1. Introduction
Coronary heart disease [CHD], which occurs as a result of coronary artery atherosclerosis is the most common cause of death in highly developed countries. Detailed studies to identify risk factors for CHD have been conducted for many years. Age, gender, hypertension, diabetes, obesity, elevated total cholesterol and LDL cholesterol, low HDL cholesterol [HDL-X], Smoking, and a history of premature cardiovascular disease are considered as independent risk factors for CHD [
1]. Based on studies in a large population, it is reported that a low concentration of cholesterol transported with the HDL fraction is one of the most important risk factors for CHD, and a high concentration of HDL in serum plays a protective role [
2].
In recent years, the ways in which HDL protects the arteries from damage caused by free radicals and prevents the development of atherosclerotic plaques have been intensively studied. Increased oxidative stress is considered as a factor affecting the development of atherosclerosis. An important role in the antioxidant capacity is assigned to specific enzymes associated with the HDL particle, such as paraoxonase [PON], glutathione peroxidase [GPX], or platelet-activating factor acetylhydrolase [PAF-AH] [
3,
4,
5].
Paraoxonase 1 is one of three enzymes [PON1, PON2, and PON3] encoded by a family of genes localized on chromosome 7 [
6]. In vitro studies show that this enzyme hydrolyzes peroxide phospholipids, but the exact mechanisms of PON's antioxidant function remain unclear [
7,
8].Some studies show that decreased PON1 activity increases the risk of developing atherosclerosis and can be considered as an additional strong risk factor for CHD [
9,
10].The results of other studies show, that a decrease in the activity of PON1-paraoxonase and arylesterase is associated with the severity of coronary artery lesions in patients with coronary heart disease [
11]. High PON1 activity reduces recurrence of CHD symptoms and improves prognosis after coronary artery bypass grafting [
12]. It has been found that the activity of PON1 is affected by genetic polymorphism, and research has been conducted to find out which polymorphic form of PON1 can predict CHD. It is reported that the Q-allo-enzyme of the coding region has a higher ability to hydrolyze peroxide lipids and protects LDL particles from peroxidation processes more effectively than the R-allo-enzyme. Several studies have been conducted to prove whether people with the PON1 192R isoenzyme are more susceptible to coronary artery disease, than people with the form PON1 192Q. Some experiments confirmed the supposed connection [
13,
14], while others received opposite conclusions [
15,
16].
Some authors reported a link between the appearance of the 55L allele and atherosclerosis [
13,
17], while other researchers denied this relationship [
18,
19]. In a meta-analysis concerning the Association between PON2 311 C/S polymorphism and CHD, the authors found a link between the estimated polymorphism and the prevalence of CHD in Caucasians, but not in Asian and Hispanic populations [
20]. We published the results of a meta-analysis on potential links between the polymorphism of the PON1 gene at positions -108, 55, 192 and the PON2 gene at position 311 of the coding region and the risk of CHD. Only a weak association was found between the appearance of the PON1 192R PON1 allele and CHD [
21]. In the prospective Heartwick Park Heart II study, no correlation was reported between the studied polymorphisms [PON1 55L / M, 192Q / R, PON2 311C / S, PON3 99A / A] and the occurrence of a documented acute cardiovascular incident. However, it was noted that individuals with the PON1 55LM or 55MM genotype and PON2 311CC were 3.5 times more susceptible to cardiovascular disease than people with any other combination of haplotypes [
22]. In a large case-control study that assessed the possible effect of PON1 status on CHD, no Association was found between C-108T and G-909C promoter polymorphism and the presence of CHD. However, the authors noted significantly lower activity and concentration of PON1 in patients with CHD compared to a healthy population, regardless of their genotype [
23]. Many environmental factors affect the activity of Paraoxonase, as well as the decrease in the concentration of the enzyme and activity observed independently of the genotype in disorders that accelerate the development of atherosclerosis, such as diabetes, hypercholesterolemia or kidney failure. Therefore, it was suggested that a joint assessment of the activity of the genotype and the enzyme in serum can be considered as a potential indicator of CHD [
24,
25].
The purpose of this study was to assess the relationship between genetic polymorphism PON1, enzyme activity, and other established risk factors for CHD, such as hypertension, elevated total cholesterol and LDL, low HDL concentration, Smoking, family history of premature CHD, and age in patients with confirmed atherosclerosis.
2. Materials and Methods
This study is a clinical and genetic study, study design: case-control. A total of 257 people were examined, including 149 men and 108 women. The subjects were the indigenous population of Kazakh nationality of both sexes aged 45-80 years, living in the region of Karaganda, hospitalized in city-level clinics. The study was performed in the Laboratory of collective use of «Karaganda Medical University» non-commercial joint-stock company in Karaganda. The genetic research was conducted at the Laboratory of genomic and personalized medicine, National Laboratory Astana, Nazarbayev University, Astana, Kazakhstan. Clinical examination of patients was carried out according to the generally accepted method with filling in primary documentation on carefully collected anamnesis, objective examination of patients and laboratory and instrumental examination of patients.
All 257 patients of the main and control groups after PCI, as well as with practically healthy individuals, were genotyped using the allelic discrimination of the variant PON1-rs854560 [L55M] by polymerase chain reaction, TaqMan Array. As a biological material, Whole blood obtained from the ulnar vein of the subjects. DNA extraction from whole blood was performed using Gene JET Genomic DNA Purification Kit [Thermo Scientific]. Sample preparation was performed using a ready-made mixture for real-time PCR TaqMan ® OpenArray ® Genotyping Master Mix [Applied Biosystems]. The gene polymorphism was determined using the QuantStudio TM 12K Flex Real-Time PCR [Applied Biosystems] system on TaqMan ® OpenArray ® Genotyping Plate, Custom Format 64 QuantStudio TM 12K Flex [Applied Biosystems] tablets. The tablets were filled with a reaction mixture using an automated QuantStudio TM 12K Flex Accufill System [Applied Biosystems]. Data analysis was performed using the TaqMan Genotyper Software V. 1. 3 software package.
Statistical processing of the obtained data was performed using the SPSS software package.20. To check the Hardy-Weinberg equilibrium Chi-square test was used. To analyze the frequency distribution of alleles and genotypes for each polymorphism separately and combined genotypes, we used the Chi-square test, the exact Fisher criterion, and the relative risk method [RR] with the 95% confidence interval [CI].
3. Results
During the study, all patients [n=257] were divided into 4 groups: The Gender characteristics of the groups are presented in
Table 1.
The average age of the subjects was 63.08 ± 8.86 [minimum 45 years, maximum 80 years]. The distribution of patients in groups corresponded to the normal distribution of Shapiro-Wilk. The number of men surveyed was higher than that of women [57.9% and 42%, respectively].
Table 2.
Clinical signs of the study participants.
Table 2.
Clinical signs of the study participants.
| Parameters |
Av |
Me |
Mode |
SD |
Variance |
Percentiles |
| Q1 |
Q2 |
Q3 |
| Age |
62,0 |
61,0 |
63 |
10.1 |
103,3 |
55 |
61 |
69 |
| Weight |
77.3 |
76,0 |
80 |
14,4 |
208,8 |
66,5 |
76 |
85 |
| Height |
165,8 |
165,0 |
165 |
8,016 |
64,3 |
160 |
165 |
172 |
| Abdominal circumference |
96,6 |
94 |
88 |
17,3 |
299,7 |
84 |
94 |
108 |
| The index of the smoker |
8,7 |
,00 |
,00 |
16,0 |
257,9 |
,0 |
,0 |
15 |
| Systolic blood pressure |
133,6 |
130 |
120 |
22,6 |
512,3 |
120 |
130 |
140 |
| Diastolic blood pressure |
83,3 |
80 |
80 |
10,2 |
103,7 |
80 |
80 |
90 |
| Av- Average; Me – Median; SD - Standard deviation; Q1-25; Q2-50; Q3- 75 |
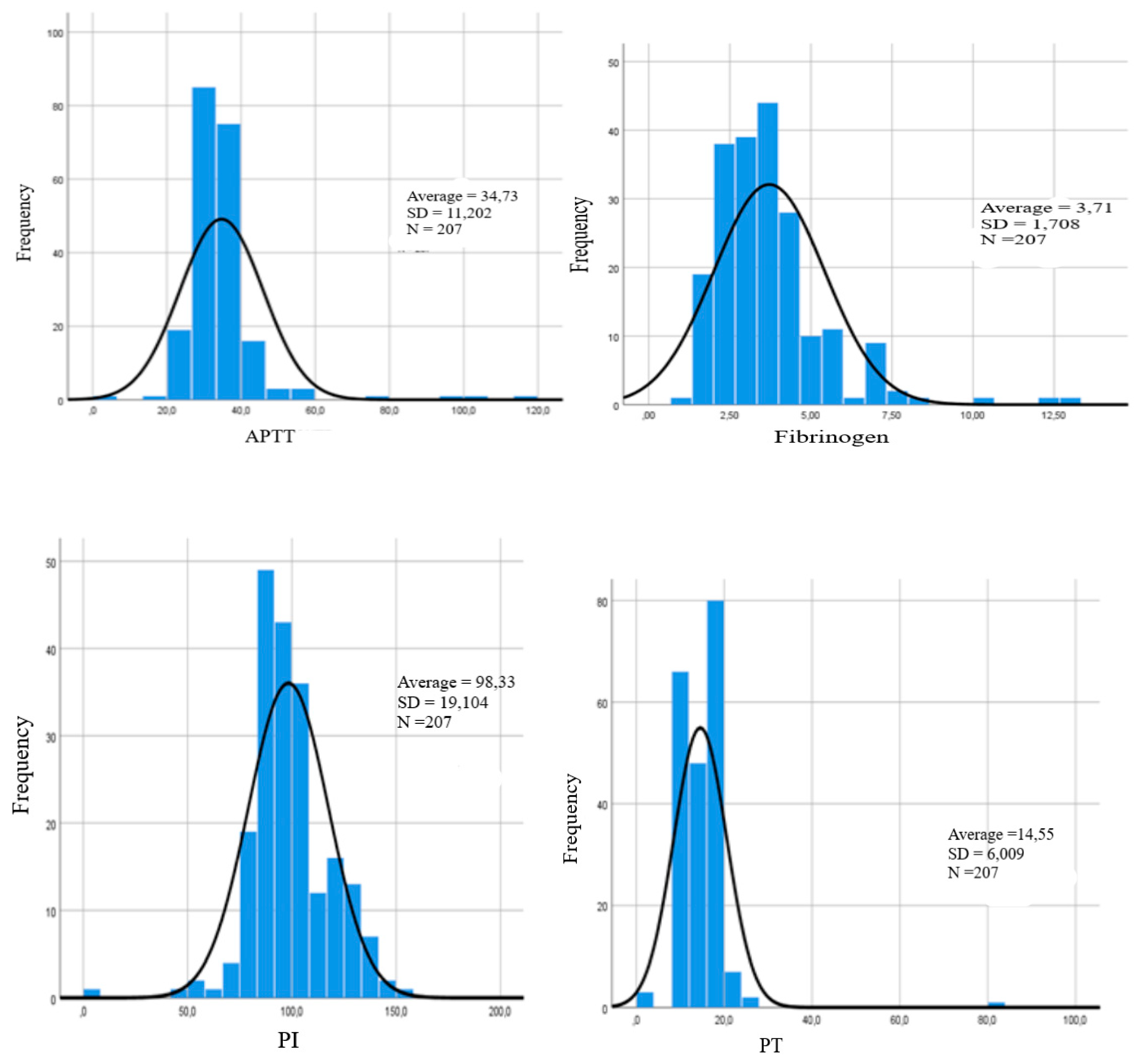
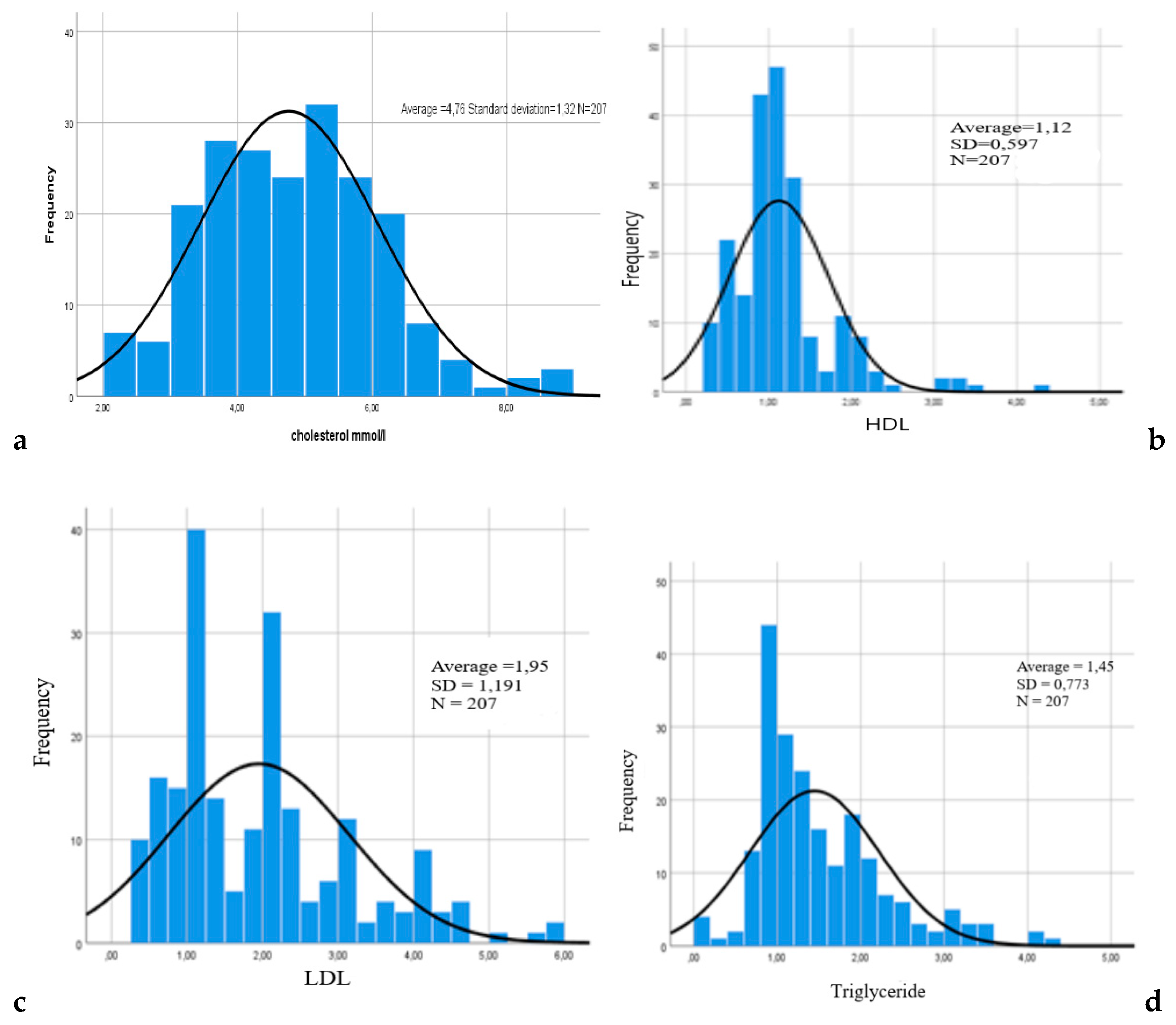
General clinical and laboratory characteristics of the subjects are presented in the form of an assessment of the lipid spectrum, coagulogram and peripheral hemogram [
Figure 1].
When evaluating lipid metabolism indicators among both men and women, the HDL content was within the limits of the physiological norm. At the same time, the level of HDL was statistically significantly lower compared to other indicators. Analysis of the values of other components of lipid metabolism did not reveal statistically significant differences.
Table 3 presents data on the comparative analysis of peripheral hemograms in the subjects. Based on the analysis of laboratory indicators, it should be noted that all the examined patients had average values within the limits of the physiological norm, but the hemoglobin and red blood cell indicators were shifted to the upper limit of the norm.
Figure 2.
Comparative analysis of coagulogram indicators
Figure 2.
Comparative analysis of coagulogram indicators
It should be noted that according to the results of this analysis, there is a tendency to a higher level of PTI and fibrinogen compared to other indicators of the coagulogram [APTT, PV, SFMC, INR]. However, there are no statistically significant differences in these parameters.
According to the results of the genetic study, the polymorphism of the PON1-rs854560 [L55M] a/a homozygous allele was detected in 54.5% of all the examined individuals. The frequency of occurrence of the PON1-rs854560 [L55M] gene polymorphism is shown in
Table 4.
It is known that the polymorphism of the PON1-rs854560 [L55M] A/A homozygous allele increases the risk of developing atherosclerosis, which allows us to consider it as an additional strong risk factor in the development of CHD [
9,
10].
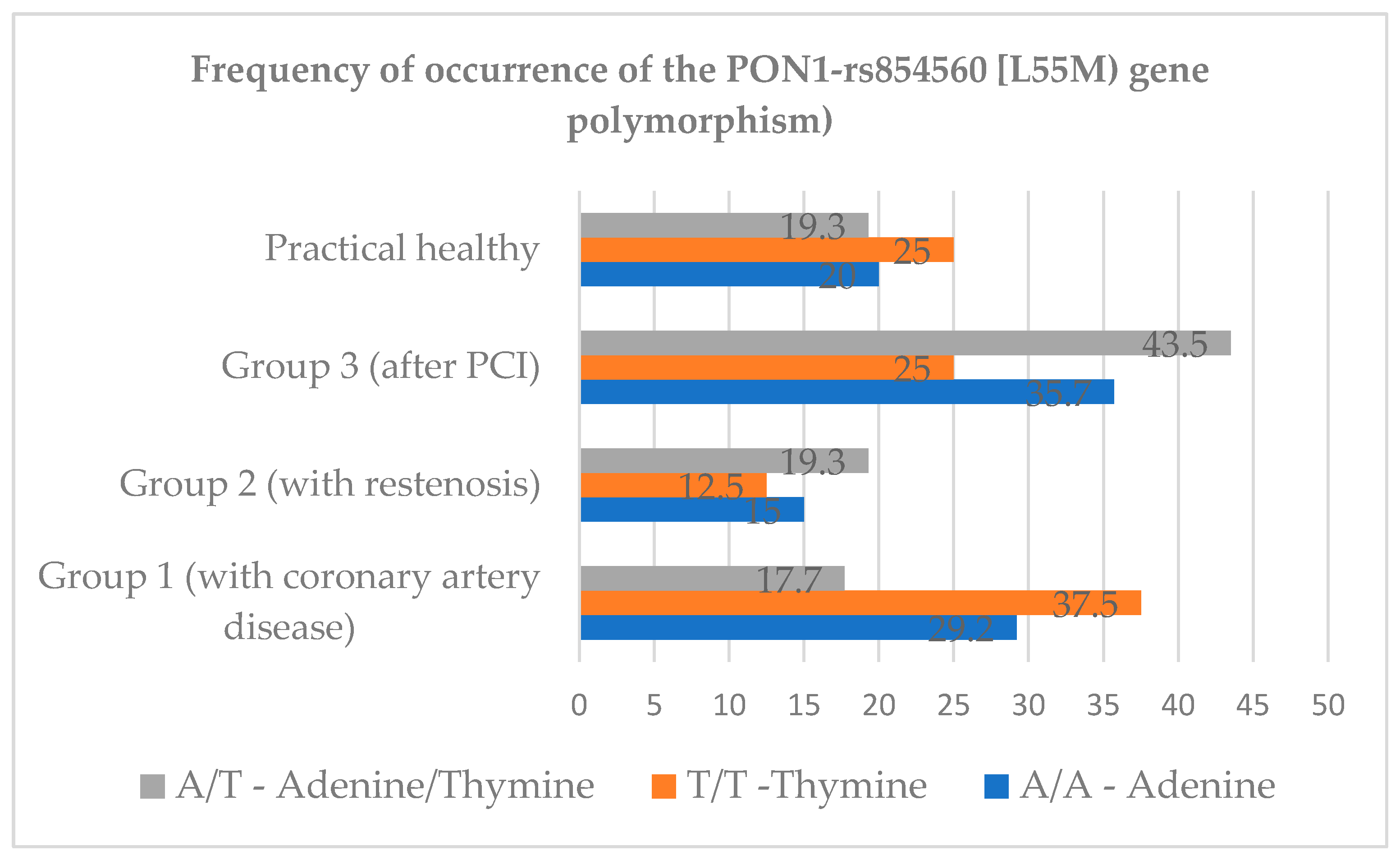
The distribution of genotypes of the PON1-rs854560 [L55M] gene polymorphism across the study groups is shown in
Figure 3.
According to the results of this analysis, there is a tendency to a higher level of homozygous genotype A/A in group III [group of patients with a history of PCI] compared to group II patients [with developed restenosis] and group I patients [with CHD without a history of PCI]. Evaluation of gender characteristics of genotypes of the PON1-rs854560 [L55M] gene polymorphism. shown in
Table 5.
Genotype A/A was detected in 57.1% of men and 42.9% of women. The ratio of men and women in A/A was 3: 2 [57.1% and 42.9%]. In the men studied, The A/T genotype was detected in 63% of cases, while in women it was significantly less [in 37% of women]. The distribution structure of the A/T genotype differed by gender, and men were more likely to have a heterozygous genotype than women.
4. Discussion
The results show that traditional risk factors for atherosclerosis and CHD, such as dyslipidemia, hypertension, smoking increased body mass index does not always make a significant contribution to the development of cardiovascular complications after PCI in patients with CHD, which is consistent with the data of a number of authors who conducted similar studies [
23]. Based on the evaluation of clinical and laboratory data, patients after PCI showed a tendency to higher levels of PTI and fibrinogen compared to other coagulogram indicators. According to the genotype study, the polymorphism of the PON1-rs854560 [L55M] a/a homozygous allele was detected in 54.5 % of the examined individuals.
The analysis showed a tendency to a more frequent level of homozygous genotype A/A in group III [group of patients with a history of PCI] in comparison with group II patients [ with developed coronary artery restenosis] and group I patients [with CHD without a history of PCI]. It was found that the ratio of men and women in A/A was approximately 3:2 [57.1% and 42.9%]. Of the 62 men surveyed, the A/T genotype was detected more often in men [63%] than in women [37%]. At the same time, men were more likely to have a heterozygous type of genotype compared to women.
5. Conclusions
Thus, the polymorphism of the PON1 gene, which encodes an enzyme of the body's antioxidant defense system, correlates with the development of CHD. At the same time, carriers of the homozygous genotype of the PON1-rs854560 [L55M] a/A gene polymorphism were more often found in men after coronary artery stenting and with the development of coronary artery restenosis. The obtained results make it necessary to conduct further research on the contribution of various risk factors in the development of CHD and possible mechanisms of sexual differences in the distribution of genotypes and alleles of the studied genes.
Author Contributions
Conceptualization, D.T. and R.B.; methodology, D.T., R.B., A.K. and A.A.; software, D.T., R.B., A.K. and A.A.; validation, D.T., R.B., A.K. and A.A.; formal analysis, D.T., R.B., A.K. and A.A; investigation, D.T., R.B., A.K. and A.A; resources, D.T., R.B., A.K. and A.A; data curation, D.T., R.B., A.K. and A.A; writing—original draft preparation, D.T., R.B., A.K. and A.A; writing—review and editing, D.T., R.B., A.K. and A.A; visualization, D.T., R.B., A.K. and A.A; supervision, D.T and A.A.; project administration, D.T. and A.A.; funding acquisition D.T., R.B., A.K. and A.A; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The scientific study was approved by the Ethics Committee of Karaganda Medical University NC JSC (Protocol No. 14 dated 14.04.2020).
Informed Consent Statement
Written informed consents were obtained from all study participants. All personal information had been encoded and anonymized.
Data Availability Statement
Data supporting the reported results are not publicly available due to privacy and ethical restrictions but can be made available by the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CHD |
Coronary heart disease |
| LDL |
low density lipoprotein |
| HDL |
High density lipoprotein |
| PON |
paraoxonase |
| GPX |
glutathione peroxidase |
| PAF-AH |
platelet-activating factor acetylhydrolase |
| PCR |
Polymerase chain reaction |
| PCI |
percutaneous intervention |
| DNA |
deoxyribonucleic acid |
| PTI |
prothrombin index |
References
- Piepoli, M.F.; Hoes, A.W.; et al. 2016 European Guidelines on cardiovascular disease in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and other Societies on Cardiovascular Disease Prevention in Clinical Practic [constituted by representatives of 10 societies and by invited experts]. Developed with the special contribution of the European Association for Cardiovascular Prevention& Rehabilitation [EACPR]. Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar]
- Li, Y.; Liang, G.; Shi, L.; Liang, X.; Long, B.; Qin, J.; Zhang, Z. Paraoxonase-1 [PON1] rs662 Polymorphism and Its Association with Serum Lipid Levels and Longevity in the Bama Zhuang Population. Med. Sci. Monit. 2016, 22, 5154–5162. [Google Scholar]
- Rye, K.A.; Barter, P.J. Cardioprotective functions of HDLs. J. Lipid Res. 2014, 55, 168–169. [Google Scholar] [CrossRef] [PubMed]
- Mussabekova, SA. Possibilities of semen stain identification after clothing and bedding washing in investigating cases of sexual assault. Periodico Tche Quimica. 2020, 17, 93–111. [Google Scholar] [CrossRef]
- Brites, F.; Martin, M.; Guillas, I.; Kontush, A. Antioxidative activity of high- density lipoprotein [HDL]: Mechanistic insights into potential clinical benefit. BBA Clin. 2017, 8, 66–77. [Google Scholar]
- Passaro, A.; Vigna, G.B.; Romani, A.; Sanz, J.M.; Cavvichio, C.; et al. Distribution of paraoxonase [PON-1] and lipoprotein Phospholipase A2[Lp-PLA2] across lipoprotein subclasses in Subjects with type 2 diabetes. Oxid. Med. Cell. Longev. 2018.
- Nurpisova T., T.; Taizhanova, D.Zh.; Abildinova G., Zh. Diagnostic and prognostic biomarkers in idiopathic pulmonary arterial hypertension. Medicine and Ecology 2024, 113, 68–77. [Google Scholar] [CrossRef]
- Draganov, D.J.; Teiber, J.F.; Speelman, A.; Osawa, Y.; Sunhara, R.; La Du, B.N. Human paraoxonases [PON1, PON2, and PON3] are lactonases with overlapping and distinct substrate specifies. J. Lip. Res. 2005, 46, 1239–1247. [Google Scholar] [CrossRef]
- Yildiz, A.; Gur, M.; Yilmaz, R.; Demirbag, R.; Polat, M.; Selek, S. Association of paraoxonase activity and coronary blood flow. J. Atherosclerosis. 2008, 197, 257–263. [Google Scholar] [CrossRef]
- Tang, W.H.; Hartiala, J.; Fan, Y.; Wu, Y.; Stewart, A.F.; Erdmann, J. Clinical and genetic association of serum paraoxonase and arylesterase activities with cardiovascular risk. J. Arterioscler. ThrombVasc. Biol. 2012, 32, 2803–2812. [Google Scholar] [CrossRef]
- Ding, J.; Chen, Q.; Zhuang, X.; Feng, Z.; Xu, L.; Chen, F. Low Paraoxonase 1 Arylesterase Activity and High von Willebrand Factor Levels are Associated with Severe Coronary Atherosclerosis in Patients with Non-Diabetic Stable Coronary Artery Disease. Med. SciMonit. 2014, 20, 2421–2429. [Google Scholar]
- Wysocka, A.; Cybulski, M.; Berbeć, H.; Wysokiński, A.; Stążka, J.; Zapolski, T. Prognostic value of paraoxonase 1 in patients undergoing coronary artery bypass grafting surgery. Med. Sci. Monit. 2014, 20, 594–600. [Google Scholar] [PubMed]
- Rivera-Mancia, S.; Jimenez-Osorio, A.S.; Medina-Campos, O.N.; Colin-Ramirez, E.; et al. Acitivity of antioxidant enzymes and their association with lipid profile in Mexican people without cardiovascular disease: An analysis of interactions. Int. J. Environ. Res. Public Health. 2018, 15, 2687. [Google Scholar] [PubMed]
- Sanghera, D.K.; Saha, N.; Aston, C.E.; Kamboh, M.I. Genetic polymorphism of paraoxonase and the risk of coronary heart disease. J. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 1067–1073. [Google Scholar] [CrossRef]
- Antikainen, M.; Murtomki, S.; Syvnne, M.; Pahlman, R.; Tahvanainen, E.; Jauhiainen, M.; et al. The Gln-Arg polymorphism of human paraoxonase gene [HUMPONA] is not associated with the risk of coronary artery disease in Finns. J. Clin. Investig. 1996, 191, 883–885. [Google Scholar] [CrossRef]
- Ombres, D.; Pannitteri, G.; Moutali, A.; Candeloro, A.; Seccareccia, F.; Campagna, F.; et al. The Gln-Arg 192 polymorphism of the human paraoxonase gene is not associated with coronary artery disease in Italian patients. J. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 1611–1616. [Google Scholar]
- Mussabekova, S.A.; Mkhitaryan, X.E.; Abdikadirova, K.R. Domestic violence in Kazakhstan: Forensic-medical and medical-social aspects. Forensic Science International: Reports. 2024, 9, 100356. [Google Scholar] [CrossRef]
- Watzinger, N.; Schmidt, H.; Schumacher, M.; Schmidt, R.; Eber, B.; Fruhwald, F.M.; Zweiker, R.; Kostner, G.M.; Klein, W. Human paraoxonase 1 gene polymorphisms and the risk of coronary heart disease: A community-based study. J. Cardiology. 2002, 98, 116–122. [Google Scholar] [CrossRef]
- Sanghera, D.K.; Saha, N.; Kamboh, M.I. The codon 55 polymorphism of the paraoxonase 1 gene is not associated with risk of coronary heart disease in Asian Indians and Chinese. J. Atherosclerosis. 1998, 136, 217–223. [Google Scholar]
- Chen, M.L.; Zhao, H.; Liao, N.; Xie, Z.F. Association between paraoxonase 2 Ser311 Cys polymorphism and Coronary Heart Disease Risk: A meta-analysis. J.Med. Sci. Monit. 2016, 22, 3196–3201. [Google Scholar] [CrossRef]
- Wheeler, J.G.; Keavney, B.D.; Watkins, H.; Collins, R.; Danesh, J. Four paraoxonase gene polymorphisms in 11212 cases of coronary heart disease and 12786 controls: Meta-analysis of 43 studies. J. Lancet. 2004, 363, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Robertson, K.S.; Hawe, E.; Miller, G.J.; Talmud, P.J.; Humphries, S.E. Human paraoxonase gene cluster polymorphism as a predictors of coronary heart disease risk in the prospective Northwick Park Heart Study, I.I. Biochim. Biophys. [BBA]/Mol. Basis Dis. 2003, 1639, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Mackness, B.; Turkie, W.; Mackness, M. Paraoxonase-1 [PON1] promoter region polymorphisms serum PON1 status and coronary heart disease. J. Arch. Med. Sci. 2013, 9, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Mackness, B.; Davies, G.K.; Turkie, W.; Lee, E.; Roberts, D.H.; Hill, E.; Roberts, C.; et al. Paraoxonase status in coronary heart disease: Are activity and concentration more important than genotype? J. Arterioscler. ThrombVasc. Biol. 2001, 21, 1451–1457. [Google Scholar] [CrossRef]
- Taizhanova, D.; Kalimbetova, A.; Bodaubay, R.; et al. Genetic Predictors of the Development of Complications after Coronary Stenting. J. Pers. Med. 2023, 13, 14. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).