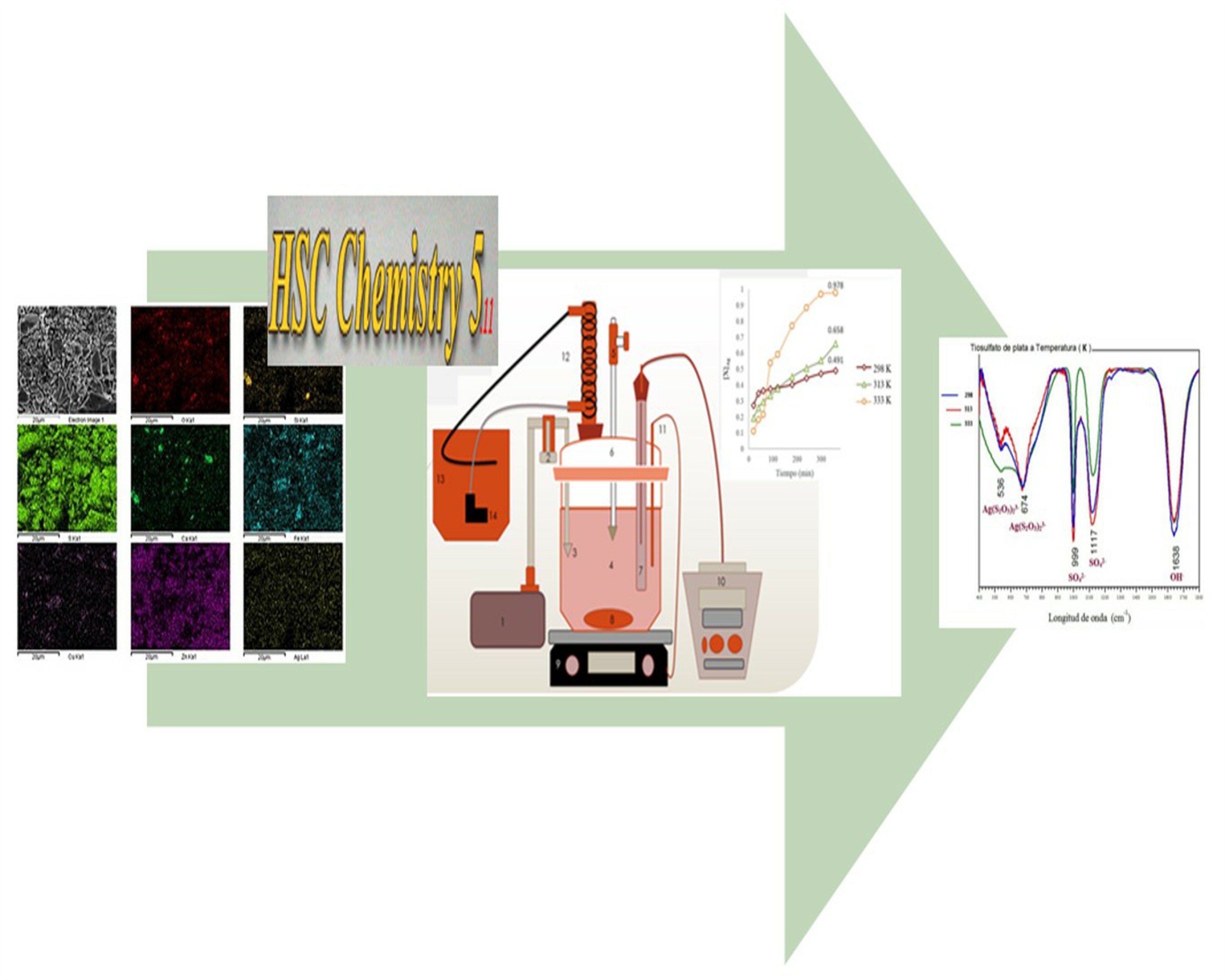
Metallic elements of higher economic value occurring in the mineralogy of Zimapán are Pb, Zn, Cu and Fe, said elements are sold as concentrates, which, even after processing, generally include significant concentrations of Mo, Cd, Sb, Ag and As that could recover through different leaching methods. In this work the influence of temperature was studied in the complexation of silver in the S2O32--O2 system. Chemical and mineralogical characterization of concentrated Zn from the state of Hidalgo confirmed the presence of the silver contained in a sulfide of silver arsenic (AgAsS2) through the techniques of Atomic Absorption Spectrophotometry (AAS), X-Ray Diffraction (XRD) and Scanning Electron Microscopy-Energy-Dispersive X-Ray Spectroscopy (SEM-EDS). The mineralogical species identified allowed the construction mineralogical species Pourbaix diagrams in the range of 298 K to 333 K, through which the Eh-pH conditions to obtain silver in solution were determined. The formation of Ag(S2O3)23- complex was confirmed by characterizing liquors leached using the technique Infrared Spectroscopy Fourier Transform (FTIR).