure 2):
(A) No T2HI
(B) Focal T2HI (limited to one intervertebral space and adjacent vertebral bodies)
(C) Multi-segmental T2HI (Extending beyond two intervertebral spaces)
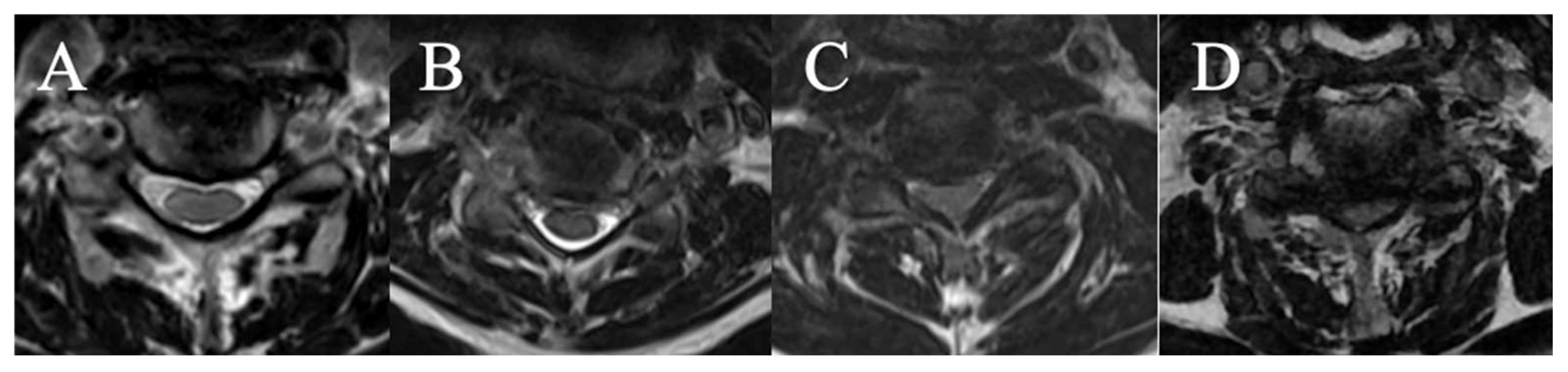
Cerebrospinal Fluid Cisterns
4 grades have been selected on axial slice (
Figure 3)
(A) CSF visible anteriorly and posteriorly
(B) CSF erased anteriorly or posteriorly
(C) CSF erased anteriorly and posteriorly but root cisterns still visible
(D) Totally erased cisterns – no CSF visible on the slice
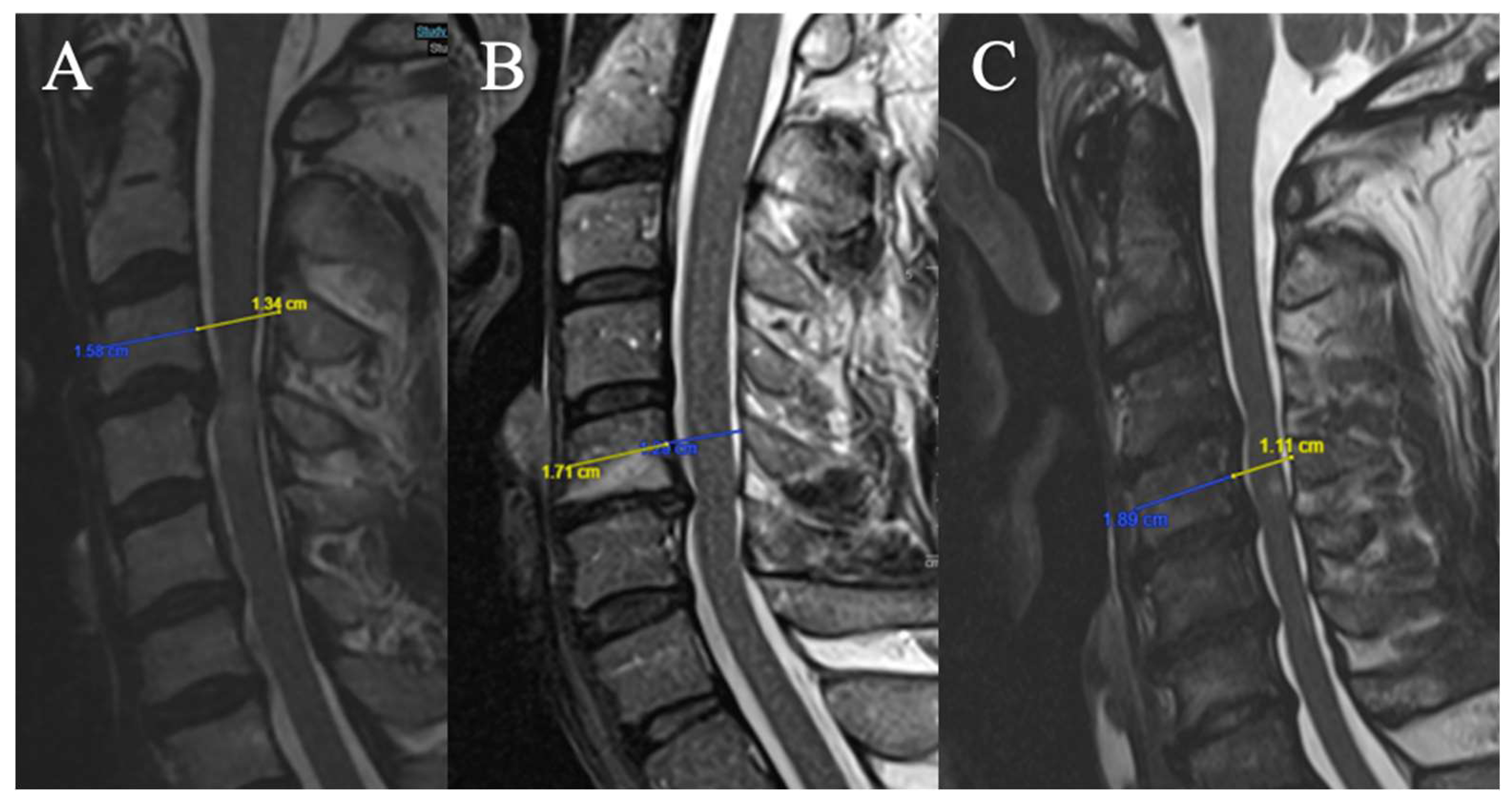
Torg-Pavlov Ratio
3 grades have been selected (based on T2-weighted sequence on the median sagittal sections at the middle vertebral level overlying the compression) (
Figure 4):
(A) TPR ≥ 0.8
(B) 0.6 ≤ TPR < 0.8
(C) TPR < 0.6
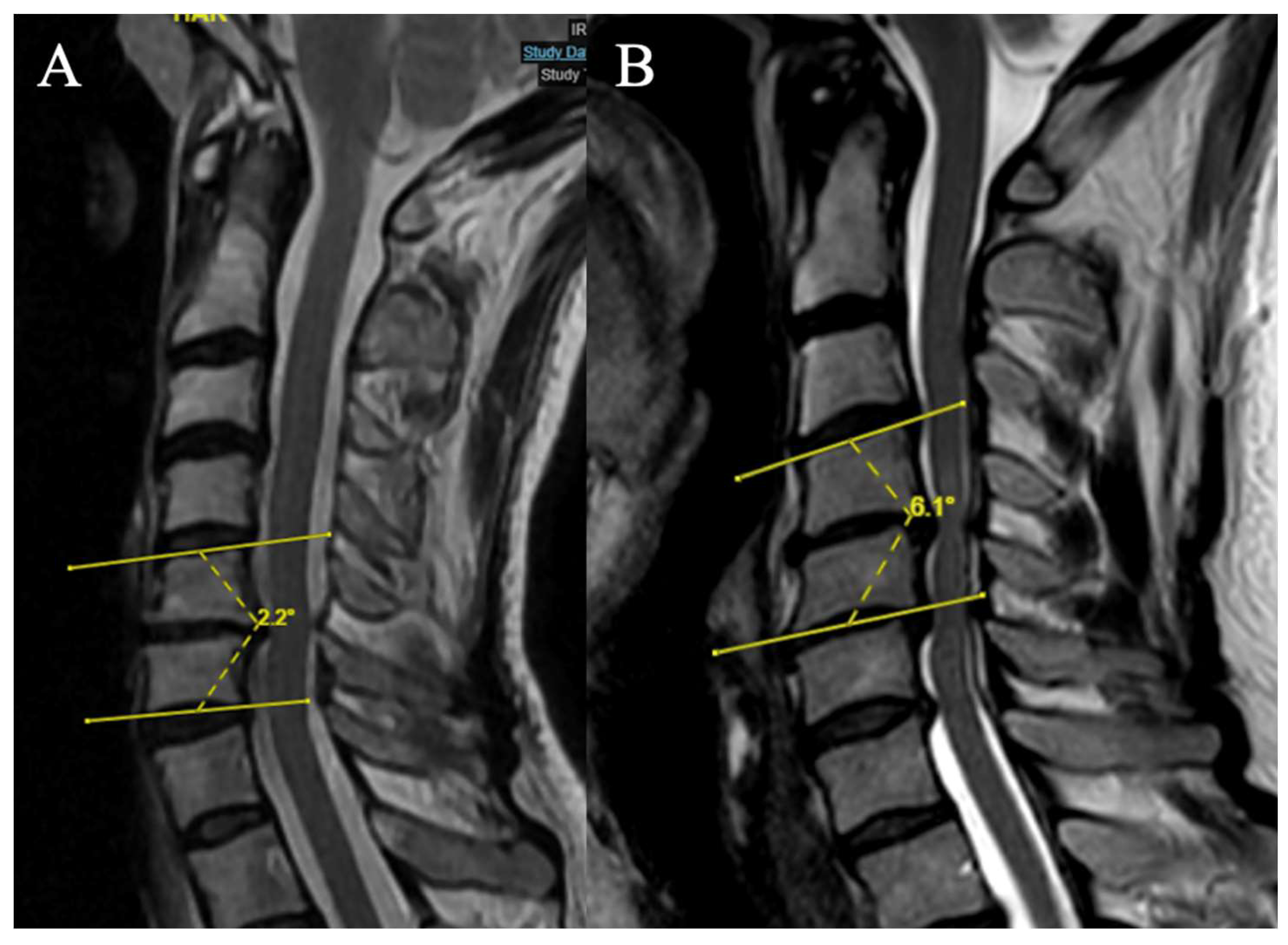
Local Kyphosis
2 grades have been selected (Focal angle measured on sagittal T2-weighted MRI sequences at the level of maximum compression) (
Figure 5):
(A) Lordosis or LK <4°
(B) LK ≥ 4°
Number of Stenotic Level(s)
3 grades have been selected (Any level (in addition to the maximum compression level) for which the FR in axial section on T2-weighted sequences is < 0.40 is considered as an additional compression level):
(A) 1 stenotic level
(B) 2 stenotic levels
(C) ≥ 3 stenotic levels
Intraobserver and Interobserver Reliability
Intraobserver reliability was measured by comparing the measurements of 2 cases obtained by two observers (GP and DC) 10 days apart. Conversely, the interobserver reliability was measured by comparing the measurements of 2 cases obtained by the two observers in the same day.
General Statistical Analysis
Quantitative data were described by the mean, standard deviation, median, first and third quartiles, as well as the minimum and maximum values.
Qualitative data were described by the numbers and proportions (percentages).
For mJOA, the 3 following categories were used [
1]: mild (score ≥15), moderate (12≤ score ≤14), severe (score ≤11).
Comparisons of mean mJOA scores for each subgroup of each variable were performed by Student t test.
Associations between quantitative variables were quantified by Spearman’s correlation coefficient, with associated 95% confidence interval.
Linear regression models were used to assess the association between each of the categorized criteria to the mJOA score. The association was quantified by the coefficient of determination (R2).
Univariate analyses were followed by multivariable regression analyses.
The final choice of criteria to be included in the SIMS score was based on the values of the R2 coefficients obtained in univariate analysis, on the results of the different multivariate models, and on clinical considerations. Conditional on explanatory variables, the mJOA score was normally distributed.
The final association between the selected SIMS score and the mJOA score was quantified by the Spearman correlation coefficient (95% confidence interval), and by describing the SIMS score values according to the 3 mJOA categories.
The analyses were performed with the R software.
The intraclass correlation coefficient (ICC) was utilized to measure the intra- and interobserver agreement for different scores, with a confidence interval (CI) of 95%. ICC values of 0.00 to 0.20 were considered to be in slight agreement; 0.21 to 0.40, fair agreement; 0.41 to 0.60, moderate agreement; 0.61 to 0.80, substantial agreement; and 0.81 to 1.00, almost perfect agreement. The analyses were performed with the JASP software ((Version 0.19.1) [Computer software] /
https://jasp-stats.org/download/).
3. Results
3.1. Characteristics of the Cohort (Table 1)
99 patients met all inclusion criteria, including 41 women (41.4%) and 58 men (58.6%). The mean age at the time of visit was 62.9 years. The minimum age was 28 years, the maximum 84 years. The most common level was C5C6 (41.4%). In 17 patients (17.2%), there was no indication for surgery (
Table 1).
The mean mJOA score is 14.9 ± 2.7. The minimum and maximum observed are 7 and 18 respectively. 62 patients (62.6%) presented with mild myelopathy, 22 (22.2%) with moderate myelopathy and 15 (15.2%) with severe myelopathy [
1].
Table 1.
Characteristics of the cohort.
Table 1.
Characteristics of the cohort.
| |
N |
% |
| Age (yrs) |
|
|
- ◆
< 60 |
40 |
40.4 |
- ◆
≥ 60 |
59 |
59.6 |
| Sex |
|
|
- ◆
F |
41 |
41.4 |
- ◆
M |
58 |
58.6 |
| FR |
99 |
|
- ◆
A |
8 |
8.1 |
- ◆
B |
33 |
33.3 |
- ◆
C |
37 |
37.4 |
- ◆
D |
21 |
21.2 |
| T2HI |
99 |
|
- ◆
A |
44 |
44.4 |
- ◆
B |
33 |
33.3 |
- ◆
C |
22 |
22.2 |
| CSF |
99 |
|
- ◆
A |
6 |
6.1 |
- ◆
B |
43 |
43.4 |
- ◆
C |
21 |
21.2 |
- ◆
D |
29 |
29.3 |
| TPR |
99 |
|
- ◆
A |
49 |
49.5 |
- ◆
B |
43 |
43.4 |
- ◆
C |
7 |
7.1 |
| LK |
99 |
|
- ◆
A |
74 |
74.7 |
- ◆
B |
25 |
25.3 |
| NSL |
99 |
|
- ◆
A |
53 |
53.5 |
- ◆
B |
27 |
27.3 |
- ◆
C |
19 |
19.2 |
3.2. Correlation Between Each Variable of the SIMS and mJOA
3.2.1. Fujiwara Ratio
The mean mJOA score was significantly lower for Grade C (15.1) compared with Grade A (16.9) (p < 0.05) and for Grade D (12.9) compared with all other grades (
Table 2).
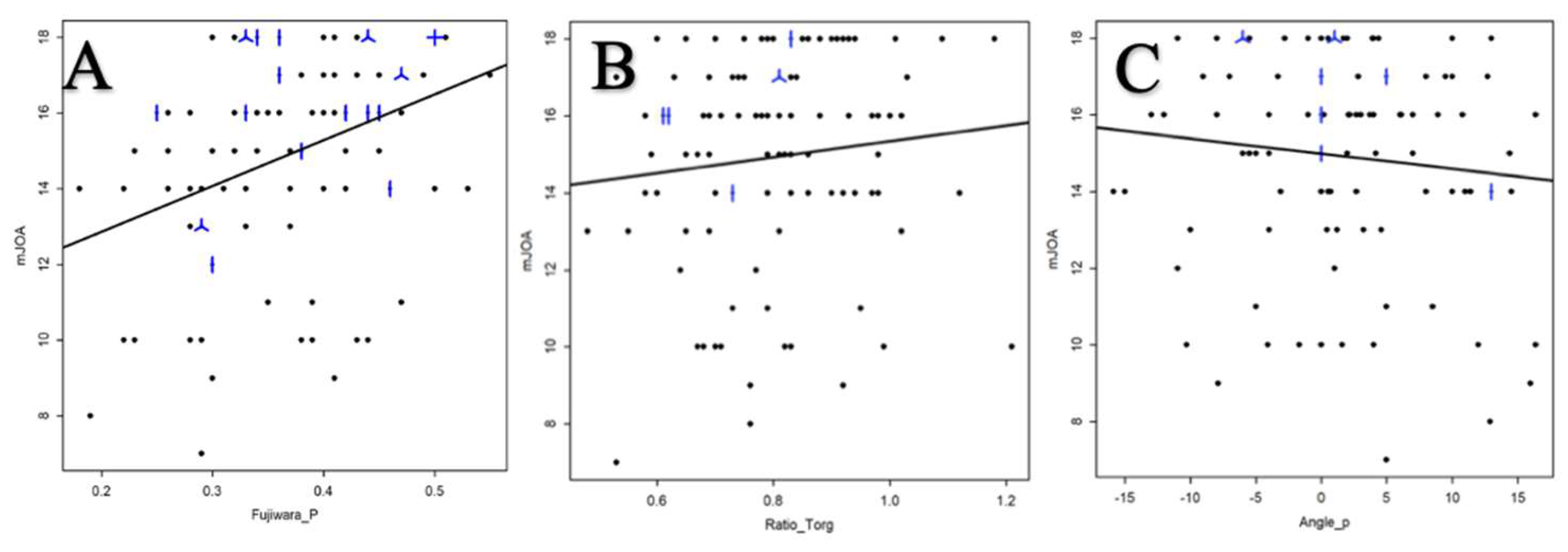
The correlation between mJOA score and FR was analyzed by Spearman’s correlation coefficient, it was 0.38 95% CI [0.20, 0.54] (
Figure 6A).
3.2.2. T2-Weight Intramedullary Hyperintensity
The mean mJOA score was significantly lower for grades C (13.1) and B (13.9) than for grade A (16.6) (p < 0.05) (
Table 2).
3.2.3. CSF Cisterns
The mean mJOA score was significantly lower for all grades compared to less severe grades, with a mean score of 18.0, 16.1, 14.5 and 12.9 for respectively grade A, B, C and D (p < 0.05) (
Table 2).
3.2.4. Torg-Pavlov Ratio
There was no statistically significant difference in the mJOA score between the different grades with respectively a score of 15.4, 14.6 and 13.6 (
Table 2). The association between TPR and mJOA score was analyzed from Spearman’s correlation coefficient, and was 0.15 95% CI [-0.05, 0.34] (
Figure 6B).
3.2.5. Local Kyphosis
There was no statistically significant difference between grades A and B regarding the mean mJOA score (respectively 15.0 and 14.9) (
Table 2).
The relationship between the degree of LK and the mJOA score was analyzed by Spearman’s correlation coefficient, and was -0.101 95% CI [-0.293, 0.098] (
Figure 6C).
Figure 6.
Relationship between the mJOA score and the FR (A) ; the TPR (B) and the LK(C).
Figure 6.
Relationship between the mJOA score and the FR (A) ; the TPR (B) and the LK(C).
*A dot represents a single patient, a line 2 patients, a helix 3 patients and a cross 4 patients
3.2.6. Number of Stenotic Level(s)
The mean mJOA score of Grade B (14.6) was significantly lower than Grade A (16.0), and the mean mJOA score of Grade C (12.4) was significantly lower than Grade A and B (p < 0.05) (
Table 2).
3.3. Ponderation of the Different Criteria
A linear regression model was used to modelize the mJOA as a function of the different variables proposed in the SIMS. First, each variable was included separately in the linear model (
Table 3). Four variables correlated significantly with the SIMS (p < 0.005): FR, CSF, T2HI, and NSL.
Secondary, a multivariate linear regression model was performed (
Table 4). Three variables correlated significantly with the SIMS (p<0.005): CSF, T2HI, and NSL.
This model allows us therefore to quantify the importance associated with each grade for each variable and thus to reproduce their relative importance. Indeed, the choice of the points associated with each subscore is made on the basis of the closest unit of the correlation coefficient (except for local kyphosis for which grade B will be given a score of 1 point), calculated as the difference the mJOA score for each grade compared to the reference grade A (
Table 5).
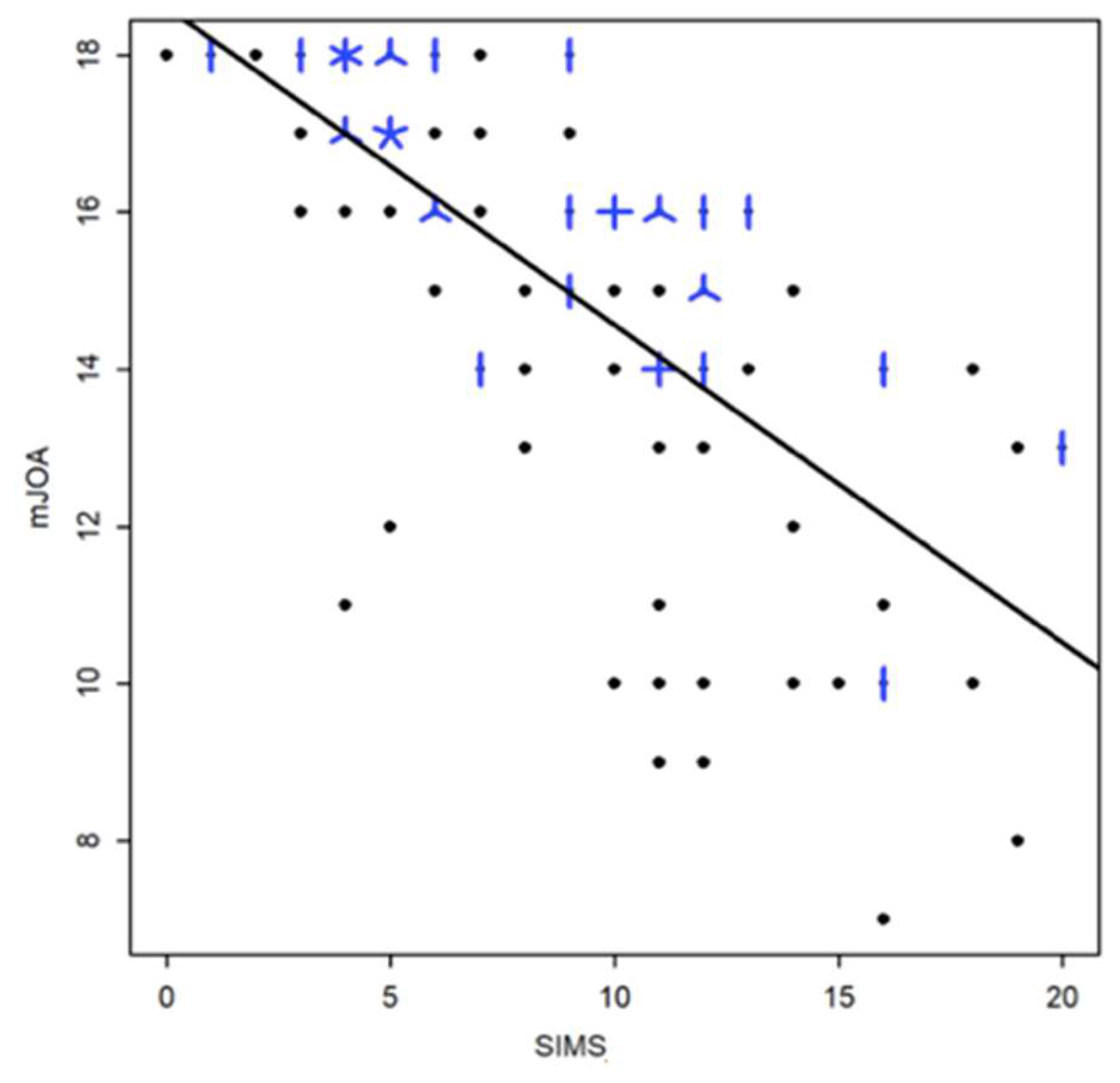
Finally, the strength of the relationship between the SIMS and the mJOA was quantified by the Spearman correlation coefficient of 0.75 (95% CI [-0823, -0.64]) (
Figure 7).
3.4. Intraobserver and Interobserver Reliability
Table 6 presents the results of the two assessments conducted by the two observers.
Table 7 and
Table 8 presents the intraobserver reliability and interobserver reliability both for the total score and each individual part respectively.
Concerning intraobserver reliability, the ICC value was above 0.80, indicating an almost perfect agreement.
Concerning intraobserver reliability, the ICC value was above 0.80, except for the LK, indicating an almost perfect agreement.
4. Discussion
At the present time, the most commonly used parameters to assess spinal cord compression in cervical myelopathy on MRI are the appearance of T2HI and the disappearance of peri-medullary fluid spaces. Although T2HI is a marker of disease severity and poor prognosis, it usually occurs at a late stage of the disease [
12,
13,
14,
15,
16,
17].
Some classifications have been published, such as those of Nagata et al., Muhle et al. or Kang et al., based on obliteration of the subarachnoid spaces, the degree of spinal cord compression or the presence of a change in spinal cord intensity. But these criteria are subjective, evaluate in sagittal plane alone and the correlation with clinical involvement has not been clearly established [
18,
19,
20].
Finally, Wang et al. have also established a new MRI score for assessing compression in patients with posterior longitudinal ligament ossification, following the same model as ours, but without weighting the variables according to their relative importance and there was no evaluation of the correlation with the mJOA [
21].
The choice to maintain the T2HI, the CSF and the NSL was natural since they were significantly associated with the JOA score (p < 0.001) and are the most useful parameters for routine practice. The results are consistent with those of the literature. For example, Watabe et al. showed that the decrease in CSF flow was significantly associated with the severity of the myelopathy assessed by the JOA [
21,
22] and Wang et al. showed a significant correlation between different grades of CSF obliteration and JOA score. Fehlings et al.,for their part, found in their prospective surgical cohort an average of 3.86 decompressed levels and showed that the mJOA was inversely proportional to the number of levels affected [
4].
In order to be used in clinical routine, MRI parameters must be easily and quickly measurable.
The study of the FR in function of the SIMS showed a significant difference between the different subscores in the univariate modeling but not in the multivariate analysis. The small number of patients with a FR <0.3 may explain the lack of power to detect differences between categories. Nevertheless, this variable is easily measurable in clinical routine with parameters that have a good interobserver and intraobserver correlation, so it seemed useful to keep in the score [
23].
In the same way, the TPR has been integrated into the initial SIMS. Chrispin [
11] and Lee found that a ratio of less than 0.85 was a risk factor, which is consistent with the results of Ehni et al. and Pavlov et al. who found ratios of 0.80 and 0.82 respectively. Yue et al. [
24] found a limit of 0.72 in their retrospective cohort of 88 patients. Unfortunately, the small number of patients with a TPR <0.6 (n=6) in our series does not allow statistical analyses to be performed with correct power and the fact that TPR was initially described on plain radiographs has to be keep in mind for interpretation of results.
Finally, Wu et al. showed that the JOA score had a large and significant negative correlation with focal kyphosis [
25]. The results in this study showed an inverse correlation between the degree of kyphosis and the mJOA, but which was weak and not significant. Nevertheless, given the data in the literature which show an association of cervical sagittal alignment with the severity of myelopathy and the need to take into account the kyphotic deformity of the cervical spine in the surgical consideration, we decided to keep this parameter in the final score in order to study its relevance more widely in future studies.
The intra- et interobserver reliability of the SIMS is almost perfect demonstrating that its use could be implemented in clinical routine.
The SIMS was strongly correlated with the clinical presentation (evaluated by mJOA) supporting the interest to use a multiparametric score to assess the severity of DCM in clinical practice. Although very encouraging, these results need to be consolidated on a larger, prospective cohort. In addition, inter-observer and intra-observer reliability must be analyzed, both between surgeons specializing in the spine and spinal cord, and with other practitioners involved in this pathology (general practitioners, neurologists, rehabilitation specialists, etc.), as this score is intended to be used widely. The second benefit of the prospective evaluation would be to demonstrate the potential prognostic value of this score.
This study had some limitations. First, it was a retrospective study, with some missing data. Then, even if already large with a series of 99 patients, the number of patients could be greater to improve the power of the study, especially concerning moderate and severe myelopathy. Finally, regarding the constitution of the score itself, the maintenance of some variables that was not significantly correlated to the mJOA score on multivariate analyses can be criticized, and their interest should be confirmed.
5. Conclusions
SIMS scoring system, based-on 6 morphological parameters, represents a coherent and relevant way to characterize the severity of DCM on MRI evaluating the degree of spinal cord compression in sagittal and axial views. The T2-weighted intramedullary change(s), the disappearance of peri-medullary fluid spaces and the number of stenotic levels being the most important factors to be taken into account. It may allow standardization of MRI image analyses for DCM and could facilitate comparisons between studies providing a useful evaluation tool.
Author Contributions
Conceptualization, Alexis Morgado and Cédric Barrey; Data curation, Alexis Morgado; Formal analysis, Fabien Subtil; Investigation, Alexis Morgado, Julien Berthiller, Fabien Subtil, Donato Creatura, Gildas Patet, Nathalie André-Obadia and Cédric Barrey; Methodology, Alexis Morgado, Julien Berthiller, Fabien Subtil, Nathalie André-Obadia and Cédric Barrey; Supervision, Cédric Barrey; Writing – original draft, Alexis Morgado; Writing – review & editing, Alexis Morgado, Julien Berthiller, Fabien Subtil, Nathalie André-Obadia and Cédric Barrey.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by Ethical Committee of French College of Neurosurgery (IRB00011687).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to ethical reason.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| SIMS |
Severity on Imaging Myelopathy Score |
| DCM |
Degenerative Cervical Myelopathy |
| MRI |
Magnetic Resonance Imaging |
| mJOA |
Modified Japanese Orthopaedic Association score |
| FR |
Fujiwara Ratio |
| T2IH |
T2-weighted Intramedullary Hyperintensity |
| CSF |
Cerebrospinal Fluid Cisterns |
| TPR |
Torg-Pavlov Ratio |
| LK |
Local Kyphosis |
| NSL |
Number of Stenotic Level |
| ICC |
IntraClass Correlation |
References
- Bernhardt M, Hynes RA, Blume HW, White AA. Cervical spondylotic myelopathy. J Bone Joint Surg Am. janv 1993;75(1):119-28.
- Benzel EC, Lancon J, Kesterson L, Hadden T. Cervical Laminectomy and Dentate Ligament Section for Cervical Spondylotic Myelopathy: Journal of Spinal Disorders. sept 1991;4(3):286-95.
- Chiles BW, Leonard MA, Choudhri HF, Cooper PR. Cervical spondylotic myelopathy: patterns of neurological deficit and recovery after anterior cervical decompression. Neurosurgery. avr 1999;44(4):762-9; discussion 769-770. [CrossRef]
- Fehlings MG, Wilson JR, Kopjar B, Yoon ST, Arnold PM, Massicotte EM, et al. Efficacy and safety of surgical decompression in patients with cervical spondylotic myelopathy: results of the AOSpine North America prospective multi-center study. J Bone Joint Surg Am. 18 sept 2013;95(18):1651-8. [CrossRef]
- Kovalova I, Kerkovsky M, Kadanka Z, Kadanka Z, Nemec M, Jurova B, et al. Prevalence and Imaging Characteristics of Nonmyelopathic and Myelopathic Spondylotic Cervical Cord Compression. Spine (Phila Pa 1976). 15 déc 2016;41(24):1908-16. [CrossRef]
- Yu L, Zhang Z, Ding Q, Li Y, Liu Y, Yin G. Relationship Between Signal Changes on T2-weighted Magnetic Resonance Images and Cervical Dynamics in Cervical Spondylotic Myelopathy. J Spinal Disord Tech. juill 2015;28(6):E365-367. [CrossRef]
- Mastronardi L, Elsawaf A, Roperto R, Bozzao A, Caroli M, Ferrante M, et al. Prognostic relevance of the postoperative evolution of intramedullary spinal cord changes in signal intensity on magnetic resonance imaging after anterior decompression for cervical spondylotic myelopathy. J Neurosurg Spine. déc 2007;7(6):615-22. [CrossRef]
- Mehalic TF, Pezzuti RT, Applebaum BI. Magnetic resonance imaging and cervical spondylotic myelopathy. Neurosurgery. févr 1990;26(2):217-226 discussion 226-227. [CrossRef]
- Alafifi T, Kern R, Fehlings M. Clinical and MRI predictors of outcome after surgical intervention for cervical spondylotic myelopathy. J Neuroimaging. oct 2007;17(4):315-22. [CrossRef]
- Fujiwara K, Yonenobu K, Hiroshima K, Ebara S, Yamashita K, Ono K. Morphometry of the Cervical Spinal Cord and its Relation to Pathology in Cases with Compression Myelopathy. Spine. nov 1988;13(11):1212. [CrossRef]
- Chrispin AR, Lees F. The spinal canal in cervical spondylosis. J Neurol Neurosurg Psychiatry. avr 1963;26(2):166-70. [CrossRef]
- Cao JM, Zhang JT, Yang DL, Yang YP, Xia HH, Yang L. Imaging Factors that Distinguish Between Patients with Asymptomatic and Symptomatic Cervical Spondylotic Myelopathy with Mild to Moderate Cervical Spinal Cord Compression. Med Sci Monit. 13 oct 2017;23:4901-8. [CrossRef]
- Nouri A, Martin AR, Mikulis D, Fehlings MG. Magnetic resonance imaging assessment of degenerative cervical myelopathy: a review of structural changes and measurement techniques. Neurosurg Focus. juin 2016;40(6):E5. [CrossRef]
- Chen CJ, Lyu RK, Lee ST, Wong YC, Wang LJ. Intramedullary high signal intensity on T2-weighted MR images in cervical spondylotic myelopathy: prediction of prognosis with type of intensity. Radiology. déc 2001;221(3):789-94. [CrossRef]
- Takahashi M, Sakamoto Y, Miyawaki M, Bussaka H. Increased MR signal intensity secondary to chronic cervical cord compression. Neuroradiology. 1987;29(6):550-6. [CrossRef]
- Harrop JS, Naroji S, Maltenfort M, Anderson DG, Albert T, Ratliff JK, et al. Cervical Myelopathy: A Clinical and Radiographic Evaluation and Correlation to Cervical Spondylotic Myelopathy. Spine. mars 2010;35(6):620-4. [CrossRef]
- Zhang YZ, Shen Y, Wang LF, Ding WY, Xu JX, He J. Magnetic resonance T2 image signal intensity ratio and clinical manifestation predict prognosis after surgical intervention for cervical spondylotic myelopathy. Spine (Phila Pa 1976). 1 mai 2010;35(10):E396-399. [CrossRef]
- Nagata K, Kiyonaga K, Ohashi T, Sagara M, Miyazaki S, Inoue A. Clinical Value of Magnetic Resonance Imaging for Cervical Myelopathy: Spine. nov 1990;15(11):1088-96. [CrossRef]
- Muhle C, Metzner J, Weinert D, Falliner A, Brinkmann G, Mehdorn MH, et al. Classification system based on kinematic MR imaging in cervical spondylitic myelopathy. AJNR Am J Neuroradiol. oct 1998;19(9):1763-71.
- Kang Y, Lee JW, Koh YH, Hur S, Kim SJ, Chai JW, et al. New MRI Grading System for the Cervical Canal Stenosis. American Journal of Roentgenology. 1 juill 2011;197(1):W134-40. [CrossRef]
- Wang H, Wang Z, Xu T, Huang B, Zheng H, Xu W, et al. A Novel Scoring System for Ossification of Posterior Longitudinal Ligament of the Cervical Spine Based on Magnetic Resonance Imaging—CSFM Scoring System. World Neurosurgery. 1 juin 2021;150:e466-73. [CrossRef]
- Watabe N, Tominaga T, Shimizu H, Koshu K, Yoshimoto T. Quantitative analysis of cerebrospinal fluid flow in patients with cervical spondylosis using cine phase-contrast magnetic resonance imaging. Neurosurgery. avr 1999;44(4):779-84. [CrossRef]
- Rüegg TB, Wicki AG, Aebli N, Wisianowsky C, Krebs J. The diagnostic value of magnetic resonance imaging measurements for assessing cervical spinal canal stenosis. J Neurosurg Spine. mars 2015;22(3):230-6. [CrossRef]
- Yue WM, Tan SB, Tan MH, Koh DC, Tan CT. The Torg--Pavlov ratio in cervical spondylotic myelopathy: a comparative study between patients with cervical spondylotic myelopathy and a nonspondylotic, nonmyelopathic population. Spine (Phila Pa 1976). 15 août 2001;26(16):1760-4.
- Wu B, Liu B, Sang D, Cui W, Wang D. The association between cervical focal kyphosis and myelopathy severity in patients with cervical spondylotic myelopathy before surgery. Eur Spine J. juin 2021;30(6):1501-8. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).